Bioquell | RBDS - CapellaScience
Bioquell | RBDS - CapellaScience
Bioquell | RBDS - CapellaScience
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3017711200000310202E710500EE<strong>Bioquell</strong> | <strong>RBDS</strong>SHRS.S. DOORWATER BARRIERS.S. BENCHINNER CHN'GROOMOUTER CHN'GROOM4 NOS. COAT HOOKSEESCAPECORRIDOR1150 594BSC(BY OWNER)1212EXT'G DOOREXIT SIGNEXIT AIR LOCKWATERBARRIERBSC(BY OWNER)BSL3 4 BSL3 5IVC (BY OWNER)DARGARD PARTITION DARGARD PARTITIONS.S. WORKBENCHDARGARD PARTITIONEXT'GEXIT SIGN12001210FT FTPRESSURE DISPLAY PANEL &FUMIGATION PORT BELOWFTS.S. WORKBENCHIVC (BY OWNER)BOX UP FORNEW EYEWASHCORRIDORNEW EMERGENCYSHOWERPRESSURE DISPLAY PANEL &FUMIGATION PORT BELOWS.S. SLOPING PANELS.S. PARTITION EXTENDTO FALSE CEILINGEXT'G DOORFUMIGATION PORTABOVE THE DOOREXT'G EXIT SIGNWATER BARRIERBOX UP FOR 2 NOSOF COPPER PIPEFTFT4 NOS. COAT HOOKSPASS-THRUSTERILIZERENTRY AIR LOCKS.S. FINISHES AL ROUNDS.S. BENCHS.S. OPEN SHELVE780ANTE RMDARGARD PARTITIONFUMIGATION PORTABOVE THE DOORDOORQUARAN52197DOOREXT'G PARTITIONAND DOOREXT'G EXIT SIGNE85830DARGARD PARTITIONPRESSURE DISPLAY PANEL &FUMIGATION PORT BELOWS.S. WORKBENCHBSL3 3 BSL3 2IVC (BY OWNER)FUMIGATION PORTPRESSURE DISPLAY PANELFTFT FTS.S. WORKBENCH S.S. WORKBENCHIVC (BY OWNER)BSL3 1BSC(BY OWNER)PROCEDUREROOM DQIVC (BY OWNER)BSC(BY OWNER)BSC(BY OWNER)HR• Fully managed hydrogen peroxide vapour (HPV)bio-decontamination service• Rapid return to normal operation• From small rooms to entire buildings• Fully documented and verified cycles
<strong>Bioquell</strong> Room and Facility Bio-Decontamination Service<strong>Bioquell</strong> <strong>RBDS</strong> is a unique service providing hydrogen peroxide vapour(HPV) bio-decontamination for any enclosed space; from individualrooms to entire buildings. Each cycle uses independently enumeratedbiological indicators ensuring the <strong>Bioquell</strong> <strong>RBDS</strong> process is verified in achallenging and repeatable way.<strong>Bioquell</strong> <strong>RBDS</strong> – Key benefits• Safe to use on sensitive electrical and electronic equipment• Proven and published efficacy against a wide range ofmicro-organisms• Carried out by highly trained <strong>Bioquell</strong> engineers• High level verification using biological indicators (BIs), 6-logGeobacillus stearothermophilus spores - the same challenge usedto validate autoclave cycles• Infinitely scaleable - entire building bio-decontamination• Rapid - 100m 3 in under 3 hours, 8,000m 3 in under 24 hours• Fully documented service including risk assessment, methodstatement, detailed final report and bio-decontamination certificateThe technology<strong>RBDS</strong> utilises <strong>Bioquell</strong>’s state-of-the-art, patent protected, lowtemperature, “residue-free” bio-decontamination technology.It is now used effectively in the pharmaceutical, biotechnology,biomedical, healthcare and military markets.The technology utilises free radicals released by hydrogenperoxide vapour to bio-deactivate micro-organisms. Once thebio-decontamination process has been completed, the hydrogenperoxide vapour is catalytically converted to water vapour andoxygen, leaving the area residue-free. Any equipment, such ascomputers or other sensitive equipment, may be left in the roommeaning the area is reusable with the minimum possible downtime.083/10
<strong>Bioquell</strong> <strong>RBDS</strong> process1. Site survey• Area assessment- Volumes- Layout(topography)- Materials- Fire exits- HVAC system- Access- Area integrity2. Proposal• Full details of area, logistics, costsand timescales3. Risk assessment andmethod statement• Full documentation prepared and supplied4. Site briefing note• Emergency action and advice• Health and safety information• Restricted access signage includingdates/times5. <strong>Bioquell</strong> <strong>RBDS</strong> deployment• Supply of engineers, equipment andconsumables• Area preparation and equipment placement• Bio-decontamination cycle activates• Active vapour purge to safe levels• Continuous safety monitoring of HPV levelsinside bio-decontamination area and outside• Recovery of BIs and equipment• Culture BIs6. Post bio-decontamination• Preliminary report• Discussion of results (as required)7. Full bio-decontamination report(7 days)• Full biological results• Full cycle parameters and data• Layout of equipment and BI placements• Engineer’s discussion: observation,issues, improvements• Bio-decontamination certificate
What is <strong>Bioquell</strong> <strong>RBDS</strong>?<strong>Bioquell</strong> <strong>RBDS</strong> comprises a number of elements whichare summarised below:• The provision of sophisticated equipment to carry out the roombio-decontamination, complete with systems to monitor andcontrol the bio-decontamination process• The provision of highly trained <strong>RBDS</strong> engineers who are able tocarry out the <strong>RBDS</strong>, taking into account the conditions andtopography of the areas as well as the health and safetyrequirements of the site• The provision of all necessary consumables required to carry outthe <strong>RBDS</strong>• The provision of BIs. Further information on the use of thesebiological indicators is set out belowVerification of bio-decontamination – use of BIsIn order to demonstrate the efficacy of the <strong>RBDS</strong> process, <strong>Bioquell</strong>uses Geobacillus stearothermophilus BIs consisting of a dried sporeculture sealed within a Tyvek ® pouch. After bio-decontamination hasbeen completed, the BIs are retrieved and incubated by <strong>Bioquell</strong>.The BIs are reviewed after 24 hours and then 7 days. A reportconfirming 100% bio-deactivation of the BIs will then be issued.It is widely accepted that the use of BIs is an appropriate means ofverifying the efficacy of process.Optimisation<strong>Bioquell</strong>’s <strong>RBDS</strong> technology uses HPV to bio-deactivate microorganismssuch as bacteria, viruses and fungi. The process is a highlevel surface bio-decontamination – although HPV will permeatethrough certain materials.The process also relies on high kineticenergy to ensure that the HPV is distributed efficiently and able toachieve bio-deactivation even within an area containing a significantamount of equipment. After 20,000 deployments, <strong>Bioquell</strong> hasoptimised the <strong>RBDS</strong> process and is highly experienced at achievingrapid and consistent results.083/10
Hydrogen peroxide vapour (HPV) technologyHPV concentrationt=0*123Inactivation of micro-organismsGASSING DWELL AERATIONTIME* Conditioning phase not shown (vaporiser reaches temperature)Note: “features” on graph change e.g. depending on size ofchamber (& related temperature effect)45Schematic of <strong>Bioquell</strong> HPV concentration/time graph1. HPV introduced - initial rapid increase in HPV concentration2. HPV saturation/“dew point” achieved – onset of rapid bio-decontamination3. HPV gassing plateau – sustained micro-condensation effecting bio-deactivation4. Dwell period (contact time)5. Aeration – removal of HPV from the atmosphere typically by catalyticconversion to water vapour and oxygen, leaving no residuesSchematic of dew point and <strong>Bioquell</strong> kill dynamics1. Injection of HPV into the enclosure starts (t=0)2. Only slight decline in biological indicator population prior to dew point3. Rapid micro-biological kill occurs immediately after saturation/“dew point” is achieved –process optimised to achieve reliable 6-log sporicidal reduction on all exposed surfaces4. Onset of micro-condensation correlates with rapid bio-deactivation5. Bio-deactivation achieved via micro-condensation (invisible layer) of hydrogenperoxide (2-6µm)* Typical BI: Tyvek ® pouched 6-logGeobacillus stearothermophilus sporesKey benefits of HPV• Excellent material compatibilityincluding sensitive electronics• Environmentally friendly -“residue-free” (breaks down towater vapour and oxygen)• Effective against a wide rangeof micro-organisms• High level disinfection achieved(6-log sporicidal reduction)HPV applications• Emergency following contamination• Routine environmental controlin clean areas• Routine bio-decontamination incontainment laboratories• Facility pre-occupation anddecommissioning• Emergency contingency planning• Pre/post maintenance
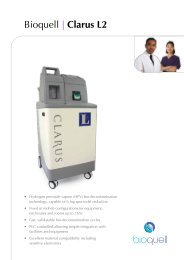
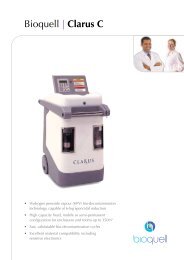
Other unique bio-decontaminationsolutions from <strong>Bioquell</strong><strong>Bioquell</strong> Clarus L2Multi function HPV generator for small rooms, enclosureand equipment.• Fixed or mobile configurations up to 75m 3• Capable of 6-log sporicidal reduction• Fast, validatable bio-decontamination<strong>Bioquell</strong> Clarus C HPV multi-function generatorLarge scale bio-decontamination capabilities designed forGMP manufacturing compliance:• Room bio-decontamination up to 350m 3• Large isolators and chambers• Permanent, semi-permanent and fixed solutions available<strong>Bioquell</strong> Z HPV generatorState-of-the-art bio-decontamination equipment offering:• Large scale room/zone bio-decontamination• Parametric cycle control• Simple to operate “plug and play”Disclaimer: <strong>Bioquell</strong> UK Ltd or its affiliates, distributors, agents or licensees(together “<strong>Bioquell</strong>”) reserves the right to change the specification of the <strong>Bioquell</strong><strong>RBDS</strong> without notice. In addition, <strong>Bioquell</strong> makes no claims as to the guarantee ofsterilisation using these products; however, <strong>Bioquell</strong> recommends that customersensure that the requisite level of bio-decontamination is achieved using standardbiological indicators such as 6-log Geobacillus stearothermophilus spores; and the<strong>Bioquell</strong> <strong>RBDS</strong>, subject to appropriate cycle development, is designed to be able toprovide such levels of bio-deactivation.Your local distributor:Clarus / <strong>Bioquell</strong> / <strong>RBDS</strong> are registered trade marks of <strong>Bioquell</strong> UK Ltd.© <strong>Bioquell</strong> UK Ltd (2010). All rights reserved.For further information on <strong>Bioquell</strong>’s technology:E: info@bioquell.comW: www.bioquell.com<strong>Bioquell</strong> UK LtdT: +44 (0)1264 835 835<strong>Bioquell</strong> IrelandT: +353 (0)61 603 622<strong>Bioquell</strong> IncT: +1 (215) 682 0225<strong>Bioquell</strong> SAST: +33 (0)1 43 78 15 94<strong>Bioquell</strong> Asia Pacific Pte LtdT: +65 6592 5145083/10