Energy Transfer
Energy Transfer
Energy Transfer
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
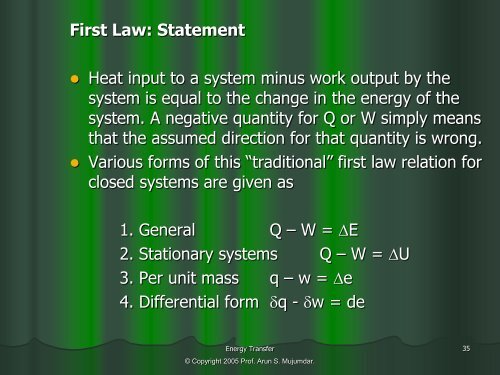
First Law: Statement• Heat input to a system minus work output by thesystem is equal to the change in the energy of thesystem. A negative quantity for Q or W simply meansthat the assumed direction for that quantity is wrong.• Various forms of this “traditional” first law relation forclosed systems are given as1. General Q – W = E2. Stationary systems Q – W = U3. Per unit mass q – w = e4. Differential form q - w = de<strong>Energy</strong> <strong>Transfer</strong> 35© Copyright 2005 Prof. Arun S. Mujumdar.