Template protocol for Investigational Medicinal product(IMP)
Template protocol for Investigational Medicinal product(IMP)
Template protocol for Investigational Medicinal product(IMP)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
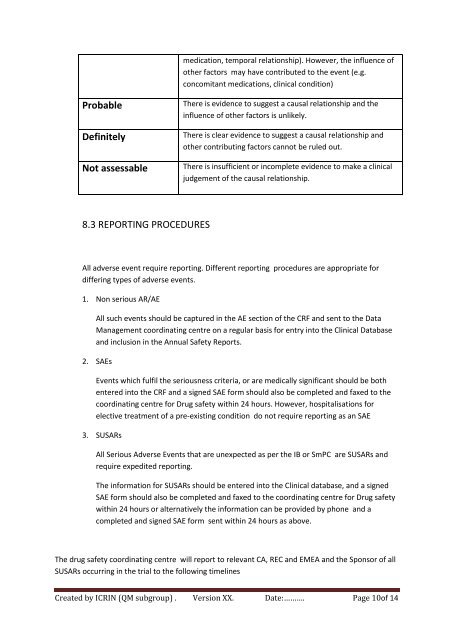
medication, temporal relationship). However, the influence ofother factors may have contributed to the event (e.g.concomitant medications, clinical condition)ProbableDefinitelyNot assessableThere is evidence to suggest a causal relationship and theinfluence of other factors is unlikely.There is clear evidence to suggest a causal relationship andother contributing factors cannot be ruled out.There is insufficient or incomplete evidence to make a clinicaljudgement of the causal relationship.8.3 REPORTING PROCEDURESAll adverse event require reporting. Different reporting procedures are appropriate <strong>for</strong>differing types of adverse events.1. Non serious AR/AEAll such events should be captured in the AE section of the CRF and sent to the DataManagement coordinating centre on a regular basis <strong>for</strong> entry into the Clinical Databaseand inclusion in the Annual Safety Reports.2. SAEsEvents which fulfil the seriousness criteria, or are medically significant should be bothentered into the CRF and a signed SAE <strong>for</strong>m should also be completed and faxed to thecoordinating centre <strong>for</strong> Drug safety within 24 hours. However, hospitalisations <strong>for</strong>elective treatment of a pre-existing condition do not require reporting as an SAE3. SUSARsAll Serious Adverse Events that are unexpected as per the IB or SmPC are SUSARs andrequire expedited reporting.The in<strong>for</strong>mation <strong>for</strong> SUSARs should be entered into the Clinical database, and a signedSAE <strong>for</strong>m should also be completed and faxed to the coordinating centre <strong>for</strong> Drug safetywithin 24 hours or alternatively the in<strong>for</strong>mation can be provided by phone and acompleted and signed SAE <strong>for</strong>m sent within 24 hours as above.The drug safety coordinating centre will report to relevant CA, REC and EMEA and the Sponsor of allSUSARs occurring in the trial to the following timelinesCreated by ICRIN (QM subgroup) . Version XX. Date:………. Page 10of 14