Download - COLA
Download - COLA
Download - COLA
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
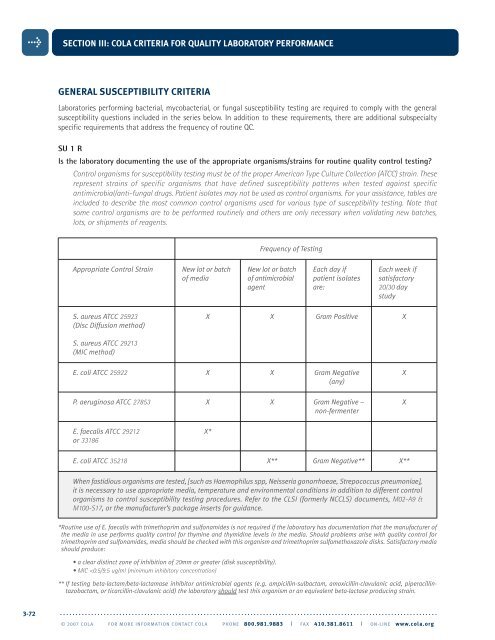
SECTION III: <strong>COLA</strong> CRITERIA FOR QUALITY LABORATORY PERFORMANCEGENERAL SUSCEPTIBILITY CRITERIALaboratories performing bacterial, mycobacterial, or fungal susceptibility testing are required to comply with the generalsusceptibility questions included in the series below. In addition to these requirements, there are additional subspecialtyspecific requirements that address the frequency of routine QC.SU 1 RIs the laboratory documenting the use of the appropriate organisms/strains for routine quality control testing?Control organisms for susceptibility testing must be of the proper American Type Culture Collection (ATCC) strain. Theserepresent strains of specific organisms that have defined susceptibility patterns when tested against specificantimicrobial/anti-fungal drugs. Patient isolates may not be used as control organisms. For your assistance, tables areincluded to describe the most common control organisms used for various type of susceptibility testing. Note thatsome control organisms are to be performed routinely and others are only necessary when validating new batches,lots, or shipments of reagents.Frequency of TestingAppropriate Control Strain New lot or batch New lot or batch Each day if Each week ifof media of antimicrobial patient isolates satisfactoryagent are: 20/30 daystudyS. aureus ATCC 25923 X X Gram Positive X(Disc Diffusion method)S. aureus ATCC 29213(MIC method)E. coli ATCC 25922 X X Gram Negative X(any)P. aeruginosa ATCC 27853 X X Gram Negative – Xnon-fermenterE. faecalis ATCC 29212 X*or 33186E. coli ATCC 35218 X** Gram Negative** X**When fastidious organisms are tested, [such as Haemophilus spp, Neisseria gonorrhoeae, Strepococcus pneumoniae],it is necessary to use appropriate media, temperature and environmental conditions in addition to different controlorganisms to control susceptibility testing procedures. Refer to the CLSI (formerly NCCLS) documents, M02-A9 &M100-S17, or the manufacturer’s package inserts for guidance.*Routine use of E. faecalis with trimethoprim and sulfonamides is not required if the laboratory has documentation that the manufacturer ofthe media in use performs quality control for thymine and thymidine levels in the media. Should problems arise with quality control fortrimethoprim and sulfonamides, media should be checked with this organism and trimethoprim sulfamethoxazole disks. Satisfactory mediashould produce:• a clear distinct zone of inhibition of 20mm or greater (disk susceptibility).• MIC