1. Directory of Clinical Research Centres/Facilities in Ireland
1. Directory of Clinical Research Centres/Facilities in Ireland
1. Directory of Clinical Research Centres/Facilities in Ireland
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
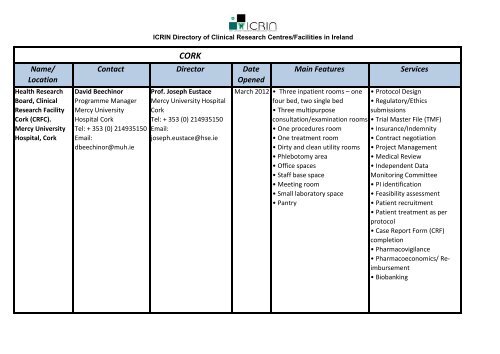
Name/LocationNational Children's<strong>Research</strong> Centre.Our Lady'sHospital, Cruml<strong>in</strong>,Dubl<strong>in</strong> L<strong>in</strong>k toWebsiteICRIN <strong>Directory</strong> <strong>of</strong> <strong>Cl<strong>in</strong>ical</strong> <strong>Research</strong> <strong>Centres</strong>/<strong>Facilities</strong> <strong>in</strong> <strong>Ireland</strong>DUBLINContact Director DateOpenedDr. S<strong>in</strong>éad Nic An Fhailí(Nally)<strong>Cl<strong>in</strong>ical</strong> <strong>Research</strong> ProjectManagerNational Children’s<strong>Research</strong> CentreOur Lady’s Children'sHospital, Cruml<strong>in</strong>Dubl<strong>in</strong>Tel: +353 1 409 6806Email:s<strong>in</strong>ead.nally@ncrc.iePr<strong>of</strong>. Carlos BlancoDirector <strong>of</strong> <strong>Research</strong>National Children’s<strong>Research</strong> CentreOur Lady’s Children'sHospital, Cruml<strong>in</strong>Dubl<strong>in</strong>Tel: +353 1 4096585Email:carlos.blanco@ncrc.ieDr. Jac<strong>in</strong>ta KellyDeputy Director <strong>of</strong><strong>Research</strong>Tel: +353 1 4096583Email:jac<strong>in</strong>ta.kelly@ncrc.ieDr. Colm O'Donnell<strong>Cl<strong>in</strong>ical</strong> DirectorTel: + 353 1 6373186codonnell@nmh.ieMa<strong>in</strong> Features1965 • Two-bed cl<strong>in</strong>ical assessmentroom (e.g. for use <strong>in</strong> medic<strong>in</strong>esfor children studies)• Patient Proximate SamplePreparation Room• <strong>Research</strong> Laboratory (tissueculture suite, PCR room,microbiology room, confocalmicroscopy suite, flowcytometry etc)• Access to support staff (cl<strong>in</strong>icalresearch management, datamanagement, researchnurses/assistants, cl<strong>in</strong>ical trialspharmacist, medical sciencelaboratory personnel)Office and Read<strong>in</strong>g Space for<strong>Cl<strong>in</strong>ical</strong> and Translational<strong>Research</strong>ersServices• Paediatric <strong>Research</strong> Support• Protocol Design• CRF Design• Regulatory/EthicsSubmissions• Informed Consent /AssentAdvice• Trial Master File (TMF)Ma<strong>in</strong>tenance• Project ManagementSupport• Data Management Support• Pharmacy Support• Statistical Support• Medical Review• Independent DataMonitor<strong>in</strong>g Committee• PI identification• Feasibility assessment• Patient recruitment• Pharmacovigilance• Biobank<strong>in</strong>g• Case Cohort - <strong>Cl<strong>in</strong>ical</strong>Investigations - <strong>Cl<strong>in</strong>ical</strong> Trials
ICRIN <strong>Directory</strong> <strong>of</strong> <strong>Cl<strong>in</strong>ical</strong> <strong>Research</strong> <strong>Centres</strong>/<strong>Facilities</strong> <strong>in</strong> <strong>Ireland</strong>Name/LocationRoyal College <strong>of</strong>Surgeons <strong>Ireland</strong>(RCSI) <strong>Cl<strong>in</strong>ical</strong><strong>Research</strong> Centre.BeaumontHospital, Dubl<strong>in</strong>L<strong>in</strong>k to WebsiteDUBLINContact Director DateOpenedDeirdre HylandDirector <strong>of</strong> Nurs<strong>in</strong>g<strong>Cl<strong>in</strong>ical</strong> <strong>Research</strong> Centre,Smurfit Build<strong>in</strong>g,Beaumont HospitalDubl<strong>in</strong> 9Telephone: +353 (1)8093785Email: dhyland@rcsi.iePr<strong>of</strong>. Dermot Kenny<strong>Cl<strong>in</strong>ical</strong> <strong>Research</strong> Centre,Smurfit Build<strong>in</strong>g,Beaumont HospitalDubl<strong>in</strong> 9Telephone: +353 (1)8093780Email: dkenny@rcsi.ieMa<strong>in</strong> Features2001 • Four consultation/cl<strong>in</strong>icalassessment rooms• Four Bed Inpatient Unit• Two Inpatient rooms• Sample Process<strong>in</strong>g Laboratory• Biobank<strong>in</strong>g Facility• Gene Therapy UnitServices• Phase I volunteer studies• Protocol Design• Regulatory/Ethicssubmissions• Trial Master File (TMF)• Insurance/Indemnity• Contract negotiation• Project Management• Medical Review• Independent DataMonitor<strong>in</strong>g Committee• PI identification• Feasibility assessment• Patient recruitment• Patient treatment as perprotocol• Nurse <strong>Research</strong> Education• Case Report Form (CRF)completion• Pharmacovigilance• Pharmacoeconomics/ Reimbursement• Biobank<strong>in</strong>g
ICRIN <strong>Directory</strong> <strong>of</strong> <strong>Cl<strong>in</strong>ical</strong> <strong>Research</strong> <strong>Centres</strong>/<strong>Facilities</strong> <strong>in</strong> <strong>Ireland</strong>Name/LocationWellcome Trust -HRB <strong>Cl<strong>in</strong>ical</strong><strong>Research</strong> Facility,Tr<strong>in</strong>ity CollegeDubl<strong>in</strong>St James'sHospital, Dubl<strong>in</strong>DUBLINContact Director DateOpenedJeremy TownsProgramme ManagerTr<strong>in</strong>ity Centre for HealthSciencesSt James's HospitalDubl<strong>in</strong> 8Tel: +353 (1) 8964537Email: townsj@tcd.iePr<strong>of</strong>. Michael Gill <strong>Cl<strong>in</strong>ical</strong>DirectorWellcome Trust/HRB CRFat St James's HospitalTr<strong>in</strong>ity Centre for HealthSciencesSt James's HospitalDubl<strong>in</strong> 8Tel: +353 (1) 896 2241Fax: +353 (1) 896 3405Email: mgill@tcd.iePr<strong>of</strong>. Colm Berg<strong>in</strong>Associate Director<strong>Cl<strong>in</strong>ical</strong> Pr<strong>of</strong>essor <strong>of</strong>Medic<strong>in</strong>e, St James'sHospitalDubl<strong>in</strong> 8Tel: +353 (1) 4162507Email: cberg<strong>in</strong> @stjames.ieMa<strong>in</strong> Features2013 • Four <strong>in</strong>patient rooms• Six-Bed Day Unit• Four Multipurposeconsultation/exam<strong>in</strong>ation rooms• Neurophysiology Suite• <strong>Research</strong> Pharmacy/ AsepticClean room for Gene Therapypreparation• Sample process<strong>in</strong>g laboratory• Office Space• Bioeng<strong>in</strong>eer<strong>in</strong>g Workshop• Sem<strong>in</strong>ar and Meet<strong>in</strong>g rooms• Staff chang<strong>in</strong>g rooms• Adjacent to 3T research MRI(CAMI)Services• Protocol Design• Regulatory/Ethicssubmissions• Trial Master File (TMF)• Insurance/Indemnity• Contract negotiation• Project Management• Medical Review• Independent DataMonitor<strong>in</strong>g Committee• PI identification• Feasibility assessment• Patient recruitment• Patient treatment as perprotocol• Case Report Form (CRF)completion• Pharmacovigilance• Pharmacoeconomics/ Reimbursement• Biobank<strong>in</strong>g• Medical Imag<strong>in</strong>g
Name/LocationUniversity CollegeDubl<strong>in</strong>, <strong>Cl<strong>in</strong>ical</strong><strong>Research</strong> Centre.(1) MaterMisericordiaeHospital Dubl<strong>in</strong>,(2) St V<strong>in</strong>cent'sHospital, Dubl<strong>in</strong>L<strong>in</strong>k to WebsiteICRIN <strong>Directory</strong> <strong>of</strong> <strong>Cl<strong>in</strong>ical</strong> <strong>Research</strong> <strong>Centres</strong>/<strong>Facilities</strong> <strong>in</strong> <strong>Ireland</strong>DUBLINScientific Director Director DateOpenedDr Peter DoranDirector UCD CRCsUCD CRC MaterMisericordiae UniversityHospital21 Nelson StreetDubl<strong>in</strong> 7UCD CRCSt V<strong>in</strong>cent's UniversityHospitalElm ParkDubl<strong>in</strong> 4Tel: +353 1 7164587Email:peter.doran@ucd.iePr<strong>of</strong>. Patrick MurraySchool <strong>of</strong> Medic<strong>in</strong>e &Medical ScienceMater HospitalEccles StreetDubl<strong>in</strong> 7Tel: +353 (1) 7166319Email:patrick.murray@ucd.iePr<strong>of</strong>. Michael KeaneSt V<strong>in</strong>cent's HospitalElm ParkDubl<strong>in</strong> 4Tel: +353 (1) 2214574Email:michael.p.keane@ucd.ieMa<strong>in</strong> Features2006 • Four consultation/cl<strong>in</strong>icalassessment rooms• Two procedure rooms• IMP storage facilities• Sample process<strong>in</strong>g laboratory• Cell culture suite• General laboratory• Biobank<strong>in</strong>g facility• Dexa scanner• Data Centre2007 • Four consultation/cl<strong>in</strong>icalassessment rooms• Two Procedure Rooms• IMP storage facilities• Sample process<strong>in</strong>g laboratory• Biobank<strong>in</strong>g facility• Biomarker Core Lab• Endoscopy Suite• Recovery room• Remote Monitor<strong>in</strong>g roomServices• Protocol Design• Regulatory/Ethicssubmissions• Trial Master File (TMF)• Insurance/Indemnity• Contract negotiation• Project Management• Medical Review• Independent DataMonitor<strong>in</strong>g Committee• PI identification• Feasibility assessment• Patient recruitment• Patient treatment as perprotocol• Case Report Form (CRF)completion• Pharmacovigilance• Pharmacoeconomics/ Reimbursement• Biobank<strong>in</strong>g
ICRIN <strong>Directory</strong> <strong>of</strong> <strong>Cl<strong>in</strong>ical</strong> <strong>Research</strong> <strong>Centres</strong>/<strong>Facilities</strong> <strong>in</strong> <strong>Ireland</strong>Name/LocationHealth <strong>Research</strong>Board, <strong>Cl<strong>in</strong>ical</strong><strong>Research</strong> FacilityGalway (CRFG),University HospitalGalway L<strong>in</strong>k toWebsiteGALWAYContact Director DateOpenedLisa DalyProgramme ManagerGeata an Eolais,University Road, Galway.Telephone +353 91495891Email:lisa.daly@nuigalway.iePr<strong>of</strong>. Frank GilesDirectorGeata an Eolais, UniversityRoad, Galway.Telephone +353 91 495970Email:frank.giles@nuigalway.ie2008(Currentlysupport<strong>in</strong>gcl<strong>in</strong>icalresearchand cl<strong>in</strong>icaltrials)Ma<strong>in</strong> FeaturesNew build<strong>in</strong>g completionscheduled 2013 with follow<strong>in</strong>gfeatures:• 4 <strong>in</strong>patient rooms: 2 2-bed, 2s<strong>in</strong>gle bed• Day ward with 5 beds• Recovery area with 3 beds• <strong>Research</strong> Pharmacy• Endoscopy Suite• Endocr<strong>in</strong>e Suite• Non-Invasive cardiology Suite• Pulmonary function test<strong>in</strong>g Suite• M<strong>in</strong>or Procedures room• Phlebotomy room• Cell/gene therapy preparationroom• 7 consultation/exam rooms• Dietetics Kitchen• Specimen sort<strong>in</strong>g area• Laboratory/ biobank<strong>in</strong>g facilities• Cell culture laboratory• <strong>Cl<strong>in</strong>ical</strong> research laboratory• Biometrics Unit• Office and sem<strong>in</strong>ar spaceServices• Protocol Design• Regulatory/Ethics submissions• Trial Master File (TMF)• Insurance/Indemnity• Contract negotiation• Project Management• Medical Review• Independent Data Monitor<strong>in</strong>gCommittee• PI identification• Feasibility assessment• Patient recruitment• Patient treatment as perprotocol• Case Report Form (CRF)completion• <strong>Research</strong> Pharmacist• Pharmacovigilance• Pharmacoeconomics/ Reimbursement• Biobank<strong>in</strong>g• Data Management• eCRF design• Statistics