Palladium-Catalyzed Cross-Coupling - A Historical Contextual Perspective to the 2010 Nobel Prize
Palladium-Catalyzed Cross-Coupling - A Historical Contextual Perspective to the 2010 Nobel Prize
Palladium-Catalyzed Cross-Coupling - A Historical Contextual Perspective to the 2010 Nobel Prize
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
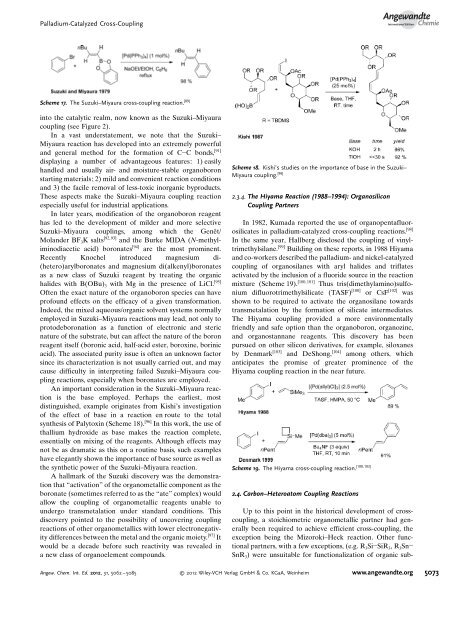
<strong>Palladium</strong>-<strong>Catalyzed</strong> <strong>Cross</strong>-<strong>Coupling</strong>AngewandteChemieScheme 17. The Suzuki–Miyaura cross-coupling reaction. [89]in<strong>to</strong> <strong>the</strong> catalytic realm, now known as <strong>the</strong> Suzuki–Miyauracoupling (see Figure 2).In a vast understatement, we note that <strong>the</strong> Suzuki–Miyaura reaction has developed in<strong>to</strong> an extremely powerfuland general method for <strong>the</strong> formation of C C bonds, [91]displaying a number of advantageous features: 1) easilyhandled and usually air- and moisture-stable organoboronstarting materials; 2) mild and convenient reaction conditionsand 3) <strong>the</strong> facile removal of less-<strong>to</strong>xic inorganic byproducts.These aspects make <strong>the</strong> Suzuki–Miyaura coupling reactionespecially useful for industrial applications.In later years, modification of <strong>the</strong> organoboron reagenthas led <strong>to</strong> <strong>the</strong> development of milder and more selectiveSuzuki–Miyaura couplings, among which <strong>the</strong> GenÞt/Molander BF 3 K salts [92,93] and <strong>the</strong> Burke MIDA (N-methyliminodiaceticacid) boronates [94] are <strong>the</strong> most prominent.Recently Knochel introduced magnesium di-(hetero)arylboronates and magnesium di(alkenyl)boronatesas a new class of Suzuki reagent by treating <strong>the</strong> organichalides with B(OBu) 3 with Mg in <strong>the</strong> presence of LiCl. [95]Often <strong>the</strong> exact nature of <strong>the</strong> organoboron species can haveprofound effects on <strong>the</strong> efficacy of a given transformation.Indeed, <strong>the</strong> mixed aqueous/organic solvent systems normallyemployed in Suzuki–Miyaura reactions may lead, not only <strong>to</strong>pro<strong>to</strong>deboronation as a function of electronic and stericnature of <strong>the</strong> substrate, but can affect <strong>the</strong> nature of <strong>the</strong> boronreagent itself (boronic acid, half-acid ester, boroxine, borinicacid). The associated purity issue is often an unknown fac<strong>to</strong>rsince its characterization is not usually carried out, and maycause difficulty in interpreting failed Suzuki–Miyaura couplingreactions, especially when boronates are employed.An important consideration in <strong>the</strong> Suzuki–Miyaura reactionis <strong>the</strong> base employed. Perhaps <strong>the</strong> earliest, mostdistinguished, example originates from Kishis investigationof <strong>the</strong> effect of base in a reaction en route <strong>to</strong> <strong>the</strong> <strong>to</strong>talsyn<strong>the</strong>sis of Paly<strong>to</strong>xin (Scheme 18). [96] In this work, <strong>the</strong> use ofthallium hydroxide as base makes <strong>the</strong> reaction complete,essentially on mixing of <strong>the</strong> reagents. Although effects maynot be as dramatic as this on a routine basis, such exampleshave elegantly shown <strong>the</strong> importance of base source as well as<strong>the</strong> syn<strong>the</strong>tic power of <strong>the</strong> Suzuki–Miyaura reaction.A hallmark of <strong>the</strong> Suzuki discovery was <strong>the</strong> demonstrationthat “activation” of <strong>the</strong> organometallic component as <strong>the</strong>boronate (sometimes referred <strong>to</strong> as <strong>the</strong> “ate” complex) wouldallow <strong>the</strong> coupling of organometallic reagents unable <strong>to</strong>undergo transmetalation under standard conditions. Thisdiscovery pointed <strong>to</strong> <strong>the</strong> possibility of uncovering couplingreactions of o<strong>the</strong>r organometallics with lower electronegativitydifferences between <strong>the</strong> metal and <strong>the</strong> organic moiety. [97] Itwould be a decade before such reactivity was revealed ina new class of organoelement compounds.Scheme 18. Kishi’s studies on <strong>the</strong> importance of base in <strong>the</strong> Suzuki–Miyaura coupling. [96]2.3.4. The Hiyama Reaction (1988–1994): Organosilicon<strong>Coupling</strong> PartnersIn 1982, Kumada reported <strong>the</strong> use of organopentafluorosilicatesin palladium-catalyzed cross-coupling reactions. [98]In <strong>the</strong> same year, Hallberg disclosed <strong>the</strong> coupling of vinyltrimethylsilane.[99] Building on <strong>the</strong>se reports, in 1988 Hiyamaand co-workers described <strong>the</strong> palladium- and nickel-catalyzedcoupling of organosilanes with aryl halides and triflatesactivated by <strong>the</strong> inclusion of a fluoride source in <strong>the</strong> reaction[100, 101]mixture (Scheme 19). Thus tris(dimethylamino)sulfoniumdifluorotrimethylsilicate (TASF) [100] or CsF [102] wasshown <strong>to</strong> be required <strong>to</strong> activate <strong>the</strong> organosilane <strong>to</strong>wardstransmetalation by <strong>the</strong> formation of silicate intermediates.The Hiyama coupling provided a more environmentallyfriendly and safe option than <strong>the</strong> organoboron, organozinc,and organostannane reagents. This discovery has beenpursued on o<strong>the</strong>r silicon derivatives, for example, siloxanesby Denmark [103] and DeShong, [104] among o<strong>the</strong>rs, whichanticipates <strong>the</strong> promise of greater prominence of <strong>the</strong>Hiyama coupling reaction in <strong>the</strong> near future.Scheme 19. The Hiyama cross-coupling reaction. [100,103]2.4. Carbon–Heteroa<strong>to</strong>m <strong>Coupling</strong> ReactionsUp <strong>to</strong> this point in <strong>the</strong> his<strong>to</strong>rical development of crosscoupling,a s<strong>to</strong>ichiometric organometallic partner had generallybeen required <strong>to</strong> achieve efficient cross-coupling, <strong>the</strong>exception being <strong>the</strong> Mizoroki–Heck reaction. O<strong>the</strong>r functionalpartners, with a few exceptions, (e.g. R 3 Si SiR 3 ,R 3 SnSnR 3 ) were unsuitable for functionalization of organic sub-Angew. Chem. Int. Ed. 2012, 51, 5062 – 5085 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.angewandte.org5073