ethylene Glycol MEGlobal_MEG.pdf
ethylene Glycol MEGlobal_MEG.pdf
ethylene Glycol MEGlobal_MEG.pdf
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
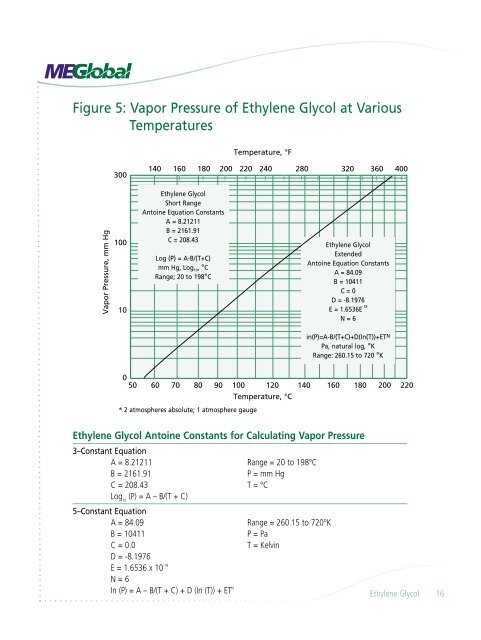
Figure 5: Vapor Pressure of Ethylene <strong>Glycol</strong> at VariousTemperaturesEthylene <strong>Glycol</strong> Antoine Constants for Calculating Vapor Pressure3–Constant EquationA = 8.21211 Range = 20 to 198°CB = 2161.91P = mm HgC = 208.43 T = °CLog 10(P) = A – B/(T + C)5–Constant EquationA = 84.09 Range = 260.15 to 720°KB = 10411P = PaC = 0.0T = KelvinD = -8.1976E = 1.6536 x 10 -18N = 6In (P) = A – B/(T + C) + D (In (T)) + ET NEthylene <strong>Glycol</strong> 16



![ratings & DEFINITIONS452-467_Technical Information[1].pdf](https://img.yumpu.com/49871719/1/190x245/ratings-definitions452-467-technical-information1pdf.jpg?quality=85)











