Proton Nuclear Magnetic Resonance Spectroscopy
Proton Nuclear Magnetic Resonance Spectroscopy
Proton Nuclear Magnetic Resonance Spectroscopy
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
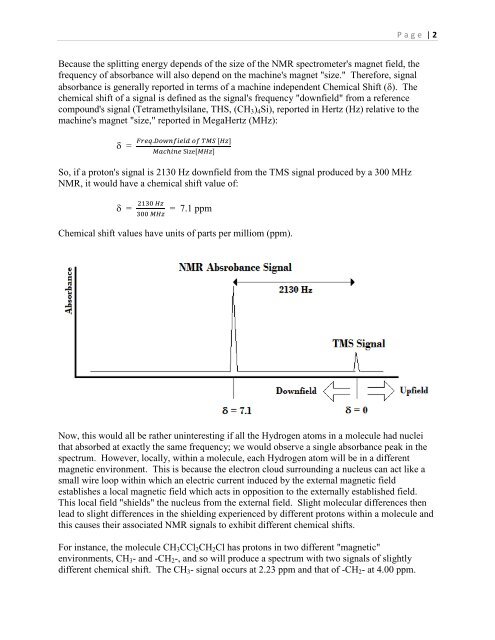
P a g e | 2Because the splitting energy depends of the size of the NMR spectrometer's magnet field, thefrequency of absorbance will also depend on the machine's magnet "size." Therefore, signalabsorbance is generally reported in terms of a machine independent Chemical Shift (). Thechemical shift of a signal is defined as the signal's frequency "downfield" from a referencecompound's signal (Tetramethylsilane, THS, (CH 3 ) 4 Si), reported in Hertz (Hz) relative to themachine's magnet "size," reported in MegaHertz (MHz): =So, if a proton's signal is 2130 Hz downfield from the TMS signal produced by a 300 MHzNMR, it would have a chemical shift value of: == 7.1 ppmChemical shift values have units of parts per milliom (ppm).Now, this would all be rather uninteresting if all the Hydrogen atoms in a molecule had nucleithat absorbed at exactly the same frequency; we would observe a single absorbance peak in thespectrum. However, locally, within a molecule, each Hydrogen atom will be in a differentmagnetic environment. This is because the electron cloud surrounding a nucleus can act like asmall wire loop within which an electric current induced by the external magnetic fieldestablishes a local magnetic field which acts in opposition to the externally established field.This local field "shields" the nucleus from the external field. Slight molecular differences thenlead to slight differences in the shielding experienced by different protons within a molecule andthis causes their associated NMR signals to exhibit different chemical shifts.For instance, the molecule CH 3 CCl 2 CH 2 Cl has protons in two different "magnetic"environments, CH 3 - and -CH 2 -, and so will produce a spectrum with two signals of slightlydifferent chemical shift. The CH 3 - signal occurs at 2.23 ppm and that of -CH 2 - at 4.00 ppm.