Formation of atomic point contacts and molecular junctions with a ...
Formation of atomic point contacts and molecular junctions with a ...
Formation of atomic point contacts and molecular junctions with a ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
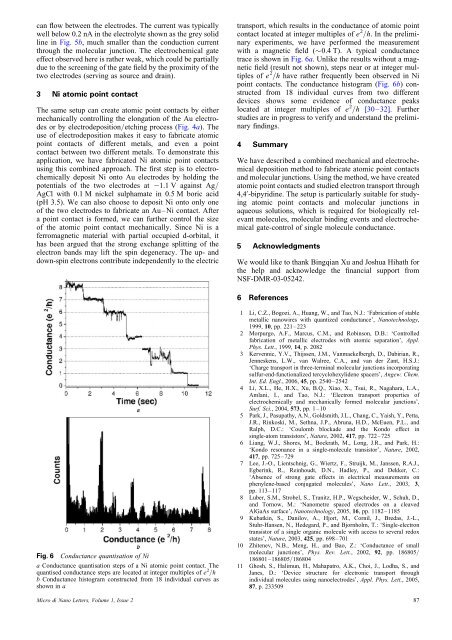
can flow between the electrodes. The current was typicallywell below 0.2 nA in the electrolyte shown as the grey solidline in Fig. 5b, much smaller than the conduction currentthrough the <strong>molecular</strong> junction. The electrochemical gateeffect observed here is rather weak, which could be partiallydue to the screening <strong>of</strong> the gate field by the proximity <strong>of</strong> thetwo electrodes (serving as source <strong>and</strong> drain).3 Ni <strong>atomic</strong> <strong>point</strong> contactThe same setup can create <strong>atomic</strong> <strong>point</strong> <strong>contacts</strong> by eithermechanically controlling the elongation <strong>of</strong> the Au electrodesor by electrodeposition/etching process (Fig. 4a). Theuse <strong>of</strong> electrodeposition makes it easy to fabricate <strong>atomic</strong><strong>point</strong> <strong>contacts</strong> <strong>of</strong> different metals, <strong>and</strong> even a <strong>point</strong>contact between two different metals. To demonstrate thisapplication, we have fabricated Ni <strong>atomic</strong> <strong>point</strong> <strong>contacts</strong>using this combined approach. The first step is to electrochemicallydeposit Ni onto Au electrodes by holding thepotentials <strong>of</strong> the two electrodes at 21.1 V against Ag/AgCl <strong>with</strong> 0.1 M nickel sulphamate in 0.5 M boric acid(pH 3.5). We can also choose to deposit Ni onto only one<strong>of</strong> the two electrodes to fabricate an Au–Ni contact. Aftera <strong>point</strong> contact is formed, we can further control the size<strong>of</strong> the <strong>atomic</strong> <strong>point</strong> contact mechanically. Since Ni is aferromagnetic material <strong>with</strong> partial occupied d-orbital, ithas been argued that the strong exchange splitting <strong>of</strong> theelectron b<strong>and</strong>s may lift the spin degeneracy. The up- <strong>and</strong>down-spin electrons contribute independently to the electrictransport, which results in the conductance <strong>of</strong> <strong>atomic</strong> <strong>point</strong>contact located at integer multiples <strong>of</strong> e 2 /h. In the preliminaryexperiments, we have performed the measurement<strong>with</strong> a magnetic field (0.4 T). A typical conductancetrace is shown in Fig. 6a. Unlike the results <strong>with</strong>out a magneticfield (result not shown), steps near or at integer multiples<strong>of</strong> e 2 /h have rather frequently been observed in Ni<strong>point</strong> <strong>contacts</strong>. The conductance histogram (Fig. 6b) constructedfrom 18 individual curves from two differentdevices shows some evidence <strong>of</strong> conductance peakslocated at integer multiples <strong>of</strong> e 2 /h [30–32]. Furtherstudies are in progress to verify <strong>and</strong> underst<strong>and</strong> the preliminaryfindings.4 SummaryWe have described a combined mechanical <strong>and</strong> electrochemicaldeposition method to fabricate <strong>atomic</strong> <strong>point</strong> <strong>contacts</strong><strong>and</strong> <strong>molecular</strong> <strong>junctions</strong>. Using the method, we have created<strong>atomic</strong> <strong>point</strong> <strong>contacts</strong> <strong>and</strong> studied electron transport through4,4 0 -bipyridine. The setup is particularly suitable for studying<strong>atomic</strong> <strong>point</strong> <strong>contacts</strong> <strong>and</strong> <strong>molecular</strong> <strong>junctions</strong> inaqueous solutions, which is required for biologically relevantmolecules, <strong>molecular</strong> binding events <strong>and</strong> electrochemicalgate-control <strong>of</strong> single molecule conductance.5 AcknowledgmentsWe would like to thank Bingqian Xu <strong>and</strong> Joshua Hihath forthe help <strong>and</strong> acknowledge the financial support fromNSF-DMR-03-05242.6 ReferencesFig. 6 Conductance quantisation <strong>of</strong> Nia Conductance quantisation steps <strong>of</strong> a Ni <strong>atomic</strong> <strong>point</strong> contact. Thequantised conductance steps are located at integer multiples <strong>of</strong> e 2 /hb Conductance histogram constructed from 18 individual curves asshown in a1 Li, C.Z., Bogozi, A., Huang, W., <strong>and</strong> Tao, N.J.: ‘Fabrication <strong>of</strong> stablemetallic nanowires <strong>with</strong> quantized conductance’, Nanotechnology,1999, 10, pp. 221–2232 Morpurgo, A.F., Marcus, C.M., <strong>and</strong> Robinson, D.B.: ‘Controlledfabrication <strong>of</strong> metallic electrodes <strong>with</strong> <strong>atomic</strong> separation’, Appl.Phys. Lett., 1999, 14, p. 20823 Kervennic, Y.V., Thijssen, J.M., Vanmaekelbergh, D., Dabirian, R.,Jenneskens, L.W., van Walree, C.A., <strong>and</strong> van der Zant, H.S.J.:‘Charge transport in three-terminal <strong>molecular</strong> <strong>junctions</strong> incorporatingsulfur-end-functionalized tercyclohexylidene spacers’, Angew. Chem.Int. Ed. Engl., 2006, 45, pp. 2540–25424 Li, X.L., He, H.X., Xu, B.Q., Xiao, X., Tsui, R., Nagahara, L.A.,Amlani, I., <strong>and</strong> Tao, N.J.: ‘Electron transport properties <strong>of</strong>electrochemically <strong>and</strong> mechanically formed <strong>molecular</strong> <strong>junctions</strong>’,Surf. Sci., 2004, 573, pp. 1–105 Park, J., Pasupathy, A.N., Goldsmith, J.L., Chang, C., Yaish, Y., Petta,J.R., Rinkoski, M., Sethna, J.P., Abruna, H.D., McEuen, P.L., <strong>and</strong>Ralph, D.C.: ‘Coulomb blockade <strong>and</strong> the Kondo effect insingle-atom transistors’, Nature, 2002, 417, pp. 722–7256 Liang, W.J., Shores, M., Bockrath, M., Long, J.R., <strong>and</strong> Park, H.:‘Kondo resonance in a single-molecule transistor’, Nature, 2002,417, pp. 725–7297 Lee, J.-O., Lientschnig, G., Wiertz, F., Struijk, M., Janssen, R.A.J.,Egberink, R., Reinhoudt, D.N., Hadley, P., <strong>and</strong> Dekker, C.:‘Absence <strong>of</strong> strong gate effects in electrical measurements onphenylene-based conjugated molecules’, Nano Lett., 2003, 3,pp. 113–1178 Luber, S.M., Strobel, S., Tranitz, H.P., Wegscheider, W., Schuh, D.,<strong>and</strong> Tornow, M.: ‘Nanometre spaced electrodes on a cleavedAlGaAs surface’, Nanotechnology, 2005, 16, pp. 1182–11859 Kubatkin, S., Danilov, A., Hjort, M., Cornil, J., Bredas, J.-L.,Stuhr-Hansen, N., Hedegard, P., <strong>and</strong> Bjornholm, T.: ‘Single-electrontransistor <strong>of</strong> a single organic molecule <strong>with</strong> access to several redoxstates’, Nature, 2003, 425, pp. 698–70110 Zhitenev, N.B., Meng, H., <strong>and</strong> Bao, Z.: ‘Conductance <strong>of</strong> small<strong>molecular</strong> <strong>junctions</strong>’, Phys. Rev. Lett., 2002, 92, pp. 186805/186801–186805/18680411 Ghosh, S., Halimun, H., Mahapatro, A.K., Choi, J., Lodha, S., <strong>and</strong>Janes, D.: ‘Device structure for electronic transport throughindividual molecules using nanoelectrodes’, Appl. Phys. Lett., 2005,87, p. 233509Micro & Nano Letters, Volume 1, Issue 2 87