NEW YORK STATE DEPARTMENT OF HEALTH 08/06 ... - eMedNY
NEW YORK STATE DEPARTMENT OF HEALTH 08/06 ... - eMedNY
NEW YORK STATE DEPARTMENT OF HEALTH 08/06 ... - eMedNY
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
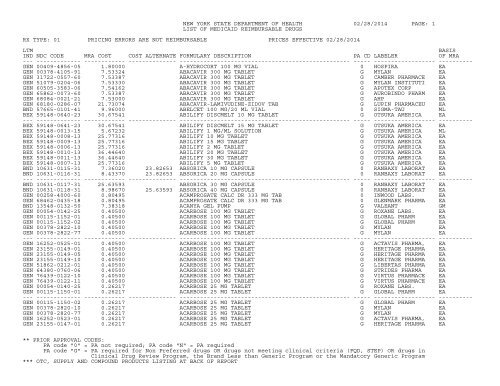
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 4LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00430-0478-01 187.16500 ACTONEL 150 MG TABLET G ACTAVIS PHARMA, EABND 00430-0478-02 187.16776 ACTONEL 150 MG TABLET G ACTAVIS PHARMA, EABND 00430-0470-15 43.19652 ACTONEL 30 MG TABLET G ACTAVIS PHARMA, EABND 00149-0472-01 27.46677 ACTONEL 35 MG TABLET G WC PR<strong>OF</strong> PRODS EABND 00149-0472-04 27.46746 ACTONEL 35 MG TABLET G WC PR<strong>OF</strong> PRODS EABND 00430-0472-03 43.19735 ACTONEL 35 MG TABLET G ACTAVIS PHARMA, EABND 00430-0472-07 43.19666 ACTONEL 35 MG TABLET G ACTAVIS PHARMA, EABND 00430-0471-15 6.17520 ACTONEL 5 MG TABLET G ACTAVIS PHARMA, EABND 64764-0510-30 7.<strong>06</strong>302 ACTOPLUS MET XR 15-1,000 MG TB G TAKEDA PHARMACE EABND 64764-0310-30 14.00016 ACTOPLUS MET XR 30-1,000 MG TB G TAKEDA PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64764-0155-18 3.00753 4.47157 ACTOPLUS MET 15 MG-500 MG TAB G TAKEDA PHARMACE EABND 64764-0155-60 3.00753 6.52297 ACTOPLUS MET 15 MG-500 MG TAB G TAKEDA PHARMACE EABND 64764-0158-18 3.00753 4.47157 ACTOPLUS MET 15 MG-850 MG TAB G TAKEDA PHARMACE EABND 64764-0158-60 3.00753 6.52297 ACTOPLUS MET 15 MG-850 MG TAB G TAKEDA PHARMACE EABND 64764-0151-04 3.61300 8.58579 ACTOS 15 MG TABLET G TAKEDA PHARMACE EABND 64764-0151-05 3.61300 8.58505 ACTOS 15 MG TABLET G TAKEDA PHARMACE EABND 64764-0301-14 3.68630 13.12<strong>06</strong>4 ACTOS 30 MG TABLET G TAKEDA PHARMACE EABND 64764-0301-15 3.68630 13.12045 ACTOS 30 MG TABLET G TAKEDA PHARMACE EABND 64764-0301-16 3.68630 8.99416 ACTOS 30 MG TABLET G TAKEDA PHARMACE EABND 64764-0451-24 3.99980 14.23145 ACTOS 45 MG TABLET G TAKEDA PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64764-0451-25 3.99980 14.23274 ACTOS 45 MG TABLET G TAKEDA PHARMACE EABND 64764-0451-26 3.99980 9.75660 ACTOS 45 MG TABLET G TAKEDA PHARMACE EABND 00023-9277-05 2.07504 35.94896 ACULAR LS 0.4% OPHTH SOL G ALLERGAN INC. MLBND 00023-2181-05 1.30500 34.96790 ACULAR 0.5% EYE DROPS G ALLERGAN INC. MLBND 00023-2181-10 1.30500 20.91185 ACULAR 0.5% EYE DROPS G ALLERGAN INC. MLBND 00023-3507-30 6.46459 ACUVAIL 0.45% OPHTH SOLUTION G ALLERGAN INC. EABND 55390-<strong>06</strong>13-20 9.11340 ACYCLOVIR SODIUM 1 GM VIAL 0 BEDFORD LABS EAGEN 55390-<strong>06</strong>12-10 4.05000 ACYCLOVIR SODIUM 500 MG VIAL 0 BEDFORD LABS EAGEN 63323-0105-10 4.64230 ACYCLOVIR SODIUM 500 MG VIAL 0 APP PHARMACEUTI EABND 63323-0325-20 1.39440 ACYCLOVIR 1,000 MG/20 ML VIAL 0 APP PHARMACEUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00093-8940-01 0.14780 ACYCLOVIR 200 MG CAPSULE 0 TEVA USA EAGUL 00093-8940-05 0.14780 ACYCLOVIR 200 MG CAPSULE 0 TEVA USA EAGUL 23155-0146-01 0.14780 ACYCLOVIR 200 MG CAPSULE 0 HERITAGE PHARMA EAGUL 60505-0042-<strong>06</strong> 0.14780 ACYCLOVIR 200 MG CAPSULE 0 APOTEX CORP EAGUL 61442-0111-01 0.14780 ACYCLOVIR 200 MG CAPSULE 0 CARLSBAD TECH EAGEN 61442-0111-05 0.<strong>08</strong>910 ACYCLOVIR 200 MG CAPSULE 0 CARLSBAD TECH EAGUL 63304-<strong>06</strong>52-01 0.14780 ACYCLOVIR 200 MG CAPSULE 0 RANBAXY PHARMAC EAGUL 67253-0100-10 0.14780 ACYCLOVIR 200 MG CAPSULE 0 DAVA PHARMACEUT EAGUL 67253-0100-11 0.14780 ACYCLOVIR 200 MG CAPSULE 0 DAVA PHARMACEUT EAGEN 68<strong>08</strong>4-0107-01 0.<strong>08</strong>910 ACYCLOVIR 200 MG CAPSULE 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0107-11 0.<strong>08</strong>910 ACYCLOVIR 200 MG CAPSULE 0 AHP EAGEN 00472-0<strong>08</strong>2-16 0.19040 ACYCLOVIR 200 MG/5 ML SUSP 0 ACTAVIS PHARMA, MLGEN 50383-<strong>08</strong>10-16 0.19040 ACYCLOVIR 200 MG/5 ML SUSP 0 HI-TECH PHARMAC MLGUL 00093-8943-01 0.23340 ACYCLOVIR 400 MG TABLET 0 TEVA USA EAGUL 00093-8943-05 0.23340 ACYCLOVIR 400 MG TABLET 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 5LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-0253-01 0.23340 ACYCLOVIR 400 MG TABLET 0 MYLAN EAGUL 00904-6121-61 0.23340 ACYCLOVIR 400 MG TABLET 0 MAJOR PHARMACEU EAGUL 23155-0227-01 0.23340 ACYCLOVIR 400 MG TABLET 0 HERITAGE PHARMA EAGUL 23155-0227-05 0.23340 ACYCLOVIR 400 MG TABLET 0 HERITAGE PHARMA EAGUL 31722-0777-01 0.23340 ACYCLOVIR 400 MG TABLET 0 CAMBER PHARMACE EAGUL 31722-0777-05 0.23340 ACYCLOVIR 400 MG TABLET 0 CAMBER PHARMACE EAGUL 60505-53<strong>06</strong>-01 0.23340 ACYCLOVIR 400 MG TABLET 0 APOTEX CORP EAGUL 60505-53<strong>06</strong>-<strong>08</strong> 0.23340 ACYCLOVIR 400 MG TABLET 0 APOTEX CORP EAGUL 61442-0112-01 0.23340 ACYCLOVIR 400 MG TABLET 0 CARLSBAD TECH EAGUL 63304-0504-01 0.23340 ACYCLOVIR 400 MG TABLET 0 RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 67253-0101-10 0.23340 ACYCLOVIR 400 MG TABLET 0 DAVA PHARMACEUT EAGUL 67253-0101-11 0.23340 ACYCLOVIR 400 MG TABLET 0 DAVA PHARMACEUT EAGUL 68<strong>08</strong>4-01<strong>08</strong>-01 0.23340 ACYCLOVIR 400 MG TABLET 0 AHP EAGUL 68<strong>08</strong>4-01<strong>08</strong>-11 0.23340 ACYCLOVIR 400 MG TABLET 0 AHP EAGEN 00378-8700-<strong>06</strong> 19.94000 ACYCLOVIR 5% OINTMENT 0 MYLAN GMGEN 00378-8700-49 19.93974 ACYCLOVIR 5% OINTMENT 0 MYLAN GMGEN 51079-0550-68 21.05625 ACYCLOVIR 5% OINTMENT 0 MYLAN INSTITUTI GMGEN 68682-0995-95 19.20000 ACYCLOVIR 5% OINTMENT 0 OCEANSIDE PHARM GMBND 63323-0325-10 1.49400 ACYCLOVIR 500 MG/10 ML VIAL 0 APP PHARMACEUTI MLGEN 00093-8947-01 0.30250 ACYCLOVIR 800 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-8947-05 0.30250 ACYCLOVIR 800 MG TABLET 0 TEVA USA EAGEN 00378-0302-01 0.30250 ACYCLOVIR 800 MG TABLET 0 MYLAN EAGEN 23155-0228-01 0.30250 ACYCLOVIR 800 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0228-05 0.30250 ACYCLOVIR 800 MG TABLET 0 HERITAGE PHARMA EAGEN 31722-0778-01 0.30250 ACYCLOVIR 800 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0778-05 0.30250 ACYCLOVIR 800 MG TABLET 0 CAMBER PHARMACE EAGEN 60505-5307-01 0.30250 ACYCLOVIR 800 MG TABLET 0 APOTEX CORP EAGEN 60505-5307-05 0.30250 ACYCLOVIR 800 MG TABLET 0 APOTEX CORP EAGEN 61442-0113-01 0.30250 ACYCLOVIR 800 MG TABLET 0 CARLSBAD TECH EAGEN 63304-0505-01 0.30250 ACYCLOVIR 800 MG TABLET 0 RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0109-01 0.30250 ACYCLOVIR 800 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0109-11 0.30250 ACYCLOVIR 800 MG TABLET 0 AHP EABND 57665-0001-01 2592.25600 ADAGEN 250 UNITS/ML VIAL 0 SIGMA-TAU MLBND 50419-0701-05 0.46980 1.33912 ADALAT CC 30 MG TABLET G BAYER,PHARM DIV EABND 50419-0701-10 0.46980 1.33922 ADALAT CC 30 MG TABLET G BAYER,PHARM DIV EABND 50419-0702-05 1.02120 2.38591 ADALAT CC 60 MG TABLET G BAYER,PHARM DIV EABND 50419-0702-10 1.02120 2.38561 ADALAT CC 60 MG TABLET G BAYER,PHARM DIV EABND 50419-0703-05 1.72120 2.79577 ADALAT CC 90 MG TABLET G BAYER,PHARM DIV EABND 66302-0467-60 31.52340 ADCIRCA 20 MG TABLET G ELI LILLY & CO. EAGEN 42794-0003-<strong>08</strong> 28.42100 ADEFOVIR DIPIVOXIL 10 MG TAB G SIGMAPHARM LABO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50419-0250-01 83.00000 ADEMPAS 0.5 MG TABLET 0 BAYER,PHARM DIV EABND 50419-0250-03 83.00000 ADEMPAS 0.5 MG TABLET 0 BAYER,PHARM DIV EABND 50419-0251-01 83.00000 ADEMPAS 1 MG TABLET 0 BAYER,PHARM DIV EABND 50419-0251-03 83.00000 ADEMPAS 1 MG TABLET 0 BAYER,PHARM DIV EABND 50419-0252-01 83.00000 ADEMPAS 1.5 MG TABLET 0 BAYER,PHARM DIV EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 6LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50419-0252-03 83.00000 ADEMPAS 1.5 MG TABLET 0 BAYER,PHARM DIV EABND 50419-0253-01 83.00000 ADEMPAS 2 MG TABLET 0 BAYER,PHARM DIV EABND 50419-0253-03 83.00000 ADEMPAS 2 MG TABLET 0 BAYER,PHARM DIV EABND 50419-0254-01 83.00000 ADEMPAS 2.5 MG TABLET 0 BAYER,PHARM DIV EABND 50419-0254-03 83.00000 ADEMPAS 2.5 MG TABLET 0 BAYER,PHARM DIV EAGEN 10337-<strong>08</strong>15-<strong>06</strong> 17.82360 ADOXA 150 MG CAPSULE G SANDOZ EABND 52054-<strong>08</strong>03-02 151.34635 ADRENACLICK 0.15 MG AUTO-INJCT 0 AMEDRA PHARMACE EABND 52054-<strong>08</strong>04-02 143.87635 ADRENACLICK 0.3 MG AUTO-INJECT 0 AMEDRA PHARMACE EABND 42023-0101-01 0.22400 0.26560 ADRENALIN CL 1 MG/ML VIAL 0 JHP PHARMACEUTI MLBND 55390-0231-10 7.28750 10.95600 ADRIAMYCIN 10 MG VIAL 0 BEDFORD LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55390-0236-10 1.98000 ADRIAMYCIN 2 MG/ML VIAL 0 BEDFORD LABS MLGEN 55390-0237-01 1.99800 ADRIAMYCIN 2 MG/ML VIAL 0 BEDFORD LABS MLBND 55390-0232-10 21.91200 ADRIAMYCIN 20 MG VIAL 0 BEDFORD LABS EAGEN 55390-0238-01 1.98000 ADRIAMYCIN 200 MG/100 ML VIAL 0 BEDFORD LABS MLBND 55390-0233-01 19.23175 55.27800 ADRIAMYCIN 50 MG VIAL 0 BEDFORD LABS EAGEN 00703-3018-12 0.24480 ADRUCIL 50 MG/ML VIAL 0 TEVA PARENTERAL MLBND 00173-0716-20 23.54986 ADVAIR HFA 115-21 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-0716-22 19.05368 ADVAIR HFA 115-21 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-0717-20 30.97421 ADVAIR HFA 230-21 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-0717-22 28.25735 ADVAIR HFA 230-21 MCG INHALER G GLAXOSMITHKLINE GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-0715-20 18.95373 ADVAIR HFA 45-21 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-0715-22 19.05368 ADVAIR HFA 45-21 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-<strong>06</strong>95-00 3.79075 ADVAIR 100-50 DISKUS G GLAXOSMITHKLINE EABND 00173-<strong>06</strong>96-00 4.70997 ADVAIR 250-50 DISKUS G GLAXOSMITHKLINE EABND 00173-<strong>06</strong>97-00 6.19484 ADVAIR 500-50 DISKUS G GLAXOSMITHKLINE EABND 00944-2924-02 1.09500 ADVATE 1,201-1,800 UNITS VIAL 0 BAXTER BIOSCIENBND 00944-2944-10 1.09500 ADVATE 1,201-1,800 UNITS VIAL 0 BAXTER BIOSCIENBND 00944-2945-10 1.09500 ADVATE 1,801-2,400 UNITS VIAL 0 BAXTER BIOSCIENBND 00944-2964-10 1.09500 ADVATE 1,801-2,400 UNITS VIAL 0 BAXTER BIOSCIENBND 00944-2946-10 1.09500 ADVATE 2,401-3,600 UNITS VIAL 0 BAXTER BIOSCIEN--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00944-2965-10 1.09500 ADVATE 2,401-3,600 UNITS VIAL 0 BAXTER BIOSCIENBND 00944-2921-02 1.09500 ADVATE 200-400 UNITS VIAL 0 BAXTER BIOSCIENBND 00944-2941-10 1.09500 ADVATE 200-400 UNITS VIAL 0 BAXTER BIOSCIENBND 00944-2948-10 1.09500 ADVATE 3,601-4,800 UNITS VIAL 0 BAXTER BIOSCIENBND 00944-2922-02 1.09500 ADVATE 401-800 UNITS VIAL 0 BAXTER BIOSCIENBND 00944-2942-10 1.09500 ADVATE 401-800 UNITS VIAL 0 BAXTER BIOSCIENBND 00944-2923-02 1.09500 ADVATE 801-1,200 UNITS VIAL 0 BAXTER BIOSCIENBND 00944-2943-10 1.09500 ADVATE 801-1,200 UNITS VIAL 0 BAXTER BIOSCIENBND 00074-3007-90 6.42078 ADVICOR 1,000 MG-20 MG TABLET G ABBVIE US LLC EABND 00074-3010-90 7.43311 ADVICOR 1,000 MG-40 MG TABLET G ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00074-3005-90 5.58368 ADVICOR 500 MG-20 MG TABLET G ABBVIE US LLC EABND 00074-3072-90 5.98918 ADVICOR 750 MG-20 MG TABLET G ABBVIE US LLC EABND 75989-0550-12 18.<strong>06</strong>881 AEROSPAN 80 MCG INHALER G MEDA PHARMACEUT GMGEN 00591-3193-01 0.46980 AFEDITAB CR 30 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-3193-05 0.46980 AFEDITAB CR 30 MG TABLET 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 7LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3194-01 1.02120 AFEDITAB CR 60 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-3194-05 1.02120 AFEDITAB CR 60 MG TABLET 0 ACTAVIS PHARMA, EABND 00078-<strong>06</strong>26-51 301.<strong>06</strong>590 AFINITOR DISPERZ 2 MG TABLET 0 NOVARTIS EABND 00078-<strong>06</strong>27-51 304.<strong>08</strong>176 AFINITOR DISPERZ 3 MG TABLET 0 NOVARTIS EABND 00078-<strong>06</strong>28-51 316.48788 AFINITOR DISPERZ 5 MG TABLET 0 NOVARTIS EABND 00078-0567-51 325.13648 AFINITOR 10 MG TABLET 0 NOVARTIS EABND 00078-0567-61 325.11100 AFINITOR 10 MG TABLET 0 NOVARTIS EABND 00078-0594-51 302.57442 AFINITOR 2.5 MG TABLET 0 NOVARTIS EABND 00078-0594-61 302.57650 AFINITOR 2.5 MG TABLET 0 NOVARTIS EABND 00078-0566-51 316.48788 AFINITOR 5 MG TABLET 0 NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0566-61 316.51220 AFINITOR 5 MG TABLET 0 NOVARTIS EABND 00078-<strong>06</strong>20-51 325.13648 AFINITOR 7.5 MG TABLET 0 NOVARTIS EABND 00078-<strong>06</strong>20-61 325.13590 AFINITOR 7.5 MG TABLET 0 NOVARTIS EABND 00597-0001-60 5.05318 AGGRENOX 25 MG-200 MG CAPSULE 0 BOEHRINGER ING. EABND 54092-0<strong>06</strong>3-01 0.23490 8.23592 AGRYLIN 0.5 MG CAPSULE G SHIRE US INC. EAGEN 17478-0238-35 3.04<strong>08</strong>8 AK-POLY-BAC EYE OINTMENT 0 AKORN INC. GMBND 13548-0030-25 5.1<strong>08</strong>15 AKNE-MYCIN 2% OINTMENT G VALEANT GMBND 17478-0792-10 4.4<strong>08</strong>96 AKTEN 3.5% GEL DROPS 0 AKORN INC. MLBND 52054-0550-22 101.58370 ALBENZA 200 MG TABLET 0 AMEDRA PHARMACE EABND 52054-0550-28 101.57955 ALBENZA 200 MG TABLET 0 AMEDRA PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00053-7680-32 1.86750 ALBUMINAR-25 IV SOLUTION 0 CSL BEHRING LLC MLBND 00053-7680-33 1.86750 ALBUMINAR-25 IV SOLUTION 0 CSL BEHRING LLC MLGEN 00378-6991-52 0.39299 ALBUTEROL SUL 0.63 MG/3 ML SOL 0 MYLAN MLGEN 00487-0301-01 0.39299 ALBUTEROL SUL 0.63 MG/3 ML SOL 0 NEPHRON CORP MLGEN 00487-0301-02 0.39299 ALBUTEROL SUL 0.63 MG/3 ML SOL 0 NEPHRON CORP MLGEN 00591-3467-53 0.39299 ALBUTEROL SUL 0.63 MG/3 ML SOL 0 ACTAVIS PHARMA, MLGEN 00378-6992-52 0.38651 ALBUTEROL SUL 1.25 MG/3 ML SOL 0 MYLAN MLGEN 00487-9904-01 0.38651 ALBUTEROL SUL 1.25 MG/3 ML SOL 0 NEPHRON CORP MLGEN 00487-9904-02 0.38651 ALBUTEROL SUL 1.25 MG/3 ML SOL 0 NEPHRON CORP MLGEN 00487-9904-25 0.38651 ALBUTEROL SUL 1.25 MG/3 ML SOL 0 NEPHRON CORP ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3468-53 0.38651 ALBUTEROL SUL 1.25 MG/3 ML SOL 0 ACTAVIS PHARMA, MLGEN 00093-<strong>06</strong>61-16 0.01350 ALBUTEROL SULF 2 MG/5 ML SYRUP 0 TEVA USA MLGEN 0<strong>06</strong>03-10<strong>08</strong>-58 0.01350 ALBUTEROL SULF 2 MG/5 ML SYRUP 0 QUALITEST MLGEN 50383-0740-16 0.01350 ALBUTEROL SULF 2 MG/5 ML SYRUP 0 HI-TECH PHARMAC MLGEN 00378-4122-01 1.11480 ALBUTEROL SULFATE ER 4 MG TAB 0 MYLAN EAGEN 68774-0400-01 1.05172 ALBUTEROL SULFATE ER 4 MG TAB 0 DAVA PHARMACEUT EAGEN 00378-4124-01 2.09040 ALBUTEROL SULFATE ER 8 MG TAB 0 MYLAN EAGEN 68774-0401-01 1.97205 ALBUTEROL SULFATE ER 8 MG TAB 0 DAVA PHARMACEUT EAGEN 00378-0255-01 4.56651 ALBUTEROL SULFATE 2 MG TAB 0 MYLAN EAGEN 00378-0255-05 4.56651 ALBUTEROL SULFATE 2 MG TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-<strong>06</strong>57-20 4.56651 ALBUTEROL SULFATE 2 MG TAB 0 MYLAN INSTITUTI EAGEN 53489-0176-01 4.4<strong>06</strong>25 ALBUTEROL SULFATE 2 MG TAB 0 MUTUAL PHARM CO EAGEN 53489-0176-05 4.4<strong>06</strong>25 ALBUTEROL SULFATE 2 MG TAB 0 MUTUAL PHARM CO EAGUL 00378-0572-01 0.14250 ALBUTEROL SULFATE 4 MG TAB 0 MYLAN EAGUL 00378-0572-05 0.14250 ALBUTEROL SULFATE 4 MG TAB 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 8LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51079-<strong>06</strong>58-20 0.14250 ALBUTEROL SULFATE 4 MG TAB 0 MYLAN INSTITUTI EAGUL 53489-0177-01 0.14250 ALBUTEROL SULFATE 4 MG TAB 0 MUTUAL PHARM CO EAGUL 53489-0177-05 0.14250 ALBUTEROL SULFATE 4 MG TAB 0 MUTUAL PHARM CO EAGEN 00378-6990-52 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 MYLAN MLGEN 00378-6990-58 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 MYLAN MLGEN 00378-6990-93 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 MYLAN MLGEN 00378-8270-52 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 MYLAN MLGEN 00487-9501-01 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 NEPHRON CORP MLGEN 00487-9501-02 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 NEPHRON CORP MLGEN 00487-9501-03 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 NEPHRON CORP ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00487-9501-25 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 NEPHRON CORP MLGEN 00487-9501-60 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 NEPHRON CORP MLGEN 00591-3797-30 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 ACTAVIS PHARMA, MLGEN 00591-3797-60 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 ACTAVIS PHARMA, MLGEN 00591-3797-83 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 ACTAVIS PHARMA, MLGEN 00781-7155-29 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 SANDOZ MLGEN 00781-7155-64 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 SANDOZ MLGEN 00781-7155-86 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 SANDOZ MLGEN 76204-0200-25 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 RITEDOSE PHARMA MLGEN 76204-0200-30 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 RITEDOSE PHARMA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 76204-0200-60 0.05279 ALBUTEROL 0.<strong>08</strong>3% INHAL SOLN 0 RITEDOSE PHARMA MLBND 00487-9901-02 0.83000 ALBUTEROL 2.5 MG/0.5 ML SOL 0 NEPHRON CORP EABND 00487-9901-30 0.74700 ALBUTEROL 2.5 MG/0.5 ML SOL 0 NEPHRON CORP EAGUL 242<strong>08</strong>-0347-20 0.23330 ALBUTEROL 5 MG/ML SOLUTION 0 VALEANT MLGUL 50383-0741-20 0.23330 ALBUTEROL 5 MG/ML SOLUTION 0 HI-TECH PHARMAC MLGEN 00998-0016-15 0.74530 ALCAINE 0.5% EYE DROPS 0 ALCON (P.R.) MLGUL 00168-0264-15 0.82830 ALCLOMETASONE DIPR 0.05% OINT G SANDOZ GMGEN 00168-0264-45 0.49700 ALCLOMETASONE DIPR 0.05% OINT G SANDOZ GMGEN 00168-0264-60 0.47199 ALCLOMETASONE DIPR 0.05% OINT G SANDOZ GMGUL 51672-1316-01 0.82830 ALCLOMETASONE DIPR 0.05% OINT G TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-1316-03 0.82830 ALCLOMETASONE DIPR 0.05% OINT G TARO PHARM USA GMGUL 51672-1316-<strong>06</strong> 0.82830 ALCLOMETASONE DIPR 0.05% OINT G TARO PHARM USA GMGUL 68462-0299-17 0.82830 ALCLOMETASONE DIPR 0.05% OINT G GLENMARK PHARMA GMGUL 68462-0299-47 0.82830 ALCLOMETASONE DIPR 0.05% OINT G GLENMARK PHARMA GMGUL 68462-0299-65 0.82830 ALCLOMETASONE DIPR 0.05% OINT G GLENMARK PHARMA GMGUL 00168-0263-15 0.82830 ALCLOMETASONE DIPRO 0.05% CRM G SANDOZ GMGUL 00168-0263-45 0.82830 ALCLOMETASONE DIPRO 0.05% CRM G SANDOZ GMGUL 00168-0263-60 0.82830 ALCLOMETASONE DIPRO 0.05% CRM G SANDOZ GMGUL 51672-13<strong>06</strong>-01 0.82830 ALCLOMETASONE DIPRO 0.05% CRM G TARO PHARM USA GMGUL 51672-13<strong>06</strong>-03 0.82830 ALCLOMETASONE DIPRO 0.05% CRM G TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-13<strong>06</strong>-<strong>06</strong> 0.82830 ALCLOMETASONE DIPRO 0.05% CRM G TARO PHARM USA GMGUL 68462-0300-17 0.82830 ALCLOMETASONE DIPRO 0.05% CRM G GLENMARK PHARMA GMGUL 68462-0300-47 0.82830 ALCLOMETASONE DIPRO 0.05% CRM G GLENMARK PHARMA GMGUL 68462-0300-65 0.82830 ALCLOMETASONE DIPRO 0.05% CRM G GLENMARK PHARMA GMBND 00517-8571-10 10.37500 ALCOHOL,DEHYDRATED 98% AMPULE 0 AMER. REGENT ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 9LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 17478-0503-05 8.69939 ALCOHOL,DEHYDRATED 98% VIAL 0 AKORN INC. MLBUL 00025-1011-31 0.34630 1.32551 ALDACTAZIDE 25-25 TABLET G PHARMACIA/UPJHN EABND 00025-1021-31 2.44459 ALDACTAZIDE 50-50 TABLET 0 PHARMACIA/UPJHN EABND 00025-1031-31 0.45550 3.80189 ALDACTONE 100 MG TABLET G PHARMACIA/UPJHN EABND 00025-1001-31 0.<strong>06</strong>510 1.29131 ALDACTONE 25 MG TABLET G PHARMACIA/UPJHN EABND 00025-1041-31 0.24800 2.26789 ALDACTONE 50 MG TABLET G PHARMACIA/UPJHN EABND 51224-0301-10 0.25937 ALENDRONATE SOD 70 MG/75 ML G TAGI PHARMA MLGEN 00093-5141-01 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 TEVA USA EAGEN 00093-5141-56 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 TEVA USA EAGEN 00378-3567-01 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-<strong>06</strong>31-01 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>31-02 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 NORTHSTAR RX LL EAGEN 41616-<strong>06</strong>36-83 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 SUN PHARMA GLOB EAGEN 41616-<strong>06</strong>36-88 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 SUN PHARMA GLOB EAGEN 51079-0941-01 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0941-05 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 MYLAN INSTITUTI EAGEN 60505-2593-01 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 APOTEX CORP EAGEN 60505-2593-03 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 APOTEX CORP EAGEN 65862-0327-30 0.17130 ALENDRONATE SODIUM 10 MG TAB 0 AUROBINDO PHARM EAGEN 00093-5172-19 0.64130 ALENDRONATE SODIUM 35 MG TAB G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-5172-20 0.64130 ALENDRONATE SODIUM 35 MG TAB G TEVA USA EAGEN 00093-5172-29 0.64130 ALENDRONATE SODIUM 35 MG TAB G TEVA USA EAGEN 00093-5172-44 0.64130 ALENDRONATE SODIUM 35 MG TAB G TEVA USA EAGEN 00378-3568-99 0.64130 ALENDRONATE SODIUM 35 MG TAB G MYLAN EAGEN 16252-0599-02 0.64130 ALENDRONATE SODIUM 35 MG TAB G ACTAVIS PHARMA, EAGEN 16252-0599-44 0.64130 ALENDRONATE SODIUM 35 MG TAB G ACTAVIS PHARMA, EAGEN 16714-<strong>06</strong>32-01 0.64130 ALENDRONATE SODIUM 35 MG TAB G NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>32-02 0.64130 ALENDRONATE SODIUM 35 MG TAB G NORTHSTAR RX LL EAGEN 24658-0162-71 0.64130 ALENDRONATE SODIUM 35 MG TAB G BLU PHARMACEUTI EAGEN 24658-0162-73 0.64130 ALENDRONATE SODIUM 35 MG TAB G BLU PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 41616-<strong>06</strong>37-68 0.64130 ALENDRONATE SODIUM 35 MG TAB G SUN PHARMA GLOB EAGEN 59746-0242-02 0.64130 ALENDRONATE SODIUM 35 MG TAB G CADISTA PHARMAC EAGEN 60505-2594-04 0.64130 ALENDRONATE SODIUM 35 MG TAB G APOTEX CORP EAGEN 65862-0328-04 0.64130 ALENDRONATE SODIUM 35 MG TAB G AUROBINDO PHARM EAGEN 67877-0240-31 0.64130 ALENDRONATE SODIUM 35 MG TAB G ASCEND LABORATO EAGEN 67877-0240-33 0.64130 ALENDRONATE SODIUM 35 MG TAB G ASCEND LABORATO EAGEN 76439-0130-04 0.64130 ALENDRONATE SODIUM 35 MG TAB G VIRTUS PHARMACE EAGEN 76439-0130-12 0.64130 ALENDRONATE SODIUM 35 MG TAB G VIRTUS PHARMACE EABND 00093-5142-56 5.38460 5.47689 ALENDRONATE SODIUM 40 MG TAB 0 TEVA USA EAGEN 00093-5140-01 0.17861 ALENDRONATE SODIUM 5 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-5140-56 0.17861 ALENDRONATE SODIUM 5 MG TABLET 0 TEVA USA EAGEN 00378-3566-01 0.17861 ALENDRONATE SODIUM 5 MG TABLET 0 MYLAN EAGEN 41616-<strong>06</strong>35-83 0.17861 ALENDRONATE SODIUM 5 MG TABLET 0 SUN PHARMA GLOB EAGEN 41616-<strong>06</strong>35-88 0.17861 ALENDRONATE SODIUM 5 MG TABLET 0 SUN PHARMA GLOB EAGEN 60505-2592-01 0.17861 ALENDRONATE SODIUM 5 MG TABLET 0 APOTEX CORP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 10LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2592-03 0.17861 ALENDRONATE SODIUM 5 MG TABLET 0 APOTEX CORP EAGEN 00093-5171-20 0.69530 ALENDRONATE SODIUM 70 MG TAB G TEVA USA EAGEN 00093-5171-29 0.69530 ALENDRONATE SODIUM 70 MG TAB G TEVA USA EAGEN 00093-5171-44 0.69530 ALENDRONATE SODIUM 70 MG TAB G TEVA USA EAGEN 00378-3569-99 0.69530 ALENDRONATE SODIUM 70 MG TAB G MYLAN EAGEN 16252-<strong>06</strong>01-02 0.69530 ALENDRONATE SODIUM 70 MG TAB G ACTAVIS PHARMA, EAGEN 16252-<strong>06</strong>01-44 0.69530 ALENDRONATE SODIUM 70 MG TAB G ACTAVIS PHARMA, EAGEN 16714-<strong>06</strong>33-01 0.69530 ALENDRONATE SODIUM 70 MG TAB G NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>33-02 0.69530 ALENDRONATE SODIUM 70 MG TAB G NORTHSTAR RX LL EAGEN 24658-0163-71 0.69530 ALENDRONATE SODIUM 70 MG TAB G BLU PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24658-0163-73 0.69530 ALENDRONATE SODIUM 70 MG TAB G BLU PHARMACEUTI EAGEN 41616-<strong>06</strong>38-68 0.69530 ALENDRONATE SODIUM 70 MG TAB G SUN PHARMA GLOB EAGEN 51079-0942-01 0.69530 ALENDRONATE SODIUM 70 MG TAB G MYLAN INSTITUTI EAGEN 51079-0942-05 0.69530 ALENDRONATE SODIUM 70 MG TAB G MYLAN INSTITUTI EAGEN 59746-0244-02 0.69530 ALENDRONATE SODIUM 70 MG TAB G CADISTA PHARMAC EAGEN 60505-2596-02 0.69530 ALENDRONATE SODIUM 70 MG TAB G APOTEX CORP EAGEN 60505-2596-04 0.69530 ALENDRONATE SODIUM 70 MG TAB G APOTEX CORP EAGEN 60505-2596-<strong>08</strong> 0.69530 ALENDRONATE SODIUM 70 MG TAB G APOTEX CORP EAGEN 65862-0329-04 0.69530 ALENDRONATE SODIUM 70 MG TAB G AUROBINDO PHARM EAGEN 67877-0241-31 0.69530 ALENDRONATE SODIUM 70 MG TAB G ASCEND LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67877-0241-33 0.69530 ALENDRONATE SODIUM 70 MG TAB G ASCEND LABORATO EAGEN 68<strong>08</strong>4-0322-11 0.69530 ALENDRONATE SODIUM 70 MG TAB G AHP EAGEN 68<strong>08</strong>4-0322-94 0.69530 ALENDRONATE SODIUM 70 MG TAB G AHP EAGEN 76439-0131-04 0.69530 ALENDRONATE SODIUM 70 MG TAB G VIRTUS PHARMACE EAGEN 76439-0131-12 0.69530 ALENDRONATE SODIUM 70 MG TAB G VIRTUS PHARMACE EAGEN 76439-0131-20 0.69530 ALENDRONATE SODIUM 70 MG TAB G VIRTUS PHARMACE EABND 54746-0001-01 599.99870 ALFERON N 5 MILLION UNITS VIAL 0 HEMISPHERX BIOP MLGEN 00378-5005-05 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 MYLAN EAGEN 00378-5005-77 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 MYLAN EAGEN 13668-0021-01 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-0021-05 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0021-71 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 TORRENT PHARMAC EAGEN 31722-0302-01 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0302-05 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 CAMBER PHARMACE EAGEN 47335-0956-18 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 SUN PHARMA GLOB EAGEN 47335-0956-81 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 SUN PHARMA GLOB EAGEN 47335-0956-88 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 SUN PHARMA GLOB EAGEN 60505-2850-01 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 APOTEX CORP EAGEN 64679-0738-02 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0738-03 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0249-01 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 AUROBINDO PHARM EAGEN 76282-0302-01 0.25370 ALFUZOSIN HCL ER 10 MG TABLET 0 EXELAN PHARMACE EABND 27437-01<strong>06</strong>-01 2.36398 ALINIA 100 MG/5 ML SUSPENSION G LUPIN PHARMA MLBND 67546-0212-21 1.51724 ALINIA 100 MG/5 ML SUSPENSION G ROMARK PHARM MLBND 67546-0111-12 25.76320 ALINIA 500 MG TABLET G ROMARK PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 11LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 52609-0001-05 9.10510 ALKERAN 2 MG TABLET 0 APOPHARMA USA I EABND 52609-3001-00 1568.76000 1636.52760 ALKERAN 50 MG VIAL 0 APOPHARMA USA I EAGEN 00378-0137-01 0.03416 ALLOPURINOL 100 MG TABLET 0 MYLAN EAGEN 00378-0137-10 0.03416 ALLOPURINOL 100 MG TABLET 0 MYLAN EAGEN 00591-5543-01 0.03416 ALLOPURINOL 100 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5543-10 0.03416 ALLOPURINOL 100 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-2115-02 0.03416 ALLOPURINOL 100 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2115-04 0.03416 ALLOPURINOL 100 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2115-21 0.03416 ALLOPURINOL 100 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2115-32 0.03416 ALLOPURINOL 100 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-2115-93 0.03416 ALLOPURINOL 100 MG TABLET 0 QUALITEST EAGEN 16714-0041-01 0.03416 ALLOPURINOL 100 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0041-04 0.03416 ALLOPURINOL 100 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0041-<strong>06</strong> 0.03416 ALLOPURINOL 100 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0041-07 0.03416 ALLOPURINOL 100 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0041-10 0.03416 ALLOPURINOL 100 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0041-12 0.03416 ALLOPURINOL 100 MG TABLET 0 NORTHSTAR RX LL EAGEN 51079-0205-20 0.03416 ALLOPURINOL 100 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0156-01 0.03416 ALLOPURINOL 100 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0156-05 0.03416 ALLOPURINOL 100 MG TABLET 0 MUTUAL PHARM CO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53489-0156-10 0.03416 ALLOPURINOL 100 MG TABLET 0 MUTUAL PHARM CO EAGEN 60505-2516-02 0.03416 ALLOPURINOL 100 MG TABLET 0 APOTEX CORP EAGEN 60505-2516-03 0.03416 ALLOPURINOL 100 MG TABLET 0 APOTEX CORP EAGEN 63304-0539-01 0.03416 ALLOPURINOL 100 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-0539-10 0.03416 ALLOPURINOL 100 MG TABLET 0 RANBAXY PHARMAC EAGEN 00378-0181-01 0.05900 ALLOPURINOL 300 MG TABLET 0 MYLAN EAGEN 00378-0181-05 0.05900 ALLOPURINOL 300 MG TABLET 0 MYLAN EAGEN 00591-5544-01 0.05900 ALLOPURINOL 300 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5544-05 0.05900 ALLOPURINOL 300 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-2116-02 0.05900 ALLOPURINOL 300 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-2116-21 0.05900 ALLOPURINOL 300 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2116-28 0.05900 ALLOPURINOL 300 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2116-32 0.05900 ALLOPURINOL 300 MG TABLET 0 QUALITEST EAGEN 16714-0042-01 0.05900 ALLOPURINOL 300 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0042-04 0.05900 ALLOPURINOL 300 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0042-05 0.05900 ALLOPURINOL 300 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0042-07 0.05900 ALLOPURINOL 300 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0042-10 0.05900 ALLOPURINOL 300 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0042-11 0.05900 ALLOPURINOL 300 MG TABLET 0 NORTHSTAR RX LL EAGEN 51079-02<strong>06</strong>-20 0.05900 ALLOPURINOL 300 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53489-0157-01 0.05900 ALLOPURINOL 300 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0157-05 0.05900 ALLOPURINOL 300 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0157-10 0.05900 ALLOPURINOL 300 MG TABLET 0 MUTUAL PHARM CO EAGEN 60505-2517-02 0.05900 ALLOPURINOL 300 MG TABLET 0 APOTEX CORP EAGEN 60505-2517-03 0.05900 ALLOPURINOL 300 MG TABLET 0 APOTEX CORP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 12LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62584-0713-01 0.05900 ALLOPURINOL 300 MG TABLET 0 AHP EAGEN 62584-0713-11 0.05900 ALLOPURINOL 300 MG TABLET 0 AHP EAGEN 63304-0540-01 0.05900 ALLOPURINOL 300 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-0540-05 0.05900 ALLOPURINOL 300 MG TABLET 0 RANBAXY PHARMAC EABND 00023-8842-05 26.83556 ALOCRIL 2% EYE DROPS 0 ALLERGAN INC. MLBND 00<strong>06</strong>5-0345-10 12.40020 ALOMIDE 0.1% EYE DROPS 0 ALCON LABS. MLBND 52544-<strong>08</strong>84-<strong>08</strong> 7.94828 ALORA 0.025 MG PATCH 0 ACTAVIS PHARMA, EABND 52544-0471-<strong>08</strong> 8.70462 ALORA 0.05 MG PATCH 0 ACTAVIS PHARMA, EABND 52544-0471-54 8.7<strong>06</strong>70 ALORA 0.05 MG PATCH 0 ACTAVIS PHARMA, EABND 52544-0472-<strong>08</strong> 8.88411 ALORA 0.075 MG PATCH 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 52544-0473-<strong>08</strong> 9.<strong>08</strong>331 ALORA 0.1 MG PATCH 0 ACTAVIS PHARMA, EABND 52544-0473-54 9.<strong>08</strong>020 ALORA 0.1 MG PATCH 0 ACTAVIS PHARMA, EABND 00023-9321-05 18.12886 ALPHAGAN P 0.1% DROPS 0 ALLERGAN INC. MLBND 00023-9321-10 18.12139 ALPHAGAN P 0.1% DROPS 0 ALLERGAN INC. MLBND 00023-9321-15 18.12443 ALPHAGAN P 0.1% DROPS 0 ALLERGAN INC. MLBND 00023-9177-05 19.9<strong>08</strong>38 19.9<strong>08</strong>38 ALPHAGAN P 0.15% EYE DROPS 0 ALLERGAN INC. MLBND 00023-9177-10 19.90340 19.90340 ALPHAGAN P 0.15% EYE DROPS 0 ALLERGAN INC. MLBND 00023-9177-15 19.90450 19.90450 ALPHAGAN P 0.15% EYE DROPS 0 ALLERGAN INC. MLBND 68516-4603-02 0.85500 ALPHANATE 1,000-400 UNIT VIAL 0 GRIFOLSBND 68516-4604-02 0.85500 ALPHANATE 1,500-600 UNIT VIAL 0 GRIFOLS--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 68516-4601-01 0.85500 ALPHANATE 250-100 UNIT VIAL 0 GRIFOLSBND 68516-4602-01 0.85500 ALPHANATE 500-200 UNIT VIAL 0 GRIFOLSBND 68516-3602-02 0.83500 ALPHANINE SD 1,000 UNITS VIAL 0 GRIFOLSBND 68516-3603-02 0.83500 ALPHANINE SD 1,500 UNITS VIAL 0 GRIFOLSBND 68516-3601-02 0.83500 ALPHANINE SD 500 UNITS VIAL 0 GRIFOLSBND 242<strong>08</strong>-0353-05 30.17714 ALREX 0.2% EYE DROPS 0 VALEANT MLBND 242<strong>08</strong>-0353-10 30.17548 ALREX 0.2% EYE DROPS 0 VALEANT MLBND 00007-5180-22 7.84792 ALTABAX 1% OINTMENT 0 GLAXOSMITHKLINE GMBND 00007-5180-25 7.47387 ALTABAX 1% OINTMENT 0 GLAXOSMITHKLINE GMBND 61570-0110-01 0.17861 2.56835 ALTACE 1.25 MG CAPSULE G MONARCH PHRM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 61570-0120-01 0.05890 3.72196 ALTACE 10 MG CAPSULE G MONARCH PHRM EABND 61570-0111-01 0.04790 3.03149 ALTACE 2.5 MG CAPSULE G MONARCH PHRM EABND 61570-0112-01 0.05040 3.18072 ALTACE 5 MG CAPSULE G MONARCH PHRM EAGEX 00781-5583-15 0.81150 ALTAVERA-28 TABLET 0 SANDOZ EABND 59630-<strong>06</strong>28-30 15.38211 ALTOPREV 20 MG TABLET G SHIONOGI PHARMA EABND 59630-<strong>06</strong>29-30 15.38211 ALTOPREV 40 MG TABLET G SHIONOGI PHARMA EABND 59630-<strong>06</strong>30-30 15.38211 ALTOPREV 60 MG TABLET G SHIONOGI PHARMA EABND 63402-0712-01 28.38600 ALVESCO 160 MCG INHALER G SUNOVION PHARMA GMBND 63402-0711-01 28.38600 ALVESCO 80 MCG INHALER G SUNOVION PHARMA GMGEX 68462-0394-29 0.73890 ALYACEN 1-35-28 TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68462-0556-29 0.83640 ALYACEN 7-7-7-28 TABLET 0 GLENMARK PHARMA EAGEN 00527-1704-01 1.51425 AMANTADINE 100 MG CAPSULE 0 LANNETT CO. INC EAGEN 00527-1704-05 1.51425 AMANTADINE 100 MG CAPSULE 0 LANNETT CO. INC EAGEN 00781-2048-01 1.51425 AMANTADINE 100 MG CAPSULE 0 SANDOZ EAGEN 00781-2048-05 1.51425 AMANTADINE 100 MG CAPSULE 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 13LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>08</strong>32-1015-00 1.51425 AMANTADINE 100 MG CAPSULE 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1015-50 1.51425 AMANTADINE 100 MG CAPSULE 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-2012-00 1.51425 AMANTADINE 100 MG CAPSULE 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-2012-50 1.51425 AMANTADINE 100 MG CAPSULE 0 UPSHER SMITH EAGEN 1<strong>08</strong>88-50<strong>06</strong>-02 1.45365 AMANTADINE 100 MG CAPSULE 0 BANNER PHARMACA EAGEN 1<strong>08</strong>88-50<strong>06</strong>-03 1.45368 AMANTADINE 100 MG CAPSULE 0 BANNER PHARMACA EAGEN 51079-0247-20 1.98750 AMANTADINE 100 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 68<strong>08</strong>4-<strong>06</strong>11-01 1.53600 AMANTADINE 100 MG CAPSULE 0 AHP EABND 0<strong>08</strong>32-0111-00 1.89513 AMANTADINE 100 MG TABLET 0 UPSHER SMITH EABND 0<strong>08</strong>32-0111-50 1.89507 AMANTADINE 100 MG TABLET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00121-<strong>06</strong>46-10 0.02133 AMANTADINE 50 MG/5 ML SYRUP 0 PHARMACEU ASSOC MLGEN 00121-<strong>06</strong>46-16 0.02133 AMANTADINE 50 MG/5 ML SYRUP 0 PHARMACEU ASSOC MLGEN 50383-<strong>08</strong>07-16 0.02133 AMANTADINE 50 MG/5 ML SYRUP 0 HI-TECH PHARMAC MLGEN 60432-0093-16 0.02133 AMANTADINE 50 MG/5 ML SYRUP 0 MORTON GROVE PH MLBND 00039-0221-10 0.02930 0.82178 AMARYL 1 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00039-0222-10 0.04347 1.33198 AMARYL 2 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00039-0223-10 0.<strong>06</strong>910 2.51207 AMARYL 4 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00469-3051-30 162.88750 AMBISOME 50 MG VIAL 0 ASTELLAS PHARMA EAGEN 00168-0278-15 7.56000 AMCINONIDE 0.1% CREAM 0 SANDOZ GMGEN 00168-0278-30 5.67000 AMCINONIDE 0.1% CREAM 0 SANDOZ GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00168-0278-60 4.86000 AMCINONIDE 0.1% CREAM 0 SANDOZ GMGEN 51672-4054-01 1.29999 AMCINONIDE 0.1% CREAM 0 TARO PHARM USA GMGEN 51672-4054-02 0.97200 AMCINONIDE 0.1% CREAM 0 TARO PHARM USA GMGEN 51672-4054-03 0.83124 AMCINONIDE 0.1% CREAM 0 TARO PHARM USA GMBND 00168-0280-60 4.50592 AMCINONIDE 0.1% LOTION 0 SANDOZ MLBND 00168-0279-60 0.94450 5.37840 AMCINONIDE 0.1% OINTMENT 0 SANDOZ GMBND 00173-0561-00 5.72429 33.21291 AMERGE 1 MG TABLET G GLAXOSMITHKLINE EABND 00173-0562-00 4.76000 33.21291 AMERGE 2.5 MG TABLET G GLAXOSMITHKLINE EAGEX 52544-0228-29 2.21332 AMETHIA LO TABLET 0 ACTAVIS PHARMA, EAGEX 52544-0268-29 2.21332 AMETHIA 0.15-0.03-0.01 MG TAB 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 52544-0295-28 1.66320 1.76078 AMETHYST 90-20 MCG TABLET 0 ACTAVIS PHARMA, EABEX 52544-0295-31 1.66320 1.76078 AMETHYST 90-20 MCG TABLET 0 ACTAVIS PHARMA, EAGEN 55390-0226-04 3.03750 AMIKACIN SULF 1 GRAM/4 ML VIAL 0 BEDFORD LABS MLGEN 55390-0226-02 3.01500 AMIKACIN SULFATE 500 MG/2 ML 0 BEDFORD LABS MLGEN 00703-9040-03 3.67650 AMIKACIN 1,000 MG/4 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-9032-03 3.28300 AMIKACIN 500 MG/2 ML 0 TEVA PARENTERAL MLGEN 00574-0292-01 0.68189 AMILORIDE HCL 5 MG TABLET 0 PADDOCK LABS. EAGEN 49884-0117-01 0.68189 AMILORIDE HCL 5 MG TABLET 0 PAR PHARM. EAGEN 49884-0117-10 0.68189 AMILORIDE HCL 5 MG TABLET 0 PAR PHARM. EAGEN 64980-0151-01 0.68189 AMILORIDE HCL 5 MG TABLET 0 RISING PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-0577-01 0.<strong>06</strong>750 AMILORIDE HCL-HCTZ 5-50 MG TAB 0 MYLAN EAGUL 00378-0577-05 0.<strong>06</strong>750 AMILORIDE HCL-HCTZ 5-50 MG TAB 0 MYLAN EAGUL 00555-0483-02 0.<strong>06</strong>750 AMILORIDE HCL-HCTZ 5-50 MG TAB 0 BARR EAGUL 00555-0483-05 0.<strong>06</strong>750 AMILORIDE HCL-HCTZ 5-50 MG TAB 0 BARR EABND 61748-0046-01 18.61939 AMINOCAPROIC ACID 1,000 MG TAB 0 VERSA PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 14LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 61748-0044-<strong>08</strong> 0.88020 3.19963 AMINOCAPROIC ACID 25% SOLUTION 0 VERSA PHARMACEU MLBND 61748-0044-16 0.88020 3.33461 AMINOCAPROIC ACID 25% SOLUTION 0 VERSA PHARMACEU MLGEN 00517-9120-25 0.07550 AMINOCAPROIC ACID 250 MG/ML 0 AMER. REGENT MLBND 61748-0045-01 1.45760 6.48877 AMINOCAPROIC ACID 500 MG TAB 0 VERSA PHARMACEU EAGEN 51672-4055-<strong>06</strong> 5.57225 AMIODARONE HCL 100 MG TABLET 0 TARO PHARM USA EAGEN 00093-9133-<strong>06</strong> 0.15390 AMIODARONE HCL 200 MG TABLET 0 TEVA USA EAGEN 00093-9133-52 0.15390 AMIODARONE HCL 200 MG TABLET 0 TEVA USA EAGEN 00185-0144-05 0.15390 AMIODARONE HCL 200 MG TABLET 0 SANDOZ EAGEN 00185-0144-09 0.15390 AMIODARONE HCL 200 MG TABLET 0 SANDOZ EAGEN 00185-0144-60 0.15390 AMIODARONE HCL 200 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-09<strong>06</strong>-17 0.15390 AMIODARONE HCL 200 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-09<strong>06</strong>-19 0.15390 AMIODARONE HCL 200 MG TABLET 0 MYLAN INSTITUTI EAGEN 51672-4025-04 0.15390 AMIODARONE HCL 200 MG TABLET 0 TARO PHARM USA EAGEN 63739-0387-10 0.15390 AMIODARONE HCL 200 MG TABLET 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0371-01 0.15390 AMIODARONE HCL 200 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0371-11 0.15390 AMIODARONE HCL 200 MG TABLET 0 AHP EAGEN 68382-0227-05 0.15390 AMIODARONE HCL 200 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0227-14 0.15390 AMIODARONE HCL 200 MG TABLET 0 ZYDUS PHARMACEU EAGEN 51672-4057-<strong>06</strong> 3.96875 AMIODARONE HCL 400 MG TABLET 0 TARO PHARM USA EAGEN 51862-0156-30 5.6<strong>06</strong>25 AMIODARONE HCL 400 MG TABLET 0 LIBERTAS PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64764-0240-60 4.56223 AMITIZA 24 MCG CAPSULES G TAKEDA PHARMACE EABND 64764-0<strong>08</strong>0-60 4.56223 AMITIZA 8 MCG CAPSULE G TAKEDA PHARMACE EAGEX 38779-0189-04 13.18140 AMITRIPTYLINE HCL POWDER 0 MEDISCA INC. GMGEX 38779-0189-05 13.18125 AMITRIPTYLINE HCL POWDER 0 MEDISCA INC. GMGEX 38779-0189-09 13.18125 AMITRIPTYLINE HCL POWDER 0 MEDISCA INC. GMGEX 00378-2610-01 0.01980 AMITRIPTYLINE HCL 10 MG TAB 0 MYLAN EAGEX 00378-2610-10 0.01980 AMITRIPTYLINE HCL 10 MG TAB 0 MYLAN EAGEX 0<strong>06</strong>03-2212-02 0.01980 AMITRIPTYLINE HCL 10 MG TAB 0 QUALITEST EAGEX 0<strong>06</strong>03-2212-16 0.01980 AMITRIPTYLINE HCL 10 MG TAB 0 QUALITEST EAGEX 0<strong>06</strong>03-2212-21 0.01980 AMITRIPTYLINE HCL 10 MG TAB 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-2212-32 0.01980 AMITRIPTYLINE HCL 10 MG TAB 0 QUALITEST EAGEX 00781-1486-01 0.01980 AMITRIPTYLINE HCL 10 MG TAB 0 SANDOZ EAGEX 00781-1486-10 0.01980 AMITRIPTYLINE HCL 10 MG TAB 0 SANDOZ EAGEX 51079-0131-20 0.01980 AMITRIPTYLINE HCL 10 MG TAB 0 MYLAN INSTITUTI EAGEX 51079-0131-63 0.01980 AMITRIPTYLINE HCL 10 MG TAB 0 MYLAN INSTITUTI EAGEX 00378-2685-01 0.05520 AMITRIPTYLINE HCL 100 MG TAB 0 MYLAN EAGEX 00378-2685-93 0.05520 AMITRIPTYLINE HCL 100 MG TAB 0 MYLAN EAGEX 0<strong>06</strong>03-2216-21 0.05520 AMITRIPTYLINE HCL 100 MG TAB 0 QUALITEST EAGEX 0<strong>06</strong>03-2216-25 0.05520 AMITRIPTYLINE HCL 100 MG TAB 0 QUALITEST EAGEX 00781-1490-01 0.05520 AMITRIPTYLINE HCL 100 MG TAB 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0563-20 0.05520 AMITRIPTYLINE HCL 100 MG TAB 0 MYLAN INSTITUTI EAGEX 00378-2695-01 0.1<strong>08</strong>40 AMITRIPTYLINE HCL 150 MG TAB 0 MYLAN EAGEX 00378-2695-93 0.1<strong>08</strong>40 AMITRIPTYLINE HCL 150 MG TAB 0 MYLAN EAGEX 0<strong>06</strong>03-2217-21 0.1<strong>08</strong>40 AMITRIPTYLINE HCL 150 MG TAB 0 QUALITEST EAGEX 00781-1491-01 0.1<strong>08</strong>40 AMITRIPTYLINE HCL 150 MG TAB 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 15LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-2625-01 0.02201 AMITRIPTYLINE HCL 25 MG TAB 0 MYLAN EAGEX 00378-2625-10 0.02201 AMITRIPTYLINE HCL 25 MG TAB 0 MYLAN EAGEX 0<strong>06</strong>03-2213-02 0.02201 AMITRIPTYLINE HCL 25 MG TAB 0 QUALITEST EAGEX 0<strong>06</strong>03-2213-21 0.02201 AMITRIPTYLINE HCL 25 MG TAB 0 QUALITEST EAGEX 0<strong>06</strong>03-2213-32 0.02201 AMITRIPTYLINE HCL 25 MG TAB 0 QUALITEST EAGEX 00781-1487-01 0.02201 AMITRIPTYLINE HCL 25 MG TAB 0 SANDOZ EAGEX 00781-1487-10 0.02201 AMITRIPTYLINE HCL 25 MG TAB 0 SANDOZ EAGEX 51079-0107-20 0.02201 AMITRIPTYLINE HCL 25 MG TAB 0 MYLAN INSTITUTI EAGEX 51079-0107-63 0.02201 AMITRIPTYLINE HCL 25 MG TAB 0 MYLAN INSTITUTI EAGEX 00378-2650-01 0.02590 AMITRIPTYLINE HCL 50 MG TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-2650-10 0.02590 AMITRIPTYLINE HCL 50 MG TAB 0 MYLAN EAGEX 0<strong>06</strong>03-2214-21 0.02590 AMITRIPTYLINE HCL 50 MG TAB 0 QUALITEST EAGEX 0<strong>06</strong>03-2214-32 0.02590 AMITRIPTYLINE HCL 50 MG TAB 0 QUALITEST EAGEX 00781-1488-01 0.02590 AMITRIPTYLINE HCL 50 MG TAB 0 SANDOZ EAGEX 00781-1488-10 0.02590 AMITRIPTYLINE HCL 50 MG TAB 0 SANDOZ EAGEX 51079-0133-20 0.02590 AMITRIPTYLINE HCL 50 MG TAB 0 MYLAN INSTITUTI EAGEX 51079-0133-63 0.02590 AMITRIPTYLINE HCL 50 MG TAB 0 MYLAN INSTITUTI EAGEX 00378-2675-01 0.04750 AMITRIPTYLINE HCL 75 MG TAB 0 MYLAN EAGEX 00378-2675-93 0.04750 AMITRIPTYLINE HCL 75 MG TAB 0 MYLAN EAGEX 0<strong>06</strong>03-2215-21 0.04750 AMITRIPTYLINE HCL 75 MG TAB 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-2215-25 0.04750 AMITRIPTYLINE HCL 75 MG TAB 0 QUALITEST EAGEX 00781-1489-01 0.04750 AMITRIPTYLINE HCL 75 MG TAB 0 SANDOZ EAGEX 51079-0147-20 0.04750 AMITRIPTYLINE HCL 75 MG TAB 0 MYLAN INSTITUTI EAGEN 00054-0102-22 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 ROXANE LABS. EAGEN 00054-0102-28 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 ROXANE LABS. EAGEN 00093-7168-98 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 TEVA USA EAGEN 00143-9961-09 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 WEST-WARD,INC. EAGEN 00378-5210-05 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 MYLAN EAGEN 00378-5210-77 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 MYLAN EAGEN 0<strong>06</strong>03-2110-02 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-2110-16 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-2110-28 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-2110-32 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-2110-33 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-2110-60 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 QUALITEST EAGEN 00904-5993-61 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 MAJOR PHARMACEU EAGEN 00904-6371-61 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 MAJOR PHARMACEU EAGEN 13668-0024-05 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 TORRENT PHARMAC EAGEN 13668-0024-10 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 TORRENT PHARMAC EAGEN 31722-0239-10 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0239-90 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 CAMBER PHARMACE EAGEN 428<strong>06</strong>-0057-05 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0057-09 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0057-10 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 EPIC PHARMA LLC EAGEN 43547-0232-09 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 SOLCO <strong>HEALTH</strong>CAR EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 16LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0452-01 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0452-17 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0452-19 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0452-20 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0452-56 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 MYLAN INSTITUTI EAGEN 54458-<strong>08</strong>95-02 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 INTERNATIONAL L EAGEN 57664-0057-13 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 CARACO PHARM EAGEN 57664-0057-18 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 CARACO PHARM EAGEN 57664-0057-99 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 CARACO PHARM EAGEN 57664-0570-13 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 CARACO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 57664-0570-18 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 CARACO PHARM EAGEN 57664-0570-99 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 CARACO PHARM EAGEN 58517-0120-30 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 <strong>NEW</strong> HORIZON RX EAGEN 59762-1540-01 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 GREENSTONE LLC. EAGEN 59762-1540-02 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 GREENSTONE LLC. EAGEN 59762-1540-03 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 GREENSTONE LLC. EAGEN 60505-0195-02 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 APOTEX CORP EAGEN 60505-0195-03 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 APOTEX CORP EAGEN 64679-0423-01 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 WOCKHARDT USA L EAGEN 64679-0423-02 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-00<strong>08</strong>-09 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 AMNEAL PHARMACE EAGEN 65162-00<strong>08</strong>-50 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 AMNEAL PHARMACE EAGEN 65862-0103-05 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0103-90 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0103-99 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 AUROBINDO PHARM EAGEN 67877-0199-05 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 ASCEND LABORATO EAGEN 67877-0199-10 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 ASCEND LABORATO EAGEN 67877-0199-90 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 ASCEND LABORATO EAGEN 68<strong>08</strong>4-0260-01 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0260-11 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-05<strong>06</strong>-01 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-05<strong>06</strong>-11 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 AHP EAGEN 68180-0752-03 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 LUPIN PHARMACEU EAGEN 68180-0752-09 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 LUPIN PHARMACEU EAGEN 68382-0123-05 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 ZYDUS PHARMACEU EAGEN 68382-0123-16 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 ZYDUS PHARMACEU EAGEN 68645-0445-70 0.02550 AMLODIPINE BESYLATE 10 MG TAB 0 LEGACY PHARMACE EAGEN 00054-0100-22 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 ROXANE LABS. EAGEN 00093-0<strong>08</strong>3-98 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 TEVA USA EAGEN 00143-9959-09 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-52<strong>08</strong>-05 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 MYLAN EAGEN 00378-52<strong>08</strong>-77 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 MYLAN EAGEN 0<strong>06</strong>03-21<strong>08</strong>-02 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-21<strong>08</strong>-25 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-21<strong>08</strong>-28 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 QUALITEST EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 17LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-21<strong>08</strong>-32 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 QUALITEST EAGEN 00904-5991-61 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 MAJOR PHARMACEU EAGEN 00904-6369-61 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 MAJOR PHARMACEU EAGEN 13668-0022-03 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 TORRENT PHARMAC EAGEN 13668-0022-05 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 TORRENT PHARMAC EAGEN 31722-0237-10 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 CAMBER PHARMACE EAGEN 31722-0237-90 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 CAMBER PHARMACE EAGEN 428<strong>06</strong>-0055-05 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0055-09 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0055-10 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 EPIC PHARMA LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0450-01 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0450-20 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0450-63 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0450-66 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 MYLAN INSTITUTI EAGEN 57664-0055-18 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 CARACO PHARM EAGEN 57664-0055-99 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 CARACO PHARM EAGEN 57664-0568-13 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 CARACO PHARM EAGEN 57664-0568-18 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 CARACO PHARM EAGEN 57664-0568-99 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 CARACO PHARM EAGEN 59762-1520-01 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-1520-02 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 GREENSTONE LLC. EAGEN 60505-0193-02 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 APOTEX CORP EAGEN 60505-0193-03 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 APOTEX CORP EAGEN 64679-0421-01 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 WOCKHARDT USA L EAGEN 65162-00<strong>06</strong>-09 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 AMNEAL PHARMACE EAGEN 65162-00<strong>06</strong>-50 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 AMNEAL PHARMACE EAGEN 65862-0101-05 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0101-90 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0101-99 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 AUROBINDO PHARM EAGEN 67877-0197-05 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 ASCEND LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67877-0197-10 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 ASCEND LABORATO EAGEN 67877-0197-90 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 ASCEND LABORATO EAGEN 68<strong>08</strong>4-0258-01 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0258-11 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0498-01 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0498-11 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 AHP EAGEN 68180-0750-09 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 LUPIN PHARMACEU EAGEN 68382-0121-05 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 ZYDUS PHARMACEU EAGEN 68382-0121-16 0.03540 AMLODIPINE BESYLATE 2.5 MG TAB 0 ZYDUS PHARMACEU EAGEN 00054-0101-22 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0101-28 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 ROXANE LABS. EAGEN 00093-7167-55 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 TEVA USA EAGEN 00093-7167-98 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 TEVA USA EAGEN 00143-9960-09 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 WEST-WARD,INC. EAGEN 00378-5209-05 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 18LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5209-77 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 MYLAN EAGEN 0<strong>06</strong>03-2109-02 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-2109-25 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-2109-28 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-2109-32 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-2109-34 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 QUALITEST EAGEN 00904-5992-61 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 MAJOR PHARMACEU EAGEN 00904-6370-61 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 MAJOR PHARMACEU EAGEN 13668-0023-03 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 TORRENT PHARMAC EAGEN 31722-0238-10 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0238-90 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 CAMBER PHARMACE EAGEN 428<strong>06</strong>-0056-05 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0056-09 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0056-10 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 EPIC PHARMA LLC EAGEN 43547-0231-09 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 51079-0451-01 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0451-17 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0451-19 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0451-20 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0451-56 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0451-69 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 MYLAN INSTITUTI EAGEN 54458-0904-02 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 INTERNATIONAL L EAGEN 57664-0056-13 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 CARACO PHARM EAGEN 57664-0056-18 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 CARACO PHARM EAGEN 57664-0056-99 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 CARACO PHARM EAGEN 57664-0569-13 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 CARACO PHARM EAGEN 57664-0569-18 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 CARACO PHARM EAGEN 57664-0569-99 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 CARACO PHARM EAGEN 59762-1530-01 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 GREENSTONE LLC. EAGEN 59762-1530-02 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-1530-03 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 GREENSTONE LLC. EAGEN 59762-1530-04 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 GREENSTONE LLC. EAGEN 59762-1530-05 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 GREENSTONE LLC. EAGEN 59762-1530-<strong>06</strong> 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 GREENSTONE LLC. EAGEN 60505-0194-02 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 APOTEX CORP EAGEN 60505-0194-03 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 APOTEX CORP EAGEN 64679-0422-01 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 WOCKHARDT USA L EAGEN 64679-0422-02 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 WOCKHARDT USA L EAGEN 65162-0007-09 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 AMNEAL PHARMACE EAGEN 65162-0007-50 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0102-05 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0102-90 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0102-99 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 AUROBINDO PHARM EAGEN 67877-0198-05 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 ASCEND LABORATO EAGEN 67877-0198-10 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 ASCEND LABORATO EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 19LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67877-0198-90 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 ASCEND LABORATO EAGEN 68<strong>08</strong>4-0505-01 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0505-11 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 AHP EAGEN 68180-0751-03 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 LUPIN PHARMACEU EAGEN 68180-0751-09 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 LUPIN PHARMACEU EAGEN 68382-0122-05 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 ZYDUS PHARMACEU EAGEN 68382-0122-16 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 ZYDUS PHARMACEU EAGEN 68645-0444-70 0.03740 AMLODIPINE BESYLATE 5 MG TAB 0 LEGACY PHARMACE EAGEN 00378-6168-05 4.55754 AMLODIPINE-ATORVAST 10-10 MG G MYLAN EAGEN 00378-6168-93 4.55754 AMLODIPINE-ATORVAST 10-10 MG G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63304-0590-30 4.55754 AMLODIPINE-ATORVAST 10-10 MG G RANBAXY PHARMAC EAGEN 00378-6169-05 6.53713 AMLODIPINE-ATORVAST 10-20 MG G MYLAN EAGEN 00378-6169-93 6.53713 AMLODIPINE-ATORVAST 10-20 MG G MYLAN EAGEN 63304-0591-30 6.53713 AMLODIPINE-ATORVAST 10-20 MG G RANBAXY PHARMAC EAGEN 00378-4519-93 7.02849 AMLODIPINE-ATORVAST 10-40 MG G MYLAN EAGEN 00378-6170-05 7.02838 AMLODIPINE-ATORVAST 10-40 MG G MYLAN EAGEN 00378-6170-93 7.02849 AMLODIPINE-ATORVAST 10-40 MG G MYLAN EAGEN 63304-0500-30 7.02849 AMLODIPINE-ATORVAST 10-40 MG G RANBAXY PHARMAC EAGEN 00378-6171-93 5.99538 AMLODIPINE-ATORVAST 10-80 MG G MYLAN EAGEN 63304-<strong>06</strong>03-30 5.99538 AMLODIPINE-ATORVAST 10-80 MG G RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6161-93 4.55754 AMLODIPINE-ATORVAST 2.5-10 MG G MYLAN EAGEN 63304-0501-30 4.55754 AMLODIPINE-ATORVAST 2.5-10 MG G RANBAXY PHARMAC EAGEN 00378-6162-93 5.99538 AMLODIPINE-ATORVAST 2.5-20 MG G MYLAN EAGEN 63304-0502-30 5.99538 AMLODIPINE-ATORVAST 2.5-20 MG G RANBAXY PHARMAC EAGEN 00378-6163-93 5.99538 AMLODIPINE-ATORVAST 2.5-40 MG G MYLAN EAGEN 63304-0503-30 5.99538 AMLODIPINE-ATORVAST 2.5-40 MG G RANBAXY PHARMAC EAGEN 00378-6164-05 4.96899 AMLODIPINE-ATORVAST 5-10 MG G MYLAN EAGEN 00378-6164-93 4.96899 AMLODIPINE-ATORVAST 5-10 MG G MYLAN EAGEN 63304-0587-30 4.96899 AMLODIPINE-ATORVAST 5-10 MG G RANBAXY PHARMAC EAGEN 00378-6165-05 6.11963 AMLODIPINE-ATORVAST 5-20 MG G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6165-93 6.11963 AMLODIPINE-ATORVAST 5-20 MG G MYLAN EAGEN 63304-0588-30 6.11963 AMLODIPINE-ATORVAST 5-20 MG G RANBAXY PHARMAC EAGEN 00378-6166-05 5.01000 AMLODIPINE-ATORVAST 5-40 MG G MYLAN EAGEN 00378-6166-93 5.01000 AMLODIPINE-ATORVAST 5-40 MG G MYLAN EAGEN 63304-0589-30 5.01000 AMLODIPINE-ATORVAST 5-40 MG G RANBAXY PHARMAC EAGEN 00378-6167-93 5.99538 AMLODIPINE-ATORVAST 5-80 MG G MYLAN EAGEN 63304-0499-30 5.99538 AMLODIPINE-ATORVAST 5-80 MG G RANBAXY PHARMAC EAGEN 00093-7373-01 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 TEVA USA EAGEN 00093-7373-10 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 TEVA USA EAGEN 00378-6898-01 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6898-05 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 MYLAN EAGEN 00591-3760-01 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 ACTAVIS PHARMA, EAGEN 00591-3760-05 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 ACTAVIS PHARMA, EAGEN 00781-2274-01 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 SANDOZ EAGEN 00781-2274-10 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 20LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0932-01 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 PAR PHARM. EAGEN 49884-0932-05 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 PAR PHARM. EAGEN 55111-0341-01 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 DR.REDDY'S LAB EAGEN 55111-0341-05 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 DR.REDDY'S LAB EAGEN 65862-0586-01 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 AUROBINDO PHARM EAGEN 65862-0586-05 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 AUROBINDO PHARM EAGEN 68180-0758-01 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 LUPIN PHARMACEU EAGEN 68180-0758-02 0.57710 AMLODIPINE-BENAZEPRIL 10-20 MG 0 LUPIN PHARMACEU EAGEN 00093-7671-01 0.98960 AMLODIPINE-BENAZEPRIL 10-40 MG 0 TEVA USA EAGEN 00378-6900-01 0.98960 AMLODIPINE-BENAZEPRIL 10-40 MG 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3762-01 0.98960 AMLODIPINE-BENAZEPRIL 10-40 MG 0 ACTAVIS PHARMA, EAGEN 00781-2279-01 0.98960 AMLODIPINE-BENAZEPRIL 10-40 MG 0 SANDOZ EAGEN 49884-0953-01 0.98960 AMLODIPINE-BENAZEPRIL 10-40 MG 0 PAR PHARM. EAGEN 49884-0953-05 0.98960 AMLODIPINE-BENAZEPRIL 10-40 MG 0 PAR PHARM. EAGEN 55111-0586-01 0.98960 AMLODIPINE-BENAZEPRIL 10-40 MG 0 DR.REDDY'S LAB EAGEN 65862-0587-01 0.98960 AMLODIPINE-BENAZEPRIL 10-40 MG 0 AUROBINDO PHARM EAGEN 65862-0587-05 0.98960 AMLODIPINE-BENAZEPRIL 10-40 MG 0 AUROBINDO PHARM EAGEN 68180-0760-01 0.98960 AMLODIPINE-BENAZEPRIL 10-40 MG 0 LUPIN PHARMACEU EAGEN 00093-7370-01 0.69420 AMLODIPINE-BENAZEPRIL 2.5-10 0 TEVA USA EAGEN 00378-6895-01 0.69420 AMLODIPINE-BENAZEPRIL 2.5-10 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3757-01 0.69420 AMLODIPINE-BENAZEPRIL 2.5-10 0 ACTAVIS PHARMA, EAGEN 00781-2271-01 0.69420 AMLODIPINE-BENAZEPRIL 2.5-10 0 SANDOZ EAGEN 49884-0929-01 0.69420 AMLODIPINE-BENAZEPRIL 2.5-10 0 PAR PHARM. EAGEN 49884-0929-05 0.69420 AMLODIPINE-BENAZEPRIL 2.5-10 0 PAR PHARM. EAGEN 55111-0338-01 0.69420 AMLODIPINE-BENAZEPRIL 2.5-10 0 DR.REDDY'S LAB EAGEN 65862-0582-01 0.69420 AMLODIPINE-BENAZEPRIL 2.5-10 0 AUROBINDO PHARM EAGEN 65862-0582-05 0.69420 AMLODIPINE-BENAZEPRIL 2.5-10 0 AUROBINDO PHARM EAGEN 68180-0755-01 0.69420 AMLODIPINE-BENAZEPRIL 2.5-10 0 LUPIN PHARMACEU EAGEN 00093-7371-01 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 TEVA USA EAGEN 00093-7371-10 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6896-01 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 MYLAN EAGEN 00378-6896-05 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 MYLAN EAGEN 00591-3758-01 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 ACTAVIS PHARMA, EAGEN 00591-3758-05 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 ACTAVIS PHARMA, EAGEN 00781-2272-01 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 SANDOZ EAGEN 00781-2272-10 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 SANDOZ EAGEN 49884-0930-01 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 PAR PHARM. EAGEN 49884-0930-05 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 PAR PHARM. EAGEN 55111-0339-01 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 DR.REDDY'S LAB EAGEN 55111-0339-05 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0583-01 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 AUROBINDO PHARM EAGEN 65862-0583-05 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 AUROBINDO PHARM EAGEN 68180-0756-01 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 LUPIN PHARMACEU EAGEN 68180-0756-02 0.47840 AMLODIPINE-BENAZEPRIL 5-10 MG 0 LUPIN PHARMACEU EAGEN 00093-7372-01 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 21LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7372-10 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 TEVA USA EAGEN 00378-6897-01 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 MYLAN EAGEN 00378-6897-05 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 MYLAN EAGEN 00591-3759-01 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 ACTAVIS PHARMA, EAGEN 00591-3759-05 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 ACTAVIS PHARMA, EAGEN 00781-2273-01 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 SANDOZ EAGEN 00781-2273-10 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 SANDOZ EAGEN 49884-0931-01 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 PAR PHARM. EAGEN 49884-0931-05 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 PAR PHARM. EAGEN 55111-0340-01 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0340-05 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 DR.REDDY'S LAB EAGEN 65862-0584-01 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 AUROBINDO PHARM EAGEN 65862-0584-05 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 AUROBINDO PHARM EAGEN 68180-0757-01 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 LUPIN PHARMACEU EAGEN 68180-0757-02 0.45364 AMLODIPINE-BENAZEPRIL 5-20 MG 0 LUPIN PHARMACEU EAGEN 00093-7670-01 0.81297 AMLODIPINE-BENAZEPRIL 5-40 MG 0 TEVA USA EAGEN 00378-6899-01 0.81297 AMLODIPINE-BENAZEPRIL 5-40 MG 0 MYLAN EAGEN 00591-3761-01 0.81297 AMLODIPINE-BENAZEPRIL 5-40 MG 0 ACTAVIS PHARMA, EAGEN 00781-2277-01 0.81297 AMLODIPINE-BENAZEPRIL 5-40 MG 0 SANDOZ EAGEN 49884-0952-01 0.81297 AMLODIPINE-BENAZEPRIL 5-40 MG 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0952-05 0.81297 AMLODIPINE-BENAZEPRIL 5-40 MG 0 PAR PHARM. EAGEN 55111-0587-01 0.81297 AMLODIPINE-BENAZEPRIL 5-40 MG 0 DR.REDDY'S LAB EAGEN 65862-0585-01 0.81297 AMLODIPINE-BENAZEPRIL 5-40 MG 0 AUROBINDO PHARM EAGEN 65862-0585-05 0.81297 AMLODIPINE-BENAZEPRIL 5-40 MG 0 AUROBINDO PHARM EAGEN 68180-0759-01 0.81297 AMLODIPINE-BENAZEPRIL 5-40 MG 0 LUPIN PHARMACEU EAGEN 00591-2157-38 0.04653 AMMONIUM LACTATE 12% CREAM 0 ACTAVIS PHARMA, GMGEN 00591-2157-80 0.04653 AMMONIUM LACTATE 12% CREAM 0 ACTAVIS PHARMA, GMGEN 45802-0493-26 0.04653 AMMONIUM LACTATE 12% CREAM 0 PERRIGO CO. GMGEN 45802-0493-83 0.04653 AMMONIUM LACTATE 12% CREAM 0 PERRIGO CO. GMGEN 51672-1301-00 0.04653 AMMONIUM LACTATE 12% CREAM 0 TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-1301-04 0.04653 AMMONIUM LACTATE 12% CREAM 0 TARO PHARM USA GMGEN 00591-2158-22 0.03170 AMMONIUM LACTATE 12% LOTION 0 ACTAVIS PHARMA, GMGEN 00591-2158-46 0.03170 AMMONIUM LACTATE 12% LOTION 0 ACTAVIS PHARMA, GMGEN 45802-0419-26 0.03170 AMMONIUM LACTATE 12% LOTION 0 PERRIGO CO. GMGEN 45802-0419-54 0.03170 AMMONIUM LACTATE 12% LOTION 0 PERRIGO CO. GMGEN 51672-1300-05 0.03170 AMMONIUM LACTATE 12% LOTION 0 TARO PHARM USA GMGEN 51672-1300-09 0.03170 AMMONIUM LACTATE 12% LOTION 0 TARO PHARM USA GMGEN 00378-6611-93 7.36020 AMNESTEEM 10 MG CAPSULE 0 MYLAN EAGEN 00378-6612-93 8.43370 AMNESTEEM 20 MG CAPSULE 0 MYLAN EAGEN 00378-6614-93 8.98670 AMNESTEEM 40 MG CAPSULE 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-2270-34 1.<strong>08</strong>560 AMOX TR-K CLV 200-28.5 TAB CHW 0 TEVA USA EAGEN 00781-1619-66 1.<strong>08</strong>560 AMOX TR-K CLV 200-28.5 TAB CHW 0 SANDOZ EAGEN 00093-2277-73 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 TEVA USA MLGEN 00143-9981-01 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 WEST-WARD,INC. MLGEN 00143-9981-75 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 WEST-WARD,INC. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 22LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0292-01 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 NORTHSTAR RX LL MLGEN 16714-0292-02 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 NORTHSTAR RX LL MLGEN 16714-0292-03 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 NORTHSTAR RX LL MLGEN 43598-0213-50 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 DR.REDDY'S LAB MLGEN 43598-0213-51 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 DR.REDDY'S LAB MLGEN 43598-0213-52 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 DR.REDDY'S LAB MLGEN 65862-0533-01 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 AUROBINDO PHARM MLGEN 65862-0533-50 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 AUROBINDO PHARM MLGEN 65862-0533-75 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 AUROBINDO PHARM MLGEN 66685-1011-00 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 66685-1011-01 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 SANDOZ MLGEN 66685-1011-02 0.10409 AMOX TR-K CLV 200-28.5/5 SUSP 0 SANDOZ MLGEN 00781-1874-31 4.43874 AMOX TR-K CLV 250-125 MG TAB 0 SANDOZ EAGEN 60505-2539-03 3.<strong>08</strong>750 AMOX TR-K CLV 250-125 MG TAB 0 APOTEX CORP EAGEN 65862-0501-30 4.43874 AMOX TR-K CLV 250-125 MG TAB 0 AUROBINDO PHARM EAGEN 43598-0204-53 0.78770 AMOX TR-K CLV 250-62.5/5 SUSP 0 DR.REDDY'S LAB MLGEN 60432-0<strong>06</strong>5-00 0.64822 AMOX TR-K CLV 250-62.5/5 SUSP 0 MORTON GROVE PH MLGEN 60432-0<strong>06</strong>5-47 0.63500 AMOX TR-K CLV 250-62.5/5 SUSP 0 MORTON GROVE PH MLGEN 60432-0<strong>06</strong>5-75 0.64730 AMOX TR-K CLV 250-62.5/5 SUSP 0 MORTON GROVE PH MLGEN 00093-2272-34 1.69760 AMOX TR-K CLV 400-57 TAB CHEW 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-1643-66 1.69760 AMOX TR-K CLV 400-57 TAB CHEW 0 SANDOZ EAGEN 00093-2279-73 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 TEVA USA MLGEN 00143-9982-01 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 WEST-WARD,INC. MLGEN 00143-9982-50 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 WEST-WARD,INC. MLGEN 00143-9982-75 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 WEST-WARD,INC. MLGEN 16714-0293-01 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 NORTHSTAR RX LL MLGEN 16714-0293-02 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 NORTHSTAR RX LL MLGEN 16714-0293-03 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 NORTHSTAR RX LL MLGEN 43598-02<strong>08</strong>-50 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 DR.REDDY'S LAB MLGEN 43598-02<strong>08</strong>-51 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 DR.REDDY'S LAB ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43598-02<strong>08</strong>-52 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 DR.REDDY'S LAB MLGEN 65862-0534-01 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 AUROBINDO PHARM MLGEN 65862-0534-50 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 AUROBINDO PHARM MLGEN 65862-0534-75 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 AUROBINDO PHARM MLGEN 66685-1012-00 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 SANDOZ MLGEN 66685-1012-01 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 SANDOZ MLGEN 66685-1012-02 0.15188 AMOX TR-K CLV 400-57/5 SUSP 0 SANDOZ MLGEN 00093-2274-34 0.98700 AMOX TR-K CLV 500-125 MG TAB 0 TEVA USA EAGEN 16714-0296-01 0.98700 AMOX TR-K CLV 500-125 MG TAB 0 NORTHSTAR RX LL EAGEN 43598-02<strong>06</strong>-14 0.98700 AMOX TR-K CLV 500-125 MG TAB 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2540-02 0.98700 AMOX TR-K CLV 500-125 MG TAB 0 APOTEX CORP EAGEN 65862-0502-20 0.98700 AMOX TR-K CLV 500-125 MG TAB 0 AUROBINDO PHARM EAGEN 66685-1002-00 0.98700 AMOX TR-K CLV 500-125 MG TAB 0 SANDOZ EAGEN 66685-1002-02 0.98700 AMOX TR-K CLV 500-125 MG TAB 0 SANDOZ EAGEN 00093-8675-74 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 TEVA USA ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 23LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-8675-75 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 TEVA USA MLGEN 00093-8675-78 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 TEVA USA MLGEN 00143-9853-16 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 WEST-WARD,INC. MLGEN 00143-9853-24 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 WEST-WARD,INC. MLGEN 00143-9853-75 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 WEST-WARD,INC. MLGEN 00781-6139-48 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 SANDOZ MLGEN 00781-6139-54 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 SANDOZ MLGEN 00781-6139-57 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 SANDOZ MLGEN 16714-0294-01 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 NORTHSTAR RX LL MLGEN 16714-0294-02 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 NORTHSTAR RX LL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0294-03 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 NORTHSTAR RX LL MLGEN 43598-0203-51 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 DR.REDDY'S LAB MLGEN 43598-0203-54 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 DR.REDDY'S LAB MLGEN 43598-0203-69 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 DR.REDDY'S LAB MLGEN 65862-0535-02 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 AUROBINDO PHARM MLGEN 65862-0535-13 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 AUROBINDO PHARM MLGEN 65862-0535-75 0.14260 AMOX TR-K CLV 600-42.9/5 SUSP 0 AUROBINDO PHARM MLGUL 00093-2275-34 2.53200 AMOX TR-K CLV 875-125 MG TAB 0 TEVA USA EAGUL 00781-1852-20 2.53200 AMOX TR-K CLV 875-125 MG TAB 0 SANDOZ EAGUL 16714-0297-01 2.53200 AMOX TR-K CLV 875-125 MG TAB 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 16714-0297-02 2.53200 AMOX TR-K CLV 875-125 MG TAB 0 NORTHSTAR RX LL EAGUL 43598-0221-14 2.53200 AMOX TR-K CLV 875-125 MG TAB 0 DR.REDDY'S LAB EAGUL 60505-2541-02 2.53200 AMOX TR-K CLV 875-125 MG TAB 0 APOTEX CORP EAGUL 65862-0503-01 2.53200 AMOX TR-K CLV 875-125 MG TAB 0 AUROBINDO PHARM EAGUL 65862-0503-20 2.53200 AMOX TR-K CLV 875-125 MG TAB 0 AUROBINDO PHARM EAGUL 66685-1001-00 2.53200 AMOX TR-K CLV 875-125 MG TAB 0 SANDOZ EAGUL 66685-1001-01 2.53200 AMOX TR-K CLV 875-125 MG TAB 0 SANDOZ EABEX 00591-5715-01 1.38568 AMOXAPINE 100 MG TABLET 0 ACTAVIS PHARMA, EABEX 00591-5716-30 2.18539 AMOXAPINE 150 MG TABLET 0 ACTAVIS PHARMA, EABEX 00591-5713-01 0.51053 AMOXAPINE 25 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00591-5714-01 0.829<strong>08</strong> AMOXAPINE 50 MG TABLET 0 ACTAVIS PHARMA, EABND 00093-2267-01 0.22393 AMOXICILLIN 125 MG TAB CHEW 0 TEVA USA EAGEN 00093-4150-73 0.01674 AMOXICILLIN 125 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-4150-79 0.01674 AMOXICILLIN 125 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-4150-80 0.01674 AMOXICILLIN 125 MG/5 ML SUSP 0 TEVA USA MLGEN 00143-9888-15 0.01674 AMOXICILLIN 125 MG/5 ML SUSP 0 WEST-WARD,INC. MLGEN 00143-9888-80 0.01674 AMOXICILLIN 125 MG/5 ML SUSP 0 WEST-WARD,INC. MLGEN 00781-6039-46 0.01674 AMOXICILLIN 125 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6039-55 0.01674 AMOXICILLIN 125 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6039-58 0.01674 AMOXICILLIN 125 MG/5 ML SUSP 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43598-0222-52 0.01674 AMOXICILLIN 125 MG/5 ML SUSP 0 DR.REDDY'S LAB MLGEN 43598-0222-53 0.01674 AMOXICILLIN 125 MG/5 ML SUSP 0 DR.REDDY'S LAB MLGEN 43598-0222-80 0.01674 AMOXICILLIN 125 MG/5 ML SUSP 0 DR.REDDY'S LAB MLGEN 00093-4160-73 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-4160-76 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 TEVA USA ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 24LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-4160-78 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 TEVA USA MLGEN 00143-9886-01 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 WEST-WARD,INC. MLGEN 00143-9886-50 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 WEST-WARD,INC. MLGEN 00143-9886-75 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 WEST-WARD,INC. MLGEN 00781-6156-46 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6156-52 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6156-57 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 SANDOZ MLGEN 43598-0223-50 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 DR.REDDY'S LAB MLGEN 43598-0223-51 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 DR.REDDY'S LAB MLGEN 43598-0223-52 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 DR.REDDY'S LAB ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-1022-02 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 GREENSTONE LLC. MLGEN 59762-1022-04 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 GREENSTONE LLC. MLGEN 59762-1022-07 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 GREENSTONE LLC. MLGEN 65862-0070-01 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 65862-0070-50 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 65862-0070-75 0.02430 AMOXICILLIN 200 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 00093-3107-01 0.05481 AMOXICILLIN 250 MG CAPSULE 0 TEVA USA EAGEN 00093-3107-05 0.05481 AMOXICILLIN 250 MG CAPSULE 0 TEVA USA EAGEN 00143-9938-01 0.05481 AMOXICILLIN 250 MG CAPSULE 0 WEST-WARD,INC. EAGEN 00143-9938-05 0.05481 AMOXICILLIN 250 MG CAPSULE 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-2020-01 0.05481 AMOXICILLIN 250 MG CAPSULE 0 SANDOZ EAGEN 00781-2020-05 0.05481 AMOXICILLIN 250 MG CAPSULE 0 SANDOZ EAGEN 00781-2020-31 0.05481 AMOXICILLIN 250 MG CAPSULE 0 SANDOZ EAGEN 00781-2020-76 0.05481 AMOXICILLIN 250 MG CAPSULE 0 SANDOZ EAGEN 16714-0298-01 0.05481 AMOXICILLIN 250 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 16714-0298-02 0.05481 AMOXICILLIN 250 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 43598-0225-01 0.05481 AMOXICILLIN 250 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 43598-0225-05 0.05481 AMOXICILLIN 250 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 59762-1020-01 0.05481 AMOXICILLIN 250 MG CAPSULE 0 GREENSTONE LLC. EAGEN 59762-1020-03 0.05481 AMOXICILLIN 250 MG CAPSULE 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0016-01 0.05481 AMOXICILLIN 250 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0016-05 0.05481 AMOXICILLIN 250 MG CAPSULE 0 AUROBINDO PHARM EABND 00093-2268-01 0.44786 AMOXICILLIN 250 MG TAB CHEW 0 TEVA USA EABND 00093-2268-05 0.43438 AMOXICILLIN 250 MG TAB CHEW 0 TEVA USA EAGEN 00093-4155-73 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-4155-79 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-4155-80 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 TEVA USA MLGEN 00143-9889-01 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 WEST-WARD,INC. MLGEN 00143-9889-15 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 WEST-WARD,INC. MLGEN 00143-9889-80 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-6041-46 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6041-55 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6041-58 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 SANDOZ MLGEN 43598-0209-52 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 DR.REDDY'S LAB MLGEN 43598-0209-53 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 DR.REDDY'S LAB ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 25LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43598-0209-80 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 DR.REDDY'S LAB MLGEN 67253-0143-10 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 DAVA PHARMACEUT MLGEN 67253-0143-15 0.02187 AMOXICILLIN 250 MG/5 ML SUSP 0 DAVA PHARMACEUT MLGEN 00093-4161-73 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-4161-76 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-4161-78 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 TEVA USA MLGEN 00143-9887-01 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 WEST-WARD,INC. MLGEN 00143-9887-50 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 WEST-WARD,INC. MLGEN 00143-9887-75 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 WEST-WARD,INC. MLGEN 00781-6157-46 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-6157-52 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6157-57 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 SANDOZ MLGEN 43598-0207-50 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 DR.REDDY'S LAB MLGEN 43598-0207-51 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 DR.REDDY'S LAB MLGEN 43598-0207-52 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 DR.REDDY'S LAB MLGEN 59762-1023-04 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 GREENSTONE LLC. MLGEN 59762-1023-05 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 GREENSTONE LLC. MLGEN 59762-1023-<strong>06</strong> 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 GREENSTONE LLC. MLGEN 65862-0071-01 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 65862-0071-50 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 AUROBINDO PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0071-75 0.02714 AMOXICILLIN 400 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 00093-3109-05 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 TEVA USA EAGEN 00093-3109-53 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 TEVA USA EAGEN 00143-9939-01 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 WEST-WARD,INC. EAGEN 00143-9939-05 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 WEST-WARD,INC. EAGEN 00781-2613-01 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 SANDOZ EAGEN 00781-2613-05 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 SANDOZ EAGEN 00781-2613-31 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 SANDOZ EAGEN 00781-2613-76 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 SANDOZ EAGEN 15749-<strong>08</strong>25-10 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 AMERICAN ANTIBI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0299-02 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 16714-0299-03 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 16714-0299-04 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 43598-0205-01 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 43598-0205-05 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 59762-1021-01 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 GREENSTONE LLC. EAGEN 59762-1021-07 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 GREENSTONE LLC. EAGEN 65862-0017-01 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0017-05 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 AUROBINDO PHARM EAGEN 67253-0141-10 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 DAVA PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67253-0141-50 0.<strong>08</strong>980 AMOXICILLIN 500 MG CAPSULE 0 DAVA PHARMACEUT EAGEN 00093-2263-01 0.29270 AMOXICILLIN 500 MG TABLET 0 TEVA USA EAGEN 00781-5<strong>06</strong>0-20 0.29270 AMOXICILLIN 500 MG TABLET 0 SANDOZ EAGEN 43598-0224-01 0.29270 AMOXICILLIN 500 MG TABLET 0 DR.REDDY'S LAB EAGEN 65862-0014-01 0.29270 AMOXICILLIN 500 MG TABLET 0 AUROBINDO PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 26LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-2264-01 0.13500 AMOXICILLIN 875 MG TABLET 0 TEVA USA EAGEN 00143-9951-01 0.13500 AMOXICILLIN 875 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-9951-20 0.13500 AMOXICILLIN 875 MG TABLET 0 WEST-WARD,INC. EAGEN 00781-5<strong>06</strong>1-01 0.13500 AMOXICILLIN 875 MG TABLET 0 SANDOZ EAGEN 00781-5<strong>06</strong>1-20 0.13500 AMOXICILLIN 875 MG TABLET 0 SANDOZ EAGEN 43598-0219-01 0.13500 AMOXICILLIN 875 MG TABLET 0 DR.REDDY'S LAB EAGEN 43598-0219-14 0.13500 AMOXICILLIN 875 MG TABLET 0 DR.REDDY'S LAB EAGEN 59762-1050-02 0.13500 AMOXICILLIN 875 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-1050-05 0.13500 AMOXICILLIN 875 MG TABLET 0 GREENSTONE LLC. EAGEN 65862-0015-01 0.13500 AMOXICILLIN 875 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-1943-39 2.90925 AMOXICILLIN-CLAV ER 1,000-62.5 0 SANDOZ EAGEN 00781-1943-82 2.90946 AMOXICILLIN-CLAV ER 1,000-62.5 0 SANDOZ EAGEN 51927-1726-00 94.59750 AMPHOTERICIN B POWDER 0 PR<strong>OF</strong>ESSIONAL CO GMBND 39822-1055-05 37.84800 AMPHOTERICIN B 50 MG VIAL 0 X-GEN PHARMACEU EAGEN 00781-2144-01 0.09801 AMPICILLIN TR 250 MG CAPSULE 0 SANDOZ EAGEN 67253-0180-10 0.09801 AMPICILLIN TR 250 MG CAPSULE 0 DAVA PHARMACEUT EAGEN 67253-0180-50 0.09801 AMPICILLIN TR 250 MG CAPSULE 0 DAVA PHARMACEUT EAGEN 00781-2145-01 0.15404 AMPICILLIN TR 500 MG CAPSULE 0 SANDOZ EAGEN 67253-0181-10 0.15404 AMPICILLIN TR 500 MG CAPSULE 0 DAVA PHARMACEUT EABND 00781-3412-92 8.29000 14.86779 AMPICILLIN 1 GM A-V VIAL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00781-9412-15 8.29000 13.58710 AMPICILLIN 1 GM A-V VIAL 0 SANDOZ/NOVAPLUS EABND 00781-9412-92 8.29000 14.86779 AMPICILLIN 1 GM A-V VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-3404-85 2.92815 AMPICILLIN 1 GM VIAL 0 SANDOZ EAGEN 00781-3404-95 2.92815 AMPICILLIN 1 GM VIAL 0 SANDOZ EAGEN 00781-9404-85 2.92815 AMPICILLIN 1 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9404-95 2.92815 AMPICILLIN 1 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0136-10 2.92815 AMPICILLIN 1 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-0102-10 2.92815 AMPICILLIN 1 GM VIAL 0 WG CRITICAL CAR EAGEN 63323-0389-10 2.92815 AMPICILLIN 1 GM VIAL 0 APP PHARMACEUTI EAGEN 00781-3409-95 53.33330 AMPICILLIN 10 GM VIAL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-9409-95 53.33330 AMPICILLIN 10 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0138-99 53.33330 AMPICILLIN 10 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-0104-10 53.33330 AMPICILLIN 10 GM VIAL 0 WG CRITICAL CAR EABND 00781-3400-78 3.33040 4.33260 AMPICILLIN 125 MG VIAL 0 SANDOZ EABND 00781-9401-78 3.33040 4.33260 AMPICILLIN 125 MG VIAL 0 SANDOZ/NOVAPLUS EABND 00781-9401-95 3.33040 4.33260 AMPICILLIN 125 MG VIAL 0 SANDOZ/NOVAPLUS EABND 67253-0182-10 0.07918 AMPICILLIN 125 MG/5 ML SUSP 0 DAVA PHARMACEUT MLBND 00781-3413-92 16.<strong>08</strong>000 28.84<strong>08</strong>4 AMPICILLIN 2 GM A-V VIAL 0 SANDOZ EABND 00781-9413-15 16.<strong>08</strong>000 26.36<strong>08</strong>0 AMPICILLIN 2 GM A-V VIAL 0 SANDOZ/NOVAPLUS EABND 00781-9413-92 16.<strong>08</strong>000 28.84<strong>08</strong>4 AMPICILLIN 2 GM A-V VIAL 0 SANDOZ/NOVAPLUS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-34<strong>08</strong>-80 6.57000 AMPICILLIN 2 GM VIAL 0 SANDOZ EAGEN 00781-34<strong>08</strong>-95 6.57000 AMPICILLIN 2 GM VIAL 0 SANDOZ EAGEN 00781-94<strong>08</strong>-80 6.57000 AMPICILLIN 2 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-94<strong>08</strong>-95 6.57000 AMPICILLIN 2 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0137-20 6.57000 AMPICILLIN 2 GM VIAL 0 SAGENT PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 27LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 44567-0103-10 6.57000 AMPICILLIN 2 GM VIAL 0 WG CRITICAL CAR EAGEN 55150-0114-20 6.57000 AMPICILLIN 2 GM VIAL 0 AUROMEDICS PHAR EAGEN 63323-0399-23 6.57000 AMPICILLIN 2 GM VIAL 0 APP PHARMACEUTI EAGEN 00781-3402-78 1.66900 AMPICILLIN 250 MG VIAL 0 SANDOZ EAGEN 00781-3402-95 1.66900 AMPICILLIN 250 MG VIAL 0 SANDOZ EAGEN 00781-9402-78 1.66900 AMPICILLIN 250 MG VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9402-95 1.66900 AMPICILLIN 250 MG VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0134-10 1.66900 AMPICILLIN 250 MG VIAL 0 SAGENT PHARMACE EAGEN 44567-0100-10 1.66900 AMPICILLIN 250 MG VIAL 0 WG CRITICAL CAR EAGEN 63323-0387-10 1.66900 AMPICILLIN 250 MG VIAL 0 APP PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 67253-0183-20 0.11686 AMPICILLIN 250 MG/5 ML SUSP 0 DAVA PHARMACEUT MLGEN 00781-3407-78 1.63215 AMPICILLIN 500 MG VIAL 0 SANDOZ EAGEN 00781-3407-95 1.63215 AMPICILLIN 500 MG VIAL 0 SANDOZ EAGEN 00781-9407-78 1.63215 AMPICILLIN 500 MG VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9407-95 1.63215 AMPICILLIN 500 MG VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0135-10 1.63215 AMPICILLIN 500 MG VIAL 0 SAGENT PHARMACE EAGEN 44567-0101-10 1.63215 AMPICILLIN 500 MG VIAL 0 WG CRITICAL CAR EAGEN 63323-0388-10 1.63215 AMPICILLIN 500 MG VIAL 0 APP PHARMACEUTI EABND 00409-2987-03 6.79272 AMPICILLIN-SULB 3 GM ADD VIAL 0 HOSPIRA EABND 00409-2987-13 6.00588 AMPICILLIN-SULB 3 GM ADD VIAL 0 HOSPIRA/NOVA+ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-2689-01 3.02800 4.09356 AMPICILLIN-SULBACTAM 1.5 GM VL 0 HOSPIRA EABND 00409-2689-11 3.02800 3.83460 AMPICILLIN-SULBACTAM 1.5 GM VL 0 HOSPIRA/NOVA+ EAGEN 00409-2988-01 1.66500 AMPICILLIN-SULBACTAM 1.5 GM VL 0 HOSPIRA EAGEN 0<strong>06</strong>41-6116-01 2.88850 AMPICILLIN-SULBACTAM 1.5 GM VL 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>41-6116-10 2.88850 AMPICILLIN-SULBACTAM 1.5 GM VL 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>41-6119-01 2.88850 AMPICILLIN-SULBACTAM 1.5 GM VL 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>41-6119-10 2.88850 AMPICILLIN-SULBACTAM 1.5 GM VL 0 WEST-WARD,INC. EAGEN 00781-3032-95 2.88850 AMPICILLIN-SULBACTAM 1.5 GM VL 0 SANDOZ EAGEN 25021-0142-20 2.70000 AMPICILLIN-SULBACTAM 1.5 GM VL 0 SAGENT PHARMACE EAGEN 55150-0116-20 2.88850 AMPICILLIN-SULBACTAM 1.5 GM VL G AUROMEDICS PHAR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0368-20 2.88850 AMPICILLIN-SULBACTAM 1.5 GM VL 0 APP PHARMACEUTI EAGEN 67457-0230-00 2.88850 AMPICILLIN-SULBACTAM 1.5 GM VL 0 MYLAN INSTITUTI EAGEN 67457-0230-10 2.88850 AMPICILLIN-SULBACTAM 1.5 GM VL 0 MYLAN INSTITUTI EAGEN 00409-2687-15 19.67250 AMPICILLIN-SULBACTAM 15 GM VL 0 HOSPIRA EAGEN 0<strong>06</strong>41-6121-01 29.70000 AMPICILLIN-SULBACTAM 15 GM VL 0 WEST-WARD,INC. EAGEN 25021-0144-99 32.39420 AMPICILLIN-SULBACTAM 15 GM VL 0 SAGENT PHARMACE EAGEN 44567-0212-01 29.70000 AMPICILLIN-SULBACTAM 15 GM VL 0 WG CRITICAL CAR EAGEN 00409-2998-03 3.24000 AMPICILLIN-SULBACTAM 3 GM VIAL 0 HOSPIRA EAGEN 0<strong>06</strong>41-6117-01 3.36825 AMPICILLIN-SULBACTAM 3 GM VIAL 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>41-6117-10 3.36825 AMPICILLIN-SULBACTAM 3 GM VIAL 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>41-6120-01 3.36825 AMPICILLIN-SULBACTAM 3 GM VIAL 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>41-6120-10 3.36825 AMPICILLIN-SULBACTAM 3 GM VIAL 0 WEST-WARD,INC. EAGEN 00781-3033-70 3.36825 AMPICILLIN-SULBACTAM 3 GM VIAL 0 SANDOZ EAGEN 00781-3033-95 3.36825 AMPICILLIN-SULBACTAM 3 GM VIAL 0 SANDOZ EAGEN 25021-0143-30 3.36825 AMPICILLIN-SULBACTAM 3 GM VIAL 0 SAGENT PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 28LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 44567-0211-10 3.36825 AMPICILLIN-SULBACTAM 3 GM VIAL 0 WG CRITICAL CAR EAGEN 55150-0117-20 3.36825 AMPICILLIN-SULBACTAM 3 GM VIAL 0 AUROMEDICS PHAR EAGEN 63323-0369-20 3.36825 AMPICILLIN-SULBACTAM 3 GM VIAL 0 APP PHARMACEUTI EAGEN 67457-0226-15 3.36825 AMPICILLIN-SULBACTAM 3 GM VIAL 0 MYLAN INSTITUTI EABND 10144-0427-60 26.58475 AMPYRA ER 10 MG TABLET 0 ACORDA THERAPEU EABND 63459-0700-60 6.77780 21.86220 AMRIX ER 15 MG CAPSULE G TEVA USA EABND 63459-0701-60 6.77780 21.86220 AMRIX ER 30 MG CAPSULE G TEVA USA EABND 00078-<strong>06</strong>10-15 3.13269 AMTURNIDE 150-5-12.5 MG TAB G NOVARTIS EABND 00078-<strong>06</strong>13-15 3.95384 AMTURNIDE 300-10-12.5 MG TAB G NOVARTIS EABND 00078-<strong>06</strong>14-15 3.95384 AMTURNIDE 300-10-25 MG TAB G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-<strong>06</strong>11-15 3.95384 AMTURNIDE 300-5-12.5 MG TAB G NOVARTIS EABND 00078-<strong>06</strong>12-15 3.95384 AMTURNIDE 300-5-25 MG TAB G NOVARTIS EABUX 004<strong>06</strong>-99<strong>06</strong>-03 0.37500 13.56441 ANAFRANIL 25 MG CAPSULE G MALLINCKRODT PH EABUX 004<strong>06</strong>-9907-03 0.50360 13.82<strong>06</strong>0 ANAFRANIL 50 MG CAPSULE G MALLINCKRODT PH EABUX 004<strong>06</strong>-99<strong>08</strong>-03 0.66230 14.07624 ANAFRANIL 75 MG CAPSULE G MALLINCKRODT PH EAGEN 00172-5241-60 0.23490 ANAGRELIDE HCL 0.5 MG CAPSULE 0 TEVA USA EABND 00172-5240-60 0.31090 13.42558 ANAGRELIDE HCL 1 MG CAPSULE 0 IVAX PHARMACEUT EABND 00004-6203-01 0.11950 4.25341 ANAPROX DS 550 MG TABLET G ROCHE LABS. EABND 00004-6202-01 0.09356 2.73186 ANAPROX 275 MG TABLET G ROCHE LABS. EABND 00225-0295-15 0.21414 ANASPAZ 0.125 MG TABLET ODT 0 B.F ASCHER & CO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00225-0295-20 0.20716 ANASPAZ 0.125 MG TABLET ODT 0 B.F ASCHER & CO EAGEN 00054-0164-13 0.27030 ANASTROZOLE 1 MG TABLET 0 ROXANE LABS. EAGEN 00093-7536-56 0.27030 ANASTROZOLE 1 MG TABLET 0 TEVA USA EAGEN 00378-6034-05 0.27030 ANASTROZOLE 1 MG TABLET 0 MYLAN EAGEN 00378-6034-77 0.27030 ANASTROZOLE 1 MG TABLET 0 MYLAN EAGEN 00781-5356-31 0.27030 ANASTROZOLE 1 MG TABLET 0 SANDOZ EAGEN 00904-6195-46 0.27030 ANASTROZOLE 1 MG TABLET 0 MAJOR PHARMACEU EAGEN 16571-0421-03 0.27030 ANASTROZOLE 1 MG TABLET 0 PACK PHARMACEUT EAGEN 16729-0035-10 0.27030 ANASTROZOLE 1 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0035-15 0.27030 ANASTROZOLE 1 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16729-0035-16 0.27030 ANASTROZOLE 1 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 42043-0180-03 0.27030 ANASTROZOLE 1 MG TABLET 0 KARALEX PHARMA, EAGEN 51079-0323-01 0.27030 ANASTROZOLE 1 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0323-<strong>06</strong> 0.27030 ANASTROZOLE 1 MG TABLET 0 MYLAN INSTITUTI EAGEN 51991-<strong>06</strong>20-10 0.27030 ANASTROZOLE 1 MG TABLET 0 BRECKENRIDGE EAGEN 51991-<strong>06</strong>20-33 0.27030 ANASTROZOLE 1 MG TABLET 0 BRECKENRIDGE EAGEN 60505-2985-03 0.27030 ANASTROZOLE 1 MG TABLET 0 APOTEX CORP EAGEN 62756-0250-13 0.27030 ANASTROZOLE 1 MG TABLET 0 SUN PHARMACEUTI EAGEN 62756-0250-83 0.27030 ANASTROZOLE 1 MG TABLET 0 SUN PHARMACEUTI EAGEN 63323-0129-30 0.23850 ANASTROZOLE 1 MG TABLET 0 APP PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 66435-0415-30 0.27030 ANASTROZOLE 1 MG TABLET 0 KADMON PHARMACE EAGEN 68<strong>08</strong>4-0448-11 0.27030 ANASTROZOLE 1 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0448-21 0.27030 ANASTROZOLE 1 MG TABLET 0 AHP EAGEN 68382-0209-<strong>06</strong> 0.27030 ANASTROZOLE 1 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0209-10 0.27030 ANASTROZOLE 1 MG TABLET 0 ZYDUS PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 29LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00187-3554-10 17.61269 59.21004 ANCOBON 250 MG CAPSULE 0 VALEANT EABND 00187-3555-10 29.88000 114.57228 ANCOBON 500 MG CAPSULE 0 VALEANT EABND 50419-0482-01 3.57967 ANGELIQ 0.25 MG-0.5 MG TABLET 0 BAYER,PHARM DIV EABND 50419-0482-03 3.57967 ANGELIQ 0.25 MG-0.5 MG TABLET 0 BAYER,PHARM DIV EABND 50419-0483-03 3.57967 ANGELIQ 0.5 MG-1 MG TABLET 0 BAYER,PHARM DIV EAGEN 51285-0523-02 2.46632 ANTABUSE 250 MG TABLET 0 DURAMED/BARR EAGEN 51285-0524-02 4.71117 ANTABUSE 500 MG TABLET 0 DURAMED/BARR EABND 27437-0110-01 5.42430 5.55411 ANTARA 130 MG CAPSULE G LUPIN PHARMA EABND 27437-0110-<strong>06</strong> 5.42430 6.38768 ANTARA 130 MG CAPSULE G LUPIN PHARMA EABND 27437-0107-<strong>06</strong> 2.16962 ANTARA 30 MG CAPSULE G LUPIN PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 27437-0109-<strong>06</strong> 1.84240 2.16962 ANTARA 43 MG CAPSULE G LUPIN PHARMA EABND 27437-01<strong>08</strong>-<strong>06</strong> 6.38768 ANTARA 90 MG CAPSULE G LUPIN PHARMA EAGEN 242<strong>08</strong>-0561-62 0.6<strong>08</strong>80 ANTIPYRINE-BENZOCAINE EAR DROP 0 VALEANT MLGEN 42192-07<strong>08</strong>-10 0.6<strong>08</strong>80 ANTIPYRINE-BENZOCAINE EAR DROP 0 ACELLA PHARMACE MLGEN 42192-0710-15 0.6<strong>08</strong>80 ANTIPYRINE-BENZOCAINE EAR DROP 0 ACELLA PHARMACE MLGEN 43199-0016-15 0.6<strong>08</strong>80 ANTIPYRINE-BENZOCAINE EAR DROP 0 COUNTY LINE PHA MLGEN 64376-0438-15 0.6<strong>08</strong>80 ANTIPYRINE-BENZOCAINE EAR DROP 0 BOCA PHARMACAL MLGEN 76439-0305-10 0.6<strong>08</strong>80 ANTIPYRINE-BENZOCAINE EAR DROP 0 VIRTUS PHARMACE MLGEN 76439-0305-15 0.6<strong>08</strong>80 ANTIPYRINE-BENZOCAINE EAR DROP 0 VIRTUS PHARMACE MLBND 00049-2100-66 0.04621 0.76758 ANTIVERT 12.5 MG TABLET 0 PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00049-2110-66 0.27050 1.21395 ANTIVERT 25 MG TABLET 0 PFIZER US PHARM EABND 00<strong>08</strong>8-1203-05 71.46964 ANZEMET 100 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00<strong>08</strong>8-12<strong>06</strong>-32 8.77476 ANZEMET 20 MG/ML VIAL 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00<strong>08</strong>8-12<strong>08</strong>-<strong>06</strong> 28.11376 ANZEMET 20 MG/ML VIAL 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00<strong>08</strong>8-1209-26 8.77409 ANZEMET 20 MG/ML VIAL 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00<strong>08</strong>8-1202-05 53.92012 ANZEMET 50 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00462-0395-60 4.94250 APEXICON E 0.05% CREAM G SANDOZ GMBND 10337-0395-30 13.31458 APEXICON E 0.05% CREAM G SANDOZ GMBND 10337-0395-60 13.3<strong>08</strong>49 APEXICON E 0.05% CREAM G SANDOZ GMBND 00<strong>08</strong>8-2502-05 20.09319 APIDRA SOLOSTAR 100 UNITS/ML 0 SAN<strong>OF</strong>I-AVENTIS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>8-2500-33 15.61313 APIDRA 100 UNITS/ML VIAL 0 SAN<strong>OF</strong>I-AVENTIS MLBEX 00024-5810-30 11.22021 APLENZIN ER 174 MG TABLET 0 SAN<strong>OF</strong>I-AVENTIS EABEX 00024-5811-30 14.79<strong>08</strong>7 APLENZIN ER 348 MG TABLET 0 SAN<strong>OF</strong>I-AVENTIS EABEX 00024-5812-30 33.65982 APLENZIN ER 522 MG TABLET 0 SAN<strong>OF</strong>I-AVENTIS EABND 15054-0211-01 330.34000 APOKYN 30 MG/3 ML CARTRIDGE 0 US WORLDMEDS MLBND 15054-0211-05 330.34000 APOKYN 30 MG/3 ML CARTRIDGE 0 US WORLDMEDS MLGEN 17478-0716-10 12.33540 APRACLONIDINE HCL 0.5% DROPS G AKORN INC. MLGEN 17478-0716-11 12.33540 APRACLONIDINE HCL 0.5% DROPS G AKORN INC. MLGEN 61314-<strong>06</strong>65-05 12.33540 APRACLONIDINE HCL 0.5% DROPS G SANDOZ MLGEN 61314-<strong>06</strong>65-10 12.33540 APRACLONIDINE HCL 0.5% DROPS G SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00555-9043-58 0.73800 APRI 28 DAY TABLET 0 BARR EABND 65649-0103-02 2.66727 APRISO ER 0.375 GRAM CAPSULE 0 SALIX PHARMACEU EABND 00597-0002-01 4.36877 APTIVUS 100 MG/ML SOLUTION G BOEHRINGER ING. MLBND 00597-0003-02 10.37617 APTIVUS 250 MG CAPSULE G BOEHRINGER ING. EABND 00944-2802-02 0.46480 ARALAST NP 1,000 MG VIAL 0 BAXTER BIOSCIEN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 30LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00944-2802-01 0.46480 ARALAST NP 500 MG VIAL 0 BAXTER BIOSCIEN EAGEX 00555-9<strong>06</strong>6-67 0.99160 ARANELLE 28 TABLET 0 BARR EABND 55513-0005-01 640.62720 ARANESP 100 MCG/ML VIAL 0 AMGEN MLBND 55513-0005-04 640.62720 ARANESP 100 MCG/ML VIAL 0 AMGEN MLBND 55513-0025-01 1281.25440 ARANESP 100 MCG/0.5 ML SYRINGE 0 AMGEN MLBND 55513-0025-04 1281.25440 ARANESP 100 MCG/0.5 ML SYRINGE 0 AMGEN MLBND 55513-0027-01 3203.13600 ARANESP 150 MCG/0.3 ML SYRINGE 0 AMGEN MLBND 55513-0027-04 3203.13600 ARANESP 150 MCG/0.3 ML SYRINGE 0 AMGEN MLBND 55513-0053-01 1281.25440 ARANESP 150 MCG/0.75 ML VIAL 0 AMGEN MLBND 55513-0053-04 1281.25440 ARANESP 150 MCG/0.75 ML VIAL 0 AMGEN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 55513-00<strong>06</strong>-01 1281.25440 ARANESP 200 MCG/ML VIAL 0 AMGEN MLBND 55513-0028-01 3203.13600 ARANESP 200 MCG/0.4 ML SYRINGE 0 AMGEN MLBND 55513-0002-01 160.15680 ARANESP 25 MCG/ML VIAL 0 AMGEN MLBND 55513-0002-04 160.15680 ARANESP 25 MCG/ML VIAL 0 AMGEN MLBND 55513-0057-01 381.32571 ARANESP 25 MCG/0.42 ML SYRING 0 AMGEN MLBND 55513-0057-04 381.32571 ARANESP 25 MCG/0.42 ML SYRING 0 AMGEN MLBND 55513-0110-01 1921.88160 ARANESP 300 MCG/ML VIAL 0 AMGEN MLBND 55513-0111-01 3203.13600 ARANESP 300 MCG/0.6 ML SYRINGE 0 AMGEN MLBND 55513-0003-01 256.27<strong>08</strong>0 ARANESP 40 MCG/ML VIAL 0 AMGEN MLBND 55513-0003-04 256.27<strong>08</strong>0 ARANESP 40 MCG/ML VIAL 0 AMGEN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 55513-0021-04 640.67700 ARANESP 40 MCG/0.4 ML SYRINGE 0 AMGEN MLBND 55513-0032-01 3203.13600 ARANESP 500 MCG/1 ML SYRINGE 0 AMGEN MLBND 55513-0004-01 384.35640 ARANESP 60 MCG/ML VIAL 0 AMGEN MLBND 55513-0004-04 384.35640 ARANESP 60 MCG/ML VIAL 0 AMGEN MLBND 55513-0023-01 1281.18800 ARANESP 60 MCG/0.3 ML SYRINGE 0 AMGEN MLBND 55513-0023-04 1281.18800 ARANESP 60 MCG/0.3 ML SYRINGE 0 AMGEN MLBND 00<strong>08</strong>8-2160-30 0.53070 32.18740 ARAVA 10 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00<strong>08</strong>8-2161-30 0.71321 32.18740 ARAVA 20 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 61755-0001-01 4980.00000 ARCALYST 220 MG INJECTION 0 REGENERON PHARM EABND 00078-<strong>06</strong>19-15 6.<strong>08</strong>777 ARCAPTA NEOHALER 75 MCG CAP G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-<strong>06</strong>19-61 6.09220 ARCAPTA NEOHALER 75 MCG CAP G NOVARTIS EABND 62856-<strong>08</strong>32-30 0.28310 12.66248 ARICEPT ODT 10 MG TABLET G EISAI INC. EABND 62856-<strong>08</strong>31-30 0.28310 12.66248 ARICEPT ODT 5 MG TABLET G EISAI INC. EABND 62856-0246-11 0.07398 7.98813 ARICEPT 10 MG TABLET G EISAI INC. EABND 62856-0246-30 0.07398 12.66248 ARICEPT 10 MG TABLET G EISAI INC. EABND 62856-0246-90 0.07398 11.72577 ARICEPT 10 MG TABLET G EISAI INC. EABND 62856-0247-30 11.31151 ARICEPT 23 MG TABLET G EISAI INC. EABND 62856-0247-90 9.69891 ARICEPT 23 MG TABLET G EISAI INC. EABND 62856-0245-30 0.05940 12.66248 ARICEPT 5 MG TABLET G EISAI INC. EABND 62856-0245-90 0.05940 12.66377 ARICEPT 5 MG TABLET G EISAI INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00310-0201-30 0.27030 14.40769 ARIMIDEX 1 MG TABLET 0 ASTRAZENECA EABND 00781-3<strong>08</strong>5-71 14.15980 ARISTOSPAN 20 MG/ML VIAL 0 SANDOZ MLBND 00781-3<strong>08</strong>5-75 7.10314 ARISTOSPAN 20 MG/ML VIAL 0 SANDOZ MLBND 00781-3<strong>08</strong>4-75 3.53580 ARISTOSPAN 5 MG/ML VIAL 0 SANDOZ MLBND 00007-3236-02 90.25640 155.682<strong>06</strong> ARIXTRA 10 MG SYRINGE G GLAXOSMITHKLINE ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 31LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00007-3236-11 90.25640 148.26<strong>08</strong>2 ARIXTRA 10 MG SYRINGE G GLAXOSMITHKLINE MLBND 00007-3230-02 31.15640 105.83330 ARIXTRA 2.5 MG SYRINGE G GLAXOSMITHKLINE MLBND 00007-3230-11 31.15640 100.80350 ARIXTRA 2.5 MG SYRINGE G GLAXOSMITHKLINE MLBND 00007-3232-11 180.51280 296.52165 ARIXTRA 5 MG SYRINGE G GLAXOSMITHKLINE MLBND 00007-3234-01 151.91010 207.58300 ARIXTRA 7.5 MG SYRINGE G GLAXOSMITHKLINE MLBND 00007-3234-02 151.91010 207.576<strong>08</strong> ARIXTRA 7.5 MG SYRINGE G GLAXOSMITHKLINE MLBND 00007-3234-11 151.91010 197.68110 ARIXTRA 7.5 MG SYRINGE G GLAXOSMITHKLINE MLBND 00456-0461-01 0.26900 ARMOUR THYROID 120 MG TABLET 0 FOREST PHARMACE EABND 00456-0457-01 0.11163 ARMOUR THYROID 15 MG TABLET 0 FOREST PHARMACE EABND 00456-0462-01 0.42686 ARMOUR THYROID 180 MG TABLET 0 FOREST PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00456-0463-01 0.63984 ARMOUR THYROID 240 MG TABLET 0 FOREST PHARMACE EABND 00456-0458-01 0.10210 0.13105 ARMOUR THYROID 30 MG TABLET 0 FOREST PHARMACE EABND 00456-0464-01 0.79323 ARMOUR THYROID 300 MG TABLET 0 FOREST PHARMACE EABND 00456-0459-01 0.14549 ARMOUR THYROID 60 MG TABLET 0 FOREST PHARMACE EABND 00456-0460-01 0.14610 0.22957 ARMOUR THYROID 90 MG TABLET 0 FOREST PHARMACE EABND 00009-7663-04 5.23905 17.913<strong>06</strong> AROMASIN 25 MG TABLET 0 PHARMACIA/UPJHN EABND 00025-1411-60 2.62870 4.00419 ARTHROTEC 50 MG-200 MCG TAB G PHARMACIA/UPJHN EABND 00025-1411-90 2.62870 4.00392 ARTHROTEC 50 MG-200 MCG TAB G PHARMACIA/UPJHN EABND 00025-1421-60 2.62870 4.00419 ARTHROTEC 75 MG-200 MCG TAB G PHARMACIA/UPJHN EABND 00430-0752-27 2.09418 ASACOL EC 400 MG TABLET 0 WC PR<strong>OF</strong> PRODS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00430-0783-27 4.56168 ASACOL HD DR 800 MG TABLET G ACTAVIS PHARMA, EABND 00<strong>08</strong>5-1461-02 131.05700 ASMANEX TWISTHALER 110 MCG #30 G MERCK SHARP & D EABND 00<strong>08</strong>5-1341-03 141.49010 ASMANEX TWISTHALER 220 MCG #30 G MERCK SHARP & D EABND 00<strong>08</strong>5-1341-07 141.49010 ASMANEX TWISTHALER 220 MCG #30 G MERCK SHARP & D EABND 00<strong>08</strong>5-1341-02 166.26560 ASMANEX TWISTHALER 220 MCG #60 G MERCK SHARP & D EABND 00<strong>08</strong>5-1341-01 238.28470 ASMANEX TWISTHALR 220 MCG #120 G MERCK SHARP & D EABND 00469-<strong>06</strong>47-73 1.97401 ASTAGRAF XL 0.5 MG CAPSULE 0 ASTELLAS PHARMA EABND 00469-<strong>06</strong>77-73 3.94803 ASTAGRAF XL 1 MG CAPSULE 0 ASTELLAS PHARMA EABND 00469-<strong>06</strong>87-73 19.74072 ASTAGRAF XL 5 MG CAPSULE 0 ASTELLAS PHARMA EABND 00037-0241-30 4.47978 4.47978 ASTELIN 137 MCG NASAL SPRAY 0 MEDA PHARMACEUT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00037-0243-30 4.28003 ASTEPRO 0.15% NASAL SPRAY 0 MEDA PHARMACEUT MLBND 00186-0162-54 1.80000 3.96168 ATACAND HCT 16-12.5 MG TAB G ASTRAZENECA EABND 00186-0322-54 1.63560 4.04053 ATACAND HCT 32-12.5 MG TAB G ASTRAZENECA EABND 00186-0324-54 3.53710 4.37363 ATACAND HCT 32-25 MG TABLET G ASTRAZENECA EABND 00186-0016-31 2.92630 ATACAND 16 MG TABLET G ASTRAZENECA EABND 00186-0016-54 2.92870 ATACAND 16 MG TABLET G ASTRAZENECA EABND 00186-0032-31 3.96242 ATACAND 32 MG TABLET G ASTRAZENECA EABND 00186-0032-54 3.96168 ATACAND 32 MG TABLET G ASTRAZENECA EABND 00186-0004-31 2.92630 ATACAND 4 MG TABLET G ASTRAZENECA EABND 00186-00<strong>08</strong>-31 2.92630 ATACAND 8 MG TABLET G ASTRAZENECA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00430-0979-03 38.51615 ATELVIA DR 35 MG TABLET G ACTAVIS PHARMA, EAGEN 00093-0753-01 0.02590 ATENOLOL 100 MG TABLET 0 TEVA USA EAGEN 00093-0753-05 0.02590 ATENOLOL 100 MG TABLET 0 TEVA USA EAGEN 00378-0757-01 0.02590 ATENOLOL 100 MG TABLET 0 MYLAN EAGEN 00378-0757-10 0.02590 ATENOLOL 100 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 32LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0757-93 0.02590 ATENOLOL 100 MG TABLET 0 MYLAN EAGEN 00781-1507-01 0.02590 ATENOLOL 100 MG TABLET 0 SANDOZ EAGEN 00781-1507-10 0.02590 ATENOLOL 100 MG TABLET 0 SANDOZ EAGEN 00781-5229-01 0.02590 ATENOLOL 100 MG TABLET 0 SANDOZ EAGEN 00781-5229-10 0.02590 ATENOLOL 100 MG TABLET 0 SANDOZ EAGEN 16571-0441-11 0.02590 ATENOLOL 100 MG TABLET 0 PACK PHARMACEUT EAGEN 16714-0033-04 0.02590 ATENOLOL 100 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0033-<strong>06</strong> 0.02590 ATENOLOL 100 MG TABLET 0 NORTHSTAR RX LL EAGEN 50742-0103-01 0.02590 ATENOLOL 100 MG TABLET 0 INGENUS PHARMAC EAGEN 50742-0103-10 0.02590 ATENOLOL 100 MG TABLET 0 INGENUS PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-<strong>06</strong>85-20 0.02590 ATENOLOL 100 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0530-01 0.02590 ATENOLOL 100 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0530-10 0.02590 ATENOLOL 100 MG TABLET 0 MUTUAL PHARM CO EAGEN 62584-0715-01 0.02590 ATENOLOL 100 MG TABLET 0 AHP EAGEN 63304-<strong>06</strong>23-01 0.02590 ATENOLOL 100 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>06</strong>23-10 0.02590 ATENOLOL 100 MG TABLET 0 RANBAXY PHARMAC EAGEN 65862-0170-01 0.02590 ATENOLOL 100 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0170-99 0.02590 ATENOLOL 100 MG TABLET 0 AUROBINDO PHARM EAGEN 68382-0024-01 0.02590 ATENOLOL 100 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0024-10 0.02590 ATENOLOL 100 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-0787-01 0.01340 ATENOLOL 25 MG TABLET 0 TEVA USA EAGEN 00093-0787-10 0.01340 ATENOLOL 25 MG TABLET 0 TEVA USA EAGEN 00378-0218-01 0.01340 ATENOLOL 25 MG TABLET 0 MYLAN EAGEN 00378-0218-10 0.01340 ATENOLOL 25 MG TABLET 0 MYLAN EAGEN 00781-1078-01 0.01340 ATENOLOL 25 MG TABLET 0 SANDOZ EAGEN 00781-1078-10 0.01340 ATENOLOL 25 MG TABLET 0 SANDOZ EAGEN 00781-5220-01 0.01340 ATENOLOL 25 MG TABLET 0 SANDOZ EAGEN 00781-5220-10 0.01340 ATENOLOL 25 MG TABLET 0 SANDOZ EAGEN 16571-0430-11 0.01340 ATENOLOL 25 MG TABLET 0 PACK PHARMACEUT EAGEN 16714-0031-04 0.01340 ATENOLOL 25 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0031-<strong>06</strong> 0.01340 ATENOLOL 25 MG TABLET 0 NORTHSTAR RX LL EAGEN 50742-0101-01 0.01340 ATENOLOL 25 MG TABLET 0 INGENUS PHARMAC EAGEN 50742-0101-10 0.01340 ATENOLOL 25 MG TABLET 0 INGENUS PHARMAC EAGEN 51079-0759-20 0.01340 ATENOLOL 25 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0759-63 0.01340 ATENOLOL 25 MG TABLET 0 MYLAN INSTITUTI EAGEN 63304-<strong>06</strong>21-01 0.01340 ATENOLOL 25 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>06</strong>21-10 0.01340 ATENOLOL 25 MG TABLET 0 RANBAXY PHARMAC EAGEN 65862-0168-01 0.01340 ATENOLOL 25 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0168-99 0.01340 ATENOLOL 25 MG TABLET 0 AUROBINDO PHARM EAGEN 68382-0022-01 0.01340 ATENOLOL 25 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0022-10 0.01340 ATENOLOL 25 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00093-0752-01 0.01530 ATENOLOL 50 MG TABLET 0 TEVA USA EAGEN 00093-0752-10 0.01530 ATENOLOL 50 MG TABLET 0 TEVA USA EAGEN 00378-0231-01 0.01530 ATENOLOL 50 MG TABLET 0 MYLAN EAGEN 00378-0231-10 0.01530 ATENOLOL 50 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 33LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-15<strong>06</strong>-01 0.01530 ATENOLOL 50 MG TABLET 0 SANDOZ EAGEN 00781-15<strong>06</strong>-10 0.01530 ATENOLOL 50 MG TABLET 0 SANDOZ EAGEN 00781-5225-01 0.01530 ATENOLOL 50 MG TABLET 0 SANDOZ EAGEN 00781-5225-10 0.01530 ATENOLOL 50 MG TABLET 0 SANDOZ EAGEN 16571-0431-11 0.01530 ATENOLOL 50 MG TABLET 0 PACK PHARMACEUT EAGEN 16714-0032-04 0.01530 ATENOLOL 50 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0032-<strong>06</strong> 0.01530 ATENOLOL 50 MG TABLET 0 NORTHSTAR RX LL EAGEN 50742-0102-01 0.01530 ATENOLOL 50 MG TABLET 0 INGENUS PHARMAC EAGEN 50742-0102-10 0.01530 ATENOLOL 50 MG TABLET 0 INGENUS PHARMAC EAGEN 51079-<strong>06</strong>84-20 0.01530 ATENOLOL 50 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-<strong>06</strong>84-63 0.01530 ATENOLOL 50 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0529-10 0.01530 ATENOLOL 50 MG TABLET 0 MUTUAL PHARM CO EAGEN 62584-0467-01 0.01530 ATENOLOL 50 MG TABLET 0 AHP EAGEN 62584-0467-11 0.01530 ATENOLOL 50 MG TABLET 0 AHP EAGEN 63304-<strong>06</strong>22-01 0.01530 ATENOLOL 50 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>06</strong>22-10 0.01530 ATENOLOL 50 MG TABLET 0 RANBAXY PHARMAC EAGEN 65862-0169-01 0.01530 ATENOLOL 50 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0169-99 0.01530 ATENOLOL 50 MG TABLET 0 AUROBINDO PHARM EAGEN 68382-0023-01 0.01530 ATENOLOL 50 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0023-10 0.01530 ATENOLOL 50 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68645-0310-54 0.01530 ATENOLOL 50 MG TABLET 0 LEGACY PHARMACE EAGEN 00378-2<strong>06</strong>3-01 0.07000 ATENOLOL-CHLORTHAL 50-25 TB 0 MYLAN EAGEN 00591-5782-01 0.07000 ATENOLOL-CHLORTHAL 50-25 TB 0 ACTAVIS PHARMA, EAGEN 53489-0531-01 0.07000 ATENOLOL-CHLORTHAL 50-25 TB 0 MUTUAL PHARM CO EAGEN 00378-2<strong>06</strong>4-01 0.19967 ATENOLOL-CHLORTHALIDONE 100-25 0 MYLAN EAGEN 00378-2<strong>06</strong>4-93 0.19967 ATENOLOL-CHLORTHALIDONE 100-25 0 MYLAN EAGEN 00591-5783-01 0.19967 ATENOLOL-CHLORTHALIDONE 100-25 0 ACTAVIS PHARMA, EABND 00009-7224-01 161.53792 ATGAM 50 MG/ML AMPUL 0 PHARMACIA/UPJHN MLBND 00009-7224-02 161.53758 ATGAM 50 MG/ML AMPUL 0 PHARMACIA/UPJHN MLGEN 00378-2015-05 0.17807 ATORVASTATIN 10 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-2015-77 0.17807 ATORVASTATIN 10 MG TABLET 0 MYLAN EAGEN 00378-3950-05 0.17807 ATORVASTATIN 10 MG TABLET 0 MYLAN EAGEN 00378-3950-77 0.17807 ATORVASTATIN 10 MG TABLET 0 MYLAN EAGEN 00591-3774-10 0.17807 ATORVASTATIN 10 MG TABLET 0 WATSON LABS EAGEN 00591-3774-19 0.17807 ATORVASTATIN 10 MG TABLET 0 WATSON LABS EAGEN 00781-5381-92 0.17807 ATORVASTATIN 10 MG TABLET 0 SANDOZ EAGEN 00904-6290-61 0.17807 ATORVASTATIN 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 51079-0409-20 0.17807 ATORVASTATIN 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 55111-0121-05 0.17807 ATORVASTATIN 10 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0121-90 0.17807 ATORVASTATIN 10 MG TABLET 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-0155-01 0.17807 ATORVASTATIN 10 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-0155-02 0.17807 ATORVASTATIN 10 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-2578-<strong>08</strong> 0.17807 ATORVASTATIN 10 MG TABLET 0 APOTEX CORP EAGEN 60505-2578-09 0.17807 ATORVASTATIN 10 MG TABLET 0 APOTEX CORP EAGEN 62175-<strong>08</strong>90-43 0.17807 ATORVASTATIN 10 MG TABLET 0 KREMERS URBAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 34LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62175-<strong>08</strong>90-46 0.17807 ATORVASTATIN 10 MG TABLET 0 KREMERS URBAN EAGEN 63304-<strong>08</strong>27-05 0.17807 ATORVASTATIN 10 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>08</strong>27-90 0.17807 ATORVASTATIN 10 MG TABLET 0 RANBAXY PHARMAC EAGEN 68<strong>08</strong>4-0097-01 0.17807 ATORVASTATIN 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0097-11 0.17807 ATORVASTATIN 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0564-01 0.17807 ATORVASTATIN 10 MG TABLET 0 AHP EAGEN 68645-0402-70 0.17807 ATORVASTATIN 10 MG TABLET 0 LEGACY PHARMACE EAGEN 68645-0458-70 0.17807 ATORVASTATIN 10 MG TABLET 0 LEGACY PHARMACE EAGEN 00378-2017-05 0.22424 ATORVASTATIN 20 MG TABLET 0 MYLAN EAGEN 00378-2017-77 0.22424 ATORVASTATIN 20 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3951-05 0.22424 ATORVASTATIN 20 MG TABLET 0 MYLAN EAGEN 00378-3951-77 0.22424 ATORVASTATIN 20 MG TABLET 0 MYLAN EAGEN 00591-3775-10 0.22424 ATORVASTATIN 20 MG TABLET 0 WATSON LABS EAGEN 00591-3775-19 0.22424 ATORVASTATIN 20 MG TABLET 0 WATSON LABS EAGEN 00781-5382-92 0.22424 ATORVASTATIN 20 MG TABLET 0 SANDOZ EAGEN 00904-6291-61 0.22424 ATORVASTATIN 20 MG TABLET 0 MAJOR PHARMACEU EAGEN 51079-0410-20 0.22424 ATORVASTATIN 20 MG TABLET 0 MYLAN INSTITUTI EAGEN 55111-0122-05 0.22424 ATORVASTATIN 20 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0122-90 0.22424 ATORVASTATIN 20 MG TABLET 0 DR.REDDY'S LAB EAGEN 59762-0156-01 0.22424 ATORVASTATIN 20 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-0156-02 0.22424 ATORVASTATIN 20 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-2579-<strong>08</strong> 0.22424 ATORVASTATIN 20 MG TABLET 0 APOTEX CORP EAGEN 60505-2579-09 0.22424 ATORVASTATIN 20 MG TABLET 0 APOTEX CORP EAGEN 62175-<strong>08</strong>91-43 0.22424 ATORVASTATIN 20 MG TABLET 0 KREMERS URBAN EAGEN 62175-<strong>08</strong>91-46 0.22424 ATORVASTATIN 20 MG TABLET 0 KREMERS URBAN EAGEN 63304-<strong>08</strong>28-05 0.22424 ATORVASTATIN 20 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>08</strong>28-90 0.22424 ATORVASTATIN 20 MG TABLET 0 RANBAXY PHARMAC EAGEN 68645-0403-70 0.22424 ATORVASTATIN 20 MG TABLET 0 LEGACY PHARMACE EAGEN 68645-0459-70 0.22424 ATORVASTATIN 20 MG TABLET 0 LEGACY PHARMACE EAGEN 00378-2121-05 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-2121-77 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 MYLAN EAGEN 00378-3952-05 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 MYLAN EAGEN 00378-3952-77 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 MYLAN EAGEN 00591-3776-05 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 WATSON LABS EAGEN 00591-3776-19 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 WATSON LABS EAGEN 00781-5384-92 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 SANDOZ EAGEN 51079-0411-20 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 MYLAN INSTITUTI EAGEN 55111-0123-05 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0123-90 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 DR.REDDY'S LAB EAGEN 58517-0001-30 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 <strong>NEW</strong> HORIZON RX EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-0157-01 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-0157-02 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-2580-<strong>08</strong> 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 APOTEX CORP EAGEN 60505-2580-09 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 APOTEX CORP EAGEN 62175-<strong>08</strong>92-41 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 KREMERS URBAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 35LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62175-<strong>08</strong>92-46 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 KREMERS URBAN EAGEN 63304-<strong>08</strong>29-05 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>08</strong>29-90 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 RANBAXY PHARMAC EAGEN 68<strong>08</strong>4-0099-01 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0589-01 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0589-11 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 AHP EAGEN 68645-0417-54 0.19<strong>06</strong>2 ATORVASTATIN 40 MG TABLET 0 LEGACY PHARMACE EAGEN 00378-2122-05 0.25974 ATORVASTATIN 80 MG TABLET 0 MYLAN EAGEN 00378-2122-77 0.25974 ATORVASTATIN 80 MG TABLET 0 MYLAN EAGEN 00378-3953-05 0.25974 ATORVASTATIN 80 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3953-77 0.25974 ATORVASTATIN 80 MG TABLET 0 MYLAN EAGEN 00591-3777-05 0.25974 ATORVASTATIN 80 MG TABLET 0 WATSON LABS EAGEN 00591-3777-19 0.25974 ATORVASTATIN 80 MG TABLET 0 WATSON LABS EAGEN 00781-5388-92 0.25974 ATORVASTATIN 80 MG TABLET 0 SANDOZ EAGEN 51079-0412-03 0.25974 ATORVASTATIN 80 MG TABLET 0 MYLAN INSTITUTI EAGEN 55111-0124-05 0.25974 ATORVASTATIN 80 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0124-90 0.25974 ATORVASTATIN 80 MG TABLET 0 DR.REDDY'S LAB EAGEN 59762-0158-01 0.25974 ATORVASTATIN 80 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-0158-02 0.25974 ATORVASTATIN 80 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-2671-<strong>08</strong> 0.25974 ATORVASTATIN 80 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2671-09 0.25974 ATORVASTATIN 80 MG TABLET 0 APOTEX CORP EAGEN 62175-<strong>08</strong>97-41 0.25974 ATORVASTATIN 80 MG TABLET 0 KREMERS URBAN EAGEN 62175-<strong>08</strong>97-46 0.25974 ATORVASTATIN 80 MG TABLET 0 KREMERS URBAN EAGEN 63304-<strong>08</strong>30-05 0.25974 ATORVASTATIN 80 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>08</strong>30-90 0.25974 ATORVASTATIN 80 MG TABLET 0 RANBAXY PHARMAC EAGEN 68<strong>08</strong>4-0590-25 0.25974 ATORVASTATIN 80 MG TABLET 0 AHP EAGEN 68645-0418-54 0.25974 ATORVASTATIN 80 MG TABLET 0 LEGACY PHARMACE EAGEN 68645-0461-54 0.25974 ATORVASTATIN 80 MG TABLET 0 LEGACY PHARMACE EAGEN 66993-0<strong>06</strong>0-02 5.44507 ATOVAQUONE-PROGUANIL 250-100 0 PRASCO LABS EAGEN 66993-0<strong>06</strong>0-27 5.55594 ATOVAQUONE-PROGUANIL 250-100 0 PRASCO LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0404-01 5.36032 ATOVAQUONE-PROGUANIL 250-100 0 GLENMARK PHARMA EAGEN 68462-0404-67 5.36031 ATOVAQUONE-PROGUANIL 250-100 0 GLENMARK PHARMA EAGEN 68462-0563-01 1.98262 ATOVAQUONE-PROGUANIL 62.5-25 0 GLENMARK PHARMA EABND 15584-0101-01 66.45644 ATRIPLA TABLET G BMS/GILEAD EABND 17478-0214-20 2.13980 2.48170 ATROPINE CARE 1% EYE DROPS 0 AKORN INC. MLGEN 00517-0401-25 0.43700 ATROPINE 0.4 MG/ML VIAL 0 AMER. REGENT MLGEN 00517-1010-25 1.28800 ATROPINE 1 MG/ML VIAL 0 AMER. REGENT MLBND 00<strong>06</strong>5-0702-12 2.13980 3.04263 ATROPINE 1% EYE DROPS 0 ALCON SURGICAL MLGEN 242<strong>08</strong>-0750-<strong>06</strong> 1.89249 ATROPINE 1% EYE DROPS 0 VALEANT MLGEN 242<strong>08</strong>-0750-60 2.13980 ATROPINE 1% EYE DROPS 0 VALEANT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61314-0303-01 2.13980 ATROPINE 1% EYE DROPS 0 SANDOZ MLGEN 61314-0303-02 1.61000 ATROPINE 1% EYE DROPS 0 SANDOZ MLBND 242<strong>08</strong>-<strong>08</strong>25-55 4.71913 ATROPINE 1% EYE OINTMENT 0 VALEANT GMBND 00597-0<strong>08</strong>7-17 18.25871 ATROVENT HFA INHALER 0 BOEHRINGER ING. GMBND 00597-0<strong>08</strong>1-30 0.88789 3.77290 ATROVENT 0.03% SPRAY G BOEHRINGER ING. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 36LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 58468-0210-02 162.84718 AUBAGIO 14 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 58468-0211-01 162.84718 AUBAGIO 7 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EAGEX 50102-0120-01 0.26035 AUBRA-28 TABLET 0 AFAXYS, INC. EABND 43598-0003-51 0.14260 0.70361 AUGMENTIN ES-600 SUSPENSION 0 DR.REDDY'S LAB MLBND 43598-0003-54 0.14260 0.66370 AUGMENTIN ES-600 SUSPENSION 0 DR.REDDY'S LAB MLBND 43598-0003-69 0.14260 0.67621 AUGMENTIN ES-600 SUSPENSION 0 DR.REDDY'S LAB MLBND 43598-0012-51 1.1<strong>08</strong>88 AUGMENTIN 125-31.25 MG/5 ML 0 DR.REDDY'S LAB MLBND 43598-0012-52 1.10796 AUGMENTIN 125-31.25 MG/5 ML 0 DR.REDDY'S LAB MLBND 43598-0012-53 1.<strong>08</strong>591 AUGMENTIN 125-31.25 MG/5 ML 0 DR.REDDY'S LAB MLBND 43598-0004-52 0.78770 1.62646 AUGMENTIN 250-62.5 MG/5 ML 0 DR.REDDY'S LAB ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 43598-0021-14 2.53200 12.823<strong>08</strong> AUGMENTIN 875-125 TABLET 0 DR.REDDY'S LAB EAGEN 00904-0793-10 0.6<strong>08</strong>80 AURODEX OTIC SOLUTION 0 MAJOR PHARMACEU MLGEN 00904-6317-10 0.6<strong>08</strong>80 AURODEX OTIC SOLUTION 0 MAJOR PHARMACEU MLGEN 66424-0520-35 0.60099 AUROGUARD OTIC SOLUTION 0 SDA LABS MLBND 00024-5831-02 166.23240 AUVI-Q 0.15 MG AUTO-INJECTOR 0 SAN<strong>OF</strong>I-AVENTIS EABND 00024-5833-02 166.23240 AUVI-Q 0.3 MG AUTO-INJECTOR 0 SAN<strong>OF</strong>I-AVENTIS EABND 00024-5855-30 0.19319 4.25734 AVALIDE 150-12.5 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00024-5855-90 0.19319 4.25633 AVALIDE 150-12.5 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00<strong>08</strong>7-2875-31 0.19319 3.42679 AVALIDE 150-12.5 MG TABLET G BMS PRIMARYCARE EABND 00<strong>08</strong>7-2875-32 0.19319 3.42605 AVALIDE 150-12.5 MG TABLET G BMS PRIMARYCARE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00024-5856-30 0.31941 4.63693 AVALIDE 300-12.5 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00024-5856-90 0.31941 4.63794 AVALIDE 300-12.5 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00<strong>08</strong>7-2876-31 0.31941 3.73278 AVALIDE 300-12.5 MG TABLET G BMS PRIMARYCARE EABND 00<strong>08</strong>7-2876-32 0.31941 3.73324 AVALIDE 300-12.5 MG TABLET G BMS PRIMARYCARE EABND 00173-<strong>08</strong>38-18 2.28679 AVANDAMET 2 MG-1,000 MG TAB G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>37-18 2.28679 AVANDAMET 2 MG-500 MG TABLET G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>40-18 3.87429 AVANDAMET 4 MG-1,000 MG TABLET G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>39-18 3.87429 AVANDAMET 4 MG-500 MG TABLET G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>41-13 4.33370 AVANDARYL 4 MG-1 MG TABLET G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>42-13 4.33370 AVANDARYL 4 MG-2 MG TABLET G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-<strong>08</strong>43-13 4.33370 AVANDARYL 4 MG-4 MG TABLET G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>44-13 7.45478 AVANDARYL 8 MG-2 MG TABLET G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>45-13 7.45478 AVANDARYL 8 MG-4 MG TABLET G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>34-18 2.61796 AVANDIA 2 MG TABLET G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>35-13 3.88578 AVANDIA 4 MG TABLET G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>36-13 7.05970 AVANDIA 8 MG TABLET G GLAXOSMITHKLINE EABND 00024-5851-30 0.24000 3.51864 AVAPRO 150 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00024-5851-90 0.24000 3.51984 AVAPRO 150 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00<strong>08</strong>7-2772-15 0.24000 2.83310 AVAPRO 150 MG TABLET G BMS PRIMARYCARE EABND 00<strong>08</strong>7-2772-31 0.24000 2.83223 AVAPRO 150 MG TABLET G BMS PRIMARYCARE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>7-2772-32 0.24000 2.83325 AVAPRO 150 MG TABLET G BMS PRIMARYCARE EABND 00024-5852-30 0.34500 4.231<strong>06</strong> AVAPRO 300 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00024-5852-90 0.34500 4.23<strong>06</strong>0 AVAPRO 300 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00<strong>08</strong>7-2773-15 0.34500 3.40524 AVAPRO 300 MG TABLET G BMS PRIMARYCARE EABND 00<strong>08</strong>7-2773-31 0.34500 3.40576 AVAPRO 300 MG TABLET G BMS PRIMARYCARE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 37LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>7-2773-32 0.34500 3.40530 AVAPRO 300 MG TABLET G BMS PRIMARYCARE EABND 00024-5850-30 0.16950 3.34379 AVAPRO 75 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00024-5850-90 0.16950 3.34370 AVAPRO 75 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00<strong>08</strong>7-2771-31 0.16950 2.69141 AVAPRO 75 MG TABLET G BMS PRIMARYCARE EABND 00<strong>08</strong>7-2771-32 0.16950 2.69141 AVAPRO 75 MG TABLET G BMS PRIMARYCARE EABND 66663-0103-04 1.33678 AVC 15% CREAM 0 MEDA PHARMACEUT GMBND 00<strong>08</strong>5-1733-03 25.10750 AVELOX ABC PACK 400 MG TAB G MERCK SHARP & D EABND 00<strong>08</strong>5-1737-01 0.16841 AVELOX IV 400 MG/250 ML 0 MERCK SHARP & D MLBND 00<strong>08</strong>5-1733-01 25.10750 AVELOX 400 MG TABLET G MERCK SHARP & D EAGEX 00555-9045-58 0.85280 AVIANE-28 TABLET 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-0712-04 4.55965 AVODART 0.5 MG S<strong>OF</strong>TGEL G GLAXOSMITHKLINE EABND 00173-0712-15 4.56029 AVODART 0.5 MG S<strong>OF</strong>TGEL G GLAXOSMITHKLINE EABND 59627-0001-03 1131.70500 AVONEX ADMIN PACK 30 MCG VL 0 BIOGEN-IDEC EABND 59627-0001-04 1131.70500 AVONEX ADMIN PACK 30 MCG VL 0 BIOGEN-IDEC EABND 59627-0003-04 4526.82000 AVONEX PEN 30 MCG/0.5 ML 0 BIOGEN-IDEC EABND 59627-0002-05 4526.82000 AVONEX PREFILLED SYR 30 MCG 0 BIOGEN-IDEC EABND 00<strong>06</strong>2-2<strong>08</strong>5-12 26.497<strong>06</strong> AXERT 12.5 MG TABLET G ORTHO PHARM. EABND 50458-0210-01 33.57003 AXERT 12.5 MG TABLET G JANSSEN PHARM. EABND 50458-0211-01 33.56381 AXERT 6.25 MG TABLET G JANSSEN PHARM. EABND 52268-0147-62 0.54020 0.72886 AXID 15 MG/ML ORAL SOLUTION 0 BRAINTREE LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 51285-0424-10 1.81640 3.66528 AYGESTIN 5 MG TABLET G DURAMED/BARR EAGEN 00781-3253-94 395.28000 AZACITIDINE 100 MG VIAL 0 SANDOZ EAGEN 43598-0305-62 395.28000 AZACITIDINE 100 MG VIAL 0 DR.REDDY'S LAB EABND 00003-2560-16 28.88400 AZACTAM 1 GM VIAL 0 BMS PRIMARYCARE EABND 00003-2570-16 57.76800 AZACTAM 2 GM VIAL 0 BMS PRIMARYCARE EABND 00003-2230-11 0.66275 AZACTAM-ISO-OSMOT 1 GM/50 ML 0 BMS PRIMARYCARE MLBND 00003-2240-11 1.32733 AZACTAM-ISO-OSMOT 2 GM/50 ML 0 BMS PRIMARYCARE MLBND 65649-0241-41 5.53717 AZASAN 100 MG TABLET 0 SALIX PHARMACEU EABND 65649-0231-41 4.13838 AZASAN 75 MG TABLET 0 SALIX PHARMACEU EABND 31357-0040-03 42.57568 AZASITE 1% EYE DROPS G MERCK SHARP & D ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 31357-0040-25 38.70456 AZASITE 1% EYE DROPS G MERCK SHARP & D MLGEN 00054-4<strong>08</strong>4-25 0.34913 AZATHIOPRINE 50 MG TABLET 0 ROXANE LABS. EAGEN 00378-1005-01 0.34913 AZATHIOPRINE 50 MG TABLET 0 MYLAN EAGEN 51079-<strong>06</strong>20-01 0.34913 AZATHIOPRINE 50 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-<strong>06</strong>20-<strong>06</strong> 0.34913 AZATHIOPRINE 50 MG TABLET 0 MYLAN INSTITUTI EAGEN 68<strong>08</strong>4-0229-01 0.34913 AZATHIOPRINE 50 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0229-11 0.34913 AZATHIOPRINE 50 MG TABLET 0 AHP EAGEN 68382-0003-01 0.34913 AZATHIOPRINE 50 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0003-05 0.34913 AZATHIOPRINE 50 MG TABLET 0 ZYDUS PHARMACEU EAGEN 47335-0938-90 8.33750 AZELASTINE HCL 0.05% DROPS G SUN PHARMA GLOB ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51525-7815-<strong>06</strong> 8.33750 AZELASTINE HCL 0.05% DROPS G WALLACE PHARMAC MLGEN 60505-0578-04 8.33750 AZELASTINE HCL 0.05% DROPS G APOTEX CORP MLGEN 61314-03<strong>08</strong>-02 8.33750 AZELASTINE HCL 0.05% DROPS G SANDOZ MLGEN 47335-0779-91 2.63000 AZELASTINE HCL 0.1% NASAL SPRY G SUN PHARMA GLOB MLGEN 51525-0294-03 2.63000 AZELASTINE 137 MCG NASAL SPRAY G WALLACE PHARMAC ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 38LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-<strong>08</strong>33-05 2.63100 AZELASTINE 137 MCG NASAL SPRAY G APOTEX CORP MLBND 68546-0142-56 15.86960 AZILECT 0.5 MG TABLET 0 TEVA NEUROSCIEN EABND 68546-0229-56 15.86960 AZILECT 1 MG TABLET 0 TEVA NEUROSCIEN EABND 00409-0144-11 6.65540 9.20304 AZITHROMYCIN I.V. 500 MG VIAL 0 HOSPIRA EAGEN 25021-0112-10 3.91500 AZITHROMYCIN I.V. 500 MG VIAL 0 SAGENT PHARMACE EAGEN 50111-0794-78 3.91500 AZITHROMYCIN I.V. 500 MG VIAL 0 PLIVA, INC EAGEN 60505-6076-04 3.91500 AZITHROMYCIN I.V. 500 MG VIAL 0 APOTEX CORP EAGEN 62756-0512-44 3.91500 AZITHROMYCIN I.V. 500 MG VIAL 0 SUN PHARMACEUTI EAGEN 63323-0398-10 3.91500 AZITHROMYCIN I.V. 500 MG VIAL 0 APP PHARMACEUTI EAGEN 38779-2246-04 27.78750 AZITHROMYCIN POWDER 0 MEDISCA INC. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-3051-01 21.84750 AZITHROMYCIN 1 GM PWD PACKET 0 GREENSTONE LLC. EAGEN 59762-3051-02 21.84500 AZITHROMYCIN 1 GM PWD PACKET 0 GREENSTONE LLC. EAGEN 00093-2027-23 0.76230 AZITHROMYCIN 100 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-7148-23 0.76230 AZITHROMYCIN 100 MG/5 ML SUSP 0 TEVA USA MLGEN 59762-3110-01 0.76230 AZITHROMYCIN 100 MG/5 ML SUSP 0 GREENSTONE LLC. MLGEN 00093-2026-23 0.79380 AZITHROMYCIN 200 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-2026-31 0.79380 AZITHROMYCIN 200 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-2026-94 0.79380 AZITHROMYCIN 200 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-7149-23 0.79380 AZITHROMYCIN 200 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-7149-31 0.79380 AZITHROMYCIN 200 MG/5 ML SUSP 0 TEVA USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7149-94 0.79380 AZITHROMYCIN 200 MG/5 ML SUSP 0 TEVA USA MLGEN 00185-72<strong>06</strong>-70 0.79380 AZITHROMYCIN 200 MG/5 ML SUSP 0 SANDOZ MLGEN 59762-3120-01 0.79380 AZITHROMYCIN 200 MG/5 ML SUSP 0 GREENSTONE LLC. MLGEN 59762-3130-01 0.79380 AZITHROMYCIN 200 MG/5 ML SUSP 0 GREENSTONE LLC. MLGEN 59762-3140-01 0.79380 AZITHROMYCIN 200 MG/5 ML SUSP 0 GREENSTONE LLC. MLGEN 00093-7146-18 0.61650 AZITHROMYCIN 250 MG TABLET 0 TEVA USA EAGEN 00093-7146-56 0.61650 AZITHROMYCIN 250 MG TABLET 0 TEVA USA EAGEN 00781-1496-31 0.61650 AZITHROMYCIN 250 MG TABLET 0 SANDOZ EAGEN 00781-1496-68 0.61650 AZITHROMYCIN 250 MG TABLET 0 SANDOZ EAGEN 00781-1496-69 0.61650 AZITHROMYCIN 250 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6010-04 0.61650 AZITHROMYCIN 250 MG TABLET 0 MAJOR PHARMACEU EAGEN 50111-0787-51 0.61650 AZITHROMYCIN 250 MG TABLET 0 TEVA USA EAGEN 50111-0787-66 0.61650 AZITHROMYCIN 250 MG TABLET 0 TEVA USA EAGEN 59762-3<strong>06</strong>0-01 0.61650 AZITHROMYCIN 250 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-3<strong>06</strong>0-02 0.61650 AZITHROMYCIN 250 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-3<strong>06</strong>0-03 0.61650 AZITHROMYCIN 250 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-2581-00 0.61650 AZITHROMYCIN 250 MG TABLET 0 APOTEX CORP EAGEN 60505-2581-02 0.61650 AZITHROMYCIN 250 MG TABLET 0 APOTEX CORP EAGEN 60505-2581-03 0.61650 AZITHROMYCIN 250 MG TABLET 0 APOTEX CORP EAGEN 60505-2581-05 0.61650 AZITHROMYCIN 250 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63739-0575-09 0.61650 AZITHROMYCIN 250 MG TABLET 0 MCKESSON PACKAG EAGEN 63739-0575-10 0.61650 AZITHROMYCIN 250 MG TABLET 0 MCKESSON PACKAG EAGEN 64679-0961-01 0.61650 AZITHROMYCIN 250 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0961-04 0.61650 AZITHROMYCIN 250 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0961-05 0.61650 AZITHROMYCIN 250 MG TABLET 0 WOCKHARDT USA L EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 39LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0278-01 0.61650 AZITHROMYCIN 250 MG TABLET 0 AHP EAGEN 00093-7169-33 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 TEVA USA EAGEN 00093-7169-56 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 TEVA USA EAGEN 00093-7169-90 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 TEVA USA EAGEN 00781-1941-31 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 SANDOZ EAGEN 00781-1941-33 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 SANDOZ EAGEN 50111-0788-52 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 PLIVA, INC EAGEN 50111-0788-55 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 TEVA USA EAGEN 59762-3070-01 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-3070-02 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2582-00 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 APOTEX CORP EAGEN 60505-2582-02 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 APOTEX CORP EAGEN 60505-2582-03 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 APOTEX CORP EAGEN 64679-0964-01 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0964-03 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0964-05 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 WOCKHARDT USA L EAGEN 68<strong>08</strong>4-0279-11 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0279-21 4.<strong>06</strong>795 AZITHROMYCIN 500 MG TABLET 0 AHP EAGEN 00093-7147-56 3.38405 AZITHROMYCIN 600 MG TABLET 0 TEVA USA EAGEN 00781-1497-31 3.38405 AZITHROMYCIN 600 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-3<strong>08</strong>0-01 3.38405 AZITHROMYCIN 600 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-2583-03 3.38405 AZITHROMYCIN 600 MG TABLET 0 APOTEX CORP EAGEN 64679-0962-01 3.38405 AZITHROMYCIN 600 MG TABLET 0 WOCKHARDT USA L EAGEN 68<strong>08</strong>4-0464-11 3.38405 AZITHROMYCIN 600 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0464-21 3.38405 AZITHROMYCIN 600 MG TABLET 0 AHP EABND 00<strong>06</strong>5-0275-10 14.19300 AZOPT 1% EYE DROPS 0 ALCON LABS. MLBND 00<strong>06</strong>5-0275-15 14.18968 AZOPT 1% EYE DROPS 0 ALCON LABS. MLBND 65597-0111-30 4.42224 AZOR 10-20 MG TABLET G DAIICHI SANKYO, EABND 65597-0111-90 4.42224 AZOR 10-20 MG TABLET G DAIICHI SANKYO, EABND 65597-0113-30 5.59752 AZOR 10-40 MG TABLET G DAIICHI SANKYO, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 65597-0113-90 5.59752 AZOR 10-40 MG TABLET G DAIICHI SANKYO, EABND 65597-0110-30 4.42224 AZOR 5-20 MG TABLET G DAIICHI SANKYO, EABND 65597-0110-90 4.42224 AZOR 5-20 MG TABLET G DAIICHI SANKYO, EABND 65597-0112-30 5.59752 AZOR 5-40 MG TABLET G DAIICHI SANKYO, EABND 65597-0112-90 5.59752 AZOR 5-40 MG TABLET G DAIICHI SANKYO, EAGEN 63323-0401-20 29.65500 AZTREONAM 1 GM VIAL 0 APP PHARMACEUTI EAGEN 63323-0402-20 60.25500 AZTREONAM 2 GM VIAL 0 APP PHARMACEUTI EABND 00013-0102-01 0.24030 1.00239 AZULFIDINE ENTAB 500 MG G PHARMACIA/UPJHN EABND 00013-0102-20 0.24030 1.00236 AZULFIDINE ENTAB 500 MG G PHARMACIA/UPJHN EABND 00013-0101-01 0.15600 0.76733 AZULFIDINE 500 MG TABLET G PHARMACIA/UPJHN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00013-0101-20 0.15600 0.76741 AZULFIDINE 500 MG TABLET G PHARMACIA/UPJHN EAGEX 52544-0940-28 1.51100 AZURETTE 28 DAY TABLET 0 ACTAVIS PHARMA, EAGEN 00009-0233-01 9.98100 BACITRACIN 50,000 UNITS VIAL 0 PHARMACIA/UPJHN EAGEN 00009-0234-01 9.98250 BACITRACIN 50,000 UNITS VIAL 0 PFIZER/NOVAPLUS EAGEN 00009-0234-02 9.98100 BACITRACIN 50,000 UNITS VIAL 0 PFIZER/NOVAPLUS EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 40LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 25021-0116-30 10.38750 BACITRACIN 50,000 UNITS VIAL 0 SAGENT PHARMACE EAGEN 25021-0116-31 9.98100 BACITRACIN 50,000 UNITS VIAL 0 SAGENT PHARMACE EAGEN 63323-0329-30 8.10000 BACITRACIN 50,000 UNITS VIAL 0 APP PHARMACEUTI EAGEN 63323-0329-31 8.10000 BACITRACIN 50,000 UNITS VIAL 0 APP PHARMACEUTI EABND 48102-0007-11 23.47516 BACITRACIN 500 UNIT/GM OPHTH G PERRIGO CO. GMBND 48102-0007-13 23.47516 BACITRACIN 500 UNIT/GM OPHTH G PERRIGO CO. GMBND 48102-0007-35 16.44348 BACITRACIN 500 UNIT/GM OPHTH G PERRIGO CO. GMGEN 242<strong>08</strong>-0555-55 3.04<strong>08</strong>8 BACITRACIN-POLYMYXIN EYE OINT 0 VALEANT GMGEN 38779-0388-<strong>08</strong> 25.65000 BACL<strong>OF</strong>EN POWDER 0 MEDISCA INC. GMGEN 38779-0388-09 25.65000 BACL<strong>OF</strong>EN POWDER 0 MEDISCA INC. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00172-4096-60 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 IVAX PHARMACEUT EAGUL 00172-4096-80 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 IVAX PHARMACEUT EAGUL 00378-3023-01 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 MYLAN EAGUL 00378-3023-10 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 MYLAN EAGUL 0<strong>06</strong>03-24<strong>06</strong>-02 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-24<strong>06</strong>-21 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-24<strong>06</strong>-28 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-24<strong>06</strong>-30 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-24<strong>06</strong>-32 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 QUALITEST EAGUL 0<strong>08</strong>32-1024-00 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 0<strong>08</strong>32-1024-09 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 UPSHER SMITH EAGUL 0<strong>08</strong>32-1024-10 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 UPSHER SMITH EAGUL 0<strong>08</strong>32-1024-50 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 UPSHER SMITH EAGUL 16714-0071-04 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 NORTHSTAR RX LL EAGUL 16714-0071-<strong>06</strong> 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 NORTHSTAR RX LL EAGUL 68<strong>08</strong>4-0599-01 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 AHP EAGUL 68<strong>08</strong>4-0599-11 0.05250 BACL<strong>OF</strong>EN 10 MG TABLET 0 AHP EAGUL 00172-4097-60 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 IVAX PHARMACEUT EAGUL 00172-4097-80 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 IVAX PHARMACEUT EAGUL 00378-3024-01 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-3024-05 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 MYLAN EAGUL 0<strong>06</strong>03-2407-21 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-2407-28 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-2407-32 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 QUALITEST EAGUL 0<strong>08</strong>32-1025-00 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 UPSHER SMITH EAGUL 0<strong>08</strong>32-1025-09 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 UPSHER SMITH EAGUL 0<strong>08</strong>32-1025-10 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 UPSHER SMITH EAGUL 0<strong>08</strong>32-1025-50 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 UPSHER SMITH EAGUL 16714-0072-04 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 NORTHSTAR RX LL EAGUL 16714-0072-05 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 68<strong>08</strong>4-<strong>06</strong>00-01 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 AHP EAGUL 68<strong>08</strong>4-<strong>06</strong>00-11 0.<strong>08</strong>930 BACL<strong>OF</strong>EN 20 MG TABLET 0 AHP EAGEN 63323-0259-30 0.07100 BACTERIOSTATIC SALINE VIAL 0 APP PHARMACEUTI MLGEN 63323-0924-10 0.07100 BACTERIOSTATIC SALINE VIAL 0 APP PHARMACEUTI MLGEN 63323-0924-30 0.<strong>06</strong>240 BACTERIOSTATIC SALINE VIAL 0 APP PHARMACEUTI ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 41LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-3977-03 0.01760 0.03353 BACTERIOSTATIC WATER VIAL 0 HOSPIRA MLBND 63323-0249-30 0.<strong>06</strong>241 BACTERIOSTATIC WATER VIAL 0 APP PHARMACEUTI MLBND 00029-1526-11 12.56122 BACTROBAN NASAL 2% OINTMENT G GLAXOSMITHKLINE GMBND 00029-1527-22 5.10726 5.10726 BACTROBAN 2% CREAM 0 GLAXOSMITHKLINE GMBND 00029-1527-25 4.32762 4.32762 BACTROBAN 2% CREAM 0 GLAXOSMITHKLINE GMBND 00029-1525-44 0.40930 4.21640 BACTROBAN 2% OINTMENT G GLAXOSMITHKLINE GMGEN 00054-0079-28 0.24476 BALSALAZIDE DISODIUM 750 MG CP G ROXANE LABS. EAGEN 00378-6750-82 0.24476 BALSALAZIDE DISODIUM 750 MG CP G MYLAN EAGEN 60505-2575-07 0.24476 BALSALAZIDE DISODIUM 750 MG CP G APOTEX CORP EAGEX 00555-9034-58 1.12980 BALZIVA 28 TABLET 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 62856-0582-30 3.5<strong>06</strong>47 BANZEL 200 MG TABLET G EISAI INC. EABEX 62856-0582-52 5.61910 BANZEL 200 MG TABLET G EISAI INC. EABEX 62856-0584-46 1.63041 BANZEL 40 MG/ML SUSPENSION G EISAI INC. MLBEX 62856-0583-52 11.23820 BANZEL 400 MG TABLET G EISAI INC. EABND 00003-1614-12 3.86586 BARACLUDE 0.05 MG/ML SOLUTION 0 BMS PRIMARYCARE MLBND 00003-1611-12 38.65946 BARACLUDE 0.5 MG TABLET 0 BMS PRIMARYCARE EABND 00003-1612-12 38.65946 BARACLUDE 1 MG TABLET 0 BMS PRIMARYCARE EABND 64193-0445-02 0.97940 BEBULIN 200-1,200 UNITS VIAL 0 BAXTER BIOSCIENBND 00173-0388-79 6.49989 BECONASE AQ 0.042% SPRAY G GLAXOSMITHKLINE GMGEN 00093-5125-01 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-5125-05 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 TEVA USA EAGEN 00185-0053-01 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 SANDOZ EAGEN 00185-0053-05 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 SANDOZ EAGEN 00378-0443-01 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 MYLAN EAGEN 13811-<strong>06</strong>28-10 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 TRIGEN LABORATO EAGEN 13811-<strong>06</strong>28-50 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 TRIGEN LABORATO EAGEN 51079-0145-20 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 60505-0266-01 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 APOTEX CORP EAGEN 60505-0266-05 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 APOTEX CORP EAGEN 63304-0338-01 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63304-0338-05 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 RANBAXY PHARMAC EAGEN 65162-0752-10 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 AMNEAL PHARMACE EAGEN 65862-0116-01 0.05<strong>06</strong>3 BENAZEPRIL HCL 10 MG TABLET 0 AUROBINDO PHARM EAGEN 00093-5126-01 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 TEVA USA EAGEN 00093-5126-05 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 TEVA USA EAGEN 00185-<strong>08</strong>20-01 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 SANDOZ EAGEN 00185-<strong>08</strong>20-05 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 SANDOZ EAGEN 00378-0444-01 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 MYLAN EAGEN 13811-<strong>06</strong>29-10 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 TRIGEN LABORATO EAGEN 13811-<strong>06</strong>29-50 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 TRIGEN LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 54458-0957-10 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 INTERNATIONAL L EAGEN 60505-0267-01 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 APOTEX CORP EAGEN 60505-0267-05 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 APOTEX CORP EAGEN 63304-0339-01 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-0339-05 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 RANBAXY PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 42LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-0753-10 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 AMNEAL PHARMACE EAGEN 65862-0117-01 0.05549 BENAZEPRIL HCL 20 MG TABLET 0 AUROBINDO PHARM EAGEN 00093-5127-01 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 TEVA USA EAGEN 00185-0048-01 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 SANDOZ EAGEN 00185-0048-05 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 SANDOZ EAGEN 00378-0447-01 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 MYLAN EAGEN 13811-<strong>06</strong>30-10 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 TRIGEN LABORATO EAGEN 13811-<strong>06</strong>30-50 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 TRIGEN LABORATO EAGEN 54458-<strong>08</strong>93-10 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 INTERNATIONAL L EAGEN 60505-0268-01 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0268-05 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 APOTEX CORP EAGEN 63304-0340-01 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-0340-05 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 RANBAXY PHARMAC EAGEN 65162-0754-10 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 AMNEAL PHARMACE EAGEN 65862-0118-01 0.<strong>06</strong>993 BENAZEPRIL HCL 40 MG TABLET 0 AUROBINDO PHARM EAGEN 00093-5124-01 0.04928 BENAZEPRIL HCL 5 MG TABLET 0 TEVA USA EAGEN 00185-0505-01 0.04928 BENAZEPRIL HCL 5 MG TABLET 0 SANDOZ EAGEN 00185-0505-05 0.04928 BENAZEPRIL HCL 5 MG TABLET 0 SANDOZ EAGEN 00378-0441-01 0.04928 BENAZEPRIL HCL 5 MG TABLET 0 MYLAN EAGEN 13811-<strong>06</strong>27-10 0.04928 BENAZEPRIL HCL 5 MG TABLET 0 TRIGEN LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0265-01 0.04928 BENAZEPRIL HCL 5 MG TABLET 0 APOTEX CORP EAGEN 63304-0337-01 0.04928 BENAZEPRIL HCL 5 MG TABLET 0 RANBAXY PHARMAC EAGEN 65162-0751-10 0.04928 BENAZEPRIL HCL 5 MG TABLET 0 AMNEAL PHARMACE EAGUL 00185-0204-01 0.49580 BENAZEPRIL-HCTZ 10-12.5 MG TAB 0 SANDOZ EAGUL 00378-4735-01 0.49580 BENAZEPRIL-HCTZ 10-12.5 MG TAB 0 MYLAN EAGUL 00185-0211-01 0.49580 BENAZEPRIL-HCTZ 20-12.5 MG TAB 0 SANDOZ EAGUL 00378-4745-01 0.49580 BENAZEPRIL-HCTZ 20-12.5 MG TAB 0 MYLAN EAGUL 54868-39<strong>06</strong>-02 0.49580 BENAZEPRIL-HCTZ 20-12.5 MG TAB 0 PHYSICIANS TC. EAGEN 00185-0277-01 0.38134 BENAZEPRIL-HCTZ 20-25 MG TAB 0 SANDOZ EAGEN 00378-4775-01 0.38134 BENAZEPRIL-HCTZ 20-25 MG TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00185-0124-01 0.49580 BENAZEPRIL-HCTZ 5-6.25 MG TAB 0 SANDOZ EAGUL 00378-4725-01 0.49580 BENAZEPRIL-HCTZ 5-6.25 MG TAB 0 MYLAN EABND 58394-<strong>06</strong>35-03 1.18690 BENEFIX 1,000 UNIT KIT 0 WYETH PHARMBND 58394-<strong>06</strong>36-03 1.18690 BENEFIX 2,000 UNIT KIT 0 WYETH PHARMBND 58394-<strong>06</strong>33-03 1.18690 BENEFIX 250 UNIT KIT 0 WYETH PHARMBND 58394-<strong>06</strong>37-03 1.18690 BENEFIX 3,000 UNIT KIT 0 WYETH PHARMBND 58394-<strong>06</strong>34-03 1.18690 BENEFIX 500 UNIT KIT 0 WYETH PHARMBND 65597-0105-30 3.54576 BENICAR HCT 20-12.5 MG TABLET G DAIICHI SANKYO, EABND 65597-0105-90 3.54576 BENICAR HCT 20-12.5 MG TABLET G DAIICHI SANKYO, EABND 65597-01<strong>06</strong>-30 4.94016 BENICAR HCT 40-12.5 MG TABLET G DAIICHI SANKYO, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 65597-01<strong>06</strong>-90 4.94016 BENICAR HCT 40-12.5 MG TABLET G DAIICHI SANKYO, EABND 65597-0107-30 4.94016 BENICAR HCT 40-25 MG TABLET G DAIICHI SANKYO, EABND 65597-0107-90 4.94016 BENICAR HCT 40-25 MG TABLET G DAIICHI SANKYO, EABND 65597-0103-30 3.54576 BENICAR 20 MG TABLET G DAIICHI SANKYO, EABND 65597-0103-90 3.54576 BENICAR 20 MG TABLET G DAIICHI SANKYO, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 43LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 65597-0104-30 4.94016 BENICAR 40 MG TABLET G DAIICHI SANKYO, EABND 65597-0104-90 4.94016 BENICAR 40 MG TABLET G DAIICHI SANKYO, EABND 65597-0101-30 2.89836 BENICAR 5 MG TABLET G DAIICHI SANKYO, EABND 00<strong>06</strong>6-0494-25 2.76850 8.52509 BENZACLIN GEL G VALEANT GMBND 00187-5190-25 2.76850 9.29234 BENZACLIN GEL G VALEANT GMBND 00<strong>06</strong>6-0494-35 8.52742 BENZACLIN GEL 35G PUMP G VALEANT GMBND 00187-5190-35 9.29505 BENZACLIN GEL 35G PUMP G VALEANT GMBND 00<strong>06</strong>6-0494-55 7.27345 BENZACLIN GEL 50G PUMP G VALEANT GMBND 00187-5190-50 7.92799 BENZACLIN GEL 50G PUMP G VALEANT GMBND 00<strong>06</strong>6-0510-46 1.24<strong>08</strong>0 7.22973 BENZAMYCIN GEL G VALEANT GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00187-5205-46 1.24<strong>08</strong>0 7.88036 BENZAMYCIN GEL G VALEANT GMBND 00<strong>06</strong>6-0577-60 3.82229 BENZAMYCINPAK GEL G VALEANT EAGEN 00904-5904-60 0.09140 BENZONATATE 100 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 50111-<strong>08</strong>51-01 0.09140 BENZONATATE 100 MG CAPSULE 0 PLIVA, INC EAGEN 50111-<strong>08</strong>51-02 0.09140 BENZONATATE 100 MG CAPSULE 0 PLIVA, INC EAGEN 57664-0133-88 0.09140 BENZONATATE 100 MG CAPSULE 0 CARACO PHARM EAGEN 65162-0536-10 0.09140 BENZONATATE 100 MG CAPSULE 0 AMNEAL PHARMACE EAGEN 65162-0536-50 0.09140 BENZONATATE 100 MG CAPSULE 0 AMNEAL PHARMACE EAGEN 67877-0105-01 0.09140 BENZONATATE 100 MG CAPSULE 0 ASCEND LABORATO EAGEN 67877-0105-05 0.09140 BENZONATATE 100 MG CAPSULE 0 ASCEND LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0214-01 0.09140 BENZONATATE 100 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-0214-11 0.09140 BENZONATATE 100 MG CAPSULE 0 AHP EAGEN 68382-0247-01 0.09140 BENZONATATE 100 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 68382-0247-05 0.09140 BENZONATATE 100 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 00555-1883-02 0.13410 BENZONATATE 200 MG CAPSULE 0 BARR EAGEN 00904-6254-60 0.13410 BENZONATATE 200 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 57664-0134-88 0.13410 BENZONATATE 200 MG CAPSULE 0 CARACO PHARM EAGEN 65162-0537-10 0.13410 BENZONATATE 200 MG CAPSULE 0 AMNEAL PHARMACE EAGEN 65162-0537-50 0.13410 BENZONATATE 200 MG CAPSULE 0 AMNEAL PHARMACE EAGEN 67877-01<strong>06</strong>-01 0.13410 BENZONATATE 200 MG CAPSULE 0 ASCEND LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67877-01<strong>06</strong>-05 0.13410 BENZONATATE 200 MG CAPSULE 0 ASCEND LABORATO EAGEN 68382-0248-01 0.13410 BENZONATATE 200 MG CAPSULE 0 ZYDUS PHARMACEU EAGUX 0<strong>06</strong>03-2433-21 0.07470 BENZTROPINE MES 0.5 MG TAB 0 QUALITEST EAGUX 0<strong>06</strong>03-2433-32 0.07470 BENZTROPINE MES 0.5 MG TAB 0 QUALITEST EAGUX 0<strong>08</strong>32-1<strong>08</strong>0-00 0.07470 BENZTROPINE MES 0.5 MG TAB 0 UPSHER SMITH EAGUX 31722-0218-01 0.07470 BENZTROPINE MES 0.5 MG TAB 0 CAMBER PHARMACE EAGUX 50111-0393-01 0.07470 BENZTROPINE MES 0.5 MG TAB 0 PLIVA, INC EAGUX 68<strong>08</strong>4-0381-01 0.07470 BENZTROPINE MES 0.5 MG TAB 0 AHP EAGUX 68<strong>08</strong>4-0381-11 0.07470 BENZTROPINE MES 0.5 MG TAB 0 AHP EAGUX 0<strong>06</strong>03-2434-21 0.<strong>08</strong>480 BENZTROPINE MES 1 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 0<strong>06</strong>03-2434-32 0.<strong>08</strong>480 BENZTROPINE MES 1 MG TABLET 0 QUALITEST EAGUX 0<strong>08</strong>32-1<strong>08</strong>1-00 0.<strong>08</strong>480 BENZTROPINE MES 1 MG TABLET 0 UPSHER SMITH EAGUX 0<strong>08</strong>32-1<strong>08</strong>1-10 0.<strong>08</strong>480 BENZTROPINE MES 1 MG TABLET 0 UPSHER SMITH EAGUX 31722-0219-01 0.<strong>08</strong>480 BENZTROPINE MES 1 MG TABLET 0 CAMBER PHARMACE EAGUX 31722-0219-10 0.<strong>08</strong>480 BENZTROPINE MES 1 MG TABLET 0 CAMBER PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 44LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 50111-0394-01 0.<strong>08</strong>480 BENZTROPINE MES 1 MG TABLET 0 PLIVA, INC EAGUX 50111-0394-03 0.<strong>08</strong>480 BENZTROPINE MES 1 MG TABLET 0 PLIVA, INC EAGUX 64125-0137-01 0.<strong>08</strong>480 BENZTROPINE MES 1 MG TABLET 0 EXCELLIUM PHARM EAGUX 68<strong>08</strong>4-0388-01 0.<strong>08</strong>480 BENZTROPINE MES 1 MG TABLET 0 AHP EAGUX 68<strong>08</strong>4-0388-11 0.<strong>08</strong>480 BENZTROPINE MES 1 MG TABLET 0 AHP EAGEX 0<strong>06</strong>03-2435-21 0.09810 BENZTROPINE MES 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2435-32 0.09810 BENZTROPINE MES 2 MG TABLET 0 QUALITEST EAGEX 0<strong>08</strong>32-1<strong>08</strong>2-00 0.09810 BENZTROPINE MES 2 MG TABLET 0 UPSHER SMITH EAGEX 0<strong>08</strong>32-1<strong>08</strong>2-10 0.09810 BENZTROPINE MES 2 MG TABLET 0 UPSHER SMITH EAGEX 31722-0220-01 0.09810 BENZTROPINE MES 2 MG TABLET 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 31722-0220-10 0.09810 BENZTROPINE MES 2 MG TABLET 0 CAMBER PHARMACE EAGEX 50111-0395-01 0.09810 BENZTROPINE MES 2 MG TABLET 0 PLIVA, INC EAGEX 50111-0395-03 0.09810 BENZTROPINE MES 2 MG TABLET 0 PLIVA, INC EAGEX 64125-0138-01 0.09810 BENZTROPINE MES 2 MG TABLET 0 EXCELLIUM PHARM EAGEX 68<strong>08</strong>4-0389-01 0.09810 BENZTROPINE MES 2 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0389-11 0.09810 BENZTROPINE MES 2 MG TABLET 0 AHP EAGEX 17478-0012-02 22.03125 BENZTROPINE 2 MG/2 ML AMPULE 0 AKORN INC. MLBND 67425-0007-50 27.27380 BEPREVE 1.5% EYE DROPS G BAUSCH & LOMB MLBND 67425-0007-75 25.84454 BEPREVE 1.5% EYE DROPS G BAUSCH & LOMB MLBND 242<strong>08</strong>-0446-05 23.19352 BESIVANCE 0.6% SUSP G VALEANT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00023-4385-05 0.99670 8.45936 BETAGAN 0.5% EYE DROPS G ALLERGAN INC. MLBND 00023-4385-10 0.99670 8.79634 BETAGAN 0.5% EYE DROPS G ALLERGAN INC. MLBND 00023-4385-15 0.99670 8.54568 BETAGAN 0.5% EYE DROPS G ALLERGAN INC. MLGEN 00115-1472-52 0.31320 BETAMETHASONE DP AUG 0.05% CRM G GLOBAL PHARM GMGEN 00115-1472-56 0.31320 BETAMETHASONE DP AUG 0.05% CRM G GLOBAL PHARM GMGEN 00168-0265-15 0.31320 BETAMETHASONE DP AUG 0.05% CRM G SANDOZ GMGEN 00168-0265-50 0.31320 BETAMETHASONE DP AUG 0.05% CRM G SANDOZ GMGEN 00781-7074-27 0.31320 BETAMETHASONE DP AUG 0.05% CRM G SANDOZ GMGEN 00781-7074-50 0.31320 BETAMETHASONE DP AUG 0.05% CRM G SANDOZ GMGEN 45802-0376-32 0.31320 BETAMETHASONE DP AUG 0.05% CRM G PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0376-35 0.31320 BETAMETHASONE DP AUG 0.05% CRM G PERRIGO CO. GMGEN 51672-1310-01 0.31320 BETAMETHASONE DP AUG 0.05% CRM G TARO PHARM USA GMGEN 51672-1310-03 0.31320 BETAMETHASONE DP AUG 0.05% CRM G TARO PHARM USA GMGEN 68462-0290-17 0.31320 BETAMETHASONE DP AUG 0.05% CRM G GLENMARK PHARMA GMGEN 68462-0290-52 0.31320 BETAMETHASONE DP AUG 0.05% CRM G GLENMARK PHARMA GMBND 51672-1309-01 1.77070 3.40189 BETAMETHASONE DP AUG 0.05% GEL G TARO PHARM USA GMBND 51672-1309-03 1.77070 2.26805 BETAMETHASONE DP AUG 0.05% GEL G TARO PHARM USA GMGEN 00168-0267-30 1.30980 BETAMETHASONE DP AUG 0.05% LOT G SANDOZ MLGEN 00168-0267-60 1.30980 BETAMETHASONE DP AUG 0.05% LOT G SANDOZ MLGEN 51672-1340-03 1.30980 BETAMETHASONE DP AUG 0.05% LOT G TARO PHARM USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-1340-04 1.30980 BETAMETHASONE DP AUG 0.05% LOT G TARO PHARM USA MLGEN 00168-0268-15 3.01220 BETAMETHASONE DP AUG 0.05% OIN G SANDOZ GMGEN 00168-0268-50 2.13090 BETAMETHASONE DP AUG 0.05% OIN G SANDOZ GMGEN 00472-0382-15 3.01220 BETAMETHASONE DP AUG 0.05% OIN G ACTAVIS PHARMA, GMGEN 00472-0382-45 2.12850 BETAMETHASONE DP AUG 0.05% OIN G ACTAVIS PHARMA, GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 45LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-1317-01 1.50000 BETAMETHASONE DP AUG 0.05% OIN G TARO PHARM USA GMGEN 51672-1317-03 1.09500 BETAMETHASONE DP AUG 0.05% OIN G TARO PHARM USA GMGEN 66993-<strong>08</strong>97-15 3.01220 BETAMETHASONE DP AUG 0.05% OIN G PRASCO LABS GMGEN 66993-<strong>08</strong>97-49 2.13090 BETAMETHASONE DP AUG 0.05% OIN G PRASCO LABS GMGUL 00168-0055-15 0.23000 BETAMETHASONE DP 0.05% CRM G SANDOZ GMGUL 00168-0055-46 0.23000 BETAMETHASONE DP 0.05% CRM G SANDOZ GMGUL 00472-0380-15 0.23000 BETAMETHASONE DP 0.05% CRM G ACTAVIS PHARMA, GMGUL 00472-0380-45 0.23000 BETAMETHASONE DP 0.05% CRM G ACTAVIS PHARMA, GMGUL 51672-1274-01 0.23000 BETAMETHASONE DP 0.05% CRM G TARO PHARM USA GMGUL 51672-1274-<strong>06</strong> 0.23000 BETAMETHASONE DP 0.05% CRM G TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00168-0057-60 0.15000 BETAMETHASONE DP 0.05% LOT G SANDOZ MLGUL 45802-0021-46 0.15000 BETAMETHASONE DP 0.05% LOT G PERRIGO CO. MLGEN 00168-0056-15 2.16099 BETAMETHASONE DP 0.05% OINT G SANDOZ GMGEN 00168-0056-46 1.45017 BETAMETHASONE DP 0.05% OINT G SANDOZ GMGEN 00472-0381-15 2.52249 BETAMETHASONE DP 0.05% OINT G ACTAVIS PHARMA, GMGEN 00472-0381-45 1.69299 BETAMETHASONE DP 0.05% OINT G ACTAVIS PHARMA, GMGUL 00168-0040-15 0.11970 BETAMETHASONE VA 0.1% CREAM G SANDOZ GMGUL 00168-0040-46 0.11970 BETAMETHASONE VA 0.1% CREAM G SANDOZ GMGUL 00472-0370-15 0.11970 BETAMETHASONE VA 0.1% CREAM G ACTAVIS PHARMA, GMGUL 00472-0370-45 0.11970 BETAMETHASONE VA 0.1% CREAM G ACTAVIS PHARMA, GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-1269-01 0.11970 BETAMETHASONE VA 0.1% CREAM G TARO PHARM USA GMGUL 51672-1269-<strong>06</strong> 0.11970 BETAMETHASONE VA 0.1% CREAM G TARO PHARM USA GMGEN 00168-0041-60 0.90000 BETAMETHASONE VA 0.1% LOTION G SANDOZ MLGEN 00168-0033-15 0.72902 BETAMETHASONE VALER 0.1% OINTM G SANDOZ GMGEN 00168-0033-46 0.69216 BETAMETHASONE VALER 0.1% OINTM G SANDOZ GMGEN 00472-0371-15 0.72902 BETAMETHASONE VALER 0.1% OINTM G ACTAVIS PHARMA, GMGEN 00472-0371-45 0.71100 BETAMETHASONE VALER 0.1% OINTM G ACTAVIS PHARMA, GMGEN 45802-0053-01 3.55350 BETAMETHASONE VALER 0.12% FOAM G PERRIGO CO. GMGEN 45802-0053-02 3.3<strong>06</strong>97 BETAMETHASONE VALER 0.12% FOAM G PERRIGO CO. GMBND 50419-0119-<strong>06</strong> 0.19548 4.78978 BETAPACE AF 120 MG TABLET G BAYER,PHARM DIV EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50419-0116-<strong>06</strong> 0.47500 5.99079 BETAPACE AF 160 MG TABLET G BAYER,PHARM DIV EABND 50419-0115-<strong>06</strong> 0.09504 3.58975 BETAPACE AF 80 MG TABLET G BAYER,PHARM DIV EABND 50419-0109-10 0.19548 5.24360 BETAPACE 120 MG TABLET G BAYER,PHARM DIV EABND 50419-01<strong>06</strong>-10 0.47500 6.55409 BETAPACE 160 MG TABLET G BAYER,PHARM DIV EABND 50419-0107-10 0.36680 7.35994 BETAPACE 240 MG TABLET G BAYER,PHARM DIV EABND 50419-0105-10 0.09504 3.92963 BETAPACE 80 MG TABLET G BAYER,PHARM DIV EABND 50419-0523-09 318.86110 BETASERON 0.3 MG KIT 0 BAYER,PHARM DIV EABND 50419-0523-35 318.85872 BETASERON 0.3 MG KIT 0 BAYER,PHARM DIV EABND 50419-0524-35 318.85872 BETASERON 0.3 MG KIT 0 BAYER,PHARM DIV EAGEN 17478-0705-10 9.51150 BETAXOLOL HCL 0.5% EYE DROP 0 AKORN INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 17478-0705-11 8.84325 BETAXOLOL HCL 0.5% EYE DROP 0 AKORN INC. MLGEN 61314-0245-01 9.94050 BETAXOLOL HCL 0.5% EYE DROP 0 SANDOZ MLGEN 61314-0245-02 9.19400 BETAXOLOL HCL 0.5% EYE DROP 0 SANDOZ MLGEN 61314-0245-03 9.24600 BETAXOLOL HCL 0.5% EYE DROP 0 SANDOZ MLGEN 10702-0013-01 0.89210 BETAXOLOL 10 MG TABLET G KVK-TECH, INC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 46LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10702-0014-01 1.21461 BETAXOLOL 20 MG TABLET G KVK-TECH, INC. EAGEN 428<strong>06</strong>-0039-01 1.21461 BETAXOLOL 20 MG TABLET G EPIC PHARMA LLC EAGEN 00115-9522-01 0.20360 BETHANECHOL 10 MG TABLET 0 GLOBAL PHARM EAGEN 0<strong>08</strong>32-0511-00 0.20360 BETHANECHOL 10 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-0511-01 0.20360 BETHANECHOL 10 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-0511-89 0.20360 BETHANECHOL 10 MG TABLET 0 UPSHER SMITH EAGEN 50111-0324-01 0.20360 BETHANECHOL 10 MG TABLET 0 PLIVA, INC EAGEN 64679-0966-01 0.20360 BETHANECHOL 10 MG TABLET 0 WOCKHARDT USA L EAGEN 65162-0572-10 0.20360 BETHANECHOL 10 MG TABLET 0 AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-0166-01 0.20360 BETHANECHOL 10 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0166-11 0.20360 BETHANECHOL 10 MG TABLET 0 AHP EAGEN 00115-9533-01 0.17890 BETHANECHOL 25 MG TABLET 0 GLOBAL PHARM EAGEN 00527-1356-01 0.17890 BETHANECHOL 25 MG TABLET 0 LANNETT CO. INC EAGEN 0<strong>08</strong>32-0512-00 0.17890 BETHANECHOL 25 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-0512-01 0.17890 BETHANECHOL 25 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-0512-50 0.17890 BETHANECHOL 25 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-0512-89 0.17890 BETHANECHOL 25 MG TABLET 0 UPSHER SMITH EAGEN 00904-6178-60 0.17890 BETHANECHOL 25 MG TABLET 0 MAJOR PHARMACEU EAGEN 50111-0325-01 0.17890 BETHANECHOL 25 MG TABLET 0 PLIVA, INC EAGEN 64679-0967-01 0.17890 BETHANECHOL 25 MG TABLET 0 WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-0573-10 0.17890 BETHANECHOL 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 00115-9511-01 0.10746 BETHANECHOL 5 MG TABLET 0 GLOBAL PHARM EAGEN 0<strong>08</strong>32-0510-00 0.10746 BETHANECHOL 5 MG TABLET 0 UPSHER SMITH EAGEN 50111-0323-01 0.10746 BETHANECHOL 5 MG TABLET 0 PLIVA, INC EAGEN 64679-0965-01 0.10746 BETHANECHOL 5 MG TABLET 0 WOCKHARDT USA L EAGEN 65162-0571-10 0.10746 BETHANECHOL 5 MG TABLET 0 AMNEAL PHARMACE EAGEN 00115-9544-01 0.43899 BETHANECHOL 50 MG TABLET 0 GLOBAL PHARM EAGEN 0<strong>08</strong>32-0513-00 0.43899 BETHANECHOL 50 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-0513-01 0.43899 BETHANECHOL 50 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-0513-89 0.43899 BETHANECHOL 50 MG TABLET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50111-0326-01 0.43899 BETHANECHOL 50 MG TABLET 0 PLIVA, INC EAGEN 64679-0968-01 0.43899 BETHANECHOL 50 MG TABLET 0 WOCKHARDT USA L EAGEN 65162-0574-10 0.43899 BETHANECHOL 50 MG TABLET 0 AMNEAL PHARMACE EABND 10122-<strong>08</strong>20-04 25.23407 BETHKIS 300 MG/4 ML AMPULE 0 CORNERSTONE THE MLBND 10122-<strong>08</strong>20-56 25.23348 BETHKIS 300 MG/4 ML AMPULE 0 CORNERSTONE THE MLBND 68669-0522-05 12.16116 BETIMOL 0.25% EYE DROPS 0 VISTAKON PHARMA MLBND 68669-0525-05 13.426<strong>08</strong> BETIMOL 0.5% EYE DROPS 0 VISTAKON PHARMA MLBND 68669-0525-10 12.89820 BETIMOL 0.5% EYE DROPS 0 VISTAKON PHARMA MLBND 68669-0525-15 12.9<strong>08</strong>16 BETIMOL 0.5% EYE DROPS 0 VISTAKON PHARMA MLBND 00<strong>06</strong>5-0246-10 17.14614 BETOPTIC S 0.25% EYE DROPS 0 ALCON LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>5-0246-15 17.14780 BETOPTIC S 0.25% EYE DROPS 0 ALCON LABS. MLBEX 50419-0407-03 3.67117 BEYAZ 28 TABLET 0 BAYER,PHARM DIV EABND 00074-3165-41 3.65<strong>06</strong>7 7.81178 BIAXIN XL 500 MG TABLET G ABBVIE US LLC EABND 00074-3165-60 3.65<strong>06</strong>7 7.87767 BIAXIN XL 500 MG TABLET G ABBVIE US LLC EABUL 00074-3368-60 2.37250 7.36348 BIAXIN 250 MG TABLET G ABBVIE US LLC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 47LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00074-3188-13 1.21379 BIAXIN 250 MG/5 ML SUSPENSION 0 ABBVIE US LLC MLBND 00074-3188-50 1.31057 BIAXIN 250 MG/5 ML SUSPENSION 0 ABBVIE US LLC MLBUL 00074-2586-60 0.86250 7.36348 BIAXIN 500 MG TABLET G ABBVIE US LLC EAGEN 00093-0220-01 0.30200 BICALUTAMIDE 50 MG TABLET 0 TEVA USA EAGEN 00093-0220-56 0.30200 BICALUTAMIDE 50 MG TABLET 0 TEVA USA EAGEN 00378-7017-05 0.30200 BICALUTAMIDE 50 MG TABLET 0 MYLAN EAGEN 00378-7017-93 0.30200 BICALUTAMIDE 50 MG TABLET 0 MYLAN EAGEN 00781-5409-01 0.30200 BICALUTAMIDE 50 MG TABLET 0 SANDOZ EAGEN 00781-5409-31 0.30200 BICALUTAMIDE 50 MG TABLET 0 SANDOZ EAGEN 00781-5409-64 0.30200 BICALUTAMIDE 50 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6019-46 0.30200 BICALUTAMIDE 50 MG TABLET 0 MAJOR PHARMACEU EAGEN 16714-0571-01 0.30200 BICALUTAMIDE 50 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0571-02 0.30200 BICALUTAMIDE 50 MG TABLET 0 NORTHSTAR RX LL EAGEN 16729-0023-01 0.30200 BICALUTAMIDE 50 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0023-10 0.30200 BICALUTAMIDE 50 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 41616-0485-83 0.30200 BICALUTAMIDE 50 MG TABLET 0 SUN PHARMA GLOB EAGEN 41616-0485-88 0.30200 BICALUTAMIDE 50 MG TABLET 0 SUN PHARMA GLOB EAGEN 51079-<strong>06</strong>92-01 0.30200 BICALUTAMIDE 50 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-<strong>06</strong>92-03 0.30200 BICALUTAMIDE 50 MG TABLET 0 MYLAN INSTITUTI EAGEN 51991-0560-01 0.30200 BICALUTAMIDE 50 MG TABLET 0 BRECKENRIDGE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2642-01 0.30200 BICALUTAMIDE 50 MG TABLET 0 APOTEX CORP EAGEN 60505-2642-03 0.30200 BICALUTAMIDE 50 MG TABLET 0 APOTEX CORP EAGEN 68<strong>08</strong>4-0374-11 0.30200 BICALUTAMIDE 50 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>12-21 0.30200 BICALUTAMIDE 50 MG TABLET 0 AHP EAGEN 68382-0224-01 0.30200 BICALUTAMIDE 50 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0224-<strong>06</strong> 0.30200 BICALUTAMIDE 50 MG TABLET 0 ZYDUS PHARMACEU EABND 60793-<strong>06</strong>00-10 27.85978 BICILLIN C-R 1.2 MILLION UNIT 0 PFIZER US PHARM MLBND 60793-<strong>06</strong>01-10 27.85978 BICILLIN C-R 1.2 MILLION UNIT 0 PFIZER US PHARM MLBND 60793-<strong>06</strong>02-10 28.99895 BICILLIN C-R 900-300 SYRINGE 0 PFIZER US PHARM MLBND 60793-0701-02 34.95130 BICILLIN L-A 1,200,000 UNITS 0 PFIZER US PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 60793-0701-10 34.94964 BICILLIN L-A 1,200,000 UNITS 0 PFIZER US PHARM MLBND 60793-0702-10 35.8<strong>08</strong>48 BICILLIN L-A 2,400,000 UNITS 0 PFIZER US PHARM MLBND 60793-0700-10 40.35792 BICILLIN L-A 600,000 UNIT/ML 0 PFIZER US PHARM MLBND 00015-3012-60 170.72270 BICNU 100 MG VIAL 0 HERITAGE PHARMA EABND 23155-0261-41 1365.78160 BICNU 100 MG VIAL 0 HERITAGE PHARMA EABND 24338-0010-18 2.23279 BIDIL TABLET 0 ARBOR PHARMACEU EABND 50419-0747-01 14.33410 BILTRICIDE 600 MG TABLET 0 BAYER,PHARM DIV EABND 00178-0101-02 34.86000 BINOSTO 70 MG TABLET EFF G MISSION PHARM. EAGEN 00093-5271-56 0.45846 BISOPROLOL FUMARATE 10 MG TAB G TEVA USA EAGEN 00185-0774-01 0.45846 BISOPROLOL FUMARATE 10 MG TAB G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0774-30 0.45846 BISOPROLOL FUMARATE 10 MG TAB G SANDOZ EAGEN 00378-0524-01 0.45846 BISOPROLOL FUMARATE 10 MG TAB G MYLAN EAGEN 00378-0524-93 0.45846 BISOPROLOL FUMARATE 10 MG TAB G MYLAN EAGEN 29300-0127-01 0.45846 BISOPROLOL FUMARATE 10 MG TAB G UNICHEM PHARMAC EAGEN 29300-0127-13 0.45846 BISOPROLOL FUMARATE 10 MG TAB G UNICHEM PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 48LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-1261-01 0.45846 BISOPROLOL FUMARATE 10 MG TAB G GREENSTONE LLC. EAGEN 59762-1261-02 0.45846 BISOPROLOL FUMARATE 10 MG TAB G GREENSTONE LLC. EAGEN 65862-0<strong>08</strong>7-01 0.45846 BISOPROLOL FUMARATE 10 MG TAB G AUROBINDO PHARM EAGEN 65862-0<strong>08</strong>7-30 0.45846 BISOPROLOL FUMARATE 10 MG TAB G AUROBINDO PHARM EAGEN 00093-5270-56 0.45846 BISOPROLOL FUMARATE 5 MG TAB G TEVA USA EAGEN 00185-0771-01 0.45846 BISOPROLOL FUMARATE 5 MG TAB G SANDOZ EAGEN 00185-0771-30 0.45846 BISOPROLOL FUMARATE 5 MG TAB G SANDOZ EAGEN 00378-0523-01 0.45846 BISOPROLOL FUMARATE 5 MG TAB G MYLAN EAGEN 00378-0523-93 0.45846 BISOPROLOL FUMARATE 5 MG TAB G MYLAN EAGEN 29300-0126-01 0.45846 BISOPROLOL FUMARATE 5 MG TAB G UNICHEM PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 29300-0126-13 0.45846 BISOPROLOL FUMARATE 5 MG TAB G UNICHEM PHARMAC EAGEN 59762-1258-01 0.45846 BISOPROLOL FUMARATE 5 MG TAB G GREENSTONE LLC. EAGEN 59762-1258-02 0.45846 BISOPROLOL FUMARATE 5 MG TAB G GREENSTONE LLC. EAGEN 65862-0<strong>08</strong>6-01 0.45846 BISOPROLOL FUMARATE 5 MG TAB G AUROBINDO PHARM EAGEN 65862-0<strong>08</strong>6-30 0.45846 BISOPROLOL FUMARATE 5 MG TAB G AUROBINDO PHARM EAGEN 00185-0707-01 0.<strong>06</strong>480 BISOPROLOL-HCTZ 10-6.25 MG TAB 0 SANDOZ EAGEN 00378-0505-01 0.<strong>06</strong>480 BISOPROLOL-HCTZ 10-6.25 MG TAB 0 MYLAN EAGEN 29300-0189-01 0.<strong>06</strong>480 BISOPROLOL-HCTZ 10-6.25 MG TAB 0 UNICHEM PHARMAC EAGEN 29300-0189-05 0.<strong>06</strong>480 BISOPROLOL-HCTZ 10-6.25 MG TAB 0 UNICHEM PHARMAC EAGEN 29300-0189-13 0.<strong>06</strong>480 BISOPROLOL-HCTZ 10-6.25 MG TAB 0 UNICHEM PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0701-01 0.<strong>06</strong>386 BISOPROLOL-HCTZ 2.5-6.25 MG TB 0 SANDOZ EAGEN 00378-0501-01 0.<strong>06</strong>386 BISOPROLOL-HCTZ 2.5-6.25 MG TB 0 MYLAN EAGEN 29300-0187-01 0.<strong>06</strong>386 BISOPROLOL-HCTZ 2.5-6.25 MG TB 0 UNICHEM PHARMAC EAGEN 29300-0187-05 0.<strong>06</strong>386 BISOPROLOL-HCTZ 2.5-6.25 MG TB 0 UNICHEM PHARMAC EAGEN 29300-0187-13 0.<strong>06</strong>386 BISOPROLOL-HCTZ 2.5-6.25 MG TB 0 UNICHEM PHARMAC EAGEN 00185-0704-01 0.<strong>06</strong>480 BISOPROLOL-HCTZ 5-6.25 MG TAB 0 SANDOZ EAGEN 00378-0503-01 0.<strong>06</strong>480 BISOPROLOL-HCTZ 5-6.25 MG TAB 0 MYLAN EAGEN 00378-0503-10 0.<strong>06</strong>480 BISOPROLOL-HCTZ 5-6.25 MG TAB 0 MYLAN EAGEN 29300-0188-01 0.<strong>06</strong>480 BISOPROLOL-HCTZ 5-6.25 MG TAB 0 UNICHEM PHARMAC EAGEN 29300-0188-05 0.<strong>06</strong>480 BISOPROLOL-HCTZ 5-6.25 MG TAB 0 UNICHEM PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 29300-0188-13 0.<strong>06</strong>480 BISOPROLOL-HCTZ 5-6.25 MG TAB 0 UNICHEM PHARMAC EABND 59730-6502-01 12.12729 BIVIGAM LIQUID 10% VIAL 0 BIOTEST PHARMAC MLBND 59730-6503-01 12.12721 BIVIGAM LIQUID 10% VIAL 0 BIOTEST PHARMAC MLGEN 00703-3154-01 40.23750 BLEOMYCIN SULFATE 15 UNIT VIAL 0 TEVA PARENTERAL EAGEN 61703-0332-18 22.62000 BLEOMYCIN SULFATE 15 UNIT VIAL 0 HOSPIRA EAGEN 00703-3155-01 80.47500 BLEOMYCIN SULFATE 30 UNIT VIAL 0 TEVA PARENTERAL EAGEN 61703-0323-22 48.19500 BLEOMYCIN SULFATE 30 UNIT VIAL 0 HOSPIRA EAGUL 11980-0011-05 0.16900 BLEPH-10 10% EYE DROPS G ALLERGAN INC. MLBND 11980-0022-05 17.72880 BLEPHAMIDE EYE DROPS 0 ALLERGAN INC. MLBND 11980-0022-10 17.72880 BLEPHAMIDE EYE DROPS 0 ALLERGAN INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00023-0313-04 25.32686 BLEPHAMIDE EYE OINTMENT 0 ALLERGAN INC. GMBND 00004-0186-82 71.60500 152.44886 BONIVA 150 MG TABLET G ROCHE LABS. EABND 00<strong>06</strong>9-0135-01 71.29955 BOSULIF 100 MG TABLET 0 PFIZER US PHARM EABND 00<strong>06</strong>9-0136-01 285.19823 BOSULIF 500 MG TABLET 0 PFIZER US PHARM EAGEN 42192-0324-30 0.64780 BP FOLINATAL PLUS B TABLET 0 ACELLA PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 49LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-<strong>08</strong>59-10 4.44326 BREO ELLIPTA 100-25 MCG INH G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>59-14 3.66741 BREO ELLIPTA 100-25 MCG INH G GLAXOSMITHKLINE EABEX 52544-0254-28 0.90440 2.21155 BREVICON 28 TABLET 0 ACTAVIS PHARMA, EAGEX 68462-0316-29 1.12980 BRIELLYN TABLET 0 GLENMARK PHARMA EABND 00186-0777-28 BRILINTA 90 MG TABLET G ASTRAZENECA EABND 00186-0777-39 4.05679 BRILINTA 90 MG TABLET G ASTRAZENECA EABND 00186-0777-60 4.05689 BRILINTA 90 MG TABLET G ASTRAZENECA EAGEN 61314-0144-05 15.88650 BRIMONIDINE TARTRATE 0.15% DRP G SANDOZ MLGEN 61314-0144-10 15.88725 BRIMONIDINE TARTRATE 0.15% DRP G SANDOZ MLGEN 61314-0144-15 15.88400 BRIMONIDINE TARTRATE 0.15% DRP G SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 17478-0715-10 1.05210 BRIMONIDINE 0.2% EYE DROP 0 AKORN INC. MLGEN 17478-0715-11 1.05210 BRIMONIDINE 0.2% EYE DROP 0 AKORN INC. MLGEN 17478-0715-12 1.05210 BRIMONIDINE 0.2% EYE DROP 0 AKORN INC. MLGEN 242<strong>08</strong>-0411-05 1.05210 BRIMONIDINE 0.2% EYE DROP 0 VALEANT MLGEN 242<strong>08</strong>-0411-10 1.05210 BRIMONIDINE 0.2% EYE DROP 0 VALEANT MLGEN 242<strong>08</strong>-0411-15 1.05210 BRIMONIDINE 0.2% EYE DROP 0 VALEANT MLGEN 60758-<strong>08</strong>66-05 1.05210 BRIMONIDINE 0.2% EYE DROP 0 PACIFIC PHARMA MLGEN 60758-<strong>08</strong>66-10 1.05210 BRIMONIDINE 0.2% EYE DROP 0 PACIFIC PHARMA MLGEN 61314-0143-05 1.05210 BRIMONIDINE 0.2% EYE DROP 0 SANDOZ MLGEN 61314-0143-10 1.05210 BRIMONIDINE 0.2% EYE DROP 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61314-0143-15 1.05210 BRIMONIDINE 0.2% EYE DROP 0 SANDOZ MLBEX 64764-0560-30 7.24092 BRINTELLIX 10 MG TABLET G TAKEDA PHARMACE EABEX 64764-0580-30 7.24092 BRINTELLIX 20 MG TABLET G TAKEDA PHARMACE EABEX 64764-0550-30 7.24092 BRINTELLIX 5 MG TABLET G TAKEDA PHARMACE EABEX 68968-9075-03 4.47204 BRISDELLE 7.5 MG CAPSULE G NOVEN THERAPEUT EABND 242<strong>08</strong>-0099-03 42.65845 94.76402 BROMDAY 0.09% EYE DROP TWINPAK G BAUSCH & LOMB MLBND 67425-0999-34 42.65845 94.76402 BROMDAY 0.09% EYE DROP TWINPAK G BAUSCH & LOMB MLBND 242<strong>08</strong>-0099-01 42.65845 99.97105 BROMDAY 0.09% EYE DROPS G BAUSCH & LOMB MLBND 67425-0999-17 42.65845 99.97105 BROMDAY 0.09% EYE DROPS G BAUSCH & LOMB MLGEN 00378-7109-35 41.29800 BROMFENAC SODIUM 0.09% EYE DRP G MYLAN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-7110-35 42.65845 BROMFENAC SODIUM 0.09% EYE DRP G MYLAN MLGEN 242<strong>08</strong>-0439-01 42.65845 BROMFENAC SODIUM 0.09% EYE DRP G BAUSCH & LOMB P MLGEN 50383-0249-71 42.65845 BROMFENAC SODIUM 0.09% EYE DRP G HI-TECH PHARMAC MLGEN 00378-2042-01 4.70100 BROMOCRIPTINE 2.5 MG TABLET 0 MYLAN EAGEN 00378-2042-93 4.70100 BROMOCRIPTINE 2.5 MG TABLET 0 MYLAN EAGEN 00574-01<strong>06</strong>-01 1.63717 BROMOCRIPTINE 2.5 MG TABLET 0 PADDOCK LABS. EAGEN 00574-01<strong>06</strong>-03 1.64150 BROMOCRIPTINE 2.5 MG TABLET 0 PADDOCK LABS. EAGEN 00781-5325-01 4.68802 BROMOCRIPTINE 2.5 MG TABLET 0 SANDOZ EAGEN 00781-5325-31 4.70100 BROMOCRIPTINE 2.5 MG TABLET 0 SANDOZ EAGEN 00378-7096-01 6.98280 BROMOCRIPTINE 5 MG CAPSULE 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-7096-93 6.98274 BROMOCRIPTINE 5 MG CAPSULE 0 MYLAN EAGEN 68382-0110-01 6.98280 BROMOCRIPTINE 5 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 68382-0110-<strong>06</strong> 6.98274 BROMOCRIPTINE 5 MG CAPSULE 0 ZYDUS PHARMACEU EABND 63402-0911-30 4.29276 BROVANA 15 MCG/2 ML SOLUTION G SUNOVION PHARMA MLBND 63402-0911-64 4.29276 BROVANA 15 MCG/2 ML SOLUTION G SUNOVION PHARMA ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 50LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-5501-01 0.24840 BUDEPRION SR 100 MG TABLET 0 TEVA USA EAGEX 00093-5502-01 0.28390 BUDEPRION SR 150 MG TABLET 0 TEVA USA EAGEX 00093-5351-56 0.71766 BUDEPRION XL 300 MG TABLET 0 TEVA USA EAGEN 00378-7155-01 12.52790 BUDESONIDE EC 3 MG CAPSULE 0 MYLAN EAGEN 00378-7155-05 12.52790 BUDESONIDE EC 3 MG CAPSULE 0 MYLAN EAGEN 49884-0501-01 12.52790 BUDESONIDE EC 3 MG CAPSULE G PAR PHARM. EAGEN 51079-0020-01 12.52790 BUDESONIDE EC 3 MG CAPSULE G MYLAN INSTITUTI EAGEN 51079-0020-03 12.52790 BUDESONIDE EC 3 MG CAPSULE G MYLAN INSTITUTI EAGEN 00093-6815-73 3.53025 BUDESONIDE 0.25 MG/2 ML SUSP G TEVA USA MLGEN 00093-6816-73 4.15462 BUDESONIDE 0.5 MG/2 ML SUSP G TEVA USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-1412-49 0.27220 BUMETANIDE 0.25 MG/ML VIAL 0 HOSPIRA/NOVATIO MLGEN 00409-1412-50 0.27220 BUMETANIDE 0.25 MG/ML VIAL 0 HOSPIRA/NOVATIO MLGEN 55390-0500-02 0.27220 BUMETANIDE 0.25 MG/ML VIAL 0 BEDFORD LABS MLGEN 55390-0500-05 0.27220 BUMETANIDE 0.25 MG/ML VIAL 0 BEDFORD LABS MLGEN 55390-0500-10 0.27220 BUMETANIDE 0.25 MG/ML VIAL 0 BEDFORD LABS MLGEN 00093-4232-01 0.12272 BUMETANIDE 0.5 MG TABLET 0 IVAX PHARMACEUT EAGEN 00185-0128-01 0.12272 BUMETANIDE 0.5 MG TABLET 0 SANDOZ EAGEN 00185-0128-05 0.12272 BUMETANIDE 0.5 MG TABLET 0 SANDOZ EAGEN 00093-4233-01 0.12339 BUMETANIDE 1 MG TABLET 0 IVAX PHARMACEUT EAGEN 00093-4233-10 0.12339 BUMETANIDE 1 MG TABLET 0 IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0129-01 0.12339 BUMETANIDE 1 MG TABLET 0 SANDOZ EAGEN 00185-0129-05 0.12339 BUMETANIDE 1 MG TABLET 0 SANDOZ EAGEN 00093-4234-01 0.15570 BUMETANIDE 2 MG TABLET 0 IVAX PHARMACEUT EAGEN 00093-4234-10 0.15570 BUMETANIDE 2 MG TABLET 0 TEVA USA EAGEN 00185-0130-01 0.15570 BUMETANIDE 2 MG TABLET 0 SANDOZ EAGEN 00185-0130-05 0.15570 BUMETANIDE 2 MG TABLET 0 SANDOZ EABND 00944-0490-01 1.84426 BUMINATE 25% IV SOLUTION 0 BAXTER BIOSCIEN MLBND 00944-0490-03 1.81927 BUMINATE 25% IV SOLUTION 0 BAXTER BIOSCIEN MLBND 00944-0491-01 0.36885 BUMINATE 5% IV SOLUTION 0 BAXTER BIOSCIEN MLBND 00944-0491-02 0.36883 BUMINATE 5% IV SOLUTION 0 BAXTER BIOSCIEN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00095-0240-01 0.30200 BUPAP TABLET 0 ECR PHARM. EABND 00095-3000-01 3.48600 BUPAP 50 MG-300 MG TABLET 0 ECR PHARM. EABND 62592-0188-64 17.63851 20.70029 BUPHENYL POWDER 0 HYPERION GMBND 62592-0496-03 10.350<strong>06</strong> BUPHENYL 500 MG TABLET 0 HYPERION EAGEN 55150-0167-10 0.14415 BUPIVACAINE 0.25% VIAL 0 AUROMEDICS PHAR MLGEN 55150-0168-30 0.04925 BUPIVACAINE 0.25% VIAL 0 AUROMEDICS PHAR MLGEN 00409-1162-01 0.16650 BUPIVACAINE 0.5% VIAL 0 HOSPIRA MLGEN 55150-0169-10 0.16560 BUPIVACAINE 0.5% VIAL 0 AUROMEDICS PHAR MLGEN 55150-0170-30 0.05435 BUPIVACAINE 0.5% VIAL 0 AUROMEDICS PHAR MLGEN 00409-1165-01 0.16560 BUPIVACAINE 0.75% VIAL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55150-0171-10 0.16470 BUPIVACAINE 0.75% VIAL 0 AUROMEDICS PHAR MLGEN 55150-0172-30 0.09759 BUPIVACAINE 0.75% VIAL 0 AUROMEDICS PHAR MLGEN 00093-5703-01 0.43350 BUPROBAN 150 MG TABLET 0 TEVA USA EAGEX 00185-0410-01 0.24840 BUPROPION HCL SR 100 MG TABLET 0 SANDOZ EAGEX 00185-0410-05 0.24840 BUPROPION HCL SR 100 MG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 51LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00185-0410-60 0.24840 BUPROPION HCL SR 100 MG TABLET 0 SANDOZ EAGEX 00378-3411-01 0.24840 BUPROPION HCL SR 100 MG TABLET 0 MYLAN EAGEX 00378-3411-05 0.24840 BUPROPION HCL SR 100 MG TABLET 0 MYLAN EAGEX 00591-3540-05 0.24840 BUPROPION HCL SR 100 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-3540-60 0.24840 BUPROPION HCL SR 100 MG TABLET 0 ACTAVIS PHARMA, EAGEX 10370-0159-<strong>06</strong> 0.24840 BUPROPION HCL SR 100 MG TABLET 0 PAR PHARM. EAGEX 47335-0736-13 0.24840 BUPROPION HCL SR 100 MG TABLET 0 SUN PHARMA GLOB EAGEX 47335-0736-86 0.24840 BUPROPION HCL SR 100 MG TABLET 0 SUN PHARMA GLOB EAGEX 47335-0736-88 0.24840 BUPROPION HCL SR 100 MG TABLET 0 SUN PHARMA GLOB EAGEX 51079-0391-01 0.24840 BUPROPION HCL SR 100 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0391-20 0.24840 BUPROPION HCL SR 100 MG TABLET 0 MYLAN INSTITUTI EAGEX 64679-0101-02 0.24840 BUPROPION HCL SR 100 MG TABLET 0 WOCKHARDT USA L EAGEX 64679-0101-03 0.24840 BUPROPION HCL SR 100 MG TABLET 0 WOCKHARDT USA L EAGEX 67767-0171-60 0.24840 BUPROPION HCL SR 100 MG TABLET 0 ACTAVIS PHARMA, EAGEX 68<strong>08</strong>4-0470-01 0.24840 BUPROPION HCL SR 100 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0470-11 0.24840 BUPROPION HCL SR 100 MG TABLET 0 AHP EAGEX 00115-5445-13 0.49170 BUPROPION HCL SR 200 MG TAB 0 GLOBAL PHARM EAGEX 00185-1111-60 0.49170 BUPROPION HCL SR 200 MG TAB 0 SANDOZ EAGEX 00378-3413-01 0.49170 BUPROPION HCL SR 200 MG TAB 0 MYLAN EAGEX 00378-3413-05 0.49170 BUPROPION HCL SR 200 MG TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00591-3542-60 0.49170 BUPROPION HCL SR 200 MG TAB 0 ACTAVIS PHARMA, EAGEX 47335-0738-86 0.49170 BUPROPION HCL SR 200 MG TAB 0 SUN PHARMA GLOB EAGEX 64679-0107-02 0.49170 BUPROPION HCL SR 200 MG TAB 0 WOCKHARDT USA L EAGEX 64679-0107-03 0.49170 BUPROPION HCL SR 200 MG TAB 0 WOCKHARDT USA L EAGEX 67767-0135-60 0.49170 BUPROPION HCL SR 200 MG TAB 0 ACTAVIS PHARMA, EAGEX 00115-6811-<strong>08</strong> 0.65250 BUPROPION HCL XL 150 MG TABLET 0 GLOBAL PHARM EAGEX 00115-6811-10 0.65250 BUPROPION HCL XL 150 MG TABLET 0 GLOBAL PHARM EAGEX 00378-20<strong>08</strong>-77 0.65250 BUPROPION HCL XL 150 MG TABLET 0 MYLAN EAGEX 00591-3331-05 0.65250 BUPROPION HCL XL 150 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-3331-19 0.65250 BUPROPION HCL XL 150 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00591-3331-30 0.65250 BUPROPION HCL XL 150 MG TABLET 0 ACTAVIS PHARMA, EAGEX 10370-0101-00 0.65250 BUPROPION HCL XL 150 MG TABLET 0 PAR PHARM. EAGEX 10370-0101-03 0.65250 BUPROPION HCL XL 150 MG TABLET 0 PAR PHARM. EAGEX 10370-0101-50 0.65250 BUPROPION HCL XL 150 MG TABLET 0 PAR PHARM. EAGEX 51079-0047-01 0.65250 BUPROPION HCL XL 150 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0047-20 0.65250 BUPROPION HCL XL 150 MG TABLET 0 MYLAN INSTITUTI EAGEX 64679-0102-01 0.65250 BUPROPION HCL XL 150 MG TABLET 0 WOCKHARDT USA L EAGEX 64679-0102-02 0.65250 BUPROPION HCL XL 150 MG TABLET 0 WOCKHARDT USA L EAGEX 67767-0141-30 0.65250 BUPROPION HCL XL 150 MG TABLET 0 ACTAVIS PHARMA, EAGEX 67767-0141-90 0.65250 BUPROPION HCL XL 150 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0251-01 0.65250 BUPROPION HCL XL 150 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0251-11 0.65250 BUPROPION HCL XL 150 MG TABLET 0 AHP EAGEX 00378-2009-05 0.71766 BUPROPION HCL XL 300 MG TABLET 0 MYLAN EAGEX 00591-3332-05 0.71766 BUPROPION HCL XL 300 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-3332-30 0.71766 BUPROPION HCL XL 300 MG TABLET 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 52LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 10370-0102-03 0.71766 BUPROPION HCL XL 300 MG TABLET 0 PAR PHARM. EAGEX 10370-0102-50 0.71766 BUPROPION HCL XL 300 MG TABLET 0 PAR PHARM. EAGEX 51079-0109-01 0.71766 BUPROPION HCL XL 300 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0109-03 0.71766 BUPROPION HCL XL 300 MG TABLET 0 MYLAN INSTITUTI EAGEX 63739-0450-10 0.71766 BUPROPION HCL XL 300 MG TABLET 0 MCKESSON PACKAG EAGEX 67767-0142-05 0.71766 BUPROPION HCL XL 300 MG TABLET 0 ACTAVIS PHARMA, EAGEX 67767-0142-30 0.71766 BUPROPION HCL XL 300 MG TABLET 0 ACTAVIS PHARMA, EAGEX 67767-0142-90 0.71766 BUPROPION HCL XL 300 MG TABLET 0 ACTAVIS PHARMA, EAGEX 68<strong>08</strong>4-0252-11 0.71766 BUPROPION HCL XL 300 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0252-21 0.71766 BUPROPION HCL XL 300 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-0435-01 0.36100 BUPROPION HCL 100 MG TABLET 0 MYLAN EAGEX 00378-0435-05 0.36100 BUPROPION HCL 100 MG TABLET 0 MYLAN EAGEX 00781-1<strong>06</strong>4-01 0.36100 BUPROPION HCL 100 MG TABLET 0 SANDOZ EAGEX 00781-1<strong>06</strong>4-10 0.36100 BUPROPION HCL 100 MG TABLET 0 SANDOZ EAGEX 00904-6129-61 0.36100 BUPROPION HCL 100 MG TABLET 0 MAJOR PHARMACEU EAGEX 60505-0157-01 0.36100 BUPROPION HCL 100 MG TABLET 0 APOTEX CORP EAGEX 60505-0157-05 0.36100 BUPROPION HCL 100 MG TABLET 0 APOTEX CORP EAGEX 60505-0157-09 0.36100 BUPROPION HCL 100 MG TABLET 0 APOTEX CORP EAGEX 00378-0433-01 0.59572 BUPROPION HCL 75 MG TABLET 0 MYLAN EAGEX 00378-0433-05 0.59575 BUPROPION HCL 75 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-1053-01 0.54000 BUPROPION HCL 75 MG TABLET 0 SANDOZ EAGEX 00781-1053-10 0.54000 BUPROPION HCL 75 MG TABLET 0 SANDOZ EAGEX 00904-6093-61 0.60937 BUPROPION HCL 75 MG TABLET 0 MAJOR PHARMACEU EAGEX 51079-0943-30 0.59574 BUPROPION HCL 75 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0943-56 0.59572 BUPROPION HCL 75 MG TABLET 0 MYLAN INSTITUTI EAGEX 60505-0158-01 0.54037 BUPROPION HCL 75 MG TABLET 0 APOTEX CORP EAGEX 60505-0158-05 0.54037 BUPROPION HCL 75 MG TABLET 0 APOTEX CORP EAGEX 60505-0158-09 0.54075 BUPROPION HCL 75 MG TABLET 0 APOTEX CORP EAGEX 00185-0415-01 0.28390 BUPROPION SR 150 MG TABLET 0 SANDOZ EAGEX 00185-0415-05 0.28390 BUPROPION SR 150 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00185-0415-52 0.28390 BUPROPION SR 150 MG TABLET 0 SANDOZ EAGEX 00185-0415-60 0.28390 BUPROPION SR 150 MG TABLET 0 SANDOZ EAGEX 00378-3412-01 0.28390 BUPROPION SR 150 MG TABLET 0 MYLAN EAGEX 00378-3412-05 0.28390 BUPROPION SR 150 MG TABLET 0 MYLAN EAGEN 00378-5521-01 0.43350 BUPROPION SR 150 MG TABLET 0 MYLAN EAGEX 00591-3541-05 0.28390 BUPROPION SR 150 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-3541-25 0.28390 BUPROPION SR 150 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-3541-60 0.28390 BUPROPION SR 150 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-3543-60 0.43350 BUPROPION SR 150 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-3543-76 0.43350 BUPROPION SR 150 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-5169-60 0.43350 BUPROPION SR 150 MG TABLET 0 SANDOZ EAGEX 10370-0160-50 0.28390 BUPROPION SR 150 MG TABLET 0 PAR PHARM. EAGEX 47335-0737-13 0.28390 BUPROPION SR 150 MG TABLET 0 SUN PHARMA GLOB EAGEX 47335-0737-86 0.28390 BUPROPION SR 150 MG TABLET 0 SUN PHARMA GLOB EAGEX 47335-0737-88 0.28390 BUPROPION SR 150 MG TABLET 0 SUN PHARMA GLOB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 53LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0392-01 0.28390 BUPROPION SR 150 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0392-20 0.28390 BUPROPION SR 150 MG TABLET 0 MYLAN INSTITUTI EAGEX 64679-0105-02 0.28390 BUPROPION SR 150 MG TABLET 0 WOCKHARDT USA L EAGEX 64679-0105-03 0.28390 BUPROPION SR 150 MG TABLET 0 WOCKHARDT USA L EAGEN 67767-0117-60 0.43350 BUPROPION SR 150 MG TABLET 0 SANDOZ EAGEX 67767-0133-05 0.28390 BUPROPION SR 150 MG TABLET 0 ACTAVIS PHARMA, EAGEX 67767-0133-25 0.28390 BUPROPION SR 150 MG TABLET 0 ACTAVIS PHARMA, EAGEX 67767-0133-60 0.28390 BUPROPION SR 150 MG TABLET 0 ACTAVIS PHARMA, EAGEX 68<strong>08</strong>4-0471-01 0.28390 BUPROPION SR 150 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0471-11 0.28390 BUPROPION SR 150 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 00093-0054-01 0.07140 BUSPIRONE HCL 10 MG TABLET 0 TEVA USA EAGUX 00093-0054-05 0.07140 BUSPIRONE HCL 10 MG TABLET 0 TEVA USA EAGUX 00378-1150-01 0.07140 BUSPIRONE HCL 10 MG TABLET 0 MYLAN EAGUX 00591-<strong>06</strong>58-01 0.07140 BUSPIRONE HCL 10 MG TABLET 0 ACTAVIS PHARMA, EAGUX 00591-<strong>06</strong>58-05 0.07140 BUSPIRONE HCL 10 MG TABLET 0 ACTAVIS PHARMA, EAGUX 00591-<strong>06</strong>58-10 0.07140 BUSPIRONE HCL 10 MG TABLET 0 ACTAVIS PHARMA, EAGUX 51079-0986-20 0.07140 BUSPIRONE HCL 10 MG TABLET 0 MYLAN INSTITUTI EAGUX 51079-0986-56 0.07140 BUSPIRONE HCL 10 MG TABLET 0 MYLAN INSTITUTI EAGUX 58517-0340-30 0.07140 BUSPIRONE HCL 10 MG TABLET 0 <strong>NEW</strong> HORIZON RX EAGUX 68<strong>08</strong>4-0029-01 0.07140 BUSPIRONE HCL 10 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 68<strong>08</strong>4-0029-11 0.07140 BUSPIRONE HCL 10 MG TABLET 0 AHP EAGUX 00093-1003-01 0.10280 BUSPIRONE HCL 15 MG TABLET 0 TEVA USA EAGUX 00093-1003-05 0.10280 BUSPIRONE HCL 15 MG TABLET 0 TEVA USA EAGUX 00378-1165-05 0.10280 BUSPIRONE HCL 15 MG TABLET 0 MYLAN EAGUX 00378-1165-80 0.10280 BUSPIRONE HCL 15 MG TABLET 0 MYLAN EAGUX 00378-1165-91 0.10280 BUSPIRONE HCL 15 MG TABLET 0 MYLAN EAGUX 00591-0718-05 0.10280 BUSPIRONE HCL 15 MG TABLET 0 ACTAVIS PHARMA, EAGUX 00591-0718-18 0.10280 BUSPIRONE HCL 15 MG TABLET 0 ACTAVIS PHARMA, EAGUX 00591-0718-60 0.10280 BUSPIRONE HCL 15 MG TABLET 0 ACTAVIS PHARMA, EAGUX 51079-0960-56 0.10280 BUSPIRONE HCL 15 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 68<strong>08</strong>4-0030-01 0.10280 BUSPIRONE HCL 15 MG TABLET 0 AHP EAGUX 68<strong>08</strong>4-0030-11 0.10280 BUSPIRONE HCL 15 MG TABLET 0 AHP EAGEX 00093-5200-05 0.86900 BUSPIRONE HCL 30 MG TABLET 0 TEVA USA EAGEX 00093-5200-<strong>06</strong> 0.86900 BUSPIRONE HCL 30 MG TABLET 0 TEVA USA EAGEX 00378-1175-91 0.86900 BUSPIRONE HCL 30 MG TABLET 0 MYLAN EAGEX 51079-0994-01 0.86900 BUSPIRONE HCL 30 MG TABLET 0 MYLAN INSTITUTI EAGEX 00093-0053-01 0.04620 BUSPIRONE HCL 5 MG TABLET 0 TEVA USA EAGEX 00093-0053-05 0.04620 BUSPIRONE HCL 5 MG TABLET 0 TEVA USA EAGEX 00378-1140-01 0.04620 BUSPIRONE HCL 5 MG TABLET 0 MYLAN EAGEX 00378-1140-05 0.04620 BUSPIRONE HCL 5 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00591-<strong>06</strong>57-01 0.04620 BUSPIRONE HCL 5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-<strong>06</strong>57-05 0.04620 BUSPIRONE HCL 5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-<strong>06</strong>57-10 0.04620 BUSPIRONE HCL 5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 51079-0985-01 0.04620 BUSPIRONE HCL 5 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0985-20 0.04620 BUSPIRONE HCL 5 MG TABLET 0 MYLAN INSTITUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 54LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0028-01 0.04620 BUSPIRONE HCL 5 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0028-11 0.04620 BUSPIRONE HCL 5 MG TABLET 0 AHP EAGEX 00378-1145-01 0.81810 BUSPIRONE HCL 7.5 MG TABLET 0 MYLAN EAGEX 49884-0725-01 0.90900 BUSPIRONE HCL 7.5 MG TABLET 0 PAR PHARM. EAGEX 49884-0725-05 0.90900 BUSPIRONE HCL 7.5 MG TABLET 0 PAR PHARM. EABND 00591-2640-01 2.03482 BUTALB-ACETAMIN-CAFF 50-300-40 0 ACTAVIS PHARMA, EABND 00591-2640-05 2.03482 BUTALB-ACETAMIN-CAFF 50-300-40 0 ACTAVIS PHARMA, EAGEN 00143-1787-01 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 WEST-WARD,INC. EAGEN 00143-1787-05 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 WEST-WARD,INC. EAGEN 00527-1695-01 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 LANNETT CO. INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00527-1695-05 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 LANNETT CO. INC EAGEN 00591-3369-01 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 ACTAVIS PHARMA, EAGEN 00591-3369-05 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-2544-02 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 QUALITEST EAGEN 0<strong>06</strong>03-2544-20 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 QUALITEST EAGEN 0<strong>06</strong>03-2544-21 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 QUALITEST EAGEN 0<strong>06</strong>03-2544-28 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 QUALITEST EAGEN 0<strong>06</strong>03-2544-32 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 QUALITEST EAGEN 46672-0053-10 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 MIKART, INC EAGEN 46672-0053-50 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 MIKART, INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0396-01 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 AHP EAGEN 68<strong>08</strong>4-0396-11 0.<strong>06</strong>030 BUTALB-ACETAMIN-CAFF 50-325-40 0 AHP EAGEN 51862-0179-01 0.48480 BUTALBIT-ACETAMINOPHEN-CAFF CP 0 LIBERTAS PHARMA EABND 66780-0219-04 101.45505 BYDUREON 2 MG VIAL G AMYLIN PHARMACE EABND 66780-0226-01 101.45090 BYDUREON 2 MG VIAL G AMYLIN PHARMACE EABND 66780-0212-01 164.11175 BYETTA 10 MCG DOSE PEN INJ G AMYLIN PHARMACE MLBND 66780-0210-07 328.22350 BYETTA 5 MCG DOSE PEN INJ G AMYLIN PHARMACE MLBND 00456-1410-01 2.613<strong>08</strong> BYSTOLIC 10 MG TABLET G FOREST PHARMACE EABND 00456-1410-30 2.61339 BYSTOLIC 10 MG TABLET G FOREST PHARMACE EABND 00456-1410-63 2.66313 BYSTOLIC 10 MG TABLET G FOREST PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00456-1402-30 2.61339 BYSTOLIC 2.5 MG TABLET G FOREST PHARMACE EABND 00456-1420-30 2.654<strong>06</strong> BYSTOLIC 20 MG TABLET G FOREST PHARMACE EABND 00456-1405-01 2.613<strong>08</strong> BYSTOLIC 5 MG TABLET G FOREST PHARMACE EABND 00456-1405-30 2.61339 BYSTOLIC 5 MG TABLET G FOREST PHARMACE EABND 00456-1405-63 2.66313 BYSTOLIC 5 MG TABLET G FOREST PHARMACE EAGEN 00093-5420-88 12.39150 CABERGOLINE 0.5 MG TABLET 0 TEVA USA EAGEN 00378-2800-26 12.39150 CABERGOLINE 0.5 MG TABLET 0 MYLAN EAGEN 16252-0536-<strong>08</strong> 12.39150 CABERGOLINE 0.5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 49884-<strong>06</strong>73-14 12.39150 CABERGOLINE 0.5 MG TABLET 0 PAR PHARM. EAGEN 60505-2597-02 12.39150 CABERGOLINE 0.5 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>9-2160-30 4.55754 6.89287 CADUET 10 MG-10 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2180-30 6.53713 9.43046 CADUET 10 MG-20 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2250-30 7.347<strong>06</strong> 9.43046 CADUET 10 MG-40 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2270-30 5.99538 9.43046 CADUET 10 MG-80 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2960-30 4.55754 6.89287 CADUET 2.5 MG-10 MG TABLET G PFIZER US PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 55LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>9-2970-30 5.99538 9.43046 CADUET 2.5 MG-20 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2980-30 5.99538 9.43046 CADUET 2.5 MG-40 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2150-30 4.96899 6.89287 CADUET 5 MG-10 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2170-30 6.11963 9.43046 CADUET 5 MG-20 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2190-30 5.01000 9.43046 CADUET 5 MG-40 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2260-30 5.99538 9.43046 CADUET 5 MG-80 MG TABLET G PFIZER US PHARM EABND 55390-0358-03 4.77529 6.64000 CAFCIT 20 MG/ML ORAL SOLN G BEDFORD LABS MLGEN 00781-5405-01 0.67640 CAFERGOT TABLET 0 SANDOZ EABND 00517-2502-10 5.18750 CAFF-SOD BENZOATE 500 MG VL 0 AMER. REGENT MLGEN 00574-0152-10 4.77529 CAFFEINE CIT 60 MG/3 ML ORAL 0 PADDOCK LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00574-0152-80 4.77529 CAFFEINE CIT 60 MG/3 ML ORAL 0 PADDOCK/NOVAPLU MLGEN 25021-<strong>06</strong>02-03 4.77529 CAFFEINE CIT 60 MG/3 ML ORAL 0 SAGENT PHARMACE MLGEN 47335-0290-44 4.77529 CAFFEINE CIT 60 MG/3 ML ORAL 0 SUN PHARMA GLOB MLGEN 63323-04<strong>06</strong>-03 4.77529 CAFFEINE CIT 60 MG/3 ML ORAL 0 APP PHARMACEUTI MLGEN 00517-0020-10 5.62500 CAFFEINE CIT 60 MG/3 ML VIAL 0 AMER. REGENT MLGEN 00574-<strong>08</strong>23-01 6.36000 CAFFEINE CIT 60 MG/3 ML VIAL 0 PADDOCK LABS. MLGEN 00574-<strong>08</strong>23-81 5.00000 CAFFEINE CIT 60 MG/3 ML VIAL 0 PADDOCK/NOVAPLU MLGEN 25021-<strong>06</strong>01-03 6.00000 CAFFEINE CIT 60 MG/3 ML VIAL 0 SAGENT PHARMACE MLBND 00025-1901-31 0.27419 3.10254 CALAN SR 120 MG CAPLET G PHARMACIA/UPJHN EABND 00025-1911-31 0.17037 3.93229 CALAN SR 180 MG CAPLET G PHARMACIA/UPJHN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00025-1891-31 0.14594 4.49826 CALAN SR 240 MG CAPLET G PHARMACIA/UPJHN EABND 00025-1891-51 0.14594 4.49835 CALAN SR 240 MG CAPLET G PHARMACIA/UPJHN EABND 00025-1861-31 0.<strong>08</strong>630 2.31628 CALAN 120 MG TABLET G PHARMACIA/UPJHN EABND 00025-1851-31 0.05630 1.71262 CALAN 80 MG TABLET G PHARMACIA/UPJHN EAGEN 00781-7117-35 5.52287 CALCIPOTRIENE 0.005% CREAM G SANDOZ GMGEN 00781-7117-83 5.28<strong>06</strong>9 CALCIPOTRIENE 0.005% CREAM G SANDOZ GMGEN 66993-<strong>08</strong>77-61 5.29612 CALCIPOTRIENE 0.005% CREAM G PRASCO LABS GMGEN 66993-<strong>08</strong>77-78 5.<strong>06</strong>381 CALCIPOTRIENE 0.005% CREAM G PRASCO LABS GMGEN 51672-4154-03 4.52362 CALCIPOTRIENE 0.005% OINTMENT 0 TARO PHARM USA GMGEN 66993-<strong>08</strong>78-61 4.52362 CALCIPOTRIENE 0.005% OINTMENT 0 PRASCO LABS GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 66993-<strong>08</strong>78-78 4.52362 CALCIPOTRIENE 0.005% OINTMENT 0 PRASCO LABS GMGEN 00115-1475-55 3.93420 CALCIPOTRIENE 0.005% SOLUTION 0 GLOBAL PHARM MLGEN 00168-0400-60 3.93420 CALCIPOTRIENE 0.005% SOLUTION 0 SANDOZ MLGEN 00713-0318-53 3.93420 CALCIPOTRIENE 0.005% SOLUTION 0 G & W LABS. MLGEN 00781-6320-79 12.21825 CALCITONIN-SALMON 200 UNITS SP 0 SANDOZ MLGEN 49884-0161-11 12.21825 CALCITONIN-SALMON 200 UNITS SP 0 PAR PHARM. MLGEN 60505-<strong>08</strong>23-<strong>06</strong> 12.21825 CALCITONIN-SALMON 200 UNITS SP 0 APOTEX CORP MLGEN 51672-5278-03 4.78687 CALCITRENE 0.005% OINTMENT G TARO PHARM USA GMGEN 00054-0007-13 0.53370 CALCITRIOL 0.25 MCG CAPSULE 0 ROXANE LABS. EAGEN 00054-0007-25 0.53370 CALCITRIOL 0.25 MCG CAPSULE 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-<strong>06</strong>57-01 0.53370 CALCITRIOL 0.25 MCG CAPSULE 0 TEVA USA EAGEN 23155-0118-01 0.53370 CALCITRIOL 0.25 MCG CAPSULE 0 HERITAGE PHARMA EAGEN 23155-0118-03 0.53370 CALCITRIOL 0.25 MCG CAPSULE 0 HERITAGE PHARMA EAGEN 63304-0239-01 0.53370 CALCITRIOL 0.25 MCG CAPSULE 0 RANBAXY PHARMAC EAGEN 63304-0239-30 0.53370 CALCITRIOL 0.25 MCG CAPSULE 0 RANBAXY PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 56LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-<strong>06</strong>58-01 1.40020 CALCITRIOL 0.5 MCG CAPSULE 0 TEVA USA EAGEN 23155-0119-01 1.40020 CALCITRIOL 0.5 MCG CAPSULE 0 HERITAGE PHARMA EAGEN 63304-0240-01 1.40020 CALCITRIOL 0.5 MCG CAPSULE 0 RANBAXY PHARMAC EAGEN 63323-0731-01 4.18320 CALCITRIOL 1 MCG/ML AMPUL 0 APP PHARMACEUTI MLGEN 00054-3120-41 7.01660 CALCITRIOL 1 MCG/ML SOLUTION 0 ROXANE LABS. MLGEN 63304-0241-59 7.01660 CALCITRIOL 1 MCG/ML SOLUTION 0 RANBAXY PHARMAC MLGEN 45802-<strong>06</strong><strong>08</strong>-01 4.80187 CALCITRIOL 3 MCG/G OINTMENT G PERRIGO CO. GMGEN 00054-0<strong>08</strong>8-13 0.70497 CALCIUM ACETATE 667 MG CAPSULE 0 ROXANE LABS. EAGEN 00054-0<strong>08</strong>8-26 0.70497 CALCIUM ACETATE 667 MG CAPSULE 0 ROXANE LABS. EAGEN 31722-0377-02 0.70497 CALCIUM ACETATE 667 MG CAPSULE 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0479-01 0.63157 CALCIUM ACETATE 667 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-0479-11 0.63157 CALCIUM ACETATE 667 MG CAPSULE 0 AHP EAGEN 00781-2<strong>08</strong>1-02 0.59220 CALCIUM ACETATE 667 MG GELCAP 0 SANDOZ EAGEN 00781-2672-02 0.59220 CALCIUM ACETATE 667 MG GELCAP 0 SANDOZ EAGEN 00574-0113-02 0.<strong>08</strong>200 CALCIUM ACETATE 667 MG TABLET 0 PADDOCK LABS. EAGEN 00517-3900-25 0.05859 CALCIUM GLUCONATE 10% VIAL 0 AMER. REGENT MLGEN 00517-3950-25 0.<strong>06</strong>562 CALCIUM GLUCONATE 10% VIAL 0 AMER. REGENT MLGEN 63323-0311-10 0.29880 CALCIUM GLUCONATE 10% VIAL 0 APP PHARMACEUTI MLGEN 63323-0311-50 0.118<strong>08</strong> CALCIUM GLUCONATE 10% VIAL 0 APP PHARMACEUTI MLGEN 63323-0311-61 0.12138 CALCIUM GLUCONATE 10% VIAL 0 APP PHARMACEUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50192-0113-09 30.36785 CAMBIA 50 MG POWDER PACKET G DEPOMED, INC. EAGEX 00555-0715-58 0.69917 CAMILA TABLET 0 BARR EABND 00456-3330-01 0.80495 1.26805 CAMPRAL DR 333 MG TABLET 0 FOREST PHARMACE EABND 00009-1111-02 12.45000 CAMPTOSAR 100 MG/5 ML VIAL G PFIZER/NOVAPLUS MLBND 00009-7529-03 12.45000 CAMPTOSAR 100 MG/5 ML VIAL G PHARMACIA/UPJHN MLBND 00009-7529-05 12.45000 CAMPTOSAR 300 MG/15 ML VIAL G PHARMACIA/UPJHN MLBND 00009-1111-01 12.45000 CAMPTOSAR 40 MG/2 ML VIAL G PFIZER/NOVAPLUS MLBND 00009-7529-04 12.45000 CAMPTOSAR 40 MG/2 ML VIAL G PHARMACIA/UPJHN MLBEX 00093-6148-82 2.44941 CAMRESE LO TABLET 0 TEVA USA EABEX 00093-3134-82 2.44941 CAMRESE 0.15-0.03-0.01 MG TAB 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00093-3134-91 2.44941 CAMRESE 0.15-0.03-0.01 MG TAB 0 TEVA USA EABND 58914-0501-42 21.42348 CANASA 1,000 MG SUPPOSITORY 0 APTALIS PHARMA EABND 58914-0501-56 21.42340 CANASA 1,000 MG SUPPOSITORY 0 APTALIS PHARMA EABND 000<strong>06</strong>-3822-10 336.35750 CANCIDAS IV 50 MG VIAL 0 MERCK SHARP & D EABND 000<strong>06</strong>-3823-10 349.47980 CANCIDAS IV 70 MG VIAL 0 MERCK SHARP & D EAGEN 00378-3001-05 1.80000 CANDESARTAN-HCTZ 16-12.5 MG TB G MYLAN EAGEN 00378-3001-77 1.80000 CANDESARTAN-HCTZ 16-12.5 MG TB G MYLAN EAGEN 49884-<strong>06</strong>62-09 1.80000 CANDESARTAN-HCTZ 16-12.5 MG TB G PAR PHARM. EAGEN 60505-3758-05 1.80000 CANDESARTAN-HCTZ 16-12.5 MG TB G APOTEX CORP EAGEN 60505-3758-09 1.80000 CANDESARTAN-HCTZ 16-12.5 MG TB G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3002-05 1.63560 CANDESARTAN-HCTZ 32-12.5 MG TB G MYLAN EAGEN 00378-3002-77 1.63560 CANDESARTAN-HCTZ 32-12.5 MG TB G MYLAN EAGEN 49884-<strong>06</strong>63-09 1.63560 CANDESARTAN-HCTZ 32-12.5 MG TB G PAR PHARM. EAGEN 60505-3759-05 1.63560 CANDESARTAN-HCTZ 32-12.5 MG TB G APOTEX CORP EAGEN 60505-3759-09 1.63560 CANDESARTAN-HCTZ 32-12.5 MG TB G APOTEX CORP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 57LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3003-05 3.38376 CANDESARTAN-HCTZ 32-25 MG TAB G MYLAN EAGEN 00378-3003-77 3.38375 CANDESARTAN-HCTZ 32-25 MG TAB G MYLAN EAGEN 49884-<strong>06</strong>64-09 3.38733 CANDESARTAN-HCTZ 32-25 MG TAB G PAR PHARM. EAGEN 60505-3760-09 3.38733 CANDESARTAN-HCTZ 32-25 MG TAB G APOTEX CORP EABND 00<strong>06</strong>8-0037-01 2.10197 CANTIL 25 MG TABLET 0 SAN<strong>OF</strong>I-AVENTIS EAGEN 58407-0534-01 0.22462 CAPACET CAPSULE 0 MAGNA PHARM EABEX 17478-0<strong>08</strong>0-50 154.34680 CAPASTAT SULFATE 1 GM VIAL 0 AKORN INC. EABND 00310-7820-30 181.11928 CAPRELSA 100 MG TABLET 0 ASTRAZENECA EABND 00310-7840-30 362.23828 CAPRELSA 300 MG TABLET 0 ASTRAZENECA EAGEN 00143-1174-01 0.07920 CAPTOPRIL 100 MG TABLET 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3022-01 0.07920 CAPTOPRIL 100 MG TABLET 0 MYLAN EAGEN 00781-1839-01 0.07920 CAPTOPRIL 100 MG TABLET 0 SANDOZ EAGEN 60505-00<strong>06</strong>-<strong>06</strong> 0.07920 CAPTOPRIL 100 MG TABLET 0 APOTEX CORP EAGEN 60505-00<strong>06</strong>-09 0.07920 CAPTOPRIL 100 MG TABLET 0 APOTEX CORP EAGEN 64679-0905-01 0.07920 CAPTOPRIL 100 MG TABLET 0 WOCKHARDT USA L EAGEN 68645-0163-59 0.07920 CAPTOPRIL 100 MG TABLET 0 LEGACY PHARMACE EAGEN 00143-1171-01 0.01580 CAPTOPRIL 12.5 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1171-10 0.01580 CAPTOPRIL 12.5 MG TABLET 0 WEST-WARD,INC. EAGEN 00378-3007-01 0.01580 CAPTOPRIL 12.5 MG TABLET 0 MYLAN EAGEN 00378-3007-10 0.01580 CAPTOPRIL 12.5 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0003-<strong>06</strong> 0.01580 CAPTOPRIL 12.5 MG TABLET 0 APOTEX CORP EAGEN 60505-0003-09 0.01580 CAPTOPRIL 12.5 MG TABLET 0 APOTEX CORP EAGEN 64679-0902-01 0.01580 CAPTOPRIL 12.5 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0902-02 0.01580 CAPTOPRIL 12.5 MG TABLET 0 WOCKHARDT USA L EAGEN 68645-0160-59 0.01580 CAPTOPRIL 12.5 MG TABLET 0 LEGACY PHARMACE EAGEN 00143-1172-01 0.01855 CAPTOPRIL 25 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1172-10 0.01855 CAPTOPRIL 25 MG TABLET 0 WEST-WARD,INC. EAGEN 00378-3012-01 0.01855 CAPTOPRIL 25 MG TABLET 0 MYLAN EAGEN 00378-3012-10 0.01855 CAPTOPRIL 25 MG TABLET 0 MYLAN EAGEN 60505-0004-<strong>06</strong> 0.01855 CAPTOPRIL 25 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0004-09 0.01855 CAPTOPRIL 25 MG TABLET 0 APOTEX CORP EAGEN 64679-0903-01 0.01855 CAPTOPRIL 25 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0903-02 0.01855 CAPTOPRIL 25 MG TABLET 0 WOCKHARDT USA L EAGEN 68645-0161-59 0.01855 CAPTOPRIL 25 MG TABLET 0 LEGACY PHARMACE EAGEN 00143-1173-01 0.02756 CAPTOPRIL 50 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1173-10 0.02756 CAPTOPRIL 50 MG TABLET 0 WEST-WARD,INC. EAGEN 00378-3017-01 0.02756 CAPTOPRIL 50 MG TABLET 0 MYLAN EAGEN 00378-3017-10 0.02756 CAPTOPRIL 50 MG TABLET 0 MYLAN EAGEN 60505-0005-<strong>06</strong> 0.02756 CAPTOPRIL 50 MG TABLET 0 APOTEX CORP EAGEN 60505-0005-09 0.02756 CAPTOPRIL 50 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0904-01 0.02756 CAPTOPRIL 50 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0904-02 0.02756 CAPTOPRIL 50 MG TABLET 0 WOCKHARDT USA L EAGEN 68645-0162-59 0.02756 CAPTOPRIL 50 MG TABLET 0 LEGACY PHARMACE EAGEN 00093-0176-01 0.<strong>06</strong>890 CAPTOPRIL-HCTZ 25-15 MG TABLET 0 TEVA USA EABND 00378-0<strong>08</strong>1-01 0.<strong>06</strong>890 0.81647 CAPTOPRIL-HCTZ 25-15 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 58LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-0177-01 0.<strong>06</strong>890 CAPTOPRIL-HCTZ 25-25 MG TABLET 0 TEVA USA EABND 00378-0<strong>08</strong>3-01 0.<strong>06</strong>890 0.81647 CAPTOPRIL-HCTZ 25-25 MG TABLET 0 MYLAN EAGEN 00093-0181-01 0.49637 CAPTOPRIL-HCTZ 50-15 MG TABLET 0 TEVA USA EABND 00378-0<strong>08</strong>4-01 0.49637 1.40145 CAPTOPRIL-HCTZ 50-15 MG TABLET 0 MYLAN EAGUL 00093-0182-01 0.37020 CAPTOPRIL-HCTZ 50-25 MG TABLET 0 TEVA USA EABUL 00378-0<strong>08</strong>6-01 0.37020 1.40145 CAPTOPRIL-HCTZ 50-25 MG TABLET 0 MYLAN EABND 00<strong>06</strong>6-7150-30 16.48214 CARAC CREAM 0 VALEANT GMBND 00187-5200-30 21.26183 CARAC 0.5% CREAM 0 VALEANT GMBND 58914-0171-10 0.22450 2.02777 CARAFATE 1 GM TABLET G APTALIS PHARMA EABND 58914-0170-14 0.260<strong>06</strong> CARAFATE 1 GM/10 ML SUSP 0 APTALIS PHARMA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 52276-0312-60 160.67472 CARBAGLU 200 MG DISPER TABLET 0 ORPHAN EUROPE S EAGEX 29033-0019-12 1.34049 CARBAMAZEPINE ER 100 MG CAP G NOSTRUM LABORAT EAGEX 51672-4151-01 1.43600 CARBAMAZEPINE ER 100 MG CAP G TARO PHARM USA EAGEX 60505-2805-07 1.43600 CARBAMAZEPINE ER 100 MG CAP G APOTEX CORP EAGEX 66993-0407-32 1.34049 CARBAMAZEPINE ER 100 MG CAP G PRASCO LABS EAGEX 29033-0020-12 1.34049 CARBAMAZEPINE ER 200 MG CAP G NOSTRUM LABORAT EAGEX 51672-4150-01 1.43600 CARBAMAZEPINE ER 200 MG CAP G TARO PHARM USA EAGEX 60505-28<strong>06</strong>-07 1.43600 CARBAMAZEPINE ER 200 MG CAP G APOTEX CORP EAGEX 66993-04<strong>08</strong>-32 1.34049 CARBAMAZEPINE ER 200 MG CAP G PRASCO LABS EAGEX 00781-5987-01 1.04962 CARBAMAZEPINE ER 200 MG TABLET G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 29033-0004-12 1.34049 CARBAMAZEPINE ER 300 MG CAP G NOSTRUM LABORAT EAGEX 51672-4149-01 1.43600 CARBAMAZEPINE ER 300 MG CAP G TARO PHARM USA EAGEX 60505-2807-07 1.43600 CARBAMAZEPINE ER 300 MG CAP G APOTEX CORP EAGEX 66993-0409-32 1.34049 CARBAMAZEPINE ER 300 MG CAP G PRASCO LABS EAGEX 00781-5988-01 2.09767 CARBAMAZEPINE ER 400 MG TABLET G SANDOZ EAGEX 51672-4124-01 1.04955 CARBAMAZEPINE XR 200 MG TABLET G TARO PHARM USA EAGEX 68<strong>08</strong>4-0561-11 1.64250 CARBAMAZEPINE XR 200 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0561-21 1.64900 CARBAMAZEPINE XR 200 MG TABLET G AHP EAGEX 51672-4125-01 2.09767 CARBAMAZEPINE XR 400 MG TABLET G TARO PHARM USA EAGEX 00093-0778-01 0.<strong>06</strong>840 CARBAMAZEPINE 100 MG TAB CHEW 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00904-3854-61 0.<strong>06</strong>840 CARBAMAZEPINE 100 MG TAB CHEW 0 MAJOR PHARMACEU EAGEX 13668-0271-01 0.<strong>06</strong>840 CARBAMAZEPINE 100 MG TAB CHEW 0 TORRENT PHARMAC EAGEX 13668-0271-05 0.<strong>06</strong>840 CARBAMAZEPINE 100 MG TAB CHEW 0 TORRENT PHARMAC EAGEX 51079-<strong>08</strong>70-20 0.<strong>06</strong>840 CARBAMAZEPINE 100 MG TAB CHEW 0 MYLAN INSTITUTI EAGEX 51672-4041-01 0.<strong>06</strong>840 CARBAMAZEPINE 100 MG TAB CHEW 0 TARO PHARM USA EAGEX 51672-4041-02 0.<strong>06</strong>840 CARBAMAZEPINE 100 MG TAB CHEW 0 TARO PHARM USA EAGUX 51672-4047-09 0.14765 CARBAMAZEPINE 100 MG/5 ML SUSP G TARO PHARM USA MLGUX 60432-0129-16 0.12462 CARBAMAZEPINE 100 MG/5 ML SUSP G MORTON GROVE PH MLGEX 00093-0109-01 0.04960 CARBAMAZEPINE 200 MG TABLET 0 TEVA USA EAGEX 00093-0109-10 0.04960 CARBAMAZEPINE 200 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00904-6172-61 0.04960 CARBAMAZEPINE 200 MG TABLET 0 MAJOR PHARMACEU EAGEX 13668-0268-01 0.04960 CARBAMAZEPINE 200 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0268-05 0.04960 CARBAMAZEPINE 200 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0268-10 0.04960 CARBAMAZEPINE 200 MG TABLET 0 TORRENT PHARMAC EAGEX 51079-0385-17 0.04960 CARBAMAZEPINE 200 MG TABLET 0 MYLAN INSTITUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 59LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0385-20 0.04960 CARBAMAZEPINE 200 MG TABLET 0 MYLAN INSTITUTI EAGEX 51672-4005-01 0.04960 CARBAMAZEPINE 200 MG TABLET 0 TARO PHARM USA EAGEX 51672-4005-02 0.04960 CARBAMAZEPINE 200 MG TABLET 0 TARO PHARM USA EAGEX 51672-4005-03 0.04960 CARBAMAZEPINE 200 MG TABLET 0 TARO PHARM USA EAGEX 60505-0183-00 0.04960 CARBAMAZEPINE 200 MG TABLET 0 APOTEX CORP EAGEX 60505-0183-01 0.04960 CARBAMAZEPINE 200 MG TABLET 0 APOTEX CORP EAGEX 63739-0045-01 0.04960 CARBAMAZEPINE 200 MG TABLET 0 MCKESSON PACKAG EAGUX 68094-0214-59 0.<strong>08</strong>370 CARBAMAZEPINE 200 MG/10 ML LIQ G PRECISION DOSE MLBEX 54092-0171-12 1.76575 1.76575 CARBATROL ER 100 MG CAPSULE 0 SHIRE US INC. EABEX 54092-0172-12 1.76575 1.76575 CARBATROL ER 200 MG CAPSULE 0 SHIRE US INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 54092-0173-12 1.76575 1.76575 CARBATROL ER 300 MG CAPSULE 0 SHIRE US INC. EAGEN 00115-3922-02 0.33075 CARBIDOPA-LEVO ER 25-100 TAB 0 GLOBAL PHARM EAGEN 00378-0<strong>08</strong>8-01 0.33075 CARBIDOPA-LEVO ER 25-100 TAB 0 MYLAN EAGEN 16729-0078-17 0.33075 CARBIDOPA-LEVO ER 25-100 TAB 0 ACCORD <strong>HEALTH</strong>CA EAGEN 51079-0978-01 0.33075 CARBIDOPA-LEVO ER 25-100 TAB 0 MYLAN INSTITUTI EAGEN 60505-0131-00 0.33075 CARBIDOPA-LEVO ER 25-100 TAB 0 APOTEX CORP EAGEN 60505-0131-01 0.33075 CARBIDOPA-LEVO ER 25-100 TAB 0 APOTEX CORP EAGEN 62756-0461-88 0.33075 CARBIDOPA-LEVO ER 25-100 TAB 0 SUN PHARMACEUTI EAGEN 68<strong>08</strong>4-0281-01 0.33075 CARBIDOPA-LEVO ER 25-100 TAB 0 AHP EAGEN 68<strong>08</strong>4-0281-11 0.33075 CARBIDOPA-LEVO ER 25-100 TAB 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00115-3911-02 0.64260 CARBIDOPA-LEVO ER 50-200 TAB 0 GLOBAL PHARM EAGEN 00378-0094-01 0.64260 CARBIDOPA-LEVO ER 50-200 TAB 0 MYLAN EAGEN 16729-0079-17 0.64260 CARBIDOPA-LEVO ER 50-200 TAB 0 ACCORD <strong>HEALTH</strong>CA EAGEN 60505-0132-00 0.64260 CARBIDOPA-LEVO ER 50-200 TAB 0 APOTEX CORP EAGEN 60505-0132-01 0.64260 CARBIDOPA-LEVO ER 50-200 TAB 0 APOTEX CORP EAGEN 62756-0457-88 0.64260 CARBIDOPA-LEVO ER 50-200 TAB 0 SUN PHARMACEUTI EAGEN 68<strong>08</strong>4-0282-01 0.64260 CARBIDOPA-LEVO ER 50-200 TAB 0 AHP EAGEN 68<strong>08</strong>4-0282-11 0.64260 CARBIDOPA-LEVO ER 50-200 TAB 0 AHP EAGEN 00378-5051-01 0.62190 CARBIDOPA-LEVO 10-100 MG ODT 0 MYLAN EAGEN 62756-0186-88 0.62190 CARBIDOPA-LEVO 10-100 MG ODT 0 SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5052-01 0.86042 CARBIDOPA-LEVO 25-100 MG ODT 0 MYLAN EAGEN 62756-0187-88 0.86042 CARBIDOPA-LEVO 25-100 MG ODT 0 SUN PHARMACEUTI EAGEN 00378-5053-01 1.09613 CARBIDOPA-LEVO 25-250 MG ODT 0 MYLAN EAGEN 62756-0188-88 1.09613 CARBIDOPA-LEVO 25-250 MG ODT 0 SUN PHARMACEUTI EAGEN 00093-0292-01 0.14364 CARBIDOPA-LEVODOPA 10-100 TAB 0 TEVA USA EAGEN 00093-0292-05 0.14364 CARBIDOPA-LEVODOPA 10-100 TAB 0 TEVA USA EAGEN 00228-2538-10 0.14364 CARBIDOPA-LEVODOPA 10-100 TAB 0 ACTAVIS PHARMA, EAGEN 00228-2538-50 0.14364 CARBIDOPA-LEVODOPA 10-100 TAB 0 ACTAVIS PHARMA, EAGEN 00378-0078-01 0.14364 CARBIDOPA-LEVODOPA 10-100 TAB 0 MYLAN EAGEN 62756-0517-13 0.14364 CARBIDOPA-LEVODOPA 10-100 TAB 0 SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62756-0517-88 0.14364 CARBIDOPA-LEVODOPA 10-100 TAB 0 SUN PHARMACEUTI EAGEN 63739-0046-10 0.14364 CARBIDOPA-LEVODOPA 10-100 TAB 0 MCKESSON PACKAG EAGEN 00093-0293-01 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 TEVA USA EAGEN 00093-0293-05 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 TEVA USA EAGEN 00093-0293-10 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 60LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-2539-10 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 ACTAVIS PHARMA, EAGEN 00228-2539-50 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 ACTAVIS PHARMA, EAGEN 00228-2539-96 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 ACTAVIS PHARMA, EAGEN 00378-0<strong>08</strong>5-01 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 MYLAN EAGEN 00904-6237-61 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 MAJOR PHARMACEU EAGEN 51079-<strong>08</strong>84-01 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 MYLAN INSTITUTI EAGEN 51079-<strong>08</strong>84-19 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 MYLAN INSTITUTI EAGEN 51079-<strong>08</strong>84-20 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 MYLAN INSTITUTI EAGEN 60505-0129-01 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 APOTEX CORP EAGEN 60505-0129-02 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62756-0518-13 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 SUN PHARMACEUTI EAGEN 62756-0518-18 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 SUN PHARMACEUTI EAGEN 62756-0518-88 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 SUN PHARMACEUTI EAGEN 63739-0047-10 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0093-01 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 AHP EAGEN 68<strong>08</strong>4-0093-11 0.13240 CARBIDOPA-LEVODOPA 25-100 TAB 0 AHP EAGEN 00093-0294-01 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 TEVA USA EAGEN 00093-0294-05 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 TEVA USA EAGEN 00093-0294-10 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 TEVA USA EAGEN 00228-2540-10 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-2540-50 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 ACTAVIS PHARMA, EAGEN 00228-2540-96 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 ACTAVIS PHARMA, EAGEN 00378-1133-01 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 MYLAN EAGEN 00904-6238-61 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 MAJOR PHARMACEU EAGEN 62756-0519-13 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 SUN PHARMACEUTI EAGEN 62756-0519-88 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 SUN PHARMACEUTI EAGEN 68<strong>08</strong>4-0094-01 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 AHP EAGEN 68<strong>08</strong>4-0094-11 0.18170 CARBIDOPA-LEVODOPA 25-250 TAB 0 AHP EAGEN 00378-8302-01 2.81310 CARBIDOPA-LEVODOPA-ENTA 100 MG 0 MYLAN EAGEN 47335-0003-88 2.80987 CARBIDOPA-LEVODOPA-ENTA 100 MG 0 SUN PHARMA GLOB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0784-02 2.81310 CARBIDOPA-LEVODOPA-ENTA 100 MG 0 WOCKHARDT USA L EAGEN 00378-8303-01 2.81310 CARBIDOPA-LEVODOPA-ENTA 125 MG 0 MYLAN EAGEN 47335-0004-88 2.80987 CARBIDOPA-LEVODOPA-ENTA 125 MG 0 SUN PHARMA GLOB EAGEN 64679-0785-02 2.81310 CARBIDOPA-LEVODOPA-ENTA 125 MG 0 WOCKHARDT USA L EAGEN 00378-8304-01 2.81310 CARBIDOPA-LEVODOPA-ENTA 150 MG 0 MYLAN EAGEN 47335-0005-88 2.80987 CARBIDOPA-LEVODOPA-ENTA 150 MG 0 SUN PHARMA GLOB EAGEN 64679-0786-02 2.81310 CARBIDOPA-LEVODOPA-ENTA 150 MG 0 WOCKHARDT USA L EAGEN 00378-8305-01 2.71958 CARBIDOPA-LEVODOPA-ENTA 200 MG 0 MYLAN EAGEN 47335-00<strong>06</strong>-88 2.71958 CARBIDOPA-LEVODOPA-ENTA 200 MG 0 SUN PHARMA GLOB EAGEN 64679-0787-02 2.71958 CARBIDOPA-LEVODOPA-ENTA 200 MG 0 WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 47335-0001-88 2.80987 CARBIDOPA-LEVODOPA-ENTA 50 MG 0 SUN PHARMA GLOB EAGEN 64679-0782-02 2.81310 CARBIDOPA-LEVODOPA-ENTA 50 MG 0 WOCKHARDT USA L EAGEN 00378-8301-01 2.81310 CARBIDOPA-LEVODOPA-ENTA 75 MG 0 MYLAN EAGEN 47335-0002-88 2.80987 CARBIDOPA-LEVODOPA-ENTA 75 MG 0 SUN PHARMA GLOB EAGEN 64679-0783-02 2.81310 CARBIDOPA-LEVODOPA-ENTA 75 MG 0 WOCKHARDT USA L EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 61LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51991-0334-04 0.09005 CARBINOXAMINE 4 MG/5 ML LIQUID 0 BRECKENRIDGE MLGEN 51991-0334-16 0.09005 CARBINOXAMINE 4 MG/5 ML LIQUID 0 BRECKENRIDGE MLGEN 64376-<strong>06</strong>12-16 0.09005 CARBINOXAMINE 4 MG/5 ML LIQUID 0 BOCA PHARMACAL MLGEN 64376-<strong>06</strong>12-40 0.09005 CARBINOXAMINE 4 MG/5 ML LIQUID 0 BOCA PHARMACAL MLBND 63323-0167-21 47.50000 214.14000 CARBOPLATIN 150 MG VIAL 0 APP PHARMACEUTI EAGEN 25021-0202-15 1.50000 CARBOPLATIN 150 MG/15 ML VIAL 0 SAGENT PHARMACE MLGEN 61703-0360-22 0.95100 CARBOPLATIN 150 MG/15 ML VIAL 0 HOSPIRA MLGEN 00703-4248-01 1.29999 CARBOPLATIN 450 MG/45 ML VIAL 0 TEVA PARENTERAL MLGEN 25021-0202-45 1.50000 CARBOPLATIN 450 MG/45 ML VIAL 0 SAGENT PHARMACE MLGEN 61703-0339-50 0.76116 CARBOPLATIN 450 MG/45 ML VIAL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61703-0360-50 0.75366 CARBOPLATIN 450 MG/45 ML VIAL 0 HOSPIRA MLGEN 25021-0202-05 1.80000 CARBOPLATIN 50 MG/5 ML VIAL 0 SAGENT PHARMACE MLGEN 61703-0339-18 1.66350 CARBOPLATIN 50 MG/5 ML VIAL 0 HOSPIRA MLGEN 61703-0360-18 1.64700 CARBOPLATIN 50 MG/5 ML VIAL 0 HOSPIRA MLGEN 25021-0202-51 1.50000 CARBOPLATIN 600 MG/60 ML VIAL 0 SAGENT PHARMACE MLGEN 61703-0339-56 0.77562 CARBOPLATIN 600 MG/60 ML VIAL 0 HOSPIRA MLGEN 63323-0172-60 1.62000 CARBOPLATIN 600 MG/60 ML VIAL 0 APP PHARMACEUTI MLGEN 66758-0047-04 2.25000 CARBOPLATIN 600 MG/60 ML VIAL 0 SANDOZ MLBND 24477-0515-01 1.65516 CARDENE SR 30 MG CAPSULE G CORNERSTONE THE EABND 24477-0516-01 3.14555 CARDENE SR 60 MG CAPSULE G CORNERSTONE THE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00187-0795-30 0.42110 7.39585 CARDIZEM CD 120 MG CAPSULE G VALEANT EABND 00187-0795-42 0.42110 7.23907 CARDIZEM CD 120 MG CAPSULE G VALEANT EABND 64455-0795-30 0.42110 4.218<strong>06</strong> CARDIZEM CD 120 MG CAPSULE G VALEANT EABND 64455-0795-42 0.42110 4.12860 CARDIZEM CD 120 MG CAPSULE G VALEANT EABND 00187-0796-30 0.41240 9.32975 CARDIZEM CD 180 MG CAPSULE G VALEANT EABND 00187-0796-42 0.41240 8.90553 CARDIZEM CD 180 MG CAPSULE G VALEANT EABND 00187-0797-30 0.59454 12.64809 CARDIZEM CD 240 MG CAPSULE G VALEANT EABND 00187-0797-42 0.59454 12.63333 CARDIZEM CD 240 MG CAPSULE G VALEANT EABND 64455-0797-30 0.59454 7.21380 CARDIZEM CD 240 MG CAPSULE G VALEANT EABND 64455-0798-30 0.85887 9.44927 CARDIZEM CD 300 MG CAPSULE G VALEANT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00187-0799-42 7.46000 24.17245 CARDIZEM CD 360 MG CAPSULE 0 VALEANT EABND 64455-0799-42 7.46000 13.78630 CARDIZEM CD 360 MG CAPSULE 0 VALEANT EABND 00074-3045-30 3.78867 CARDIZEM LA 120 MG TABLET 0 ABBVIE US LLC EABND 00074-3045-90 3.78332 CARDIZEM LA 120 MG TABLET 0 ABBVIE US LLC EABND 00074-3<strong>06</strong>1-30 2.33270 4.00253 CARDIZEM LA 180 MG TABLET 0 ABBVIE US LLC EABND 00074-3<strong>06</strong>1-90 2.33270 4.00299 CARDIZEM LA 180 MG TABLET 0 ABBVIE US LLC EABND 00074-3<strong>06</strong>2-30 2.61450 4.48670 CARDIZEM LA 240 MG TABLET 0 ABBVIE US LLC EABND 00074-3<strong>06</strong>2-90 2.61450 4.48633 CARDIZEM LA 240 MG TABLET 0 ABBVIE US LLC EABND 00074-3<strong>06</strong>3-30 2.77658 5.83683 CARDIZEM LA 300 MG TABLET 0 ABBVIE US LLC EABND 00074-3<strong>06</strong>3-90 2.77658 5.83609 CARDIZEM LA 300 MG TABLET 0 ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00074-3<strong>06</strong>4-30 3.65930 6.27480 CARDIZEM LA 360 MG TABLET 0 ABBVIE US LLC EABND 00074-3<strong>06</strong>9-30 3.23860 6.80074 CARDIZEM LA 420 MG TABLET 0 ABBVIE US LLC EABND 00074-3<strong>06</strong>9-90 3.23860 6.81374 CARDIZEM LA 420 MG TABLET 0 ABBVIE US LLC EABND 00187-0792-47 0.13690 7.98584 CARDIZEM 120 MG TABLET G VALEANT EABND 64455-0792-47 0.13690 4.55462 CARDIZEM 120 MG TABLET G VALEANT EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 62LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00187-0771-47 0.<strong>06</strong>129 2.76497 CARDIZEM 30 MG TABLET G VALEANT EABND 64455-0771-55 0.<strong>06</strong>129 2.38452 CARDIZEM 30 MG TABLET G VALEANT EABND 00187-0772-47 0.1<strong>06</strong>92 4.33824 CARDIZEM 60 MG TABLET G VALEANT EABND 64455-0791-47 0.09135 5.35300 CARDIZEM 90 MG TABLET G VALEANT EABND 00049-2710-30 2.50521 CARDURA XL 4 MG TABLET 0 PFIZER US PHARM EABND 00049-2720-30 2.63165 CARDURA XL 8 MG TABLET 0 PFIZER US PHARM EABND 00049-2750-66 0.05170 2.17958 CARDURA 1 MG TABLET G PFIZER US PHARM EABUL 00049-2760-66 0.59180 2.17958 CARDURA 2 MG TABLET G PFIZER US PHARM EABUL 00049-2770-66 0.62100 2.28739 CARDURA 4 MG TABLET G PFIZER US PHARM EABUL 00049-2780-66 0.65180 2.40218 CARDURA 8 MG TABLET G PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 442<strong>06</strong>-0418-12 1055.76000 CARIMUNE NF 12 GM VIAL 0 CSL BEHRING LLC EABND 442<strong>06</strong>-0416-03 263.94000 CARIMUNE NF 3 GM VIAL 0 CSL BEHRING LLC EABND 442<strong>06</strong>-0417-<strong>06</strong> 527.88000 CARIMUNE NF 6 GM VIAL 0 CSL BEHRING LLC EABND 54482-0148-01 0.24302 CARNITOR SF 100 MG/ML ORAL SOL 0 SIGMA-TAU MLBND 54482-0147-01 6.59750 CARNITOR 1 GM/5 ML VIAL G SIGMA-TAU MLBND 54482-0145-<strong>08</strong> 0.16990 0.24302 CARNITOR 100 MG/ML ORAL SOLN 0 SIGMA-TAU MLBND 54482-0144-07 0.58740 0.83009 CARNITOR 330 MG TABLET 0 SIGMA-TAU EAGEN 242<strong>08</strong>-0367-05 1.18<strong>08</strong>5 CARTEOLOL HCL 1% EYE DROPS 0 VALEANT MLGEN 242<strong>08</strong>-0367-10 1.18<strong>08</strong>5 CARTEOLOL HCL 1% EYE DROPS 0 VALEANT MLGEN 242<strong>08</strong>-0367-15 1.18<strong>08</strong>5 CARTEOLOL HCL 1% EYE DROPS 0 VALEANT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61314-0238-05 1.18<strong>08</strong>5 CARTEOLOL HCL 1% EYE DROPS 0 SANDOZ MLGEN 61314-0238-10 1.18<strong>08</strong>5 CARTEOLOL HCL 1% EYE DROPS 0 SANDOZ MLGEN 61314-0238-15 1.18<strong>08</strong>5 CARTEOLOL HCL 1% EYE DROPS 0 SANDOZ MLGEN 62037-0597-05 0.42110 CARTIA XT 120 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0597-90 0.42110 CARTIA XT 120 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0598-05 0.41240 CARTIA XT 180 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0598-90 0.41240 CARTIA XT 180 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0599-05 0.59454 CARTIA XT 240 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0599-90 0.59454 CARTIA XT 240 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-<strong>06</strong>00-05 0.85887 CARTIA XT 300 MG CAPSULE 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62037-<strong>06</strong>00-90 0.85887 CARTIA XT 300 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00093-7295-01 0.03848 CARVEDILOL 12.5 MG TABLET 0 TEVA USA EAGEN 00093-7295-05 0.03848 CARVEDILOL 12.5 MG TABLET 0 TEVA USA EAGEN 00378-3633-01 0.03848 CARVEDILOL 12.5 MG TABLET 0 MYLAN EAGEN 00378-3633-02 0.03848 CARVEDILOL 12.5 MG TABLET 0 MYLAN EAGEN 00378-3633-05 0.03848 CARVEDILOL 12.5 MG TABLET 0 MYLAN EAGEN 00378-3633-07 0.03848 CARVEDILOL 12.5 MG TABLET 0 MYLAN EAGEN 00781-5223-01 0.03848 CARVEDILOL 12.5 MG TABLET 0 SANDOZ EAGEN 00904-6302-61 0.03848 CARVEDILOL 12.5 MG TABLET 0 MAJOR PHARMACEU EAGEN 13107-0144-01 0.03848 CARVEDILOL 12.5 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13107-0144-05 0.03848 CARVEDILOL 12.5 MG TABLET 0 AUROBINDO PHARM EAGEN 43547-0256-10 0.03848 CARVEDILOL 12.5 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0256-50 0.03848 CARVEDILOL 12.5 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 51079-0931-01 0.03848 CARVEDILOL 12.5 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0931-17 0.03848 CARVEDILOL 12.5 MG TABLET 0 MYLAN INSTITUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 63LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0931-19 0.03848 CARVEDILOL 12.5 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0931-20 0.03848 CARVEDILOL 12.5 MG TABLET 0 MYLAN INSTITUTI EAGEN 52343-0028-01 0.02422 CARVEDILOL 12.5 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0028-05 0.02338 CARVEDILOL 12.5 MG TABLET 0 GEN-SOURCE RX EAGEN 57664-0245-13 0.03848 CARVEDILOL 12.5 MG TABLET 0 CARACO PHARM EAGEN 57664-0245-18 0.03848 CARVEDILOL 12.5 MG TABLET 0 CARACO PHARM EAGEN 57664-0245-88 0.03848 CARVEDILOL 12.5 MG TABLET 0 CARACO PHARM EAGEN 60505-26<strong>08</strong>-01 0.03848 CARVEDILOL 12.5 MG TABLET 0 APOTEX CORP EAGEN 60505-26<strong>08</strong>-<strong>08</strong> 0.03848 CARVEDILOL 12.5 MG TABLET 0 APOTEX CORP EAGEN 65862-0144-01 0.03848 CARVEDILOL 12.5 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0144-05 0.03848 CARVEDILOL 12.5 MG TABLET 0 AUROBINDO PHARM EAGEN 68001-0151-00 0.03848 CARVEDILOL 12.5 MG TABLET 0 BLUEPOINT LABOR EAGEN 68001-0151-03 0.03848 CARVEDILOL 12.5 MG TABLET 0 BLUEPOINT LABOR EAGEN 68<strong>08</strong>4-0263-01 0.03848 CARVEDILOL 12.5 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0263-11 0.03848 CARVEDILOL 12.5 MG TABLET 0 AHP EAGEN 68382-0094-01 0.03848 CARVEDILOL 12.5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0094-05 0.03848 CARVEDILOL 12.5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0164-01 0.03848 CARVEDILOL 12.5 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0164-05 0.03848 CARVEDILOL 12.5 MG TABLET 0 GLENMARK PHARMA EAGEN 68645-0351-59 0.03848 CARVEDILOL 12.5 MG TABLET 0 LEGACY PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7296-01 0.03915 CARVEDILOL 25 MG TABLET 0 TEVA USA EAGEN 00093-7296-05 0.03915 CARVEDILOL 25 MG TABLET 0 TEVA USA EAGEN 00378-3634-01 0.03915 CARVEDILOL 25 MG TABLET 0 MYLAN EAGEN 00378-3634-02 0.03915 CARVEDILOL 25 MG TABLET 0 MYLAN EAGEN 00378-3634-05 0.03915 CARVEDILOL 25 MG TABLET 0 MYLAN EAGEN 00378-3634-07 0.03915 CARVEDILOL 25 MG TABLET 0 MYLAN EAGEN 00781-5224-01 0.03915 CARVEDILOL 25 MG TABLET 0 SANDOZ EAGEN 00904-6303-61 0.03915 CARVEDILOL 25 MG TABLET 0 MAJOR PHARMACEU EAGEN 13107-0145-01 0.03915 CARVEDILOL 25 MG TABLET 0 AUROBINDO PHARM EAGEN 13107-0145-05 0.03915 CARVEDILOL 25 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43547-0257-10 0.03915 CARVEDILOL 25 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0257-50 0.03915 CARVEDILOL 25 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 51079-0932-01 0.03915 CARVEDILOL 25 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0932-20 0.03915 CARVEDILOL 25 MG TABLET 0 MYLAN INSTITUTI EAGEN 52343-0029-01 0.02422 CARVEDILOL 25 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0029-05 0.02338 CARVEDILOL 25 MG TABLET 0 GEN-SOURCE RX EAGEN 57664-0247-13 0.03915 CARVEDILOL 25 MG TABLET 0 CARACO PHARM EAGEN 57664-0247-18 0.03915 CARVEDILOL 25 MG TABLET 0 CARACO PHARM EAGEN 57664-0247-88 0.03915 CARVEDILOL 25 MG TABLET 0 CARACO PHARM EAGEN 60505-2609-01 0.03915 CARVEDILOL 25 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2609-<strong>08</strong> 0.03915 CARVEDILOL 25 MG TABLET 0 APOTEX CORP EAGEN 65862-0145-01 0.03915 CARVEDILOL 25 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0145-05 0.03915 CARVEDILOL 25 MG TABLET 0 AUROBINDO PHARM EAGEN 68001-0152-00 0.03915 CARVEDILOL 25 MG TABLET 0 BLUEPOINT LABOR EAGEN 68001-0152-03 0.03915 CARVEDILOL 25 MG TABLET 0 BLUEPOINT LABOR EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 64LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0264-01 0.03915 CARVEDILOL 25 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0264-11 0.03915 CARVEDILOL 25 MG TABLET 0 AHP EAGEN 68382-0095-01 0.03915 CARVEDILOL 25 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0095-05 0.03915 CARVEDILOL 25 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0165-01 0.03915 CARVEDILOL 25 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0165-05 0.03915 CARVEDILOL 25 MG TABLET 0 GLENMARK PHARMA EAGEN 68645-0352-59 0.03915 CARVEDILOL 25 MG TABLET 0 LEGACY PHARMACE EAGEN 68645-0468-59 0.03915 CARVEDILOL 25 MG TABLET 0 LEGACY PHARMACE EAGEN 00093-0051-01 0.03848 CARVEDILOL 3.125 MG TABLET 0 TEVA USA EAGEN 00093-0051-05 0.03848 CARVEDILOL 3.125 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3631-01 0.03848 CARVEDILOL 3.125 MG TABLET 0 MYLAN EAGEN 00378-3631-02 0.03848 CARVEDILOL 3.125 MG TABLET 0 MYLAN EAGEN 00378-3631-05 0.03848 CARVEDILOL 3.125 MG TABLET 0 MYLAN EAGEN 00378-3631-07 0.03848 CARVEDILOL 3.125 MG TABLET 0 MYLAN EAGEN 00781-5221-01 0.03848 CARVEDILOL 3.125 MG TABLET 0 SANDOZ EAGEN 00904-6300-61 0.03848 CARVEDILOL 3.125 MG TABLET 0 MAJOR PHARMACEU EAGEN 13107-0142-01 0.03848 CARVEDILOL 3.125 MG TABLET 0 AUROBINDO PHARM EAGEN 13107-0142-05 0.03848 CARVEDILOL 3.125 MG TABLET 0 AUROBINDO PHARM EAGEN 43547-0254-10 0.03848 CARVEDILOL 3.125 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0254-50 0.03848 CARVEDILOL 3.125 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0771-01 0.03848 CARVEDILOL 3.125 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0771-17 0.03848 CARVEDILOL 3.125 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0771-19 0.03848 CARVEDILOL 3.125 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0771-20 0.03848 CARVEDILOL 3.125 MG TABLET 0 MYLAN INSTITUTI EAGEN 52343-0026-01 0.02422 CARVEDILOL 3.125 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0026-05 0.02338 CARVEDILOL 3.125 MG TABLET 0 GEN-SOURCE RX EAGEN 57664-0242-13 0.03848 CARVEDILOL 3.125 MG TABLET 0 CARACO PHARM EAGEN 57664-0242-18 0.03848 CARVEDILOL 3.125 MG TABLET 0 CARACO PHARM EAGEN 57664-0242-88 0.03848 CARVEDILOL 3.125 MG TABLET 0 CARACO PHARM EAGEN 60505-26<strong>06</strong>-01 0.03848 CARVEDILOL 3.125 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-26<strong>06</strong>-<strong>08</strong> 0.03848 CARVEDILOL 3.125 MG TABLET 0 APOTEX CORP EAGEN 65862-0142-01 0.03848 CARVEDILOL 3.125 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0142-05 0.03848 CARVEDILOL 3.125 MG TABLET 0 AUROBINDO PHARM EAGEN 68001-0153-00 0.03848 CARVEDILOL 3.125 MG TABLET 0 BLUEPOINT LABOR EAGEN 68001-0153-03 0.03848 CARVEDILOL 3.125 MG TABLET 0 BLUEPOINT LABOR EAGEN 68<strong>08</strong>4-0261-01 0.03848 CARVEDILOL 3.125 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0261-11 0.03848 CARVEDILOL 3.125 MG TABLET 0 AHP EAGEN 68382-0092-01 0.03848 CARVEDILOL 3.125 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0092-05 0.03848 CARVEDILOL 3.125 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0162-01 0.03848 CARVEDILOL 3.125 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0162-05 0.03848 CARVEDILOL 3.125 MG TABLET 0 GLENMARK PHARMA EAGEN 00093-0135-01 0.05700 CARVEDILOL 6.25 MG TABLET 0 TEVA USA EAGEN 00093-0135-05 0.05700 CARVEDILOL 6.25 MG TABLET 0 TEVA USA EAGEN 00378-3632-01 0.05700 CARVEDILOL 6.25 MG TABLET 0 MYLAN EAGEN 00378-3632-02 0.05700 CARVEDILOL 6.25 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 65LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3632-05 0.05700 CARVEDILOL 6.25 MG TABLET 0 MYLAN EAGEN 00378-3632-07 0.05700 CARVEDILOL 6.25 MG TABLET 0 MYLAN EAGEN 00781-5222-01 0.05700 CARVEDILOL 6.25 MG TABLET 0 SANDOZ EAGEN 00904-6301-61 0.05700 CARVEDILOL 6.25 MG TABLET 0 MAJOR PHARMACEU EAGEN 13107-0143-01 0.05700 CARVEDILOL 6.25 MG TABLET 0 AUROBINDO PHARM EAGEN 13107-0143-05 0.05700 CARVEDILOL 6.25 MG TABLET 0 AUROBINDO PHARM EAGEN 43547-0255-10 0.05700 CARVEDILOL 6.25 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0255-50 0.05700 CARVEDILOL 6.25 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 51079-0930-01 0.05700 CARVEDILOL 6.25 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0930-17 0.05700 CARVEDILOL 6.25 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0930-19 0.05700 CARVEDILOL 6.25 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0930-20 0.05700 CARVEDILOL 6.25 MG TABLET 0 MYLAN INSTITUTI EAGEN 52343-0027-01 0.02422 CARVEDILOL 6.25 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0027-05 0.02338 CARVEDILOL 6.25 MG TABLET 0 GEN-SOURCE RX EAGEN 57664-0244-13 0.05700 CARVEDILOL 6.25 MG TABLET 0 CARACO PHARM EAGEN 57664-0244-18 0.05700 CARVEDILOL 6.25 MG TABLET 0 CARACO PHARM EAGEN 57664-0244-88 0.05700 CARVEDILOL 6.25 MG TABLET 0 CARACO PHARM EAGEN 60505-2607-01 0.05700 CARVEDILOL 6.25 MG TABLET 0 APOTEX CORP EAGEN 60505-2607-<strong>08</strong> 0.05700 CARVEDILOL 6.25 MG TABLET 0 APOTEX CORP EAGEN 65862-0143-01 0.05700 CARVEDILOL 6.25 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0143-05 0.05700 CARVEDILOL 6.25 MG TABLET 0 AUROBINDO PHARM EAGEN 68001-0154-00 0.05700 CARVEDILOL 6.25 MG TABLET 0 BLUEPOINT LABOR EAGEN 68001-0154-03 0.05700 CARVEDILOL 6.25 MG TABLET 0 BLUEPOINT LABOR EAGEN 68<strong>08</strong>4-0262-01 0.05700 CARVEDILOL 6.25 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0262-11 0.05700 CARVEDILOL 6.25 MG TABLET 0 AHP EAGEN 68382-0093-01 0.05700 CARVEDILOL 6.25 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0093-05 0.05700 CARVEDILOL 6.25 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0163-01 0.05700 CARVEDILOL 6.25 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0163-05 0.05700 CARVEDILOL 6.25 MG TABLET 0 GLENMARK PHARMA EAGEN 68645-0350-59 0.05700 CARVEDILOL 6.25 MG TABLET 0 LEGACY PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00310-0705-30 0.30200 17.43221 CASODEX 50 MG TABLET G ASTRAZENECA EABND 00078-0436-05 0.43335 5.32445 CATAFLAM 50 MG TABLET G NOVARTIS EABND 00597-00<strong>06</strong>-01 0.03380 1.84550 CATAPRES 0.1 MG TABLET G BOEHRINGER ING. EABND 00597-0007-01 0.04293 2.82349 CATAPRES 0.2 MG TABLET G BOEHRINGER ING. EABND 00597-0011-01 0.05430 3.54293 CATAPRES 0.3 MG TABLET G BOEHRINGER ING. EABND 00597-0031-34 44.73077 44.73077 CATAPRES-TTS 1 PATCH 0 BOEHRINGER ING. EABND 00597-0032-34 75.31420 75.31420 CATAPRES-TTS 2 PATCH 0 BOEHRINGER ING. EABND 00597-0033-34 104.47625 104.47625 CATAPRES-TTS 3 PATCH 0 BOEHRINGER ING. EABND 50242-0041-64 111.40260 CATHFLO ACTIVASE 2 MG VIAL 0 GENENTECH, INC. EABND 61958-0901-01 71.98214 CAYSTON 75 MG INHAL SOLUTION 0 GILEAD SCIENCES ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 52544-0959-31 0.84780 CAZIANT 28 DAY TABLET 0 ACTAVIS PHARMA, EABND 65224-<strong>08</strong>04-02 8.96302 CEDAX 180 MG/5 ML SUSPENSION G PERNIX THERAPEU MLBND 65224-<strong>08</strong>04-30 3.31668 CEDAX 180 MG/5 ML SUSPENSION G PERNIX THERAPEU MLBND 45809-0401-20 14.43411 CEDAX 400 MG CAPSULE G PERNIX THERAPEU EABND 65224-<strong>08</strong>00-22 31.87117 CEDAX 400 MG CAPSULE G PERNIX THERAPEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 66LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00015-3030-20 8.78721 CEENU 10 MG CAPSULE 0 BMS ONCO/IMMUN EABND 00015-3031-20 26.46164 CEENU 40 MG CAPSULE 0 BMS ONCO/IMMUN EABND 00093-1<strong>08</strong>7-01 5.16675 CEFACLOR ER 500 MG TABLET 0 TEVA USA EABND 16571-0070-12 0.80902 CEFACLOR 125 MG/5 ML SUSP 0 PACK PHARMACEUT MLGEN 00143-9985-01 1.19988 CEFACLOR 250 MG CAPSULE 0 WEST-WARD,INC. EAGEN 61442-0171-30 1.19988 CEFACLOR 250 MG CAPSULE 0 CARLSBAD TECH EABND 16571-0071-12 1.61794 CEFACLOR 250 MG/5 ML SUSP 0 PACK PHARMACEUT MLBND 16571-0072-11 2.42692 CEFACLOR 375 MG/5 ML SUSPEN 0 PACK PHARMACEUT MLGEN 00143-9986-01 1.62243 CEFACLOR 500 MG CAPSULE 0 WEST-WARD,INC. EAGEN 61442-0172-30 1.62243 CEFACLOR 500 MG CAPSULE 0 CARLSBAD TECH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-4059-53 5.35620 CEFADROXIL 1 GM TABLET 0 TEVA USA EAGEN 00143-9948-50 5.25000 CEFADROXIL 1 GM TABLET 0 WEST-WARD,INC. EAGEN 16714-0202-01 0.37611 CEFADROXIL 250 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0389-01 0.37611 CEFADROXIL 250 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 65862-0<strong>08</strong>3-01 0.37611 CEFADROXIL 250 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 68180-0181-02 0.37611 CEFADROXIL 250 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 00093-3196-01 0.34938 CEFADROXIL 500 MG CAPSULE 0 TEVA USA EAGEN 00093-3196-53 0.34938 CEFADROXIL 500 MG CAPSULE 0 TEVA USA EAGEN 00143-9947-01 0.34938 CEFADROXIL 500 MG CAPSULE 0 WEST-WARD,INC. EAGEN 00143-9947-50 0.34938 CEFADROXIL 500 MG CAPSULE 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-2938-50 0.34938 CEFADROXIL 500 MG CAPSULE 0 SANDOZ EAGEN 16714-0204-02 0.34938 CEFADROXIL 500 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 16714-0204-03 0.34938 CEFADROXIL 500 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 16714-0388-01 0.34938 CEFADROXIL 500 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 16714-0388-02 0.34938 CEFADROXIL 500 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 65862-0<strong>08</strong>5-01 0.34938 CEFADROXIL 500 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0<strong>08</strong>5-50 0.34938 CEFADROXIL 500 MG CAPSULE 0 AUROBINDO PHARM EAGEN 68180-0180-01 0.34938 CEFADROXIL 500 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68180-0180-<strong>08</strong> 0.34938 CEFADROXIL 500 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68820-0043-<strong>08</strong> 0.34938 CEFADROXIL 500 MG CAPSULE 0 ORCHID <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68820-0043-10 0.34938 CEFADROXIL 500 MG CAPSULE 0 ORCHID <strong>HEALTH</strong>CA EAGEN 16714-0203-01 0.62280 CEFADROXIL 500 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0203-02 0.62280 CEFADROXIL 500 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0390-01 0.62280 CEFADROXIL 500 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0390-02 0.62280 CEFADROXIL 500 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 65862-0<strong>08</strong>4-75 0.62280 CEFADROXIL 500 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 68180-0182-02 0.62280 CEFADROXIL 500 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68180-0182-03 0.62280 CEFADROXIL 500 MG/5 ML SUSP 0 LUPIN PHARMACEU MLBND 00409-2585-01 2.19120 CEFAZOLIN 1 GM ADD-VAN VIAL 0 HOSPIRA EAGEN 00143-9924-90 0.89640 CEFAZOLIN 1 GM VIAL 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-<strong>08</strong>05-01 0.48600 CEFAZOLIN 1 GM VIAL 0 HOSPIRA EAGEN 00781-3451-70 0.89640 CEFAZOLIN 1 GM VIAL 0 SANDOZ EAGEN 00781-3451-96 0.89640 CEFAZOLIN 1 GM VIAL 0 SANDOZ EAGEN 00781-9339-96 0.89640 CEFAZOLIN 1 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0101-10 0.89640 CEFAZOLIN 1 GM VIAL 0 SAGENT PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 67LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 44567-0707-25 0.89640 CEFAZOLIN 1 GM VIAL 0 WG CRITICAL CAR EAGEN 60505-0749-04 0.89640 CEFAZOLIN 1 GM VIAL 0 APOTEX CORP EAGEN 60505-0749-05 0.89640 CEFAZOLIN 1 GM VIAL 0 APOTEX CORP EAGEN 60505-6093-05 0.89640 CEFAZOLIN 1 GM VIAL 0 APOTEX/NOVAPLUS EAGEN 63323-0237-10 0.89640 CEFAZOLIN 1 GM VIAL 0 APP PHARMACEUTI EABND 00264-3103-11 4.18320 CEFAZOLIN 1 GM-D5W BAG 0 B.BRAUN EABND 00338-3503-41 0.<strong>08</strong>924 CEFAZOLIN 1 GM-D5W BAG 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-<strong>08</strong><strong>06</strong>-01 6.04800 CEFAZOLIN 10 GM VIAL 0 HOSPIRA EAGEN 00781-3452-46 7.45200 CEFAZOLIN 10 GM VIAL 0 SANDOZ EAGEN 00781-3452-95 7.45200 CEFAZOLIN 10 GM VIAL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-9337-95 7.45200 CEFAZOLIN 10 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0102-99 7.45200 CEFAZOLIN 10 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-07<strong>08</strong>-10 7.45200 CEFAZOLIN 10 GM VIAL 0 WG CRITICAL CAR EAGEN 60505-0769-00 7.45200 CEFAZOLIN 10 GM VIAL 0 APOTEX CORP EAGEN 60505-6094-00 7.45200 CEFAZOLIN 10 GM VIAL 0 APOTEX/NOVAPLUS EAGEN 63323-0238-61 7.45200 CEFAZOLIN 10 GM VIAL 0 APP PHARMACEUTI EABND 00264-3105-11 7.27<strong>08</strong>0 CEFAZOLIN 2 GM-D5W BAG 0 B.BRAUN EAGEN 44567-0709-10 22.27500 CEFAZOLIN 20 GM BULK VIAL 0 WG CRITICAL CAR EAGEN 00143-9923-90 1.43586 CEFAZOLIN 500 MG VIAL 0 WEST-WARD,INC. EAGEN 00781-3450-95 1.43586 CEFAZOLIN 500 MG VIAL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-9338-95 1.43586 CEFAZOLIN 500 MG VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0100-10 1.<strong>08</strong>000 CEFAZOLIN 500 MG VIAL 0 SAGENT PHARMACE EAGEN 44567-07<strong>06</strong>-25 1.43586 CEFAZOLIN 500 MG VIAL 0 WG CRITICAL CAR EAGEN 63323-0236-10 1.43586 CEFAZOLIN 500 MG VIAL 0 APP PHARMACEUTI EAGEN 00093-4136-64 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-4136-73 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 TEVA USA MLGEN 00781-6077-46 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6077-61 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 SANDOZ MLGEN 16714-02<strong>06</strong>-01 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-02<strong>06</strong>-02 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 NORTHSTAR RX LL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0392-01 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0392-02 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 42043-0251-38 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 KARALEX PHARMA, MLGEN 42043-0251-67 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 KARALEX PHARMA, MLGEN 65862-0218-01 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 65862-0218-60 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 68180-0722-10 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68180-0722-20 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68820-0<strong>06</strong>4-17 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 ORCHID <strong>HEALTH</strong>CA MLGEN 68820-0<strong>06</strong>4-37 0.46587 CEFDINIR 125 MG/5 ML SUSP 0 ORCHID <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-4137-64 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-4137-73 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 TEVA USA MLGEN 00781-6078-46 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6078-61 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 SANDOZ MLGEN 16714-0207-01 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 NORTHSTAR RX LL ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 68LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0207-02 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0393-01 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0393-02 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 42043-0252-38 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 KARALEX PHARMA, MLGEN 42043-0252-67 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 KARALEX PHARMA, MLGEN 65862-0219-01 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 65862-0219-60 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 68180-0723-10 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68180-0723-20 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68820-0<strong>06</strong>5-17 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 ORCHID <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68820-0<strong>06</strong>5-37 0.92000 CEFDINIR 250 MG/5 ML SUSP 0 ORCHID <strong>HEALTH</strong>CA MLGEN 00093-3160-<strong>06</strong> 1.88700 CEFDINIR 300 MG CAPSULE 0 TEVA USA EAGEN 00781-2176-60 1.88700 CEFDINIR 300 MG CAPSULE 0 SANDOZ EAGEN 16714-0205-01 1.88700 CEFDINIR 300 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 16714-0205-02 1.88700 CEFDINIR 300 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 16714-0391-01 1.88700 CEFDINIR 300 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 16714-0391-02 1.88700 CEFDINIR 300 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 65862-0177-60 1.88700 CEFDINIR 300 MG CAPSULE 0 AUROBINDO PHARM EAGEN 68180-0711-60 1.88700 CEFDINIR 300 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68820-0<strong>06</strong>3-09 1.88700 CEFDINIR 300 MG CAPSULE 0 ORCHID <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68820-0<strong>06</strong>3-19 1.88700 CEFDINIR 300 MG CAPSULE 0 NORTHSTAR RX LL EABND 24486-<strong>08</strong>01-20 12.23461 CEFDITOREN PIVOXIL 200 MG TAB G METHAPHARM INC EAGEN 00781-3222-80 4.33485 CEFEPIME HCL 1 GM VIAL 0 SANDOZ EAGEN 00781-3222-95 4.33485 CEFEPIME HCL 1 GM VIAL 0 SANDOZ EAGEN 25021-0121-20 4.33485 CEFEPIME HCL 1 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-0240-10 4.33485 CEFEPIME HCL 1 GM VIAL 0 WG CRITICAL CAR EAGEN 60505-<strong>08</strong>34-00 4.33485 CEFEPIME HCL 1 GM VIAL 0 APOTEX CORP EAGEN 60505-<strong>08</strong>34-04 4.33485 CEFEPIME HCL 1 GM VIAL 0 APOTEX CORP EAGEN 60505-6030-04 4.33485 CEFEPIME HCL 1 GM VIAL 0 APOTEX/NOVAPLUS EAGEN 00781-3223-91 9.81000 CEFEPIME HCL 2 GRAM VIAL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3223-95 9.81000 CEFEPIME HCL 2 GRAM VIAL 0 SANDOZ EAGEN 25021-0122-50 9.00000 CEFEPIME HCL 2 GRAM VIAL 0 SAGENT PHARMACE EAGEN 44567-0241-10 8.82000 CEFEPIME HCL 2 GRAM VIAL 0 WG CRITICAL CAR EAGEN 60505-<strong>06</strong>81-00 9.81000 CEFEPIME HCL 2 GRAM VIAL 0 APOTEX CORP EAGEN 60505-<strong>06</strong>81-04 9.81000 CEFEPIME HCL 2 GRAM VIAL 0 APOTEX CORP EAGEN 60505-6031-04 9.81000 CEFEPIME HCL 2 GRAM VIAL 0 APOTEX/NOVAPLUS EAGEN 63323-0340-20 8.91000 CEFEPIME HCL 2 GRAM VIAL 0 APP PHARMACEUTI EABND 00338-1301-41 0.52907 CEFEPIME 1 GM INJECTION 0 BAXTER <strong>HEALTH</strong>CA MLBND 00338-1301-48 0.42409 CEFEPIME 2 GM INJECTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00143-9931-01 1.76000 CEFOTAXIME SODIUM 1 GM VIAL 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-9931-22 1.76000 CEFOTAXIME SODIUM 1 GM VIAL 0 WEST-WARD,INC. EAGEN 00143-9931-25 1.76000 CEFOTAXIME SODIUM 1 GM VIAL 0 WEST-WARD,INC. EAGEN 00143-9935-91 18.26910 CEFOTAXIME SODIUM 10 GM VIAL 0 WEST-WARD,INC. EAGEN 00143-9933-01 4.12516 CEFOTAXIME SODIUM 2 GM VIAL 0 WEST-WARD,INC. EAGEN 00143-9933-22 4.12516 CEFOTAXIME SODIUM 2 GM VIAL 0 WEST-WARD,INC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 69LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-9933-25 4.12516 CEFOTAXIME SODIUM 2 GM VIAL 0 WEST-WARD,INC. EAGEN 64679-0948-02 4.12516 CEFOTAXIME SODIUM 2 GM VIAL 0 WOCKHARDT USA L EAGEN 00143-9930-01 1.20000 CEFOTAXIME SODIUM 500 MG VIAL 0 WEST-WARD,INC. EAGEN 00143-9930-03 1.20000 CEFOTAXIME SODIUM 500 MG VIAL 0 WEST-WARD,INC. EAGEN 00143-9930-10 1.20000 CEFOTAXIME SODIUM 500 MG VIAL 0 WEST-WARD,INC. EABND 63323-0385-10 11.33448 CEFOTETAN 1 GM VIAL 0 APP PHARMACEUTI EABND 63323-0386-20 22.66896 CEFOTETAN 2 GM VIAL 0 APP PHARMACEUTI EABND 00264-3123-11 10.20900 CEFOXITIN 1 GM PIGGYBACK BAG 0 B.BRAUN EAGEN 00143-9878-25 6.36000 CEFOXITIN 1 GM VIAL 0 WEST-WARD,INC. EAGEN 25021-0109-10 5.40000 CEFOXITIN 1 GM VIAL 0 SAGENT PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0759-05 6.36000 CEFOXITIN 1 GM VIAL 0 APOTEX CORP EAGEN 60505-6025-05 6.36000 CEFOXITIN 1 GM VIAL 0 APOTEX/NOVAPLUS EAGEN 63323-0341-25 5.74200 CEFOXITIN 1 GM VIAL 0 APP PHARMACEUTI EAGEN 00143-9876-10 41.<strong>06</strong>000 CEFOXITIN 10 GM VIAL 0 WEST-WARD,INC. EAGEN 25021-0111-99 41.<strong>06</strong>000 CEFOXITIN 10 GM VIAL 0 SAGENT PHARMACE EAGEN 60505-0761-04 41.<strong>06</strong>000 CEFOXITIN 10 GM VIAL 0 APOTEX CORP EAGEN 63323-0343-66 41.<strong>06</strong>000 CEFOXITIN 10 GM VIAL 0 APP PHARMACEUTI EABND 00264-3125-11 18.72480 CEFOXITIN 2 GM PIGGYBACK BAG 0 B.BRAUN EAGEN 00143-9877-25 9.<strong>08</strong>900 CEFOXITIN 2 GM VIAL 0 WEST-WARD,INC. EAGEN 25021-0110-20 9.00000 CEFOXITIN 2 GM VIAL 0 SAGENT PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0760-05 9.<strong>08</strong>900 CEFOXITIN 2 GM VIAL 0 APOTEX CORP EAGEN 60505-6026-05 9.<strong>08</strong>900 CEFOXITIN 2 GM VIAL 0 APOTEX/NOVAPLUS EAGEN 63323-0342-25 9.<strong>08</strong>900 CEFOXITIN 2 GM VIAL 0 APP PHARMACEUTI EAGEN 00781-5438-20 5.05275 CEFPODOXIME 100 MG TABLET 0 SANDOZ EAGEN 16714-0211-01 3.73575 CEFPODOXIME 100 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0394-01 5.05275 CEFPODOXIME 100 MG TABLET 0 NORTHSTAR RX LL EAGEN 65862-0095-01 5.28220 CEFPODOXIME 100 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0095-20 5.05275 CEFPODOXIME 100 MG TABLET 0 AUROBINDO PHARM EAGEN 00781-6169-46 1.22970 CEFPODOXIME 100 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6169-52 1.29255 CEFPODOXIME 100 MG/5 ML SUSP 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0403-01 1.29255 CEFPODOXIME 100 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0403-02 1.22970 CEFPODOXIME 100 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 65862-0141-01 1.22970 CEFPODOXIME 100 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 65862-0141-50 1.29255 CEFPODOXIME 100 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 00781-5439-01 6.34260 CEFPODOXIME 200 MG TABLET 0 SANDOZ EAGEN 00781-5439-20 6.34275 CEFPODOXIME 200 MG TABLET 0 SANDOZ EAGEN 16714-0212-01 4.80937 CEFPODOXIME 200 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0212-02 4.80937 CEFPODOXIME 200 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0395-01 6.34275 CEFPODOXIME 200 MG TABLET 0 NORTHSTAR RX LL EAGEN 65862-0096-01 7.33125 CEFPODOXIME 200 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0096-20 6.34275 CEFPODOXIME 200 MG TABLET 0 AUROBINDO PHARM EAGEN 00781-6168-46 0.64627 CEFPODOXIME 50 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6168-52 0.67920 CEFPODOXIME 50 MG/5 ML SUSP 0 SANDOZ MLGEN 16714-0402-01 0.67920 CEFPODOXIME 50 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0402-02 0.64627 CEFPODOXIME 50 MG/5 ML SUSP 0 NORTHSTAR RX LL ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 70LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0140-01 0.64627 CEFPODOXIME 50 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 65862-0140-50 0.67920 CEFPODOXIME 50 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 00093-1075-76 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 TEVA USA MLGEN 00781-6202-46 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6202-57 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6202-91 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 SANDOZ MLGEN 16714-0215-01 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0215-02 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0215-03 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 65862-0099-01 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 AUROBINDO PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0099-75 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 68180-0401-01 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68180-0401-02 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68180-0401-03 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68820-0018-15 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 ORCHID <strong>HEALTH</strong>CA MLGEN 68820-0018-16 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 ORCHID <strong>HEALTH</strong>CA MLGEN 68820-0018-17 0.29920 CEFPROZIL 125 MG/5 ML SUSP 0 ORCHID <strong>HEALTH</strong>CA MLGEN 00093-1077-01 2.<strong>06</strong>700 CEFPROZIL 250 MG TABLET 0 TEVA USA EAGEN 00781-5043-01 2.<strong>06</strong>700 CEFPROZIL 250 MG TABLET 0 SANDOZ EAGEN 16714-02<strong>08</strong>-01 2.<strong>06</strong>700 CEFPROZIL 250 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2532-01 2.<strong>06</strong>700 CEFPROZIL 250 MG TABLET 0 APOTEX CORP EAGEN 65862-0<strong>06</strong>8-01 2.<strong>06</strong>700 CEFPROZIL 250 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0403-01 2.<strong>06</strong>700 CEFPROZIL 250 MG TABLET 0 LUPIN PHARMACEU EAGEN 68820-0016-10 2.<strong>06</strong>700 CEFPROZIL 250 MG TABLET 0 ORCHID <strong>HEALTH</strong>CA EAGEN 00093-1076-76 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 TEVA USA MLGEN 00781-6203-46 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6203-57 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 SANDOZ MLGEN 00781-6203-91 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 SANDOZ MLGEN 16714-0216-01 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0216-03 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 NORTHSTAR RX LL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0397-01 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0397-02 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 16714-0397-03 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 NORTHSTAR RX LL MLGEN 65862-0100-01 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 65862-0100-75 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 AUROBINDO PHARM MLGEN 68180-0402-01 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68180-0402-02 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68180-0402-03 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68820-0019-15 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 ORCHID <strong>HEALTH</strong>CA MLGEN 68820-0019-16 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 ORCHID <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68820-0019-17 0.56000 CEFPROZIL 250 MG/5 ML SUSP 0 ORCHID <strong>HEALTH</strong>CA MLGEN 00093-1078-53 1.96670 CEFPROZIL 500 MG TABLET 0 TEVA USA EAGEN 00781-5044-01 1.96670 CEFPROZIL 500 MG TABLET 0 SANDOZ EAGEN 00781-5044-50 1.96670 CEFPROZIL 500 MG TABLET 0 SANDOZ EAGEN 16714-0209-01 1.96670 CEFPROZIL 500 MG TABLET 0 NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 71LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0399-01 1.96670 CEFPROZIL 500 MG TABLET 0 NORTHSTAR RX LL EAGEN 60505-2533-01 1.96670 CEFPROZIL 500 MG TABLET 0 APOTEX CORP EAGEN 60505-2533-05 1.96670 CEFPROZIL 500 MG TABLET 0 APOTEX CORP EAGEN 65862-0<strong>06</strong>9-01 1.96670 CEFPROZIL 500 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0<strong>06</strong>9-50 1.96670 CEFPROZIL 500 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0404-01 1.96670 CEFPROZIL 500 MG TABLET 0 LUPIN PHARMACEU EAGEN 68820-0017-<strong>08</strong> 1.96670 CEFPROZIL 500 MG TABLET 0 ORCHID <strong>HEALTH</strong>CA EAGEN 00781-3177-96 5.<strong>08</strong>800 CEFTAZIDIME 1 GM VIAL 0 SANDOZ EAGEN 25021-0127-20 4.32000 CEFTAZIDIME 1 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-0235-25 4.05000 CEFTAZIDIME 1 GM VIAL 0 WG CRITICAL CAR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3178-91 10.10130 CEFTAZIDIME 2 GM VIAL 0 SANDOZ EAGEN 00781-3178-95 10.10130 CEFTAZIDIME 2 GM VIAL 0 SANDOZ EAGEN 25021-0128-50 9.66675 CEFTAZIDIME 2 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-0236-10 9.00000 CEFTAZIDIME 2 GM VIAL 0 WG CRITICAL CAR EAGEN 00781-3179-86 24.93817 CEFTAZIDIME 6 GM VIAL 0 SANDOZ EAGEN 25021-0129-99 24.93817 CEFTAZIDIME 6 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-0237-<strong>06</strong> 21.60000 CEFTAZIDIME 6 GM VIAL 0 WG CRITICAL CAR EABND 00173-0740-00 0.85257 CEFTIN 125 MG/5 ML ORAL SUSP 0 GLAXOSMITHKLINE MLBND 00173-0387-00 0.38299 10.53477 CEFTIN 250 MG TABLET G GLAXOSMITHKLINE EABND 00173-0741-00 1.451<strong>08</strong> CEFTIN 250 MG/5 ML ORAL SUSP 0 GLAXOSMITHKLINE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-0741-10 1.28<strong>06</strong>9 CEFTIN 250 MG/5 ML ORAL SUSP 0 GLAXOSMITHKLINE MLBND 00173-0394-00 0.60000 19.19748 CEFTIN 500 MG TABLET G GLAXOSMITHKLINE EABND 00338-5002-41 0.28266 CEFTRIAXONE 1 GM PIGGYBACK 0 BAXTER <strong>HEALTH</strong>CA MLBND 60505-<strong>06</strong>79-<strong>08</strong> 13.18860 39.76530 CEFTRIAXONE 1 GM PIGGYBACK 0 APOTEX CORP EAGEN 00143-9857-25 1.59300 CEFTRIAXONE 1 GM VIAL 0 WEST-WARD,INC. EAGEN 00409-7332-01 1.14300 CEFTRIAXONE 1 GM VIAL 0 HOSPIRA EABND 00409-7333-04 4.16328 CEFTRIAXONE 1 GM VIAL 0 HOSPIRA EABND 00409-7333-49 3.88440 CEFTRIAXONE 1 GM VIAL 0 HOSPIRA/NOVA+ EAGEN 00781-32<strong>08</strong>-85 1.59300 CEFTRIAXONE 1 GM VIAL 0 SANDOZ EAGEN 00781-32<strong>08</strong>-95 1.59300 CEFTRIAXONE 1 GM VIAL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-9328-85 1.59300 CEFTRIAXONE 1 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9328-95 1.59300 CEFTRIAXONE 1 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-01<strong>06</strong>-10 1.59300 CEFTRIAXONE 1 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-0701-25 1.59300 CEFTRIAXONE 1 GM VIAL 0 WG CRITICAL CAR EAGEN 55390-0311-10 1.59300 CEFTRIAXONE 1 GM VIAL 0 BEDFORD LABS EAGEN 60505-0752-04 1.59300 CEFTRIAXONE 1 GM VIAL 0 APOTEX CORP EAGEN 63323-0346-10 1.59300 CEFTRIAXONE 1 GM VIAL 0 APP PHARMACEUTI EAGEN 64679-0983-02 1.59300 CEFTRIAXONE 1 GM VIAL 0 WOCKHARDT USA L EAGEN 00409-7334-10 14.90250 CEFTRIAXONE 10 GM VIAL 0 HOSPIRA EAGEN 00781-3210-46 19.48975 CEFTRIAXONE 10 GM VIAL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-9330-46 19.48975 CEFTRIAXONE 10 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 10019-<strong>06</strong>89-05 19.48975 CEFTRIAXONE 10 GM VIAL 0 WEST-WARD,INC. EAGEN 10019-<strong>06</strong>89-11 19.48975 CEFTRIAXONE 10 GM VIAL 0 WEST-WARD,INC. EAGEN 25021-01<strong>08</strong>-99 19.48975 CEFTRIAXONE 10 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-0703-01 19.48975 CEFTRIAXONE 10 GM VIAL 0 WG CRITICAL CAR EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 72LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-<strong>06</strong>79-05 19.48975 CEFTRIAXONE 10 GM VIAL 0 APOTEX CORP EAGEN 60505-6103-<strong>06</strong> 19.48975 CEFTRIAXONE 10 GM VIAL 0 APOTEX/NOVAPLUS EAGEN 63323-0348-61 19.48975 CEFTRIAXONE 10 GM VIAL 0 APP PHARMACEUTI EABND 00409-7336-04 7.84848 CEFTRIAXONE 2 GM ADD VIAL 0 HOSPIRA EABND 00409-7336-49 7.32<strong>06</strong>0 CEFTRIAXONE 2 GM ADD VIAL 0 HOSPIRA/NOVA+ EABND 00338-5003-41 0.66951 CEFTRIAXONE 2 GM PIGGYBACK 0 BAXTER <strong>HEALTH</strong>CA MLBND 60505-<strong>06</strong>79-09 25.89780 78.16110 CEFTRIAXONE 2 GM PIGGYBACK 0 APOTEX CORP EAGEN 00143-9856-25 3.56265 CEFTRIAXONE 2 GM VIAL 0 WEST-WARD,INC. EAGEN 00409-7335-03 2.47500 CEFTRIAXONE 2 GM VIAL 0 HOSPIRA EAGEN 00781-3209-90 3.56265 CEFTRIAXONE 2 GM VIAL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3209-95 3.56265 CEFTRIAXONE 2 GM VIAL 0 SANDOZ EAGEN 00781-9329-90 3.56265 CEFTRIAXONE 2 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9329-95 3.56265 CEFTRIAXONE 2 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 10019-<strong>06</strong>88-04 3.56265 CEFTRIAXONE 2 GM VIAL 0 WEST-WARD,INC. EAGEN 25021-0107-20 3.56265 CEFTRIAXONE 2 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-0702-25 3.56265 CEFTRIAXONE 2 GM VIAL 0 WG CRITICAL CAR EAGEN 55390-0312-10 3.56265 CEFTRIAXONE 2 GM VIAL 0 BEDFORD LABS EAGEN 60505-0753-04 3.56265 CEFTRIAXONE 2 GM VIAL 0 APOTEX CORP EAGEN 60505-6102-04 3.56265 CEFTRIAXONE 2 GM VIAL 0 APOTEX/NOVAPLUS EAGEN 63323-0347-20 3.56265 CEFTRIAXONE 2 GM VIAL 0 APP PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0703-01 3.56265 CEFTRIAXONE 2 GM VIAL 0 WOCKHARDT USA L EAGEN 00143-9859-25 2.28150 CEFTRIAXONE 250 MG VIAL 0 WEST-WARD,INC. EAGEN 00409-7337-01 0.67500 CEFTRIAXONE 250 MG VIAL 0 HOSPIRA EAGEN 00781-32<strong>06</strong>-85 2.28150 CEFTRIAXONE 250 MG VIAL 0 SANDOZ EAGEN 00781-32<strong>06</strong>-95 2.28150 CEFTRIAXONE 250 MG VIAL 0 SANDOZ EAGEN 00781-9326-85 2.28150 CEFTRIAXONE 250 MG VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9326-95 2.28150 CEFTRIAXONE 250 MG VIAL 0 SANDOZ/NOVAPLUS EAGEN 55390-0309-10 1.12500 CEFTRIAXONE 250 MG VIAL 0 BEDFORD LABS EAGEN 60505-0750-00 2.28150 CEFTRIAXONE 250 MG VIAL 0 APOTEX CORP EAGEN 60505-0750-04 2.28150 CEFTRIAXONE 250 MG VIAL 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-6104-04 2.28150 CEFTRIAXONE 250 MG VIAL 0 APOTEX/NOVAPLUS EAGEN 63323-0344-10 1.42200 CEFTRIAXONE 250 MG VIAL 0 APP PHARMACEUTI EAGEN 64679-0701-02 2.28150 CEFTRIAXONE 250 MG VIAL 0 WOCKHARDT USA L EAGEN 00143-9858-25 1.32500 CEFTRIAXONE 500 MG VIAL 0 WEST-WARD,INC. EAGEN 00409-7338-01 0.85500 CEFTRIAXONE 500 MG VIAL 0 HOSPIRA EAGEN 00781-3207-85 1.32500 CEFTRIAXONE 500 MG VIAL 0 SANDOZ EAGEN 00781-3207-95 1.32500 CEFTRIAXONE 500 MG VIAL 0 SANDOZ EAGEN 00781-9327-85 1.32500 CEFTRIAXONE 500 MG VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9327-95 1.32500 CEFTRIAXONE 500 MG VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0105-10 1.32500 CEFTRIAXONE 500 MG VIAL 0 SAGENT PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 44567-0700-25 1.32500 CEFTRIAXONE 500 MG VIAL 0 WG CRITICAL CAR EAGEN 55390-0310-10 1.12500 CEFTRIAXONE 500 MG VIAL 0 BEDFORD LABS EAGEN 60505-0751-00 1.32500 CEFTRIAXONE 500 MG VIAL 0 APOTEX CORP EAGEN 60505-0751-04 1.32500 CEFTRIAXONE 500 MG VIAL 0 APOTEX CORP EAGEN 63323-0345-10 1.32500 CEFTRIAXONE 500 MG VIAL 0 APP PHARMACEUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 73LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0702-02 1.32500 CEFTRIAXONE 500 MG VIAL 0 WOCKHARDT USA L EAGEN 16714-0232-01 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 NORTHSTAR RX LL EAGEN 16714-0232-02 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 NORTHSTAR RX LL EAGEN 52343-0046-20 0.31500 CEFUROXIME AXETIL 250 MG TAB 0 GEN-SOURCE RX EAGEN 52343-0046-60 0.28650 CEFUROXIME AXETIL 250 MG TAB 0 GEN-SOURCE RX EAGEN 60505-2681-02 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 APOTEX CORP EAGEN 60505-2681-<strong>06</strong> 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 APOTEX CORP EAGEN 64679-0921-01 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 WOCKHARDT USA L EAGEN 64679-0921-02 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 WOCKHARDT USA L EAGEN 65862-0034-20 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0034-60 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 AUROBINDO PHARM EAGEN 67877-0215-20 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 ASCEND LABORATO EAGEN 67877-0215-60 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 ASCEND LABORATO EAGEN 68180-0302-20 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 LUPIN PHARMACEU EAGEN 68180-0302-60 0.38299 CEFUROXIME AXETIL 250 MG TAB 0 LUPIN PHARMACEU EAGEN 16714-0233-01 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 NORTHSTAR RX LL EAGEN 16714-0233-02 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 NORTHSTAR RX LL EAGEN 52343-0047-20 0.54675 CEFUROXIME AXETIL 500 MG TAB 0 GEN-SOURCE RX EAGEN 52343-0047-60 0.51750 CEFUROXIME AXETIL 500 MG TAB 0 GEN-SOURCE RX EAGEN 60505-2682-02 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2682-<strong>06</strong> 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 APOTEX CORP EAGEN 64679-0922-01 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 WOCKHARDT USA L EAGEN 64679-0922-02 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 WOCKHARDT USA L EAGEN 65862-0035-20 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0035-60 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 AUROBINDO PHARM EAGEN 67877-0216-20 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 ASCEND LABORATO EAGEN 67877-0216-60 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 ASCEND LABORATO EAGEN 68180-0303-20 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 LUPIN PHARMACEU EAGEN 68180-0303-60 0.60000 CEFUROXIME AXETIL 500 MG TAB 0 LUPIN PHARMACEU EAGEN 00409-<strong>08</strong>02-01 2.36700 CEFUROXIME SOD 1.5 GM VIAL 0 HOSPIRA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-<strong>08</strong>03-01 14.17500 CEFUROXIME SOD 7.5 GM VIAL 0 HOSPIRA EABND 00025-1520-31 3.80588 CELEBREX 100 MG CAPSULE G PFIZER US PHARM EABND 00025-1520-51 3.80593 CELEBREX 100 MG CAPSULE G PFIZER US PHARM EABND 00025-1525-31 6.24251 CELEBREX 200 MG CAPSULE G PFIZER US PHARM EABND 00025-1525-51 6.24259 CELEBREX 200 MG CAPSULE G PFIZER US PHARM EABND 00025-1530-02 9.364<strong>06</strong> CELEBREX 400 MG CAPSULE G PFIZER US PHARM EABND 00025-1515-01 1.77869 CELEBREX 50 MG CAPSULE G PFIZER US PHARM EABEX 00456-4010-01 0.026<strong>06</strong> 4.80926 CELEXA 10 MG TABLET G FOREST PHARMACE EABEX 00456-4020-01 0.03710 5.01270 CELEXA 20 MG TABLET G FOREST PHARMACE EABEX 00456-4040-01 0.04930 5.23090 CELEXA 40 MG TABLET G FOREST PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00004-0261-29 6.72180 CELLCEPT 200 MG/ML ORAL SUSP 0 GENENTECH, INC. MLBND 00004-0259-01 0.27378 6.52712 CELLCEPT 250 MG CAPSULE G GENENTECH, INC. EABND 00004-0259-05 0.27378 6.527<strong>08</strong> CELLCEPT 250 MG CAPSULE G GENENTECH, INC. EABND 00004-0259-43 0.27378 6.527<strong>08</strong> CELLCEPT 250 MG CAPSULE G GENENTECH, INC. EABND 00004-0260-01 0.47210 13.05415 CELLCEPT 500 MG TABLET G GENENTECH, INC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 74LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00004-0260-43 0.47210 13.05417 CELLCEPT 500 MG TABLET G GENENTECH, INC. EABEX 00071-0525-24 2.16713 CELONTIN 300 MG KAPSEAL 0 PFIZER US PHARM EABND 51285-0441-02 3.62544 CENESTIN 0.3 MG TABLET 0 DURAMED/BARR EABND 51285-0446-02 3.62544 CENESTIN 0.45 MG TABLET 0 DURAMED/BARR EABND 51285-0442-02 3.62544 CENESTIN 0.625 MG TABLET 0 DURAMED/BARR EABND 51285-0443-02 3.62544 CENESTIN 0.9 MG TABLET 0 DURAMED/BARR EABND 51285-0444-02 3.466<strong>08</strong> CENESTIN 1.25 MG TABLET 0 DURAMED/BARR EABND 43538-0300-30 0.40930 5.13548 CENTANY 2% OINTMENT G MEDIMETRIKS PHA GMGEN 00093-4175-73 0.05680 CEPHALEXIN 125 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-4175-74 0.05680 CEPHALEXIN 125 MG/5 ML SUSP 0 TEVA USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42043-0142-38 0.05680 CEPHALEXIN 125 MG/5 ML SUSP 0 KARALEX PHARMA, MLGEN 42043-0142-58 0.05680 CEPHALEXIN 125 MG/5 ML SUSP 0 KARALEX PHARMA, MLGEN 68180-0123-01 0.05680 CEPHALEXIN 125 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68180-0123-02 0.05680 CEPHALEXIN 125 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 00093-3145-01 0.09511 CEPHALEXIN 250 MG CAPSULE 0 TEVA USA EAGEN 00093-3145-05 0.09511 CEPHALEXIN 250 MG CAPSULE 0 TEVA USA EAGEN 00143-9898-01 0.09511 CEPHALEXIN 250 MG CAPSULE 0 WEST-WARD,INC. EAGEN 00143-9898-05 0.09511 CEPHALEXIN 250 MG CAPSULE 0 WEST-WARD,INC. EAGEN 16714-<strong>06</strong>41-02 0.09511 CEPHALEXIN 250 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>41-03 0.09511 CEPHALEXIN 250 MG CAPSULE 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42043-0140-01 0.09511 CEPHALEXIN 250 MG CAPSULE 0 KARALEX PHARMA, EAGEN 42043-0140-05 0.09511 CEPHALEXIN 250 MG CAPSULE 0 KARALEX PHARMA, EAGEN 61442-0161-01 0.09511 CEPHALEXIN 250 MG CAPSULE 0 CARLSBAD TECH EAGEN 61442-0161-05 0.09511 CEPHALEXIN 250 MG CAPSULE 0 CARLSBAD TECH EAGEN 62756-0293-13 0.09511 CEPHALEXIN 250 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 62756-0293-88 0.09511 CEPHALEXIN 250 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 65862-0018-01 0.09511 CEPHALEXIN 250 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0018-05 0.09511 CEPHALEXIN 250 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0018-40 0.09511 CEPHALEXIN 250 MG CAPSULE 0 AUROBINDO PHARM EAGEN 67877-0220-01 0.09511 CEPHALEXIN 250 MG CAPSULE 0 ASCEND LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67877-0220-05 0.09511 CEPHALEXIN 250 MG CAPSULE 0 ASCEND LABORATO EAGEN 68180-0121-01 0.09511 CEPHALEXIN 250 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68180-0121-02 0.09511 CEPHALEXIN 250 MG CAPSULE 0 LUPIN PHARMACEU EABND 00093-2238-01 2.22207 CEPHALEXIN 250 MG TABLET 0 TEVA USA EAGEN 00093-4177-73 0.07182 CEPHALEXIN 250 MG/5 ML SUSP 0 TEVA USA MLGEN 00093-4177-74 0.07182 CEPHALEXIN 250 MG/5 ML SUSP 0 TEVA USA MLGEN 42043-0143-38 0.07182 CEPHALEXIN 250 MG/5 ML SUSP 0 KARALEX PHARMA, MLGEN 42043-0143-58 0.07182 CEPHALEXIN 250 MG/5 ML SUSP 0 KARALEX PHARMA, MLGEN 68180-0124-01 0.07182 CEPHALEXIN 250 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68180-0124-02 0.07182 CEPHALEXIN 250 MG/5 ML SUSP 0 LUPIN PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-3147-01 0.10100 CEPHALEXIN 500 MG CAPSULE 0 TEVA USA EAGEN 00093-3147-05 0.10100 CEPHALEXIN 500 MG CAPSULE 0 TEVA USA EAGEN 00143-9897-01 0.10100 CEPHALEXIN 500 MG CAPSULE 0 WEST-WARD,INC. EAGEN 00143-9897-05 0.10100 CEPHALEXIN 500 MG CAPSULE 0 WEST-WARD,INC. EAGEN 16714-<strong>06</strong>42-02 0.10100 CEPHALEXIN 500 MG CAPSULE 0 NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 75LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-<strong>06</strong>42-03 0.10100 CEPHALEXIN 500 MG CAPSULE 0 NORTHSTAR RX LL EAGEN 42043-0141-01 0.10100 CEPHALEXIN 500 MG CAPSULE 0 KARALEX PHARMA, EAGEN 42043-0141-05 0.10100 CEPHALEXIN 500 MG CAPSULE 0 KARALEX PHARMA, EAGEN 61442-0162-01 0.10100 CEPHALEXIN 500 MG CAPSULE 0 CARLSBAD TECH EAGEN 61442-0162-05 0.10100 CEPHALEXIN 500 MG CAPSULE 0 CARLSBAD TECH EAGEN 62250-<strong>08</strong>02-<strong>08</strong> 0.10100 CEPHALEXIN 500 MG CAPSULE 0 BELCHER PHARMAC EAGEN 62756-0294-13 0.10100 CEPHALEXIN 500 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 62756-0294-88 0.10100 CEPHALEXIN 500 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 65862-0019-01 0.10100 CEPHALEXIN 500 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0019-05 0.10100 CEPHALEXIN 500 MG CAPSULE 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0019-40 0.10100 CEPHALEXIN 500 MG CAPSULE 0 AUROBINDO PHARM EAGEN 67877-0219-01 0.10100 CEPHALEXIN 500 MG CAPSULE 0 ASCEND LABORATO EAGEN 67877-0219-05 0.10100 CEPHALEXIN 500 MG CAPSULE 0 ASCEND LABORATO EAGEN 67877-0219-10 0.10100 CEPHALEXIN 500 MG CAPSULE 0 ASCEND LABORATO EAGEN 68180-0122-01 0.10100 CEPHALEXIN 500 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68180-0122-02 0.10100 CEPHALEXIN 500 MG CAPSULE 0 LUPIN PHARMACEU EABND 00093-2240-01 4.36687 CEPHALEXIN 500 MG TABLET 0 TEVA USA EABND 58468-4663-01 1579.65600 CEREZYME 400 UNITS VIAL 0 GENZYME EAGEN 55390-0281-10 62.55000 CERUBIDINE 20 MG VIAL 0 BEDFORD LABS EAGEN 0<strong>06</strong>03-9<strong>06</strong>3-54 0.22812 CETIRIZINE HCL 1 MG/ML SYRUP G QUALITEST ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51991-<strong>08</strong>37-04 0.22812 CETIRIZINE HCL 1 MG/ML SYRUP G BRECKENRIDGE MLGEN 51991-<strong>08</strong>37-16 0.22804 CETIRIZINE HCL 1 MG/ML SYRUP G BRECKENRIDGE MLGEN 00054-0334-25 2.37510 CEVIMELINE HCL 30 MG CAPSULE 0 ROXANE LABS. EAGEN 60505-3145-01 2.37510 CEVIMELINE HCL 30 MG CAPSULE 0 APOTEX CORP EAGEN 60505-3145-05 2.37510 CEVIMELINE HCL 30 MG CAPSULE 0 APOTEX CORP EAGEN 63304-0479-01 2.37510 CEVIMELINE HCL 30 MG CAPSULE 0 RANBAXY PHARMAC EABND 00<strong>06</strong>9-0471-02 3.96238 CHANTIX STARTING MONTH BOX 0 PFIZER US PHARM EABND 00<strong>06</strong>9-0468-56 3.75011 CHANTIX 0.5 MG TABLET 0 PFIZER US PHARM EABND 00<strong>06</strong>9-0469-12 3.75011 CHANTIX 1 MG CONT MONTH BOX 0 PFIZER US PHARM EABND 00<strong>06</strong>9-0469-97 2.81296 CHANTIX 1 MG CONT MONTH PAK 0 PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>9-0469-56 3.75011 CHANTIX 1 MG TABLET 0 PFIZER US PHARM EAGEX 50102-0130-01 0.26035 CHATEAL-28 TABLET 0 AFAXYS, INC. EABND 55292-0201-11 8.20961 CHEMET 100 MG CAPSULE 0 RECORDATI RARE EABND 67386-0201-11 8.20961 CHEMET 100 MG CAPSULE 0 RECORDATI RARE EABND 45043-<strong>08</strong>76-40 94.22160 CHENODAL 250 MG TABLET 0 MANCHESTER PHAR EABND 63323-0011-15 29.82024 CHLORAMPHEN NA SUCC 1 GM VL 0 APP PHARMACEUTI EAGEN 51927-1484-00 13.19250 CHLORAMPHENICOL POWDER 0 PR<strong>OF</strong>ESSIONAL CO GMGEN 38779-0205-<strong>08</strong> 0.16875 CHLORHEXIDINE GLUC 20% SOLN 0 MEDISCA INC. MLGEN 00116-2001-16 0.0<strong>06</strong>50 CHLORHEXIDINE 0.12% RINSE 0 XTTRIUM LABS. MLGEN 50383-0720-15 0.0<strong>06</strong>50 CHLORHEXIDINE 0.12% RINSE 0 HI-TECH PHARMAC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50383-0720-16 0.0<strong>06</strong>50 CHLORHEXIDINE 0.12% RINSE 0 HI-TECH PHARMAC MLGEN 50383-0720-17 0.0<strong>06</strong>50 CHLORHEXIDINE 0.12% RINSE 0 HI-TECH PHARMAC MLGEN 50383-0720-19 0.0<strong>06</strong>50 CHLORHEXIDINE 0.12% RINSE 0 HI-TECH PHARMAC MLGEN 00115-2790-<strong>06</strong> 0.89721 CHLOROQUINE PH 250 MG TABLET 0 GLOBAL PHARM EAGEN 00143-1195-50 0.89721 CHLOROQUINE PH 250 MG TABLET 0 WEST-WARD,INC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 76LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63304-0460-50 0.89721 CHLOROQUINE PH 250 MG TABLET 0 RANBAXY PHARMAC EAGEN 64980-0177-50 0.89721 CHLOROQUINE PH 250 MG TABLET 0 RISING PHARM EAGEN 00115-7010-09 2.05416 CHLOROQUINE PH 500 MG TABLET 0 GLOBAL PHARM EAGEN 00143-2125-22 2.05416 CHLOROQUINE PH 500 MG TABLET 0 WEST-WARD,INC. EAGEN 63304-0461-26 2.05416 CHLOROQUINE PH 500 MG TABLET 0 RANBAXY PHARMAC EAGEN 64980-0178-02 2.05416 CHLOROQUINE PH 500 MG TABLET 0 RISING PHARM EABND 00378-0150-01 0.16954 0.17952 CHLOROTHIAZIDE 250 MG TABLET 0 MYLAN EAGEN 00143-1210-01 0.18937 CHLOROTHIAZIDE 500 MG TABLET 0 WEST-WARD,INC. EAGEN 00378-0162-01 0.29032 CHLOROTHIAZIDE 500 MG TABLET 0 MYLAN EAGEX 00781-1715-01 0.52500 CHLORPROMAZINE 10 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-5913-01 0.7<strong>08</strong>75 CHLORPROMAZINE 10 MG TABLET 0 SANDOZ EAGEX 0<strong>08</strong>32-0300-00 0.90030 CHLORPROMAZINE 10 MG TABLET 0 UPSHER SMITH EAGEX 0<strong>08</strong>32-0300-10 0.84297 CHLORPROMAZINE 10 MG TABLET 0 UPSHER SMITH EAGEX 51079-0518-20 0.80205 CHLORPROMAZINE 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 68<strong>08</strong>4-0420-01 0.69787 CHLORPROMAZINE 10 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0420-11 0.69750 CHLORPROMAZINE 10 MG TABLET 0 AHP EAGEX 00781-1718-01 1.52677 CHLORPROMAZINE 100 MG TABLET 0 SANDOZ EAGEX 00781-5916-01 2.<strong>06</strong>115 CHLORPROMAZINE 100 MG TABLET 0 SANDOZ EAGEX 0<strong>08</strong>32-0303-00 3.19005 CHLORPROMAZINE 100 MG TABLET 0 UPSHER SMITH EAGEX 0<strong>08</strong>32-0303-10 2.46121 CHLORPROMAZINE 100 MG TABLET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0516-20 2.44785 CHLORPROMAZINE 100 MG TABLET 0 MYLAN INSTITUTI EAGEX 00781-1719-01 2.18115 CHLORPROMAZINE 200 MG TABLET 0 SANDOZ EAGEX 00781-5917-01 2.94450 CHLORPROMAZINE 200 MG TABLET 0 SANDOZ EAGEX 0<strong>08</strong>32-0304-00 4.89472 CHLORPROMAZINE 200 MG TABLET 0 UPSHER SMITH EAGEX 0<strong>08</strong>32-0304-10 4.72440 CHLORPROMAZINE 200 MG TABLET 0 UPSHER SMITH EAGEX 51079-0517-20 2.93745 CHLORPROMAZINE 200 MG TABLET 0 MYLAN INSTITUTI EAGEX 68<strong>08</strong>4-0422-01 3.72562 CHLORPROMAZINE 200 MG TABLET 0 AHP EAGEX 00781-5914-01 1.01250 CHLORPROMAZINE 25 MG TABLET 0 SANDOZ EAGEX 0<strong>08</strong>32-0301-00 1.63732 CHLORPROMAZINE 25 MG TABLET 0 UPSHER SMITH EAGEX 0<strong>08</strong>32-0301-10 1.54407 CHLORPROMAZINE 25 MG TABLET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0519-20 1.09372 CHLORPROMAZINE 25 MG TABLET 0 MYLAN INSTITUTI EABEX 0<strong>06</strong>41-1397-35 19.12320 CHLORPROMAZINE 25 MG/ML AMP 0 WEST-WARD,INC. MLBEX 0<strong>06</strong>41-1398-35 10.95600 CHLORPROMAZINE 25 MG/ML AMP 0 WEST-WARD,INC. MLGEX 00781-1717-01 1.<strong>06</strong>875 CHLORPROMAZINE 50 MG TABLET 0 SANDOZ EAGEX 00781-5915-01 1.44277 CHLORPROMAZINE 50 MG TABLET 0 SANDOZ EAGEX 0<strong>08</strong>32-0302-00 2.22315 CHLORPROMAZINE 50 MG TABLET 0 UPSHER SMITH EAGEX 0<strong>08</strong>32-0302-10 2.<strong>08</strong>986 CHLORPROMAZINE 50 MG TABLET 0 UPSHER SMITH EAGEX 51079-0130-20 1.55205 CHLORPROMAZINE 50 MG TABLET 0 MYLAN INSTITUTI EABND 00378-0197-01 0.15920 0.51169 CHLORPROPAMIDE 100 MG TABLET G MYLAN EABND 00378-0210-01 0.33640 1.<strong>08</strong>124 CHLORPROPAMIDE 250 MG TABLET G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0222-01 0.41377 CHLORTHALIDONE 25 MG TABLET 0 MYLAN EAGEN 00378-0222-10 0.41375 CHLORTHALIDONE 25 MG TABLET 0 MYLAN EAGEN 51079-0058-20 0.43650 CHLORTHALIDONE 25 MG TABLET 0 MYLAN INSTITUTI EABND 00378-0213-01 0.56473 CHLORTHALIDONE 50 MG TABLET 0 MYLAN EABND 00378-0213-10 0.56464 CHLORTHALIDONE 50 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 77LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00591-2520-01 0.07570 CHLORZOXAZONE 500 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00591-2520-05 0.07570 CHLORZOXAZONE 500 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00185-0939-98 1.10090 CHOLESTYRAMINE LIGHT PACKET 0 SANDOZ EAGEN 49884-0466-65 1.10090 CHOLESTYRAMINE LIGHT PACKET 0 PAR PHARM. EAGEN 00185-0939-97 0.26973 CHOLESTYRAMINE LIGHT POWDER 0 SANDOZ GMGUL 00185-0940-98 1.27670 CHOLESTYRAMINE PACKET 0 SANDOZ EAGUL 00245-0536-60 1.27670 CHOLESTYRAMINE PACKET 0 UPSHER SMITH EAGUL 49884-0465-65 1.27670 CHOLESTYRAMINE PACKET 0 PAR PHARM. EAGEN 00185-0940-97 0.09320 CHOLESTYRAMINE POWDER 0 SANDOZ GMGEN 00245-0536-37 0.09320 CHOLESTYRAMINE POWDER 0 UPSHER SMITH GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0465-66 0.09320 CHOLESTYRAMINE POWDER 0 PAR PHARM. GMBND 54838-0522-70 0.15233 CHOLINE MAG TRISAL LIQUID 0 SILARX PHARM MLGEN 63323-0025-10 207.04500 CHORIONIC GONAD 10,000 UNIT VL 0 APP PHARMACEUTI EAGEN 43538-0520-90 0.15<strong>08</strong>0 CICLODAN 0.77% CREAM G MEDIMETRIKS PHA GMGEN 00713-<strong>06</strong>38-15 0.15<strong>08</strong>0 CICLOPIROX 0.77% CREAM G G & W LABS. GMGEN 00713-<strong>06</strong>38-18 0.15<strong>08</strong>0 CICLOPIROX 0.77% CREAM G G & W LABS. GMGEN 00713-<strong>06</strong>38-31 0.15<strong>08</strong>0 CICLOPIROX 0.77% CREAM G G & W LABS. GMGEN 45802-0138-11 0.15<strong>08</strong>0 CICLOPIROX 0.77% CREAM G PERRIGO CO. GMGEN 45802-0138-18 0.15<strong>08</strong>0 CICLOPIROX 0.77% CREAM G PERRIGO CO. GMGEN 45802-0138-35 0.15<strong>08</strong>0 CICLOPIROX 0.77% CREAM G PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0297-17 0.15<strong>08</strong>0 CICLOPIROX 0.77% CREAM G GLENMARK PHARMA GMGEN 68462-0297-35 0.15<strong>08</strong>0 CICLOPIROX 0.77% CREAM G GLENMARK PHARMA GMGEN 68462-0297-92 0.15<strong>08</strong>0 CICLOPIROX 0.77% CREAM G GLENMARK PHARMA GMGEN 00168-0407-30 1.37850 CICLOPIROX 0.77% GEL G SANDOZ GMGEN 00168-0407-46 1.37850 CICLOPIROX 0.77% GEL G SANDOZ GMGEN 00168-0407-99 1.37850 CICLOPIROX 0.77% GEL G SANDOZ GMGEN 00574-2<strong>06</strong>1-01 1.37850 CICLOPIROX 0.77% GEL G PADDOCK LABS. GMGEN 00574-2<strong>06</strong>1-30 1.37850 CICLOPIROX 0.77% GEL G PADDOCK LABS. GMGEN 00574-2<strong>06</strong>1-45 1.37850 CICLOPIROX 0.77% GEL G PADDOCK LABS. GMGEN 68462-0455-35 1.37850 CICLOPIROX 0.77% GEL G GLENMARK PHARMA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0455-47 1.37850 CICLOPIROX 0.77% GEL G GLENMARK PHARMA GMGEN 68462-0455-94 1.37850 CICLOPIROX 0.77% GEL G GLENMARK PHARMA GMGEN 00168-0314-30 0.54270 CICLOPIROX 0.77% TOPICAL SUSP G SANDOZ MLGEN 00168-0314-60 0.54270 CICLOPIROX 0.77% TOPICAL SUSP G SANDOZ MLGEN 45802-0400-46 0.54270 CICLOPIROX 0.77% TOPICAL SUSP G PERRIGO CO. MLGEN 45802-0400-49 0.54270 CICLOPIROX 0.77% TOPICAL SUSP G PERRIGO CO. MLGEN 51672-1323-03 0.54270 CICLOPIROX 0.77% TOPICAL SUSP G TARO PHARM USA MLGEN 51672-1323-04 0.54270 CICLOPIROX 0.77% TOPICAL SUSP G TARO PHARM USA MLGEN 00713-0317-88 4.80367 CICLOPIROX 8% SOLUTION 0 G & W LABS. MLGEN 00781-71<strong>06</strong>-60 4.80367 CICLOPIROX 8% SOLUTION 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0141-67 4.80367 CICLOPIROX 8% SOLUTION 0 PERRIGO CO. MLGEN 50383-0419-<strong>06</strong> 4.80367 CICLOPIROX 8% SOLUTION 0 HI-TECH PHARMAC MLGEN 61748-0200-<strong>06</strong> 4.80367 CICLOPIROX 8% SOLUTION 0 VERSA PHARMACEU MLGEN 67405-0450-66 4.80367 CICLOPIROX 8% SOLUTION 0 HARRIS PHARM MLGEN 23155-0216-31 133.20000 CID<strong>OF</strong>OVIR 375 MG/5 ML VIAL 0 HERITAGE PHARMA ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 78LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67457-0210-05 119.88000 CID<strong>OF</strong>OVIR 375 MG/5 ML VIAL 0 MYLAN INSTITUTI MLGEN 00054-0044-21 0.13595 CILOSTAZOL 100 MG TABLET 0 ROXANE LABS. EAGEN 00054-0044-29 0.13595 CILOSTAZOL 100 MG TABLET 0 ROXANE LABS. EAGEN 00093-2<strong>06</strong>4-<strong>06</strong> 0.13595 CILOSTAZOL 100 MG TABLET 0 TEVA USA EAGEN 00093-2<strong>06</strong>4-50 0.13595 CILOSTAZOL 100 MG TABLET 0 TEVA USA EAGEN 00185-0223-05 0.13595 CILOSTAZOL 100 MG TABLET 0 SANDOZ EAGEN 00185-0223-60 0.13595 CILOSTAZOL 100 MG TABLET 0 SANDOZ EAGEN 00378-2980-91 0.13595 CILOSTAZOL 100 MG TABLET 0 MYLAN EAGEN 51991-0168-<strong>06</strong> 0.13595 CILOSTAZOL 100 MG TABLET 0 BRECKENRIDGE EAGEN 60505-2522-01 0.13595 CILOSTAZOL 100 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 66993-0009-60 0.13595 CILOSTAZOL 100 MG TABLET 0 PRASCO LABS EAGEN 00054-0028-21 0.16538 CILOSTAZOL 50 MG TABLET 0 ROXANE LABS. EAGEN 00093-2<strong>06</strong>5-<strong>06</strong> 0.16538 CILOSTAZOL 50 MG TABLET 0 TEVA USA EAGEN 00185-0123-60 0.16538 CILOSTAZOL 50 MG TABLET 0 SANDOZ EAGEN 00378-2979-91 0.16538 CILOSTAZOL 50 MG TABLET 0 MYLAN EAGEN 51991-0167-<strong>06</strong> 0.16538 CILOSTAZOL 50 MG TABLET 0 BRECKENRIDGE EAGEN 60505-2521-01 0.16538 CILOSTAZOL 50 MG TABLET 0 APOTEX CORP EABND 00<strong>06</strong>5-<strong>06</strong>56-05 0.90070 17.58936 CILOXAN 0.3% EYE DROPS G ALCON LABS. MLBND 00<strong>06</strong>5-<strong>06</strong>54-35 34.36200 CILOXAN 0.3% OINTMENT G ALCON LABS. GMGEN 00378-0053-01 0.10940 CIMETIDINE 200 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0018-<strong>06</strong> 0.10940 CIMETIDINE 200 MG TABLET 0 APOTEX CORP EAGUL 00378-0317-01 0.13130 CIMETIDINE 300 MG TABLET 0 MYLAN EAGUL 60505-0019-04 0.13130 CIMETIDINE 300 MG TABLET 0 APOTEX CORP EAGUL 60505-0019-<strong>06</strong> 0.13130 CIMETIDINE 300 MG TABLET 0 APOTEX CORP EAGEN 50383-0050-<strong>08</strong> 0.07344 CIMETIDINE 300 MG/5 ML SOLN 0 HI-TECH PHARMAC MLGEN 60432-0007-<strong>08</strong> 0.07344 CIMETIDINE 300 MG/5 ML SOLN 0 MORTON GROVE PH MLGUL 00093-8204-01 0.15480 CIMETIDINE 400 MG TABLET 0 TEVA USA EAGUL 00093-8204-05 0.15480 CIMETIDINE 400 MG TABLET 0 TEVA USA EAGUL 00378-0372-01 0.15480 CIMETIDINE 400 MG TABLET 0 MYLAN EAGUL 00378-0372-05 0.15480 CIMETIDINE 400 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 60505-0020-04 0.15480 CIMETIDINE 400 MG TABLET 0 APOTEX CORP EAGUL 60505-0020-<strong>06</strong> 0.15480 CIMETIDINE 400 MG TABLET 0 APOTEX CORP EAGUL 60505-0020-<strong>08</strong> 0.15480 CIMETIDINE 400 MG TABLET 0 APOTEX CORP EAGUL 00378-0541-01 0.27750 CIMETIDINE 800 MG TABLET 0 MYLAN EAGUL 60505-0021-03 0.27750 CIMETIDINE 800 MG TABLET 0 APOTEX CORP EABND 50474-0700-62 2758.07340 CIMZIA 200 MG VIAL KIT G UCB PHARMA EABND 50474-0710-79 2758.07340 CIMZIA 200 MG/ML SYRINGE KIT G UCB PHARMA EABND 00<strong>06</strong>5-8531-10 18.157<strong>08</strong> CIPRO HC OTIC SUSPENSION G ALCON LABS. MLBND 50419-0759-01 0.01296 0.12450 CIPRO I.V. 400 MG/200 ML D5W 0 BAYER,PHARM DIV MLBND 50419-0789-01 7.14000 9.88928 CIPRO XR 1,000 MG TABLET G BAYER,PHARM DIV EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50419-0788-01 7.4<strong>08</strong>99 8.68611 CIPRO XR 500 MG TABLET G BAYER,PHARM DIV EABND 50419-0773-01 1.19752 CIPRO 10% SUSPENSION 0 BAYER,PHARM DIV MLBND 50419-0758-01 0.14378 4.44157 CIPRO 250 MG TABLET G BAYER,PHARM DIV EABND 50419-0777-01 1.02289 CIPRO 5% SUSPENSION 0 BAYER,PHARM DIV MLBND 00<strong>08</strong>5-1754-01 0.16335 4.95128 CIPRO 500 MG TABLET G SCHERING CORP. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 79LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50419-0754-01 0.16335 5.19870 CIPRO 500 MG TABLET G BAYER,PHARM DIV EABND 00<strong>06</strong>5-8533-02 20.13248 CIPRODEX OTIC SUSPENSION 0 ALCON LABS. MLGEN 00378-1745-89 7.14000 CIPR<strong>OF</strong>LOXACIN ER 1,000 MG TAB G MYLAN EAGEN 10370-01<strong>08</strong>-05 7.14000 CIPR<strong>OF</strong>LOXACIN ER 1,000 MG TAB G PAR PHARM. EAGEN 00378-1743-89 7.34910 CIPR<strong>OF</strong>LOXACIN ER 500 MG TABLET G MYLAN EAGEN 10370-0107-05 7.34910 CIPR<strong>OF</strong>LOXACIN ER 500 MG TABLET G PAR PHARM. EABND 55111-0125-<strong>06</strong> 2.79710 CIPR<strong>OF</strong>LOXACIN HCL 100 MG TAB 0 DR.REDDY'S LAB EAGEN 00143-9927-01 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 WEST-WARD,INC. EAGEN 00172-5311-60 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 TEVA USA EAGEN 16252-0514-01 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16571-0411-10 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 PACK PHARMACEUT EAGEN 16714-<strong>06</strong>51-02 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 NORTHSTAR RX LL EAGEN 55111-0126-01 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 DR.REDDY'S LAB EAGEN 55111-0126-05 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 DR.REDDY'S LAB EAGEN 60505-13<strong>08</strong>-01 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 APOTEX CORP EAGEN 61442-0222-01 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 CARLSBAD TECH EAGEN 65862-0076-01 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0<strong>06</strong>9-01 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0<strong>06</strong>9-11 0.14378 CIPR<strong>OF</strong>LOXACIN HCL 250 MG TAB 0 AHP EAGEN 00143-2037-01 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-9928-01 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 WEST-WARD,INC. EAGEN 00172-5312-60 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 TEVA USA EAGEN 00172-5312-70 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 TEVA USA EAGEN 00378-7098-01 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 MYLAN EAGEN 13107-0077-01 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 AUROBINDO PHARM EAGEN 16252-0515-01 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 ACTAVIS PHARMA, EAGEN 16571-0412-10 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 PACK PHARMACEUT EAGEN 16714-<strong>06</strong>52-02 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>52-04 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 NORTHSTAR RX LL EAGEN 24658-0250-05 0.15187 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 BLU PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24658-0250-60 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 BLU PHARMACEUTI EAGEN 51079-0182-01 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0182-20 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 MYLAN INSTITUTI EAGEN 55111-0127-01 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 DR.REDDY'S LAB EAGEN 55111-0127-05 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 DR.REDDY'S LAB EAGEN 60505-1309-01 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 APOTEX CORP EAGEN 61442-0223-01 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 CARLSBAD TECH EAGEN 61442-0223-05 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 CARLSBAD TECH EAGEN 63739-0559-10 0.16147 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 MCKESSON PACKAG EAGEN 65862-0077-01 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0070-01 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0070-11 0.16335 CIPR<strong>OF</strong>LOXACIN HCL 500 MG TAB 0 AHP EAGEN 00143-9929-50 0.28<strong>06</strong>7 CIPR<strong>OF</strong>LOXACIN HCL 750 MG TAB 0 WEST-WARD,INC. EAGEN 00172-5313-60 0.28<strong>06</strong>7 CIPR<strong>OF</strong>LOXACIN HCL 750 MG TAB 0 TEVA USA EAGEN 16252-0516-05 0.28<strong>06</strong>7 CIPR<strong>OF</strong>LOXACIN HCL 750 MG TAB 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 80LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16571-0413-05 0.28<strong>06</strong>7 CIPR<strong>OF</strong>LOXACIN HCL 750 MG TAB 0 PACK PHARMACEUT EAGEN 16714-<strong>06</strong>53-01 0.28<strong>06</strong>7 CIPR<strong>OF</strong>LOXACIN HCL 750 MG TAB 0 NORTHSTAR RX LL EAGEN 55111-0128-50 0.28<strong>06</strong>7 CIPR<strong>OF</strong>LOXACIN HCL 750 MG TAB 0 DR.REDDY'S LAB EAGEN 60505-1310-01 0.28<strong>06</strong>7 CIPR<strong>OF</strong>LOXACIN HCL 750 MG TAB 0 APOTEX CORP EAGEN 60505-1310-04 0.28<strong>06</strong>7 CIPR<strong>OF</strong>LOXACIN HCL 750 MG TAB 0 APOTEX CORP EAGEN 65862-0078-50 0.28<strong>06</strong>7 CIPR<strong>OF</strong>LOXACIN HCL 750 MG TAB 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0071-01 0.28<strong>06</strong>7 CIPR<strong>OF</strong>LOXACIN HCL 750 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0071-11 0.28<strong>06</strong>7 CIPR<strong>OF</strong>LOXACIN HCL 750 MG TAB 0 AHP EAGEN 16571-0120-25 0.90070 CIPR<strong>OF</strong>LOXACIN 0.3% EYE DROP G PACK PHARMACEUT MLGEN 16571-0120-50 0.90070 CIPR<strong>OF</strong>LOXACIN 0.3% EYE DROP G PACK PHARMACEUT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 17478-0714-10 0.90070 CIPR<strong>OF</strong>LOXACIN 0.3% EYE DROP G AKORN INC. MLGEN 17478-0714-25 0.90070 CIPR<strong>OF</strong>LOXACIN 0.3% EYE DROP G AKORN INC. MLGEN 50383-0282-02 0.90070 CIPR<strong>OF</strong>LOXACIN 0.3% EYE DROP G HI-TECH PHARMAC MLGEN 50383-0282-05 0.90070 CIPR<strong>OF</strong>LOXACIN 0.3% EYE DROP G HI-TECH PHARMAC MLGEN 50383-0282-10 0.90070 CIPR<strong>OF</strong>LOXACIN 0.3% EYE DROP G HI-TECH PHARMAC MLGEN 61314-<strong>06</strong>56-05 0.90070 CIPR<strong>OF</strong>LOXACIN 0.3% EYE DROP G SANDOZ MLGEN 61314-<strong>06</strong>56-10 0.90070 CIPR<strong>OF</strong>LOXACIN 0.3% EYE DROP G SANDOZ MLGEN 61314-<strong>06</strong>56-25 0.90070 CIPR<strong>OF</strong>LOXACIN 0.3% EYE DROP G SANDOZ MLGEN 00409-4765-86 0.11212 CIPR<strong>OF</strong>LOXACIN 200 MG/20 ML VL 0 HOSPIRA MLGEN 00409-4778-86 0.07837 CIPR<strong>OF</strong>LOXACIN 400 MG/40 ML VL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-4777-23 0.01823 CIPR<strong>OF</strong>LOXACN-D5W 200 MG/100 ML 0 HOSPIRA MLGEN 00781-3239-09 0.01823 CIPR<strong>OF</strong>LOXACN-D5W 200 MG/100 ML 0 SANDOZ MLGEN 00781-3239-46 0.01823 CIPR<strong>OF</strong>LOXACN-D5W 200 MG/100 ML 0 SANDOZ MLGEN 00409-4777-02 0.01197 CIPR<strong>OF</strong>LOXACN-D5W 400 MG/200 ML 0 HOSPIRA MLGEN 00781-3240-09 0.01296 CIPR<strong>OF</strong>LOXACN-D5W 400 MG/200 ML 0 SANDOZ MLGEN 00781-3240-48 0.01296 CIPR<strong>OF</strong>LOXACN-D5W 400 MG/200 ML 0 SANDOZ MLGEN 25021-0114-87 0.01296 CIPR<strong>OF</strong>LOXACN-D5W 400 MG/200 ML 0 SAGENT PHARMACE MLGEN 00703-5748-11 0.36270 CISPLATIN 100 MG/100 ML VIAL 0 TEVA PARENTERAL MLGEN 44567-0510-01 0.27000 CISPLATIN 100 MG/100 ML VIAL 0 WG CRITICAL CAR MLGEN 63323-0103-65 0.32490 CISPLATIN 100 MG/100 ML VIAL 0 APP PHARMACEUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0103-64 0.32490 CISPLATIN 200 MG/200 ML VIAL 0 APP PHARMACEUTI MLGEN 00703-5747-11 0.36270 CISPLATIN 50 MG/50 ML VIAL 0 TEVA PARENTERAL MLGEN 44567-0509-01 0.27000 CISPLATIN 50 MG/50 ML VIAL 0 WG CRITICAL CAR MLGEN 63323-0103-51 0.32490 CISPLATIN 50 MG/50 ML VIAL 0 APP PHARMACEUTI MLGEX 00093-4740-01 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 TEVA USA EAGEX 00093-4740-10 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 TEVA USA EAGEX 00185-0371-01 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 SANDOZ EAGEX 00378-6231-01 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 MYLAN EAGEX 00378-6231-05 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 MYLAN EAGEX 00904-6<strong>08</strong>4-61 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13107-0005-01 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0005-05 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 AUROBINDO PHARM EAGEX 13668-0009-01 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0009-05 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0009-09 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 81LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0009-30 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 TORRENT PHARMAC EAGEX 31722-02<strong>06</strong>-01 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 CAMBER PHARMACE EAGEX 31722-02<strong>06</strong>-05 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 CAMBER PHARMACE EAGEX 428<strong>06</strong>-0019-01 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 EPIC PHARMA LLC EAGEX 428<strong>06</strong>-0019-10 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 EPIC PHARMA LLC EAGEX 54458-0981-10 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 INTERNATIONAL L EAGEX 55111-0342-01 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 DR.REDDY'S LAB EAGEX 55111-0342-30 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 DR.REDDY'S LAB EAGEX 57664-0507-13 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 CARACO PHARM EAGEX 57664-0507-18 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 CARACO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 57664-0507-88 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 CARACO PHARM EAGEX 60505-2518-01 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 APOTEX CORP EAGEX 60505-2518-03 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 APOTEX CORP EAGEX 60505-2518-04 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 APOTEX CORP EAGEX 60505-2518-<strong>08</strong> 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 APOTEX CORP EAGEX 64720-0170-03 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 COREPHARMA LLC EAGEX 65162-0052-03 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 AMNEAL PHARMACE EAGEX 65162-0052-10 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 AMNEAL PHARMACE EAGEX 65162-0052-50 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 AMNEAL PHARMACE EAGEX 65862-0005-01 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0005-05 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 AUROBINDO PHARM EAGEX 76282-02<strong>06</strong>-01 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-02<strong>06</strong>-05 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-02<strong>06</strong>-10 0.026<strong>06</strong> CITALOPRAM HBR 10 MG TABLET 0 EXELAN PHARMACE EAGEX 00054-0<strong>06</strong>2-58 0.17910 CITALOPRAM HBR 10 MG/5 ML SOLN 0 ROXANE LABS. MLGEX 54838-0540-70 0.17910 CITALOPRAM HBR 10 MG/5 ML SOLN 0 SILARX PHARM MLGEX 65862-0074-24 0.17910 CITALOPRAM HBR 10 MG/5 ML SOLN 0 AUROBINDO PHARM MLGEX 00093-4741-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 TEVA USA EAGEX 00093-4741-05 0.03710 CITALOPRAM HBR 20 MG TABLET 0 TEVA USA EAGEX 00093-4741-50 0.03710 CITALOPRAM HBR 20 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00185-0372-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 SANDOZ EAGEX 00378-6232-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 MYLAN EAGEX 00378-6232-05 0.03710 CITALOPRAM HBR 20 MG TABLET 0 MYLAN EAGEX 00904-6<strong>08</strong>5-61 0.03710 CITALOPRAM HBR 20 MG TABLET 0 MAJOR PHARMACEU EAGEX 13107-00<strong>06</strong>-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-00<strong>06</strong>-05 0.03710 CITALOPRAM HBR 20 MG TABLET 0 AUROBINDO PHARM EAGEX 13668-0010-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0010-05 0.03710 CITALOPRAM HBR 20 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0010-<strong>06</strong> 0.03710 CITALOPRAM HBR 20 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0010-30 0.03710 CITALOPRAM HBR 20 MG TABLET 0 TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 31722-0207-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 CAMBER PHARMACE EAGEX 31722-0207-05 0.03710 CITALOPRAM HBR 20 MG TABLET 0 CAMBER PHARMACE EAGEX 31722-0207-10 0.03710 CITALOPRAM HBR 20 MG TABLET 0 CAMBER PHARMACE EAGEX 428<strong>06</strong>-0020-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 EPIC PHARMA LLC EAGEX 428<strong>06</strong>-0020-10 0.03710 CITALOPRAM HBR 20 MG TABLET 0 EPIC PHARMA LLC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 82LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 54458-<strong>08</strong>96-02 0.03710 CITALOPRAM HBR 20 MG TABLET 0 INTERNATIONAL L EAGEX 54458-0912-02 0.03710 CITALOPRAM HBR 20 MG TABLET 0 INTERNATIONAL L EAGEX 54458-0980-10 0.03710 CITALOPRAM HBR 20 MG TABLET 0 INTERNATIONAL L EAGEX 55111-0343-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 DR.REDDY'S LAB EAGEX 55111-0343-30 0.03710 CITALOPRAM HBR 20 MG TABLET 0 DR.REDDY'S LAB EAGEX 57664-05<strong>08</strong>-13 0.03710 CITALOPRAM HBR 20 MG TABLET 0 CARACO PHARM EAGEX 57664-05<strong>08</strong>-18 0.03710 CITALOPRAM HBR 20 MG TABLET 0 CARACO PHARM EAGEX 57664-05<strong>08</strong>-83 0.03710 CITALOPRAM HBR 20 MG TABLET 0 CARACO PHARM EAGEX 57664-05<strong>08</strong>-88 0.03710 CITALOPRAM HBR 20 MG TABLET 0 CARACO PHARM EAGEX 59762-4801-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-4801-03 0.03710 CITALOPRAM HBR 20 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-4801-05 0.03710 CITALOPRAM HBR 20 MG TABLET 0 GREENSTONE LLC. EAGEX 60505-2519-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 APOTEX CORP EAGEX 60505-2519-03 0.03710 CITALOPRAM HBR 20 MG TABLET 0 APOTEX CORP EAGEX 60505-2519-04 0.03710 CITALOPRAM HBR 20 MG TABLET 0 APOTEX CORP EAGEX 60505-2519-<strong>08</strong> 0.03710 CITALOPRAM HBR 20 MG TABLET 0 APOTEX CORP EAGEX 64720-0171-03 0.03710 CITALOPRAM HBR 20 MG TABLET 0 COREPHARMA LLC EAGEX 65162-0053-03 0.03710 CITALOPRAM HBR 20 MG TABLET 0 AMNEAL PHARMACE EAGEX 65162-0053-10 0.03710 CITALOPRAM HBR 20 MG TABLET 0 AMNEAL PHARMACE EAGEX 65162-0053-50 0.03710 CITALOPRAM HBR 20 MG TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-00<strong>06</strong>-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-00<strong>06</strong>-05 0.03710 CITALOPRAM HBR 20 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0<strong>06</strong>6-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0<strong>06</strong>6-11 0.03710 CITALOPRAM HBR 20 MG TABLET 0 AHP EAGEX 76282-0207-01 0.03710 CITALOPRAM HBR 20 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-0207-05 0.03710 CITALOPRAM HBR 20 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-0207-10 0.03710 CITALOPRAM HBR 20 MG TABLET 0 EXELAN PHARMACE EAGEX 00093-4742-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 TEVA USA EAGEX 00093-4742-05 0.04930 CITALOPRAM HBR 40 MG TABLET 0 TEVA USA EAGEX 00093-4742-50 0.04930 CITALOPRAM HBR 40 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00185-0373-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 SANDOZ EAGEX 00378-6233-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 MYLAN EAGEX 00378-6233-05 0.04930 CITALOPRAM HBR 40 MG TABLET 0 MYLAN EAGEX 00904-6<strong>08</strong>6-61 0.04930 CITALOPRAM HBR 40 MG TABLET 0 MAJOR PHARMACEU EAGEX 13107-0007-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0007-05 0.04930 CITALOPRAM HBR 40 MG TABLET 0 AUROBINDO PHARM EAGEX 13668-0011-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0011-05 0.04930 CITALOPRAM HBR 40 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0011-<strong>08</strong> 0.04930 CITALOPRAM HBR 40 MG TABLET 0 TORRENT PHARMAC EAGEX 31722-02<strong>08</strong>-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 31722-02<strong>08</strong>-05 0.04930 CITALOPRAM HBR 40 MG TABLET 0 CAMBER PHARMACE EAGEX 428<strong>06</strong>-0021-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 EPIC PHARMA LLC EAGEX 428<strong>06</strong>-0021-10 0.04930 CITALOPRAM HBR 40 MG TABLET 0 EPIC PHARMA LLC EAGEX 55111-0344-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 DR.REDDY'S LAB EAGEX 55111-0344-05 0.04930 CITALOPRAM HBR 40 MG TABLET 0 DR.REDDY'S LAB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 83LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 55111-0344-30 0.04930 CITALOPRAM HBR 40 MG TABLET 0 DR.REDDY'S LAB EAGEX 57664-0509-13 0.04930 CITALOPRAM HBR 40 MG TABLET 0 CARACO PHARM EAGEX 57664-0509-18 0.04930 CITALOPRAM HBR 40 MG TABLET 0 CARACO PHARM EAGEX 57664-0509-83 0.04930 CITALOPRAM HBR 40 MG TABLET 0 CARACO PHARM EAGEX 57664-0509-88 0.04930 CITALOPRAM HBR 40 MG TABLET 0 CARACO PHARM EAGEX 58517-0100-30 0.04930 CITALOPRAM HBR 40 MG TABLET 0 <strong>NEW</strong> HORIZON RX EAGEX 59762-4802-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-4802-03 0.04930 CITALOPRAM HBR 40 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-4802-05 0.04930 CITALOPRAM HBR 40 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-4802-<strong>06</strong> 0.04930 CITALOPRAM HBR 40 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-2520-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 APOTEX CORP EAGEX 60505-2520-03 0.04930 CITALOPRAM HBR 40 MG TABLET 0 APOTEX CORP EAGEX 60505-2520-04 0.04930 CITALOPRAM HBR 40 MG TABLET 0 APOTEX CORP EAGEX 60505-2520-<strong>08</strong> 0.04930 CITALOPRAM HBR 40 MG TABLET 0 APOTEX CORP EAGEX 64720-0172-03 0.04930 CITALOPRAM HBR 40 MG TABLET 0 COREPHARMA LLC EAGEX 65162-0054-03 0.04930 CITALOPRAM HBR 40 MG TABLET 0 AMNEAL PHARMACE EAGEX 65162-0054-10 0.04930 CITALOPRAM HBR 40 MG TABLET 0 AMNEAL PHARMACE EAGEX 65162-0054-50 0.04930 CITALOPRAM HBR 40 MG TABLET 0 AMNEAL PHARMACE EAGEX 65862-0007-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0007-05 0.04930 CITALOPRAM HBR 40 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68645-0252-54 0.04930 CITALOPRAM HBR 40 MG TABLET 0 LEGACY PHARMACE EAGEX 68645-0282-54 0.04930 CITALOPRAM HBR 40 MG TABLET 0 LEGACY PHARMACE EAGEX 68645-0467-54 0.04930 CITALOPRAM HBR 40 MG TABLET 0 LEGACY PHARMACE EAGEX 76282-02<strong>08</strong>-01 0.04930 CITALOPRAM HBR 40 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-02<strong>08</strong>-05 0.04930 CITALOPRAM HBR 40 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-02<strong>08</strong>-10 0.04930 CITALOPRAM HBR 40 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-02<strong>08</strong>-30 0.04930 CITALOPRAM HBR 40 MG TABLET 0 EXELAN PHARMACE EABND 00178-<strong>08</strong>59-90 0.27300 1.67042 CITRANATAL RX TABLET 0 MISSION PHARM. EAGEN 38779-0<strong>06</strong>8-01 0.49875 CITRIC ACID POWDER 0 MEDISCA INC. GMGEN 00<strong>06</strong>9-0201-01 31.50000 CLADRIBINE 10 MG/10 ML VIAL 0 PFIZER/NOVAPLUS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55390-0124-01 36.09000 CLADRIBINE 10 MG/10 ML VIAL 0 BEDFORD LABS MLGEN 63323-0140-10 39.15000 CLADRIBINE 10 MG/10 ML VIAL 0 APP PHARMACEUTI MLBND 00039-0023-25 3.35652 CLAFORAN 1 GM ADD-VANTAGE VL 0 HOSPIRA EABND 00039-0018-10 1.71312 CLAFORAN 1 GM VIAL G HOSPIRA EABND 00039-0024-25 6.07560 CLAFORAN 2 GM ADD-VANTAGE VL 0 HOSPIRA EABND 00039-0019-10 4.12516 4.19316 CLAFORAN 2 GM VIAL G HOSPIRA EABND 00039-0017-10 1.20000 1.49400 CLAFORAN 500 MG VIAL G SAN<strong>OF</strong>I-AVENTIS EAGEN 00555-1054-56 7.36020 CLARAVIS 10 MG CAPSULE 0 BARR EAGEN 00555-1054-86 7.36020 CLARAVIS 10 MG CAPSULE 0 BARR EAGEN 00555-1055-56 8.43370 CLARAVIS 20 MG CAPSULE 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00555-1055-86 8.43370 CLARAVIS 20 MG CAPSULE 0 BARR EAGEN 00555-1056-86 12.33875 CLARAVIS 30 MG CAPSULE 0 BARR EAGEN 00555-1057-56 8.98670 CLARAVIS 40 MG CAPSULE 0 BARR EAGEN 00555-1057-86 8.98670 CLARAVIS 40 MG CAPSULE 0 BARR EABND 00<strong>08</strong>5-1334-01 0.58043 CLARINEX 0.5 MG/ML (2.5 MG/5) G MERCK SHARP & D ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 84LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>5-1264-01 1.04400 5.61602 CLARINEX 5 MG TABLET G MERCK SHARP & D EABND 00<strong>08</strong>5-1264-02 1.04400 5.61664 CLARINEX 5 MG TABLET G MERCK SHARP & D EABND 00<strong>08</strong>5-1322-01 3.85991 CLARINEX-D 12 HOUR TABLET G MERCK SHARP & D EABND 00<strong>08</strong>5-1317-01 6.02148 CLARINEX-D 24 HOUR TABLET G MERCK SHARP & D EAGEN 00093-7244-<strong>06</strong> 3.65<strong>06</strong>7 CLARITHROMYCIN ER 500 MG TAB 0 TEVA USA EAGEN 62037-0777-60 3.65<strong>06</strong>7 CLARITHROMYCIN ER 500 MG TAB 0 ACTAVIS PHARMA, EAGEN 68382-0763-14 3.65<strong>06</strong>7 CLARITHROMYCIN ER 500 MG TAB 0 ZYDUS PHARMACEU EAGEN 00781-6022-46 0.38842 CLARITHROMYCIN 125 MG/5 ML SUS 0 SANDOZ MLGEN 00781-6022-52 0.41990 CLARITHROMYCIN 125 MG/5 ML SUS 0 SANDOZ MLGEN 68382-0764-05 0.41990 CLARITHROMYCIN 125 MG/5 ML SUS 0 ZYDUS PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0764-<strong>06</strong> 0.38842 CLARITHROMYCIN 125 MG/5 ML SUS 0 ZYDUS PHARMACEU MLGUL 00093-7157-<strong>06</strong> 2.37250 CLARITHROMYCIN 250 MG TABLET 0 TEVA USA EAGUL 00781-1961-60 2.37250 CLARITHROMYCIN 250 MG TABLET 0 SANDOZ EAGUL 64679-0954-01 2.37250 CLARITHROMYCIN 250 MG TABLET 0 WOCKHARDT USA L EAGUL 65862-0225-60 2.37250 CLARITHROMYCIN 250 MG TABLET 0 AUROBINDO PHARM EAGUL 68382-0761-14 2.37250 CLARITHROMYCIN 250 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00781-6023-46 0.74025 CLARITHROMYCIN 250 MG/5 ML SUS 0 SANDOZ MLGEN 00781-6023-52 0.79950 CLARITHROMYCIN 250 MG/5 ML SUS 0 SANDOZ MLGEN 68382-0765-05 0.79950 CLARITHROMYCIN 250 MG/5 ML SUS 0 ZYDUS PHARMACEU MLGEN 68382-0765-<strong>06</strong> 0.74025 CLARITHROMYCIN 250 MG/5 ML SUS 0 ZYDUS PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00054-0037-21 0.86250 CLARITHROMYCIN 500 MG TABLET 0 ROXANE LABS. EAGUL 00093-7158-<strong>06</strong> 0.86250 CLARITHROMYCIN 500 MG TABLET 0 TEVA USA EAGUL 00781-1962-60 0.86250 CLARITHROMYCIN 500 MG TABLET 0 SANDOZ EAGUL 64679-0949-01 0.86250 CLARITHROMYCIN 500 MG TABLET 0 WOCKHARDT USA L EAGUL 65862-0226-60 0.86250 CLARITHROMYCIN 500 MG TABLET 0 AUROBINDO PHARM EAGUL 68<strong>08</strong>4-0437-11 0.86250 CLARITHROMYCIN 500 MG TABLET 0 AHP EAGUL 68<strong>08</strong>4-0437-65 0.86250 CLARITHROMYCIN 500 MG TABLET 0 AHP EAGUL 68382-0762-14 0.86250 CLARITHROMYCIN 500 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00093-03<strong>08</strong>-01 0.2<strong>08</strong>35 CLEMASTINE FUM 2.68 MG TAB 0 TEVA USA EAGEN 00781-1359-01 0.2<strong>08</strong>35 CLEMASTINE FUM 2.68 MG TAB 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00093-0309-12 0.04390 0.16170 CLEMASTINE 0.5 MG/5 ML SYRUP 0 TEVA USA MLBND 00009-0225-02 0.<strong>08</strong>860 4.30703 CLEOCIN HCL 150 MG CAPSULE G PHARMACIA/UPJHN EABND 00009-0225-03 0.<strong>08</strong>860 3.72055 CLEOCIN HCL 150 MG CAPSULE G PHARMACIA/UPJHN EABND 00009-0395-14 0.28850 8.74836 CLEOCIN HCL 300 MG CAPSULE G PHARMACIA/UPJHN EABND 00009-0331-02 0.24400 2.19377 CLEOCIN HCL 75 MG CAPSULE 0 PHARMACIA/UPJHN EABND 00009-0728-09 0.35870 1.58814 CLEOCIN PHOS 150 MG/ML VIAL G PHARMACIA/UPJHN MLBND 00009-0775-26 0.35870 2.11849 CLEOCIN PHOS 150 MG/ML VIAL G PHARMACIA/UPJHN MLBND 00009-<strong>08</strong>70-26 0.35870 2.03516 CLEOCIN PHOS 150 MG/ML VIAL G PHARMACIA/UPJHN MLBND 00009-0902-18 0.35870 1.85090 CLEOCIN PHOS 150 MG/ML VIAL G PHARMACIA/UPJHN MLBND 00009-3447-03 1.79877 CLEOCIN PHOS 150 MG/ML VIAL 0 PHARMACIA/UPJHN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00009-3331-01 0.76470 2.29578 CLEOCIN T 1% GEL G PHARMACIA/UPJHN GMBUL 00009-3331-02 0.76470 2.54920 CLEOCIN T 1% GEL G PHARMACIA/UPJHN GMBUL 00009-3329-01 0.79880 1.77343 CLEOCIN T 1% LOTION G PHARMACIA/UPJHN MLBND 00009-3116-14 0.39030 1.680<strong>06</strong> CLEOCIN T 1% PLEDGETS G PHARMACIA/UPJHN EABUL 00009-3116-01 0.2<strong>06</strong>00 1.48182 CLEOCIN T 1% SOLUTION G PHARMACIA/UPJHN ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 85LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00009-3116-02 0.2<strong>06</strong>00 1.44766 CLEOCIN T 1% SOLUTION G PHARMACIA/UPJHN MLBND 00009-7667-01 29.96853 CLEOCIN 100 MG VAGINAL OVULE 0 PHARMACIA/UPJHN EABND 00009-3124-03 2.25760 CLEOCIN 150 MG/ML ADDVN VIAL 0 PHARMACIA/UPJHN MLBND 00009-3448-01 1.57588 2.40368 CLEOCIN 2% VAGINAL CREAM G PHARMACIA/UPJHN GMBND 00009-3381-02 0.09820 0.22697 CLEOCIN 300 MG-D5W-GALAXY 0 PHARMACIA/UPJHN MLBND 00009-3375-02 0.14780 0.34745 CLEOCIN 600 MG-D5W-GALAXY 0 PHARMACIA/UPJHN MLGEN 00009-0760-04 0.42471 CLEOCIN 75 MG/5 ML GRANULES 0 PHARMACIA/UPJHN MLBND 00009-3382-02 0.17760 0.42451 CLEOCIN 900 MG-D5W-GALAXY 0 PHARMACIA/UPJHN MLBND 50419-0491-04 28.43580 CLIMARA PRO PATCH 0 BAYER,PHARM DIV EABND 50419-0454-04 11.21110 23.54710 CLIMARA 0.025 MG/DAY PATCH G BAYER,PHARM DIV EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50419-0456-04 10.74000 23.54710 CLIMARA 0.0375 MG/DAY PATCH G BAYER,PHARM DIV EABND 50419-0451-01 11.37750 23.54710 CLIMARA 0.05 MG/DAY PATCH G BAYER,PHARM DIV EABND 50419-0451-04 11.37750 23.54710 CLIMARA 0.05 MG/DAY PATCH G BAYER,PHARM DIV EABND 50419-0459-04 11.49568 23.54710 CLIMARA 0.<strong>06</strong>/MG DAY PATCH G BAYER,PHARM DIV EABND 50419-0453-04 11.46550 23.54710 CLIMARA 0.075 MG/DAY PATCH G BAYER,PHARM DIV EABND 50419-0452-01 17.36394 23.54710 CLIMARA 0.1 MG/DAY PATCH G BAYER,PHARM DIV EABND 50419-0452-04 17.36394 23.54710 CLIMARA 0.1 MG/DAY PATCH G BAYER,PHARM DIV EAGEN 43538-0172-60 0.39030 CLINDACIN ETZ 1% PLEDGET G MEDIMETRIKS PHA EAGEN 43538-0170-69 0.39030 CLINDACIN P 1% PLEDGETS G MEDIMETRIKS PHA EABND 00299-4500-75 6.89165 CLINDAGEL 1% GEL G ONSET DERMATOLO ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-3171-01 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 TEVA USA EAGEN 00527-1382-01 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 LANNETT CO. INC EAGEN 00591-57<strong>08</strong>-01 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00904-5959-61 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 51079-0598-17 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 51079-0598-19 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 59762-3328-01 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 GREENSTONE LLC. EAGEN 63304-<strong>06</strong>92-01 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 RANBAXY PHARMAC EAGEN 63304-<strong>06</strong>92-05 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 RANBAXY PHARMAC EAGEN 65862-0185-01 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0185-05 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0243-01 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-0243-11 0.<strong>08</strong>860 CLINDAMYCIN HCL 150 MG CAPSULE 0 AHP EAGEN 00093-5256-01 0.28850 CLINDAMYCIN HCL 300 MG CAPSULE 0 TEVA USA EAGEN 00093-5256-68 0.28850 CLINDAMYCIN HCL 300 MG CAPSULE 0 TEVA USA EAGEN 00527-1383-01 0.28850 CLINDAMYCIN HCL 300 MG CAPSULE 0 LANNETT CO. INC EAGEN 00527-1383-02 0.28850 CLINDAMYCIN HCL 300 MG CAPSULE 0 LANNETT CO. INC EAGEN 00591-3120-01 0.28850 CLINDAMYCIN HCL 300 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 59762-5010-01 0.28850 CLINDAMYCIN HCL 300 MG CAPSULE 0 GREENSTONE LLC. EAGEN 59762-5010-02 0.28850 CLINDAMYCIN HCL 300 MG CAPSULE 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63304-<strong>06</strong>93-01 0.28850 CLINDAMYCIN HCL 300 MG CAPSULE 0 RANBAXY PHARMAC EAGEN 63304-<strong>06</strong>93-16 0.28850 CLINDAMYCIN HCL 300 MG CAPSULE 0 RANBAXY PHARMAC EAGEN 65862-0186-01 0.28850 CLINDAMYCIN HCL 300 MG CAPSULE 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0244-01 0.28850 CLINDAMYCIN HCL 300 MG CAPSULE 0 AHP EAGEN 00527-1381-04 0.24400 CLINDAMYCIN HCL 75 MG CAPSULE 0 LANNETT CO. INC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 86LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64980-0511-10 0.42471 CLINDAMYCIN PEDIATR 75 MG/5 ML 0 RISING PHARM MLGEN 65862-0596-01 0.42471 CLINDAMYCIN PEDIATR 75 MG/5 ML 0 AUROBINDO PHARM MLGUL 00168-0202-30 0.76470 CLINDAMYCIN PH 1% GEL G SANDOZ GMGUL 00168-0202-60 0.76470 CLINDAMYCIN PH 1% GEL G SANDOZ GMGUL 59762-3743-01 0.76470 CLINDAMYCIN PH 1% GEL G GREENSTONE LLC. GMGUL 59762-3743-02 0.76470 CLINDAMYCIN PH 1% GEL G GREENSTONE LLC. GMGUL 00168-0201-60 0.2<strong>06</strong>00 CLINDAMYCIN PH 1% SOLUTION 0 SANDOZ MLGUL 45802-0562-01 0.2<strong>06</strong>00 CLINDAMYCIN PH 1% SOLUTION 0 PERRIGO CO. MLGUL 45802-0562-02 0.2<strong>06</strong>00 CLINDAMYCIN PH 1% SOLUTION 0 PERRIGO CO. MLGUL 51672-4<strong>08</strong>1-03 0.2<strong>06</strong>00 CLINDAMYCIN PH 1% SOLUTION 0 TARO PHARM USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-4<strong>08</strong>1-04 0.2<strong>06</strong>00 CLINDAMYCIN PH 1% SOLUTION 0 TARO PHARM USA MLGUL 59762-3728-01 0.2<strong>06</strong>00 CLINDAMYCIN PH 1% SOLUTION 0 GREENSTONE LLC. MLGUL 59762-3728-02 0.2<strong>06</strong>00 CLINDAMYCIN PH 1% SOLUTION 0 GREENSTONE LLC. MLGEN 00409-4050-01 0.35870 CLINDAMYCIN PH 300 MG/2 ML VL 0 HOSPIRA MLGEN 25021-0115-02 0.35870 CLINDAMYCIN PH 300 MG/2 ML VL 0 SAGENT PHARMACE MLGEN 00409-4051-01 0.35870 CLINDAMYCIN PH 600 MG/4 ML VL 0 HOSPIRA MLGEN 25021-0115-04 0.35870 CLINDAMYCIN PH 600 MG/4 ML VL 0 SAGENT PHARMACE MLGEN 63323-0282-04 0.35870 CLINDAMYCIN PH 600 MG/4 ML VL 0 APP PHARMACEUTI MLGEN 00409-4197-01 0.25962 CLINDAMYCIN PH 9 G/60 ML VIAL 0 HOSPIRA MLGEN 25021-0115-51 0.34500 CLINDAMYCIN PH 9 G/60 ML VIAL 0 SAGENT PHARMACE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0282-60 0.35187 CLINDAMYCIN PH 9 G/60 ML VIAL 0 APP PHARMACEUTI MLGEN 00409-4052-01 0.35870 CLINDAMYCIN PH 900 MG/6 ML VL 0 HOSPIRA MLGEN 25021-0115-<strong>06</strong> 0.35870 CLINDAMYCIN PH 900 MG/6 ML VL 0 SAGENT PHARMACE MLGEN 63323-0282-<strong>06</strong> 0.35870 CLINDAMYCIN PH 900 MG/6 ML VL 0 APP PHARMACEUTI MLGEN 45802-0263-37 0.39030 CLINDAMYCIN PHOS 1% PLEDGET G PERRIGO CO. EAGEN 59762-3728-03 0.39030 CLINDAMYCIN PHOS 1% PLEDGET G GREENSTONE LLC. EAGEN 61748-0201-60 0.39030 CLINDAMYCIN PHOS 1% PLEDGET G VERSA PHARMACEU EAGUL 00168-0203-60 0.79880 CLINDAMYCIN PHOSP 1% LOTION 0 SANDOZ MLGUL 59762-3744-01 0.79880 CLINDAMYCIN PHOSP 1% LOTION 0 GREENSTONE LLC. MLGEN 38779-00<strong>06</strong>-05 22.44375 CLINDAMYCIN PHOSPHATE POWDER 0 MEDISCA INC. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-<strong>06</strong>60-32 3.93270 CLINDAMYCIN PHOSPHATE 1% FOAM G PERRIGO CO. GMGEN 45802-<strong>06</strong>60-33 3.00952 CLINDAMYCIN PHOSPHATE 1% FOAM G PERRIGO CO. GMBND 00409-4053-03 1.73802 CLINDAMYCIN 150 MG/ML ADDVAN G HOSPIRA MLGEN 00409-4054-03 0.81675 CLINDAMYCIN 150 MG/ML ADDVAN 0 HOSPIRA MLGEN 00409-4055-03 0.64350 CLINDAMYCIN 150 MG/ML ADDVAN 0 HOSPIRA MLGEN 00168-0277-40 1.57588 CLINDAMYCIN 2% VAGINAL CREAM 0 SANDOZ GMGEN 59762-5009-01 1.57588 CLINDAMYCIN 2% VAGINAL CREAM 0 GREENSTONE LLC. GMGEN 00574-0129-01 0.42471 CLINDAMYCIN 75 MG/5 ML SOLN 0 PADDOCK LABS. MLGEN 59762-0016-01 0.42471 CLINDAMYCIN 75 MG/5 ML SOLN 0 GREENSTONE LLC. MLGEN 00378-8688-35 2.76850 CLINDAMYCIN-BENZOYL PEROX GEL G MYLAN GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-8688-54 2.76850 CLINDAMYCIN-BENZOYL PEROX GEL G MYLAN GMGEN 00781-3288-09 0.09820 CLINDAMYCIN-D5W 300 MG/50 ML 0 SANDOZ MLGEN 17478-0120-50 0.<strong>08</strong>250 CLINDAMYCIN-D5W 300 MG/50 ML 0 AKORN INC. MLGEN 00781-3289-91 0.14780 CLINDAMYCIN-D5W 600 MG/50 ML 0 SANDOZ MLGEN 17478-0121-50 0.12375 CLINDAMYCIN-D5W 600 MG/50 ML 0 AKORN INC. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 87LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 17478-0122-50 0.14430 CLINDAMYCIN-D5W 900 MG/50 ML 0 AKORN INC. MLBND 64011-0124-<strong>08</strong> 14.59655 CLINDESSE 2% VAGINAL CREAM 0 THER-RX GMGEN 00168-0301-15 0.35970 CLOBETASOL EMOLLIENT 0.05% CRM 0 SANDOZ GMGEN 00168-0301-30 0.35970 CLOBETASOL EMOLLIENT 0.05% CRM 0 SANDOZ GMGEN 00168-0301-60 0.35970 CLOBETASOL EMOLLIENT 0.05% CRM 0 SANDOZ GMGEN 50383-0270-15 0.35970 CLOBETASOL EMOLLIENT 0.05% CRM 0 HI-TECH PHARMAC GMGEN 50383-0270-30 0.35970 CLOBETASOL EMOLLIENT 0.05% CRM 0 HI-TECH PHARMAC GMGEN 50383-0270-60 0.35970 CLOBETASOL EMOLLIENT 0.05% CRM 0 HI-TECH PHARMAC GMGEN 51672-1297-01 0.35970 CLOBETASOL EMOLLIENT 0.05% CRM 0 TARO PHARM USA GMGEN 51672-1297-02 0.35970 CLOBETASOL EMOLLIENT 0.05% CRM 0 TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-1297-03 0.35970 CLOBETASOL EMOLLIENT 0.05% CRM 0 TARO PHARM USA GMGEN 45802-<strong>06</strong>37-32 4.14071 CLOBETASOL EMOLLNT 0.05% FOAM G PERRIGO CO. GMGEN 45802-<strong>06</strong>37-33 3.9<strong>08</strong>70 CLOBETASOL EMOLLNT 0.05% FOAM G PERRIGO CO. GMGEN 40<strong>08</strong>5-<strong>08</strong>93-00 4.<strong>06</strong>035 CLOBETASOL EMULSION 0.05% FOAM G RENAISSANCE PHA GMGEN 40<strong>08</strong>5-<strong>08</strong>93-50 4.14071 CLOBETASOL EMULSION 0.05% FOAM G RENAISSANCE PHA GMGEN 66993-<strong>08</strong>93-49 3.58980 CLOBETASOL EMULSION 0.05% FOAM G PRASCO LABS GMGEN 66993-<strong>08</strong>93-65 3.3<strong>08</strong>85 CLOBETASOL EMULSION 0.05% FOAM G PRASCO LABS GMGUL 40<strong>08</strong>5-<strong>08</strong>88-00 2.97960 CLOBETASOL PROP 0.05% FOAM G RENAISSANCE PHA GMGUL 40<strong>08</strong>5-<strong>08</strong>88-50 2.97960 CLOBETASOL PROP 0.05% FOAM G RENAISSANCE PHA GMGUL 45802-0437-32 2.97960 CLOBETASOL PROP 0.05% FOAM G PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 45802-0437-33 2.97960 CLOBETASOL PROP 0.05% FOAM G PERRIGO CO. GMGEN 66993-<strong>08</strong>88-65 2.35365 CLOBETASOL PROP 0.05% FOAM G PRASCO LABS GMGUL 00168-0163-15 0.18250 CLOBETASOL 0.05% CREAM 0 SANDOZ GMGUL 00168-0163-30 0.18250 CLOBETASOL 0.05% CREAM 0 SANDOZ GMGUL 00168-0163-46 0.18250 CLOBETASOL 0.05% CREAM 0 SANDOZ GMGUL 00168-0163-60 0.18250 CLOBETASOL 0.05% CREAM 0 SANDOZ GMGUL 50383-0267-15 0.18250 CLOBETASOL 0.05% CREAM 0 HI-TECH PHARMAC GMGUL 50383-0267-30 0.18250 CLOBETASOL 0.05% CREAM 0 HI-TECH PHARMAC GMGUL 50383-0267-45 0.18250 CLOBETASOL 0.05% CREAM 0 HI-TECH PHARMAC GMGUL 50383-0267-60 0.18250 CLOBETASOL 0.05% CREAM 0 HI-TECH PHARMAC GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-1258-01 0.18250 CLOBETASOL 0.05% CREAM 0 TARO PHARM USA GMGUL 51672-1258-02 0.18250 CLOBETASOL 0.05% CREAM 0 TARO PHARM USA GMGUL 51672-1258-03 0.18250 CLOBETASOL 0.05% CREAM 0 TARO PHARM USA GMGUL 51672-1258-<strong>06</strong> 0.18250 CLOBETASOL 0.05% CREAM 0 TARO PHARM USA GMGEN 00168-0293-15 0.36423 CLOBETASOL 0.05% GEL 0 SANDOZ GMGEN 00168-0293-30 0.36423 CLOBETASOL 0.05% GEL 0 SANDOZ GMGEN 00168-0293-60 0.36423 CLOBETASOL 0.05% GEL 0 SANDOZ GMGEN 45802-0925-14 0.36423 CLOBETASOL 0.05% GEL 0 PERRIGO CO. GMGEN 45802-0925-94 0.36423 CLOBETASOL 0.05% GEL 0 PERRIGO CO. GMGEN 45802-0925-96 0.36423 CLOBETASOL 0.05% GEL 0 PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50383-0269-15 0.36423 CLOBETASOL 0.05% GEL 0 HI-TECH PHARMAC GMGEN 50383-0269-30 0.36423 CLOBETASOL 0.05% GEL 0 HI-TECH PHARMAC GMGEN 50383-0269-60 0.36423 CLOBETASOL 0.05% GEL 0 HI-TECH PHARMAC GMGEN 51672-1294-01 0.36423 CLOBETASOL 0.05% GEL 0 TARO PHARM USA GMGEN 51672-1294-02 0.36423 CLOBETASOL 0.05% GEL 0 TARO PHARM USA GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 88LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-1294-03 0.36423 CLOBETASOL 0.05% GEL 0 TARO PHARM USA GMGUL 00168-0162-15 0.19400 CLOBETASOL 0.05% OINTMENT 0 SANDOZ GMGUL 00168-0162-30 0.19400 CLOBETASOL 0.05% OINTMENT 0 SANDOZ GMGUL 00168-0162-46 0.19400 CLOBETASOL 0.05% OINTMENT 0 SANDOZ GMGUL 00168-0162-60 0.19400 CLOBETASOL 0.05% OINTMENT 0 SANDOZ GMGUL 50383-0268-15 0.19400 CLOBETASOL 0.05% OINTMENT 0 HI-TECH PHARMAC GMGUL 50383-0268-30 0.19400 CLOBETASOL 0.05% OINTMENT 0 HI-TECH PHARMAC GMGUL 50383-0268-45 0.19400 CLOBETASOL 0.05% OINTMENT 0 HI-TECH PHARMAC GMGUL 50383-0268-60 0.19400 CLOBETASOL 0.05% OINTMENT 0 HI-TECH PHARMAC GMGUL 51672-1259-01 0.19400 CLOBETASOL 0.05% OINTMENT 0 TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-1259-02 0.19400 CLOBETASOL 0.05% OINTMENT 0 TARO PHARM USA GMGUL 51672-1259-03 0.19400 CLOBETASOL 0.05% OINTMENT 0 TARO PHARM USA GMGUL 51672-1259-<strong>06</strong> 0.19400 CLOBETASOL 0.05% OINTMENT 0 TARO PHARM USA GMGEN 00168-0269-50 0.22470 CLOBETASOL 0.05% SOLUTION 0 SANDOZ MLGEN 50383-0266-25 0.22470 CLOBETASOL 0.05% SOLUTION 0 HI-TECH PHARMAC MLGEN 50383-0266-50 0.22470 CLOBETASOL 0.05% SOLUTION 0 HI-TECH PHARMAC MLGEN 51672-1293-02 0.22470 CLOBETASOL 0.05% SOLUTION 0 TARO PHARM USA MLGEN 51672-1293-03 0.22470 CLOBETASOL 0.05% SOLUTION 0 TARO PHARM USA MLGEN 60432-0133-25 0.22470 CLOBETASOL 0.05% SOLUTION 0 MORTON GROVE PH MLGEN 60432-0133-50 0.22470 CLOBETASOL 0.05% SOLUTION 0 MORTON GROVE PH ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00472-0404-92 3.56130 CLOBETASOL 0.05% TOPICAL LOTN G ACTAVIS PHARMA, MLGEN 00472-0404-94 3.35949 CLOBETASOL 0.05% TOPICAL LOTN G ACTAVIS PHARMA, MLGEN 00574-2103-02 3.56130 CLOBETASOL 0.05% TOPICAL LOTN G PADDOCK LABS. MLGEN 00574-2103-04 3.36324 CLOBETASOL 0.05% TOPICAL LOTN G PADDOCK LABS. MLGEN 51672-1350-04 3.52373 CLOBETASOL 0.05% TOPICAL LOTN G TARO PHARM USA MLGEN 51672-1350-<strong>08</strong> 3.22284 CLOBETASOL 0.05% TOPICAL LOTN G TARO PHARM USA MLBND 00299-3849-02 5.15893 CLOBEX 0.05% SPRAY G GALDERMA LABORA MLBND 00299-3849-04 4.38040 CLOBEX 0.05% SPRAY G GALDERMA LABORA MLBND 00299-3848-02 3.56130 6.6<strong>06</strong>51 CLOBEX 0.05% TOPICAL LOTION G GALDERMA LABORA MLBND 00299-3848-04 3.56130 6.64661 CLOBEX 0.05% TOPICAL LOTION G GALDERMA LABORA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 67857-<strong>08</strong>04-45 6.6<strong>08</strong>64 CLODERM 0.1% CREAM G PROMIUS PHARMA GMBND 67857-<strong>08</strong>04-90 6.6<strong>08</strong>64 CLODERM 0.1% CREAM G PROMIUS PHARMA GMBND 67857-<strong>08</strong>04-30 6.6<strong>08</strong>73 CLODERM 0.1% CREAM PUMP G PROMIUS PHARMA GMBND 67857-<strong>08</strong>04-51 6.6<strong>08</strong>68 CLODERM 0.1% CREAM PUMP G PROMIUS PHARMA GMBND 00<strong>06</strong>8-0226-30 1.02<strong>06</strong>0 13.54<strong>08</strong>9 CLOMID 50 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EAGEN 00591-0781-30 1.02<strong>06</strong>0 CLOMIPHENE CITRATE 50 MG TAB 0 ACTAVIS PHARMA, EAGEN 49884-0701-54 1.02<strong>06</strong>0 CLOMIPHENE CITRATE 50 MG TAB 0 PAR PHARM. EAGUX 00378-3025-01 0.37500 CLOMIPRAMINE 25 MG CAPSULE 0 MYLAN EAGUX 00781-2027-01 0.37500 CLOMIPRAMINE 25 MG CAPSULE 0 SANDOZ EAGUX 51672-4011-05 0.37500 CLOMIPRAMINE 25 MG CAPSULE 0 TARO PHARM USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 51672-4011-<strong>06</strong> 0.37500 CLOMIPRAMINE 25 MG CAPSULE 0 TARO PHARM USA EAGUX 00378-3050-01 0.50360 CLOMIPRAMINE 50 MG CAPSULE 0 MYLAN EAGUX 00781-2037-01 0.50360 CLOMIPRAMINE 50 MG CAPSULE 0 SANDOZ EAGUX 51672-4012-05 0.50360 CLOMIPRAMINE 50 MG CAPSULE 0 TARO PHARM USA EAGUX 51672-4012-<strong>06</strong> 0.50360 CLOMIPRAMINE 50 MG CAPSULE 0 TARO PHARM USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 89LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 00378-3075-01 0.66230 CLOMIPRAMINE 75 MG CAPSULE 0 MYLAN EAGUX 00781-2047-01 0.66230 CLOMIPRAMINE 75 MG CAPSULE 0 SANDOZ EAGUX 51672-4013-05 0.66230 CLOMIPRAMINE 75 MG CAPSULE 0 TARO PHARM USA EAGUX 51672-4013-<strong>06</strong> 0.66230 CLOMIPRAMINE 75 MG CAPSULE 0 TARO PHARM USA EAGEN 38779-0561-03 146.<strong>06</strong>250 CLONIDINE HCL POWDER 0 MEDISCA INC. GMGEN 38779-0561-05 146.<strong>06</strong>250 CLONIDINE HCL POWDER 0 MEDISCA INC. GMGEN 00228-2127-10 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00228-2127-50 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00378-0152-01 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 MYLAN EAGEN 00378-0152-10 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-2957-02 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2957-04 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2957-21 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2957-28 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2957-32 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 QUALITEST EAGEN 16714-0341-02 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0341-03 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0341-04 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 NORTHSTAR RX LL EAGEN 29300-0135-01 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 UNICHEM PHARMAC EAGEN 29300-0135-10 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 UNICHEM PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0299-17 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0299-20 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0299-66 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0215-01 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0215-10 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 MUTUAL PHARM CO EAGEN 61442-0321-01 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0321-05 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0321-10 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 CARLSBAD TECH EAGEN 62584-<strong>06</strong>57-11 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 AHP EAGEN 63739-0<strong>06</strong>0-10 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 MCKESSON PACKAG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67253-0263-10 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 DAVA PHARMACEUT EAGEN 67253-0263-11 0.03380 CLONIDINE HCL 0.1 MG TABLET 0 DAVA PHARMACEUT EAGEN 00228-2128-10 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00228-2128-50 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00378-0186-01 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 MYLAN EAGEN 00378-0186-10 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-2958-21 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2958-28 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2958-32 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 QUALITEST EAGEN 16714-0342-03 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0342-04 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 NORTHSTAR RX LL EAGEN 29300-0136-01 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 UNICHEM PHARMAC EAGEN 29300-0136-10 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 UNICHEM PHARMAC EAGEN 51079-0300-17 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0300-20 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 MYLAN INSTITUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 90LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0300-30 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0300-56 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0300-66 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0216-01 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0216-10 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 MUTUAL PHARM CO EAGEN 61442-0322-01 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0322-05 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0322-10 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 CARLSBAD TECH EAGEN 62584-0339-11 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 AHP EAGEN 67253-0264-10 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 DAVA PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67253-0264-11 0.04293 CLONIDINE HCL 0.2 MG TABLET 0 DAVA PHARMACEUT EAGEN 00228-2129-10 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00378-0199-01 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-2959-21 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-2959-28 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 QUALITEST EAGEN 16714-0343-03 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0343-04 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 NORTHSTAR RX LL EAGEN 29300-0137-01 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 UNICHEM PHARMAC EAGEN 51079-0301-20 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0301-63 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0301-66 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0217-01 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 MUTUAL PHARM CO EAGEN 61442-0323-01 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0323-05 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0323-10 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 CARLSBAD TECH EAGEN 62584-<strong>06</strong>59-11 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 AHP EAGEN 67253-0265-10 0.05430 CLONIDINE HCL 0.3 MG TABLET 0 DAVA PHARMACEUT EAGEN 00378-<strong>08</strong>71-99 24.84187 CLONIDINE 0.1 MG/DAY PATCH G MYLAN EAGEN 00555-1009-16 24.86812 CLONIDINE 0.1 MG/DAY PATCH G BARR EAGEN 00378-<strong>08</strong>72-99 41.82375 CLONIDINE 0.2 MG/DAY PATCH G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00555-1010-16 41.87<strong>06</strong>2 CLONIDINE 0.2 MG/DAY PATCH G BARR EAGEN 00378-<strong>08</strong>73-99 58.02000 CLONIDINE 0.3 MG/DAY PATCH G MYLAN EAGEN 00555-1011-16 58.<strong>08</strong>562 CLONIDINE 0.3 MG/DAY PATCH G BARR EAGEN 00143-9724-01 2.77330 CLONIDINE 1,000 MCG/10 ML VIAL 0 WEST-WARD,INC. MLGEN 00517-0730-01 2.77330 CLONIDINE 1,000 MCG/10 ML VIAL 0 AMER. REGENT MLGEN 00143-9723-01 11.66660 CLONIDINE 5,000 MCG/10 ML VIAL 0 WEST-WARD,INC. MLGEN 00517-0731-01 11.66660 CLONIDINE 5,000 MCG/10 ML VIAL 0 AMER. REGENT MLGEN 00093-7314-05 0.13017 CLOPIDOGREL 75 MG TABLET 0 TEVA USA EAGEN 00093-7314-56 0.13017 CLOPIDOGREL 75 MG TABLET 0 TEVA USA EAGEN 00093-7314-98 0.13017 CLOPIDOGREL 75 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3627-05 0.13017 CLOPIDOGREL 75 MG TABLET 0 MYLAN EAGEN 00378-3627-77 0.13017 CLOPIDOGREL 75 MG TABLET 0 MYLAN EAGEN 00378-3627-93 0.13017 CLOPIDOGREL 75 MG TABLET 0 MYLAN EAGEN 00904-6294-61 0.13017 CLOPIDOGREL 75 MG TABLET 0 MAJOR PHARMACEU EAGEN 13668-0141-05 0.13017 CLOPIDOGREL 75 MG TABLET 0 TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 91LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-0141-30 0.13017 CLOPIDOGREL 75 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0141-90 0.13017 CLOPIDOGREL 75 MG TABLET 0 TORRENT PHARMAC EAGEN 16729-0218-10 0.13017 CLOPIDOGREL 75 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0218-15 0.13017 CLOPIDOGREL 75 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0218-16 0.13017 CLOPIDOGREL 75 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 47335-<strong>08</strong>94-13 0.13017 CLOPIDOGREL 75 MG TABLET 0 SUN PHARMA GLOB EAGEN 47335-<strong>08</strong>94-81 0.13017 CLOPIDOGREL 75 MG TABLET 0 SUN PHARMA GLOB EAGEN 47335-<strong>08</strong>94-83 0.13017 CLOPIDOGREL 75 MG TABLET 0 SUN PHARMA GLOB EAGEN 51079-0557-01 0.13017 CLOPIDOGREL 75 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0557-20 0.13017 CLOPIDOGREL 75 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0196-05 0.13017 CLOPIDOGREL 75 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0196-30 0.13017 CLOPIDOGREL 75 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0196-90 0.13017 CLOPIDOGREL 75 MG TABLET 0 DR.REDDY'S LAB EAGEN 60505-0253-01 0.13017 CLOPIDOGREL 75 MG TABLET 0 APOTEX CORP EAGEN 60505-0253-02 0.13017 CLOPIDOGREL 75 MG TABLET 0 APOTEX CORP EAGEN 60505-0253-03 0.13017 CLOPIDOGREL 75 MG TABLET 0 APOTEX CORP EAGEN 60505-3992-03 0.13017 CLOPIDOGREL 75 MG TABLET 0 APOTEX CORP EAGEN 64679-0314-00 0.13017 CLOPIDOGREL 75 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0314-01 0.13017 CLOPIDOGREL 75 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0314-02 0.13017 CLOPIDOGREL 75 MG TABLET 0 WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0314-03 0.13017 CLOPIDOGREL 75 MG TABLET 0 WOCKHARDT USA L EAGEN 65862-0357-05 0.13017 CLOPIDOGREL 75 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0357-30 0.13017 CLOPIDOGREL 75 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0357-90 0.13017 CLOPIDOGREL 75 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0357-99 0.13017 CLOPIDOGREL 75 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-<strong>06</strong>09-01 0.13017 CLOPIDOGREL 75 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>09-11 0.13017 CLOPIDOGREL 75 MG TABLET 0 AHP EAGEN 68645-0443-70 0.13017 CLOPIDOGREL 75 MG TABLET 0 LEGACY PHARMACE EABND 00378-0072-01 3.36399 CLORPRES 0.3-15 TABLET 0 MYLAN EAGEN 00168-0133-15 0.74400 CLOTRIMAZOLE 1% CREAM G SANDOZ GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00168-0133-30 0.74400 CLOTRIMAZOLE 1% CREAM G SANDOZ GMGEN 00168-0133-46 0.74400 CLOTRIMAZOLE 1% CREAM G SANDOZ GMGEN 51672-1275-01 0.74400 CLOTRIMAZOLE 1% CREAM G TARO PHARM USA GMGEN 51672-1275-02 0.74400 CLOTRIMAZOLE 1% CREAM G TARO PHARM USA GMGEN 51672-1275-<strong>06</strong> 0.74400 CLOTRIMAZOLE 1% CREAM G TARO PHARM USA GMGEN 51672-1275-07 0.54000 CLOTRIMAZOLE 1% CREAM G TARO PHARM USA GMGEN 68462-0181-17 0.74400 CLOTRIMAZOLE 1% CREAM G GLENMARK PHARMA GMGEN 00093-0248-31 0.38799 CLOTRIMAZOLE 1% SOLUTION G TEVA USA MLGUL 00093-0248-43 0.47250 CLOTRIMAZOLE 1% SOLUTION G TEVA USA MLGEN 51672-1260-03 0.38799 CLOTRIMAZOLE 1% SOLUTION G TARO PHARM USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-4146-22 0.38450 CLOTRIMAZOLE 10 MG TROCHE 0 ROXANE LABS. EAGEN 00054-4146-23 0.38450 CLOTRIMAZOLE 10 MG TROCHE 0 ROXANE LABS. EAGEN 00054-8146-22 0.38450 CLOTRIMAZOLE 10 MG TROCHE 0 ROXANE LABS. EAGEN 00574-0107-14 0.38450 CLOTRIMAZOLE 10 MG TROCHE 0 PADDOCK LABS. EAGEN 00574-0107-70 0.38450 CLOTRIMAZOLE 10 MG TROCHE 0 PADDOCK LABS. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 92LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00168-0258-15 0.82300 CLOTRIMAZOLE-BETAMETHASONE CRM G SANDOZ GMGUL 00168-0258-46 0.82300 CLOTRIMAZOLE-BETAMETHASONE CRM G SANDOZ GMGUL 00472-0379-15 0.82300 CLOTRIMAZOLE-BETAMETHASONE CRM G ACTAVIS PHARMA, GMGUL 00472-0379-45 0.82300 CLOTRIMAZOLE-BETAMETHASONE CRM G ACTAVIS PHARMA, GMGUL 51672-4048-01 0.82300 CLOTRIMAZOLE-BETAMETHASONE CRM G TARO PHARM USA GMGUL 51672-4048-<strong>06</strong> 0.82300 CLOTRIMAZOLE-BETAMETHASONE CRM G TARO PHARM USA GMGUL 66993-<strong>08</strong>98-15 0.82300 CLOTRIMAZOLE-BETAMETHASONE CRM G PRASCO LABS GMGUL 66993-<strong>08</strong>98-45 0.82300 CLOTRIMAZOLE-BETAMETHASONE CRM G PRASCO LABS GMGUL 00168-0370-30 1.81150 CLOTRIMAZOLE-BETAMETHASONE LOT G SANDOZ MLGUL 51672-13<strong>08</strong>-03 1.81150 CLOTRIMAZOLE-BETAMETHASONE LOT G TARO PHARM USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-3010-01 6.13095 CLOZAPINE ODT 100 MG TABLET G TEVA USA EAGEX 00093-3010-84 6.13125 CLOZAPINE ODT 100 MG TABLET G TEVA USA EAGEX 00093-3011-01 1.67<strong>06</strong>2 CLOZAPINE ODT 12.5 MG TABLET G TEVA USA EAGEX 00093-3012-01 2.24775 CLOZAPINE ODT 25 MG TABLET G TEVA USA EAGEX 00093-3012-84 2.24828 CLOZAPINE ODT 25 MG TABLET G TEVA USA EAGEX 00093-7772-01 1.09400 CLOZAPINE 100 MG TABLET G TEVA USA EAGEX 00093-7772-05 1.09400 CLOZAPINE 100 MG TABLET G TEVA USA EAGEX 00093-7772-19 1.09400 CLOZAPINE 100 MG TABLET G TEVA USA EAGEX 00093-7772-93 1.09400 CLOZAPINE 100 MG TABLET G TEVA USA EAGEX 00378-<strong>08</strong>60-01 1.09400 CLOZAPINE 100 MG TABLET G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-<strong>08</strong>60-05 1.09400 CLOZAPINE 100 MG TABLET G MYLAN EAGEX 00093-4405-01 3.51140 CLOZAPINE 200 MG TABLET G TEVA USA EAGEX 00093-4405-05 3.51140 CLOZAPINE 200 MG TABLET G TEVA USA EAGEX 00378-0973-01 3.51140 CLOZAPINE 200 MG TABLET G MYLAN EAGEX 51079-0749-01 3.51140 CLOZAPINE 200 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0749-20 3.51140 CLOZAPINE 200 MG TABLET G MYLAN INSTITUTI EAGEX 00093-4359-01 0.47880 CLOZAPINE 25 MG TABLET G TEVA USA EAGEX 00093-4359-05 0.47880 CLOZAPINE 25 MG TABLET G TEVA USA EAGEX 00378-<strong>08</strong>25-01 0.47880 CLOZAPINE 25 MG TABLET G MYLAN EAGEX 00093-4404-01 1.23750 CLOZAPINE 50 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-4404-05 1.23750 CLOZAPINE 50 MG TABLET G TEVA USA EAGEX 00378-0972-01 1.23750 CLOZAPINE 50 MG TABLET G MYLAN EABEX 00078-0127-05 1.09400 10.32719 CLOZARIL 100 MG TABLET G NOVARTIS EABEX 00078-0126-05 0.47880 3.98566 CLOZARIL 25 MG TABLET G NOVARTIS EABND 00078-0568-45 3.83391 COARTEM TABLETS 0 NOVARTIS EABND 65649-0101-02 0.24476 1.41594 COLAZAL 750 MG CAPSULE G SALIX PHARMACEU EABND 13310-0119-01 4.83<strong>06</strong>0 COLCRYS 0.6 MG TABLET 0 AR SCIENTIFIC EABND 13310-0119-07 4.83<strong>06</strong>0 COLCRYS 0.6 MG TABLET 0 AR SCIENTIFIC EABND 64764-0119-01 5.12433 COLCRYS 0.6 MG TABLET 0 TAKEDA PHARMACE EABND 64764-0119-07 5.12442 COLCRYS 0.6 MG TABLET 0 TAKEDA PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-0370-03 3.74523 COLESTID FLAVORED GRANULES G PHARMACIA/UPJHN EABND 00009-0370-05 0.18360 0.34946 COLESTID FLAVORED GRANULES G PHARMACIA/UPJHN GMBND 00009-0260-02 0.18360 0.4<strong>06</strong>53 COLESTID GRANULES G PHARMACIA/UPJHN GMBND 00009-0260-17 0.18360 0.4<strong>06</strong>53 COLESTID GRANULES G PHARMACIA/UPJHN GMBND 00009-0260-01 2.00300 3.414<strong>06</strong> COLESTID GRANULES PACKET G PHARMACIA/UPJHN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 93LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-0260-04 2.00300 3.41342 COLESTID GRANULES PACKET G PHARMACIA/UPJHN EABND 00009-0450-03 0.54220 1.07138 COLESTID 1 GM TABLET 0 PHARMACIA/UPJHN EAGEN 00115-5213-02 0.18360 COLESTIPOL HCL GRANULES G GLOBAL PHARM GMGEN 00115-5212-18 1.87775 COLESTIPOL HCL GRANULES PACKET G GLOBAL PHARM EAGEN 00115-5212-29 1.87775 COLESTIPOL HCL GRANULES PACKET G GLOBAL PHARM EAGEN 00115-5211-16 0.49337 COLESTIPOL HCL 1 GM TABLET 0 GLOBAL PHARM EAGEN 59762-0450-01 0.54220 COLESTIPOL MICRONIZED 1 GM TAB 0 GREENSTONE LLC. EAGEN 00574-<strong>08</strong>58-01 12.4<strong>06</strong>10 COLISTIMETHATE 150 MG VIAL 0 PADDOCK LABS. EAGEN 23155-0193-31 12.4<strong>06</strong>10 COLISTIMETHATE 150 MG VIAL 0 HERITAGE PHARMA EAGEN 39822-<strong>06</strong>15-01 12.4<strong>06</strong>10 COLISTIMETHATE 150 MG VIAL 0 X-GEN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42023-0131-01 12.4<strong>06</strong>10 COLISTIMETHATE 150 MG VIAL 0 JHP PHARMACEUTI EAGEN 42023-0131-<strong>06</strong> 12.4<strong>06</strong>10 COLISTIMETHATE 150 MG VIAL 0 JHP PHARMACEUTI EAGEN 63323-0393-<strong>06</strong> 12.4<strong>06</strong>10 COLISTIMETHATE 150 MG VIAL 0 APP PHARMACEUTI EAGEN 00574-2020-01 0.<strong>08</strong>681 COLOCORT 100 MG ENEMA 0 PADDOCK LABS. MLGEN 00574-2020-07 0.<strong>08</strong>681 COLOCORT 100 MG ENEMA 0 PADDOCK LABS. MLBND 42023-01<strong>08</strong>-01 7.533<strong>08</strong> COLY-MYCIN S EAR DROPS 0 JHP PHARMACEUTI MLBND 00091-7036-23 0.00260 0.00727 COLYTE WITH FLAVOR PACKETS G MEDA PHARMACEUT MLBND 68220-0130-04 0.00260 0.01019 COLYTE WITH FLAVOR PACKETS G MEDA PHARMACEUT MLBND 68220-0133-01 0.01055 COLYTE WITH FLAVOR PACKS G MEDA PHARMACEUT MLBND 00023-9211-05 19.983<strong>08</strong> COMBIGAN EYE DROPS 0 ALLERGAN INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00023-9211-10 19.98557 COMBIGAN EYE DROPS 0 ALLERGAN INC. MLBND 00078-0377-42 10.02225 COMBIPATCH 0.05-0.14 MG PTCH 0 NOVARTIS EABND 00078-0377-62 10.00980 COMBIPATCH 0.05-0.14 MG PTCH 0 NOVARTIS EABND 00078-0378-42 10.00980 COMBIPATCH 0.05-0.25 MG PTCH 0 NOVARTIS EABND 00078-0378-62 10.00150 COMBIPATCH 0.05-0.25 MG PTCH 0 NOVARTIS EABND 00597-0024-02 65.53265 COMBIVENT RESPIMAT INHAL SPRAY 0 BOEHRINGER ING. GMBND 00173-0595-00 13.73622 13.73622 COMBIVIR TABLET G GLAXOSMITHKLINE EABND 49702-0202-18 14.96351 14.96351 COMBIVIR TABLET G VIIV <strong>HEALTH</strong>CARE EABND 49702-0202-29 14.96358 14.96358 COMBIVIR TABLET G VIIV <strong>HEALTH</strong>CARE EABND 42388-0012-14 184.88250 COMETRIQ 100 MG DAILY-DOSE PK 0 EXELIXIS, INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 42388-0011-14 92.44125 COMETRIQ 140 MG DAILY-DOSE PK 0 EXELIXIS, INC. EABND 42388-0013-14 123.25500 COMETRIQ 60 MG DAILY-DOSE PACK 0 EXELIXIS, INC. EAGEN 66213-0200-12 14.81250 COMPAZINE 25 MG SUPPOSITORY 0 PBM PHARMA. EABND 61958-1101-01 68.15323 COMPLERA TABLET G GILEAD SCIENCES EAGEN 00574-7226-12 9.43687 COMPRO 25 MG SUPPOSITORY 0 PADDOCK LABS. EABND 00078-0327-05 3.10050 4.60591 COMTAN 200 MG TABLET 0 NOVARTIS EABND 52747-<strong>06</strong>21-30 0.61520 0.94122 CONCEPT DHA CAPSULE 0 US PHARMACEUTIC EABND 52747-<strong>06</strong>20-30 0.56960 0.87150 CONCEPT OB CAPSULE 0 US PHARMACEUTIC EABND 52544-0045-13 111.265<strong>06</strong> CONDYLOX 0.5% GEL 0 ACTAVIS PHARMA, GMBND 52544-0046-13 24.72000 51.83231 CONDYLOX 0.5% TOPICAL SOLN G ACTAVIS PHARMA, ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45963-0439-63 0.01400 CONSTULOSE 10 GM/15 ML SOLN 0 ACTAVIS PHARMA, MLGEN 45963-0439-65 0.01400 CONSTULOSE 10 GM/15 ML SOLN 0 ACTAVIS PHARMA, MLBND 68025-0053-30 6.42392 CONZIP 100 MG CAPSULE G VERTICAL PHARM EABND 68025-0055-30 8.01752 CONZIP 200 MG CAPSULE G VERTICAL PHARM EABND 68546-0317-30 5040.09200 COPAXONE 20 MG INJECTION KIT 0 TEVA NEUROSCIEN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 94LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 68546-0325-12 385.223<strong>06</strong> COPAXONE 40 MG/ML SYRINGE 0 TEVA NEUROSCIEN MLBND 00004-0<strong>08</strong>6-94 0.65250 17.79984 COPEGUS 200 MG TABLET G GENENTECH, INC. EABND 00409-4092-01 1.56870 COPPER CHLORIDE 4 MG/10 ML VL 0 HOSPIRA MLBND 000<strong>08</strong>-4188-04 0.15390 4.<strong>08</strong>664 CORDARONE 200 MG TABLET G WYETH PHARM EABND 52544-0044-24 177.72790 CORDRAN 4 MCG/SQ CM TAPE G ACTAVIS PHARMA, EABND 52544-0044-80 381.36010 CORDRAN 4 MCG/SQ CM TAPE G ACTAVIS PHARMA, EABND 00007-3370-13 5.33662 COREG CR 10 MG CAPSULE G GLAXOSMITHKLINE EABND 00007-3371-13 5.33662 COREG CR 20 MG CAPSULE G GLAXOSMITHKLINE EABND 00007-3372-13 5.33662 COREG CR 40 MG CAPSULE G GLAXOSMITHKLINE EABND 00007-3373-13 5.33662 COREG CR 80 MG CAPSULE G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00007-4141-20 0.03848 2.65732 COREG 12.5 MG TABLET G GLAXOSMITHKLINE EABND 00007-4142-20 0.03915 2.65732 COREG 25 MG TABLET G GLAXOSMITHKLINE EABND 00007-4139-20 0.03848 2.65732 COREG 3.125 MG TABLET G GLAXOSMITHKLINE EABND 00007-4140-20 0.05700 2.65732 COREG 6.25 MG TABLET G GLAXOSMITHKLINE EABUL 60793-<strong>08</strong>00-01 0.46500 3.34689 CORGARD 20 MG TABLET G PFIZER US PHARM EABUL 60793-<strong>08</strong>01-01 0.42890 3.91577 CORGARD 40 MG TABLET G PFIZER US PHARM EABUL 60793-<strong>08</strong>02-01 0.80250 5.37972 CORGARD 80 MG TABLET G PFIZER US PHARM EAGEN 00095-0049-50 0.22470 CORMAX 0.05% SOLUTION G ECR PHARM. MLBND 00009-0031-01 0.45836 0.78277 CORTEF 10 MG TABLET G PHARMACIA/UPJHN EABND 00009-0044-01 0.64071 1.48370 CORTEF 20 MG TABLET G PHARMACIA/UPJHN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-0012-01 0.46347 CORTEF 5 MG TABLET G PHARMACIA/UPJHN EABND 62559-1110-07 0.<strong>08</strong>681 0.16578 CORTENEMA 100 MG ENEMA G ANI PHARMACEUTI MLBND 62559-0111-11 0.<strong>08</strong>681 0.17181 CORTENEMA 100 MG/60 ML ENEMA G ANI PHARMACEUTI MLBND 62559-1110-01 0.<strong>08</strong>681 0.17181 CORTENEMA 100 MG/60 ML ENEMA G ANI PHARMACEUTI MLBND 68220-0140-15 18.83546 CORTIFOAM 10% AEROSOL 0 MEDA PHARMACEUT GMBND 00143-1202-01 2.59375 CORTISONE 25 MG TABLET 0 WEST-WARD,INC. EABND 61570-0032-75 7.87614 CORTISPORIN CREAM 0 MONARCH PHRM GMBND 61570-0034-10 1.82437 9.04036 CORTISPORIN EAR SOLUTION G MONARCH PHRM MLBND 61570-0031-50 5.38559 CORTISPORIN OINTMENT 0 MONARCH PHRM GMBND 42023-0109-01 6.88734 CORTISPORIN-TC EAR SUSP 0 JHP PHARMACEUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 60793-0283-01 1.82730 4.04575 CORZIDE 40-5 TABLET G PFIZER US PHARM EABND 60793-0284-01 1.96820 5.338<strong>06</strong> CORZIDE 80-5 TABLET G PFIZER US PHARM EABND 000<strong>06</strong>-3628-36 1.83465 14.01787 COSOPT EYE DROPS 0 MERCK SHARP & D MLBND 000<strong>06</strong>-3629-60 1.42096 COSOPT PF EYE DROPS 0 MERCK SHARP & D EABND 000<strong>06</strong>-3629-62 1.42096 COSOPT PF EYE DROPS 0 MERCK SHARP & D EABND 00781-3052-71 99.52530 COSYNTROPIN 0.25 MG/ML 0 SANDOZ MLBND 00781-3052-95 99.52281 COSYNTROPIN 0.25 MG/ML 0 SANDOZ MLBND 00056-0169-70 0.<strong>06</strong>520 1.45250 COUMADIN 1 MG TABLET 0 BMS EABND 00056-0169-90 0.<strong>06</strong>520 1.45281 COUMADIN 1 MG TABLET 0 BMS EABND 00056-0174-70 0.07180 2.25311 COUMADIN 10 MG TABLET 0 BMS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00056-0170-70 0.<strong>06</strong>520 1.51541 COUMADIN 2 MG TABLET 0 BMS EABND 00056-0170-90 0.<strong>06</strong>520 1.51601 COUMADIN 2 MG TABLET 0 BMS EABND 00056-0176-70 0.<strong>06</strong>520 1.56363 COUMADIN 2.5 MG TABLET 0 BMS EABND 00056-0176-90 0.<strong>06</strong>520 1.56258 COUMADIN 2.5 MG TABLET 0 BMS EABND 00056-0188-70 0.<strong>06</strong>520 1.56969 COUMADIN 3 MG TABLET 0 BMS EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 95LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00056-0168-70 0.<strong>06</strong>520 1.57384 COUMADIN 4 MG TABLET 0 BMS EABND 00056-0168-90 0.<strong>06</strong>520 1.30250 COUMADIN 4 MG TABLET 0 BMS EABND 00056-0172-70 0.<strong>06</strong>520 1.62962 COUMADIN 5 MG TABLET 0 BMS EABND 00056-0172-90 0.<strong>06</strong>520 1.62991 COUMADIN 5 MG TABLET 0 BMS EABND 00590-0324-35 38.92561 COUMADIN 5 MG VIAL 0 BMS EABND 00590-0324-96 38.92700 COUMADIN 5 MG VIAL 0 BMS EABND 00056-0189-70 0.07180 2.09956 COUMADIN 6 MG TABLET 0 BMS EABND 00056-0189-90 0.07180 1.98487 COUMADIN 6 MG TABLET 0 BMS EABND 00056-0173-70 0.07200 2.17227 COUMADIN 7.5 MG TABLET 0 BMS EABND 000<strong>06</strong>-0960-31 0.33000 3.88772 COZAAR 100 MG TABLET G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-0960-54 0.33000 3.88762 COZAAR 100 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0960-82 0.33000 3.88776 COZAAR 100 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0951-54 0.19000 2.12258 COZAAR 25 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0951-82 0.19000 2.12246 COZAAR 25 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0951-87 0.19000 2.12247 COZAAR 25 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0952-31 0.24000 2.85381 COZAAR 50 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0952-54 0.24000 2.85409 COZAAR 50 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0952-82 0.24000 2.85417 COZAAR 50 MG TABLET G MERCK SHARP & D EABND 00032-1212-01 2.12031 CREON DR 12,000 UNITS CAPSULE 0 ABBVIE US LLC EABND 00032-1212-07 2.09853 CREON DR 12,000 UNITS CAPSULE 0 ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00032-1224-01 4.16411 CREON DR 24,000 UNITS CAPSULE 0 ABBVIE US LLC EABND 00032-1224-07 4.07729 CREON DR 24,000 UNITS CAPSULE 0 ABBVIE US LLC EABND 00032-1203-70 0.98011 CREON DR 3,000 UNITS CAPSULE 0 ABBVIE US LLC EABND 00032-3016-13 6.55849 CREON DR 36,000 UNITS CAPSULE 0 ABBVIE US LLC EABND 00032-3016-28 6.42177 CREON DR 36,000 UNITS CAPSULE 0 ABBVIE US LLC EABND 00032-12<strong>06</strong>-01 1.13610 CREON DR 6,000 UNITS CAPSULE 0 ABBVIE US LLC EABND 00032-12<strong>06</strong>-07 1.12365 CREON DR 6,000 UNITS CAPSULE 0 ABBVIE US LLC EABND 00310-0751-90 6.02958 CRESTOR 10 MG TABLET G ASTRAZENECA EABND 00310-0752-90 6.02958 CRESTOR 20 MG TABLET G ASTRAZENECA EABND 00310-0754-30 6.02967 CRESTOR 40 MG TABLET G ASTRAZENECA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00310-0755-90 6.02958 CRESTOR 5 MG TABLET G ASTRAZENECA EABND 000<strong>06</strong>-0571-43 1.26369 CRIXIVAN 200 MG CAPSULE G MERCK SHARP & D EABND 000<strong>06</strong>-0573-54 2.52744 CRIXIVAN 400 MG CAPSULE G MERCK SHARP & D EABND 000<strong>06</strong>-0573-62 2.52744 CRIXIVAN 400 MG CAPSULE G MERCK SHARP & D EAGEN 16571-<strong>06</strong>00-96 0.94218 CROMOLYN SODIUM 100 MG/5 ML 0 PACK PHARMACEUT MLGEN 66993-0470-96 0.94178 CROMOLYN SODIUM 100 MG/5 ML 0 PRASCO LABS MLBND 00172-64<strong>06</strong>-49 0.79714 CROMOLYN 20 MG/2 ML NEB SOLN 0 IVAX PHARMACEUT MLBND 00172-64<strong>06</strong>-59 0.74340 CROMOLYN 20 MG/2 ML NEB SOLN 0 IVAX PHARMACEUT MLGEN 00093-1389-43 0.70470 CROMOLYN 4% EYE DROPS 0 TEVA USA MLGEN 17478-0291-11 0.70470 CROMOLYN 4% EYE DROPS 0 AKORN INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61314-0237-10 0.70470 CROMOLYN 4% EYE DROPS 0 SANDOZ MLGEX 00555-9049-58 0.80097 CRYSELLE-28 TABLET 0 BARR EABND 67919-0011-01 334.88840 CUBICIN 500 MG VIAL 0 CUBIST PHARMACE EABND 25010-0705-15 23.55025 CUPRIMINE 250 MG CAPSULE 0 VALEANT EABND 10337-0332-30 0.62890 3.75630 CUTIVATE 0.05% CREAM G SANDOZ GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 96LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 10337-0332-60 0.62890 3.75519 CUTIVATE 0.05% CREAM G SANDOZ GMBND 10337-0434-04 5.07624 6.76277 CUTIVATE 0.05% LOTION G SANDOZ MLBND 00259-0501-16 0.81768 CUVPOSA 1 MG/5 ML SOLUTION 0 MERZ MLBND 59630-02<strong>06</strong>-16 0.81768 CUVPOSA 1 MG/5 ML SOLUTION 0 MERZ MLGEN 00517-0031-25 2.28750 CYANOCOBALAMIN 1,000 MCG/ML 0 AMER. REGENT MLGEN 00517-0032-25 0.37500 CYANOCOBALAMIN 1,000 MCG/ML 0 AMER. REGENT MLGEN 00517-0130-05 0.31250 CYANOCOBALAMIN 1,000 MCG/ML 0 AMER. REGENT MLGEN 63323-0044-01 2.81700 CYANOCOBALAMIN 1,000 MCG/ML 0 APP PHARMACEUTI MLGEX 0<strong>06</strong>03-7521-17 0.73890 CYCLAFEM 1-35-28 TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-7521-49 0.73890 CYCLAFEM 1-35-28 TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-7525-17 0.83640 CYCLAFEM 7-7-7-28 TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-7525-49 0.83640 CYCLAFEM 7-7-7-28 TABLET 0 QUALITEST EABEX 00052-0283-<strong>06</strong> 0.84780 1.93676 CYCLESSA 28 DAY TABLET G ORGANON PHARM. EAGEN 51927-2501-00 36.57750 CYCLOBENZAPRINE HCL POWDER 0 PR<strong>OF</strong>ESSIONAL CO GMGEN 00378-0751-01 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 MYLAN EAGEN 00378-0751-10 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 MYLAN EAGEN 00378-0751-93 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 MYLAN EAGEN 00591-5658-01 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5658-05 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5658-10 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-3079-02 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3079-03 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3079-04 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3079-21 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3079-28 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3079-32 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3079-34 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 QUALITEST EAGEN 10702-0007-01 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 KVK-TECH, INC. EAGEN 10702-0007-05 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 KVK-TECH, INC. EAGEN 10702-0007-10 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 KVK-TECH, INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10702-0007-50 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 KVK-TECH, INC. EAGEN 31722-0283-01 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0283-10 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 CAMBER PHARMACE EAGEN 50111-0563-01 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 PLIVA, INC EAGEN 50111-0563-02 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 PLIVA, INC EAGEN 50111-0563-03 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 PLIVA, INC EAGEN 51079-<strong>06</strong>44-17 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-<strong>06</strong>44-20 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 51991-0468-01 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 BRECKENRIDGE EAGEN 51991-0468-10 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 BRECKENRIDGE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 58517-0<strong>08</strong>0-30 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 <strong>NEW</strong> HORIZON RX EAGEN 59746-0177-<strong>06</strong> 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 CADISTA PHARMAC EAGEN 59746-0177-10 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 CADISTA PHARMAC EAGEN 63739-0531-10 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 MCKESSON PACKAG EAGEN 65162-0541-10 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 AMNEAL PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 97LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-0541-11 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0541-50 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 AMNEAL PHARMACE EAGEN 65862-0191-01 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0191-05 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0191-99 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0397-01 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0397-11 0.03038 CYCLOBENZAPRINE 10 MG TABLET 0 AHP EAGEN 00378-0771-01 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 MYLAN EAGEN 00378-0771-05 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 MYLAN EAGEN 00378-0771-93 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3256-01 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-3078-21 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3078-28 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 QUALITEST EAGEN 10702-00<strong>06</strong>-01 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 KVK-TECH, INC. EAGEN 10702-00<strong>06</strong>-03 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 KVK-TECH, INC. EAGEN 10702-00<strong>06</strong>-10 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 KVK-TECH, INC. EAGEN 10702-00<strong>06</strong>-50 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 KVK-TECH, INC. EAGEN 31722-0282-01 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 CAMBER PHARMACE EAGEN 51991-0467-01 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 BRECKENRIDGE EAGEN 59746-0211-<strong>06</strong> 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 CADISTA PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59746-0211-10 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 CADISTA PHARMAC EAGEN 65862-0190-01 0.05090 CYCLOBENZAPRINE 5 MG TABLET 0 AUROBINDO PHARM EABND 00<strong>06</strong>5-0395-15 4.04044 CYCLOGYL 0.5% EYE DROPS 0 ALCON LABS. MLGEN 00<strong>06</strong>5-0396-02 1.66455 CYCLOGYL 1% EYE DROPS 0 ALCON LABS. MLGEN 00<strong>06</strong>5-0396-05 1.66455 CYCLOGYL 1% EYE DROPS 0 ALCON LABS. MLGEN 00<strong>06</strong>5-0396-15 1.66455 CYCLOGYL 1% EYE DROPS 0 ALCON LABS. MLGEN 00<strong>06</strong>5-0397-02 12.55500 CYCLOGYL 2% EYE DROPS 0 ALCON LABS. MLGEN 00<strong>06</strong>5-0397-05 8.28900 CYCLOGYL 2% EYE DROPS 0 ALCON LABS. MLGEN 00<strong>06</strong>5-0397-15 4.59000 CYCLOGYL 2% EYE DROPS 0 ALCON LABS. MLBND 00<strong>06</strong>5-0359-02 11.<strong>08</strong>050 CYCLOMYDRIL EYE DROPS 0 ALCON LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>5-0359-05 7.77876 CYCLOMYDRIL EYE DROPS 0 ALCON LABS. MLGEN 17478-0097-02 11.<strong>06</strong>625 CYCLOPENTOLATE HCL 2% DROPS 0 AKORN INC. MLGEN 17478-0097-12 4.05249 CYCLOPENTOLATE HCL 2% DROPS 0 AKORN INC. MLGEN 17478-0100-02 1.66455 CYCLOPENTOLATE 1% EYE DROPS 0 AKORN INC. MLGEN 17478-0100-12 1.66455 CYCLOPENTOLATE 1% EYE DROPS 0 AKORN INC. MLGEN 242<strong>08</strong>-0735-01 1.66455 CYCLOPENTOLATE 1% EYE DROPS 0 VALEANT MLGEN 242<strong>08</strong>-0735-<strong>06</strong> 1.66455 CYCLOPENTOLATE 1% EYE DROPS 0 VALEANT MLGEN 61314-0396-01 1.66455 CYCLOPENTOLATE 1% EYE DROPS 0 SANDOZ MLGEN 61314-0396-03 1.66455 CYCLOPENTOLATE 1% EYE DROPS 0 SANDOZ MLBND 10019-0956-01 729.57000 CYCLOPHOSPHAMIDE 1 GM VIAL 0 BAXTER <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 10019-0957-01 1459.14000 CYCLOPHOSPHAMIDE 2 GM VIAL 0 BAXTER <strong>HEALTH</strong>CA EABND 10019-0957-11 1459.14000 CYCLOPHOSPHAMIDE 2 GM VIAL 0 BAXTER <strong>HEALTH</strong>CA EABND 00054-4129-25 2.31246 CYCLOPHOSPHAMIDE 25 MG TAB 0 ROXANE LABS. EABND 00054-4130-25 4.24379 CYCLOPHOSPHAMIDE 50 MG TABLET 0 ROXANE LABS. EABND 10019-0955-01 364.78500 CYCLOPHOSPHAMIDE 500 MG VIAL 0 BAXTER <strong>HEALTH</strong>CA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 98LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 13845-1202-02 11.95200 CYCLOSERINE 250 MG CAPSULE 0 THE CHAO CENTER EAGEN 00172-7312-46 4.12224 CYCLOSPORINE MODIFIED 100 MG 0 IVAX PHARMACEUT EAGEN 00185-0933-30 4.12224 CYCLOSPORINE MODIFIED 100 MG 0 SANDOZ EAGEN 00591-2223-15 4.12200 CYCLOSPORINE MODIFIED 100 MG 0 ACTAVIS PHARMA, EAGEN 00172-7310-46 1.03125 CYCLOSPORINE MODIFIED 25 MG 0 IVAX PHARMACEUT EAGEN 00185-0932-30 1.03125 CYCLOSPORINE MODIFIED 25 MG 0 SANDOZ EAGEN 00591-2222-15 1.03149 CYCLOSPORINE MODIFIED 25 MG 0 ACTAVIS PHARMA, EABND 00172-7311-00 2.27281 CYCLOSPORINE MODIFIED 50 MG 0 TEVA USA EABND 00172-7311-46 2.27281 CYCLOSPORINE MODIFIED 50 MG 0 TEVA USA EAGEN 60505-0134-00 5.67500 CYCLOSPORINE 100 MG CAPSULE 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00172-7313-20 4.38400 CYCLOSPORINE 100 MG/ML SOLN 0 IVAX PHARMACEUT MLGEN 00591-2224-55 4.38400 CYCLOSPORINE 100 MG/ML SOLN 0 ACTAVIS PHARMA, MLGEN 60505-0133-00 1.83000 CYCLOSPORINE 25 MG CAPSULE 0 APOTEX CORP EAGEN 00574-<strong>08</strong>66-10 4.<strong>08</strong>750 CYCLOSPORINE 50 MG/ML AMPUL 0 PADDOCK LABS. MLBND 00013-1114-10 8.67623 CYKLOKAPRON 100 MG/ML AMPUL 0 PHARMACIA/UPJHN MLBEX 00002-3235-60 5.<strong>08</strong>020 6.454<strong>08</strong> CYMBALTA 20 MG CAPSULE G ELI LILLY & CO. EABEX 00002-3240-01 6.21790 7.23760 CYMBALTA 30 MG CAPSULE G ELI LILLY & CO. EABEX 00002-3240-30 7.24092 CYMBALTA 30 MG CAPSULE G ELI LILLY & CO. EABEX 00002-3240-90 6.21790 7.24092 CYMBALTA 30 MG CAPSULE G ELI LILLY & CO. EABEX 00002-3270-01 6.21790 7.23760 CYMBALTA 60 MG CAPSULE G ELI LILLY & CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00002-3270-04 6.21790 7.24092 CYMBALTA 60 MG CAPSULE G ELI LILLY & CO. EABEX 00002-3270-30 6.21790 7.24092 CYMBALTA 60 MG CAPSULE G ELI LILLY & CO. EABEX 00002-3270-33 6.21790 7.24092 CYMBALTA 60 MG CAPSULE G ELI LILLY & CO. EAGEN 00472-1400-16 0.09790 CYPROHEPTADINE 2 MG/5 ML SYRUP 0 ACTAVIS PHARMA, MLGEN 64980-0504-48 0.09790 CYPROHEPTADINE 2 MG/5 ML SYRUP 0 RISING PHARM MLGEN 00093-2929-01 0.26300 CYPROHEPTADINE 4 MG TABLET 0 TEVA USA EAGEN 00093-2929-10 0.26300 CYPROHEPTADINE 4 MG TABLET 0 TEVA USA EAGEN 51991-<strong>08</strong>38-01 0.26300 CYPROHEPTADINE 4 MG TABLET 0 BRECKENRIDGE EAGEN 51991-<strong>08</strong>38-10 0.26300 CYPROHEPTADINE 4 MG TABLET 0 BRECKENRIDGE EAGEN 60258-<strong>08</strong>50-01 0.26300 CYPROHEPTADINE 4 MG TABLET 0 BRECKENRIDGE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60258-<strong>08</strong>50-10 0.26300 CYPROHEPTADINE 4 MG TABLET 0 BRECKENRIDGE EABND 66621-4000-01 5.20520 CYSTADANE POWDER 0 RARE DISEASE GMBND 00378-9045-01 1.<strong>06</strong>198 CYSTAGON 150 MG CAPSULE 0 MYLAN EABND 00378-9045-05 0.92486 CYSTAGON 150 MG CAPSULE 0 MYLAN EABND 00378-9040-01 0.36478 CYSTAGON 50 MG CAPSULE 0 MYLAN EABND 00378-9040-05 0.31689 CYSTAGON 50 MG CAPSULE 0 MYLAN EABND 55390-0133-01 23.15700 CYTARABINE 1 GM VIAL 0 BEDFORD LABS EABND 55390-<strong>08</strong><strong>08</strong>-01 23.15700 CYTARABINE 1 GM VIAL 0 BEDFORD/NOVAPLU EABND 55390-0131-10 3.98400 CYTARABINE 100 MG VIAL 0 BEDFORD LABS EAGEN 61703-0319-22 0.82725 CYTARABINE 100 MG/ML VIAL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0120-20 0.83600 CYTARABINE 100 MG/ML VIAL 0 APP PHARMACEUTI MLBND 61703-0303-46 0.32000 0.42462 CYTARABINE 20 MG/ML VIAL 0 HOSPIRA MLBND 61703-0304-36 0.25360 0.46944 CYTARABINE 20 MG/ML VIAL 0 HOSPIRA MLBND 61703-0305-38 0.38000 1.11352 CYTARABINE 20 MG/ML VIAL 0 HOSPIRA MLBND 60793-0116-01 0.70144 1.28035 CYTOMEL 25 MCG TABLET 0 PFIZER US PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 99LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 60793-0115-01 0.49923 0.97458 CYTOMEL 5 MCG TABLET 0 PFIZER US PHARM EABND 60793-0117-01 1.05489 1.95581 CYTOMEL 50 MCG TABLET 0 PFIZER US PHARM EABND 00025-1451-20 0.58428 1.924<strong>08</strong> CYTOTEC 100 MCG TABLET G PHARMACIA/UPJHN EABND 00025-1451-60 0.58428 1.92379 CYTOTEC 100 MCG TABLET G PHARMACIA/UPJHN EABND 00025-1461-31 2.80241 CYTOTEC 200 MCG TABLET G PHARMACIA/UPJHN EABND 00025-1461-60 2.80276 CYTOTEC 200 MCG TABLET G PHARMACIA/UPJHN EABND 00004-6940-03 94.97324 CYTOVENE 500 MG VIAL 0 ROCHE LABS. EABND 60258-0005-01 1.41100 CYTRA-K CRYSTALS PACKET 0 CYPRESS PHARM. EABND 60258-0003-16 0.<strong>08</strong>923 CYTRA-K ORAL SOLUTION 0 CYPRESS PHARM. MLGEN 60258-0001-16 0.00700 CYTRA-2 ORAL SOLUTION 0 CYPRESS PHARM. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60258-0002-16 0.01940 CYTRA-3 SYRUP 0 CYPRESS PHARM. MLBND 66490-0041-01 199.72373 D.H.E.45 1 MG/ML AMPUL G VALEANT MLBND 63323-0127-10 9.41220 DACARBAZINE 100 MG VIAL 0 APP PHARMACEUTI EAGEN 00703-5075-01 9.53250 DACARBAZINE 200 MG VIAL 0 TEVA PARENTERAL EAGEN 00703-5075-03 9.53100 DACARBAZINE 200 MG VIAL 0 TEVA PARENTERAL EAGEN 61703-0327-22 15.64500 DACARBAZINE 200 MG VIAL 0 HOSPIRA EAGEN 63323-0128-20 10.80000 DACARBAZINE 200 MG VIAL 0 APP PHARMACEUTI EABND 00456-0095-30 7.18005 DALIRESP 500 MCG TABLET G FOREST PHARMACE EABND 00456-0095-63 7.18024 DALIRESP 500 MCG TABLET G FOREST PHARMACE EABND 00456-0095-90 7.18014 DALIRESP 500 MCG TABLET G FOREST PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00527-1368-01 2.14837 DANAZOL 100 MG CAPSULE 0 LANNETT CO. INC EAGEN 00555-<strong>06</strong>34-02 2.14837 DANAZOL 100 MG CAPSULE 0 BARR EAGEN 00527-1369-01 3.57997 DANAZOL 200 MG CAPSULE 0 LANNETT CO. INC EAGEN 00527-1369-<strong>06</strong> 3.66850 DANAZOL 200 MG CAPSULE 0 LANNETT CO. INC EAGEN 00555-<strong>06</strong>35-02 3.57997 DANAZOL 200 MG CAPSULE 0 BARR EAGEN 00555-<strong>06</strong>35-09 3.66850 DANAZOL 200 MG CAPSULE 0 BARR EAGEN 68<strong>08</strong>4-0074-11 3.61625 DANAZOL 200 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-0074-21 3.61625 DANAZOL 200 MG CAPSULE 0 AHP EAGEN 00527-1392-01 1.43182 DANAZOL 50 MG CAPSULE 0 LANNETT CO. INC EAGEN 00555-<strong>06</strong>33-02 1.43182 DANAZOL 50 MG CAPSULE 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 42023-0126-01 1.6<strong>08</strong>20 1.94917 DANTRIUM 100 MG CAPSULE G JHP PHARMACEUTI EABND 42023-0124-01 0.79350 1.<strong>06</strong>572 DANTRIUM 25 MG CAPSULE G JHP PHARMACEUTI EABND 42023-0125-01 1.28240 1.66332 DANTRIUM 50 MG CAPSULE G JHP PHARMACEUTI EAGEN 00115-4433-01 1.6<strong>08</strong>20 DANTROLENE SODIUM 100 MG CAP 0 GLOBAL PHARM EAGEN 00115-4411-01 0.79350 DANTROLENE SODIUM 25 MG CAP 0 GLOBAL PHARM EAGEN 00115-4411-03 0.79350 DANTROLENE SODIUM 25 MG CAP 0 GLOBAL PHARM EAGEN 42023-0144-01 0.79350 DANTROLENE SODIUM 25 MG CAP 0 JHP PHARMACEUTI EAGEN 68<strong>08</strong>4-0300-11 0.79350 DANTROLENE SODIUM 25 MG CAP 0 AHP EAGEN 68<strong>08</strong>4-0300-21 0.79350 DANTROLENE SODIUM 25 MG CAP 0 AHP EAGEN 00115-4422-01 1.28240 DANTROLENE SODIUM 50 MG CAP 0 GLOBAL PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42023-0145-01 1.28240 DANTROLENE SODIUM 50 MG CAP 0 JHP PHARMACEUTI EABND 49938-0101-30 1.07900 DAPSONE 100 MG TABLET 0 JACOBUS PHARM. EABND 49938-0102-30 0.87980 DAPSONE 25 MG TABLET 0 JACOBUS PHARM. EABND 52054-0330-10 13.50<strong>06</strong>1 DARAPRIM 25 MG TABLET 0 AMEDRA PHARMACE EAGEX 16714-0348-01 0.73890 DASETTA 1-35-28 TABLET 0 NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 100LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16714-0346-01 0.83640 DASETTA 7/7/7-28 TABLET 0 NORTHSTAR RX LL EAGEN 63323-0119-<strong>08</strong> 94.50000 DAUNORUBICIN 20 MG VIAL 0 APP PHARMACEUTI EAGEN 00703-5233-13 10.19690 DAUNORUBICIN 20 MG/4 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-5233-91 10.19690 DAUNORUBICIN 20 MG/4 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-5233-93 10.19690 DAUNORUBICIN 20 MG/4 ML VIAL 0 TEVA PARENTERAL MLGEN 55390-01<strong>08</strong>-10 10.19690 DAUNORUBICIN 20 MG/4 ML VIAL 0 BEDFORD LABS MLGEN 55390-01<strong>08</strong>-01 10.19690 DAUNORUBICIN 50 MG/10 ML VIAL 0 BEDFORD LABS MLBUL 00025-1381-31 0.67580 3.98607 DAYPRO 600 MG CAPLET G PHARMACIA/UPJHN EABND 00075-2452-01 38.60220 64.<strong>06</strong>438 DDAVP 0.01% NASAL SPRAY G SAN<strong>OF</strong>I-AVENTIS MLBND 00075-2450-01 74.09576 DDAVP 0.01% SOLUTION 0 SAN<strong>OF</strong>I-AVENTIS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00075-0016-00 1.27<strong>06</strong>0 5.63578 DDAVP 0.1 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00075-0026-00 1.33760 8.11955 DDAVP 0.2 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00075-2451-01 6.42600 54.62147 DDAVP 4 MCG/ML AMPUL G SAN<strong>OF</strong>I-AVENTIS MLBND 00075-2451-53 6.81000 55.29958 DDAVP 4 MCG/ML VIAL G SAN<strong>OF</strong>I-AVENTIS MLGEN 00409-2337-25 27.88125 DEFEROXAMINE 2 GRAM VIAL 0 HOSPIRA EAGEN 00555-1131-11 41.60190 DEFEROXAMINE 2 GRAM VIAL 0 BARR EAGEN 63323-0599-30 37.<strong>08</strong>000 DEFEROXAMINE 2 GRAM VIAL 0 APP PHARMACEUTI EAGEN 00409-2336-10 7.03875 DEFEROXAMINE 500 MG VIAL 0 HOSPIRA EAGEN 00555-1132-12 11.24625 DEFEROXAMINE 500 MG VIAL 0 BARR EAGEN 55390-0263-10 12.69100 DEFEROXAMINE 500 MG VIAL 0 BEDFORD LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0597-10 11.65500 DEFEROXAMINE 500 MG VIAL 0 APP PHARMACEUTI EABND 42023-0111-01 26.61644 DELESTROGEN 20 MG/ML VIAL 0 JHP PHARMACEUTI MLBND 42023-0112-01 44.15268 DELESTROGEN 40 MG/ML VIAL G JHP PHARMACEUTI MLBND 00430-0753-27 2.20116 DELZICOL DR 400 MG CAPSULE G ACTAVIS PHARMA, EABND 00037-5010-01 0.10351 2.04113 DEMADEX 10 MG TABLET G MEDA PHARMACEUT EABND 00037-5020-01 0.12730 2.27577 DEMADEX 20 MG TABLET G MEDA PHARMACEUT EABND 00037-5005-01 0.12240 1.84168 DEMADEX 5 MG TABLET G MEDA PHARMACEUT EAGEN 00115-2111-01 5.73930 DEMECLOCYCLINE 150 MG TABLET 0 GLOBAL PHARM EAGEN 00555-0701-02 5.73930 DEMECLOCYCLINE 150 MG TABLET 0 BARR EAGEN 62584-0159-01 5.73930 DEMECLOCYCLINE 150 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62584-0159-11 5.73930 DEMECLOCYCLINE 150 MG TABLET 0 AHP EAGEN 65162-0554-10 5.73930 DEMECLOCYCLINE 150 MG TABLET 0 AMNEAL PHARMACE EAGEN 00115-2122-14 11.00010 DEMECLOCYCLINE 300 MG TABLET 0 GLOBAL PHARM EAGEN 00555-0702-84 11.00010 DEMECLOCYCLINE 300 MG TABLET 0 BARR EAGEN 61748-0113-48 11.00010 DEMECLOCYCLINE 300 MG TABLET 0 VERSA PHARMACEU EAGEN 62584-0163-11 11.00010 DEMECLOCYCLINE 300 MG TABLET 0 AHP EAGEN 62584-0163-65 11.00010 DEMECLOCYCLINE 300 MG TABLET 0 AHP EAGEN 65162-0555-48 11.00010 DEMECLOCYCLINE 300 MG TABLET 0 AMNEAL PHARMACE EABND 25010-0305-15 99.39266 DEMSER 250 MG CAPSULE 0 VALEANT EABND 40076-<strong>06</strong>24-05 85.34392 DENAVIR 1% CREAM G PRESTIUM PHARMA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 5<strong>08</strong>16-<strong>06</strong>24-01 63.81040 DENAVIR 1% CREAM G PRESTIUM PHARMA GMBND 5<strong>08</strong>16-<strong>06</strong>24-05 64.65036 DENAVIR 1% CREAM G PRESTIUM PHARMA GMBEX 00074-1564-10 4.69995 DEPACON 500 MG VIAL G ABBVIE US LLC MLBEX 00074-5681-13 0.16830 3.68810 DEPAKENE 250 MG CAPSULE G ABBVIE US LLC EABEX 00074-5682-16 0.01660 0.78537 DEPAKENE 250 MG/5 ML SOLUTION G ABBVIE US LLC ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 101LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00074-6212-13 0.05220 1.31588 DEPAKOTE DR 125 MG TABLET G ABBVIE US LLC EABEX 00074-6214-13 0.<strong>08</strong>897 2.58470 DEPAKOTE DR 250 MG TABLET G ABBVIE US LLC EABEX 00074-6214-53 0.<strong>08</strong>897 2.58471 DEPAKOTE DR 250 MG TABLET G ABBVIE US LLC EABEX 00074-6215-13 0.15593 4.76644 DEPAKOTE DR 500 MG TABLET G ABBVIE US LLC EABEX 00074-6215-53 0.15593 4.76645 DEPAKOTE DR 500 MG TABLET G ABBVIE US LLC EABEX 00074-3826-13 2.35<strong>06</strong>4 DEPAKOTE ER 250 MG TABLET G ABBVIE US LLC EABEX 00074-7126-13 4.13456 DEPAKOTE ER 500 MG TABLET G ABBVIE US LLC EABEX 00074-7126-53 4.13466 DEPAKOTE ER 500 MG TABLET G ABBVIE US LLC EABEX 00074-6114-13 1.25446 1.25446 DEPAKOTE 125 MG SPRINKLE CAP 0 ABBVIE US LLC EABND 00037-4401-01 22.30932 DEPEN 250 MG TITRATAB 0 MEDA PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-0271-01 9.74918 DEPO-ESTRADIOL 5 MG/ML VIAL 0 PHARMACIA/UPJHN MLBND 00009-0274-01 3.76654 DEPO-MEDROL 20 MG/ML VIAL 0 PHARMACIA/UPJHN MLBND 00009-0280-02 3.65050 5.84984 DEPO-MEDROL 40 MG/ML VIAL 0 PHARMACIA/UPJHN MLBND 00009-0280-03 3.65050 5.32777 DEPO-MEDROL 40 MG/ML VIAL 0 PHARMACIA/UPJHN MLBND 00009-0280-51 3.65050 5.85050 DEPO-MEDROL 40 MG/ML VIAL 0 PHARMACIA/UPJHN MLBND 00009-0280-52 3.65050 5.32793 DEPO-MEDROL 40 MG/ML VIAL 0 PHARMACIA/UPJHN MLBND 00009-3073-01 3.65050 8.25020 DEPO-MEDROL 40 MG/ML VIAL 0 PHARMACIA/UPJHN MLBND 00009-3073-03 3.65050 8.25020 DEPO-MEDROL 40 MG/ML VIAL 0 PHARMACIA/UPJHN MLBND 00009-03<strong>06</strong>-02 6.94784 10.65554 DEPO-MEDROL 80 MG/ML VIAL 0 PHARMACIA/UPJHN MLBND 00009-03<strong>06</strong>-12 6.94784 10.65587 DEPO-MEDROL 80 MG/ML VIAL 0 PHARMACIA/UPJHN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-3475-01 6.94784 14.30920 DEPO-MEDROL 80 MG/ML VIAL 0 PHARMACIA/UPJHN MLBND 00009-3475-03 6.94784 14.3<strong>08</strong>53 DEPO-MEDROL 80 MG/ML VIAL 0 PHARMACIA/UPJHN MLBEX 00009-7376-07 84.32800 DEPO-PROVERA 150 MG/ML SYRINGE G PFIZER US PHARM MLBEX 00009-7376-11 117.23750 DEPO-PROVERA 150 MG/ML SYRINGE G PFIZER US PHARM MLBEX 00009-0746-30 40.77000 122.52460 DEPO-PROVERA 150 MG/ML VIAL G PHARMACIA/UPJHN MLBEX 00009-0746-35 40.77000 122.53190 DEPO-PROVERA 150 MG/ML VIAL G PHARMACIA/UPJHN MLBND 00009-<strong>06</strong>26-01 105.18092 DEPO-PROVERA 400 MG/ML VIAL 0 PHARMACIA/UPJHN MLBEX 00009-4709-13 182.57446 DEPO-SUBQ PROVERA 104 SYRINGE 0 PFIZER US PHARM MLBND 00<strong>06</strong>6-0507-60 0.71380 1.67577 DERMATOP 0.1% CREAM G VALEANT GMBND 00<strong>06</strong>6-05<strong>08</strong>-60 1.13900 1.59594 DERMATOP 0.1% OINTMENT G VALEANT GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0467-61 12.69100 29.39030 DESFERAL MESYLATE 500 MG VL G NOVARTIS EABND 00078-0467-91 12.69100 29.38200 DESFERAL MESYLATE 500 MG VL G NOVARTIS EABND 00078-0347-51 41.60190 114.21837 DESFERAL 2 GRAM VIAL 0 NOVARTIS EABND 00078-0347-61 41.60190 114.22460 DESFERAL 2 GRAM VIAL 0 NOVARTIS EAGEX 00781-1971-01 0.84277 DESIPRAMINE 10 MG TABLET 0 SANDOZ EAGEX 45963-0341-02 0.88100 DESIPRAMINE 10 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00781-1975-01 3.18780 DESIPRAMINE 100 MG TABLET 0 SANDOZ EAGEX 45963-0345-02 3.35955 DESIPRAMINE 100 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00781-1976-50 4.61895 DESIPRAMINE 150 MG TABLET 0 SANDOZ EAGEX 45963-0346-50 4.86765 DESIPRAMINE 150 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-1972-01 0.87210 DESIPRAMINE 25 MG TABLET 0 SANDOZ EAGEX 45963-0342-02 0.87210 DESIPRAMINE 25 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00781-1973-01 1.76120 DESIPRAMINE 50 MG TABLET 0 SANDOZ EAGEX 45963-0343-02 1.76120 DESIPRAMINE 50 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00781-1974-01 2.42595 DESIPRAMINE 75 MG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 102LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 45963-0344-02 2.32620 DESIPRAMINE 75 MG TABLET 0 ACTAVIS PHARMA, EABND 55111-0551-31 4.83475 DESLORATADINE 2.5 MG ODT G DR.REDDY'S LAB EABND 55111-0360-31 4.83475 DESLORATADINE 5 MG ODT G DR.REDDY'S LAB EAGEN 00378-4017-01 1.04400 DESLORATADINE 5 MG TABLET G MYLAN EAGEN 00378-4017-05 1.04400 DESLORATADINE 5 MG TABLET G MYLAN EAGEN 00574-9838-01 0.45000 DESLORATADINE 5 MG TABLET G PADDOCK LABS. EAGEN 00781-5226-01 1.04400 DESLORATADINE 5 MG TABLET G SANDOZ EAGEN 00781-5226-05 1.04400 DESLORATADINE 5 MG TABLET G SANDOZ EAGEN 16714-0339-01 1.04400 DESLORATADINE 5 MG TABLET G NORTHSTAR RX LL EAGEN 16714-0339-02 1.04400 DESLORATADINE 5 MG TABLET G NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62756-0523-13 1.04400 DESLORATADINE 5 MG TABLET G SUN PHARMACEUTI EAGEN 62756-0523-88 1.04400 DESLORATADINE 5 MG TABLET G SUN PHARMACEUTI EAGEN 68180-0153-01 1.04400 DESLORATADINE 5 MG TABLET G LUPIN PHARMACEU EAGEN 68180-0153-02 1.04400 DESLORATADINE 5 MG TABLET G LUPIN PHARMACEU EAGEN 76439-0107-10 1.04400 DESLORATADINE 5 MG TABLET G VIRTUS PHARMACE EAGEN 76439-0107-50 1.04400 DESLORATADINE 5 MG TABLET G VIRTUS PHARMACE EAGEN 00409-2265-01 6.42600 DESMOPRESSIN AC 4 MCG/ML AMPUL 0 HOSPIRA MLGEN 55566-5030-01 6.42600 DESMOPRESSIN AC 4 MCG/ML AMPUL 0 FERRING PH INC MLGEN 00703-5051-03 5.53125 DESMOPRESSIN AC 4 MCG/ML VIAL 0 TEVA PARENTERAL MLGEN 00703-5054-01 5.53125 DESMOPRESSIN AC 4 MCG/ML VIAL 0 TEVA PARENTERAL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55566-5040-01 6.81000 DESMOPRESSIN AC 4 MCG/ML VIAL 0 FERRING PH INC MLGEN 00093-7316-01 1.27<strong>06</strong>0 DESMOPRESSIN ACETATE 0.1 MG TB 0 TEVA USA EAGEN 00591-2464-01 1.27<strong>06</strong>0 DESMOPRESSIN ACETATE 0.1 MG TB 0 ACTAVIS PHARMA, EAGEN 55566-5<strong>06</strong>0-01 0.99885 DESMOPRESSIN ACETATE 0.1 MG TB 0 FERRING PH INC EAGEN 60505-0257-01 1.27<strong>06</strong>0 DESMOPRESSIN ACETATE 0.1 MG TB 0 APOTEX CORP EAGEN 68<strong>08</strong>4-<strong>06</strong><strong>06</strong>-21 1.27<strong>06</strong>0 DESMOPRESSIN ACETATE 0.1 MG TB 0 AHP EAGEN 00093-7317-01 1.33760 DESMOPRESSIN ACETATE 0.2 MG TB 0 TEVA USA EAGEN 00591-2465-01 1.33760 DESMOPRESSIN ACETATE 0.2 MG TB 0 ACTAVIS PHARMA, EAGEN 51079-0446-01 1.33760 DESMOPRESSIN ACETATE 0.2 MG TB 0 MYLAN INSTITUTI EAGEN 51079-0446-03 1.33760 DESMOPRESSIN ACETATE 0.2 MG TB 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55566-5<strong>06</strong>1-01 1.27785 DESMOPRESSIN ACETATE 0.2 MG TB 0 FERRING PH INC EAGEN 60505-0258-01 1.33760 DESMOPRESSIN ACETATE 0.2 MG TB 0 APOTEX CORP EAGEN 68<strong>08</strong>4-<strong>06</strong>04-21 1.33760 DESMOPRESSIN ACETATE 0.2 MG TB 0 AHP EAGEN 242<strong>08</strong>-0342-05 34.23910 DESMOPRESSIN 0.01% SOLUTION 0 VALEANT MLGEN 62756-0161-91 34.23910 DESMOPRESSIN 0.01% SPRAY 0 SUN PHARMACEUTI MLGEN 55566-5020-01 27.12600 DESMOPRESSIN 0.1 MG/ML SOL 0 FERRING PH INC MLGEN 60505-<strong>08</strong>15-00 36.93750 DESMOPRESSIN 0.1 MG/ML SPRAY 0 APOTEX CORP MLGEN 62756-0529-40 6.81000 DESMOPRESSIN 40 MCG/10 ML VIAL 0 SUN PHARMACEUTI MLBEX 00052-0261-<strong>06</strong> 0.73800 1.69507 DESOGEN 28 DAY TABLET G ORGANON PHARM. EAGEX 16714-0367-01 0.73800 DESOGESTREL-ETHINYL ESTRAD TAB 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16714-0367-04 0.73800 DESOGESTREL-ETHINYL ESTRAD TAB 0 NORTHSTAR RX LL EABND 10922-<strong>08</strong>28-<strong>06</strong> 6.21338 DESONATE 0.05% GEL G INTENDIS INC. GMBND 50419-<strong>08</strong>28-<strong>06</strong> 6.64830 DESONATE 0.05% GEL G BAYER,PHARM DIV GMBND 50419-<strong>08</strong>28-12 5.67056 DESONATE 0.05% GEL G BAYER,PHARM DIV GMGUL 00472-<strong>08</strong>04-15 0.23370 DESONIDE 0.05% CREAM G ACTAVIS PHARMA, GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 103LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00472-<strong>08</strong>04-60 0.23370 DESONIDE 0.05% CREAM G ACTAVIS PHARMA, GMGUL 45802-0422-35 0.23370 DESONIDE 0.05% CREAM G PERRIGO CO. GMGUL 45802-0422-37 0.23370 DESONIDE 0.05% CREAM G PERRIGO CO. GMGUL 51672-1280-01 0.23370 DESONIDE 0.05% CREAM G TARO PHARM USA GMGUL 51672-1280-03 0.23370 DESONIDE 0.05% CREAM G TARO PHARM USA GMGUL 00168-0310-02 0.54410 DESONIDE 0.05% LOTION G SANDOZ MLGUL 00168-0310-04 0.54410 DESONIDE 0.05% LOTION G SANDOZ MLGUL 00472-<strong>08</strong>03-02 0.54410 DESONIDE 0.05% LOTION G ACTAVIS PHARMA, MLGUL 00472-<strong>08</strong>03-04 0.54410 DESONIDE 0.05% LOTION G ACTAVIS PHARMA, MLGUL 00168-0309-15 0.40770 DESONIDE 0.05% OINTMENT G SANDOZ GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00168-0309-60 0.40770 DESONIDE 0.05% OINTMENT G SANDOZ GMGUL 45802-0423-35 0.40770 DESONIDE 0.05% OINTMENT G PERRIGO CO. GMGUL 45802-0423-37 0.40770 DESONIDE 0.05% OINTMENT G PERRIGO CO. GMGUL 51672-1281-01 0.40770 DESONIDE 0.05% OINTMENT G TARO PHARM USA GMGUL 51672-1281-03 0.40770 DESONIDE 0.05% OINTMENT G TARO PHARM USA GMBND 51672-1271-01 2.25180 3.45446 DESOXIMETASONE 0.05% CREAM G TARO PHARM USA GMBND 51672-1271-03 2.25180 3.84275 DESOXIMETASONE 0.05% CREAM G TARO PHARM USA GMBND 51672-1271-<strong>08</strong> 1.61924 DESOXIMETASONE 0.05% CREAM G TARO PHARM USA GMGEN 51672-1261-01 3.97076 DESOXIMETASONE 0.05% GEL G TARO PHARM USA GMGEN 51672-1261-03 3.50274 DESOXIMETASONE 0.05% GEL G TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61748-0205-15 3.97076 DESOXIMETASONE 0.05% GEL G VERSA PHARMACEU GMGEN 61748-0205-60 3.50250 DESOXIMETASONE 0.05% GEL G VERSA PHARMACEU GMBND 51672-1352-03 4.03490 DESOXIMETASONE 0.05% OINTMENT G TARO PHARM USA GMBND 51672-1352-07 2.48095 DESOXIMETASONE 0.05% OINTMENT G TARO PHARM USA GMGEN 45802-0495-35 1.57780 DESOXIMETASONE 0.25% CREAM G PERRIGO CO. GMGEN 45802-0495-37 1.57780 DESOXIMETASONE 0.25% CREAM G PERRIGO CO. GMGEN 51672-1270-01 1.57780 DESOXIMETASONE 0.25% CREAM G TARO PHARM USA GMGEN 51672-1270-03 1.57780 DESOXIMETASONE 0.25% CREAM G TARO PHARM USA GMGEN 51672-1270-07 1.57780 DESOXIMETASONE 0.25% CREAM G TARO PHARM USA GMGEN 51672-1270-09 1.43512 DESOXIMETASONE 0.25% CREAM G TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00168-0151-15 4.43450 DESOXIMETASONE 0.25% OINTMENT G SANDOZ GMGEN 00168-0151-60 3.68175 DESOXIMETASONE 0.25% OINTMENT G SANDOZ GMGEN 51672-1262-01 5.31249 DESOXIMETASONE 0.25% OINTMENT G TARO PHARM USA GMGEN 51672-1262-03 4.41037 DESOXIMETASONE 0.25% OINTMENT G TARO PHARM USA GMGEN 51672-1262-07 2.71537 DESOXIMETASONE 0.25% OINTMENT G TARO PHARM USA GMGEN 68462-0531-17 5.31249 DESOXIMETASONE 0.25% OINTMENT G GLENMARK PHARMA GMGEN 68462-0531-65 4.41037 DESOXIMETASONE 0.25% OINTMENT G GLENMARK PHARMA GMBEX 63304-0192-30 4.81787 DESVENLAFAXINE ER 100 MG TAB G RANBAXY PHARMAC EABEX 63304-0192-90 4.81787 DESVENLAFAXINE ER 100 MG TAB G RANBAXY PHARMAC EABEX 63304-0191-30 4.81787 DESVENLAFAXINE ER 50 MG TAB G RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 63304-0191-90 4.81787 DESVENLAFAXINE ER 50 MG TAB G RANBAXY PHARMAC EABND 00009-5190-01 7.41051 DETROL LA 2 MG CAPSULE G PHARMACIA/UPJHN EABND 00009-5190-02 7.41<strong>06</strong>0 DETROL LA 2 MG CAPSULE G PHARMACIA/UPJHN EABND 00009-5190-03 7.41047 DETROL LA 2 MG CAPSULE G PHARMACIA/UPJHN EABND 00009-5191-01 7.41051 DETROL LA 4 MG CAPSULE G PHARMACIA/UPJHN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 104LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-5191-02 7.41<strong>06</strong>0 DETROL LA 4 MG CAPSULE G PHARMACIA/UPJHN EABND 00009-5191-03 7.41047 DETROL LA 4 MG CAPSULE G PHARMACIA/UPJHN EABND 00009-4541-02 1.11132 4.35999 DETROL 1 MG TABLET G PHARMACIA/UPJHN EABND 00009-4541-03 1.11132 4.36010 DETROL 1 MG TABLET G PHARMACIA/UPJHN EABND 00009-4544-02 1.14050 4.47521 DETROL 2 MG TABLET G PHARMACIA/UPJHN EABND 00009-4544-03 1.14050 4.47526 DETROL 2 MG TABLET G PHARMACIA/UPJHN EABND 00054-3176-44 0.72237 DEXAMETHASONE INTENSOL 1MG/1ML G ROXANE LABS. MLGEN 242<strong>08</strong>-0720-02 3.17850 DEXAMETHASONE 0.1% EYE DROP 0 VALEANT MLGEN 61314-0294-05 3.16500 DEXAMETHASONE 0.1% EYE DROP 0 SANDOZ MLGEN 00054-4179-25 0.<strong>06</strong>300 DEXAMETHASONE 0.5 MG TABLET 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-8179-25 0.<strong>06</strong>300 DEXAMETHASONE 0.5 MG TABLET 0 ROXANE LABS. EAGEN 0<strong>06</strong>03-1147-56 0.18954 DEXAMETHASONE 0.5 MG/5 ML ELX G QUALITEST MLGEN 60432-0466-<strong>08</strong> 0.18954 DEXAMETHASONE 0.5 MG/5 ML ELX G MORTON GROVE PH MLGEN 64980-0509-24 0.18954 DEXAMETHASONE 0.5 MG/5 ML ELX G RISING PHARM MLBND 00054-3177-57 0.22026 DEXAMETHASONE 0.5 MG/5 ML LIQ 0 ROXANE LABS. MLBND 00054-3177-63 0.03969 DEXAMETHASONE 0.5 MG/5 ML LIQ 0 ROXANE LABS. MLGEN 00054-4180-25 0.11360 DEXAMETHASONE 0.75 MG TABLET 0 ROXANE LABS. EAGEN 00054-8180-25 0.11360 DEXAMETHASONE 0.75 MG TABLET 0 ROXANE LABS. EABND 00054-4181-25 0.29050 DEXAMETHASONE 1 MG TABLET 0 ROXANE LABS. EABND 00054-8174-25 0.31374 DEXAMETHASONE 1 MG TABLET 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-4182-25 0.07722 DEXAMETHASONE 1.5 MG TABLET 0 ROXANE LABS. EAGEN 49884-0<strong>08</strong>6-01 0.07722 DEXAMETHASONE 1.5 MG TABLET 0 PAR PHARM. EAGEN 0<strong>06</strong>41-0367-25 0.843<strong>08</strong> DEXAMETHASONE 10 MG/ML VIAL 0 WEST-WARD,INC. MLBND 63323-05<strong>06</strong>-01 4.02384 DEXAMETHASONE 10 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 00<strong>06</strong>9-0177-01 0.35985 DEXAMETHASONE 100 MG/10 ML VL 0 PFIZER/NOVAPLUS MLGEN 00<strong>06</strong>9-0177-02 0.35985 DEXAMETHASONE 100 MG/10 ML VL 0 PFIZER/NOVAPLUS MLGEN 00<strong>06</strong>9-4541-01 0.35985 DEXAMETHASONE 100 MG/10 ML VL 0 PFIZER US PHARM MLGEN 00<strong>06</strong>9-0192-01 0.31500 DEXAMETHASONE 120 MG/30 ML VL 0 PFIZER/NOVAPLUS MLGEN 00<strong>06</strong>9-0192-02 0.31500 DEXAMETHASONE 120 MG/30 ML VL 0 PFIZER/NOVAPLUS MLGEN 00<strong>06</strong>9-4545-01 0.31500 DEXAMETHASONE 120 MG/30 ML VL 0 PFIZER US PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00517-4930-25 0.23250 DEXAMETHASONE 120 MG/30 ML VL 0 AMER. REGENT MLGEN 63323-0165-30 0.65649 DEXAMETHASONE 120 MG/30 ML VL 0 APP PHARMACEUTI MLBND 00054-4183-25 0.56888 DEXAMETHASONE 2 MG TABLET 0 ROXANE LABS. EABND 00054-8176-25 0.59303 DEXAMETHASONE 2 MG TABLET 0 ROXANE LABS. EAGEN 00<strong>06</strong>9-0178-01 0.35100 DEXAMETHASONE 20 MG/5 ML VIAL 0 PFIZER/NOVAPLUS MLGEN 00<strong>06</strong>9-0178-02 0.35100 DEXAMETHASONE 20 MG/5 ML VIAL 0 PFIZER/NOVAPLUS MLGEN 00<strong>06</strong>9-4543-01 0.35100 DEXAMETHASONE 20 MG/5 ML VIAL 0 PFIZER US PHARM MLGEN 00517-4905-25 0.31500 DEXAMETHASONE 20 MG/5 ML VIAL 0 AMER. REGENT MLGEN 63323-0165-05 0.73<strong>08</strong>0 DEXAMETHASONE 20 MG/5 ML VIAL 0 APP PHARMACEUTI MLGEN 00054-4184-25 0.12430 DEXAMETHASONE 4 MG TABLET 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-8175-25 0.12430 DEXAMETHASONE 4 MG TABLET 0 ROXANE LABS. EAGEN 49884-0<strong>08</strong>7-01 0.12430 DEXAMETHASONE 4 MG TABLET 0 PAR PHARM. EAGEN 00<strong>06</strong>9-0179-01 0.74280 DEXAMETHASONE 4 MG/ML VIAL 0 PFIZER/NOVAPLUS MLGEN 00<strong>06</strong>9-0179-02 0.74280 DEXAMETHASONE 4 MG/ML VIAL 0 PFIZER/NOVAPLUS MLGEN 00<strong>06</strong>9-4547-01 0.74280 DEXAMETHASONE 4 MG/ML VIAL 0 PFIZER US PHARM ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 105LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00517-4901-25 0.78300 DEXAMETHASONE 4 MG/ML VIAL 0 AMER. REGENT MLGEN 63323-0165-01 0.83098 DEXAMETHASONE 4 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 67457-0423-00 0.74280 DEXAMETHASONE 4 MG/ML VIAL 0 MYLAN INSTITUTI MLGEN 67457-0423-12 0.74280 DEXAMETHASONE 4 MG/ML VIAL 0 MYLAN INSTITUTI MLGEN 00054-4186-25 0.42110 DEXAMETHASONE 6 MG TABLET 0 ROXANE LABS. EAGEN 00054-8183-25 0.42110 DEXAMETHASONE 6 MG TABLET 0 ROXANE LABS. EAGEN 49884-0129-01 0.42110 DEXAMETHASONE 6 MG TABLET 0 PAR PHARM. EABND 00517-0234-10 18.67500 DEXFERRUM 100 MG/2 ML VIAL 0 AMER. REGENT MLBND 00517-0134-10 18.67500 DEXFERRUM 50 MG/ML VIAL 0 AMER. REGENT MLBND 64764-0171-30 5.55491 DEXILANT DR 30 MG CAPSULE G TAKEDA PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64764-0175-30 5.55491 DEXILANT DR 60 MG CAPSULE G TAKEDA PHARMACE EABND 64764-0175-90 5.55472 DEXILANT DR 60 MG CAPSULE G TAKEDA PHARMACE EABND 00095-0<strong>08</strong>7-35 0.57140 1.99200 DEXPAK 10 DAY 1.5 MG TABLET G ECR PHARM. EABND 00095-0<strong>08</strong>8-51 0.53180 1.99200 DEXPAK 13 DAY 1.5 MG TABLET G ECR PHARM. EABND 00095-0<strong>08</strong>9-21 0.87540 1.99200 DEXPAK 6 DAY 1.5 MG TABLET G ECR PHARM. EABND 00517-0131-25 1.61850 DEXPANTHENOL 250 MG/ML VIAL 0 AMER. REGENT MLGEN 38779-0355-01 8.72850 DEXTROMETHORPHAN HBR POWDER 0 MEDISCA INC. GMGEN 38779-0355-05 8.72812 DEXTROMETHORPHAN HBR POWDER 0 MEDISCA INC. GMGEN 51927-1339-00 10.03500 DEXTROMETHORPHAN HBR POWDER 0 PR<strong>OF</strong>ESSIONAL CO GMBND 00264-7520-00 0.00174 DEXTROSE 10%-WATER IV SOLUTION 0 B.BRAUN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00264-7520-10 0.00340 DEXTROSE 10%-WATER IV SOLUTION 0 B.BRAUN MLGEN 00338-0023-02 0.00270 DEXTROSE 10%-WATER IV SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0023-03 0.00270 DEXTROSE 10%-WATER IV SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0023-04 0.00234 DEXTROSE 10%-WATER IV SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-7930-02 0.00270 DEXTROSE 10%-WATER IV SOLUTION 0 HOSPIRA MLBND 00409-4862-02 0.02350 DEXTROSE 10%-1/4NS IV SOLN 0 HOSPIRA MLGEN 00338-0073-04 0.00210 DEXTROSE 2.5%-1/2NS IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00264-7751-00 0.00157 DEXTROSE 5%-LR IV SOLUTION 0 B.BRAUN MLGEN 00264-7751-10 0.00176 DEXTROSE 5%-LR IV SOLUTION 0 B.BRAUN MLGEN 00338-0125-03 0.00176 DEXTROSE 5%-LR IV SOLUTION 0 BAXTER <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00338-0125-04 0.00169 DEXTROSE 5%-LR IV SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-7929-09 0.00172 DEXTROSE 5%-LR IV SOLUTION 0 HOSPIRA MLGEN 00264-7610-00 0.00157 DEXTROSE 5%-NS IV SOLUTION 0 B.BRAUN MLGEN 00264-7610-10 0.00189 DEXTROSE 5%-NS IV SOLUTION 0 B.BRAUN MLGEN 00264-7610-20 0.00189 DEXTROSE 5%-NS IV SOLUTION 0 B.BRAUN MLGEN 00338-0<strong>08</strong>9-03 0.00189 DEXTROSE 5%-NS IV SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0<strong>08</strong>9-04 0.00158 DEXTROSE 5%-NS IV SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-7941-02 0.00189 DEXTROSE 5%-NS IV SOLUTION 0 HOSPIRA MLGEN 00409-7941-03 0.00189 DEXTROSE 5%-NS IV SOLUTION 0 HOSPIRA MLGEN 00409-7941-09 0.00179 DEXTROSE 5%-NS IV SOLUTION 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00264-7781-00 0.00171 DEXTROSE 5%-RINGERS IV SOLN 0 B.BRAUN MLGEN 00338-0077-02 0.00210 DEXTROSE 5%-SOD CHLORIDE 0.2% 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0077-03 0.00210 DEXTROSE 5%-SOD CHLORIDE 0.2% 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0077-04 0.00192 DEXTROSE 5%-SOD CHLORIDE 0.2% 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00264-1510-31 0.00190 DEXTROSE 5%-WATER IV SOLN 0 B.BRAUN ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 1<strong>06</strong>LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00264-1510-32 0.00190 DEXTROSE 5%-WATER IV SOLN 0 B.BRAUN MLGEN 00264-1510-36 0.00190 DEXTROSE 5%-WATER IV SOLN 0 B.BRAUN MLGEN 00264-7510-00 0.00157 DEXTROSE 5%-WATER IV SOLN 0 B.BRAUN MLGEN 00264-7510-10 0.00190 DEXTROSE 5%-WATER IV SOLN 0 B.BRAUN MLGEN 00264-7510-20 0.00190 DEXTROSE 5%-WATER IV SOLN 0 B.BRAUN MLGEN 00338-0017-01 0.00190 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0017-02 0.00190 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0017-03 0.00190 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0017-04 0.00156 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0017-10 0.00190 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00338-0017-11 0.00190 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0017-18 0.00190 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0017-31 0.00190 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0017-38 0.00190 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0017-41 0.00190 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0017-48 0.00190 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLBND 00338-0551-11 0.09900 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLBND 00338-0551-18 0.02371 0.04950 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-6346-02 0.00190 DEXTROSE 5%-WATER IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLBND 00409-7100-66 0.02390 DEXTROSE 5%-WATER IV SOLN 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-7100-67 0.01822 DEXTROSE 5%-WATER IV SOLN 0 HOSPIRA MLGEN 00409-7922-02 0.00190 DEXTROSE 5%-WATER IV SOLN 0 HOSPIRA MLGEN 00409-7922-03 0.00190 DEXTROSE 5%-WATER IV SOLN 0 HOSPIRA MLGEN 00409-7922-09 0.00162 DEXTROSE 5%-WATER IV SOLN 0 HOSPIRA MLGEN 00409-7922-53 0.00190 DEXTROSE 5%-WATER IV SOLN 0 HOSPIRA MLGEN 00409-7922-55 0.00190 DEXTROSE 5%-WATER IV SOLN 0 HOSPIRA MLGEN 00409-7922-61 0.00190 DEXTROSE 5%-WATER IV SOLN 0 HOSPIRA MLGEN 00409-7923-36 0.00190 DEXTROSE 5%-WATER IV SOLN 0 HOSPIRA MLGEN 00409-7923-37 0.00190 DEXTROSE 5%-WATER IV SOLN 0 HOSPIRA MLGEN 00264-7612-00 0.00157 DEXTROSE 5%-1/2NS IV SOLUTION 0 B.BRAUN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00338-0<strong>08</strong>5-03 0.00189 DEXTROSE 5%-1/2NS IV SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0<strong>08</strong>5-04 0.00168 DEXTROSE 5%-1/2NS IV SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-7926-02 0.00189 DEXTROSE 5%-1/2NS IV SOLUTION 0 HOSPIRA MLGEN 00409-7926-03 0.00189 DEXTROSE 5%-1/2NS IV SOLUTION 0 HOSPIRA MLGEN 00409-7926-09 0.00152 DEXTROSE 5%-1/2NS IV SOLUTION 0 HOSPIRA MLGEN 00338-0<strong>08</strong>1-03 0.00190 DEXTROSE 5%-1/3NS IV SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-7925-03 0.00190 DEXTROSE 5%-1/3NS IV SOLUTION 0 HOSPIRA MLGEN 00409-7925-09 0.00168 DEXTROSE 5%-1/3NS IV SOLUTION 0 HOSPIRA MLGEN 00409-7924-02 0.00210 DEXTROSE 5%-1/4NS IV SOLUTION 0 HOSPIRA MLGEN 00409-7924-09 0.00160 DEXTROSE 5%-1/4NS IV SOLUTION 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00264-1280-55 0.00580 DEXTROSE 50%-WATER IV SOLN 0 B.BRAUN MLGEN 00409-7517-16 0.09640 DEXTROSE 50%-WATER SYRINGE 0 HOSPIRA MLGEN 76329-3301-01 0.09640 DEXTROSE 50%-WATER SYRINGE 0 INTERNATIONAL M MLBND 00409-6648-02 0.02020 0.04<strong>08</strong>3 DEXTROSE 50%-WATER VIAL 0 HOSPIRA MLBND 00039-0053-05 0.12440 0.4<strong>06</strong>53 DIABETA 1.25 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 107LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00039-0051-10 0.14877 0.89922 DIABETA 2.5 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00039-0052-10 0.22570 1.64896 DIABETA 5 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 51285-0754-02 3.03510 6.86758 DIAMOX SEQUELS ER 500 MG CAP 0 DURAMED/BARR EABND 55494-0100-10 4.73100 DICLEGIS DR 10-10 MG TABLET 0 DUCHESNAY USA, EAGEN 00093-0948-01 0.43335 DICL<strong>OF</strong>ENAC POT 50 MG TABLET 0 TEVA USA EAGEN 00093-0948-05 0.43335 DICL<strong>OF</strong>ENAC POT 50 MG TABLET 0 TEVA USA EAGEN 00378-2474-01 0.43335 DICL<strong>OF</strong>ENAC POT 50 MG TABLET 0 MYLAN EAGEN 60505-0135-00 0.43335 DICL<strong>OF</strong>ENAC POT 50 MG TABLET 0 APOTEX CORP EAGEN 00378-6280-01 0.36710 DICL<strong>OF</strong>ENAC SOD DR 50 MG TAB 0 MYLAN EAGEN 00378-6280-10 0.36710 DICL<strong>OF</strong>ENAC SOD DR 50 MG TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-1787-01 0.36710 DICL<strong>OF</strong>ENAC SOD DR 50 MG TAB 0 SANDOZ EAGEN 00781-1787-10 0.36710 DICL<strong>OF</strong>ENAC SOD DR 50 MG TAB 0 SANDOZ EAGEN 00781-1787-60 0.36710 DICL<strong>OF</strong>ENAC SOD DR 50 MG TAB 0 SANDOZ EAGEN 00378-6281-01 0.27972 DICL<strong>OF</strong>ENAC SOD DR 75 MG TAB 0 MYLAN EAGEN 00378-6281-10 0.27972 DICL<strong>OF</strong>ENAC SOD DR 75 MG TAB 0 MYLAN EAGEN 61442-0103-05 0.27972 DICL<strong>OF</strong>ENAC SOD DR 75 MG TAB 0 CARLSBAD TECH EAGEN 68<strong>08</strong>4-0333-01 0.27972 DICL<strong>OF</strong>ENAC SOD DR 75 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0333-11 0.27972 DICL<strong>OF</strong>ENAC SOD DR 75 MG TAB 0 AHP EAGEN 00781-1785-01 1.<strong>06</strong>635 DICL<strong>OF</strong>ENAC SOD EC 25 MG TAB 0 SANDOZ EAGEN 16571-0203-10 1.04360 DICL<strong>OF</strong>ENAC SOD EC 25 MG TAB 0 PACK PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-2550-<strong>06</strong> 0.36710 DICL<strong>OF</strong>ENAC SOD EC 50 MG TAB 0 ACTAVIS PHARMA, EAGEN 00228-2550-11 0.36710 DICL<strong>OF</strong>ENAC SOD EC 50 MG TAB 0 ACTAVIS PHARMA, EAGEN 00228-2550-96 0.36710 DICL<strong>OF</strong>ENAC SOD EC 50 MG TAB 0 ACTAVIS PHARMA, EAGEN 00591-0338-01 0.36710 DICL<strong>OF</strong>ENAC SOD EC 50 MG TAB 0 WATSON LABS EAGEN 00591-0338-10 0.36710 DICL<strong>OF</strong>ENAC SOD EC 50 MG TAB 0 ACTAVIS PHARMA, EAGEN 00591-0338-60 0.36710 DICL<strong>OF</strong>ENAC SOD EC 50 MG TAB 0 WATSON LABS EAGEN 16571-0202-<strong>06</strong> 0.36710 DICL<strong>OF</strong>ENAC SOD EC 50 MG TAB 0 PACK PHARMACEUT EAGEN 16571-0202-10 0.36710 DICL<strong>OF</strong>ENAC SOD EC 50 MG TAB 0 PACK PHARMACEUT EAGEN 16571-0202-11 0.36710 DICL<strong>OF</strong>ENAC SOD EC 50 MG TAB 0 PACK PHARMACEUT EAGEN 51079-0466-01 0.36710 DICL<strong>OF</strong>ENAC SOD EC 50 MG TAB 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0466-20 0.36710 DICL<strong>OF</strong>ENAC SOD EC 50 MG TAB 0 MYLAN INSTITUTI EAGEN 00228-2551-<strong>06</strong> 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 ACTAVIS PHARMA, EAGEN 00228-2551-11 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 ACTAVIS PHARMA, EAGEN 00228-2551-96 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 ACTAVIS PHARMA, EAGEN 00591-0339-05 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 WATSON LABS EAGEN 00591-0339-10 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 WATSON LABS EAGEN 00591-0339-60 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 WATSON LABS EAGEN 00781-1789-01 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 SANDOZ EAGEN 00781-1789-05 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 SANDOZ EAGEN 00781-1789-10 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-1789-60 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 SANDOZ EAGEN 16571-0201-<strong>06</strong> 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 PACK PHARMACEUT EAGEN 16571-0201-10 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 PACK PHARMACEUT EAGEN 16571-0201-11 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 PACK PHARMACEUT EAGEN 16571-0201-50 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 PACK PHARMACEUT EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 1<strong>08</strong>LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0224-01 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0224-20 0.27972 DICL<strong>OF</strong>ENAC SOD EC 75 MG TAB 0 MYLAN INSTITUTI EAGEN 00093-1041-01 0.51910 DICL<strong>OF</strong>ENAC SOD ER 100 MG TAB 0 TEVA USA EAGEN 00228-2717-11 0.51910 DICL<strong>OF</strong>ENAC SOD ER 100 MG TAB 0 ACTAVIS PHARMA, EAGEN 00378-0355-01 0.51910 DICL<strong>OF</strong>ENAC SOD ER 100 MG TAB 0 MYLAN EAGEN 00591-<strong>06</strong>76-01 0.51910 DICL<strong>OF</strong>ENAC SOD ER 100 MG TAB 0 ACTAVIS PHARMA, EAGEN 00781-1381-01 0.51910 DICL<strong>OF</strong>ENAC SOD ER 100 MG TAB 0 SANDOZ EAGEN 38779-2683-<strong>08</strong> 10.68750 DICL<strong>OF</strong>ENAC SODIUM POWDER 0 MEDISCA INC. GMGEN 00115-1483-61 8.49210 DICL<strong>OF</strong>ENAC SODIUM 3% GEL G GLOBAL PHARM GMGEN 00168-<strong>08</strong>03-01 8.84595 DICL<strong>OF</strong>ENAC SODIUM 3% GEL G SANDOZ GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16571-0101-25 2.11050 DICL<strong>OF</strong>ENAC 0.1% EYE DROPS 0 PACK PHARMACEUT MLGEN 16571-0101-50 2.11050 DICL<strong>OF</strong>ENAC 0.1% EYE DROPS 0 PACK PHARMACEUT MLGEN 242<strong>08</strong>-0457-05 2.11050 DICL<strong>OF</strong>ENAC 0.1% EYE DROPS 0 VALEANT MLGEN 242<strong>08</strong>-0457-25 2.11050 DICL<strong>OF</strong>ENAC 0.1% EYE DROPS 0 VALEANT MLGEN 61314-0014-05 2.11050 DICL<strong>OF</strong>ENAC 0.1% EYE DROPS 0 SANDOZ MLGEN 61314-0014-25 2.11050 DICL<strong>OF</strong>ENAC 0.1% EYE DROPS 0 SANDOZ MLGEN 00591-0397-60 2.51462 DICL<strong>OF</strong>ENAC-MISOPROST 50-0.2 TB G ACTAVIS PHARMA, EAGEN 59762-0028-01 2.51462 DICL<strong>OF</strong>ENAC-MISOPROST 50-200 TB G GREENSTONE LLC. EAGEN 59762-0028-02 2.51442 DICL<strong>OF</strong>ENAC-MISOPROST 50-200 TB G GREENSTONE LLC. EAGEN 59762-0028-04 2.51460 DICL<strong>OF</strong>ENAC-MISOPROST 50-200 TB G GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-0398-60 2.51462 DICL<strong>OF</strong>ENAC-MISOPROST 75-0.2 TB G ACTAVIS PHARMA, EAGEN 59762-0029-01 2.51462 DICL<strong>OF</strong>ENAC-MISOPROST 75-200 TB G GREENSTONE LLC. EAGEN 59762-0029-03 2.51460 DICL<strong>OF</strong>ENAC-MISOPROST 75-200 TB G GREENSTONE LLC. EAGEN 00093-3123-01 0.29363 DICLOXACILLIN 250 MG CAPSULE 0 TEVA USA EAGEN 00781-2248-01 0.29363 DICLOXACILLIN 250 MG CAPSULE 0 SANDOZ EAGEN 00093-3125-01 0.48830 DICLOXACILLIN 500 MG CAPSULE 0 TEVA USA EAGEN 00781-2258-01 0.48830 DICLOXACILLIN 500 MG CAPSULE 0 SANDOZ EAGEN 00143-3126-01 0.04266 DICYCLOMINE 10 MG CAPSULE 0 WEST-WARD,INC. EAGEN 00143-3126-10 0.04266 DICYCLOMINE 10 MG CAPSULE 0 WEST-WARD,INC. EAGEN 00378-1610-01 0.04266 DICYCLOMINE 10 MG CAPSULE 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-1610-05 0.04266 DICYCLOMINE 10 MG CAPSULE 0 MYLAN EAGEN 00527-0586-01 0.04266 DICYCLOMINE 10 MG CAPSULE 0 LANNETT CO. INC EAGEN 00527-0586-10 0.04266 DICYCLOMINE 10 MG CAPSULE 0 LANNETT CO. INC EAGEN 00591-0794-01 0.04266 DICYCLOMINE 10 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00591-0794-10 0.04266 DICYCLOMINE 10 MG CAPSULE 0 ACTAVIS PHARMA, EABND 0<strong>06</strong>03-1161-58 0.07750 0.19090 DICYCLOMINE 10 MG/5 ML SOLN 0 QUALITEST MLGUL 00143-1227-01 0.04050 DICYCLOMINE 20 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-1227-10 0.04050 DICYCLOMINE 20 MG TABLET 0 WEST-WARD,INC. EAGUL 00378-1620-01 0.04050 DICYCLOMINE 20 MG TABLET 0 MYLAN EAGUL 00378-1620-05 0.04050 DICYCLOMINE 20 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00527-1282-01 0.04050 DICYCLOMINE 20 MG TABLET 0 LANNETT CO. INC EAGUL 00527-1282-10 0.04050 DICYCLOMINE 20 MG TABLET 0 LANNETT CO. INC EAGUL 00591-0795-01 0.04050 DICYCLOMINE 20 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00591-0795-10 0.04050 DICYCLOMINE 20 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00904-0195-60 0.04050 DICYCLOMINE 20 MG TABLET 0 MAJOR PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 109LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-8886-93 2.72370 DIDANOSINE DR 125 MG CAPSULE G MYLAN EAGEN 65862-0310-30 2.72370 DIDANOSINE DR 125 MG CAPSULE G AUROBINDO PHARM EAGEN 00378-8887-93 4.35740 DIDANOSINE DR 200 MG CAPSULE G MYLAN EAGEN 00555-0588-01 4.35740 DIDANOSINE DR 200 MG CAPSULE G BARR EAGEN 65862-0311-30 4.35740 DIDANOSINE DR 200 MG CAPSULE G AUROBINDO PHARM EAGEN 00378-8888-93 5.55240 DIDANOSINE DR 250 MG CAPSULE G MYLAN EAGEN 00555-0589-01 5.55240 DIDANOSINE DR 250 MG CAPSULE G BARR EAGEN 65862-0312-30 5.55240 DIDANOSINE DR 250 MG CAPSULE G AUROBINDO PHARM EAGEN 00378-8889-93 8.67230 DIDANOSINE DR 400 MG CAPSULE G MYLAN EAGEN 00555-0590-01 8.67230 DIDANOSINE DR 400 MG CAPSULE G BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0313-30 8.67230 DIDANOSINE DR 400 MG CAPSULE G AUROBINDO PHARM EABND 52015-0<strong>08</strong>0-01 147.29595 DIFICID 200 MG TABLET G OPTIMER PHARMAC EABND 51672-1296-01 4.68175 DIFLORASONE 0.05% CREAM G TARO PHARM USA GMBND 51672-1296-02 4.68203 DIFLORASONE 0.05% CREAM G TARO PHARM USA GMBND 51672-1296-03 4.68230 DIFLORASONE 0.05% CREAM G TARO PHARM USA GMGEN 00168-0243-60 1.25199 DIFLORASONE 0.05% OINTMENT G SANDOZ GMBND 51672-1295-01 4.96727 DIFLORASONE 0.05% OINTMENT G TARO PHARM USA GMBND 51672-1295-02 4.96727 DIFLORASONE 0.05% OINTMENT G TARO PHARM USA GMBND 51672-1295-03 4.96782 DIFLORASONE 0.05% OINTMENT G TARO PHARM USA GMBND 00049-3440-19 0.30200 2.15894 DIFLUCAN 10 MG/ML SUSPENSION G PFIZER US PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00049-3420-30 0.88250 17.63611 DIFLUCAN 100 MG TABLET G PFIZER US PHARM EABND 00049-3500-79 2.74400 28.<strong>06</strong>991 DIFLUCAN 150 MG TABLET G PFIZER US PHARM EABUL 00049-3430-30 1.40750 28.86048 DIFLUCAN 200 MG TABLET G PFIZER US PHARM EABND 00049-3450-19 0.70470 7.46809 DIFLUCAN 40 MG/ML SUSPENSION G PFIZER US PHARM MLBUL 00049-3410-30 0.50000 11.22436 DIFLUCAN 50 MG TABLET G PFIZER US PHARM EABND 00049-3372-26 0.07220 0.77917 DIFLUCAN-SALINE 400 MG/200 ML G PFIZER US PHARM MLGEN 00093-0755-01 0.95500 DIFLUNISAL 500 MG TABLET G TEVA USA EAGEN 00093-0755-05 0.95500 DIFLUNISAL 500 MG TABLET G TEVA USA EAGEN 00093-0755-<strong>06</strong> 0.95500 DIFLUNISAL 500 MG TABLET G TEVA USA EAGEN 64980-0181-01 0.95500 DIFLUNISAL 500 MG TABLET G RISING PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64980-0181-05 0.95500 DIFLUNISAL 500 MG TABLET G RISING PHARM EAGEN 64980-0181-<strong>06</strong> 0.95500 DIFLUNISAL 500 MG TABLET G RISING PHARM EAGUL 00527-1324-01 0.21320 DIGOX 125 MCG TABLET 0 LANNETT CO. INC EAGUL 00527-1324-10 0.21320 DIGOX 125 MCG TABLET 0 LANNETT CO. INC EAGUL 00527-1325-01 0.21320 DIGOX 250 MCG TABLET 0 LANNETT CO. INC EAGUL 00527-1325-10 0.21320 DIGOX 250 MCG TABLET 0 LANNETT CO. INC EAGEN 0<strong>06</strong>41-1410-35 1.40770 DIGOXIN 0.25 MG/ML AMPUL 0 WEST-WARD,INC. MLGEN 68094-0752-59 0.75600 DIGOXIN 0.25 MG/5 ML SOLUTION 0 PRECISION DOSE MLGEN 68094-0752-62 0.75600 DIGOXIN 0.25 MG/5 ML SOLUTION 0 PRECISION DOSE MLGUL 00115-9811-01 0.21320 DIGOXIN 125 MCG TABLET 0 GLOBAL PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00115-9811-03 0.21320 DIGOXIN 125 MCG TABLET 0 GLOBAL PHARM EAGEN 00143-1240-01 0.16275 DIGOXIN 125 MCG TABLET 0 WEST-WARD,INC. EAGEN 00143-1240-10 0.12618 DIGOXIN 125 MCG TABLET 0 WEST-WARD,INC. EAGEN 00143-1240-51 0.10962 DIGOXIN 125 MCG TABLET 0 WEST-WARD,INC. EAGUL 49884-0514-01 0.21320 DIGOXIN 125 MCG TABLET 0 PAR PHARM. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 110LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 49884-0514-10 0.21320 DIGOXIN 125 MCG TABLET 0 PAR PHARM. EAGUL 00115-9822-01 0.21320 DIGOXIN 250 MCG TABLET 0 GLOBAL PHARM EAGUL 00115-9822-03 0.21320 DIGOXIN 250 MCG TABLET 0 GLOBAL PHARM EAGEN 00143-1241-01 0.16275 DIGOXIN 250 MCG TABLET 0 WEST-WARD,INC. EAGEN 00143-1241-10 0.12618 DIGOXIN 250 MCG TABLET 0 WEST-WARD,INC. EAGEN 00143-1241-51 0.10962 DIGOXIN 250 MCG TABLET 0 WEST-WARD,INC. EAGUL 49884-0494-01 0.21320 DIGOXIN 250 MCG TABLET 0 PAR PHARM. EAGUL 49884-0494-10 0.21320 DIGOXIN 250 MCG TABLET 0 PAR PHARM. EAGEN 57664-0441-18 0.12825 DIGOXIN 250 MCG TABLET 0 CARACO PHARM EABND 00054-0057-46 0.64<strong>06</strong>1 DIGOXIN 50 MCG/ML SOLUTION 0 ROXANE LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00574-<strong>08</strong>50-05 59.10750 DIHYDROERGOTAMINE 1 MG/ML AM 0 PADDOCK LABS. MLGEN 00574-<strong>08</strong>50-10 56.15250 DIHYDROERGOTAMINE 1 MG/ML AM 0 PADDOCK LABS. MLBND 55390-0013-10 27.88800 DIHYDROERGOTAMINE 1 MG/ML VL 0 BEDFORD LABS MLGEN 52544-0484-01 0.73265 DILACOR XR 240 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00071-0369-24 0.09<strong>06</strong>0 DILANTIN 100 MG CAPSULE 0 PFIZER US PHARM EAGEX 00071-0369-32 0.09<strong>06</strong>0 DILANTIN 100 MG CAPSULE 0 PFIZER US PHARM EABEX 00071-2214-20 0.<strong>08</strong>830 0.31568 DILANTIN 125 MG/5 ML SUSP 0 PFIZER US PHARM MLBEX 00071-3740-66 0.51742 DILANTIN 30 MG CAPSULE 0 PFIZER US PHARM EAGEX 00071-0007-24 0.4<strong>06</strong>50 DILANTIN 50 MG INFATAB 0 PFIZER US PHARM EABND 52244-0920-10 2.74539 DILATRATE-SR 40 MG CAPSULE 0 AUXILIUM PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0014-<strong>06</strong> 0.53200 DILT XR 120 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0014-<strong>08</strong> 0.53200 DILT XR 120 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0015-<strong>06</strong> 0.66515 DILT XR 180 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0015-<strong>08</strong> 0.66515 DILT XR 180 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0016-<strong>06</strong> 0.73265 DILT XR 240 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0016-<strong>08</strong> 0.73265 DILT XR 240 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0010-04 0.85887 DILT-CD ER 300 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0010-<strong>08</strong> 0.85887 DILT-CD ER 300 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0007-04 0.42110 DILT-CD 120 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0007-<strong>08</strong> 0.42110 DILT-CD 120 MG CAPSULE 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-00<strong>08</strong>-04 0.41240 DILT-CD 180 MG CAPSULE 0 APOTEX CORP EAGEN 60505-00<strong>08</strong>-<strong>08</strong> 0.41240 DILT-CD 180 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0009-04 0.59454 DILT-CD 240 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0009-<strong>08</strong> 0.59454 DILT-CD 240 MG CAPSULE 0 APOTEX CORP EAGEN 62037-0548-01 0.53200 DILTIA XT 120 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0548-10 0.53200 DILTIA XT 120 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0549-01 0.66515 DILTIA XT 180 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0549-10 0.66515 DILTIA XT 180 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0550-01 0.73265 DILTIA XT 240 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0550-10 0.73265 DILTIA XT 240 MG CAPSULE 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00258-3687-90 0.62210 DILTIAZEM ER 120 MG CAPSULE 0 INWOOD LABS. EAGEN 00378-5220-01 0.53200 DILTIAZEM ER 120 MG CAPSULE 0 MYLAN EAGEN 00378-5220-05 0.53200 DILTIAZEM ER 120 MG CAPSULE 0 MYLAN EAGEN 47335-<strong>06</strong>69-13 0.62210 DILTIAZEM ER 120 MG CAPSULE 0 SUN PHARMA GLOB EAGEN 47335-<strong>06</strong>69-81 0.62210 DILTIAZEM ER 120 MG CAPSULE 0 SUN PHARMA GLOB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 111LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 47335-<strong>06</strong>69-83 0.62210 DILTIAZEM ER 120 MG CAPSULE 0 SUN PHARMA GLOB EAGEN 51079-0947-<strong>08</strong> 0.53200 DILTIAZEM ER 120 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 00378-6120-01 1.46812 DILTIAZEM ER 120 MG 12-HR CAP 0 MYLAN EAGEN 00258-3688-90 0.81330 DILTIAZEM ER 180 MG CAPSULE 0 INWOOD LABS. EAGEN 00378-5280-01 0.66515 DILTIAZEM ER 180 MG CAPSULE 0 MYLAN EAGEN 00378-5280-05 0.66515 DILTIAZEM ER 180 MG CAPSULE 0 MYLAN EAGEN 47335-<strong>06</strong>70-13 0.81330 DILTIAZEM ER 180 MG CAPSULE 0 SUN PHARMA GLOB EAGEN 47335-<strong>06</strong>70-81 0.81330 DILTIAZEM ER 180 MG CAPSULE 0 SUN PHARMA GLOB EAGEN 47335-<strong>06</strong>70-83 0.81330 DILTIAZEM ER 180 MG CAPSULE 0 SUN PHARMA GLOB EAGEN 00378-5340-01 0.73265 DILTIAZEM ER 240 MG CAPSULE 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5340-05 0.73265 DILTIAZEM ER 240 MG CAPSULE 0 MYLAN EAGEN 00378-6<strong>06</strong>0-01 0.80020 DILTIAZEM ER 60 MG 12-HR CAP 0 MYLAN EAGEN 00378-6090-01 0.88190 DILTIAZEM ER 90 MG 12-HR CAP 0 MYLAN EAGEN 00781-2452-05 0.62210 DILTIAZEM HCL ER 120 MG CAP 0 SANDOZ EAGEN 00781-2452-31 0.62210 DILTIAZEM HCL ER 120 MG CAP 0 SANDOZ EAGEN 00781-2452-92 0.62210 DILTIAZEM HCL ER 120 MG CAP 0 SANDOZ EAGEN 00228-1113-03 0.81330 DILTIAZEM HCL ER 180 MG CAP 0 SANDOZ EAGEN 00228-1113-09 0.81330 DILTIAZEM HCL ER 180 MG CAP 0 SANDOZ EAGEN 00781-2453-05 0.81330 DILTIAZEM HCL ER 180 MG CAP 0 SANDOZ EAGEN 00781-2453-31 0.81330 DILTIAZEM HCL ER 180 MG CAP 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-2453-92 0.81330 DILTIAZEM HCL ER 180 MG CAP 0 SANDOZ EAGEN 00258-3689-90 1.16285 DILTIAZEM HCL ER 240 MG CAP 0 INWOOD LABS. EAGEN 00781-2454-05 1.16285 DILTIAZEM HCL ER 240 MG CAP 0 SANDOZ EAGEN 00781-2454-31 1.16285 DILTIAZEM HCL ER 240 MG CAP 0 SANDOZ EAGEN 00781-2454-92 1.16285 DILTIAZEM HCL ER 240 MG CAP 0 SANDOZ EAGEN 47335-<strong>06</strong>71-13 1.16285 DILTIAZEM HCL ER 240 MG CAP 0 SUN PHARMA GLOB EAGEN 47335-<strong>06</strong>71-81 1.16285 DILTIAZEM HCL ER 240 MG CAP 0 SUN PHARMA GLOB EAGEN 47335-<strong>06</strong>71-83 1.16285 DILTIAZEM HCL ER 240 MG CAP 0 SUN PHARMA GLOB EAGEN 00258-3690-90 1.38010 DILTIAZEM HCL ER 300 MG CAP 0 INWOOD LABS. EAGEN 00781-2455-05 1.38010 DILTIAZEM HCL ER 300 MG CAP 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-2455-31 1.38010 DILTIAZEM HCL ER 300 MG CAP 0 SANDOZ EAGEN 00781-2455-92 1.38010 DILTIAZEM HCL ER 300 MG CAP 0 SANDOZ EAGEN 47335-<strong>06</strong>72-13 1.38010 DILTIAZEM HCL ER 300 MG CAP 0 SUN PHARMA GLOB EAGEN 47335-<strong>06</strong>72-81 1.38010 DILTIAZEM HCL ER 300 MG CAP 0 SUN PHARMA GLOB EAGEN 47335-<strong>06</strong>72-83 1.38010 DILTIAZEM HCL ER 300 MG CAP 0 SUN PHARMA GLOB EAGEN 00258-3691-90 1.54115 DILTIAZEM HCL ER 360 MG CAP 0 INWOOD LABS. EAGEN 00781-2456-05 1.54115 DILTIAZEM HCL ER 360 MG CAP 0 SANDOZ EAGEN 00781-2456-31 1.54115 DILTIAZEM HCL ER 360 MG CAP 0 SANDOZ EAGEN 00781-2456-92 1.54115 DILTIAZEM HCL ER 360 MG CAP 0 SANDOZ EAGEN 47335-<strong>06</strong>73-13 1.54115 DILTIAZEM HCL ER 360 MG CAP 0 SUN PHARMA GLOB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 47335-<strong>06</strong>73-81 1.54115 DILTIAZEM HCL ER 360 MG CAP 0 SUN PHARMA GLOB EAGEN 47335-<strong>06</strong>73-83 1.54115 DILTIAZEM HCL ER 360 MG CAP 0 SUN PHARMA GLOB EAGEN 00258-3692-90 1.76920 DILTIAZEM HCL ER 420 MG CAP 0 INWOOD LABS. EAGEN 00781-2457-05 1.76920 DILTIAZEM HCL ER 420 MG CAP 0 SANDOZ EAGEN 00781-2457-31 1.76920 DILTIAZEM HCL ER 420 MG CAP 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 112LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-2457-92 1.76920 DILTIAZEM HCL ER 420 MG CAP 0 SANDOZ EAGEN 38779-0227-05 9.66330 DILTIAZEM HCL POWDER 0 MEDISCA INC. GMGEN 00093-0321-01 0.13690 DILTIAZEM 120 MG TABLET 0 TEVA USA EAGEN 00378-0525-01 0.13690 DILTIAZEM 120 MG TABLET 0 MYLAN EAGEN 00228-2588-03 0.42110 DILTIAZEM 24HR CD 120 MG CAP 0 ACTAVIS PHARMA, EAGEN 00228-2588-09 0.42110 DILTIAZEM 24HR CD 120 MG CAP 0 ACTAVIS PHARMA, EAGEN 00228-2588-50 0.42110 DILTIAZEM 24HR CD 120 MG CAP 0 ACTAVIS PHARMA, EAGEN 49884-<strong>08</strong>29-05 0.42110 DILTIAZEM 24HR CD 120 MG CAP 0 PAR PHARM. EAGEN 49884-<strong>08</strong>29-09 0.42110 DILTIAZEM 24HR CD 120 MG CAP 0 PAR PHARM. EAGEN 49884-<strong>08</strong>29-11 0.42110 DILTIAZEM 24HR CD 120 MG CAP 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63739-0283-10 0.42110 DILTIAZEM 24HR CD 120 MG CAP 0 MCKESSON PACKAG EAGEN 63739-0283-31 0.42110 DILTIAZEM 24HR CD 120 MG CAP 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0052-01 0.32452 DILTIAZEM 24HR CD 120 MG CAP 0 AHP EAGEN 68<strong>08</strong>4-0052-30 0.42110 DILTIAZEM 24HR CD 120 MG CAP 0 AHP EAGEN 68<strong>08</strong>4-0052-90 0.42110 DILTIAZEM 24HR CD 120 MG CAP 0 AHP EAGEN 00228-2577-03 0.41240 DILTIAZEM 24HR CD 180 MG CAP 0 ACTAVIS PHARMA, EAGEN 00228-2577-09 0.41240 DILTIAZEM 24HR CD 180 MG CAP 0 ACTAVIS PHARMA, EAGEN 00228-2577-50 0.41240 DILTIAZEM 24HR CD 180 MG CAP 0 ACTAVIS PHARMA, EAGEN 49884-<strong>08</strong>30-05 0.41240 DILTIAZEM 24HR CD 180 MG CAP 0 PAR PHARM. EAGEN 49884-<strong>08</strong>30-09 0.41240 DILTIAZEM 24HR CD 180 MG CAP 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-<strong>08</strong>30-11 0.41240 DILTIAZEM 24HR CD 180 MG CAP 0 PAR PHARM. EAGEN 63739-0284-10 0.41240 DILTIAZEM 24HR CD 180 MG CAP 0 MCKESSON PACKAG EAGEN 63739-0284-31 0.41240 DILTIAZEM 24HR CD 180 MG CAP 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0053-01 0.38220 DILTIAZEM 24HR CD 180 MG CAP 0 AHP EAGEN 00228-2578-03 0.59454 DILTIAZEM 24HR CD 240 MG CAP 0 ACTAVIS PHARMA, EAGEN 00228-2578-09 0.59454 DILTIAZEM 24HR CD 240 MG CAP 0 ACTAVIS PHARMA, EAGEN 00228-2578-50 0.59454 DILTIAZEM 24HR CD 240 MG CAP 0 ACTAVIS PHARMA, EAGEN 49884-<strong>08</strong>31-05 0.59454 DILTIAZEM 24HR CD 240 MG CAP 0 PAR PHARM. EAGEN 49884-<strong>08</strong>31-09 0.59454 DILTIAZEM 24HR CD 240 MG CAP 0 PAR PHARM. EAGEN 49884-<strong>08</strong>31-11 0.59454 DILTIAZEM 24HR CD 240 MG CAP 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63739-0285-10 0.59454 DILTIAZEM 24HR CD 240 MG CAP 0 MCKESSON PACKAG EAGEN 63739-0285-31 0.59454 DILTIAZEM 24HR CD 240 MG CAP 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0054-30 0.59454 DILTIAZEM 24HR CD 240 MG CAP 0 AHP EAGEN 68<strong>08</strong>4-0054-90 0.59454 DILTIAZEM 24HR CD 240 MG CAP 0 AHP EAGEN 00228-2579-03 0.85887 DILTIAZEM 24HR CD 300 MG CAP 0 ACTAVIS PHARMA, EAGEN 00228-2579-09 0.85887 DILTIAZEM 24HR CD 300 MG CAP 0 ACTAVIS PHARMA, EAGEN 00228-2579-50 0.85887 DILTIAZEM 24HR CD 300 MG CAP 0 ACTAVIS PHARMA, EAGEN 49884-<strong>08</strong>32-05 0.85887 DILTIAZEM 24HR CD 300 MG CAP 0 PAR PHARM. EAGEN 49884-<strong>08</strong>32-09 0.85887 DILTIAZEM 24HR CD 300 MG CAP 0 PAR PHARM. EAGEN 49884-<strong>08</strong>32-11 0.85887 DILTIAZEM 24HR CD 300 MG CAP 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0055-30 0.85887 DILTIAZEM 24HR CD 300 MG CAP 0 AHP EAGEN 68<strong>08</strong>4-0055-90 0.85887 DILTIAZEM 24HR CD 300 MG CAP 0 AHP EAGEN 68682-0521-01 7.36883 DILTIAZEM 24HR CD 360 MG CAP 0 OCEANSIDE PHARM EAGEN 00093-5112-98 0.42110 DILTIAZEM 24HR ER 120 MG CAP 0 TEVA USA EAGEN 47335-<strong>06</strong>75-13 0.42110 DILTIAZEM 24HR ER 120 MG CAP 0 SUN PHARMA GLOB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 113LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 47335-<strong>06</strong>75-81 0.42110 DILTIAZEM 24HR ER 120 MG CAP 0 SUN PHARMA GLOB EAGEN 62584-0974-11 0.42110 DILTIAZEM 24HR ER 120 MG CAP 0 AHP EAGEN 62584-0974-30 0.42110 DILTIAZEM 24HR ER 120 MG CAP 0 AHP EAGEN 62584-0974-90 0.42110 DILTIAZEM 24HR ER 120 MG CAP 0 AHP EAGEN 00093-5117-98 0.41240 DILTIAZEM 24HR ER 180 MG CAP 0 TEVA USA EAGEN 47335-<strong>06</strong>76-13 0.41240 DILTIAZEM 24HR ER 180 MG CAP 0 SUN PHARMA GLOB EAGEN 47335-<strong>06</strong>76-81 0.41240 DILTIAZEM 24HR ER 180 MG CAP 0 SUN PHARMA GLOB EAGEN 62584-0975-11 0.41240 DILTIAZEM 24HR ER 180 MG CAP 0 AHP EAGEN 62584-0975-30 0.41240 DILTIAZEM 24HR ER 180 MG CAP 0 AHP EAGEN 62584-0975-90 0.41240 DILTIAZEM 24HR ER 180 MG CAP 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-5118-98 0.59454 DILTIAZEM 24HR ER 240 MG CAP 0 TEVA USA EAGEN 47335-<strong>06</strong>77-13 0.59454 DILTIAZEM 24HR ER 240 MG CAP 0 SUN PHARMA GLOB EAGEN 47335-<strong>06</strong>77-81 0.59454 DILTIAZEM 24HR ER 240 MG CAP 0 SUN PHARMA GLOB EAGEN 62584-0976-11 0.59454 DILTIAZEM 24HR ER 240 MG CAP 0 AHP EAGEN 62584-0976-30 0.59454 DILTIAZEM 24HR ER 240 MG CAP 0 AHP EAGEN 62584-0976-90 0.59454 DILTIAZEM 24HR ER 240 MG CAP 0 AHP EAGEN 00093-5119-98 0.85887 DILTIAZEM 24HR ER 300 MG CAP 0 TEVA USA EAGEN 47335-<strong>06</strong>78-13 0.85887 DILTIAZEM 24HR ER 300 MG CAP 0 SUN PHARMA GLOB EAGEN 47335-<strong>06</strong>78-81 0.85887 DILTIAZEM 24HR ER 300 MG CAP 0 SUN PHARMA GLOB EAGEN 62584-0977-11 0.85887 DILTIAZEM 24HR ER 300 MG CAP 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62584-0977-30 0.85887 DILTIAZEM 24HR ER 300 MG CAP 0 AHP EAGEN 62584-0977-90 0.85887 DILTIAZEM 24HR ER 300 MG CAP 0 AHP EAGEN 00228-2918-09 7.46000 DILTIAZEM 24HR ER 360 MG CAP 0 ACTAVIS PHARMA, EAGEN 47335-<strong>06</strong>79-81 7.46000 DILTIAZEM 24HR ER 360 MG CAP 0 SUN PHARMA GLOB EAGEN 00093-0318-01 0.<strong>06</strong>129 DILTIAZEM 30 MG TABLET 0 TEVA USA EAGEN 00093-0318-05 0.<strong>06</strong>129 DILTIAZEM 30 MG TABLET 0 TEVA USA EAGEN 00378-0023-01 0.<strong>06</strong>129 DILTIAZEM 30 MG TABLET 0 MYLAN EAGEN 00378-0023-05 0.<strong>06</strong>129 DILTIAZEM 30 MG TABLET 0 MYLAN EAGEN 51079-0745-17 0.<strong>06</strong>129 DILTIAZEM 30 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0745-20 0.<strong>06</strong>129 DILTIAZEM 30 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63739-0079-10 0.<strong>06</strong>129 DILTIAZEM 30 MG TABLET 0 MCKESSON PACKAG EAGEN 00093-0319-01 0.1<strong>06</strong>92 DILTIAZEM 60 MG TABLET 0 TEVA USA EAGEN 00093-0319-05 0.1<strong>06</strong>92 DILTIAZEM 60 MG TABLET 0 TEVA USA EAGEN 00378-0045-01 0.1<strong>06</strong>92 DILTIAZEM 60 MG TABLET 0 MYLAN EAGEN 00378-0045-05 0.1<strong>06</strong>92 DILTIAZEM 60 MG TABLET 0 MYLAN EAGEN 51079-0746-17 0.1<strong>06</strong>92 DILTIAZEM 60 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0746-20 0.1<strong>06</strong>92 DILTIAZEM 60 MG TABLET 0 MYLAN INSTITUTI EAGEN 63739-0<strong>08</strong>0-10 0.1<strong>06</strong>92 DILTIAZEM 60 MG TABLET 0 MCKESSON PACKAG EAGEN 00093-0320-01 0.09135 DILTIAZEM 90 MG TABLET 0 TEVA USA EAGEN 00378-0135-01 0.09135 DILTIAZEM 90 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0135-05 0.09135 DILTIAZEM 90 MG TABLET 0 MYLAN EAGEN 51079-0747-20 0.09135 DILTIAZEM 90 MG TABLET 0 MYLAN INSTITUTI EAGEN 60505-0210-03 0.62210 DILTZAC ER 120 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0210-09 0.62210 DILTZAC ER 120 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0211-03 0.81330 DILTZAC ER 180 MG CAPSULE 0 APOTEX CORP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 114LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0211-09 0.81330 DILTZAC ER 180 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0212-03 1.16285 DILTZAC ER 240 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0212-09 1.16285 DILTZAC ER 240 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0213-03 1.38010 DILTZAC ER 300 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0213-09 1.38010 DILTZAC ER 300 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0214-03 1.54115 DILTZAC ER 360 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0214-09 1.54115 DILTZAC ER 360 MG CAPSULE 0 APOTEX CORP EAGEN 00703-9258-09 0.15750 DILUENT FOR EPOPROSTENOL VIAL 0 TEVA PARENTERAL MLBND 00173-0518-01 0.28037 DILUENT FOR FLOLAN VIAL 0 GLAXOSMITHKLINE MLGEN 38779-<strong>06</strong>14-01 1.01531 DIMETHYL SULFOXIDE LIQUID 0 MEDISCA INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0315-15 5.00102 5.00102 DIOVAN HCT 160-12.5 MG TAB G NOVARTIS EABND 00078-0315-17 3.78797 3.78797 DIOVAN HCT 160-12.5 MG TAB G NOVARTIS EABND 00078-0315-34 5.00<strong>08</strong>4 5.00<strong>08</strong>4 DIOVAN HCT 160-12.5 MG TAB G NOVARTIS EABND 00078-0315-67 5.00107 5.00107 DIOVAN HCT 160-12.5 MG TAB G NOVARTIS EABND 00078-0383-15 5.67111 5.67111 DIOVAN HCT 160-25 MG TABLET G NOVARTIS EABND 00078-0383-17 4.29573 4.29573 DIOVAN HCT 160-25 MG TABLET G NOVARTIS EABND 00078-0383-34 5.67129 5.67129 DIOVAN HCT 160-25 MG TABLET G NOVARTIS EABND 00078-0383-67 5.67111 5.67111 DIOVAN HCT 160-25 MG TABLET G NOVARTIS EABND 00078-0471-11 4.79901 4.79901 DIOVAN HCT 320-12.5 MG TAB G NOVARTIS EABND 00078-0471-15 6.33566 6.33566 DIOVAN HCT 320-12.5 MG TAB G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0471-34 6.33585 6.33585 DIOVAN HCT 320-12.5 MG TAB G NOVARTIS EABND 00078-0471-67 6.33548 6.33548 DIOVAN HCT 320-12.5 MG TAB G NOVARTIS EABND 00078-0472-11 5.44457 5.44457 DIOVAN HCT 320-25 MG TABLET G NOVARTIS EABND 00078-0472-15 7.18752 7.18752 DIOVAN HCT 320-25 MG TABLET G NOVARTIS EABND 00078-0472-34 7.18798 7.18798 DIOVAN HCT 320-25 MG TABLET G NOVARTIS EABND 00078-0472-67 7.18826 7.18826 DIOVAN HCT 320-25 MG TABLET G NOVARTIS EABND 00078-0314-33 3.48153 3.48153 DIOVAN HCT 80-12.5 MG TABLET G NOVARTIS EABND 00078-0314-34 4.59617 4.59617 DIOVAN HCT 80-12.5 MG TABLET G NOVARTIS EABND 00078-0359-17 3.48<strong>06</strong>0 DIOVAN 160 MG TABLET G NOVARTIS EABND 00078-0359-34 4.61480 DIOVAN 160 MG TABLET G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0360-11 4.40301 DIOVAN 320 MG TABLET G NOVARTIS EABND 00078-0360-34 5.83803 DIOVAN 320 MG TABLET G NOVARTIS EABND 00078-0423-15 3.59002 DIOVAN 40 MG TABLET G NOVARTIS EABND 00078-0358-33 3.23690 DIOVAN 80 MG TABLET G NOVARTIS EABND 00078-0358-34 4.29165 DIOVAN 80 MG TABLET G NOVARTIS EABND 68220-0160-10 6.27189 DIPENTUM 250 MG CAPSULE 0 MEDA PHARMACEUT EAGEX 00555-0059-02 0.02660 DIPHENHYDRAMINE 50 MG CAPSULE 0 BARR EAGEX 00555-0059-05 0.02660 DIPHENHYDRAMINE 50 MG CAPSULE 0 BARR EAGEX 0<strong>06</strong>41-0376-21 0.73500 DIPHENHYDRAMINE 50 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-0376-25 0.73800 DIPHENHYDRAMINE 50 MG/ML VIAL 0 WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 63323-<strong>06</strong>64-01 0.83300 DIPHENHYDRAMINE 50 MG/ML VIAL 0 APP PHARMACEUTI MLBND 00<strong>08</strong>5-0517-01 0.31320 4.63693 DIPROLENE AF 0.05% CREAM G MERCK SHARP & D GMBND 00<strong>08</strong>5-0517-04 0.31320 3.11167 DIPROLENE AF 0.05% CREAM G MERCK SHARP & D GMBND 00<strong>08</strong>5-0962-01 1.30980 2.66042 DIPROLENE 0.05% LOTION G MERCK SHARP & D MLBND 00<strong>08</strong>5-0962-02 1.30980 2.62169 DIPROLENE 0.05% LOTION G MERCK SHARP & D ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 115LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>5-0575-02 3.01220 4.63693 DIPROLENE 0.05% OINTMENT G MERCK SHARP & D GMBND 00<strong>08</strong>5-0575-05 3.01220 3.11167 DIPROLENE 0.05% OINTMENT G MERCK SHARP & D GMGEN 00054-0434-25 0.14760 DIPYRIDAMOLE 25 MG TABLET 0 ROXANE LABS. EAGEN 00115-1070-01 0.14760 DIPYRIDAMOLE 25 MG TABLET 0 GLOBAL PHARM EAGEN 00115-1070-03 0.14760 DIPYRIDAMOLE 25 MG TABLET 0 GLOBAL PHARM EAGEN 00555-0252-02 0.14760 DIPYRIDAMOLE 25 MG TABLET 0 BARR EAGEN 00555-0252-05 0.14760 DIPYRIDAMOLE 25 MG TABLET 0 BARR EAGEN 64980-0133-01 0.14760 DIPYRIDAMOLE 25 MG TABLET 0 RISING PHARM EAGEN 64980-0133-10 0.14760 DIPYRIDAMOLE 25 MG TABLET 0 RISING PHARM EAGEN 68382-0187-01 0.14760 DIPYRIDAMOLE 25 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0187-05 0.14760 DIPYRIDAMOLE 25 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00054-0435-25 0.17630 DIPYRIDAMOLE 50 MG TABLET 0 ROXANE LABS. EAGEN 00115-1071-01 0.17630 DIPYRIDAMOLE 50 MG TABLET 0 GLOBAL PHARM EAGEN 00115-1071-03 0.17630 DIPYRIDAMOLE 50 MG TABLET 0 GLOBAL PHARM EAGEN 00555-0285-02 0.17630 DIPYRIDAMOLE 50 MG TABLET 0 BARR EAGEN 00555-0285-05 0.17630 DIPYRIDAMOLE 50 MG TABLET 0 BARR EAGEN 64980-0134-01 0.17630 DIPYRIDAMOLE 50 MG TABLET 0 RISING PHARM EAGEN 64980-0134-10 0.17630 DIPYRIDAMOLE 50 MG TABLET 0 RISING PHARM EAGEN 68382-0188-01 0.17630 DIPYRIDAMOLE 50 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0188-05 0.17630 DIPYRIDAMOLE 50 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0436-25 0.36500 DIPYRIDAMOLE 75 MG TABLET 0 ROXANE LABS. EAGEN 00115-1072-01 0.36500 DIPYRIDAMOLE 75 MG TABLET 0 GLOBAL PHARM EAGEN 00115-1072-03 0.36500 DIPYRIDAMOLE 75 MG TABLET 0 GLOBAL PHARM EAGEN 00555-0286-02 0.36500 DIPYRIDAMOLE 75 MG TABLET 0 BARR EAGEN 00555-0286-05 0.36500 DIPYRIDAMOLE 75 MG TABLET 0 BARR EAGEN 64980-0135-01 0.36500 DIPYRIDAMOLE 75 MG TABLET 0 RISING PHARM EAGEN 64980-0135-10 0.36500 DIPYRIDAMOLE 75 MG TABLET 0 RISING PHARM EAGEN 68382-0189-01 0.36500 DIPYRIDAMOLE 75 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0189-05 0.36500 DIPYRIDAMOLE 75 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00093-3127-01 0.31000 DISOPYRAMIDE 100 MG CAPSULE 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-5560-01 0.31000 DISOPYRAMIDE 100 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00093-3129-01 0.41<strong>08</strong>2 DISOPYRAMIDE 150 MG CAPSULE 0 TEVA USA EAGEN 00591-5561-01 0.41<strong>08</strong>2 DISOPYRAMIDE 150 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00093-5035-01 2.46632 DISULFIRAM 250 MG TABLET 0 TEVA USA EAGEN 47781-<strong>06</strong>07-30 2.46632 DISULFIRAM 250 MG TABLET 0 ALVOGEN INC EAGEN 64980-0171-01 2.46632 DISULFIRAM 250 MG TABLET 0 RISING PHARM EAGEN 00093-5036-01 4.71117 DISULFIRAM 500 MG TABLET 0 TEVA USA EAGEN 64980-0172-01 4.71117 DISULFIRAM 500 MG TABLET 0 RISING PHARM EABND 50458-<strong>08</strong>10-01 1.290<strong>06</strong> 5.24576 DITROPAN XL 10 MG TABLET G JANSSEN PHARM. EABND 50458-<strong>08</strong>15-01 2.17500 5.37698 DITROPAN XL 15 MG TABLET G JANSSEN PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50458-<strong>08</strong>05-01 1.24956 5.24037 DITROPAN XL 5 MG TABLET G JANSSEN PHARM. EABND 65649-0311-12 0.22189 DIURIL 250 MG/5 ML ORAL SUSP 0 SALIX PHARMACEU MLGEX 00093-7439-01 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 TEVA USA EAGEX 00245-0180-01 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 UPSHER SMITH EAGEX 00245-0180-11 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 UPSHER SMITH EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 116LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00245-0180-15 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 UPSHER SMITH EAGEX 00245-0180-89 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 UPSHER SMITH EAGEX 00378-1043-01 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 MYLAN EAGEX 0<strong>06</strong>03-3441-21 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 QUALITEST EAGEX 0<strong>06</strong>03-3441-25 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 QUALITEST EAGEX 16714-0511-01 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 NORTHSTAR RX LL EAGEX 29300-0138-01 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 UNICHEM PHARMAC EAGEX 29300-0138-05 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 UNICHEM PHARMAC EAGEX 60505-3<strong>06</strong>5-01 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 APOTEX CORP EAGEX 62756-0796-13 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62756-0796-88 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 SUN PHARMACEUTI EAGEX 65862-0401-01 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 AUROBINDO PHARM EAGEX 65862-0401-05 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 AUROBINDO PHARM EAGEX 68180-0265-01 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 LUPIN PHARMACEU EAGEX 68382-0031-01 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 ZYDUS PHARMACEU EAGEX 68382-0031-05 0.05220 DIVALPROEX SOD DR 125 MG TAB 0 ZYDUS PHARMACEU EAGEX 00093-7440-01 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 TEVA USA EAGEX 00093-7440-05 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 TEVA USA EAGEX 00245-0181-11 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 UPSHER SMITH EAGEX 00245-0181-15 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-1044-01 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 MYLAN EAGEX 0<strong>06</strong>03-3442-21 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 QUALITEST EAGEX 0<strong>06</strong>03-3442-28 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 QUALITEST EAGEX 16714-0512-01 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 NORTHSTAR RX LL EAGEX 16714-0512-02 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 NORTHSTAR RX LL EAGEX 29300-0139-01 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 UNICHEM PHARMAC EAGEX 29300-0139-05 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 UNICHEM PHARMAC EAGEX 51079-0474-01 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 MYLAN INSTITUTI EAGEX 51079-0474-20 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 MYLAN INSTITUTI EAGEX 60505-3<strong>06</strong>6-01 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-3<strong>06</strong>6-05 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 APOTEX CORP EAGEX 62756-0797-13 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 SUN PHARMACEUTI EAGEX 62756-0797-88 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 SUN PHARMACEUTI EAGEX 63739-0553-10 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 MCKESSON PACKAG EAGEX 65862-0402-01 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 AUROBINDO PHARM EAGEX 65862-0402-05 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0314-01 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 AHP EAGEX 68<strong>08</strong>4-0314-11 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 AHP EAGEX 68180-0266-01 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 LUPIN PHARMACEU EAGEX 68180-0266-02 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68382-0032-01 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 ZYDUS PHARMACEU EAGEX 68382-0032-05 0.<strong>08</strong>897 DIVALPROEX SOD DR 250 MG TAB 0 ZYDUS PHARMACEU EAGEX 00093-7441-01 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 TEVA USA EAGEX 00093-7441-05 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 TEVA USA EAGEX 00245-0182-11 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 UPSHER SMITH EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 117LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00245-0182-15 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 UPSHER SMITH EAGEX 00245-0182-89 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 UPSHER SMITH EAGEX 00378-1045-01 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 MYLAN EAGEX 0<strong>06</strong>03-3443-21 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 QUALITEST EAGEX 0<strong>06</strong>03-3443-28 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 QUALITEST EAGEX 16714-0513-01 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 NORTHSTAR RX LL EAGEX 16714-0513-02 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 NORTHSTAR RX LL EAGEX 29300-0140-01 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 UNICHEM PHARMAC EAGEX 29300-0140-05 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 UNICHEM PHARMAC EAGEX 51079-0475-<strong>08</strong> 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-3<strong>06</strong>7-01 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 APOTEX CORP EAGEX 60505-3<strong>06</strong>7-05 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 APOTEX CORP EAGEX 62756-0798-13 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 SUN PHARMACEUTI EAGEX 62756-0798-88 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 SUN PHARMACEUTI EAGEX 63739-0554-10 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 MCKESSON PACKAG EAGEX 65862-0403-01 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0315-01 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 AHP EAGEX 68<strong>08</strong>4-0315-11 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 AHP EAGEX 68180-0267-01 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 LUPIN PHARMACEU EAGEX 68180-0267-02 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68382-0033-01 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 ZYDUS PHARMACEU EAGEX 68382-0033-05 0.15593 DIVALPROEX SOD DR 500 MG TAB 0 ZYDUS PHARMACEU EAGEX 00115-6911-01 1.26<strong>08</strong>2 DIVALPROEX SOD ER 250 MG TAB 0 GLOBAL PHARM EAGEX 00115-6911-02 1.19778 DIVALPROEX SOD ER 250 MG TAB 0 GLOBAL PHARM EAGEX 00378-0472-01 1.84110 DIVALPROEX SOD ER 250 MG TAB 0 MYLAN EAGEX 00378-0472-05 1.84110 DIVALPROEX SOD ER 250 MG TAB 0 MYLAN EAGEX 00378-0472-77 1.841<strong>08</strong> DIVALPROEX SOD ER 250 MG TAB 0 MYLAN EAGEX 00904-5990-61 1.59682 DIVALPROEX SOD ER 250 MG TAB 0 MAJOR PHARMACEU EAGEX 10370-0510-10 1.84110 DIVALPROEX SOD ER 250 MG TAB 0 PAR PHARM. EAGEX 10370-0510-50 1.84110 DIVALPROEX SOD ER 250 MG TAB 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0766-01 1.83750 DIVALPROEX SOD ER 250 MG TAB 0 MYLAN INSTITUTI EAGEX 51079-0766-<strong>08</strong> 1.841<strong>06</strong> DIVALPROEX SOD ER 250 MG TAB 0 MYLAN INSTITUTI EAGEX 55111-0533-01 1.84110 DIVALPROEX SOD ER 250 MG TAB 0 DR.REDDY'S LAB EAGEX 55111-0533-05 1.84110 DIVALPROEX SOD ER 250 MG TAB 0 DR.REDDY'S LAB EAGEX 64679-0724-01 1.32725 DIVALPROEX SOD ER 250 MG TAB 0 WOCKHARDT USA L EAGEX 64679-0724-02 1.26<strong>08</strong>2 DIVALPROEX SOD ER 250 MG TAB 0 WOCKHARDT USA L EAGEX 64679-0724-03 1.19778 DIVALPROEX SOD ER 250 MG TAB 0 WOCKHARDT USA L EAGEX 68001-0105-00 1.84110 DIVALPROEX SOD ER 250 MG TAB 0 BLUEPOINT LABOR EAGEX 68001-0105-03 1.84110 DIVALPROEX SOD ER 250 MG TAB 0 BLUEPOINT LABOR EAGEX 68<strong>08</strong>4-0310-01 1.83525 DIVALPROEX SOD ER 250 MG TAB 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0316-01 1.79250 DIVALPROEX SOD ER 250 MG TAB 0 AHP EAGEX 68<strong>08</strong>4-0316-11 1.79250 DIVALPROEX SOD ER 250 MG TAB 0 AHP EAGEX 68382-0314-01 1.84110 DIVALPROEX SOD ER 250 MG TAB 0 ZYDUS PHARMACEU EAGEX 00115-6922-01 2.21520 DIVALPROEX SOD ER 500 MG TAB 0 GLOBAL PHARM EAGEX 00115-6922-02 2.21520 DIVALPROEX SOD ER 500 MG TAB 0 GLOBAL PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 118LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-0473-01 3.05617 DIVALPROEX SOD ER 500 MG TAB 0 MYLAN EAGEX 00378-0473-05 3.05617 DIVALPROEX SOD ER 500 MG TAB 0 MYLAN EAGEX 00378-0473-77 3.05616 DIVALPROEX SOD ER 500 MG TAB 0 MYLAN EAGEX 00904-6073-61 3.30465 DIVALPROEX SOD ER 500 MG TAB 0 MAJOR PHARMACEU EAGEX 10370-0511-10 3.05617 DIVALPROEX SOD ER 500 MG TAB 0 PAR PHARM. EAGEX 10370-0511-50 3.05617 DIVALPROEX SOD ER 500 MG TAB 0 PAR PHARM. EAGEX 51079-0767-01 3.05250 DIVALPROEX SOD ER 500 MG TAB 0 MYLAN INSTITUTI EAGEX 51079-0767-<strong>08</strong> 3.05616 DIVALPROEX SOD ER 500 MG TAB 0 MYLAN INSTITUTI EAGEX 51079-0767-30 3.05250 DIVALPROEX SOD ER 500 MG TAB 0 MYLAN INSTITUTI EAGEX 51079-0767-56 3.05615 DIVALPROEX SOD ER 500 MG TAB 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 55111-0534-01 3.05617 DIVALPROEX SOD ER 500 MG TAB 0 DR.REDDY'S LAB EAGEX 55111-0534-05 3.05617 DIVALPROEX SOD ER 500 MG TAB 0 DR.REDDY'S LAB EAGEX 64679-0725-01 2.21775 DIVALPROEX SOD ER 500 MG TAB 0 WOCKHARDT USA L EAGEX 64679-0725-02 2.21775 DIVALPROEX SOD ER 500 MG TAB 0 WOCKHARDT USA L EAGEX 64679-0725-03 2.21776 DIVALPROEX SOD ER 500 MG TAB 0 WOCKHARDT USA L EAGEX 68001-01<strong>06</strong>-00 3.05617 DIVALPROEX SOD ER 500 MG TAB 0 BLUEPOINT LABOR EAGEX 68001-01<strong>06</strong>-03 3.05617 DIVALPROEX SOD ER 500 MG TAB 0 BLUEPOINT LABOR EAGEX 68<strong>08</strong>4-0317-01 2.87400 DIVALPROEX SOD ER 500 MG TAB 0 AHP EAGEX 68<strong>08</strong>4-0317-11 2.87250 DIVALPROEX SOD ER 500 MG TAB 0 AHP EAGEX 68<strong>08</strong>4-0415-01 2.87400 DIVALPROEX SOD ER 500 MG TAB 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68382-0315-01 3.05617 DIVALPROEX SOD ER 500 MG TAB 0 ZYDUS PHARMACEU EAGEX 68382-0315-05 3.05617 DIVALPROEX SOD ER 500 MG TAB 0 ZYDUS PHARMACEU EAGEX 00378-80<strong>08</strong>-01 0.67237 DIVALPROEX SODIUM 125 MG CAP G MYLAN EAGEX 00378-80<strong>08</strong>-05 0.67237 DIVALPROEX SODIUM 125 MG CAP G MYLAN EAGEX 51079-0765-01 0.67237 DIVALPROEX SODIUM 125 MG CAP G MYLAN INSTITUTI EAGEX 51079-0765-<strong>08</strong> 0.67237 DIVALPROEX SODIUM 125 MG CAP G MYLAN INSTITUTI EAGEX 51079-0765-30 0.67237 DIVALPROEX SODIUM 125 MG CAP G MYLAN INSTITUTI EAGEX 51079-0765-56 0.67237 DIVALPROEX SODIUM 125 MG CAP G MYLAN INSTITUTI EAGEX 55111-0532-01 0.67237 DIVALPROEX SODIUM 125 MG CAP G DR.REDDY'S LAB EAGEX 55111-0532-05 0.67237 DIVALPROEX SODIUM 125 MG CAP G DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0313-01 0.59595 DIVALPROEX SODIUM 125 MG CAP G AHP EAGEX 68<strong>08</strong>4-0313-11 0.59595 DIVALPROEX SODIUM 125 MG CAP G AHP EAGEX 68382-01<strong>06</strong>-01 0.67207 DIVALPROEX SODIUM 125 MG CAP G ZYDUS PHARMACEU EAGEX 68382-01<strong>06</strong>-10 0.67207 DIVALPROEX SODIUM 125 MG CAP G ZYDUS PHARMACEU EABND 00245-<strong>08</strong>80-30 3.0<strong>08</strong>75 DIVIGEL 0.25 MG GEL PACKET 0 UPSHER SMITH EABND 00245-<strong>08</strong>81-30 3.0<strong>08</strong>75 DIVIGEL 0.5 MG GEL PACKET 0 UPSHER SMITH EABND 00245-<strong>08</strong>82-30 3.0<strong>08</strong>75 DIVIGEL 1 MG GEL PACKET 0 UPSHER SMITH GMGEN 00338-1077-02 0.07387 DOBUTAMINE 1 GM-D5W 250 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-2344-02 0.19845 DOBUTAMINE 12.5 MG/ML VIAL 0 HOSPIRA MLGEN 55390-0560-90 0.17325 DOBUTAMINE 12.5 MG/ML VIAL 0 BEDFORD LABS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00338-1073-02 0.05396 DOBUTAMINE 250 MG-D5W 250 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-1075-02 0.05470 DOBUTAMINE 500 MG-D5W 250 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 66758-0050-03 72.65610 DOCETAXEL 160 MG/16 ML VIAL 0 SANDOZ MLBND 16729-0267-65 303.07450 DOCETAXEL 160 MG/8 ML VIAL 0 ACCORD <strong>HEALTH</strong>CA MLGEN 16729-0267-63 112.69875 DOCETAXEL 20 MG/ML VIAL 0 ACCORD <strong>HEALTH</strong>CA ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 119LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 25021-0222-01 112.69875 DOCETAXEL 20 MG/ML VIAL 0 SAGENT PHARMACE MLGEN 66758-0050-01 63.9<strong>08</strong>75 DOCETAXEL 20 MG/2 ML VIAL 0 SANDOZ MLGEN 16729-0267-64 112.82431 DOCETAXEL 80 MG/4 ML VIAL 0 ACCORD <strong>HEALTH</strong>CA MLGEN 25021-0222-04 112.82431 DOCETAXEL 80 MG/4 ML VIAL 0 SAGENT PHARMACE MLGEN 66758-0050-02 64.40075 DOCETAXEL 80 MG/8 ML VIAL 0 SANDOZ MLBND 59630-0074-10 5.98737 DOLGIC PLUS TABLET 0 SHIONOGI PHARMA EABND 68453-0074-10 4.33865 DOLGIC PLUS TABLET 0 SHIONOGI PHARMA EAGEN 00781-5277-64 0.28310 DONEPEZIL HCL ODT 10 MG TABLET 0 SANDOZ EAGEN 33342-0030-07 0.28310 DONEPEZIL HCL ODT 10 MG TABLET 0 MACLEODS PHARMA EAGEN 59762-0252-01 0.28310 DONEPEZIL HCL ODT 10 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63739-<strong>06</strong>53-10 0.28310 DONEPEZIL HCL ODT 10 MG TABLET 0 MCKESSON PACKAG EAGEN 68382-0347-<strong>06</strong> 0.28310 DONEPEZIL HCL ODT 10 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00781-5276-64 0.28310 DONEPEZIL HCL ODT 5 MG TABLET 0 SANDOZ EAGEN 33342-0029-07 0.28310 DONEPEZIL HCL ODT 5 MG TABLET 0 MACLEODS PHARMA EAGEN 59762-0250-01 0.28310 DONEPEZIL HCL ODT 5 MG TABLET 0 GREENSTONE LLC. EAGEN 63739-<strong>06</strong>52-10 0.28310 DONEPEZIL HCL ODT 5 MG TABLET 0 MCKESSON PACKAG EAGEN 68382-0346-<strong>06</strong> 0.28310 DONEPEZIL HCL ODT 5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00093-0739-05 0.07398 DONEPEZIL HCL 10 MG TABLET 0 TEVA USA EAGEN 00093-0739-56 0.07398 DONEPEZIL HCL 10 MG TABLET 0 TEVA USA EAGEN 00093-0739-98 0.07398 DONEPEZIL HCL 10 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-9748-09 0.07398 DONEPEZIL HCL 10 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-9748-30 0.07398 DONEPEZIL HCL 10 MG TABLET 0 WEST-WARD,INC. EAGEN 00781-5275-10 0.07398 DONEPEZIL HCL 10 MG TABLET 0 SANDOZ EAGEN 00781-5275-13 0.07398 DONEPEZIL HCL 10 MG TABLET 0 SANDOZ EAGEN 00781-5275-31 0.07398 DONEPEZIL HCL 10 MG TABLET 0 SANDOZ EAGEN 00781-5275-92 0.07398 DONEPEZIL HCL 10 MG TABLET 0 SANDOZ EAGEN 00904-6243-61 0.07398 DONEPEZIL HCL 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6355-61 0.07398 DONEPEZIL HCL 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 13668-0103-10 0.07398 DONEPEZIL HCL 10 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0103-26 0.07398 DONEPEZIL HCL 10 MG TABLET 0 TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-0103-30 0.07398 DONEPEZIL HCL 10 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0103-90 0.07398 DONEPEZIL HCL 10 MG TABLET 0 TORRENT PHARMAC EAGEN 43547-0276-11 0.07398 DONEPEZIL HCL 10 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 55111-0357-05 0.07398 DONEPEZIL HCL 10 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0357-10 0.07398 DONEPEZIL HCL 10 MG TABLET 0 DR.REDDY'S LAB EAGEN 59746-0330-30 0.07398 DONEPEZIL HCL 10 MG TABLET 0 CADISTA PHARMAC EAGEN 59746-0330-90 0.07398 DONEPEZIL HCL 10 MG TABLET 0 CADISTA PHARMAC EAGEN 59762-0246-01 0.07398 DONEPEZIL HCL 10 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-0246-02 0.07398 DONEPEZIL HCL 10 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-0246-03 0.07398 DONEPEZIL HCL 10 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-0246-04 0.07398 DONEPEZIL HCL 10 MG TABLET 0 GREENSTONE LLC. EAGEN 62756-0445-18 0.07398 DONEPEZIL HCL 10 MG TABLET 0 SUN PHARMACEUTI EAGEN 62756-0445-81 0.07398 DONEPEZIL HCL 10 MG TABLET 0 SUN PHARMACEUTI EAGEN 62756-0445-83 0.07398 DONEPEZIL HCL 10 MG TABLET 0 SUN PHARMACEUTI EAGEN 63304-0129-10 0.07398 DONEPEZIL HCL 10 MG TABLET 0 RANBAXY PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 120LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63304-0129-30 0.07398 DONEPEZIL HCL 10 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-0129-90 0.07398 DONEPEZIL HCL 10 MG TABLET 0 RANBAXY PHARMAC EAGEN 63739-<strong>06</strong>68-10 0.07398 DONEPEZIL HCL 10 MG TABLET 0 MCKESSON PACKAG EAGEN 64679-0312-01 0.07398 DONEPEZIL HCL 10 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0312-03 0.07398 DONEPEZIL HCL 10 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0312-05 0.07398 DONEPEZIL HCL 10 MG TABLET 0 WOCKHARDT USA L EAGEN 65862-0326-30 0.07398 DONEPEZIL HCL 10 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0326-90 0.07398 DONEPEZIL HCL 10 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0326-99 0.07398 DONEPEZIL HCL 10 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0478-01 0.07398 DONEPEZIL HCL 10 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0478-11 0.07398 DONEPEZIL HCL 10 MG TABLET 0 AHP EAGEN 00093-0738-05 0.05940 DONEPEZIL HCL 5 MG TABLET 0 TEVA USA EAGEN 00093-0738-56 0.05940 DONEPEZIL HCL 5 MG TABLET 0 TEVA USA EAGEN 00093-0738-98 0.05940 DONEPEZIL HCL 5 MG TABLET 0 TEVA USA EAGEN 00143-9747-09 0.05940 DONEPEZIL HCL 5 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-9747-30 0.05940 DONEPEZIL HCL 5 MG TABLET 0 WEST-WARD,INC. EAGEN 00781-5274-10 0.05940 DONEPEZIL HCL 5 MG TABLET 0 SANDOZ EAGEN 00781-5274-13 0.05940 DONEPEZIL HCL 5 MG TABLET 0 SANDOZ EAGEN 00781-5274-31 0.05940 DONEPEZIL HCL 5 MG TABLET 0 SANDOZ EAGEN 00781-5274-92 0.05940 DONEPEZIL HCL 5 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6242-61 0.05940 DONEPEZIL HCL 5 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6354-61 0.05940 DONEPEZIL HCL 5 MG TABLET 0 MAJOR PHARMACEU EAGEN 13668-0102-05 0.05940 DONEPEZIL HCL 5 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0102-10 0.05940 DONEPEZIL HCL 5 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0102-30 0.05940 DONEPEZIL HCL 5 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0102-90 0.05940 DONEPEZIL HCL 5 MG TABLET 0 TORRENT PHARMAC EAGEN 55111-0356-05 0.05940 DONEPEZIL HCL 5 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0356-10 0.05940 DONEPEZIL HCL 5 MG TABLET 0 DR.REDDY'S LAB EAGEN 59746-0329-30 0.05940 DONEPEZIL HCL 5 MG TABLET 0 CADISTA PHARMAC EAGEN 59746-0329-90 0.05940 DONEPEZIL HCL 5 MG TABLET 0 CADISTA PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-0245-01 0.05940 DONEPEZIL HCL 5 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-0245-02 0.05940 DONEPEZIL HCL 5 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-0245-03 0.05940 DONEPEZIL HCL 5 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-0245-04 0.05940 DONEPEZIL HCL 5 MG TABLET 0 GREENSTONE LLC. EAGEN 62756-0440-18 0.05940 DONEPEZIL HCL 5 MG TABLET 0 SUN PHARMACEUTI EAGEN 62756-0440-81 0.05940 DONEPEZIL HCL 5 MG TABLET 0 SUN PHARMACEUTI EAGEN 62756-0440-83 0.05940 DONEPEZIL HCL 5 MG TABLET 0 SUN PHARMACEUTI EAGEN 63304-0128-10 0.05940 DONEPEZIL HCL 5 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-0128-30 0.05940 DONEPEZIL HCL 5 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-0128-90 0.05940 DONEPEZIL HCL 5 MG TABLET 0 RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63739-<strong>06</strong>67-10 0.05940 DONEPEZIL HCL 5 MG TABLET 0 MCKESSON PACKAG EAGEN 64679-0311-01 0.05940 DONEPEZIL HCL 5 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0311-03 0.05940 DONEPEZIL HCL 5 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0311-05 0.05940 DONEPEZIL HCL 5 MG TABLET 0 WOCKHARDT USA L EAGEN 65862-0325-30 0.05940 DONEPEZIL HCL 5 MG TABLET 0 AUROBINDO PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 121LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0325-90 0.05940 DONEPEZIL HCL 5 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0325-99 0.05940 DONEPEZIL HCL 5 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0477-01 0.05940 DONEPEZIL HCL 5 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0477-11 0.05940 DONEPEZIL HCL 5 MG TABLET 0 AHP EAGEN 00338-1005-02 0.03240 DOPAMINE 200 MG-D5W 250 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00517-1805-25 0.31500 DOPAMINE 40 MG/ML VIAL 0 AMER. REGENT MLGEN 00338-1007-02 0.03589 DOPAMINE 400 MG-D5W 250 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-1005-03 0.01971 DOPAMINE 400 MG-D5W 500 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-1009-02 0.05004 DOPAMINE 800 MG-D5W 250 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-1007-03 0.03205 DOPAMINE 800 MG-D5W 500 ML 0 BAXTER <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59630-0309-01 20.99568 DORIBAX 250 MG VIAL 0 SHIONOGI PHARMA EABND 59630-0309-10 20.99568 DORIBAX 250 MG VIAL 0 SHIONOGI PHARMA EABND 50458-0401-02 38.17668 DORIBAX 500 MG VIAL 0 SHIONOGI PHARMA EABND 59630-0320-01 38.17668 DORIBAX 500 MG VIAL 0 SHIONOGI PHARMA EABND 59630-0320-10 38.17668 DORIBAX 500 MG VIAL 0 SHIONOGI PHARMA EABND 00430-0115-20 13.16703 19.02125 DORYX DR 150 MG TABLET G ACTAVIS PHARMA, EABND 00430-0114-20 25.35539 DORYX DR 200 MG TABLET G ACTAVIS PHARMA, EAGEN 00093-7618-43 2.27450 DORZOLAMIDE HCL 2% EYE DROPS 0 TEVA USA MLGEN 242<strong>08</strong>-0485-10 2.27450 DORZOLAMIDE HCL 2% EYE DROPS 0 VALEANT MLGEN 50383-0232-10 2.27450 DORZOLAMIDE HCL 2% EYE DROPS 0 HI-TECH PHARMAC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61314-0019-10 2.27450 DORZOLAMIDE HCL 2% EYE DROPS 0 SANDOZ MLGEN 66993-0175-20 2.27450 DORZOLAMIDE HCL 2% EYE DROPS 0 PRASCO LABS MLGEN 242<strong>08</strong>-0486-10 1.83465 DORZOLAMIDE-TIMOLOL EYE DROPS 0 VALEANT MLGEN 50383-0233-10 1.83465 DORZOLAMIDE-TIMOLOL EYE DROPS 0 HI-TECH PHARMAC MLGEN 61314-0030-02 1.83465 DORZOLAMIDE-TIMOLOL EYE DROPS 0 SANDOZ MLBND 50222-0260-<strong>06</strong> 7.81777 7.81777 DOVONEX 0.005% CREAM 0 LEO PHARMA INC. GMBND 50222-0260-12 7.47449 7.47449 DOVONEX 0.005% CREAM 0 LEO PHARMA INC. GMGEN 00093-8120-01 0.05170 DOXAZOSIN MESYLATE 1 MG TAB 0 TEVA USA EAGEN 00378-4021-01 0.05170 DOXAZOSIN MESYLATE 1 MG TAB 0 MYLAN EAGEN 60505-0093-00 0.05170 DOXAZOSIN MESYLATE 1 MG TAB 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0093-01 0.05170 DOXAZOSIN MESYLATE 1 MG TAB 0 APOTEX CORP EAGUL 00093-8121-01 0.59180 DOXAZOSIN MESYLATE 2 MG TAB 0 TEVA USA EAGUL 00093-8121-19 0.59180 DOXAZOSIN MESYLATE 2 MG TAB 0 TEVA USA EAGUL 00093-8121-93 0.59180 DOXAZOSIN MESYLATE 2 MG TAB 0 TEVA USA EAGUL 00378-4022-01 0.59180 DOXAZOSIN MESYLATE 2 MG TAB 0 MYLAN EAGUL 60505-0094-00 0.59180 DOXAZOSIN MESYLATE 2 MG TAB 0 APOTEX CORP EAGUL 60505-0094-01 0.59180 DOXAZOSIN MESYLATE 2 MG TAB 0 APOTEX CORP EAGUL 60505-0094-<strong>08</strong> 0.59180 DOXAZOSIN MESYLATE 2 MG TAB 0 APOTEX CORP EAGUL 68645-0463-70 0.59180 DOXAZOSIN MESYLATE 2 MG TAB 0 LEGACY PHARMACE EAGUL 00093-8122-01 0.62100 DOXAZOSIN MESYLATE 4 MG TAB 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-4024-01 0.62100 DOXAZOSIN MESYLATE 4 MG TAB 0 MYLAN EAGUL 60505-0095-00 0.62100 DOXAZOSIN MESYLATE 4 MG TAB 0 APOTEX CORP EAGUL 60505-0095-01 0.62100 DOXAZOSIN MESYLATE 4 MG TAB 0 APOTEX CORP EAGUL 60505-0095-05 0.62100 DOXAZOSIN MESYLATE 4 MG TAB 0 APOTEX CORP EAGUL 68645-0464-70 0.62100 DOXAZOSIN MESYLATE 4 MG TAB 0 LEGACY PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 122LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00093-8123-01 0.65180 DOXAZOSIN MESYLATE 8 MG TAB 0 TEVA USA EAGUL 00378-4028-01 0.65180 DOXAZOSIN MESYLATE 8 MG TAB 0 MYLAN EAGUL 60505-0096-00 0.65180 DOXAZOSIN MESYLATE 8 MG TAB 0 APOTEX CORP EAGUL 60505-0096-01 0.65180 DOXAZOSIN MESYLATE 8 MG TAB 0 APOTEX CORP EAGUL 60505-0096-<strong>08</strong> 0.65180 DOXAZOSIN MESYLATE 8 MG TAB 0 APOTEX CORP EAGUL 68645-0465-70 0.65180 DOXAZOSIN MESYLATE 8 MG TAB 0 LEGACY PHARMACE EAGUX 00378-1049-01 0.<strong>08</strong>910 DOXEPIN 10 MG CAPSULE 0 MYLAN EAGUX 00378-1049-10 0.<strong>08</strong>910 DOXEPIN 10 MG CAPSULE 0 MYLAN EAGUX 51079-0436-20 0.<strong>08</strong>910 DOXEPIN 10 MG CAPSULE 0 MYLAN INSTITUTI EAGEX 00093-9612-12 0.<strong>06</strong>740 DOXEPIN 10 MG/ML ORAL CONC 0 TEVA USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 54838-0512-40 0.<strong>06</strong>740 DOXEPIN 10 MG/ML ORAL CONC 0 SILARX PHARM MLGEX 60432-<strong>06</strong>51-04 0.<strong>06</strong>740 DOXEPIN 10 MG/ML ORAL CONC 0 MORTON GROVE PH MLGUX 00378-6410-01 0.41740 DOXEPIN 100 MG CAPSULE 0 MYLAN EAGUX 00378-6410-10 0.41740 DOXEPIN 100 MG CAPSULE 0 MYLAN EAGUX 51079-<strong>06</strong>51-20 0.41740 DOXEPIN 100 MG CAPSULE 0 MYLAN INSTITUTI EABEX 49884-0222-01 2.76199 DOXEPIN 150 MG CAPSULE 0 PAR PHARM. EABEX 49884-0222-03 2.81834 DOXEPIN 150 MG CAPSULE 0 PAR PHARM. EABEX 49884-0222-05 2.62381 DOXEPIN 150 MG CAPSULE 0 PAR PHARM. EAGUX 00378-3125-01 0.18220 DOXEPIN 25 MG CAPSULE 0 MYLAN EAGUX 00378-3125-10 0.18220 DOXEPIN 25 MG CAPSULE 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 51079-0437-20 0.18220 DOXEPIN 25 MG CAPSULE 0 MYLAN INSTITUTI EAGUX 00378-4250-01 0.14470 DOXEPIN 50 MG CAPSULE 0 MYLAN EAGUX 00378-4250-10 0.14470 DOXEPIN 50 MG CAPSULE 0 MYLAN EAGUX 51079-0438-20 0.14470 DOXEPIN 50 MG CAPSULE 0 MYLAN INSTITUTI EABEX 00378-5375-01 0.10970 1.03160 DOXEPIN 75 MG CAPSULE 0 MYLAN EABEX 00378-5375-10 0.10970 1.0<strong>08</strong>98 DOXEPIN 75 MG CAPSULE 0 MYLAN EAGEN 00781-3305-95 5.27362 DOXERCALCIFEROL 4 MCG/2 ML VL 0 SANDOZ MLBND 59676-0960-02 76.74480 107.35884 DOXIL 2 MG/ML VIAL 0 JANSSEN PRODUCT MLGEN 47335-0049-40 76.74480 DOXORUBICIN LIPOSOME 20MG/10ML 0 SUN PHARMA GLOB MLGEN 47335-0050-40 76.74480 DOXORUBICIN LIPOSOME 50MG/25ML 0 SUN PHARMA GLOB ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55390-0241-10 7.28750 DOXORUBICIN 10 MG VIAL 0 BEDFORD/NOVAPLU EAGEN 00<strong>06</strong>9-4030-01 1.80000 DOXORUBICIN 10 MG/5 ML VIAL 0 PFIZER/NOVAPLUS MLGEN 00703-5043-01 2.17800 DOXORUBICIN 10 MG/5 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-5043-03 2.17800 DOXORUBICIN 10 MG/5 ML VIAL 0 TEVA PARENTERAL MLGEN 53150-0320-10 1.80000 DOXORUBICIN 10 MG/5 ML VIAL 0 AMNEAL-AGILA, L MLGEN 00<strong>06</strong>9-4031-01 1.80000 DOXORUBICIN 20 MG/10 ML VIAL 0 PFIZER/NOVAPLUS MLGEN 53150-0314-10 1.80000 DOXORUBICIN 20 MG/10 ML VIAL 0 AMNEAL-AGILA, L MLGEN 00703-5040-01 1.26735 DOXORUBICIN 200 MG/100 ML VIAL 0 TEVA PARENTERAL MLGEN 53150-0317-01 1.26000 DOXORUBICIN 200 MG/100 ML VIAL 0 AMNEAL-AGILA, L MLGEN 62756-<strong>08</strong>27-40 1.2<strong>06</strong>00 DOXORUBICIN 200 MG/100 ML VIAL 0 SUN PHARMACEUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0101-61 1.19977 DOXORUBICIN 200 MG/100 ML VIAL 0 APP PHARMACEUTI MLGEN 55390-0243-01 19.23175 DOXORUBICIN 50 MG VIAL 0 BEDFORD/NOVAPLU EAGEN 00<strong>06</strong>9-4032-01 1.26000 DOXORUBICIN 50 MG/25 ML VIAL 0 PFIZER/NOVAPLUS MLGEN 00703-5046-01 1.69200 DOXORUBICIN 50 MG/25 ML VIAL 0 TEVA PARENTERAL MLGEN 53150-0315-01 1.26000 DOXORUBICIN 50 MG/25 ML VIAL 0 AMNEAL-AGILA, L ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 123LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62756-<strong>08</strong>26-40 1.2<strong>06</strong>00 DOXORUBICIN 50 MG/25 ML VIAL 0 SUN PHARMACEUTI MLGEN 63323-0130-11 14.44263 DOXY 100 VIAL 0 APP PHARMACEUTI EAGEN 63323-0130-13 12.60000 DOXY 100 VIAL 0 APP/NOVAPLUS EAGEN 00228-2896-11 9.86122 DOXYCYCLINE HYC DR 100 MG TAB G ACTAVIS PHARMA, EAGEN 00378-4532-01 9.86122 DOXYCYCLINE HYC DR 100 MG TAB G MYLAN EAGEN 23155-0142-01 9.86122 DOXYCYCLINE HYC DR 100 MG TAB G HERITAGE PHARMA EAGEN 683<strong>08</strong>-0710-10 9.84000 DOXYCYCLINE HYC DR 100 MG TAB G MIDLOTHIAN LABO EAGEN 00378-3030-01 13.16703 DOXYCYCLINE HYC DR 150 MG TAB G MYLAN EAGEN 23155-0143-01 13.16703 DOXYCYCLINE HYC DR 150 MG TAB G HERITAGE PHARMA EAGEN 683<strong>08</strong>-0715-10 13.16703 DOXYCYCLINE HYC DR 150 MG TAB G MIDLOTHIAN LABO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-2895-<strong>06</strong> 6.54346 DOXYCYCLINE HYC DR 75 MG TAB G ACTAVIS PHARMA, EAGEN 00378-4531-91 6.54346 DOXYCYCLINE HYC DR 75 MG TAB G MYLAN EAGEN 23155-0141-01 6.54346 DOXYCYCLINE HYC DR 75 MG TAB G HERITAGE PHARMA EAGEN 23155-0141-<strong>06</strong> 6.54346 DOXYCYCLINE HYC DR 75 MG TAB G HERITAGE PHARMA EAGEN 683<strong>08</strong>-0775-60 6.54346 DOXYCYCLINE HYC DR 75 MG TAB G MIDLOTHIAN LABO EAGUL 00143-3142-05 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 WEST-WARD,INC. EAGUL 00143-3142-50 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 WEST-WARD,INC. EAGUL 00143-9803-05 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 WEST-WARD,INC. EAGUL 00143-9803-50 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 WEST-WARD,INC. EAGUL 00591-5440-05 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00591-5440-50 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 ACTAVIS PHARMA, EAGUL 0<strong>06</strong>03-3481-28 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 QUALITEST EAGUL 00904-0428-40 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 MAJOR PHARMACEU EAGUL 15338-0100-14 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 APACE PACKAGING EAGUL 53489-0119-02 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 MUTUAL PHARM CO EAGUL 53489-0119-05 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 MUTUAL PHARM CO EAGUL 61748-0111-14 0.14910 DOXYCYCLINE HYCLATE 100 MG CAP 0 VERSA PHARMACEU EAGUL 00143-2112-05 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 WEST-WARD,INC. EAGUL 00143-2112-50 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 WEST-WARD,INC. EAGUL 00172-3626-48 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00172-3626-70 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 IVAX PHARMACEUT EAGUL 00591-5553-05 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 ACTAVIS PHARMA, EAGUL 00591-5553-50 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 ACTAVIS PHARMA, EAGUL 00904-0430-40 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 MAJOR PHARMACEU EAGUL 24658-0312-05 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 BLU PHARMACEUTI EAGUL 24658-0312-20 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 BLU PHARMACEUTI EAGUL 24658-0312-50 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 BLU PHARMACEUTI EAGUL 53489-0120-02 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 MUTUAL PHARM CO EAGUL 53489-0120-05 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 MUTUAL PHARM CO EAGUL 62584-<strong>06</strong>93-11 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 62584-<strong>06</strong>93-21 0.12870 DOXYCYCLINE HYCLATE 100 MG TAB 0 AHP EAGEN 00172-4626-60 0.36290 DOXYCYCLINE HYCLATE 20 MG TAB 0 IVAX PHARMACEUT EAGEN 00172-4626-70 0.36290 DOXYCYCLINE HYCLATE 20 MG TAB 0 IVAX PHARMACEUT EAGEN 00527-1336-01 0.36290 DOXYCYCLINE HYCLATE 20 MG TAB 0 LANNETT CO. INC EAGEN 53489-<strong>06</strong>47-01 0.36290 DOXYCYCLINE HYCLATE 20 MG TAB 0 MUTUAL PHARM CO EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 124LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68047-0714-01 0.36290 DOXYCYCLINE HYCLATE 20 MG TAB 0 LARKEN LABS EAGEN 68047-0714-60 0.36290 DOXYCYCLINE HYCLATE 20 MG TAB 0 LARKEN LABS EAGUL 00143-3141-50 0.13170 DOXYCYCLINE HYCLATE 50 MG CAP 0 WEST-WARD,INC. EAGUL 00591-5535-50 0.13170 DOXYCYCLINE HYCLATE 50 MG CAP 0 ACTAVIS PHARMA, EAGUL 00904-0427-51 0.13170 DOXYCYCLINE HYCLATE 50 MG CAP 0 MAJOR PHARMACEU EAGUL 53489-0118-02 0.13170 DOXYCYCLINE HYCLATE 50 MG CAP 0 MUTUAL PHARM CO EAGUL 53489-0118-05 0.13170 DOXYCYCLINE HYCLATE 50 MG CAP 0 MUTUAL PHARM CO EAGEN 00591-0411-50 1.45380 DOXYCYCLINE MONO 100 MG CAP G ACTAVIS PHARMA, EAGEN 49884-0727-03 1.77675 DOXYCYCLINE MONO 100 MG CAP G PAR PHARM. EAGEN 49884-0727-04 1.70787 DOXYCYCLINE MONO 100 MG CAP G PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0216-52 1.11550 DOXYCYCLINE MONO 100 MG TABLET G SANDOZ EAGEN 00185-0216-53 1.11550 DOXYCYCLINE MONO 100 MG TABLET G SANDOZ EAGEN 00378-6023-89 1.11550 DOXYCYCLINE MONO 100 MG TABLET G MYLAN EAGEN 00527-1338-25 1.11550 DOXYCYCLINE MONO 100 MG TABLET G LANNETT CO. INC EAGEN 00527-1338-50 1.11550 DOXYCYCLINE MONO 100 MG TABLET G LANNETT CO. INC EAGEN 23155-0135-25 1.11550 DOXYCYCLINE MONO 100 MG TABLET G HERITAGE PHARMA EAGEN 49884-0093-03 1.11550 DOXYCYCLINE MONO 100 MG TABLET G PAR PHARM. EAGEN 49884-0093-04 1.11550 DOXYCYCLINE MONO 100 MG TABLET G PAR PHARM. EAGEN 63304-0132-50 1.11550 DOXYCYCLINE MONO 100 MG TABLET G RANBAXY PHARMAC EAGEN 00115-1327-13 17.82360 DOXYCYCLINE MONO 150 MG CAP G GLOBAL PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5475-91 17.82360 DOXYCYCLINE MONO 150 MG CAP G MYLAN EAGEN 49884-0305-02 17.82360 DOXYCYCLINE MONO 150 MG CAP G PAR PHARM. EAGEN 00378-6124-93 3.88665 DOXYCYCLINE MONO 150 MG TABLET G MYLAN EAGEN 00527-1537-30 3.88665 DOXYCYCLINE MONO 150 MG TABLET G LANNETT CO. INC EAGEN 23155-0136-03 3.88665 DOXYCYCLINE MONO 150 MG TABLET G HERITAGE PHARMA EAGEN 49884-0236-11 3.88665 DOXYCYCLINE MONO 150 MG TABLET G PAR PHARM. EAGEN 63304-0173-30 3.88665 DOXYCYCLINE MONO 150 MG TABLET G RANBAXY PHARMAC EAGEN 00591-0410-01 0.89055 DOXYCYCLINE MONO 50 MG CAP G ACTAVIS PHARMA, EAGEN 49884-0726-01 1.<strong>08</strong>825 DOXYCYCLINE MONO 50 MG CAP G PAR PHARM. EAGEN 00185-0036-01 1.19760 DOXYCYCLINE MONO 50 MG TABLET G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6021-01 1.19760 DOXYCYCLINE MONO 50 MG TABLET G MYLAN EAGEN 00527-1335-01 1.19760 DOXYCYCLINE MONO 50 MG TABLET G LANNETT CO. INC EAGEN 23155-0133-01 1.19760 DOXYCYCLINE MONO 50 MG TABLET G HERITAGE PHARMA EAGEN 49884-0091-01 1.19760 DOXYCYCLINE MONO 50 MG TABLET G PAR PHARM. EAGEN 63304-<strong>06</strong>15-01 12.29827 DOXYCYCLINE MONO 75 MG CAPSULE G RANBAXY PHARMAC EAGEN 00378-6022-01 1.02938 DOXYCYCLINE MONO 75 MG TABLET G MYLAN EAGEN 00527-1535-01 1.02938 DOXYCYCLINE MONO 75 MG TABLET G LANNETT CO. INC EAGEN 23155-0134-01 1.02938 DOXYCYCLINE MONO 75 MG TABLET G HERITAGE PHARMA EAGEN 49884-0092-01 1.02938 DOXYCYCLINE MONO 75 MG TABLET G PAR PHARM. EAGEN 63304-0131-01 1.02938 DOXYCYCLINE MONO 75 MG TABLET G RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-<strong>06</strong>57-01 0.28462 DOXYCYCLINE 25 MG/5 ML SUSP G LUPIN PHARMACEU MLBND 00024-0392-02 0.28026 2.56702 DRISDOL 50,000 UNITS CAPSULE G SAN<strong>OF</strong>I-AVENTIS EABND 00024-0393-10 0.28026 2.89927 DRISDOL 50,000 UNITS CAPSULE G SAN<strong>OF</strong>I-AVENTIS EABND 00003-6335-17 0.75378 DROXIA 200 MG CAPSULE 0 BMS ONCO/IMMUN EABND 00003-6336-17 0.75378 DROXIA 300 MG CAPSULE 0 BMS ONCO/IMMUN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 125LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00003-6337-17 0.8<strong>06</strong>61 DROXIA 400 MG CAPSULE 0 BMS ONCO/IMMUN EABND 00145-2371-05 5.64326 DUAC 1.2-5% GEL G STIEFEL LABS. GMBND 64764-0302-30 13.12<strong>06</strong>4 DUETACT 30-2 MG TABLET G TAKEDA PHARMACE EABND 64764-0304-30 13.12<strong>06</strong>4 DUETACT 30-4 MG TABLET G TAKEDA PHARMACE EABND 75987-0010-03 8.84448 DUEXIS 800-26.6 MG TABLET G HORIZON PHARMA EABND 00<strong>08</strong>5-72<strong>06</strong>-01 18.36470 DULERA 100 MCG/5 MCG INHALER G MERCK SHARP & D GMBND 00<strong>08</strong>5-72<strong>06</strong>-07 18.36375 DULERA 100 MCG/5 MCG INHALER G MERCK SHARP & D GMBND 00<strong>08</strong>5-4610-01 18.36470 DULERA 200 MCG/5 MCG INHALER G MERCK SHARP & D GMBND 00<strong>08</strong>5-4610-05 18.36375 DULERA 200 MCG/5 MCG INHALER G MERCK SHARP & D GMBND 49502-<strong>06</strong>72-30 0.09666 0.74589 DUONEB 0.5 MG-3 MG/3 ML SOLN G MYLAN SPECIALTY ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 49502-<strong>06</strong>72-60 0.09666 0.74584 DUONEB 0.5 MG-3 MG/3 ML SOLN G MYLAN SPECIALTY MLBND 67457-0218-10 2.77330 4.38240 DURACLON 100 MCG/ML VIAL 0 MYLAN INSTITUTI MLBND 67457-0219-10 11.66660 21.91200 DURACLON 500 MCG/ML VIAL 0 MYLAN INSTITUTI MLBND 00<strong>06</strong>5-9240-05 21.35424 DUREZOL 0.05% EYE DROPS 0 ALCON LABS. MLBND 00<strong>06</strong>5-9240-07 23.98368 DUREZOL 0.05% EYE DROPS 0 ALCON LABS. MLBND 00310-1097-30 0.52788 DUTOPROL 100-12.5 MG TABLET G ASTRAZENECA EABND 00310-1<strong>08</strong>7-30 0.52788 DUTOPROL 25-12.5 MG TABLET G ASTRAZENECA EABND 00310-1095-30 0.52788 DUTOPROL 50-12.5 MG TABLET G ASTRAZENECA EABUL 00007-3650-22 0.31770 1.26301 DYAZIDE 37.5-25 CAPSULE G GLAXOSMITHKLINE EABUL 00007-3650-30 0.31770 1.21350 DYAZIDE 37.5-25 CAPSULE G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00037-0245-23 6.27011 DYMISTA NASAL SPRAY G MEDA PHARMACEUT GMGEN 49884-0098-03 3.<strong>06</strong>288 DYNACIN 100 MG TABLET G PAR PHARM. EABND 24338-0134-02 1.34709 E.E.S. 200 MG/5 ML GRANULES 0 ARBOR PHARMACEU MLBND 24338-0136-10 0.81937 E.E.S. 200 MG/5 ML GRANULES 0 ARBOR PHARMACEU MLBND 24338-0100-13 3.24911 E.E.S. 400 FILMTAB 0 ARBOR PHARMACEU EAGEN 00168-0312-15 0.22015 ECONAZOLE NITRATE 1% CREAM G SANDOZ GMGEN 00168-0312-30 0.22015 ECONAZOLE NITRATE 1% CREAM G SANDOZ GMGEN 00168-0312-85 0.22015 ECONAZOLE NITRATE 1% CREAM G SANDOZ GMGEN 45802-0466-11 0.22015 ECONAZOLE NITRATE 1% CREAM G PERRIGO CO. GMGEN 45802-0466-35 0.22015 ECONAZOLE NITRATE 1% CREAM G PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0466-53 0.22015 ECONAZOLE NITRATE 1% CREAM G PERRIGO CO. GMGEN 51672-1303-01 0.22015 ECONAZOLE NITRATE 1% CREAM G TARO PHARM USA GMGEN 51672-1303-02 0.22015 ECONAZOLE NITRATE 1% CREAM G TARO PHARM USA GMGEN 51672-1303-<strong>08</strong> 0.22015 ECONAZOLE NITRATE 1% CREAM G TARO PHARM USA GMGEN 52565-0022-15 0.22015 ECONAZOLE NITRATE 1% CREAM G IGI LABS, INC. GMGEN 52565-0022-30 0.22015 ECONAZOLE NITRATE 1% CREAM G IGI LABS, INC. GMGEN 52565-0022-85 0.22015 ECONAZOLE NITRATE 1% CREAM G IGI LABS, INC. GMGEN 66993-<strong>08</strong>79-15 0.22015 ECONAZOLE NITRATE 1% CREAM G PRASCO LABS GMGEN 66993-<strong>08</strong>79-31 0.22015 ECONAZOLE NITRATE 1% CREAM G PRASCO LABS GMGEN 66993-<strong>08</strong>79-85 0.22015 ECONAZOLE NITRATE 1% CREAM G PRASCO LABS GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00485-0<strong>08</strong>2-01 0.40950 ED-SPAZ 0.125 MG ODT 0 EDWARDS PHARMAC EABND 64764-<strong>08</strong>44-30 3.13269 EDARBI 40 MG TABLET G ARBOR PHARMACEU EABND 64764-<strong>08</strong>84-30 3.13269 EDARBI 80 MG TABLET G ARBOR PHARMACEU EABND 64764-0944-30 3.21403 EDARBYCLOR 40-12.5 MG TABLET G ARBOR PHARMACEU EABND 64764-0994-30 3.21403 EDARBYCLOR 40-25 MG TABLET G ARBOR PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 126LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 25010-0205-15 3.91145 EDECRIN 25 MG TABLET 0 VALEANT EABND 25010-0215-15 6.60746 EDECRIN 25 MG TABLET 0 VALEANT EABND 59676-0278-01 25.54933 EDURANT 25 MG TABLET G JANSSEN PRODUCT EAGEN 51801-0005-30 0.15110 EFFER-K 25 MEQ TABLET EFF 0 NOMAX INC EAGEN 51801-00<strong>06</strong>-30 0.15110 EFFER-K 25 MEQ TABLET EFF 0 NOMAX INC EAGEN 51801-0007-30 0.15110 EFFER-K 25 MEQ TABLET EFF 0 NOMAX INC EABEX 000<strong>08</strong>-<strong>08</strong>36-02 0.74240 7.47830 EFFEXOR XR 150 MG CAPSULE G WYETH PHARM EABEX 000<strong>08</strong>-<strong>08</strong>36-20 0.74240 7.47996 EFFEXOR XR 150 MG CAPSULE G WYETH PHARM EABEX 000<strong>08</strong>-<strong>08</strong>36-21 0.74240 7.48051 EFFEXOR XR 150 MG CAPSULE G WYETH PHARM EABEX 000<strong>08</strong>-<strong>08</strong>36-22 0.74240 7.48<strong>06</strong>9 EFFEXOR XR 150 MG CAPSULE G WYETH PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 000<strong>08</strong>-<strong>08</strong>37-20 0.29270 6.12761 EFFEXOR XR 37.5 MG CAPSULE G WYETH PHARM EABEX 000<strong>08</strong>-<strong>08</strong>37-21 0.29270 6.12844 EFFEXOR XR 37.5 MG CAPSULE G WYETH PHARM EABEX 000<strong>08</strong>-<strong>08</strong>37-22 0.29270 6.12890 EFFEXOR XR 37.5 MG CAPSULE G WYETH PHARM EABEX 000<strong>08</strong>-<strong>08</strong>33-20 0.34670 6.86686 EFFEXOR XR 75 MG CAPSULE G WYETH PHARM EABEX 000<strong>08</strong>-<strong>08</strong>33-21 0.34670 6.86133 EFFEXOR XR 75 MG CAPSULE G WYETH PHARM EABEX 000<strong>08</strong>-<strong>08</strong>33-22 0.34670 6.86705 EFFEXOR XR 75 MG CAPSULE G WYETH PHARM EABND 00002-5123-30 8.22696 EFFIENT 10 MG TABLET 0 ELI LILLY & CO. EABND 00002-5121-52 8.22702 EFFIENT 5 MG TABLET 0 ELI LILLY & CO. EABND 00187-3204-47 5.35250 7.23822 EFUDEX 5% CREAM 0 VALEANT GMBND 49502-0420-60 1.64150 3.318<strong>06</strong> ELDEPRYL 5 MG CAPSULE G MYLAN SPECIALTY EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00023-9201-05 9.26440 31.82552 ELESTAT 0.05% EYE DROPS G ALLERGAN INC. MLBND 18860-0480-01 2.99789 ELESTRIN 0.<strong>06</strong>% GEL 0 MEDA PHARMACEUT GMBND 18860-0480-60 2.99789 ELESTRIN 0.<strong>06</strong>% GEL 0 MEDA PHARMACEUT GMBND 00187-5100-01 6.2<strong>08</strong>67 ELIDEL 1% CREAM G VALEANT GMBND 00187-5101-02 6.2<strong>08</strong>67 ELIDEL 1% CREAM G VALEANT GMBND 00187-5102-03 6.2<strong>08</strong>56 ELIDEL 1% CREAM G VALEANT GMGEX 16714-0365-01 0.80097 ELINEST-28 TABLET 0 NORTHSTAR RX LL EAGEX 16714-0365-04 0.80097 ELINEST-28 TABLET 0 NORTHSTAR RX LL EAGEN 63717-0910-02 0.<strong>08</strong>200 ELIPHOS 667 MG TABLET 0 HAWTHORN PHARM EABND 00003-<strong>08</strong>93-21 4.40549 ELIQUIS 2.5 MG TABLET G BMS PRIMARYCARE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00003-<strong>08</strong>93-31 4.40547 ELIQUIS 2.5 MG TABLET G BMS PRIMARYCARE EABND 00003-<strong>08</strong>94-21 4.40549 ELIQUIS 5 MG TABLET G BMS PRIMARYCARE EABND 00003-<strong>08</strong>94-31 4.40547 ELIQUIS 5 MG TABLET G BMS PRIMARYCARE EABND 497<strong>08</strong>-<strong>06</strong>44-90 0.16012 ELIXOPHYLLIN 80 MG/15 ML ELIX 0 CARACO PHARMA I MLBEX 52544-0238-54 35.60700 ELLA 30 MG TABLET 0 ACTAVIS PHARMA, EABND 50458-0098-01 6.15494 ELMIRON 100 MG CAPSULE 0 JANSSEN PHARM. EABND 00<strong>08</strong>5-0567-01 0.46670 2.97<strong>08</strong>4 ELOCON 0.1% CREAM G MERCK SHARP & D GMBND 00<strong>08</strong>5-0567-02 0.46670 1.81382 ELOCON 0.1% CREAM G MERCK SHARP & D GMBND 00<strong>08</strong>5-3149-01 0.46670 2.97<strong>08</strong>4 ELOCON 0.1% CREAM G MERCK SHARP & D GMBND 00<strong>08</strong>5-3149-03 0.46670 1.63244 ELOCON 0.1% CREAM G MERCK SHARP & D GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>5-<strong>08</strong>54-01 0.23<strong>08</strong>5 1.61020 ELOCON 0.1% LOTION G MERCK SHARP & D MLBND 00<strong>08</strong>5-<strong>08</strong>54-02 0.23<strong>08</strong>5 1.53854 ELOCON 0.1% LOTION G MERCK SHARP & D MLBND 00<strong>08</strong>5-0370-01 0.26490 2.97<strong>08</strong>4 ELOCON 0.1% OINTMENT G MERCK SHARP & D GMBND 00<strong>08</strong>5-0370-02 0.26490 1.81382 ELOCON 0.1% OINTMENT G MERCK SHARP & D GMBND 00<strong>06</strong>5-0325-05 19.22280 EMADINE 0.05% EYE DROPS G ALCON LABS. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 127LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00013-0132-02 8.15010 EMCYT 140 MG CAPSULE 0 PHARMACIA/UPJHN EABND 000<strong>06</strong>-3862-03 134.305<strong>06</strong> EMEND TRIFOLD PACK 0 MERCK SHARP & D EABND 000<strong>06</strong>-0462-<strong>06</strong> 177.86070 EMEND 125 MG CAPSULE 0 MERCK SHARP & D EABND 000<strong>06</strong>-0464-05 61.41336 EMEND 40 MG CAPSULE 0 MERCK SHARP & D EABND 000<strong>06</strong>-0464-10 61.41170 EMEND 40 MG CAPSULE 0 MERCK SHARP & D EABND 000<strong>06</strong>-0461-02 113.83450 EMEND 80 MG CAPSULE 0 MERCK SHARP & D EABND 000<strong>06</strong>-0461-<strong>06</strong> 113.82758 EMEND 80 MG CAPSULE 0 MERCK SHARP & D EAGEX 0<strong>06</strong>03-7540-17 0.73800 EMOQUETTE 28 DAY TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-7540-49 0.73800 EMOQUETTE 28 DAY TABLET 0 QUALITEST EABEX 49502-0902-30 32.94380 EMSAM 12 MG/24 HOURS PATCH 0 MYLAN SPECIALTY EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 49502-0900-30 32.94380 EMSAM 6 MG/24 HOURS PATCH 0 MYLAN SPECIALTY EABEX 49502-0901-30 32.94380 EMSAM 9 MG/24 HOURS PATCH 0 MYLAN SPECIALTY EABND 61958-<strong>06</strong>02-01 0.69436 EMTRIVA 10 MG/ML SOLUTION G GILEAD SCIENCES MLBND 61958-<strong>06</strong>01-01 16.66280 EMTRIVA 200 MG CAPSULE G GILEAD SCIENCES EABND 00078-0420-15 4.95150 ENABLEX 15 MG TABLET G WC PR<strong>OF</strong> PRODS EABND 00078-0420-34 4.95141 ENABLEX 15 MG TABLET G WC PR<strong>OF</strong> PRODS EABND 00430-0171-15 6.38436 ENABLEX 15 MG TABLET G ACTAVIS PHARMA, EABND 00430-0171-23 6.38436 ENABLEX 15 MG TABLET G ACTAVIS PHARMA, EABND 00078-0419-15 4.95150 ENABLEX 7.5 MG TABLET G WC PR<strong>OF</strong> PRODS EABND 00078-0419-34 4.95141 ENABLEX 7.5 MG TABLET G WC PR<strong>OF</strong> PRODS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00430-0170-15 6.38436 ENABLEX 7.5 MG TABLET G ACTAVIS PHARMA, EABND 00430-0170-23 6.38436 ENABLEX 7.5 MG TABLET G ACTAVIS PHARMA, EAGEN 38779-0514-04 19.45140 ENALAPRIL MALEATE POWDER 0 MEDISCA INC. GMGEN 38779-0514-05 19.45125 ENALAPRIL MALEATE POWDER 0 MEDISCA INC. GMGUL 00093-0028-01 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 TEVA USA EAGUL 00093-0028-10 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 TEVA USA EAGUL 00093-0028-50 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 TEVA USA EAGUL 00378-1053-01 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 MYLAN EAGUL 00378-1053-10 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 MYLAN EAGUL 15338-0220-30 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 APACE PACKAGING EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 49158-0502-01 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 TARO PHARM USA EAGUL 49158-0502-10 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 TARO PHARM USA EAGUL 51672-4039-01 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 TARO PHARM USA EAGUL 51672-4039-03 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 TARO PHARM USA EAGUL 60505-0051-07 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 APOTEX CORP EAGUL 60505-0051-09 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 APOTEX CORP EAGUL 64679-0925-02 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 WOCKHARDT USA L EAGUL 64679-0925-03 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 WOCKHARDT USA L EAGUL 68<strong>08</strong>4-0391-01 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 AHP EAGUL 68<strong>08</strong>4-0391-11 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 68645-0456-90 0.07320 ENALAPRIL MALEATE 10 MG TAB 0 LEGACY PHARMACE EAGUL 00093-0026-01 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 TEVA USA EAGUL 00093-0026-10 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 TEVA USA EAGUL 00378-1051-01 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 MYLAN EAGUL 15338-0200-30 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 APACE PACKAGING EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 128LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 49158-0500-01 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 TARO PHARM USA EAGUL 51672-4037-01 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 TARO PHARM USA EAGUL 51672-4037-03 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 TARO PHARM USA EAGUL 60505-0049-07 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 APOTEX CORP EAGUL 60505-0049-09 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 APOTEX CORP EAGUL 64679-0923-02 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 WOCKHARDT USA L EAGUL 64679-0923-03 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 WOCKHARDT USA L EAGUL 68645-0454-90 0.04730 ENALAPRIL MALEATE 2.5 MG TAB 0 LEGACY PHARMACE EAGEN 00093-0029-01 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 TEVA USA EAGEN 00093-0029-10 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-0029-50 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 TEVA USA EAGEN 00378-1054-01 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 MYLAN EAGEN 00378-1054-05 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 MYLAN EAGEN 15338-0233-30 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 APACE PACKAGING EAGEN 49158-0503-01 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 TARO PHARM USA EAGEN 51672-4040-01 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 TARO PHARM USA EAGEN 51672-4040-03 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 TARO PHARM USA EAGEN 60505-0052-09 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 APOTEX CORP EAGEN 64679-0926-02 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 WOCKHARDT USA L EAGEN 64679-0926-03 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0392-01 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0392-11 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 AHP EAGEN 68645-0457-90 0.04470 ENALAPRIL MALEATE 20 MG TAB 0 LEGACY PHARMACE EAGUL 00093-0027-01 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 TEVA USA EAGUL 00093-0027-50 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 TEVA USA EAGUL 00378-1052-01 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 MYLAN EAGUL 00378-1052-10 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 MYLAN EAGUL 15338-0211-30 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 APACE PACKAGING EAGUL 49158-0501-01 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 TARO PHARM USA EAGUL 49158-0501-10 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 TARO PHARM USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-4038-01 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 TARO PHARM USA EAGUL 51672-4038-03 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 TARO PHARM USA EAGUL 60505-0050-07 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 APOTEX CORP EAGUL 60505-0050-09 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 APOTEX CORP EAGUL 64679-0924-02 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 WOCKHARDT USA L EAGUL 64679-0924-03 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 WOCKHARDT USA L EAGUL 68<strong>08</strong>4-0390-01 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 AHP EAGUL 68<strong>08</strong>4-0390-11 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 AHP EAGUL 68645-0455-90 0.05700 ENALAPRIL MALEATE 5 MG TABLET 0 LEGACY PHARMACE EAGEN 00093-1052-01 0.11813 ENALAPRIL-HCTZ 10-25 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0723-01 0.11813 ENALAPRIL-HCTZ 10-25 MG TABLET 0 MYLAN EAGEN 51672-4046-01 0.11813 ENALAPRIL-HCTZ 10-25 MG TABLET 0 TARO PHARM USA EAGEN 60505-0209-01 0.11813 ENALAPRIL-HCTZ 10-25 MG TABLET 0 APOTEX CORP EAGEN 00093-1044-01 0.1<strong>06</strong>11 ENALAPRIL-HCTZ 5-12.5 MG TAB 0 TEVA USA EAGEN 00378-0712-01 0.1<strong>06</strong>11 ENALAPRIL-HCTZ 5-12.5 MG TAB 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 129LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-4045-01 0.1<strong>06</strong>11 ENALAPRIL-HCTZ 5-12.5 MG TAB 0 TARO PHARM USA EAGEN 60505-02<strong>08</strong>-01 0.1<strong>06</strong>11 ENALAPRIL-HCTZ 5-12.5 MG TAB 0 APOTEX CORP EABND 584<strong>06</strong>-0425-34 314.54717 ENBREL 25 MG KIT G AMGEN EABND 584<strong>06</strong>-0425-41 314.54510 ENBREL 25 MG KIT G AMGEN EABND 584<strong>06</strong>-0455-01 616.75509 ENBREL 25 MG/0.5 ML SYRINGE G AMGEN MLBND 584<strong>06</strong>-0455-04 616.75916 ENBREL 25 MG/0.5 ML SYRINGE G AMGEN MLBND 584<strong>06</strong>-0445-01 641.92877 ENBREL 50 MG/ML SURECLICK SYR G AMGEN MLBND 584<strong>06</strong>-0445-04 641.93301 ENBREL 50 MG/ML SURECLICK SYR G AMGEN MLBND 584<strong>06</strong>-0435-01 641.92877 ENBREL 50 MG/ML SYRINGE G AMGEN MLBND 584<strong>06</strong>-0435-04 641.93301 ENBREL 50 MG/ML SYRINGE G AMGEN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 51285-04<strong>06</strong>-02 2.38542 ENJUVIA 0.3 MG TABLET 0 DURAMED/BARR EABND 51285-0407-02 2.38542 ENJUVIA 0.45 MG TABLET 0 DURAMED/BARR EABND 51285-04<strong>08</strong>-02 2.38542 ENJUVIA 0.625 MG TABLET 0 DURAMED/BARR EABND 51285-0409-02 2.38542 ENJUVIA 0.9 MG TABLET 0 DURAMED/BARR EABND 51285-0410-02 2.38542 ENJUVIA 1.25 MG TABLET 0 DURAMED/BARR EAGEN 00548-5635-00 49.50000 ENOXAPARIN 100 MG/ML SYRINGE G AMPHASTAR PHARM MLGEN 00781-3500-05 60.96000 ENOXAPARIN 100 MG/ML SYRINGE G SANDOZ MLGEN 00781-3500-69 60.95925 ENOXAPARIN 100 MG/ML SYRINGE G SANDOZ MLGEN 00955-1010-10 60.89400 ENOXAPARIN 100 MG/ML SYRINGE G WINTHROP US MLGEN 62037-<strong>08</strong>63-20 60.95925 ENOXAPARIN 100 MG/ML SYRINGE G ACTAVIS PHARMA, ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00548-5636-00 74.25000 ENOXAPARIN 120 MG/0.8 ML SYR G AMPHASTAR PHARM MLGEN 00781-3612-04 91.47187 ENOXAPARIN 120 MG/0.8 ML SYR G SANDOZ MLGEN 00781-3612-68 91.47093 ENOXAPARIN 120 MG/0.8 ML SYR G SANDOZ MLGEN 00955-1012-10 91.37<strong>06</strong>2 ENOXAPARIN 120 MG/0.8 ML SYR G WINTHROP US MLGEN 62037-<strong>08</strong>64-20 91.47093 ENOXAPARIN 120 MG/0.8 ML SYR G ACTAVIS PHARMA, MLGEN 00548-5637-00 74.25000 ENOXAPARIN 150 MG/ML SYRINGE G AMPHASTAR PHARM MLGEN 00781-3655-05 91.47750 ENOXAPARIN 150 MG/ML SYRINGE G SANDOZ MLGEN 00781-3655-69 91.47600 ENOXAPARIN 150 MG/ML SYRINGE G SANDOZ MLGEN 00955-1015-10 91.37175 ENOXAPARIN 150 MG/ML SYRINGE G WINTHROP US MLGEN 62037-<strong>08</strong>66-20 91.47600 ENOXAPARIN 150 MG/ML SYRINGE G ACTAVIS PHARMA, ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00548-5601-00 49.50000 ENOXAPARIN 30 MG/0.3 ML SYR G AMPHASTAR PHARM MLGEN 00548-5631-00 49.50000 ENOXAPARIN 30 MG/0.3 ML SYR G AMPHASTAR PHARM MLGEN 00781-3133-01 60.87500 ENOXAPARIN 30 MG/0.3 ML SYR G SANDOZ MLGEN 00781-3133-63 60.88500 ENOXAPARIN 30 MG/0.3 ML SYR G SANDOZ MLGEN 00955-1003-10 60.82500 ENOXAPARIN 30 MG/0.3 ML SYR G WINTHROP US MLGEN 62037-<strong>08</strong>39-20 60.88500 ENOXAPARIN 30 MG/0.3 ML SYR G ACTAVIS PHARMA, MLGEN 00781-3122-93 63.42999 ENOXAPARIN 300 MG/3 ML VIAL G SANDOZ MLGEN 00955-1016-01 66.90999 ENOXAPARIN 300 MG/3 ML VIAL G WINTHROP US MLGEN 00548-5602-00 49.50000 ENOXAPARIN 40 MG/0.4 ML SYR G AMPHASTAR PHARM MLGEN 00548-5632-00 49.50000 ENOXAPARIN 40 MG/0.4 ML SYR G AMPHASTAR PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3224-02 60.88125 ENOXAPARIN 40 MG/0.4 ML SYR G SANDOZ MLGEN 00781-3224-64 60.88500 ENOXAPARIN 40 MG/0.4 ML SYR G SANDOZ MLGEN 00955-1004-10 60.82125 ENOXAPARIN 40 MG/0.4 ML SYR G WINTHROP US MLGEN 62037-<strong>08</strong>49-20 60.88500 ENOXAPARIN 40 MG/0.4 ML SYR G ACTAVIS PHARMA, MLGEN 00548-5633-00 49.50000 ENOXAPARIN 60 MG/0.6 ML SYR G AMPHASTAR PHARM ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 130LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3356-03 60.96249 ENOXAPARIN 60 MG/0.6 ML SYR G SANDOZ MLGEN 00781-3356-66 60.96375 ENOXAPARIN 60 MG/0.6 ML SYR G SANDOZ MLGEN 00955-10<strong>06</strong>-10 60.89375 ENOXAPARIN 60 MG/0.6 ML SYR G WINTHROP US MLGEN 62037-<strong>08</strong>61-20 60.96375 ENOXAPARIN 60 MG/0.6 ML SYR G ACTAVIS PHARMA, MLGEN 00548-5634-00 49.50000 ENOXAPARIN 80 MG/0.8 ML SYR G AMPHASTAR PHARM MLGEN 00781-3428-04 60.96562 ENOXAPARIN 80 MG/0.8 ML SYR G SANDOZ MLGEN 00781-3428-68 60.96093 ENOXAPARIN 80 MG/0.8 ML SYR G SANDOZ MLGEN 00955-10<strong>08</strong>-10 60.89531 ENOXAPARIN 80 MG/0.8 ML SYR G WINTHROP US MLGEN 62037-<strong>08</strong>62-20 60.96093 ENOXAPARIN 80 MG/0.8 ML SYR G ACTAVIS PHARMA, MLGEX 00555-9047-58 0.73260 ENPRESSE-28 TABLET 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-9<strong>08</strong>0-01 2.96610 ENTACAPONE 200 MG TABLET 0 MYLAN EAGEN 51079-0273-03 3.05775 ENTACAPONE 200 MG TABLET 0 MYLAN INSTITUTI EAGEN 64679-0711-02 2.96610 ENTACAPONE 200 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0781-02 2.96610 ENTACAPONE 200 MG TABLET 0 WOCKHARDT USA L EAGEN 45963-0438-64 0.01500 ENULOSE 10 GM/15 ML SOLUTION 0 ACTAVIS PHARMA, MLBND 52652-1001-01 1.89240 EPANED 1 MG/ML SOLUTION G SILVERGATE PHAR MLGEN 00574-4005-05 9.26440 EPINASTINE HCL 0.05% EYE DROPS G PADDOCK LABS. MLGEN 51991-<strong>08</strong>36-75 9.26440 EPINASTINE HCL 0.05% EYE DROPS G BRECKENRIDGE MLGEN 60258-<strong>08</strong>58-07 9.26440 EPINASTINE HCL 0.05% EYE DROPS G CYPRESS PHARM. MLGEN 60505-0584-01 9.26440 EPINASTINE HCL 0.05% EYE DROPS G APOTEX CORP ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62756-0329-90 9.26440 EPINASTINE HCL 0.05% EYE DROPS G SUN PHARMACEUTI MLGEN 54505-0101-02 121.71000 EPINEPHRINE 0.15 MG AUTO-INJCT 0 LINEAGE THERAPE EAGEN 54505-0102-02 121.71000 EPINEPHRINE 0.3 MG AUTO-INJECT 0 LINEAGE THERAPE EABND 00409-7241-01 1.07100 2.09160 EPINEPHRINE 1 MG/ML AMPUL 0 HOSPIRA MLGEN 00517-1071-25 0.46000 EPINEPHRINE 1 MG/ML AMPUL 0 AMER. REGENT MLBND 49502-0501-02 151.34635 EPIPEN JR 2-PAK 0.15 MG INJCTR 0 MYLAN SPECIALTY EABND 49502-0500-02 151.34635 EPIPEN 2-PAK 0.3 MG AUTO-INJCT 0 MYLAN SPECIALTY EAGEX 00093-0090-01 0.04960 EPITOL 200 MG TABLET 0 TEVA USA EABND 00173-<strong>06</strong>62-00 14.85810 EPIVIR HBV 100 MG TABLET 0 GLAXOSMITHKLINE EABND 00173-<strong>06</strong>63-00 0.74288 EPIVIR HBV 25 MG/5 ML SOLN 0 GLAXOSMITHKLINE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 49702-0205-48 0.46009 EPIVIR 10 MG/ML ORAL SOLN G VIIV <strong>HEALTH</strong>CARE MLBND 00173-0470-01 6.33524 6.33524 EPIVIR 150 MG TABLET G GLAXOSMITHKLINE EABND 49702-0203-18 6.90130 6.90130 EPIVIR 150 MG TABLET G VIIV <strong>HEALTH</strong>CARE EABND 49702-0204-13 13.80262 13.80262 EPIVIR 300 MG TABLET G VIIV <strong>HEALTH</strong>CARE EAGEN 00185-5368-09 2.35805 EPLERENONE 25 MG TABLET 0 SANDOZ EAGEN 00185-5368-30 2.35805 EPLERENONE 25 MG TABLET 0 SANDOZ EAGEN 59762-1710-02 2.35805 EPLERENONE 25 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-1710-03 2.35805 EPLERENONE 25 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-2651-03 2.35805 EPLERENONE 25 MG TABLET 0 APOTEX CORP EAGEN 60505-2651-05 2.35805 EPLERENONE 25 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2651-09 2.35805 EPLERENONE 25 MG TABLET 0 APOTEX CORP EAGEN 00185-5369-09 2.40570 EPLERENONE 50 MG TABLET 0 SANDOZ EAGEN 00185-5369-30 2.40570 EPLERENONE 50 MG TABLET 0 SANDOZ EAGEN 59762-1720-01 2.40570 EPLERENONE 50 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-1720-02 2.40570 EPLERENONE 50 MG TABLET 0 GREENSTONE LLC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 131LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2652-03 2.40570 EPLERENONE 50 MG TABLET 0 APOTEX CORP EAGEN 60505-2652-09 2.40570 EPLERENONE 50 MG TABLET 0 APOTEX CORP EABND 55513-0144-01 137.24880 EPOGEN 10,000 UNITS/ML VIAL G AMGEN MLBND 55513-0144-10 137.24880 EPOGEN 10,000 UNITS/ML VIAL G AMGEN MLBND 55513-0126-01 27.44810 EPOGEN 2,000 UNITS/ML VIAL G AMGEN MLBND 55513-0126-10 27.44976 EPOGEN 2,000 UNITS/ML VIAL G AMGEN MLBND 55513-0478-01 274.49760 EPOGEN 20,000 UNITS/ML VIAL G AMGEN MLBND 55513-0478-10 274.49760 EPOGEN 20,000 UNITS/ML VIAL G AMGEN MLBND 55513-0283-01 137.24880 EPOGEN 20,000 UNITS/2 ML VIAL G AMGEN MLBND 55513-0283-10 137.24880 EPOGEN 20,000 UNITS/2 ML VIAL G AMGEN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 55513-0267-01 41.17630 EPOGEN 3,000 UNITS/ML VIAL G AMGEN MLBND 55513-0267-10 41.17464 EPOGEN 3,000 UNITS/ML VIAL G AMGEN MLBND 55513-0148-01 54.89620 EPOGEN 4,000 UNITS/ML VIAL G AMGEN MLBND 55513-0148-10 54.89952 EPOGEN 4,000 UNITS/ML VIAL G AMGEN MLGEN 00703-1985-01 10.84500 EPOPROSTENOL SODIUM 0.5 MG VL 0 TEVA PARENTERAL EAGEN 00703-1995-01 26.19750 EPOPROSTENOL SODIUM 1.5 MG VL 0 TEVA PARENTERAL EAGEN 00378-6629-93 2.56950 EPROSARTAN MESYLATE 600 MG TAB G MYLAN EABND 49702-02<strong>06</strong>-13 36.65639 EPZICOM TABLET G VIIV <strong>HEALTH</strong>CARE EABEX 3<strong>06</strong>98-0419-12 1.85920 EQUETRO 100 MG CAPSULE 0 VALIDUS PHARMAC EABEX 3<strong>06</strong>98-0421-12 2.14140 EQUETRO 200 MG CAPSULE 0 VALIDUS PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 3<strong>06</strong>98-0423-12 2.40700 EQUETRO 300 MG CAPSULE 0 VALIDUS PHARMAC EABND 00049-0116-28 179.28000 ERAXIS(WATER DIL) 100 MG VIAL 0 PFIZER US PHARM EABND 00049-0114-28 89.64000 ERAXIS(WATER DIL) 50 MG VIAL 0 PFIZER US PHARM EABND 53489-0281-01 6.72640 ERGOLOID MESYLATES 1 MG TAB 0 MUTUAL PHARM CO EABND 50242-0140-01 294.13125 ERIVEDGE 150 MG CAPSULE 0 GENENTECH, INC. EAGEX 00555-0344-58 0.69917 ERRIN 0.35 MG TABLET 0 BARR EABND 00<strong>06</strong>2-1650-02 5.07655 ERTACZO 2% CREAM G VALEANT GMBND 00187-5115-60 5.07655 ERTACZO 2% CREAM G VALEANT GMGEN 45802-0962-72 1.18575 ERY 2% PADS 0 PERRIGO CO. EABND 24338-0122-13 2.44858 ERY-TAB EC 250 MG TABLET 0 ARBOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 24338-0124-13 2.6<strong>08</strong>10 ERY-TAB EC 333 MG TABLET 0 ARBOR PHARMACEU EABND 24338-0126-13 2.81311 ERY-TAB EC 500 MG TABLET 0 ARBOR PHARMACEU EAGUL 40076-0315-30 0.62500 ERYGEL 2% GEL G PRESTIUM PHARMA GMGUL 40076-0315-60 0.62500 ERYGEL 2% GEL G PRESTIUM PHARMA GMBND 00074-6302-13 0.36287 ERYPED 200 MG/5 ML SUSPENSION 0 ARBOR PHARMACEU MLBND 24338-0132-13 1.41515 ERYPED 200 MG/5 ML SUSPENSION 0 ARBOR PHARMACEU MLBND 00074-6305-13 0.99483 ERYPED 400 MG/5 ML SUSPENSION 0 ARBOR PHARMACEU MLBND 24338-0130-13 1.81056 ERYPED 400 MG/5 ML SUSPENSION 0 ARBOR PHARMACEU MLBND 24338-01<strong>06</strong>-20 2.95621 ERYTHROCIN 250 MG FILMTAB 0 ARBOR PHARMACEU EABND 00409-6476-44 44.13276 ERYTHROCIN 500 MG ADDVNT VL 0 HOSPIRA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 683<strong>08</strong>-0250-10 2.58750 ERYTHROMYCIN DR 250 MG CAP 0 MIDLOTHIAN LABO EAGEN 24338-0120-13 2.76000 ERYTHROMYCIN EC 250 MG CAP 0 ARBOR PHARMACEU EABND 24338-0110-13 3.38449 ERYTHROMYCIN ES 400 MG TAB 0 ARBOR PHARMACEU EAGEN 17478-0070-35 3.02<strong>06</strong>0 ERYTHROMYCIN 0.5% EYE OINTMENT 0 AKORN INC. GMGEN 17478-<strong>08</strong>24-01 3.02<strong>06</strong>0 ERYTHROMYCIN 0.5% EYE OINTMENT 0 AKORN INC. GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 132LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 17478-<strong>08</strong>24-35 3.02<strong>06</strong>0 ERYTHROMYCIN 0.5% EYE OINTMENT 0 AKORN INC. GMGEN 242<strong>08</strong>-0910-19 3.02<strong>06</strong>0 ERYTHROMYCIN 0.5% EYE OINTMENT 0 VALEANT GMGEN 242<strong>08</strong>-0910-55 3.02<strong>06</strong>0 ERYTHROMYCIN 0.5% EYE OINTMENT 0 VALEANT GMGEN 48102-00<strong>08</strong>-35 3.02<strong>06</strong>0 ERYTHROMYCIN 0.5% EYE OINTMENT 0 PERRIGO CO. GMGUL 45802-0966-94 0.62500 ERYTHROMYCIN 2% GEL 0 PERRIGO CO. GMGUL 45802-0966-96 0.62500 ERYTHROMYCIN 2% GEL 0 PERRIGO CO. GMGEN 61748-0202-60 1.18187 ERYTHROMYCIN 2% PLEDGETS G VERSA PHARMACEU EAGEN 00168-0215-60 0.59537 ERYTHROMYCIN 2% SOLUTION 0 SANDOZ MLGEN 45802-0038-46 0.62499 ERYTHROMYCIN 2% SOLUTION 0 PERRIGO CO. MLGEN 60432-<strong>06</strong>71-60 0.62499 ERYTHROMYCIN 2% SOLUTION 0 MORTON GROVE PH ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 24338-0102-13 3.52434 ERYTHROMYCIN 250 MG FILMTAB 0 ARBOR PHARMACEU EABND 24338-0104-13 4.47394 ERYTHROMYCIN 500 MG FILMTAB 0 ARBOR PHARMACEU EAGEN 00781-7054-49 1.24<strong>08</strong>0 ERYTHROMYCIN-BENZOYL GEL G SANDOZ GMGEN 00781-7094-59 1.24<strong>08</strong>0 ERYTHROMYCIN-BENZOYL GEL G SANDOZ GMGEN 45802-0<strong>08</strong>3-02 1.24<strong>08</strong>0 ERYTHROMYCIN-BENZOYL GEL G PERRIGO CO. GMGEN 45802-0<strong>08</strong>3-86 1.24<strong>08</strong>0 ERYTHROMYCIN-BENZOYL GEL G PERRIGO CO. GMBND 51285-0445-21 0.25558 ERYTHROMYCIN-SULFISOX SUSP 0 DURAMED/BARR MLBND 51285-0445-22 0.25829 ERYTHROMYCIN-SULFISOX SUSP 0 DURAMED/BARR MLBND 51285-0445-23 0.25165 ERYTHROMYCIN-SULFISOX SUSP 0 DURAMED/BARR MLBND 58809-<strong>08</strong>77-50 1.64124 ESCAVITE LQ DROPS 0 G.M. PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 31722-0569-24 0.59437 ESCITALOPRAM OXALATE 5 MG/5 ML 0 CAMBER PHARMACE MLGEX 51672-1348-01 0.59437 ESCITALOPRAM OXALATE 5 MG/5 ML 0 TARO PHARM USA MLGEX 54838-0551-70 0.62130 ESCITALOPRAM OXALATE 5 MG/5 ML 0 SILARX PHARM MLGEX 65162-0705-88 0.59437 ESCITALOPRAM OXALATE 5 MG/5 ML 0 AMNEAL PHARMACE MLGEX 65862-0248-24 0.59437 ESCITALOPRAM OXALATE 5 MG/5 ML 0 AUROBINDO PHARM MLGEX 00093-5851-01 0.12000 ESCITALOPRAM 10 MG TABLET 0 TEVA USA EAGEX 00093-5851-05 0.12000 ESCITALOPRAM 10 MG TABLET 0 TEVA USA EAGEX 00143-98<strong>08</strong>-01 0.12000 ESCITALOPRAM 10 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-98<strong>08</strong>-05 0.12000 ESCITALOPRAM 10 MG TABLET 0 WEST-WARD,INC. EAGEX 00378-3856-10 0.12000 ESCITALOPRAM 10 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-3856-77 0.12000 ESCITALOPRAM 10 MG TABLET 0 MYLAN EAGEX 00378-3856-93 0.12000 ESCITALOPRAM 10 MG TABLET 0 MYLAN EAGEX 00904-6314-61 0.12000 ESCITALOPRAM 10 MG TABLET 0 MAJOR PHARMACEU EAGEX 13668-0136-01 0.12000 ESCITALOPRAM 10 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0136-05 0.12000 ESCITALOPRAM 10 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0136-10 0.12000 ESCITALOPRAM 10 MG TABLET 0 TORRENT PHARMAC EAGEX 16729-0169-01 0.12000 ESCITALOPRAM 10 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0169-17 0.12000 ESCITALOPRAM 10 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 31722-0250-90 0.12000 ESCITALOPRAM 10 MG TABLET 0 CAMBER PHARMACE EAGEX 33342-0037-11 0.12000 ESCITALOPRAM 10 MG TABLET 0 MACLEODS PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0543-01 0.12000 ESCITALOPRAM 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0543-20 0.12000 ESCITALOPRAM 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0543-30 0.12000 ESCITALOPRAM 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0543-56 0.12000 ESCITALOPRAM 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 54458-<strong>08</strong>92-10 0.12000 ESCITALOPRAM 10 MG TABLET 0 INTERNATIONAL L EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 133LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59746-0280-01 0.12000 ESCITALOPRAM 10 MG TABLET 0 CADISTA PHARMAC EAGEX 60505-2781-01 0.12000 ESCITALOPRAM 10 MG TABLET 0 APOTEX CORP EAGEX 60505-2781-<strong>08</strong> 0.12000 ESCITALOPRAM 10 MG TABLET 0 APOTEX CORP EAGEX 65862-0374-01 0.12000 ESCITALOPRAM 10 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0374-05 0.12000 ESCITALOPRAM 10 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-<strong>06</strong>17-01 0.12000 ESCITALOPRAM 10 MG TABLET 0 AHP EAGEX 68180-0135-01 0.12000 ESCITALOPRAM 10 MG TABLET 0 LUPIN PHARMACEU EAGEX 68645-0448-70 0.12000 ESCITALOPRAM 10 MG TABLET 0 LEGACY PHARMACE EAGEX 76282-0250-30 0.12000 ESCITALOPRAM 10 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-0250-90 0.12000 ESCITALOPRAM 10 MG TABLET 0 EXELAN PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-5852-01 0.16850 ESCITALOPRAM 20 MG TABLET 0 TEVA USA EAGEX 00093-5852-05 0.16850 ESCITALOPRAM 20 MG TABLET 0 TEVA USA EAGEX 00143-9807-01 0.16850 ESCITALOPRAM 20 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-9807-05 0.16850 ESCITALOPRAM 20 MG TABLET 0 WEST-WARD,INC. EAGEX 00378-3857-10 0.16850 ESCITALOPRAM 20 MG TABLET 0 MYLAN EAGEX 00378-3857-77 0.16850 ESCITALOPRAM 20 MG TABLET 0 MYLAN EAGEX 00378-3857-93 0.16850 ESCITALOPRAM 20 MG TABLET 0 MYLAN EAGEX 00904-6315-61 0.16850 ESCITALOPRAM 20 MG TABLET 0 MAJOR PHARMACEU EAGEX 13668-0137-01 0.16850 ESCITALOPRAM 20 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0137-10 0.16850 ESCITALOPRAM 20 MG TABLET 0 TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16729-0170-01 0.16850 ESCITALOPRAM 20 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 31722-0251-90 0.16850 ESCITALOPRAM 20 MG TABLET 0 CAMBER PHARMACE EAGEX 33342-0038-11 0.16850 ESCITALOPRAM 20 MG TABLET 0 MACLEODS PHARMA EAGEX 51079-0544-01 0.16850 ESCITALOPRAM 20 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0544-20 0.16850 ESCITALOPRAM 20 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0544-30 0.16850 ESCITALOPRAM 20 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0544-56 0.16850 ESCITALOPRAM 20 MG TABLET 0 MYLAN INSTITUTI EAGEX 54458-<strong>08</strong>91-10 0.16850 ESCITALOPRAM 20 MG TABLET 0 INTERNATIONAL L EAGEX 59746-0281-01 0.16850 ESCITALOPRAM 20 MG TABLET 0 CADISTA PHARMAC EAGEX 60505-2782-01 0.16850 ESCITALOPRAM 20 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-2782-<strong>08</strong> 0.16850 ESCITALOPRAM 20 MG TABLET 0 APOTEX CORP EAGEX 65862-0375-01 0.16850 ESCITALOPRAM 20 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0375-05 0.16850 ESCITALOPRAM 20 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-<strong>06</strong>18-01 0.16850 ESCITALOPRAM 20 MG TABLET 0 AHP EAGEX 68180-0136-01 0.16850 ESCITALOPRAM 20 MG TABLET 0 LUPIN PHARMACEU EAGEX 68645-0447-70 0.16850 ESCITALOPRAM 20 MG TABLET 0 LEGACY PHARMACE EAGEX 76282-0251-30 0.16850 ESCITALOPRAM 20 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-0251-90 0.16850 ESCITALOPRAM 20 MG TABLET 0 EXELAN PHARMACE EAGEX 00093-5850-01 0.11430 ESCITALOPRAM 5 MG TABLET 0 TEVA USA EAGEX 00093-5850-05 0.11430 ESCITALOPRAM 5 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-5850-19 0.11430 ESCITALOPRAM 5 MG TABLET 0 TEVA USA EAGEX 00093-5850-93 0.11430 ESCITALOPRAM 5 MG TABLET 0 TEVA USA EAGEX 00143-9809-01 0.11430 ESCITALOPRAM 5 MG TABLET 0 WEST-WARD,INC. EAGEX 00378-3855-77 0.11430 ESCITALOPRAM 5 MG TABLET 0 MYLAN EAGEX 00378-3855-93 0.11430 ESCITALOPRAM 5 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 134LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0135-01 0.11430 ESCITALOPRAM 5 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0135-05 0.11430 ESCITALOPRAM 5 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0135-10 0.11430 ESCITALOPRAM 5 MG TABLET 0 TORRENT PHARMAC EAGEX 16729-0168-01 0.11430 ESCITALOPRAM 5 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0168-17 0.11430 ESCITALOPRAM 5 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 31722-0249-90 0.11430 ESCITALOPRAM 5 MG TABLET 0 CAMBER PHARMACE EAGEX 33342-0036-11 0.11430 ESCITALOPRAM 5 MG TABLET 0 MACLEODS PHARMA EAGEX 59746-0279-01 0.11430 ESCITALOPRAM 5 MG TABLET 0 CADISTA PHARMAC EAGEX 60505-2780-01 0.11430 ESCITALOPRAM 5 MG TABLET 0 APOTEX CORP EAGEX 60505-2780-<strong>08</strong> 0.11430 ESCITALOPRAM 5 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0373-01 0.11430 ESCITALOPRAM 5 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0373-05 0.11430 ESCITALOPRAM 5 MG TABLET 0 AUROBINDO PHARM EAGEX 68180-0137-01 0.11430 ESCITALOPRAM 5 MG TABLET 0 LUPIN PHARMACEU EAGEX 76282-0249-10 0.11430 ESCITALOPRAM 5 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-0249-30 0.11430 ESCITALOPRAM 5 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-0249-90 0.11430 ESCITALOPRAM 5 MG TABLET 0 EXELAN PHARMACE EAGEN 00535-0012-01 0.48480 ESGIC CAPSULE 0 FOREST PHARMACE EAGEN 00535-0011-01 0.<strong>06</strong>030 ESGIC 50-325-40 MG TABLET 0 FOREST PHARMACE EABND 00430-3754-14 3.55591 ESTRACE 0.01% CREAM 0 ACTAVIS PHARMA, GMGEN 00430-0720-24 0.13896 ESTRACE 0.5 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00430-0721-24 0.<strong>08</strong>748 ESTRACE 1 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00430-0722-24 0.12137 ESTRACE 2 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00378-3349-99 11.21110 ESTRADIOL TDS 0.025 MG/DAY 0 MYLAN EAGEN 00378-3360-99 10.74000 ESTRADIOL TDS 0.0375 MG/DAY 0 MYLAN EAGEN 00378-3350-99 11.37750 ESTRADIOL TDS 0.05 MG/DAY 0 MYLAN EAGEN 00378-3361-99 11.49568 ESTRADIOL TDS 0.<strong>06</strong> MG/DAY 0 MYLAN EAGEN 00378-3351-99 11.46550 ESTRADIOL TDS 0.075 MG/DAY 0 MYLAN EAGEN 00378-3352-99 17.36394 ESTRADIOL TDS 0.1 MG/DAY 0 MYLAN EAGEN 00574-<strong>08</strong>70-05 21.64650 ESTRADIOL VALERATE 20 MG/ML VL 0 PADDOCK LABS. MLGEN 00574-<strong>08</strong>72-05 35.90700 ESTRADIOL VALERATE 40 MG/ML VL 0 PADDOCK LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 47781-0204-04 11.21110 ESTRADIOL 0.025 MG/DAY PATCH 0 ALVOGEN INC EAGEN 47781-0205-04 10.74000 ESTRADIOL 0.0375 MG/DAY PATCH 0 ALVOGEN INC EAGEN 47781-02<strong>06</strong>-04 11.37750 ESTRADIOL 0.05 MG/DAY PATCH 0 ALVOGEN INC EAGEN 47781-0207-04 11.49568 ESTRADIOL 0.<strong>06</strong> MG/DAY PATCH 0 ALVOGEN INC EAGEN 47781-02<strong>08</strong>-04 11.46550 ESTRADIOL 0.075 MG/DAY PATCH 0 ALVOGEN INC EAGEN 47781-0209-04 16.57500 ESTRADIOL 0.1 MG/DAY PATCH 0 ALVOGEN INC EAGEN 00378-1452-01 0.13896 ESTRADIOL 0.5 MG TABLET 0 MYLAN EAGEN 00378-1452-05 0.13896 ESTRADIOL 0.5 MG TABLET 0 MYLAN EAGEN 00555-<strong>08</strong>99-02 0.13896 ESTRADIOL 0.5 MG TABLET 0 BARR EAGEN 00591-0528-01 0.13896 ESTRADIOL 0.5 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-1454-01 0.<strong>08</strong>748 ESTRADIOL 1 MG TABLET 0 MYLAN EAGEN 00378-1454-05 0.<strong>08</strong>748 ESTRADIOL 1 MG TABLET 0 MYLAN EAGEN 00555-<strong>08</strong>86-02 0.<strong>08</strong>748 ESTRADIOL 1 MG TABLET 0 BARR EAGEN 00555-<strong>08</strong>86-04 0.<strong>08</strong>748 ESTRADIOL 1 MG TABLET 0 BARR EAGEN 00591-0487-01 0.<strong>08</strong>748 ESTRADIOL 1 MG TABLET 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 135LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-0487-05 0.<strong>08</strong>748 ESTRADIOL 1 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00378-1458-01 0.12137 ESTRADIOL 2 MG TABLET 0 MYLAN EAGEN 00378-1458-05 0.12137 ESTRADIOL 2 MG TABLET 0 MYLAN EAGEN 00378-1458-77 0.12137 ESTRADIOL 2 MG TABLET 0 MYLAN EAGEN 00555-<strong>08</strong>87-02 0.12137 ESTRADIOL 2 MG TABLET 0 BARR EAGEN 00555-<strong>08</strong>87-04 0.12137 ESTRADIOL 2 MG TABLET 0 BARR EAGEN 00591-0488-01 0.12137 ESTRADIOL 2 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-0488-05 0.12137 ESTRADIOL 2 MG TABLET 0 ACTAVIS PHARMA, EAGEN 51991-<strong>06</strong>23-28 3.33294 ESTRADIOL-NORETH 0.5-0.1 MG TB 0 BRECKENRIDGE EAGEN 51991-0474-28 3.22934 ESTRADIOL-NORETH 1-0.5 MG TAB 0 BRECKENRIDGE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00013-2150-36 222.53960 ESTRING 2 MG VAGINAL RING 0 PHARMACIA/UPJHN EABND 00591-0414-01 0.10040 0.35814 ESTROPIPATE 0.625(0.75 MG) TAB 0 ACTAVIS PHARMA, EABND 00591-0415-01 0.11640 0.51460 ESTROPIPATE 1.25(1.5 MG) TAB 0 ACTAVIS PHARMA, EAGEN 00591-0416-01 0.50<strong>06</strong>4 ESTROPIPATE 2.5(3 MG) TAB 0 ACTAVIS PHARMA, EABEX 00430-0570-45 1.514<strong>08</strong> 3.65536 ESTROSTEP FE-28 TABLET 0 ACTAVIS PHARMA, EAGEX 54879-0001-00 0.43200 ETHAMBUTOL HCL 100 MG TABLET 0 STI PHARMA, LLC EAGEX 54879-0001-01 0.34192 ETHAMBUTOL HCL 100 MG TABLET 0 STI PHARMA, LLC EAGEX 68180-0280-01 0.44400 ETHAMBUTOL HCL 100 MG TABLET 0 LUPIN PHARMACEU EAGEX 68850-0004-01 0.44377 ETHAMBUTOL HCL 100 MG TABLET 0 G & W LABS. EAGEX 00555-0923-02 1.<strong>08</strong>070 ETHAMBUTOL HCL 400 MG TABLET 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 54879-0002-00 1.<strong>08</strong>070 ETHAMBUTOL HCL 400 MG TABLET 0 STI PHARMA, LLC EAGEX 54879-0002-01 1.<strong>08</strong>070 ETHAMBUTOL HCL 400 MG TABLET 0 STI PHARMA, LLC EAGEX 68<strong>08</strong>4-0280-01 1.<strong>08</strong>070 ETHAMBUTOL HCL 400 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0280-11 1.<strong>08</strong>070 ETHAMBUTOL HCL 400 MG TABLET 0 AHP EAGEX 68180-0281-01 1.<strong>08</strong>070 ETHAMBUTOL HCL 400 MG TABLET 0 LUPIN PHARMACEU EABND 00517-8575-10 7.37040 ETHANOL 98% AMPUL 0 AMER. REGENT MLGEX 50111-0901-01 0.93650 ETHOSUXIMIDE 250 MG CAPSULE 0 BARR EAGEX 61748-0025-01 0.93650 ETHOSUXIMIDE 250 MG CAPSULE 0 VERSA PHARMACEU EAGEX 00093-9660-16 0.22000 ETHOSUXIMIDE 250 MG/5 ML SOLN 0 TEVA USA MLGEX 61748-0024-16 0.30919 ETHOSUXIMIDE 250 MG/5 ML SOLN 0 VERSA PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00378-3286-91 3.58628 ETIDRONATE DISODIUM 200 MG TAB 0 MYLAN EABND 00378-3288-91 7.17092 ETIDRONATE DISODIUM 400 MG TAB 0 MYLAN EAGEN 00093-1122-01 2.17597 ETODOLAC ER 400 MG TABLET G TEVA USA EAGEN 51672-4051-01 2.17590 ETODOLAC ER 400 MG TABLET G TARO PHARM USA EAGEN 51672-4051-04 2.21949 ETODOLAC ER 400 MG TABLET G TARO PHARM USA EAGEN 00093-7172-01 2.31150 ETODOLAC ER 500 MG TABLET G TEVA USA EAGEN 51672-4052-01 2.31157 ETODOLAC ER 500 MG TABLET G TARO PHARM USA EAGEN 51672-4052-04 2.35775 ETODOLAC ER 500 MG TABLET G TARO PHARM USA EAGEN 00093-1118-01 2.45557 ETODOLAC ER 600 MG TABLET G TEVA USA EAGEN 51672-4053-01 2.45557 ETODOLAC ER 600 MG TABLET G TARO PHARM USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-4053-04 2.50475 ETODOLAC ER 600 MG TABLET G TARO PHARM USA EAGUL 51672-4016-01 0.58500 ETODOLAC 200 MG CAPSULE 0 TARO PHARM USA EAGUL 60505-0039-01 0.58500 ETODOLAC 200 MG CAPSULE 0 APOTEX CORP EAGEN 51672-4017-01 1.21260 ETODOLAC 300 MG CAPSULE 0 TARO PHARM USA EAGEN 60505-0040-01 1.21260 ETODOLAC 300 MG CAPSULE 0 APOTEX CORP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 136LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00093-<strong>08</strong>92-01 0.39230 ETODOLAC 400 MG TABLET 0 TEVA USA EAGUL 00185-0140-01 0.39230 ETODOLAC 400 MG TABLET 0 SANDOZ EAGUL 00185-0140-10 0.39230 ETODOLAC 400 MG TABLET 0 SANDOZ EAGEN 49158-0507-10 0.17377 ETODOLAC 400 MG TABLET 0 TARO PHARM USA EAGUL 51672-4018-01 0.39230 ETODOLAC 400 MG TABLET 0 TARO PHARM USA EAGUL 60505-0041-01 0.39230 ETODOLAC 400 MG TABLET 0 APOTEX CORP EAGUL 00093-1893-01 0.75000 ETODOLAC 500 MG TABLET 0 TEVA USA EAGUL 00185-0139-01 0.75000 ETODOLAC 500 MG TABLET 0 SANDOZ EAGUL 00185-0139-05 0.75000 ETODOLAC 500 MG TABLET 0 SANDOZ EAGEN 49158-05<strong>08</strong>-10 0.40305 ETODOLAC 500 MG TABLET 0 TARO PHARM USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-4036-01 0.75000 ETODOLAC 500 MG TABLET 0 TARO PHARM USA EAGUL 60505-0102-01 0.75000 ETODOLAC 500 MG TABLET 0 APOTEX CORP EABND 00015-3404-20 132.42650 ETOPOPHOS 100 MG VIAL 0 BMS ONCO/IMMUN EAGEN 55390-0293-01 1.58400 ETOPOSIDE 1,000 MG/50 ML VIAL 0 BEDFORD LABS MLGEN 63323-0104-50 2.23890 ETOPOSIDE 1,000 MG/50 ML VIAL 0 APP PHARMACEUTI MLGEN 16729-0114-31 1.70550 ETOPOSIDE 100 MG/5 ML VIAL 0 ACCORD <strong>HEALTH</strong>CA MLGEN 55390-0291-01 1.49400 ETOPOSIDE 100 MG/5 ML VIAL 0 BEDFORD LABS MLGEN 55390-0491-01 1.49400 ETOPOSIDE 100 MG/5 ML VIAL 0 BEDFORD/NOVAPLU MLGEN 63323-0104-05 2.23920 ETOPOSIDE 100 MG/5 ML VIAL 0 APP PHARMACEUTI MLBND 00378-3266-94 58.81338 ETOPOSIDE 50 MG CAPSULE 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55390-0292-01 1.58400 ETOPOSIDE 500 MG/25 ML VIAL 0 BEDFORD LABS MLGEN 63323-0104-25 2.23890 ETOPOSIDE 500 MG/25 ML VIAL 0 APP PHARMACEUTI MLBND 64011-0215-41 11.16913 EVAMIST 1.53 MG/SPRAY 0 THER-RX MLBND 00002-4165-02 6.57360 EVISTA 60 MG TABLET 0 ELI LILLY & CO. EABND 00002-4165-07 6.57360 EVISTA 60 MG TABLET 0 ELI LILLY & CO. EABND 00002-4165-30 6.57360 EVISTA 60 MG TABLET 0 ELI LILLY & CO. EABND 00145-0<strong>06</strong>1-00 4.25574 EVOCLIN 1% FOAM G PRESTIUM PHARMA GMBND 00145-0<strong>06</strong>1-50 5.56100 EVOCLIN 1% FOAM G PRESTIUM PHARMA GMBND 63395-0201-13 2.37510 3.81468 EVOXAC 30 MG CAPSULE 0 DAIICHI SANKYO, EABND 1<strong>06</strong>31-0101-15 3.43343 EXELDERM 1% CREAM G RANBAXY LABORAT GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 1<strong>06</strong>31-0101-30 4.53041 EXELDERM 1% CREAM G RANBAXY LABORAT GMBND 1<strong>06</strong>31-0101-60 3.75228 EXELDERM 1% CREAM G RANBAXY LABORAT GMBND 1<strong>06</strong>31-0100-04 4.53097 EXELDERM 1% SOLUTION G RANBAXY LABORAT MLBND 1<strong>06</strong>31-0100-30 4.53<strong>06</strong>9 EXELDERM 1% SOLUTION G RANBAXY LABORAT MLBND 00078-0323-44 2.04166 5.19469 EXELON 1.5 MG CAPSULE G NOVARTIS EABND 00078-0503-15 10.33018 EXELON 13.3 MG/24HR PATCH 0 NOVARTIS EABND 00078-0339-31 3.86607 EXELON 2 MG/ML ORAL SOLUTION 0 NOVARTIS MLBND 00078-0324-44 1.95740 5.19469 EXELON 3 MG CAPSULE G NOVARTIS EABND 00078-0325-44 2.<strong>06</strong>618 5.19469 EXELON 4.5 MG CAPSULE G NOVARTIS EABND 00078-0501-15 10.33018 EXELON 4.6 MG/24HR PATCH 0 NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0501-61 10.32520 EXELON 4.6 MG/24HR PATCH 0 NOVARTIS EABND 00078-0326-44 2.04160 5.19469 EXELON 6 MG CAPSULE G NOVARTIS EABND 00078-0502-15 10.33018 EXELON 9.5 MG/24HR PATCH 0 NOVARTIS EABND 00078-0502-61 10.32520 EXELON 9.5 MG/24HR PATCH 0 NOVARTIS EAGEN 00054-0<strong>08</strong>0-13 5.23905 EXEMESTANE 25 MG TABLET 0 ROXANE LABS. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 137LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-2858-01 5.23905 EXEMESTANE 25 MG TABLET 0 GREENSTONE LLC. EABND 00078-0561-15 4.97834 EXFORGE HCT 10-160-12.5 MG TAB G NOVARTIS EABND 00078-0562-15 4.97834 EXFORGE HCT 10-160-25 MG TAB G NOVARTIS EABND 00078-0563-15 6.31962 EXFORGE HCT 10-320-25 MG TAB G NOVARTIS EABND 00078-0559-15 4.38848 EXFORGE HCT 5-160-12.5 MG TAB G NOVARTIS EABND 00078-0560-15 4.38848 EXFORGE HCT 5-160-25 MG TAB G NOVARTIS EABND 00078-0489-15 4.97834 EXFORGE 10-160 MG TABLET G NOVARTIS EABND 00078-0491-15 6.31962 EXFORGE 10-320 MG TABLET G NOVARTIS EABND 00078-0488-15 4.38848 EXFORGE 5-160 MG TABLET G NOVARTIS EABND 00078-0490-15 5.56625 EXFORGE 5-320 MG TABLET G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0468-15 23.36256 EXJADE 125 MG TABLET 0 NOVARTIS EABND 00078-0469-15 46.72402 EXJADE 250 MG TABLET 0 NOVARTIS EABND 00078-0470-15 93.44638 EXJADE 500 MG TABLET 0 NOVARTIS EABND 00078-0569-12 290.480<strong>08</strong> EXTAVIA 0.3 MG KIT G NOVARTIS EABND 00078-0569-99 290.49170 EXTAVIA 0.3 MG KIT G NOVARTIS EABND 00078-0569-61 290.49170 EXTAVIA 0.3 MG VIAL G NOVARTIS EABND 40076-0051-00 5.68<strong>06</strong>0 EXTINA 2% FOAM G PRESTIUM PHARMA GMBND 40076-0051-50 6.74607 EXTINA 2% FOAM G PRESTIUM PHARMA GMBND 63032-0051-00 5.68<strong>06</strong>0 EXTINA 2% FOAM G PRESTIUM PHARMA GMBND 63032-0051-50 6.74607 EXTINA 2% FOAM G PRESTIUM PHARMA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 10122-0321-05 39.70720 FACTIVE 320 MG TABLET G METHAPHARM INC EABND 10122-0321-07 39.7<strong>06</strong>01 FACTIVE 320 MG TABLET G METHAPHARM INC EAGEX 16714-0359-01 0.85280 FALMINA-28 TABLET 0 NORTHSTAR RX LL EAGEX 16714-0359-04 0.85280 FALMINA-28 TABLET 0 NORTHSTAR RX LL EAGEN 00054-0196-13 2.98390 FAMCICLOVIR 125 MG TABLET G ROXANE LABS. EAGEN 00093-8117-56 2.98390 FAMCICLOVIR 125 MG TABLET G TEVA USA EAGEN 00378-4490-93 2.98390 FAMCICLOVIR 125 MG TABLET G MYLAN EAGEN 00591-3271-30 2.98390 FAMCICLOVIR 125 MG TABLET G ACTAVIS PHARMA, EAGEN 00781-5620-31 2.98390 FAMCICLOVIR 125 MG TABLET G SANDOZ EAGEN 16714-0300-01 2.98390 FAMCICLOVIR 125 MG TABLET G NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-2700-01 2.98390 FAMCICLOVIR 125 MG TABLET G GREENSTONE LLC. EAGEN 60505-3245-03 2.98390 FAMCICLOVIR 125 MG TABLET G APOTEX CORP EAGEN 65862-0465-30 2.98390 FAMCICLOVIR 125 MG TABLET G AUROBINDO PHARM EAGEN 00054-0197-13 1.22175 FAMCICLOVIR 250 MG TABLET G ROXANE LABS. EAGEN 00093-8118-56 1.22175 FAMCICLOVIR 250 MG TABLET G TEVA USA EAGEN 00378-4491-93 1.22175 FAMCICLOVIR 250 MG TABLET G MYLAN EAGEN 00591-3272-30 1.22175 FAMCICLOVIR 250 MG TABLET G ACTAVIS PHARMA, EAGEN 00781-5621-31 1.22175 FAMCICLOVIR 250 MG TABLET G SANDOZ EAGEN 16714-0304-01 1.22175 FAMCICLOVIR 250 MG TABLET G NORTHSTAR RX LL EAGEN 59762-2701-02 1.22175 FAMCICLOVIR 250 MG TABLET G GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-3246-03 1.22175 FAMCICLOVIR 250 MG TABLET G APOTEX CORP EAGEN 65862-0466-30 1.22175 FAMCICLOVIR 250 MG TABLET G AUROBINDO PHARM EAGEN 00054-0198-13 1.86530 FAMCICLOVIR 500 MG TABLET G ROXANE LABS. EAGEN 00093-8119-56 1.86530 FAMCICLOVIR 500 MG TABLET G TEVA USA EAGEN 00378-4492-93 1.86530 FAMCICLOVIR 500 MG TABLET G MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 138LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3273-30 1.86530 FAMCICLOVIR 500 MG TABLET G ACTAVIS PHARMA, EAGEN 00781-5622-31 1.86530 FAMCICLOVIR 500 MG TABLET G SANDOZ EAGEN 16714-0305-01 1.86530 FAMCICLOVIR 500 MG TABLET G NORTHSTAR RX LL EAGEN 59762-2703-01 1.86530 FAMCICLOVIR 500 MG TABLET G GREENSTONE LLC. EAGEN 60505-3247-03 1.86530 FAMCICLOVIR 500 MG TABLET G APOTEX CORP EAGEN 65862-0467-30 1.86530 FAMCICLOVIR 500 MG TABLET G AUROBINDO PHARM EAGEN 63323-0738-09 0.40500 FAMOTIDINE 10 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0738-20 0.40500 FAMOTIDINE 10 MG/ML VIAL 0 APP PHARMACEUTI MLBND 00338-5197-41 0.07771 FAMOTIDINE 20 MG PIGGYBACK 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00172-5728-60 0.07620 FAMOTIDINE 20 MG TABLET 0 IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00172-5728-70 0.07620 FAMOTIDINE 20 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-5728-80 0.07620 FAMOTIDINE 20 MG TABLET 0 IVAX PHARMACEUT EAGEN 00378-3020-01 0.07620 FAMOTIDINE 20 MG TABLET 0 MYLAN EAGEN 00378-3020-05 0.07620 FAMOTIDINE 20 MG TABLET 0 MYLAN EAGEN 16714-0361-01 0.07620 FAMOTIDINE 20 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0361-04 0.07620 FAMOTIDINE 20 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0361-05 0.07620 FAMOTIDINE 20 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0361-<strong>06</strong> 0.07620 FAMOTIDINE 20 MG TABLET 0 NORTHSTAR RX LL EAGEN 51079-0966-17 0.07620 FAMOTIDINE 20 MG TABLET 0 MYLAN INSTITUTI EAGEN 55111-0119-01 0.07620 FAMOTIDINE 20 MG TABLET 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0119-10 0.07620 FAMOTIDINE 20 MG TABLET 0 DR.REDDY'S LAB EAGEN 61442-0121-01 0.07620 FAMOTIDINE 20 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0121-10 0.07620 FAMOTIDINE 20 MG TABLET 0 CARLSBAD TECH EAGEN 64679-0936-02 0.07620 FAMOTIDINE 20 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0936-03 0.07620 FAMOTIDINE 20 MG TABLET 0 WOCKHARDT USA L EAGEN 68<strong>08</strong>4-0172-01 0.07620 FAMOTIDINE 20 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0172-11 0.07620 FAMOTIDINE 20 MG TABLET 0 AHP EAGEN 68645-0140-59 0.07620 FAMOTIDINE 20 MG TABLET 0 LEGACY PHARMACE EAGEN 10019-0045-02 0.33195 FAMOTIDINE 20 MG/2 ML VIAL 0 WEST-WARD,INC. MLGEN 55390-0029-10 0.47700 FAMOTIDINE 20 MG/2 ML VIAL 0 BEDFORD LABS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0739-12 0.40500 FAMOTIDINE 20 MG/2 ML VIAL 0 APP PHARMACEUTI MLGEN 0<strong>06</strong>41-6021-01 0.25875 FAMOTIDINE 200 MG/20 ML VIAL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6021-10 0.25875 FAMOTIDINE 200 MG/20 ML VIAL 0 WEST-WARD,INC. MLGEN 55390-0027-01 0.43030 FAMOTIDINE 200 MG/20 ML VIAL 0 BEDFORD LABS MLGEN 00172-5729-60 0.07425 FAMOTIDINE 40 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-5729-70 0.07425 FAMOTIDINE 40 MG TABLET 0 IVAX PHARMACEUT EAGEN 00378-3040-01 0.07425 FAMOTIDINE 40 MG TABLET 0 MYLAN EAGEN 16714-0362-01 0.07425 FAMOTIDINE 40 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0362-04 0.07425 FAMOTIDINE 40 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0362-05 0.07425 FAMOTIDINE 40 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0362-<strong>06</strong> 0.07425 FAMOTIDINE 40 MG TABLET 0 NORTHSTAR RX LL EAGEN 55111-0120-01 0.07425 FAMOTIDINE 40 MG TABLET 0 DR.REDDY'S LAB EAGEN 61442-0122-01 0.07425 FAMOTIDINE 40 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0122-10 0.07425 FAMOTIDINE 40 MG TABLET 0 CARLSBAD TECH EAGEN 64679-0937-01 0.07425 FAMOTIDINE 40 MG TABLET 0 WOCKHARDT USA L EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 139LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0937-02 0.07425 FAMOTIDINE 40 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0937-03 0.07425 FAMOTIDINE 40 MG TABLET 0 WOCKHARDT USA L EAGEN 68645-0141-54 0.07425 FAMOTIDINE 40 MG TABLET 0 LEGACY PHARMACE EAGEN 0<strong>06</strong>41-6023-01 0.43030 FAMOTIDINE 40 MG/4 ML VIAL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6023-25 0.43030 FAMOTIDINE 40 MG/4 ML VIAL 0 WEST-WARD,INC. MLGEN 55390-0028-10 0.43030 FAMOTIDINE 40 MG/4 ML VIAL 0 BEDFORD LABS MLGEN 00574-0147-04 1.34150 FAMOTIDINE 40 MG/5 ML SUSP 0 PADDOCK LABS. MLGEN 68180-0150-01 1.34150 FAMOTIDINE 40 MG/5 ML SUSP 0 LUPIN PHARMACEU MLGEN 68382-0444-05 1.34150 FAMOTIDINE 40 MG/5 ML SUSP 0 ZYDUS PHARMACEU MLGEN 55390-0026-01 0.43030 FAMOTIDINE 500 MG/50 ML VIAL 0 BEDFORD LABS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0366-15 2.98390 7.82219 FAMVIR 125 MG TABLET G NOVARTIS EABND 00078-0367-15 1.22175 8.50473 FAMVIR 250 MG TABLET G NOVARTIS EABND 00078-0368-15 1.86530 17.07974 FAMVIR 500 MG TABLET G NOVARTIS EABEX 00078-<strong>06</strong>02-<strong>08</strong> 12.71352 FANAPT TITRATION PACK G NOVARTIS EABEX 00078-0595-20 12.71338 FANAPT 1 MG TABLET G NOVARTIS EABEX 00078-<strong>06</strong>00-20 12.71338 FANAPT 10 MG TABLET G NOVARTIS EABEX 00078-<strong>06</strong>01-20 12.71338 FANAPT 12 MG TABLET G NOVARTIS EABEX 00078-0596-20 12.71338 FANAPT 2 MG TABLET G NOVARTIS EABEX 00078-0597-20 12.71338 FANAPT 4 MG TABLET G NOVARTIS EABEX 00078-0598-20 12.71338 FANAPT 6 MG TABLET G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00078-0599-20 12.71338 FANAPT 8 MG TABLET G NOVARTIS EABND 11399-0005-30 25.64700 FARESTON 60 MG TABLET 0 PROSTRAKAN INC. EABND 00003-1428-11 9.60144 FARXIGA 10 MG TABLET G BMS PRIMARYCARE EABND 00003-1427-11 9.60144 FARXIGA 5 MG TABLET G BMS PRIMARYCARE EABEX 18860-0104-01 10.83737 FAZACLO 100 MG ODT G JAZZ PHARMACEUT EABEX 18860-0104-10 10.837<strong>06</strong> FAZACLO 100 MG ODT G JAZZ PHARMACEUT EABEX 18860-0101-10 2.36242 FAZACLO 12.5 MG ODT G JAZZ PHARMACEUT EABEX 18860-0105-01 12.86915 FAZACLO 150 MG ODT G JAZZ PHARMACEUT EABEX 18860-0105-10 12.86898 FAZACLO 150 MG ODT G JAZZ PHARMACEUT EABEX 18860-01<strong>06</strong>-01 17.15852 FAZACLO 200 MG ODT G JAZZ PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 18860-01<strong>06</strong>-10 17.15867 FAZACLO 200 MG ODT G JAZZ PHARMACEUT EABEX 18860-0102-01 3.17907 FAZACLO 25 MG ODT G JAZZ PHARMACEUT EABEX 18860-0102-10 3.17856 FAZACLO 25 MG ODT G JAZZ PHARMACEUT EAGEN 64376-<strong>08</strong>02-01 0.18480 FE C PLUS TABLET 0 BOCA PHARMACAL EABND 64193-0424-02 1.58500 FEIBA NF 1,000 UNIT (NOMINAL) 0 BAXTER BIOSCIENBND 64193-0225-02 1.58500 FEIBA NF 1,750-3,250 UNIT VIAL 0 BAXTER BIOSCIENBND 64193-0425-02 1.58500 FEIBA NF 2,500 UNIT (NOMINAL) 0 BAXTER BIOSCIENBND 64193-0223-02 1.58500 FEIBA NF 400-650 UNIT VIAL 0 BAXTER BIOSCIENBND 64193-0423-02 1.58500 FEIBA NF 500 UNIT (NOMINAL) 0 BAXTER BIOSCIENBND 64193-0224-02 1.58500 FEIBA NF 651-1,200 UNIT VIAL 0 BAXTER BIOSCIEN--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64193-0222-03 1.52500 FEIBA VH IMMUNO 400-650 UNITS 0 BAXTER BIOSCIENBND 64193-0222-04 1.52500 FEIBA VH IMMUNO 651-1,200 UNIT 0 BAXTER BIOSCIENGEX 51525-0430-01 3.87615 FELBAMATE 400 MG TABLET G WALLACE PHARMAC EAGEX 65162-0734-03 3.91524 FELBAMATE 400 MG TABLET G AMNEAL PHARMACE EAGEX 65162-0734-09 3.91524 FELBAMATE 400 MG TABLET G AMNEAL PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 140LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51525-0431-01 4.44232 FELBAMATE 600 MG TABLET G WALLACE PHARMAC EAGEX 65162-0735-03 4.48725 FELBAMATE 600 MG TABLET G AMNEAL PHARMACE EAGEX 65162-0735-09 4.48716 FELBAMATE 600 MG TABLET G AMNEAL PHARMACE EAGEX 65162-0735-18 4.48716 FELBAMATE 600 MG TABLET G AMNEAL PHARMACE EAGEX 51525-0442-03 1.94176 FELBAMATE 600 MG/5 ML SUSP G WALLACE PHARMAC MLGEX 51525-0442-<strong>08</strong> 2.010<strong>06</strong> FELBAMATE 600 MG/5 ML SUSP G WALLACE PHARMAC MLGEX 65162-<strong>06</strong>86-88 2.00503 FELBAMATE 600 MG/5 ML SUSP G AMNEAL PHARMACE MLGEX 65162-<strong>06</strong>86-90 1.95942 FELBAMATE 600 MG/5 ML SUSP G AMNEAL PHARMACE MLBEX 00037-0430-01 5.85855 5.85855 FELBATOL 400 MG TABLET 0 MEDA PHARMACEUT EABEX 00037-0431-01 6.71411 6.71411 FELBATOL 600 MG TABLET 0 MEDA PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00037-0442-17 2.93482 FELBATOL 600 MG/5 ML SUSP 0 MEDA PHARMACEUT MLBEX 00037-0442-67 3.038<strong>08</strong> FELBATOL 600 MG/5 ML SUSP 0 MEDA PHARMACEUT MLBUL 00<strong>06</strong>9-3220-66 0.<strong>08</strong>910 3.92631 FELDENE 10 MG CAPSULE G PFIZER US PHARM EABUL 00<strong>06</strong>9-3230-66 0.11310 6.71909 FELDENE 20 MG CAPSULE G PFIZER US PHARM EAGEN 00378-5013-01 0.77004 FELODIPINE ER 10 MG TABLET 0 MYLAN EAGEN 00378-5013-05 0.77004 FELODIPINE ER 10 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-3583-21 0.77004 FELODIPINE ER 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3583-28 0.77004 FELODIPINE ER 10 MG TABLET 0 QUALITEST EAGEN 13668-0134-01 0.77004 FELODIPINE ER 10 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0134-05 0.77004 FELODIPINE ER 10 MG TABLET 0 TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-0134-10 0.77004 FELODIPINE ER 10 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0134-90 0.77004 FELODIPINE ER 10 MG TABLET 0 TORRENT PHARMAC EAGEN 51079-0468-01 0.77004 FELODIPINE ER 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0468-20 0.77004 FELODIPINE ER 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0370-01 0.77004 FELODIPINE ER 10 MG TABLET 0 MUTUAL PHARM CO EAGEN 63304-0437-01 0.77004 FELODIPINE ER 10 MG TABLET 0 RANBAXY PHARMAC EAGEN 65862-<strong>06</strong>75-01 0.77004 FELODIPINE ER 10 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-<strong>06</strong>75-99 0.77004 FELODIPINE ER 10 MG TABLET 0 AUROBINDO PHARM EAGEN 68462-0235-01 0.77004 FELODIPINE ER 10 MG TABLET 0 GLENMARK PHARMA EAGEN 00378-5011-01 0.56430 FELODIPINE ER 2.5 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5011-05 0.56430 FELODIPINE ER 2.5 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-3581-21 0.56430 FELODIPINE ER 2.5 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3581-28 0.56430 FELODIPINE ER 2.5 MG TABLET 0 QUALITEST EAGEN 13668-0132-01 0.56430 FELODIPINE ER 2.5 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0132-05 0.56430 FELODIPINE ER 2.5 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0132-10 0.56430 FELODIPINE ER 2.5 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0132-90 0.56430 FELODIPINE ER 2.5 MG TABLET 0 TORRENT PHARMAC EAGEN 53489-0368-01 0.56430 FELODIPINE ER 2.5 MG TABLET 0 MUTUAL PHARM CO EAGEN 63304-0435-01 0.56430 FELODIPINE ER 2.5 MG TABLET 0 RANBAXY PHARMAC EAGEN 65862-<strong>06</strong>73-01 0.56430 FELODIPINE ER 2.5 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-<strong>06</strong>73-99 0.56430 FELODIPINE ER 2.5 MG TABLET 0 AUROBINDO PHARM EAGEN 68462-0233-01 0.56430 FELODIPINE ER 2.5 MG TABLET 0 GLENMARK PHARMA EAGEN 00378-5012-01 0.54824 FELODIPINE ER 5 MG TABLET 0 MYLAN EAGEN 00378-5012-05 0.54824 FELODIPINE ER 5 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-3582-21 0.54824 FELODIPINE ER 5 MG TABLET 0 QUALITEST EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 141LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-3582-28 0.54824 FELODIPINE ER 5 MG TABLET 0 QUALITEST EAGEN 13668-0133-01 0.54824 FELODIPINE ER 5 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0133-05 0.54824 FELODIPINE ER 5 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0133-10 0.54824 FELODIPINE ER 5 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0133-90 0.54824 FELODIPINE ER 5 MG TABLET 0 TORRENT PHARMAC EAGEN 51079-0467-01 0.54824 FELODIPINE ER 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0467-20 0.54824 FELODIPINE ER 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0369-01 0.54824 FELODIPINE ER 5 MG TABLET 0 MUTUAL PHARM CO EAGEN 63304-0436-01 0.54824 FELODIPINE ER 5 MG TABLET 0 RANBAXY PHARMAC EAGEN 65862-<strong>06</strong>74-01 0.54824 FELODIPINE ER 5 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-<strong>06</strong>74-99 0.54824 FELODIPINE ER 5 MG TABLET 0 AUROBINDO PHARM EAGEN 68462-0234-01 0.54824 FELODIPINE ER 5 MG TABLET 0 GLENMARK PHARMA EABND 00078-0249-15 0.15300 20.19943 FEMARA 2.5 MG TABLET 0 NOVARTIS EABEX 00430-0482-14 3.18720 FEMCON FE TABLET 0 ACTAVIS PHARMA, EABND 00430-0145-14 2.87844 FEMHRT 0.5 MG-2.5 MCG TABLET 0 ACTAVIS PHARMA, EABND 00430-6201-40 227.76860 FEMRING 0.05 MG VAGINAL RING 0 ACTAVIS PHARMA, EABND 00430-6202-40 242.71690 FEMRING 0.10 MG VAGINAL RING 0 ACTAVIS PHARMA, EAGEN 00378-6<strong>08</strong>9-77 5.18900 FEN<strong>OF</strong>IBRATE 130 MG CAPSULE G MYLAN EAGEN 00378-6<strong>08</strong>9-93 5.18900 FEN<strong>OF</strong>IBRATE 130 MG CAPSULE G MYLAN EAGEN 60505-3121-03 5.19474 FEN<strong>OF</strong>IBRATE 130 MG CAPSULE G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-3121-09 5.19474 FEN<strong>OF</strong>IBRATE 130 MG CAPSULE G APOTEX CORP EAGEN 68180-0131-<strong>06</strong> 5.19474 FEN<strong>OF</strong>IBRATE 130 MG CAPSULE G LUPIN PHARMACEU EAGEN 00115-0522-01 1.34388 FEN<strong>OF</strong>IBRATE 134 MG CAPSULE G GLOBAL PHARM EAGEN 00115-0522-02 1.34388 FEN<strong>OF</strong>IBRATE 134 MG CAPSULE G GLOBAL PHARM EAGEN 00378-8629-05 1.34388 FEN<strong>OF</strong>IBRATE 134 MG CAPSULE G MYLAN EAGEN 00378-8629-77 1.34388 FEN<strong>OF</strong>IBRATE 134 MG CAPSULE G MYLAN EAGEN 00093-2<strong>06</strong>0-98 4.29650 FEN<strong>OF</strong>IBRATE 145 MG TABLET G TEVA USA EAGEN 00378-3<strong>06</strong>6-77 4.29650 FEN<strong>OF</strong>IBRATE 145 MG TABLET G MYLAN EAGEN 51079-<strong>06</strong><strong>08</strong>-20 3.86250 FEN<strong>OF</strong>IBRATE 145 MG TABLET G MYLAN INSTITUTI EAGEN 68<strong>08</strong>4-<strong>06</strong>36-25 3.86250 FEN<strong>OF</strong>IBRATE 145 MG TABLET G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0361-02 4.29648 FEN<strong>OF</strong>IBRATE 145 MG TABLET G LUPIN PHARMACEU EAGEN 68180-0361-09 4.29650 FEN<strong>OF</strong>IBRATE 145 MG TABLET G LUPIN PHARMACEU EAGEN 68682-0528-01 4.10000 FEN<strong>OF</strong>IBRATE 145 MG TABLET G PERRIGO CO. EAGEN 00115-5522-02 1.34973 FEN<strong>OF</strong>IBRATE 160 MG TABLET G GLOBAL PHARM EAGEN 00115-5522-10 1.34973 FEN<strong>OF</strong>IBRATE 160 MG TABLET G GLOBAL PHARM EAGEN 00378-7101-77 1.34973 FEN<strong>OF</strong>IBRATE 160 MG TABLET G MYLAN EAGEN 63304-0901-90 1.34973 FEN<strong>OF</strong>IBRATE 160 MG TABLET G RANBAXY PHARMAC EAGEN 68<strong>08</strong>4-0328-11 1.34973 FEN<strong>OF</strong>IBRATE 160 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0328-21 1.34973 FEN<strong>OF</strong>IBRATE 160 MG TABLET G AHP EAGEN 00115-0533-01 2.17020 FEN<strong>OF</strong>IBRATE 200 MG CAPSULE G GLOBAL PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00115-0533-02 2.17020 FEN<strong>OF</strong>IBRATE 200 MG CAPSULE G GLOBAL PHARM EAGEN 00378-8630-05 2.17020 FEN<strong>OF</strong>IBRATE 200 MG CAPSULE G MYLAN EAGEN 00378-8630-77 2.17020 FEN<strong>OF</strong>IBRATE 200 MG CAPSULE G MYLAN EAGEN 68<strong>08</strong>4-0329-21 2.17020 FEN<strong>OF</strong>IBRATE 200 MG CAPSULE G AHP EAGEN 00378-6<strong>08</strong>8-93 1.76250 FEN<strong>OF</strong>IBRATE 43 MG CAPSULE G MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 142LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-3120-03 1.76450 FEN<strong>OF</strong>IBRATE 43 MG CAPSULE G APOTEX CORP EAGEN 68180-0130-<strong>06</strong> 1.76450 FEN<strong>OF</strong>IBRATE 43 MG CAPSULE G LUPIN PHARMACEU EAGEN 00093-2<strong>06</strong>1-98 1.43217 FEN<strong>OF</strong>IBRATE 48 MG TABLET G TEVA USA EAGEN 51079-0599-20 1.43227 FEN<strong>OF</strong>IBRATE 48 MG TABLET G MYLAN INSTITUTI EAGEN 68<strong>08</strong>4-<strong>06</strong>35-21 1.43225 FEN<strong>OF</strong>IBRATE 48 MG TABLET G AHP EAGEN 68180-0360-09 1.43217 FEN<strong>OF</strong>IBRATE 48 MG TABLET G LUPIN PHARMACEU EAGEN 68682-0525-01 1.35000 FEN<strong>OF</strong>IBRATE 48 MG TABLET G PERRIGO CO. EAGEN 00115-5511-10 0.594<strong>08</strong> FEN<strong>OF</strong>IBRATE 54 MG TABLET G GLOBAL PHARM EAGEN 00378-7100-77 0.594<strong>08</strong> FEN<strong>OF</strong>IBRATE 54 MG TABLET G MYLAN EAGEN 63304-0900-90 0.594<strong>08</strong> FEN<strong>OF</strong>IBRATE 54 MG TABLET G RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00115-0511-01 0.72470 FEN<strong>OF</strong>IBRATE 67 MG CAPSULE G GLOBAL PHARM EAGEN 00378-8628-77 0.72470 FEN<strong>OF</strong>IBRATE 67 MG CAPSULE G MYLAN EAGEN 00115-1460-10 4.00133 FEN<strong>OF</strong>IBRIC ACID DR 135 MG CAP G GLOBAL PHARM EAGEN 00378-2590-05 4.49643 FEN<strong>OF</strong>IBRIC ACID DR 135 MG CAP G MYLAN EAGEN 00378-2590-77 4.49641 FEN<strong>OF</strong>IBRIC ACID DR 135 MG CAP G MYLAN EAGEN 10370-0210-09 4.50117 FEN<strong>OF</strong>IBRIC ACID DR 135 MG CAP G PAR PHARM. EAGEN 51079-0196-03 4.49649 FEN<strong>OF</strong>IBRIC ACID DR 135 MG CAP G MYLAN INSTITUTI EAGEN 68180-0129-09 4.50133 FEN<strong>OF</strong>IBRIC ACID DR 135 MG CAP G LUPIN PHARMACEU EAGEN 00115-1459-10 1.42441 FEN<strong>OF</strong>IBRIC ACID DR 45 MG CAP G GLOBAL PHARM EAGEN 00378-2589-77 1.49874 FEN<strong>OF</strong>IBRIC ACID DR 45 MG CAP G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10370-0209-09 1.50033 FEN<strong>OF</strong>IBRIC ACID DR 45 MG CAP G PAR PHARM. EAGEN 51079-0195-03 1.49874 FEN<strong>OF</strong>IBRIC ACID DR 45 MG CAP G MYLAN INSTITUTI EAGEN 68180-0128-09 1.50042 FEN<strong>OF</strong>IBRIC ACID DR 45 MG CAP G LUPIN PHARMACEU EAGEN 53489-<strong>06</strong>78-07 2.25249 FEN<strong>OF</strong>IBRIC ACID 105 MG TABLET G MUTUAL PHARM CO EAGEN 53489-<strong>06</strong>78-90 2.25241 FEN<strong>OF</strong>IBRIC ACID 105 MG TABLET G MUTUAL PHARM CO EAGEN 53489-<strong>06</strong>77-07 0.75075 FEN<strong>OF</strong>IBRIC ACID 35 MG TABLET G MUTUAL PHARM CO EABND 00378-0471-01 1.82110 FENOPR<strong>OF</strong>EN 600 MG TABLET G MYLAN EABND 52609-00<strong>06</strong>-01 37.41474 FERRIPROX 500 MG TABLET 0 APOPHARMA USA I EABND 00024-2791-50 6.33456 FERRLECIT 62.5 MG/5 ML AMPUL 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00024-2792-10 5.15220 6.33456 FERRLECIT 62.5 MG/5 ML VIAL 0 SAN<strong>OF</strong>I-AVENTIS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00456-2212-30 6.72300 FETZIMA ER 120 MG CAPSULE G FOREST PHARMACE EABEX 00456-2220-30 6.72300 FETZIMA ER 20 MG CAPSULE G FOREST PHARMACE EABEX 00456-2240-30 6.72300 FETZIMA ER 40 MG CAPSULE G FOREST PHARMACE EABEX 00456-2280-30 6.72300 FETZIMA ER 80 MG CAPSULE G FOREST PHARMACE EABEX 00456-2202-28 6.72300 FETZIMA 20-40 MG TITRATION PAK G FOREST PHARMACE EAGEN 59630-0950-10 3.07660 FEXMID 7.5 MG TABLET G SHIONOGI PHARMA EABND 13310-0102-07 2.40270 3.41849 FIBRICOR 105 MG TABLET G AR SCIENTIFIC EABND 13310-0102-90 2.40270 3.41849 FIBRICOR 105 MG TABLET G AR SCIENTIFIC EABND 13310-0101-07 0.80<strong>08</strong>0 1.13931 FIBRICOR 35 MG TABLET G AR SCIENTIFIC EABND 10922-<strong>08</strong>25-02 3.85418 FINACEA 15% GEL 0 INTENDIS INC. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50419-<strong>08</strong>25-02 4.35517 FINACEA 15% GEL 0 BAYER,PHARM DIV GMGEN 38779-2410-04 317.<strong>06</strong>250 FINASTERIDE POWDER 0 MEDISCA INC. GMGEN 00093-7355-05 0.18000 FINASTERIDE 5 MG TABLET 0 TEVA USA EAGEN 00093-7355-56 0.18000 FINASTERIDE 5 MG TABLET 0 TEVA USA EAGEN 00093-7355-98 0.18000 FINASTERIDE 5 MG TABLET 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 143LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3151-77 0.18000 FINASTERIDE 5 MG TABLET 0 MYLAN EAGEN 00378-3151-93 0.18000 FINASTERIDE 5 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-3633-02 0.18000 FINASTERIDE 5 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3633-16 0.18000 FINASTERIDE 5 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3633-21 0.18000 FINASTERIDE 5 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3633-28 0.18000 FINASTERIDE 5 MG TABLET 0 QUALITEST EAGEN 16714-0522-01 0.18000 FINASTERIDE 5 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0522-03 0.18000 FINASTERIDE 5 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0522-04 0.18000 FINASTERIDE 5 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0522-05 0.18000 FINASTERIDE 5 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0522-10 0.18000 FINASTERIDE 5 MG TABLET 0 NORTHSTAR RX LL EAGEN 16729-0090-01 0.18000 FINASTERIDE 5 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0090-10 0.18000 FINASTERIDE 5 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0090-15 0.18000 FINASTERIDE 5 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0090-16 0.18000 FINASTERIDE 5 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 31722-0525-01 0.18000 FINASTERIDE 5 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0525-10 0.18000 FINASTERIDE 5 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0525-30 0.18000 FINASTERIDE 5 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0525-90 0.18000 FINASTERIDE 5 MG TABLET 0 CAMBER PHARMACE EAGEN 43598-0303-30 0.18000 FINASTERIDE 5 MG TABLET 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43598-0303-90 0.18000 FINASTERIDE 5 MG TABLET 0 DR.REDDY'S LAB EAGEN 45963-0500-02 0.18000 FINASTERIDE 5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 45963-0500-<strong>08</strong> 0.18000 FINASTERIDE 5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 45963-0500-30 0.18000 FINASTERIDE 5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 47335-0715-13 0.18000 FINASTERIDE 5 MG TABLET 0 SUN PHARMA GLOB EAGEN 47335-0715-81 0.18000 FINASTERIDE 5 MG TABLET 0 SUN PHARMA GLOB EAGEN 47335-0715-83 0.18000 FINASTERIDE 5 MG TABLET 0 SUN PHARMA GLOB EAGEN 50742-0123-30 0.18000 FINASTERIDE 5 MG TABLET 0 INGENUS PHARMAC EAGEN 51079-0520-01 0.18000 FINASTERIDE 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0520-20 0.18000 FINASTERIDE 5 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0520-30 0.18000 FINASTERIDE 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0520-56 0.18000 FINASTERIDE 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 55111-0172-30 0.18000 FINASTERIDE 5 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0172-90 0.18000 FINASTERIDE 5 MG TABLET 0 DR.REDDY'S LAB EAGEN 58517-0200-30 0.18000 FINASTERIDE 5 MG TABLET 0 <strong>NEW</strong> HORIZON RX EAGEN 59762-<strong>08</strong>50-02 0.18000 FINASTERIDE 5 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-<strong>08</strong>50-03 0.18000 FINASTERIDE 5 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-<strong>08</strong>50-07 0.18000 FINASTERIDE 5 MG TABLET 0 GREENSTONE LLC. EAGEN 65862-0149-01 0.18000 FINASTERIDE 5 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0149-05 0.18000 FINASTERIDE 5 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0149-30 0.18000 FINASTERIDE 5 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0149-90 0.18000 FINASTERIDE 5 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0399-01 0.18000 FINASTERIDE 5 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0399-11 0.18000 FINASTERIDE 5 MG TABLET 0 AHP EAGEN 68645-0446-70 0.18000 FINASTERIDE 5 MG TABLET 0 LEGACY PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 144LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 76282-0412-05 0.18000 FINASTERIDE 5 MG TABLET 0 EXELAN PHARMACE EAGEN 76282-0412-30 0.18000 FINASTERIDE 5 MG TABLET 0 EXELAN PHARMACE EAGEN 76282-0412-90 0.18000 FINASTERIDE 5 MG TABLET 0 EXELAN PHARMACE EABND 52544-0<strong>08</strong>0-01 2.26092 FIORICET 50-300-40 MG CAPSULE 0 ACTAVIS PHARMA, EABND 54092-0702-02 2657.66000 FIRAZYR 30 MG/3 ML SYRINGE 0 SHIRE US INC. MLBND 54092-0702-03 2657.66000 FIRAZYR 30 MG/3 ML SYRINGE 0 SHIRE US INC. MLBND 00025-1961-30 14.73222 FLAGYL ER 750 MG TABLET G PHARMACIA/UPJHN EABUL 00025-1831-31 0.<strong>08</strong>490 4.36613 FLAGYL 250 MG TABLET G PHARMACIA/UPJHN EABUL 00025-1831-50 0.<strong>08</strong>490 4.36646 FLAGYL 250 MG TABLET G PHARMACIA/UPJHN EABND 00025-1942-50 3.59960 6.07759 FLAGYL 375 CAPSULE G PHARMACIA/UPJHN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00025-1821-31 0.21840 7.79967 FLAGYL 500 MG TABLET G PHARMACIA/UPJHN EABUL 00025-1821-50 0.21840 7.80034 FLAGYL 500 MG TABLET G PHARMACIA/UPJHN EABND 00<strong>06</strong>5-0096-05 10.14924 FLAREX 0.1% EYE DROPS 0 ALCON LABS. MLGEN 00115-1811-01 0.95400 FLAVOXATE HCL 100 MG TABLET 0 GLOBAL PHARM EAGEN 00574-0115-01 0.95400 FLAVOXATE HCL 100 MG TABLET 0 PADDOCK LABS. EAGEN 428<strong>06</strong>-0058-01 0.86550 FLAVOXATE HCL 100 MG TABLET 0 EPIC PHARMA LLC EABND 61953-0005-01 8.35444 FLEBOGAMMA DIF 10% VIAL 0 GRIFOLS MLBND 61953-0005-02 8.35444 FLEBOGAMMA DIF 10% VIAL 0 GRIFOLS MLBND 61953-0005-03 8.35444 FLEBOGAMMA DIF 10% VIAL 0 GRIFOLS MLBND 61953-0005-04 8.35444 FLEBOGAMMA DIF 10% VIAL 0 GRIFOLS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 61953-0005-05 8.35444 FLEBOGAMMA DIF 10% VIAL 0 GRIFOLS MLBND 61953-0005-<strong>06</strong> 8.35444 FLEBOGAMMA DIF 10% VIAL 0 GRIFOLS MLBND 61953-0004-00 4.17722 FLEBOGAMMA DIF 5% VIAL 0 GRIFOLS MLBND 61953-0004-01 4.17739 FLEBOGAMMA DIF 5% VIAL 0 GRIFOLS MLBND 61953-0004-02 4.17722 FLEBOGAMMA DIF 5% VIAL 0 GRIFOLS MLBND 61953-0004-03 4.17722 FLEBOGAMMA DIF 5% VIAL 0 GRIFOLS MLBND 61953-0004-04 4.17722 FLEBOGAMMA DIF 5% VIAL 0 GRIFOLS MLBND 61953-0004-05 4.17722 FLEBOGAMMA DIF 5% VIAL 0 GRIFOLS MLBND 61953-0004-<strong>06</strong> 4.17739 FLEBOGAMMA DIF 5% VIAL 0 GRIFOLS MLBND 61953-0004-07 4.17722 FLEBOGAMMA DIF 5% VIAL 0 GRIFOLS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 61953-0004-<strong>08</strong> 4.17722 FLEBOGAMMA DIF 5% VIAL 0 GRIFOLS MLBND 61953-0004-09 4.17722 FLEBOGAMMA DIF 5% VIAL 0 GRIFOLS MLGEN 00054-0011-21 0.29170 FLECAINIDE ACETATE 100 MG TAB 0 ROXANE LABS. EAGEN 00054-0011-25 0.29170 FLECAINIDE ACETATE 100 MG TAB 0 ROXANE LABS. EAGEN 00555-<strong>08</strong>60-02 0.29170 FLECAINIDE ACETATE 100 MG TAB 0 BARR EAGEN 65162-<strong>06</strong>42-10 0.29170 FLECAINIDE ACETATE 100 MG TAB 0 AMNEAL PHARMACE EAGEN 00054-0012-21 0.54972 FLECAINIDE ACETATE 150 MG TAB 0 ROXANE LABS. EAGEN 00054-0012-25 0.54972 FLECAINIDE ACETATE 150 MG TAB 0 ROXANE LABS. EAGEN 00555-<strong>08</strong>61-02 0.54972 FLECAINIDE ACETATE 150 MG TAB 0 BARR EAGEN 65162-<strong>06</strong>43-10 0.54972 FLECAINIDE ACETATE 150 MG TAB 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0010-21 0.19750 FLECAINIDE ACETATE 50 MG TAB 0 ROXANE LABS. EAGEN 00054-0010-25 0.19750 FLECAINIDE ACETATE 50 MG TAB 0 ROXANE LABS. EAGEN 00555-<strong>08</strong>59-02 0.19750 FLECAINIDE ACETATE 50 MG TAB 0 BARR EAGEN 65162-<strong>06</strong>41-10 0.19750 FLECAINIDE ACETATE 50 MG TAB 0 AMNEAL PHARMACE EABND 60793-0411-30 7.16483 FLECTOR 1.3% PATCH G PFIZER US PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 145LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 51672-1338-03 3.45197 FLO-PRED 16.7(15) MG/5 ML SUSP G TARO PHARM USA MLBND 00173-0517-00 18.61690 FLOLAN 0.5 MG VIAL 0 GLAXOSMITHKLINE EABND 00173-0519-00 44.96110 FLOLAN 1.5 MG VIAL 0 GLAXOSMITHKLINE EABND 00597-0058-01 0.17210 5.69048 FLOMAX 0.4 MG CAPSULE G BOEHRINGER ING. EABND 00173-0453-01 1.70280 5.56618 FLONASE 0.05% NASAL SPRAY G GLAXOSMITHKLINE GMBND 00173-0719-20 15.09493 FLOVENT HFA 110 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-0720-20 23.44611 FLOVENT HFA 220 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-0718-20 12.76398 FLOVENT HFA 44 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-<strong>06</strong>02-00 2.98236 FLOVENT 100 MCG DISKUS G GLAXOSMITHKLINE EABND 00173-<strong>06</strong>02-02 2.25496 FLOVENT 100 MCG DISKUS G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-<strong>06</strong>01-00 3.99318 FLOVENT 250 MCG DISKUS G GLAXOSMITHKLINE EABND 00173-<strong>06</strong>01-02 3.01898 FLOVENT 250 MCG DISKUS G GLAXOSMITHKLINE EABND 00173-<strong>06</strong>00-02 2.13835 FLOVENT 50 MCG DISKUS G GLAXOSMITHKLINE EAGEN 63323-0145-07 107.10000 FLOXURIDINE 500 MG VIAL 0 APP PHARMACEUTI EAGEN 38779-2442-09 52.72500 FLUCONAZOLE POWDER 0 MEDISCA INC. GMGEN 00054-0002-85 0.30200 FLUCONAZOLE 10 MG/ML SUSP 0 ROXANE LABS. MLGEN 00093-5414-95 0.30200 FLUCONAZOLE 10 MG/ML SUSP 0 TEVA USA MLGEN 16714-<strong>06</strong>95-01 0.30200 FLUCONAZOLE 10 MG/ML SUSP 0 NORTHSTAR RX LL MLGEN 59762-5029-01 0.30200 FLUCONAZOLE 10 MG/ML SUSP 0 GREENSTONE LLC. MLGUL 00172-5411-46 0.88250 FLUCONAZOLE 100 MG TABLET 0 IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00172-5411-60 0.88250 FLUCONAZOLE 100 MG TABLET 0 IVAX PHARMACEUT EAGUL 00378-2516-93 0.88250 FLUCONAZOLE 100 MG TABLET 0 MYLAN EAGUL 57237-0004-30 0.88250 FLUCONAZOLE 100 MG TABLET 0 CITRON PHARMA L EAGUL 59762-5016-01 0.88250 FLUCONAZOLE 100 MG TABLET 0 GREENSTONE LLC. EAGUL 65862-0059-30 0.88250 FLUCONAZOLE 100 MG TABLET 0 AUROBINDO PHARM EAGUL 68462-0102-30 0.88250 FLUCONAZOLE 100 MG TABLET 0 GLENMARK PHARMA EAGEN 00172-5412-11 2.74400 FLUCONAZOLE 150 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-5412-79 2.74400 FLUCONAZOLE 150 MG TABLET 0 IVAX PHARMACEUT EAGEN 16714-<strong>06</strong>92-11 2.74400 FLUCONAZOLE 150 MG TABLET 0 NORTHSTAR RX LL EAGEN 57237-0005-11 2.74400 FLUCONAZOLE 150 MG TABLET 0 CITRON PHARMA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-5017-01 2.74400 FLUCONAZOLE 150 MG TABLET 0 GREENSTONE LLC. EAGEN 65862-0<strong>06</strong>0-11 2.74400 FLUCONAZOLE 150 MG TABLET 0 AUROBINDO PHARM EAGEN 68462-0103-40 2.74400 FLUCONAZOLE 150 MG TABLET 0 GLENMARK PHARMA EAGUL 00172-5413-46 1.40750 FLUCONAZOLE 200 MG TABLET 0 IVAX PHARMACEUT EAGUL 00172-5413-60 1.40750 FLUCONAZOLE 200 MG TABLET 0 IVAX PHARMACEUT EAGUL 00378-2520-93 1.40750 FLUCONAZOLE 200 MG TABLET 0 MYLAN EAGUL 57237-00<strong>06</strong>-30 1.40750 FLUCONAZOLE 200 MG TABLET 0 CITRON PHARMA L EAGUL 59762-5018-01 1.40750 FLUCONAZOLE 200 MG TABLET 0 GREENSTONE LLC. EAGUL 65862-0<strong>06</strong>1-30 1.40750 FLUCONAZOLE 200 MG TABLET 0 AUROBINDO PHARM EAGUL 68462-0104-30 1.40750 FLUCONAZOLE 200 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0003-85 0.70470 FLUCONAZOLE 40 MG/ML SUSP 0 ROXANE LABS. MLGEN 00093-5415-95 0.70470 FLUCONAZOLE 40 MG/ML SUSP 0 TEVA USA MLGEN 59762-5030-01 0.70470 FLUCONAZOLE 40 MG/ML SUSP 0 GREENSTONE LLC. MLGUL 00172-5410-46 0.50000 FLUCONAZOLE 50 MG TABLET 0 IVAX PHARMACEUT EAGUL 00172-5410-60 0.50000 FLUCONAZOLE 50 MG TABLET 0 IVAX PHARMACEUT EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 146LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-2514-93 0.50000 FLUCONAZOLE 50 MG TABLET 0 MYLAN EAGUL 57237-0003-30 0.50000 FLUCONAZOLE 50 MG TABLET 0 CITRON PHARMA L EAGUL 59762-5015-01 0.50000 FLUCONAZOLE 50 MG TABLET 0 GREENSTONE LLC. EAGUL 65862-0058-30 0.50000 FLUCONAZOLE 50 MG TABLET 0 AUROBINDO PHARM EAGUL 68462-0101-30 0.50000 FLUCONAZOLE 50 MG TABLET 0 GLENMARK PHARMA EAGEN 00143-9667-01 0.10170 FLUCONAZOLE-DEXT 200 MG/100 ML 0 WEST-WARD,INC. MLGEN 00143-9667-<strong>06</strong> 0.10170 FLUCONAZOLE-DEXT 200 MG/100 ML 0 WEST-WARD,INC. MLGEN 00409-4684-23 0.1<strong>08</strong>81 FLUCONAZOLE-DEXT 200 MG/100 ML 0 HOSPIRA MLGEN 00<strong>06</strong>9-0038-02 0.05481 FLUCONAZOLE-NACL 200 MG/100 ML 0 PFIZER US PHARM MLGEN 00<strong>06</strong>9-0038-03 0.05481 FLUCONAZOLE-NACL 200 MG/100 ML 0 PFIZER US PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-9669-01 0.05481 FLUCONAZOLE-NACL 200 MG/100 ML 0 WEST-WARD,INC. MLGEN 00143-9669-<strong>06</strong> 0.05481 FLUCONAZOLE-NACL 200 MG/100 ML 0 WEST-WARD,INC. MLGEN 00<strong>06</strong>9-0045-02 0.04290 FLUCONAZOLE-NACL 400 MG/200 ML 0 PFIZER US PHARM MLGEN 00<strong>06</strong>9-0045-03 0.04290 FLUCONAZOLE-NACL 400 MG/200 ML 0 PFIZER US PHARM MLGEN 00143-9668-01 0.04290 FLUCONAZOLE-NACL 400 MG/200 ML 0 WEST-WARD,INC. MLGEN 00143-9668-<strong>06</strong> 0.04290 FLUCONAZOLE-NACL 400 MG/200 ML 0 WEST-WARD,INC. MLGEN 00143-9899-91 0.05481 FLUCONAZOLE-NS 200 MG/100 ML 0 WEST-WARD,INC. MLGEN 00338-6046-48 0.05481 FLUCONAZOLE-NS 200 MG/100 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-4688-23 0.05481 FLUCONAZOLE-NS 200 MG/100 ML 0 HOSPIRA MLGEN 00703-1029-30 0.05481 FLUCONAZOLE-NS 200 MG/100 ML 0 TEVA PARENTERAL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 25021-0113-82 0.05481 FLUCONAZOLE-NS 200 MG/100 ML 0 SAGENT PHARMACE MLGEN 55390-0227-01 0.05481 FLUCONAZOLE-NS 200 MG/100 ML 0 BEDFORD LABS MLGEN 00338-6045-37 0.04290 FLUCONAZOLE-NS 400 MG/200 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-4688-02 0.03357 FLUCONAZOLE-NS 400 MG/200 ML 0 HOSPIRA MLGEN 00409-4688-34 0.02893 FLUCONAZOLE-NS 400 MG/200 ML 0 HOSPIRA/NOVA+ MLGEN 00703-1020-30 0.04290 FLUCONAZOLE-NS 400 MG/200 ML 0 TEVA PARENTERAL MLGEN 25021-0113-87 0.04290 FLUCONAZOLE-NS 400 MG/200 ML 0 SAGENT PHARMACE MLGEN 55390-0228-01 0.04290 FLUCONAZOLE-NS 400 MG/200 ML 0 BEDFORD LABS MLGEN 64980-0179-01 17.37000 FLUCYTOSINE 250 MG CAPSULE 0 RISING PHARM EAGEN 68682-0355-10 17.37000 FLUCYTOSINE 250 MG CAPSULE 0 OCEANSIDE PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64980-0180-01 29.88000 FLUCYTOSINE 500 MG CAPSULE 0 RISING PHARM EAGEN 68682-0356-10 29.88000 FLUCYTOSINE 500 MG CAPSULE 0 OCEANSIDE PHARM EAGEN 00703-5854-01 85.05000 FLUDARABINE 50 MG VIAL 0 TEVA PARENTERAL EAGEN 63323-0196-<strong>06</strong> 258.<strong>06</strong>750 FLUDARABINE 50 MG VIAL 0 APP PHARMACEUTI EAGEN 66758-0046-01 131.25000 FLUDARABINE 50 MG/2 ML VIAL 0 SANDOZ INC. MLGEN 67457-0238-02 93.75000 FLUDARABINE 50 MG/2 ML VIAL 0 MYLAN INSTITUTI MLGEN 00115-7033-01 0.5<strong>06</strong>50 FLUDROCORTISONE 0.1 MG TABLET 0 GLOBAL PHARM EAGEN 00115-7033-02 0.5<strong>06</strong>50 FLUDROCORTISONE 0.1 MG TABLET 0 GLOBAL PHARM EAGEN 00555-0997-02 0.5<strong>06</strong>50 FLUDROCORTISONE 0.1 MG TABLET 0 BARR EAGEN 68<strong>08</strong>4-0288-01 0.5<strong>06</strong>50 FLUDROCORTISONE 0.1 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0288-11 0.5<strong>06</strong>50 FLUDROCORTISONE 0.1 MG TABLET 0 AHP EAGEN 242<strong>08</strong>-0344-25 2.<strong>06</strong>250 FLUNISOLIDE 0.025% SPRAY G VALEANT MLGEN 64980-0510-25 2.<strong>06</strong>250 FLUNISOLIDE 0.025% SPRAY G RISING PHARM MLBND 60505-<strong>08</strong>24-00 1.26640 1.82467 FLUNISOLIDE 29 MCG-0.025% SPR G APOTEX CORP MLGEN 13925-05<strong>08</strong>-20 8.2<strong>08</strong>75 FLUOCINOLONE OIL 0.01% EAR DRP 0 SETON PHARMACEU ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 147LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-0702-94 8.19375 FLUOCINOLONE OIL 0.01% EAR DRP 0 AMNEAL PHARMACE MLGEN 13925-05<strong>06</strong>-04 1.39974 FLUOCINOLONE 0.01% BODY OIL G SETON PHARMACEU MLGEN 65162-0704-86 1.39689 FLUOCINOLONE 0.01% BODY OIL G AMNEAL PHARMACE MLGEN 00168-0058-15 2.22849 FLUOCINOLONE 0.01% CREAM G SANDOZ GMGEN 00168-0058-60 1.67<strong>08</strong>7 FLUOCINOLONE 0.01% CREAM G SANDOZ GMGEN 13925-0507-04 1.41433 FLUOCINOLONE 0.01% SCALP OIL G SETON PHARMACEU MLGEN 65162-0703-86 1.41262 FLUOCINOLONE 0.01% SCALP OIL G AMNEAL PHARMACE MLGEN 00168-0059-60 2.25000 FLUOCINOLONE 0.01% SOLUTION G SANDOZ MLGEN 00168-0<strong>06</strong>0-15 1.68849 FLUOCINOLONE 0.025% CREAM G SANDOZ GMGEN 00168-0<strong>06</strong>0-60 1.26587 FLUOCINOLONE 0.025% CREAM G SANDOZ GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00713-0324-15 1.38645 FLUOCINOLONE 0.025% CREAM G G & W LABS. GMGEN 00713-0324-60 1.26774 FLUOCINOLONE 0.025% CREAM G G & W LABS. GMGEN 52565-0020-60 1.27125 FLUOCINOLONE 0.025% CREAM G IGI LABS, INC. GMGEN 00168-0<strong>06</strong>4-15 1.68849 FLUOCINOLONE 0.025% OINTMENT G SANDOZ GMGEN 00168-0<strong>06</strong>4-60 1.26587 FLUOCINOLONE 0.025% OINTMENT G SANDOZ GMGEN 52565-0013-15 1.38705 FLUOCINOLONE 0.025% OINTMENT G IGI LABS, INC. GMGEN 52565-0013-60 1.27125 FLUOCINOLONE 0.025% OINTMENT G IGI LABS, INC. GMGUL 00093-0262-15 0.11870 FLUOCINONIDE 0.05% CREAM 0 TEVA USA GMGUL 00093-0262-30 0.11870 FLUOCINONIDE 0.05% CREAM 0 TEVA USA GMGUL 00093-0262-92 0.11870 FLUOCINONIDE 0.05% CREAM 0 TEVA USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-1253-01 0.11870 FLUOCINONIDE 0.05% CREAM 0 TARO PHARM USA GMGUL 51672-1253-02 0.11870 FLUOCINONIDE 0.05% CREAM 0 TARO PHARM USA GMGUL 51672-1253-03 0.11870 FLUOCINONIDE 0.05% CREAM 0 TARO PHARM USA GMGUL 51672-1253-04 0.11870 FLUOCINONIDE 0.05% CREAM 0 TARO PHARM USA GMGUL 00093-0265-92 0.49650 FLUOCINONIDE 0.05% GEL 0 TEVA USA GMGUL 00168-0135-15 0.49650 FLUOCINONIDE 0.05% GEL 0 SANDOZ GMGEN 00168-0135-60 0.48350 FLUOCINONIDE 0.05% GEL 0 SANDOZ GMGUL 51672-1279-01 0.49650 FLUOCINONIDE 0.05% GEL 0 TARO PHARM USA GMGUL 51672-1279-02 0.49650 FLUOCINONIDE 0.05% GEL 0 TARO PHARM USA GMGUL 51672-1279-03 0.49650 FLUOCINONIDE 0.05% GEL 0 TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-0264-15 0.58730 FLUOCINONIDE 0.05% OINTMENT 0 TEVA USA GMGEN 00093-0264-30 0.58730 FLUOCINONIDE 0.05% OINTMENT 0 TEVA USA GMGEN 00093-0264-92 0.58730 FLUOCINONIDE 0.05% OINTMENT 0 TEVA USA GMGEN 00168-0140-15 0.58730 FLUOCINONIDE 0.05% OINTMENT 0 SANDOZ GMGEN 00168-0140-30 0.58730 FLUOCINONIDE 0.05% OINTMENT 0 SANDOZ GMGEN 00168-0140-60 0.58730 FLUOCINONIDE 0.05% OINTMENT 0 SANDOZ GMGEN 51672-1264-01 0.58730 FLUOCINONIDE 0.05% OINTMENT 0 TARO PHARM USA GMGEN 51672-1264-02 0.58730 FLUOCINONIDE 0.05% OINTMENT 0 TARO PHARM USA GMGEN 51672-1264-03 0.58730 FLUOCINONIDE 0.05% OINTMENT 0 TARO PHARM USA GMGUL 00168-0134-60 0.26400 FLUOCINONIDE 0.05% SOLUTION 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00472-<strong>08</strong>29-02 0.26400 FLUOCINONIDE 0.05% SOLUTION 0 ACTAVIS PHARMA, MLGUL 51672-1273-02 0.26400 FLUOCINONIDE 0.05% SOLUTION 0 TARO PHARM USA MLGUL 51672-1273-04 0.26400 FLUOCINONIDE 0.05% SOLUTION 0 TARO PHARM USA MLGEN 45802-0151-53 10.323<strong>06</strong> FLUOCINONIDE 0.1% CREAM G PERRIGO CO. GMGEN 45802-0151-94 12.61275 FLUOCINONIDE 0.1% CREAM G PERRIGO CO. GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 148LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0151-96 10.86624 FLUOCINONIDE 0.1% CREAM G PERRIGO CO. GMGEN 68682-0992-10 9.90900 FLUOCINONIDE 0.1% CREAM G OCEANSIDE PHARM GMGEN 68682-0992-30 12.1<strong>06</strong>74 FLUOCINONIDE 0.1% CREAM G OCEANSIDE PHARM GMGEN 68682-0992-60 10.43037 FLUOCINONIDE 0.1% CREAM G OCEANSIDE PHARM GMGUL 00093-0263-15 0.24530 FLUOCINONIDE-E 0.05% CREAM 0 TEVA USA GMGUL 00093-0263-30 0.24530 FLUOCINONIDE-E 0.05% CREAM 0 TEVA USA GMGUL 00093-0263-92 0.24530 FLUOCINONIDE-E 0.05% CREAM 0 TEVA USA GMGUL 51672-1254-01 0.24530 FLUOCINONIDE-E 0.05% CREAM 0 TARO PHARM USA GMGUL 51672-1254-02 0.24530 FLUOCINONIDE-E 0.05% CREAM 0 TARO PHARM USA GMGUL 51672-1254-03 0.24530 FLUOCINONIDE-E 0.05% CREAM 0 TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00168-0246-15 0.24530 FLUOCINONIDE-EMOL 0.05% CREAM 0 SANDOZ GMGUL 00168-0246-30 0.24530 FLUOCINONIDE-EMOL 0.05% CREAM 0 SANDOZ GMGUL 00168-0246-60 0.24530 FLUOCINONIDE-EMOL 0.05% CREAM 0 SANDOZ GMBND 24338-<strong>06</strong>56-61 1.53218 FLUOR-A-DAY 2.5 MG/ML DROPS 0 ARBOR PHARMACEU MLGEN 51862-0171-10 0.02450 FLUORIDE 0.5 MG TABLET CHEW 0 LIBERTAS PHARMA EAGEN 51862-0171-12 0.02450 FLUORIDE 0.5 MG TABLET CHEW 0 LIBERTAS PHARMA EAGEN 60758-<strong>08</strong>80-05 7.92000 FLUOROMETHOLONE 0.1% DROPS 0 PACIFIC PHARMA MLGEN 60758-<strong>08</strong>80-10 7.92000 FLUOROMETHOLONE 0.1% DROPS 0 PACIFIC PHARMA MLGEN 60758-<strong>08</strong>80-15 7.92000 FLUOROMETHOLONE 0.1% DROPS 0 PACIFIC PHARMA MLBND 00023-<strong>08</strong>12-30 11.62000 FLUOROPLEX 1% CREAM 0 AQUA PHARMACEUT GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10139-0<strong>06</strong>3-12 0.22000 FLUOROURACIL 1,000 MG/20 ML VL 0 MYLAN INSTITUTI MLGEN 10139-0<strong>06</strong>3-20 0.22000 FLUOROURACIL 1,000 MG/20 ML VL 0 MYLAN INSTITUTI MLGEN 63323-0117-20 0.22000 FLUOROURACIL 1,000 MG/20 ML VL 0 APP PHARMACEUTI MLGEN 10139-0<strong>06</strong>3-50 0.22950 FLUOROURACIL 2,500 MG/50 ML VL 0 MYLAN INSTITUTI MLGEN 63323-0117-51 0.23130 FLUOROURACIL 2,500 MG/50 ML VL 0 APP PHARMACEUTI MLGEN 43547-0259-01 5.18616 FLUOROURACIL 2% TOPICAL SOLN 0 SOLCO <strong>HEALTH</strong>CAR MLGEN 51672-4<strong>06</strong>2-01 5.18616 FLUOROURACIL 2% TOPICAL SOLN 0 TARO PHARM USA MLGEN 63323-0117-61 0.23130 FLUOROURACIL 5,000 MG/100 ML 0 APP PHARMACEUTI MLGEN 00378-4791-<strong>06</strong> 4.63293 FLUOROURACIL 5% CREAM 0 MYLAN GMGEN 51672-4118-<strong>06</strong> 5.35250 FLUOROURACIL 5% CREAM 0 TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 66530-0249-40 4.826<strong>06</strong> FLUOROURACIL 5% CREAM 0 SPEAR DERM PROD GMGEN 68682-0004-31 4.63293 FLUOROURACIL 5% CREAM 0 MYLAN GMGEN 43547-0258-01 7.63714 FLUOROURACIL 5% TOP SOLUTION 0 SOLCO <strong>HEALTH</strong>CAR MLGEN 51672-4<strong>06</strong>3-01 7.63714 FLUOROURACIL 5% TOP SOLUTION 0 TARO PHARM USA MLGEN 10139-0<strong>06</strong>3-10 0.24750 FLUOROURACIL 500 MG/10 ML VIAL 0 MYLAN INSTITUTI MLGEN 63323-0117-10 0.26400 FLUOROURACIL 500 MG/10 ML VIAL 0 APP PHARMACEUTI MLGEX 55111-0284-48 24.92000 FLUOXETINE DR 90 MG CAPSULE G DR.REDDY'S LAB EAGEX 00378-5410-28 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 MYLAN EAGEX 00781-2823-01 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 SANDOZ EAGEX 00781-2827-<strong>08</strong> 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16714-0351-01 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 16714-0351-02 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 16714-0351-03 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 50111-<strong>06</strong>47-01 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 PLIVA, INC EAGEX 50111-<strong>06</strong>47-02 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 PLIVA, INC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 149LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 50111-<strong>06</strong>47-03 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 PLIVA, INC EAGEX 55111-0147-01 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 DR.REDDY'S LAB EAGEX 65862-0192-01 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 AUROBINDO PHARM EAGEX 65862-0192-05 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 AUROBINDO PHARM EAGEX 65862-0192-99 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 AUROBINDO PHARM EAGEX 68645-0131-54 0.03250 FLUOXETINE HCL 10 MG CAPSULE 0 LEGACY PHARMACE EAGEX 00093-7188-56 0.05170 FLUOXETINE HCL 10 MG TABLET 0 TEVA USA EAGEX 00378-0734-01 0.05170 FLUOXETINE HCL 10 MG TABLET 0 MYLAN EAGEX 00378-0734-93 0.05170 FLUOXETINE HCL 10 MG TABLET 0 MYLAN EAGEX 49884-0734-01 0.05170 FLUOXETINE HCL 10 MG TABLET 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 49884-0734-10 0.05170 FLUOXETINE HCL 10 MG TABLET 0 PAR PHARM. EAGEX 49884-0734-11 0.05170 FLUOXETINE HCL 10 MG TABLET 0 PAR PHARM. EAGEX 00093-4356-19 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 TEVA USA EAGEX 00093-4356-93 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 TEVA USA EAGEX 00378-5420-28 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 MYLAN EAGEX 00781-2822-01 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 SANDOZ EAGEX 00781-2822-10 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 SANDOZ EAGEX 16714-0352-01 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 16714-0352-02 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 16714-0352-03 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 50111-<strong>06</strong>48-01 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 PLIVA, INC EAGEX 50111-<strong>06</strong>48-02 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 TEVA USA EAGEX 50111-<strong>06</strong>48-03 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 PLIVA, INC EAGEX 50111-<strong>06</strong>48-44 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 PLIVA, INC EAGEX 55111-0148-01 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 DR.REDDY'S LAB EAGEX 55111-0148-10 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 DR.REDDY'S LAB EAGEX 65862-0193-01 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 AUROBINDO PHARM EAGEX 65862-0193-05 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 AUROBINDO PHARM EAGEX 65862-0193-99 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-<strong>06</strong>05-01 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68645-0130-54 0.02500 FLUOXETINE HCL 20 MG CAPSULE 0 LEGACY PHARMACE EAGEX 00378-0735-01 0.72553 FLUOXETINE HCL 20 MG TABLET 0 MYLAN EAGEX 00378-0735-93 0.72553 FLUOXETINE HCL 20 MG TABLET 0 MYLAN EAGEX 49884-0735-01 0.72553 FLUOXETINE HCL 20 MG TABLET 0 PAR PHARM. EAGEX 49884-0735-10 0.72553 FLUOXETINE HCL 20 MG TABLET 0 PAR PHARM. EAGEX 49884-0735-11 0.72553 FLUOXETINE HCL 20 MG TABLET 0 PAR PHARM. EAGEX 00093-4346-56 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 TEVA USA EAGEX 00093-7198-01 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 TEVA USA EAGEX 00093-7198-56 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 TEVA USA EAGEX 00781-2824-01 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-2824-10 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 SANDOZ EAGEX 00781-2824-31 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 SANDOZ EAGEX 16714-0353-01 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 16714-0353-02 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 16714-0353-03 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 150LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16714-0353-04 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 49884-<strong>08</strong>72-01 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 PAR PHARM. EAGEX 49884-<strong>08</strong>72-05 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 PAR PHARM. EAGEX 49884-<strong>08</strong>72-11 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 PAR PHARM. EAGEX 55111-0149-01 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 DR.REDDY'S LAB EAGEX 55111-0149-30 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 DR.REDDY'S LAB EAGEX 63304-<strong>06</strong>32-01 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 RANBAXY PHARMAC EAGEX 63304-<strong>06</strong>32-30 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 RANBAXY PHARMAC EAGEX 65862-0194-01 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 AUROBINDO PHARM EAGEX 65862-0194-05 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0194-30 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 AUROBINDO PHARM EAGEX 65862-0194-99 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 AUROBINDO PHARM EAGEX 68001-0129-00 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 BLUEPOINT LABOR EAGEX 68001-0129-03 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 BLUEPOINT LABOR EAGEX 68001-0129-04 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 BLUEPOINT LABOR EAGEX 68<strong>08</strong>4-0101-01 0.49033 FLUOXETINE HCL 40 MG CAPSULE 0 AHP EABEX 49909-0005-30 3.28680 FLUOXETINE HCL 60 MG TABLET G EDGEMONT PHARMA EAGEX 00093-61<strong>08</strong>-12 0.03830 FLUOXETINE 20 MG/5 ML SOLUTION 0 TEVA USA MLGEX 00121-0721-04 0.03830 FLUOXETINE 20 MG/5 ML SOLUTION 0 PHARMACEU ASSOC MLGEX 00121-4721-05 0.03830 FLUOXETINE 20 MG/5 ML SOLUTION 0 PHARMACEU ASSOC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 54838-0523-40 0.03830 FLUOXETINE 20 MG/5 ML SOLUTION 0 SILARX PHARM MLGEX 60505-0352-01 0.03830 FLUOXETINE 20 MG/5 ML SOLUTION 0 APOTEX CORP MLBEX 63323-0272-05 26.84386 FLUPHENAZINE DEC 25 MG/ML VL 0 APP PHARMACEUTI MLGEX 00378-6004-01 0.13398 FLUPHENAZINE 1 MG TABLET 0 MYLAN EAGEX 00378-6004-05 0.13398 FLUPHENAZINE 1 MG TABLET 0 MYLAN EAGEX 00527-1788-01 0.13398 FLUPHENAZINE 1 MG TABLET 0 LANNETT CO. INC EAGEX 00527-1788-05 0.13398 FLUPHENAZINE 1 MG TABLET 0 LANNETT CO. INC EAGEX 00781-1436-01 0.13398 FLUPHENAZINE 1 MG TABLET 0 SANDOZ EAGEX 51079-0485-20 0.13398 FLUPHENAZINE 1 MG TABLET 0 MYLAN INSTITUTI EAGEX 00378-6097-01 0.31316 FLUPHENAZINE 10 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-6097-05 0.31316 FLUPHENAZINE 10 MG TABLET 0 MYLAN EAGEX 00527-1791-01 0.31316 FLUPHENAZINE 10 MG TABLET 0 LANNETT CO. INC EAGEX 00527-1791-05 0.31316 FLUPHENAZINE 10 MG TABLET 0 LANNETT CO. INC EAGEX 00781-1439-01 0.31316 FLUPHENAZINE 10 MG TABLET 0 SANDOZ EAGEX 51079-0488-20 0.31316 FLUPHENAZINE 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 00378-6009-01 0.18900 FLUPHENAZINE 2.5 MG TABLET 0 MYLAN EAGEX 00378-6009-05 0.18900 FLUPHENAZINE 2.5 MG TABLET 0 MYLAN EAGEX 00527-1789-01 0.18900 FLUPHENAZINE 2.5 MG TABLET 0 LANNETT CO. INC EAGEX 00527-1789-05 0.18900 FLUPHENAZINE 2.5 MG TABLET 0 LANNETT CO. INC EAGEX 00781-1437-01 0.18900 FLUPHENAZINE 2.5 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0486-20 0.18900 FLUPHENAZINE 2.5 MG TABLET 0 MYLAN INSTITUTI EABEX 63323-0281-10 15.84221 FLUPHENAZINE 2.5 MG/ML VIAL 0 APP PHARMACEUTI MLBEX 00121-<strong>06</strong>54-02 0.4<strong>08</strong>36 FLUPHENAZINE 2.5 MG/5 ML ELIX 0 PHARMACEU ASSOC MLBEX 00121-<strong>06</strong>54-16 0.33691 FLUPHENAZINE 2.5 MG/5 ML ELIX 0 PHARMACEU ASSOC MLGEX 00378-6074-01 0.22181 FLUPHENAZINE 5 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 151LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-6074-05 0.22181 FLUPHENAZINE 5 MG TABLET 0 MYLAN EAGEX 00527-1790-01 0.22181 FLUPHENAZINE 5 MG TABLET 0 LANNETT CO. INC EAGEX 00527-1790-05 0.22181 FLUPHENAZINE 5 MG TABLET 0 LANNETT CO. INC EAGEX 00781-1438-01 0.22181 FLUPHENAZINE 5 MG TABLET 0 SANDOZ EAGEX 51079-0487-20 0.22181 FLUPHENAZINE 5 MG TABLET 0 MYLAN INSTITUTI EABEX 00121-<strong>06</strong>53-04 0.96203 FLUPHENAZINE 5 MG/ML CONC 0 PHARMACEU ASSOC MLBND 58223-<strong>06</strong>84-24 0.22617 FLURA-DROPS 0.25 MG/DROP 0 KIRKMAN SALES MLGEN 242<strong>08</strong>-0314-25 1.21045 FLURBIPR<strong>OF</strong>EN 0.03% EYE DROP 0 VALEANT MLGEN 60758-0910-03 1.21045 FLURBIPR<strong>OF</strong>EN 0.03% EYE DROP 0 PACIFIC PHARMA MLGUL 00093-0711-01 0.24380 FLURBIPR<strong>OF</strong>EN 100 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00093-0711-05 0.24380 FLURBIPR<strong>OF</strong>EN 100 MG TABLET 0 TEVA USA EAGUL 00378-0093-01 0.24380 FLURBIPR<strong>OF</strong>EN 100 MG TABLET 0 MYLAN EABND 00378-0076-01 0.11420 0.68674 FLURBIPR<strong>OF</strong>EN 50 MG TABLET 0 MYLAN EAGEN 00172-4960-58 0.72310 FLUTAMIDE 125 MG CAPSULE 0 IVAX PHARMACEUT EAGEN 00172-4960-70 0.72310 FLUTAMIDE 125 MG CAPSULE 0 IVAX PHARMACEUT EAGEN 00591-2466-18 0.72310 FLUTAMIDE 125 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 49884-0753-13 0.72310 FLUTAMIDE 125 MG CAPSULE 0 PAR PHARM. EAGEN 00713-<strong>06</strong>32-15 0.49<strong>08</strong>0 FLUTICASONE PROP 0.005% OINT G G & W LABS. GMGEN 00713-<strong>06</strong>32-31 0.49<strong>08</strong>0 FLUTICASONE PROP 0.005% OINT G G & W LABS. GMGEN 00713-<strong>06</strong>32-60 0.49<strong>08</strong>0 FLUTICASONE PROP 0.005% OINT G G & W LABS. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0221-11 0.49<strong>08</strong>0 FLUTICASONE PROP 0.005% OINT G PERRIGO CO. GMGEN 45802-0221-35 0.49<strong>08</strong>0 FLUTICASONE PROP 0.005% OINT G PERRIGO CO. GMGEN 45802-0221-37 0.49<strong>08</strong>0 FLUTICASONE PROP 0.005% OINT G PERRIGO CO. GMGEN 00115-1473-45 0.62890 FLUTICASONE PROP 0.05% CREAM G GLOBAL PHARM GMGEN 00115-1473-52 0.62890 FLUTICASONE PROP 0.05% CREAM G GLOBAL PHARM GMGEN 00115-1473-58 0.62890 FLUTICASONE PROP 0.05% CREAM G GLOBAL PHARM GMGEN 00168-0332-15 0.62890 FLUTICASONE PROP 0.05% CREAM G SANDOZ GMGEN 00168-0332-30 0.62890 FLUTICASONE PROP 0.05% CREAM G SANDOZ GMGEN 00168-0332-60 0.62890 FLUTICASONE PROP 0.05% CREAM G SANDOZ GMGEN 00713-<strong>06</strong>31-15 0.62890 FLUTICASONE PROP 0.05% CREAM G G & W LABS. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00713-<strong>06</strong>31-31 0.62890 FLUTICASONE PROP 0.05% CREAM G G & W LABS. GMGEN 00713-<strong>06</strong>31-60 0.62890 FLUTICASONE PROP 0.05% CREAM G G & W LABS. GMGEN 45802-0222-11 0.62890 FLUTICASONE PROP 0.05% CREAM G PERRIGO CO. GMGEN 45802-0222-35 0.62890 FLUTICASONE PROP 0.05% CREAM G PERRIGO CO. GMGEN 45802-0222-37 0.62890 FLUTICASONE PROP 0.05% CREAM G PERRIGO CO. GMGEN 00168-0434-04 5.07624 FLUTICASONE PROP 0.05% LOTION G SANDOZ MLGEN 00168-0434-60 4.87437 FLUTICASONE PROP 0.05% LOTION G SANDOZ MLGEN 45802-0441-02 4.87437 FLUTICASONE PROP 0.05% LOTION G PERRIGO CO. MLGEN 68462-0427-02 4.87437 FLUTICASONE PROP 0.05% LOTION G GLENMARK PHARMA MLGEN 00054-3270-99 1.70280 FLUTICASONE PROP 50 MCG SPRAY G ROXANE LABS. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50383-0700-16 1.70280 FLUTICASONE PROP 50 MCG SPRAY G HI-TECH PHARMAC GMGEN 60432-0264-15 1.70280 FLUTICASONE PROP 50 MCG SPRAY G MORTON GROVE PH GMGEN 60505-<strong>08</strong>29-01 1.70280 FLUTICASONE PROP 50 MCG SPRAY G APOTEX CORP GMGEN 60505-<strong>08</strong>47-03 1.70280 FLUTICASONE PROP 50 MCG SPRAY G APOTEX/NOVAPLUS GMGEN 66993-0024-52 1.70280 FLUTICASONE PROP 50 MCG SPRAY G PRASCO LABS GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 152LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7442-01 2.83020 FLUVASTATIN SODIUM 20 MG CAP G TEVA USA EAGEN 00093-7442-56 2.83500 FLUVASTATIN SODIUM 20 MG CAP G TEVA USA EAGEN 00378-8020-77 3.20544 FLUVASTATIN SODIUM 20 MG CAP G MYLAN EAGEN 00378-8020-93 3.20544 FLUVASTATIN SODIUM 20 MG CAP G MYLAN EAGEN 00093-7443-01 2.64297 FLUVASTATIN SODIUM 40 MG CAP G TEVA USA EAGEN 00093-7443-56 2.64297 FLUVASTATIN SODIUM 40 MG CAP G TEVA USA EAGEN 00378-8021-77 2.64297 FLUVASTATIN SODIUM 40 MG CAP G MYLAN EAGEN 00378-8021-93 2.64297 FLUVASTATIN SODIUM 40 MG CAP G MYLAN EAGEX 00185-0157-01 0.34570 FLUVOXAMINE MALEATE 100 MG TAB G SANDOZ EAGEX 00185-0157-05 0.34570 FLUVOXAMINE MALEATE 100 MG TAB G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-0414-01 0.34570 FLUVOXAMINE MALEATE 100 MG TAB G MYLAN EAGEX 42769-1221-00 0.34570 FLUVOXAMINE MALEATE 100 MG TAB G BAYPHARMA INC. EAGEX 51079-0993-01 0.34570 FLUVOXAMINE MALEATE 100 MG TAB G MYLAN INSTITUTI EAGEX 60505-0166-01 0.34570 FLUVOXAMINE MALEATE 100 MG TAB G APOTEX CORP EAGEX 62559-0160-01 0.34570 FLUVOXAMINE MALEATE 100 MG TAB G ANI PHARMACEUTI EAGUX 00378-0407-01 1.<strong>08</strong>830 FLUVOXAMINE MALEATE 25 MG TAB G MYLAN EAGUX 60505-0164-01 1.<strong>08</strong>830 FLUVOXAMINE MALEATE 25 MG TAB G APOTEX CORP EAGUX 62559-0158-01 1.<strong>08</strong>830 FLUVOXAMINE MALEATE 25 MG TAB G ANI PHARMACEUTI EAGEX 00185-0027-01 0.31347 FLUVOXAMINE MALEATE 50 MG TAB G SANDOZ EAGEX 00185-0027-05 0.31347 FLUVOXAMINE MALEATE 50 MG TAB G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-0412-01 0.31347 FLUVOXAMINE MALEATE 50 MG TAB G MYLAN EAGEX 42769-1225-00 0.31347 FLUVOXAMINE MALEATE 50 MG TAB G BAYPHARMA INC. EAGEX 51079-0992-01 0.31347 FLUVOXAMINE MALEATE 50 MG TAB G MYLAN INSTITUTI EAGEX 60505-0165-01 0.31347 FLUVOXAMINE MALEATE 50 MG TAB G APOTEX CORP EAGEX 62559-0159-01 0.31347 FLUVOXAMINE MALEATE 50 MG TAB G ANI PHARMACEUTI EABND 11980-0228-05 17.72880 FML FORTE 0.25% EYE DROPS 0 ALLERGAN INC. MLBND 11980-0228-10 17.72880 FML FORTE 0.25% EYE DROPS 0 ALLERGAN INC. MLBND 11980-0211-05 7.92000 17.72880 FML LIQUIFILM 0.1% EYE DROP G ALLERGAN INC. MLBND 11980-0211-10 7.92000 17.72880 FML LIQUIFILM 0.1% EYE DROP G ALLERGAN INC. MLBND 00023-0316-04 25.32686 FML S.O.P. 0.1% OINTMENT 0 ALLERGAN INC. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51991-0077-33 0.64780 FOLBECAL TABLET 0 BRECKENRIDGE EAGEN 00143-1248-01 0.01590 FOLIC ACID 1 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1248-10 0.01590 FOLIC ACID 1 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-9717-01 0.01590 FOLIC ACID 1 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-9717-10 0.01590 FOLIC ACID 1 MG TABLET 0 WEST-WARD,INC. EAGEN 00591-5216-01 0.01590 FOLIC ACID 1 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5216-10 0.01590 FOLIC ACID 1 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-3162-02 0.01590 FOLIC ACID 1 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3162-21 0.01590 FOLIC ACID 1 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3162-30 0.01590 FOLIC ACID 1 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-3162-32 0.01590 FOLIC ACID 1 MG TABLET 0 QUALITEST EAGEN 10267-0120-01 0.01590 FOLIC ACID 1 MG TABLET 0 CONTRACT PHARM EAGEN 10267-0120-04 0.01590 FOLIC ACID 1 MG TABLET 0 CONTRACT PHARM EAGEN 15338-0170-00 0.01590 FOLIC ACID 1 MG TABLET 0 APACE PACKAGING EAGEN 51079-0105-01 0.01590 FOLIC ACID 1 MG TABLET 0 MYLAN INSTITUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 153LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0105-17 0.01590 FOLIC ACID 1 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0105-19 0.01590 FOLIC ACID 1 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0105-20 0.01590 FOLIC ACID 1 MG TABLET 0 MYLAN INSTITUTI EAGEN 59746-0012-10 0.01590 FOLIC ACID 1 MG TABLET 0 CADISTA PHARMAC EAGEN 62584-<strong>08</strong>97-11 0.01590 FOLIC ACID 1 MG TABLET 0 AHP EAGEN 63739-0537-10 0.01590 FOLIC ACID 1 MG TABLET 0 MCKESSON PACKAG EAGEN 64125-0127-01 0.01590 FOLIC ACID 1 MG TABLET 0 EXCELLIUM PHARM EAGEN 64125-0127-10 0.01590 FOLIC ACID 1 MG TABLET 0 EXCELLIUM PHARM EAGEN 65162-0361-10 0.01590 FOLIC ACID 1 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0361-11 0.01590 FOLIC ACID 1 MG TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 76282-0210-01 0.01590 FOLIC ACID 1 MG TABLET 0 EXELAN PHARMACE EAGEN 76282-0210-10 0.01590 FOLIC ACID 1 MG TABLET 0 EXELAN PHARMACE EABND 63323-0184-10 2.65932 FOLIC ACID 5 MG/ML VIAL 0 APP PHARMACEUTI MLBND 63323-0184-11 2.65932 FOLIC ACID 5 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 13811-0535-30 0.56960 FOLIVANE-OB CAPSULE 0 TRIGEN LABORATO EABND 13811-0544-30 1.28982 FOLIVANE-PRX DHA NF CAPSULE 0 TRIGEN LABORATO EAGEN 55111-<strong>06</strong>81-02 90.25640 FONDAPARINUX 10 MG/0.8 ML SYR G DR.REDDY'S LAB MLGEN 55111-<strong>06</strong>81-10 90.25640 FONDAPARINUX 10 MG/0.8 ML SYR G DR.REDDY'S LAB MLGEN 60505-6<strong>08</strong>1-00 90.25640 FONDAPARINUX 10 MG/0.8 ML SYR G APOTEX CORP MLGEN 60505-6<strong>08</strong>1-04 90.25640 FONDAPARINUX 10 MG/0.8 ML SYR G APOTEX CORP ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-<strong>06</strong>78-02 31.15640 FONDAPARINUX 2.5 MG/0.5 ML SYR G DR.REDDY'S LAB MLGEN 55111-<strong>06</strong>78-10 31.15640 FONDAPARINUX 2.5 MG/0.5 ML SYR G DR.REDDY'S LAB MLGEN 60505-6078-00 31.15640 FONDAPARINUX 2.5 MG/0.5 ML SYR G APOTEX CORP MLGEN 60505-6078-04 31.15640 FONDAPARINUX 2.5 MG/0.5 ML SYR G APOTEX CORP MLGEN 55111-<strong>06</strong>79-02 180.51280 FONDAPARINUX 5 MG/0.4 ML SYR G DR.REDDY'S LAB MLGEN 55111-<strong>06</strong>79-10 180.51280 FONDAPARINUX 5 MG/0.4 ML SYR G DR.REDDY'S LAB MLGEN 60505-6079-00 180.51280 FONDAPARINUX 5 MG/0.4 ML SYR G APOTEX CORP MLGEN 60505-6079-04 180.51280 FONDAPARINUX 5 MG/0.4 ML SYR G APOTEX CORP MLGEN 55111-<strong>06</strong>80-02 151.91010 FONDAPARINUX 7.5 MG/0.6 ML SYR G DR.REDDY'S LAB MLGEN 55111-<strong>06</strong>80-10 151.91010 FONDAPARINUX 7.5 MG/0.6 ML SYR G DR.REDDY'S LAB ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-6<strong>08</strong>0-00 151.91010 FONDAPARINUX 7.5 MG/0.6 ML SYR G APOTEX CORP MLGEN 60505-6<strong>08</strong>0-04 151.91010 FONDAPARINUX 7.5 MG/0.6 ML SYR G APOTEX CORP MLBND 00<strong>08</strong>5-1401-01 3.33923 FORADIL AEROLIZER 12 MCG CAP G MERCK SHARP & D EABND 00<strong>08</strong>5-1402-01 4.12510 FORADIL AEROLIZER 12 MCG CAP G MERCK SHARP & D EABND 59630-0575-60 7.32850 27.11693 FORTAMET ER 1,000 MG TABLET G SHIONOGI PHARMA EABND 59630-0574-60 4.21031 27.11693 FORTAMET ER 500 MG TABLET G SHIONOGI PHARMA EABND 00173-0434-00 12.2<strong>06</strong>97 FORTAZ 1 GM ADD-VANTAGE VIAL 0 COVIS PHARMACEU EABND 24987-0434-00 12.9<strong>08</strong>16 FORTAZ 1 GM TWISTVIAL 0 COVIS PHARMACEU EABND 00173-0378-10 5.<strong>08</strong>800 11.81256 FORTAZ 1 GM VIAL 0 COVIS PHARMACEU EABND 24987-0378-10 5.<strong>08</strong>800 11.81256 FORTAZ 1 GM VIAL 0 COVIS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-0435-00 18.7<strong>08</strong>00 24.01439 FORTAZ 2 GM ADD-VANTAGE VIAL 0 COVIS PHARMACEU EABND 24987-0435-00 18.7<strong>08</strong>00 25.39800 FORTAZ 2 GM TWISTVIAL 0 COVIS PHARMACEU EABND 00173-0379-34 10.10130 23.61599 FORTAZ 2 GM VIAL 0 COVIS PHARMACEU EABND 24987-0379-34 10.10130 23.61599 FORTAZ 2 GM VIAL 0 COVIS PHARMACEU EABND 00173-0382-37 24.93817 68.72400 FORTAZ 6 GM VIAL 0 COVIS PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 154LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-0413-00 0.59760 FORTAZ-ISO-OSMOT 2 GM/50 ML 0 COVIS PHARMACEU MLBND 24987-0413-00 0.65570 FORTAZ-ISO-OSMOT 2 GM/50 ML 0 COVIS PHARMACEU MLBND 00173-0412-00 0.32453 FORTAZ-ISO-OSMOTIC 1 GM/50 ML 0 COVIS PHARMACEU MLBND 24987-0412-00 0.35607 FORTAZ-ISO-OSMOTIC 1 GM/50 ML 0 COVIS PHARMACEU MLBND 00002-8400-01 588.22100 FORTEO 600 MCG/2.4 ML PEN INJ G ELI LILLY & CO. MLBND 00245-00<strong>08</strong>-35 12.21825 23.<strong>08</strong>073 FORTICAL 200 UNITS NASAL SPRAY G UPSHER SMITH MLBND 000<strong>06</strong>-0710-44 31.85540 FOSAMAX PLUS D 70 MG-2,800 IU G MERCK SHARP & D EABND 000<strong>06</strong>-0270-44 31.85540 FOSAMAX PLUS D 70 MG-5,600 IU G MERCK SHARP & D EABND 000<strong>06</strong>-0031-44 0.69530 25.29632 FOSAMAX 70 MG TABLET G MERCK SHARP & D EABND 00409-3863-02 0.30294 FOSCARNET 24 MG/ML INFUS BTTL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-3863-05 0.30029 FOSCARNET 24 MG/ML INFUS BTTL 0 HOSPIRA MLGEN 00093-7222-10 0.15724 FOSINOPRIL SODIUM 10 MG TAB G TEVA USA EAGEN 00093-7222-98 0.15724 FOSINOPRIL SODIUM 10 MG TAB G TEVA USA EAGEN 00185-0041-09 0.15724 FOSINOPRIL SODIUM 10 MG TAB G SANDOZ EAGEN 00185-0041-10 0.15724 FOSINOPRIL SODIUM 10 MG TAB G SANDOZ EAGEN 31722-0200-10 0.15724 FOSINOPRIL SODIUM 10 MG TAB G CAMBER PHARMACE EAGEN 31722-0200-90 0.15724 FOSINOPRIL SODIUM 10 MG TAB G CAMBER PHARMACE EAGEN 60505-2510-02 0.15724 FOSINOPRIL SODIUM 10 MG TAB G APOTEX CORP EAGEN 60505-2510-04 0.15724 FOSINOPRIL SODIUM 10 MG TAB G APOTEX CORP EAGEN 76282-0200-10 0.15724 FOSINOPRIL SODIUM 10 MG TAB G EXELAN PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 76282-0200-90 0.15724 FOSINOPRIL SODIUM 10 MG TAB G EXELAN PHARMACE EAGEN 00093-7223-10 0.15316 FOSINOPRIL SODIUM 20 MG TAB G TEVA USA EAGEN 00093-7223-98 0.15316 FOSINOPRIL SODIUM 20 MG TAB G TEVA USA EAGEN 00185-0042-09 0.15316 FOSINOPRIL SODIUM 20 MG TAB G SANDOZ EAGEN 00185-0042-10 0.15316 FOSINOPRIL SODIUM 20 MG TAB G SANDOZ EAGEN 31722-0201-10 0.15316 FOSINOPRIL SODIUM 20 MG TAB G CAMBER PHARMACE EAGEN 31722-0201-90 0.15316 FOSINOPRIL SODIUM 20 MG TAB G CAMBER PHARMACE EAGEN 60505-2511-02 0.15316 FOSINOPRIL SODIUM 20 MG TAB G APOTEX CORP EAGEN 60505-2511-04 0.15316 FOSINOPRIL SODIUM 20 MG TAB G APOTEX CORP EAGEN 76282-0201-10 0.15316 FOSINOPRIL SODIUM 20 MG TAB G EXELAN PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 76282-0201-90 0.15316 FOSINOPRIL SODIUM 20 MG TAB G EXELAN PHARMACE EAGEN 00093-7224-10 0.21965 FOSINOPRIL SODIUM 40 MG TAB G TEVA USA EAGEN 00093-7224-98 0.21965 FOSINOPRIL SODIUM 40 MG TAB G TEVA USA EAGEN 00185-0047-09 0.21965 FOSINOPRIL SODIUM 40 MG TAB G SANDOZ EAGEN 00185-0047-10 0.21965 FOSINOPRIL SODIUM 40 MG TAB G SANDOZ EAGEN 31722-0202-10 0.21965 FOSINOPRIL SODIUM 40 MG TAB G CAMBER PHARMACE EAGEN 31722-0202-90 0.21965 FOSINOPRIL SODIUM 40 MG TAB G CAMBER PHARMACE EAGEN 60505-2512-02 0.21965 FOSINOPRIL SODIUM 40 MG TAB G APOTEX CORP EAGEN 60505-2512-<strong>08</strong> 0.21965 FOSINOPRIL SODIUM 40 MG TAB G APOTEX CORP EAGEN 76282-0202-10 0.21965 FOSINOPRIL SODIUM 40 MG TAB G EXELAN PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 76282-0202-90 0.21965 FOSINOPRIL SODIUM 40 MG TAB G EXELAN PHARMACE EAGEN 00185-0341-01 0.88460 FOSINOPRIL-HCTZ 10-12.5 MG TAB G SANDOZ EAGEN 59762-5250-01 0.88460 FOSINOPRIL-HCTZ 10-12.5 MG TAB G GREENSTONE LLC. EAGEN 59762-5250-04 0.88460 FOSINOPRIL-HCTZ 10-12.5 MG TAB G GREENSTONE LLC. EAGEN 65862-03<strong>08</strong>-01 0.88460 FOSINOPRIL-HCTZ 10-12.5 MG TAB G AUROBINDO PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 155LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0554-01 0.88460 FOSINOPRIL-HCTZ 10-12.5 MG TAB G GLENMARK PHARMA EAGEN 00185-0342-01 0.88460 FOSINOPRIL-HCTZ 20-12.5 MG TAB G SANDOZ EAGEN 59762-5251-01 0.88460 FOSINOPRIL-HCTZ 20-12.5 MG TAB G GREENSTONE LLC. EAGEN 59762-5251-04 0.88460 FOSINOPRIL-HCTZ 20-12.5 MG TAB G GREENSTONE LLC. EAGEN 65862-0309-01 0.88460 FOSINOPRIL-HCTZ 20-12.5 MG TAB G AUROBINDO PHARM EAGEN 68462-0555-01 0.88460 FOSINOPRIL-HCTZ 20-12.5 MG TAB G GLENMARK PHARMA EABND 54092-0254-90 8.61982 FOSRENOL 1,000 MG TABLET CHEW 0 SHIRE US INC. EABND 54092-0252-90 8.61982 FOSRENOL 500 MG TABLET CHEW 0 SHIRE US INC. EABND 54092-0253-15 8.61982 FOSRENOL 750 MG TABLET CHEW 0 SHIRE US INC. EABND 54092-0253-90 8.61982 FOSRENOL 750 MG TABLET CHEW 0 SHIRE US INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 62856-0101-01 70.61640 FRAGMIN 10,000 UNITS SYRINGE 0 EISAI INC. MLBND 62856-0101-10 70.618<strong>06</strong> FRAGMIN 10,000 UNITS SYRINGE 0 EISAI INC. MLBND 62856-0125-01 176.55760 FRAGMIN 12,500 UNITS SYRINGE 0 EISAI INC. MLBND 62856-0125-10 176.54764 FRAGMIN 12,500 UNITS SYRINGE 0 EISAI INC. MLBND 62856-0150-01 176.54100 FRAGMIN 15,000 UNITS SYRINGE 0 EISAI INC. MLBND 62856-0150-10 176.54791 FRAGMIN 15,000 UNITS SYRINGE 0 EISAI INC. MLBND 62856-0180-10 176.54330 FRAGMIN 18,000 UNITS SYRINGE 0 EISAI INC. MLBND 62856-0250-01 1<strong>08</strong>.81300 FRAGMIN 2,500 UNITS SYRINGE 0 EISAI INC. MLBND 62856-0250-10 1<strong>08</strong>.83375 FRAGMIN 2,500 UNITS SYRINGE 0 EISAI INC. MLBND 62856-0251-01 159.73131 FRAGMIN 25,000 UNITS/ML VIAL 0 EISAI INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 62856-0500-01 176.54100 FRAGMIN 5,000 UNITS SYRINGE 0 EISAI INC. MLBND 62856-0500-10 176.54930 FRAGMIN 5,000 UNITS SYRINGE 0 EISAI INC. MLBND 62856-0750-10 176.55760 FRAGMIN 7,500 UNITS SYRINGE 0 EISAI INC. MLBND 63481-0025-09 33.93685 FROVA 2.5 MG TABLET G ENDO PHARM INC. EABND 65649-<strong>08</strong>02-02 8.96400 FULYZAQ 125 MG DR TABLET 0 SALIX PHARMACEU EABND 59630-0450-<strong>08</strong> 2.07480 3.94<strong>06</strong>9 FURADANTIN 25 MG/5 ML SUSP 0 SHIONOGI PHARMA MLGEN 00054-3294-46 0.11170 FUROSEMIDE 10 MG/ML SOLUTION 0 ROXANE LABS. MLGEN 00054-3294-50 0.11170 FUROSEMIDE 10 MG/ML SOLUTION 0 ROXANE LABS. MLGEN 60432-<strong>06</strong>13-04 0.11170 FUROSEMIDE 10 MG/ML SOLUTION 0 MORTON GROVE PH MLGEN 60432-<strong>06</strong>13-60 0.11170 FUROSEMIDE 10 MG/ML SOLUTION 0 MORTON GROVE PH ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-6102-02 0.64350 FUROSEMIDE 10 MG/ML VIAL 0 HOSPIRA MLGEN 00409-6102-04 0.39600 FUROSEMIDE 10 MG/ML VIAL 0 HOSPIRA MLGEN 00409-6102-10 0.18000 FUROSEMIDE 10 MG/ML VIAL 0 HOSPIRA MLGEN 00517-5702-25 0.70320 FUROSEMIDE 10 MG/ML VIAL 0 AMER. REGENT MLGEN 00517-5704-25 0.41017 FUROSEMIDE 10 MG/ML VIAL 0 AMER. REGENT MLGEN 00517-5710-25 0.21096 FUROSEMIDE 10 MG/ML VIAL 0 AMER. REGENT MLGEN 63323-0280-02 2.78100 FUROSEMIDE 10 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0280-04 2.79675 FUROSEMIDE 10 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0280-10 2.10240 FUROSEMIDE 10 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 00054-4297-25 0.01013 FUROSEMIDE 20 MG TABLET 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-4297-31 0.01013 FUROSEMIDE 20 MG TABLET 0 ROXANE LABS. EAGEN 00054-8297-25 0.01013 FUROSEMIDE 20 MG TABLET 0 ROXANE LABS. EAGEN 00172-29<strong>08</strong>-60 0.01013 FUROSEMIDE 20 MG TABLET 0 TEVA USA EAGEN 00172-29<strong>08</strong>-80 0.01013 FUROSEMIDE 20 MG TABLET 0 TEVA USA EAGEN 00378-02<strong>08</strong>-01 0.01013 FUROSEMIDE 20 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 156LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-02<strong>08</strong>-10 0.01013 FUROSEMIDE 20 MG TABLET 0 MYLAN EAGEN 00378-02<strong>08</strong>-93 0.01013 FUROSEMIDE 20 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-3739-21 0.01013 FUROSEMIDE 20 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3739-32 0.01013 FUROSEMIDE 20 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3739-34 0.01013 FUROSEMIDE 20 MG TABLET 0 QUALITEST EAGEN 00781-1818-01 0.01013 FUROSEMIDE 20 MG TABLET 0 SANDOZ EAGEN 00781-1818-10 0.01013 FUROSEMIDE 20 MG TABLET 0 SANDOZ EAGEN 00904-5796-61 0.01013 FUROSEMIDE 20 MG TABLET 0 MAJOR PHARMACEU EAGEN 50742-0104-01 0.01013 FUROSEMIDE 20 MG TABLET 0 INGENUS PHARMAC EAGEN 50742-0104-10 0.01013 FUROSEMIDE 20 MG TABLET 0 INGENUS PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0072-20 0.01013 FUROSEMIDE 20 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0072-30 0.01013 FUROSEMIDE 20 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0072-56 0.01013 FUROSEMIDE 20 MG TABLET 0 MYLAN INSTITUTI EAGEN 63304-<strong>06</strong>24-01 0.01013 FUROSEMIDE 20 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>06</strong>24-10 0.01013 FUROSEMIDE 20 MG TABLET 0 RANBAXY PHARMAC EAGEN 64125-0116-01 0.01013 FUROSEMIDE 20 MG TABLET 0 EXCELLIUM PHARM EAGEN 64125-0116-10 0.01013 FUROSEMIDE 20 MG TABLET 0 EXCELLIUM PHARM EAGEN 68<strong>08</strong>4-0014-11 0.01013 FUROSEMIDE 20 MG TABLET 0 AHP EAGEN 00054-4299-25 0.01170 FUROSEMIDE 40 MG TABLET 0 ROXANE LABS. EAGEN 00054-4299-31 0.01170 FUROSEMIDE 40 MG TABLET 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-8299-25 0.01170 FUROSEMIDE 40 MG TABLET 0 ROXANE LABS. EAGEN 00172-2907-60 0.01170 FUROSEMIDE 40 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-2907-80 0.01170 FUROSEMIDE 40 MG TABLET 0 IVAX PHARMACEUT EAGEN 00378-0216-01 0.01170 FUROSEMIDE 40 MG TABLET 0 MYLAN EAGEN 00378-0216-10 0.01170 FUROSEMIDE 40 MG TABLET 0 MYLAN EAGEN 00378-0216-93 0.01170 FUROSEMIDE 40 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-3740-21 0.01170 FUROSEMIDE 40 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3740-32 0.01170 FUROSEMIDE 40 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3740-34 0.01170 FUROSEMIDE 40 MG TABLET 0 QUALITEST EAGEN 00781-1966-01 0.01170 FUROSEMIDE 40 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-1966-10 0.01170 FUROSEMIDE 40 MG TABLET 0 SANDOZ EAGEN 00904-5797-61 0.01170 FUROSEMIDE 40 MG TABLET 0 MAJOR PHARMACEU EAGEN 50742-0105-10 0.01170 FUROSEMIDE 40 MG TABLET 0 INGENUS PHARMAC EAGEN 51079-0073-20 0.01170 FUROSEMIDE 40 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0073-30 0.01170 FUROSEMIDE 40 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0073-56 0.01170 FUROSEMIDE 40 MG TABLET 0 MYLAN INSTITUTI EAGEN 58517-0400-30 0.01170 FUROSEMIDE 40 MG TABLET 0 <strong>NEW</strong> HORIZON RX EAGEN 63304-<strong>06</strong>25-01 0.01170 FUROSEMIDE 40 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>06</strong>25-10 0.01170 FUROSEMIDE 40 MG TABLET 0 RANBAXY PHARMAC EAGEN 63739-0542-01 0.01170 FUROSEMIDE 40 MG TABLET 0 MCKESSON PACKAG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63739-0542-04 0.01170 FUROSEMIDE 40 MG TABLET 0 MCKESSON PACKAG EAGEN 63739-0542-10 0.01170 FUROSEMIDE 40 MG TABLET 0 MCKESSON PACKAG EAGEN 64125-0117-01 0.01170 FUROSEMIDE 40 MG TABLET 0 EXCELLIUM PHARM EAGEN 64125-0117-10 0.01170 FUROSEMIDE 40 MG TABLET 0 EXCELLIUM PHARM EABND 00054-3298-63 0.07242 FUROSEMIDE 40 MG/5 ML SOLN 0 ROXANE LABS. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 157LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-4301-25 0.02730 FUROSEMIDE 80 MG TABLET 0 ROXANE LABS. EAGEN 00054-4301-29 0.02730 FUROSEMIDE 80 MG TABLET 0 ROXANE LABS. EAGEN 00054-8301-25 0.02730 FUROSEMIDE 80 MG TABLET 0 ROXANE LABS. EAGEN 00378-0232-01 0.02730 FUROSEMIDE 80 MG TABLET 0 MYLAN EAGEN 00378-0232-05 0.02730 FUROSEMIDE 80 MG TABLET 0 MYLAN EAGEN 00378-0232-93 0.02730 FUROSEMIDE 80 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-3741-02 0.02730 FUROSEMIDE 80 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3741-04 0.02730 FUROSEMIDE 80 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3741-21 0.02730 FUROSEMIDE 80 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-3741-28 0.02730 FUROSEMIDE 80 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-3741-32 0.02730 FUROSEMIDE 80 MG TABLET 0 QUALITEST EAGEN 00781-1446-01 0.02730 FUROSEMIDE 80 MG TABLET 0 SANDOZ EAGEN 00781-1446-05 0.02730 FUROSEMIDE 80 MG TABLET 0 SANDOZ EAGEN 00904-5798-61 0.02730 FUROSEMIDE 80 MG TABLET 0 MAJOR PHARMACEU EAGEN 50742-01<strong>06</strong>-01 0.02730 FUROSEMIDE 80 MG TABLET 0 INGENUS PHARMAC EAGEN 50742-01<strong>06</strong>-05 0.02730 FUROSEMIDE 80 MG TABLET 0 INGENUS PHARMAC EAGEN 51079-0527-20 0.02730 FUROSEMIDE 80 MG TABLET 0 MYLAN INSTITUTI EAGEN 63304-<strong>06</strong>26-01 0.02730 FUROSEMIDE 80 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>06</strong>26-05 0.02730 FUROSEMIDE 80 MG TABLET 0 RANBAXY PHARMAC EAGEN 64125-0118-01 0.02730 FUROSEMIDE 80 MG TABLET 0 EXCELLIUM PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64125-0118-05 0.02730 FUROSEMIDE 80 MG TABLET 0 EXCELLIUM PHARM EAGEN 68<strong>08</strong>4-0017-11 0.02730 FUROSEMIDE 80 MG TABLET 0 AHP EABND 00004-0381-40 48.60328 FUZEON 90 MG VIAL G GENENTECH, INC. EAGEX 38779-2461-<strong>08</strong> 39.18750 GABAPENTIN POWDER 0 MEDISCA INC. GMGEX 00143-9992-01 0.05090 GABAPENTIN 100 MG CAPSULE 0 WEST-WARD,INC. EAGEX 00228-2665-11 0.05090 GABAPENTIN 100 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00228-2665-50 0.05090 GABAPENTIN 100 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00378-5426-01 0.05090 GABAPENTIN 100 MG CAPSULE 0 MYLAN EAGEX 00378-5426-05 0.05090 GABAPENTIN 100 MG CAPSULE 0 MYLAN EAGEX 00904-5631-61 0.05090 GABAPENTIN 100 MG CAPSULE 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00904-5631-93 0.05090 GABAPENTIN 100 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-6078-61 0.05090 GABAPENTIN 100 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 14550-0511-02 0.05090 GABAPENTIN 100 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 14550-0511-04 0.05090 GABAPENTIN 100 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 16714-<strong>06</strong>61-01 0.05090 GABAPENTIN 100 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>61-02 0.05090 GABAPENTIN 100 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 31722-0221-01 0.05090 GABAPENTIN 100 MG CAPSULE 0 CAMBER PHARMACE EAGEX 31722-0221-05 0.05090 GABAPENTIN 100 MG CAPSULE 0 CAMBER PHARMACE EAGEX 51991-0337-01 0.05090 GABAPENTIN 100 MG CAPSULE 0 BRECKENRIDGE EAGEX 52343-0030-01 0.02257 GABAPENTIN 100 MG CAPSULE 0 GEN-SOURCE RX EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 52343-0030-18 0.02545 GABAPENTIN 100 MG CAPSULE 0 GEN-SOURCE RX EAGEX 52343-0030-27 0.02347 GABAPENTIN 100 MG CAPSULE 0 GEN-SOURCE RX EAGEX 52343-0030-90 0.02283 GABAPENTIN 100 MG CAPSULE 0 GEN-SOURCE RX EAGEX 52343-0030-99 0.01974 GABAPENTIN 100 MG CAPSULE 0 GEN-SOURCE RX EAGEX 53746-0101-01 0.05090 GABAPENTIN 100 MG CAPSULE 0 AMNEAL PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 158LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 53746-0101-05 0.05090 GABAPENTIN 100 MG CAPSULE 0 AMNEAL PHARMACE EAGEX 53746-0101-10 0.05090 GABAPENTIN 100 MG CAPSULE 0 AMNEAL PHARMACE EAGUX 59762-5026-01 0.<strong>08</strong>250 GABAPENTIN 100 MG CAPSULE 0 GREENSTONE LLC. EAGEX 60505-0112-00 0.05090 GABAPENTIN 100 MG CAPSULE 0 APOTEX CORP EAGEX 60505-0112-01 0.05090 GABAPENTIN 100 MG CAPSULE 0 APOTEX CORP EAGEX 60505-0112-07 0.05090 GABAPENTIN 100 MG CAPSULE 0 APOTEX CORP EAGEX 60505-0112-<strong>08</strong> 0.05090 GABAPENTIN 100 MG CAPSULE 0 APOTEX CORP EAGEX 62756-0137-02 0.05090 GABAPENTIN 100 MG CAPSULE 0 SUN PHARMACEUTI EAGEX 62756-0137-05 0.05090 GABAPENTIN 100 MG CAPSULE 0 SUN PHARMACEUTI EAGEX 63739-0374-10 0.05090 GABAPENTIN 100 MG CAPSULE 0 MCKESSON PACKAG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0198-01 0.05090 GABAPENTIN 100 MG CAPSULE 0 AUROBINDO PHARM EAGEX 65862-0198-05 0.05090 GABAPENTIN 100 MG CAPSULE 0 AUROBINDO PHARM EAGEX 65862-0198-99 0.05090 GABAPENTIN 100 MG CAPSULE 0 AUROBINDO PHARM EAGEX 67877-0222-01 0.05090 GABAPENTIN 100 MG CAPSULE 0 ASCEND LABORATO EAGEX 67877-0222-05 0.05090 GABAPENTIN 100 MG CAPSULE 0 ASCEND LABORATO EAGEX 67877-0222-10 0.05090 GABAPENTIN 100 MG CAPSULE 0 ASCEND LABORATO EAGEX 68<strong>08</strong>4-0594-01 0.05090 GABAPENTIN 100 MG CAPSULE 0 AHP EAGEX 76282-0321-01 0.05090 GABAPENTIN 100 MG CAPSULE 0 EXELAN PHARMACE EAGEX 50383-0311-47 0.23260 GABAPENTIN 250 MG/5 ML SOLN 0 HI-TECH PHARMAC MLGEX 59762-5025-01 0.23260 GABAPENTIN 250 MG/5 ML SOLN 0 GREENSTONE LLC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00143-9993-01 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 WEST-WARD,INC. EAGEX 00228-2666-11 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00228-2666-50 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00378-5427-01 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MYLAN EAGEX 00378-5427-05 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MYLAN EAGEX 00904-5632-40 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-5632-46 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-5632-52 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-5632-53 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-5632-61 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00904-5632-89 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-5632-93 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-6079-61 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 14550-0512-02 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 14550-0512-04 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 16714-<strong>06</strong>62-01 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>62-02 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 31722-0222-01 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 CAMBER PHARMACE EAGEX 31722-0222-05 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 CAMBER PHARMACE EAGEX 51991-0338-01 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 BRECKENRIDGE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 52343-0031-01 0.02985 GABAPENTIN 300 MG CAPSULE 0 GEN-SOURCE RX EAGEX 52343-0031-18 0.02854 GABAPENTIN 300 MG CAPSULE 0 GEN-SOURCE RX EAGEX 52343-0031-27 0.02980 GABAPENTIN 300 MG CAPSULE 0 GEN-SOURCE RX EAGEX 52343-0031-30 0.03399 GABAPENTIN 300 MG CAPSULE 0 GEN-SOURCE RX EAGEX 52343-0031-60 0.04074 GABAPENTIN 300 MG CAPSULE 0 GEN-SOURCE RX EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 159LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 52343-0031-90 0.02958 GABAPENTIN 300 MG CAPSULE 0 GEN-SOURCE RX EAGEX 53746-0102-01 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 AMNEAL PHARMACE EAGEX 53746-0102-05 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 AMNEAL PHARMACE EAGEX 53746-0102-10 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 AMNEAL PHARMACE EAGEX 58517-0<strong>06</strong>0-30 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 <strong>NEW</strong> HORIZON RX EAGUX 59762-5027-01 0.12380 GABAPENTIN 300 MG CAPSULE 0 GREENSTONE LLC. EAGEX 59762-5027-02 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 GREENSTONE LLC. EAGEX 60505-0113-00 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 APOTEX CORP EAGEX 60505-0113-01 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 APOTEX CORP EAGEX 60505-0113-07 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-0113-<strong>08</strong> 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 APOTEX CORP EAGEX 62756-0138-02 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 SUN PHARMACEUTI EAGEX 62756-0138-05 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 SUN PHARMACEUTI EAGEX 63739-0375-10 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MCKESSON PACKAG EAGEX 63739-<strong>08</strong>09-41 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MCKESSON PACKAG EAGEX 63739-<strong>08</strong>09-42 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MCKESSON PACKAG EAGEX 63739-<strong>08</strong>09-43 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MCKESSON PACKAG EAGEX 63739-<strong>08</strong>09-45 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MCKESSON PACKAG EAGEX 63739-<strong>08</strong>09-47 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 MCKESSON PACKAG EAGEX 65862-0199-01 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0199-05 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 AUROBINDO PHARM EAGEX 65862-0199-99 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 AUROBINDO PHARM EAGEX 67877-0223-01 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 ASCEND LABORATO EAGEX 67877-0223-05 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 ASCEND LABORATO EAGEX 67877-0223-10 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 ASCEND LABORATO EAGEX 68<strong>08</strong>4-0563-01 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 AHP EAGEX 68<strong>08</strong>4-0563-11 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 AHP EAGEX 68<strong>08</strong>4-0563-65 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 AHP EAGEX 76282-0322-01 0.<strong>06</strong>642 GABAPENTIN 300 MG CAPSULE 0 EXELAN PHARMACE EAGEX 00143-9994-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00228-2667-11 0.09977 GABAPENTIN 400 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00228-2667-50 0.09977 GABAPENTIN 400 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00378-5428-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 MYLAN EAGEX 00378-5428-05 0.09977 GABAPENTIN 400 MG CAPSULE 0 MYLAN EAGEX 00904-5633-40 0.09977 GABAPENTIN 400 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-5633-61 0.09977 GABAPENTIN 400 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-6105-61 0.09977 GABAPENTIN 400 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 14550-0513-02 0.09977 GABAPENTIN 400 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 14550-0513-04 0.09977 GABAPENTIN 400 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 16714-<strong>06</strong>63-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16714-<strong>06</strong>63-02 0.09977 GABAPENTIN 400 MG CAPSULE 0 NORTHSTAR RX LL EAGEX 31722-0223-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 CAMBER PHARMACE EAGEX 31722-0223-05 0.09977 GABAPENTIN 400 MG CAPSULE 0 CAMBER PHARMACE EAGEX 51991-0339-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 BRECKENRIDGE EAGEX 51991-0339-05 0.09977 GABAPENTIN 400 MG CAPSULE 0 BRECKENRIDGE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 160LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 52343-0032-01 0.03540 GABAPENTIN 400 MG CAPSULE 0 GEN-SOURCE RX EAGEX 52343-0032-05 0.03312 GABAPENTIN 400 MG CAPSULE 0 GEN-SOURCE RX EAGEX 52343-0032-27 0.03650 GABAPENTIN 400 MG CAPSULE 0 GEN-SOURCE RX EAGEX 52343-0032-90 0.03650 GABAPENTIN 400 MG CAPSULE 0 GEN-SOURCE RX EAGEX 53746-0103-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 AMNEAL PHARMACE EAGEX 53746-0103-05 0.09977 GABAPENTIN 400 MG CAPSULE 0 AMNEAL PHARMACE EAGEX 59762-5028-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 GREENSTONE LLC. EAGEX 60505-0114-00 0.09977 GABAPENTIN 400 MG CAPSULE 0 APOTEX CORP EAGEX 60505-0114-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 APOTEX CORP EAGEX 60505-0114-05 0.09977 GABAPENTIN 400 MG CAPSULE 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-0114-07 0.09977 GABAPENTIN 400 MG CAPSULE 0 APOTEX CORP EAGEX 62756-0139-02 0.09977 GABAPENTIN 400 MG CAPSULE 0 SUN PHARMACEUTI EAGEX 62756-0139-05 0.09977 GABAPENTIN 400 MG CAPSULE 0 SUN PHARMACEUTI EAGEX 63739-0376-10 0.09977 GABAPENTIN 400 MG CAPSULE 0 MCKESSON PACKAG EAGEX 65862-0200-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 AUROBINDO PHARM EAGEX 65862-0200-05 0.09977 GABAPENTIN 400 MG CAPSULE 0 AUROBINDO PHARM EAGEX 67877-0224-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 ASCEND LABORATO EAGEX 67877-0224-05 0.09977 GABAPENTIN 400 MG CAPSULE 0 ASCEND LABORATO EAGEX 67877-0224-10 0.09977 GABAPENTIN 400 MG CAPSULE 0 ASCEND LABORATO EAGEX 68<strong>08</strong>4-0595-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0595-65 0.09977 GABAPENTIN 400 MG CAPSULE 0 AHP EAGEX 76282-0323-01 0.09977 GABAPENTIN 400 MG CAPSULE 0 EXELAN PHARMACE EAGEX 00093-4443-01 0.30150 GABAPENTIN 600 MG TABLET G TEVA USA EAGEX 00093-4443-05 0.30150 GABAPENTIN 600 MG TABLET G TEVA USA EAGEX 00093-4443-10 0.30150 GABAPENTIN 600 MG TABLET G TEVA USA EAGEX 00228-2636-11 0.30150 GABAPENTIN 600 MG TABLET G ACTAVIS PHARMA, EAGEX 00228-2636-50 0.30150 GABAPENTIN 600 MG TABLET G ACTAVIS PHARMA, EAGEX 16714-0330-01 0.30150 GABAPENTIN 600 MG TABLET G NORTHSTAR RX LL EAGEX 16714-0330-02 0.30150 GABAPENTIN 600 MG TABLET G NORTHSTAR RX LL EAGEX 31722-0405-01 0.30150 GABAPENTIN 600 MG TABLET G CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 31722-0405-05 0.30150 GABAPENTIN 600 MG TABLET G CAMBER PHARMACE EAGEX 59762-5023-01 0.30150 GABAPENTIN 600 MG TABLET G GREENSTONE LLC. EAGEX 60505-2551-01 0.30150 GABAPENTIN 600 MG TABLET G APOTEX CORP EAGEX 60505-2551-05 0.30150 GABAPENTIN 600 MG TABLET G APOTEX CORP EAGEX 62756-0202-01 0.30150 GABAPENTIN 600 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0202-03 0.30150 GABAPENTIN 600 MG TABLET G SUN PHARMACEUTI EAGEX 63739-0391-10 0.30150 GABAPENTIN 600 MG TABLET G MCKESSON PACKAG EAGEX 65862-0523-01 0.30150 GABAPENTIN 600 MG TABLET G AUROBINDO PHARM EAGEX 65862-0523-05 0.30150 GABAPENTIN 600 MG TABLET G AUROBINDO PHARM EAGEX 68001-00<strong>06</strong>-00 0.30150 GABAPENTIN 600 MG TABLET G BLUEPOINT LABOR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68001-00<strong>06</strong>-03 0.30150 GABAPENTIN 600 MG TABLET G BLUEPOINT LABOR EAGEX 68<strong>08</strong>4-<strong>06</strong>24-01 0.30150 GABAPENTIN 600 MG TABLET G AHP EAGEX 68<strong>08</strong>4-<strong>06</strong>24-11 0.30150 GABAPENTIN 600 MG TABLET G AHP EAGEX 68382-0204-01 0.30150 GABAPENTIN 600 MG TABLET G ZYDUS PHARMACEU EAGEX 68382-0204-05 0.30150 GABAPENTIN 600 MG TABLET G ZYDUS PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 161LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68462-0126-01 0.30150 GABAPENTIN 600 MG TABLET G GLENMARK PHARMA EAGEX 68462-0126-05 0.30150 GABAPENTIN 600 MG TABLET G GLENMARK PHARMA EAGEX 76282-0405-01 0.30150 GABAPENTIN 600 MG TABLET G EXELAN PHARMACE EAGEX 76282-0405-05 0.30150 GABAPENTIN 600 MG TABLET G EXELAN PHARMACE EAGEX 76282-0405-90 0.30150 GABAPENTIN 600 MG TABLET G EXELAN PHARMACE EAGEX 00093-4444-01 0.30510 GABAPENTIN 800 MG TABLET G TEVA USA EAGEX 00093-4444-05 0.30510 GABAPENTIN 800 MG TABLET G TEVA USA EAGEX 00228-2637-11 0.30510 GABAPENTIN 800 MG TABLET G ACTAVIS PHARMA, EAGEX 00228-2637-50 0.30510 GABAPENTIN 800 MG TABLET G ACTAVIS PHARMA, EAGEX 31722-04<strong>06</strong>-01 0.30510 GABAPENTIN 800 MG TABLET G CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 31722-04<strong>06</strong>-05 0.30510 GABAPENTIN 800 MG TABLET G CAMBER PHARMACE EAGEX 59762-5024-01 0.30510 GABAPENTIN 800 MG TABLET G GREENSTONE LLC. EAGEX 60505-2552-01 0.30510 GABAPENTIN 800 MG TABLET G APOTEX CORP EAGEX 60505-2552-05 0.30510 GABAPENTIN 800 MG TABLET G APOTEX CORP EAGEX 62756-0204-01 0.30510 GABAPENTIN 800 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0204-03 0.30510 GABAPENTIN 800 MG TABLET G SUN PHARMACEUTI EAGEX 65862-0524-01 0.30510 GABAPENTIN 800 MG TABLET G AUROBINDO PHARM EAGEX 65862-0524-05 0.30510 GABAPENTIN 800 MG TABLET G AUROBINDO PHARM EAGEX 68001-0007-00 0.30510 GABAPENTIN 800 MG TABLET G BLUEPOINT LABOR EAGEX 68001-0007-03 0.30510 GABAPENTIN 800 MG TABLET G BLUEPOINT LABOR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-<strong>06</strong>25-01 0.30510 GABAPENTIN 800 MG TABLET G AHP EAGEX 68382-0205-01 0.30510 GABAPENTIN 800 MG TABLET G ZYDUS PHARMACEU EAGEX 68382-0205-05 0.30510 GABAPENTIN 800 MG TABLET G ZYDUS PHARMACEU EAGEX 68462-0127-01 0.30510 GABAPENTIN 800 MG TABLET G GLENMARK PHARMA EAGEX 68462-0127-05 0.30510 GABAPENTIN 800 MG TABLET G GLENMARK PHARMA EAGEX 76282-04<strong>06</strong>-01 0.30510 GABAPENTIN 800 MG TABLET G EXELAN PHARMACE EAGEX 76282-04<strong>06</strong>-05 0.30510 GABAPENTIN 800 MG TABLET G EXELAN PHARMACE EAGEX 76282-04<strong>06</strong>-90 0.30510 GABAPENTIN 800 MG TABLET G EXELAN PHARMACE EABEX 63459-0412-30 7.96800 GABITRIL 12 MG TABLET G CEPHALON,INC.-T EABEX 63459-0416-30 10.42480 GABITRIL 16 MG TABLET G CEPHALON,INC.-T EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 63459-0402-30 6.17520 6.17520 GABITRIL 2 MG TABLET 0 CEPHALON,INC.-T EABEX 63459-0404-30 6.17520 6.17520 GABITRIL 4 MG TABLET 0 CEPHALON,INC.-T EABND 45945-0155-02 10.70700 GABL<strong>OF</strong>EN 10,000 MCG/20 ML VIAL 0 CNS THERAPEUTIC MLBND 45945-0156-02 21.41400 GABL<strong>OF</strong>EN 20,000 MCG/20 ML VIAL 0 CNS THERAPEUTIC MLBND 45945-0157-01 46.<strong>06</strong>500 GABL<strong>OF</strong>EN 40,000 MCG/20 ML SYRG 0 CNS THERAPEUTIC MLBND 45945-0157-02 42.82800 GABL<strong>OF</strong>EN 40,000 MCG/20 ML VIAL 0 CNS THERAPEUTIC MLBND 45945-0151-01 79.68000 GABL<strong>OF</strong>EN 50 MCG/ML SYRINGE 0 CNS THERAPEUTIC MLGEN 00115-1121-<strong>08</strong> 2.01866 GALANTAMINE ER 16 MG CAPSULE 0 GLOBAL PHARM EAGEN 00378-81<strong>06</strong>-93 2.01866 GALANTAMINE ER 16 MG CAPSULE 0 MYLAN EAGEN 00555-1021-01 2.01866 GALANTAMINE ER 16 MG CAPSULE 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10147-<strong>08</strong>92-03 2.01866 GALANTAMINE ER 16 MG CAPSULE 0 PATRIOT PHARMAC EAGEN 47335-<strong>08</strong>36-83 2.01866 GALANTAMINE ER 16 MG CAPSULE 0 SUN PHARMA GLOB EAGEN 00115-1122-<strong>08</strong> 4.77350 GALANTAMINE ER 24 MG CAPSULE 0 GLOBAL PHARM EAGEN 00378-8107-93 4.77350 GALANTAMINE ER 24 MG CAPSULE 0 MYLAN EAGEN 10147-<strong>08</strong>93-03 4.57800 GALANTAMINE ER 24 MG CAPSULE 0 PATRIOT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 162LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 47335-<strong>08</strong>37-83 4.77350 GALANTAMINE ER 24 MG CAPSULE 0 SUN PHARMA GLOB EAGEN 00115-1120-<strong>08</strong> 3.13700 GALANTAMINE ER 8 MG CAPSULE 0 GLOBAL PHARM EAGEN 00378-8105-93 3.13700 GALANTAMINE ER 8 MG CAPSULE 0 MYLAN EAGEN 00555-1020-01 3.13700 GALANTAMINE ER 8 MG CAPSULE 0 BARR EAGEN 00591-3496-30 3.13700 GALANTAMINE ER 8 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 10147-<strong>08</strong>91-03 3.13700 GALANTAMINE ER 8 MG CAPSULE 0 PATRIOT PHARMAC EAGEN 47335-<strong>08</strong>35-83 3.13700 GALANTAMINE ER 8 MG CAPSULE 0 SUN PHARMA GLOB EAGEN 00378-2723-91 1.87151 GALANTAMINE HBR 12 MG TABLET 0 MYLAN EAGEN 00555-0140-09 1.87151 GALANTAMINE HBR 12 MG TABLET 0 BARR EAGEN 10147-<strong>08</strong>83-<strong>06</strong> 1.87151 GALANTAMINE HBR 12 MG TABLET 0 PATRIOT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-<strong>08</strong>54-01 1.87151 GALANTAMINE HBR 12 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-<strong>08</strong>54-03 1.87151 GALANTAMINE HBR 12 MG TABLET 0 MYLAN INSTITUTI EAGEN 59762-0010-01 1.87151 GALANTAMINE HBR 12 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-2544-<strong>06</strong> 1.87151 GALANTAMINE HBR 12 MG TABLET 0 APOTEX CORP EAGEN 65862-0460-60 1.87151 GALANTAMINE HBR 12 MG TABLET 0 AUROBINDO PHARM EAGEN 68382-0179-14 1.87151 GALANTAMINE HBR 12 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00378-2721-91 1.87407 GALANTAMINE HBR 4 MG TABLET 0 MYLAN EAGEN 00555-0138-09 1.87407 GALANTAMINE HBR 4 MG TABLET 0 BARR EAGEN 10147-<strong>08</strong>81-<strong>06</strong> 1.87407 GALANTAMINE HBR 4 MG TABLET 0 PATRIOT PHARMAC EAGEN 51079-<strong>08</strong>52-01 1.87407 GALANTAMINE HBR 4 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-<strong>08</strong>52-03 1.87407 GALANTAMINE HBR 4 MG TABLET 0 MYLAN INSTITUTI EAGEN 59762-00<strong>08</strong>-01 1.87407 GALANTAMINE HBR 4 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-2542-<strong>06</strong> 1.87407 GALANTAMINE HBR 4 MG TABLET 0 APOTEX CORP EAGEN 65862-0458-60 1.87407 GALANTAMINE HBR 4 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0492-11 1.87407 GALANTAMINE HBR 4 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0492-21 1.87407 GALANTAMINE HBR 4 MG TABLET 0 AHP EAGEN 68382-0177-14 1.87407 GALANTAMINE HBR 4 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00378-2722-91 1.86638 GALANTAMINE HBR 8 MG TABLET 0 MYLAN EAGEN 00555-0139-09 1.86638 GALANTAMINE HBR 8 MG TABLET 0 BARR EAGEN 10147-<strong>08</strong>82-<strong>06</strong> 1.86638 GALANTAMINE HBR 8 MG TABLET 0 PATRIOT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-<strong>08</strong>53-01 1.86638 GALANTAMINE HBR 8 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-<strong>08</strong>53-03 1.86638 GALANTAMINE HBR 8 MG TABLET 0 MYLAN INSTITUTI EAGEN 59762-0009-01 1.86638 GALANTAMINE HBR 8 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-2543-<strong>06</strong> 1.86638 GALANTAMINE HBR 8 MG TABLET 0 APOTEX CORP EAGEN 65862-0459-60 1.86638 GALANTAMINE HBR 8 MG TABLET 0 AUROBINDO PHARM EAGEN 68382-0178-14 1.86638 GALANTAMINE HBR 8 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00054-0137-49 2.20072 GALANTAMINE 4 MG/ML ORAL SOLN 0 ROXANE LABS. MLBND 57844-0215-52 1.24154 GALZIN 25 MG CAPSULE 0 GATE PHARM EABND 57844-02<strong>08</strong>-52 2.<strong>06</strong>922 GALZIN 50 MG CAPSULE 0 GATE PHARM EABND 13533-<strong>06</strong>35-12 28.36774 GAMASTAN S-D VIAL 0 GRIFOLS THERAPE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00944-2700-02 12.39107 GAMMAGARD LIQUID 10% VIAL 0 BAXTER BIOSCIEN MLBND 00944-2700-03 12.39156 GAMMAGARD LIQUID 10% VIAL 0 BAXTER BIOSCIEN MLBND 00944-2700-04 12.39123 GAMMAGARD LIQUID 10% VIAL 0 BAXTER BIOSCIEN MLBND 00944-2700-05 12.39123 GAMMAGARD LIQUID 10% VIAL 0 BAXTER BIOSCIEN MLBND 00944-2700-<strong>06</strong> 12.39123 GAMMAGARD LIQUID 10% VIAL 0 BAXTER BIOSCIEN ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 163LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00944-2700-07 12.39123 GAMMAGARD LIQUID 10% VIAL 0 BAXTER BIOSCIEN MLBND 00944-2655-04 1411.92960 GAMMAGARD S-D 10 G (IGA
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 164LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-0185-01 8.04000 GEMCITABINE HCL 200 MG VIAL 0 HOSPIRA EAGEN 00703-5775-01 8.37000 GEMCITABINE HCL 200 MG VIAL 0 TEVA PARENTERAL EAGEN 00781-3282-75 8.90100 GEMCITABINE HCL 200 MG VIAL 0 SANDOZ EAGEN 16729-0092-03 8.90100 GEMCITABINE HCL 200 MG VIAL 0 ACCORD <strong>HEALTH</strong>CA EAGEN 25021-02<strong>08</strong>-10 8.90100 GEMCITABINE HCL 200 MG VIAL 0 SAGENT PHARMACE EAGEN 47335-0153-40 8.90100 GEMCITABINE HCL 200 MG VIAL 0 SUN PHARMA GLOB EAGEN 55111-<strong>06</strong>86-07 8.90100 GEMCITABINE HCL 200 MG VIAL 0 DR.REDDY'S LAB EAGEN 55390-0391-10 8.90100 GEMCITABINE HCL 200 MG VIAL 0 BEDFORD LABS EAGEN 63323-0102-13 8.90100 GEMCITABINE HCL 200 MG VIAL 0 APP PHARMACEUTI EAGUL 00093-<strong>06</strong>70-05 0.13500 GEMFIBROZIL 600 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00093-<strong>06</strong>70-<strong>06</strong> 0.13500 GEMFIBROZIL 600 MG TABLET 0 TEVA USA EAGUL 00115-9911-13 0.13500 GEMFIBROZIL 600 MG TABLET 0 GLOBAL PHARM EAGUL 00143-9130-05 0.13500 GEMFIBROZIL 600 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-9130-60 0.13500 GEMFIBROZIL 600 MG TABLET 0 WEST-WARD,INC. EAGUL 00904-5379-40 0.13500 GEMFIBROZIL 600 MG TABLET 0 MAJOR PHARMACEU EAGUL 00904-5379-52 0.13500 GEMFIBROZIL 600 MG TABLET 0 MAJOR PHARMACEU EAGUL 16714-0101-02 0.13500 GEMFIBROZIL 600 MG TABLET 0 NORTHSTAR RX LL EAGUL 16714-0101-05 0.13500 GEMFIBROZIL 600 MG TABLET 0 NORTHSTAR RX LL EAGUL 24658-0260-90 0.13500 GEMFIBROZIL 600 MG TABLET 0 BLU PHARMACEUTI EAGUL 31722-0225-01 0.13500 GEMFIBROZIL 600 MG TABLET 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 31722-0225-05 0.13500 GEMFIBROZIL 600 MG TABLET 0 CAMBER PHARMACE EAGUL 31722-0225-60 0.13500 GEMFIBROZIL 600 MG TABLET 0 CAMBER PHARMACE EAGUL 60505-0034-00 0.13500 GEMFIBROZIL 600 MG TABLET 0 APOTEX CORP EAGUL 60505-0034-04 0.13500 GEMFIBROZIL 600 MG TABLET 0 APOTEX CORP EAGUL 60505-0034-<strong>06</strong> 0.13500 GEMFIBROZIL 600 MG TABLET 0 APOTEX CORP EAGUL 60505-0034-<strong>08</strong> 0.13500 GEMFIBROZIL 600 MG TABLET 0 APOTEX CORP EAGUL 68<strong>08</strong>4-0473-01 0.13500 GEMFIBROZIL 600 MG TABLET 0 AHP EAGUL 68<strong>08</strong>4-0473-11 0.13500 GEMFIBROZIL 600 MG TABLET 0 AHP EABND 00002-7502-01 39.63675 737.98620 GEMZAR 1 GRAM VIAL 0 ELI LILLY & CO. EABND 00002-7501-01 8.90100 147.59890 GEMZAR 200 MG VIAL 0 ELI LILLY & CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 52544-0204-31 3.19717 GENERESS FE CHEWABLE TABLET 0 ACTAVIS PHARMA, EAGEN 60432-0038-16 0.01500 GENERLAC 10 GM/15 ML SOLUTION 0 MORTON GROVE PH MLGEN 60432-0038-64 0.01500 GENERLAC 10 GM/15 ML SOLUTION 0 MORTON GROVE PH MLGEN 00074-6479-32 4.3<strong>08</strong>60 GENGRAF 100 MG CAPSULE 0 ABBVIE US LLC EAGEN 00074-7269-50 4.38400 GENGRAF 100 MG/ML SOLUTION 0 ABBVIE US LLC MLGEN 00074-6463-32 1.07750 GENGRAF 25 MG CAPSULE 0 ABBVIE US LLC EABND 00013-2649-02 19.116<strong>08</strong> GENOTROPIN MINIQUICK 0.2 MG G PHARMACIA/UPJHN EABND 00013-2650-02 38.23453 GENOTROPIN MINIQUICK 0.4 MG G PHARMACIA/UPJHN EABND 00013-2651-02 57.35<strong>06</strong>2 GENOTROPIN MINIQUICK 0.6 MG G PHARMACIA/UPJHN EABND 00013-2652-02 76.46671 GENOTROPIN MINIQUICK 0.8 MG G PHARMACIA/UPJHN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00013-2653-02 95.58753 GENOTROPIN MINIQUICK 1 MG G PHARMACIA/UPJHN EABND 00013-2654-02 114.70362 GENOTROPIN MINIQUICK 1.2 MG G PHARMACIA/UPJHN EABND 00013-2655-02 133.81852 GENOTROPIN MINIQUICK 1.4 MG G PHARMACIA/UPJHN EABND 00013-2656-02 152.93342 GENOTROPIN MINIQUICK 1.6 MG G PHARMACIA/UPJHN EABND 00013-2657-02 172.05426 GENOTROPIN MINIQUICK 1.8 MG G PHARMACIA/UPJHN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 165LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00013-2658-02 191.16916 GENOTROPIN MINIQUICK 2 MG G PHARMACIA/UPJHN EABND 00013-2646-81 1052.35700 GENOTROPIN 12 MG CARTRIDGE G PHARMACIA/UPJHN EABND 00013-2626-81 438.47240 GENOTROPIN 5 MG CARTRIDGE G PHARMACIA/UPJHN EAGEN 17478-0284-35 4.21500 GENTAK 3 MG/GM EYE OINTMENT 0 AKORN INC. GMGUL 17478-0283-10 0.57000 GENTAK 3 MG/ML EYE DROPS 0 AKORN INC. MLBND 63323-0173-02 1.96212 GENTAMICIN PED 20 MG/2 ML VIAL 0 APP PHARMACEUTI MLBND 63323-0173-95 0.85656 GENTAMICIN PED 20 MG/2 ML VIAL 0 APP/NOVAPLUS MLBND 45802-0056-11 0.<strong>08</strong>730 1.82157 GENTAMICIN 0.1% CREAM 0 PERRIGO CO. GMBND 45802-0056-35 0.<strong>08</strong>730 1.82157 GENTAMICIN 0.1% CREAM 0 PERRIGO CO. GMBND 45802-0046-11 0.<strong>08</strong>640 1.82157 GENTAMICIN 0.1% OINTMENT 0 PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 45802-0046-35 0.<strong>08</strong>640 1.82157 GENTAMICIN 0.1% OINTMENT 0 PERRIGO CO. GMBND 00409-3401-01 0.18550 GENTAMICIN 10 MG/ML VIAL 0 HOSPIRA MLBND 00409-3402-01 0.14840 GENTAMICIN 10 MG/ML VIAL 0 HOSPIRA MLBND 63323-0513-02 2.07500 GENTAMICIN 20 MG/2 ML VIAL 0 APP PHARMACEUTI MLGEN 00574-4102-35 4.28142 GENTAMICIN 3 MG/GM EYE OINT 0 PERRIGO CO. GMGEN 48102-0102-35 4.28142 GENTAMICIN 3 MG/GM EYE OINT 0 PERRIGO CO. GMGUL 242<strong>08</strong>-0580-60 0.57000 GENTAMICIN 3 MG/ML EYE DROPS 0 VALEANT MLGUL 242<strong>08</strong>-0580-64 0.57000 GENTAMICIN 3 MG/ML EYE DROPS 0 VALEANT MLGUL 60758-0188-05 0.57000 GENTAMICIN 3 MG/ML EYE DROPS 0 PACIFIC PHARMA MLGUL 61314-<strong>06</strong>33-05 0.57000 GENTAMICIN 3 MG/ML EYE DROPS 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0010-20 0.72900 GENTAMICIN 40 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0010-95 0.44100 GENTAMICIN 40 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 00409-7879-13 0.<strong>08</strong>622 GENTAMICIN 60 MG/NS 50 ML PB 0 HOSPIRA MLGEN 00409-7883-13 0.<strong>06</strong>570 GENTAMICIN 80 MG/NS 50 ML PB 0 HOSPIRA MLGEN 00409-1207-03 0.47250 GENTAMICIN 80 MG/2 ML VIAL 0 HOSPIRA MLGEN 63323-0010-02 1.16100 GENTAMICIN 80 MG/2 ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0010-94 0.37800 GENTAMICIN 80 MG/2 ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0010-96 0.46170 GENTAMICIN 800 MG/20 ML VIAL 0 APP/NOVAPLUS MLBEX 00049-3960-60 3.37750 10.70519 GEODON 20 MG CAPSULE G PFIZER US PHARM EABEX 00049-3920-83 21.95516 GEODON 20 MG/ML VIAL G PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00049-3970-60 2.39850 10.70519 GEODON 40 MG CAPSULE G PFIZER US PHARM EABEX 00049-3980-60 3.85300 12.99130 GEODON 60 MG CAPSULE G PFIZER US PHARM EABEX 00049-3990-60 4.26398 12.99130 GEODON 80 MG CAPSULE G PFIZER US PHARM EABEX 00093-5423-28 2.04356 2.09911 GIANVI 3 MG-0.02 MG TABLET 0 TEVA USA EABEX 00093-5423-58 2.04356 2.09911 GIANVI 3 MG-0.02 MG TABLET 0 TEVA USA EABND 65649-0102-02 4.15000 GIAZO 1.1 GM TABLET G SALIX PHARMACEU EAGEX 0<strong>06</strong>03-3590-17 1.12980 GILDAGIA 0.4 MG-0.035 MG TAB 0 QUALITEST EAGEX 0<strong>06</strong>03-76<strong>08</strong>-17 0.72384 GILDESS FE 1.5-30 TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-7609-17 0.71880 GILDESS FE 1-20 TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-7607-15 1.00280 GILDESS 1 MG-20 MCG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-7607-48 1.00280 GILDESS 1 MG-20 MCG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-76<strong>06</strong>-15 1.00290 GILDESS 1.5 MG-30 MCG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-76<strong>06</strong>-48 1.00290 GILDESS 1.5 MG-30 MCG TABLET 0 QUALITEST EABND 00078-<strong>06</strong>07-51 173.12436 GILENYA 0.5 MG CAPSULE G NOVARTIS EABND 00597-0141-30 199.00<strong>08</strong>0 GILOTRIF 20 MG TABLET 0 BOEHRINGER ING. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 166LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00597-0137-30 199.00<strong>08</strong>0 GILOTRIF 30 MG TABLET 0 BOEHRINGER ING. EABND 00597-0138-30 199.00<strong>08</strong>0 GILOTRIF 40 MG TABLET 0 BOEHRINGER ING. EABND 00944-2884-01 0.48140 GLASSIA 1 GM/50 ML VIAL 0 BAXTER BIOSCIEN EABND 00078-0401-34 70.58301 GLEEVEC 100 MG TABLET 0 NOVARTIS EABND 00078-0438-15 254.33717 GLEEVEC 400 MG TABLET 0 NOVARTIS EAGEN 00093-7254-01 0.02930 GLIMEPIRIDE 1 MG TABLET G TEVA USA EAGEN 00378-4011-01 0.02930 GLIMEPIRIDE 1 MG TABLET G MYLAN EAGEN 0<strong>06</strong>03-3744-21 0.02930 GLIMEPIRIDE 1 MG TABLET G QUALITEST EAGEN 0<strong>06</strong>03-3744-28 0.02930 GLIMEPIRIDE 1 MG TABLET G QUALITEST EAGEN 16729-0001-01 0.02930 GLIMEPIRIDE 1 MG TABLET G ACCORD <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42571-0100-05 0.02930 GLIMEPIRIDE 1 MG TABLET G MICRO LABS USA, EAGEN 45802-0770-78 0.02930 GLIMEPIRIDE 1 MG TABLET G PERRIGO CO. EAGEN 54458-0968-10 0.02930 GLIMEPIRIDE 1 MG TABLET G INTERNATIONAL L EAGEN 55111-0320-01 0.02930 GLIMEPIRIDE 1 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0320-05 0.02930 GLIMEPIRIDE 1 MG TABLET G DR.REDDY'S LAB EAGEN 76439-0123-10 0.02930 GLIMEPIRIDE 1 MG TABLET G VIRTUS PHARMACE EAGEN 00093-7255-01 0.04347 GLIMEPIRIDE 2 MG TABLET G TEVA USA EAGEN 00378-4012-01 0.04347 GLIMEPIRIDE 2 MG TABLET G MYLAN EAGEN 0<strong>06</strong>03-3745-21 0.04347 GLIMEPIRIDE 2 MG TABLET G QUALITEST EAGEN 0<strong>06</strong>03-3745-28 0.04347 GLIMEPIRIDE 2 MG TABLET G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16729-0002-01 0.04347 GLIMEPIRIDE 2 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 42571-0101-05 0.04347 GLIMEPIRIDE 2 MG TABLET G MICRO LABS USA, EAGEN 45802-<strong>08</strong>22-78 0.04347 GLIMEPIRIDE 2 MG TABLET G PERRIGO CO. EAGEN 51079-0425-01 0.04347 GLIMEPIRIDE 2 MG TABLET G MYLAN INSTITUTI EAGEN 51079-0425-20 0.04347 GLIMEPIRIDE 2 MG TABLET G MYLAN INSTITUTI EAGEN 54458-0967-10 0.04347 GLIMEPIRIDE 2 MG TABLET G INTERNATIONAL L EAGEN 55111-0321-01 0.04347 GLIMEPIRIDE 2 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0321-05 0.04347 GLIMEPIRIDE 2 MG TABLET G DR.REDDY'S LAB EAGEN 66993-0163-02 0.04347 GLIMEPIRIDE 2 MG TABLET G PRASCO LABS EAGEN 68<strong>08</strong>4-0326-01 0.04347 GLIMEPIRIDE 2 MG TABLET G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0326-11 0.04347 GLIMEPIRIDE 2 MG TABLET G AHP EAGEN 76439-0124-10 0.04347 GLIMEPIRIDE 2 MG TABLET G VIRTUS PHARMACE EAGEN 00093-7256-01 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G TEVA USA EAGEN 00093-7256-52 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G TEVA USA EAGEN 00378-4013-01 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G MYLAN EAGEN 0<strong>06</strong>03-3746-21 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G QUALITEST EAGEN 0<strong>06</strong>03-3746-28 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G QUALITEST EAGEN 16729-0003-01 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 42571-0103-05 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G MICRO LABS USA, EAGEN 45802-0947-78 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G PERRIGO CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0426-01 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G MYLAN INSTITUTI EAGEN 51079-0426-20 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G MYLAN INSTITUTI EAGEN 54458-0966-10 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G INTERNATIONAL L EAGEN 55111-0322-01 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0322-05 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G DR.REDDY'S LAB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 167LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61442-0117-01 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G CARLSBAD TECH EAGEN 66993-0164-02 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G PRASCO LABS EAGEN 68<strong>08</strong>4-0327-01 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0327-11 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G AHP EAGEN 76439-0125-10 0.<strong>06</strong>910 GLIMEPIRIDE 4 MG TABLET G VIRTUS PHARMACE EAGEN 00591-<strong>08</strong>45-01 0.36194 GLIPIZIDE ER 10 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-<strong>08</strong>45-10 0.36194 GLIPIZIDE ER 10 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-<strong>08</strong>45-15 0.36194 GLIPIZIDE ER 10 MG TABLET G ACTAVIS PHARMA, EAGEN 49884-0746-01 0.36194 GLIPIZIDE ER 10 MG TABLET G PAR PHARM. EAGEN 49884-0746-05 0.36194 GLIPIZIDE ER 10 MG TABLET G PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0112-01 0.36194 GLIPIZIDE ER 10 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0112-11 0.36194 GLIPIZIDE ER 10 MG TABLET G AHP EAGEN 00591-0900-30 0.28350 GLIPIZIDE ER 2.5 MG TABLET G ACTAVIS PHARMA, EAGEN 68<strong>08</strong>4-0295-11 0.28350 GLIPIZIDE ER 2.5 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0295-21 0.28350 GLIPIZIDE ER 2.5 MG TABLET G AHP EAGEN 00591-<strong>08</strong>44-01 0.18720 GLIPIZIDE ER 5 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-<strong>08</strong>44-10 0.18720 GLIPIZIDE ER 5 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-<strong>08</strong>44-15 0.18720 GLIPIZIDE ER 5 MG TABLET G ACTAVIS PHARMA, EAGEN 49884-0745-01 0.18720 GLIPIZIDE ER 5 MG TABLET G PAR PHARM. EAGEN 49884-0745-05 0.18720 GLIPIZIDE ER 5 MG TABLET G PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0111-01 0.18720 GLIPIZIDE ER 5 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0111-11 0.18720 GLIPIZIDE ER 5 MG TABLET G AHP EAGEN 59762-5033-01 0.36194 GLIPIZIDE XL 10 MG TABLET G GREENSTONE LLC. EAGEN 59762-5033-02 0.36194 GLIPIZIDE XL 10 MG TABLET G GREENSTONE LLC. EAGEN 59762-5031-01 0.28350 GLIPIZIDE XL 2.5 MG TABLET G GREENSTONE LLC. EAGEN 59762-0541-01 0.18720 GLIPIZIDE XL 5 MG TABLET G GREENSTONE LLC. EAGEN 59762-0541-02 0.18720 GLIPIZIDE XL 5 MG TABLET G GREENSTONE LLC. EAGEN 59762-5032-01 0.18720 GLIPIZIDE XL 5 MG TABLET G GREENSTONE LLC. EAGEN 59762-5032-02 0.18720 GLIPIZIDE XL 5 MG TABLET G GREENSTONE LLC. EAGEN 00172-3650-60 0.05265 GLIPIZIDE 10 MG TABLET G IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00172-3650-70 0.05265 GLIPIZIDE 10 MG TABLET G IVAX PHARMACEUT EAGEN 00378-1110-01 0.05265 GLIPIZIDE 10 MG TABLET G MYLAN EAGEN 00378-1110-05 0.05265 GLIPIZIDE 10 MG TABLET G MYLAN EAGEN 00591-0461-01 0.05265 GLIPIZIDE 10 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-0461-05 0.05265 GLIPIZIDE 10 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-0461-10 0.05265 GLIPIZIDE 10 MG TABLET G ACTAVIS PHARMA, EAGEN 00781-1453-01 0.05265 GLIPIZIDE 10 MG TABLET G SANDOZ EAGEN 00781-1453-10 0.05265 GLIPIZIDE 10 MG TABLET G SANDOZ EAGEN 00904-6123-61 0.05265 GLIPIZIDE 10 MG TABLET G MAJOR PHARMACEU EAGEN 16729-0140-00 0.05265 GLIPIZIDE 10 MG TABLET G ACCORD <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16729-0140-16 0.05265 GLIPIZIDE 10 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 60505-0142-00 0.05265 GLIPIZIDE 10 MG TABLET G APOTEX CORP EAGEN 60505-0142-01 0.05265 GLIPIZIDE 10 MG TABLET G APOTEX CORP EAGEN 60505-0142-02 0.05265 GLIPIZIDE 10 MG TABLET G APOTEX CORP EAGEN 60505-0142-04 0.05265 GLIPIZIDE 10 MG TABLET G APOTEX CORP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 168LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68645-0151-59 0.05265 GLIPIZIDE 10 MG TABLET G LEGACY PHARMACE EAGEN 00172-3649-60 0.01931 GLIPIZIDE 5 MG TABLET G IVAX PHARMACEUT EAGEN 00378-1105-01 0.01931 GLIPIZIDE 5 MG TABLET G MYLAN EAGEN 00378-1105-05 0.01931 GLIPIZIDE 5 MG TABLET G MYLAN EAGEN 00591-0460-01 0.01931 GLIPIZIDE 5 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-0460-05 0.01931 GLIPIZIDE 5 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-0460-10 0.01931 GLIPIZIDE 5 MG TABLET G ACTAVIS PHARMA, EAGEN 00781-1452-01 0.01931 GLIPIZIDE 5 MG TABLET G SANDOZ EAGEN 00781-1452-10 0.01931 GLIPIZIDE 5 MG TABLET G SANDOZ EAGEN 00904-6124-61 0.01931 GLIPIZIDE 5 MG TABLET G MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16729-0139-00 0.01931 GLIPIZIDE 5 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0139-16 0.01931 GLIPIZIDE 5 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 60505-0141-00 0.01931 GLIPIZIDE 5 MG TABLET G APOTEX CORP EAGEN 60505-0141-01 0.01931 GLIPIZIDE 5 MG TABLET G APOTEX CORP EAGEN 60505-0141-02 0.01931 GLIPIZIDE 5 MG TABLET G APOTEX CORP EAGEN 60505-0141-<strong>08</strong> 0.01931 GLIPIZIDE 5 MG TABLET G APOTEX CORP EAGEN 68645-0150-54 0.01931 GLIPIZIDE 5 MG TABLET G LEGACY PHARMACE EAGEN 00093-7455-01 0.41567 GLIPIZIDE-METFORMIN 2.5-250 MG 0 TEVA USA EAGEN 00378-3131-01 0.41567 GLIPIZIDE-METFORMIN 2.5-250 MG 0 MYLAN EAGEN 00591-3971-01 0.41567 GLIPIZIDE-METFORMIN 2.5-250 MG 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 23155-0115-01 0.41567 GLIPIZIDE-METFORMIN 2.5-250 MG 0 HERITAGE PHARMA EAGEN 00093-7456-01 0.41570 GLIPIZIDE-METFORMIN 2.5-500 MG 0 TEVA USA EAGEN 00378-3132-01 0.41570 GLIPIZIDE-METFORMIN 2.5-500 MG 0 MYLAN EAGEN 00591-3972-01 0.41570 GLIPIZIDE-METFORMIN 2.5-500 MG 0 ACTAVIS PHARMA, EAGEN 00093-7457-01 0.41690 GLIPIZIDE-METFORMIN 5-500 MG 0 TEVA USA EAGEN 00378-3133-01 0.41690 GLIPIZIDE-METFORMIN 5-500 MG 0 MYLAN EAGEN 00591-3973-01 0.41690 GLIPIZIDE-METFORMIN 5-500 MG 0 ACTAVIS PHARMA, EAGEN 23155-0117-01 0.41690 GLIPIZIDE-METFORMIN 5-500 MG 0 HERITAGE PHARMA EABND 00169-7<strong>06</strong>5-15 164.34000 GLUCAGEN 1 MG HYPOKIT 0 NOVO NORDISK EABND 00169-7<strong>06</strong>5-21 164.34000 GLUCAGEN 1 MG HYPOKIT 2-PACK 0 NOVO NORDISK EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 55390-0004-01 118.52400 GLUCAGEN 1 MG VIAL 0 BEDFORD LABS EABND 55390-0004-10 118.52400 GLUCAGEN 1 MG VIAL 0 BEDFORD LABS EABND 00002-8031-01 164.34000 GLUCAGON 1 MG EMERGENCY KIT 0 ELI LILLY & CO. EAGEN 49452-3285-01 0.59587 GLUCONOLACTONE POWDER 0 SPECTRUM GMBND 00<strong>08</strong>7-6<strong>06</strong>3-13 0.04185 0.94686 GLUCOPHAGE XR 500 MG TAB G BMS PRIMARYCARE EABND 00<strong>08</strong>7-6<strong>06</strong>4-13 0.20480 1.42046 GLUCOPHAGE XR 750 MG TAB G BMS PRIMARYCARE EABND 00<strong>08</strong>7-6071-11 0.04104 1.91090 GLUCOPHAGE 1,000 MG TABLET G BMS PRIMARYCARE EABND 00<strong>08</strong>7-6<strong>06</strong>0-05 0.02633 0.92769 GLUCOPHAGE 500 MG TABLET G BMS PRIMARYCARE EABND 00<strong>08</strong>7-6<strong>06</strong>0-10 0.02633 0.92760 GLUCOPHAGE 500 MG TABLET G BMS PRIMARYCARE EABND 00<strong>08</strong>7-6070-05 0.04077 1.577<strong>08</strong> GLUCOPHAGE 850 MG TABLET G BMS PRIMARYCARE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00049-0178-07 0.36194 1.61791 GLUCOTROL XL 10 MG TABLET G PFIZER US PHARM EABND 00049-1560-66 0.36194 1.41315 GLUCOTROL XL 10 MG TABLET G PFIZER US PHARM EABND 00049-1560-73 0.36194 1.34591 GLUCOTROL XL 10 MG TABLET G PFIZER US PHARM EABND 00049-0170-01 0.28350 0.83553 GLUCOTROL XL 2.5 MG TABLET G PFIZER US PHARM EABND 00049-0174-02 0.18720 0.83572 GLUCOTROL XL 5 MG TABLET G PFIZER US PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 169LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00049-1550-66 0.18720 0.78103 GLUCOTROL XL 5 MG TABLET G PFIZER US PHARM EABND 00049-1550-73 0.18720 0.83546 GLUCOTROL XL 5 MG TABLET G PFIZER US PHARM EABND 00049-4120-66 0.05265 1.56704 GLUCOTROL 10 MG TABLET G PFIZER US PHARM EABND 00049-4110-66 0.01931 0.83805 GLUCOTROL 5 MG TABLET G PFIZER US PHARM EABND 00<strong>08</strong>7-6073-11 0.<strong>08</strong>978 1.22001 GLUCOVANCE 2.5-500 MG TABLET 0 BMS PRIMARYCARE EABND 00<strong>08</strong>7-6074-11 0.<strong>08</strong>860 1.22001 GLUCOVANCE 5-500 MG TABLET 0 BMS PRIMARYCARE EABND 13913-0002-13 3.53580 GLUMETZA ER 500 MG TABLET G SANTARUS INC. EAGEN 00093-5710-01 0.09220 GLYBURID-METFORMIN 1.25-250 MG 0 TEVA USA EAGEN 00093-5710-05 0.09220 GLYBURID-METFORMIN 1.25-250 MG 0 TEVA USA EAGEN 00228-2751-11 0.09220 GLYBURID-METFORMIN 1.25-250 MG 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-2751-50 0.09220 GLYBURID-METFORMIN 1.25-250 MG 0 ACTAVIS PHARMA, EAGEN 23155-0233-01 0.09220 GLYBURID-METFORMIN 1.25-250 MG 0 HERITAGE PHARMA EAGEN 23155-0233-05 0.09220 GLYBURID-METFORMIN 1.25-250 MG 0 HERITAGE PHARMA EAGEN 55111-<strong>06</strong>95-01 0.09220 GLYBURID-METFORMIN 1.25-250 MG 0 DR.REDDY'S LAB EAGEN 65862-0<strong>08</strong>0-01 0.09220 GLYBURID-METFORMIN 1.25-250 MG 0 AUROBINDO PHARM EAGEN 65862-0<strong>08</strong>0-05 0.09220 GLYBURID-METFORMIN 1.25-250 MG 0 AUROBINDO PHARM EAGEN 00093-8034-01 0.03010 GLYBURIDE MICRO 1.5 MG TAB G TEVA USA EAGEN 00143-9918-01 0.03010 GLYBURIDE MICRO 1.5 MG TAB G WEST-WARD,INC. EAGEN 00378-1113-01 0.03010 GLYBURIDE MICRO 1.5 MG TAB G MYLAN EAGEN 67253-0460-10 0.03010 GLYBURIDE MICRO 1.5 MG TAB G DAVA PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-8035-01 0.03092 GLYBURIDE MICRO 3 MG TABLET G TEVA USA EAGEN 00093-8035-05 0.03092 GLYBURIDE MICRO 3 MG TABLET G TEVA USA EAGEN 00143-9919-01 0.03092 GLYBURIDE MICRO 3 MG TABLET G WEST-WARD,INC. EAGEN 00143-9919-05 0.03092 GLYBURIDE MICRO 3 MG TABLET G WEST-WARD,INC. EAGEN 00378-1125-01 0.03092 GLYBURIDE MICRO 3 MG TABLET G MYLAN EAGEN 67253-0461-10 0.03092 GLYBURIDE MICRO 3 MG TABLET G DAVA PHARMACEUT EAGEN 67253-0461-11 0.03092 GLYBURIDE MICRO 3 MG TABLET G DAVA PHARMACEUT EAGEN 67253-0461-50 0.03092 GLYBURIDE MICRO 3 MG TABLET G DAVA PHARMACEUT EAGEN 00093-8036-01 0.<strong>06</strong>380 GLYBURIDE MICRO 6 MG TABLET G TEVA USA EAGEN 00143-9920-01 0.<strong>06</strong>380 GLYBURIDE MICRO 6 MG TABLET G WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-9920-05 0.<strong>06</strong>380 GLYBURIDE MICRO 6 MG TABLET G WEST-WARD,INC. EAGEN 00143-9920-10 0.<strong>06</strong>380 GLYBURIDE MICRO 6 MG TABLET G WEST-WARD,INC. EAGEN 00378-1142-01 0.<strong>06</strong>380 GLYBURIDE MICRO 6 MG TABLET G MYLAN EAGEN 67253-0462-10 0.<strong>06</strong>380 GLYBURIDE MICRO 6 MG TABLET G DAVA PHARMACEUT EAGEN 67253-0462-11 0.<strong>06</strong>380 GLYBURIDE MICRO 6 MG TABLET G DAVA PHARMACEUT EAGUL 00093-8342-01 0.12440 GLYBURIDE 1.25 MG TABLET G TEVA USA EAGEN 00093-9477-53 0.12440 GLYBURIDE 1.25 MG TABLET G TEVA USA EAGUL 23155-0056-01 0.12440 GLYBURIDE 1.25 MG TABLET G HERITAGE PHARMA EAGUL 59762-7020-09 0.12440 GLYBURIDE 1.25 MG TABLET G GREENSTONE LLC. EAGUL 64720-0123-10 0.12440 GLYBURIDE 1.25 MG TABLET G COREPHARMA LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 65862-0028-01 0.12440 GLYBURIDE 1.25 MG TABLET G AUROBINDO PHARM EAGEN 00093-8343-01 0.14877 GLYBURIDE 2.5 MG TABLET G TEVA USA EAGEN 00093-8343-05 0.14877 GLYBURIDE 2.5 MG TABLET G TEVA USA EAGEN 00093-8343-10 0.14877 GLYBURIDE 2.5 MG TABLET G TEVA USA EAGEN 00093-8343-98 0.14877 GLYBURIDE 2.5 MG TABLET G TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 170LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-9433-01 0.14877 GLYBURIDE 2.5 MG TABLET G TEVA USA EAGEN 00093-9433-05 0.14877 GLYBURIDE 2.5 MG TABLET G TEVA USA EAGEN 23155-0057-01 0.14877 GLYBURIDE 2.5 MG TABLET G HERITAGE PHARMA EAGEN 59762-7021-05 0.14877 GLYBURIDE 2.5 MG TABLET G GREENSTONE LLC. EAGEN 59762-7021-09 0.14877 GLYBURIDE 2.5 MG TABLET G GREENSTONE LLC. EAGEN 64720-0124-10 0.14877 GLYBURIDE 2.5 MG TABLET G COREPHARMA LLC EAGEN 65862-0029-01 0.14877 GLYBURIDE 2.5 MG TABLET G AUROBINDO PHARM EAGEN 65862-0029-05 0.14877 GLYBURIDE 2.5 MG TABLET G AUROBINDO PHARM EAGEN 68645-0210-54 0.14877 GLYBURIDE 2.5 MG TABLET G LEGACY PHARMACE EAGEN 00093-8344-01 0.22570 GLYBURIDE 5 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-8344-05 0.22570 GLYBURIDE 5 MG TABLET G TEVA USA EAGEN 00093-8344-10 0.22570 GLYBURIDE 5 MG TABLET G TEVA USA EAGEN 00093-8344-98 0.22570 GLYBURIDE 5 MG TABLET G TEVA USA EAGEN 00093-9364-01 0.22570 GLYBURIDE 5 MG TABLET G TEVA USA EAGEN 00093-9364-05 0.22570 GLYBURIDE 5 MG TABLET G TEVA USA EAGEN 00093-9364-10 0.22570 GLYBURIDE 5 MG TABLET G TEVA USA EAGEN 23155-0058-01 0.22570 GLYBURIDE 5 MG TABLET G HERITAGE PHARMA EAGEN 23155-0058-10 0.22570 GLYBURIDE 5 MG TABLET G HERITAGE PHARMA EAGEN 59762-7022-05 0.22570 GLYBURIDE 5 MG TABLET G GREENSTONE LLC. EAGEN 59762-7022-09 0.22570 GLYBURIDE 5 MG TABLET G GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63739-0119-10 0.22570 GLYBURIDE 5 MG TABLET G MCKESSON PACKAG EAGEN 64720-0125-10 0.22570 GLYBURIDE 5 MG TABLET G COREPHARMA LLC EAGEN 64720-0125-11 0.22570 GLYBURIDE 5 MG TABLET G COREPHARMA LLC EAGEN 65862-0030-01 0.22570 GLYBURIDE 5 MG TABLET G AUROBINDO PHARM EAGEN 65862-0030-99 0.22570 GLYBURIDE 5 MG TABLET G AUROBINDO PHARM EAGEN 68645-0211-54 0.22570 GLYBURIDE 5 MG TABLET G LEGACY PHARMACE EAGEN 00093-5711-01 0.<strong>08</strong>978 GLYBURIDE-METFORMIN 2.5-500 MG 0 TEVA USA EAGEN 00093-5711-05 0.<strong>08</strong>978 GLYBURIDE-METFORMIN 2.5-500 MG 0 TEVA USA EAGEN 00228-2752-11 0.<strong>08</strong>978 GLYBURIDE-METFORMIN 2.5-500 MG 0 ACTAVIS PHARMA, EAGEN 00228-2752-50 0.<strong>08</strong>978 GLYBURIDE-METFORMIN 2.5-500 MG 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 23155-0234-01 0.<strong>08</strong>978 GLYBURIDE-METFORMIN 2.5-500 MG 0 HERITAGE PHARMA EAGEN 23155-0234-05 0.<strong>08</strong>978 GLYBURIDE-METFORMIN 2.5-500 MG 0 HERITAGE PHARMA EAGEN 59762-2331-<strong>06</strong> 0.<strong>08</strong>978 GLYBURIDE-METFORMIN 2.5-500 MG 0 GREENSTONE LLC. EAGEN 59762-2331-<strong>08</strong> 0.<strong>08</strong>978 GLYBURIDE-METFORMIN 2.5-500 MG 0 GREENSTONE LLC. EAGEN 65862-0<strong>08</strong>1-01 0.<strong>08</strong>978 GLYBURIDE-METFORMIN 2.5-500 MG 0 AUROBINDO PHARM EAGEN 65862-0<strong>08</strong>1-05 0.<strong>08</strong>978 GLYBURIDE-METFORMIN 2.5-500 MG 0 AUROBINDO PHARM EAGEN 00093-5712-01 0.<strong>08</strong>860 GLYBURIDE-METFORMIN 5-500 MG 0 TEVA USA EAGEN 00093-5712-05 0.<strong>08</strong>860 GLYBURIDE-METFORMIN 5-500 MG 0 TEVA USA EAGEN 00228-2753-11 0.<strong>08</strong>860 GLYBURIDE-METFORMIN 5-500 MG 0 ACTAVIS PHARMA, EAGEN 00228-2753-50 0.<strong>08</strong>860 GLYBURIDE-METFORMIN 5-500 MG 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 23155-0235-01 0.<strong>08</strong>860 GLYBURIDE-METFORMIN 5-500 MG 0 HERITAGE PHARMA EAGEN 23155-0235-05 0.<strong>08</strong>860 GLYBURIDE-METFORMIN 5-500 MG 0 HERITAGE PHARMA EAGEN 59762-2332-<strong>06</strong> 0.<strong>08</strong>860 GLYBURIDE-METFORMIN 5-500 MG 0 GREENSTONE LLC. EAGEN 59762-2332-<strong>08</strong> 0.<strong>08</strong>860 GLYBURIDE-METFORMIN 5-500 MG 0 GREENSTONE LLC. EAGEN 65862-0<strong>08</strong>2-01 0.<strong>08</strong>860 GLYBURIDE-METFORMIN 5-500 MG 0 AUROBINDO PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 171LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0<strong>08</strong>2-05 0.<strong>08</strong>860 GLYBURIDE-METFORMIN 5-500 MG 0 AUROBINDO PHARM EAGEN 38779-2<strong>08</strong>7-03 374.<strong>06</strong>250 GLYCOPYRROLATE POWDER 0 MEDISCA INC. GMGEN 38779-2<strong>08</strong>7-<strong>06</strong> 374.<strong>06</strong>250 GLYCOPYRROLATE POWDER 0 MEDISCA INC. GMGEN 00143-9682-01 0.64000 GLYCOPYRROLATE 0.2 MG/ML VIAL 0 WEST-WARD,INC. MLGEN 00143-9682-25 0.64000 GLYCOPYRROLATE 0.2 MG/ML VIAL 0 WEST-WARD,INC. MLGEN 00517-4605-25 0.45180 GLYCOPYRROLATE 0.2 MG/ML VIAL 0 AMER. REGENT MLGEN 00517-4620-25 0.22455 GLYCOPYRROLATE 0.2 MG/ML VIAL 0 AMER. REGENT MLGEN 0<strong>06</strong>41-6033-01 0.64000 GLYCOPYRROLATE 0.2 MG/ML VIAL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6033-25 0.64000 GLYCOPYRROLATE 0.2 MG/ML VIAL 0 WEST-WARD,INC. MLGEN 10019-0016-02 0.54000 GLYCOPYRROLATE 0.2 MG/ML VIAL 0 WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10019-0016-29 0.54000 GLYCOPYRROLATE 0.2 MG/ML VIAL 0 WEST-WARD,INC. MLGEN 00143-9681-01 0.64000 GLYCOPYRROLATE 0.4 MG/2 ML VL 0 WEST-WARD,INC. MLGEN 00143-9681-25 0.64000 GLYCOPYRROLATE 0.4 MG/2 ML VL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6034-01 0.64000 GLYCOPYRROLATE 0.4 MG/2 ML VL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6034-25 0.64000 GLYCOPYRROLATE 0.4 MG/2 ML VL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>03-3180-21 0.86720 GLYCOPYRROLATE 1 MG TABLET 0 QUALITEST EAGEN 49884-0<strong>06</strong>5-01 0.86720 GLYCOPYRROLATE 1 MG TABLET 0 PAR PHARM. EAGEN 51079-0700-01 0.86720 GLYCOPYRROLATE 1 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0700-20 0.86720 GLYCOPYRROLATE 1 MG TABLET 0 MYLAN INSTITUTI EAGEN 55111-<strong>06</strong>48-01 0.86720 GLYCOPYRROLATE 1 MG TABLET 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-9680-01 0.64000 GLYCOPYRROLATE 1 MG/5 ML VIAL 0 WEST-WARD,INC. MLGEN 00143-9680-25 0.64000 GLYCOPYRROLATE 1 MG/5 ML VIAL 0 WEST-WARD,INC. MLGEN 00143-1251-01 1.52190 GLYCOPYRROLATE 2 MG TABLET 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>03-3181-21 1.52190 GLYCOPYRROLATE 2 MG TABLET 0 QUALITEST EAGEN 49884-0<strong>06</strong>6-01 1.52190 GLYCOPYRROLATE 2 MG TABLET 0 PAR PHARM. EAGEN 55111-<strong>06</strong>49-01 1.52190 GLYCOPYRROLATE 2 MG TABLET 0 DR.REDDY'S LAB EAGEN 00143-9679-01 0.64000 GLYCOPYRROLATE 4 MG/20 ML VIAL 0 WEST-WARD,INC. MLGEN 00143-9679-10 0.64000 GLYCOPYRROLATE 4 MG/20 ML VIAL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6036-01 0.54000 GLYCOPYRROLATE 4 MG/20 ML VIAL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6036-10 0.54000 GLYCOPYRROLATE 4 MG/20 ML VIAL 0 WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-0341-01 0.03010 0.9<strong>08</strong>93 GLYNASE 1.5 MG PRESTAB G PHARMACIA/UPJHN EABND 00009-0352-01 0.03092 1.53699 GLYNASE 3 MG PRESTAB G PHARMACIA/UPJHN EABND 00009-3449-01 0.<strong>06</strong>380 2.42360 GLYNASE 6 MG PRESTAB G PHARMACIA/UPJHN EABND 00009-3449-03 0.<strong>06</strong>380 2.42356 GLYNASE 6 MG PRESTAB G PHARMACIA/UPJHN EABND 00009-5014-01 1.811<strong>06</strong> GLYSET 100 MG TABLET G PHARMACIA/UPJHN EABND 00009-5012-01 1.39597 GLYSET 25 MG TABLET G PHARMACIA/UPJHN EABND 00009-5013-01 1.53500 GLYSET 50 MG TABLET G PHARMACIA/UPJHN EABND 52268-0700-01 12.63260 GOLYTELY PACKET G BRAINTREE LABS. EABND 52268-0100-01 0.00370 0.00477 GOLYTELY SOLUTION G BRAINTREE LABS. MLBND 52268-0101-01 0.00370 0.00509 GOLYTELY SOLUTION G BRAINTREE LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 13913-0004-13 2.73900 GRALISE ER 300 MG TABLET 0 DEPOMED, INC. EABND 13913-0004-19 3.37644 GRALISE ER 300 MG TABLET 0 DEPOMED, INC. EABND 13913-0005-19 3.37644 GRALISE ER 600 MG TABLET 0 DEPOMED, INC. EABND 13913-00<strong>06</strong>-16 3.37639 GRALISE 30-DAY STARTER PACK 0 DEPOMED, INC. EAGEN 25021-0778-01 2.88000 GRANISETRON HCL 0.1 MG/ML VIAL 0 SAGENT PHARMACE ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 172LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 25021-0778-66 2.16000 GRANISETRON HCL 0.1 MG/ML VIAL 0 SAGENT PHARMACE MLGEN 00054-0143-<strong>08</strong> 5.60000 GRANISETRON HCL 1 MG TABLET G ROXANE LABS. EAGEN 00054-0143-87 5.60000 GRANISETRON HCL 1 MG TABLET G ROXANE LABS. EAGEN 00093-7485-12 5.60000 GRANISETRON HCL 1 MG TABLET G TEVA USA EAGEN 00093-7485-19 5.60000 GRANISETRON HCL 1 MG TABLET G TEVA USA EAGEN 00093-7485-20 5.60000 GRANISETRON HCL 1 MG TABLET G TEVA USA EAGEN 16714-0221-01 5.25000 GRANISETRON HCL 1 MG TABLET G NORTHSTAR RX LL EAGEN 16714-0221-30 5.60000 GRANISETRON HCL 1 MG TABLET G NORTHSTAR RX LL EAGEN 16714-0221-32 5.60000 GRANISETRON HCL 1 MG TABLET G NORTHSTAR RX LL EAGEN 51672-4138-<strong>06</strong> 5.25000 GRANISETRON HCL 1 MG TABLET G TARO PHARM USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51991-0735-20 5.60000 GRANISETRON HCL 1 MG TABLET G BRECKENRIDGE EAGEN 51991-0735-32 5.60000 GRANISETRON HCL 1 MG TABLET G BRECKENRIDGE EAGEN 00143-9744-10 14.72750 GRANISETRON HCL 1 MG/ML VIAL 0 WEST-WARD,INC. MLGEN 00703-7971-03 14.72750 GRANISETRON HCL 1 MG/ML VIAL 0 TEVA PARENTERAL MLGEN 17478-0546-02 14.72750 GRANISETRON HCL 1 MG/ML VIAL 0 AKORN INC. MLGEN 25021-0779-01 9.00000 GRANISETRON HCL 1 MG/ML VIAL 0 SAGENT PHARMACE MLGEN 63323-0318-01 13.<strong>06</strong>800 GRANISETRON HCL 1 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 64679-<strong>06</strong>61-03 13.<strong>06</strong>800 GRANISETRON HCL 1 MG/ML VIAL 0 WOCKHARDT USA L MLGEN 66758-0035-01 14.72750 GRANISETRON HCL 1 MG/ML VIAL 0 SANDOZ INC. MLGEN 00143-9745-05 8.63390 GRANISETRON HCL 4 MG/4 ML VIAL 0 WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00703-7973-01 8.63390 GRANISETRON HCL 4 MG/4 ML VIAL 0 TEVA PARENTERAL MLGEN 17478-0546-05 8.63390 GRANISETRON HCL 4 MG/4 ML VIAL 0 AKORN INC. MLGEN 25021-0781-04 5.62500 GRANISETRON HCL 4 MG/4 ML VIAL 0 SAGENT PHARMACE MLGEN 63323-0319-04 8.63390 GRANISETRON HCL 4 MG/4 ML VIAL 0 APP PHARMACEUTI MLGEN 64679-<strong>06</strong>61-02 8.63390 GRANISETRON HCL 4 MG/4 ML VIAL 0 WOCKHARDT USA L MLGEN 66758-0036-01 8.63390 GRANISETRON HCL 4 MG/4 ML VIAL 0 SANDOZ INC. MLBND 63459-0910-11 476.<strong>08</strong>800 GRANIX 300 MCG/0.5 ML SYRINGE 0 CEPHALON,INC.-T MLBND 63459-0910-15 476.<strong>08</strong>800 GRANIX 300 MCG/0.5 ML SYRINGE 0 CEPHALON,INC.-T MLBND 63459-0912-11 476.21250 GRANIX 480 MCG/0.8 ML SYRINGE 0 CEPHALON,INC.-T MLBND 63459-0912-15 476.21250 GRANIX 480 MCG/0.8 ML SYRINGE 0 CEPHALON,INC.-T ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00<strong>06</strong>2-0214-60 5.25280 GRIFULVIN V 500 MG TABLET G VALEANT EABND 0<strong>08</strong>84-0763-04 3.91020 5.14815 GRIS-PEG 125 MG TABLET G VALEANT EABND 0<strong>08</strong>84-0773-04 4.99800 6.57102 GRIS-PEG 250 MG TABLET G VALEANT EAGEN 00781-5515-01 5.25280 GRISE<strong>OF</strong>ULVIN MICRO 500 MG TAB G SANDOZ EAGEN 64980-0186-01 5.25280 GRISE<strong>OF</strong>ULVIN MICRO 500 MG TAB G RISING PHARM EAGEN 64980-0184-01 3.91020 GRISE<strong>OF</strong>ULVIN ULTRA 125 MG TAB 0 RISING PHARM EAGEN 68682-0519-01 3.59092 GRISE<strong>OF</strong>ULVIN ULTRA 125 MG TAB 0 OCEANSIDE PHARM EAGEN 64980-0185-01 4.99800 GRISE<strong>OF</strong>ULVIN ULTRA 250 MG TAB 0 RISING PHARM EAGEN 68682-0520-01 4.58992 GRISE<strong>OF</strong>ULVIN ULTRA 250 MG TAB 0 OCEANSIDE PHARM EAGEN 00093-7102-12 0.12501 GRISE<strong>OF</strong>ULVIN 125 MG/5 ML SUSP 0 TEVA USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00472-0013-04 0.12501 GRISE<strong>OF</strong>ULVIN 125 MG/5 ML SUSP 0 ACTAVIS PHARMA, MLGEN 0<strong>06</strong>03-9171-54 0.12501 GRISE<strong>OF</strong>ULVIN 125 MG/5 ML SUSP 0 QUALITEST MLGEN 45802-0968-26 0.12501 GRISE<strong>OF</strong>ULVIN 125 MG/5 ML SUSP 0 PERRIGO CO. MLGEN 38779-<strong>06</strong>96-05 0.67687 GUAIFENESIN POWDER 0 MEDISCA INC. GMGEN 51927-1147-00 0.63000 GUAIFENESIN POWDER 0 PR<strong>OF</strong>ESSIONAL CO GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 173LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-1160-01 0.<strong>08</strong>180 GUANFACINE 1 MG TABLET 0 MYLAN EAGEN 00591-0444-01 0.<strong>08</strong>180 GUANFACINE 1 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00904-6183-60 0.<strong>08</strong>180 GUANFACINE 1 MG TABLET 0 MAJOR PHARMACEU EAGEN 65162-0711-10 0.<strong>08</strong>180 GUANFACINE 1 MG TABLET 0 AMNEAL PHARMACE EAGEN 00378-1190-01 0.11800 GUANFACINE 2 MG TABLET 0 MYLAN EAGEN 00591-0453-01 0.11800 GUANFACINE 2 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00904-6184-60 0.11800 GUANFACINE 2 MG TABLET 0 MAJOR PHARMACEU EAGEN 65162-0713-10 0.11800 GUANFACINE 2 MG TABLET 0 AMNEAL PHARMACE EABND 00<strong>08</strong>5-0492-01 0.21015 GUANIDINE HCL 125 MG TABLET 0 MERCK SHARP & D EABEX 50458-0254-14 136.88360 HALDOL DECANOATE 100 AMPUL 0 JANSSEN PHARM. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 50458-0253-03 71.8<strong>06</strong><strong>06</strong> HALDOL DECANOATE 50 AMPUL 0 JANSSEN PHARM. MLBEX 50458-0255-01 6.14000 17.01666 HALDOL 5 MG/ML AMPUL 0 JANSSEN PHARM. MLBND 52268-0523-02 65.29610 HALFLYTELY-BISACODYL BOWEL KIT G BRAINTREE LABS. EAGUL 00168-0355-50 0.48000 HALOBETASOL PROP 0.05% CREAM 0 SANDOZ GMGUL 00713-<strong>06</strong>40-15 0.48000 HALOBETASOL PROP 0.05% CREAM 0 G & W LABS. GMGUL 00713-<strong>06</strong>40-86 0.48000 HALOBETASOL PROP 0.05% CREAM 0 G & W LABS. GMGUL 45802-0129-32 0.48000 HALOBETASOL PROP 0.05% CREAM 0 PERRIGO CO. GMGUL 45802-0129-35 0.48000 HALOBETASOL PROP 0.05% CREAM 0 PERRIGO CO. GMGUL 00713-<strong>06</strong>39-15 0.53250 HALOBETASOL PROP 0.05% OINTMNT 0 G & W LABS. GMGUL 00713-<strong>06</strong>39-86 0.53250 HALOBETASOL PROP 0.05% OINTMNT 0 G & W LABS. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 45802-0131-32 0.53250 HALOBETASOL PROP 0.05% OINTMNT 0 PERRIGO CO. GMGUL 45802-0131-35 0.53250 HALOBETASOL PROP 0.05% OINTMNT 0 PERRIGO CO. GMBND 1<strong>06</strong>31-0094-20 4.79159 HALOG 0.1% CREAM G RANBAXY LABORAT GMBND 1<strong>06</strong>31-0094-76 3.11219 HALOG 0.1% CREAM G RANBAXY LABORAT GMBND 1<strong>06</strong>31-0096-20 4.79159 HALOG 0.1% OINTMENT G RANBAXY LABORAT GMBND 1<strong>06</strong>31-0096-30 4.07419 HALOG 0.1% OINTMENT G RANBAXY LABORAT GMGEX 10147-0922-05 44.41500 HALOPERIDOL DEC 100 MG/ML AMP 0 PATRIOT PHARMAC MLGEX 00703-7021-03 5.62500 HALOPERIDOL DEC 100 MG/ML VIAL 0 TEVA PARENTERAL MLGEX 00703-7023-01 3.56250 HALOPERIDOL DEC 100 MG/ML VIAL 0 TEVA PARENTERAL MLGEX 53150-0485-05 39.60000 HALOPERIDOL DEC 100 MG/ML VIAL 0 AMNEAL-AGILA, L ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 53150-0489-05 36.00000 HALOPERIDOL DEC 100 MG/ML VIAL 0 AMNEAL-AGILA, L MLGEX 60505-0703-01 37.<strong>08</strong>750 HALOPERIDOL DEC 100 MG/ML VIAL 0 APOTEX CORP MLGEX 63323-0471-01 35.64000 HALOPERIDOL DEC 100 MG/ML VIAL 0 APP PHARMACEUTI MLGEX 63323-0471-05 35.64000 HALOPERIDOL DEC 100 MG/ML VIAL 0 APP PHARMACEUTI MLGEX 00703-7011-03 5.71875 HALOPERIDOL DEC 50 MG/ML VIAL 0 TEVA PARENTERAL MLGEX 00703-7013-01 4.78200 HALOPERIDOL DEC 50 MG/ML VIAL 0 TEVA PARENTERAL MLGEX 53150-0415-10 19.80000 HALOPERIDOL DEC 50 MG/ML VIAL 0 AMNEAL-AGILA, L MLGEX 53150-0422-05 19.80000 HALOPERIDOL DEC 50 MG/ML VIAL 0 AMNEAL-AGILA, L MLGEX 60505-0702-01 21.00000 HALOPERIDOL DEC 50 MG/ML VIAL 0 APOTEX CORP MLGEX 60505-6020-02 21.00000 HALOPERIDOL DEC 50 MG/ML VIAL 0 APOTEX/NOVAPLUS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 63323-0469-01 19.44000 HALOPERIDOL DEC 50 MG/ML VIAL 0 APP PHARMACEUTI MLGEX 63323-0469-05 19.44000 HALOPERIDOL DEC 50 MG/ML VIAL 0 APP PHARMACEUTI MLGEX 10147-0921-03 23.30000 HALOPERIDOL DECAN 50 MG/ML AMP 0 PATRIOT PHARMAC MLGEX 00093-9604-12 0.05360 HALOPERIDOL LAC 2 MG/ML CONC 0 TEVA USA MLGEX 00093-9604-23 0.05360 HALOPERIDOL LAC 2 MG/ML CONC 0 TEVA USA ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 174LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00121-0581-04 0.05360 HALOPERIDOL LAC 2 MG/ML CONC 0 PHARMACEU ASSOC MLGEX 00121-0581-05 0.05360 HALOPERIDOL LAC 2 MG/ML CONC 0 PHARMACEU ASSOC MLGEX 54838-0501-15 0.05360 HALOPERIDOL LAC 2 MG/ML CONC 0 SILARX PHARM MLGEX 54838-0501-40 0.05360 HALOPERIDOL LAC 2 MG/ML CONC 0 SILARX PHARM MLGEX 00703-7045-01 1.15695 HALOPERIDOL LAC 5 MG/ML VIAL 0 TEVA PARENTERAL MLGEX 25021-<strong>08</strong><strong>06</strong>-01 1.15695 HALOPERIDOL LAC 5 MG/ML VIAL 0 SAGENT PHARMACE MLGEX 55390-0147-01 1.15695 HALOPERIDOL LAC 5 MG/ML VIAL 0 BEDFORD LABS MLGEX 55390-0147-10 1.15695 HALOPERIDOL LAC 5 MG/ML VIAL 0 BEDFORD LABS MLGEX 63323-0474-01 1.15695 HALOPERIDOL LAC 5 MG/ML VIAL 0 APP PHARMACEUTI MLGEX 63323-0474-10 1.15695 HALOPERIDOL LAC 5 MG/ML VIAL 0 APP PHARMACEUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 25021-<strong>08</strong>23-10 1.15695 HALOPERIDOL LAC 50 MG/10 ML VL 0 SAGENT PHARMACE MLGEX 00378-0351-01 0.22046 HALOPERIDOL 0.5 MG TABLET 0 MYLAN EAGEX 00378-0351-10 0.22046 HALOPERIDOL 0.5 MG TABLET 0 MYLAN EAGEX 00781-1391-01 0.22046 HALOPERIDOL 0.5 MG TABLET 0 SANDOZ EAGEX 51079-0733-01 0.22046 HALOPERIDOL 0.5 MG TABLET 0 MYLAN INSTITUTI EAGEX 00378-0257-01 0.34109 HALOPERIDOL 1 MG TABLET 0 MYLAN EAGEX 00378-0257-10 0.34109 HALOPERIDOL 1 MG TABLET 0 MYLAN EAGEX 00781-1392-01 0.34109 HALOPERIDOL 1 MG TABLET 0 SANDOZ EAGEX 00904-5923-61 0.32370 HALOPERIDOL 1 MG TABLET 0 MAJOR PHARMACEU EAGEX 51079-0734-01 0.34109 HALOPERIDOL 1 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-0334-01 0.83700 HALOPERIDOL 10 MG TABLET 0 MYLAN EAGEX 00781-1397-01 0.83700 HALOPERIDOL 10 MG TABLET 0 SANDOZ EAGEX 51079-0431-01 0.83700 HALOPERIDOL 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0431-20 0.83700 HALOPERIDOL 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 68<strong>08</strong>4-0249-01 0.83700 HALOPERIDOL 10 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0249-11 0.83700 HALOPERIDOL 10 MG TABLET 0 AHP EAGEX 68382-0<strong>08</strong>0-01 0.83700 HALOPERIDOL 10 MG TABLET 0 ZYDUS PHARMACEU EAGEX 00378-0214-01 0.47400 HALOPERIDOL 2 MG TABLET 0 MYLAN EAGEX 00378-0214-10 0.47398 HALOPERIDOL 2 MG TABLET 0 MYLAN EAGEX 00781-1393-01 0.47400 HALOPERIDOL 2 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00904-5924-61 0.44340 HALOPERIDOL 2 MG TABLET 0 MAJOR PHARMACEU EAGEX 51079-0735-01 0.50250 HALOPERIDOL 2 MG TABLET 0 MYLAN INSTITUTI EAGEX 00378-0335-01 1.43300 HALOPERIDOL 20 MG TABLET 0 MYLAN EAGEX 00781-1398-01 1.43300 HALOPERIDOL 20 MG TABLET 0 SANDOZ EAGEX 68<strong>08</strong>4-0250-11 1.43300 HALOPERIDOL 20 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0250-21 1.43300 HALOPERIDOL 20 MG TABLET 0 AHP EAGEX 68382-0<strong>08</strong>1-01 1.43300 HALOPERIDOL 20 MG TABLET 0 ZYDUS PHARMACEU EAGEX 00378-0327-01 0.67517 HALOPERIDOL 5 MG TABLET 0 MYLAN EAGEX 00378-0327-10 0.67517 HALOPERIDOL 5 MG TABLET 0 MYLAN EAGEX 00781-1396-01 0.67517 HALOPERIDOL 5 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00904-5925-61 0.67517 HALOPERIDOL 5 MG TABLET 0 MAJOR PHARMACEU EAGEX 51079-0736-01 0.67517 HALOPERIDOL 5 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0736-56 0.67517 HALOPERIDOL 5 MG TABLET 0 MYLAN INSTITUTI EAGEX 68462-0303-29 0.69917 HEATHER TABLET 0 GLENMARK PHARMA EAGEN 00078-<strong>06</strong>16-05 1.63545 HECORIA 0.5 MG CAPSULE G NOVARTIS EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 175LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00078-<strong>06</strong>17-05 3.27105 HECORIA 1 MG CAPSULE G NOVARTIS EAGEN 00078-<strong>06</strong>18-05 16.35525 HECORIA 5 MG CAPSULE G NOVARTIS EABND 58468-0120-01 14.47669 HECTOROL 0.5 MCG CAPSULE 0 GENZYME EABND 58468-0124-01 28.95239 HECTOROL 1 MCG CAPSULE 0 GENZYME EABND 58468-0126-01 6.22500 HECTOROL 2 MCG/ML VIAL 0 GENZYME MLBND 58468-0121-01 33.53897 HECTOROL 2.5 MCG CAPSULE 0 GENZYME EABND 58468-0123-01 6.22500 HECTOROL 4 MCG/2 ML VIAL 0 GENZYME MLBND 65483-0495-14 3.45376 HELIDAC THERAPY 0 PROMETHEUS EABND 00053-8133-02 1.01500 HELIXATE FS 1,000 UNIT VIAL 0 CSL BEHRING LLCBND 00053-8134-02 1.01500 HELIXATE FS 2,000 UNIT VIAL 0 CSL BEHRING LLC--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00053-8131-02 1.01500 HELIXATE FS 250 UNIT VIAL 0 CSL BEHRING LLCBND 00053-8135-02 1.01500 HELIXATE FS 3,000 UNITS VIAL 0 CSL BEHRING LLCBND 00053-8132-02 1.01500 HELIXATE FS 500 UNIT VIAL 0 CSL BEHRING LLCBND 00009-<strong>08</strong>56-<strong>08</strong> 150.71057 HEMABATE 250 MCG/ML AMPUL 0 PHARMACIA/UPJHN MLBND 00944-2932-01 0.90500 HEM<strong>OF</strong>IL M 1,000 UNIT NOMINAL 0 BAXTER BIOSCIENBND 00944-3944-02 0.90500 HEM<strong>OF</strong>IL M 1,000 UNIT NOMINAL 0 BAXTER BIOSCIENBND 00944-2933-01 0.90500 HEM<strong>OF</strong>IL M 1,700 UNIT NOMINAL 0 BAXTER BIOSCIENBND 00944-3946-02 0.90500 HEM<strong>OF</strong>IL M 1,700 UNIT NOMINAL 0 BAXTER BIOSCIENBND 00944-2930-01 0.90500 HEM<strong>OF</strong>IL M 250 UNIT NOMINAL 0 BAXTER BIOSCIENBND 00944-3940-02 0.90500 HEM<strong>OF</strong>IL M 250 UNIT NOMINAL 0 BAXTER BIOSCIEN--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00944-2931-01 0.90500 HEM<strong>OF</strong>IL M 500 UNIT NOMINAL 0 BAXTER BIOSCIENBND 00944-3942-02 0.90500 HEM<strong>OF</strong>IL M 500 UNIT NOMINAL 0 BAXTER BIOSCIENGEN 00409-1280-31 0.68190 HEPARIN LOCK FLUSH 10 UNITS/ML 0 HOSPIRA MLBND 00409-1281-31 1.49400 HEPARIN LOCK FLUSH 100 UNIT/ML 0 HOSPIRA MLGEN 00409-2720-01 1.11600 HEPARIN SOD 1,000 UNIT/ML VIAL 0 HOSPIRA MLGEN 0<strong>06</strong>41-0391-37 2.35800 HEPARIN SOD 1,000 UNIT/ML VIAL 0 WEST-WARD,INC. MLGEN 25021-0400-01 2.16000 HEPARIN SOD 1,000 UNIT/ML VIAL 0 SAGENT PHARMACE MLGEN 63323-0540-01 3.60930 HEPARIN SOD 1,000 UNIT/ML VIAL 0 APP PHARMACEUTI MLGEN 00<strong>06</strong>9-0<strong>06</strong>2-02 2.87000 HEPARIN SOD 10,000 UNIT/ML VL 0 PFIZER US PHARM MLGEN 00409-2721-01 2.01600 HEPARIN SOD 10,000 UNIT/ML VL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>41-0410-37 2.87000 HEPARIN SOD 10,000 UNIT/ML VL 0 WEST-WARD,INC. MLGEN 25021-0403-01 2.87000 HEPARIN SOD 10,000 UNIT/ML VL 0 SAGENT PHARMACE MLGEN 63323-0542-01 6.56250 HEPARIN SOD 10,000 UNIT/ML VL 0 APP PHARMACEUTI MLGEN 25021-0404-01 9.00000 HEPARIN SOD 20,000 UNIT/ML VL 0 SAGENT PHARMACE MLGEN 63323-0915-01 15.37090 HEPARIN SOD 20,000 UNIT/ML VL 0 APP PHARMACEUTI MLBND 63323-0543-02 10.35840 HEPARIN SOD 5,000 UNIT/ 0.5 ML 0 APP PHARMACEUTI MLBND 00409-1402-12 2.50992 HEPARIN SOD 5,000 UNIT/ML SYR 0 HOSPIRA MLGEN 00<strong>06</strong>9-0059-03 1.11360 HEPARIN SOD 5,000 UNIT/ML VIAL 0 PFIZER US PHARM MLGEN 00<strong>06</strong>9-0059-04 1.11360 HEPARIN SOD 5,000 UNIT/ML VIAL 0 PFIZER US PHARM MLGEN 00409-2723-01 0.99900 HEPARIN SOD 5,000 UNIT/ML VIAL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>41-0400-37 1.11360 HEPARIN SOD 5,000 UNIT/ML VIAL 0 WEST-WARD,INC. MLGEN 25021-0402-01 1.11360 HEPARIN SOD 5,000 UNIT/ML VIAL 0 SAGENT PHARMACE MLGEN 63323-0262-01 1.11360 HEPARIN SOD 5,000 UNIT/ML VIAL 0 APP PHARMACEUTI MLBND 00409-1316-32 5.65728 HEPARIN SOD 5,000 UNIT/0.5 ML 0 HOSPIRA MLGEN 00409-1152-70 0.<strong>08</strong>730 HEPARIN 1,000 UNIT/10 (100/ML) 0 HOSPIRA ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 176LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00<strong>06</strong>9-0058-02 0.57600 HEPARIN 10,000 UNIT/10 ML VIAL 0 PFIZER US PHARM MLGEN 00409-2720-02 0.18450 HEPARIN 10,000 UNIT/10 ML VIAL 0 HOSPIRA MLGEN 25021-0400-10 0.32400 HEPARIN 10,000 UNIT/10 ML VIAL 0 SAGENT PHARMACE MLGEN 63323-0540-11 0.68154 HEPARIN 10,000 UNIT/10 ML VIAL 0 APP PHARMACEUTI MLGEN 00409-1151-70 0.05940 HEPARIN 100 UNIT/10 ML (10/ML) 0 HOSPIRA MLGEN 00<strong>06</strong>9-0043-02 4.16000 HEPARIN 2,000 UNIT/2 ML VIAL 0 PFIZER US PHARM MLGEN 25021-0401-02 4.16000 HEPARIN 2,000 UNIT/2 ML VIAL 0 SAGENT PHARMACE MLGEN 63323-0276-02 4.16000 HEPARIN 2,000 UNIT/2 ML VIAL 0 APP PHARMACEUTI MLGEN 00409-1280-32 0.67500 HEPARIN 20 UNITS/2 ML (10/ML) 0 HOSPIRA MLBND 00409-1281-32 0.76692 HEPARIN 200 UNIT/2 ML (100/ML) 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-1152-78 0.04320 HEPARIN 3,000 UNIT/30 (100/ML) 0 HOSPIRA MLGEN 00409-1280-33 0.57900 HEPARIN 30 UNIT/3 ML (10/ML) 0 HOSPIRA MLGEN 00<strong>06</strong>9-0137-01 0.21300 HEPARIN 30,000 UNIT/30 ML VIAL 0 PFIZER US PHARM MLGEN 00<strong>06</strong>9-0137-03 0.21300 HEPARIN 30,000 UNIT/30 ML VIAL 0 PFIZER US PHARM MLGEN 00409-2720-03 0.16530 HEPARIN 30,000 UNIT/30 ML VIAL 0 HOSPIRA MLGEN 0<strong>06</strong>41-2450-30 0.26340 HEPARIN 30,000 UNIT/30 ML VIAL 0 WEST-WARD,INC. MLGEN 25021-0400-30 0.20400 HEPARIN 30,000 UNIT/30 ML VIAL 0 SAGENT PHARMACE MLGEN 63323-0540-31 0.21390 HEPARIN 30,000 UNIT/30 ML VIAL 0 APP PHARMACEUTI MLBND 00409-1281-33 0.60092 HEPARIN 300 UNIT/3 ML (100/ML) 0 HOSPIRA MLGEN 25021-0403-04 1.71000 HEPARIN 40,000 UNITS/4 ML VIAL 0 SAGENT PHARMACE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0459-09 1.63125 HEPARIN 40,000 UNITS/4 ML VIAL 0 APP PHARMACEUTI MLGEN 00409-1280-35 0.36540 HEPARIN 50 UNITS/5 ML (10/ML) 0 HOSPIRA MLGEN 0<strong>06</strong>41-2460-30 1.00170 HEPARIN 50,000 UNIT/10 ML VIAL 0 WEST-WARD,INC. MLGEN 00<strong>06</strong>9-0059-02 1.11360 HEPARIN 50,000 UNITS/10 ML VL 0 PFIZER US PHARM MLGEN 00409-2723-02 0.55260 HEPARIN 50,000 UNITS/10 ML VL 0 HOSPIRA MLGEN 25021-0402-10 0.75600 HEPARIN 50,000 UNITS/10 ML VL 0 SAGENT PHARMACE MLGEN 63323-0047-10 1.11360 HEPARIN 50,000 UNITS/10 ML VL 0 APP PHARMACEUTI MLGEN 63323-0542-07 4.23900 HEPARIN 50,000 UNITS/5 ML VIAL 0 APP PHARMACEUTI MLGEN 00409-1281-35 0.35280 HEPARIN 500 UNIT/5 ML (100/ML) 0 HOSPIRA MLBND 00409-7794-62 0.02764 HEPARIN-D5W 12,500 UNIT/250 ML 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00264-9567-10 0.01320 HEPARIN-D5W 20,000 UNIT/500 ML 0 B.BRAUN MLGEN 00409-7760-03 0.00984 HEPARIN-D5W 20,000 UNIT/500 ML 0 HOSPIRA MLGEN 00264-9587-20 0.03000 HEPARIN-D5W 25,000 UNIT/250 ML 0 B.BRAUN MLGEN 00409-7793-62 0.03070 HEPARIN-D5W 25,000 UNIT/250 ML 0 HOSPIRA MLGEN 00264-9577-10 0.01650 HEPARIN-D5W 25,000 UNIT/500 ML 0 B.BRAUN MLGEN 00409-7761-03 0.01033 HEPARIN-D5W 25,000 UNIT/500 ML 0 HOSPIRA MLGEN 00264-9872-10 0.0<strong>06</strong>00 HEPARIN-NS 1,000 UNITS/500 ML 0 B.BRAUN MLGEN 00409-7620-03 0.00710 HEPARIN-NS 1,000 UNITS/500 ML 0 HOSPIRA MLGEN 00409-7620-59 0.00430 HEPARIN-NS 2,000 UNIT/1,000 ML 0 HOSPIRA MLBND 00409-7651-62 0.02421 HEPARIN-1/2NS 12,500 UNITS/250 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-7650-62 0.02792 HEPARIN-1/2NS 25,000 UNITS/250 0 HOSPIRA MLBND 00409-7651-03 0.01047 HEPARIN-1/2NS 25,000 UNITS/500 0 HOSPIRA MLBND 61958-0501-01 36.65971 HEPSERA 10 MG TABLET 0 GILEAD SCIENCES EABND 62856-0001-10 13.56552 HEXALEN 50 MG CAPSULE 0 EISAI INC. EABND 00<strong>06</strong>8-0277-61 1.67330 2.93654 HIPREX 1 GM TABLET G SAN<strong>OF</strong>I-AVENTIS EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 177LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 442<strong>06</strong>-0451-01 27.20076 HIZENTRA 1 GRAM/5 ML VIAL 0 CSL BEHRING LLC MLBND 442<strong>06</strong>-0455-10 27.20076 HIZENTRA 10 GRAM/ 50 ML VIAL 0 CSL BEHRING LLC MLBND 442<strong>06</strong>-0452-02 27.20076 HIZENTRA 2 GRAM/10 ML VIAL 0 CSL BEHRING LLC MLBND 442<strong>06</strong>-0454-04 27.20076 HIZENTRA 4 GRAM/20 ML VIAL 0 CSL BEHRING LLC MLBND 53451-0103-01 5.38<strong>06</strong>1 HORIZANT ER 300 MG TABLET 0 XENOPORT, INC. EABND 00173-<strong>08</strong><strong>06</strong>-01 3.79752 HORIZANT ER 600 MG TABLET 0 GLAXOSMITHKLINE EABND 53451-0101-01 5.38<strong>06</strong>1 HORIZANT ER 600 MG TABLET 0 XENOPORT, INC. EABND 63004-7731-01 5952.34500 HP ACTHAR GEL 80 UNIT/ML VIAL G QUESTCOR MLBND 63004-8710-01 6299.89920 HP ACTHAR GEL 80 UNIT/ML VIAL G QUESTCOR MLBND 00002-8798-01 21.51<strong>08</strong>3 HUMALOG MIX 50-50 KWIKPEN 0 ELI LILLY & CO. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00002-8798-59 21.51028 HUMALOG MIX 50-50 KWIKPEN 0 ELI LILLY & CO. MLBND 00002-7512-01 17.30550 HUMALOG MIX 50-50 VIAL 0 ELI LILLY & CO. MLBND 00002-8797-01 21.51<strong>08</strong>3 HUMALOG MIX 75-25 KWIKPEN 0 ELI LILLY & CO. MLBND 00002-8797-59 21.51028 HUMALOG MIX 75-25 KWIKPEN 0 ELI LILLY & CO. MLBND 00002-7511-01 17.30550 HUMALOG MIX 75-25 VIAL 0 ELI LILLY & CO. MLBND 00002-7516-01 20.70020 HUMALOG 100 UNITS/ML CARTRIDGE 0 ELI LILLY & CO. MLBND 00002-7516-59 20.70020 HUMALOG 100 UNITS/ML CARTRIDGE 0 ELI LILLY & CO. MLBND 00002-8799-01 21.51<strong>08</strong>3 HUMALOG 100 UNITS/ML KWIKPEN 0 ELI LILLY & CO. MLBND 00002-8799-59 21.51028 HUMALOG 100 UNITS/ML KWIKPEN 0 ELI LILLY & CO. MLBND 00002-7510-01 16.70292 HUMALOG 100 UNITS/ML VIAL 0 ELI LILLY & CO. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00002-7510-17 16.70236 HUMALOG 100 UNITS/ML VIAL 0 ELI LILLY & CO. MLGEN 61953-0002-01 1.62000 HUMAN ALBUMIN 25% IV SOLUTION 0 GRIFOLS MLGEN 61953-0002-02 1.62000 HUMAN ALBUMIN 25% IV SOLUTION 0 GRIFOLS MLBND 63833-<strong>06</strong>16-02 0.86500 HUMATE-P 1,200 UNIT VWF:RCO 0 CSL BEHRING, LLBND 63833-<strong>06</strong>17-02 0.86500 HUMATE-P 2,400 UNIT VWF:RCO 0 CSL BEHRING, LLBND 63833-<strong>06</strong>15-02 0.86500 HUMATE-P 600 UNIT VWF:RCO 0 CSL BEHRING, LLBND 00002-8148-01 1<strong>06</strong>3.13040 HUMATROPE 12 MG CARTRIDGE G ELI LILLY & CO. EABND 00002-8149-01 2126.26<strong>08</strong>0 HUMATROPE 24 MG CARTRIDGE G ELI LILLY & CO. EABND 00002-7335-11 442.97100 HUMATROPE 5 MG VIAL G ELI LILLY & CO. EABND 00002-7349-01 HUMATROPE 5 MG VIAL G ELI LILLY & CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00002-8147-01 531.56520 HUMATROPE 6 MG CARTRIDGE G ELI LILLY & CO. EABND 00074-4339-<strong>06</strong> 1246.30033 HUMIRA CROHN'S STARTER PACK G ABBVIE US LLC EABND 00074-4339-07 1246.29895 HUMIRA PSORIASIS STARTER PACK G ABBVIE US LLC EABND 00074-9374-02 1246.29895 HUMIRA 20 MG/0.4 ML SYRINGE G ABBVIE US LLC EABND 00074-4339-02 1246.29895 HUMIRA 40 MG/0.8 ML PEN G ABBVIE US LLC EABND 00074-3799-02 1246.29895 HUMIRA 40 MG/0.8 ML SYRINGE G ABBVIE US LLC EABND 00002-8501-01 45.21840 HUMULIN R 500 UNITS/ML VIAL 0 ELI LILLY & CO. MLBND 00007-4205-11 91.59714 HYCAMTIN 0.25 MG CAPSULE 0 GLAXOSMITHKLINE EABND 00007-4207-11 366.38275 HYCAMTIN 1 MG CAPSULE 0 GLAXOSMITHKLINE EAGEN 23155-0001-01 0.05750 HYDRALAZINE 10 MG TABLET 0 HERITAGE PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 23155-0001-10 0.05750 HYDRALAZINE 10 MG TABLET 0 HERITAGE PHARMA EAGEN 31722-0519-01 0.05750 HYDRALAZINE 10 MG TABLET 0 CAMBER PHARMACE EAGEN 49884-0029-01 0.05750 HYDRALAZINE 10 MG TABLET 0 PAR PHARM. EAGEN 49884-0029-10 0.05750 HYDRALAZINE 10 MG TABLET 0 PAR PHARM. EAGEN 50111-0398-01 0.05750 HYDRALAZINE 10 MG TABLET 0 PLIVA, INC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 178LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50111-0398-03 0.05750 HYDRALAZINE 10 MG TABLET 0 PLIVA, INC EAGEN 51079-0074-01 0.05750 HYDRALAZINE 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 68<strong>08</strong>4-0447-01 0.05750 HYDRALAZINE 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0447-11 0.05750 HYDRALAZINE 10 MG TABLET 0 AHP EAGEN 68462-0341-01 0.05750 HYDRALAZINE 10 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0341-05 0.05750 HYDRALAZINE 10 MG TABLET 0 GLENMARK PHARMA EAGEN 23155-0004-01 0.19580 HYDRALAZINE 100 MG TABLET 0 HERITAGE PHARMA EAGEN 31722-0522-01 0.19580 HYDRALAZINE 100 MG TABLET 0 CAMBER PHARMACE EAGEN 49884-0121-01 0.19580 HYDRALAZINE 100 MG TABLET 0 PAR PHARM. EAGEN 49884-0121-10 0.19580 HYDRALAZINE 100 MG TABLET 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50111-0397-01 0.19580 HYDRALAZINE 100 MG TABLET 0 PLIVA, INC EAGEN 64380-0736-<strong>06</strong> 0.19580 HYDRALAZINE 100 MG TABLET 0 STRIDES PHARMA EAGEN 68462-0344-01 0.19580 HYDRALAZINE 100 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0344-05 0.19580 HYDRALAZINE 100 MG TABLET 0 GLENMARK PHARMA EAGEN 23155-0002-01 0.09138 HYDRALAZINE 25 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0002-10 0.09138 HYDRALAZINE 25 MG TABLET 0 HERITAGE PHARMA EAGEN 31722-0520-01 0.09138 HYDRALAZINE 25 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0520-10 0.09138 HYDRALAZINE 25 MG TABLET 0 CAMBER PHARMACE EAGEN 49884-0027-01 0.09138 HYDRALAZINE 25 MG TABLET 0 PAR PHARM. EAGEN 49884-0027-10 0.09138 HYDRALAZINE 25 MG TABLET 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50111-0327-01 0.09138 HYDRALAZINE 25 MG TABLET 0 TEVA USA EAGEN 50111-0327-03 0.09138 HYDRALAZINE 25 MG TABLET 0 PLIVA, INC EAGEN 51079-0075-01 0.09138 HYDRALAZINE 25 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0075-17 0.09138 HYDRALAZINE 25 MG TABLET 0 MYLAN INSTITUTI EAGEN 62584-0733-01 0.09138 HYDRALAZINE 25 MG TABLET 0 AHP EAGEN 62584-0733-11 0.09138 HYDRALAZINE 25 MG TABLET 0 AHP EAGEN 63739-0126-10 0.09138 HYDRALAZINE 25 MG TABLET 0 MCKESSON PACKAG EAGEN 64380-0734-<strong>06</strong> 0.09138 HYDRALAZINE 25 MG TABLET 0 STRIDES PHARMA EAGEN 68462-0342-01 0.09138 HYDRALAZINE 25 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0342-05 0.09138 HYDRALAZINE 25 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 23155-0003-01 0.11420 HYDRALAZINE 50 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0003-10 0.11420 HYDRALAZINE 50 MG TABLET 0 HERITAGE PHARMA EAGEN 31722-0521-01 0.11420 HYDRALAZINE 50 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0521-10 0.11420 HYDRALAZINE 50 MG TABLET 0 CAMBER PHARMACE EAGEN 49884-0028-01 0.11420 HYDRALAZINE 50 MG TABLET 0 PAR PHARM. EAGEN 49884-0028-10 0.11420 HYDRALAZINE 50 MG TABLET 0 PAR PHARM. EAGEN 50111-0328-01 0.11420 HYDRALAZINE 50 MG TABLET 0 PLIVA, INC EAGEN 50111-0328-03 0.11420 HYDRALAZINE 50 MG TABLET 0 PLIVA, INC EAGEN 51079-0076-01 0.11420 HYDRALAZINE 50 MG TABLET 0 MYLAN INSTITUTI EAGEN 62584-0734-01 0.11420 HYDRALAZINE 50 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62584-0734-11 0.11420 HYDRALAZINE 50 MG TABLET 0 AHP EAGEN 63739-0127-10 0.11420 HYDRALAZINE 50 MG TABLET 0 MCKESSON PACKAG EAGEN 64380-0735-<strong>06</strong> 0.11420 HYDRALAZINE 50 MG TABLET 0 STRIDES PHARMA EAGEN 68462-0343-01 0.11420 HYDRALAZINE 50 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0343-05 0.11420 HYDRALAZINE 50 MG TABLET 0 GLENMARK PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 179LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00003-<strong>08</strong>30-50 0.38480 1.28268 HYDREA 500 MG CAPSULE G BMS ONCO/IMMUN EAGEN 00093-2<strong>08</strong>0-01 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 TEVA USA EAGEN 00093-2<strong>08</strong>0-10 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 TEVA USA EAGEN 00143-3125-01 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 WEST-WARD,INC. EAGEN 00143-3125-05 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 WEST-WARD,INC. EAGEN 00378-<strong>08</strong>10-01 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 MYLAN EAGEN 00378-<strong>08</strong>10-05 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 MYLAN EAGEN 00378-<strong>08</strong>10-93 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 MYLAN EAGEN 00591-0347-01 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 ACTAVIS PHARMA, EAGEN 00591-0347-05 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-3855-21 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 QUALITEST EAGEN 0<strong>06</strong>03-3855-25 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 QUALITEST EAGEN 0<strong>06</strong>03-3855-32 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 QUALITEST EAGEN 0<strong>06</strong>03-3855-93 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 QUALITEST EAGEN 23155-0045-01 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 HERITAGE PHARMA EAGEN 23155-0045-05 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 HERITAGE PHARMA EAGEN 29300-0130-01 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 UNICHEM PHARMAC EAGEN 29300-0130-05 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 UNICHEM PHARMAC EAGEN 29300-0130-10 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 UNICHEM PHARMAC EAGEN 51079-0776-01 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0776-20 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 MYLAN INSTITUTI EAGEN 57237-0002-01 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 CITRON PHARMA L EAGEN 57237-0002-99 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 CITRON PHARMA L EAGEN 59746-0382-<strong>06</strong> 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 CADISTA PHARMAC EAGEN 59746-0382-10 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 CADISTA PHARMAC EAGEN 59762-1735-02 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 GREENSTONE LLC. EAGEN 65862-0113-01 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 AUROBINDO PHARM EAGEN 65862-0113-99 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0398-01 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 AHP EAGEN 68<strong>08</strong>4-0398-11 0.03900 HYDROCHLOROTHIAZIDE 12.5 MG CP 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-2820-11 0.13028 HYDROCHLOROTHIAZIDE 12.5 MG TB 0 ACTAVIS PHARMA, EAGEN 16729-0182-01 0.13028 HYDROCHLOROTHIAZIDE 12.5 MG TB 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0182-17 0.13028 HYDROCHLOROTHIAZIDE 12.5 MG TB 0 ACCORD <strong>HEALTH</strong>CA EAGEN 23155-0137-01 0.13028 HYDROCHLOROTHIAZIDE 12.5 MG TB 0 HERITAGE PHARMA EAGEN 00143-1256-10 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 WEST-WARD,INC. EAGEN 00143-1256-51 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 WEST-WARD,INC. EAGEN 00172-2<strong>08</strong>3-60 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 TEVA USA EAGEN 00172-2<strong>08</strong>3-80 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 TEVA USA EAGEN 00527-1413-01 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 LANNETT CO. INC EAGEN 00527-1413-10 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 LANNETT CO. INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-3856-21 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-3856-32 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-3856-34 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 QUALITEST EAGEN 16729-0183-01 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0183-17 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 ACCORD <strong>HEALTH</strong>CA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 180LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 23155-0047-01 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 HERITAGE PHARMA EAGEN 23155-0047-10 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 HERITAGE PHARMA EAGEN 29300-0128-01 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 UNICHEM PHARMAC EAGEN 29300-0128-10 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 UNICHEM PHARMAC EAGEN 54458-0930-10 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 INTERNATIONAL L EAGEN 59746-0125-10 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 CADISTA PHARMAC EAGEN 60505-2640-01 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 APOTEX CORP EAGEN 60505-2640-07 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 APOTEX CORP EAGEN 60505-2640-<strong>08</strong> 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 APOTEX CORP EAGEN 63739-0128-10 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 MCKESSON PACKAG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64125-0131-01 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 EXCELLIUM PHARM EAGEN 64125-0131-10 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 EXCELLIUM PHARM EAGEN 65862-0133-01 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0133-99 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0<strong>08</strong>6-01 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0<strong>08</strong>6-11 0.01161 HYDROCHLOROTHIAZIDE 25 MG TAB 0 AHP EAGEN 00143-1257-10 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 WEST-WARD,INC. EAGEN 00143-1257-51 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 WEST-WARD,INC. EAGEN 00172-2<strong>08</strong>9-60 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 IVAX PHARMACEUT EAGEN 00172-2<strong>08</strong>9-80 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00172-2<strong>08</strong>9-85 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 IVAX PHARMACEUT EAGEN 00527-1414-01 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 LANNETT CO. INC EAGEN 00527-1414-10 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 LANNETT CO. INC EAGEN 0<strong>06</strong>03-3857-21 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-3857-32 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 QUALITEST EAGEN 16729-0184-01 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0184-17 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 ACCORD <strong>HEALTH</strong>CA EAGEN 23155-0046-01 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 HERITAGE PHARMA EAGEN 23155-0046-10 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 HERITAGE PHARMA EAGEN 29300-0129-01 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 UNICHEM PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 29300-0129-10 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 UNICHEM PHARMAC EAGEN 54458-0929-10 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 INTERNATIONAL L EAGEN 59746-0127-01 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 CADISTA PHARMAC EAGEN 59746-0127-10 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 CADISTA PHARMAC EAGEN 59762-1737-07 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 GREENSTONE LLC. EAGEN 60505-2641-01 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 APOTEX CORP EAGEN 60505-2641-07 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 APOTEX CORP EAGEN 60505-2641-<strong>08</strong> 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 APOTEX CORP EAGEN 64125-0130-01 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 EXCELLIUM PHARM EAGEN 64125-0130-10 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 EXCELLIUM PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0134-01 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0134-99 0.03710 HYDROCHLOROTHIAZIDE 50 MG TAB 0 AUROBINDO PHARM EAGEN 498<strong>08</strong>-0384-45 6.40733 HYDROCORT BUTY 0.1% LIPID CRM G METACON LABS GMGEN 498<strong>08</strong>-0384-60 6.40712 HYDROCORT BUTY 0.1% LIPID CRM G METACON LABS GMGEN 68462-0464-47 6.74566 HYDROCORT BUTY 0.1% LIPO CREAM G GLENMARK PHARMA GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 181LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0464-65 6.74550 HYDROCORT BUTY 0.1% LIPO CREAM G GLENMARK PHARMA GMGEN 50383-0901-10 11.62240 HYDROCORTISON-ACETIC ACID SOLN 0 HI-TECH PHARMAC MLGEN 51672-3007-01 11.62240 HYDROCORTISON-ACETIC ACID SOLN 0 TARO PHARM USA MLGEN 00574-0421-25 2.70000 HYDROCORTISONE ACETATE POWDER 0 PADDOCK LABS. GMGEN 51927-1110-00 13.47750 HYDROCORTISONE ACETATE POWDER 0 PR<strong>OF</strong>ESSIONAL CO GMGUL 43478-0270-15 1.11770 HYDROCORTISONE BUTY 0.1% CREAM G ROUSES POINT PH GMGEN 43478-0270-45 0.91683 HYDROCORTISONE BUTY 0.1% CREAM G ROUSES POINT PH GMGUL 51672-4074-01 1.11770 HYDROCORTISONE BUTY 0.1% CREAM G TARO PHARM USA GMGUL 51672-4074-<strong>06</strong> 1.11770 HYDROCORTISONE BUTY 0.1% CREAM G TARO PHARM USA GMGEN 43478-0271-15 1.28700 HYDROCORTISONE BUTYR 0.1% OINT 0 ROUSES POINT PH GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43478-0271-45 0.91683 HYDROCORTISONE BUTYR 0.1% OINT 0 ROUSES POINT PH GMGEN 51672-4<strong>08</strong>3-01 1.51580 HYDROCORTISONE BUTYR 0.1% OINT 0 TARO PHARM USA GMGEN 51672-4<strong>08</strong>3-<strong>06</strong> 1.43400 HYDROCORTISONE BUTYR 0.1% OINT 0 TARO PHARM USA GMGEN 00574-0420-01 2.57812 HYDROCORTISONE POWDER 0 PADDOCK LABS. GMGEN 00574-0420-10 3.46875 HYDROCORTISONE POWDER 0 PADDOCK LABS. GMGEN 00574-0420-25 2.70000 HYDROCORTISONE POWDER 0 PADDOCK LABS. GMGUL 45802-0455-35 0.65830 HYDROCORTISONE VAL 0.2% CREAM 0 PERRIGO CO. GMGUL 45802-0455-37 0.65830 HYDROCORTISONE VAL 0.2% CREAM 0 PERRIGO CO. GMGUL 45802-0455-42 0.65830 HYDROCORTISONE VAL 0.2% CREAM 0 PERRIGO CO. GMGUL 51672-1290-01 0.65830 HYDROCORTISONE VAL 0.2% CREAM 0 TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-1290-03 0.65830 HYDROCORTISONE VAL 0.2% CREAM 0 TARO PHARM USA GMGUL 51672-1290-<strong>06</strong> 0.65830 HYDROCORTISONE VAL 0.2% CREAM 0 TARO PHARM USA GMGUL 51672-1292-01 0.65830 HYDROCORTISONE VAL 0.2% OINTMT 0 TARO PHARM USA GMGUL 51672-1292-03 0.65830 HYDROCORTISONE VAL 0.2% OINTMT 0 TARO PHARM USA GMGUL 51672-1292-<strong>06</strong> 0.65830 HYDROCORTISONE VAL 0.2% OINTMT 0 TARO PHARM USA GMGEN 43478-0273-61 0.18600 HYDROCORTISONE 0.1% SOLN 0 ROUSES POINT PH MLGUL 51672-4<strong>06</strong>1-02 0.37880 HYDROCORTISONE 0.1% SOLN 0 TARO PHARM USA MLGUL 51672-4<strong>06</strong>1-04 0.37880 HYDROCORTISONE 0.1% SOLN 0 TARO PHARM USA MLGEN 0<strong>06</strong>03-3900-21 0.42975 HYDROCORTISONE 10 MG TABLET 0 QUALITEST EAGEN 54505-0332-10 0.42975 HYDROCORTISONE 10 MG TABLET 0 LINEAGE THERAPE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-0074-01 0.42975 HYDROCORTISONE 10 MG TABLET 0 GREENSTONE LLC. EAGEN 64720-0332-10 0.42975 HYDROCORTISONE 10 MG TABLET 0 COREPHARMA LLC EAGEN 68<strong>08</strong>4-0469-01 0.42757 HYDROCORTISONE 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0469-11 0.42757 HYDROCORTISONE 10 MG TABLET 0 AHP EAGEN 51991-0728-67 0.<strong>08</strong>681 HYDROCORTISONE 100 MG/60 ML 0 BRECKENRIDGE MLGEN 62559-0138-07 0.<strong>08</strong>681 HYDROCORTISONE 100 MG/60 ML 0 ANI PHARMACEUTI MLGEN 00168-0<strong>08</strong>0-16 0.10030 HYDROCORTISONE 2.5% CREAM 0 SANDOZ GMGEN 00168-0<strong>08</strong>0-31 0.10030 HYDROCORTISONE 2.5% CREAM 0 SANDOZ GMGEN 00472-0337-20 0.10030 HYDROCORTISONE 2.5% CREAM 0 ACTAVIS PHARMA, GMGEN 00472-0337-30 0.10030 HYDROCORTISONE 2.5% CREAM 0 ACTAVIS PHARMA, GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-7781-78 0.10030 HYDROCORTISONE 2.5% CREAM 0 QUALITEST GMGEN 45802-0004-02 0.10030 HYDROCORTISONE 2.5% CREAM 0 PERRIGO CO. GMGEN 45802-0004-03 0.10030 HYDROCORTISONE 2.5% CREAM 0 PERRIGO CO. GMGEN 00168-0288-02 0.25150 HYDROCORTISONE 2.5% LOTION 0 SANDOZ MLGEN 0<strong>06</strong>03-7785-52 0.25150 HYDROCORTISONE 2.5% LOTION 0 QUALITEST ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 182LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0937-16 0.25150 HYDROCORTISONE 2.5% LOTION 0 PERRIGO CO. MLGEN 45802-0937-26 0.25150 HYDROCORTISONE 2.5% LOTION 0 PERRIGO CO. MLGEN 00168-0146-16 0.11407 HYDROCORTISONE 2.5% OINTMENT 0 SANDOZ GMGEN 00168-0146-30 0.11407 HYDROCORTISONE 2.5% OINTMENT 0 SANDOZ GMGEN 45802-0014-02 0.11407 HYDROCORTISONE 2.5% OINTMENT 0 PERRIGO CO. GMGEN 00143-1254-01 0.29700 HYDROCORTISONE 20 MG TABLET 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>03-3901-21 0.64071 HYDROCORTISONE 20 MG TABLET 0 QUALITEST EAGEN 00904-2674-60 0.59107 HYDROCORTISONE 20 MG TABLET 0 MAJOR PHARMACEU EAGEN 54505-0333-10 0.64071 HYDROCORTISONE 20 MG TABLET 0 LINEAGE THERAPE EAGEN 59762-0075-01 0.64071 HYDROCORTISONE 20 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64720-0333-10 0.64071 HYDROCORTISONE 20 MG TABLET 0 COREPHARMA LLC EAGEN 0<strong>06</strong>03-3899-19 0.25425 HYDROCORTISONE 5 MG TABLET 0 QUALITEST EAGEN 54505-0331-05 0.25440 HYDROCORTISONE 5 MG TABLET 0 LINEAGE THERAPE EAGEN 59762-0073-01 0.25425 HYDROCORTISONE 5 MG TABLET 0 GREENSTONE LLC. EAGEN 64720-0331-05 0.25425 HYDROCORTISONE 5 MG TABLET 0 COREPHARMA LLC EAGEN 38779-2533-03 615.60000 HYDROXOCOBALAMIN POWDER 0 MEDISCA INC. GMBND 00591-2888-30 1.05852 HYDROXOCOBALAMIN 1,000 MCG/ML 0 ACTAVIS PHARMA, MLGEN 00143-2128-01 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 WEST-WARD,INC. EAGEN 00378-0373-01 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 MYLAN EAGEN 00591-<strong>06</strong>98-01 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-<strong>06</strong>98-05 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 ACTAVIS PHARMA, EAGEN 00781-1407-01 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 SANDOZ EAGEN 00781-1407-05 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 SANDOZ EAGEN 63304-0296-01 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 RANBAXY PHARMAC EAGEN 63304-0296-05 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 RANBAXY PHARMAC EAGEN 68<strong>08</strong>4-0269-01 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0269-11 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 AHP EAGEN 68382-0096-01 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 ZYDUS PHARMACEU EAGEN 68382-0096-05 0.1<strong>08</strong>00 HYDROXYCHLOROQUINE 200 MG TAB 0 ZYDUS PHARMACEU EAGEN 38779-2102-05 61.27500 HYDROXYPROGESTERONE CAP POWDER 0 MEDISCA INC. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00555-<strong>08</strong>82-02 0.38480 HYDROXYUREA 500 MG CAPSULE 0 BARR EAGEN 49884-0724-01 0.38480 HYDROXYUREA 500 MG CAPSULE 0 PAR PHARM. EAGEN 68<strong>08</strong>4-0284-01 0.38480 HYDROXYUREA 500 MG CAPSULE 0 AHP EAGEX 00093-5<strong>06</strong>0-01 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 TEVA USA EAGEX 00093-5<strong>06</strong>0-05 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 TEVA USA EAGEX 00093-5<strong>06</strong>0-10 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 TEVA USA EAGEX 00378-2586-01 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 MYLAN EAGEX 00378-2586-10 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 MYLAN EAGEX 0<strong>06</strong>03-3967-21 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3967-28 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-3967-32 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 QUALITEST EAGEX 10702-0010-01 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 KVK-TECH, INC. EAGEX 10702-0010-10 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 KVK-TECH, INC. EAGEX 10702-0010-50 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 KVK-TECH, INC. EAGEX 16714-0<strong>08</strong>1-04 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 183LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16714-0<strong>08</strong>1-05 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-0<strong>08</strong>1-11 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 NORTHSTAR RX LL EAGEX 23155-0105-01 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 HERITAGE PHARMA EAGEX 23155-0105-05 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 HERITAGE PHARMA EAGEX 23155-0105-10 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 HERITAGE PHARMA EAGEX 51079-0796-01 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0796-20 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 63739-0483-10 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 MCKESSON PACKAG EAGEX 67405-0575-10 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 HARRIS PHARM EAGEX 67405-0575-50 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 HARRIS PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 67405-0575-96 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 HARRIS PHARM EAGEX 68<strong>08</strong>4-0253-01 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0253-11 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 AHP EAGEX 68462-0360-01 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 GLENMARK PHARMA EAGEX 68462-0360-05 0.09300 HYDROXYZINE HCL 10 MG TABLET 0 GLENMARK PHARMA EAGEX 00093-5<strong>06</strong>1-01 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 TEVA USA EAGEX 00093-5<strong>06</strong>1-05 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 TEVA USA EAGEX 00093-5<strong>06</strong>1-10 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 TEVA USA EAGEX 00378-2587-01 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 MYLAN EAGEX 00378-2587-10 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-3968-21 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3968-28 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3968-32 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 QUALITEST EAGEX 10702-0011-01 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 KVK-TECH, INC. EAGEX 10702-0011-10 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 KVK-TECH, INC. EAGEX 10702-0011-50 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 KVK-TECH, INC. EAGEX 16714-0<strong>08</strong>2-04 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-0<strong>08</strong>2-05 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-0<strong>08</strong>2-<strong>06</strong> 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-0<strong>08</strong>2-10 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 23155-01<strong>06</strong>-01 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 HERITAGE PHARMA EAGEX 23155-01<strong>06</strong>-05 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 HERITAGE PHARMA EAGEX 23155-01<strong>06</strong>-10 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 HERITAGE PHARMA EAGEX 51079-<strong>08</strong><strong>06</strong>-01 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-<strong>08</strong><strong>06</strong>-20 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 MYLAN INSTITUTI EAGEX 63739-0486-10 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 MCKESSON PACKAG EAGEX 67405-<strong>06</strong>71-10 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 HARRIS PHARM EAGEX 67405-<strong>06</strong>71-50 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 HARRIS PHARM EAGEX 67405-<strong>06</strong>71-96 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 HARRIS PHARM EAGEX 68<strong>08</strong>4-0254-01 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0254-11 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 AHP EAGEX 68462-0361-01 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 GLENMARK PHARMA EAGEX 68462-0361-05 0.17270 HYDROXYZINE HCL 25 MG TABLET 0 GLENMARK PHARMA EAGEX 00093-5<strong>06</strong>2-01 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 TEVA USA EAGEX 00093-5<strong>06</strong>2-05 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 184LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-5<strong>06</strong>2-10 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 TEVA USA EAGEX 00378-2588-01 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 MYLAN EAGEX 00378-2588-10 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 MYLAN EAGEX 0<strong>06</strong>03-3969-21 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3969-28 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 QUALITEST EAGEX 10702-0012-01 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 KVK-TECH, INC. EAGEX 10702-0012-10 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 KVK-TECH, INC. EAGEX 10702-0012-50 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 KVK-TECH, INC. EAGEX 16714-0<strong>08</strong>3-04 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-0<strong>08</strong>3-05 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 23155-0107-01 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 HERITAGE PHARMA EAGEX 23155-0107-05 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 HERITAGE PHARMA EAGEX 23155-0107-10 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 HERITAGE PHARMA EAGEX 51079-<strong>08</strong>16-01 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-<strong>08</strong>16-20 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 MYLAN INSTITUTI EAGEX 67405-0577-10 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 HARRIS PHARM EAGEX 67405-0577-50 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 HARRIS PHARM EAGEX 68<strong>08</strong>4-0255-01 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0255-11 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 AHP EAGEX 68462-0362-01 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68462-0362-05 0.11100 HYDROXYZINE HCL 50 MG TABLET 0 GLENMARK PHARMA EABEX 00555-0324-02 0.52032 HYDROXYZINE PAM 100 MG CAP 0 BARR EAGEX 00185-<strong>06</strong>13-01 0.<strong>06</strong>670 HYDROXYZINE PAM 25 MG CAP 0 SANDOZ EAGEX 00185-<strong>06</strong>13-05 0.<strong>06</strong>670 HYDROXYZINE PAM 25 MG CAP 0 SANDOZ EAGEX 00555-0323-02 0.<strong>06</strong>670 HYDROXYZINE PAM 25 MG CAP 0 BARR EAGEX 00555-0323-04 0.<strong>06</strong>670 HYDROXYZINE PAM 25 MG CAP 0 BARR EAGEX 00591-<strong>08</strong>00-01 0.<strong>06</strong>670 HYDROXYZINE PAM 25 MG CAP 0 ACTAVIS PHARMA, EAGEX 00591-<strong>08</strong>00-05 0.<strong>06</strong>670 HYDROXYZINE PAM 25 MG CAP 0 ACTAVIS PHARMA, EAGEX 51079-0077-20 0.<strong>06</strong>670 HYDROXYZINE PAM 25 MG CAP 0 MYLAN INSTITUTI EAGEX 62584-0739-11 0.<strong>06</strong>670 HYDROXYZINE PAM 25 MG CAP 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64980-0169-01 0.<strong>06</strong>670 HYDROXYZINE PAM 25 MG CAP 0 RISING PHARM EAGEX 64980-0169-05 0.<strong>06</strong>670 HYDROXYZINE PAM 25 MG CAP 0 RISING PHARM EAGEX 00185-<strong>06</strong>15-01 0.<strong>08</strong>750 HYDROXYZINE PAM 50 MG CAP 0 SANDOZ EAGEX 00185-<strong>06</strong>15-05 0.<strong>08</strong>750 HYDROXYZINE PAM 50 MG CAP 0 SANDOZ EAGEX 00555-0302-02 0.<strong>08</strong>750 HYDROXYZINE PAM 50 MG CAP 0 BARR EAGEX 00555-0302-04 0.<strong>08</strong>750 HYDROXYZINE PAM 50 MG CAP 0 BARR EAGEX 00591-<strong>08</strong>01-01 0.<strong>08</strong>750 HYDROXYZINE PAM 50 MG CAP 0 ACTAVIS PHARMA, EAGEX 00591-<strong>08</strong>01-05 0.<strong>08</strong>750 HYDROXYZINE PAM 50 MG CAP 0 ACTAVIS PHARMA, EAGEX 51079-0078-20 0.<strong>08</strong>750 HYDROXYZINE PAM 50 MG CAP 0 MYLAN INSTITUTI EAGEX 62584-0741-11 0.<strong>08</strong>750 HYDROXYZINE PAM 50 MG CAP 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64980-0170-01 0.<strong>08</strong>750 HYDROXYZINE PAM 50 MG CAP 0 RISING PHARM EAGEX 64980-0170-05 0.<strong>08</strong>750 HYDROXYZINE PAM 50 MG CAP 0 RISING PHARM EAGEX 10702-0052-16 0.<strong>06</strong>250 HYDROXYZINE 10 MG/5 ML SYRUP 0 KVK-TECH, INC. MLGEX 50383-0796-16 0.<strong>06</strong>250 HYDROXYZINE 10 MG/5 ML SYRUP 0 HI-TECH PHARMAC MLGEX 60432-0150-04 0.<strong>06</strong>250 HYDROXYZINE 10 MG/5 ML SYRUP 0 MORTON GROVE PH ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 185LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60432-0150-16 0.<strong>06</strong>250 HYDROXYZINE 10 MG/5 ML SYRUP 0 MORTON GROVE PH MLBEX 00517-5610-25 0.72420 0.88189 HYDROXYZINE 50 MG/ML VIAL 0 AMER. REGENT MLGEN 00574-0246-01 0.48<strong>06</strong>0 HYOSCYAMINE SULF 0.125 MG TAB 0 PADDOCK LABS. EAGEN 42192-0340-01 0.43500 HYOSCYAMINE SULF 0.125 MG TAB 0 ACELLA PHARMACE EAGEN 43199-0013-01 0.48<strong>06</strong>0 HYOSCYAMINE SULF 0.125 MG TAB 0 COUNTY LINE PHA EAGEN 00574-0247-01 0.40950 HYOSCYAMINE 0.125 MG ODT 0 PADDOCK LABS. EAGEN 42192-0338-01 0.40950 HYOSCYAMINE 0.125 MG ODT 0 ACELLA PHARMACE EAGEN 42192-0338-05 0.26100 HYOSCYAMINE 0.125 MG ODT 0 ACELLA PHARMACE EAGEN 43199-0012-01 0.40950 HYOSCYAMINE 0.125 MG ODT 0 COUNTY LINE PHA EAGEN 76439-0307-10 0.40950 HYOSCYAMINE 0.125 MG ODT 0 VIRTUS PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00574-0250-01 0.63750 HYOSCYAMINE 0.125 MG TAB SL 0 PADDOCK LABS. EAGEN 42192-0339-01 0.43500 HYOSCYAMINE 0.125 MG TAB SL 0 ACELLA PHARMACE EAGEN 42192-0339-05 0.26100 HYOSCYAMINE 0.125 MG TAB SL 0 ACELLA PHARMACE EAGEN 43199-0011-01 0.63750 HYOSCYAMINE 0.125 MG TAB SL 0 COUNTY LINE PHA EAGEN 76439-0309-10 0.72367 HYOSCYAMINE 0.125 MG TAB SL 0 VIRTUS PHARMACE EAGEN 60258-<strong>08</strong>02-15 0.99160 HYOSCYAMINE 0.125 MG/ML DROP 0 CYPRESS PHARM. MLGEN 60258-<strong>08</strong>01-16 0.05550 HYOSCYAMINE 125 MCG/5 ML ELIX 0 CYPRESS PHARM. MLGEN 54838-05<strong>06</strong>-15 0.99160 HYOSYNE 0.125 MG/ML DROP 0 SILARX PHARM MLBND 000<strong>06</strong>-0745-31 0.14000 4.29884 HYZAAR 100-12.5 TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0745-54 0.14000 4.29893 HYZAAR 100-12.5 TABLET G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-0745-82 0.14000 4.29888 HYZAAR 100-12.5 TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0747-31 0.18240 4.29884 HYZAAR 100-25 TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0747-54 0.18240 4.29893 HYZAAR 100-25 TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0747-82 0.18240 4.29888 HYZAAR 100-25 TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0717-31 0.09844 3.15593 HYZAAR 50-12.5 TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0717-54 0.09844 3.15602 HYZAAR 50-12.5 TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0717-82 0.09844 3.15596 HYZAAR 50-12.5 TABLET G MERCK SHARP & D EAGEN 00378-5215-32 71.60500 IBANDRONATE SODIUM 150 MG TAB G MYLAN EAGEN 00378-5215-53 71.60500 IBANDRONATE SODIUM 150 MG TAB G MYLAN EAGEN 00591-3770-31 71.60500 IBANDRONATE SODIUM 150 MG TAB G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0575-03 71.60500 IBANDRONATE SODIUM 150 MG TAB G DR.REDDY'S LAB EAGEN 55111-0575-11 71.60500 IBANDRONATE SODIUM 150 MG TAB G DR.REDDY'S LAB EAGEN 60505-2795-00 71.60500 IBANDRONATE SODIUM 150 MG TAB G APOTEX CORP EAGEN 38779-0299-<strong>08</strong> 1.69575 IBUPR<strong>OF</strong>EN POWDER 0 MEDISCA INC. GMGUL 00904-1748-40 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 MAJOR PHARMACEU EAGUL 00904-1748-60 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 MAJOR PHARMACEU EAGUL 00904-5853-60 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 MAJOR PHARMACEU EAGUL 52605-0121-01 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 POLYGEN PHARMAC EAGUL 52605-0121-05 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 POLYGEN PHARMAC EAGUL 53746-0464-01 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 53746-0464-05 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 AMNEAL PHARMACE EAGUL 55111-<strong>06</strong>82-01 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 DR.REDDY'S LAB EAGUL 55111-<strong>06</strong>82-05 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 DR.REDDY'S LAB EAGUL 63739-0442-01 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 MCKESSON PACKAG EAGUL 63739-0442-10 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 MCKESSON PACKAG EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 186LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 67877-0294-01 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 ASCEND LABORATO EAGUL 67877-0294-05 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 ASCEND LABORATO EAGUL 68<strong>08</strong>4-<strong>06</strong>58-01 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 AHP EAGUL 68645-0220-90 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 LEGACY PHARMACE EAGUL 68645-0474-90 0.03450 IBUPR<strong>OF</strong>EN 400 MG TABLET 0 LEGACY PHARMACE EAGUL 00904-5186-40 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 MAJOR PHARMACEU EAGUL 00904-5186-60 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 MAJOR PHARMACEU EAGUL 00904-5854-61 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 MAJOR PHARMACEU EAGUL 52605-0122-01 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 POLYGEN PHARMAC EAGUL 52605-0122-05 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 POLYGEN PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 53746-0465-01 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 AMNEAL PHARMACE EAGUL 53746-0465-05 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 AMNEAL PHARMACE EAGUL 53746-0465-50 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 AMNEAL PHARMACE EAGUL 53746-0465-60 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 AMNEAL PHARMACE EAGUL 53746-0465-90 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 AMNEAL PHARMACE EAGUL 55111-<strong>06</strong>83-01 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 DR.REDDY'S LAB EAGUL 55111-<strong>06</strong>83-05 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 DR.REDDY'S LAB EAGUL 55111-<strong>06</strong>83-09 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 DR.REDDY'S LAB EAGUL 55111-<strong>06</strong>83-30 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 DR.REDDY'S LAB EAGUL 55111-<strong>06</strong>83-50 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 62584-0747-11 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 AHP EAGUL 63739-0443-01 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 MCKESSON PACKAG EAGUL 63739-0443-10 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 MCKESSON PACKAG EAGUL 67877-0120-05 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 ASCEND LABORATO EAGUL 67877-0295-01 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 ASCEND LABORATO EAGUL 67877-0295-05 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 ASCEND LABORATO EAGUL 67877-0295-30 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 ASCEND LABORATO EAGUL 67877-0295-50 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 ASCEND LABORATO EAGUL 68<strong>08</strong>4-0703-01 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 AHP EAGUL 68645-0221-59 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 LEGACY PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 68645-0475-59 0.04170 IBUPR<strong>OF</strong>EN 600 MG TABLET 0 LEGACY PHARMACE EAGUL 00904-5187-40 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 MAJOR PHARMACEU EAGUL 00904-5187-60 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 MAJOR PHARMACEU EAGUL 00904-5855-61 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 MAJOR PHARMACEU EAGUL 49884-0779-05 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 PAR PHARM. EAGUL 52605-0123-05 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 POLYGEN PHARMAC EAGUL 53746-0466-01 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 AMNEAL PHARMACE EAGUL 53746-0466-05 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 AMNEAL PHARMACE EAGUL 53746-0466-90 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 AMNEAL PHARMACE EAGUL 55111-<strong>06</strong>84-01 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 55111-<strong>06</strong>84-05 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 DR.REDDY'S LAB EAGUL 55111-<strong>06</strong>84-09 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 DR.REDDY'S LAB EAGUL 55111-<strong>06</strong>84-30 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 DR.REDDY'S LAB EAGUL 55111-<strong>06</strong>84-50 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 DR.REDDY'S LAB EAGUL 55111-<strong>06</strong>84-60 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 DR.REDDY'S LAB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 187LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 62584-0748-11 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 AHP EAGUL 63739-0444-01 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 MCKESSON PACKAG EAGUL 63739-0444-10 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 MCKESSON PACKAG EAGUL 67877-0121-05 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 ASCEND LABORATO EAGUL 67877-0296-01 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 ASCEND LABORATO EAGUL 67877-0296-05 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 ASCEND LABORATO EAGUL 67877-0296-30 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 ASCEND LABORATO EAGUL 67877-0296-50 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 ASCEND LABORATO EAGUL 68<strong>08</strong>4-0772-01 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 AHP EAGUL 68645-0222-54 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 LEGACY PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 68645-0476-54 0.<strong>06</strong>380 IBUPR<strong>OF</strong>EN 800 MG TABLET 0 LEGACY PHARMACE EABND 00338-3991-01 66.11780 IFEX 1 GM VIAL 0 BAXTER <strong>HEALTH</strong>CA EABND 00338-3993-01 104.21480 IFEX 3 GM VIAL 0 BAXTER <strong>HEALTH</strong>CA EABND 00<strong>06</strong>5-1750-07 92.27647 ILEVRO 0.3% OPHTH DROPS G ALCON LABS. MLBND 57962-0140-09 90.74666 IMBRUVICA 140 MG CAPSULE 0 PHARMACYCLICS, EABND 57962-0140-12 90.74666 IMBRUVICA 140 MG CAPSULE 0 PHARMACYCLICS, EABND 00<strong>08</strong>5-0091-01 0.45023 4.37559 IMDUR ER 120 MG TABLET G MERCK SHARP & D EAGEN 00<strong>08</strong>5-1374-01 0.22194 IMDUR ER 30 MG TABLET G MERCK SHARP & D EAGEN 00<strong>08</strong>5-2028-01 0.24530 IMDUR ER 60 MG TABLET G MERCK SHARP & D EAGEN 00409-35<strong>08</strong>-01 4.72500 IMIPENEM-CILASTATIN 250 MG VL 0 HOSPIRA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-35<strong>08</strong>-10 4.25700 IMIPENEM-CILASTATIN 250 MG VL 0 HOSPIRA/NOVA+ EAGEN 63323-0349-20 5.14500 IMIPENEM-CILASTATIN 250 MG VL 0 APP PHARMACEUTI EAGEN 63323-0349-25 5.14500 IMIPENEM-CILASTATIN 250 MG VL 0 APP PHARMACEUTI EAGEN 00409-3507-01 9.45000 IMIPENEM-CILASTATIN 500 MG VL 0 HOSPIRA EAGEN 00409-3507-10 8.50500 IMIPENEM-CILASTATIN 500 MG VL 0 HOSPIRA/NOVA+ EAGEN 63323-0322-20 11.13000 IMIPENEM-CILASTATIN 500 MG VL 0 APP PHARMACEUTI EAGEN 63323-0322-25 11.13000 IMIPENEM-CILASTATIN 500 MG VL 0 APP PHARMACEUTI EAGEX 00781-1762-01 0.12825 IMIPRAMINE HCL 10 MG TABLET 0 SANDOZ EAGEX 49884-0054-01 0.12825 IMIPRAMINE HCL 10 MG TABLET 0 PAR PHARM. EAGEX 49884-0054-10 0.12825 IMIPRAMINE HCL 10 MG TABLET 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 53489-0330-01 0.12825 IMIPRAMINE HCL 10 MG TABLET 0 MUTUAL PHARM CO EAGEX 64125-0133-01 0.12825 IMIPRAMINE HCL 10 MG TABLET 0 EXCELLIUM PHARM EAGEX 64125-0133-10 0.12825 IMIPRAMINE HCL 10 MG TABLET 0 EXCELLIUM PHARM EAGEX 68180-0311-01 0.12825 IMIPRAMINE HCL 10 MG TABLET 0 LUPIN PHARMACEU EAGEX 00781-1764-01 0.17280 IMIPRAMINE HCL 25 MG TABLET 0 SANDOZ EAGEX 00781-1764-10 0.17280 IMIPRAMINE HCL 25 MG TABLET 0 SANDOZ EAGEX 49884-0055-01 0.17280 IMIPRAMINE HCL 25 MG TABLET 0 PAR PHARM. EAGEX 49884-0055-10 0.17280 IMIPRAMINE HCL 25 MG TABLET 0 PAR PHARM. EAGEX 53489-0331-01 0.17280 IMIPRAMINE HCL 25 MG TABLET 0 MUTUAL PHARM CO EAGEX 64125-0134-01 0.17280 IMIPRAMINE HCL 25 MG TABLET 0 EXCELLIUM PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64125-0134-10 0.17280 IMIPRAMINE HCL 25 MG TABLET 0 EXCELLIUM PHARM EAGEX 68180-0312-01 0.17280 IMIPRAMINE HCL 25 MG TABLET 0 LUPIN PHARMACEU EAGEX 00781-1766-01 0.23420 IMIPRAMINE HCL 50 MG TABLET 0 SANDOZ EAGEX 00781-1766-10 0.23420 IMIPRAMINE HCL 50 MG TABLET 0 SANDOZ EAGEX 49884-0056-01 0.23420 IMIPRAMINE HCL 50 MG TABLET 0 PAR PHARM. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 188LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 49884-0056-10 0.23420 IMIPRAMINE HCL 50 MG TABLET 0 PAR PHARM. EAGEX 53489-0332-01 0.23420 IMIPRAMINE HCL 50 MG TABLET 0 MUTUAL PHARM CO EAGEX 64125-0135-01 0.23420 IMIPRAMINE HCL 50 MG TABLET 0 EXCELLIUM PHARM EAGEX 64125-0135-10 0.23420 IMIPRAMINE HCL 50 MG TABLET 0 EXCELLIUM PHARM EAGEX 68180-0313-01 0.23420 IMIPRAMINE HCL 50 MG TABLET 0 LUPIN PHARMACEU EAGEX 00054-0274-13 11.37900 IMIPRAMINE PAMOATE 100 MG CAP 0 ROXANE LABS. EAGEX 004<strong>06</strong>-9932-03 11.56432 IMIPRAMINE PAMOATE 100 MG CAP 0 MALLINCKRODT PH EAGEX 68180-0315-<strong>06</strong> 11.56432 IMIPRAMINE PAMOATE 100 MG CAP 0 LUPIN PHARMACEU EAGEX 00054-0275-13 9.20420 IMIPRAMINE PAMOATE 125 MG CAP 0 ROXANE LABS. EAGEX 004<strong>06</strong>-9933-03 9.20420 IMIPRAMINE PAMOATE 125 MG CAP 0 MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68180-0316-<strong>06</strong> 9.20420 IMIPRAMINE PAMOATE 125 MG CAP 0 LUPIN PHARMACEU EAGEX 00054-0276-13 11.35617 IMIPRAMINE PAMOATE 150 MG CAP 0 ROXANE LABS. EAGEX 004<strong>06</strong>-9934-03 11.35617 IMIPRAMINE PAMOATE 150 MG CAP 0 MALLINCKRODT PH EAGEX 68180-0317-<strong>06</strong> 11.35617 IMIPRAMINE PAMOATE 150 MG CAP 0 LUPIN PHARMACEU EAGEX 00054-0273-13 8.26440 IMIPRAMINE PAMOATE 75 MG CAP 0 ROXANE LABS. EAGEX 004<strong>06</strong>-9931-03 8.26440 IMIPRAMINE PAMOATE 75 MG CAP 0 MALLINCKRODT PH EAGEX 68180-0314-<strong>06</strong> 8.26440 IMIPRAMINE PAMOATE 75 MG CAP 0 LUPIN PHARMACEU EAGEN 00093-6126-19 16.23000 IMIQUIMOD 5% CREAM PACKET 0 TEVA USA EAGEN 00093-6126-64 16.23000 IMIQUIMOD 5% CREAM PACKET 0 TEVA USA EAGEN 00115-1476-23 16.23000 IMIQUIMOD 5% CREAM PACKET 0 GLOBAL PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00168-0432-24 16.23000 IMIQUIMOD 5% CREAM PACKET 0 SANDOZ EAGEN 00781-7152-09 16.23000 IMIQUIMOD 5% CREAM PACKET 0 SANDOZ EAGEN 45802-0368-62 16.23000 IMIQUIMOD 5% CREAM PACKET 0 PERRIGO CO. EAGEN 51672-4145-<strong>06</strong> 7.81250 IMIQUIMOD 5% CREAM PACKET 0 TARO PHARM USA EAGEN 51672-4145-<strong>08</strong> 7.81500 IMIQUIMOD 5% CREAM PACKET 0 TARO PHARM USA EAGEN 60505-0501-05 16.23000 IMIQUIMOD 5% CREAM PACKET 0 APOTEX CORP EAGEN 68462-0536-70 16.23000 IMIQUIMOD 5% CREAM PACKET 0 GLENMARK PHARMA EABND 00173-0737-01 1.00790 35.12467 IMITREX 100 MG TABLET G GLAXOSMITHKLINE EABND 00173-0523-00 35.82500 43.84<strong>06</strong>0 IMITREX 20 MG NASAL SPRAY G GLAXOSMITHKLINE EABND 00173-0735-00 0.84470 27.59565 IMITREX 25 MG TABLET G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-0739-02 147.55200 207.95650 IMITREX 4 MG/0.5 ML CARTRIDGES G GLAXOSMITHKLINE MLBND 00173-0739-00 155.78400 219.55990 IMITREX 4 MG/0.5 ML PEN INJECT G GLAXOSMITHKLINE MLBND 00173-0524-00 31.88<strong>06</strong>0 43.84<strong>06</strong>0 IMITREX 5 MG NASAL SPRAY G GLAXOSMITHKLINE EABND 00173-0736-01 1.15000 35.12467 IMITREX 50 MG TABLET G GLAXOSMITHKLINE EABND 00173-0478-00 131.17000 207.95650 IMITREX 6 MG/0.5 ML CARTRIDGES G GLAXOSMITHKLINE MLBND 00173-0479-00 147.52000 219.55990 IMITREX 6 MG/0.5 ML PEN INJECT G GLAXOSMITHKLINE MLBND 00173-0449-02 75.98740 204.824<strong>08</strong> IMITREX 6 MG/0.5 ML VIAL G GLAXOSMITHKLINE MLBND 65483-0590-10 0.34913 6.28841 IMURAN 50 MG TABLET G PROMETHEUS EAGEN 63044-0154-01 0.07900 INATAL ULTRA TABLET 0 NNODUM CORP EABND 51167-0100-01 130.73507 INCIVEK 375 MG TABLET G VERTEX PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 51167-0100-03 130.73507 INCIVEK 375 MG TABLET G VERTEX PHARMACE EABND 15054-1040-05 331.49370 INCRELEX 40 MG/4 ML VIAL 0 IPSEN BIOPHARMA MLGUL 00228-2597-11 0.10350 INDAPAMIDE 1.25 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00228-2597-96 0.10350 INDAPAMIDE 1.25 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00378-0<strong>06</strong>9-01 0.10350 INDAPAMIDE 1.25 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 189LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-0<strong>06</strong>9-05 0.10350 INDAPAMIDE 1.25 MG TABLET 0 MYLAN EAGUL 00228-2571-11 0.11250 INDAPAMIDE 2.5 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00228-2571-96 0.11250 INDAPAMIDE 2.5 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00378-0<strong>08</strong>0-01 0.11250 INDAPAMIDE 2.5 MG TABLET 0 MYLAN EAGUL 00378-0<strong>08</strong>0-10 0.11250 INDAPAMIDE 2.5 MG TABLET 0 MYLAN EABND 24090-0473-88 1.31963 14.73341 INDERAL LA 120 MG CAPSULE G AKRIMAX PHARMAC EABND 24090-0479-88 1.62650 17.94393 INDERAL LA 160 MG CAPSULE G AKRIMAX PHARMAC EABND 24090-0470-88 0.80784 10.48431 INDERAL LA 60 MG CAPSULE G AKRIMAX PHARMAC EABND 24090-0471-88 1.<strong>06</strong>529 12.24590 INDERAL LA 80 MG CAPSULE G AKRIMAX PHARMAC EABND 42211-0101-11 1.21961 INDOCIN 25 MG/5 ML SUSPENSION G IROKO PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0720-01 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 SANDOZ EAGEN 00185-0720-05 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 SANDOZ EAGEN 00185-0720-60 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 SANDOZ EAGEN 00574-0193-01 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 PADDOCK LABS. EAGEN 00574-0193-60 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 PADDOCK LABS. EAGEN 10702-0016-01 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 KVK-TECH, INC. EAGEN 10702-0016-03 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 KVK-TECH, INC. EAGEN 10702-0016-<strong>06</strong> 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 KVK-TECH, INC. EAGEN 10702-0016-10 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 KVK-TECH, INC. EAGEN 10702-0016-50 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 KVK-TECH, INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0565-01 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 CAMBER PHARMACE EAGEN 31722-0565-60 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 CAMBER PHARMACE EAGEN 45963-<strong>08</strong>11-<strong>06</strong> 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 65162-05<strong>06</strong>-03 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 AMNEAL PHARMACE EAGEN 65162-05<strong>06</strong>-<strong>06</strong> 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 AMNEAL PHARMACE EAGEN 65162-05<strong>06</strong>-09 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 AMNEAL PHARMACE EAGEN 65162-05<strong>06</strong>-50 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-0411-11 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-0411-21 1.19030 INDOMETHACIN ER 75 MG CAPSULE 0 AHP EAGEN 38779-0076-05 4.65705 INDOMETHACIN POWDER 0 MEDISCA INC. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51927-1<strong>08</strong>3-00 3.67500 INDOMETHACIN POWDER 0 PR<strong>OF</strong>ESSIONAL CO GMGEN 00093-4029-01 0.13325 INDOMETHACIN 25 MG CAPSULE 0 TEVA USA EAGEN 00093-4029-10 0.13325 INDOMETHACIN 25 MG CAPSULE 0 TEVA USA EAGEN 00378-0143-01 0.13325 INDOMETHACIN 25 MG CAPSULE 0 MYLAN EAGEN 00378-0143-10 0.13325 INDOMETHACIN 25 MG CAPSULE 0 MYLAN EAGEN 00781-2325-01 0.13325 INDOMETHACIN 25 MG CAPSULE 0 SANDOZ EAGEN 00781-2325-10 0.13325 INDOMETHACIN 25 MG CAPSULE 0 SANDOZ EAGEN 23155-0010-01 0.13325 INDOMETHACIN 25 MG CAPSULE 0 HERITAGE PHARMA EAGEN 23155-0010-10 0.13325 INDOMETHACIN 25 MG CAPSULE 0 HERITAGE PHARMA EAGEN 31722-0542-01 0.13325 INDOMETHACIN 25 MG CAPSULE 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0190-20 0.13325 INDOMETHACIN 25 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 51079-0190-56 0.13325 INDOMETHACIN 25 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 68462-04<strong>06</strong>-01 0.13325 INDOMETHACIN 25 MG CAPSULE 0 GLENMARK PHARMA EAGEN 68462-04<strong>06</strong>-10 0.13325 INDOMETHACIN 25 MG CAPSULE 0 GLENMARK PHARMA EAGEN 00093-4030-01 0.16430 INDOMETHACIN 50 MG CAPSULE 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 190LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-4030-05 0.16430 INDOMETHACIN 50 MG CAPSULE 0 TEVA USA EAGEN 00378-0147-01 0.16430 INDOMETHACIN 50 MG CAPSULE 0 MYLAN EAGEN 00378-0147-05 0.16430 INDOMETHACIN 50 MG CAPSULE 0 MYLAN EAGEN 00781-2350-01 0.16430 INDOMETHACIN 50 MG CAPSULE 0 SANDOZ EAGEN 00781-2350-05 0.16430 INDOMETHACIN 50 MG CAPSULE 0 SANDOZ EAGEN 23155-0011-01 0.16430 INDOMETHACIN 50 MG CAPSULE 0 HERITAGE PHARMA EAGEN 23155-0011-05 0.16430 INDOMETHACIN 50 MG CAPSULE 0 HERITAGE PHARMA EAGEN 31722-0543-01 0.16430 INDOMETHACIN 50 MG CAPSULE 0 CAMBER PHARMACE EAGEN 51079-0191-20 0.16430 INDOMETHACIN 50 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 51079-0191-56 0.16430 INDOMETHACIN 50 MG CAPSULE 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0302-01 0.16430 INDOMETHACIN 50 MG CAPSULE 0 GLENMARK PHARMA EAGEN 68462-0302-05 0.16430 INDOMETHACIN 50 MG CAPSULE 0 GLENMARK PHARMA EABND 52544-0931-02 15.64716 INFED 100 MG/2 ML VIAL 0 ACTAVIS PHARMA, MLBND 52544-0931-07 15.64716 INFED 100 MG/2 ML VIAL 0 ACTAVIS PHARMA, MLBND 66435-0201-15 288.49140 INFERGEN 15 MCG/0.5 ML VIAL 0 KADMON PHARMACE MLBND 66435-0202-09 480.81900 INFERGEN 9 MCG/0.3 ML VIAL 0 KADMON PHARMACE MLBND 66435-0202-95 480.81900 INFERGEN 9 MCG/0.3 ML VIAL 0 KADMON PHARMACE MLBND 00<strong>06</strong>9-0145-01 53.80152 INLYTA 1 MG TABLET 0 PFIZER US PHARM EABND 00<strong>06</strong>9-0151-11 161.40456 INLYTA 5 MG TABLET 0 PFIZER US PHARM EABND 00173-0791-01 10.95600 INNOPRAN XL 120 MG CAPSULE G AKRIMAX PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-0791-02 13.12528 INNOPRAN XL 120 MG CAPSULE G AKRIMAX PHARMAC EABND 24090-0451-85 13.12534 INNOPRAN XL 120 MG CAPSULE G AKRIMAX PHARMAC EABND 00173-0790-01 10.95600 INNOPRAN XL 80 MG CAPSULE G AKRIMAX PHARMAC EABND 00173-0790-02 13.12528 INNOPRAN XL 80 MG CAPSULE G AKRIMAX PHARMAC EABND 24090-0450-85 13.12534 INNOPRAN XL 80 MG CAPSULE G AKRIMAX PHARMAC EABND 00025-1710-01 2.35805 5.79533 INSPRA 25 MG TABLET G PFIZER US PHARM EABND 00025-1710-02 2.35805 5.79552 INSPRA 25 MG TABLET G PFIZER US PHARM EABND 00025-1720-01 2.40570 5.79552 INSPRA 50 MG TABLET G PFIZER US PHARM EABND 00025-1720-03 2.40570 5.79533 INSPRA 50 MG TABLET G PFIZER US PHARM EABND 59676-0570-01 7.77101 INTELENCE 100 MG TABLET G JANSSEN PRODUCT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59676-0571-01 15.54202 INTELENCE 200 MG TABLET G JANSSEN PRODUCT EABND 59676-0572-01 1.94275 INTELENCE 25 MG TABLET G JANSSEN PRODUCT EABND 00<strong>08</strong>5-1133-01 186.43875 INTRON A 10 MILLION UNIT/ML 0 MERCK SHARP & D MLBND 00<strong>08</strong>5-0571-02 185.43860 INTRON A 10 MILLION UNITS VIAL 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1110-01 333.80110 INTRON A 18 MILLION UNITS VIAL 0 MERCK SHARP & D EABND 00<strong>08</strong>5-0539-01 927.31750 INTRON A 50 MILLION UNITS VIAL 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1168-01 113.03071 INTRON A 6 MILLION UNIT/ML VL 0 MERCK SHARP & D MLGEX 00781-5584-36 1.24580 INTROVALE 0.15-0.03 MG TABLET 0 SANDOZ EAGEX 00781-5584-91 1.24580 INTROVALE 0.15-0.03 MG TABLET 0 SANDOZ EABND 54092-0513-02 7.99854 INTUNIV ER 1 MG TABLET G SHIRE US INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 54092-0515-02 7.99854 INTUNIV ER 2 MG TABLET G SHIRE US INC. EABND 54092-0517-02 7.99854 INTUNIV ER 3 MG TABLET 0 SHIRE US INC. EABND 54092-0519-02 7.99854 INTUNIV ER 4 MG TABLET 0 SHIRE US INC. EABND 000<strong>06</strong>-3845-71 77.66974 INVANZ 1 GM ADD-VANTAGE VIAL 0 MERCK SHARP & D EABND 000<strong>06</strong>-3843-71 73.97790 INVANZ 1 GM VIAL 0 MERCK SHARP & D EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 191LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 50458-0554-01 22.03926 INVEGA ER 1.5 MG TABLET G JANSSEN PHARM. EABEX 50458-0550-01 22.03926 INVEGA ER 3 MG TABLET G JANSSEN PHARM. EABEX 50458-0550-10 22.03940 INVEGA ER 3 MG TABLET G JANSSEN PHARM. EABEX 50458-0551-01 22.03926 INVEGA ER 6 MG TABLET G JANSSEN PHARM. EABEX 50458-0551-10 22.03940 INVEGA ER 6 MG TABLET G JANSSEN PHARM. EABEX 50458-0552-01 33.05890 INVEGA ER 9 MG TABLET G JANSSEN PHARM. EABEX 50458-0552-10 33.05939 INVEGA ER 9 MG TABLET G JANSSEN PHARM. EABND 00004-0245-15 3.54231 INVIRASE 200 MG CAPSULE G GENENTECH, INC. EABND 00004-0244-51 8.14493 INVIRASE 500 MG TABLET G GENENTECH, INC. EABND 50458-0140-30 9.59950 INVOKANA 100 MG TABLET G JANSSEN PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50458-0140-90 9.59941 INVOKANA 100 MG TABLET G JANSSEN PHARM. EABND 50458-0141-30 9.59950 INVOKANA 300 MG TABLET G JANSSEN PHARM. EABND 50458-0141-90 9.59941 INVOKANA 300 MG TABLET G JANSSEN PHARM. EABND 00<strong>06</strong>5-<strong>06</strong>65-05 12.33540 24.18288 IOPIDINE 0.5% EYE DROPS G ALCON LABS. MLBND 00<strong>06</strong>5-<strong>06</strong>65-10 12.33540 23.99364 IOPIDINE 0.5% EYE DROPS G ALCON LABS. MLBND 00<strong>06</strong>5-<strong>06</strong>60-10 20.09430 IOPIDINE 1% EYE DROPS G ALCON LABS. EAGEN 00093-6723-73 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 TEVA USA MLGEN 00093-6723-74 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 TEVA USA MLGEN 00185-7322-30 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 SANDOZ MLGEN 00185-7322-60 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6988-58 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 MYLAN MLGEN 00378-6988-91 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 MYLAN MLGEN 00378-6988-93 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 MYLAN MLGEN 00378-9671-58 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 MYLAN MLGEN 00378-9671-93 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 MYLAN MLGEN 00487-0201-01 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 NEPHRON CORP MLGEN 00487-0201-02 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 NEPHRON CORP MLGEN 00487-0201-03 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 NEPHRON CORP MLGEN 00487-0201-60 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 NEPHRON CORP MLGEN 00591-3433-30 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 WATSON LABS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3433-60 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 WATSON LABS MLGEN 00591-3817-30 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 ACTAVIS PHARMA, MLGEN 00591-3817-60 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 ACTAVIS PHARMA, MLGEN 00781-7146-29 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 SANDOZ MLGEN 00781-7146-64 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 SANDOZ MLGEN 16252-0547-33 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 WATSON LABS MLGEN 16252-0547-66 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 WATSON LABS MLGEN 76204-<strong>06</strong>00-05 0.09666 IPRAT-ALBUT 0.5-3(2.5) MG/3 ML 0 RITEDOSE PHARMA MLGEN 00378-6989-93 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 MYLAN MLGEN 00378-7970-52 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 MYLAN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-7970-55 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 MYLAN MLGEN 00487-9801-01 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 NEPHRON CORP MLGEN 00487-9801-02 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 NEPHRON CORP MLGEN 00487-9801-25 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 NEPHRON CORP MLGEN 00487-9801-30 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 NEPHRON CORP ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 192LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00487-9801-60 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 NEPHRON CORP MLGEN 00591-3798-30 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 ACTAVIS PHARMA, MLGEN 00591-3798-60 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 ACTAVIS PHARMA, MLGEN 00591-3798-83 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 ACTAVIS PHARMA, MLGEN 00781-7157-29 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 SANDOZ MLGEN 00781-7157-64 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 SANDOZ MLGEN 00781-7157-86 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 SANDOZ MLGEN 76204-0100-25 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 RITEDOSE PHARMA MLGEN 76204-0100-30 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 RITEDOSE PHARMA MLGEN 76204-0100-60 0.<strong>06</strong>170 IPRATROPIUM BR 0.02% SOLN 0 RITEDOSE PHARMA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0045-44 0.88789 IPRATROPIUM 0.03% SPRAY 0 ROXANE LABS. MLGEN 242<strong>08</strong>-0398-30 0.88789 IPRATROPIUM 0.03% SPRAY 0 VALEANT MLGEN 00054-0251-13 0.24000 IRBESARTAN 150 MG TABLET G ROXANE LABS. EAGEN 00054-0251-22 0.24000 IRBESARTAN 150 MG TABLET G ROXANE LABS. EAGEN 00093-7465-05 0.24000 IRBESARTAN 150 MG TABLET G TEVA USA EAGEN 00093-7465-56 0.24000 IRBESARTAN 150 MG TABLET G TEVA USA EAGEN 00093-7465-98 0.24000 IRBESARTAN 150 MG TABLET G TEVA USA EAGEN 00378-2023-05 0.24000 IRBESARTAN 150 MG TABLET G MYLAN EAGEN 00378-2023-77 0.24000 IRBESARTAN 150 MG TABLET G MYLAN EAGEN 00378-2023-93 0.24000 IRBESARTAN 150 MG TABLET G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-2783-19 0.24000 IRBESARTAN 150 MG TABLET G WATSON LABS EAGEN 00591-2783-30 0.24000 IRBESARTAN 150 MG TABLET G WATSON LABS EAGEN 00955-1041-90 0.24000 IRBESARTAN 150 MG TABLET G WINTHROP US EAGEN 31722-0730-30 0.24000 IRBESARTAN 150 MG TABLET G CAMBER PHARMACE EAGEN 31722-0730-90 0.24000 IRBESARTAN 150 MG TABLET G CAMBER PHARMACE EAGEN 33342-0048-10 0.24000 IRBESARTAN 150 MG TABLET G MACLEODS PHARMA EAGEN 43547-0278-03 0.24000 IRBESARTAN 150 MG TABLET G SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0278-09 0.24000 IRBESARTAN 150 MG TABLET G SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0278-50 0.24000 IRBESARTAN 150 MG TABLET G SOLCO <strong>HEALTH</strong>CAR EAGEN 60505-3548-03 0.24000 IRBESARTAN 150 MG TABLET G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-3548-05 0.24000 IRBESARTAN 150 MG TABLET G APOTEX CORP EAGEN 60505-3548-09 0.24000 IRBESARTAN 150 MG TABLET G APOTEX CORP EAGEN 65862-<strong>06</strong>38-30 0.24000 IRBESARTAN 150 MG TABLET G AUROBINDO PHARM EAGEN 65862-<strong>06</strong>38-90 0.24000 IRBESARTAN 150 MG TABLET G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0007-21 0.24000 IRBESARTAN 150 MG TABLET G AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>44-21 0.24000 IRBESARTAN 150 MG TABLET G AHP EAGEN 68180-0411-<strong>06</strong> 0.24000 IRBESARTAN 150 MG TABLET G LUPIN PHARMACEU EAGEN 68180-0411-09 0.24000 IRBESARTAN 150 MG TABLET G LUPIN PHARMACEU EAGEN 68645-0404-70 0.24000 IRBESARTAN 150 MG TABLET G LEGACY PHARMACE EAGEN 00054-0252-13 0.34500 IRBESARTAN 300 MG TABLET G ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0252-22 0.34500 IRBESARTAN 300 MG TABLET G ROXANE LABS. EAGEN 00093-7466-05 0.34500 IRBESARTAN 300 MG TABLET G TEVA USA EAGEN 00093-7466-56 0.34500 IRBESARTAN 300 MG TABLET G TEVA USA EAGEN 00093-7466-98 0.34500 IRBESARTAN 300 MG TABLET G TEVA USA EAGEN 00093-7514-05 0.34500 IRBESARTAN 300 MG TABLET G TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 193LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7514-56 0.34500 IRBESARTAN 300 MG TABLET G TEVA USA EAGEN 00378-2024-05 0.34500 IRBESARTAN 300 MG TABLET G MYLAN EAGEN 00378-2024-77 0.34500 IRBESARTAN 300 MG TABLET G MYLAN EAGEN 00378-2024-93 0.34500 IRBESARTAN 300 MG TABLET G MYLAN EAGEN 00591-2784-19 0.34500 IRBESARTAN 300 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-2784-30 0.34500 IRBESARTAN 300 MG TABLET G ACTAVIS PHARMA, EAGEN 00955-1042-30 0.34500 IRBESARTAN 300 MG TABLET G WINTHROP US EAGEN 00955-1042-90 0.34500 IRBESARTAN 300 MG TABLET G WINTHROP US EAGEN 31722-0731-30 0.34500 IRBESARTAN 300 MG TABLET G CAMBER PHARMACE EAGEN 31722-0731-90 0.34500 IRBESARTAN 300 MG TABLET G CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 33342-0049-10 0.34500 IRBESARTAN 300 MG TABLET G MACLEODS PHARMA EAGEN 43547-0279-03 0.34500 IRBESARTAN 300 MG TABLET G SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0279-09 0.34500 IRBESARTAN 300 MG TABLET G SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0279-50 0.34500 IRBESARTAN 300 MG TABLET G SOLCO <strong>HEALTH</strong>CAR EAGEN 60505-3549-03 0.34500 IRBESARTAN 300 MG TABLET G APOTEX CORP EAGEN 60505-3549-05 0.34500 IRBESARTAN 300 MG TABLET G APOTEX CORP EAGEN 60505-3549-09 0.34500 IRBESARTAN 300 MG TABLET G APOTEX CORP EAGEN 65862-<strong>06</strong>39-05 0.34500 IRBESARTAN 300 MG TABLET G AUROBINDO PHARM EAGEN 65862-<strong>06</strong>39-30 0.34500 IRBESARTAN 300 MG TABLET G AUROBINDO PHARM EAGEN 65862-<strong>06</strong>39-90 0.34500 IRBESARTAN 300 MG TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0412-<strong>06</strong> 0.34500 IRBESARTAN 300 MG TABLET G LUPIN PHARMACEU EAGEN 68180-0412-09 0.34500 IRBESARTAN 300 MG TABLET G LUPIN PHARMACEU EAGEN 00054-0250-13 0.16950 IRBESARTAN 75 MG TABLET G ROXANE LABS. EAGEN 00054-0250-22 0.16950 IRBESARTAN 75 MG TABLET G ROXANE LABS. EAGEN 00093-7464-56 0.16950 IRBESARTAN 75 MG TABLET G TEVA USA EAGEN 00093-7464-98 0.16950 IRBESARTAN 75 MG TABLET G TEVA USA EAGEN 00378-2022-05 0.16950 IRBESARTAN 75 MG TABLET G MYLAN EAGEN 00378-2022-77 0.16950 IRBESARTAN 75 MG TABLET G MYLAN EAGEN 00591-2782-19 0.16950 IRBESARTAN 75 MG TABLET G WATSON LABS EAGEN 00591-2782-30 0.16950 IRBESARTAN 75 MG TABLET G WATSON LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00955-1040-90 0.16950 IRBESARTAN 75 MG TABLET G WINTHROP US EAGEN 31722-0729-30 0.16950 IRBESARTAN 75 MG TABLET G CAMBER PHARMACE EAGEN 31722-0729-90 0.16950 IRBESARTAN 75 MG TABLET G CAMBER PHARMACE EAGEN 33342-0047-10 0.16950 IRBESARTAN 75 MG TABLET G MACLEODS PHARMA EAGEN 43547-0277-03 0.16950 IRBESARTAN 75 MG TABLET G SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0277-09 0.16950 IRBESARTAN 75 MG TABLET G SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0277-50 0.16950 IRBESARTAN 75 MG TABLET G SOLCO <strong>HEALTH</strong>CAR EAGEN 60505-3547-03 0.16950 IRBESARTAN 75 MG TABLET G APOTEX CORP EAGEN 60505-3547-09 0.16950 IRBESARTAN 75 MG TABLET G APOTEX CORP EAGEN 65862-<strong>06</strong>37-30 0.16950 IRBESARTAN 75 MG TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-<strong>06</strong>37-90 0.16950 IRBESARTAN 75 MG TABLET G AUROBINDO PHARM EAGEN 68180-0410-<strong>06</strong> 0.16950 IRBESARTAN 75 MG TABLET G LUPIN PHARMACEU EAGEN 68180-0410-09 0.16950 IRBESARTAN 75 MG TABLET G LUPIN PHARMACEU EAGEN 00054-0254-13 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G ROXANE LABS. EAGEN 00054-0254-22 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G ROXANE LABS. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 194LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-8238-98 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G TEVA USA EAGEN 00378-3033-77 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G MYLAN EAGEN 00378-3033-93 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G MYLAN EAGEN 00591-2785-19 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G ACTAVIS PHARMA, EAGEN 00591-2785-30 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-4<strong>08</strong>8-02 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G QUALITEST EAGEN 0<strong>06</strong>03-4<strong>08</strong>8-16 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G QUALITEST EAGEN 00955-1045-30 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G WINTHROP US EAGEN 00955-1045-90 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G WINTHROP US EAGEN 60505-3603-03 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-3603-09 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G APOTEX CORP EAGEN 68180-0413-<strong>06</strong> 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G LUPIN PHARMACEU EAGEN 68180-0413-09 0.19319 IRBESARTAN-HCTZ 150-12.5 MG TB G LUPIN PHARMACEU EAGEN 00054-0255-13 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G ROXANE LABS. EAGEN 00054-0255-22 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G ROXANE LABS. EAGEN 00093-8232-56 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G TEVA USA EAGEN 00093-8232-98 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G TEVA USA EAGEN 00378-3034-77 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G MYLAN EAGEN 00378-3034-93 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G MYLAN EAGEN 00591-2786-19 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-2786-30 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-4<strong>08</strong>9-02 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G QUALITEST EAGEN 0<strong>06</strong>03-4<strong>08</strong>9-16 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G QUALITEST EAGEN 00955-1046-30 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G WINTHROP US EAGEN 00955-1046-90 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G WINTHROP US EAGEN 60505-3604-03 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G APOTEX CORP EAGEN 60505-3604-09 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G APOTEX CORP EAGEN 68180-0414-<strong>06</strong> 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G LUPIN PHARMACEU EAGEN 68180-0414-09 0.31941 IRBESARTAN-HCTZ 300-12.5 MG TB G LUPIN PHARMACEU EAGEN 00143-9701-01 11.25000 IRINOTECAN HCL 100 MG/5 ML VL 0 WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00703-4434-11 6.35400 IRINOTECAN HCL 100 MG/5 ML VL 0 TEVA PARENTERAL MLGEN 25021-0214-05 11.70000 IRINOTECAN HCL 100 MG/5 ML VL 0 SAGENT PHARMACE MLGEN 61703-0349-09 5.07450 IRINOTECAN HCL 100 MG/5 ML VL 0 HOSPIRA MLGEN 63323-0193-05 6.12000 IRINOTECAN HCL 100 MG/5 ML VL 0 APP PHARMACEUTI MLGEN 63323-0193-55 6.12000 IRINOTECAN HCL 100 MG/5 ML VL 0 APP PHARMACEUTI MLGEN 66758-0048-02 17.77600 IRINOTECAN HCL 100 MG/5 ML VL 0 SANDOZ INC. MLGEN 00143-9702-01 11.25000 IRINOTECAN HCL 40 MG/2 ML VIAL 0 WEST-WARD,INC. MLGEN 00703-4432-11 6.83250 IRINOTECAN HCL 40 MG/2 ML VIAL 0 TEVA PARENTERAL MLGEN 25021-0214-02 13.50000 IRINOTECAN HCL 40 MG/2 ML VIAL 0 SAGENT PHARMACE MLGEN 61703-0349-16 5.55375 IRINOTECAN HCL 40 MG/2 ML VIAL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0193-02 7.20000 IRINOTECAN HCL 40 MG/2 ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0193-52 7.20000 IRINOTECAN HCL 40 MG/2 ML VIAL 0 APP PHARMACEUTI MLGEN 66758-0048-01 18.37000 IRINOTECAN HCL 40 MG/2 ML VIAL 0 SANDOZ INC. MLBND 61703-0349-36 5.34022 IRINOTECAN HCL 500 MG/25 ML VL 0 HOSPIRA MLBND 000<strong>06</strong>-0477-61 4.67622 ISENTRESS 100 MG TABLET CHEW G MERCK SHARP & D EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 195LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-0473-61 1.16919 ISENTRESS 25 MG TABLET CHEW G MERCK SHARP & D EABND 000<strong>06</strong>-0227-61 18.70336 ISENTRESS 400 MG TABLET G MERCK SHARP & D EAGEN 00338-0505-48 0.03141 ISO GENTAMICIN 100 MG/100 ML 0 BAXTER <strong>HEALTH</strong>CA MLBND 00338-0507-48 0.03386 ISO GENTAMICIN 120 MG/100 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 52054-0183-10 1.<strong>08</strong>600 ISODITRATE ER 40 MG TABLET 0 AMEDRA PHARMACE EAGUX 00185-4351-01 0.05610 ISONIAZID 100 MG TABLET 0 SANDOZ EAGUX 00185-4351-10 0.05610 ISONIAZID 100 MG TABLET 0 SANDOZ EAGUX 00185-4351-30 0.05610 ISONIAZID 100 MG TABLET 0 SANDOZ EAGUX 00555-0<strong>06</strong>6-02 0.05610 ISONIAZID 100 MG TABLET 0 BARR EAGUX 00555-0<strong>06</strong>6-05 0.05610 ISONIAZID 100 MG TABLET 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00781-3056-70 29.22098 ISONIAZID 100 MG/ML VIAL 0 SANDOZ MLGUX 00143-1261-01 0.<strong>08</strong>900 ISONIAZID 300 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-1261-10 0.<strong>08</strong>681 ISONIAZID 300 MG TABLET 0 WEST-WARD,INC. EAGUX 00185-4350-01 0.<strong>08</strong>900 ISONIAZID 300 MG TABLET 0 SANDOZ EAGUX 00185-4350-10 0.<strong>08</strong>900 ISONIAZID 300 MG TABLET 0 SANDOZ EAGUX 00185-4350-30 0.<strong>08</strong>900 ISONIAZID 300 MG TABLET 0 SANDOZ EAGUX 00555-0071-01 0.<strong>08</strong>900 ISONIAZID 300 MG TABLET 0 BARR EAGUX 00555-0071-02 0.<strong>08</strong>900 ISONIAZID 300 MG TABLET 0 BARR EAGUX 00555-0071-05 0.<strong>08</strong>900 ISONIAZID 300 MG TABLET 0 BARR EAGUX 51079-0<strong>08</strong>3-20 0.<strong>08</strong>900 ISONIAZID 300 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 46287-0009-01 0.26531 ISONIAZID 50 MG/5 ML SOLUTION 0 CAROLINA MED. MLBND 00998-0303-05 2.13980 6.50388 ISOPTO ATROPINE 1% EYE DROPS 0 ALCON (P.R.) MLBND 00998-0303-15 2.13980 2.94816 ISOPTO ATROPINE 1% EYE DROPS 0 ALCON (P.R.) MLBND 00998-0225-15 4.24960 ISOPTO CARBACHOL 3% DROPS 0 ALCON (P.R.) MLBND 00998-0203-15 4.37633 4.94016 ISOPTO CARPINE 1% EYE DROPS 0 ALCON (P.R.) MLBND 00998-0204-15 2.54090 5.05304 ISOPTO CARPINE 2% EYE DROPS 0 ALCON (P.R.) MLBND 00998-02<strong>06</strong>-15 5.29540 ISOPTO CARPINE 4% EYE DROPS 0 ALCON (P.R.) MLBND 00998-0331-05 6.41424 ISOPTO HYOSCINE 0.25% DROPS 0 ALCON (P.R.) MLBUL 64455-0152-01 0.04880 1.<strong>08</strong>771 ISORDIL TITRADOSE 5 MG TAB G VALEANT EABND 00187-0192-01 2.677<strong>08</strong> ISORDIL 40 MG TABLET 0 VALEANT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64455-0192-01 2.39438 ISORDIL 40 MG TABLET 0 VALEANT EAGEN 57664-<strong>06</strong>00-88 1.01812 ISOSORBIDE DN ER 40 MG TABLET 0 CARACO PHARM EAGUL 00143-1771-01 0.05250 ISOSORBIDE DN 10 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-1771-10 0.05250 ISOSORBIDE DN 10 MG TABLET 0 WEST-WARD,INC. EAGUL 00781-1556-01 0.05250 ISOSORBIDE DN 10 MG TABLET 0 SANDOZ EAGUL 00781-1556-10 0.05250 ISOSORBIDE DN 10 MG TABLET 0 SANDOZ EAGUL 49884-0021-01 0.05250 ISOSORBIDE DN 10 MG TABLET 0 PAR PHARM. EAGUL 49884-0021-10 0.05250 ISOSORBIDE DN 10 MG TABLET 0 PAR PHARM. EAGUL 68<strong>08</strong>4-0<strong>08</strong>2-01 0.05250 ISOSORBIDE DN 10 MG TABLET 0 AHP EABND 00143-1765-01 0.04600 0.<strong>06</strong>366 ISOSORBIDE DN 2.5 MG TAB SL 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00143-1772-01 0.05630 ISOSORBIDE DN 20 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-1772-10 0.05630 ISOSORBIDE DN 20 MG TABLET 0 WEST-WARD,INC. EAGUL 00781-1695-01 0.05630 ISOSORBIDE DN 20 MG TABLET 0 SANDOZ EAGUL 00781-1695-10 0.05630 ISOSORBIDE DN 20 MG TABLET 0 SANDOZ EAGUL 49884-0022-01 0.05630 ISOSORBIDE DN 20 MG TABLET 0 PAR PHARM. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 196LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 49884-0022-10 0.05630 ISOSORBIDE DN 20 MG TABLET 0 PAR PHARM. EAGUL 68<strong>08</strong>4-0<strong>08</strong>3-01 0.05630 ISOSORBIDE DN 20 MG TABLET 0 AHP EAGEN 00143-1773-01 0.78260 ISOSORBIDE DN 30 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1773-10 0.78260 ISOSORBIDE DN 30 MG TABLET 0 WEST-WARD,INC. EABND 49884-0009-01 0.78260 1.<strong>08</strong>821 ISOSORBIDE DN 30 MG TABLET 0 PAR PHARM. EABND 49884-0009-10 0.78260 1.<strong>08</strong>821 ISOSORBIDE DN 30 MG TABLET 0 PAR PHARM. EAGUL 00143-1769-01 0.04880 ISOSORBIDE DN 5 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-1769-10 0.04880 ISOSORBIDE DN 5 MG TABLET 0 WEST-WARD,INC. EAGUL 00781-1635-01 0.04880 ISOSORBIDE DN 5 MG TABLET 0 SANDOZ EAGUL 00781-1635-10 0.04880 ISOSORBIDE DN 5 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 49884-0020-01 0.04880 ISOSORBIDE DN 5 MG TABLET 0 PAR PHARM. EAGUL 49884-0020-10 0.04880 ISOSORBIDE DN 5 MG TABLET 0 PAR PHARM. EABND 00143-1767-01 0.04690 0.<strong>06</strong>366 ISOSORBIDE DN 5 MG TABLET SL 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>03-4112-21 0.45023 ISOSORBIDE MN ER 120 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-4112-25 0.45023 ISOSORBIDE MN ER 120 MG TAB 0 QUALITEST EAGEN 13668-01<strong>06</strong>-01 0.45023 ISOSORBIDE MN ER 120 MG TAB 0 TORRENT PHARMAC EAGEN 13668-01<strong>06</strong>-05 0.45023 ISOSORBIDE MN ER 120 MG TAB 0 TORRENT PHARMAC EAGEN 13668-01<strong>06</strong>-10 0.45023 ISOSORBIDE MN ER 120 MG TAB 0 TORRENT PHARMAC EAGEN 13668-01<strong>06</strong>-59 0.45023 ISOSORBIDE MN ER 120 MG TAB 0 TORRENT PHARMAC EAGEN 13668-01<strong>06</strong>-70 0.45023 ISOSORBIDE MN ER 120 MG TAB 0 TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-01<strong>06</strong>-71 0.45023 ISOSORBIDE MN ER 120 MG TAB 0 TORRENT PHARMAC EAGEN 62175-0129-37 0.45023 ISOSORBIDE MN ER 120 MG TAB 0 KREMERS URBAN EAGEN 00143-2230-01 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-2230-10 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>03-4110-21 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4110-28 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4110-32 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 QUALITEST EAGEN 13668-0104-01 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0104-10 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0104-71 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62175-0128-37 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 KREMERS URBAN EAGEN 62175-0128-41 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 KREMERS URBAN EAGEN 62175-0128-43 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 KREMERS URBAN EAGEN 62175-0128-46 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 KREMERS URBAN EAGEN 68<strong>08</strong>4-0435-01 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0435-11 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0591-01 0.22194 ISOSORBIDE MN ER 30 MG TABLET 0 AHP EAGEN 00143-2260-01 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-2260-10 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>03-4111-21 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-4111-28 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4111-32 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 QUALITEST EAGEN 13668-0105-01 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0105-05 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0105-10 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 197LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-0105-20 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0105-71 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 TORRENT PHARMAC EAGEN 47781-0275-05 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 ALVOGEN INC EAGEN 47781-0275-09 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 ALVOGEN INC EAGEN 62175-0119-37 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 KREMERS URBAN EAGEN 62175-0119-41 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 KREMERS URBAN EAGEN 62175-0119-43 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 KREMERS URBAN EAGEN 62175-0119-46 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 KREMERS URBAN EAGEN 68<strong>08</strong>4-0436-01 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0436-11 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0592-01 0.24530 ISOSORBIDE MN ER 60 MG TABLET 0 AHP EAGEN 00228-2631-11 0.38637 ISOSORBIDE MN 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 62175-01<strong>06</strong>-01 0.38182 ISOSORBIDE MN 10 MG TABLET 0 KREMERS URBAN EAGEN 00143-1333-01 0.09250 ISOSORBIDE MN 20 MG TABLET 0 WEST-WARD,INC. EAGEN 00228-2620-11 0.09250 ISOSORBIDE MN 20 MG TABLET 0 ACTAVIS PHARMA, EAGEN 62175-0107-01 0.09250 ISOSORBIDE MN 20 MG TABLET 0 KREMERS URBAN EABND 00338-0511-41 0.<strong>06</strong>752 ISOTON GENTAMICIN 100 MG/50 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0507-41 0.05634 ISOTON GENTAMICIN 60 MG/50 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0503-48 0.02934 ISOTON GENTAMICIN 80 MG/100 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0509-41 0.05976 ISOTON GENTAMICIN 80 MG/50 ML 0 BAXTER <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 16252-0539-01 0.93240 1.13286 ISRADIPINE 2.5 MG CAPSULE 0 ACTAVIS PHARMA, EABND 16252-0540-01 1.65659 ISRADIPINE 5 MG CAPSULE 0 ACTAVIS PHARMA, EABND 242<strong>08</strong>-0004-01 43.32268 ISTALOL 0.5% EYE DROPS 0 VALEANT MLBND 242<strong>08</strong>-0004-03 43.32268 ISTALOL 0.5% EYE DROPS 0 VALEANT MLBND 67425-0003-12 36.97152 ISTALOL 0.5% EYE DROPS 0 BAUSCH & LOMB MLBND 67425-0003-50 36.97152 ISTALOL 0.5% EYE DROPS 0 BAUSCH & LOMB MLGEN 38779-1931-05 15.30000 ITRACONAZOLE POWDER 0 MEDISCA INC. GMGEN 38779-2467-05 140.36250 ITRACONAZOLE POWDER 0 MEDISCA INC. GMGEN 00185-0550-30 6.95825 ITRACONAZOLE 100 MG CAPSULE G SANDOZ EAGEN 00185-0550-83 6.97767 ITRACONAZOLE 100 MG CAPSULE G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5100-93 7.27350 ITRACONAZOLE 100 MG CAPSULE G MYLAN EAGEN 10147-1700-03 6.95775 ITRACONAZOLE 100 MG CAPSULE G PATRIOT PHARMAC EAGEN 10147-1700-07 6.97687 ITRACONAZOLE 100 MG CAPSULE G PATRIOT PHARMAC EABND 5<strong>08</strong>81-0010-60 138.69300 JAKAFI 10 MG TABLET 0 INCYTE CORPORAT EABND 5<strong>08</strong>81-0015-60 138.69300 JAKAFI 15 MG TABLET 0 INCYTE CORPORAT EABND 5<strong>08</strong>81-0020-60 138.69300 JAKAFI 20 MG TABLET 0 INCYTE CORPORAT EABND 5<strong>08</strong>81-0025-60 138.69300 JAKAFI 25 MG TABLET 0 INCYTE CORPORAT EABND 5<strong>08</strong>81-0005-60 138.69300 JAKAFI 5 MG TABLET 0 INCYTE CORPORAT EABND 00173-<strong>08</strong>09-13 4.56029 JALYN 0.5-0.4 MG CAPSULE G GLAXOSMITHKLINE EABND 00173-<strong>08</strong>09-59 4.55965 JALYN 0.5-0.4 MG CAPSULE G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>08</strong>32-1211-00 0.<strong>06</strong>520 JANTOVEN 1 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1211-10 0.<strong>06</strong>520 JANTOVEN 1 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1211-89 0.<strong>06</strong>520 JANTOVEN 1 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1219-00 0.07180 JANTOVEN 10 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1219-50 0.07180 JANTOVEN 10 MG TABLET 0 UPSHER SMITH EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 198LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>08</strong>32-1219-89 0.07180 JANTOVEN 10 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1212-00 0.<strong>06</strong>520 JANTOVEN 2 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1212-10 0.<strong>06</strong>520 JANTOVEN 2 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1212-89 0.<strong>06</strong>520 JANTOVEN 2 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1213-00 0.<strong>06</strong>520 JANTOVEN 2.5 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1213-10 0.<strong>06</strong>520 JANTOVEN 2.5 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1213-89 0.<strong>06</strong>520 JANTOVEN 2.5 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1214-00 0.<strong>06</strong>520 JANTOVEN 3 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1214-10 0.<strong>06</strong>520 JANTOVEN 3 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1214-89 0.<strong>06</strong>520 JANTOVEN 3 MG TABLET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>08</strong>32-1215-00 0.<strong>06</strong>520 JANTOVEN 4 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1215-10 0.<strong>06</strong>520 JANTOVEN 4 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1215-89 0.<strong>06</strong>520 JANTOVEN 4 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1216-00 0.<strong>06</strong>520 JANTOVEN 5 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1216-10 0.<strong>06</strong>520 JANTOVEN 5 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1216-89 0.<strong>06</strong>520 JANTOVEN 5 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1217-00 0.07180 JANTOVEN 6 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1217-01 0.07180 JANTOVEN 6 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1217-10 0.07180 JANTOVEN 6 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1217-89 0.07180 JANTOVEN 6 MG TABLET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>08</strong>32-1218-00 0.07200 JANTOVEN 7.5 MG TABLET 0 UPSHER SMITH EAGEN 0<strong>08</strong>32-1218-50 0.07200 JANTOVEN 7.5 MG TABLET 0 UPSHER SMITH EABND 000<strong>06</strong>-0<strong>08</strong>1-31 9.42354 JANUMET XR 100-1,000 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0<strong>08</strong>1-54 9.42262 JANUMET XR 100-1,000 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0<strong>08</strong>1-82 9.42262 JANUMET XR 100-1,000 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0<strong>08</strong>0-61 4.71176 JANUMET XR 50-1,000 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0<strong>08</strong>0-62 4.71131 JANUMET XR 50-1,000 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0<strong>08</strong>0-82 4.71129 JANUMET XR 50-1,000 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0078-61 4.71176 JANUMET XR 50-500 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0078-62 4.71131 JANUMET XR 50-500 MG TABLET G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-0078-82 4.71129 JANUMET XR 50-500 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0577-61 4.71176 JANUMET 50-1,000 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0577-62 4.71131 JANUMET 50-1,000 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0577-82 4.71129 JANUMET 50-1,000 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0575-61 4.71176 JANUMET 50-500 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0575-62 4.71131 JANUMET 50-500 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0575-82 4.71129 JANUMET 50-500 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0277-28 9.42249 JANUVIA 100 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0277-31 9.42354 JANUVIA 100 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0277-54 9.42262 JANUVIA 100 MG TABLET G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-0277-82 9.42262 JANUVIA 100 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0221-28 9.42249 JANUVIA 25 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0221-31 9.42354 JANUVIA 25 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0221-54 9.42262 JANUVIA 25 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0112-28 9.42249 JANUVIA 50 MG TABLET G MERCK SHARP & D EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 199LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-0112-31 9.42354 JANUVIA 50 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0112-54 9.42262 JANUVIA 50 MG TABLET G MERCK SHARP & D EAGEX 68180-<strong>08</strong>77-11 0.69917 JENCYCLA 0.35 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-<strong>08</strong>77-13 0.69917 JENCYCLA 0.35 MG TABLET 0 LUPIN PHARMACEU EABND 00597-0148-18 4.71154 JENTADUETO 2.5 MG-1000 MG TAB G BOEHRINGER ING. EABND 00597-0148-60 4.71191 JENTADUETO 2.5 MG-1000 MG TAB G BOEHRINGER ING. EABND 00597-0146-18 4.71154 JENTADUETO 2.5 MG-500 MG TAB G BOEHRINGER ING. EABND 00597-0146-60 4.71191 JENTADUETO 2.5 MG-500 MG TAB G BOEHRINGER ING. EABND 00597-0147-18 4.71154 JENTADUETO 2.5 MG-850 MG TAB G BOEHRINGER ING. EABND 00597-0147-60 4.71191 JENTADUETO 2.5 MG-850 MG TAB G BOEHRINGER ING. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00093-3122-42 1.83690 1.94486 JINTELI 1 MG-5 MCG TABLET 0 TEVA USA EABND 00093-3122-98 1.83690 1.94450 JINTELI 1 MG-5 MCG TABLET 0 TEVA USA EABEX 00555-9123-66 1.24580 1.46548 JOLESSA 0.15 MG-0.03 MG TABLET 0 BARR EABEX 52544-<strong>08</strong>92-28 0.69917 1.09441 JOLIVETTE TABLET 0 ACTAVIS PHARMA, EAGEX 00555-9026-58 0.71880 JUNEL FE 1 MG-20 MCG TABLET 0 BARR EAGEX 00555-9028-58 0.72384 JUNEL FE 1.5 MG-30 MCG TABLET 0 BARR EAGEX 00555-9025-42 1.00280 JUNEL 1 MG-20 MCG TABLET 0 BARR EAGEX 00555-9027-42 1.00290 JUNEL 1.5 MG-30 MCG TABLET 0 BARR EABND 000<strong>06</strong>-0753-31 8.57445 JUVISYNC 100-10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0753-54 8.57380 JUVISYNC 100-10 MG TABLET G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-0757-31 8.57445 JUVISYNC 100-20 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0757-54 8.57380 JUVISYNC 100-20 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0773-31 8.57445 JUVISYNC 100-40 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0773-54 8.57380 JUVISYNC 100-40 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0773-82 8.57381 JUVISYNC 100-40 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0533-31 8.57445 JUVISYNC 50-10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0533-54 8.57380 JUVISYNC 50-10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0535-31 8.57445 JUVISYNC 50-20 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0535-54 8.57380 JUVISYNC 50-20 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0537-31 8.57445 JUVISYNC 50-40 MG TABLET G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-0537-54 8.57380 JUVISYNC 50-40 MG TABLET G MERCK SHARP & D EAGEN 0<strong>06</strong>03-4170-16 0.15110 K EFFERVESCENT 25 MEQ TABLET 0 QUALITEST EABND 00486-1134-01 0.67852 K-PHOS #2 TABLET 0 BEACH PRODUCTS EABND 00486-1125-01 0.50215 K-PHOS NEUTRAL TABLET 0 BEACH PRODUCTS EABND 00486-1125-05 0.478<strong>08</strong> K-PHOS NEUTRAL TABLET 0 BEACH PRODUCTS EABND 00486-1111-01 0.36105 K-PHOS ORIGINAL TABLET 0 BEACH PRODUCTS EABND 00486-1111-05 0.33117 K-PHOS ORIGINAL TABLET 0 BEACH PRODUCTS EABND 00074-7804-13 0.79597 K-TAB ER 10 MEQ TABLET 0 ABBVIE US LLC EABND 00074-7804-19 0.75622 K-TAB ER 10 MEQ TABLET 0 ABBVIE US LLC EABND 00074-0522-60 3.37961 KALETRA 100-25 MG TABLET G ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00074-6799-22 6.75910 KALETRA 200-50 MG TABLET G ABBVIE US LLC EABND 00074-3956-46 2.53461 KALETRA 400-100/5 ML ORAL SOLU G ABBVIE US LLC MLBND 51167-0200-02 425.00980 KALYDECO 150 MG TABLET 0 VERTEX PHARMACE EABND 59630-<strong>06</strong>58-60 4.35750 KAPVAY ER 0.1 MG TABLET G CONCORDIA PHARM EABND 59630-<strong>06</strong>70-60 5.07130 KAPVAY 0.1-0.2 MG DOSEPACK G CONCORDIA PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 200LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00555-9050-58 1.51100 KARIVA 28 DAY TABLET 0 BARR EABND 00024-1075-01 0.24230 1.<strong>06</strong>274 KAYEXALATE POWDER G COVIS PHARMACEU GMBND 64764-0337-60 4.71218 KAZANO 12.5-1,000 MG TABLET G TAKEDA PHARMACE EABND 64764-0335-60 4.71218 KAZANO 12.5-500 MG TABLET G TAKEDA PHARMACE EAGEN 00264-7635-00 0.00210 KCL 20 MEQ IN D5W-1/2 NS 0 B.BRAUN MLGEN 00338-<strong>06</strong>71-04 0.00183 KCL 20 MEQ IN D5W-1/2 NS 0 BAXTER <strong>HEALTH</strong>CA MLBND 76179-0025-01 0.99600 KEDBUMIN 25% VIAL 0 KEDRION BIOPHAR MLBND 76179-0025-02 0.99600 KEDBUMIN 25% VIAL 0 KEDRION BIOPHAR MLBND 76179-0025-03 0.93375 KEDBUMIN 25% VIAL 0 KEDRION BIOPHAR MLBND 76179-0025-04 0.93375 KEDBUMIN 25% VIAL 0 KEDRION BIOPHAR ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59630-0112-10 0.09511 7.95015 KEFLEX 250 MG CAPSULE G SHIONOGI PHARMA EABND 68453-0112-10 0.09511 7.09840 KEFLEX 250 MG CAPSULE G SHIONOGI PHARMA EABND 68453-0113-10 0.10100 7.95015 KEFLEX 500 MG CAPSULE G SHIONOGI PHARMA EABND 59630-0115-05 7.95023 KEFLEX 750 MG CAPSULE G SHIONOGI PHARMA EABND 68453-0115-05 4.92140 KEFLEX 750 MG CAPSULE G SHIONOGI PHARMA EAGEX 00555-9<strong>06</strong>4-58 0.77620 KELNOR 1-35 28 TABLET 0 BARR EABND 1<strong>06</strong>31-0093-07 3.58966 KENALOG AEROSOL SPRAY G RANBAXY LABORAT GMBND 1<strong>06</strong>31-0093-62 3.58968 KENALOG AEROSOL SPRAY G RANBAXY LABORAT GMBND 00003-0494-20 2.19950 KENALOG-10 10 MG/ML VIAL 0 BMS PRIMARYCARE MLBND 00003-0293-05 8.51580 KENALOG-40 40 MG/ML VIAL 0 BMS PRIMARYCARE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00003-0293-20 8.64362 KENALOG-40 40 MG/ML VIAL 0 BMS PRIMARYCARE MLBND 00003-0293-28 6.454<strong>08</strong> KENALOG-40 40 MG/ML VIAL 0 BMS PRIMARYCARE MLBEX 50474-0598-66 0.51000 5.69145 KEPPRA XR 500 MG TABLET G UCB PHARMA EABEX 50474-0599-66 0.88870 8.54582 KEPPRA XR 750 MG TABLET G UCB PHARMA EABEX 50474-0597-66 0.41490 12.55638 KEPPRA 1,000 MG TABLET G UCB PHARMA EABEX 50474-0001-48 0.07840 1.21077 KEPPRA 100 MG/ML ORAL SOLN G UCB PHARMA MLBEX 50474-0594-40 0.09980 5.13680 KEPPRA 250 MG TABLET G UCB PHARMA EABEX 50474-0595-40 0.24840 6.27818 KEPPRA 500 MG TABLET G UCB PHARMA EABEX 50474-0002-63 1.30491 9.30330 KEPPRA 500 MG/5 ML VIAL G UCB PHARMA MLBEX 50474-0596-40 0.22248 8.50569 KEPPRA 750 MG TABLET G UCB PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>8-2225-41 16.19717 KETEK 400 MG TABLET 0 SAN<strong>OF</strong>I-AVENTIS EAGEN 00093-<strong>08</strong>40-15 0.18435 KETOCONAZOLE 2% CREAM G TEVA USA GMGEN 00093-<strong>08</strong>40-30 0.18435 KETOCONAZOLE 2% CREAM G TEVA USA GMGEN 00093-<strong>08</strong>40-92 0.18435 KETOCONAZOLE 2% CREAM G TEVA USA GMGEN 00168-0099-15 0.18435 KETOCONAZOLE 2% CREAM G SANDOZ GMGEN 00168-0099-30 0.18435 KETOCONAZOLE 2% CREAM G SANDOZ GMGEN 00168-0099-60 0.18435 KETOCONAZOLE 2% CREAM G SANDOZ GMGEN 51672-1298-01 0.18435 KETOCONAZOLE 2% CREAM G TARO PHARM USA GMGEN 51672-1298-02 0.18435 KETOCONAZOLE 2% CREAM G TARO PHARM USA GMGEN 51672-1298-03 0.18435 KETOCONAZOLE 2% CREAM G TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0532-32 3.23430 KETOCONAZOLE 2% FOAM G PERRIGO CO. GMGEN 45802-0532-33 3.01275 KETOCONAZOLE 2% FOAM G PERRIGO CO. GMGEN 00093-0900-01 0.21155 KETOCONAZOLE 200 MG TABLET 0 TEVA USA EAGEN 00093-0900-05 0.21155 KETOCONAZOLE 200 MG TABLET 0 TEVA USA EAGEN 00378-0261-01 0.21155 KETOCONAZOLE 200 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 201LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-4026-01 0.21155 KETOCONAZOLE 200 MG TABLET 0 TARO PHARM USA EAGEN 51672-4026-<strong>06</strong> 0.21155 KETOCONAZOLE 200 MG TABLET 0 TARO PHARM USA EAGEN 60505-0092-00 0.21155 KETOCONAZOLE 200 MG TABLET 0 APOTEX CORP EAGEN 60505-0092-02 0.21155 KETOCONAZOLE 200 MG TABLET 0 APOTEX CORP EAGEN 68<strong>08</strong>4-0552-11 0.21155 KETOCONAZOLE 200 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0552-21 0.21155 KETOCONAZOLE 200 MG TABLET 0 AHP EAGEN 43538-0530-10 3.56820 KETODAN 2% FOAM G MEDIMETRIKS PHA GMBND 00378-8200-01 6.412<strong>08</strong> KETOPR<strong>OF</strong>EN ER 200 MG CAPSULE G MYLAN EAGEN 38779-0078-04 7.83750 KETOPR<strong>OF</strong>EN POWDER 0 MEDISCA INC. GMGEN 38779-0078-09 7.83750 KETOPR<strong>OF</strong>EN POWDER 0 MEDISCA INC. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-3193-01 0.21854 KETOPR<strong>OF</strong>EN 50 MG CAPSULE 0 TEVA USA EAGEN 00378-4070-01 0.21854 KETOPR<strong>OF</strong>EN 50 MG CAPSULE 0 MYLAN EAGEN 00093-3195-01 0.24294 KETOPR<strong>OF</strong>EN 75 MG CAPSULE 0 TEVA USA EAGEN 00093-3195-05 0.24294 KETOPR<strong>OF</strong>EN 75 MG CAPSULE 0 TEVA USA EAGEN 00378-5750-01 0.24294 KETOPR<strong>OF</strong>EN 75 MG CAPSULE 0 MYLAN EAGEN 17478-02<strong>08</strong>-10 2.07504 KETOROLAC 0.4% OPHTH SOLUTION 0 AKORN INC. MLGEN 60505-0570-01 2.07504 KETOROLAC 0.4% OPHTH SOLUTION 0 APOTEX CORP MLGEN 60758-0773-05 2.07504 KETOROLAC 0.4% OPHTH SOLUTION 0 PACIFIC PHARMA MLGEN 61314-0018-05 2.07504 KETOROLAC 0.4% OPHTH SOLUTION 0 SANDOZ MLGEN 17478-0209-10 1.30500 KETOROLAC 0.5% OPHTH SOLUTION 0 AKORN INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 17478-0209-11 1.30500 KETOROLAC 0.5% OPHTH SOLUTION 0 AKORN INC. MLGEN 41616-0219-90 1.30500 KETOROLAC 0.5% OPHTH SOLUTION 0 SUN PHARMA GLOB MLGEN 41616-0220-90 1.30500 KETOROLAC 0.5% OPHTH SOLUTION 0 SUN PHARMA GLOB MLGEN 41616-0221-90 1.30500 KETOROLAC 0.5% OPHTH SOLUTION 0 SUN PHARMA GLOB MLGEN 60505-1003-01 1.30500 KETOROLAC 0.5% OPHTH SOLUTION 0 APOTEX CORP MLGEN 60505-1003-02 1.30500 KETOROLAC 0.5% OPHTH SOLUTION 0 APOTEX CORP MLGEN 61314-0126-05 1.30500 KETOROLAC 0.5% OPHTH SOLUTION 0 SANDOZ MLGEN 61314-0126-10 1.30500 KETOROLAC 0.5% OPHTH SOLUTION 0 SANDOZ MLGEN 00093-0314-01 0.66182 KETOROLAC 10 MG TABLET 0 TEVA USA EAGEN 00378-1134-01 0.66182 KETOROLAC 10 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-2288-31 1.90236 KETOROLAC 15 MG/ML CARPUJECT 0 HOSPIRA MLGEN 00409-3793-01 0.69300 KETOROLAC 15 MG/ML VIAL 0 HOSPIRA MLGEN 00409-3793-49 0.72900 KETOROLAC 15 MG/ML VIAL 0 HOSPIRA MLGEN 55390-0480-01 0.85860 KETOROLAC 15 MG/ML VIAL 0 BEDFORD LABS MLGEN 63323-0161-01 0.85860 KETOROLAC 15 MG/ML VIAL 0 APP PHARMACEUTI MLBND 00409-2287-31 1.62000 2.09160 KETOROLAC 30 MG/ML CARPUJECT 0 HOSPIRA MLGEN 00409-3795-01 0.75600 KETOROLAC 30 MG/ML VIAL 0 HOSPIRA MLGEN 00409-3795-49 0.74700 KETOROLAC 30 MG/ML VIAL 0 HOSPIRA/NOVA+ MLGEN 55390-0481-01 0.85914 KETOROLAC 30 MG/ML VIAL 0 BEDFORD LABS MLGEN 63323-0162-01 0.85914 KETOROLAC 30 MG/ML VIAL 0 APP PHARMACEUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 55390-0481-10 0.84162 KETOROLAC 300 MG/10 ML VIAL 0 BEDFORD LABS MLGEN 00409-3796-01 0.39600 KETOROLAC 60 MG/2 ML VIAL 0 HOSPIRA MLGEN 00409-3796-49 0.39150 KETOROLAC 60 MG/2 ML VIAL 0 HOSPIRA/NOVA+ MLGEN 55390-0481-02 0.43120 KETOROLAC 60 MG/2 ML VIAL 0 BEDFORD LABS MLGEN 63323-0162-02 0.43120 KETOROLAC 60 MG/2 ML VIAL 0 APP PHARMACEUTI ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 202LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 49884-0375-09 4.43220 KHEDEZLA ER 100 MG TABLET G PAR PHARMACEUTI EABEX 49884-0375-11 4.43220 KHEDEZLA ER 100 MG TABLET G PAR PHARMACEUTI EABEX 49884-0374-09 4.43220 KHEDEZLA ER 50 MG TABLET G PAR PHARMACEUTI EABEX 49884-0374-11 4.43220 KHEDEZLA ER 50 MG TABLET G PAR PHARMACEUTI EABND 66658-0234-01 149.30<strong>08</strong>9 KINERET 100 MG/0.67 ML SYRINGE G SOBI-SWEDISH OR MLBND 66658-0234-07 149.29381 KINERET 100 MG/0.67 ML SYRINGE G SOBI-SWEDISH OR MLBND 66658-0234-28 149.29381 KINERET 100 MG/0.67 ML SYRINGE G SOBI-SWEDISH OR MLGEN 00574-2004-16 0.24230 KIONEX POWDER 0 PADDOCK LABS. GMGEN 00574-2002-16 0.11718 KIONEX 15 GM/60 ML SUSPENSION 0 PADDOCK LABS. MLGUL 00245-0057-10 0.25380 KLOR-CON M10 TABLET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00245-0057-11 0.25380 KLOR-CON M10 TABLET 0 UPSHER SMITH EAGUL 00245-0057-90 0.25380 KLOR-CON M10 TABLET 0 UPSHER SMITH EAGUL 15338-0122-30 0.25380 KLOR-CON M10 TABLET 0 APACE PACKAGING EABND 00245-0150-11 0.50771 KLOR-CON M15 TABLET 0 UPSHER SMITH EAGEN 00245-0058-01 0.35424 KLOR-CON M20 TABLET 0 UPSHER SMITH EAGEN 00245-0058-10 0.35424 KLOR-CON M20 TABLET 0 UPSHER SMITH EAGEN 00245-0058-11 0.35424 KLOR-CON M20 TABLET 0 UPSHER SMITH EAGEN 00245-0058-15 0.35424 KLOR-CON M20 TABLET 0 UPSHER SMITH EAGEN 00245-0058-89 0.35424 KLOR-CON M20 TABLET 0 UPSHER SMITH EAGEN 00245-0058-90 0.35424 KLOR-CON M20 TABLET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 15338-0133-30 0.35424 KLOR-CON M20 TABLET 0 APACE PACKAGING EABND 00245-0041-01 0.53966 KLOR-CON 10 MEQ TABLET 0 UPSHER SMITH EABND 00245-0041-11 0.46861 KLOR-CON 10 MEQ TABLET 0 UPSHER SMITH EABND 00245-0041-15 0.46861 KLOR-CON 10 MEQ TABLET 0 UPSHER SMITH EABND 00245-0041-55 0.46861 KLOR-CON 10 MEQ TABLET 0 UPSHER SMITH EABND 00245-0041-89 0.53950 KLOR-CON 10 MEQ TABLET 0 UPSHER SMITH EABND 00245-0035-01 4.67191 5.63628 KLOR-CON 20 MEQ PACKET 0 UPSHER SMITH EABND 00245-0035-30 4.67191 5.63680 KLOR-CON 20 MEQ PACKET 0 UPSHER SMITH EABND 00245-0037-01 0.76800 6.70042 KLOR-CON 25 MEQ PACKET 0 UPSHER SMITH EABND 00245-0037-30 0.76800 6.70<strong>08</strong>6 KLOR-CON 25 MEQ PACKET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00245-0040-01 0.41380 0.54796 KLOR-CON 8 MEQ TABLET 0 UPSHER SMITH EABND 00245-0040-11 0.41380 0.44521 KLOR-CON 8 MEQ TABLET 0 UPSHER SMITH EABND 00245-0040-15 0.41380 0.44521 KLOR-CON 8 MEQ TABLET 0 UPSHER SMITH EABND 00245-0040-55 0.41380 0.44521 KLOR-CON 8 MEQ TABLET 0 UPSHER SMITH EABND 00245-0040-89 0.41380 0.54780 KLOR-CON 8 MEQ TABLET 0 UPSHER SMITH EABUL 68645-0201-54 0.10440 0.44520 KLOR-CON 8 MEQ TABLET 0 LEGACY PHARMACE EAGEN 00245-0039-01 0.15110 KLOR-CON-EF 25 MEQ TAB EFF 0 UPSHER SMITH EAGEN 00245-0039-30 0.15110 KLOR-CON-EF 25 MEQ TAB EFF 0 UPSHER SMITH EABND 76125-<strong>06</strong>67-50 0.78500 KOATE-DVI 1,000 UNITS VIAL 0 KEDRION BIOPHARBND 76125-0250-20 0.78500 KOATE-DVI 250 UNITS VIAL 0 KEDRION BIOPHAR--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 76125-0500-30 0.78500 KOATE-DVI 500 UNITS KIT 0 KEDRION BIOPHARBND 00026-3785-55 0.98800 KOGENATE FS 1,000 UNIT VIAL 0 BAYER,PHARM DIVBND 00026-3785-50 0.98800 KOGENATE FS 1,000 UNITS VIAL 0 BAYER,PHARM DIVBND 00026-3795-50 0.98800 KOGENATE FS 1,000 UNITS VIAL 0 BAYER,PHARM DIVBND 00026-3786-60 0.98800 KOGENATE FS 2,000 UNIT VIAL 0 BAYER,PHARM DIV** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 203LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00026-3786-65 0.98800 KOGENATE FS 2,000 UNIT VIAL 0 BAYER,PHARM DIVBND 00026-3796-60 0.98800 KOGENATE FS 2,000 UNIT VIAL 0 BAYER,PHARM DIVBND 00026-3782-20 0.98800 KOGENATE FS 250 UNIT VIAL 0 BAYER,PHARM DIVBND 00026-3782-25 0.98800 KOGENATE FS 250 UNIT VIAL 0 BAYER,PHARM DIVBND 00026-3792-20 0.98800 KOGENATE FS 250 UNIT VIAL 0 BAYER,PHARM DIVBND 00026-3787-75 0.98800 KOGENATE FS 3,000 UNIT VIAL 0 BAYER,PHARM DIVBND 00026-3787-70 0.98800 KOGENATE FS 3,000 UNITS VIAL 0 BAYER,PHARM DIVBND 00026-3797-70 0.98800 KOGENATE FS 3,000 UNITS VIAL 0 BAYER,PHARM DIVBND 00026-3783-30 0.98800 KOGENATE FS 500 UNIT VIAL 0 BAYER,PHARM DIVBND 00026-3783-35 0.98800 KOGENATE FS 500 UNIT VIAL 0 BAYER,PHARM DIV--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00026-3793-30 0.98800 KOGENATE FS 500 UNIT VIAL 0 BAYER,PHARM DIVBND 00003-4222-16 4.62974 KOMBIGLYZE XR 2.5-1,000 MG TAB G BMS PRIMARYCARE EABND 00003-4223-11 9.25948 KOMBIGLYZE XR 5-1,000 MG TAB G BMS PRIMARYCARE EABND 00003-4221-11 9.25948 KOMBIGLYZE XR 5-500 MG TABLET G BMS PRIMARYCARE EABND 76346-0073-01 221.11200 KORLYM 300 MG TABLET 0 CORCEPT THERAPE EABND 76346-0073-02 221.11200 KORLYM 300 MG TABLET 0 CORCEPT THERAPE EABND 66220-0719-30 5.97904 KRISTALOSE 10 GM PACKET 0 CUMBERLAND PHAR EABND 66220-0729-30 6.33207 KRISTALOSE 20 GM PACKET 0 CUMBERLAND PHAR EAGEX 68180-<strong>08</strong>44-11 0.81150 KURVELO TABLET 0 LUPIN PHARMACEU EAGEX 68180-<strong>08</strong>44-13 0.81150 KURVELO TABLET 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 68135-0300-02 32.15420 KUVAN 100 MG TABLET 0 BIOMARIN PHARMA EAGUL 00172-4364-10 0.21570 LABETALOL HCL 100 MG TABLET 0 TEVA USA EAGUL 00172-4364-60 0.21570 LABETALOL HCL 100 MG TABLET 0 IVAX PHARMACEUT EAGUL 00172-4364-70 0.21570 LABETALOL HCL 100 MG TABLET 0 IVAX PHARMACEUT EAGUL 00185-0010-01 0.21570 LABETALOL HCL 100 MG TABLET 0 SANDOZ EAGUL 00185-0010-05 0.21570 LABETALOL HCL 100 MG TABLET 0 SANDOZ EAGUL 00591-<strong>06</strong>05-01 0.21570 LABETALOL HCL 100 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00591-<strong>06</strong>05-05 0.21570 LABETALOL HCL 100 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00904-5928-61 0.21570 LABETALOL HCL 100 MG TABLET 0 MAJOR PHARMACEU EAGUL 49884-0122-01 0.21570 LABETALOL HCL 100 MG TABLET 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 49884-0122-05 0.21570 LABETALOL HCL 100 MG TABLET 0 PAR PHARM. EAGUL 00172-4365-60 0.35820 LABETALOL HCL 200 MG TABLET 0 IVAX PHARMACEUT EAGUL 00172-4365-70 0.35820 LABETALOL HCL 200 MG TABLET 0 IVAX PHARMACEUT EAGUL 00185-0117-01 0.35820 LABETALOL HCL 200 MG TABLET 0 SANDOZ EAGUL 00185-0117-05 0.35820 LABETALOL HCL 200 MG TABLET 0 SANDOZ EAGUL 00591-<strong>06</strong><strong>06</strong>-01 0.35820 LABETALOL HCL 200 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00591-<strong>06</strong><strong>06</strong>-05 0.35820 LABETALOL HCL 200 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00904-5929-61 0.35820 LABETALOL HCL 200 MG TABLET 0 MAJOR PHARMACEU EAGUL 49884-0123-01 0.35820 LABETALOL HCL 200 MG TABLET 0 PAR PHARM. EAGUL 49884-0123-05 0.35820 LABETALOL HCL 200 MG TABLET 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 63739-0366-10 0.35820 LABETALOL HCL 200 MG TABLET 0 MCKESSON PACKAG EAGUL 68<strong>08</strong>4-0456-01 0.35820 LABETALOL HCL 200 MG TABLET 0 AHP EAGUL 68<strong>08</strong>4-0456-11 0.35820 LABETALOL HCL 200 MG TABLET 0 AHP EAGUL 00172-4366-60 0.53630 LABETALOL HCL 300 MG TABLET 0 IVAX PHARMACEUT EAGUL 00185-0118-01 0.53630 LABETALOL HCL 300 MG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 204LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00185-0118-05 0.53630 LABETALOL HCL 300 MG TABLET 0 SANDOZ EAGUL 00591-<strong>06</strong>07-01 0.53630 LABETALOL HCL 300 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00904-5930-61 0.53630 LABETALOL HCL 300 MG TABLET 0 MAJOR PHARMACEU EAGUL 49884-0124-01 0.53630 LABETALOL HCL 300 MG TABLET 0 PAR PHARM. EAGUL 49884-0124-05 0.53630 LABETALOL HCL 300 MG TABLET 0 PAR PHARM. EAGUL 68<strong>08</strong>4-0457-01 0.53630 LABETALOL HCL 300 MG TABLET 0 AHP EAGUL 68<strong>08</strong>4-0457-11 0.53630 LABETALOL HCL 300 MG TABLET 0 AHP EABND 25010-<strong>08</strong>05-68 5.51175 LACRISERT 5 MG EYE INSERT 0 VALEANT EAGEN 00264-7750-00 0.00141 LACTATED RINGERS INJECTION 0 B.BRAUN MLGEN 00338-0117-03 0.00180 LACTATED RINGERS INJECTION 0 BAXTER <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00338-0117-04 0.00160 LACTATED RINGERS INJECTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-7953-09 0.00148 LACTATED RINGERS INJECTION 0 HOSPIRA MLGEN 00409-7953-30 0.00180 LACTATED RINGERS INJECTION 0 HOSPIRA MLGEN 00409-7953-48 0.00180 LACTATED RINGERS INJECTION 0 HOSPIRA MLGEN 00264-2203-00 0.00310 LACTATED RINGERS IRRIGATION 0 B.BRAUN MLGEN 00338-0137-27 0.00255 LACTATED RINGERS IRRIGATION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0137-29 0.00264 LACTATED RINGERS IRRIGATION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 38779-0565-<strong>08</strong> 0.41325 LACTIC ACID LIQUID 0 MEDISCA INC. MLGEN 00054-3486-63 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 ROXANE LABS. MLGEN 00121-0577-<strong>08</strong> 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 PHARMACEU ASSOC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00121-0577-16 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 PHARMACEU ASSOC MLGEN 00121-0577-32 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 PHARMACEU ASSOC MLGUL 00121-4577-15 0.02210 LACTULOSE 10 GM/15 ML SOLUTION 0 PHARMACEU ASSOC MLGEN 0<strong>06</strong>03-1378-56 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 QUALITEST MLGEN 0<strong>06</strong>03-1378-58 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 QUALITEST MLGEN 0<strong>06</strong>03-1378-59 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 QUALITEST MLGEN 0<strong>06</strong>03-1378-65 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 QUALITEST MLGEN 50383-0779-<strong>08</strong> 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 HI-TECH PHARMAC MLGEN 50383-0779-16 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 HI-TECH PHARMAC MLGUL 50383-0779-17 0.02210 LACTULOSE 10 GM/15 ML SOLUTION 0 HI-TECH PHARMAC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50383-0779-32 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 HI-TECH PHARMAC MLGEN 50383-0795-16 0.01500 LACTULOSE 10 GM/15 ML SOLUTION 0 HI-TECH PHARMAC MLGEN 60432-0037-<strong>08</strong> 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 MORTON GROVE PH MLGEN 60432-0037-32 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 MORTON GROVE PH MLGEN 60505-0360-00 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 APOTEX CORP MLGEN 60505-0360-01 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 APOTEX CORP MLGEN 60505-0360-02 0.01400 LACTULOSE 10 GM/15 ML SOLUTION 0 APOTEX CORP MLGUL 66689-0039-01 0.02210 LACTULOSE 10 GM/15 ML SOLUTION 0 VISTAPHARM MLGUL 66689-0039-50 0.02210 LACTULOSE 10 GM/15 ML SOLUTION 0 VISTAPHARM MLGUL 00121-4577-30 0.02210 LACTULOSE 20 GM/30 ML SOLUTION 0 PHARMACEU ASSOC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00121-4577-35 0.02037 LACTULOSE 20 GM/30 ML SOLUTION 0 PHARMACEU ASSOC MLGUL 50383-0779-30 0.02210 LACTULOSE 20 GM/30 ML SOLUTION 0 HI-TECH PHARMAC MLGUL 50383-0779-31 0.02210 LACTULOSE 20 GM/30 ML SOLUTION 0 HI-TECH PHARMAC MLGUL 50383-0779-33 0.02210 LACTULOSE 20 GM/30 ML SOLUTION 0 HI-TECH PHARMAC MLGUL 66689-0038-01 0.02210 LACTULOSE 20 GM/30 ML SOLUTION 0 VISTAPHARM ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 205LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 66689-0038-50 0.02210 LACTULOSE 20 GM/30 ML SOLUTION 0 VISTAPHARM MLBEX 00173-0779-00 8.30266 LAMICTAL ODT START KIT (BLUE) G GLAXOSMITHKLINE EABEX 00173-0780-00 11.85951 LAMICTAL ODT START KIT (GREEN) G GLAXOSMITHKLINE EABEX 00173-0778-00 9.48784 LAMICTAL ODT START KT (ORANGE) G GLAXOSMITHKLINE EABEX 00173-0776-02 7.58647 LAMICTAL ODT 100 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0777-02 9.053<strong>08</strong> LAMICTAL ODT 200 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0772-02 6.64276 LAMICTAL ODT 25 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0774-02 7.11476 LAMICTAL ODT 50 MG TABLET G GLAXOSMITHKLINE EABUX 00173-<strong>06</strong>33-10 0.30350 6.83659 LAMICTAL TAB START KIT (BLUE) G GLAXOSMITHKLINE EABEX 00173-<strong>08</strong>17-28 6.97504 LAMICTAL TAB START KIT (GREEN) G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00173-0594-02 6.97504 LAMICTAL TB START KIT (ORANGE) G GLAXOSMITHKLINE EABEX 00173-0758-00 7.54796 LAMICTAL XR START KIT (BLUE) G GLAXOSMITHKLINE EABEX 00173-0759-00 17.25047 LAMICTAL XR START KIT (GREEN) G GLAXOSMITHKLINE EABEX 00173-0760-00 8.62536 LAMICTAL XR START KIT (ORANGE) G GLAXOSMITHKLINE EABEX 00173-0756-00 9.27015 12.93527 LAMICTAL XR 100 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0757-00 9.88540 13.79432 LAMICTAL XR 200 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0754-00 4.77449 6.03880 LAMICTAL XR 25 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0781-00 18.81<strong>08</strong>4 LAMICTAL XR 250 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0761-00 13.84815 20.69190 LAMICTAL XR 300 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0755-00 8.65410 12.07622 LAMICTAL XR 50 MG TABLET G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00173-<strong>06</strong>42-55 0.<strong>06</strong>900 7.8<strong>08</strong>64 LAMICTAL 100 MG TABLET G GLAXOSMITHKLINE EABEX 00173-<strong>06</strong>43-60 0.<strong>08</strong>262 8.55840 LAMICTAL 150 MG TABLET G GLAXOSMITHKLINE EABEX 00173-<strong>06</strong>99-00 LAMICTAL 2 MG DISPER TABLET G GLAXOSMITHKLINE EABEX 00173-<strong>06</strong>44-60 0.11930 9.31689 LAMICTAL 200 MG TABLET G GLAXOSMITHKLINE EABUX 00173-0527-00 0.69230 7.101<strong>06</strong> LAMICTAL 25 MG DISPER TABLET G GLAXOSMITHKLINE EABEX 00173-<strong>06</strong>33-02 0.19825 6.83612 LAMICTAL 25 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0526-00 0.36340 6.61476 LAMICTAL 5 MG DISPER TABLET G GLAXOSMITHKLINE EABND 00078-0499-58 11.20737 LAMISIL 125 MG GRANULES PACKET 0 NOVARTIS EABND 00078-0499-59 11.20717 LAMISIL 125 MG GRANULES PACKET 0 NOVARTIS EABND 00078-0499-62 11.19670 LAMISIL 125 MG GRANULES PACKET 0 NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0500-58 16.81165 LAMISIL 187.5 MG GRANULES PACK 0 NOVARTIS EABND 00078-0500-59 16.81125 LAMISIL 187.5 MG GRANULES PACK 0 NOVARTIS EABND 00078-0500-62 16.81580 LAMISIL 187.5 MG GRANULES PACK 0 NOVARTIS EABND 00078-0179-15 0.14554 20.39614 LAMISIL 250 MG TABLET G NOVARTIS EAGEN 60505-3251-<strong>06</strong> 5.37075 LAMIVUDINE 150 MG TABLET G APOTEX CORP EAGEN 65862-0552-60 5.36487 LAMIVUDINE 150 MG TABLET G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0578-11 6.12500 LAMIVUDINE 150 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0578-21 6.12500 LAMIVUDINE 150 MG TABLET G AHP EAGEN 60505-3252-03 10.74150 LAMIVUDINE 300 MG TABLET G APOTEX CORP EAGEN 65862-0553-30 10.72974 LAMIVUDINE 300 MG TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-5385-<strong>06</strong> 11.64512 LAMIVUDINE-ZIDOVUDINE TABLET G TEVA USA EAGEN 65862-0597-60 10.97312 LAMIVUDINE-ZIDOVUDINE TABLET G AUROBINDO PHARM EAGEN 68180-0284-07 11.64512 LAMIVUDINE-ZIDOVUDINE TABLET G LUPIN PHARMACEU EAGEX 49884-0563-11 9.27015 LAMOTRIGINE ER 100 MG TABLET G PAR PHARM. EAGEX 55111-0719-30 9.27015 LAMOTRIGINE ER 100 MG TABLET G DR.REDDY'S LAB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 2<strong>06</strong>LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64679-0273-01 9.27015 LAMOTRIGINE ER 100 MG TABLET G WOCKHARDT USA L EAGEX 64679-0273-02 9.27015 LAMOTRIGINE ER 100 MG TABLET G WOCKHARDT USA L EAGEX 49884-0564-11 9.88540 LAMOTRIGINE ER 200 MG TABLET G PAR PHARM. EAGEX 55111-0720-30 9.88540 LAMOTRIGINE ER 200 MG TABLET G DR.REDDY'S LAB EAGEX 64679-0272-01 9.88540 LAMOTRIGINE ER 200 MG TABLET G WOCKHARDT USA L EAGEX 64679-0272-02 9.88540 LAMOTRIGINE ER 200 MG TABLET G WOCKHARDT USA L EAGEX 49884-0561-11 4.77449 LAMOTRIGINE ER 25 MG TABLET G PAR PHARM. EAGEX 55111-0717-30 4.77449 LAMOTRIGINE ER 25 MG TABLET G DR.REDDY'S LAB EAGEX 64679-0271-01 4.77449 LAMOTRIGINE ER 25 MG TABLET G WOCKHARDT USA L EAGEX 64679-0271-02 4.77449 LAMOTRIGINE ER 25 MG TABLET G WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 49884-<strong>06</strong>04-11 15.29700 LAMOTRIGINE ER 250 MG TABLET G PAR PHARM. EAGEX 49884-<strong>06</strong>05-11 13.84815 LAMOTRIGINE ER 300 MG TABLET G PAR PHARM. EAGEX 55111-0428-30 13.84815 LAMOTRIGINE ER 300 MG TABLET G DR.REDDY'S LAB EAGEX 64679-0275-01 13.84815 LAMOTRIGINE ER 300 MG TABLET G WOCKHARDT USA L EAGEX 49884-0562-11 8.65410 LAMOTRIGINE ER 50 MG TABLET G PAR PHARM. EAGEX 55111-0718-30 8.65410 LAMOTRIGINE ER 50 MG TABLET G DR.REDDY'S LAB EAGEX 64679-0274-01 8.65410 LAMOTRIGINE ER 50 MG TABLET G WOCKHARDT USA L EAGEX 64679-0274-02 8.65410 LAMOTRIGINE ER 50 MG TABLET G WOCKHARDT USA L EAGEX 00093-0463-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 TEVA USA EAGEX 00093-0463-05 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-4252-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 MYLAN EAGEX 00378-4252-05 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 MYLAN EAGEX 13668-0047-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0047-05 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0047-30 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 TORRENT PHARMAC EAGEX 16714-0372-02 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 NORTHSTAR RX LL EAGEX 29300-0112-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 UNICHEM PHARMAC EAGEX 29300-0112-05 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 UNICHEM PHARMAC EAGEX 29300-0112-10 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 UNICHEM PHARMAC EAGEX 51079-0499-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0499-20 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 MYLAN INSTITUTI EAGEX 51672-4131-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 TARO PHARM USA EAGEX 51672-4131-03 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 TARO PHARM USA EAGEX 55111-0221-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 DR.REDDY'S LAB EAGEX 59746-0246-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 CADISTA PHARMAC EAGEX 59746-0246-10 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 CADISTA PHARMAC EAGEX 60505-2664-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 APOTEX CORP EAGEX 60505-2664-05 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 APOTEX CORP EAGEX 63739-0516-10 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 MCKESSON PACKAG EAGEX 65862-0228-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0319-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0319-11 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 AHP EAGEX 68382-00<strong>08</strong>-01 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-00<strong>08</strong>-10 0.<strong>06</strong>900 LAMOTRIGINE 100 MG TABLET 0 ZYDUS PHARMACEU EAGEX 00093-7247-05 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 207LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-7247-<strong>06</strong> 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 TEVA USA EAGEX 00378-4253-05 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 MYLAN EAGEX 00378-4253-91 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 MYLAN EAGEX 13668-0048-05 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0048-60 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 TORRENT PHARMAC EAGEX 16714-0373-04 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 NORTHSTAR RX LL EAGEX 29300-0113-05 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 UNICHEM PHARMAC EAGEX 29300-0113-16 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 UNICHEM PHARMAC EAGEX 51079-<strong>08</strong>65-01 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-<strong>08</strong>65-20 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51672-4132-04 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 TARO PHARM USA EAGEX 55111-0222-60 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 DR.REDDY'S LAB EAGEX 59746-0247-05 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 CADISTA PHARMAC EAGEX 59746-0247-60 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 CADISTA PHARMAC EAGEX 60505-2665-05 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 APOTEX CORP EAGEX 60505-2665-<strong>06</strong> 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 APOTEX CORP EAGEX 65862-0229-01 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0229-60 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0320-11 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0320-21 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68382-0009-05 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0009-14 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68462-0245-60 0.<strong>08</strong>262 LAMOTRIGINE 150 MG TABLET 0 GLENMARK PHARMA EAGEX 00093-7248-05 0.11930 LAMOTRIGINE 200 MG TABLET 0 TEVA USA EAGEX 00093-7248-<strong>06</strong> 0.11930 LAMOTRIGINE 200 MG TABLET 0 TEVA USA EAGEX 00378-4254-05 0.11930 LAMOTRIGINE 200 MG TABLET 0 MYLAN EAGEX 00378-4254-91 0.11930 LAMOTRIGINE 200 MG TABLET 0 MYLAN EAGEX 13668-0049-05 0.11930 LAMOTRIGINE 200 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0049-60 0.11930 LAMOTRIGINE 200 MG TABLET 0 TORRENT PHARMAC EAGEX 16714-0374-04 0.11930 LAMOTRIGINE 200 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 29300-0114-05 0.11930 LAMOTRIGINE 200 MG TABLET 0 UNICHEM PHARMAC EAGEX 29300-0114-16 0.11930 LAMOTRIGINE 200 MG TABLET 0 UNICHEM PHARMAC EAGEX 51079-<strong>08</strong>66-01 0.11930 LAMOTRIGINE 200 MG TABLET 0 MYLAN INSTITUTI EAGEX 51672-4133-03 0.11930 LAMOTRIGINE 200 MG TABLET 0 TARO PHARM USA EAGEX 51672-4133-04 0.11930 LAMOTRIGINE 200 MG TABLET 0 TARO PHARM USA EAGEX 55111-0223-60 0.11930 LAMOTRIGINE 200 MG TABLET 0 DR.REDDY'S LAB EAGEX 59746-0248-05 0.11930 LAMOTRIGINE 200 MG TABLET 0 CADISTA PHARMAC EAGEX 59746-0248-60 0.11930 LAMOTRIGINE 200 MG TABLET 0 CADISTA PHARMAC EAGEX 60505-2680-05 0.11930 LAMOTRIGINE 200 MG TABLET 0 APOTEX CORP EAGEX 60505-2680-<strong>06</strong> 0.11930 LAMOTRIGINE 200 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0230-01 0.11930 LAMOTRIGINE 200 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0230-60 0.11930 LAMOTRIGINE 200 MG TABLET 0 AUROBINDO PHARM EAGEX 68382-0010-05 0.11930 LAMOTRIGINE 200 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0010-14 0.11930 LAMOTRIGINE 200 MG TABLET 0 ZYDUS PHARMACEU EAGUX 00093-0132-01 0.69230 LAMOTRIGINE 25 MG DISPER TAB 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 2<strong>08</strong>LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-6925-01 0.41904 LAMOTRIGINE 25 MG DISPER TAB 0 MYLAN EAGEX 16252-0598-01 0.41904 LAMOTRIGINE 25 MG DISPER TAB 0 ACTAVIS PHARMA, EAGEX 55111-0226-05 0.41904 LAMOTRIGINE 25 MG DISPER TAB 0 DR.REDDY'S LAB EAGEX 65862-0362-01 0.41904 LAMOTRIGINE 25 MG DISPER TAB 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0335-11 0.41904 LAMOTRIGINE 25 MG DISPER TAB 0 AHP EAGEX 68<strong>08</strong>4-0335-21 0.41904 LAMOTRIGINE 25 MG DISPER TAB 0 AHP EAGEX 68382-0109-01 0.41904 LAMOTRIGINE 25 MG DISPER TAB 0 ZYDUS PHARMACEU EAGEX 68462-0229-01 0.41904 LAMOTRIGINE 25 MG DISPER TAB 0 GLENMARK PHARMA EAGEX 00093-0039-01 0.19825 LAMOTRIGINE 25 MG TABLET 0 TEVA USA EAGEX 00093-0039-05 0.19825 LAMOTRIGINE 25 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-4251-01 0.19825 LAMOTRIGINE 25 MG TABLET 0 MYLAN EAGEX 00378-4251-05 0.19825 LAMOTRIGINE 25 MG TABLET 0 MYLAN EAGEX 13668-0045-01 0.19825 LAMOTRIGINE 25 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0045-05 0.19825 LAMOTRIGINE 25 MG TABLET 0 TORRENT PHARMAC EAGEX 16714-0371-02 0.19825 LAMOTRIGINE 25 MG TABLET 0 NORTHSTAR RX LL EAGEX 29300-0111-01 0.19825 LAMOTRIGINE 25 MG TABLET 0 UNICHEM PHARMAC EAGEX 29300-0111-05 0.19825 LAMOTRIGINE 25 MG TABLET 0 UNICHEM PHARMAC EAGEX 29300-0111-10 0.19825 LAMOTRIGINE 25 MG TABLET 0 UNICHEM PHARMAC EAGEX 51079-0498-01 0.19825 LAMOTRIGINE 25 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0498-20 0.19825 LAMOTRIGINE 25 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51672-4130-01 0.19825 LAMOTRIGINE 25 MG TABLET 0 TARO PHARM USA EAGEX 55111-0220-01 0.19825 LAMOTRIGINE 25 MG TABLET 0 DR.REDDY'S LAB EAGEX 59746-0245-01 0.19825 LAMOTRIGINE 25 MG TABLET 0 CADISTA PHARMAC EAGEX 59746-0245-05 0.19825 LAMOTRIGINE 25 MG TABLET 0 CADISTA PHARMAC EAGEX 60505-2663-01 0.19825 LAMOTRIGINE 25 MG TABLET 0 APOTEX CORP EAGEX 60505-2663-05 0.19825 LAMOTRIGINE 25 MG TABLET 0 APOTEX CORP EAGEX 65862-0227-01 0.19825 LAMOTRIGINE 25 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0318-01 0.11145 LAMOTRIGINE 25 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0318-11 0.11145 LAMOTRIGINE 25 MG TABLET 0 AHP EAGEX 68382-00<strong>06</strong>-01 0.19825 LAMOTRIGINE 25 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68382-00<strong>06</strong>-10 0.19825 LAMOTRIGINE 25 MG TABLET 0 ZYDUS PHARMACEU EAGEX 00093-<strong>06</strong>88-01 0.36340 LAMOTRIGINE 5 MG DISPER TABLET 0 TEVA USA EAGEX 00378-6905-01 0.36340 LAMOTRIGINE 5 MG DISPER TABLET 0 MYLAN EAGEX 16252-0597-01 0.36340 LAMOTRIGINE 5 MG DISPER TABLET 0 ACTAVIS PHARMA, EAGEX 65862-0361-01 0.36340 LAMOTRIGINE 5 MG DISPER TABLET 0 AUROBINDO PHARM EAGEX 68382-01<strong>08</strong>-01 0.36340 LAMOTRIGINE 5 MG DISPER TABLET 0 ZYDUS PHARMACEU EAGEX 68462-0228-01 0.36340 LAMOTRIGINE 5 MG DISPER TABLET 0 GLENMARK PHARMA EABND 00173-0262-10 5.97517 LANOXIN PED 0.1 MG/ML AMPUL 0 COVIS PHARMACEU MLBUL 00173-0242-55 0.21320 0.63744 LANOXIN 125 MCG TABLET 0 COVIS PHARMACEU EABUL 00173-0242-75 0.21320 0.63046 LANOXIN 125 MCG TABLET 0 COVIS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 24987-0242-55 0.21320 2.39040 LANOXIN 125 MCG TABLET 0 COVIS PHARMACEU EABUL 24987-0242-75 0.21320 1.972<strong>08</strong> LANOXIN 125 MCG TABLET 0 COVIS PHARMACEU EABND 24987-0245-55 2.39040 LANOXIN 187.5 MCG TABLET 0 COVIS PHARMACEU EABUL 00173-0249-55 0.21320 0.63744 LANOXIN 250 MCG TABLET 0 COVIS PHARMACEU EABUL 00173-0249-75 0.21320 0.63046 LANOXIN 250 MCG TABLET 0 COVIS PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 209LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00173-0249-80 0.21320 1.972<strong>08</strong> LANOXIN 250 MCG TABLET 0 COVIS PHARMACEU EABUL 24987-0249-56 0.21320 2.39040 LANOXIN 250 MCG TABLET 0 COVIS PHARMACEU EABND 00173-0260-10 1.40770 2.98758 LANOXIN 500 MCG/2 ML AMPULE 0 COVIS PHARMACEU MLBND 24987-0260-10 2.98758 LANOXIN 500 MCG/2 ML AMPULE 0 COVIS PHARMACEU MLBND 24987-0240-55 2.39040 LANOXIN 62.5 MCG TABLET 0 COVIS PHARMACEU EAGEN 00093-8055-14 3.84964 LANSOPRAZOL-AMOXICIL-CLARITHRO G TEVA USA EAGEN 00093-7350-56 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G TEVA USA EAGEN 00378-8015-10 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G MYLAN EAGEN 00378-8015-93 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G MYLAN EAGEN 00781-2147-10 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45963-0460-03 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G ACTAVIS PHARMA, EAGEN 47335-0923-83 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G SUN PHARMA GLOB EAGEN 51991-0771-33 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G BRECKENRIDGE EAGEN 55111-0398-30 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G DR.REDDY'S LAB EAGEN 55111-0398-90 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G DR.REDDY'S LAB EAGEN 63739-0574-10 1.66777 LANSOPRAZOLE DR 15 MG CAPSULE G MCKESSON PACKAG EAGEN 64679-<strong>06</strong>69-01 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G WOCKHARDT USA L EAGEN 64679-<strong>06</strong>69-05 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G WOCKHARDT USA L EAGEN 68<strong>08</strong>4-0467-11 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G AHP EAGEN 68<strong>08</strong>4-0467-21 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0543-<strong>06</strong> 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G ZYDUS PHARMACEU EAGEN 68382-0543-10 2.<strong>06</strong>070 LANSOPRAZOLE DR 15 MG CAPSULE G ZYDUS PHARMACEU EAGEN 00093-7351-56 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G TEVA USA EAGEN 00378-8030-05 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G MYLAN EAGEN 00378-8030-77 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G MYLAN EAGEN 00378-8030-93 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G MYLAN EAGEN 00781-2148-01 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G SANDOZ EAGEN 00781-2148-10 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G SANDOZ EAGEN 00781-2148-92 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G SANDOZ EAGEN 45963-0461-03 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45963-0461-10 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G ACTAVIS PHARMA, EAGEN 45963-0461-96 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G ACTAVIS PHARMA, EAGEN 47335-0924-18 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G SUN PHARMA GLOB EAGEN 47335-0924-83 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G SUN PHARMA GLOB EAGEN 47335-0924-88 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G SUN PHARMA GLOB EAGEN 51991-0772-05 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G BRECKENRIDGE EAGEN 51991-0772-33 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G BRECKENRIDGE EAGEN 51991-0772-90 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G BRECKENRIDGE EAGEN 55111-0399-05 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G DR.REDDY'S LAB EAGEN 55111-0399-90 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-<strong>06</strong>70-01 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G WOCKHARDT USA L EAGEN 64679-<strong>06</strong>70-05 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G WOCKHARDT USA L EAGEN 64679-<strong>06</strong>70-07 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G WOCKHARDT USA L EAGEN 68<strong>08</strong>4-0468-01 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G AHP EAGEN 68<strong>08</strong>4-0468-11 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 210LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0544-01 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G ZYDUS PHARMACEU EAGEN 68382-0544-<strong>06</strong> 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G ZYDUS PHARMACEU EAGEN 68382-0544-10 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G ZYDUS PHARMACEU EAGEN 68382-0544-16 0.76950 LANSOPRAZOLE DR 30 MG CAPSULE G ZYDUS PHARMACEU EAGEN 38779-2289-04 28.89630 LANSOPRAZOLE POWDER 0 MEDISCA INC. GMGEN 38779-2289-05 28.89630 LANSOPRAZOLE POWDER 0 MEDISCA INC. GMBND 00<strong>08</strong>8-2219-05 20.12694 LANTUS SOLOSTAR 100 UNITS/ML 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00<strong>08</strong>8-2220-33 19.05182 LANTUS 100 UNITS/ML VIAL 0 SAN<strong>OF</strong>I-AVENTIS MLGEX 16714-0405-01 0.72384 LARIN FE 1.5-30 TABLET 0 NORTHSTAR RX LL EAGEX 16714-0405-04 0.72384 LARIN FE 1.5-30 TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16714-04<strong>06</strong>-01 0.71880 LARIN FE 1-20 TABLET 0 NORTHSTAR RX LL EAGEX 16714-04<strong>06</strong>-04 0.71880 LARIN FE 1-20 TABLET 0 NORTHSTAR RX LL EABND 00039-0<strong>06</strong>7-10 0.01013 0.44620 LASIX 20 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00039-0<strong>06</strong>7-70 0.01013 0.31<strong>08</strong>3 LASIX 20 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00039-0<strong>06</strong>0-13 0.01170 0.62532 LASIX 40 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00039-0<strong>06</strong>0-50 0.01170 0.448<strong>08</strong> LASIX 40 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00039-0<strong>06</strong>0-70 0.01170 0.43770 LASIX 40 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00039-0<strong>06</strong>6-05 0.02730 1.01110 LASIX 80 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00023-4290-03 42.99123 LASTACAFT 0.25% EYE DROPS G ALLERGAN INC. MLGEN 00378-9645-32 3.66296 LATANOPROST 0.005% EYE DROPS 0 MYLAN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00517-<strong>08</strong>30-01 3.66296 LATANOPROST 0.005% EYE DROPS 0 AMER. REGENT MLGEN 17478-<strong>06</strong>25-12 3.66296 LATANOPROST 0.005% EYE DROPS 0 AKORN INC. MLGEN 242<strong>08</strong>-0463-25 3.66296 LATANOPROST 0.005% EYE DROPS 0 VALEANT MLGEN 59762-0333-02 3.66296 LATANOPROST 0.005% EYE DROPS 0 GREENSTONE LLC. MLGEN 60505-0565-00 3.66296 LATANOPROST 0.005% EYE DROPS 0 APOTEX CORP MLGEN 60505-0565-01 3.66296 LATANOPROST 0.005% EYE DROPS 0 APOTEX CORP MLGEN 61314-0547-01 3.66296 LATANOPROST 0.005% EYE DROPS 0 SANDOZ MLGEN 61314-0547-03 3.66296 LATANOPROST 0.005% EYE DROPS 0 SANDOZ MLBEX 63402-0312-30 33.07716 LATUDA 120 MG TABLET G SUNOVION PHARMA EABEX 63402-0302-30 22.16100 LATUDA 20 MG TABLET G SUNOVION PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 63402-0304-01 22.16100 LATUDA 40 MG TABLET G SUNOVION PHARMA EABEX 63402-0304-30 22.16100 LATUDA 40 MG TABLET G SUNOVION PHARMA EABEX 63402-03<strong>06</strong>-30 22.16100 LATUDA 60 MG TABLET G SUNOVION PHARMA EABEX 63402-03<strong>08</strong>-01 22.16100 LATUDA 80 MG TABLET G SUNOVION PHARMA EABEX 63402-03<strong>08</strong>-30 22.16100 LATUDA 80 MG TABLET G SUNOVION PHARMA EAGEN 00093-0173-56 0.53070 LEFLUNOMIDE 10 MG TABLET 0 TEVA USA EAGEN 00781-5056-31 0.53070 LEFLUNOMIDE 10 MG TABLET 0 SANDOZ EAGEN 16714-0321-01 0.53070 LEFLUNOMIDE 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 23155-0043-03 0.53070 LEFLUNOMIDE 10 MG TABLET 0 HERITAGE PHARMA EAGEN 60505-2502-01 0.53070 LEFLUNOMIDE 10 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2502-03 0.53070 LEFLUNOMIDE 10 MG TABLET 0 APOTEX CORP EAGEN 66993-0160-30 0.53070 LEFLUNOMIDE 10 MG TABLET 0 PRASCO LABS EAGEN 00093-0174-56 0.71321 LEFLUNOMIDE 20 MG TABLET 0 TEVA USA EAGEN 00781-5057-31 0.71321 LEFLUNOMIDE 20 MG TABLET 0 SANDOZ EAGEN 16714-0331-01 0.71321 LEFLUNOMIDE 20 MG TABLET 0 NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 211LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 23155-0044-03 0.71321 LEFLUNOMIDE 20 MG TABLET 0 HERITAGE PHARMA EAGEN 60505-2503-01 0.71321 LEFLUNOMIDE 20 MG TABLET 0 APOTEX CORP EAGEN 60505-2503-03 0.71321 LEFLUNOMIDE 20 MG TABLET 0 APOTEX CORP EAGEN 66993-0161-30 0.71321 LEFLUNOMIDE 20 MG TABLET 0 PRASCO LABS EABND 00078-0354-05 6.18872 LESCOL XL 80 MG TABLET G NOVARTIS EABND 00078-0354-15 6.18875 LESCOL XL 80 MG TABLET G NOVARTIS EABND 00078-0176-05 3.20544 4.61430 LESCOL 20 MG CAPSULE G NOVARTIS EABND 00078-0176-15 3.20544 4.62199 LESCOL 20 MG CAPSULE G NOVARTIS EABND 00078-0234-05 2.64297 4.61430 LESCOL 40 MG CAPSULE G NOVARTIS EABND 00078-0234-15 2.64297 4.62199 LESCOL 40 MG CAPSULE G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00555-9014-67 0.85280 LESSINA-28 TABLET 0 BARR EABND 61958-<strong>08</strong>02-01 228.85756 LETAIRIS 10 MG TABLET 0 GILEAD SCIENCES EABND 61958-<strong>08</strong>02-02 228.85756 LETAIRIS 10 MG TABLET 0 GILEAD SCIENCES EABND 61958-<strong>08</strong>02-03 228.85756 LETAIRIS 10 MG TABLET 0 GILEAD SCIENCES EABND 61958-<strong>08</strong>01-01 228.85756 LETAIRIS 5 MG TABLET 0 GILEAD SCIENCES EABND 61958-<strong>08</strong>01-02 228.85756 LETAIRIS 5 MG TABLET 0 GILEAD SCIENCES EABND 61958-<strong>08</strong>01-03 228.85756 LETAIRIS 5 MG TABLET 0 GILEAD SCIENCES EAGEN 00054-0269-13 0.15300 LETROZOLE 2.5 MG TABLET 0 ROXANE LABS. EAGEN 00093-7620-56 0.15300 LETROZOLE 2.5 MG TABLET 0 TEVA USA EAGEN 00378-2071-05 0.15300 LETROZOLE 2.5 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-2071-93 0.15300 LETROZOLE 2.5 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-4180-16 0.15300 LETROZOLE 2.5 MG TABLET 0 QUALITEST EAGEN 16729-0034-10 0.15300 LETROZOLE 2.5 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 51991-0759-10 0.15300 LETROZOLE 2.5 MG TABLET 0 BRECKENRIDGE EAGEN 51991-0759-33 0.15300 LETROZOLE 2.5 MG TABLET 0 BRECKENRIDGE EAGEN 60505-3255-03 0.15300 LETROZOLE 2.5 MG TABLET 0 APOTEX CORP EAGEN 60505-3255-<strong>08</strong> 0.15300 LETROZOLE 2.5 MG TABLET 0 APOTEX CORP EAGEN 62756-0511-83 0.15300 LETROZOLE 2.5 MG TABLET 0 SUN PHARMACEUTI EAGEN 63323-0772-30 0.15300 LETROZOLE 2.5 MG TABLET 0 APP PHARMACEUTI EABND 55390-0009-01 0.83664 LEUCOVORIN CAL 500 MG/50 ML VL 0 BEDFORD LABS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00054-4497-05 6.28932 LEUCOVORIN CALCIUM 10 MG TAB 0 ROXANE LABS. EABND 00054-4497-10 6.22672 LEUCOVORIN CALCIUM 10 MG TAB 0 ROXANE LABS. EAGEN 00703-5140-01 8.52000 LEUCOVORIN CALCIUM 100 MG VIAL 0 TEVA PARENTERAL EAGEN 25021-<strong>08</strong>14-67 7.92000 LEUCOVORIN CALCIUM 100 MG VIAL 0 SAGENT/PREMIERP EAGEN 55390-0052-10 7.02000 LEUCOVORIN CALCIUM 100 MG VIAL 0 BEDFORD LABS EAGEN 55390-<strong>08</strong>18-10 4.18500 LEUCOVORIN CALCIUM 100 MG VIAL 0 BEDFORD/NOVAPLU EABND 00054-4498-10 8.80561 LEUCOVORIN CALCIUM 15 MG TAB 0 ROXANE LABS. EAGEN 25021-<strong>08</strong>15-67 13.86000 LEUCOVORIN CALCIUM 200 MG VIAL 0 SAGENT/PREMIERP EAGEN 55390-0053-01 17.17200 LEUCOVORIN CALCIUM 200 MG VIAL 0 BEDFORD LABS EAGEN 55390-<strong>08</strong>24-01 14.85000 LEUCOVORIN CALCIUM 200 MG VIAL 0 BEDFORD/NOVAPLU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-4499-11 7.51090 LEUCOVORIN CALCIUM 25 MG TAB 0 ROXANE LABS. EAGEN 00555-0485-27 7.51090 LEUCOVORIN CALCIUM 25 MG TAB 0 BARR EAGEN 51079-0582-05 7.51090 LEUCOVORIN CALCIUM 25 MG TAB 0 MYLAN INSTITUTI EAGEN 00703-5145-01 17.05500 LEUCOVORIN CALCIUM 350 MG VIAL 0 TEVA PARENTERAL EAGEN 25021-<strong>08</strong>16-67 15.84000 LEUCOVORIN CALCIUM 350 MG VIAL 0 SAGENT PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 212LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55390-0054-01 27.90000 LEUCOVORIN CALCIUM 350 MG VIAL 0 BEDFORD LABS EAGEN 00054-4496-13 0.91840 LEUCOVORIN CALCIUM 5 MG TAB 0 ROXANE LABS. EAGEN 00054-4496-25 0.91840 LEUCOVORIN CALCIUM 5 MG TAB 0 ROXANE LABS. EAGEN 00555-0484-01 0.91840 LEUCOVORIN CALCIUM 5 MG TAB 0 BARR EAGEN 00555-0484-02 0.91840 LEUCOVORIN CALCIUM 5 MG TAB 0 BARR EAGEN 51079-0581-<strong>06</strong> 0.91840 LEUCOVORIN CALCIUM 5 MG TAB 0 MYLAN INSTITUTI EAGEN 25021-<strong>08</strong>13-66 3.96000 LEUCOVORIN CALCIUM 50 MG VIAL 0 SAGENT/PREMIERP EAGEN 55390-0051-10 5.85000 LEUCOVORIN CALCIUM 50 MG VIAL 0 BEDFORD LABS EABND 63323-0711-00 52.43940 LEUCOVORIN CALCIUM 500 MG VL 0 APP PHARMACEUTI EABND 00173-<strong>06</strong>35-35 4.62144 LEUKERAN 2 MG TABLET 0 PRASCO LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 76388-<strong>06</strong>35-50 9.24288 LEUKERAN 2 MG TABLET 0 PRASCO LABS EABND 00024-5843-05 217.64260 LEUKINE 250 MCG VIAL 0 SAN<strong>OF</strong>I-AVENTIS EABND 58468-0180-02 217.64260 LEUKINE 250 MCG VIAL 0 GENZYME EABND 58468-0181-01 366.58610 LEUKINE 500 MCG/ML VIAL 0 GENZYME MLBND 58468-0181-02 366.58610 LEUKINE 500 MCG/ML VIAL 0 GENZYME MLGEN 00703-4014-18 1<strong>08</strong>.00000 LEUPROLIDE 2WK 1 MG/0.2 ML KIT 0 TEVA PARENTERAL EAGEN 00781-4003-32 160.13575 LEUPROLIDE 2WK 1 MG/0.2 ML KIT 0 SANDOZ EAGEN 41616-0936-40 160.13575 LEUPROLIDE 2WK 1 MG/0.2 ML KIT 0 SUN PHARMA GLOB EAGEN 00378-6993-93 3.25720 LEVALBUTEROL CONC 1.25 MG/0.5 G MYLAN EAGEN 00093-4145-04 0.82107 LEVALBUTEROL 0.31 MG/3 ML SOL G TEVA USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-4145-64 0.82107 LEVALBUTEROL 0.31 MG/3 ML SOL G TEVA USA MLGEN 00591-2918-23 0.82107 LEVALBUTEROL 0.31 MG/3 ML SOL G ACTAVIS PHARMA, MLGEN 66993-0021-27 0.82107 LEVALBUTEROL 0.31 MG/3 ML SOL G PRASCO LABS MLGEN 00093-4146-64 1.01350 LEVALBUTEROL 0.63 MG/3 ML SOL G TEVA USA MLGEN 00591-2919-23 1.01350 LEVALBUTEROL 0.63 MG/3 ML SOL G ACTAVIS PHARMA, MLGEN 66993-0022-27 1.01350 LEVALBUTEROL 0.63 MG/3 ML SOL G PRASCO LABS MLGEN 00093-4148-04 1.03760 LEVALBUTEROL 1.25 MG/3 ML SOL G TEVA USA MLGEN 00093-4148-64 1.03760 LEVALBUTEROL 1.25 MG/3 ML SOL G TEVA USA MLGEN 00591-2920-23 1.03760 LEVALBUTEROL 1.25 MG/3 ML SOL G ACTAVIS PHARMA, MLGEN 66993-0023-27 1.03760 LEVALBUTEROL 1.25 MG/3 ML SOL G PRASCO LABS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50458-0170-01 0.99210 1.54568 LEVAQUIN 25 MG/ML SOLUTION G JANSSEN PHARM. MLBND 50458-0920-10 0.23220 21.61129 LEVAQUIN 250 MG TABLET G JANSSEN PHARM. EABND 50458-0920-50 0.23220 21.61120 LEVAQUIN 250 MG TABLET G JANSSEN PHARM. EABND 50458-0925-50 0.29860 24.76836 LEVAQUIN 500 MG TABLET G JANSSEN PHARM. EABND 50458-0930-10 0.79460 46.37990 LEVAQUIN 750 MG TABLET G JANSSEN PHARM. EABND 50458-0930-20 0.79460 46.37915 LEVAQUIN 750 MG TABLET G JANSSEN PHARM. EABND 50458-0167-01 0.13250 0.21231 LEVAQUIN-D5W 250 MG/50 ML BAG 0 JANSSEN PHARM. MLBND 50458-0168-01 0.1<strong>08</strong>65 0.20949 LEVAQUIN-D5W 500 MG/100 ML BAG 0 JANSSEN PHARM. MLBND 50458-0166-01 0.05400 0.18564 LEVAQUIN-D5W 750 MG/150 ML BAG 0 JANSSEN PHARM. MLBND 52244-0450-10 3.37644 LEVATOL 20 MG TABLET G AUXILIUM PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00169-6439-10 20.12694 LEVEMIR FLEXPEN 100 UNITS/ML 0 NOVO NORDISK MLBND 00169-3687-12 19.05182 LEVEMIR 100 UNITS/ML VIAL 0 NOVO NORDISK MLGEX 00093-7795-<strong>06</strong> 0.51000 LEVETIRACETAM ER 500 MG TABLET 0 TEVA USA EAGEX 00228-4417-<strong>06</strong> 0.51000 LEVETIRACETAM ER 500 MG TABLET 0 ACTAVIS ELIZABE EAGEX 00228-4417-50 0.51000 LEVETIRACETAM ER 500 MG TABLET 0 ACTAVIS ELIZABE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 213LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00591-3635-60 0.51000 LEVETIRACETAM ER 500 MG TABLET 0 ACTAVIS PHARMA, EAGEX 0<strong>06</strong>03-4186-20 0.51000 LEVETIRACETAM ER 500 MG TABLET 0 QUALITEST EAGEX 13668-0272-60 0.51000 LEVETIRACETAM ER 500 MG TABLET 0 TORRENT PHARMAC EAGEX 47335-0573-86 0.51000 LEVETIRACETAM ER 500 MG TABLET 0 SUN PHARMA GLOB EAGEX 49884-0204-02 0.51000 LEVETIRACETAM ER 500 MG TABLET 0 PAR PHARM. EAGEX 60505-3280-<strong>06</strong> 0.51000 LEVETIRACETAM ER 500 MG TABLET 0 APOTEX CORP EAGEX 68180-0117-07 0.51000 LEVETIRACETAM ER 500 MG TABLET 0 LUPIN PHARMACEU EAGEX 00093-7796-<strong>06</strong> 0.88870 LEVETIRACETAM ER 750 MG TABLET 0 TEVA USA EAGEX 00228-2975-<strong>06</strong> 0.88870 LEVETIRACETAM ER 750 MG TABLET 0 ACTAVIS ELIZABE EAGEX 00591-3699-60 0.88870 LEVETIRACETAM ER 750 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-4187-20 0.88870 LEVETIRACETAM ER 750 MG TABLET 0 QUALITEST EAGEX 13668-0300-60 0.88870 LEVETIRACETAM ER 750 MG TABLET 0 TORRENT PHARMAC EAGEX 47335-0576-86 0.88870 LEVETIRACETAM ER 750 MG TABLET 0 SUN PHARMA GLOB EAGEX 49884-0205-02 0.88870 LEVETIRACETAM ER 750 MG TABLET 0 PAR PHARM. EAGEX 60505-3517-<strong>06</strong> 0.88870 LEVETIRACETAM ER 750 MG TABLET 0 APOTEX CORP EAGEX 68180-0118-07 0.88870 LEVETIRACETAM ER 750 MG TABLET 0 LUPIN PHARMACEU EAGEX 00093-7493-<strong>06</strong> 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 TEVA USA EAGEX 00378-5619-18 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 MYLAN EAGEX 00378-5619-91 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 MYLAN EAGEX 13668-0017-05 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0017-12 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0017-25 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0017-60 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 TORRENT PHARMAC EAGEX 16714-0357-01 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 NORTHSTAR RX LL EAGEX 16729-0<strong>06</strong>7-12 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 31722-0539-60 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 CAMBER PHARMACE EAGEX 42043-0193-<strong>06</strong> 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 KARALEX PHARMA, EAGEX 43547-0224-<strong>06</strong> 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEX 51079-<strong>08</strong>60-01 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-<strong>08</strong>60-<strong>06</strong> 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 55111-0248-60 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 DR.REDDY'S LAB EAGEX 68<strong>08</strong>4-0356-01 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0356-11 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 AHP EAGEX 68180-0115-07 0.41490 LEVETIRACETAM 1,000 MG TABLET 0 LUPIN PHARMACEU EAGEX 00054-0224-63 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 ROXANE LABS. MLGEX 00472-0235-16 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 ACTAVIS PHARMA, MLGEX 0<strong>06</strong>03-1384-58 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 QUALITEST MLGEX 00781-6141-16 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 SANDOZ MLGEX 16714-0358-01 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 NORTHSTAR RX LL MLGEX 50383-0241-16 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 HI-TECH PHARMAC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51672-4136-09 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 TARO PHARM USA MLGEX 51991-<strong>06</strong>51-16 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 BRECKENRIDGE MLGEX 54838-0548-80 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 SILARX PHARM MLGEX 60258-<strong>08</strong>65-16 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 CYPRESS PHARM. MLGEX 60432-<strong>08</strong>31-16 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 MORTON GROVE PH ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 214LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65162-<strong>06</strong>85-90 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 AMNEAL PHARMACE MLGEX 65862-0250-47 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 AUROBINDO PHARM MLGEX 68180-0116-01 0.07840 LEVETIRACETAM 100 MG/ML SOLN 0 LUPIN PHARMACEU MLGEX 00093-7285-89 0.09980 LEVETIRACETAM 250 MG TABLET 0 TEVA USA EAGEX 00378-5613-05 0.09980 LEVETIRACETAM 250 MG TABLET 0 MYLAN EAGEX 00378-5613-07 0.09980 LEVETIRACETAM 250 MG TABLET 0 MYLAN EAGEX 00378-5613-12 0.09980 LEVETIRACETAM 250 MG TABLET 0 MYLAN EAGEX 00378-5613-78 0.09980 LEVETIRACETAM 250 MG TABLET 0 MYLAN EAGEX 00904-6001-61 0.09980 LEVETIRACETAM 250 MG TABLET 0 MAJOR PHARMACEU EAGEX 00904-6051-61 0.09980 LEVETIRACETAM 250 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0014-05 0.09980 LEVETIRACETAM 250 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0014-12 0.09980 LEVETIRACETAM 250 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0014-25 0.09980 LEVETIRACETAM 250 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0014-60 0.09980 LEVETIRACETAM 250 MG TABLET 0 TORRENT PHARMAC EAGEX 16714-0354-01 0.09980 LEVETIRACETAM 250 MG TABLET 0 NORTHSTAR RX LL EAGEX 16729-0<strong>06</strong>4-16 0.09980 LEVETIRACETAM 250 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0<strong>06</strong>4-29 0.09980 LEVETIRACETAM 250 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 31722-0536-05 0.09980 LEVETIRACETAM 250 MG TABLET 0 CAMBER PHARMACE EAGEX 31722-0536-12 0.09980 LEVETIRACETAM 250 MG TABLET 0 CAMBER PHARMACE EAGEX 42043-0190-04 0.09980 LEVETIRACETAM 250 MG TABLET 0 KARALEX PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 42043-0190-05 0.09980 LEVETIRACETAM 250 MG TABLET 0 KARALEX PHARMA, EAGEX 43547-0221-15 0.09980 LEVETIRACETAM 250 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEX 51079-<strong>08</strong>20-01 0.09980 LEVETIRACETAM 250 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-<strong>08</strong>20-20 0.09980 LEVETIRACETAM 250 MG TABLET 0 MYLAN INSTITUTI EAGEX 55111-0181-04 0.09980 LEVETIRACETAM 250 MG TABLET 0 DR.REDDY'S LAB EAGEX 64376-0136-05 0.09980 LEVETIRACETAM 250 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-0136-12 0.09980 LEVETIRACETAM 250 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-0136-90 0.09980 LEVETIRACETAM 250 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-0136-99 0.09980 LEVETIRACETAM 250 MG TABLET 0 BOCA PHARMACAL EAGEX 68<strong>08</strong>4-0336-01 0.09980 LEVETIRACETAM 250 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0336-11 0.09980 LEVETIRACETAM 250 MG TABLET 0 AHP EAGEX 68180-0112-02 0.09980 LEVETIRACETAM 250 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0112-09 0.09980 LEVETIRACETAM 250 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0112-16 0.09980 LEVETIRACETAM 250 MG TABLET 0 LUPIN PHARMACEU EAGEX 76282-0246-12 0.09980 LEVETIRACETAM 250 MG TABLET 0 EXELAN PHARMACE EAGEX 00093-7286-89 0.24840 LEVETIRACETAM 500 MG TABLET 0 TEVA USA EAGEX 00378-5615-05 0.24840 LEVETIRACETAM 500 MG TABLET 0 MYLAN EAGEX 00378-5615-07 0.24840 LEVETIRACETAM 500 MG TABLET 0 MYLAN EAGEX 00378-5615-12 0.24840 LEVETIRACETAM 500 MG TABLET 0 MYLAN EAGEX 00378-5615-78 0.24840 LEVETIRACETAM 500 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00904-6052-61 0.24840 LEVETIRACETAM 500 MG TABLET 0 MAJOR PHARMACEU EAGEX 13668-0015-05 0.24840 LEVETIRACETAM 500 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0015-12 0.24840 LEVETIRACETAM 500 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0015-25 0.24840 LEVETIRACETAM 500 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0015-60 0.24840 LEVETIRACETAM 500 MG TABLET 0 TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 215LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13811-<strong>06</strong>21-12 0.24840 LEVETIRACETAM 500 MG TABLET 0 TRIGEN LABORATO EAGEX 13811-<strong>06</strong>21-50 0.24840 LEVETIRACETAM 500 MG TABLET 0 TRIGEN LABORATO EAGEX 16714-0355-01 0.24840 LEVETIRACETAM 500 MG TABLET 0 NORTHSTAR RX LL EAGEX 16729-0<strong>06</strong>5-16 0.24840 LEVETIRACETAM 500 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0<strong>06</strong>5-29 0.24840 LEVETIRACETAM 500 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 31722-0537-05 0.24840 LEVETIRACETAM 500 MG TABLET 0 CAMBER PHARMACE EAGEX 31722-0537-12 0.24840 LEVETIRACETAM 500 MG TABLET 0 CAMBER PHARMACE EAGEX 42043-0191-04 0.24840 LEVETIRACETAM 500 MG TABLET 0 KARALEX PHARMA, EAGEX 42043-0191-05 0.24840 LEVETIRACETAM 500 MG TABLET 0 KARALEX PHARMA, EAGEX 43547-0222-15 0.24840 LEVETIRACETAM 500 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-<strong>08</strong>21-01 0.24840 LEVETIRACETAM 500 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-<strong>08</strong>21-17 0.24840 LEVETIRACETAM 500 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-<strong>08</strong>21-19 0.24840 LEVETIRACETAM 500 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-<strong>08</strong>21-20 0.24840 LEVETIRACETAM 500 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-<strong>08</strong>21-30 0.24840 LEVETIRACETAM 500 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-<strong>08</strong>21-56 0.24840 LEVETIRACETAM 500 MG TABLET 0 MYLAN INSTITUTI EAGEX 55111-0182-04 0.24840 LEVETIRACETAM 500 MG TABLET 0 DR.REDDY'S LAB EAGEX 64376-0137-05 0.24840 LEVETIRACETAM 500 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-0137-12 0.24840 LEVETIRACETAM 500 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-0137-90 0.24840 LEVETIRACETAM 500 MG TABLET 0 BOCA PHARMACAL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64376-0137-99 0.24840 LEVETIRACETAM 500 MG TABLET 0 BOCA PHARMACAL EAGEX 65162-0529-16 0.24840 LEVETIRACETAM 500 MG TABLET 0 AMNEAL PHARMACE EAGEX 65862-0246-<strong>08</strong> 0.24840 LEVETIRACETAM 500 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0337-01 0.24840 LEVETIRACETAM 500 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0337-11 0.24840 LEVETIRACETAM 500 MG TABLET 0 AHP EAGEX 68180-0113-02 0.24840 LEVETIRACETAM 500 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0113-09 0.24840 LEVETIRACETAM 500 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0113-16 0.24840 LEVETIRACETAM 500 MG TABLET 0 LUPIN PHARMACEU EAGEX 76282-0247-12 0.24840 LEVETIRACETAM 500 MG TABLET 0 EXELAN PHARMACE EAGUX 63739-0539-50 0.34880 LEVETIRACETAM 500 MG/5 ML SOLN 0 MCKESSON PACKAG ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00143-9673-10 1.30491 LEVETIRACETAM 500 MG/5 ML VIAL 0 WEST-WARD,INC. MLGEX 00409-1886-02 0.87840 LEVETIRACETAM 500 MG/5 ML VIAL 0 HOSPIRA MLGEX 00517-3605-01 1.30491 LEVETIRACETAM 500 MG/5 ML VIAL 0 AMER. REGENT MLGEX 00517-3605-25 1.30491 LEVETIRACETAM 500 MG/5 ML VIAL 0 AMER. REGENT MLGEX 62756-0513-40 1.30491 LEVETIRACETAM 500 MG/5 ML VIAL 0 SUN PHARMACEUTI MLGEX 62756-0513-44 1.30491 LEVETIRACETAM 500 MG/5 ML VIAL 0 SUN PHARMACEUTI MLGEX 63323-0400-09 1.30491 LEVETIRACETAM 500 MG/5 ML VIAL 0 APP PHARMACEUTI MLGEX 00093-7287-89 0.22248 LEVETIRACETAM 750 MG TABLET 0 TEVA USA EAGEX 00378-5617-05 0.22248 LEVETIRACETAM 750 MG TABLET 0 MYLAN EAGEX 00378-5617-07 0.22248 LEVETIRACETAM 750 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-5617-12 0.22248 LEVETIRACETAM 750 MG TABLET 0 MYLAN EAGEX 00378-5617-78 0.22248 LEVETIRACETAM 750 MG TABLET 0 MYLAN EAGEX 00904-6002-61 0.22248 LEVETIRACETAM 750 MG TABLET 0 MAJOR PHARMACEU EAGEX 00904-6053-61 0.22248 LEVETIRACETAM 750 MG TABLET 0 MAJOR PHARMACEU EAGEX 13668-0016-05 0.22248 LEVETIRACETAM 750 MG TABLET 0 TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 216LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0016-12 0.22248 LEVETIRACETAM 750 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0016-25 0.22248 LEVETIRACETAM 750 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0016-60 0.22248 LEVETIRACETAM 750 MG TABLET 0 TORRENT PHARMAC EAGEX 16714-0356-01 0.22248 LEVETIRACETAM 750 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-0356-02 0.22248 LEVETIRACETAM 750 MG TABLET 0 NORTHSTAR RX LL EAGEX 16729-0<strong>06</strong>6-16 0.22248 LEVETIRACETAM 750 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0<strong>06</strong>6-29 0.22248 LEVETIRACETAM 750 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 31722-0538-05 0.22248 LEVETIRACETAM 750 MG TABLET 0 CAMBER PHARMACE EAGEX 31722-0538-12 0.22248 LEVETIRACETAM 750 MG TABLET 0 CAMBER PHARMACE EAGEX 42043-0192-04 0.22248 LEVETIRACETAM 750 MG TABLET 0 KARALEX PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 42043-0192-05 0.22248 LEVETIRACETAM 750 MG TABLET 0 KARALEX PHARMA, EAGEX 43547-0223-15 0.22248 LEVETIRACETAM 750 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEX 51079-<strong>08</strong>22-01 0.22248 LEVETIRACETAM 750 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-<strong>08</strong>22-20 0.22248 LEVETIRACETAM 750 MG TABLET 0 MYLAN INSTITUTI EAGEX 55111-0183-04 0.22248 LEVETIRACETAM 750 MG TABLET 0 DR.REDDY'S LAB EAGEX 64376-0138-05 0.22248 LEVETIRACETAM 750 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-0138-12 0.22248 LEVETIRACETAM 750 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-0138-90 0.22248 LEVETIRACETAM 750 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-0138-99 0.22248 LEVETIRACETAM 750 MG TABLET 0 BOCA PHARMACAL EAGEX 65162-0538-16 0.22248 LEVETIRACETAM 750 MG TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0247-<strong>08</strong> 0.22248 LEVETIRACETAM 750 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0338-01 0.22248 LEVETIRACETAM 750 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0338-11 0.22248 LEVETIRACETAM 750 MG TABLET 0 AHP EAGEX 68180-0114-02 0.22248 LEVETIRACETAM 750 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0114-09 0.22248 LEVETIRACETAM 750 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0114-16 0.22248 LEVETIRACETAM 750 MG TABLET 0 LUPIN PHARMACEU EAGEX 76282-0248-12 0.22248 LEVETIRACETAM 750 MG TABLET 0 EXELAN PHARMACE EABEX 67457-0265-00 0.45416 LEVETIRACETAM-NACL 1,000MG/100 0 MYLAN INSTITUTI MLBEX 67457-0265-10 0.45416 LEVETIRACETAM-NACL 1,000MG/100 0 MYLAN INSTITUTI MLBEX 67457-0266-00 0.67964 LEVETIRACETAM-NACL 1,500MG/100 0 MYLAN INSTITUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 67457-0266-10 0.67964 LEVETIRACETAM-NACL 1,500MG/100 0 MYLAN INSTITUTI MLBEX 67457-0255-00 0.27249 LEVETIRACETAM-NACL 500 MG/100 0 MYLAN INSTITUTI MLBEX 67457-0255-10 0.27249 LEVETIRACETAM-NACL 500 MG/100 0 MYLAN INSTITUTI MLBND 60758-0<strong>06</strong>3-05 0.89570 2.33396 LEVOBUNOLOL 0.25% EYE DROPS 0 PACIFIC PHARMA MLGEN 242<strong>08</strong>-0505-05 0.99670 LEVOBUNOLOL 0.5% EYE DROPS 0 VALEANT MLGEN 242<strong>08</strong>-0505-10 0.99670 LEVOBUNOLOL 0.5% EYE DROPS 0 VALEANT MLGEN 242<strong>08</strong>-0505-15 0.99670 LEVOBUNOLOL 0.5% EYE DROPS 0 VALEANT MLGEN 60758-0<strong>06</strong>0-05 0.99670 LEVOBUNOLOL 0.5% EYE DROPS 0 PACIFIC PHARMA MLGEN 60758-0<strong>06</strong>0-10 0.99670 LEVOBUNOLOL 0.5% EYE DROPS 0 PACIFIC PHARMA MLGEN 61314-0229-05 0.99670 LEVOBUNOLOL 0.5% EYE DROPS 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61314-0229-10 0.99670 LEVOBUNOLOL 0.5% EYE DROPS 0 SANDOZ MLGEN 61314-0229-15 0.99670 LEVOBUNOLOL 0.5% EYE DROPS 0 SANDOZ MLGEN 50383-0171-04 0.16990 LEVOCARNITINE 100 MG/ML SOLN 0 HI-TECH PHARMAC MLGEN 64980-0503-12 0.16990 LEVOCARNITINE 100 MG/ML SOLN 0 RISING PHARM MLGEN 00517-1045-25 1.98000 LEVOCARNITINE 200 MG/ML VIAL 0 AMER. REGENT ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 217LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00703-0404-02 1.9<strong>08</strong>00 LEVOCARNITINE 200 MG/ML VIAL 0 TEVA PARENTERAL MLGEN 00703-0405-02 1.72800 LEVOCARNITINE 200 MG/ML VIAL 0 TEVA PARENTERAL MLGEN 55390-0136-05 2.16000 LEVOCARNITINE 200 MG/ML VIAL 0 BEDFORD LABS MLGEN 50383-0172-90 0.58740 LEVOCARNITINE 330 MG TABLET 0 HI-TECH PHARMAC EAGEN 64980-0130-09 0.58740 LEVOCARNITINE 330 MG TABLET 0 RISING PHARM EAGEN 00955-1026-21 0.46768 LEVOCETIRIZINE 2.5 MG/5 ML SOL G WINTHROP US MLGEN 45802-<strong>06</strong>80-28 0.46773 LEVOCETIRIZINE 2.5 MG/5 ML SOL G PERRIGO CO. MLGEN 00093-7701-98 0.23112 LEVOCETIRIZINE 5 MG TABLET G TEVA USA EAGEN 00955-1025-90 0.23112 LEVOCETIRIZINE 5 MG TABLET G WINTHROP US EAGEN 31722-0551-90 0.23112 LEVOCETIRIZINE 5 MG TABLET G CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42571-0122-90 0.23112 LEVOCETIRIZINE 5 MG TABLET G MICRO LABS USA, EAGEN 45802-0594-65 0.23112 LEVOCETIRIZINE 5 MG TABLET G PERRIGO CO. EAGEN 45802-0594-75 0.23112 LEVOCETIRIZINE 5 MG TABLET G PERRIGO CO. EAGEN 45802-0594-87 0.23112 LEVOCETIRIZINE 5 MG TABLET G PERRIGO CO. EAGEN 47335-0175-81 0.23112 LEVOCETIRIZINE 5 MG TABLET G SUN PHARMA GLOB EAGEN 55111-0282-90 0.23112 LEVOCETIRIZINE 5 MG TABLET G DR.REDDY'S LAB EAGEN 68462-0346-90 0.23112 LEVOCETIRIZINE 5 MG TABLET G GLENMARK PHARMA EAGEN 16571-0150-50 7.56010 LEV<strong>OF</strong>LOXACIN 0.5% EYE DROPS G PACK PHARMACEUT MLGEN 17478-01<strong>06</strong>-10 7.56010 LEV<strong>OF</strong>LOXACIN 0.5% EYE DROPS G AKORN INC. MLGEN 50383-0283-05 7.56010 LEV<strong>OF</strong>LOXACIN 0.5% EYE DROPS G HI-TECH PHARMAC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10147-0941-<strong>06</strong> 0.90534 LEV<strong>OF</strong>LOXACIN 25 MG/ML SOLUTION G PATRIOT PHARMAC MLGEN 50383-0286-04 0.95250 LEV<strong>OF</strong>LOXACIN 25 MG/ML SOLUTION G HI-TECH PHARMAC MLGEN 50383-0286-<strong>08</strong> 0.95250 LEV<strong>OF</strong>LOXACIN 25 MG/ML SOLUTION G HI-TECH PHARMAC MLGEN 50383-0286-16 0.94296 LEV<strong>OF</strong>LOXACIN 25 MG/ML SOLUTION G HI-TECH PHARMAC MLGEN 00093-7291-53 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 TEVA USA EAGEN 00781-5790-01 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 SANDOZ EAGEN 00781-5790-50 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 SANDOZ EAGEN 00904-6249-61 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 MAJOR PHARMACEU EAGEN 13668-0<strong>08</strong>2-01 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0<strong>08</strong>2-50 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-0<strong>08</strong>2-71 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 TORRENT PHARMAC EAGEN 33342-0021-<strong>08</strong> 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 MACLEODS PHARMA EAGEN 55111-0279-50 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 DR.REDDY'S LAB EAGEN 59762-0278-02 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 GREENSTONE LLC. EAGEN 64679-0544-02 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0544-03 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 WOCKHARDT USA L EAGEN 65862-0536-50 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0481-01 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0481-11 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 AHP EAGEN 68180-0240-01 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0240-<strong>08</strong> 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 LUPIN PHARMACEU EAGEN 68382-0015-01 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0015-18 0.23220 LEV<strong>OF</strong>LOXACIN 250 MG TABLET 0 ZYDUS PHARMACEU EABND 50383-0286-10 1.25641 LEV<strong>OF</strong>LOXACIN 250 MG/10 ML SOLN G HI-TECH PHARMAC MLBND 50383-0286-11 1.25641 LEV<strong>OF</strong>LOXACIN 250 MG/10 ML SOLN G HI-TECH PHARMAC ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 218LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7292-53 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 TEVA USA EAGEN 00781-5791-01 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 SANDOZ EAGEN 00781-5791-50 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 SANDOZ EAGEN 00904-6250-61 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6352-61 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 13668-0<strong>08</strong>3-01 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0<strong>08</strong>3-50 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0<strong>08</strong>3-71 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 TORRENT PHARMAC EAGEN 33342-0022-<strong>08</strong> 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 MACLEODS PHARMA EAGEN 55111-0280-50 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 58517-0220-20 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 <strong>NEW</strong> HORIZON RX EAGEN 59762-0279-02 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 GREENSTONE LLC. EAGEN 64679-0545-02 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0545-03 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 WOCKHARDT USA L EAGEN 65862-0537-50 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0482-01 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0482-11 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 AHP EAGEN 68180-0241-01 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0241-02 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0241-<strong>08</strong> 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0016-01 0.29860 LEV<strong>OF</strong>LOXACIN 500 MG TABLET 0 ZYDUS PHARMACEU EABND 50383-0286-20 1.25527 LEV<strong>OF</strong>LOXACIN 500 MG/20 ML SOLN G HI-TECH PHARMAC MLBND 50383-0286-21 1.25527 LEV<strong>OF</strong>LOXACIN 500 MG/20 ML SOLN G HI-TECH PHARMAC MLGEN 17478-0107-20 0.55715 LEV<strong>OF</strong>LOXACIN 500 MG/20 ML VIAL 0 AKORN INC. MLGEN 23155-0201-31 0.55715 LEV<strong>OF</strong>LOXACIN 500 MG/20 ML VIAL 0 HERITAGE PHARMA MLGEN 25021-0130-20 0.55715 LEV<strong>OF</strong>LOXACIN 500 MG/20 ML VIAL 0 SAGENT PHARMACE MLGEN 55150-0156-20 0.55715 LEV<strong>OF</strong>LOXACIN 500 MG/20 ML VIAL 0 AUROMEDICS PHAR MLGEN 00093-7293-53 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 TEVA USA EAGEN 00781-5792-20 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 SANDOZ EAGEN 00781-5792-50 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6251-61 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6353-61 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 MAJOR PHARMACEU EAGEN 13668-0<strong>08</strong>4-01 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0<strong>08</strong>4-21 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0<strong>08</strong>4-50 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0<strong>08</strong>4-71 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 TORRENT PHARMAC EAGEN 33342-0023-32 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 MACLEODS PHARMA EAGEN 55111-0281-30 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 DR.REDDY'S LAB EAGEN 59762-0280-03 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 GREENSTONE LLC. EAGEN 64679-0547-01 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0547-03 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 WOCKHARDT USA L EAGEN 65862-0538-20 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0483-01 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0483-11 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 AHP EAGEN 68180-0242-01 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 LUPIN PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 219LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0242-20 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 LUPIN PHARMACEU EAGEN 68382-0017-01 0.79460 LEV<strong>OF</strong>LOXACIN 750 MG TABLET 0 ZYDUS PHARMACEU EAGEN 17478-0107-30 0.55715 LEV<strong>OF</strong>LOXACIN 750 MG/30 ML VIAL 0 AKORN INC. MLGEN 25021-0130-30 0.55715 LEV<strong>OF</strong>LOXACIN 750 MG/30 ML VIAL 0 SAGENT PHARMACE MLGEN 55150-0157-30 0.55715 LEV<strong>OF</strong>LOXACIN 750 MG/30 ML VIAL 0 AUROMEDICS PHAR MLGEN 00143-9722-01 0.12996 LEV<strong>OF</strong>LOXACIN-D5W 250 MG/50 ML 0 WEST-WARD,INC. MLGEN 00143-9722-24 0.12996 LEV<strong>OF</strong>LOXACIN-D5W 250 MG/50 ML 0 WEST-WARD,INC. MLGEN 00781-3341-09 0.12996 LEV<strong>OF</strong>LOXACIN-D5W 250 MG/50 ML 0 SANDOZ MLGEN 25021-0132-81 0.12600 LEV<strong>OF</strong>LOXACIN-D5W 250 MG/50 ML 0 SAGENT PHARMACE MLGEN 25021-<strong>08</strong>25-81 0.1<strong>08</strong>00 LEV<strong>OF</strong>LOXACIN-D5W 250 MG/50 ML 0 SAGENT PHARMACE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0355-50 0.11250 LEV<strong>OF</strong>LOXACIN-D5W 250 MG/50 ML 0 APP PHARMACEUTI MLGEN 00143-9721-01 0.1<strong>08</strong>65 LEV<strong>OF</strong>LOXACIN-D5W 500 MG/100 ML 0 WEST-WARD,INC. MLGEN 00143-9721-24 0.1<strong>08</strong>65 LEV<strong>OF</strong>LOXACIN-D5W 500 MG/100 ML 0 WEST-WARD,INC. MLGEN 00781-3342-09 0.1<strong>08</strong>65 LEV<strong>OF</strong>LOXACIN-D5W 500 MG/100 ML 0 SANDOZ MLGEN 25021-0132-67 0.05400 LEV<strong>OF</strong>LOXACIN-D5W 500 MG/100 ML 0 SAGENT/PREMIERP MLGEN 25021-0132-82 0.09900 LEV<strong>OF</strong>LOXACIN-D5W 500 MG/100 ML 0 SAGENT PHARMACE MLGEN 25021-<strong>08</strong>25-82 0.05400 LEV<strong>OF</strong>LOXACIN-D5W 500 MG/100 ML 0 SAGENT PHARMACE MLGEN 63323-0355-65 0.09225 LEV<strong>OF</strong>LOXACIN-D5W 500 MG/100 ML 0 APP PHARMACEUTI MLGEN 00143-9720-01 0.05400 LEV<strong>OF</strong>LOXACIN-D5W 750 MG/150 ML 0 WEST-WARD,INC. MLGEN 00143-9720-24 0.05400 LEV<strong>OF</strong>LOXACIN-D5W 750 MG/150 ML 0 WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3343-09 0.05400 LEV<strong>OF</strong>LOXACIN-D5W 750 MG/150 ML 0 SANDOZ MLGEN 25021-0132-68 0.03600 LEV<strong>OF</strong>LOXACIN-D5W 750 MG/150 ML 0 SAGENT/PREMIERP MLGEN 25021-0132-83 0.05400 LEV<strong>OF</strong>LOXACIN-D5W 750 MG/150 ML 0 SAGENT PHARMACE MLGEN 25021-<strong>08</strong>25-83 0.03600 LEV<strong>OF</strong>LOXACIN-D5W 750 MG/150 ML 0 SAGENT PHARMACE MLGEN 63323-0355-60 0.05400 LEV<strong>OF</strong>LOXACIN-D5W 750 MG/150 ML 0 APP PHARMACEUTI MLGEX 16714-0340-01 0.73260 LEVONEST-28 TABLET 0 NORTHSTAR RX LL EAGEX 68180-<strong>08</strong>54-11 0.85280 LEVONOR-ETH ESTRAD 0.1-0.02 MG 0 LUPIN PHARMACEU EAGEX 00378-6550-53 0.81150 LEVONOR-ETH ESTRAD 0.15-0.03 0 MYLAN EAGEX 68180-<strong>08</strong>43-11 1.24580 LEVONOR-ETH ESTRAD 0.15-0.03 0 LUPIN PHARMACEU EAGEX 68180-<strong>08</strong>43-13 1.24580 LEVONOR-ETH ESTRAD 0.15-0.03 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68180-<strong>08</strong>48-13 2.21332 LEVONORG-ETH ESTRAD ETH ESTRAD 0 LUPIN PHARMACEU EABEX 45802-<strong>08</strong>40-54 14.62330 15.17240 LEVONORGESTREL 0.75 MG TABLET 0 PERRIGO CO. EAGEX 63704-0009-01 27.42000 LEVONORGESTREL 1.5 MG TABLET 0 PHARMACIST PHAR EAGEX 52544-0279-28 0.81150 LEVORA-28 TABLET 0 ACTAVIS PHARMA, EABND 00456-1323-00 0.22453 LEVOTHROID 100 MCG TABLET 0 FOREST PHARMACE EABND 00456-1330-00 0.14602 LEVOTHROID 112 MCG TABLET 0 FOREST PHARMACE EABND 00456-1324-00 0.15617 LEVOTHROID 125 MCG TABLET 0 FOREST PHARMACE EABND 00456-1331-00 0.15890 LEVOTHROID 137 MCG TABLET 0 FOREST PHARMACE EABND 00456-1325-00 0.26810 LEVOTHROID 150 MCG TABLET 0 FOREST PHARMACE EABND 00456-1326-00 0.18175 LEVOTHROID 175 MCG TABLET 0 FOREST PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00456-1327-00 0.33209 LEVOTHROID 200 MCG TABLET 0 FOREST PHARMACE EABND 00456-1320-00 0.10707 LEVOTHROID 25 MCG TABLET 0 FOREST PHARMACE EABND 00456-1328-00 0.27710 LEVOTHROID 300 MCG TABLET 0 FOREST PHARMACE EABND 00456-1321-00 0.19667 LEVOTHROID 50 MCG TABLET 0 FOREST PHARMACE EABND 00456-1322-00 0.13011 LEVOTHROID 75 MCG TABLET 0 FOREST PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 220LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00456-1329-00 0.13333 LEVOTHROID 88 MCG TABLET 0 FOREST PHARMACE EAGUL 00378-1809-01 0.29850 LEVOTHYROXINE 100 MCG TABLET 0 MYLAN EAGUL 00378-1809-10 0.29850 LEVOTHYROXINE 100 MCG TABLET 0 MYLAN EAGUL 00527-1345-01 0.29850 LEVOTHYROXINE 100 MCG TABLET 0 LANNETT CO. INC EAGUL 00527-1345-10 0.29850 LEVOTHYROXINE 100 MCG TABLET 0 LANNETT CO. INC EAGUL 00781-5184-10 0.29850 LEVOTHYROXINE 100 MCG TABLET 0 SANDOZ EAGUL 00781-5184-92 0.29850 LEVOTHYROXINE 100 MCG TABLET 0 SANDOZ EAGUL 51079-0442-01 0.29850 LEVOTHYROXINE 100 MCG TABLET 0 MYLAN INSTITUTI EAGUL 51079-0442-20 0.29850 LEVOTHYROXINE 100 MCG TABLET 0 MYLAN INSTITUTI EAGUL 00378-1811-01 0.34430 LEVOTHYROXINE 112 MCG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-1811-10 0.34430 LEVOTHYROXINE 112 MCG TABLET 0 MYLAN EAGUL 00527-1346-01 0.34430 LEVOTHYROXINE 112 MCG TABLET 0 LANNETT CO. INC EAGUL 00527-1346-10 0.34430 LEVOTHYROXINE 112 MCG TABLET 0 LANNETT CO. INC EAGUL 00781-5185-92 0.34430 LEVOTHYROXINE 112 MCG TABLET 0 SANDOZ EAGUL 00378-1813-01 0.34950 LEVOTHYROXINE 125 MCG TABLET 0 MYLAN EAGUL 00378-1813-10 0.34950 LEVOTHYROXINE 125 MCG TABLET 0 MYLAN EAGUL 00527-1347-01 0.34950 LEVOTHYROXINE 125 MCG TABLET 0 LANNETT CO. INC EAGUL 00527-1347-10 0.34950 LEVOTHYROXINE 125 MCG TABLET 0 LANNETT CO. INC EAGUL 00781-5186-10 0.34950 LEVOTHYROXINE 125 MCG TABLET 0 SANDOZ EAGUL 00781-5186-92 0.34950 LEVOTHYROXINE 125 MCG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51079-0443-01 0.34950 LEVOTHYROXINE 125 MCG TABLET 0 MYLAN INSTITUTI EAGUL 51079-0443-20 0.34950 LEVOTHYROXINE 125 MCG TABLET 0 MYLAN INSTITUTI EAGEN 00378-1823-01 0.36020 LEVOTHYROXINE 137 MCG TABLET 0 MYLAN EAGEN 00378-1823-10 0.36020 LEVOTHYROXINE 137 MCG TABLET 0 MYLAN EAGEN 00527-1638-01 0.36020 LEVOTHYROXINE 137 MCG TABLET 0 LANNETT CO. INC EAGEN 00527-1638-10 0.36020 LEVOTHYROXINE 137 MCG TABLET 0 LANNETT CO. INC EAGEN 00781-5191-92 0.36020 LEVOTHYROXINE 137 MCG TABLET 0 SANDOZ EAGUL 00378-1815-01 0.36000 LEVOTHYROXINE 150 MCG TABLET 0 MYLAN EAGUL 00378-1815-10 0.36000 LEVOTHYROXINE 150 MCG TABLET 0 MYLAN EAGUL 00527-1349-01 0.36000 LEVOTHYROXINE 150 MCG TABLET 0 LANNETT CO. INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00527-1349-10 0.36000 LEVOTHYROXINE 150 MCG TABLET 0 LANNETT CO. INC EAGUL 00781-5187-10 0.36000 LEVOTHYROXINE 150 MCG TABLET 0 SANDOZ EAGUL 00781-5187-92 0.36000 LEVOTHYROXINE 150 MCG TABLET 0 SANDOZ EAGUL 51079-0445-01 0.36000 LEVOTHYROXINE 150 MCG TABLET 0 MYLAN INSTITUTI EAGUL 51079-0445-20 0.36000 LEVOTHYROXINE 150 MCG TABLET 0 MYLAN INSTITUTI EAGUL 00378-1817-01 0.42750 LEVOTHYROXINE 175 MCG TABLET 0 MYLAN EAGUL 00378-1817-10 0.42750 LEVOTHYROXINE 175 MCG TABLET 0 MYLAN EAGUL 00527-1350-01 0.42750 LEVOTHYROXINE 175 MCG TABLET 0 LANNETT CO. INC EAGUL 00527-1350-10 0.42750 LEVOTHYROXINE 175 MCG TABLET 0 LANNETT CO. INC EAGUL 00781-5188-92 0.42750 LEVOTHYROXINE 175 MCG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-1819-01 0.43551 LEVOTHYROXINE 200 MCG TABLET 0 MYLAN EAGEN 00378-1819-10 0.43551 LEVOTHYROXINE 200 MCG TABLET 0 MYLAN EAGEN 00527-1351-01 0.43551 LEVOTHYROXINE 200 MCG TABLET 0 LANNETT CO. INC EAGEN 00527-1351-10 0.43551 LEVOTHYROXINE 200 MCG TABLET 0 LANNETT CO. INC EAGEN 00781-5189-92 0.43551 LEVOTHYROXINE 200 MCG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 221LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-1800-01 0.23180 LEVOTHYROXINE 25 MCG TABLET 0 MYLAN EAGUL 00378-1800-10 0.23180 LEVOTHYROXINE 25 MCG TABLET 0 MYLAN EAGUL 00527-1341-01 0.23180 LEVOTHYROXINE 25 MCG TABLET 0 LANNETT CO. INC EAGUL 00527-1341-10 0.23180 LEVOTHYROXINE 25 MCG TABLET 0 LANNETT CO. INC EAGUL 00781-5180-10 0.23180 LEVOTHYROXINE 25 MCG TABLET 0 SANDOZ EAGUL 00781-5180-92 0.23180 LEVOTHYROXINE 25 MCG TABLET 0 SANDOZ EAGUL 51079-0444-01 0.23180 LEVOTHYROXINE 25 MCG TABLET 0 MYLAN INSTITUTI EAGUL 51079-0444-20 0.23180 LEVOTHYROXINE 25 MCG TABLET 0 MYLAN INSTITUTI EAGEN 00378-1821-01 0.58580 LEVOTHYROXINE 300 MCG TABLET 0 MYLAN EAGEN 00527-1352-01 0.58580 LEVOTHYROXINE 300 MCG TABLET 0 LANNETT CO. INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-5190-92 0.58580 LEVOTHYROXINE 300 MCG TABLET 0 SANDOZ EAGUL 00378-1803-01 0.26330 LEVOTHYROXINE 50 MCG TABLET 0 MYLAN EAGUL 00378-1803-10 0.26330 LEVOTHYROXINE 50 MCG TABLET 0 MYLAN EAGUL 00527-1342-01 0.26330 LEVOTHYROXINE 50 MCG TABLET 0 LANNETT CO. INC EAGUL 00527-1342-10 0.26330 LEVOTHYROXINE 50 MCG TABLET 0 LANNETT CO. INC EAGUL 00781-5181-10 0.26330 LEVOTHYROXINE 50 MCG TABLET 0 SANDOZ EAGUL 00781-5181-92 0.26330 LEVOTHYROXINE 50 MCG TABLET 0 SANDOZ EAGUL 51079-0440-01 0.26330 LEVOTHYROXINE 50 MCG TABLET 0 MYLAN INSTITUTI EAGUL 51079-0440-20 0.26330 LEVOTHYROXINE 50 MCG TABLET 0 MYLAN INSTITUTI EAGUL 00378-1805-01 0.29100 LEVOTHYROXINE 75 MCG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-1805-10 0.29100 LEVOTHYROXINE 75 MCG TABLET 0 MYLAN EAGUL 00527-1343-01 0.29100 LEVOTHYROXINE 75 MCG TABLET 0 LANNETT CO. INC EAGUL 00527-1343-10 0.29100 LEVOTHYROXINE 75 MCG TABLET 0 LANNETT CO. INC EAGUL 00781-5182-10 0.29100 LEVOTHYROXINE 75 MCG TABLET 0 SANDOZ EAGUL 00781-5182-92 0.29100 LEVOTHYROXINE 75 MCG TABLET 0 SANDOZ EAGUL 51079-0441-01 0.29100 LEVOTHYROXINE 75 MCG TABLET 0 MYLAN INSTITUTI EAGUL 51079-0441-20 0.29100 LEVOTHYROXINE 75 MCG TABLET 0 MYLAN INSTITUTI EAGUL 00378-1807-01 0.29550 LEVOTHYROXINE 88 MCG TABLET 0 MYLAN EAGUL 00378-1807-10 0.29550 LEVOTHYROXINE 88 MCG TABLET 0 MYLAN EAGUL 00527-1344-01 0.29550 LEVOTHYROXINE 88 MCG TABLET 0 LANNETT CO. INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00527-1344-10 0.29550 LEVOTHYROXINE 88 MCG TABLET 0 LANNETT CO. INC EAGUL 00781-5183-92 0.29550 LEVOTHYROXINE 88 MCG TABLET 0 SANDOZ EABUL 60793-<strong>08</strong>54-01 0.29850 0.46629 LEVOXYL 100 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>54-10 0.29850 0.46633 LEVOXYL 100 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>55-01 0.34430 0.53925 LEVOXYL 112 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>55-10 0.34430 0.53925 LEVOXYL 112 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>56-01 0.34950 0.54672 LEVOXYL 125 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>56-10 0.34950 0.54673 LEVOXYL 125 MCG TABLET 0 PFIZER US PHARM EABND 60793-<strong>08</strong>57-01 0.36020 0.55427 LEVOXYL 137 MCG TABLET 0 PFIZER US PHARM EABND 60793-<strong>08</strong>57-10 0.36020 0.55433 LEVOXYL 137 MCG TABLET 0 PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 60793-<strong>08</strong>58-01 0.36000 0.56274 LEVOXYL 150 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>58-10 0.36000 0.56267 LEVOXYL 150 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>59-01 0.42750 0.66881 LEVOXYL 175 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>59-10 0.42750 0.66880 LEVOXYL 175 MCG TABLET 0 PFIZER US PHARM EABND 60793-<strong>08</strong>60-01 0.43551 0.67014 LEVOXYL 200 MCG TABLET 0 PFIZER US PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 222LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 60793-<strong>08</strong>60-10 0.43551 0.67015 LEVOXYL 200 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>50-01 0.23180 0.36262 LEVOXYL 25 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>50-10 0.23180 0.36265 LEVOXYL 25 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>51-01 0.26330 0.41168 LEVOXYL 50 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>51-10 0.26330 0.41168 LEVOXYL 50 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>52-01 0.29100 0.45500 LEVOXYL 75 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>52-10 0.29100 0.45493 LEVOXYL 75 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>53-01 0.29550 0.46272 LEVOXYL 88 MCG TABLET 0 PFIZER US PHARM EABUL 60793-<strong>08</strong>53-10 0.29550 0.46278 LEVOXYL 88 MCG TABLET 0 PFIZER US PHARM EABND 68220-0112-10 0.48<strong>06</strong>0 1.54255 LEVSIN 0.125 MG TABLET 0 MEDA PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 68220-0113-10 1.54255 LEVSIN-SL 0.125 MG TABLET SL 0 MEDA PHARMACEUT EABEX 00456-2010-01 0.12000 5.63221 LEXAPRO 10 MG TABLET G FOREST PHARMACE EABEX 00456-2020-01 0.16850 5.877<strong>06</strong> LEXAPRO 20 MG TABLET G FOREST PHARMACE EABEX 00456-2005-01 0.11430 5.38678 LEXAPRO 5 MG TABLET G FOREST PHARMACE EABEX 00456-2101-<strong>08</strong> 0.62130 1.11963 LEXAPRO 5 MG/5 ML SOLUTION G FOREST PHARMACE MLBND 49702-02<strong>08</strong>-53 0.63677 LEXIVA 50 MG/ML SUSPENSION G VIIV <strong>HEALTH</strong>CARE MLBND 49702-0207-18 15.58588 LEXIVA 700 MG TABLET G VIIV <strong>HEALTH</strong>CARE EABND 54092-0476-12 6.72763 LIALDA DR 1.2 GM TABLET G SHIRE US INC. EAGEN 38779-0<strong>08</strong>2-<strong>08</strong> 2.85000 LIDOCAINE HCL POWDER 0 MEDISCA INC. GMBND 00409-9137-05 1.00596 LIDOCAINE HCL 1% SYRINGE 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0201-02 0.12180 LIDOCAINE HCL 1% VIAL 0 APP PHARMACEUTI MLGEN 63323-0201-10 0.12180 LIDOCAINE HCL 1% VIAL 0 APP PHARMACEUTI MLGEN 00093-9200-31 0.33750 LIDOCAINE HCL 2% JELLY 0 TEVA USA MLGEN 17478-0711-10 0.33750 LIDOCAINE HCL 2% JELLY 0 AKORN INC. MLGEN 17478-0711-30 0.33750 LIDOCAINE HCL 2% JELLY 0 AKORN INC. MLGEN 17478-<strong>08</strong>11-10 0.33750 LIDOCAINE HCL 2% JELLY 0 AKORN/NOVAPLUS MLGEN 17478-<strong>08</strong>11-30 0.33750 LIDOCAINE HCL 2% JELLY 0 AKORN/NOVAPLUS MLGEN 17478-<strong>08</strong>40-05 0.33750 LIDOCAINE HCL 2% JELLY 0 AKORN INC. MLGEN 17478-<strong>08</strong>40-30 0.33750 LIDOCAINE HCL 2% JELLY 0 AKORN INC. MLBND 76329-3015-05 0.38445 LIDOCAINE HCL 2% JELLY 0 INTERNATIONAL M ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-4283-01 0.63540 LIDOCAINE HCL 4% AMPUL 0 HOSPIRA MLGEN 00054-3505-47 0.<strong>08</strong>289 LIDOCAINE HCL 4% SOLUTION 0 ROXANE LABS. MLGEN 0<strong>06</strong>03-1394-47 0.<strong>08</strong>289 LIDOCAINE HCL 4% SOLUTION 0 QUALITEST MLGEN 60432-0465-50 0.<strong>08</strong>289 LIDOCAINE HCL 4% SOLUTION 0 MORTON GROVE PH MLGEN 00264-9594-10 0.01110 LIDOCAINE 0.4% IN D5W SOLN 0 B.BRAUN MLGEN 00264-9594-20 0.01110 LIDOCAINE 0.4% IN D5W SOLN 0 B.BRAUN MLGEN 00264-9598-20 0.01917 LIDOCAINE 0.8% IN D5W SOLN 0 B.BRAUN MLGEN 00054-3500-49 0.02187 LIDOCAINE 2% VISCOUS SOLN 0 ROXANE LABS. MLGEN 0<strong>06</strong>03-1393-64 0.02187 LIDOCAINE 2% VISCOUS SOLN 0 QUALITEST MLGEN 50383-0775-04 0.02187 LIDOCAINE 2% VISCOUS SOLN 0 HI-TECH PHARMAC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50383-0775-15 0.02187 LIDOCAINE 2% VISCOUS SOLN 0 HI-TECH PHARMAC MLGEN 50383-0775-17 0.02187 LIDOCAINE 2% VISCOUS SOLN 0 HI-TECH PHARMAC MLGEN 60432-0464-00 0.02187 LIDOCAINE 2% VISCOUS SOLN 0 MORTON GROVE PH MLGEN 13925-0159-01 1.54365 LIDOCAINE 3% CREAM 0 SETON PHARMACEU GMGEN 59<strong>08</strong>8-0997-03 1.36749 LIDOCAINE 3% CREAM 0 PURETEK CORPORA GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 223LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59<strong>08</strong>8-0997-07 1.<strong>08</strong>105 LIDOCAINE 3% CREAM 0 PURETEK CORPORA GMGEN 00168-0204-37 1.52370 LIDOCAINE 5% OINTMENT 0 SANDOZ GMGEN 50383-0933-35 1.52370 LIDOCAINE 5% OINTMENT 0 HI-TECH PHARMAC GMGEN 50383-0933-55 1.44750 LIDOCAINE 5% OINTMENT 0 HI-TECH PHARMAC GMGEN 51672-30<strong>08</strong>-03 1.48335 LIDOCAINE 5% OINTMENT 0 TARO PHARM USA GMGEN 51672-3020-02 1.52375 LIDOCAINE 5% OINTMENT 0 TARO PHARM USA GMGEN 00591-3525-30 7.02024 LIDOCAINE 5% PATCH G ACTAVIS PHARMA, EABND 13925-0163-07 14.88605 LIDOCAINE-HC 3-1% CREAM 0 SETON PHARMACEU EABND 13925-0164-07 14.88605 LIDOCAINE-HYDROCORT 3-2.5% GEL 0 SETON PHARMACEU EAGEN 00115-1468-45 0.66920 LIDOCAINE-PRILOCAINE CREAM 0 GLOBAL PHARM GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00115-1468-60 0.66920 LIDOCAINE-PRILOCAINE CREAM 0 GLOBAL PHARM GMGEN 00168-0357-30 0.66920 LIDOCAINE-PRILOCAINE CREAM 0 SANDOZ GMGEN 00168-0357-55 0.66920 LIDOCAINE-PRILOCAINE CREAM 0 SANDOZ GMBND 00168-0357-56 1.12170 31.87200 LIDOCAINE-PRILOCAINE CREAM 0 SANDOZ EAGEN 00781-7058-03 0.66920 LIDOCAINE-PRILOCAINE CREAM 0 SANDOZ GMGEN 00781-7058-39 0.66920 LIDOCAINE-PRILOCAINE CREAM 0 SANDOZ GMGEN 17478-0190-05 0.66920 LIDOCAINE-PRILOCAINE CREAM 0 AKORN INC. GMGEN 17478-0190-55 0.66920 LIDOCAINE-PRILOCAINE CREAM 0 AKORN INC. GMGEN 50383-<strong>06</strong>67-30 0.66920 LIDOCAINE-PRILOCAINE CREAM 0 HI-TECH PHARMAC GMBND 63481-<strong>06</strong>87-<strong>06</strong> 8.63227 8.63227 LIDODERM 5% PATCH G ENDO PHARM INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-0555-01 11.13860 LINCOCIN 300 MG/ML VIAL 0 PHARMACIA/UPJHN MLBND 00009-0555-02 8.42616 LINCOCIN 300 MG/ML VIAL 0 PHARMACIA/UPJHN MLGEN 60432-<strong>08</strong>33-60 1.71075 LINDANE 1% LOTION 0 MORTON GROVE PH MLGEN 61748-0401-02 1.65937 LINDANE 1% LOTION 0 VERSA PHARMACEU MLGEN 60432-<strong>08</strong>34-60 1.71075 LINDANE 1% SHAMPOO 0 MORTON GROVE PH MLGEN 61748-0400-02 1.65937 LINDANE 1% SHAMPOO 0 VERSA PHARMACEU MLBND 00456-1201-30 7.67279 LINZESS 145 MCG CAPSULE G FOREST PHARMACE EABND 00456-1202-30 7.67279 LINZESS 290 MCG CAPSULE G FOREST PHARMACE EABND 58281-0560-01 10.70700 LIORESAL IT 10 MG/20 ML KIT 0 MEDTRONIC MLBND 58281-0560-02 10.70700 LIORESAL IT 10 MG/20 ML KIT 0 MEDTRONIC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 58281-0561-02 42.82800 LIORESAL IT 10 MG/5 ML KIT 0 MEDTRONIC MLBND 58281-0563-01 42.82800 LIORESAL IT 40 MG/20 ML KIT 0 MEDTRONIC MLBND 58281-0563-02 42.82800 LIORESAL IT 40 MG/20 ML KIT 0 MEDTRONIC MLGEN 00378-3612-01 0.70144 LIOTHYRONINE SOD 25 MCG TAB 0 MYLAN EAGEN 00378-3612-10 0.70144 LIOTHYRONINE SOD 25 MCG TAB 0 MYLAN EAGEN 00574-0222-01 0.70144 LIOTHYRONINE SOD 25 MCG TAB 0 PADDOCK LABS. EAGEN 42794-0019-02 0.70144 LIOTHYRONINE SOD 25 MCG TAB 0 SIGMAPHARM LABO EAGEN 42794-0019-<strong>06</strong> 0.70144 LIOTHYRONINE SOD 25 MCG TAB 0 SIGMAPHARM LABO EAGEN 00378-3611-01 0.49923 LIOTHYRONINE SOD 5 MCG TAB 0 MYLAN EAGEN 00378-3611-10 0.49923 LIOTHYRONINE SOD 5 MCG TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00574-0220-01 0.49923 LIOTHYRONINE SOD 5 MCG TAB 0 PADDOCK LABS. EAGEN 42794-0018-02 0.49923 LIOTHYRONINE SOD 5 MCG TAB 0 SIGMAPHARM LABO EAGEN 42794-0018-<strong>06</strong> 0.49923 LIOTHYRONINE SOD 5 MCG TAB 0 SIGMAPHARM LABO EAGEN 00378-3613-01 1.05489 LIOTHYRONINE SOD 50 MCG TAB 0 MYLAN EAGEN 00574-0223-01 1.05489 LIOTHYRONINE SOD 50 MCG TAB 0 PADDOCK LABS. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 224LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42794-0020-02 1.05489 LIOTHYRONINE SOD 50 MCG TAB 0 SIGMAPHARM LABO EABND 00071-0155-23 0.17807 5.07581 LIPITOR 10 MG TABLET G PFIZER US PHARM EABND 00071-0155-34 0.17807 5.07587 LIPITOR 10 MG TABLET G PFIZER US PHARM EABND 00071-0156-23 0.22424 7.24036 LIPITOR 20 MG TABLET G PFIZER US PHARM EABND 00071-0156-94 0.22424 7.24023 LIPITOR 20 MG TABLET G PFIZER US PHARM EABND 00071-0157-23 0.19<strong>06</strong>2 7.24036 LIPITOR 40 MG TABLET G PFIZER US PHARM EABND 00071-0157-73 0.19<strong>06</strong>2 7.24023 LIPITOR 40 MG TABLET G PFIZER US PHARM EABND 00071-0157-88 0.19<strong>06</strong>2 7.24023 LIPITOR 40 MG TABLET G PFIZER US PHARM EABND 00071-0158-23 0.25974 7.24036 LIPITOR 80 MG TABLET G PFIZER US PHARM EABND 00071-0158-73 0.25974 7.24023 LIPITOR 80 MG TABLET G PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00071-0158-88 0.25974 7.24023 LIPITOR 80 MG TABLET G PFIZER US PHARM EABND 66869-0147-30 4.83668 LIP<strong>OF</strong>EN 150 MG CAPSULE G KOWA PHARMACEUT EABND 66869-0137-30 2.2<strong>06</strong>14 LIP<strong>OF</strong>EN 50 MG CAPSULE G KOWA PHARMACEUT EABND 66582-0320-30 5.47800 LIPTRUZET 10-10 MG TABLET G MERCK SHARP & D EABND 66582-0320-54 5.47800 LIPTRUZET 10-10 MG TABLET G MERCK SHARP & D EABND 66582-0321-30 5.47800 LIPTRUZET 10-20 MG TABLET G MERCK SHARP & D EABND 66582-0321-54 5.47800 LIPTRUZET 10-20 MG TABLET G MERCK SHARP & D EABND 66582-0322-30 5.47800 LIPTRUZET 10-40 MG TABLET G MERCK SHARP & D EABND 66582-0322-54 5.47800 LIPTRUZET 10-40 MG TABLET G MERCK SHARP & D EAGEN 00172-3759-60 0.02687 LISINOPRIL 10 MG TABLET 0 IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00172-3759-70 0.02687 LISINOPRIL 10 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-3759-80 0.02687 LISINOPRIL 10 MG TABLET 0 TEVA USA EAGEN 00185-0101-01 0.02687 LISINOPRIL 10 MG TABLET 0 SANDOZ EAGEN 00185-0101-10 0.02687 LISINOPRIL 10 MG TABLET 0 SANDOZ EAGEN 00378-2074-01 0.02687 LISINOPRIL 10 MG TABLET 0 MYLAN EAGEN 00378-2074-10 0.02687 LISINOPRIL 10 MG TABLET 0 MYLAN EAGEN 00591-0407-01 0.02687 LISINOPRIL 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-0407-10 0.02687 LISINOPRIL 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-4211-02 0.02687 LISINOPRIL 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4211-21 0.02687 LISINOPRIL 10 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-4211-28 0.02687 LISINOPRIL 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4211-32 0.02687 LISINOPRIL 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4211-34 0.02687 LISINOPRIL 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4211-60 0.02687 LISINOPRIL 10 MG TABLET 0 QUALITEST EAGEN 00781-1666-92 0.02687 LISINOPRIL 10 MG TABLET 0 SANDOZ EAGEN 00904-58<strong>08</strong>-43 0.02687 LISINOPRIL 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-58<strong>08</strong>-46 0.02687 LISINOPRIL 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-58<strong>08</strong>-80 0.02687 LISINOPRIL 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-58<strong>08</strong>-89 0.02687 LISINOPRIL 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 31722-0419-01 0.02687 LISINOPRIL 10 MG TABLET 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0419-10 0.02687 LISINOPRIL 10 MG TABLET 0 CAMBER PHARMACE EAGEN 51079-0982-17 0.02687 LISINOPRIL 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0982-19 0.02687 LISINOPRIL 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0982-30 0.02687 LISINOPRIL 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0982-56 0.02687 LISINOPRIL 10 MG TABLET 0 MYLAN INSTITUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 225LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 54458-0997-10 0.02687 LISINOPRIL 10 MG TABLET 0 INTERNATIONAL L EAGEN 60505-0186-00 0.02687 LISINOPRIL 10 MG TABLET 0 APOTEX CORP EAGEN 60505-0186-01 0.02687 LISINOPRIL 10 MG TABLET 0 APOTEX CORP EAGEN 63304-0533-01 0.02687 LISINOPRIL 10 MG TABLET 0 RANBAXY PHARMAC EAGEN 63739-0349-10 0.02687 LISINOPRIL 10 MG TABLET 0 MCKESSON PACKAG EAGEN 64679-0929-01 0.02687 LISINOPRIL 10 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0929-05 0.02687 LISINOPRIL 10 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0929-<strong>06</strong> 0.02687 LISINOPRIL 10 MG TABLET 0 WOCKHARDT USA L EAGEN 65862-0039-01 0.02687 LISINOPRIL 10 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0039-05 0.02687 LISINOPRIL 10 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0039-99 0.02687 LISINOPRIL 10 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0<strong>06</strong>1-01 0.02687 LISINOPRIL 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0<strong>06</strong>1-11 0.02687 LISINOPRIL 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0197-01 0.02687 LISINOPRIL 10 MG TABLET 0 AHP EAGEN 68180-0514-01 0.02687 LISINOPRIL 10 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0514-03 0.02687 LISINOPRIL 10 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0514-09 0.02687 LISINOPRIL 10 MG TABLET 0 LUPIN PHARMACEU EAGEN 00143-1265-01 0.01810 LISINOPRIL 2.5 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1265-10 0.01810 LISINOPRIL 2.5 MG TABLET 0 WEST-WARD,INC. EAGEN 00172-3757-60 0.01810 LISINOPRIL 2.5 MG TABLET 0 IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00172-3757-70 0.01810 LISINOPRIL 2.5 MG TABLET 0 IVAX PHARMACEUT EAGEN 00185-0025-01 0.01810 LISINOPRIL 2.5 MG TABLET 0 SANDOZ EAGEN 00185-0025-10 0.01810 LISINOPRIL 2.5 MG TABLET 0 SANDOZ EAGEN 00378-2072-01 0.01810 LISINOPRIL 2.5 MG TABLET 0 MYLAN EAGEN 00591-0405-01 0.01810 LISINOPRIL 2.5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-0405-05 0.01810 LISINOPRIL 2.5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-4209-21 0.01810 LISINOPRIL 2.5 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4209-28 0.01810 LISINOPRIL 2.5 MG TABLET 0 QUALITEST EAGEN 31722-0417-01 0.01810 LISINOPRIL 2.5 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0417-10 0.01810 LISINOPRIL 2.5 MG TABLET 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 54458-0999-09 0.01810 LISINOPRIL 2.5 MG TABLET 0 INTERNATIONAL L EAGEN 60505-0184-00 0.01810 LISINOPRIL 2.5 MG TABLET 0 APOTEX CORP EAGEN 63304-0531-01 0.01810 LISINOPRIL 2.5 MG TABLET 0 RANBAXY PHARMAC EAGEN 64679-0927-01 0.01810 LISINOPRIL 2.5 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0927-05 0.01810 LISINOPRIL 2.5 MG TABLET 0 WOCKHARDT USA L EAGEN 65862-0037-01 0.01810 LISINOPRIL 2.5 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0037-05 0.01810 LISINOPRIL 2.5 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0037-99 0.01810 LISINOPRIL 2.5 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0512-01 0.01810 LISINOPRIL 2.5 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0512-02 0.01810 LISINOPRIL 2.5 MG TABLET 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0512-09 0.01810 LISINOPRIL 2.5 MG TABLET 0 LUPIN PHARMACEU EAGEN 00143-1268-01 0.03310 LISINOPRIL 20 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1268-10 0.03310 LISINOPRIL 20 MG TABLET 0 WEST-WARD,INC. EAGEN 00172-3760-60 0.03310 LISINOPRIL 20 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-3760-70 0.03310 LISINOPRIL 20 MG TABLET 0 IVAX PHARMACEUT EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 226LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00172-3760-80 0.03310 LISINOPRIL 20 MG TABLET 0 IVAX PHARMACEUT EAGEN 00185-0102-01 0.03310 LISINOPRIL 20 MG TABLET 0 SANDOZ EAGEN 00185-0102-10 0.03310 LISINOPRIL 20 MG TABLET 0 SANDOZ EAGEN 00378-2075-01 0.03310 LISINOPRIL 20 MG TABLET 0 MYLAN EAGEN 00378-2075-10 0.03310 LISINOPRIL 20 MG TABLET 0 MYLAN EAGEN 00591-04<strong>08</strong>-01 0.03310 LISINOPRIL 20 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-04<strong>08</strong>-10 0.03310 LISINOPRIL 20 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-4212-02 0.03310 LISINOPRIL 20 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4212-21 0.03310 LISINOPRIL 20 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4212-28 0.03310 LISINOPRIL 20 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-4212-32 0.03310 LISINOPRIL 20 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4212-34 0.03310 LISINOPRIL 20 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4212-60 0.03310 LISINOPRIL 20 MG TABLET 0 QUALITEST EAGEN 00781-1667-92 0.03310 LISINOPRIL 20 MG TABLET 0 SANDOZ EAGEN 00904-5809-80 0.03310 LISINOPRIL 20 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5809-89 0.03310 LISINOPRIL 20 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5809-93 0.03310 LISINOPRIL 20 MG TABLET 0 MAJOR PHARMACEU EAGEN 31722-0420-01 0.03310 LISINOPRIL 20 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0420-10 0.03310 LISINOPRIL 20 MG TABLET 0 CAMBER PHARMACE EAGEN 51079-0983-19 0.03310 LISINOPRIL 20 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0983-30 0.03310 LISINOPRIL 20 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0983-56 0.03310 LISINOPRIL 20 MG TABLET 0 MYLAN INSTITUTI EAGEN 54458-0996-10 0.03310 LISINOPRIL 20 MG TABLET 0 INTERNATIONAL L EAGEN 60505-0187-00 0.03310 LISINOPRIL 20 MG TABLET 0 APOTEX CORP EAGEN 60505-0187-01 0.03310 LISINOPRIL 20 MG TABLET 0 APOTEX CORP EAGEN 63304-0534-01 0.03310 LISINOPRIL 20 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-0534-10 0.03310 LISINOPRIL 20 MG TABLET 0 RANBAXY PHARMAC EAGEN 63739-0350-10 0.03310 LISINOPRIL 20 MG TABLET 0 MCKESSON PACKAG EAGEN 64679-0941-01 0.03310 LISINOPRIL 20 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0941-<strong>06</strong> 0.03310 LISINOPRIL 20 MG TABLET 0 WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0040-01 0.03310 LISINOPRIL 20 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0040-05 0.03310 LISINOPRIL 20 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0040-99 0.03310 LISINOPRIL 20 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0<strong>06</strong>2-01 0.03310 LISINOPRIL 20 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0<strong>06</strong>2-11 0.03310 LISINOPRIL 20 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0198-01 0.03310 LISINOPRIL 20 MG TABLET 0 AHP EAGEN 68180-0515-01 0.03310 LISINOPRIL 20 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0515-03 0.03310 LISINOPRIL 20 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0515-09 0.03310 LISINOPRIL 20 MG TABLET 0 LUPIN PHARMACEU EAGEN 68645-0469-54 0.03310 LISINOPRIL 20 MG TABLET 0 LEGACY PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-1280-01 0.09113 LISINOPRIL 30 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1280-10 0.09113 LISINOPRIL 30 MG TABLET 0 WEST-WARD,INC. EAGEN 00172-3762-60 0.09113 LISINOPRIL 30 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-3762-70 0.09113 LISINOPRIL 30 MG TABLET 0 IVAX PHARMACEUT EAGEN 00185-0103-01 0.09113 LISINOPRIL 30 MG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 227LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0103-10 0.09113 LISINOPRIL 30 MG TABLET 0 SANDOZ EAGEN 00378-2077-01 0.09113 LISINOPRIL 30 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-4213-28 0.09113 LISINOPRIL 30 MG TABLET 0 QUALITEST EAGEN 31722-0421-01 0.09113 LISINOPRIL 30 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0421-10 0.09113 LISINOPRIL 30 MG TABLET 0 CAMBER PHARMACE EAGEN 54458-0995-10 0.09113 LISINOPRIL 30 MG TABLET 0 INTERNATIONAL L EAGEN 60505-0188-00 0.09113 LISINOPRIL 30 MG TABLET 0 APOTEX CORP EAGEN 63304-0599-01 0.09113 LISINOPRIL 30 MG TABLET 0 RANBAXY PHARMAC EAGEN 64679-0953-01 0.09113 LISINOPRIL 30 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0953-05 0.09113 LISINOPRIL 30 MG TABLET 0 WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0041-01 0.09113 LISINOPRIL 30 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0041-05 0.09113 LISINOPRIL 30 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0041-99 0.09113 LISINOPRIL 30 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0516-01 0.09113 LISINOPRIL 30 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0516-02 0.09113 LISINOPRIL 30 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0516-09 0.09113 LISINOPRIL 30 MG TABLET 0 LUPIN PHARMACEU EAGEN 00143-1270-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1270-10 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 WEST-WARD,INC. EAGEN 00172-3761-60 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-3761-70 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0104-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 SANDOZ EAGEN 00185-0104-10 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 SANDOZ EAGEN 00378-2076-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 MYLAN EAGEN 00378-2076-05 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 MYLAN EAGEN 00591-0409-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-0409-05 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-4214-02 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4214-04 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4214-21 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4214-28 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-4214-30 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4214-32 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4214-60 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 QUALITEST EAGEN 00781-1668-92 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 SANDOZ EAGEN 00904-5810-43 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5810-46 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5810-52 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5810-80 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5810-89 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5810-93 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0422-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0422-10 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 CAMBER PHARMACE EAGEN 51079-0984-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 MYLAN INSTITUTI EAGEN 54458-0905-02 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 INTERNATIONAL L EAGEN 54458-0994-10 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 INTERNATIONAL L EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 228LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0189-00 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 APOTEX CORP EAGEN 60505-0189-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 APOTEX CORP EAGEN 63304-0535-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 RANBAXY PHARMAC EAGEN 64679-0942-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0942-02 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0942-05 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 WOCKHARDT USA L EAGEN 65862-0042-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0042-05 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0042-99 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0<strong>06</strong>4-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0199-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 AHP EAGEN 68180-0517-01 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0517-03 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0517-09 0.<strong>06</strong>872 LISINOPRIL 40 MG TABLET 0 LUPIN PHARMACEU EAGEN 00143-1266-01 0.01958 LISINOPRIL 5 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1266-10 0.01958 LISINOPRIL 5 MG TABLET 0 WEST-WARD,INC. EAGEN 00172-3758-60 0.01958 LISINOPRIL 5 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-3758-70 0.01958 LISINOPRIL 5 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-3758-80 0.01958 LISINOPRIL 5 MG TABLET 0 IVAX PHARMACEUT EAGEN 00185-5400-01 0.01958 LISINOPRIL 5 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-5400-10 0.01958 LISINOPRIL 5 MG TABLET 0 SANDOZ EAGEN 00378-2073-01 0.01958 LISINOPRIL 5 MG TABLET 0 MYLAN EAGEN 00378-2073-10 0.01958 LISINOPRIL 5 MG TABLET 0 MYLAN EAGEN 00591-04<strong>06</strong>-01 0.01958 LISINOPRIL 5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-04<strong>06</strong>-10 0.01958 LISINOPRIL 5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-4210-02 0.01958 LISINOPRIL 5 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4210-16 0.01958 LISINOPRIL 5 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4210-21 0.01958 LISINOPRIL 5 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4210-28 0.01958 LISINOPRIL 5 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4210-30 0.01958 LISINOPRIL 5 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-4210-32 0.01958 LISINOPRIL 5 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4210-60 0.01958 LISINOPRIL 5 MG TABLET 0 QUALITEST EAGEN 00781-1665-92 0.01958 LISINOPRIL 5 MG TABLET 0 SANDOZ EAGEN 00904-5811-43 0.01958 LISINOPRIL 5 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5811-80 0.01958 LISINOPRIL 5 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5811-89 0.01958 LISINOPRIL 5 MG TABLET 0 MAJOR PHARMACEU EAGEN 31722-0418-01 0.01958 LISINOPRIL 5 MG TABLET 0 CAMBER PHARMACE EAGEN 51079-0981-30 0.01958 LISINOPRIL 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0981-40 0.01958 LISINOPRIL 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0981-56 0.01958 LISINOPRIL 5 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 54458-09<strong>06</strong>-02 0.01958 LISINOPRIL 5 MG TABLET 0 INTERNATIONAL L EAGEN 54458-0998-09 0.01958 LISINOPRIL 5 MG TABLET 0 INTERNATIONAL L EAGEN 60505-0185-00 0.01958 LISINOPRIL 5 MG TABLET 0 APOTEX CORP EAGEN 60505-0185-01 0.01958 LISINOPRIL 5 MG TABLET 0 APOTEX CORP EAGEN 63304-0532-01 0.01958 LISINOPRIL 5 MG TABLET 0 RANBAXY PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 229LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63304-0532-10 0.01958 LISINOPRIL 5 MG TABLET 0 RANBAXY PHARMAC EAGEN 64679-0928-01 0.01958 LISINOPRIL 5 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0928-<strong>06</strong> 0.01958 LISINOPRIL 5 MG TABLET 0 WOCKHARDT USA L EAGEN 65862-0038-01 0.01958 LISINOPRIL 5 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0038-05 0.01958 LISINOPRIL 5 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0038-99 0.01958 LISINOPRIL 5 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0<strong>06</strong>0-01 0.01958 LISINOPRIL 5 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0<strong>06</strong>0-11 0.01958 LISINOPRIL 5 MG TABLET 0 AHP EAGEN 68180-0513-01 0.01958 LISINOPRIL 5 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0513-03 0.01958 LISINOPRIL 5 MG TABLET 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0513-09 0.01958 LISINOPRIL 5 MG TABLET 0 LUPIN PHARMACEU EAGEN 00143-1262-01 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 WEST-WARD,INC. EAGEN 00143-1262-10 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 WEST-WARD,INC. EAGEN 00172-5033-60 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 IVAX PHARMACEUT EAGEN 00172-5033-70 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 IVAX PHARMACEUT EAGEN 00185-7100-01 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 SANDOZ EAGEN 00185-7100-10 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 SANDOZ EAGEN 00591-<strong>08</strong>60-01 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 ACTAVIS PHARMA, EAGEN 00591-<strong>08</strong>60-05 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 ACTAVIS PHARMA, EAGEN 00781-1848-01 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 54458-0907-02 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 INTERNATIONAL L EAGEN 65862-0043-01 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0043-05 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 AUROBINDO PHARM EAGEN 68180-0518-01 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 LUPIN PHARMACEU EAGEN 68180-0518-02 0.03186 LISINOPRIL-HCTZ 10-12.5 MG TAB 0 LUPIN PHARMACEU EAGEN 00143-1263-01 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 WEST-WARD,INC. EAGEN 00143-1263-10 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 WEST-WARD,INC. EAGEN 00172-5034-60 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 IVAX PHARMACEUT EAGEN 00172-5034-70 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 IVAX PHARMACEUT EAGEN 00185-0152-01 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0152-10 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 SANDOZ EAGEN 00591-<strong>08</strong>61-01 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 ACTAVIS PHARMA, EAGEN 00591-<strong>08</strong>61-05 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 ACTAVIS PHARMA, EAGEN 51079-<strong>06</strong>98-40 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 MYLAN INSTITUTI EAGEN 54458-0992-10 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 INTERNATIONAL L EAGEN 65862-0044-01 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0044-05 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 AUROBINDO PHARM EAGEN 68180-0519-01 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 LUPIN PHARMACEU EAGEN 68180-0519-02 0.04170 LISINOPRIL-HCTZ 20-12.5 MG TAB 0 LUPIN PHARMACEU EAGEN 00143-1264-01 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-1264-10 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 WEST-WARD,INC. EAGEN 00172-5032-60 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 IVAX PHARMACEUT EAGEN 00172-5032-70 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 IVAX PHARMACEUT EAGEN 00185-0173-01 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 SANDOZ EAGEN 00185-0173-10 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 230LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-<strong>08</strong>62-01 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 ACTAVIS PHARMA, EAGEN 00591-<strong>08</strong>62-05 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 ACTAVIS PHARMA, EAGEN 00781-1178-01 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 SANDOZ EAGEN 51079-<strong>06</strong>99-40 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 MYLAN INSTITUTI EAGEN 54458-0991-10 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 INTERNATIONAL L EAGEN 65862-0045-01 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0045-05 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 AUROBINDO PHARM EAGEN 68180-0520-01 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 LUPIN PHARMACEU EAGEN 68180-0520-02 0.05120 LISINOPRIL-HCTZ 20-25 MG TAB 0 LUPIN PHARMACEU EAGEX 00054-0021-25 0.25569 LITHIUM CARBONATE ER 300 MG TB 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00054-0021-29 0.25569 LITHIUM CARBONATE ER 300 MG TB 0 ROXANE LABS. EAGEX 00378-1300-01 0.25569 LITHIUM CARBONATE ER 300 MG TB 0 MYLAN EAGEX 00378-1300-05 0.25569 LITHIUM CARBONATE ER 300 MG TB 0 MYLAN EAGEX 51079-0180-20 0.25569 LITHIUM CARBONATE ER 300 MG TB 0 MYLAN INSTITUTI EAGEX 68462-0223-01 0.25569 LITHIUM CARBONATE ER 300 MG TB 0 GLENMARK PHARMA EAGEX 00054-2526-25 0.<strong>06</strong>440 LITHIUM CARBONATE 150 MG CAP 0 ROXANE LABS. EAGEX 00054-8526-25 0.<strong>06</strong>440 LITHIUM CARBONATE 150 MG CAP 0 ROXANE LABS. EAGEX 00143-3188-01 0.<strong>06</strong>440 LITHIUM CARBONATE 150 MG CAP 0 WEST-WARD,INC. EAGEX 00904-6205-60 0.<strong>06</strong>440 LITHIUM CARBONATE 150 MG CAP 0 MAJOR PHARMACEU EAGEX 31722-0544-01 0.<strong>06</strong>440 LITHIUM CARBONATE 150 MG CAP 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68462-0220-01 0.<strong>06</strong>440 LITHIUM CARBONATE 150 MG CAP 0 GLENMARK PHARMA EAGEX 00054-2527-25 0.03170 LITHIUM CARBONATE 300 MG CAP 0 ROXANE LABS. EAGEX 00054-2527-31 0.03170 LITHIUM CARBONATE 300 MG CAP 0 ROXANE LABS. EAGEX 00054-8527-25 0.03170 LITHIUM CARBONATE 300 MG CAP 0 ROXANE LABS. EAGEX 00143-3189-01 0.03170 LITHIUM CARBONATE 300 MG CAP 0 WEST-WARD,INC. EAGEX 00143-3189-10 0.03170 LITHIUM CARBONATE 300 MG CAP 0 WEST-WARD,INC. EAGEX 31722-0545-01 0.03170 LITHIUM CARBONATE 300 MG CAP 0 CAMBER PHARMACE EAGEX 31722-0545-10 0.03170 LITHIUM CARBONATE 300 MG CAP 0 CAMBER PHARMACE EAGEX 60505-2504-01 0.03170 LITHIUM CARBONATE 300 MG CAP 0 APOTEX CORP EAGEX 60505-2504-02 0.03170 LITHIUM CARBONATE 300 MG CAP 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-2504-03 0.03170 LITHIUM CARBONATE 300 MG CAP 0 APOTEX CORP EAGEX 63739-0265-10 0.03170 LITHIUM CARBONATE 300 MG CAP 0 MCKESSON PACKAG EAGEX 68462-0221-01 0.03170 LITHIUM CARBONATE 300 MG CAP 0 GLENMARK PHARMA EAGEX 68462-0221-10 0.02686 LITHIUM CARBONATE 300 MG CAP 0 GLENMARK PHARMA EAGEX 00054-4527-25 0.16140 LITHIUM CARBONATE 300 MG TAB 0 ROXANE LABS. EAGEX 00054-4527-31 0.16128 LITHIUM CARBONATE 300 MG TAB 0 ROXANE LABS. EAGEX 00054-8528-25 0.19365 LITHIUM CARBONATE 300 MG TAB 0 ROXANE LABS. EAGEX 00143-1300-01 0.15300 LITHIUM CARBONATE 300 MG TAB 0 WEST-WARD,INC. EAGEX 00143-1300-10 0.15390 LITHIUM CARBONATE 300 MG TAB 0 WEST-WARD,INC. EAGEX 62756-0430-18 0.15562 LITHIUM CARBONATE 300 MG TAB 0 SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62756-0430-88 0.15562 LITHIUM CARBONATE 300 MG TAB 0 SUN PHARMACEUTI EAGEX 00054-2531-25 0.13740 LITHIUM CARBONATE 600 MG CAP 0 ROXANE LABS. EAGEX 00054-8531-25 0.13740 LITHIUM CARBONATE 600 MG CAP 0 ROXANE LABS. EAGEX 31722-0546-01 0.13740 LITHIUM CARBONATE 600 MG CAP 0 CAMBER PHARMACE EAGEX 68462-0222-01 0.13740 LITHIUM CARBONATE 600 MG CAP 0 GLENMARK PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 231LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00054-0020-25 0.28040 LITHIUM ER 450 MG TABLET 0 ROXANE LABS. EAGEX 00378-1450-01 0.28040 LITHIUM ER 450 MG TABLET 0 MYLAN EAGEX 51079-0142-20 0.28040 LITHIUM ER 450 MG TABLET 0 MYLAN INSTITUTI EAGEX 68<strong>08</strong>4-0548-01 0.28040 LITHIUM ER 450 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0548-11 0.28040 LITHIUM ER 450 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-<strong>06</strong>55-01 0.28040 LITHIUM ER 450 MG TABLET 0 AHP EABEX 00054-3527-63 0.07270 0.15316 LITHIUM 8 MEQ/5 ML SOLUTION 0 ROXANE LABS. MLGEX 68094-0757-59 0.21630 LITHIUM 8 MEQ/5 ML SOLUTION 0 PRECISION DOSE MLGEX 68094-0757-62 0.21630 LITHIUM 8 MEQ/5 ML SOLUTION 0 PRECISION DOSE MLBEX 68968-4492-01 0.25569 3.00260 LITHOBID 300 MG TABLET SA G NOVEN THERAPEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00178-0500-01 1.64937 LITHOSTAT 250 MG TABLET 0 MISSION PHARM. EABND 00002-4770-90 4.54176 LIVALO 1 MG TABLET G ELI LILLY & CO. EABND 66869-0104-90 4.98000 LIVALO 1 MG TABLET G KOWA PHARMACEUT EABND 00002-4771-90 4.54176 LIVALO 2 MG TABLET G ELI LILLY & CO. EABND 66869-0204-90 4.98000 LIVALO 2 MG TABLET G KOWA PHARMACEUT EABND 00002-4772-90 4.54176 LIVALO 4 MG TABLET G ELI LILLY & CO. EABND 66869-0404-90 4.98000 LIVALO 4 MG TABLET G KOWA PHARMACEUT EABEX 00430-0420-14 2.95231 LO LOESTRIN FE 1-10 TABLET 0 ACTAVIS PHARMA, EABEX 00430-0537-14 2.95231 LO MINASTRIN FE TABLET CHEW 0 ACTAVIS PHARMA, EABND 25010-0711-15 10.<strong>08</strong>632 LODOSYN 25 MG TABLET 0 VALEANT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51285-0<strong>08</strong>3-70 0.72384 LOESTRIN FE 1.5-30 TABLET 0 DURAMED/BARR EAGEX 51285-0<strong>08</strong>4-98 0.72384 LOESTRIN FE 1.5-30 TABLET 0 DURAMED/BARR EABEX 51285-0<strong>08</strong>0-70 0.71880 3.32237 LOESTRIN FE 1-20 TABLET G DURAMED/BARR EAGEX 51285-0<strong>08</strong>1-98 0.71880 LOESTRIN FE 1-20 TABLET 0 DURAMED/BARR EAGEX 51285-0<strong>08</strong>2-97 1.00290 LOESTRIN 21 1.5-30 TABLET 0 DURAMED/BARR EAGEX 51285-0079-97 1.00280 LOESTRIN 21 1-20 TABLET 0 DURAMED/BARR EABEX 00430-0530-14 2.95231 LOESTRIN 24 FE TABLET 0 WC PR<strong>OF</strong> PRODS EAGEN 57844-0323-01 1.34388 L<strong>OF</strong>IBRA 134 MG CAPSULE G GATE PHARM EAGEN 57844-<strong>06</strong>92-98 1.34973 L<strong>OF</strong>IBRA 160 MG TABLET G GATE PHARM EAGEN 57844-0324-01 2.17020 L<strong>OF</strong>IBRA 200 MG CAPSULE G GATE PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 57844-<strong>06</strong>91-98 0.62100 L<strong>OF</strong>IBRA 54 MG TABLET G GATE PHARM EAGEN 57844-0322-01 0.72470 L<strong>OF</strong>IBRA 67 MG CAPSULE G GATE PHARM EAGEN 00093-0311-01 0.16025 LOPERAMIDE 2 MG CAPSULE 0 TEVA USA EAGEN 00093-0311-05 0.16025 LOPERAMIDE 2 MG CAPSULE 0 TEVA USA EAGEN 00378-2100-01 0.16025 LOPERAMIDE 2 MG CAPSULE 0 MYLAN EAGEN 00378-2100-05 0.16025 LOPERAMIDE 2 MG CAPSULE 0 MYLAN EAGEN 51079-<strong>06</strong>90-20 0.16025 LOPERAMIDE 2 MG CAPSULE 0 MYLAN INSTITUTI EABUL 00071-0737-20 0.13500 3.41738 LOPID 600 MG TABLET G PFIZER US PHARM EABUL 00071-0737-30 0.13500 3.41727 LOPID 600 MG TABLET G PFIZER US PHARM EABND 00078-0460-05 1.04361 2.<strong>06</strong>221 LOPRESSOR HCT 50-25 TABLET G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 3<strong>06</strong>98-0460-01 1.04361 2.<strong>06</strong>221 LOPRESSOR HCT 50-25 TABLET G VALIDUS PHARMAC EABND 00078-0459-05 0.03240 2.70023 LOPRESSOR 100 MG TABLET G NOVARTIS EABND 00078-0458-05 0.02120 1.79844 LOPRESSOR 50 MG TABLET G NOVARTIS EABND 99207-0013-01 1.37850 5.209<strong>08</strong> LOPROX 0.77% GEL G VALEANT GMGEX 00781-5656-15 1.89669 LORYNA 3 MG-0.02 MG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 232LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 68025-0046-10 3.93959 LORZONE 375 MG TABLET G VERTICAL PHARM EABND 68025-0047-10 4.40497 LORZONE 750 MG TABLET G VERTICAL PHARM EAGEN 00054-0125-22 0.33000 LOSARTAN POTASSIUM 100 MG TAB G ROXANE LABS. EAGEN 00093-7366-10 0.33000 LOSARTAN POTASSIUM 100 MG TAB G TEVA USA EAGEN 00093-7366-56 0.33000 LOSARTAN POTASSIUM 100 MG TAB G TEVA USA EAGEN 00093-7366-98 0.33000 LOSARTAN POTASSIUM 100 MG TAB G TEVA USA EAGEN 00245-0195-90 0.33000 LOSARTAN POTASSIUM 100 MG TAB G UPSHER SMITH EAGEN 00378-4043-77 0.33000 LOSARTAN POTASSIUM 100 MG TAB G MYLAN EAGEN 00378-4043-93 0.33000 LOSARTAN POTASSIUM 100 MG TAB G MYLAN EAGEN 0<strong>06</strong>03-4226-02 0.33000 LOSARTAN POTASSIUM 100 MG TAB G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-4226-16 0.33000 LOSARTAN POTASSIUM 100 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-4226-32 0.33000 LOSARTAN POTASSIUM 100 MG TAB G QUALITEST EAGEN 00781-5702-10 0.33000 LOSARTAN POTASSIUM 100 MG TAB G SANDOZ EAGEN 00781-5702-31 0.33000 LOSARTAN POTASSIUM 100 MG TAB G SANDOZ EAGEN 00781-5702-92 0.33000 LOSARTAN POTASSIUM 100 MG TAB G SANDOZ EAGEN 13668-0115-10 0.33000 LOSARTAN POTASSIUM 100 MG TAB G TORRENT PHARMAC EAGEN 13668-0115-30 0.33000 LOSARTAN POTASSIUM 100 MG TAB G TORRENT PHARMAC EAGEN 13668-0115-90 0.33000 LOSARTAN POTASSIUM 100 MG TAB G TORRENT PHARMAC EAGEN 16571-0502-11 0.33000 LOSARTAN POTASSIUM 100 MG TAB G PACK PHARMACEUT EAGEN 16714-0583-01 0.33000 LOSARTAN POTASSIUM 100 MG TAB G NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0583-02 0.33000 LOSARTAN POTASSIUM 100 MG TAB G NORTHSTAR RX LL EAGEN 16714-0583-03 0.33000 LOSARTAN POTASSIUM 100 MG TAB G NORTHSTAR RX LL EAGEN 42571-0112-10 0.33000 LOSARTAN POTASSIUM 100 MG TAB G MICRO LABS USA, EAGEN 42571-0112-90 0.33000 LOSARTAN POTASSIUM 100 MG TAB G MICRO LABS USA, EAGEN 59746-0335-10 0.23187 LOSARTAN POTASSIUM 100 MG TAB G CADISTA PHARMAC EAGEN 59746-0335-30 0.23225 LOSARTAN POTASSIUM 100 MG TAB G CADISTA PHARMAC EAGEN 59746-0335-90 0.23166 LOSARTAN POTASSIUM 100 MG TAB G CADISTA PHARMAC EAGEN 60505-3162-03 0.33000 LOSARTAN POTASSIUM 100 MG TAB G APOTEX CORP EAGEN 60505-3162-09 0.33000 LOSARTAN POTASSIUM 100 MG TAB G APOTEX CORP EAGEN 63739-0515-10 0.33000 LOSARTAN POTASSIUM 100 MG TAB G MCKESSON PACKAG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0203-30 0.33000 LOSARTAN POTASSIUM 100 MG TAB G AUROBINDO PHARM EAGEN 65862-0203-90 0.33000 LOSARTAN POTASSIUM 100 MG TAB G AUROBINDO PHARM EAGEN 65862-0203-99 0.33000 LOSARTAN POTASSIUM 100 MG TAB G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0348-01 0.33000 LOSARTAN POTASSIUM 100 MG TAB G AHP EAGEN 68180-0212-03 0.33000 LOSARTAN POTASSIUM 100 MG TAB G LUPIN PHARMACEU EAGEN 68180-0212-09 0.33000 LOSARTAN POTASSIUM 100 MG TAB G LUPIN PHARMACEU EAGEN 68382-0137-<strong>06</strong> 0.33000 LOSARTAN POTASSIUM 100 MG TAB G ZYDUS PHARMACEU EAGEN 68382-0137-10 0.33000 LOSARTAN POTASSIUM 100 MG TAB G ZYDUS PHARMACEU EAGEN 68382-0137-16 0.33000 LOSARTAN POTASSIUM 100 MG TAB G ZYDUS PHARMACEU EAGEN 68645-0410-70 0.33000 LOSARTAN POTASSIUM 100 MG TAB G LEGACY PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68645-0411-70 0.33000 LOSARTAN POTASSIUM 100 MG TAB G LEGACY PHARMACE EAGEN 00054-0123-22 0.19000 LOSARTAN POTASSIUM 25 MG TAB G ROXANE LABS. EAGEN 00093-7364-10 0.19000 LOSARTAN POTASSIUM 25 MG TAB G TEVA USA EAGEN 00093-7364-98 0.19000 LOSARTAN POTASSIUM 25 MG TAB G TEVA USA EAGEN 00378-4041-77 0.19000 LOSARTAN POTASSIUM 25 MG TAB G MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 233LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-4224-02 0.19000 LOSARTAN POTASSIUM 25 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-4224-32 0.19000 LOSARTAN POTASSIUM 25 MG TAB G QUALITEST EAGEN 00781-5700-10 0.19000 LOSARTAN POTASSIUM 25 MG TAB G SANDOZ EAGEN 00781-5700-92 0.19000 LOSARTAN POTASSIUM 25 MG TAB G SANDOZ EAGEN 13668-0113-10 0.19000 LOSARTAN POTASSIUM 25 MG TAB G TORRENT PHARMAC EAGEN 13668-0113-90 0.19000 LOSARTAN POTASSIUM 25 MG TAB G TORRENT PHARMAC EAGEN 16571-0500-50 0.19000 LOSARTAN POTASSIUM 25 MG TAB G PACK PHARMACEUT EAGEN 16714-0581-02 0.19000 LOSARTAN POTASSIUM 25 MG TAB G NORTHSTAR RX LL EAGEN 16714-0581-03 0.19000 LOSARTAN POTASSIUM 25 MG TAB G NORTHSTAR RX LL EAGEN 42571-0110-10 0.19000 LOSARTAN POTASSIUM 25 MG TAB G MICRO LABS USA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42571-0110-90 0.19000 LOSARTAN POTASSIUM 25 MG TAB G MICRO LABS USA, EAGEN 59746-0333-10 0.12657 LOSARTAN POTASSIUM 25 MG TAB G CADISTA PHARMAC EAGEN 59746-0333-90 0.12658 LOSARTAN POTASSIUM 25 MG TAB G CADISTA PHARMAC EAGEN 60505-3160-09 0.19000 LOSARTAN POTASSIUM 25 MG TAB G APOTEX CORP EAGEN 63739-0513-10 0.19000 LOSARTAN POTASSIUM 25 MG TAB G MCKESSON PACKAG EAGEN 65862-0201-90 0.19000 LOSARTAN POTASSIUM 25 MG TAB G AUROBINDO PHARM EAGEN 65862-0201-99 0.19000 LOSARTAN POTASSIUM 25 MG TAB G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0346-01 0.19000 LOSARTAN POTASSIUM 25 MG TAB G AHP EAGEN 68<strong>08</strong>4-0346-11 0.19000 LOSARTAN POTASSIUM 25 MG TAB G AHP EAGEN 68180-0210-03 0.19000 LOSARTAN POTASSIUM 25 MG TAB G LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0210-09 0.19000 LOSARTAN POTASSIUM 25 MG TAB G LUPIN PHARMACEU EAGEN 68382-0135-<strong>06</strong> 0.19000 LOSARTAN POTASSIUM 25 MG TAB G ZYDUS PHARMACEU EAGEN 68382-0135-10 0.19000 LOSARTAN POTASSIUM 25 MG TAB G ZYDUS PHARMACEU EAGEN 68382-0135-16 0.19000 LOSARTAN POTASSIUM 25 MG TAB G ZYDUS PHARMACEU EAGEN 68645-04<strong>06</strong>-70 0.19000 LOSARTAN POTASSIUM 25 MG TAB G LEGACY PHARMACE EAGEN 68645-0407-70 0.19000 LOSARTAN POTASSIUM 25 MG TAB G LEGACY PHARMACE EAGEN 00054-0124-22 0.24000 LOSARTAN POTASSIUM 50 MG TAB G ROXANE LABS. EAGEN 00093-7365-10 0.24000 LOSARTAN POTASSIUM 50 MG TAB G TEVA USA EAGEN 00093-7365-56 0.24000 LOSARTAN POTASSIUM 50 MG TAB G TEVA USA EAGEN 00093-7365-98 0.24000 LOSARTAN POTASSIUM 50 MG TAB G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-4042-77 0.24000 LOSARTAN POTASSIUM 50 MG TAB G MYLAN EAGEN 00378-4042-93 0.24000 LOSARTAN POTASSIUM 50 MG TAB G MYLAN EAGEN 0<strong>06</strong>03-4225-02 0.24000 LOSARTAN POTASSIUM 50 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-4225-16 0.24000 LOSARTAN POTASSIUM 50 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-4225-32 0.24000 LOSARTAN POTASSIUM 50 MG TAB G QUALITEST EAGEN 00781-5701-10 0.24000 LOSARTAN POTASSIUM 50 MG TAB G SANDOZ EAGEN 00781-5701-31 0.24000 LOSARTAN POTASSIUM 50 MG TAB G SANDOZ EAGEN 00781-5701-92 0.24000 LOSARTAN POTASSIUM 50 MG TAB G SANDOZ EAGEN 13668-0409-10 0.24000 LOSARTAN POTASSIUM 50 MG TAB G TORRENT PHARMAC EAGEN 13668-0409-30 0.24000 LOSARTAN POTASSIUM 50 MG TAB G TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-0409-90 0.24000 LOSARTAN POTASSIUM 50 MG TAB G TORRENT PHARMAC EAGEN 16571-0501-11 0.24000 LOSARTAN POTASSIUM 50 MG TAB G PACK PHARMACEUT EAGEN 16714-0582-01 0.24000 LOSARTAN POTASSIUM 50 MG TAB G NORTHSTAR RX LL EAGEN 16714-0582-02 0.24000 LOSARTAN POTASSIUM 50 MG TAB G NORTHSTAR RX LL EAGEN 16714-0582-03 0.24000 LOSARTAN POTASSIUM 50 MG TAB G NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 234LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42571-0111-10 0.24000 LOSARTAN POTASSIUM 50 MG TAB G MICRO LABS USA, EAGEN 42571-0111-90 0.24000 LOSARTAN POTASSIUM 50 MG TAB G MICRO LABS USA, EAGEN 58517-0240-30 0.24000 LOSARTAN POTASSIUM 50 MG TAB G <strong>NEW</strong> HORIZON RX EAGEN 59746-0334-10 0.17019 LOSARTAN POTASSIUM 50 MG TAB G CADISTA PHARMAC EAGEN 59746-0334-30 0.17075 LOSARTAN POTASSIUM 50 MG TAB G CADISTA PHARMAC EAGEN 59746-0334-90 0.17033 LOSARTAN POTASSIUM 50 MG TAB G CADISTA PHARMAC EAGEN 60505-3161-03 0.24000 LOSARTAN POTASSIUM 50 MG TAB G APOTEX CORP EAGEN 60505-3161-09 0.24000 LOSARTAN POTASSIUM 50 MG TAB G APOTEX CORP EAGEN 63739-0514-10 0.24000 LOSARTAN POTASSIUM 50 MG TAB G MCKESSON PACKAG EAGEN 65862-0202-30 0.24000 LOSARTAN POTASSIUM 50 MG TAB G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0202-90 0.24000 LOSARTAN POTASSIUM 50 MG TAB G AUROBINDO PHARM EAGEN 65862-0202-99 0.24000 LOSARTAN POTASSIUM 50 MG TAB G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0347-01 0.24000 LOSARTAN POTASSIUM 50 MG TAB G AHP EAGEN 68<strong>08</strong>4-0347-11 0.24000 LOSARTAN POTASSIUM 50 MG TAB G AHP EAGEN 68180-0211-03 0.24000 LOSARTAN POTASSIUM 50 MG TAB G LUPIN PHARMACEU EAGEN 68180-0211-09 0.24000 LOSARTAN POTASSIUM 50 MG TAB G LUPIN PHARMACEU EAGEN 68382-0136-<strong>06</strong> 0.24000 LOSARTAN POTASSIUM 50 MG TAB G ZYDUS PHARMACEU EAGEN 68382-0136-10 0.24000 LOSARTAN POTASSIUM 50 MG TAB G ZYDUS PHARMACEU EAGEN 68382-0136-16 0.24000 LOSARTAN POTASSIUM 50 MG TAB G ZYDUS PHARMACEU EAGEN 68645-04<strong>08</strong>-70 0.24000 LOSARTAN POTASSIUM 50 MG TAB G LEGACY PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68645-0409-70 0.24000 LOSARTAN POTASSIUM 50 MG TAB G LEGACY PHARMACE EAGEN 00093-7369-10 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G TEVA USA EAGEN 00093-7369-56 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G TEVA USA EAGEN 00093-7369-98 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G TEVA USA EAGEN 00378-1419-77 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G MYLAN EAGEN 00378-1419-93 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G MYLAN EAGEN 0<strong>06</strong>03-4229-02 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-4229-16 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-4229-32 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G QUALITEST EAGEN 00781-5204-10 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-5204-31 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G SANDOZ EAGEN 00781-5204-92 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G SANDOZ EAGEN 13668-0117-10 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G TORRENT PHARMAC EAGEN 13668-0117-30 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G TORRENT PHARMAC EAGEN 13668-0117-90 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G TORRENT PHARMAC EAGEN 16714-0224-01 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G NORTHSTAR RX LL EAGEN 16714-0224-02 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G NORTHSTAR RX LL EAGEN 60505-2916-03 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G APOTEX CORP EAGEN 60505-2916-09 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G APOTEX CORP EAGEN 63739-0528-10 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G MCKESSON PACKAG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0469-30 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G AUROBINDO PHARM EAGEN 65862-0469-90 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G AUROBINDO PHARM EAGEN 65862-0469-99 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G AUROBINDO PHARM EAGEN 68180-0216-03 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G LUPIN PHARMACEU EAGEN 68180-0216-<strong>06</strong> 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G LUPIN PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 235LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0216-09 0.14000 LOSARTAN-HCTZ 100-12.5 MG TAB G LUPIN PHARMACEU EAGEN 00054-0127-22 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G ROXANE LABS. EAGEN 00093-7368-10 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G TEVA USA EAGEN 00093-7368-56 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G TEVA USA EAGEN 00093-7368-98 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G TEVA USA EAGEN 00378-1420-77 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G MYLAN EAGEN 00378-1420-93 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G MYLAN EAGEN 0<strong>06</strong>03-4230-02 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-4230-16 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-4230-32 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-5207-10 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G SANDOZ EAGEN 00781-5207-31 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G SANDOZ EAGEN 00781-5207-92 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G SANDOZ EAGEN 13668-0118-10 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G TORRENT PHARMAC EAGEN 13668-0118-30 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G TORRENT PHARMAC EAGEN 13668-0118-90 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G TORRENT PHARMAC EAGEN 16714-0225-01 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G NORTHSTAR RX LL EAGEN 16714-0225-02 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G NORTHSTAR RX LL EAGEN 16714-0225-04 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G NORTHSTAR RX LL EAGEN 60505-2917-03 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2917-09 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G APOTEX CORP EAGEN 65862-0470-30 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G AUROBINDO PHARM EAGEN 65862-0470-90 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G AUROBINDO PHARM EAGEN 65862-0470-99 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G AUROBINDO PHARM EAGEN 68180-0217-03 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G LUPIN PHARMACEU EAGEN 68180-0217-<strong>06</strong> 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G LUPIN PHARMACEU EAGEN 68180-0217-09 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G LUPIN PHARMACEU EAGEN 68382-0143-<strong>06</strong> 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G ZYDUS PHARMACEU EAGEN 68382-0143-10 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G ZYDUS PHARMACEU EAGEN 68382-0143-16 0.18240 LOSARTAN-HCTZ 100-25 MG TAB G ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0126-22 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G ROXANE LABS. EAGEN 00093-7367-10 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G TEVA USA EAGEN 00093-7367-56 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G TEVA USA EAGEN 00093-7367-98 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G TEVA USA EAGEN 00378-1418-77 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G MYLAN EAGEN 00378-1418-93 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G MYLAN EAGEN 0<strong>06</strong>03-4228-02 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-4228-16 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-4228-32 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G QUALITEST EAGEN 00781-52<strong>06</strong>-10 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-52<strong>06</strong>-31 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G SANDOZ EAGEN 00781-52<strong>06</strong>-92 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G SANDOZ EAGEN 13668-0116-10 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G TORRENT PHARMAC EAGEN 13668-0116-30 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G TORRENT PHARMAC EAGEN 13668-0116-90 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 236LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0226-01 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G NORTHSTAR RX LL EAGEN 16714-0226-02 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G NORTHSTAR RX LL EAGEN 16714-0226-04 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G NORTHSTAR RX LL EAGEN 60505-2915-03 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G APOTEX CORP EAGEN 60505-2915-09 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G APOTEX CORP EAGEN 63739-0527-10 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G MCKESSON PACKAG EAGEN 65862-0468-30 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G AUROBINDO PHARM EAGEN 65862-0468-90 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G AUROBINDO PHARM EAGEN 65862-0468-99 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G AUROBINDO PHARM EAGEN 68180-0215-03 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0215-<strong>06</strong> 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G LUPIN PHARMACEU EAGEN 68180-0215-09 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G LUPIN PHARMACEU EAGEN 68382-0142-<strong>06</strong> 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G ZYDUS PHARMACEU EAGEN 68382-0142-10 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G ZYDUS PHARMACEU EAGEN 68382-0142-16 0.09844 LOSARTAN-HCTZ 50-12.5 MG TAB G ZYDUS PHARMACEU EABEX 51285-0092-87 2.97158 LOSEASONIQUE TABLET 0 DURAMED/BARR EABND 242<strong>08</strong>-0299-05 28.48394 LOTEMAX 0.5% EYE DROPS 0 VALEANT MLBND 242<strong>08</strong>-0299-10 28.48228 LOTEMAX 0.5% EYE DROPS 0 VALEANT MLBND 242<strong>08</strong>-0299-15 28.23051 LOTEMAX 0.5% EYE DROPS 0 VALEANT MLBND 242<strong>08</strong>-0443-35 52.50342 LOTEMAX 0.5% EYE OINTMENT 0 VALEANT GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00078-0452-05 0.49580 1.9<strong>08</strong>75 LOTENSIN HCT 10-12.5 TABLET G NOVARTIS EABUL 00078-0453-05 0.49580 1.9<strong>08</strong>75 LOTENSIN HCT 20-12.5 TABLET G NOVARTIS EABND 00078-0454-05 0.38134 1.9<strong>08</strong>75 LOTENSIN HCT 20-25 TABLET G NOVARTIS EABND 00078-0449-05 0.05549 1.9<strong>08</strong>75 LOTENSIN 20 MG TABLET G NOVARTIS EABND 3<strong>06</strong>98-0449-01 0.05549 1.9<strong>08</strong>75 LOTENSIN 20 MG TABLET G VALIDUS PHARMAC EABND 00078-0450-05 0.<strong>06</strong>993 1.9<strong>08</strong>75 LOTENSIN 40 MG TABLET G NOVARTIS EABND 3<strong>06</strong>98-0450-01 0.<strong>06</strong>993 1.9<strong>08</strong>75 LOTENSIN 40 MG TABLET G VALIDUS PHARMAC EABND 00078-0364-05 0.57710 6.56272 LOTREL 10-20 MG CAPSULE 0 NOVARTIS EABND 00078-0379-05 0.98960 7.23137 LOTREL 10-40 MG CAPSULE 0 NOVARTIS EABND 00078-0404-05 0.69420 5.24526 LOTREL 2.5-10 MG CAPSULE 0 NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0405-05 0.47840 5.34910 LOTREL 5-10 MG CAPSULE 0 NOVARTIS EABND 00078-04<strong>06</strong>-05 0.45364 5.64873 LOTREL 5-20 MG CAPSULE 0 NOVARTIS EABND 00078-0384-05 0.81297 5.98579 LOTREL 5-40 MG CAPSULE 0 NOVARTIS EABUL 00<strong>08</strong>5-0924-01 0.82300 3.00017 LOTRISONE CREAM G MERCK SHARP & D GMBUL 00<strong>08</strong>5-0924-02 0.82300 2.15283 LOTRISONE CREAM G MERCK SHARP & D GMBND 65483-<strong>08</strong>94-03 17.35834 LOTRONEX 0.5 MG TABLET 0 PROMETHEUS EABND 65483-<strong>08</strong>95-03 34.71668 LOTRONEX 1 MG TABLET 0 PROMETHEUS EAGEN 00093-0926-<strong>06</strong> 0.09518 LOVASTATIN 10 MG TABLET 0 TEVA USA EAGEN 00093-0926-10 0.09518 LOVASTATIN 10 MG TABLET 0 TEVA USA EAGEN 00185-0070-01 0.09518 LOVASTATIN 10 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0070-10 0.09518 LOVASTATIN 10 MG TABLET 0 SANDOZ EAGEN 00185-0070-60 0.09518 LOVASTATIN 10 MG TABLET 0 SANDOZ EAGEN 00228-2633-<strong>06</strong> 0.09518 LOVASTATIN 10 MG TABLET 0 ACTAVIS ELIZABE EAGEN 00228-2633-50 0.09518 LOVASTATIN 10 MG TABLET 0 ACTAVIS ELIZABE EAGEN 00378-6510-91 0.09518 LOVASTATIN 10 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 237LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45963-<strong>06</strong>33-01 0.09518 LOVASTATIN 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 45963-<strong>06</strong>33-04 0.09518 LOVASTATIN 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 54458-0916-10 0.09518 LOVASTATIN 10 MG TABLET 0 INTERNATIONAL L EAGEN 54458-0938-10 0.09518 LOVASTATIN 10 MG TABLET 0 INTERNATIONAL L EAGEN 60505-0177-00 0.09518 LOVASTATIN 10 MG TABLET 0 APOTEX CORP EAGEN 61442-0141-01 0.09518 LOVASTATIN 10 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0141-10 0.09518 LOVASTATIN 10 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0141-60 0.09518 LOVASTATIN 10 MG TABLET 0 CARLSBAD TECH EAGEN 68<strong>08</strong>4-0558-01 0.09518 LOVASTATIN 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0558-11 0.09518 LOVASTATIN 10 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0467-01 0.09518 LOVASTATIN 10 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0467-03 0.09518 LOVASTATIN 10 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0467-07 0.09518 LOVASTATIN 10 MG TABLET 0 LUPIN PHARMACEU EAGEN 00093-0576-<strong>06</strong> 0.09830 LOVASTATIN 20 MG TABLET 0 TEVA USA EAGEN 00093-0576-10 0.09830 LOVASTATIN 20 MG TABLET 0 TEVA USA EAGEN 00093-0576-19 0.09830 LOVASTATIN 20 MG TABLET 0 TEVA USA EAGEN 00093-0576-93 0.09830 LOVASTATIN 20 MG TABLET 0 TEVA USA EAGEN 00185-0072-01 0.09830 LOVASTATIN 20 MG TABLET 0 SANDOZ EAGEN 00185-0072-10 0.09830 LOVASTATIN 20 MG TABLET 0 SANDOZ EAGEN 00185-0072-60 0.09830 LOVASTATIN 20 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-2634-<strong>06</strong> 0.09830 LOVASTATIN 20 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00228-2634-50 0.09830 LOVASTATIN 20 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00378-6520-05 0.09830 LOVASTATIN 20 MG TABLET 0 MYLAN EAGEN 00378-6520-91 0.09830 LOVASTATIN 20 MG TABLET 0 MYLAN EAGEN 45963-<strong>06</strong>34-01 0.09830 LOVASTATIN 20 MG TABLET 0 ACTAVIS PHARMA, EAGEN 45963-<strong>06</strong>34-04 0.09830 LOVASTATIN 20 MG TABLET 0 ACTAVIS PHARMA, EAGEN 51079-0975-01 0.09830 LOVASTATIN 20 MG TABLET 0 MYLAN INSTITUTI EAGEN 54458-0915-10 0.09830 LOVASTATIN 20 MG TABLET 0 INTERNATIONAL L EAGEN 54458-0937-10 0.09830 LOVASTATIN 20 MG TABLET 0 INTERNATIONAL L EAGEN 60505-0178-00 0.09830 LOVASTATIN 20 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61442-0142-01 0.09830 LOVASTATIN 20 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0142-10 0.09830 LOVASTATIN 20 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0142-60 0.09830 LOVASTATIN 20 MG TABLET 0 CARLSBAD TECH EAGEN 68<strong>08</strong>4-0559-01 0.09830 LOVASTATIN 20 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0559-11 0.09830 LOVASTATIN 20 MG TABLET 0 AHP EAGEN 68180-0468-01 0.09830 LOVASTATIN 20 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0468-03 0.09830 LOVASTATIN 20 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0468-05 0.09830 LOVASTATIN 20 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0468-07 0.09830 LOVASTATIN 20 MG TABLET 0 LUPIN PHARMACEU EAGEN 00093-0928-<strong>06</strong> 0.12100 LOVASTATIN 40 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-0928-10 0.12100 LOVASTATIN 40 MG TABLET 0 TEVA USA EAGEN 00093-0928-19 0.12100 LOVASTATIN 40 MG TABLET 0 TEVA USA EAGEN 00093-0928-93 0.12100 LOVASTATIN 40 MG TABLET 0 TEVA USA EAGEN 00185-0074-01 0.12100 LOVASTATIN 40 MG TABLET 0 SANDOZ EAGEN 00185-0074-10 0.12100 LOVASTATIN 40 MG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 238LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0074-60 0.12100 LOVASTATIN 40 MG TABLET 0 SANDOZ EAGEN 00228-2635-<strong>06</strong> 0.12100 LOVASTATIN 40 MG TABLET 0 ACTAVIS ELIZABE EAGEN 00228-2635-50 0.12100 LOVASTATIN 40 MG TABLET 0 ACTAVIS ELIZABE EAGEN 00378-6540-05 0.12100 LOVASTATIN 40 MG TABLET 0 MYLAN EAGEN 00378-6540-91 0.12100 LOVASTATIN 40 MG TABLET 0 MYLAN EAGEN 45963-<strong>06</strong>35-01 0.12100 LOVASTATIN 40 MG TABLET 0 ACTAVIS PHARMA, EAGEN 45963-<strong>06</strong>35-04 0.12100 LOVASTATIN 40 MG TABLET 0 ACTAVIS PHARMA, EAGEN 54458-0914-10 0.12100 LOVASTATIN 40 MG TABLET 0 INTERNATIONAL L EAGEN 54458-0936-10 0.12100 LOVASTATIN 40 MG TABLET 0 INTERNATIONAL L EAGEN 60505-0179-00 0.12100 LOVASTATIN 40 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61442-0143-01 0.12100 LOVASTATIN 40 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0143-10 0.12100 LOVASTATIN 40 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0143-60 0.12100 LOVASTATIN 40 MG TABLET 0 CARLSBAD TECH EAGEN 68<strong>08</strong>4-0560-01 0.12100 LOVASTATIN 40 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0560-11 0.12100 LOVASTATIN 40 MG TABLET 0 AHP EAGEN 68180-0469-01 0.12100 LOVASTATIN 40 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0469-03 0.12100 LOVASTATIN 40 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0469-05 0.12100 LOVASTATIN 40 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0469-07 0.12100 LOVASTATIN 40 MG TABLET 0 LUPIN PHARMACEU EABND 00173-0783-02 1.74949 LOVAZA 1 GM CAPSULE G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00075-<strong>06</strong>23-00 82.45552 82.45552 LOVENOX 100 MG/ML SYRINGE 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00075-<strong>06</strong>23-01 82.45552 82.45552 LOVENOX 100 MG/ML SYRINGE 0 SAN<strong>OF</strong>I/NOVAPLUS MLBND 00075-8020-01 82.45552 82.45552 LOVENOX 100 MG/ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-8020-10 82.45552 82.45552 LOVENOX 100 MG/ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-2912-01 123.72395 123.72395 LOVENOX 120 MG/0.8 ML SYRINGE 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00075-2912-02 123.72395 123.72395 LOVENOX 120 MG/0.8 ML SYRINGE 0 SAN<strong>OF</strong>I/NOVAPLUS MLBND 00075-8022-01 123.72395 123.72395 LOVENOX 120 MG/0.8 ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-8022-10 123.72395 123.72395 LOVENOX 120 MG/0.8 ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-2915-01 123.72644 123.72644 LOVENOX 150 MG/ML SYRINGE 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00075-2915-02 123.72644 123.72644 LOVENOX 150 MG/ML SYRINGE 0 SAN<strong>OF</strong>I/NOVAPLUS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00075-8025-01 123.72644 123.72644 LOVENOX 150 MG/ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-8025-10 123.72644 123.72644 LOVENOX 150 MG/ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-<strong>06</strong>24-30 82.36366 82.36366 LOVENOX 30 MG/0.3 ML SYRINGE 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00075-<strong>06</strong>24-31 82.36366 82.36366 LOVENOX 30 MG/0.3 ML SYRINGE 0 SAN<strong>OF</strong>I/NOVAPLUS MLBND 00075-8013-01 82.36366 82.36366 LOVENOX 30 MG/0.3 ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-8013-10 82.36366 82.36366 LOVENOX 30 MG/0.3 ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-<strong>06</strong>26-03 82.36366 82.36366 LOVENOX 300 MG/3 ML VIAL 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00075-<strong>06</strong>26-04 82.36366 82.36366 LOVENOX 300 MG/3 ML VIAL 0 SAN<strong>OF</strong>I/NOVAPLUS MLBND 00075-8030-01 82.36366 82.36366 LOVENOX 300 MG/3 ML VIAL 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-<strong>06</strong>20-40 82.35882 82.35882 LOVENOX 40 MG/0.4 ML SYRINGE 0 SAN<strong>OF</strong>I-AVENTIS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00075-<strong>06</strong>20-41 82.35882 82.35882 LOVENOX 40 MG/0.4 ML SYRINGE 0 SAN<strong>OF</strong>I/NOVAPLUS MLBND 00075-8014-01 82.35882 82.35882 LOVENOX 40 MG/0.4 ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-8014-10 82.35882 82.35882 LOVENOX 40 MG/0.4 ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-<strong>06</strong>21-60 82.45773 82.45773 LOVENOX 60 MG/0.6 ML SYRINGE 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00075-<strong>06</strong>21-61 82.45773 82.45773 LOVENOX 60 MG/0.6 ML SYRINGE 0 SAN<strong>OF</strong>I/NOVAPLUS ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 239LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00075-8016-01 82.45773 82.45773 LOVENOX 60 MG/0.6 ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-8016-10 82.45773 82.45773 LOVENOX 60 MG/0.6 ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-<strong>06</strong>22-80 82.45738 82.45738 LOVENOX 80 MG/0.8 ML SYRINGE 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00075-<strong>06</strong>22-81 82.45738 82.45738 LOVENOX 80 MG/0.8 ML SYRINGE 0 SAN<strong>OF</strong>I/NOVAPLUS MLBND 00075-8018-01 82.45738 82.45738 LOVENOX 80 MG/0.8 ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLBND 00075-8018-10 82.45738 82.45738 LOVENOX 80 MG/0.8 ML SYRINGE 0 SAN<strong>OF</strong>I/PREMIERP MLGEX 52544-<strong>08</strong>47-28 0.80097 LOW-OGESTREL-28 TABLET 0 ACTAVIS PHARMA, EAGEX 00378-7010-01 0.53379 LOXAPINE 10 MG CAPSULE 0 MYLAN EAGEX 00527-1395-01 0.53379 LOXAPINE 10 MG CAPSULE 0 LANNETT CO. INC EAGEX 00591-0370-01 0.53379 LOXAPINE 10 MG CAPSULE 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0002-21 0.53379 LOXAPINE 10 MG CAPSULE 0 AHP EAGEX 00378-7025-01 0.77260 LOXAPINE 25 MG CAPSULE 0 MYLAN EAGEX 00527-1396-01 0.77260 LOXAPINE 25 MG CAPSULE 0 LANNETT CO. INC EAGEX 00591-0371-01 0.77260 LOXAPINE 25 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 68<strong>08</strong>4-0003-21 0.77260 LOXAPINE 25 MG CAPSULE 0 AHP EAGEX 00378-7005-01 0.37422 LOXAPINE 5 MG CAPSULE 0 MYLAN EAGEX 00527-1394-01 0.37422 LOXAPINE 5 MG CAPSULE 0 LANNETT CO. INC EAGEX 00591-0369-01 0.37422 LOXAPINE 5 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00378-7050-01 0.99130 LOXAPINE 50 MG CAPSULE 0 MYLAN EAGEX 00527-1397-01 0.99130 LOXAPINE 50 MG CAPSULE 0 LANNETT CO. INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00591-0372-01 0.99130 LOXAPINE 50 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 68<strong>08</strong>4-0004-21 0.99130 LOXAPINE 50 MG CAPSULE 0 AHP EAGEX 52544-0495-01 0.53379 LOXITANE 10 MG CAPSULE 0 WATSON PHARMA EAGEX 52544-0494-01 0.37422 LOXITANE 5 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 44946-10<strong>08</strong>-03 0.03340 LUDENT FLUORIDE 0.25 MG TB CHW 0 SANCILIO & COMP EAGEN 44946-1009-03 0.02450 LUDENT FLUORIDE 0.5 MG TB CHEW 0 SANCILIO & COMP EAGEN 44946-1009-09 0.02450 LUDENT FLUORIDE 0.5 MG TB CHEW 0 SANCILIO & COMP EAGEN 44946-1010-03 0.03190 LUDENT FLUORIDE 1 MG TAB CHEW 0 SANCILIO & COMP EABND 00037-0521-92 4.32654 LUFYLLIN 200 MG TABLET 0 MEDA PHARMACEUT EABND 00037-0731-92 6.67071 LUFYLLIN-400 TABLET 0 MEDA PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00023-3205-03 44.64736 LUMIGAN 0.01% EYE DROPS G ALLERGAN INC. MLBND 00023-3205-05 44.63076 LUMIGAN 0.01% EYE DROPS G ALLERGAN INC. MLBND 00023-3205-<strong>08</strong> 44.62744 LUMIGAN 0.01% EYE DROPS G ALLERGAN INC. MLBND 00023-9187-03 38.63816 LUMIGAN 0.03% EYE DROPS G ALLERGAN INC. MLBND 00023-9187-05 38.62156 LUMIGAN 0.03% EYE DROPS G ALLERGAN INC. MLBND 00023-9187-07 38.61934 LUMIGAN 0.03% EYE DROPS G ALLERGAN INC. MLBND 00074-1052-05 853.63010 LUPANETA PK 3.75-5 MG 1MO KIT 0 ABBVIE US LLC EABND 00074-3663-03 2560.94840 LUPRON DEPOT 11.25 MG 3MO KIT 0 ABBVIE US LLC EABND 00074-3346-03 3051.72740 LUPRON DEPOT 22.5 MG 3MO KIT 0 ABBVIE US LLC EABND 00074-3641-03 853.63010 LUPRON DEPOT 3.75 MG KIT 0 ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00074-3473-03 6103.54610 LUPRON DEPOT 45 MG 6MO KIT 0 ABBVIE US LLC EABND 00074-3642-03 1017.25630 LUPRON DEPOT 7.5 MG KIT 0 ABBVIE US LLC EABND 00074-2282-03 1864.37920 LUPRON DEPOT-PED 11.25 MG KIT 0 ABBVIE US LLC EABND 00074-3779-03 5593.15420 LUPRON DEPOT-PED 11.25 MG 3MO 0 ABBVIE US LLC EABND 00074-2440-03 2053.42000 LUPRON DEPOT-PED 15 MG KIT 0 ABBVIE US LLC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 240LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00074-9694-03 6160.29320 LUPRON DEPOT-PED 30 MG 3MO KIT 0 ABBVIE US LLC EABND 00074-21<strong>08</strong>-03 1026.92580 LUPRON DEPOT-PED 7.5 MG KIT 0 ABBVIE US LLC EABND 00074-3683-03 4<strong>06</strong>8.96710 LUPRON DEPOT-4 MONTH KIT 0 ABBVIE US LLC EAGEX 52544-0949-28 0.85280 LUTERA-28 TABLET 0 ACTAVIS PHARMA, EABEX 68727-<strong>06</strong>00-01 12.42150 LUVOX CR 100 MG CAPSULE G JAZZ PHARMACEUT EABEX 68727-<strong>06</strong>01-01 13.33007 LUVOX CR 150 MG CAPSULE G JAZZ PHARMACEUT EABND 00145-0021-00 4.67630 LUXIQ 0.12% FOAM G PRESTIUM PHARMA GMBND 00145-0021-50 5.02482 LUXIQ 0.12% FOAM G PRESTIUM PHARMA GMBND 63032-0021-00 3.44607 LUXIQ 0.12% FOAM G STIEFEL LABS. GMBND 00015-3<strong>08</strong>0-60 4.50972 LYSODREN 500 MG TABLET 0 BMS ONCO/IMMUN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 55566-2100-01 4.81400 LYSTEDA 650 MG TABLET 0 FERRING PH INC EABND 55566-2100-02 4.81400 LYSTEDA 650 MG TABLET 0 FERRING PH INC EAGEX 50102-0100-01 0.26035 LYZA 0.35 MG TABLET 0 AFAXYS, INC. EAGEX 50102-0100-48 0.26035 LYZA 0.35 MG TABLET 0 AFAXYS, INC. EABND 52427-0285-01 2.02560 14.2<strong>06</strong>11 MACROBID 100 MG CAPSULE G ALMATICA PHARMA EABND 52427-0286-01 7.78075 MACRODANTIN 25 MG CAPSULE 0 ALMATICA PHARMA EABND 00149-00<strong>08</strong>-05 0.96550 2.09732 MACRODANTIN 50 MG CAPSULE G ALMATICA PHARMA EABND 52427-0287-01 0.96550 7.34052 MACRODANTIN 50 MG CAPSULE G ALMATICA PHARMA EABEX 00409-6729-23 0.<strong>06</strong>364 MAGNESIUM SULF 4% IV SOLN 0 HOSPIRA MLGEN 00409-2168-02 0.11340 MAGNESIUM SULFATE 50% VIAL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00517-2650-25 0.04875 MAGNESIUM SULFATE 50% VIAL 0 AMER. REGENT MLGEX 63323-0<strong>06</strong>4-02 0.47700 MAGNESIUM SULFATE 50% VIAL 0 APP PHARMACEUTI MLGEX 63323-0<strong>06</strong>4-10 0.11970 MAGNESIUM SULFATE 50% VIAL 0 APP PHARMACEUTI MLGEX 63323-0<strong>06</strong>4-20 0.24885 MAGNESIUM SULFATE 50% VIAL 0 APP PHARMACEUTI MLGEX 63323-0<strong>06</strong>4-50 0.22284 MAGNESIUM SULFATE 50% VIAL 0 APP PHARMACEUTI MLBND 00173-<strong>06</strong>75-01 5.64140 6.70291 MALARONE 250-100 MG TABLET 0 GLAXOSMITHKLINE EABND 00173-<strong>06</strong>75-02 5.64140 6.83920 MALARONE 250-100 MG TABLET 0 GLAXOSMITHKLINE EABND 00173-<strong>06</strong>76-01 2.47921 MALARONE 62.5-25 MG PED TAB 0 GLAXOSMITHKLINE EAGEN 42043-0150-23 1.98876 MALATHION 0.5% LOTION 0 KARALEX PHARMA, MLGEN 51672-5277-04 2.31343 MALATHION 0.5% LOTION 0 TARO PHARM USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00264-7578-10 0.02701 MANNITOL 20% IV SOLUTION 0 B.BRAUN MLGEN 00264-7578-20 0.03700 MANNITOL 20% IV SOLUTION 0 B.BRAUN MLBEX 00378-0<strong>06</strong>0-01 0.79779 MAPROTILINE 25 MG TABLET 0 MYLAN EABEX 00378-0<strong>08</strong>7-01 1.18117 MAPROTILINE 50 MG TABLET 0 MYLAN EABEX 00378-0092-01 1.62074 MAPROTILINE 75 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>82-<strong>08</strong>04-01 0.48480 MARGESIC CAPSULE 0 MARNEL PHARM. EAGEX 68462-0388-29 0.81150 MARLISSA-28 TABLET 0 GLENMARK PHARMA EAGEX 68462-0388-84 0.81150 MARLISSA-28 TABLET 0 GLENMARK PHARMA EABND 0<strong>06</strong>82-1570-01 0.76332 MARNATAL-F CAPSULE 0 MARNEL PHARM. EABEX 3<strong>06</strong>98-0032-01 3.01788 MARPLAN 10 MG TABLET 0 VALIDUS PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10267-1991-01 0.13380 MATERNITY VITAMIN 0 CONTRACT PHARM EABND 54482-0053-01 53.4<strong>08</strong>92 MATULANE 50 MG CAPSULE 0 SIGMA-TAU EAGEN 52544-<strong>06</strong>91-19 2.23167 MATZIM LA 180 MG TABLET 0 ACTAVIS PHARMA, EAGEN 52544-<strong>06</strong>91-30 2.23149 MATZIM LA 180 MG TABLET 0 ACTAVIS PHARMA, EAGEN 52544-<strong>06</strong>92-19 2.50100 MATZIM LA 240 MG TABLET 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 241LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 52544-<strong>06</strong>92-30 2.50125 MATZIM LA 240 MG TABLET 0 ACTAVIS PHARMA, EAGEN 52544-<strong>06</strong>93-19 2.77658 MATZIM LA 300 MG TABLET 0 ACTAVIS PHARMA, EAGEN 52544-<strong>06</strong>93-30 2.77658 MATZIM LA 300 MG TABLET 0 ACTAVIS PHARMA, EAGEN 52544-<strong>06</strong>94-19 3.50<strong>06</strong>7 MATZIM LA 360 MG TABLET 0 ACTAVIS PHARMA, EAGEN 52544-<strong>06</strong>94-30 3.50075 MATZIM LA 360 MG TABLET 0 ACTAVIS PHARMA, EAGEN 52544-<strong>06</strong>95-19 3.23860 MATZIM LA 420 MG TABLET 0 ACTAVIS PHARMA, EAGEN 52544-<strong>06</strong>95-30 3.23860 MATZIM LA 420 MG TABLET 0 ACTAVIS PHARMA, EABND 00074-2278-13 0.33669 1.74897 MAVIK 1 MG TABLET G ABBVIE US LLC EABND 00074-2279-13 0.33669 1.74897 MAVIK 2 MG TABLET G ABBVIE US LLC EABND 00074-2280-13 0.33588 1.74897 MAVIK 4 MG TABLET G ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 99207-0280-40 44.55262 MAXAIR AUTOHALER 0.2 MG AERO 0 VALEANT GMBND 000<strong>06</strong>-3801-18 23.69460 34.39243 MAXALT MLT 10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-3800-18 23.69460 34.39243 MAXALT MLT 5 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0267-18 2.01590 34.39243 MAXALT 10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0266-18 1.50000 34.39243 MAXALT 5 MG TABLET G MERCK SHARP & D EABND 00998-<strong>06</strong>15-05 12.01176 MAXIDEX 0.1% EYE DROPS 0 ALCON (P.R.) MLBND 00409-0219-01 3.84456 MAXIPIME 1 GRAM VIAL G HOSPIRA EABND 00409-0220-01 7.01184 MAXIPIME 2 GRAM VIAL G HOSPIRA EAGEN 00998-<strong>06</strong>30-<strong>06</strong> 3.<strong>06</strong>220 MAXITROL EYE DROPS G ALCON (P.R.) MLBUL 00<strong>06</strong>5-<strong>06</strong>31-36 1.07140 30.71948 MAXITROL EYE OINTMENT 0 ALCON LABS. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00378-0464-01 0.16830 1.40394 MAXZIDE 37.5 MG-25 MG TABLET G MYLAN EABUL 00378-0460-01 0.04880 3.14860 MAXZIDE 75 MG-50 MG TABLET G MYLAN EABND 00093-9107-29 5.29540 MEBENDAZOLE 100 MG TAB CHEW 0 TEVA USA EAGEN 00378-5485-10 0.04621 MECLIZINE 12.5 MG TABLET 0 MYLAN EAGEN 00378-5485-77 0.04621 MECLIZINE 12.5 MG TABLET 0 MYLAN EAGEN 49884-0034-01 0.04621 MECLIZINE 12.5 MG TABLET 0 PAR PHARM. EAGEN 49884-0034-10 0.04621 MECLIZINE 12.5 MG TABLET 0 PAR PHARM. EAGEN 59746-0122-<strong>06</strong> 0.04621 MECLIZINE 12.5 MG TABLET 0 CADISTA PHARMAC EAGEN 59746-0122-10 0.04621 MECLIZINE 12.5 MG TABLET 0 CADISTA PHARMAC EAGEN 65162-0441-10 0.04621 MECLIZINE 12.5 MG TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-0441-11 0.04621 MECLIZINE 12.5 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0441-50 0.04621 MECLIZINE 12.5 MG TABLET 0 AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-0490-01 0.04621 MECLIZINE 12.5 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0490-11 0.04621 MECLIZINE 12.5 MG TABLET 0 AHP EAGEN 00378-5486-10 0.27050 MECLIZINE 25 MG TABLET 0 MYLAN EAGEN 00378-5486-77 0.27050 MECLIZINE 25 MG TABLET 0 MYLAN EAGEN 49884-0035-01 0.27050 MECLIZINE 25 MG TABLET 0 PAR PHARM. EAGEN 49884-0035-10 0.27050 MECLIZINE 25 MG TABLET 0 PAR PHARM. EAGEN 59746-0121-<strong>06</strong> 0.27050 MECLIZINE 25 MG TABLET 0 CADISTA PHARMAC EAGEN 59746-0121-10 0.27050 MECLIZINE 25 MG TABLET 0 CADISTA PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-0442-10 0.27050 MECLIZINE 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0442-11 0.27050 MECLIZINE 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0442-50 0.27050 MECLIZINE 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-0491-01 0.27050 MECLIZINE 25 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0491-11 0.27050 MECLIZINE 25 MG TABLET 0 AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 242LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00378-3000-01 4.45992 MECL<strong>OF</strong>ENAMATE 100 MG CAPSULE G MYLAN EABND 00378-2150-01 2.40251 MECL<strong>OF</strong>ENAMATE 50 MG CAPSULE G MYLAN EABND 00009-0073-01 2.39270 3.37345 MEDROL 16 MG TABLET G PHARMACIA/UPJHN EABND 00009-0020-01 0.82203 MEDROL 2 MG TABLET G PHARMACIA/UPJHN EABND 00009-0049-02 0.76824 MEDROL 2 MG TABLET G PHARMACIA/UPJHN EABND 00009-0176-01 3.55700 5.02316 MEDROL 32 MG TABLET G PHARMACIA/UPJHN EABUL 00009-0056-04 0.43040 1.55486 MEDROL 4 MG DOSEPAK G PHARMACIA/UPJHN EABUL 00009-0056-02 0.43040 1.55492 MEDROL 4 MG TABLET G PHARMACIA/UPJHN EABND 00009-0022-01 1.28670 2.18389 MEDROL 8 MG TABLET G PHARMACIA/UPJHN EAGEN 00555-0779-02 0.<strong>08</strong>168 MEDROXYPROGESTERONE 10 MG TAB 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00555-0779-04 0.<strong>08</strong>168 MEDROXYPROGESTERONE 10 MG TAB 0 TEVA USA EAGEN 59762-3742-02 0.<strong>08</strong>168 MEDROXYPROGESTERONE 10 MG TAB 0 GREENSTONE LLC. EAGEN 59762-3742-<strong>08</strong> 0.<strong>08</strong>168 MEDROXYPROGESTERONE 10 MG TAB 0 GREENSTONE LLC. EAGEX 00703-6801-01 26.10000 MEDROXYPROGESTERONE 150 MG/ML 0 TEVA PARENTERAL MLGEX 00703-6801-04 26.10000 MEDROXYPROGESTERONE 150 MG/ML 0 TEVA PARENTERAL MLGEX 59762-4537-01 40.77000 MEDROXYPROGESTERONE 150 MG/ML 0 GREENSTONE LLC. MLGEX 59762-4537-02 40.77000 MEDROXYPROGESTERONE 150 MG/ML 0 GREENSTONE LLC. MLGEX 59762-4538-02 51.53250 MEDROXYPROGESTERONE 150 MG/ML 0 GREENSTONE LLC. MLGEN 00555-<strong>08</strong>72-02 0.<strong>06</strong>183 MEDROXYPROGESTERONE 2.5 MG TAB 0 BARR EAGEN 00555-<strong>08</strong>72-04 0.<strong>06</strong>183 MEDROXYPROGESTERONE 2.5 MG TAB 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-3740-01 0.<strong>06</strong>183 MEDROXYPROGESTERONE 2.5 MG TAB 0 GREENSTONE LLC. EAGEN 59762-3740-05 0.<strong>06</strong>183 MEDROXYPROGESTERONE 2.5 MG TAB 0 GREENSTONE LLC. EAGEN 68<strong>08</strong>4-0553-11 0.<strong>06</strong>183 MEDROXYPROGESTERONE 2.5 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0553-21 0.<strong>06</strong>183 MEDROXYPROGESTERONE 2.5 MG TAB 0 AHP EAGEN 00555-<strong>08</strong>73-02 0.07776 MEDROXYPROGESTERONE 5 MG TAB 0 BARR EAGEN 00555-<strong>08</strong>73-04 0.07776 MEDROXYPROGESTERONE 5 MG TAB 0 BARR EAGEN 59762-3741-01 0.07776 MEDROXYPROGESTERONE 5 MG TAB 0 GREENSTONE LLC. EAGEN 59762-3741-04 0.07776 MEDROXYPROGESTERONE 5 MG TAB 0 GREENSTONE LLC. EAGEN 00574-0195-30 10.78300 MEFENAMIC ACID 250 MG CAPSULE G PADDOCK LABS. EAGEN 66993-0070-30 10.78300 MEFENAMIC ACID 250 MG CAPSULE G PRASCO LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0185-<strong>06</strong> 10.78300 MEFENAMIC ACID 250 MG CAPSULE G LUPIN PHARMACEU EAGEN 00054-0025-11 5.748<strong>08</strong> MEFLOQUINE HCL 250 MG TABLET 0 ROXANE LABS. EAGEN 00143-1282-22 5.748<strong>08</strong> MEFLOQUINE HCL 250 MG TABLET 0 WEST-WARD,INC. EAGEN 00555-0171-78 5.748<strong>08</strong> MEFLOQUINE HCL 250 MG TABLET 0 BARR EAGEN 00781-5076-86 5.748<strong>08</strong> MEFLOQUINE HCL 250 MG TABLET 0 SANDOZ EABND 49884-0949-69 5.02714 MEGACE ES 625 MG/5 ML SUSP G PAR PHARMACEUTI MLBND 00015-05<strong>08</strong>-42 0.09190 0.62436 MEGACE 40 MG/ML ORAL SUSP G BMS ONCO/IMMUN MLGEN 00054-3542-58 0.09190 MEGESTROL ACET 40 MG/ML SUSP 0 ROXANE LABS. MLGEN 49884-0907-38 0.09190 MEGESTROL ACET 40 MG/ML SUSP 0 PAR PHARM. MLGEN 49884-0907-61 0.09190 MEGESTROL ACET 40 MG/ML SUSP 0 PAR PHARM. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60432-0126-<strong>08</strong> 0.09190 MEGESTROL ACET 40 MG/ML SUSP 0 MORTON GROVE PH MLGEN 60505-0368-01 0.09190 MEGESTROL ACET 40 MG/ML SUSP 0 APOTEX CORP MLGEN 68094-0518-62 0.31312 MEGESTROL ACET 40 MG/ML SUSP 0 PRECISION DOSE MLGEN 00121-4776-35 0.32201 MEGESTROL ACET 400 MG/10 ML 0 PHARMACEU ASSOC MLGEN 66689-0020-01 0.31875 MEGESTROL ACET 400 MG/10 ML 0 VISTAPHARM ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 243LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 66689-0020-50 0.31860 MEGESTROL ACET 400 MG/10 ML 0 VISTAPHARM MLGEN 68094-0528-59 0.31725 MEGESTROL ACET 400 MG/10 ML 0 PRECISION DOSE MLGEN 00054-4603-25 0.14769 MEGESTROL 20 MG TABLET 0 ROXANE LABS. EAGEN 00555-<strong>06</strong><strong>06</strong>-02 0.14769 MEGESTROL 20 MG TABLET 0 BARR EAGEN 49884-0289-01 0.14769 MEGESTROL 20 MG TABLET 0 PAR PHARM. EAGEN 51079-0434-20 0.14769 MEGESTROL 20 MG TABLET 0 MYLAN INSTITUTI EAGEN 00054-4604-25 0.19629 MEGESTROL 40 MG TABLET 0 ROXANE LABS. EAGEN 00555-<strong>06</strong>07-02 0.19629 MEGESTROL 40 MG TABLET 0 BARR EAGEN 00555-<strong>06</strong>07-04 0.19629 MEGESTROL 40 MG TABLET 0 BARR EAGEN 49884-0290-01 0.19629 MEGESTROL 40 MG TABLET 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0290-04 0.19629 MEGESTROL 40 MG TABLET 0 PAR PHARM. EAGEN 49884-0290-05 0.19629 MEGESTROL 40 MG TABLET 0 PAR PHARM. EAGEN 51079-0435-20 0.19629 MEGESTROL 40 MG TABLET 0 MYLAN INSTITUTI EAGEN 63739-0165-10 0.19629 MEGESTROL 40 MG TABLET 0 MCKESSON PACKAG EABND 00173-<strong>08</strong>49-13 75.82050 MEKINIST 0.5 MG TABLET 0 GLAXOSMITHKLINE EABND 00173-<strong>08</strong>48-13 303.28200 MEKINIST 2 MG TABLET 0 GLAXOSMITHKLINE EAGEN 00093-7299-01 0.02565 MELOXICAM 15 MG TABLET 0 TEVA USA EAGEN 00378-1<strong>08</strong>9-01 0.02565 MELOXICAM 15 MG TABLET 0 MYLAN EAGEN 00378-1<strong>08</strong>9-05 0.02565 MELOXICAM 15 MG TABLET 0 MYLAN EAGEN 29300-0125-01 0.02565 MELOXICAM 15 MG TABLET 0 UNICHEM PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 29300-0125-10 0.02565 MELOXICAM 15 MG TABLET 0 UNICHEM PHARMAC EAGEN 51079-0459-01 0.02565 MELOXICAM 15 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0459-20 0.02565 MELOXICAM 15 MG TABLET 0 MYLAN INSTITUTI EAGEN 54458-0909-02 0.02565 MELOXICAM 15 MG TABLET 0 INTERNATIONAL L EAGEN 54458-0923-05 0.02565 MELOXICAM 15 MG TABLET 0 INTERNATIONAL L EAGEN 54458-0964-10 0.02565 MELOXICAM 15 MG TABLET 0 INTERNATIONAL L EAGEN 58517-0260-30 0.02565 MELOXICAM 15 MG TABLET 0 <strong>NEW</strong> HORIZON RX EAGEN 60505-2554-01 0.02565 MELOXICAM 15 MG TABLET 0 APOTEX CORP EAGEN 60505-2554-<strong>08</strong> 0.02565 MELOXICAM 15 MG TABLET 0 APOTEX CORP EAGEN 60505-3579-01 0.02565 MELOXICAM 15 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61442-0127-01 0.02565 MELOXICAM 15 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0127-10 0.02565 MELOXICAM 15 MG TABLET 0 CARLSBAD TECH EAGEN 65862-0098-01 0.02565 MELOXICAM 15 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0098-05 0.02565 MELOXICAM 15 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0502-01 0.02565 MELOXICAM 15 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0502-03 0.02565 MELOXICAM 15 MG TABLET 0 LUPIN PHARMACEU EAGEN 68382-0051-01 0.02565 MELOXICAM 15 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0051-05 0.02565 MELOXICAM 15 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0141-01 0.02565 MELOXICAM 15 MG TABLET 0 GLENMARK PHARMA EAGEN 00093-7234-01 0.02565 MELOXICAM 7.5 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-1<strong>06</strong>6-01 0.02565 MELOXICAM 7.5 MG TABLET 0 MYLAN EAGEN 00378-1<strong>06</strong>6-05 0.02565 MELOXICAM 7.5 MG TABLET 0 MYLAN EAGEN 29300-0124-01 0.02565 MELOXICAM 7.5 MG TABLET 0 UNICHEM PHARMAC EAGEN 29300-0124-10 0.02565 MELOXICAM 7.5 MG TABLET 0 UNICHEM PHARMAC EAGEN 51079-0457-01 0.02565 MELOXICAM 7.5 MG TABLET 0 MYLAN INSTITUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 244LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0457-20 0.02565 MELOXICAM 7.5 MG TABLET 0 MYLAN INSTITUTI EAGEN 54458-0965-10 0.02565 MELOXICAM 7.5 MG TABLET 0 INTERNATIONAL L EAGEN 60505-2553-01 0.02565 MELOXICAM 7.5 MG TABLET 0 APOTEX CORP EAGEN 60505-2553-<strong>08</strong> 0.02565 MELOXICAM 7.5 MG TABLET 0 APOTEX CORP EAGEN 60505-3578-01 0.02565 MELOXICAM 7.5 MG TABLET 0 APOTEX CORP EAGEN 61442-0126-01 0.02565 MELOXICAM 7.5 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0126-10 0.02565 MELOXICAM 7.5 MG TABLET 0 CARLSBAD TECH EAGEN 65862-0097-01 0.02565 MELOXICAM 7.5 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0097-05 0.02565 MELOXICAM 7.5 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0501-01 0.02565 MELOXICAM 7.5 MG TABLET 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0501-03 0.02565 MELOXICAM 7.5 MG TABLET 0 LUPIN PHARMACEU EAGEN 68382-0050-01 0.02565 MELOXICAM 7.5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0050-05 0.02565 MELOXICAM 7.5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0140-01 0.02565 MELOXICAM 7.5 MG TABLET 0 GLENMARK PHARMA EAGEN 00054-0228-49 0.86760 MELOXICAM 7.5 MG/5 ML SUSP 0 ROXANE LABS. MLBND 61570-0072-01 0.91972 MENEST 0.3 MG TABLET 0 MONARCH PHRM EABND 61570-0073-01 1.3<strong>06</strong>75 MENEST 0.625 MG TABLET 0 MONARCH PHRM EABND 61570-0074-01 1.82284 MENEST 1.25 MG TABLET 0 MONARCH PHRM EABND 61570-0075-50 3.39503 MENEST 2.5 MG TABLET 0 MONARCH PHRM EABND 50419-0455-04 25.20295 MENOSTAR 14 MCG/DAY PATCH 0 BAYER,PHARM DIV EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00378-6151-46 5.56598 MENTAX 1% CREAM G MYLAN GMBND 00378-6151-49 5.56625 MENTAX 1% CREAM G MYLAN GMBND 00187-1704-05 15.77572 MEPHYTON 5 MG TABLET 0 VALEANT EABND 25010-0405-15 10.23323 MEPHYTON 5 MG TABLET 0 VALEANT EABND 00173-<strong>06</strong>65-18 6.05915 MEPRON 750 MG/5 ML SUSPENSION 0 GLAXOSMITHKLINE MLGEN 38779-1427-04 77.3<strong>06</strong>40 MERCAPTOPURINE POWDER 0 MEDISCA INC. GMGEN 00054-4581-11 1.07450 MERCAPTOPURINE 50 MG TABLET 0 ROXANE LABS. EAGEN 00054-4581-27 1.07450 MERCAPTOPURINE 50 MG TABLET 0 ROXANE LABS. EAGEN 00093-5510-<strong>06</strong> 1.07450 MERCAPTOPURINE 50 MG TABLET 0 TEVA USA EAGEN 00378-3547-25 1.07450 MERCAPTOPURINE 50 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3547-52 1.07450 MERCAPTOPURINE 50 MG TABLET 0 MYLAN EAGEN 49884-0922-02 1.07450 MERCAPTOPURINE 50 MG TABLET 0 PAR PHARM. EAGEN 49884-0922-04 1.07450 MERCAPTOPURINE 50 MG TABLET 0 PAR PHARM. EAGEN 00409-35<strong>06</strong>-01 9.63900 MEROPENEM IV 1 GM VIAL 0 HOSPIRA EAGEN 00781-3267-90 12.51470 MEROPENEM IV 1 GM VIAL 0 SANDOZ EAGEN 00781-3267-95 12.51470 MEROPENEM IV 1 GM VIAL 0 SANDOZ EAGEN 63323-05<strong>08</strong>-30 12.51470 MEROPENEM IV 1 GM VIAL 0 APP PHARMACEUTI EAGEN 00409-3505-01 4.82400 MEROPENEM IV 500 MG VIAL 0 HOSPIRA EAGEN 00781-3265-80 5.25280 MEROPENEM IV 500 MG VIAL 0 SANDOZ EAGEN 00781-3265-95 5.25280 MEROPENEM IV 500 MG VIAL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0507-20 5.25280 MEROPENEM IV 500 MG VIAL 0 APP PHARMACEUTI EABND 00310-0321-30 12.51470 66.84488 MERREM IV 1 GM VIAL 0 ASTRAZENECA EABND 00310-0325-20 5.25280 33.42161 MERREM IV 500 MG VIAL 0 ASTRAZENECA EAGEN 51927-1078-00 2.98500 MESALAMINE POWDER 0 PR<strong>OF</strong>ESSIONAL CO GMGEN 43386-0510-87 0.21720 MESALAMINE 4 GM/60 ML ENEMA 0 GAVIS PHARMACEU ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 245LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0098-28 0.21720 MESALAMINE 4 GM/60 ML ENEMA 0 PERRIGO CO. MLGEN 45802-0098-51 0.21720 MESALAMINE 4 GM/60 ML ENEMA 0 PERRIGO CO. MLGEN 45802-0923-41 128.28750 MESALAMINE 4 GM/60 ML KIT 0 PERRIGO CO. EABND 671<strong>08</strong>-3565-09 78.27647 MESNEX 400 MG TABLET 0 BAXTER <strong>HEALTH</strong>CA EABND 00187-3013-30 6.28863 MESTINON 180 MG TIMESPAN 0 VALEANT EABND 00187-3010-30 0.28458 3.31560 MESTINON 60 MG TABLET G VALEANT EABND 00187-3012-20 0.56936 MESTINON 60 MG/5 ML SYRUP 0 VALEANT MLBND 54838-0507-80 0.02270 0.05597 METAPROTERENOL 10 MG/5 ML SYR 0 SILARX PHARM MLGEN 64720-0321-10 3.66825 METAXALONE 800 MG TABLET G COREPHARMA LLC EAGEN 65162-0553-10 3.66825 METAXALONE 800 MG TABLET G AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68001-0004-00 3.66825 METAXALONE 800 MG TABLET G BLUEPOINT LABOR EAGEN 68<strong>08</strong>4-0135-21 3.91280 METAXALONE 800 MG TABLET G AHP EAGEN 00591-2720-60 7.32850 METFORMIN HCL ER 1,000 MG TAB G ACTAVIS PHARMA, EAGEN 68180-0337-07 7.32850 METFORMIN HCL ER 1,000 MG TAB G LUPIN PHARMACEU EAGEN 00093-7267-01 0.04185 METFORMIN HCL ER 500 MG TABLET 0 TEVA USA EAGEN 00093-7267-10 0.04185 METFORMIN HCL ER 500 MG TABLET 0 TEVA USA EAGEN 00185-4416-01 0.04185 METFORMIN HCL ER 500 MG TABLET 0 SANDOZ EAGEN 00591-2719-60 4.21031 METFORMIN HCL ER 500 MG TABLET G ACTAVIS PHARMA, EAGEN 00904-6107-61 0.04185 METFORMIN HCL ER 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 29033-0018-01 0.04185 METFORMIN HCL ER 500 MG TABLET 0 NOSTRUM LABORAT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 29033-0018-05 0.04185 METFORMIN HCL ER 500 MG TABLET 0 NOSTRUM LABORAT EAGEN 29033-0018-10 0.04185 METFORMIN HCL ER 500 MG TABLET 0 NOSTRUM LABORAT EAGEN 53746-0178-01 0.04185 METFORMIN HCL ER 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0178-05 0.04185 METFORMIN HCL ER 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0178-10 0.04185 METFORMIN HCL ER 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0178-90 0.04185 METFORMIN HCL ER 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 60505-0260-01 0.04185 METFORMIN HCL ER 500 MG TABLET 0 APOTEX CORP EAGEN 60505-0260-02 0.04185 METFORMIN HCL ER 500 MG TABLET 0 APOTEX CORP EAGEN 60505-0260-07 0.04185 METFORMIN HCL ER 500 MG TABLET 0 APOTEX CORP EAGEN 62037-0571-01 0.04185 METFORMIN HCL ER 500 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62037-0571-10 0.04185 METFORMIN HCL ER 500 MG TABLET 0 ACTAVIS PHARMA, EAGEN 62756-0142-01 0.04185 METFORMIN HCL ER 500 MG TABLET 0 SUN PHARMACEUTI EAGEN 62756-0142-02 0.04185 METFORMIN HCL ER 500 MG TABLET 0 SUN PHARMACEUTI EAGEN 65862-0291-01 0.04185 METFORMIN HCL ER 500 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0291-05 0.04185 METFORMIN HCL ER 500 MG TABLET 0 AUROBINDO PHARM EAGEN 67877-0159-01 0.04185 METFORMIN HCL ER 500 MG TABLET 0 ASCEND LABORATO EAGEN 67877-0159-05 0.04185 METFORMIN HCL ER 500 MG TABLET 0 ASCEND LABORATO EAGEN 67877-0159-10 0.04185 METFORMIN HCL ER 500 MG TABLET 0 ASCEND LABORATO EAGEN 68<strong>08</strong>4-0072-01 0.04185 METFORMIN HCL ER 500 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0072-11 0.04185 METFORMIN HCL ER 500 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0336-07 4.21031 METFORMIN HCL ER 500 MG TABLET G LUPIN PHARMACEU EAGEN 00093-7212-01 0.20480 METFORMIN HCL ER 750 MG TABLET 0 TEVA USA EAGEN 29033-0021-01 0.20480 METFORMIN HCL ER 750 MG TABLET 0 NOSTRUM LABORAT EAGEN 53746-0179-01 0.20480 METFORMIN HCL ER 750 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0179-05 0.20480 METFORMIN HCL ER 750 MG TABLET 0 AMNEAL PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 246LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-1329-01 0.20480 METFORMIN HCL ER 750 MG TABLET 0 APOTEX CORP EAGEN 62037-0577-01 0.20480 METFORMIN HCL ER 750 MG TABLET 0 ACTAVIS PHARMA, EAGEN 62037-0577-10 0.20480 METFORMIN HCL ER 750 MG TABLET 0 ACTAVIS PHARMA, EAGEN 62756-0143-01 0.20480 METFORMIN HCL ER 750 MG TABLET 0 SUN PHARMACEUTI EAGEN 65862-0292-01 0.20480 METFORMIN HCL ER 750 MG TABLET 0 AUROBINDO PHARM EAGEN 00093-7214-01 0.04104 METFORMIN HCL 1,000 MG TABLET 0 TEVA USA EAGEN 00093-7214-10 0.04104 METFORMIN HCL 1,000 MG TABLET 0 TEVA USA EAGEN 00093-7214-98 0.04104 METFORMIN HCL 1,000 MG TABLET 0 TEVA USA EAGEN 00378-7187-05 0.04104 METFORMIN HCL 1,000 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-4469-21 0.04104 METFORMIN HCL 1,000 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-4469-28 0.04104 METFORMIN HCL 1,000 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4469-32 0.04104 METFORMIN HCL 1,000 MG TABLET 0 QUALITEST EAGEN 00781-5052-01 0.04104 METFORMIN HCL 1,000 MG TABLET 0 SANDOZ EAGEN 00781-5052-05 0.04104 METFORMIN HCL 1,000 MG TABLET 0 SANDOZ EAGEN 00781-5052-61 0.04104 METFORMIN HCL 1,000 MG TABLET 0 SANDOZ EAGEN 00904-5851-40 0.04104 METFORMIN HCL 1,000 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5851-52 0.04104 METFORMIN HCL 1,000 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5851-89 0.04104 METFORMIN HCL 1,000 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5851-93 0.04104 METFORMIN HCL 1,000 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6328-61 0.04104 METFORMIN HCL 1,000 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6345-40 0.04104 METFORMIN HCL 1,000 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6345-52 0.04104 METFORMIN HCL 1,000 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6345-89 0.04104 METFORMIN HCL 1,000 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6345-93 0.04104 METFORMIN HCL 1,000 MG TABLET 0 MAJOR PHARMACEU EAGEN 23155-0104-01 0.04104 METFORMIN HCL 1,000 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0104-05 0.04104 METFORMIN HCL 1,000 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0104-10 0.04104 METFORMIN HCL 1,000 MG TABLET 0 HERITAGE PHARMA EAGEN 24658-0292-05 0.04104 METFORMIN HCL 1,000 MG TABLET 0 BLU PHARMACEUTI EAGEN 24658-0292-60 0.04104 METFORMIN HCL 1,000 MG TABLET 0 BLU PHARMACEUTI EAGEN 24658-0292-90 0.04104 METFORMIN HCL 1,000 MG TABLET 0 BLU PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43547-0250-10 0.04104 METFORMIN HCL 1,000 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0250-11 0.04104 METFORMIN HCL 1,000 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0250-50 0.04104 METFORMIN HCL 1,000 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0322-50 0.04104 METFORMIN HCL 1,000 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 51079-0174-20 0.04104 METFORMIN HCL 1,000 MG TABLET 0 MYLAN INSTITUTI EAGEN 53746-0220-01 0.04104 METFORMIN HCL 1,000 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0220-05 0.04104 METFORMIN HCL 1,000 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0220-10 0.04104 METFORMIN HCL 1,000 MG TABLET 0 AMNEAL PHARMACE EAGEN 57664-0474-51 0.04104 METFORMIN HCL 1,000 MG TABLET 0 CARACO PHARM EAGEN 57664-0474-53 0.04104 METFORMIN HCL 1,000 MG TABLET 0 CARACO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 57664-0474-58 0.04104 METFORMIN HCL 1,000 MG TABLET 0 CARACO PHARM EAGEN 57664-0474-88 0.04104 METFORMIN HCL 1,000 MG TABLET 0 CARACO PHARM EAGEN 59762-4322-00 0.04104 METFORMIN HCL 1,000 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-4322-02 0.04104 METFORMIN HCL 1,000 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-0192-00 0.04104 METFORMIN HCL 1,000 MG TABLET 0 APOTEX CORP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 247LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0192-01 0.04104 METFORMIN HCL 1,000 MG TABLET 0 APOTEX CORP EAGEN 60505-0192-<strong>08</strong> 0.04104 METFORMIN HCL 1,000 MG TABLET 0 APOTEX CORP EAGEN 61442-0363-01 0.04104 METFORMIN HCL 1,000 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0363-05 0.04104 METFORMIN HCL 1,000 MG TABLET 0 CARLSBAD TECH EAGEN 65162-0177-50 0.04104 METFORMIN HCL 1,000 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0220-10 0.04104 METFORMIN HCL 1,000 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0220-11 0.04104 METFORMIN HCL 1,000 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0220-50 0.04104 METFORMIN HCL 1,000 MG TABLET 0 AMNEAL PHARMACE EAGEN 65862-0010-01 0.04104 METFORMIN HCL 1,000 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0010-05 0.04104 METFORMIN HCL 1,000 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0010-46 0.04104 METFORMIN HCL 1,000 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0010-99 0.04104 METFORMIN HCL 1,000 MG TABLET 0 AUROBINDO PHARM EAGEN 67877-0221-01 0.04104 METFORMIN HCL 1,000 MG TABLET 0 ASCEND LABORATO EAGEN 68382-0030-01 0.04104 METFORMIN HCL 1,000 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0030-05 0.04104 METFORMIN HCL 1,000 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0030-10 0.04104 METFORMIN HCL 1,000 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0161-01 0.04104 METFORMIN HCL 1,000 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0161-05 0.04104 METFORMIN HCL 1,000 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0161-10 0.04104 METFORMIN HCL 1,000 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0161-18 0.04104 METFORMIN HCL 1,000 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0161-90 0.04104 METFORMIN HCL 1,000 MG TABLET 0 GLENMARK PHARMA EAGEN 68645-0300-59 0.04104 METFORMIN HCL 1,000 MG TABLET 0 LEGACY PHARMACE EAGEN 00093-1048-01 0.02633 METFORMIN HCL 500 MG TABLET 0 TEVA USA EAGEN 00093-1048-10 0.02633 METFORMIN HCL 500 MG TABLET 0 TEVA USA EAGEN 00093-1048-98 0.02633 METFORMIN HCL 500 MG TABLET 0 TEVA USA EAGEN 00378-7185-05 0.02633 METFORMIN HCL 500 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-4467-21 0.02633 METFORMIN HCL 500 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4467-28 0.02633 METFORMIN HCL 500 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4467-32 0.02633 METFORMIN HCL 500 MG TABLET 0 QUALITEST EAGEN 00781-5050-01 0.02633 METFORMIN HCL 500 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-5050-05 0.02633 METFORMIN HCL 500 MG TABLET 0 SANDOZ EAGEN 00781-5050-10 0.02633 METFORMIN HCL 500 MG TABLET 0 SANDOZ EAGEN 00781-5050-61 0.02633 METFORMIN HCL 500 MG TABLET 0 SANDOZ EAGEN 00904-5849-14 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5849-18 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5849-40 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5849-53 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5849-54 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5849-80 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5849-89 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5849-93 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6326-61 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6343-14 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6343-18 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6343-40 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 248LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6343-52 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6343-53 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6343-54 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6343-80 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6343-89 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6343-93 0.02633 METFORMIN HCL 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 23155-0102-01 0.02633 METFORMIN HCL 500 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0102-05 0.02633 METFORMIN HCL 500 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0102-10 0.02633 METFORMIN HCL 500 MG TABLET 0 HERITAGE PHARMA EAGEN 24658-0290-05 0.02633 METFORMIN HCL 500 MG TABLET 0 BLU PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24658-0290-12 0.02633 METFORMIN HCL 500 MG TABLET 0 BLU PHARMACEUTI EAGEN 24658-0290-18 0.02633 METFORMIN HCL 500 MG TABLET 0 BLU PHARMACEUTI EAGEN 24658-0290-27 0.02633 METFORMIN HCL 500 MG TABLET 0 BLU PHARMACEUTI EAGEN 24658-0290-36 0.02633 METFORMIN HCL 500 MG TABLET 0 BLU PHARMACEUTI EAGEN 24658-0290-46 0.02633 METFORMIN HCL 500 MG TABLET 0 BLU PHARMACEUTI EAGEN 24658-0290-60 0.02633 METFORMIN HCL 500 MG TABLET 0 BLU PHARMACEUTI EAGEN 24658-0290-90 0.02633 METFORMIN HCL 500 MG TABLET 0 BLU PHARMACEUTI EAGEN 43547-0248-10 0.02633 METFORMIN HCL 500 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0248-11 0.02633 METFORMIN HCL 500 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0248-50 0.02633 METFORMIN HCL 500 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43547-0320-11 0.02633 METFORMIN HCL 500 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 51079-0172-01 0.02633 METFORMIN HCL 500 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0172-20 0.02633 METFORMIN HCL 500 MG TABLET 0 MYLAN INSTITUTI EAGEN 53746-0218-01 0.02633 METFORMIN HCL 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0218-05 0.02633 METFORMIN HCL 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0218-10 0.02633 METFORMIN HCL 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 57664-0397-51 0.02633 METFORMIN HCL 500 MG TABLET 0 CARACO PHARM EAGEN 57664-0397-53 0.02633 METFORMIN HCL 500 MG TABLET 0 CARACO PHARM EAGEN 57664-0397-58 0.02633 METFORMIN HCL 500 MG TABLET 0 CARACO PHARM EAGEN 58517-0040-90 0.02633 METFORMIN HCL 500 MG TABLET 0 <strong>NEW</strong> HORIZON RX EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-4320-00 0.02633 METFORMIN HCL 500 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-4320-02 0.02633 METFORMIN HCL 500 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-0190-00 0.02633 METFORMIN HCL 500 MG TABLET 0 APOTEX CORP EAGEN 60505-0190-01 0.02633 METFORMIN HCL 500 MG TABLET 0 APOTEX CORP EAGEN 60505-0190-<strong>08</strong> 0.02633 METFORMIN HCL 500 MG TABLET 0 APOTEX CORP EAGEN 61442-0361-01 0.02633 METFORMIN HCL 500 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0361-05 0.02633 METFORMIN HCL 500 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0361-10 0.02633 METFORMIN HCL 500 MG TABLET 0 CARLSBAD TECH EAGEN 62584-0259-11 0.02633 METFORMIN HCL 500 MG TABLET 0 AHP EAGEN 65162-0175-50 0.02633 METFORMIN HCL 500 MG TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-0218-10 0.02633 METFORMIN HCL 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0218-11 0.02633 METFORMIN HCL 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0218-50 0.02633 METFORMIN HCL 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 65862-00<strong>08</strong>-01 0.02633 METFORMIN HCL 500 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-00<strong>08</strong>-05 0.02633 METFORMIN HCL 500 MG TABLET 0 AUROBINDO PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 249LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-00<strong>08</strong>-99 0.02633 METFORMIN HCL 500 MG TABLET 0 AUROBINDO PHARM EAGEN 67877-0217-01 0.02633 METFORMIN HCL 500 MG TABLET 0 ASCEND LABORATO EAGEN 67877-0217-05 0.02633 METFORMIN HCL 500 MG TABLET 0 ASCEND LABORATO EAGEN 68382-0028-01 0.02633 METFORMIN HCL 500 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0028-05 0.02633 METFORMIN HCL 500 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0028-10 0.02633 METFORMIN HCL 500 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0159-01 0.02633 METFORMIN HCL 500 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0159-05 0.02633 METFORMIN HCL 500 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0159-10 0.02633 METFORMIN HCL 500 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0159-18 0.02633 METFORMIN HCL 500 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0159-90 0.02633 METFORMIN HCL 500 MG TABLET 0 GLENMARK PHARMA EAGEN 68645-0290-59 0.02633 METFORMIN HCL 500 MG TABLET 0 LEGACY PHARMACE EAGEN 00093-1049-01 0.04077 METFORMIN HCL 850 MG TABLET 0 TEVA USA EAGEN 00093-1049-10 0.04077 METFORMIN HCL 850 MG TABLET 0 TEVA USA EAGEN 00093-1049-98 0.04077 METFORMIN HCL 850 MG TABLET 0 TEVA USA EAGEN 00378-7186-05 0.04077 METFORMIN HCL 850 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-4468-21 0.04077 METFORMIN HCL 850 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4468-28 0.04077 METFORMIN HCL 850 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4468-32 0.04077 METFORMIN HCL 850 MG TABLET 0 QUALITEST EAGEN 00781-5051-01 0.04077 METFORMIN HCL 850 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-5051-05 0.04077 METFORMIN HCL 850 MG TABLET 0 SANDOZ EAGEN 00781-5051-61 0.04077 METFORMIN HCL 850 MG TABLET 0 SANDOZ EAGEN 00904-5850-40 0.04077 METFORMIN HCL 850 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5850-53 0.04077 METFORMIN HCL 850 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5850-89 0.04077 METFORMIN HCL 850 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5850-93 0.04077 METFORMIN HCL 850 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6091-61 0.04077 METFORMIN HCL 850 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6327-61 0.04077 METFORMIN HCL 850 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6344-40 0.04077 METFORMIN HCL 850 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6344-52 0.04077 METFORMIN HCL 850 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6344-53 0.04077 METFORMIN HCL 850 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6344-89 0.04077 METFORMIN HCL 850 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6344-93 0.04077 METFORMIN HCL 850 MG TABLET 0 MAJOR PHARMACEU EAGEN 23155-0103-01 0.04077 METFORMIN HCL 850 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0103-05 0.04077 METFORMIN HCL 850 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0103-10 0.04077 METFORMIN HCL 850 MG TABLET 0 HERITAGE PHARMA EAGEN 43547-0249-10 0.04077 METFORMIN HCL 850 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0249-11 0.04077 METFORMIN HCL 850 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0249-50 0.04077 METFORMIN HCL 850 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0321-50 0.04077 METFORMIN HCL 850 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53746-0219-01 0.04077 METFORMIN HCL 850 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0219-05 0.04077 METFORMIN HCL 850 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0219-10 0.04077 METFORMIN HCL 850 MG TABLET 0 AMNEAL PHARMACE EAGEN 57664-0435-51 0.04077 METFORMIN HCL 850 MG TABLET 0 CARACO PHARM EAGEN 57664-0435-53 0.04077 METFORMIN HCL 850 MG TABLET 0 CARACO PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 250LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 57664-0435-58 0.04077 METFORMIN HCL 850 MG TABLET 0 CARACO PHARM EAGEN 59762-4321-00 0.04077 METFORMIN HCL 850 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-4321-02 0.04077 METFORMIN HCL 850 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-0191-00 0.04077 METFORMIN HCL 850 MG TABLET 0 APOTEX CORP EAGEN 60505-0191-01 0.04077 METFORMIN HCL 850 MG TABLET 0 APOTEX CORP EAGEN 60505-0191-<strong>08</strong> 0.04077 METFORMIN HCL 850 MG TABLET 0 APOTEX CORP EAGEN 61442-0362-01 0.04077 METFORMIN HCL 850 MG TABLET 0 CARLSBAD TECH EAGEN 61442-0362-05 0.04077 METFORMIN HCL 850 MG TABLET 0 CARLSBAD TECH EAGEN 62584-0332-01 0.04077 METFORMIN HCL 850 MG TABLET 0 AHP EAGEN 62584-0332-11 0.04077 METFORMIN HCL 850 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-0174-50 0.04077 METFORMIN HCL 850 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0219-10 0.04077 METFORMIN HCL 850 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0219-11 0.04077 METFORMIN HCL 850 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0219-50 0.04077 METFORMIN HCL 850 MG TABLET 0 AMNEAL PHARMACE EAGEN 65862-0009-01 0.04077 METFORMIN HCL 850 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0009-05 0.04077 METFORMIN HCL 850 MG TABLET 0 AUROBINDO PHARM EAGEN 67877-0218-01 0.04077 METFORMIN HCL 850 MG TABLET 0 ASCEND LABORATO EAGEN 68382-0029-01 0.04077 METFORMIN HCL 850 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0029-05 0.04077 METFORMIN HCL 850 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0029-10 0.04077 METFORMIN HCL 850 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0160-01 0.04077 METFORMIN HCL 850 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0160-05 0.04077 METFORMIN HCL 850 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0160-10 0.04077 METFORMIN HCL 850 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0160-18 0.04077 METFORMIN HCL 850 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0160-90 0.04077 METFORMIN HCL 850 MG TABLET 0 GLENMARK PHARMA EAGUL 00781-1072-01 0.31500 METHAZOLAMIDE 25 MG TABLET 0 SANDOZ EAGUL 48102-0100-01 0.31500 METHAZOLAMIDE 25 MG TABLET 0 FERA PHARMACEUT EAGUL 00781-1071-01 0.46500 METHAZOLAMIDE 50 MG TABLET 0 SANDOZ EAGUL 48102-0101-01 0.46500 METHAZOLAMIDE 50 MG TABLET 0 FERA PHARMACEUT EAGEN 00955-1037-10 1.60185 METHENAMINE HIPP 1 GM TABLET 0 WINTHROP US EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43199-0020-01 1.56825 METHENAMINE HIPP 1 GM TABLET 0 COUNTY LINE PHA EAGEN 64720-0139-10 1.56870 METHENAMINE HIPP 1 GM TABLET 0 COREPHARMA LLC EAGUL 42799-01<strong>06</strong>-01 0.29230 METHENAMINE MD 1 GM TABLET 0 EDENBRIDGE PHAR EABND 42799-0105-01 0.73562 METHENAMINE MD 500 MG TABLET 0 EDENBRIDGE PHAR EABND 00078-0053-03 4.35096 7.83686 METHERGINE 0.2 MG/ML AMPUL 0 NOVARTIS MLGEN 00185-0210-01 0.30250 METHIMAZOLE 10 MG TABLET 0 SANDOZ EAGEN 00185-0210-10 0.30250 METHIMAZOLE 10 MG TABLET 0 SANDOZ EAGEN 23155-0071-01 0.30250 METHIMAZOLE 10 MG TABLET 0 HERITAGE PHARMA EAGEN 49884-<strong>06</strong>41-01 0.30250 METHIMAZOLE 10 MG TABLET 0 PAR PHARM. EAGEN 64376-<strong>06</strong>56-01 0.30250 METHIMAZOLE 10 MG TABLET 0 BOCA PHARMACAL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0276-01 0.30250 METHIMAZOLE 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0276-11 0.30250 METHIMAZOLE 10 MG TABLET 0 AHP EAGEN 00185-0205-01 0.15800 METHIMAZOLE 5 MG TABLET 0 SANDOZ EAGEN 00185-0205-10 0.15800 METHIMAZOLE 5 MG TABLET 0 SANDOZ EAGEN 23155-0070-01 0.15800 METHIMAZOLE 5 MG TABLET 0 HERITAGE PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 251LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-<strong>06</strong>40-01 0.15800 METHIMAZOLE 5 MG TABLET 0 PAR PHARM. EAGEN 49884-<strong>06</strong>40-05 0.15800 METHIMAZOLE 5 MG TABLET 0 PAR PHARM. EAGEN 64376-<strong>06</strong>55-01 0.15800 METHIMAZOLE 5 MG TABLET 0 BOCA PHARMACAL EAGEN 68<strong>08</strong>4-0275-11 0.15800 METHIMAZOLE 5 MG TABLET 0 AHP EAGEN 00143-1290-01 0.07920 METHOCARBAMOL 500 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1290-05 0.<strong>06</strong>615 METHOCARBAMOL 500 MG TABLET 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>03-4485-21 0.07920 METHOCARBAMOL 500 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4485-28 0.07920 METHOCARBAMOL 500 MG TABLET 0 QUALITEST EAGEN 31722-0533-01 0.07920 METHOCARBAMOL 500 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0533-05 0.07920 METHOCARBAMOL 500 MG TABLET 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43547-0225-10 0.07920 METHOCARBAMOL 500 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0225-50 0.07920 METHOCARBAMOL 500 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 63739-0166-10 0.07920 METHOCARBAMOL 500 MG TABLET 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0056-01 0.07920 METHOCARBAMOL 500 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0056-11 0.07920 METHOCARBAMOL 500 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0585-01 0.07920 METHOCARBAMOL 500 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0585-11 0.07920 METHOCARBAMOL 500 MG TABLET 0 AHP EAGEN 76439-0134-10 0.07920 METHOCARBAMOL 500 MG TABLET 0 VIRTUS PHARMACE EAGEN 76439-0134-50 0.07920 METHOCARBAMOL 500 MG TABLET 0 VIRTUS PHARMACE EAGEN 00143-1292-01 0.10755 METHOCARBAMOL 750 MG TABLET 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-1292-05 0.<strong>08</strong>811 METHOCARBAMOL 750 MG TABLET 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>03-4486-21 0.12530 METHOCARBAMOL 750 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4486-28 0.12530 METHOCARBAMOL 750 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-4486-32 0.12530 METHOCARBAMOL 750 MG TABLET 0 QUALITEST EAGEN 31722-0534-01 0.12530 METHOCARBAMOL 750 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0534-05 0.12530 METHOCARBAMOL 750 MG TABLET 0 CAMBER PHARMACE EAGEN 43547-0226-10 0.12530 METHOCARBAMOL 750 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0226-50 0.12530 METHOCARBAMOL 750 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 63739-0167-10 0.12530 METHOCARBAMOL 750 MG TABLET 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0057-01 0.12530 METHOCARBAMOL 750 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0057-11 0.12530 METHOCARBAMOL 750 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0586-01 0.12530 METHOCARBAMOL 750 MG TABLET 0 AHP EAGEN 76439-0135-10 0.12530 METHOCARBAMOL 750 MG TABLET 0 VIRTUS PHARMACE EAGEN 76439-0135-50 0.12530 METHOCARBAMOL 750 MG TABLET 0 VIRTUS PHARMACE EAGEN 63323-0122-50 57.24000 METHOTREXATE 1 GM VIAL 0 APP PHARMACEUTI EAGEN 67457-0221-40 0.77175 METHOTREXATE 1 GM/40 ML VIAL 0 MYLAN INSTITUTI MLGEN 00703-3678-01 0.74812 METHOTREXATE 1 GRAM/40 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-3673-01 1.<strong>06</strong>875 METHOTREXATE 100 MG/4 ML VIAL 0 TEVA PARENTERAL MLGUL 00054-4550-15 1.26370 METHOTREXATE 2.5 MG TABLET 0 ROXANE LABS. EAGUL 00054-4550-25 1.26370 METHOTREXATE 2.5 MG TABLET 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-0014-01 1.26370 METHOTREXATE 2.5 MG TABLET 0 MYLAN EAGUL 00555-0572-02 1.26370 METHOTREXATE 2.5 MG TABLET 0 BARR EAGUL 00555-0572-35 1.26370 METHOTREXATE 2.5 MG TABLET 0 BARR EAGUL 51079-<strong>06</strong>70-05 1.26370 METHOTREXATE 2.5 MG TABLET 0 MYLAN INSTITUTI EAGUL 67253-0320-10 1.26370 METHOTREXATE 2.5 MG TABLET 0 DAVA PHARMACEUT EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 252LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 67253-0320-36 1.26370 METHOTREXATE 2.5 MG TABLET 0 DAVA PHARMACEUT EAGEN 00<strong>06</strong>9-0204-01 0.95625 METHOTREXATE 200 MG/8 ML VIAL 0 PFIZER/NOVAPLUS MLGEN 00<strong>06</strong>9-0204-10 0.95625 METHOTREXATE 200 MG/8 ML VIAL 0 PFIZER/NOVAPLUS MLGEN 55390-0031-10 1.80000 METHOTREXATE 25 MG/ML VIAL 0 BEDFORD LABS MLGEN 55390-0032-10 1.12500 METHOTREXATE 25 MG/ML VIAL 0 BEDFORD LABS MLGEN 55390-0033-10 0.88312 METHOTREXATE 25 MG/ML VIAL 0 BEDFORD LABS MLGEN 55390-0034-10 0.85500 METHOTREXATE 25 MG/ML VIAL 0 BEDFORD LABS MLGEN 61703-0350-38 1.61972 METHOTREXATE 25 MG/ML VIAL 0 HOSPIRA MLGEN 63323-0121-02 1.80000 METHOTREXATE 25 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0121-<strong>08</strong> 0.95625 METHOTREXATE 25 MG/ML VIAL 0 APP PHARMACEUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0121-10 0.85500 METHOTREXATE 25 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0121-40 0.78750 METHOTREXATE 25 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0123-02 1.61972 METHOTREXATE 25 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0123-10 1.61972 METHOTREXATE 25 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 00703-3675-01 0.81300 METHOTREXATE 250 MG/10 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-3675-91 0.81300 METHOTREXATE 250 MG/10 ML VIAL 0 TEVA PARENTERAL MLGEN 10139-0<strong>06</strong>2-10 0.83775 METHOTREXATE 250 MG/10 ML VIAL 0 MYLAN INSTITUTI MLGEN 67457-0221-10 0.83775 METHOTREXATE 250 MG/10 ML VIAL 0 MYLAN INSTITUTI MLGEN 00703-3671-01 1.71000 METHOTREXATE 50 MG/2 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-3671-03 1.71000 METHOTREXATE 50 MG/2 ML VIAL 0 TEVA PARENTERAL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00703-3671-91 1.71000 METHOTREXATE 50 MG/2 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-3671-93 1.71000 METHOTREXATE 50 MG/2 ML VIAL 0 TEVA PARENTERAL MLGEN 00168-0482-99 1.63200 METHSCOPOLAMINE BROM 2.5 MG TB 0 SANDOZ EAGEN 51991-0191-01 1.63200 METHSCOPOLAMINE BROM 2.5 MG TB 0 BRECKENRIDGE EAGEN 64376-<strong>06</strong>03-01 1.63200 METHSCOPOLAMINE BROM 2.5 MG TB 0 BOCA PHARMACAL EAGEN 00168-0483-60 2.<strong>06</strong>450 METHSCOPOLAMINE BROM 5 MG TAB 0 SANDOZ EAGEN 51991-0192-<strong>06</strong> 2.<strong>06</strong>450 METHSCOPOLAMINE BROM 5 MG TAB 0 BRECKENRIDGE EAGEN 64376-<strong>06</strong>04-61 2.<strong>06</strong>450 METHSCOPOLAMINE BROM 5 MG TAB 0 BOCA PHARMACAL EABND 00378-0160-01 1.52910 METHYCLOTHIAZIDE 5 MG TABLET 0 MYLAN EAGEN 00093-2931-01 0.10193 METHYLDOPA 250 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-2931-10 0.10193 METHYLDOPA 250 MG TABLET 0 TEVA USA EAGEN 00378-<strong>06</strong>11-01 0.10193 METHYLDOPA 250 MG TABLET 0 MYLAN EAGEN 00378-<strong>06</strong>11-10 0.10193 METHYLDOPA 250 MG TABLET 0 MYLAN EAGEN 16729-0030-01 0.10193 METHYLDOPA 250 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 51079-0200-20 0.10193 METHYLDOPA 250 MG TABLET 0 MYLAN INSTITUTI EAGEN 00093-2932-01 0.17780 METHYLDOPA 500 MG TABLET 0 TEVA USA EAGEN 00093-2932-05 0.17780 METHYLDOPA 500 MG TABLET 0 TEVA USA EAGEN 00378-0421-01 0.17780 METHYLDOPA 500 MG TABLET 0 MYLAN EAGEN 00378-0421-05 0.17780 METHYLDOPA 500 MG TABLET 0 MYLAN EAGEN 16729-0031-01 0.17780 METHYLDOPA 500 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16729-0031-16 0.17780 METHYLDOPA 500 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 51079-0201-20 0.17780 METHYLDOPA 500 MG TABLET 0 MYLAN INSTITUTI EABND 00378-0507-01 0.84435 METHYLDOPA-HCTZ 250-15 MG TAB 0 MYLAN EABND 00378-0711-01 0.17600 0.99500 METHYLDOPA-HCTZ 250-25 MG TAB 0 MYLAN EABND 00517-8905-10 7.96800 METHYLDOPATE 250 MG/5 ML VIAL 0 AMER. REGENT ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 253LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43386-0140-07 20.95714 METHYLERGONOVINE 0.2 MG TABLET 0 GAVIS PHARMACEU EAGEN 43386-0140-28 20.32500 METHYLERGONOVINE 0.2 MG TABLET 0 GAVIS PHARMACEU EAGEN 63704-00<strong>06</strong>-01 13.59750 METHYLERGONOVINE 0.2 MG TABLET 0 PHARMACIST PHAR EAGEN 68<strong>08</strong>4-0554-11 1.7<strong>08</strong>25 METHYLERGONOVINE 0.2 MG TABLET 0 AHP EAGEN 17478-0501-01 4.35096 METHYLERGONOVINE 0.2 MG/ML AMP 0 AKORN INC. MLGEN 63704-0004-01 4.35096 METHYLERGONOVINE 0.2 MG/ML AMP 0 PHARMACIST PHAR MLGEN 63323-0265-30 17.45800 METHYLPREDNISOLONE SS 1 GM VL 0 APP PHARMACEUTI EAGEN 59746-0003-14 2.32935 METHYLPREDNISOLONE 16 MG TAB G CADISTA PHARMAC EAGEN 59762-0050-01 2.32935 METHYLPREDNISOLONE 16 MG TAB G GREENSTONE LLC. EAGEN 59746-0015-04 3.46860 METHYLPREDNISOLONE 32 MG TAB 0 CADISTA PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 0<strong>06</strong>03-4593-15 0.43040 METHYLPREDNISOLONE 4 MG DOSEPK 0 QUALITEST EAGUL 00781-5022-07 0.43040 METHYLPREDNISOLONE 4 MG DOSEPK 0 SANDOZ EAGUL 51991-0188-31 0.43040 METHYLPREDNISOLONE 4 MG DOSEPK 0 BRECKENRIDGE EAGUL 59746-0001-03 0.43040 METHYLPREDNISOLONE 4 MG DOSEPK 0 CADISTA PHARMAC EAGUL 59762-4440-02 0.43040 METHYLPREDNISOLONE 4 MG DOSEPK 0 GREENSTONE LLC. EAGUL 68001-0005-01 0.43040 METHYLPREDNISOLONE 4 MG DOSEPK 0 BLUEPOINT LABOR EAGUL 0<strong>06</strong>03-4593-21 0.43040 METHYLPREDNISOLONE 4 MG TABLET 0 QUALITEST EAGUL 00781-5022-01 0.43040 METHYLPREDNISOLONE 4 MG TABLET 0 SANDOZ EAGUL 59746-0001-<strong>06</strong> 0.43040 METHYLPREDNISOLONE 4 MG TABLET 0 CADISTA PHARMAC EAGUL 68<strong>08</strong>4-0149-01 0.43040 METHYLPREDNISOLONE 4 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 68<strong>08</strong>4-0149-11 0.43040 METHYLPREDNISOLONE 4 MG TABLET 0 AHP EABND 63323-0255-03 3.02360 4.66128 METHYLPREDNISOLONE 40 MG VIAL 0 APP PHARMACEUTI EAGEN 00703-0031-01 3.65050 METHYLPREDNISOLONE 40 MG/ML VL 0 TEVA PARENTERAL MLGEN 00703-0031-04 3.65050 METHYLPREDNISOLONE 40 MG/ML VL 0 TEVA PARENTERAL MLGEN 00703-0043-01 3.24000 METHYLPREDNISOLONE 40 MG/ML VL 0 TEVA PARENTERAL MLGEN 00703-0045-01 3.24000 METHYLPREDNISOLONE 40 MG/ML VL 0 TEVA PARENTERAL MLGEN 59762-0049-01 1.28670 METHYLPREDNISOLONE 8 MG TAB 0 GREENSTONE LLC. EAGEN 00703-0051-01 6.84000 METHYLPREDNISOLONE 80 MG/ML VL 0 TEVA PARENTERAL MLGEN 00703-0051-04 6.84000 METHYLPREDNISOLONE 80 MG/ML VL 0 TEVA PARENTERAL MLGEN 00703-0<strong>06</strong>3-01 6.48000 METHYLPREDNISOLONE 80 MG/ML VL 0 TEVA PARENTERAL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3132-71 6.94784 METHYLPREDNISOLONE 80 MG/ML VL 0 SANDOZ MLGEN 00781-3132-95 6.94784 METHYLPREDNISOLONE 80 MG/ML VL 0 SANDOZ MLBND 61314-0447-05 2.76410 4.92522 METIPRANOLOL 0.3% EYE DROPS 0 SANDOZ MLBND 61314-0447-10 2.76410 4.16411 METIPRANOLOL 0.3% EYE DROPS 0 SANDOZ MLGEN 00093-2203-01 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 TEVA USA EAGEN 00093-2203-05 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 TEVA USA EAGEN 00093-2203-10 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 TEVA USA EAGEN 00228-2269-10 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00228-2269-50 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-2468-01 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-2468-05 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-2468-10 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 16714-0<strong>06</strong>2-04 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0<strong>06</strong>2-05 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0<strong>06</strong>2-<strong>06</strong> 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 254LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0<strong>06</strong>2-10 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0<strong>06</strong>2-11 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0<strong>06</strong>2-12 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 49884-<strong>06</strong>85-01 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 PAR PHARM. EAGEN 49884-<strong>06</strong>85-05 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 PAR PHARM. EAGEN 51079-0283-20 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 63304-<strong>08</strong>46-01 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>08</strong>46-05 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>08</strong>46-10 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 RANBAXY PHARMAC EAGEN 63739-0482-10 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 MCKESSON PACKAG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0009-01 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0009-11 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0091-01 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0091-11 0.04620 METOCLOPRAMIDE 10 MG TABLET 0 AHP EAGEN 00409-3414-01 0.49950 METOCLOPRAMIDE 10 MG/2 ML VIAL 0 HOSPIRA MLGEN 00093-2204-01 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 TEVA USA EAGEN 00093-2204-05 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 TEVA USA EAGEN 00591-2467-01 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-2467-05 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 16714-0<strong>06</strong>1-04 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0<strong>06</strong>1-05 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0<strong>06</strong>1-10 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0<strong>06</strong>1-11 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 NORTHSTAR RX LL EAGEN 51079-<strong>06</strong>29-20 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 63304-<strong>08</strong>45-01 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 RANBAXY PHARMAC EAGEN 63304-<strong>08</strong>45-05 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 RANBAXY PHARMAC EAGEN 63739-0481-10 0.04280 METOCLOPRAMIDE 5 MG TABLET 0 MCKESSON PACKAG EAGEN 00121-0576-16 0.01360 METOCLOPRAMIDE 5 MG/5 ML SOLN 0 PHARMACEU ASSOC MLGEN 62559-0110-16 0.01360 METOCLOPRAMIDE 5 MG/5 ML SOLN 0 ANI PHARMACEUTI MLGEN 60432-<strong>06</strong>22-16 0.01360 METOCLOPRAMIDE 5 MG/5 ML SYRUP 0 MORTON GROVE PH ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62559-11<strong>06</strong>-<strong>06</strong> 0.01360 METOCLOPRAMIDE 5 MG/5 ML SYRUP 0 ANI PHARMACEUTI MLGEN 00185-5600-01 1.09625 METOLAZONE 10 MG TABLET 0 SANDOZ EAGEN 00378-6174-01 1.09625 METOLAZONE 10 MG TABLET 0 MYLAN EAGEN 65580-<strong>06</strong>45-71 1.09625 METOLAZONE 10 MG TABLET 0 UCB PHARMA EAGEN 00185-5050-01 0.80444 METOLAZONE 2.5 MG TABLET 0 SANDOZ EAGEN 00378-6172-01 0.80444 METOLAZONE 2.5 MG TABLET 0 MYLAN EAGEN 51079-0023-01 0.80444 METOLAZONE 2.5 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0023-20 0.80444 METOLAZONE 2.5 MG TABLET 0 MYLAN INSTITUTI EAGEN 65580-<strong>06</strong>43-71 0.80444 METOLAZONE 2.5 MG TABLET 0 UCB PHARMA EAGEN 00185-0055-01 0.91467 METOLAZONE 5 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0055-10 0.91467 METOLAZONE 5 MG TABLET 0 SANDOZ EAGEN 00378-6173-01 0.91467 METOLAZONE 5 MG TABLET 0 MYLAN EAGEN 51079-0024-01 0.91467 METOLAZONE 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0024-20 0.91467 METOLAZONE 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 65580-<strong>06</strong>44-71 0.91467 METOLAZONE 5 MG TABLET 0 UCB PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 255LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0418-01 0.91467 METOLAZONE 5 MG TABLET 0 AHP EAGEN 00185-0283-10 1.07736 METOPROLOL SUCC ER 100 MG TAB G SANDOZ EAGEN 00378-4597-10 1.31849 METOPROLOL SUCC ER 100 MG TAB G MYLAN EAGEN 00378-4597-77 1.31850 METOPROLOL SUCC ER 100 MG TAB G MYLAN EAGEN 00904-6171-61 1.39807 METOPROLOL SUCC ER 100 MG TAB G MAJOR PHARMACEU EAGEN 00904-6324-61 1.39807 METOPROLOL SUCC ER 100 MG TAB G MAJOR PHARMACEU EAGEN 49884-04<strong>06</strong>-01 1.18762 METOPROLOL SUCC ER 100 MG TAB G PAR PHARM. EAGEN 49884-04<strong>06</strong>-10 1.18763 METOPROLOL SUCC ER 100 MG TAB G PAR PHARM. EAGEN 51079-0171-01 1.31850 METOPROLOL SUCC ER 100 MG TAB G MYLAN INSTITUTI EAGEN 51079-0171-03 1.31850 METOPROLOL SUCC ER 100 MG TAB G MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 54458-0302-30 1.37600 METOPROLOL SUCC ER 100 MG TAB G INTERNATIONAL L EAGEN 54458-0302-31 1.37587 METOPROLOL SUCC ER 100 MG TAB G INTERNATIONAL L EAGEN 55111-0468-01 1.18762 METOPROLOL SUCC ER 100 MG TAB G DR.REDDY'S LAB EAGEN 55111-0468-05 1.18762 METOPROLOL SUCC ER 100 MG TAB G DR.REDDY'S LAB EAGEN 62037-<strong>08</strong>32-01 1.18762 METOPROLOL SUCC ER 100 MG TAB G ACTAVIS PHARMA, EAGEN 62037-<strong>08</strong>32-10 1.18762 METOPROLOL SUCC ER 100 MG TAB G ACTAVIS PHARMA, EAGEN 63739-0454-10 1.25625 METOPROLOL SUCC ER 100 MG TAB G MCKESSON PACKAG EAGEN 64679-0736-02 1.18762 METOPROLOL SUCC ER 100 MG TAB G WOCKHARDT USA L EAGEN 64679-0736-03 1.12824 METOPROLOL SUCC ER 100 MG TAB G WOCKHARDT USA L EAGEN 64679-0736-<strong>08</strong> 1.12824 METOPROLOL SUCC ER 100 MG TAB G WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0736-09 1.18762 METOPROLOL SUCC ER 100 MG TAB G WOCKHARDT USA L EAGEN 68001-0119-00 1.18762 METOPROLOL SUCC ER 100 MG TAB G BLUEPOINT LABOR EAGEN 68001-0119-03 1.12824 METOPROLOL SUCC ER 100 MG TAB G BLUEPOINT LABOR EAGEN 68<strong>08</strong>4-0301-01 1.25625 METOPROLOL SUCC ER 100 MG TAB G AHP EAGEN 68<strong>08</strong>4-0301-11 1.25625 METOPROLOL SUCC ER 100 MG TAB G AHP EAGEN 68645-0479-54 1.18762 METOPROLOL SUCC ER 100 MG TAB G LEGACY PHARMACE EAGEN 00185-0284-10 1.71416 METOPROLOL SUCC ER 200 MG TAB G SANDOZ EAGEN 00378-4598-05 2.09785 METOPROLOL SUCC ER 200 MG TAB G MYLAN EAGEN 00378-4598-77 2.09783 METOPROLOL SUCC ER 200 MG TAB G MYLAN EAGEN 49884-0407-01 1.88962 METOPROLOL SUCC ER 200 MG TAB G PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0407-10 1.88959 METOPROLOL SUCC ER 200 MG TAB G PAR PHARM. EAGEN 55111-0469-01 1.88962 METOPROLOL SUCC ER 200 MG TAB G DR.REDDY'S LAB EAGEN 55111-0469-05 1.88962 METOPROLOL SUCC ER 200 MG TAB G DR.REDDY'S LAB EAGEN 62037-<strong>08</strong>33-01 1.88962 METOPROLOL SUCC ER 200 MG TAB G ACTAVIS PHARMA, EAGEN 64679-0737-02 1.88962 METOPROLOL SUCC ER 200 MG TAB G WOCKHARDT USA L EAGEN 64679-0737-03 1.79514 METOPROLOL SUCC ER 200 MG TAB G WOCKHARDT USA L EAGEN 64679-0737-<strong>08</strong> 1.79514 METOPROLOL SUCC ER 200 MG TAB G WOCKHARDT USA L EAGEN 68001-0120-00 1.88962 METOPROLOL SUCC ER 200 MG TAB G BLUEPOINT LABOR EAGEN 68001-0120-03 1.79514 METOPROLOL SUCC ER 200 MG TAB G BLUEPOINT LABOR EAGEN 68<strong>08</strong>4-0302-21 1.99875 METOPROLOL SUCC ER 200 MG TAB G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-4595-10 0.87740 METOPROLOL SUCC ER 25 MG TAB G MYLAN EAGEN 00378-4595-77 0.87741 METOPROLOL SUCC ER 25 MG TAB G MYLAN EAGEN 00904-6169-61 0.92955 METOPROLOL SUCC ER 25 MG TAB G MAJOR PHARMACEU EAGEN 00904-6322-61 0.92955 METOPROLOL SUCC ER 25 MG TAB G MAJOR PHARMACEU EAGEN 49884-0404-01 0.79035 METOPROLOL SUCC ER 25 MG TAB G PAR PHARM. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 256LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0404-10 0.79035 METOPROLOL SUCC ER 25 MG TAB G PAR PHARM. EAGEN 51079-0169-01 0.87742 METOPROLOL SUCC ER 25 MG TAB G MYLAN INSTITUTI EAGEN 51079-0169-20 0.87742 METOPROLOL SUCC ER 25 MG TAB G MYLAN INSTITUTI EAGEN 54458-0300-30 0.99425 METOPROLOL SUCC ER 25 MG TAB G INTERNATIONAL L EAGEN 54458-0300-31 0.99420 METOPROLOL SUCC ER 25 MG TAB G INTERNATIONAL L EAGEN 55111-0466-01 0.79035 METOPROLOL SUCC ER 25 MG TAB G DR.REDDY'S LAB EAGEN 55111-0466-05 0.79035 METOPROLOL SUCC ER 25 MG TAB G DR.REDDY'S LAB EAGEN 62037-<strong>08</strong>30-01 0.79035 METOPROLOL SUCC ER 25 MG TAB G ACTAVIS PHARMA, EAGEN 62037-<strong>08</strong>30-10 0.79035 METOPROLOL SUCC ER 25 MG TAB G ACTAVIS PHARMA, EAGEN 64679-0734-02 0.79035 METOPROLOL SUCC ER 25 MG TAB G WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0734-03 0.75<strong>08</strong>4 METOPROLOL SUCC ER 25 MG TAB G WOCKHARDT USA L EAGEN 64679-0734-<strong>08</strong> 0.75<strong>08</strong>3 METOPROLOL SUCC ER 25 MG TAB G WOCKHARDT USA L EAGEN 64679-0734-09 0.79035 METOPROLOL SUCC ER 25 MG TAB G WOCKHARDT USA L EAGEN 68001-0121-00 0.79035 METOPROLOL SUCC ER 25 MG TAB G BLUEPOINT LABOR EAGEN 68001-0121-03 0.75<strong>08</strong>4 METOPROLOL SUCC ER 25 MG TAB G BLUEPOINT LABOR EAGEN 68<strong>08</strong>4-0303-01 0.83625 METOPROLOL SUCC ER 25 MG TAB G AHP EAGEN 68<strong>08</strong>4-0303-11 0.83625 METOPROLOL SUCC ER 25 MG TAB G AHP EAGEN 00378-4596-10 0.87740 METOPROLOL SUCC ER 50 MG TAB G MYLAN EAGEN 00378-4596-77 0.87741 METOPROLOL SUCC ER 50 MG TAB G MYLAN EAGEN 00904-6323-61 0.92955 METOPROLOL SUCC ER 50 MG TAB G MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0405-01 0.79035 METOPROLOL SUCC ER 50 MG TAB G PAR PHARM. EAGEN 49884-0405-10 0.79035 METOPROLOL SUCC ER 50 MG TAB G PAR PHARM. EAGEN 51079-0170-01 0.87742 METOPROLOL SUCC ER 50 MG TAB G MYLAN INSTITUTI EAGEN 51079-0170-20 0.87742 METOPROLOL SUCC ER 50 MG TAB G MYLAN INSTITUTI EAGEN 54458-0301-30 0.68975 METOPROLOL SUCC ER 50 MG TAB G INTERNATIONAL L EAGEN 54458-0301-31 0.68962 METOPROLOL SUCC ER 50 MG TAB G INTERNATIONAL L EAGEN 55111-0467-01 0.79035 METOPROLOL SUCC ER 50 MG TAB G DR.REDDY'S LAB EAGEN 55111-0467-05 0.79035 METOPROLOL SUCC ER 50 MG TAB G DR.REDDY'S LAB EAGEN 62037-<strong>08</strong>31-01 0.79035 METOPROLOL SUCC ER 50 MG TAB G ACTAVIS PHARMA, EAGEN 62037-<strong>08</strong>31-10 0.79035 METOPROLOL SUCC ER 50 MG TAB G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0735-02 0.79035 METOPROLOL SUCC ER 50 MG TAB G WOCKHARDT USA L EAGEN 64679-0735-03 0.75<strong>08</strong>4 METOPROLOL SUCC ER 50 MG TAB G WOCKHARDT USA L EAGEN 64679-0735-<strong>08</strong> 0.75<strong>08</strong>3 METOPROLOL SUCC ER 50 MG TAB G WOCKHARDT USA L EAGEN 64679-0735-09 0.79035 METOPROLOL SUCC ER 50 MG TAB G WOCKHARDT USA L EAGEN 68001-0122-00 0.79035 METOPROLOL SUCC ER 50 MG TAB G BLUEPOINT LABOR EAGEN 68001-0122-03 0.75<strong>08</strong>4 METOPROLOL SUCC ER 50 MG TAB G BLUEPOINT LABOR EAGEN 68<strong>08</strong>4-0304-01 0.83625 METOPROLOL SUCC ER 50 MG TAB G AHP EAGEN 68<strong>08</strong>4-0304-11 0.83625 METOPROLOL SUCC ER 50 MG TAB G AHP EAGEN 00143-9660-10 0.26000 METOPROLOL TART 5 MG/5 ML VIAL 0 WEST-WARD,INC. MLGEN 00143-9873-10 0.26000 METOPROLOL TART 5 MG/5 ML VIAL 0 WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3071-95 0.26000 METOPROLOL TART 5 MG/5 ML VIAL 0 SANDOZ MLGEN 00093-0734-10 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 TEVA USA EAGEN 00378-0047-01 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 MYLAN EAGEN 00378-0047-02 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 MYLAN EAGEN 00378-0047-04 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 257LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0047-10 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 MYLAN EAGEN 00591-0463-01 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 ACTAVIS PHARMA, EAGEN 00591-0463-10 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 ACTAVIS PHARMA, EAGEN 00904-6342-60 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 MAJOR PHARMACEU EAGEN 00904-6342-80 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 MAJOR PHARMACEU EAGEN 50742-0109-01 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 INGENUS PHARMAC EAGEN 50742-0109-10 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 INGENUS PHARMAC EAGEN 51079-<strong>08</strong>02-20 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 MYLAN INSTITUTI EAGEN 57664-0167-52 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 CARACO PHARM EAGEN 57664-0167-58 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 CARACO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-1302-01 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 GREENSTONE LLC. EAGEN 59762-1302-03 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 GREENSTONE LLC. EAGEN 62584-0267-01 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 AHP EAGEN 62584-0267-11 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 AHP EAGEN 63304-0581-01 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 RANBAXY PHARMAC EAGEN 63304-0581-10 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 RANBAXY PHARMAC EAGEN 65862-0<strong>06</strong>4-01 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0<strong>06</strong>4-99 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 AUROBINDO PHARM EAGEN 68645-0191-59 0.03240 METOPROLOL TARTRATE 100 MG TAB 0 LEGACY PHARMACE EAGEN 00378-0018-01 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0018-02 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 MYLAN EAGEN 00378-0018-05 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 MYLAN EAGEN 00378-0018-07 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 MYLAN EAGEN 00378-0018-91 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 MYLAN EAGEN 00904-6162-61 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 MAJOR PHARMACEU EAGEN 00904-6340-60 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 MAJOR PHARMACEU EAGEN 00904-6340-80 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 MAJOR PHARMACEU EAGEN 50742-0107-01 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 INGENUS PHARMAC EAGEN 50742-0107-05 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 INGENUS PHARMAC EAGEN 51079-0255-17 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0255-19 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 MYLAN INSTITUTI EAGEN 51079-0255-20 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 MYLAN INSTITUTI EAGEN 57664-05<strong>06</strong>-<strong>08</strong> 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 CARACO PHARM EAGEN 57664-05<strong>06</strong>-18 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 CARACO PHARM EAGEN 57664-05<strong>06</strong>-52 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 CARACO PHARM EAGEN 57664-05<strong>06</strong>-58 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 CARACO PHARM EAGEN 58517-0320-30 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 <strong>NEW</strong> HORIZON RX EAGEN 59762-1300-01 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 GREENSTONE LLC. EAGEN 59762-1300-03 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 GREENSTONE LLC. EAGEN 62584-0265-01 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62584-0265-11 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 AHP EAGEN 63304-0579-01 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 RANBAXY PHARMAC EAGEN 63304-0579-10 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 RANBAXY PHARMAC EAGEN 65862-0<strong>06</strong>2-01 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0<strong>06</strong>2-99 0.02133 METOPROLOL TARTRATE 25 MG TAB 0 AUROBINDO PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 258LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-0733-10 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 TEVA USA EAGEN 00378-0032-01 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 MYLAN EAGEN 00378-0032-02 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 MYLAN EAGEN 00378-0032-04 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 MYLAN EAGEN 00378-0032-10 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 MYLAN EAGEN 00591-0462-01 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 ACTAVIS PHARMA, EAGEN 00591-0462-10 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 ACTAVIS PHARMA, EAGEN 00904-6341-60 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 MAJOR PHARMACEU EAGEN 00904-6341-80 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 MAJOR PHARMACEU EAGEN 50742-01<strong>08</strong>-01 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 INGENUS PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50742-01<strong>08</strong>-10 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 INGENUS PHARMAC EAGEN 51079-<strong>08</strong>01-20 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 MYLAN INSTITUTI EAGEN 57664-0166-52 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 CARACO PHARM EAGEN 57664-0166-58 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 CARACO PHARM EAGEN 57664-0477-52 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 CARACO PHARM EAGEN 57664-0477-58 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 CARACO PHARM EAGEN 59762-1301-01 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 GREENSTONE LLC. EAGEN 59762-1301-03 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 GREENSTONE LLC. EAGEN 62584-0266-01 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 AHP EAGEN 63304-0580-01 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63304-0580-10 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 RANBAXY PHARMAC EAGEN 65862-0<strong>06</strong>3-01 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 AUROBINDO PHARM EAGEN 65862-0<strong>06</strong>3-99 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 AUROBINDO PHARM EAGEN 68645-0190-59 0.02120 METOPROLOL TARTRATE 50 MG TAB 0 LEGACY PHARMACE EAGEN 00378-0434-01 1.45564 METOPROLOL-HCTZ 100-25 MG TAB G MYLAN EAGEN 62756-0368-88 1.32937 METOPROLOL-HCTZ 100-25 MG TAB G SUN PHARMACEUTI EAGEN 00378-0445-01 1.74232 METOPROLOL-HCTZ 100-50 MG TAB G MYLAN EAGEN 62756-0369-88 1.40962 METOPROLOL-HCTZ 100-50 MG TAB G SUN PHARMACEUTI EAGEN 00378-0424-01 1.04361 METOPROLOL-HCTZ 50-25 MG TAB G MYLAN EAGEN 62756-0370-88 0.85050 METOPROLOL-HCTZ 50-25 MG TAB G SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 65649-0431-02 2.19120 METOZOLV ODT 5 MG TABLET G SALIX PHARMACEU EABND 00264-5535-32 0.01512 0.02075 METRO IV 500 MG/100 ML G B.BRAUN MLBUL 00299-3836-45 1.62630 9.91573 METROCREAM 0.75% CREAM 0 GALDERMA LABORA GMBND 00299-3820-60 4.47536 METROGEL TOPICAL 1% GEL 0 GALDERMA LABORA GMBND 00299-3820-01 4.88220 METROGEL TOPICAL 1% PUMP 0 GALDERMA LABORA GMBND 99207-0130-70 0.46520 0.70242 METROGEL-VAGINAL 0.75% GEL 0 VALEANT GMBUL 00299-3838-02 1.16950 8.69220 METROLOTION TOPICAL 0.75% 0 GALDERMA LABORA MLGUL 00115-1474-46 1.54170 METRONIDAZOLE TOPICAL 0.75% GL 0 GLOBAL PHARM GMGUL 00168-0275-45 1.54170 METRONIDAZOLE TOPICAL 0.75% GL 0 SANDOZ GMGUL 00713-<strong>06</strong>37-37 1.54170 METRONIDAZOLE TOPICAL 0.75% GL 0 G & W LABS. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00781-7078-19 1.54170 METRONIDAZOLE TOPICAL 0.75% GL 0 SANDOZ GMGUL 51672-4116-<strong>06</strong> 1.54170 METRONIDAZOLE TOPICAL 0.75% GL 0 TARO PHARM USA GMGEN 00781-7<strong>08</strong>0-35 3.46575 METRONIDAZOLE TOPICAL 1% GEL 0 SANDOZ GMGEN 00781-7077-87 0.46520 METRONIDAZOLE VAGINAL 0.75% GL 0 SANDOZ GMGEN 66993-0935-70 0.36117 METRONIDAZOLE VAGINAL 0.75% GL 0 PRASCO LABS GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 259LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00168-0323-46 1.62630 METRONIDAZOLE 0.75% CREAM 0 SANDOZ GMGUL 00472-0911-45 1.62630 METRONIDAZOLE 0.75% CREAM 0 ACTAVIS PHARMA, GMGUL 00713-<strong>06</strong>33-37 1.62630 METRONIDAZOLE 0.75% CREAM 0 G & W LABS. GMGUL 66993-0960-45 1.62630 METRONIDAZOLE 0.75% CREAM 0 PRASCO LABS GMGUL 67405-0110-45 1.62630 METRONIDAZOLE 0.75% CREAM 0 HARRIS PHARM GMGUL 00168-0383-60 1.16950 METRONIDAZOLE 0.75% LOTION 0 SANDOZ MLGUL 00472-0912-02 1.16950 METRONIDAZOLE 0.75% LOTION 0 ACTAVIS PHARMA, MLGUL 66993-0961-59 1.16950 METRONIDAZOLE 0.75% LOTION 0 PRASCO LABS MLGUL 00591-2521-01 0.<strong>08</strong>490 METRONIDAZOLE 250 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00591-2521-05 0.<strong>08</strong>490 METRONIDAZOLE 250 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00591-2521-25 0.<strong>08</strong>490 METRONIDAZOLE 250 MG TABLET 0 ACTAVIS PHARMA, EAGUL 23155-0<strong>06</strong>4-01 0.<strong>08</strong>490 METRONIDAZOLE 250 MG TABLET 0 HERITAGE PHARMA EAGUL 23155-0<strong>06</strong>4-05 0.<strong>08</strong>490 METRONIDAZOLE 250 MG TABLET 0 HERITAGE PHARMA EAGUL 50111-0333-01 0.<strong>08</strong>490 METRONIDAZOLE 250 MG TABLET 0 PLIVA, INC EAGUL 50111-0333-02 0.<strong>08</strong>490 METRONIDAZOLE 250 MG TABLET 0 PLIVA, INC EAGUL 50111-0333-<strong>06</strong> 0.<strong>08</strong>490 METRONIDAZOLE 250 MG TABLET 0 PLIVA, INC EAGUL 51079-0216-19 0.<strong>08</strong>490 METRONIDAZOLE 250 MG TABLET 0 MYLAN INSTITUTI EAGUL 51079-0216-20 0.<strong>08</strong>490 METRONIDAZOLE 250 MG TABLET 0 MYLAN INSTITUTI EAGEN 23155-0<strong>06</strong>6-25 3.59960 METRONIDAZOLE 375 MG CAPSULE G HERITAGE PHARMA EAGUL 00591-2522-05 0.21840 METRONIDAZOLE 500 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00591-2522-50 0.21840 METRONIDAZOLE 500 MG TABLET 0 ACTAVIS PHARMA, EAGUL 23155-0<strong>06</strong>5-01 0.21840 METRONIDAZOLE 500 MG TABLET 0 HERITAGE PHARMA EAGUL 23155-0<strong>06</strong>5-05 0.21840 METRONIDAZOLE 500 MG TABLET 0 HERITAGE PHARMA EAGUL 50111-0334-01 0.21840 METRONIDAZOLE 500 MG TABLET 0 PLIVA, INC EAGUL 50111-0334-02 0.21840 METRONIDAZOLE 500 MG TABLET 0 PLIVA, INC EAGUL 51079-0217-20 0.21840 METRONIDAZOLE 500 MG TABLET 0 MYLAN INSTITUTI EAGEN 00338-1055-48 0.01512 METRONIDAZOLE 500 MG/100 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-7811-24 0.01440 METRONIDAZOLE 500 MG/100 ML 0 HOSPIRA MLGEN 00409-7811-37 0.01260 METRONIDAZOLE 500 MG/100 ML 0 HOSPIRA MLBND 000<strong>06</strong>-0731-61 0.09830 2.61352 MEVACOR 20 MG TABLET G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00093-8739-01 1.10763 MEXILETINE 150 MG CAPSULE 0 TEVA USA EABND 00093-8740-01 0.44420 1.32219 MEXILETINE 200 MG CAPSULE 0 TEVA USA EABND 00093-8741-01 1.52811 MEXILETINE 250 MG CAPSULE 0 TEVA USA EABND 00078-0311-54 12.21825 39.90953 MIACALCIN 200 UNIT NASAL SPRAY 0 NOVARTIS MLBND 00078-0149-23 33.85570 MIACALCIN 200 UNIT/ML VIAL 0 NOVARTIS MLBND 00597-0043-37 4.88759 MICARDIS HCT 40-12.5 MG TABLET G BOEHRINGER ING. EABND 00597-0044-37 4.88759 MICARDIS HCT 80-12.5 MG TABLET G BOEHRINGER ING. EABND 00597-0042-37 4.88759 MICARDIS HCT 80-25 MG TABLET G BOEHRINGER ING. EABND 00597-0039-37 4.88759 MICARDIS 20 MG TABLET G BOEHRINGER ING. EABND 00597-0040-37 4.88759 MICARDIS 40 MG TABLET G BOEHRINGER ING. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00597-0041-37 4.88759 MICARDIS 80 MG TABLET G BOEHRINGER ING. EABND 00472-1738-03 14.54436 MICONAZOLE 3 200 MG VAG SUPP 0 ACTAVIS PHARMA, EABND 64011-0009-04 0.59333 0.87863 MICRO-K 10 MEQ EXTENCAPS G THER-RX EABND 64011-0010-04 0.73680 0.83282 MICRO-K 8 MEQ EXTENCAPS 0 THER-RX EAGEX 52544-<strong>06</strong>31-28 0.72384 MICROGESTIN FE 1.5-30 TAB 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 260LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 52544-<strong>06</strong>30-28 0.71880 MICROGESTIN FE 1-20 TABLET 0 ACTAVIS PHARMA, EAGEX 52544-0951-21 1.00290 MICROGESTIN 21 1.5-30 TAB 0 ACTAVIS PHARMA, EAGEX 52544-0950-21 1.00280 MICROGESTIN 21 1-20 TABLET 0 ACTAVIS PHARMA, EABND 52544-<strong>06</strong>22-01 0.03900 1.16532 MICROZIDE 12.5 MG CAPSULE G ACTAVIS PHARMA, EAGEN 00115-4233-01 0.93700 MIDODRINE HCL 10 MG TABLET 0 GLOBAL PHARM EAGEN 00185-0149-01 0.93700 MIDODRINE HCL 10 MG TABLET 0 SANDOZ EAGEN 00245-0213-11 0.93700 MIDODRINE HCL 10 MG TABLET 0 UPSHER SMITH EAGEN 00378-1903-01 0.93700 MIDODRINE HCL 10 MG TABLET 0 MYLAN EAGEN 60505-1325-01 0.93700 MIDODRINE HCL 10 MG TABLET 0 APOTEX CORP EAGEN 60505-1325-<strong>08</strong> 0.93700 MIDODRINE HCL 10 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00115-4211-01 0.32562 MIDODRINE HCL 2.5 MG TABLET 0 GLOBAL PHARM EAGEN 00185-0040-01 0.32562 MIDODRINE HCL 2.5 MG TABLET 0 SANDOZ EAGEN 00245-0211-11 0.32562 MIDODRINE HCL 2.5 MG TABLET 0 UPSHER SMITH EAGEN 00378-1901-01 0.32562 MIDODRINE HCL 2.5 MG TABLET 0 MYLAN EAGEN 60505-1320-01 0.32562 MIDODRINE HCL 2.5 MG TABLET 0 APOTEX CORP EAGEN 60505-1320-<strong>08</strong> 0.32562 MIDODRINE HCL 2.5 MG TABLET 0 APOTEX CORP EAGEN 68<strong>08</strong>4-0240-01 0.32562 MIDODRINE HCL 2.5 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0240-11 0.32562 MIDODRINE HCL 2.5 MG TABLET 0 AHP EAGEN 00115-4222-01 0.38340 MIDODRINE HCL 5 MG TABLET 0 GLOBAL PHARM EAGEN 00185-0043-01 0.38340 MIDODRINE HCL 5 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0043-05 0.38340 MIDODRINE HCL 5 MG TABLET 0 SANDOZ EAGEN 00245-0212-11 0.38340 MIDODRINE HCL 5 MG TABLET 0 UPSHER SMITH EAGEN 00378-1902-01 0.38340 MIDODRINE HCL 5 MG TABLET 0 MYLAN EAGEN 51079-0453-01 0.38340 MIDODRINE HCL 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0453-20 0.38340 MIDODRINE HCL 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 60505-1321-01 0.38340 MIDODRINE HCL 5 MG TABLET 0 APOTEX CORP EAGEN 60505-1321-<strong>08</strong> 0.38340 MIDODRINE HCL 5 MG TABLET 0 APOTEX CORP EAGEN 63739-0556-10 0.38340 MIDODRINE HCL 5 MG TABLET 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0241-01 0.38340 MIDODRINE HCL 5 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0241-11 0.38340 MIDODRINE HCL 5 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00713-0166-12 34.6<strong>08</strong>23 MIGERGOT SUPPOSITORY 0 G & W LABS. EABND 00187-0245-03 212.71655 MIGRANAL NASAL SPRAY 0 VALEANT MLBND 16477-0505-21 6.49376 MILLIPRED DP 5 MG DOSE PACK TB G ZYLERA EABND 16477-0505-48 6.49924 MILLIPRED DP 5 MG DOSE PACK TB G ZYLERA EABND 16477-0510-<strong>08</strong> 2.92664 MILLIPRED 10 MG/5 ML SOLUTION G ZYLERA MLBND 16477-0505-01 6.54123 MILLIPRED 5 MG TABLET G ZYLERA EAGEN 00143-9710-10 0.46400 MILRINONE LACT 10 MG/10 ML VL 0 WEST-WARD,INC. MLGEN 63323-<strong>06</strong>17-10 0.46400 MILRINONE LACT 10 MG/10 ML VL 0 APP PHARMACEUTI MLGEN 00143-9709-10 0.41310 MILRINONE LACT 20 MG/20 ML VL 0 WEST-WARD,INC. MLGEN 55390-0020-10 0.46400 MILRINONE LACT 20 MG/20 ML VL 0 BEDFORD LABS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-<strong>06</strong>17-20 0.46400 MILRINONE LACT 20 MG/20 ML VL 0 APP PHARMACEUTI MLGEN 00143-97<strong>08</strong>-01 0.46400 MILRINONE LACT 50 MG/50 ML VL 0 WEST-WARD,INC. MLGEN 63323-<strong>06</strong>17-50 0.46400 MILRINONE LACT 50 MG/50 ML VL 0 APP PHARMACEUTI MLGEN 00143-9719-10 0.13680 MILRINONE-D5W 20 MG/100 ML 0 WEST-WARD,INC. MLGEN 00338-6010-48 0.16100 MILRINONE-D5W 20 MG/100 ML 0 BAXTER <strong>HEALTH</strong>CA ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 261LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-2776-23 0.1<strong>08</strong>72 MILRINONE-D5W 20 MG/100 ML 0 HOSPIRA MLGEN 00143-9718-10 0.14355 MILRINONE-D5W 40 MG/200 ML 0 WEST-WARD,INC. MLGEN 00338-6011-37 0.15950 MILRINONE-D5W 40 MG/200 ML 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-2776-02 0.11812 MILRINONE-D5W 40 MG/200 ML 0 HOSPIRA MLGEN 00093-5455-28 3.03187 MIMVEY 1-0.5 MG TABLET 0 TEVA USA EAGEN 00093-5455-42 3.03198 MIMVEY 1-0.5 MG TABLET 0 TEVA USA EABEX 00430-0535-14 2.95231 MINASTRIN 24 FE CHEWABLE TAB 0 ACTAVIS PHARMA, EABEX 00430-0535-50 2.95231 MINASTRIN 24 FE CHEWABLE TAB 0 ACTAVIS PHARMA, EABND 00<strong>06</strong>9-4310-71 0.15510 1.11478 MINIPRESS 1 MG CAPSULE G PFIZER US PHARM EABND 00<strong>06</strong>9-4370-71 0.28090 1.55170 MINIPRESS 2 MG CAPSULE G PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>9-4380-71 0.53055 2.64540 MINIPRESS 5 MG CAPSULE G PFIZER US PHARM EAGEN 99207-0170-02 0.68250 MINITRAN 0.1 MG/HR PATCH 0 VALEANT EAGEN 99207-0171-05 0.7<strong>06</strong>30 MINITRAN 0.2 MG/HR PATCH 0 VALEANT EAGEN 99207-0172-10 0.87305 MINITRAN 0.4 MG/HR PATCH 0 VALEANT EAGEN 99207-0173-15 0.96130 MINITRAN 0.6 MG/HR PATCH 0 VALEANT EABND 68968-6637-<strong>08</strong> 9.96207 MINIVELLE 0.0375 MG PATCH 0 NOVEN THERAPEUT EABND 68968-6650-<strong>08</strong> 9.96207 MINIVELLE 0.05 MG PATCH 0 NOVEN THERAPEUT EABND 68968-6675-<strong>08</strong> 9.96207 MINIVELLE 0.075 MG PATCH 0 NOVEN THERAPEUT EABND 68968-6610-<strong>08</strong> 9.96207 MINIVELLE 0.1 MG PATCH 0 NOVEN THERAPEUT EAGEN 00115-1247-01 13.85<strong>06</strong>2 MINOCYCLINE ER 135 MG TABLET G GLOBAL PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00115-1247-<strong>08</strong> 13.85049 MINOCYCLINE ER 135 MG TABLET G GLOBAL PHARM EAGEN 00378-4298-01 17.42505 MINOCYCLINE ER 135 MG TABLET G MYLAN EAGEN 00378-4298-93 17.42475 MINOCYCLINE ER 135 MG TABLET G MYLAN EAGEN 00781-5387-01 15.12510 MINOCYCLINE ER 135 MG TABLET G SANDOZ EAGEN 00781-5387-31 15.12525 MINOCYCLINE ER 135 MG TABLET G SANDOZ EAGEN 68180-0381-<strong>06</strong> 17.42475 MINOCYCLINE ER 135 MG TABLET G LUPIN PHARMACEU EAGEN 00115-1245-01 13.85<strong>06</strong>2 MINOCYCLINE ER 45 MG TABLET G GLOBAL PHARM EAGEN 00115-1245-<strong>08</strong> 13.85049 MINOCYCLINE ER 45 MG TABLET G GLOBAL PHARM EAGEN 00378-4296-01 17.42505 MINOCYCLINE ER 45 MG TABLET G MYLAN EAGEN 00378-4296-93 17.42475 MINOCYCLINE ER 45 MG TABLET G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-5385-01 15.12510 MINOCYCLINE ER 45 MG TABLET G SANDOZ EAGEN 00781-5385-31 15.12525 MINOCYCLINE ER 45 MG TABLET G SANDOZ EAGEN 68180-0379-<strong>06</strong> 17.42475 MINOCYCLINE ER 45 MG TABLET G LUPIN PHARMACEU EAGEN 00115-1246-01 13.85<strong>06</strong>2 MINOCYCLINE ER 90 MG TABLET G GLOBAL PHARM EAGEN 00115-1246-<strong>08</strong> 13.85049 MINOCYCLINE ER 90 MG TABLET G GLOBAL PHARM EAGEN 00378-4297-01 17.42505 MINOCYCLINE ER 90 MG TABLET G MYLAN EAGEN 00378-4297-93 17.42475 MINOCYCLINE ER 90 MG TABLET G MYLAN EAGEN 00781-5386-01 15.12510 MINOCYCLINE ER 90 MG TABLET G SANDOZ EAGEN 00781-5386-31 15.12525 MINOCYCLINE ER 90 MG TABLET G SANDOZ EAGEN 68180-0380-<strong>06</strong> 17.42475 MINOCYCLINE ER 90 MG TABLET G LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0513-03 3.<strong>06</strong>288 MINOCYCLINE HCL 100 MG TABLET G PAR PHARM. EAGEN 55111-<strong>06</strong>39-60 3.<strong>06</strong>288 MINOCYCLINE HCL 100 MG TABLET G DR.REDDY'S LAB EAGEN 63304-<strong>06</strong>99-50 3.<strong>06</strong>288 MINOCYCLINE HCL 100 MG TABLET G RANBAXY PHARMAC EAGEN 49884-0511-01 2.57602 MINOCYCLINE HCL 50 MG TABLET G PAR PHARM. EAGUL 55111-<strong>06</strong>37-01 3.00000 MINOCYCLINE HCL 50 MG TABLET G DR.REDDY'S LAB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 262LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0512-01 1.74870 MINOCYCLINE HCL 75 MG TABLET G PAR PHARM. EAGEN 55111-<strong>06</strong>38-01 1.74870 MINOCYCLINE HCL 75 MG TABLET G DR.REDDY'S LAB EAGUL 00093-3167-53 1.80000 MINOCYCLINE 100 MG CAPSULE 0 TEVA USA EAGUL 00591-5695-50 1.80000 MINOCYCLINE 100 MG CAPSULE 0 ACTAVIS PHARMA, EAGUL 57664-<strong>08</strong>53-85 1.80000 MINOCYCLINE 100 MG CAPSULE 0 CARACO PHARM EAGUL 63304-<strong>06</strong>96-05 1.80000 MINOCYCLINE 100 MG CAPSULE 0 RANBAXY PHARMAC EAGUL 63304-<strong>06</strong>96-50 1.80000 MINOCYCLINE 100 MG CAPSULE 0 RANBAXY PHARMAC EAGUL 65862-0211-50 1.80000 MINOCYCLINE 100 MG CAPSULE 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-<strong>06</strong>23-11 1.25610 MINOCYCLINE 100 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>23-65 1.25610 MINOCYCLINE 100 MG CAPSULE 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-3165-01 0.15336 MINOCYCLINE 50 MG CAPSULE 0 TEVA USA EAGEN 00591-5694-01 0.15336 MINOCYCLINE 50 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00591-5694-60 0.15336 MINOCYCLINE 50 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 57664-<strong>08</strong>51-88 0.15336 MINOCYCLINE 50 MG CAPSULE 0 CARACO PHARM EAGEN 63304-<strong>06</strong>94-01 0.15336 MINOCYCLINE 50 MG CAPSULE 0 RANBAXY PHARMAC EAGEN 65862-0209-01 0.15336 MINOCYCLINE 50 MG CAPSULE 0 AUROBINDO PHARM EAGEN 00093-7300-01 0.36059 MINOCYCLINE 75 MG CAPSULE 0 TEVA USA EAGEN 00591-3153-01 0.36059 MINOCYCLINE 75 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 57664-<strong>08</strong>52-88 0.36059 MINOCYCLINE 75 MG CAPSULE 0 CARACO PHARM EAGEN 63304-<strong>06</strong>95-01 0.36059 MINOCYCLINE 75 MG CAPSULE 0 RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0210-01 0.36059 MINOCYCLINE 75 MG CAPSULE 0 AUROBINDO PHARM EAGEN 00591-5643-01 0.29376 MINOXIDIL 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5643-05 0.29376 MINOXIDIL 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 49884-0257-01 0.29376 MINOXIDIL 10 MG TABLET 0 PAR PHARM. EAGEN 49884-0257-05 0.29376 MINOXIDIL 10 MG TABLET 0 PAR PHARM. EAGEN 53489-0387-01 0.29376 MINOXIDIL 10 MG TABLET 0 MUTUAL PHARM CO EAGEN 68<strong>08</strong>4-0205-01 0.29376 MINOXIDIL 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0205-11 0.29376 MINOXIDIL 10 MG TABLET 0 AHP EAGEN 00591-5642-01 0.16940 MINOXIDIL 2.5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5642-05 0.16940 MINOXIDIL 2.5 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0256-01 0.16940 MINOXIDIL 2.5 MG TABLET 0 PAR PHARM. EAGEN 53489-0386-01 0.16940 MINOXIDIL 2.5 MG TABLET 0 MUTUAL PHARM CO EAGEN 68<strong>08</strong>4-0204-01 0.16940 MINOXIDIL 2.5 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0204-11 0.16940 MINOXIDIL 2.5 MG TABLET 0 AHP EABND 00<strong>06</strong>5-0023-15 20.27874 MIOSTAT VIAL 0 ALCON SURGICAL MLBND 00597-0109-17 13.54<strong>08</strong>6 MIRAPEX ER 0.375 MG TABLET G BOEHRINGER ING. EABND 00597-0109-30 13.54283 MIRAPEX ER 0.375 MG TABLET G BOEHRINGER ING. EABND 00597-0285-17 13.54<strong>08</strong>6 MIRAPEX ER 0.75 MG TABLET G BOEHRINGER ING. EABND 00597-0285-30 13.54283 MIRAPEX ER 0.75 MG TABLET G BOEHRINGER ING. EABND 00597-0113-17 13.54<strong>08</strong>6 MIRAPEX ER 1.5 MG TABLET G BOEHRINGER ING. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00597-0113-30 13.54283 MIRAPEX ER 1.5 MG TABLET G BOEHRINGER ING. EABND 00597-0286-30 13.54283 MIRAPEX ER 2.25 MG TABLET G BOEHRINGER ING. EABND 00597-0115-30 13.54283 MIRAPEX ER 3 MG TABLET G BOEHRINGER ING. EABND 00597-0287-30 13.54283 MIRAPEX ER 3.75 MG TABLET G BOEHRINGER ING. EABND 00597-0116-30 13.54283 MIRAPEX ER 4.5 MG TABLET G BOEHRINGER ING. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 263LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00597-0183-90 0.12987 4.77102 MIRAPEX 0.125 MG TABLET G BOEHRINGER ING. EABND 00597-0184-90 0.09720 4.77102 MIRAPEX 0.25 MG TABLET G BOEHRINGER ING. EABND 00597-0185-90 0.09720 4.77102 MIRAPEX 0.5 MG TABLET G BOEHRINGER ING. EABND 00597-0101-90 0.09274 4.77102 MIRAPEX 0.75 MG TABLET G BOEHRINGER ING. EABND 00597-0190-90 0.12740 4.77102 MIRAPEX 1 MG TABLET G BOEHRINGER ING. EABND 00597-0191-90 0.12987 4.77102 MIRAPEX 1.5 MG TABLET G BOEHRINGER ING. EABEX 51285-0114-58 1.51100 3.46821 MIRCETTE 28 DAY TABLET G DURAMED/BARR EAGEX 00093-7303-65 0.83700 MIRTAZAPINE 15 MG ODT 0 TEVA USA EAGEX 00591-2230-15 0.83700 MIRTAZAPINE 15 MG ODT 0 ACTAVIS PHARMA, EAGEX 00591-2469-15 0.83700 MIRTAZAPINE 15 MG ODT 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-1410-07 0.83700 MIRTAZAPINE 15 MG ODT 0 GREENSTONE LLC. EAGEX 65862-0021-<strong>06</strong> 0.83700 MIRTAZAPINE 15 MG ODT 0 AUROBINDO PHARM EAGEX 66993-0709-30 0.83700 MIRTAZAPINE 15 MG ODT 0 PRASCO LABS EAGEX 00093-72<strong>06</strong>-56 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 TEVA USA EAGEX 00185-0020-10 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 SANDOZ EAGEX 00185-0020-30 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 SANDOZ EAGEX 00378-3515-10 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 MYLAN EAGEX 00378-3515-93 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 MYLAN EAGEX 00591-1117-10 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-1117-30 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13107-0031-05 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0031-30 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0031-34 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 AUROBINDO PHARM EAGEX 51079-0<strong>08</strong>6-01 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0<strong>08</strong>6-56 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 MYLAN INSTITUTI EAGEX 59762-1416-03 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1416-05 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1416-09 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 GREENSTONE LLC. EAGEX 60505-0247-01 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 APOTEX CORP EAGEX 60505-0247-<strong>08</strong> 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 63739-0355-04 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 MCKESSON PACKAG EAGEX 63739-0355-10 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 MCKESSON PACKAG EAGEX 65862-0031-30 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0119-01 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0119-11 0.1<strong>08</strong>50 MIRTAZAPINE 15 MG TABLET 0 AHP EAGEX 00093-7304-65 0.86225 MIRTAZAPINE 30 MG ODT 0 TEVA USA EAGEX 00591-2231-15 0.86225 MIRTAZAPINE 30 MG ODT 0 ACTAVIS PHARMA, EAGEX 00591-2470-15 0.86225 MIRTAZAPINE 30 MG ODT 0 ACTAVIS PHARMA, EAGEX 59762-1412-07 0.86225 MIRTAZAPINE 30 MG ODT 0 GREENSTONE LLC. EAGEX 65862-0022-<strong>06</strong> 0.86225 MIRTAZAPINE 30 MG ODT 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 66993-0711-30 0.86225 MIRTAZAPINE 30 MG ODT 0 PRASCO LABS EAGEX 00093-7207-56 0.13590 MIRTAZAPINE 30 MG TABLET 0 TEVA USA EAGEX 00185-0212-10 0.13590 MIRTAZAPINE 30 MG TABLET 0 SANDOZ EAGEX 00185-0212-30 0.13590 MIRTAZAPINE 30 MG TABLET 0 SANDOZ EAGEX 00378-3530-05 0.13590 MIRTAZAPINE 30 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 264LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-3530-93 0.13590 MIRTAZAPINE 30 MG TABLET 0 MYLAN EAGEX 00591-1118-10 0.13590 MIRTAZAPINE 30 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-1118-30 0.13590 MIRTAZAPINE 30 MG TABLET 0 ACTAVIS PHARMA, EAGEX 13107-0003-05 0.13590 MIRTAZAPINE 30 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0003-30 0.13590 MIRTAZAPINE 30 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0003-34 0.13590 MIRTAZAPINE 30 MG TABLET 0 AUROBINDO PHARM EAGEX 51079-0<strong>08</strong>7-01 0.13590 MIRTAZAPINE 30 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0<strong>08</strong>7-20 0.13590 MIRTAZAPINE 30 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0<strong>08</strong>7-56 0.13590 MIRTAZAPINE 30 MG TABLET 0 MYLAN INSTITUTI EAGEX 59762-1417-03 0.13590 MIRTAZAPINE 30 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-1417-05 0.13590 MIRTAZAPINE 30 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1417-09 0.13590 MIRTAZAPINE 30 MG TABLET 0 GREENSTONE LLC. EAGEX 60505-0248-01 0.13590 MIRTAZAPINE 30 MG TABLET 0 APOTEX CORP EAGEX 60505-0248-<strong>08</strong> 0.13590 MIRTAZAPINE 30 MG TABLET 0 APOTEX CORP EAGEX 63739-0356-10 0.13590 MIRTAZAPINE 30 MG TABLET 0 MCKESSON PACKAG EAGEX 65862-0003-30 0.13590 MIRTAZAPINE 30 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0120-01 0.13590 MIRTAZAPINE 30 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0120-11 0.13590 MIRTAZAPINE 30 MG TABLET 0 AHP EAGEX 00093-7305-65 1.12415 MIRTAZAPINE 45 MG ODT 0 TEVA USA EAGEX 59762-1414-07 1.12415 MIRTAZAPINE 45 MG ODT 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0023-<strong>06</strong> 1.12415 MIRTAZAPINE 45 MG ODT 0 AUROBINDO PHARM EAGEX 66993-0712-30 1.12415 MIRTAZAPINE 45 MG ODT 0 PRASCO LABS EAGEX 00093-72<strong>08</strong>-56 0.24400 MIRTAZAPINE 45 MG TABLET 0 TEVA USA EAGEX 00185-0222-10 0.24400 MIRTAZAPINE 45 MG TABLET 0 SANDOZ EAGEX 00185-0222-30 0.24400 MIRTAZAPINE 45 MG TABLET 0 SANDOZ EAGEX 00378-3545-05 0.24400 MIRTAZAPINE 45 MG TABLET 0 MYLAN EAGEX 00378-3545-93 0.24400 MIRTAZAPINE 45 MG TABLET 0 MYLAN EAGEX 00591-1119-30 0.24400 MIRTAZAPINE 45 MG TABLET 0 ACTAVIS PHARMA, EAGEX 13107-0032-05 0.24400 MIRTAZAPINE 45 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0032-30 0.24400 MIRTAZAPINE 45 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13107-0032-34 0.24400 MIRTAZAPINE 45 MG TABLET 0 AUROBINDO PHARM EAGEX 51079-0<strong>08</strong>8-01 0.24400 MIRTAZAPINE 45 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0<strong>08</strong>8-30 0.24400 MIRTAZAPINE 45 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0<strong>08</strong>8-56 0.24400 MIRTAZAPINE 45 MG TABLET 0 MYLAN INSTITUTI EAGEX 59762-1418-03 0.24400 MIRTAZAPINE 45 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1418-05 0.24400 MIRTAZAPINE 45 MG TABLET 0 GREENSTONE LLC. EAGEX 60505-0249-01 0.24400 MIRTAZAPINE 45 MG TABLET 0 APOTEX CORP EAGEX 60505-0249-<strong>08</strong> 0.24400 MIRTAZAPINE 45 MG TABLET 0 APOTEX CORP EAGEX 65862-0032-30 0.24400 MIRTAZAPINE 45 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0121-01 0.24400 MIRTAZAPINE 45 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0121-11 0.24400 MIRTAZAPINE 45 MG TABLET 0 AHP EAGEX 13107-0001-05 0.<strong>06</strong>170 MIRTAZAPINE 7.5 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0001-30 0.<strong>06</strong>170 MIRTAZAPINE 7.5 MG TABLET 0 AUROBINDO PHARM EAGEX 57664-0510-83 1.92500 MIRTAZAPINE 7.5 MG TABLET 0 CARACO PHARM EAGEX 59762-1415-03 0.<strong>06</strong>170 MIRTAZAPINE 7.5 MG TABLET 0 GREENSTONE LLC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 265LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-1415-05 0.<strong>06</strong>170 MIRTAZAPINE 7.5 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1415-<strong>06</strong> 0.<strong>06</strong>170 MIRTAZAPINE 7.5 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1415-09 0.<strong>06</strong>170 MIRTAZAPINE 7.5 MG TABLET 0 GREENSTONE LLC. EABND 00299-5980-30 8.20704 MIRVASO 0.33% GEL 0 GALDERMA LABORA GMGEN 00172-4430-49 0.58428 MISOPROSTOL 100 MCG TABLET 0 IVAX PHARMACEUT EAGEN 00172-4430-59 0.58428 MISOPROSTOL 100 MCG TABLET 0 IVAX PHARMACEUT EAGEN 43386-0160-<strong>06</strong> 0.58428 MISOPROSTOL 100 MCG TABLET 0 GAVIS PHARMACEU EAGEN 43386-0160-12 0.58428 MISOPROSTOL 100 MCG TABLET 0 GAVIS PHARMACEU EAGEN 59762-5007-01 0.58428 MISOPROSTOL 100 MCG TABLET 0 GREENSTONE LLC. EAGEN 59762-5007-02 0.58428 MISOPROSTOL 100 MCG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0040-01 0.58428 MISOPROSTOL 100 MCG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0040-11 0.58428 MISOPROSTOL 100 MCG TABLET 0 AHP EAGEN 00172-4431-49 0.89937 MISOPROSTOL 200 MCG TABLET 0 IVAX PHARMACEUT EAGEN 00172-4431-60 0.89962 MISOPROSTOL 200 MCG TABLET 0 IVAX PHARMACEUT EAGEN 43386-0161-01 1.07212 MISOPROSTOL 200 MCG TABLET 0 GAVIS PHARMACEU EAGEN 43386-0161-<strong>06</strong> 1.07250 MISOPROSTOL 200 MCG TABLET 0 GAVIS PHARMACEU EAGEN 43393-0203-04 1.63125 MISOPROSTOL 200 MCG TABLET 0 GENBIOPRO, INC. EAGEN 59762-50<strong>08</strong>-01 0.89937 MISOPROSTOL 200 MCG TABLET 0 GREENSTONE LLC. EAGEN 59762-50<strong>08</strong>-02 0.89962 MISOPROSTOL 200 MCG TABLET 0 GREENSTONE LLC. EAGEN 63704-00<strong>08</strong>-01 1.07175 MISOPROSTOL 200 MCG TABLET 0 PHARMACIST PHAR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0041-01 0.89925 MISOPROSTOL 200 MCG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0041-11 0.89925 MISOPROSTOL 200 MCG TABLET 0 AHP EAGEN 16729-01<strong>08</strong>-11 74.46000 MITOMYCIN 20 MG VIAL 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0247-11 74.46000 MITOMYCIN 20 MG VIAL 0 ACCORD <strong>HEALTH</strong>CA EAGEN 55390-0252-01 74.46000 MITOMYCIN 20 MG VIAL 0 BEDFORD LABS EAGEN 16729-0248-38 159.35680 MITOMYCIN 40 MG VIAL 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0246-05 50.40000 MITOMYCIN 5 MG VIAL 0 ACCORD <strong>HEALTH</strong>CA EAGEN 55390-0251-01 50.40000 MITOMYCIN 5 MG VIAL 0 BEDFORD LABS EAGEN 00703-4685-01 22.76475 MITOXANTRONE 20 MG/10 ML VIAL 0 TEVA PARENTERAL MLGEN 61703-0343-18 11.81250 MITOXANTRONE 20 MG/10 ML VIAL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00703-4680-01 19.48260 MITOXANTRONE 25 MG/12.5 ML VL 0 TEVA PARENTERAL MLGEN 61703-0343-65 11.81220 MITOXANTRONE 25 MG/12.5 ML VL 0 HOSPIRA MLGEN 00703-4686-01 20.47100 MITOXANTRONE 30 MG/15 ML VIAL 0 TEVA PARENTERAL MLGEN 61703-0343-66 17.31549 MITOXANTRONE 30 MG/15 ML VIAL 0 HOSPIRA MLBND 00597-0030-01 0.02565 8.76886 MOBIC 15 MG TABLET G BOEHRINGER ING. EABND 00597-0029-01 0.02565 5.73480 MOBIC 7.5 MG TABLET G BOEHRINGER ING. EABND 00597-0034-01 1.52703 MOBIC 7.5 MG/5 ML SUSPENSION G BOEHRINGER ING. MLGEN 00074-3197-16 0.65250 MODERIBA 200 MG TABLET G ABBVIE US LLC EAGEN 00074-3224-56 11.73951 MODERIBA 200-400 MG DOSEPACK G ABBVIE US LLC EAGEN 00074-3239-56 12.35732 MODERIBA 400-400 MG DOSEPACK G ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00074-3271-56 15.44691 MODERIBA 600-400 MG DOSEPACK G ABBVIE US LLC EAGEN 00074-3282-56 18.53598 MODERIBA 600-600 MG DOSEPACK G ABBVIE US LLC EABEX 50458-0171-15 0.90440 2.07431 MODICON 28 TABLET G JANSSEN PHARM. EAGEN 00093-5150-01 0.67163 MOEXIPRIL HCL 15 MG TABLET 0 TEVA USA EAGEN 00574-0112-15 0.67163 MOEXIPRIL HCL 15 MG TABLET 0 PADDOCK LABS. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 266LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0272-01 0.67163 MOEXIPRIL HCL 15 MG TABLET 0 APOTEX CORP EAGEN 68462-02<strong>08</strong>-01 0.67163 MOEXIPRIL HCL 15 MG TABLET 0 GLENMARK PHARMA EAGEN 00093-0017-01 0.70362 MOEXIPRIL HCL 7.5 MG TABLET 0 TEVA USA EAGEN 00574-0110-01 0.70362 MOEXIPRIL HCL 7.5 MG TABLET 0 PADDOCK LABS. EAGEN 60505-0271-01 0.70362 MOEXIPRIL HCL 7.5 MG TABLET 0 APOTEX CORP EAGEN 68462-0209-01 0.70362 MOEXIPRIL HCL 7.5 MG TABLET 0 GLENMARK PHARMA EAGEN 00093-5214-01 0.64787 MOEXIPRIL-HCTZ 15-12.5 MG TAB 0 TEVA USA EAGEN 68462-02<strong>06</strong>-01 0.64787 MOEXIPRIL-HCTZ 15-12.5 MG TAB 0 GLENMARK PHARMA EAGEN 00093-5215-01 0.64787 MOEXIPRIL-HCTZ 15-25 MG TABLET 0 TEVA USA EAGEN 68462-0205-01 0.64787 MOEXIPRIL-HCTZ 15-25 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-5213-01 0.64787 MOEXIPRIL-HCTZ 7.5-12.5 MG TAB 0 TEVA USA EAGEN 68462-0207-01 0.64787 MOEXIPRIL-HCTZ 7.5-12.5 MG TAB 0 GLENMARK PHARMA EAGEN 00115-1470-46 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 GLOBAL PHARM GMGEN 00115-1470-52 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 GLOBAL PHARM GMGEN 00713-<strong>06</strong>34-15 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 G & W LABS. GMGEN 00713-<strong>06</strong>34-37 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 G & W LABS. GMGEN 00781-7<strong>06</strong>6-19 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 SANDOZ GMGEN 00781-7<strong>06</strong>6-27 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 SANDOZ GMGEN 45802-0257-35 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 PERRIGO CO. GMGEN 45802-0257-42 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67405-0100-15 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 HARRIS PHARM GMGEN 67405-0100-45 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 HARRIS PHARM GMGEN 68462-0192-17 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 GLENMARK PHARMA GMGEN 68462-0192-55 0.46670 MOMETASONE FUROATE 0.1% CREAM 0 GLENMARK PHARMA GMGEN 00115-1469-46 0.26490 MOMETASONE FUROATE 0.1% OINT 0 GLOBAL PHARM GMGEN 00115-1469-52 0.26490 MOMETASONE FUROATE 0.1% OINT 0 GLOBAL PHARM GMGEN 00168-0271-15 0.26490 MOMETASONE FUROATE 0.1% OINT 0 SANDOZ GMGEN 00168-0271-46 0.26490 MOMETASONE FUROATE 0.1% OINT 0 SANDOZ GMGEN 00713-<strong>06</strong>35-15 0.26490 MOMETASONE FUROATE 0.1% OINT 0 G & W LABS. GMGEN 00713-<strong>06</strong>35-37 0.26490 MOMETASONE FUROATE 0.1% OINT 0 G & W LABS. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-7<strong>06</strong>8-27 0.26490 MOMETASONE FUROATE 0.1% OINT 0 SANDOZ GMGEN 45802-0119-37 0.26490 MOMETASONE FUROATE 0.1% OINT 0 PERRIGO CO. GMGEN 45802-0119-42 0.26490 MOMETASONE FUROATE 0.1% OINT 0 PERRIGO CO. GMGEN 67405-0300-15 0.26490 MOMETASONE FUROATE 0.1% OINT 0 HARRIS PHARM GMGEN 67405-0300-45 0.26490 MOMETASONE FUROATE 0.1% OINT 0 HARRIS PHARM GMGEN 68462-0225-17 0.26490 MOMETASONE FUROATE 0.1% OINT 0 GLENMARK PHARMA GMGEN 68462-0225-55 0.26490 MOMETASONE FUROATE 0.1% OINT 0 GLENMARK PHARMA GMGEN 00115-1471-54 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 GLOBAL PHARM MLGEN 00115-1471-55 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 GLOBAL PHARM MLGEN 00168-0272-30 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00168-0272-60 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 SANDOZ MLGEN 00713-0701-53 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 G & W LABS. MLGEN 00713-0701-85 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 G & W LABS. MLGEN 00781-7<strong>06</strong>7-30 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 SANDOZ MLGEN 45802-0118-46 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 PERRIGO CO. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 267LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0118-59 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 PERRIGO CO. MLGEN 51672-1305-03 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 TARO PHARM USA MLGEN 51672-1305-04 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 TARO PHARM USA MLGEN 67405-0275-30 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 HARRIS PHARM MLGEN 67405-0275-60 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 HARRIS PHARM MLGEN 68462-0385-02 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 GLENMARK PHARMA MLGEN 68462-0385-37 0.23<strong>08</strong>5 MOMETASONE FUROATE 0.1% SOLN 0 GLENMARK PHARMA MLGEX 16714-0360-01 0.49260 MONO-LINYAH 28 TABLET 0 NORTHSTAR RX LL EAGEX 16714-0360-04 0.49260 MONO-LINYAH 28 TABLET 0 NORTHSTAR RX LL EABND 00053-7633-02 0.76500 MONOCLATE-P 1,000 UNITS KIT 0 CSL BEHRING LLC--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00053-7634-02 0.76500 MONOCLATE-P 1,500 UNITS KIT 0 CSL BEHRING LLCBEX 52544-0247-28 0.49260 1.01<strong>08</strong>2 MONONESSA 28 TABLET 0 ACTAVIS PHARMA, EABND 00053-6233-02 0.97500 MONONINE 1,000 UNITS KIT 0 CSL BEHRING LLCGEN 00054-0259-13 0.34250 MONTELUKAST SOD 10 MG TABLET G ROXANE LABS. EAGEN 00054-0259-22 0.34250 MONTELUKAST SOD 10 MG TABLET G ROXANE LABS. EAGEN 00093-7426-10 0.34250 MONTELUKAST SOD 10 MG TABLET G TEVA USA EAGEN 00093-7426-56 0.34250 MONTELUKAST SOD 10 MG TABLET G TEVA USA EAGEN 00093-7426-98 0.34250 MONTELUKAST SOD 10 MG TABLET G TEVA USA EAGEN 00378-5201-93 0.34250 MONTELUKAST SOD 10 MG TABLET G MYLAN EAGEN 0<strong>06</strong>03-4655-02 0.34250 MONTELUKAST SOD 10 MG TABLET G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-4655-16 0.34250 MONTELUKAST SOD 10 MG TABLET G QUALITEST EAGEN 00781-5560-31 0.34250 MONTELUKAST SOD 10 MG TABLET G SANDOZ EAGEN 00781-5560-92 0.34250 MONTELUKAST SOD 10 MG TABLET G SANDOZ EAGEN 13668-0<strong>08</strong>1-30 0.34250 MONTELUKAST SOD 10 MG TABLET G TORRENT PHARMAC EAGEN 13668-0<strong>08</strong>1-90 0.34250 MONTELUKAST SOD 10 MG TABLET G TORRENT PHARMAC EAGEN 16729-0119-10 0.34250 MONTELUKAST SOD 10 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0119-15 0.34250 MONTELUKAST SOD 10 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 51079-0223-20 0.34250 MONTELUKAST SOD 10 MG TABLET G MYLAN INSTITUTI EAGEN 54458-<strong>08</strong>90-10 0.34250 MONTELUKAST SOD 10 MG TABLET G INTERNATIONAL L EAGEN 55111-0725-10 0.34250 MONTELUKAST SOD 10 MG TABLET G DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0725-30 0.34250 MONTELUKAST SOD 10 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0725-90 0.34250 MONTELUKAST SOD 10 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0725-94 0.34250 MONTELUKAST SOD 10 MG TABLET G DR.REDDY'S LAB EAGEN 59762-0030-01 0.34250 MONTELUKAST SOD 10 MG TABLET G GREENSTONE LLC. EAGEN 59762-0030-02 0.34250 MONTELUKAST SOD 10 MG TABLET G GREENSTONE LLC. EAGEN 60505-3562-03 0.34250 MONTELUKAST SOD 10 MG TABLET G APOTEX CORP EAGEN 60505-3562-<strong>08</strong> 0.34250 MONTELUKAST SOD 10 MG TABLET G APOTEX CORP EAGEN 60505-3562-09 0.34250 MONTELUKAST SOD 10 MG TABLET G APOTEX CORP EAGEN 62175-0210-32 0.34250 MONTELUKAST SOD 10 MG TABLET G KREMERS URBAN EAGEN 62175-0210-43 0.34250 MONTELUKAST SOD 10 MG TABLET G KREMERS URBAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62175-0210-46 0.34250 MONTELUKAST SOD 10 MG TABLET G KREMERS URBAN EAGEN 65862-0574-19 0.34250 MONTELUKAST SOD 10 MG TABLET G AUROBINDO PHARM EAGEN 65862-0574-30 0.34250 MONTELUKAST SOD 10 MG TABLET G AUROBINDO PHARM EAGEN 65862-0574-90 0.34250 MONTELUKAST SOD 10 MG TABLET G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-<strong>06</strong>20-01 0.34250 MONTELUKAST SOD 10 MG TABLET G AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 268LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-<strong>06</strong>20-11 0.34250 MONTELUKAST SOD 10 MG TABLET G AHP EAGEN 68462-0392-05 0.34250 MONTELUKAST SOD 10 MG TABLET G GLENMARK PHARMA EAGEN 68462-0392-90 0.34250 MONTELUKAST SOD 10 MG TABLET G GLENMARK PHARMA EAGEN 68645-0466-54 0.34250 MONTELUKAST SOD 10 MG TABLET G LEGACY PHARMACE EAGEN 00378-6040-93 4.23825 MONTELUKAST SOD 4 MG GRANULES G MYLAN EAGEN 55111-0763-03 4.24299 MONTELUKAST SOD 4 MG GRANULES G DR.REDDY'S LAB EAGEN 66993-0416-30 4.48824 MONTELUKAST SOD 4 MG GRANULES G PRASCO LABS EAGEN 66993-0416-81 4.48824 MONTELUKAST SOD 4 MG GRANULES G PRASCO LABS EAGEN 00054-0288-13 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G ROXANE LABS. EAGEN 00054-0288-22 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7424-56 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G TEVA USA EAGEN 00093-7424-98 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G TEVA USA EAGEN 00378-5204-93 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G MYLAN EAGEN 0<strong>06</strong>03-4653-02 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G QUALITEST EAGEN 0<strong>06</strong>03-4653-16 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G QUALITEST EAGEN 00781-5554-31 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G SANDOZ EAGEN 00781-5554-92 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G SANDOZ EAGEN 13668-0079-05 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G TORRENT PHARMAC EAGEN 13668-0079-30 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G TORRENT PHARMAC EAGEN 13668-0079-90 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0593-30 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G DR.REDDY'S LAB EAGEN 55111-0593-90 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G DR.REDDY'S LAB EAGEN 59762-0045-01 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G GREENSTONE LLC. EAGEN 59762-0045-02 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G GREENSTONE LLC. EAGEN 60505-3573-03 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G APOTEX CORP EAGEN 60505-3573-<strong>08</strong> 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G APOTEX CORP EAGEN 60505-3573-09 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G APOTEX CORP EAGEN 62175-0204-32 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G KREMERS URBAN EAGEN 62175-0204-43 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G KREMERS URBAN EAGEN 62175-0204-46 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G KREMERS URBAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0567-05 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G AUROBINDO PHARM EAGEN 65862-0567-30 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G AUROBINDO PHARM EAGEN 65862-0567-90 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-<strong>06</strong>38-21 0.39290 MONTELUKAST SOD 4 MG TAB CHEW G AHP EAGEN 00054-0289-13 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G ROXANE LABS. EAGEN 00054-0289-22 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G ROXANE LABS. EAGEN 00093-7425-56 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G TEVA USA EAGEN 00093-7425-98 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G TEVA USA EAGEN 00378-5205-93 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G MYLAN EAGEN 0<strong>06</strong>03-4654-02 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-4654-16 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G QUALITEST EAGEN 0<strong>06</strong>03-4654-32 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G QUALITEST EAGEN 00781-5555-31 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G SANDOZ EAGEN 00781-5555-92 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G SANDOZ EAGEN 13668-0<strong>08</strong>0-05 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 269LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-0<strong>08</strong>0-30 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G TORRENT PHARMAC EAGEN 13668-0<strong>08</strong>0-90 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G TORRENT PHARMAC EAGEN 55111-0594-30 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G DR.REDDY'S LAB EAGEN 55111-0594-90 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G DR.REDDY'S LAB EAGEN 59762-0046-01 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G GREENSTONE LLC. EAGEN 59762-0046-02 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G GREENSTONE LLC. EAGEN 60505-3574-03 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G APOTEX CORP EAGEN 60505-3574-<strong>08</strong> 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G APOTEX CORP EAGEN 60505-3574-09 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G APOTEX CORP EAGEN 62175-0205-32 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G KREMERS URBAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62175-0205-43 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G KREMERS URBAN EAGEN 62175-0205-46 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G KREMERS URBAN EAGEN 65862-0568-05 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G AUROBINDO PHARM EAGEN 65862-0568-30 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G AUROBINDO PHARM EAGEN 65862-0568-90 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-<strong>06</strong>19-01 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>19-11 0.38840 MONTELUKAST SOD 5 MG TAB CHEW G AHP EABND 00456-4300-<strong>08</strong> 49.89683 MONUROL 3 GM SACHET 0 FOREST PHARMACE EAGUL 43538-<strong>06</strong>00-30 0.14910 MORGIDOX 100 MG CAPSULE 0 MEDIMETRIKS PHA EAGUL 43538-<strong>06</strong>10-60 0.14910 MORGIDOX 100 MG CAPSULE 0 MEDIMETRIKS PHA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 65649-0201-75 72.75780 MOVIPREP POWDER PACKET G SALIX PHARMACEU EABND 59630-0142-03 14.52223 MOXATAG ER 775 MG TABLET G SHIONOGI PHARMA EABND 00<strong>06</strong>5-00<strong>06</strong>-03 33.48220 MOXEZA 0.5% EYE DROPS G ALCON LABS. MLGEN 0<strong>06</strong>03-4382-21 0.19390 MULT-VIT-FLUOR 0.5 MG TAB CHW 0 QUALITEST EAGEN 0<strong>06</strong>03-4382-28 0.19390 MULT-VIT-FLUOR 0.5 MG TAB CHW 0 QUALITEST EABND 00024-4142-10 5.71944 MULTAQ 400 MG TABLET 0 SAN<strong>OF</strong>I-AVENTIS EABND 00024-4142-18 5.71953 MULTAQ 400 MG TABLET 0 SAN<strong>OF</strong>I-AVENTIS EABND 00024-4142-60 5.71953 MULTAQ 400 MG TABLET 0 SAN<strong>OF</strong>I-AVENTIS EAGEN 0<strong>06</strong>03-4381-21 0.19390 MULTIVIT-FLUOR 0.25 MG TAB CHW 0 QUALITEST EAGEN 0<strong>06</strong>03-4381-28 0.19390 MULTIVIT-FLUOR 0.25 MG TAB CHW 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 44946-1023-01 0.19390 MULTIVIT-FLUOR 0.25 MG TAB CHW 0 SANCILIO & COMP EAGEN 64376-<strong>08</strong>13-01 0.19390 MULTIVIT-FLUOR 0.25 MG TAB CHW 0 BOCA PHARMACAL EAGEN 0<strong>06</strong>03-1449-47 0.15000 MULTIVIT-FLUOR 0.25 MG/ML DROP 0 QUALITEST MLGEN 64376-<strong>08</strong>20-50 0.15000 MULTIVIT-FLUOR 0.25 MG/ML DROP 0 BOCA PHARMACAL MLGEN 44946-1024-01 0.19390 MULTIVIT-FLUOR 0.5 MG TAB CHEW 0 SANCILIO & COMP EAGEN 64376-<strong>08</strong>14-01 0.19390 MULTIVIT-FLUOR 0.5 MG TAB CHW 0 BOCA PHARMACAL EAGEN 0<strong>06</strong>03-1450-47 0.14840 MULTIVIT-FLUOR 0.5 MG/ML DROP 0 QUALITEST MLGEN 64376-<strong>08</strong>22-50 0.14840 MULTIVIT-FLUOR 0.5MG/ML DROP 0 BOCA PHARMACAL MLGEN 0<strong>06</strong>03-4383-21 0.19390 MULTIVIT-FLUORIDE 1 MG TAB CHW 0 QUALITEST EAGEN 0<strong>06</strong>03-4383-28 0.19390 MULTIVIT-FLUORIDE 1 MG TAB CHW 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 44946-1025-01 0.19390 MULTIVIT-FLUORIDE 1 MG TAB CHW 0 SANCILIO & COMP EAGEN 59<strong>08</strong>8-0109-59 0.14400 MULTIVIT-FLUORIDE 1 MG TAB CHW 0 PURETEK CORPORA EAGEN 64376-<strong>08</strong>15-01 0.19390 MULTIVIT-FLUORIDE 1 MG TAB CHW 0 BOCA PHARMACAL EAGEN 0<strong>06</strong>03-1452-47 0.14840 MULTIVIT-IRON-FL 0.25 MG/ML 0 QUALITEST MLGEN 64376-<strong>08</strong>21-50 0.14840 MULTIVIT-IRON-FL 0.25 MG/ML 0 BOCA PHARMACAL ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 270LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 66993-0942-15 3.46250 MUPIROCIN 2% CREAM G PRASCO LABS GMGEN 66993-0942-31 2.93349 MUPIROCIN 2% CREAM G PRASCO LABS GMGEN 68462-0564-17 3.79100 MUPIROCIN 2% CREAM G GLENMARK PHARMA GMGEN 68462-0564-35 3.21200 MUPIROCIN 2% CREAM G GLENMARK PHARMA GMGEN 00093-1010-42 0.40930 MUPIROCIN 2% OINTMENT 0 TEVA USA GMGEN 00168-0352-22 0.40930 MUPIROCIN 2% OINTMENT 0 SANDOZ GMGEN 45802-0112-22 0.40930 MUPIROCIN 2% OINTMENT 0 PERRIGO CO. GMGEN 51672-1312-00 0.40930 MUPIROCIN 2% OINTMENT 0 TARO PHARM USA GMGEN 68462-0180-22 0.40930 MUPIROCIN 2% OINTMENT 0 GLENMARK PHARMA GMBND 55292-0911-51 148.32515 MUSTARGEN 10 MG VIAL 0 RECORDATI RARE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 67386-0911-51 148.32515 MUSTARGEN 10 MG VIAL 0 RECORDATI RARE EABND 44946-1020-05 0.32757 MVC-FLUORIDE 0.25 MG TAB CHEW 0 SANCILIO & COMP EABND 44946-1021-05 0.32757 MVC-FLUORIDE 0.5 MG TAB CHEW 0 SANCILIO & COMP EABND 44946-1022-05 0.32757 MVC-FLUORIDE 1 MG TAB CHEW 0 SANCILIO & COMP EAGEX 43386-<strong>06</strong>20-30 27.42000 MY WAY 1.5 MG TABLET 0 GAVIS PHARMACEU EABND 00469-3211-10 186.25200 MYCAMINE 100 MG VIAL 0 ASTELLAS PHARMA EABND 00469-3250-10 93.12600 MYCAMINE 50 MG VIAL 0 ASTELLAS PHARMA EABEX 00013-5301-17 16.13370 MYCOBUTIN 150 MG CAPSULE 0 PHARMACIA/UPJHN EAGEN 00054-0163-25 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 ROXANE LABS. EAGEN 00054-0163-29 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7334-01 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 TEVA USA EAGEN 00093-7334-05 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 TEVA USA EAGEN 00378-2250-01 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 MYLAN EAGEN 00378-2250-05 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 MYLAN EAGEN 00781-2<strong>06</strong>7-01 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 SANDOZ EAGEN 00781-2<strong>06</strong>7-05 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 SANDOZ EAGEN 00781-2<strong>06</strong>7-89 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 SANDOZ EAGEN 16729-0094-01 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0094-16 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 ACCORD <strong>HEALTH</strong>CA EAGEN 51079-0721-01 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0721-20 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 59762-0703-01 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 GREENSTONE LLC. EAGEN 60505-2968-01 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 APOTEX CORP EAGEN 60505-2968-05 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 APOTEX CORP EAGEN 60505-2968-07 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 APOTEX CORP EAGEN 64380-0726-<strong>06</strong> 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 STRIDES PHARMA EAGEN 67877-0266-01 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 ASCEND LABORATO EAGEN 67877-0266-05 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 ASCEND LABORATO EAGEN 68<strong>08</strong>4-0587-01 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-0587-11 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0130-01 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 68382-0130-05 0.27378 MYCOPHENOLATE 250 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 00054-0166-25 0.47210 MYCOPHENOLATE 500 MG TABLET 0 ROXANE LABS. EAGEN 00054-0166-29 0.47210 MYCOPHENOLATE 500 MG TABLET 0 ROXANE LABS. EAGEN 00093-7477-01 0.47210 MYCOPHENOLATE 500 MG TABLET 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 271LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7477-05 0.47210 MYCOPHENOLATE 500 MG TABLET 0 TEVA USA EAGEN 00378-4472-01 0.47210 MYCOPHENOLATE 500 MG TABLET 0 MYLAN EAGEN 00378-4472-05 0.47210 MYCOPHENOLATE 500 MG TABLET 0 MYLAN EAGEN 00781-5175-01 0.47210 MYCOPHENOLATE 500 MG TABLET 0 SANDOZ EAGEN 00781-5175-05 0.47210 MYCOPHENOLATE 500 MG TABLET 0 SANDOZ EAGEN 16729-0019-01 0.47210 MYCOPHENOLATE 500 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0019-16 0.47210 MYCOPHENOLATE 500 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 51079-0379-01 0.47210 MYCOPHENOLATE 500 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0379-20 0.47210 MYCOPHENOLATE 500 MG TABLET 0 MYLAN INSTITUTI EAGEN 59762-0702-01 0.47210 MYCOPHENOLATE 500 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2967-01 0.47210 MYCOPHENOLATE 500 MG TABLET 0 APOTEX CORP EAGEN 60505-2967-05 0.47210 MYCOPHENOLATE 500 MG TABLET 0 APOTEX CORP EAGEN 60505-2967-07 0.47210 MYCOPHENOLATE 500 MG TABLET 0 APOTEX CORP EAGEN 64380-0725-<strong>06</strong> 0.47210 MYCOPHENOLATE 500 MG TABLET 0 STRIDES PHARMA EAGEN 68<strong>08</strong>4-0588-01 0.47210 MYCOPHENOLATE 500 MG TABLET 0 AHP EAGEN 68382-0131-01 0.47210 MYCOPHENOLATE 500 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0131-05 0.47210 MYCOPHENOLATE 500 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00378-4201-78 3.42662 MYCOPHENOLIC ACID DR 180 MG TB 0 MYLAN EAGEN 00378-4202-78 6.85312 MYCOPHENOLIC ACID DR 360 MG TB 0 MYLAN EAGEN 00<strong>06</strong>5-0342-03 2.0<strong>08</strong>50 MYDFRIN 2.5% EYE DROPS 0 ALCON LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00998-0342-05 2.0<strong>08</strong>50 MYDFRIN 2.5% EYE DROPS 0 ALCON (P.R.) MLGUL 00<strong>06</strong>5-0355-03 0.70000 MYDRIACYL 1% EYE DROPS 0 ALCON LABS. MLGUL 00998-0355-15 0.70000 MYDRIACYL 1% EYE DROPS 0 ALCON (P.R.) MLBND 00078-0385-66 4.21812 MYFORTIC 180 MG TABLET 0 NOVARTIS EABND 00078-0386-66 8.43618 MYFORTIC 360 MG TABLET 0 NOVARTIS EABND 278<strong>08</strong>-0004-04 0.19244 MYKIDZ IRON FL SUSPENSION 0 TRIS PHARMA INC MLBND 00173-0713-25 5.33856 MYLERAN 2 MG TABLET 0 PRASCO LABS EAGEN 58809-0333-15 7.04400 MYOXIN OTIC SUSPENSION 0 G.M. PHARM MLBND 00469-2601-30 7.54525 MYRBETRIQ ER 25 MG TABLET G ASTELLAS PHARMA EABND 00469-2601-71 7.54519 MYRBETRIQ ER 25 MG TABLET G ASTELLAS PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00469-2601-90 7.54525 MYRBETRIQ ER 25 MG TABLET G ASTELLAS PHARMA EABND 00469-2602-30 7.54525 MYRBETRIQ ER 50 MG TABLET G ASTELLAS PHARMA EABND 00469-2602-71 7.54519 MYRBETRIQ ER 50 MG TABLET G ASTELLAS PHARMA EABND 00469-2602-90 7.54525 MYRBETRIQ ER 50 MG TABLET G ASTELLAS PHARMA EABEX 66490-<strong>06</strong>91-10 0.18660 10.72177 MYSOLINE 250 MG TABLET G VALEANT EABEX 66490-<strong>06</strong>90-10 0.09520 3.11557 MYSOLINE 50 MG TABLET G VALEANT EAGEX 0<strong>06</strong>03-7625-17 0.73260 MYZILRA-28 TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-7625-49 0.73260 MYZILRA-28 TABLET 0 QUALITEST EAGEN 00093-1015-01 0.25640 NABUMETONE 500 MG TABLET 0 TEVA USA EAGEN 00093-1015-10 0.25640 NABUMETONE 500 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0145-01 0.25640 NABUMETONE 500 MG TABLET 0 SANDOZ EAGEN 00185-0145-05 0.25640 NABUMETONE 500 MG TABLET 0 SANDOZ EAGEN 00378-3015-01 0.25640 NABUMETONE 500 MG TABLET 0 MYLAN EAGEN 00378-3015-05 0.25640 NABUMETONE 500 MG TABLET 0 MYLAN EAGEN 00591-3670-01 0.25640 NABUMETONE 500 MG TABLET 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 272LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3670-05 0.25640 NABUMETONE 500 MG TABLET 0 ACTAVIS PHARMA, EAGEN 68<strong>08</strong>4-0051-01 0.25640 NABUMETONE 500 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0542-01 0.25640 NABUMETONE 500 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0542-11 0.25640 NABUMETONE 500 MG TABLET 0 AHP EAGEN 68180-0141-01 0.25640 NABUMETONE 500 MG TABLET 0 LUPIN PHARMACEU EAGEN 68462-0358-01 0.25640 NABUMETONE 500 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0358-05 0.25640 NABUMETONE 500 MG TABLET 0 GLENMARK PHARMA EAGEN 76282-0257-01 0.25640 NABUMETONE 500 MG TABLET 0 EXELAN PHARMACE EAGEN 00093-1016-01 0.27689 NABUMETONE 750 MG TABLET 0 TEVA USA EAGEN 00093-1016-10 0.27689 NABUMETONE 750 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-0146-01 0.27689 NABUMETONE 750 MG TABLET 0 SANDOZ EAGEN 00185-0146-05 0.27689 NABUMETONE 750 MG TABLET 0 SANDOZ EAGEN 00378-3016-01 0.27689 NABUMETONE 750 MG TABLET 0 MYLAN EAGEN 00378-3016-05 0.27689 NABUMETONE 750 MG TABLET 0 MYLAN EAGEN 00591-3671-01 0.27689 NABUMETONE 750 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-3671-05 0.27689 NABUMETONE 750 MG TABLET 0 ACTAVIS PHARMA, EAGEN 68180-0142-01 0.27689 NABUMETONE 750 MG TABLET 0 LUPIN PHARMACEU EAGEN 68462-0359-01 0.27689 NABUMETONE 750 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0359-05 0.27689 NABUMETONE 750 MG TABLET 0 GLENMARK PHARMA EAGUL 00093-4235-01 0.46500 NADOLOL 20 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-0028-01 0.46500 NADOLOL 20 MG TABLET G MYLAN EAGUL 00781-1181-01 0.46500 NADOLOL 20 MG TABLET G SANDOZ EAGUL 00781-1181-10 0.46500 NADOLOL 20 MG TABLET G SANDOZ EAGUL 00781-1181-92 0.46500 NADOLOL 20 MG TABLET G SANDOZ EAGUL 00093-4236-01 0.42890 NADOLOL 40 MG TABLET G TEVA USA EAGUL 00378-1171-01 0.42890 NADOLOL 40 MG TABLET G MYLAN EAGUL 00378-1171-10 0.42890 NADOLOL 40 MG TABLET G MYLAN EAGUL 00781-1182-01 0.42890 NADOLOL 40 MG TABLET G SANDOZ EAGUL 00781-1182-10 0.42890 NADOLOL 40 MG TABLET G SANDOZ EAGUL 00781-1182-92 0.42890 NADOLOL 40 MG TABLET G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00093-4237-01 0.80250 NADOLOL 80 MG TABLET G TEVA USA EAGUL 00378-1132-01 0.80250 NADOLOL 80 MG TABLET G MYLAN EAGUL 00378-1132-10 0.80250 NADOLOL 80 MG TABLET G MYLAN EAGUL 00781-1183-01 0.80250 NADOLOL 80 MG TABLET G SANDOZ EAGUL 00781-1183-10 0.80250 NADOLOL 80 MG TABLET G SANDOZ EAGUL 00781-1183-92 0.80250 NADOLOL 80 MG TABLET G SANDOZ EAGEN 00115-5311-01 1.82730 NADOLOL-BENDR<strong>OF</strong>LU 40-5 MG TAB G GLOBAL PHARM EAGEN 00115-5322-01 1.96820 NADOLOL-BENDR<strong>OF</strong>LU 80-5 MG TAB G GLOBAL PHARM EABND 00781-3128-15 8.29000 14.54160 NAFCILLIN 1 GM ADD-VAN VIAL 0 SANDOZ EABND 00781-3128-92 8.29000 15.98<strong>08</strong>2 NAFCILLIN 1 GM ADD-VAN VIAL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00781-9224-15 8.29000 14.54160 NAFCILLIN 1 GM ADD-VAN VIAL 0 SANDOZ/NOVAPLUS EABND 00781-9224-92 8.29000 15.98<strong>08</strong>2 NAFCILLIN 1 GM ADD-VAN VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-3124-85 10.95000 NAFCILLIN 1 GM VIAL 0 SANDOZ EAGEN 00781-9124-85 10.94250 NAFCILLIN 1 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9124-95 10.94925 NAFCILLIN 1 GM VIAL 0 SANDOZ/NOVAPLUS EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 273LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 25021-0139-10 10.12500 NAFCILLIN 1 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-0221-10 9.90000 NAFCILLIN 1 GM VIAL 0 WG CRITICAL CAR EAGEN 55150-0122-15 10.87500 NAFCILLIN 1 GM VIAL 0 AUROMEDICS PHAR EAGEN 63323-0327-10 11.46600 NAFCILLIN 1 GM VIAL 0 APP PHARMACEUTI EABND 00338-1017-41 0.28784 NAFCILLIN 1 GM/ 50 ML INJ 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00781-9126-95 104.07975 NAFCILLIN 10 GM BULK VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-3126-46 104.07750 NAFCILLIN 10 GM VIAL 0 SANDOZ EAGEN 00781-3126-95 104.07975 NAFCILLIN 10 GM VIAL 0 SANDOZ EAGEN 25021-0141-99 97.23250 NAFCILLIN 10 GM VIAL 0 SAGENT PHARMACE EAGEN 55150-0124-99 97.23250 NAFCILLIN 10 GM VIAL 0 AUROMEDICS PHAR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0330-60 97.23250 NAFCILLIN 10 GM VIAL 0 APP PHARMACEUTI EABND 00781-3129-15 28.21170 NAFCILLIN 2 GM ADD-VANT VIAL 0 SANDOZ EABND 00781-9225-92 31.00050 NAFCILLIN 2 GM ADD-VANT VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-3125-85 21.24000 NAFCILLIN 2 GM VIAL 0 SANDOZ EAGEN 00781-3125-95 21.24075 NAFCILLIN 2 GM VIAL 0 SANDOZ EAGEN 00781-9125-85 21.24000 NAFCILLIN 2 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9125-95 21.24075 NAFCILLIN 2 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0140-10 20.25000 NAFCILLIN 2 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-0222-10 19.80000 NAFCILLIN 2 GM VIAL 0 WG CRITICAL CAR EAGEN 55150-0123-15 21.2<strong>06</strong>25 NAFCILLIN 2 GM VIAL 0 AUROMEDICS PHAR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0328-20 22.17920 NAFCILLIN 2 GM VIAL 0 APP PHARMACEUTI EABND 00338-1019-48 0.207<strong>06</strong> NAFCILLIN 2 GM/ 100 ML INJ 0 BAXTER <strong>HEALTH</strong>CA MLBND 00259-4126-60 5.01403 NAFTIN 1% CREAM G MERZ GMBND 00259-4126-90 3.34268 NAFTIN 1% CREAM G MERZ GMBND 00259-4770-40 7.52104 NAFTIN 1% GEL G MERZ GMBND 00259-4770-60 5.01403 NAFTIN 1% GEL G MERZ GMBND 00259-4770-90 3.34268 NAFTIN 1% GEL G MERZ GMBND 00259-1102-45 6.50941 NAFTIN 2% CREAM G MERZ GMBND 00259-1202-45 6.50941 NAFTIN 2% GEL G MERZ GMBND 00409-1463-01 1.60700 3.29676 NALBUPHINE 10 MG/ML AMPUL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-1463-49 1.60700 3.26688 NALBUPHINE 10 MG/ML AMPUL 0 HOSPIRA/NOVATIO MLBND 00409-1464-01 1.44640 2.92126 NALBUPHINE 100 MG/10 ML VIAL 0 HOSPIRA MLGEN 00409-1465-49 3.19240 NALBUPHINE 20 MG/ML AMPUL 0 HOSPIRA/NOVATIO MLBND 00409-1782-69 15.37824 NALOXONE 0.4 MG/ML SYRINGE 0 HOSPIRA MLBND 00409-1215-01 21.90204 NALOXONE 0.4 MG/ML VIAL 0 HOSPIRA MLGEN 00185-0039-01 1.0<strong>08</strong>00 NALTREXONE 50 MG TABLET 0 SANDOZ EAGEN 004<strong>06</strong>-1170-01 1.0<strong>08</strong>00 NALTREXONE 50 MG TABLET 0 MALLINCKRODT PH EAGEN 004<strong>06</strong>-1170-03 1.0<strong>08</strong>00 NALTREXONE 50 MG TABLET 0 MALLINCKRODT PH EAGEN 00555-0902-01 1.0<strong>08</strong>00 NALTREXONE 50 MG TABLET 0 BARR EAGEN 00555-0902-02 1.0<strong>08</strong>00 NALTREXONE 50 MG TABLET 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16729-0<strong>08</strong>1-01 1.0<strong>08</strong>00 NALTREXONE 50 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0<strong>08</strong>1-10 1.0<strong>08</strong>00 NALTREXONE 50 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 47335-0326-83 1.0<strong>08</strong>00 NALTREXONE 50 MG TABLET 0 SUN PHARMA GLOB EAGEN 47335-0326-88 1.0<strong>08</strong>00 NALTREXONE 50 MG TABLET 0 SUN PHARMA GLOB EAGEN 68<strong>08</strong>4-0291-21 1.0<strong>08</strong>00 NALTREXONE 50 MG TABLET 0 AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 274LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00456-3400-29 9.<strong>08</strong>435 NAMENDA XR TITRATION PACK G FOREST PHARMACE EABND 00456-3414-33 9.<strong>08</strong>407 NAMENDA XR 14 MG CAPSULE G FOREST PHARMACE EABND 00456-3414-63 9.<strong>08</strong>293 NAMENDA XR 14 MG CAPSULE G FOREST PHARMACE EABND 00456-3414-90 9.<strong>08</strong>407 NAMENDA XR 14 MG CAPSULE G FOREST PHARMACE EABND 00456-3421-33 9.<strong>08</strong>407 NAMENDA XR 21 MG CAPSULE G FOREST PHARMACE EABND 00456-3428-33 9.<strong>08</strong>407 NAMENDA XR 28 MG CAPSULE G FOREST PHARMACE EABND 00456-3428-63 9.<strong>08</strong>293 NAMENDA XR 28 MG CAPSULE G FOREST PHARMACE EABND 00456-3428-90 9.<strong>08</strong>407 NAMENDA XR 28 MG CAPSULE G FOREST PHARMACE EABND 00456-3407-33 9.<strong>08</strong>407 NAMENDA XR 7 MG CAPSULE G FOREST PHARMACE EABND 00456-3210-60 4.78094 NAMENDA 10 MG TABLET 0 FOREST PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00456-3202-12 1.67226 NAMENDA 10 MG/5 ML SOLUTION 0 FOREST PHARMACE MLBND 00456-3205-60 4.78094 NAMENDA 5 MG TABLET 0 FOREST PHARMACE EABND 00456-3200-14 4.77995 NAMENDA 5-10 MG TITRATION PK 0 FOREST PHARMACE EABND 17478-0216-12 0.21350 0.39452 NAPHAZOLINE 0.1% EYE DROPS 0 AKORN INC. MLBND 59630-0375-10 17.92800 NAPRELAN CR 375 MG TABLET G ALMATICA PHARMA EABND 68453-0375-10 8.51140 NAPRELAN CR 375 MG TABLET G SHIONOGI PHARMA EABND 59630-<strong>08</strong>50-75 17.92800 NAPRELAN CR 500 MG TABLET G ALMATICA PHARMA EABND 68453-<strong>08</strong>50-75 8.47905 NAPRELAN CR 500 MG TABLET G SHIONOGI PHARMA EABND 59630-0777-03 17.92800 NAPRELAN CR 750 MG TABLET G ALMATICA PHARMA EABND 68453-0777-03 8.49200 NAPRELAN CR 750 MG TABLET G SHIONOGI PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00004-6416-01 0.17024 2.56104 NAPROSYN EC 500 MG TABLET G ROCHE LABS. EABND 00004-0028-28 0.1<strong>08</strong>88 0.16746 NAPROSYN 125 MG/5 ML SUSPEN G ROCHE LABS. MLBND 00004-6313-01 0.04388 1.72664 NAPROSYN 250 MG TABLET G ROCHE LABS. EABND 00004-6314-01 0.05160 2.21950 NAPROSYN 375 MG TABLET G ROCHE LABS. EABND 00004-6316-01 0.<strong>06</strong>370 2.71<strong>06</strong>9 NAPROSYN 500 MG TABLET G ROCHE LABS. EAGEN 00093-1005-01 0.15130 NAPROXEN DR 375 MG TABLET 0 TEVA USA EAGEN 00093-1005-05 0.15130 NAPROXEN DR 375 MG TABLET 0 TEVA USA EAGEN 31722-0338-01 0.15130 NAPROXEN DR 375 MG TABLET 0 CAMBER PHARMACE EAGEN 00093-10<strong>06</strong>-01 0.17024 NAPROXEN DR 500 MG TABLET 0 TEVA USA EAGEN 00093-10<strong>06</strong>-05 0.17024 NAPROXEN DR 500 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0339-01 0.17024 NAPROXEN DR 500 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0339-05 0.17024 NAPROXEN DR 500 MG TABLET 0 CAMBER PHARMACE EAGEN 00781-1646-01 0.15130 NAPROXEN EC 375 MG TABLET 0 SANDOZ EAGEN 00781-1653-01 0.17024 NAPROXEN EC 500 MG TABLET 0 SANDOZ EAGEN 00093-0536-01 0.09356 NAPROXEN SODIUM 275 MG TAB 0 TEVA USA EAGEN 00093-0536-05 0.09356 NAPROXEN SODIUM 275 MG TAB 0 TEVA USA EAGEN 00093-0536-10 0.09356 NAPROXEN SODIUM 275 MG TAB 0 TEVA USA EAGEN 00143-9916-01 0.09356 NAPROXEN SODIUM 275 MG TAB 0 WEST-WARD,INC. EAGEN 53746-0193-01 0.09356 NAPROXEN SODIUM 275 MG TAB 0 AMNEAL PHARMACE EAGEN 53746-0193-05 0.09356 NAPROXEN SODIUM 275 MG TAB 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0178-01 0.09356 NAPROXEN SODIUM 275 MG TAB 0 GLENMARK PHARMA EAGEN 68462-0178-05 0.09356 NAPROXEN SODIUM 275 MG TAB 0 GLENMARK PHARMA EAGEN 00093-0537-01 0.11950 NAPROXEN SODIUM 550 MG TAB 0 TEVA USA EAGEN 00093-0537-05 0.11950 NAPROXEN SODIUM 550 MG TAB 0 TEVA USA EAGEN 00093-0537-10 0.11950 NAPROXEN SODIUM 550 MG TAB 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 275LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-99<strong>08</strong>-01 0.11950 NAPROXEN SODIUM 550 MG TAB 0 WEST-WARD,INC. EAGEN 00143-99<strong>08</strong>-05 0.11950 NAPROXEN SODIUM 550 MG TAB 0 WEST-WARD,INC. EAGEN 53746-0194-01 0.11950 NAPROXEN SODIUM 550 MG TAB 0 AMNEAL PHARMACE EAGEN 53746-0194-05 0.11950 NAPROXEN SODIUM 550 MG TAB 0 AMNEAL PHARMACE EAGEN 58517-0300-30 0.11950 NAPROXEN SODIUM 550 MG TAB 0 <strong>NEW</strong> HORIZON RX EAGEN 68462-0179-01 0.11950 NAPROXEN SODIUM 550 MG TAB 0 GLENMARK PHARMA EAGEN 68462-0179-05 0.11950 NAPROXEN SODIUM 550 MG TAB 0 GLENMARK PHARMA EAGEN 00054-3630-63 0.07533 NAPROXEN 125 MG/5 ML SUSPEN 0 ROXANE LABS. MLGEN 68134-0363-01 0.09999 NAPROXEN 125 MG/5 ML SUSPEN 0 PALMETTO PHARMA MLGEN 00093-0147-01 0.04388 NAPROXEN 250 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-0147-05 0.04388 NAPROXEN 250 MG TABLET 0 TEVA USA EAGEN 00143-1346-01 0.04388 NAPROXEN 250 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1346-05 0.04388 NAPROXEN 250 MG TABLET 0 WEST-WARD,INC. EAGEN 00904-6<strong>06</strong>9-61 0.04388 NAPROXEN 250 MG TABLET 0 MAJOR PHARMACEU EAGEN 31722-0340-01 0.04388 NAPROXEN 250 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0340-05 0.04388 NAPROXEN 250 MG TABLET 0 CAMBER PHARMACE EAGEN 428<strong>06</strong>-0124-05 0.04388 NAPROXEN 250 MG TABLET 0 EPIC PHARMA LLC EAGEN 53746-0188-01 0.04388 NAPROXEN 250 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0188-05 0.04388 NAPROXEN 250 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0188-10 0.04388 NAPROXEN 250 MG TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0520-01 0.04388 NAPROXEN 250 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0520-05 0.04388 NAPROXEN 250 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0520-99 0.04388 NAPROXEN 250 MG TABLET 0 AUROBINDO PHARM EAGEN 68462-0188-01 0.04388 NAPROXEN 250 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0188-05 0.04388 NAPROXEN 250 MG TABLET 0 GLENMARK PHARMA EAGEN 00093-0148-01 0.05160 NAPROXEN 375 MG TABLET 0 TEVA USA EAGEN 00093-0148-05 0.05160 NAPROXEN 375 MG TABLET 0 TEVA USA EAGEN 00143-1347-01 0.05160 NAPROXEN 375 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1347-05 0.05160 NAPROXEN 375 MG TABLET 0 WEST-WARD,INC. EAGEN 31722-0341-01 0.05160 NAPROXEN 375 MG TABLET 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0341-05 0.05160 NAPROXEN 375 MG TABLET 0 CAMBER PHARMACE EAGEN 428<strong>06</strong>-0125-01 0.05160 NAPROXEN 375 MG TABLET 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0125-05 0.05160 NAPROXEN 375 MG TABLET 0 EPIC PHARMA LLC EAGEN 53746-0189-01 0.05160 NAPROXEN 375 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0189-05 0.05160 NAPROXEN 375 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0189-10 0.05160 NAPROXEN 375 MG TABLET 0 AMNEAL PHARMACE EAGEN 65862-0521-01 0.05160 NAPROXEN 375 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0521-05 0.05160 NAPROXEN 375 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0521-99 0.05160 NAPROXEN 375 MG TABLET 0 AUROBINDO PHARM EAGEN 68462-0189-01 0.05160 NAPROXEN 375 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0189-05 0.05160 NAPROXEN 375 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0189-60 0.05160 NAPROXEN 375 MG TABLET 0 GLENMARK PHARMA EAGEN 00093-0149-01 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 TEVA USA EAGEN 00093-0149-05 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 TEVA USA EAGEN 00093-0149-10 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 276LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-1348-01 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1348-05 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 WEST-WARD,INC. EAGEN 31722-0342-01 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0342-05 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 CAMBER PHARMACE EAGEN 428<strong>06</strong>-0126-01 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0126-05 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0126-10 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 EPIC PHARMA LLC EAGEN 52605-0140-10 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 POLYGEN PHARMAC EAGEN 53746-0190-01 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0190-05 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53746-0190-10 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 65862-0522-05 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0127-01 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 AHP EAGEN 68462-0190-01 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0190-05 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0190-30 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0190-50 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0190-60 0.<strong>06</strong>370 NAPROXEN 500 MG TABLET 0 GLENMARK PHARMA EAGEN 00054-0278-03 5.72429 NARATRIPTAN HCL 1 MG TABLET G ROXANE LABS. EAGEN 00093-8522-19 5.72429 NARATRIPTAN HCL 1 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-8522-90 5.72429 NARATRIPTAN HCL 1 MG TABLET G TEVA USA EAGEN 00378-4450-59 5.72429 NARATRIPTAN HCL 1 MG TABLET G MYLAN EAGEN 00574-0214-09 5.72429 NARATRIPTAN HCL 1 MG TABLET G PADDOCK LABS. EAGEN 23155-0054-19 5.72429 NARATRIPTAN HCL 1 MG TABLET G HERITAGE PHARMA EAGEN 42043-0130-09 5.72429 NARATRIPTAN HCL 1 MG TABLET G KARALEX PHARMA, EAGEN 00054-0279-03 4.76000 NARATRIPTAN HCL 2.5 MG TABLET G ROXANE LABS. EAGEN 00093-8523-19 4.76000 NARATRIPTAN HCL 2.5 MG TABLET G TEVA USA EAGEN 00378-4451-59 4.76000 NARATRIPTAN HCL 2.5 MG TABLET G MYLAN EAGEN 00574-0215-09 4.76000 NARATRIPTAN HCL 2.5 MG TABLET G PADDOCK LABS. EAGEN 23155-0055-19 4.76000 NARATRIPTAN HCL 2.5 MG TABLET G HERITAGE PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42043-0131-09 4.76000 NARATRIPTAN HCL 2.5 MG TABLET G KARALEX PHARMA, EAGEN 62756-0437-69 4.76000 NARATRIPTAN HCL 2.5 MG TABLET G SUN PHARMACEUTI EAGEN 16714-0290-01 5.72429 NARATRIPTAN 1 MG TABLET G NORTHSTAR RX LL EAGEN 16714-0290-02 5.72429 NARATRIPTAN 1 MG TABLET G NORTHSTAR RX LL EAGEN 16714-0291-01 4.76000 NARATRIPTAN 2.5 MG TABLET G NORTHSTAR RX LL EAGEN 16714-0291-02 4.76000 NARATRIPTAN 2.5 MG TABLET G NORTHSTAR RX LL EABEX 00071-0350-60 0.65460 1.22867 NARDIL 15 MG TABLET 0 PFIZER US PHARM EABND 00075-15<strong>06</strong>-16 5.28410 6.85680 NASACORT AQ NASAL SPRAY G SAN<strong>OF</strong>I-AVENTIS GMBND 49884-0270-86 276.68369 NASCOBAL 500 MCG NASAL SPRAY 0 PAR PHARMACEUTI MLBND 00<strong>08</strong>5-1288-01 9.27647 NASONEX 50 MCG NASAL SPRAY 0 MERCK SHARP & D GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>5-<strong>06</strong>45-15 17.47648 NATACYN EYE DROPS 0 ALCON LABS. MLBEX 50419-0409-03 3.67117 NATAZIA 28 TABLET 0 BAYER,PHARM DIV EAGEN 00591-3355-01 1.21270 NATEGLINIDE 120 MG TABLET G ACTAVIS PHARMA, EAGEN 49884-0985-01 1.21270 NATEGLINIDE 120 MG TABLET G PAR PHARM. EAGEN 55111-0329-90 1.21270 NATEGLINIDE 120 MG TABLET G DR.REDDY'S LAB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 277LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0459-11 1.21270 NATEGLINIDE 120 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0459-21 1.21270 NATEGLINIDE 120 MG TABLET G AHP EAGEN 00591-3354-01 0.85830 NATEGLINIDE 60 MG TABLET G ACTAVIS PHARMA, EAGEN 49884-0984-01 0.85830 NATEGLINIDE 60 MG TABLET G PAR PHARM. EAGEN 55111-0328-90 0.85830 NATEGLINIDE 60 MG TABLET G DR.REDDY'S LAB EAGEN 68<strong>08</strong>4-0458-11 0.85830 NATEGLINIDE 60 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0458-21 0.85830 NATEGLINIDE 60 MG TABLET G AHP EABND 18860-0752-01 3.969<strong>06</strong> NATELLE ONE CAPSULE 0 MEDA PHARMACEUT EABND 52246-0929-04 1.92677 NATROBA 0.9% TOPICAL SUSP 0 PARAPRO LLC MLBND 63323-<strong>08</strong>77-15 98.35500 NEBUPENT 300 MG INHAL POWDER 0 APP PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 52544-0550-28 0.86<strong>08</strong>9 NECON 0.5-35-28 TABLET 0 ACTAVIS PHARMA, EAGEX 52544-0552-28 0.73890 NECON 1-35-28 TABLET 0 ACTAVIS PHARMA, EABEX 52544-0245-31 0.82890 0.87357 NECON 1-50-28 TABLET 0 ACTAVIS PHARMA, EABEX 52544-0554-28 0.87860 0.95272 NECON 10-11-28 TABLET 0 ACTAVIS PHARMA, EABEX 52544-0936-28 0.83640 0.95440 NECON 7-7-7-28 TABLET 0 ACTAVIS PHARMA, EABEX 00093-1024-<strong>06</strong> 1.67715 NEFAZODONE HCL 100 MG TABLET 0 TEVA USA EABEX 00093-7113-<strong>06</strong> 1.7<strong>08</strong>82 NEFAZODONE HCL 150 MG TABLET 0 TEVA USA EABEX 00093-1025-<strong>06</strong> 0.44880 1.74092 NEFAZODONE HCL 200 MG TABLET 0 TEVA USA EABEX 00093-1026-<strong>06</strong> 0.46920 1.77315 NEFAZODONE HCL 250 MG TABLET 0 TEVA USA EABEX 00093-7178-01 0.48411 1.63767 NEFAZODONE HCL 50 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 17478-0719-35 13.21714 NEO-BACIT-POLY-HC EYE OINTMENT G AKORN INC. GMGEN 242<strong>08</strong>-0785-55 13.93928 NEO-BACIT-POLY-HC EYE OINTMENT G VALEANT GMGEN 48102-00<strong>06</strong>-35 10.09250 NEO-POLYCIN EYE OINTMENT G PERRIGO CO. GMGEN 48102-0005-35 12.85285 NEO-POLYCIN HC EYE OINTMENT G PERRIGO CO. GMGEN 00591-2190-45 9.10490 NEOMY-POLYMYXIN B 40 MG/ML AMP 0 ACTAVIS PHARMA, MLGEN 00591-2190-50 9.10490 NEOMY-POLYMYXIN B 40 MG/ML AMP 0 ACTAVIS PHARMA, MLGEN 00591-2190-54 9.10490 NEOMY-POLYMYXIN B 40 MG/ML AMP 0 ACTAVIS PHARMA, MLGEN 39822-1201-02 9.10490 NEOMY-POLYMYXIN B 40 MG/ML AMP 0 X-GEN PHARMACEU MLGEN 39822-1201-05 9.10490 NEOMY-POLYMYXIN B 40 MG/ML AMP 0 X-GEN PHARMACEU MLGEN 39822-1220-01 4.96000 NEOMY-POLYMYXIN B 40 MG/ML VL 0 X-GEN PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 17478-0235-35 10.09250 NEOMYC-BACIT-POLYMIX EYE OINT G AKORN INC. GMGEN 242<strong>08</strong>-0780-55 10.09250 NEOMYC-BACIT-POLYMIX EYE OINT G BAUSCH & LOMB P GMGUL 242<strong>08</strong>-0795-35 1.07140 NEOMYC-POLYM-DEXAMET EYE OINTM 0 VALEANT GMGUL 48102-0003-35 1.07140 NEOMYC-POLYM-DEXAMET EYE OINTM 0 PERRIGO CO. GMGUL 61314-<strong>06</strong>31-36 1.07140 NEOMYC-POLYM-DEXAMET EYE OINTM 0 SANDOZ GMGEN 242<strong>08</strong>-<strong>08</strong>30-60 2.97900 NEOMYC-POLYM-DEXAMETH EYE DROP 0 VALEANT MLGEN 61314-<strong>06</strong>30-<strong>06</strong> 3.<strong>06</strong>220 NEOMYC-POLYM-DEXAMETH EYE DROP 0 SANDOZ MLGEN 00574-4009-10 1.89750 NEOMYC-POLYM-GRAMICID EYE DROP 0 PADDOCK LABS. MLGUL 242<strong>08</strong>-0790-62 2.02500 NEOMYC-POLYM-GRAMICID EYE DROP 0 VALEANT MLGEN 00093-1177-01 0.94339 NEOMYCIN 500 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 39822-0310-05 0.94339 NEOMYCIN 500 MG TABLET 0 X-GEN PHARMACEU EAGEN 50383-0565-10 0.94339 NEOMYCIN 500 MG TABLET 0 HI-TECH PHARMAC EAGEN 51991-0738-01 0.94339 NEOMYCIN 500 MG TABLET 0 BRECKENRIDGE EABND 61314-<strong>06</strong>41-75 15.59514 NEOMYCIN-POLY-HC EYE DROPS G SANDOZ MLGEN 242<strong>08</strong>-<strong>06</strong>31-10 1.82437 NEOMYCIN-POLYMYXIN-HC EAR SOLN 0 VALEANT ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 278LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61314-<strong>06</strong>46-10 1.82437 NEOMYCIN-POLYMYXIN-HC EAR SOLN 0 SANDOZ MLGEN 242<strong>08</strong>-<strong>06</strong>35-62 1.80420 NEOMYCIN-POLYMYXIN-HC EAR SUSP 0 VALEANT MLGEN 61314-<strong>06</strong>45-11 1.80420 NEOMYCIN-POLYMYXIN-HC EAR SUSP 0 SANDOZ MLBND 00078-0248-15 4.3<strong>08</strong>60 5.87114 NEORAL 100 MG GELATIN CAPSULE 0 NOVARTIS EABND 00078-0274-22 4.38400 6.39963 NEORAL 100 MG/ML SOLUTION 0 NOVARTIS MLBND 00078-0246-15 1.07750 1.46937 NEORAL 25 MG GELATIN CAPSULE 0 NOVARTIS EAGUL 61570-0045-10 2.02500 NEOSPORIN EYE DROPS G MONARCH PHRM MLGEN 61570-0047-10 9.10490 NEOSPORIN GU IRR 40 MG/ML AMP 0 MONARCH PHRM MLGEN 61570-0047-50 9.10490 NEOSPORIN GU IRR 40 MG/ML AMP 0 MONARCH PHRM MLGEN 61570-0048-20 4.96000 NEOSPORIN GU IRR 40 MG/ML VIAL 0 MONARCH PHRM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59528-0317-01 0.30710 NEPHPLEX RX TABLET 0 NEPHRO-TECH EABND 59528-4456-01 0.37350 NEPHRON FA TABLET 0 NEPHRO-TECH EAGUL 48102-0014-90 0.31500 NEPTAZANE 25 MG TABLET 0 FERA PHARMACEUT EAGUL 48102-0015-90 0.46500 NEPTAZANE 50 MG TABLET 0 FERA PHARMACEUT EABND 64764-0125-30 9.42437 NESINA 12.5 MG TABLET G TAKEDA PHARMACE EABND 64764-0250-30 9.42437 NESINA 25 MG TABLET G TAKEDA PHARMACE EABND 64764-<strong>06</strong>25-30 9.42437 NESINA 6.25 MG TABLET G TAKEDA PHARMACE EABND 50967-0219-90 1.25376 NESTABS PRENATAL TABLET 0 WOMEN'S CHOICE EABND 58394-0004-<strong>08</strong> 320.91120 NEUMEGA 5 MG VIAL 0 WYETH PHARM EABND 55513-0530-01 291.42960 NEUPOGEN 300 MCG/ML VIAL 0 AMGEN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 55513-0530-10 291.42960 NEUPOGEN 300 MCG/ML VIAL 0 AMGEN MLBND 55513-0924-10 617.81880 NEUPOGEN 300 MCG/0.5 ML SYR 0 AMGEN MLBND 55513-0209-10 614.90550 NEUPOGEN 480 MCG/0.8 ML SYR 0 AMGEN MLBND 55513-0546-01 290.02275 NEUPOGEN 480 MCG/1.6 ML VIAL 0 AMGEN MLBND 55513-0546-10 290.02275 NEUPOGEN 480 MCG/1.6 ML VIAL 0 AMGEN MLBND 50474-<strong>08</strong>01-03 16.40550 NEUPRO 1 MG/24 HR PATCH G UCB PHARMA EABND 50474-<strong>08</strong>02-03 16.40550 NEUPRO 2 MG/24 HR PATCH G UCB PHARMA EABND 50474-<strong>08</strong>03-03 16.40550 NEUPRO 3 MG/24 HR PATCH G UCB PHARMA EABND 50474-<strong>08</strong>04-03 16.40550 NEUPRO 4 MG/24 HR PATCH G UCB PHARMA EABND 50474-<strong>08</strong>05-03 16.40550 NEUPRO 6 MG/24 HR PATCH G UCB PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50474-<strong>08</strong><strong>06</strong>-03 16.40550 NEUPRO 8 MG/24 HR PATCH G UCB PHARMA EABEX 00071-<strong>08</strong>03-24 0.05090 1.04596 NEURONTIN 100 MG CAPSULE G PFIZER US PHARM EABEX 00071-2012-23 0.23260 0.41507 NEURONTIN 250 MG/5 ML SOLN G PFIZER US PHARM MLBEX 00071-<strong>08</strong>05-24 0.<strong>06</strong>642 2.61549 NEURONTIN 300 MG CAPSULE G PFIZER US PHARM EABEX 00071-<strong>08</strong><strong>06</strong>-24 0.09977 3.13781 NEURONTIN 400 MG CAPSULE G PFIZER US PHARM EABEX 00071-0513-24 0.30150 4.96846 NEURONTIN 600 MG TABLET G PFIZER US PHARM EABEX 00071-0401-24 0.30510 5.96139 NEURONTIN 800 MG TABLET G PFIZER US PHARM EAGEN 60258-0158-16 0.01457 NEUTRAL SODIUM FLUORIDE 0 CYPRESS PHARM. MLBND 00<strong>06</strong>5-0002-03 52.29000 NEVANAC 0.1% DROPTAINER G ALCON LABS. MLGEN 00054-0459-21 0.17415 NEVIRAPINE 200 MG TABLET G ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-4050-91 0.17415 NEVIRAPINE 200 MG TABLET G MYLAN EAGEN 13925-0500-60 0.17415 NEVIRAPINE 200 MG TABLET G SETON PHARMACEU EAGEN 31722-0505-60 0.17415 NEVIRAPINE 200 MG TABLET G CAMBER PHARMACE EAGEN 47781-0100-60 0.17415 NEVIRAPINE 200 MG TABLET G ALVOGEN INC EAGEN 51991-0331-<strong>06</strong> 0.17415 NEVIRAPINE 200 MG TABLET G BRECKENRIDGE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 279LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-3788-<strong>06</strong> 0.17415 NEVIRAPINE 200 MG TABLET G APOTEX CORP EAGEN 65162-0209-<strong>06</strong> 0.17415 NEVIRAPINE 200 MG TABLET G AMNEAL PHARMACE EAGEN 65862-0027-60 0.17415 NEVIRAPINE 200 MG TABLET G AUROBINDO PHARM EAGEN 00054-0450-58 0.53287 NEVIRAPINE 50 MG/5 ML SUSP G ROXANE LABS. MLBND 50419-0488-58 87.35632 NEXAVAR 200 MG TABLET 0 BAYER,PHARM DIV EABND 00186-4010-01 7.85982 NEXIUM DR 10 MG PACKET G ASTRAZENECA EABND 00186-4025-01 7.85982 NEXIUM DR 2.5 MG PACKET G ASTRAZENECA EABND 00186-5020-31 7.85982 NEXIUM DR 20 MG CAPSULE G ASTRAZENECA EABND 00186-5020-54 7.85936 NEXIUM DR 20 MG CAPSULE G ASTRAZENECA EABND 00186-5020-82 7.85889 NEXIUM DR 20 MG CAPSULE G ASTRAZENECA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00186-4020-01 7.85982 NEXIUM DR 20 MG PACKET G ASTRAZENECA EABND 00186-5040-31 7.85982 NEXIUM DR 40 MG CAPSULE G ASTRAZENECA EABND 00186-5040-35 7.85982 NEXIUM DR 40 MG CAPSULE G ASTRAZENECA EABND 00186-5040-54 7.85936 NEXIUM DR 40 MG CAPSULE G ASTRAZENECA EABND 00186-5040-55 7.85936 NEXIUM DR 40 MG CAPSULE G ASTRAZENECA EABND 00186-5040-82 7.85889 NEXIUM DR 40 MG CAPSULE G ASTRAZENECA EABND 00186-5040-85 7.85889 NEXIUM DR 40 MG CAPSULE G ASTRAZENECA EABND 00186-4040-01 7.85982 NEXIUM DR 40 MG PACKET G ASTRAZENECA EABND 00186-4050-01 7.85982 NEXIUM DR 5 MG PACKET G ASTRAZENECA EABND 00186-6040-01 38.66970 NEXIUM I.V. 40 MG VIAL 0 ASTRAZENECA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 52544-0287-54 27.42000 NEXT CHOICE ONE DOSE 1.5 MG TB 0 ACTAVIS PHARMA, EAGEN 00093-7394-86 5.11383 NIACIN ER 1,000 MG TABLET G TEVA USA EAGEN 00093-7394-98 5.11383 NIACIN ER 1,000 MG TABLET G TEVA USA EAGEN 00093-7392-86 2.89133 NIACIN ER 500 MG TABLET G TEVA USA EAGEN 00093-7392-98 2.89133 NIACIN ER 500 MG TABLET G TEVA USA EAGEN 00093-7393-86 4.12416 NIACIN ER 750 MG TABLET G TEVA USA EAGEN 00093-7393-98 4.12416 NIACIN ER 750 MG TABLET G TEVA USA EABND 00074-3<strong>08</strong>0-90 6.91048 NIASPAN ER 1,000 MG TABLET 0 ABBVIE US LLC EABND 00074-3074-90 3.90727 NIASPAN ER 500 MG TABLET 0 ABBVIE US LLC EABND 00074-3079-90 5.57317 NIASPAN ER 750 MG TABLET 0 ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-1020-05 0.33750 NICARDIPINE 20 MG CAPSULE 0 MYLAN EAGUL 00378-1020-77 0.33750 NICARDIPINE 20 MG CAPSULE 0 MYLAN EAGEN 428<strong>06</strong>-0501-01 0.32220 NICARDIPINE 20 MG CAPSULE 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0501-05 0.32205 NICARDIPINE 20 MG CAPSULE 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0501-09 0.32916 NICARDIPINE 20 MG CAPSULE 0 EPIC PHARMA LLC EAGUL 00378-1430-05 0.40500 NICARDIPINE 30 MG CAPSULE 0 MYLAN EAGUL 00378-1430-77 0.40500 NICARDIPINE 30 MG CAPSULE 0 MYLAN EAGUL 428<strong>06</strong>-0502-01 0.40500 NICARDIPINE 30 MG CAPSULE 0 EPIC PHARMA LLC EAGUL 428<strong>06</strong>-0502-05 0.40500 NICARDIPINE 30 MG CAPSULE 0 EPIC PHARMA LLC EAGUL 428<strong>06</strong>-0502-09 0.40500 NICARDIPINE 30 MG CAPSULE 0 EPIC PHARMA LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-5400-01 1.346<strong>08</strong> NICOTROL CARTRIDGE INHALER 0 PHARMACIA/UPJHN EABND 00009-5401-01 5.93616 NICOTROL NS 10 MG/ML SPRAY 0 PHARMACIA/UPJHN MLGEN 00093-1023-01 1.72120 NIFEDIAC CC 90 MG TABLET 0 TEVA USA EAGEN 00093-<strong>08</strong>19-01 0.4<strong>08</strong>20 NIFEDICAL XL 30 MG TABLET 0 TEVA USA EAGEN 00093-<strong>08</strong>19-55 0.4<strong>08</strong>20 NIFEDICAL XL 30 MG TABLET 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 280LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-5173-01 0.59240 NIFEDICAL XL 60 MG TABLET 0 TEVA USA EAGEN 00093-5173-55 0.59240 NIFEDICAL XL 60 MG TABLET 0 TEVA USA EAGEN 00093-2057-01 0.46980 NIFEDIPINE ER 30 MG TABLET 0 TEVA USA EAGEN 00093-2057-55 0.46980 NIFEDIPINE ER 30 MG TABLET 0 TEVA USA EAGEN 00378-0353-01 0.46980 NIFEDIPINE ER 30 MG TABLET 0 MYLAN EAGEN 00378-0480-01 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 MYLAN EAGEN 00378-0480-30 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 MYLAN EAGEN 00378-3475-01 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 MYLAN EAGEN 00378-3475-30 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 MYLAN EAGEN 47781-0368-01 0.46980 NIFEDIPINE ER 30 MG TABLET 0 ALVOGEN INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-<strong>06</strong>77-01 0.46980 NIFEDIPINE ER 30 MG TABLET 0 PAR PHARM. EAGEN 49884-<strong>06</strong>77-05 0.46980 NIFEDIPINE ER 30 MG TABLET 0 PAR PHARM. EAGEN 51079-0400-01 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0400-20 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 MYLAN INSTITUTI EAGEN 54458-0950-10 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 INTERNATIONAL L EAGEN 59762-6690-03 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-6690-05 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 GREENSTONE LLC. EAGEN 62175-0260-37 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 KREMERS URBAN EAGEN 62175-0260-43 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 KREMERS URBAN EAGEN 62175-0260-46 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 KREMERS URBAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62175-0260-55 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 KREMERS URBAN EAGEN 67767-0153-01 0.46980 NIFEDIPINE ER 30 MG TABLET 0 PAR PHARM. EAGEN 67767-0153-05 0.46980 NIFEDIPINE ER 30 MG TABLET 0 PAR PHARM. EAGEN 68<strong>08</strong>4-0597-01 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0597-11 0.4<strong>08</strong>20 NIFEDIPINE ER 30 MG TABLET 0 AHP EAGEN 00093-2058-01 1.02120 NIFEDIPINE ER 60 MG TABLET 0 TEVA USA EAGEN 00093-2058-55 1.02120 NIFEDIPINE ER 60 MG TABLET 0 TEVA USA EAGEN 00378-0360-01 1.02120 NIFEDIPINE ER 60 MG TABLET 0 MYLAN EAGEN 00378-0481-01 0.59240 NIFEDIPINE ER 60 MG TABLET 0 MYLAN EAGEN 00378-0481-30 0.59240 NIFEDIPINE ER 60 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3482-01 0.59240 NIFEDIPINE ER 60 MG TABLET 0 MYLAN EAGEN 00378-3482-30 0.59240 NIFEDIPINE ER 60 MG TABLET 0 MYLAN EAGEN 47781-0369-01 1.02120 NIFEDIPINE ER 60 MG TABLET 0 ALVOGEN INC EAGEN 49884-<strong>06</strong>78-01 1.02120 NIFEDIPINE ER 60 MG TABLET 0 PAR PHARM. EAGEN 49884-<strong>06</strong>78-05 1.02120 NIFEDIPINE ER 60 MG TABLET 0 PAR PHARM. EAGEN 51079-<strong>08</strong>96-01 0.59240 NIFEDIPINE ER 60 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-<strong>08</strong>96-20 0.59240 NIFEDIPINE ER 60 MG TABLET 0 MYLAN INSTITUTI EAGEN 54458-0948-<strong>08</strong> 0.59240 NIFEDIPINE ER 60 MG TABLET 0 INTERNATIONAL L EAGEN 59762-6691-03 0.59240 NIFEDIPINE ER 60 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-6691-05 0.59240 NIFEDIPINE ER 60 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62175-0261-37 0.59240 NIFEDIPINE ER 60 MG TABLET 0 KREMERS URBAN EAGEN 62175-0261-43 0.59240 NIFEDIPINE ER 60 MG TABLET 0 KREMERS URBAN EAGEN 62175-0261-46 0.59240 NIFEDIPINE ER 60 MG TABLET 0 KREMERS URBAN EAGEN 62175-0261-55 0.59240 NIFEDIPINE ER 60 MG TABLET 0 KREMERS URBAN EAGEN 67767-0151-01 1.02120 NIFEDIPINE ER 60 MG TABLET 0 PAR PHARM. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 281LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0598-01 0.59240 NIFEDIPINE ER 60 MG TABLET 0 AHP EAGEN 00093-2059-01 1.72120 NIFEDIPINE ER 90 MG TABLET 0 TEVA USA EAGEN 00378-0390-01 1.72120 NIFEDIPINE ER 90 MG TABLET 0 MYLAN EAGEN 00378-0494-01 1.03275 NIFEDIPINE ER 90 MG TABLET 0 MYLAN EAGEN 00378-3495-01 1.03275 NIFEDIPINE ER 90 MG TABLET 0 MYLAN EAGEN 45963-0152-02 1.72120 NIFEDIPINE ER 90 MG TABLET 0 ACTAVIS PHARMA, EAGEN 47781-0370-01 1.72120 NIFEDIPINE ER 90 MG TABLET 0 ALVOGEN INC EAGEN 49884-<strong>06</strong>79-01 1.72120 NIFEDIPINE ER 90 MG TABLET 0 PAR PHARM. EAGEN 51079-<strong>08</strong>97-01 1.03275 NIFEDIPINE ER 90 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-<strong>08</strong>97-<strong>08</strong> 1.03275 NIFEDIPINE ER 90 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-6692-03 1.03275 NIFEDIPINE ER 90 MG TABLET 0 GREENSTONE LLC. EAGEN 62175-0262-32 1.03275 NIFEDIPINE ER 90 MG TABLET 0 KREMERS URBAN EAGEN 62175-0262-37 1.03275 NIFEDIPINE ER 90 MG TABLET 0 KREMERS URBAN EAGEN 62175-0262-46 1.03275 NIFEDIPINE ER 90 MG TABLET 0 KREMERS URBAN EAGEN 68<strong>08</strong>4-<strong>06</strong>03-11 1.03275 NIFEDIPINE ER 90 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>03-21 1.03275 NIFEDIPINE ER 90 MG TABLET 0 AHP EAGEN 00228-2497-10 0.80130 NIFEDIPINE 10 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 23155-0194-01 0.80130 NIFEDIPINE 10 MG CAPSULE 0 HERITAGE PHARMA EAGEN 43386-0440-24 0.86986 NIFEDIPINE 10 MG CAPSULE 0 GAVIS PHARMACEU EAGEN 59762-1004-01 0.80130 NIFEDIPINE 10 MG CAPSULE 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-2530-10 1.72695 NIFEDIPINE 20 MG CAPSULE 0 ACTAVIS PHARMA, EABND 00<strong>08</strong>8-1111-14 139.17440 NILANDRON 150 MG TABLET 0 COVIS PHARMACEU EAGEN 00555-0980-37 7.26575 NIMODIPINE 30 MG CAPSULE 0 BARR EAGEN 00555-0980-40 6.94222 NIMODIPINE 30 MG CAPSULE 0 BARR EAGEN 23155-01<strong>08</strong>-00 13.80750 NIMODIPINE 30 MG CAPSULE 0 HERITAGE PHARMA EAGEN 23155-01<strong>08</strong>-30 14.42499 NIMODIPINE 30 MG CAPSULE 0 HERITAGE PHARMA EAGEN 57664-0135-64 7.18575 NIMODIPINE 30 MG CAPSULE 0 CARACO PHARM EAGEN 57664-0135-65 6.86587 NIMODIPINE 30 MG CAPSULE 0 CARACO PHARM EAGEN 00378-2097-01 5.65770 NISOLDIPINE ER 17 MG TABLET G MYLAN EAGEN 66993-0473-02 5.65770 NISOLDIPINE ER 17 MG TABLET G PRASCO LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00378-2222-01 8.12819 NISOLDIPINE ER 20 MG TABLET G MYLAN EABND 00378-2098-01 6.17020 6.96768 NISOLDIPINE ER 25.5 MG TABLET G MYLAN EABND 00378-2223-01 8.86423 NISOLDIPINE ER 30 MG TABLET G MYLAN EAGEN 00378-2099-01 6.17018 NISOLDIPINE ER 34 MG TABLET G MYLAN EAGEN 66993-0475-02 6.17018 NISOLDIPINE ER 34 MG TABLET G PRASCO LABS EABND 00378-2224-01 8.86423 NISOLDIPINE ER 40 MG TABLET G MYLAN EAGEN 00378-2096-01 4.51420 NISOLDIPINE ER 8.5 MG TABLET G MYLAN EAGEN 66993-0472-02 4.51420 NISOLDIPINE ER 8.5 MG TABLET G PRASCO LABS EABND 00281-0326-30 0.82336 NITRO-BID 2% OINTMENT 0 SANDOZ GMBND 00<strong>08</strong>5-3305-30 0.68250 3.75962 NITRO-DUR 0.1 MG/HR PATCH 0 MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>5-3310-30 0.7<strong>06</strong>30 3.81634 NITRO-DUR 0.2 MG/HR PATCH 0 MERCK SHARP & D EABND 00<strong>08</strong>5-3310-35 0.7<strong>06</strong>30 3.81634 NITRO-DUR 0.2 MG/HR PATCH 0 MERCK SHARP & D EABND 00<strong>08</strong>5-3315-30 4.27643 NITRO-DUR 0.3 MG/HR PATCH 0 MERCK SHARP & D EABND 00<strong>08</strong>5-3315-35 4.27643 NITRO-DUR 0.3 MG/HR PATCH 0 MERCK SHARP & D EABND 00<strong>08</strong>5-3320-30 0.87305 4.27643 NITRO-DUR 0.4 MG/HR PATCH 0 MERCK SHARP & D EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 282LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>5-3320-35 0.87305 4.27643 NITRO-DUR 0.4 MG/HR PATCH 0 MERCK SHARP & D EABND 00<strong>08</strong>5-3330-30 0.96130 4.63693 NITRO-DUR 0.6 MG/HR PATCH 0 MERCK SHARP & D EABND 00<strong>08</strong>5-3330-35 0.96130 4.37880 NITRO-DUR 0.6 MG/HR PATCH 0 MERCK SHARP & D EABND 00<strong>08</strong>5-<strong>08</strong>19-30 4.63693 NITRO-DUR 0.8 MG/HR PATCH 0 MERCK SHARP & D EABND 00<strong>08</strong>5-<strong>08</strong>19-35 4.63693 NITRO-DUR 0.8 MG/HR PATCH 0 MERCK SHARP & D EAGEN 00093-2131-01 1.68970 NITR<strong>OF</strong>URANTOIN MCR 100 MG CAP 0 TEVA USA EAGEN 00093-2131-10 1.68970 NITR<strong>OF</strong>URANTOIN MCR 100 MG CAP 0 TEVA USA EAGEN 00378-1700-01 1.68970 NITR<strong>OF</strong>URANTOIN MCR 100 MG CAP 0 MYLAN EAGEN 00378-1700-05 1.68970 NITR<strong>OF</strong>URANTOIN MCR 100 MG CAP 0 MYLAN EAGEN 47781-03<strong>08</strong>-01 1.68970 NITR<strong>OF</strong>URANTOIN MCR 100 MG CAP 0 ALVOGEN INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0585-20 1.68970 NITR<strong>OF</strong>URANTOIN MCR 100 MG CAP 0 MYLAN INSTITUTI EAGEN 68001-0003-00 1.68970 NITR<strong>OF</strong>URANTOIN MCR 100 MG CAP 0 BLUEPOINT LABOR EAGEN 00093-2130-01 0.96550 NITR<strong>OF</strong>URANTOIN MCR 50 MG CAP 0 TEVA USA EAGEN 00093-2130-10 0.96550 NITR<strong>OF</strong>URANTOIN MCR 50 MG CAP 0 TEVA USA EAGEN 00378-1650-01 0.96550 NITR<strong>OF</strong>URANTOIN MCR 50 MG CAP 0 MYLAN EAGEN 47781-0307-01 0.96550 NITR<strong>OF</strong>URANTOIN MCR 50 MG CAP 0 ALVOGEN INC EAGEN 51079-0584-20 0.96550 NITR<strong>OF</strong>URANTOIN MCR 50 MG CAP 0 MYLAN INSTITUTI EAGEN 68001-0002-00 0.96550 NITR<strong>OF</strong>URANTOIN MCR 50 MG CAP 0 BLUEPOINT LABOR EAGEN 00185-0122-01 2.02560 NITR<strong>OF</strong>URANTOIN MONO-MCR 100 MG 0 SANDOZ EAGEN 00185-0122-10 2.02560 NITR<strong>OF</strong>URANTOIN MONO-MCR 100 MG 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3422-01 2.02560 NITR<strong>OF</strong>URANTOIN MONO-MCR 100 MG 0 MYLAN EAGEN 47781-0303-01 2.02560 NITR<strong>OF</strong>URANTOIN MONO-MCR 100 MG 0 ALVOGEN INC EAGEN 51079-0348-01 2.02560 NITR<strong>OF</strong>URANTOIN MONO-MCR 100 MG 0 MYLAN INSTITUTI EAGEN 51079-0348-20 2.02560 NITR<strong>OF</strong>URANTOIN MONO-MCR 100 MG 0 MYLAN INSTITUTI EAGEN 68001-0001-00 2.02560 NITR<strong>OF</strong>URANTOIN MONO-MCR 100 MG 0 BLUEPOINT LABOR EAGEN 68<strong>08</strong>4-0446-01 1.76587 NITR<strong>OF</strong>URANTOIN MONO-MCR 100 MG 0 AHP EAGEN 68<strong>08</strong>4-0446-11 1.76587 NITR<strong>OF</strong>URANTOIN MONO-MCR 100 MG 0 AHP EAGEN 57664-0239-32 2.07480 NITR<strong>OF</strong>URANTOIN 25 MG/5 ML SUSP 0 CARACO PHARM MLGEN 65162-<strong>06</strong>89-88 1.98482 NITR<strong>OF</strong>URANTOIN 25 MG/5 ML SUSP 0 AMNEAL PHARMACE MLGEN 66993-0471-73 2.07480 NITR<strong>OF</strong>URANTOIN 25 MG/5 ML SUSP 0 PRASCO LABS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-<strong>06</strong>43-60 0.09070 NITROGLYCERIN ER 2.5 MG CAP 0 MAJOR PHARMACEU EAGEN 00904-<strong>06</strong>44-52 0.12180 NITROGLYCERIN ER 6.5 MG CAP 0 MAJOR PHARMACEU EAGEN 00904-<strong>06</strong>44-60 0.12180 NITROGLYCERIN ER 6.5 MG CAP 0 MAJOR PHARMACEU EAGEN 00904-<strong>06</strong>47-52 0.83700 NITROGLYCERIN ER 9 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 00904-<strong>06</strong>47-60 0.7<strong>06</strong>12 NITROGLYCERIN ER 9 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 45802-0210-01 34.35765 NITROGLYCERIN LINGUAL 0.4 MG 0 PERRIGO CO. GMGEN 45802-0210-02 20.94750 NITROGLYCERIN LINGUAL 0.4 MG 0 PERRIGO CO. GMGEN 00378-9102-93 0.68250 NITROGLYCERIN 0.1 MG/HR PATCH 0 MYLAN EAGEN 47781-0296-03 0.68250 NITROGLYCERIN 0.1 MG/HR PATCH 0 ALVOGEN INC EAGEN 00378-9104-93 0.7<strong>06</strong>30 NITROGLYCERIN 0.2 MG/HR PATCH 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5495-46 0.7<strong>06</strong>30 NITROGLYCERIN 0.2 MG/HR PATCH 0 MAJOR PHARMACEU EAGEN 47781-0297-03 0.7<strong>06</strong>30 NITROGLYCERIN 0.2 MG/HR PATCH 0 ALVOGEN INC EAGEN 49730-0111-30 0.7<strong>06</strong>30 NITROGLYCERIN 0.2 MG/HR PATCH 0 HERCON EAGEN 62175-0123-01 0.63600 NITROGLYCERIN 0.2 MG/HR PATCH 0 KREMERS URBAN EAGEN 00378-9112-93 0.87305 NITROGLYCERIN 0.4 MG/HR PATCH 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 283LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5496-46 0.87305 NITROGLYCERIN 0.4 MG/HR PATCH 0 MAJOR PHARMACEU EAGEN 47781-0298-03 0.87305 NITROGLYCERIN 0.4 MG/HR PATCH 0 ALVOGEN INC EAGEN 49730-0112-30 0.87305 NITROGLYCERIN 0.4 MG/HR PATCH 0 HERCON EAGEN 62175-0124-01 0.76575 NITROGLYCERIN 0.4 MG/HR PATCH 0 KREMERS URBAN EAGEN 00378-9116-93 0.96130 NITROGLYCERIN 0.6 MG/HR PATCH 0 MYLAN EAGEN 00904-5497-46 0.96130 NITROGLYCERIN 0.6 MG/HR PATCH 0 MAJOR PHARMACEU EAGEN 47781-0299-03 0.96130 NITROGLYCERIN 0.6 MG/HR PATCH 0 ALVOGEN INC EAGEN 49730-0113-30 0.96130 NITROGLYCERIN 0.6 MG/HR PATCH 0 HERCON EAGEN 43478-0410-03 41.04878 NITROGLYCERIN 400 MCG SPRAY 0 ROUSES POINT PH GMGEN 43478-0410-07 29.54118 NITROGLYCERIN 400 MCG SPRAY 0 ROUSES POINT PH GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 24338-0300-20 28.99051 NITROLINGUAL 0.4 MG SPRAY 0 ARBOR PHARMACEU GMBND 24338-0300-65 38.17550 48.62444 NITROLINGUAL 0.4 MG SPRAY 0 ARBOR PHARMACEU GMBND 24090-0410-03 46.11965 NITROMIST 400 MCG SPRAY 0 AKRIMAX PHARMAC GMBND 24090-0410-04 81.37036 NITROMIST 400 MCG SPRAY 0 AKRIMAX PHARMAC GMBND 24090-0410-<strong>08</strong> 54.86007 NITROMIST 400 MCG SPRAY 0 AKRIMAX PHARMAC GMBND 00071-0417-24 0.19828 NITROSTAT 0.3 MG TABLET SL 0 PFIZER US PHARM EABND 00071-0418-13 0.43259 NITROSTAT 0.4 MG TABLET SL 0 PFIZER US PHARM EABND 00071-0418-24 0.19828 NITROSTAT 0.4 MG TABLET SL 0 PFIZER US PHARM EABND 00071-0419-24 0.19828 NITROSTAT 0.6 MG TABLET SL 0 PFIZER US PHARM EAGEN 10572-0147-62 0.48339 NIZATIDINE 15 MG/ML SOLUTION 0 AFFORDABLE PHAR ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-<strong>06</strong>59-90 0.54020 NIZATIDINE 15 MG/ML SOLUTION 0 AMNEAL PHARMACE MLGEN 00185-0150-05 0.28040 NIZATIDINE 150 MG CAPSULE 0 SANDOZ EAGEN 00185-0150-60 0.28040 NIZATIDINE 150 MG CAPSULE 0 SANDOZ EAGEN 00378-5150-91 0.28040 NIZATIDINE 150 MG CAPSULE 0 MYLAN EAGEN 00591-3137-60 0.28040 NIZATIDINE 150 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 55111-0310-05 0.28040 NIZATIDINE 150 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 60505-0230-04 0.28040 NIZATIDINE 150 MG CAPSULE 0 APOTEX CORP EAGEN 68462-0425-60 0.28040 NIZATIDINE 150 MG CAPSULE 0 GLENMARK PHARMA EAGEN 00185-0300-01 0.86225 NIZATIDINE 300 MG CAPSULE 0 SANDOZ EAGEN 00185-0300-30 0.86225 NIZATIDINE 300 MG CAPSULE 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5300-93 0.86225 NIZATIDINE 300 MG CAPSULE 0 MYLAN EAGEN 00591-3138-30 0.86225 NIZATIDINE 300 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 55111-0311-30 0.86225 NIZATIDINE 300 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 60505-0231-01 0.86225 NIZATIDINE 300 MG CAPSULE 0 APOTEX CORP EAGEN 68462-0426-30 0.86225 NIZATIDINE 300 MG CAPSULE 0 GLENMARK PHARMA EABEX 52544-0235-28 0.69917 2.21046 NOR-Q-D TABLET G ACTAVIS PHARMA, EABEX 52544-<strong>06</strong>29-28 0.69917 1.09441 NORA-BE TABLET 0 ACTAVIS PHARMA, EABND 00169-7705-21 580.07040 NORDITROPIN FLEXPRO 10 MG/1.5 G NOVO NORDISK MLBND 00169-77<strong>08</strong>-21 870.10560 NORDITROPIN FLEXPRO 15 MG/1.5 G NOVO NORDISK MLBND 00169-7704-21 290.03520 NORDITROPIN FLEXPRO 5 MG/1.5 G NOVO NORDISK ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00169-7703-11 870.10560 NORDITROPIN NORDIFLEX 30 MG/3 G NOVO NORDISK MLGEX 00378-7272-53 0.69917 NORETHINDRONE 0.35 MG TABLET 0 MYLAN EAGEX 00378-7292-53 0.69917 NORETHINDRONE 0.35 MG TABLET 0 MYLAN EAGEX 68180-<strong>08</strong>76-13 0.69917 NORETHINDRONE 0.35 MG TABLET 0 LUPIN PHARMACEU EAGEX 68462-0305-29 0.69917 NORETHINDRONE 0.35 MG TABLET 0 GLENMARK PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 284LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00555-0211-10 1.81640 NORETHINDRONE 5 MG TABLET 0 BARR EAGEN 65162-0475-05 1.81640 NORETHINDRONE 5 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0475-09 1.81640 NORETHINDRONE 5 MG TABLET 0 AMNEAL PHARMACE EAGEN 68462-0304-50 1.81640 NORETHINDRONE 5 MG TABLET 0 GLENMARK PHARMA EAGEX 68462-0565-29 0.46850 NORGESTIMATE-ETH ESTRADIOL TAB 0 GLENMARK PHARMA EABEX 52544-0259-28 0.73890 2.21<strong>08</strong>1 NORINYL 1+35-28 TABLET 0 ACTAVIS PHARMA, EABEX 52544-0265-31 0.82890 2.41036 NORINYL 1+50-28 TABLET G ACTAVIS PHARMA, EABND 00187-5202-60 8.75718 NORITATE 1% CREAM 0 VALEANT GMBND 000<strong>06</strong>-0705-20 4.01720 NOROXIN 400 MG TABLET G MERCK SHARP & D EABND 00025-2732-31 2.38625 NORPACE CR 100 MG CAPSULE 0 PHARMACIA/UPJHN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00025-2732-51 2.38623 NORPACE CR 100 MG CAPSULE 0 PHARMACIA/UPJHN EABND 00025-2742-31 2.82025 NORPACE CR 150 MG CAPSULE 0 PHARMACIA/UPJHN EABND 00025-2742-51 2.82038 NORPACE CR 150 MG CAPSULE 0 PHARMACIA/UPJHN EABND 00025-2752-31 0.31000 2.17891 NORPACE 100 MG CAPSULE G PHARMACIA/UPJHN EABND 00025-2762-31 0.41<strong>08</strong>2 2.57457 NORPACE 150 MG CAPSULE G PHARMACIA/UPJHN EABEX 00<strong>06</strong>8-0007-01 0.88100 1.40070 NORPRAMIN 10 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABEX 00<strong>06</strong>8-0020-01 3.41860 5.29855 NORPRAMIN 100 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABEX 00<strong>06</strong>8-0021-50 5.01150 7.67716 NORPRAMIN 150 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABEX 00<strong>06</strong>8-0011-01 0.87210 1.68274 NORPRAMIN 25 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABEX 00<strong>06</strong>8-0015-01 1.76120 3.16794 NORPRAMIN 50 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00<strong>06</strong>8-0019-01 2.43280 4.03197 NORPRAMIN 75 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EAGEX 00555-90<strong>08</strong>-67 0.86178 NORTREL 0.5-35 TABLET 0 BARR EAGEX 00555-9009-42 0.73890 NORTREL 1-35 TABLET 0 BARR EAGEX 00555-9010-58 0.73890 NORTREL 1-35 TABLET 0 BARR EAGEX 00555-9012-58 0.83640 NORTREL 7-7-7-28 TABLET 0 BARR EAGEX 00093-<strong>08</strong>10-01 0.09990 NORTRIPTYLINE HCL 10 MG CAP 0 TEVA USA EAGEX 00093-<strong>08</strong>10-05 0.09990 NORTRIPTYLINE HCL 10 MG CAP 0 TEVA USA EAGEX 00591-5786-01 0.09990 NORTRIPTYLINE HCL 10 MG CAP 0 ACTAVIS PHARMA, EAGEX 00591-5786-05 0.09990 NORTRIPTYLINE HCL 10 MG CAP 0 ACTAVIS PHARMA, EAGEX 51672-4001-02 0.09375 NORTRIPTYLINE HCL 10 MG CAP 0 TARO PHARM USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51672-4001-05 0.096<strong>08</strong> NORTRIPTYLINE HCL 10 MG CAP 0 TARO PHARM USA EAGEX 68<strong>08</strong>4-0031-11 0.09990 NORTRIPTYLINE HCL 10 MG CAP 0 AHP EAGEX 00093-<strong>08</strong>11-01 0.12210 NORTRIPTYLINE HCL 25 MG CAP 0 TEVA USA EAGEX 00093-<strong>08</strong>11-05 0.12210 NORTRIPTYLINE HCL 25 MG CAP 0 TEVA USA EAGEX 00591-5787-01 0.12210 NORTRIPTYLINE HCL 25 MG CAP 0 ACTAVIS PHARMA, EAGEX 00591-5787-05 0.12210 NORTRIPTYLINE HCL 25 MG CAP 0 ACTAVIS PHARMA, EAGEX 00591-5787-10 0.12210 NORTRIPTYLINE HCL 25 MG CAP 0 ACTAVIS PHARMA, EAGEX 51672-4002-02 0.12210 NORTRIPTYLINE HCL 25 MG CAP 0 TARO PHARM USA EAGEX 51672-4002-05 0.12210 NORTRIPTYLINE HCL 25 MG CAP 0 TARO PHARM USA EAGEX 68<strong>08</strong>4-0032-01 0.12210 NORTRIPTYLINE HCL 25 MG CAP 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0032-11 0.12210 NORTRIPTYLINE HCL 25 MG CAP 0 AHP EAGEX 00093-<strong>08</strong>12-01 0.17010 NORTRIPTYLINE HCL 50 MG CAP 0 TEVA USA EAGEX 00093-<strong>08</strong>12-05 0.17010 NORTRIPTYLINE HCL 50 MG CAP 0 TEVA USA EAGEX 00591-5788-01 0.17010 NORTRIPTYLINE HCL 50 MG CAP 0 ACTAVIS PHARMA, EAGEX 00591-5788-05 0.17010 NORTRIPTYLINE HCL 50 MG CAP 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 285LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51672-4003-02 0.15675 NORTRIPTYLINE HCL 50 MG CAP 0 TARO PHARM USA EAGEX 51672-4003-05 0.16074 NORTRIPTYLINE HCL 50 MG CAP 0 TARO PHARM USA EAGUX 00093-<strong>08</strong>13-01 0.22030 NORTRIPTYLINE HCL 75 MG CAP 0 TEVA USA EAGUX 00093-<strong>08</strong>13-05 0.22030 NORTRIPTYLINE HCL 75 MG CAP 0 TEVA USA EAGUX 00591-5789-01 0.22030 NORTRIPTYLINE HCL 75 MG CAP 0 ACTAVIS PHARMA, EAGUX 51672-4004-05 0.22030 NORTRIPTYLINE HCL 75 MG CAP 0 TARO PHARM USA EABEX 00121-<strong>06</strong>78-16 0.<strong>06</strong>300 0.32103 NORTRIPTYLINE 10 MG/5 ML SOL 0 PHARMACEU ASSOC MLBND 00<strong>06</strong>9-1540-68 0.02550 4.31138 NORVASC 10 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-1520-68 0.03540 3.14459 NORVASC 2.5 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-1530-68 0.03740 3.14459 NORVASC 5 MG TABLET G PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>9-1530-72 0.03740 3.14459 NORVASC 5 MG TABLET G PFIZER US PHARM EABND 00074-6633-30 8.53793 NORVIR 100 MG S<strong>OF</strong>TGEL CAP G ABBVIE US LLC EABND 00074-3333-30 8.53793 NORVIR 100 MG TABLET G ABBVIE US LLC EABND 00074-1940-63 5.97683 NORVIR 80 MG/ML SOLUTION G ABBVIE US LLC MLBND 00169-6339-10 21.56672 NOVOLOG FLEXPEN SYRINGE 0 NOVO NORDISK MLBND 00169-3696-19 21.56672 NOVOLOG MIX 70-30 FLEXPEN SYRN 0 NOVO NORDISK MLBND 00169-3685-12 17.37439 NOVOLOG MIX 70-30 VIAL 0 NOVO NORDISK MLBND 00169-3303-12 20.74059 NOVOLOG 100 UNIT/ML CARTRIDGE 0 NOVO NORDISK MLBND 00169-7501-11 16.74774 NOVOLOG 100 UNIT/ML VIAL 0 NOVO NORDISK MLBND 00169-7010-01 1.58800 NOVOSEVEN RT 1 MG VIAL 0 NOVO NORDISK--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00169-7201-01 1.58800 NOVOSEVEN RT 1 MG VIAL 0 NOVO NORDISKBND 00169-7020-01 1.58800 NOVOSEVEN RT 2 MG VIAL 0 NOVO NORDISKBND 00169-7202-01 1.58800 NOVOSEVEN RT 2 MG VIAL 0 NOVO NORDISKBND 00169-7050-01 1.58800 NOVOSEVEN RT 5 MG VIAL 0 NOVO NORDISKBND 00169-7205-01 1.58800 NOVOSEVEN RT 5 MG VIAL 0 NOVO NORDISKBND 00169-7040-01 1.58800 NOVOSEVEN RT 8 MG VIAL 0 NOVO NORDISKBND 00169-72<strong>08</strong>-01 1.58800 NOVOSEVEN RT 8 MG VIAL 0 NOVO NORDISKBND 00<strong>08</strong>5-4324-02 49.53537 NOXAFIL DR 100 MG TABLET 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1328-01 9.0<strong>06</strong>37 NOXAFIL 40 MG/ML SUSPENSION 0 MERCK SHARP & D MLGEN 00338-1049-02 0.02152 NTG 0.2 MG/ML IN D5W 0 BAXTER <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00338-1051-02 0.02638 NTG 100 MG/250 ML IN D5W 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-1047-02 0.02052 NTG 25 MG/250 ML IN D5W 0 BAXTER <strong>HEALTH</strong>CA MLBND 64597-0301-60 10.70700 NUEDEXTA 20-10 MG CAPSULE 0 AVANIR PHARMACE EAGEN 52544-0149-26 5.15220 NULECIT 62.5 MG/5 ML VIAL 0 ACTAVIS PHARMA, MLGEN 52544-0149-87 5.15220 NULECIT 62.5 MG/5 ML VIAL 0 ACTAVIS PHARMA, MLGEN 68220-0118-10 0.40950 NULEV 0.125 MG CHEWABLE MELT 0 MEDA PHARMACEUT EABND 52268-0400-01 0.00360 0.0<strong>06</strong>64 NULYTELY WITH FLAVOR PACKS SOL G BRAINTREE LABS. MLBND 50242-0074-01 443.58935 NUTROPIN AQ NUSPIN 10 PEN CART G GENENTECH, INC. MLBND 50242-0076-01 887.17870 NUTROPIN AQ NUSPIN 20 PEN CART G GENENTECH, INC. MLBND 50242-0075-01 221.79260 NUTROPIN AQ NUSPIN 5 PEN CART G GENENTECH, INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50242-0043-14 443.58935 NUTROPIN AQ PEN CARTRIDGE G GENENTECH, INC. MLBND 50242-0073-01 887.17870 NUTROPIN AQ 20 MG/2ML PEN CART G GENENTECH, INC. MLBEX 00052-0273-01 91.32490 NUVARING VAGINAL RING 0 ORGANON PHARM. EABEX 00052-0273-03 91.32766 NUVARING VAGINAL RING 0 ORGANON PHARM. EAGEN 0<strong>08</strong>32-0465-15 1.01130 NYAMYC 100,000 UNITS/GM POWDER 0 UPSHER SMITH GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 286LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>08</strong>32-0465-30 1.01130 NYAMYC 100,000 UNITS/GM POWDER 0 UPSHER SMITH GMGEN 0<strong>08</strong>32-0465-60 1.01130 NYAMYC 100,000 UNITS/GM POWDER 0 UPSHER SMITH GMGUL 00168-0054-15 0.09900 NYSTATIN 100,000 UNIT/GM CREAM 0 SANDOZ GMGUL 00168-0054-30 0.09900 NYSTATIN 100,000 UNIT/GM CREAM 0 SANDOZ GMGUL 00472-0163-15 0.09900 NYSTATIN 100,000 UNIT/GM CREAM 0 ACTAVIS PHARMA, GMGUL 00472-0163-30 0.09900 NYSTATIN 100,000 UNIT/GM CREAM 0 ACTAVIS PHARMA, GMGUL 0<strong>06</strong>03-7818-74 0.09900 NYSTATIN 100,000 UNIT/GM CREAM 0 QUALITEST GMGUL 0<strong>06</strong>03-7818-78 0.09900 NYSTATIN 100,000 UNIT/GM CREAM 0 QUALITEST GMGUL 45802-0059-11 0.09900 NYSTATIN 100,000 UNIT/GM CREAM 0 PERRIGO CO. GMGUL 45802-0059-35 0.09900 NYSTATIN 100,000 UNIT/GM CREAM 0 PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-1289-01 0.09900 NYSTATIN 100,000 UNIT/GM CREAM 0 TARO PHARM USA GMGUL 51672-1289-02 0.09900 NYSTATIN 100,000 UNIT/GM CREAM 0 TARO PHARM USA GMGEN 683<strong>08</strong>-0152-15 1.01130 NYSTATIN 100,000 UNIT/GM POWD 0 MIDLOTHIAN LABO GMGEN 683<strong>08</strong>-0152-30 1.01130 NYSTATIN 100,000 UNIT/GM POWD 0 MIDLOTHIAN LABO GMGEN 683<strong>08</strong>-0152-60 0.81712 NYSTATIN 100,000 UNIT/GM POWD 0 MIDLOTHIAN LABO GMGUL 00168-0007-15 0.10190 NYSTATIN 100,000 UNITS/GM OINT 0 SANDOZ GMGUL 00168-0007-30 0.10190 NYSTATIN 100,000 UNITS/GM OINT 0 SANDOZ GMGUL 00472-0166-15 0.10190 NYSTATIN 100,000 UNITS/GM OINT 0 ACTAVIS PHARMA, GMGUL 00472-0166-30 0.10190 NYSTATIN 100,000 UNITS/GM OINT 0 ACTAVIS PHARMA, GMGUL 45802-0048-11 0.10190 NYSTATIN 100,000 UNITS/GM OINT 0 PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 45802-0048-35 0.10190 NYSTATIN 100,000 UNITS/GM OINT 0 PERRIGO CO. GMGEN 0<strong>06</strong>03-1481-49 0.19290 NYSTATIN 100,000 UNITS/ML SUSP 0 QUALITEST MLGEN 0<strong>06</strong>03-1481-58 0.18393 NYSTATIN 100,000 UNITS/ML SUSP 0 QUALITEST MLGEN 50383-0587-16 0.18404 NYSTATIN 100,000 UNITS/ML SUSP 0 HI-TECH PHARMAC MLGEN 50383-0587-66 0.19290 NYSTATIN 100,000 UNITS/ML SUSP 0 HI-TECH PHARMAC MLGEN 51672-4117-04 0.19290 NYSTATIN 100,000 UNITS/ML SUSP 0 TARO PHARM USA MLGEN 51672-4117-09 0.18391 NYSTATIN 100,000 UNITS/ML SUSP 0 TARO PHARM USA MLGEN 60432-0537-16 0.18404 NYSTATIN 100,000 UNITS/ML SUSP 0 MORTON GROVE PH MLGEN 60432-0537-60 0.19290 NYSTATIN 100,000 UNITS/ML SUSP 0 MORTON GROVE PH MLGEN 66689-00<strong>08</strong>-02 0.18000 NYSTATIN 100,000 UNITS/ML SUSP 0 VISTAPHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 66689-00<strong>08</strong>-16 0.15000 NYSTATIN 100,000 UNITS/ML SUSP 0 VISTAPHARM MLGEN 66689-0037-01 0.17700 NYSTATIN 100,000 UNITS/ML SUSP 0 VISTAPHARM MLGEN 66689-0037-50 0.17640 NYSTATIN 100,000 UNITS/ML SUSP 0 VISTAPHARM MLGEN 68094-0599-59 0.19290 NYSTATIN 100,000 UNITS/ML SUSP 0 PRECISION DOSE MLBND 00574-0404-15 83.12450 NYSTATIN 150,000,000 UNITS PWD 0 PADDOCK LABS. EABND 00574-0404-05 32.37000 NYSTATIN 50,000,000 UNITS PWD 0 PADDOCK LABS. EAGEN 00093-0983-01 0.51<strong>06</strong>0 NYSTATIN 500,000 UNIT ORAL TAB 0 TEVA USA EAGEN 23155-0051-01 0.51052 NYSTATIN 500,000 UNIT ORAL TAB 0 HERITAGE PHARMA EAGEN 53489-0400-01 0.72828 NYSTATIN 500,000 UNIT ORAL TAB 0 MUTUAL PHARM CO EAGEN 00121-4785-35 0.19290 NYSTATIN 500,000 UNITS/5 ML 0 PHARMACEU ASSOC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00574-0404-50 241.07350 NYSTATIN 500,000,000 UNITS PWD 0 PADDOCK LABS. EAGUL 00168-0<strong>08</strong>1-15 0.09750 NYSTATIN-TRIAMCINOLONE CREAM 0 SANDOZ GMGUL 00168-0<strong>08</strong>1-30 0.09750 NYSTATIN-TRIAMCINOLONE CREAM 0 SANDOZ GMGUL 00168-0<strong>08</strong>1-60 0.09750 NYSTATIN-TRIAMCINOLONE CREAM 0 SANDOZ GMGUL 51672-1263-01 0.09750 NYSTATIN-TRIAMCINOLONE CREAM 0 TARO PHARM USA GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 287LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51672-1263-02 0.09750 NYSTATIN-TRIAMCINOLONE CREAM 0 TARO PHARM USA GMGUL 51672-1263-03 0.09750 NYSTATIN-TRIAMCINOLONE CREAM 0 TARO PHARM USA GMGUL 00168-0<strong>08</strong>9-15 0.09750 NYSTATIN-TRIAMCINOLONE OINTM 0 SANDOZ GMGUL 00168-0<strong>08</strong>9-30 0.09750 NYSTATIN-TRIAMCINOLONE OINTM 0 SANDOZ GMGUL 00168-0<strong>08</strong>9-60 0.09750 NYSTATIN-TRIAMCINOLONE OINTM 0 SANDOZ GMGUL 51672-1272-01 0.09750 NYSTATIN-TRIAMCINOLONE OINTM 0 TARO PHARM USA GMGUL 51672-1272-02 0.09750 NYSTATIN-TRIAMCINOLONE OINTM 0 TARO PHARM USA GMGUL 51672-1272-03 0.09750 NYSTATIN-TRIAMCINOLONE OINTM 0 TARO PHARM USA GMGEN 00574-20<strong>08</strong>-02 1.01130 NYSTOP 100,000 UNITS/GM POWDER 0 PADDOCK LABS. GMGEN 00574-20<strong>08</strong>-15 1.01130 NYSTOP 100,000 UNITS/GM POWDER 0 PADDOCK LABS. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00574-20<strong>08</strong>-30 1.01130 NYSTOP 100,000 UNITS/GM POWDER 0 PADDOCK LABS. GMBND 0<strong>08</strong>13-9316-01 0.26875 O-CAL FA TABLET 0 PHARMICS EABND 0<strong>08</strong>13-0202-01 0.12815 O-CAL PRENATAL TABLET 0 PHARMICS EABND 68025-0010-10 0.96110 1.50952 OB COMPLETE CAPLET 0 VERTICAL PHARM EABND 68025-0039-30 2.330<strong>08</strong> OB COMPLETE CHEWABLE TABLET 0 VERTICAL PHARM EABND 68025-0044-30 3.25830 OB COMPLETE ONE S<strong>OF</strong>TGEL 0 VERTICAL PHARM EABND 68025-0059-30 3.15870 OB COMPLETE PETITE S<strong>OF</strong>TGEL 0 VERTICAL PHARM EABND 68025-0043-30 2.82200 OB COMPLETE PREMIER TABLET 0 VERTICAL PHARM EABEX 00555-9131-67 2.<strong>08</strong>130 2.27311 OCELLA 3 MG-0.03 MG TABLET 0 BARR EABEX 00555-9131-79 2.<strong>08</strong>130 2.27301 OCELLA 3 MG-0.03 MG TABLET 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 67467-<strong>08</strong>43-01 5.72492 OCTAGAM 5% VIAL 0 OCTAPHARMA USA MLBND 67467-<strong>08</strong>43-02 5.72500 OCTAGAM 5% VIAL 0 OCTAPHARMA USA MLBND 67467-<strong>08</strong>43-03 5.72500 OCTAGAM 5% VIAL 0 OCTAPHARMA USA MLBND 67467-<strong>08</strong>43-04 5.72500 OCTAGAM 5% VIAL 0 OCTAPHARMA USA MLBND 67467-<strong>08</strong>43-05 5.72500 OCTAGAM 5% VIAL 0 OCTAPHARMA USA MLBND 68209-<strong>08</strong>43-01 5.72492 OCTAGAM 5% VIAL 0 OCTAPHARMA USA MLBND 68209-<strong>08</strong>43-02 5.72500 OCTAGAM 5% VIAL 0 OCTAPHARMA USA MLBND 68209-<strong>08</strong>43-03 5.72500 OCTAGAM 5% VIAL 0 OCTAPHARMA USA MLBND 68209-<strong>08</strong>43-04 5.72500 OCTAGAM 5% VIAL 0 OCTAPHARMA USA MLGEN 00781-9167-71 4.02220 OCTREOTIDE ACET 100 MCG/ML AMP 0 SANDOZ/NOVAPLUS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62756-0349-44 4.02220 OCTREOTIDE ACET 100 MCG/ML AMP 0 SUN PHARMACEUTI MLBND 67457-0245-00 7.86840 OCTREOTIDE ACET 100 MCG/ML SYR 0 MYLAN INSTITUTI MLBND 67457-0245-01 7.86840 OCTREOTIDE ACET 100 MCG/ML SYR 0 MYLAN INSTITUTI MLGEN 00703-3311-04 4.86000 OCTREOTIDE ACET 100 MCG/ML VL 0 TEVA PARENTERAL MLGEN 25021-0452-01 5.29200 OCTREOTIDE ACET 100 MCG/ML VL 0 SAGENT PHARMACE MLGEN 55390-0161-10 5.29200 OCTREOTIDE ACET 100 MCG/ML VL 0 BEDFORD LABS MLGEN 62756-0094-44 5.29200 OCTREOTIDE ACET 100 MCG/ML VL 0 SUN PHARMACEUTI MLGEN 64679-<strong>06</strong>33-02 5.29200 OCTREOTIDE ACET 100 MCG/ML VL 0 WOCKHARDT USA L MLGEN 00703-3333-01 7.78320 OCTREOTIDE ACET 200 MCG/ML VL 0 TEVA PARENTERAL MLGEN 00781-3165-75 7.78320 OCTREOTIDE ACET 200 MCG/ML VL 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55390-0163-01 7.78320 OCTREOTIDE ACET 200 MCG/ML VL 0 BEDFORD LABS MLGEN 62756-0350-40 7.78320 OCTREOTIDE ACET 200 MCG/ML VL 0 SUN PHARMACEUTI MLGEN 63323-0378-05 7.78320 OCTREOTIDE ACET 200 MCG/ML VL 0 APP PHARMACEUTI MLGEN 64679-<strong>06</strong>34-01 7.78320 OCTREOTIDE ACET 200 MCG/ML VL 0 WOCKHARDT USA L MLGEN 00781-9166-71 2.87820 OCTREOTIDE ACET 50 MCG/ML AMP 0 SANDOZ/NOVAPLUS ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 288LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62756-0348-44 2.87820 OCTREOTIDE ACET 50 MCG/ML AMP 0 SUN PHARMACEUTI MLBND 67457-0239-00 3.98400 OCTREOTIDE ACET 50 MCG/ML SYR 0 MYLAN INSTITUTI MLBND 67457-0239-01 3.98400 OCTREOTIDE ACET 50 MCG/ML SYR 0 MYLAN INSTITUTI MLGEN 00703-3301-04 2.70000 OCTREOTIDE ACET 50 MCG/ML VIAL 0 TEVA PARENTERAL MLGEN 25021-0451-01 2.75770 OCTREOTIDE ACET 50 MCG/ML VIAL 0 SAGENT PHARMACE MLGEN 55390-0160-10 2.75770 OCTREOTIDE ACET 50 MCG/ML VIAL 0 BEDFORD LABS MLGEN 63323-0365-01 2.75770 OCTREOTIDE ACET 50 MCG/ML VIAL 0 APP PHARMACEUTI MLGEN 00781-9168-71 25.69680 OCTREOTIDE ACET 500 MCG/ML AMP 0 SANDOZ/NOVAPLUS MLGEN 62756-0351-44 25.69680 OCTREOTIDE ACET 500 MCG/ML AMP 0 SUN PHARMACEUTI MLBND 67457-0246-00 39.54120 OCTREOTIDE ACET 500 MCG/ML SYR 0 MYLAN INSTITUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 67457-0246-01 39.54120 OCTREOTIDE ACET 500 MCG/ML SYR 0 MYLAN INSTITUTI MLGEN 25021-0453-01 19.11090 OCTREOTIDE ACET 500 MCG/ML VL 0 SAGENT PHARMACE MLGEN 55390-0162-10 19.11090 OCTREOTIDE ACET 500 MCG/ML VL 0 BEDFORD LABS MLGEN 64679-<strong>06</strong>35-02 19.11090 OCTREOTIDE ACET 500 MCG/ML VL 0 WOCKHARDT USA L MLGEN 00703-3343-01 50.40000 OCTREOTIDE 1,000 MCG/ML VIAL 0 TEVA PARENTERAL MLGEN 00781-3164-75 118.12500 OCTREOTIDE 1,000 MCG/ML VIAL 0 SANDOZ MLGEN 55390-0164-01 54.00000 OCTREOTIDE 1,000 MCG/ML VIAL 0 BEDFORD LABS MLGEN 62756-0352-40 119.55000 OCTREOTIDE 1,000 MCG/ML VIAL 0 SUN PHARMACEUTI MLGEN 25021-0454-05 7.78320 OCTREOTIDE 1,000 MCG/5 ML VIAL 0 SAGENT PHARMACE MLGEN 25021-0455-05 43.20000 OCTREOTIDE 5,000 MCG/5 ML VIAL 0 SAGENT PHARMACE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 11980-<strong>08</strong>01-03 1.21045 11.79928 OCUFEN 0.03% EYE DROPS G ALLERGAN INC. MLBND 11980-0779-05 0.39015 15.74842 OCUFLOX 0.3% EYE DROPS G ALLERGAN INC. MLGEN 242<strong>08</strong>-0410-05 2.76600 <strong>OF</strong>LOXACIN 0.3% EAR DROPS 0 VALEANT MLGEN 242<strong>08</strong>-0410-10 2.32050 <strong>OF</strong>LOXACIN 0.3% EAR DROPS 0 VALEANT MLGEN 50383-0025-05 2.91801 <strong>OF</strong>LOXACIN 0.3% EAR DROPS 0 HI-TECH PHARMAC MLGEN 50383-0025-10 2.91801 <strong>OF</strong>LOXACIN 0.3% EAR DROPS 0 HI-TECH PHARMAC MLGEN 60505-0363-01 2.91801 <strong>OF</strong>LOXACIN 0.3% EAR DROPS 0 APOTEX CORP MLGEN 60505-0363-02 2.91801 <strong>OF</strong>LOXACIN 0.3% EAR DROPS 0 APOTEX CORP MLGEN 61314-0015-05 2.91801 <strong>OF</strong>LOXACIN 0.3% EAR DROPS 0 SANDOZ MLGEN 61314-0015-10 2.91801 <strong>OF</strong>LOXACIN 0.3% EAR DROPS 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16571-0130-11 0.39015 <strong>OF</strong>LOXACIN 0.3% EYE DROPS G PACK PHARMACEUT MLGEN 16571-0130-50 0.39015 <strong>OF</strong>LOXACIN 0.3% EYE DROPS G PACK PHARMACEUT MLGEN 17478-0713-10 0.39015 <strong>OF</strong>LOXACIN 0.3% EYE DROPS G AKORN INC. MLGEN 17478-0713-11 0.39015 <strong>OF</strong>LOXACIN 0.3% EYE DROPS G AKORN INC. MLGEN 242<strong>08</strong>-0434-05 0.39015 <strong>OF</strong>LOXACIN 0.3% EYE DROPS G VALEANT MLGEN 242<strong>08</strong>-0434-10 0.39015 <strong>OF</strong>LOXACIN 0.3% EYE DROPS G VALEANT MLGEN 50383-0024-05 0.39015 <strong>OF</strong>LOXACIN 0.3% EYE DROPS G HI-TECH PHARMAC MLGEN 50383-0024-10 0.39015 <strong>OF</strong>LOXACIN 0.3% EYE DROPS G HI-TECH PHARMAC MLGEN 61314-0012-05 0.39015 <strong>OF</strong>LOXACIN 0.3% EYE DROPS G SANDOZ MLGEN 61314-0012-10 0.39015 <strong>OF</strong>LOXACIN 0.3% EYE DROPS G SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00093-7180-01 2.56430 3.97047 <strong>OF</strong>LOXACIN 200 MG TABLET G TEVA USA EABND 00093-7181-01 2.89040 4.72510 <strong>OF</strong>LOXACIN 300 MG TABLET G TEVA USA EAGEN 00093-7182-01 4.50270 <strong>OF</strong>LOXACIN 400 MG TABLET G TEVA USA EABEX 52544-<strong>08</strong>48-28 1.21180 1.33165 OGESTREL TABLET 0 ACTAVIS PHARMA, EAGEX 00093-5246-19 5.54000 OLANZAPINE ODT 10 MG TABLET G TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 289LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-5246-65 5.54000 OLANZAPINE ODT 10 MG TABLET G TEVA USA EAGEX 13668-0<strong>08</strong>8-30 5.54000 OLANZAPINE ODT 10 MG TABLET G TORRENT PHARMAC EAGEX 49884-0321-52 5.54000 OLANZAPINE ODT 10 MG TABLET G PAR PHARM. EAGEX 49884-0321-55 5.54000 OLANZAPINE ODT 10 MG TABLET G PAR PHARM. EAGEX 55111-0263-81 5.54000 OLANZAPINE ODT 10 MG TABLET G DR.REDDY'S LAB EAGEX 59746-0307-32 2.95475 OLANZAPINE ODT 10 MG TABLET G CADISTA PHARMAC EAGEX 60505-3276-00 5.54000 OLANZAPINE ODT 10 MG TABLET G APOTEX CORP EAGEX 60505-3276-03 5.54000 OLANZAPINE ODT 10 MG TABLET G APOTEX CORP EAGEX 62756-0754-64 5.54000 OLANZAPINE ODT 10 MG TABLET G SUN PHARMACEUTI EAGEX 66993-0054-30 5.54000 OLANZAPINE ODT 10 MG TABLET G PRASCO LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 66993-0467-30 5.54000 OLANZAPINE ODT 10 MG TABLET G PRASCO LABS EAGEX 00093-5247-19 8.15000 OLANZAPINE ODT 15 MG TABLET G TEVA USA EAGEX 00093-5247-65 8.15000 OLANZAPINE ODT 15 MG TABLET G TEVA USA EAGEX 13668-0<strong>08</strong>9-30 8.15000 OLANZAPINE ODT 15 MG TABLET G TORRENT PHARMAC EAGEX 49884-0322-52 8.15000 OLANZAPINE ODT 15 MG TABLET G PAR PHARM. EAGEX 49884-0322-55 8.15000 OLANZAPINE ODT 15 MG TABLET G PAR PHARM. EAGEX 55111-0264-81 8.15000 OLANZAPINE ODT 15 MG TABLET G DR.REDDY'S LAB EAGEX 59746-03<strong>08</strong>-32 4.35125 OLANZAPINE ODT 15 MG TABLET G CADISTA PHARMAC EAGEX 60505-3277-00 8.15000 OLANZAPINE ODT 15 MG TABLET G APOTEX CORP EAGEX 60505-3277-03 8.15000 OLANZAPINE ODT 15 MG TABLET G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62756-0755-64 8.15000 OLANZAPINE ODT 15 MG TABLET G SUN PHARMACEUTI EAGEX 66993-0055-30 8.15000 OLANZAPINE ODT 15 MG TABLET G PRASCO LABS EAGEX 66993-0468-30 8.15000 OLANZAPINE ODT 15 MG TABLET G PRASCO LABS EAGEX 00093-5248-19 23.73792 OLANZAPINE ODT 20 MG TABLET G TEVA USA EAGEX 00093-5248-65 23.73792 OLANZAPINE ODT 20 MG TABLET G TEVA USA EAGEX 13668-0090-30 23.73792 OLANZAPINE ODT 20 MG TABLET G TORRENT PHARMAC EAGEX 49884-0323-52 23.73792 OLANZAPINE ODT 20 MG TABLET G PAR PHARM. EAGEX 49884-0323-55 23.73792 OLANZAPINE ODT 20 MG TABLET G PAR PHARM. EAGEX 55111-0265-81 23.73792 OLANZAPINE ODT 20 MG TABLET G DR.REDDY'S LAB EAGEX 59746-0309-32 5.76249 OLANZAPINE ODT 20 MG TABLET G CADISTA PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-3278-00 23.73792 OLANZAPINE ODT 20 MG TABLET G APOTEX CORP EAGEX 60505-3278-03 23.73792 OLANZAPINE ODT 20 MG TABLET G APOTEX CORP EAGEX 62756-0757-64 23.73792 OLANZAPINE ODT 20 MG TABLET G SUN PHARMACEUTI EAGEX 66993-0056-30 23.73792 OLANZAPINE ODT 20 MG TABLET G PRASCO LABS EAGEX 66993-0469-30 23.73792 OLANZAPINE ODT 20 MG TABLET G PRASCO LABS EAGEX 00093-5245-65 9.68338 OLANZAPINE ODT 5 MG TABLET G TEVA USA EAGEX 13668-0<strong>08</strong>6-30 9.68338 OLANZAPINE ODT 5 MG TABLET G TORRENT PHARMAC EAGEX 49884-0320-52 9.68338 OLANZAPINE ODT 5 MG TABLET G PAR PHARM. EAGEX 49884-0320-55 9.68338 OLANZAPINE ODT 5 MG TABLET G PAR PHARM. EAGEX 55111-0262-81 9.68338 OLANZAPINE ODT 5 MG TABLET G DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59746-03<strong>06</strong>-32 2.01399 OLANZAPINE ODT 5 MG TABLET G CADISTA PHARMAC EAGEX 60505-3275-00 9.68338 OLANZAPINE ODT 5 MG TABLET G APOTEX CORP EAGEX 60505-3275-03 9.68338 OLANZAPINE ODT 5 MG TABLET G APOTEX CORP EAGEX 62756-0751-64 9.68338 OLANZAPINE ODT 5 MG TABLET G SUN PHARMACEUTI EAGEX 66993-0053-30 9.68338 OLANZAPINE ODT 5 MG TABLET G PRASCO LABS EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 290LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 66993-0466-30 9.68338 OLANZAPINE ODT 5 MG TABLET G PRASCO LABS EAGEX 00093-5770-01 0.56070 OLANZAPINE 10 MG TABLET G TEVA USA EAGEX 00093-5770-10 0.56070 OLANZAPINE 10 MG TABLET G TEVA USA EAGEX 00093-5770-56 0.56070 OLANZAPINE 10 MG TABLET G TEVA USA EAGEX 00378-5454-10 0.56070 OLANZAPINE 10 MG TABLET G MYLAN EAGEX 00378-5454-91 0.56070 OLANZAPINE 10 MG TABLET G MYLAN EAGEX 00378-5454-93 0.56070 OLANZAPINE 10 MG TABLET G MYLAN EAGEX 00904-6285-61 0.56070 OLANZAPINE 10 MG TABLET G MAJOR PHARMACEU EAGEX 13668-0169-01 0.56070 OLANZAPINE 10 MG TABLET G TORRENT PHARMAC EAGEX 13668-0169-10 0.56070 OLANZAPINE 10 MG TABLET G TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 43598-0166-05 0.56070 OLANZAPINE 10 MG TABLET G DR.REDDY'S LAB EAGEX 43598-0166-30 0.56070 OLANZAPINE 10 MG TABLET G DR.REDDY'S LAB EAGEX 51079-0154-20 0.56070 OLANZAPINE 10 MG TABLET G MYLAN INSTITUTI EAGEX 55111-0166-05 0.56070 OLANZAPINE 10 MG TABLET G DR.REDDY'S LAB EAGEX 55111-0166-30 0.56070 OLANZAPINE 10 MG TABLET G DR.REDDY'S LAB EAGEX 59762-01<strong>08</strong>-01 0.56070 OLANZAPINE 10 MG TABLET G GREENSTONE LLC. EAGEX 60505-3113-00 0.56070 OLANZAPINE 10 MG TABLET G APOTEX CORP EAGEX 60505-3113-03 0.56070 OLANZAPINE 10 MG TABLET G APOTEX CORP EAGEX 62756-0554-18 0.56070 OLANZAPINE 10 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0554-83 0.56070 OLANZAPINE 10 MG TABLET G SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62756-0554-88 0.56070 OLANZAPINE 10 MG TABLET G SUN PHARMACEUTI EAGEX 65862-0564-30 0.56070 OLANZAPINE 10 MG TABLET G AUROBINDO PHARM EAGEX 65862-0564-99 0.56070 OLANZAPINE 10 MG TABLET G AUROBINDO PHARM EAGEX 66993-0050-05 0.56070 OLANZAPINE 10 MG TABLET G PRASCO LABS EAGEX 66993-0050-30 0.56070 OLANZAPINE 10 MG TABLET G PRASCO LABS EAGEX 66993-0463-05 0.56070 OLANZAPINE 10 MG TABLET G PRASCO LABS EAGEX 66993-0463-30 0.56070 OLANZAPINE 10 MG TABLET G PRASCO LABS EAGEX 00093-5771-01 0.84150 OLANZAPINE 15 MG TABLET G TEVA USA EAGEX 00093-5771-10 0.84150 OLANZAPINE 15 MG TABLET G TEVA USA EAGEX 00093-5771-56 0.84150 OLANZAPINE 15 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-5522-10 0.84150 OLANZAPINE 15 MG TABLET G MYLAN EAGEX 00378-5522-91 0.84150 OLANZAPINE 15 MG TABLET G MYLAN EAGEX 00378-5522-93 0.84150 OLANZAPINE 15 MG TABLET G MYLAN EAGEX 00904-6286-<strong>06</strong> 0.84150 OLANZAPINE 15 MG TABLET G MAJOR PHARMACEU EAGEX 13668-0170-01 0.84150 OLANZAPINE 15 MG TABLET G TORRENT PHARMAC EAGEX 13668-0170-10 0.84150 OLANZAPINE 15 MG TABLET G TORRENT PHARMAC EAGEX 13668-0170-30 0.84150 OLANZAPINE 15 MG TABLET G TORRENT PHARMAC EAGEX 51079-0155-20 0.84150 OLANZAPINE 15 MG TABLET G MYLAN INSTITUTI EAGEX 55111-0167-05 0.84150 OLANZAPINE 15 MG TABLET G DR.REDDY'S LAB EAGEX 55111-0167-30 0.84150 OLANZAPINE 15 MG TABLET G DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-0109-01 0.84150 OLANZAPINE 15 MG TABLET G GREENSTONE LLC. EAGEX 60505-3114-00 0.84150 OLANZAPINE 15 MG TABLET G APOTEX CORP EAGEX 60505-3114-03 0.84150 OLANZAPINE 15 MG TABLET G APOTEX CORP EAGEX 60505-3114-<strong>08</strong> 0.84150 OLANZAPINE 15 MG TABLET G APOTEX CORP EAGEX 62756-0555-18 0.84150 OLANZAPINE 15 MG TABLET G SUN PHARMACEUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 291LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62756-0555-83 0.84150 OLANZAPINE 15 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0555-88 0.84150 OLANZAPINE 15 MG TABLET G SUN PHARMACEUTI EAGEX 65862-0565-30 0.84150 OLANZAPINE 15 MG TABLET G AUROBINDO PHARM EAGEX 65862-0565-99 0.84150 OLANZAPINE 15 MG TABLET G AUROBINDO PHARM EAGEX 66993-0051-05 0.84150 OLANZAPINE 15 MG TABLET G PRASCO LABS EAGEX 66993-0051-30 0.84150 OLANZAPINE 15 MG TABLET G PRASCO LABS EAGEX 66993-0464-05 0.84150 OLANZAPINE 15 MG TABLET G PRASCO LABS EAGEX 66993-0464-30 0.84150 OLANZAPINE 15 MG TABLET G PRASCO LABS EAGEX 00093-5767-01 0.30740 OLANZAPINE 2.5 MG TABLET G TEVA USA EAGEX 00093-5767-10 0.30740 OLANZAPINE 2.5 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-5767-56 0.30740 OLANZAPINE 2.5 MG TABLET G TEVA USA EAGEX 00378-5157-10 0.30740 OLANZAPINE 2.5 MG TABLET G MYLAN EAGEX 00378-5157-91 0.30740 OLANZAPINE 2.5 MG TABLET G MYLAN EAGEX 00378-5157-93 0.30740 OLANZAPINE 2.5 MG TABLET G MYLAN EAGEX 00904-6283-61 0.30740 OLANZAPINE 2.5 MG TABLET G MAJOR PHARMACEU EAGEX 13668-0166-01 0.30740 OLANZAPINE 2.5 MG TABLET G TORRENT PHARMAC EAGEX 13668-0166-10 0.30740 OLANZAPINE 2.5 MG TABLET G TORRENT PHARMAC EAGEX 13668-0166-30 0.30740 OLANZAPINE 2.5 MG TABLET G TORRENT PHARMAC EAGEX 51079-0152-20 0.30740 OLANZAPINE 2.5 MG TABLET G MYLAN INSTITUTI EAGEX 55111-0163-05 0.30740 OLANZAPINE 2.5 MG TABLET G DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 55111-0163-30 0.30740 OLANZAPINE 2.5 MG TABLET G DR.REDDY'S LAB EAGEX 59762-0105-01 0.30740 OLANZAPINE 2.5 MG TABLET G GREENSTONE LLC. EAGEX 60505-3110-00 0.30740 OLANZAPINE 2.5 MG TABLET G APOTEX CORP EAGEX 60505-3110-03 0.30740 OLANZAPINE 2.5 MG TABLET G APOTEX CORP EAGEX 60505-3110-<strong>08</strong> 0.30740 OLANZAPINE 2.5 MG TABLET G APOTEX CORP EAGEX 62756-0551-18 0.30740 OLANZAPINE 2.5 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0551-83 0.30740 OLANZAPINE 2.5 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0551-88 0.30740 OLANZAPINE 2.5 MG TABLET G SUN PHARMACEUTI EAGEX 65862-0561-30 0.30740 OLANZAPINE 2.5 MG TABLET G AUROBINDO PHARM EAGEX 65862-0561-99 0.30740 OLANZAPINE 2.5 MG TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 66993-0460-05 0.30740 OLANZAPINE 2.5 MG TABLET G PRASCO LABS EAGEX 66993-0460-30 0.30740 OLANZAPINE 2.5 MG TABLET G PRASCO LABS EAGEX 68<strong>08</strong>4-0525-01 0.30740 OLANZAPINE 2.5 MG TABLET G AHP EAGEX 00093-5105-01 1.09400 OLANZAPINE 20 MG TABLET G TEVA USA EAGEX 00093-5105-05 1.09400 OLANZAPINE 20 MG TABLET G TEVA USA EAGEX 00093-5105-56 1.09400 OLANZAPINE 20 MG TABLET G TEVA USA EAGEX 00378-5713-10 1.09400 OLANZAPINE 20 MG TABLET G MYLAN EAGEX 00378-5713-91 1.09400 OLANZAPINE 20 MG TABLET G MYLAN EAGEX 00378-5713-93 1.09400 OLANZAPINE 20 MG TABLET G MYLAN EAGEX 00904-6287-<strong>06</strong> 1.09400 OLANZAPINE 20 MG TABLET G MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0171-01 1.09400 OLANZAPINE 20 MG TABLET G TORRENT PHARMAC EAGEX 13668-0171-10 1.09400 OLANZAPINE 20 MG TABLET G TORRENT PHARMAC EAGEX 51079-0156-20 1.09400 OLANZAPINE 20 MG TABLET G MYLAN INSTITUTI EAGEX 55111-0168-05 1.09400 OLANZAPINE 20 MG TABLET G DR.REDDY'S LAB EAGEX 55111-0168-30 1.09400 OLANZAPINE 20 MG TABLET G DR.REDDY'S LAB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 292LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-0110-01 1.09400 OLANZAPINE 20 MG TABLET G GREENSTONE LLC. EAGEX 60505-3140-00 1.09400 OLANZAPINE 20 MG TABLET G APOTEX CORP EAGEX 60505-3140-03 1.09400 OLANZAPINE 20 MG TABLET G APOTEX CORP EAGEX 60505-3140-<strong>08</strong> 1.09400 OLANZAPINE 20 MG TABLET G APOTEX CORP EAGEX 62756-0556-18 1.09400 OLANZAPINE 20 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0556-83 1.09400 OLANZAPINE 20 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0556-88 1.09400 OLANZAPINE 20 MG TABLET G SUN PHARMACEUTI EAGEX 65862-0566-30 1.09400 OLANZAPINE 20 MG TABLET G AUROBINDO PHARM EAGEX 65862-0566-99 1.09400 OLANZAPINE 20 MG TABLET G AUROBINDO PHARM EAGEX 66993-0052-05 1.09400 OLANZAPINE 20 MG TABLET G PRASCO LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 66993-0052-30 1.09400 OLANZAPINE 20 MG TABLET G PRASCO LABS EAGEX 66993-0465-05 1.09400 OLANZAPINE 20 MG TABLET G PRASCO LABS EAGEX 66993-0465-30 1.09400 OLANZAPINE 20 MG TABLET G PRASCO LABS EAGEX 00093-5768-01 0.36360 OLANZAPINE 5 MG TABLET G TEVA USA EAGEX 00093-5768-10 0.36360 OLANZAPINE 5 MG TABLET G TEVA USA EAGEX 00093-5768-56 0.36360 OLANZAPINE 5 MG TABLET G TEVA USA EAGEX 00378-5212-10 0.36360 OLANZAPINE 5 MG TABLET G MYLAN EAGEX 00378-5212-91 0.36360 OLANZAPINE 5 MG TABLET G MYLAN EAGEX 00378-5212-93 0.36360 OLANZAPINE 5 MG TABLET G MYLAN EAGEX 00904-6284-61 0.36360 OLANZAPINE 5 MG TABLET G MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0167-01 0.36360 OLANZAPINE 5 MG TABLET G TORRENT PHARMAC EAGEX 43598-0164-05 0.36360 OLANZAPINE 5 MG TABLET G DR.REDDY'S LAB EAGEX 43598-0164-30 0.36360 OLANZAPINE 5 MG TABLET G DR.REDDY'S LAB EAGEX 51079-0153-20 0.36360 OLANZAPINE 5 MG TABLET G MYLAN INSTITUTI EAGEX 55111-0164-05 0.36360 OLANZAPINE 5 MG TABLET G DR.REDDY'S LAB EAGEX 55111-0164-30 0.36360 OLANZAPINE 5 MG TABLET G DR.REDDY'S LAB EAGEX 59762-01<strong>06</strong>-01 0.36360 OLANZAPINE 5 MG TABLET G GREENSTONE LLC. EAGEX 60505-3111-00 0.36360 OLANZAPINE 5 MG TABLET G APOTEX CORP EAGEX 60505-3111-03 0.36360 OLANZAPINE 5 MG TABLET G APOTEX CORP EAGEX 62756-0552-18 0.36360 OLANZAPINE 5 MG TABLET G SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62756-0552-83 0.36360 OLANZAPINE 5 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0552-88 0.36360 OLANZAPINE 5 MG TABLET G SUN PHARMACEUTI EAGEX 65862-0562-30 0.36360 OLANZAPINE 5 MG TABLET G AUROBINDO PHARM EAGEX 65862-0562-99 0.36360 OLANZAPINE 5 MG TABLET G AUROBINDO PHARM EAGEX 66993-0461-05 0.36360 OLANZAPINE 5 MG TABLET G PRASCO LABS EAGEX 66993-0461-30 0.36360 OLANZAPINE 5 MG TABLET G PRASCO LABS EAGEX 00093-5769-01 0.45270 OLANZAPINE 7.5 MG TABLET G TEVA USA EAGEX 00093-5769-10 0.45270 OLANZAPINE 7.5 MG TABLET G TEVA USA EAGEX 00093-5769-56 0.45270 OLANZAPINE 7.5 MG TABLET G TEVA USA EAGEX 00378-5335-10 0.45270 OLANZAPINE 7.5 MG TABLET G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-5335-91 0.45270 OLANZAPINE 7.5 MG TABLET G MYLAN EAGEX 00378-5335-93 0.45270 OLANZAPINE 7.5 MG TABLET G MYLAN EAGEX 13668-0168-10 0.45270 OLANZAPINE 7.5 MG TABLET G TORRENT PHARMAC EAGEX 13668-0168-30 0.45270 OLANZAPINE 7.5 MG TABLET G TORRENT PHARMAC EAGEX 43598-0165-05 0.45270 OLANZAPINE 7.5 MG TABLET G DR.REDDY'S LAB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 293LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 43598-0165-30 0.45270 OLANZAPINE 7.5 MG TABLET G DR.REDDY'S LAB EAGEX 55111-0165-05 0.45270 OLANZAPINE 7.5 MG TABLET G DR.REDDY'S LAB EAGEX 55111-0165-30 0.45270 OLANZAPINE 7.5 MG TABLET G DR.REDDY'S LAB EAGEX 59762-0107-01 0.45270 OLANZAPINE 7.5 MG TABLET G GREENSTONE LLC. EAGEX 60505-3112-00 0.45270 OLANZAPINE 7.5 MG TABLET G APOTEX CORP EAGEX 60505-3112-03 0.45270 OLANZAPINE 7.5 MG TABLET G APOTEX CORP EAGEX 60505-3112-<strong>08</strong> 0.45270 OLANZAPINE 7.5 MG TABLET G APOTEX CORP EAGEX 62756-0553-18 0.45270 OLANZAPINE 7.5 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0553-83 0.45270 OLANZAPINE 7.5 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0553-88 0.45270 OLANZAPINE 7.5 MG TABLET G SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0563-30 0.45270 OLANZAPINE 7.5 MG TABLET G AUROBINDO PHARM EAGEX 65862-0563-99 0.45270 OLANZAPINE 7.5 MG TABLET G AUROBINDO PHARM EAGEX 66993-0049-05 0.45270 OLANZAPINE 7.5 MG TABLET G PRASCO LABS EAGEX 66993-0049-30 0.45270 OLANZAPINE 7.5 MG TABLET G PRASCO LABS EAGEX 66993-0462-05 0.45270 OLANZAPINE 7.5 MG TABLET G PRASCO LABS EAGEX 66993-0462-30 0.45270 OLANZAPINE 7.5 MG TABLET G PRASCO LABS EAGEX 00093-55<strong>06</strong>-56 16.37000 OLANZAPINE-FLUOXETINE 12-25 MG G TEVA USA EAGEX 00781-2192-31 16.35825 OLANZAPINE-FLUOXETINE 12-25 MG G SANDOZ EAGEX 49884-0252-11 16.36899 OLANZAPINE-FLUOXETINE 12-25 MG G PAR PHARM. EAGEX 00093-5507-56 16.37000 OLANZAPINE-FLUOXETINE 12-50 MG G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-2194-31 16.35825 OLANZAPINE-FLUOXETINE 12-50 MG G SANDOZ EAGEX 49884-0253-11 16.36899 OLANZAPINE-FLUOXETINE 12-50 MG G PAR PHARM. EAGEX 00093-5503-56 7.94600 OLANZAPINE-FLUOXETINE 3-25 MG G TEVA USA EAGEX 00781-2195-31 8.27724 OLANZAPINE-FLUOXETINE 3-25 MG G SANDOZ EAGEX 49884-0277-11 7.94550 OLANZAPINE-FLUOXETINE 3-25 MG G PAR PHARM. EAGEX 00093-5504-56 10.86200 OLANZAPINE-FLUOXETINE 6-25 MG G TEVA USA EAGEX 00781-2191-31 10.86099 OLANZAPINE-FLUOXETINE 6-25 MG G SANDOZ EAGEX 49884-0250-11 10.86150 OLANZAPINE-FLUOXETINE 6-25 MG G PAR PHARM. EAGEX 00093-5505-56 10.86200 OLANZAPINE-FLUOXETINE 6-50 MG G TEVA USA EAGEX 00781-2193-31 10.86099 OLANZAPINE-FLUOXETINE 6-50 MG G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 49884-0251-11 10.86150 OLANZAPINE-FLUOXETINE 6-50 MG G PAR PHARM. EABEX 43595-0<strong>08</strong>0-03 3.18720 OLEPTRO ER 150 MG TABLET 0 ANGELINI LABOPH EABEX 43595-0<strong>08</strong>1-03 3.88440 OLEPTRO ER 300 MG TABLET 0 ANGELINI LABOPH EABUL 40076-0031-00 2.97960 6.00388 OLUX 0.05% FOAM G PRESTIUM PHARMA GMBUL 40076-0031-50 2.97960 6.51367 OLUX 0.05% FOAM G PRESTIUM PHARMA GMBUL 63032-0031-00 2.97960 6.00388 OLUX 0.05% FOAM G PRESTIUM PHARMA GMBUL 63032-0031-50 2.97960 6.51367 OLUX 0.05% FOAM G PRESTIUM PHARMA GMBND 63032-0101-00 4.14071 6.00388 OLUX-E 0.05% FOAM G PRESTIUM PHARMA GMBND 63032-0101-50 4.14071 6.51367 OLUX-E 0.05% FOAM G PRESTIUM PHARMA GMBND 59676-0225-28 786.84000 OLYSIO 150 MG CAPSULE G JANSSEN PRODUCT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 65224-0707-11 5.16623 OMECLAMOX-PAK COMBO PACK G PERNIX THERAPEU EAGEN 00378-5211-93 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G MYLAN EAGEN 00781-2232-01 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G SANDOZ EAGEN 00781-2232-10 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G SANDOZ EAGEN 00781-2232-31 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 294LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0157-01 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G DR.REDDY'S LAB EAGEN 55111-0157-30 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G DR.REDDY'S LAB EAGEN 60505-0145-00 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G APOTEX CORP EAGEN 60505-0145-01 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G APOTEX CORP EAGEN 60505-0145-02 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G APOTEX CORP EAGEN 62175-0114-32 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G KREMERS URBAN EAGEN 62175-0114-37 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G KREMERS URBAN EAGEN 68<strong>08</strong>4-0555-11 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G AHP EAGEN 68<strong>08</strong>4-0555-21 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G AHP EAGEN 68382-0411-01 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0411-<strong>06</strong> 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G ZYDUS PHARMACEU EAGEN 68382-0411-10 0.26514 OMEPRAZOLE DR 10 MG CAPSULE G ZYDUS PHARMACEU EAGEN 00378-6150-01 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G MYLAN EAGEN 00378-6150-10 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G MYLAN EAGEN 00378-6150-77 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G MYLAN EAGEN 00378-6150-93 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G MYLAN EAGEN 00781-2233-01 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G SANDOZ EAGEN 00781-2233-10 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G SANDOZ EAGEN 00781-2233-31 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G SANDOZ EAGEN 00781-2233-92 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0007-17 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G MYLAN INSTITUTI EAGEN 51079-0007-19 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G MYLAN INSTITUTI EAGEN 51079-0007-30 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G MYLAN INSTITUTI EAGEN 51079-0007-56 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G MYLAN INSTITUTI EAGEN 55111-0158-01 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G DR.REDDY'S LAB EAGEN 55111-0158-10 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G DR.REDDY'S LAB EAGEN 55111-0158-30 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G DR.REDDY'S LAB EAGEN 58517-0020-30 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G <strong>NEW</strong> HORIZON RX EAGEN 60505-0<strong>06</strong>5-00 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G APOTEX CORP EAGEN 60505-0<strong>06</strong>5-01 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0<strong>06</strong>5-02 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G APOTEX CORP EAGEN 60505-3952-03 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G APOTEX CORP EAGEN 62175-0118-32 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G KREMERS URBAN EAGEN 62175-0118-37 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G KREMERS URBAN EAGEN 62175-0118-43 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G KREMERS URBAN EAGEN 68<strong>08</strong>4-0128-01 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G AHP EAGEN 68<strong>08</strong>4-0128-11 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G AHP EAGEN 68382-0412-01 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G ZYDUS PHARMACEU EAGEN 68382-0412-<strong>06</strong> 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G ZYDUS PHARMACEU EAGEN 68382-0412-10 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68645-0320-54 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G LEGACY PHARMACE EAGEN 68645-0473-54 0.<strong>08</strong>735 OMEPRAZOLE DR 20 MG CAPSULE G LEGACY PHARMACE EAGEN 00378-5222-05 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G MYLAN EAGEN 00378-5222-93 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G MYLAN EAGEN 00781-2234-01 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 295LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-2234-10 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G SANDOZ EAGEN 00781-2234-31 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G SANDOZ EAGEN 00904-6144-61 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G MAJOR PHARMACEU EAGEN 55111-0159-01 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G DR.REDDY'S LAB EAGEN 55111-0159-05 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G DR.REDDY'S LAB EAGEN 55111-0159-30 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G DR.REDDY'S LAB EAGEN 60505-0146-00 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G APOTEX CORP EAGEN 60505-0146-01 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G APOTEX CORP EAGEN 60505-0146-02 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G APOTEX CORP EAGEN 60505-0146-09 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62037-<strong>06</strong>40-01 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G ACTAVIS PHARMA, EAGEN 62037-<strong>06</strong>40-10 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G ACTAVIS PHARMA, EAGEN 62037-<strong>06</strong>40-30 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G ACTAVIS PHARMA, EAGEN 62175-0136-32 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G KREMERS URBAN EAGEN 62175-0136-37 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G KREMERS URBAN EAGEN 62175-0136-43 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G KREMERS URBAN EAGEN 63739-0445-10 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0466-01 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G AHP EAGEN 68<strong>08</strong>4-0466-11 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G AHP EAGEN 68382-0500-01 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0500-<strong>06</strong> 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G ZYDUS PHARMACEU EAGEN 68382-0500-10 0.29260 OMEPRAZOLE DR 40 MG CAPSULE G ZYDUS PHARMACEU EAGEN 38779-1935-04 74.81250 OMEPRAZOLE POWDER 0 MEDISCA INC. GMGEN 38779-1935-05 74.81250 OMEPRAZOLE POWDER 0 MEDISCA INC. GMGEN 49884-0397-11 0.86400 OMEPRAZOLE-BICARB 20-1,100 CAP G PAR PHARM. EAGEN 66993-0412-30 0.86400 OMEPRAZOLE-BICARB 20-1,100 CAP G PRASCO LABS EAGEN 49884-0455-11 4.85150 OMEPRAZOLE-BICARB 40-1,100 CAP G PAR PHARM. EAGEN 66993-0413-30 9.40175 OMEPRAZOLE-BICARB 40-1,100 CAP G PRASCO LABS EABND 63402-0701-01 11.63394 OMNARIS 50 MCG NASAL SPRAY G SUNOVION PHARMA GMBUL 00<strong>06</strong>5-<strong>06</strong>38-25 1.69500 10.38828 OMNIPRED 1% EYE DROPS G ALCON LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00<strong>06</strong>5-<strong>06</strong>38-27 1.69500 13.74480 OMNIPRED 1% EYE DROPS G ALCON LABS. MLBND 00781-3004-07 541.26513 OMNITROPE 10 MG/1.5 ML CRTG G SANDOZ MLBND 00781-3004-26 541.26402 OMNITROPE 10 MG/1.5 ML CRTG G SANDOZ MLBND 00781-3001-07 270.63533 OMNITROPE 5 MG/1.5 ML CRTG G SANDOZ MLBND 00781-3001-26 270.63201 OMNITROPE 5 MG/1.5 ML CRTG G SANDOZ MLBND 00781-4004-36 270.67026 OMNITROPE 5.8 MG VIAL G SANDOZ EABND 54482-0301-01 1192.29334 ONCASPAR 750 UNIT/ML VIAL 0 SIGMA-TAU MLGEN 00093-0233-56 0.21070 ONDANSETRON HCL 4 MG TABLET 0 TEVA USA EAGEN 00378-0315-93 0.21070 ONDANSETRON HCL 4 MG TABLET 0 MYLAN EAGEN 00781-1679-31 0.21070 ONDANSETRON HCL 4 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-1679-33 0.21070 ONDANSETRON HCL 4 MG TABLET 0 SANDOZ EAGEN 00904-62<strong>08</strong>-46 0.21070 ONDANSETRON HCL 4 MG TABLET 0 MAJOR PHARMACEU EAGEN 45963-0538-30 0.21070 ONDANSETRON HCL 4 MG TABLET 0 ACTAVIS PHARMA, EAGEN 51079-0524-01 0.21070 ONDANSETRON HCL 4 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0524-20 0.21070 ONDANSETRON HCL 4 MG TABLET 0 MYLAN INSTITUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 296LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0153-30 0.21070 ONDANSETRON HCL 4 MG TABLET 0 DR.REDDY'S LAB EAGEN 59762-2990-01 0.21070 ONDANSETRON HCL 4 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-2990-02 0.21070 ONDANSETRON HCL 4 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-1311-03 0.21070 ONDANSETRON HCL 4 MG TABLET 0 APOTEX CORP EAGEN 62756-0130-01 0.21070 ONDANSETRON HCL 4 MG TABLET 0 SUN PHARMACEUTI EAGEN 63304-0458-30 0.21070 ONDANSETRON HCL 4 MG TABLET 0 RANBAXY PHARMAC EAGEN 65862-0187-03 0.21070 ONDANSETRON HCL 4 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0187-05 0.21070 ONDANSETRON HCL 4 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0187-30 0.21070 ONDANSETRON HCL 4 MG TABLET 0 AUROBINDO PHARM EAGEN 67877-0169-30 0.21070 ONDANSETRON HCL 4 MG TABLET 0 ASCEND LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0220-01 0.21070 ONDANSETRON HCL 4 MG TABLET 0 AHP EAGEN 68462-0105-30 0.21070 ONDANSETRON HCL 4 MG TABLET 0 GLENMARK PHARMA EAGEN 76045-0103-20 0.59200 ONDANSETRON HCL 4 MG/2 ML SYR 0 BD RX INC. MLGEN 00143-9891-25 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 WEST-WARD,INC. MLGEN 00409-4755-03 0.19800 ONDANSETRON HCL 4 MG/2 ML VIAL 0 HOSPIRA MLGEN 0<strong>06</strong>41-6078-01 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6078-25 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6<strong>08</strong>0-01 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6<strong>08</strong>0-25 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 WEST-WARD,INC. MLGEN 00703-7221-04 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 TEVA PARENTERAL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3010-72 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 SANDOZ MLGEN 00781-3010-95 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 SANDOZ MLGEN 25021-0777-02 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 SAGENT PHARMACE MLGEN 55150-0125-02 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 AUROMEDICS PHAR MLGEN 64679-0726-01 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 WOCKHARDT USA L MLGEN 65293-0373-02 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 MEDICINES COMP. MLGEN 65293-0373-25 0.26600 ONDANSETRON HCL 4 MG/2 ML VIAL 0 MEDICINES COMP. MLGEN 00093-7236-56 0.19040 ONDANSETRON HCL 8 MG TABLET 0 TEVA USA EAGEN 00378-0344-93 0.19040 ONDANSETRON HCL 8 MG TABLET 0 MYLAN EAGEN 00781-1681-31 0.19040 ONDANSETRON HCL 8 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-1681-33 0.19040 ONDANSETRON HCL 8 MG TABLET 0 SANDOZ EAGEN 00904-6209-46 0.19040 ONDANSETRON HCL 8 MG TABLET 0 MAJOR PHARMACEU EAGEN 45963-0539-30 0.19040 ONDANSETRON HCL 8 MG TABLET 0 ACTAVIS PHARMA, EAGEN 51079-0525-01 0.19040 ONDANSETRON HCL 8 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0525-20 0.19040 ONDANSETRON HCL 8 MG TABLET 0 MYLAN INSTITUTI EAGEN 55111-0154-30 0.19040 ONDANSETRON HCL 8 MG TABLET 0 DR.REDDY'S LAB EAGEN 59762-2993-01 0.19040 ONDANSETRON HCL 8 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-2993-02 0.19040 ONDANSETRON HCL 8 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-1312-03 0.19040 ONDANSETRON HCL 8 MG TABLET 0 APOTEX CORP EAGEN 62756-0131-01 0.19040 ONDANSETRON HCL 8 MG TABLET 0 SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63304-0459-30 0.19040 ONDANSETRON HCL 8 MG TABLET 0 RANBAXY PHARMAC EAGEN 65862-0188-03 0.19040 ONDANSETRON HCL 8 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0188-05 0.19040 ONDANSETRON HCL 8 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0188-30 0.19040 ONDANSETRON HCL 8 MG TABLET 0 AUROBINDO PHARM EAGEN 67877-0170-30 0.19040 ONDANSETRON HCL 8 MG TABLET 0 ASCEND LABORATO EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 297LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0221-01 0.19040 ONDANSETRON HCL 8 MG TABLET 0 AHP EAGEN 68462-01<strong>06</strong>-30 0.19040 ONDANSETRON HCL 8 MG TABLET 0 GLENMARK PHARMA EAGEN 00378-7732-93 0.67914 ONDANSETRON ODT 4 MG TABLET 0 MYLAN EAGEN 00781-5238-<strong>06</strong> 0.67914 ONDANSETRON ODT 4 MG TABLET 0 SANDOZ EAGEN 00781-5238-64 0.67914 ONDANSETRON ODT 4 MG TABLET 0 SANDOZ EAGEN 62756-0240-64 0.67914 ONDANSETRON ODT 4 MG TABLET 0 SUN PHARMACEUTI EAGEN 65862-0390-10 0.67914 ONDANSETRON ODT 4 MG TABLET 0 AUROBINDO PHARM EAGEN 68462-0157-13 0.67914 ONDANSETRON ODT 4 MG TABLET 0 GLENMARK PHARMA EAGEN 00378-7734-93 1.04450 ONDANSETRON ODT 8 MG TABLET 0 MYLAN EAGEN 00378-7734-97 1.04450 ONDANSETRON ODT 8 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-5239-<strong>06</strong> 1.04450 ONDANSETRON ODT 8 MG TABLET 0 SANDOZ EAGEN 00781-5239-64 1.04450 ONDANSETRON ODT 8 MG TABLET 0 SANDOZ EAGEN 00781-5239-80 1.04450 ONDANSETRON ODT 8 MG TABLET 0 SANDOZ EAGEN 62756-0356-64 1.04450 ONDANSETRON ODT 8 MG TABLET 0 SUN PHARMACEUTI EAGEN 62756-0356-66 1.04450 ONDANSETRON ODT 8 MG TABLET 0 SUN PHARMACEUTI EAGEN 65862-0391-10 1.04450 ONDANSETRON ODT 8 MG TABLET 0 AUROBINDO PHARM EAGEN 68462-0158-11 1.04450 ONDANSETRON ODT 8 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0158-13 1.04450 ONDANSETRON ODT 8 MG TABLET 0 GLENMARK PHARMA EAGEN 00054-0<strong>06</strong>4-47 1.45720 ONDANSETRON 4 MG/5 ML SOLUTION 0 ROXANE LABS. MLGEN 16714-<strong>06</strong>71-01 1.45720 ONDANSETRON 4 MG/5 ML SOLUTION 0 NORTHSTAR RX LL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-4091-03 1.45720 ONDANSETRON 4 MG/5 ML SOLUTION 0 TARO PHARM USA MLGEN 54838-0555-50 1.45720 ONDANSETRON 4 MG/5 ML SOLUTION 0 SILARX PHARM MLGEN 60505-0381-05 1.45720 ONDANSETRON 4 MG/5 ML SOLUTION 0 APOTEX CORP MLGEN 65162-<strong>06</strong>91-79 1.45720 ONDANSETRON 4 MG/5 ML SOLUTION 0 AMNEAL PHARMACE MLGEN 68094-0325-59 1.45720 ONDANSETRON 4 MG/5 ML SOLUTION 0 PRECISION DOSE MLGEN 68094-0325-62 1.45720 ONDANSETRON 4 MG/5 ML SOLUTION 0 PRECISION DOSE MLGEN 00143-9890-01 0.35325 ONDANSETRON 40 MG/20 ML VIAL 0 WEST-WARD,INC. MLGEN 00409-4759-01 0.10500 ONDANSETRON 40 MG/20 ML VIAL 0 HOSPIRA MLGEN 00703-7226-01 0.90000 ONDANSETRON 40 MG/20 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-7226-03 0.90000 ONDANSETRON 40 MG/20 ML VIAL 0 TEVA PARENTERAL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 23155-0168-31 0.93750 ONDANSETRON 40 MG/20 ML VIAL 0 HERITAGE PHARMA MLGEN 25021-0782-20 0.67500 ONDANSETRON 40 MG/20 ML VIAL 0 SAGENT PHARMACE MLGEN 55150-0126-20 0.93750 ONDANSETRON 40 MG/20 ML VIAL 0 AUROMEDICS PHAR MLGEN 65293-0374-01 0.22050 ONDANSETRON 40 MG/20 ML VIAL 0 MEDICINES COMP. MLGEN 65293-0374-20 0.22050 ONDANSETRON 40 MG/20 ML VIAL 0 MEDICINES COMP. MLBND 00003-4214-11 9.25948 ONGLYZA 2.5 MG TABLET G BMS PRIMARYCARE EABND 00003-4214-21 9.25929 ONGLYZA 2.5 MG TABLET G BMS PRIMARYCARE EABND 00003-4215-11 9.25948 ONGLYZA 5 MG TABLET G BMS PRIMARYCARE EABND 00003-4215-21 9.25929 ONGLYZA 5 MG TABLET G BMS PRIMARYCARE EABND 00003-4215-31 9.25921 ONGLYZA 5 MG TABLET G BMS PRIMARYCARE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00003-4215-41 9.25923 ONGLYZA 5 MG TABLET G BMS PRIMARYCARE EABND 00259-1420-14 29.36777 ONMEL 200 MG TABLET G MERZ EABND 00259-1420-28 29.36777 ONMEL 200 MG TABLET G MERZ EABND 66215-0501-15 227.<strong>08</strong>800 OPSUMIT 10 MG TABLET G ACTELION PHARMA EABND 66215-0501-30 227.<strong>08</strong>800 OPSUMIT 10 MG TABLET G ACTELION PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 298LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00037-7025-60 8.33750 24.09628 OPTIVAR 0.05% DROPS G MEDA PHARMACEUT MLBND 00299-3822-30 14.55820 ORACEA 40 MG CAPSULE G GALDERMA LABORA EAGEN 51672-1335-05 9.00100 ORALONE 0.1% PASTE 0 TARO PHARM USA GMBEX 57844-0151-01 1.59202 ORAP 1 MG TABLET 0 GATE PHARM EABEX 57844-0187-01 2.12247 ORAP 2 MG TABLET 0 GATE PHARM EABND 59630-0700-48 6.82743 ORAPRED ODT 10 MG TABLET G CONCORDIA PHARM EABND 59630-0701-48 10.77962 ORAPRED ODT 15 MG TABLET G CONCORDIA PHARM EABND 59630-0702-48 15.36053 ORAPRED ODT 30 MG TABLET G CONCORDIA PHARM EAGEN 59630-0710-<strong>08</strong> 0.07700 ORAPRED 15 MG/5 ML SOLUTION G CONCORDIA PHARM MLGEN 59630-0710-10 0.07700 ORAPRED 15 MG/5 ML SOLUTION G CONCORDIA PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00003-2188-11 629.21055 ORENCIA 125 MG/ML SYRINGE G BMS PRIMARYCARE MLBND 00003-2188-31 588.59865 ORENCIA 125 MG/ML SYRINGE G BMS PRIMARYCARE MLBND 66621-1010-<strong>06</strong> 346.<strong>08</strong>109 ORFADIN 10 MG CAPSULE 0 RARE DISEASE EABND 66621-1002-<strong>06</strong> 69.21646 ORFADIN 2 MG CAPSULE 0 RARE DISEASE EABND 66621-1005-<strong>06</strong> 173.04033 ORFADIN 5 MG CAPSULE 0 RARE DISEASE EABND 00185-0714-01 2.17368 ORPHENADRINE COMP FORTE TAB 0 SANDOZ EAGEN 00115-2011-01 0.39390 ORPHENADRINE ER 100 MG TABLET 0 GLOBAL PHARM EAGEN 00115-2011-02 0.39390 ORPHENADRINE ER 100 MG TABLET 0 GLOBAL PHARM EAGEN 00185-0022-01 0.39390 ORPHENADRINE ER 100 MG TABLET 0 SANDOZ EAGEN 00185-0022-10 0.39390 ORPHENADRINE ER 100 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43386-0480-24 0.39390 ORPHENADRINE ER 100 MG TABLET 0 GAVIS PHARMACEU EAGEN 43386-0480-26 0.39390 ORPHENADRINE ER 100 MG TABLET 0 GAVIS PHARMACEU EAGEN 43386-0480-28 0.39390 ORPHENADRINE ER 100 MG TABLET 0 GAVIS PHARMACEU EAGEX 0<strong>06</strong>03-7634-17 0.85280 ORSYTHIA-28 TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-7634-49 0.85280 ORSYTHIA-28 TABLET 0 QUALITEST EABEX 50458-0192-01 36.59470 ORTHO EVRA PATCH 0 JANSSEN PHARM. EABEX 50458-0192-15 36.59285 ORTHO EVRA PATCH 0 JANSSEN PHARM. EABEX 50458-0194-16 0.69917 1.35670 ORTHO MICRONOR 0.35 MG TABLET G JANSSEN PHARM. EABEX 50458-0251-15 4.11240 ORTHO TRI-CYCLEN LO TABLET 0 JANSSEN PHARM. EABEX 50458-0191-15 0.46850 1.24001 ORTHO TRI-CYCLEN 28 TABLET G JANSSEN PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 50458-0196-15 0.73800 1.90163 ORTHO-CEPT 28 DAY TABLET G JANSSEN PHARM. EABEX 50458-0197-15 0.49260 1.24001 ORTHO-CYCLEN 28 TABLET 0 JANSSEN PHARM. EABEX 50458-0176-15 0.73890 2.<strong>08</strong>982 ORTHO-NOVUM 1-35-28 TABLET G JANSSEN PHARM. EABEX 50458-0178-15 0.83640 1.12440 ORTHO-NOVUM 7-7-7-28 TABLET 0 JANSSEN PHARM. EAGEN 68047-0253-01 0.33555 OSCIMIN SL 0.125 MG TABLET 0 LARKEN LABS EAGEN 68047-0255-01 0.39640 OSCIMIN SR 0.375 MG TABLET 0 LARKEN LABS EAGEN 68047-0254-01 0.33922 OSCIMIN 0.125 MG ODT 0 LARKEN LABS EAGEN 68047-0252-01 0.33727 OSCIMIN 0.125 MG TABLET 0 LARKEN LABS EABND 64764-0121-03 9.42437 OSENI 12.5-15 MG TABLET G TAKEDA PHARMACE EABND 64764-0123-03 9.42437 OSENI 12.5-30 MG TABLET G TAKEDA PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64764-0124-03 9.42437 OSENI 12.5-45 MG TABLET G TAKEDA PHARMACE EABND 64764-0251-03 9.42437 OSENI 25-15 MG TABLET G TAKEDA PHARMACE EABND 64764-0253-03 9.42437 OSENI 25-30 MG TABLET G TAKEDA PHARMACE EABND 64764-0254-03 9.42437 OSENI 25-45 MG TABLET G TAKEDA PHARMACE EABND 65649-0701-41 3.91245 OSMOPREP TABLET G SALIX PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 299LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 28595-0420-20 5.70210 OTOZIN EAR DROPS 0 ALLEGIS PHARMAC MLBND 54436-0010-02 341.13000 OTREXUP 10 MG/0.4 ML AUTO-INJ 0 ANTARES PHARMA MLBND 54436-0010-04 341.13000 OTREXUP 10 MG/0.4 ML AUTO-INJ 0 ANTARES PHARMA MLBND 54436-0015-02 341.13000 OTREXUP 15 MG/0.4 ML AUTO-INJ 0 ANTARES PHARMA MLBND 54436-0015-04 341.13000 OTREXUP 15 MG/0.4 ML AUTO-INJ 0 ANTARES PHARMA MLBND 54436-0020-02 341.13000 OTREXUP 20 MG/0.4 ML AUTO-INJ 0 ANTARES PHARMA MLBND 54436-0020-04 341.13000 OTREXUP 20 MG/0.4 ML AUTO-INJ 0 ANTARES PHARMA MLBND 54436-0025-02 341.13000 OTREXUP 25 MG/0.4 ML AUTO-INJ 0 ANTARES PHARMA MLBND 54436-0025-04 341.13000 OTREXUP 25 MG/0.4 ML AUTO-INJ 0 ANTARES PHARMA MLGEX 00430-0580-45 1.12980 OVCON-35 28 TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 51672-5276-04 2.63940 OVIDE 0.5% LOTION G TARO PHARM USA MLBND 00781-3094-15 14.54160 OXACILLIN 1 GM ADD-VANTAGE VL 0 SANDOZ EABND 00781-3094-92 15.98<strong>08</strong>2 OXACILLIN 1 GM ADD-VANTAGE VL 0 SANDOZ EABND 00781-9110-15 14.54160 OXACILLIN 1 GM ADD-VANTAGE VL 0 SANDOZ/NOVAPLUS EABND 00781-9110-92 15.98<strong>08</strong>2 OXACILLIN 1 GM ADD-VANTAGE VL 0 SANDOZ/NOVAPLUS EAGEN 00781-3099-85 9.02000 OXACILLIN 1 GM VIAL 0 SANDOZ EAGEN 00781-3099-95 10.94925 OXACILLIN 1 GM VIAL 0 SANDOZ EAGEN 00781-9109-85 9.02000 OXACILLIN 1 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9109-95 10.94925 OXACILLIN 1 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0146-10 9.02000 OXACILLIN 1 GM VIAL 0 SAGENT PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55150-0127-15 9.02000 OXACILLIN 1 GM VIAL 0 AUROMEDICS PHAR EABND 00338-1013-41 0.28784 OXACILLIN 1 GM/ 50 ML INJ 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00781-3103-46 86.49463 OXACILLIN 10 GM VIAL 0 SANDOZ EAGEN 00781-9113-46 86.49463 OXACILLIN 10 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9113-95 104.07975 OXACILLIN 10 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0163-99 86.49463 OXACILLIN 10 GM VIAL 0 SAGENT PHARMACE EAGEN 55150-0129-99 86.49463 OXACILLIN 10 GM VIAL 0 AUROMEDICS PHAR EABND 00781-3095-15 28.21170 OXACILLIN 2 GM ADD-VANTAGE VL 0 SANDOZ EABND 00781-3095-80 21.90370 OXACILLIN 2 GM ADD-VANTAGE VL 0 SANDOZ EABND 00781-3095-92 31.00050 OXACILLIN 2 GM ADD-VANTAGE VL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00781-9112-15 28.21170 OXACILLIN 2 GM ADD-VANTAGE VL 0 SANDOZ/NOVAPLUS EABND 00781-9112-92 31.00050 OXACILLIN 2 GM ADD-VANTAGE VL 0 SANDOZ/NOVAPLUS EAGEN 00781-3101-80 17.39880 OXACILLIN 2 GM VIAL 0 SANDOZ EAGEN 00781-9111-80 17.39880 OXACILLIN 2 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9111-88 17.39880 OXACILLIN 2 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 00781-9111-95 21.24075 OXACILLIN 2 GM VIAL 0 SANDOZ/NOVAPLUS EAGEN 25021-0162-24 17.39880 OXACILLIN 2 GM VIAL 0 SAGENT PHARMACE EAGEN 55150-0128-24 17.39880 OXACILLIN 2 GM VIAL 0 AUROMEDICS PHAR EABND 00338-1015-41 0.41413 OXACILLIN 2 GM/ 50 ML INJ 0 BAXTER <strong>HEALTH</strong>CA MLGUL 59762-6002-01 0.67580 OXAPROZIN 600 MG CAPLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00093-0924-01 0.67580 OXAPROZIN 600 MG TABLET 0 TEVA USA EAGUL 00093-0924-05 0.67580 OXAPROZIN 600 MG TABLET 0 TEVA USA EAGUL 00185-0141-01 0.67580 OXAPROZIN 600 MG TABLET 0 SANDOZ EAGUL 55111-0170-01 0.67580 OXAPROZIN 600 MG TABLET 0 DR.REDDY'S LAB EAGUL 57664-0391-<strong>08</strong> 0.67580 OXAPROZIN 600 MG TABLET 0 CARACO PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 300LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 60505-0176-00 0.67580 OXAPROZIN 600 MG TABLET 0 APOTEX CORP EAGUL 60505-0176-01 0.67580 OXAPROZIN 600 MG TABLET 0 APOTEX CORP EAGEX 00054-0097-20 0.13840 OXCARBAZEPINE 150 MG TABLET 0 ROXANE LABS. EAGEX 00054-0097-25 0.13840 OXCARBAZEPINE 150 MG TABLET 0 ROXANE LABS. EAGEX 51991-0292-01 0.13840 OXCARBAZEPINE 150 MG TABLET 0 BRECKENRIDGE EAGEX 51991-0292-05 0.13840 OXCARBAZEPINE 150 MG TABLET 0 BRECKENRIDGE EAGEX 59746-0232-01 0.13840 OXCARBAZEPINE 150 MG TABLET 0 CADISTA PHARMAC EAGEX 60505-2534-00 0.13840 OXCARBAZEPINE 150 MG TABLET 0 APOTEX CORP EAGEX 60505-2534-01 0.13840 OXCARBAZEPINE 150 MG TABLET 0 APOTEX CORP EAGEX 60505-2534-05 0.13840 OXCARBAZEPINE 150 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62584-0142-01 0.13840 OXCARBAZEPINE 150 MG TABLET 0 AHP EAGEX 62584-0142-11 0.13840 OXCARBAZEPINE 150 MG TABLET 0 AHP EAGEX 62756-0183-13 0.13840 OXCARBAZEPINE 150 MG TABLET 0 SUN PHARMACEUTI EAGEX 62756-0183-18 0.13840 OXCARBAZEPINE 150 MG TABLET 0 SUN PHARMACEUTI EAGEX 62756-0183-88 0.13840 OXCARBAZEPINE 150 MG TABLET 0 SUN PHARMACEUTI EAGEX 68462-0137-01 0.13840 OXCARBAZEPINE 150 MG TABLET 0 GLENMARK PHARMA EAGEX 00054-0098-20 0.27660 OXCARBAZEPINE 300 MG TABLET 0 ROXANE LABS. EAGEX 00054-0098-25 0.27660 OXCARBAZEPINE 300 MG TABLET 0 ROXANE LABS. EAGEX 51991-0293-01 0.27660 OXCARBAZEPINE 300 MG TABLET 0 BRECKENRIDGE EAGEX 51991-0293-05 0.27660 OXCARBAZEPINE 300 MG TABLET 0 BRECKENRIDGE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59746-0233-01 0.27660 OXCARBAZEPINE 300 MG TABLET 0 CADISTA PHARMAC EAGEX 60505-2535-00 0.27660 OXCARBAZEPINE 300 MG TABLET 0 APOTEX CORP EAGEX 60505-2535-01 0.27660 OXCARBAZEPINE 300 MG TABLET 0 APOTEX CORP EAGEX 60505-2535-<strong>08</strong> 0.27660 OXCARBAZEPINE 300 MG TABLET 0 APOTEX CORP EAGEX 62584-0143-01 0.27660 OXCARBAZEPINE 300 MG TABLET 0 AHP EAGEX 62584-0143-11 0.27660 OXCARBAZEPINE 300 MG TABLET 0 AHP EAGEX 62756-0184-13 0.27660 OXCARBAZEPINE 300 MG TABLET 0 SUN PHARMACEUTI EAGEX 62756-0184-18 0.27660 OXCARBAZEPINE 300 MG TABLET 0 SUN PHARMACEUTI EAGEX 62756-0184-88 0.27660 OXCARBAZEPINE 300 MG TABLET 0 SUN PHARMACEUTI EAGEX 68462-0138-01 0.27660 OXCARBAZEPINE 300 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00054-0199-59 0.62040 OXCARBAZEPINE 300 MG/5 ML SUSP G ROXANE LABS. MLGEX 65162-<strong>06</strong>49-78 0.62040 OXCARBAZEPINE 300 MG/5 ML SUSP G AMNEAL PHARMACE MLGEX 00054-0099-20 0.55320 OXCARBAZEPINE 600 MG TABLET 0 ROXANE LABS. EAGEX 00054-0099-25 0.55320 OXCARBAZEPINE 600 MG TABLET 0 ROXANE LABS. EAGEX 51991-0294-01 0.55320 OXCARBAZEPINE 600 MG TABLET 0 BRECKENRIDGE EAGEX 51991-0294-05 0.55320 OXCARBAZEPINE 600 MG TABLET 0 BRECKENRIDGE EAGEX 59746-0234-01 0.55320 OXCARBAZEPINE 600 MG TABLET 0 CADISTA PHARMAC EAGEX 60505-2536-00 0.55320 OXCARBAZEPINE 600 MG TABLET 0 APOTEX CORP EAGEX 60505-2536-01 0.55320 OXCARBAZEPINE 600 MG TABLET 0 APOTEX CORP EAGEX 60505-2536-05 0.55320 OXCARBAZEPINE 600 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62584-0145-01 0.55320 OXCARBAZEPINE 600 MG TABLET 0 AHP EAGEX 62584-0145-11 0.55320 OXCARBAZEPINE 600 MG TABLET 0 AHP EAGEX 62756-0185-13 0.55320 OXCARBAZEPINE 600 MG TABLET 0 SUN PHARMACEUTI EAGEX 62756-0185-18 0.55320 OXCARBAZEPINE 600 MG TABLET 0 SUN PHARMACEUTI EAGEX 62756-0185-88 0.55320 OXCARBAZEPINE 600 MG TABLET 0 SUN PHARMACEUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 301LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68462-0139-01 0.55320 OXCARBAZEPINE 600 MG TABLET 0 GLENMARK PHARMA EABND 00462-0358-60 4.12856 OXISTAT 1% CREAM G SANDOZ GMBND 10337-0358-30 7.76935 OXISTAT 1% CREAM G SANDOZ GMBND 10337-0358-60 5.32113 OXISTAT 1% CREAM G SANDOZ GMBND 10337-0358-90 5.31873 OXISTAT 1% CREAM G SANDOZ GMBND 10337-0359-30 7.76935 OXISTAT 1% LOTION G SANDOZ MLBND 10337-0359-60 4.83419 OXISTAT 1% LOTION G SANDOZ MLBND 00187-<strong>06</strong>50-42 66.92057 OXSORALEN-ULTRA 10 MG CAP 0 VALEANT EABEX 17772-0121-01 3.23218 OXTELLAR XR 150 MG TABLET G SUPERNUS PHARMA EABEX 17772-0122-01 4.49046 OXTELLAR XR 300 MG TABLET G SUPERNUS PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 17772-0123-01 8.22156 OXTELLAR XR 600 MG TABLET G SUPERNUS PHARMA EAGEN 00093-5207-01 1.290<strong>06</strong> OXYBUTYNIN CL ER 10 MG TABLET G TEVA USA EAGEN 00378-6610-01 1.290<strong>06</strong> OXYBUTYNIN CL ER 10 MG TABLET G MYLAN EAGEN 00378-6610-05 1.290<strong>06</strong> OXYBUTYNIN CL ER 10 MG TABLET G MYLAN EAGEN 51079-0723-01 1.290<strong>06</strong> OXYBUTYNIN CL ER 10 MG TABLET G MYLAN INSTITUTI EAGEN 51079-0723-20 1.290<strong>06</strong> OXYBUTYNIN CL ER 10 MG TABLET G MYLAN INSTITUTI EAGEN 62175-0271-37 1.290<strong>06</strong> OXYBUTYNIN CL ER 10 MG TABLET G KREMERS URBAN EAGEN 62175-0271-41 1.290<strong>06</strong> OXYBUTYNIN CL ER 10 MG TABLET G KREMERS URBAN EAGEN 68<strong>08</strong>4-<strong>06</strong>10-21 1.290<strong>06</strong> OXYBUTYNIN CL ER 10 MG TABLET G AHP EAGEN 00093-52<strong>08</strong>-01 2.17500 OXYBUTYNIN CL ER 15 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6015-01 2.17500 OXYBUTYNIN CL ER 15 MG TABLET G MYLAN EAGEN 62175-0272-37 2.17500 OXYBUTYNIN CL ER 15 MG TABLET G KREMERS URBAN EAGEN 62175-0272-41 2.17500 OXYBUTYNIN CL ER 15 MG TABLET G KREMERS URBAN EAGEN 00093-52<strong>06</strong>-01 1.24956 OXYBUTYNIN CL ER 5 MG TABLET G TEVA USA EAGEN 00378-6605-01 1.24956 OXYBUTYNIN CL ER 5 MG TABLET G MYLAN EAGEN 00378-6605-05 1.24956 OXYBUTYNIN CL ER 5 MG TABLET G MYLAN EAGEN 51079-0722-01 1.24956 OXYBUTYNIN CL ER 5 MG TABLET G MYLAN INSTITUTI EAGEN 51079-0722-20 1.24956 OXYBUTYNIN CL ER 5 MG TABLET G MYLAN INSTITUTI EAGEN 62175-0270-37 1.24956 OXYBUTYNIN CL ER 5 MG TABLET G KREMERS URBAN EAGEN 62175-0270-41 1.24956 OXYBUTYNIN CL ER 5 MG TABLET G KREMERS URBAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0480-01 1.24956 OXYBUTYNIN CL ER 5 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0480-11 1.24956 OXYBUTYNIN CL ER 5 MG TABLET G AHP EAGUL 0<strong>06</strong>03-4975-02 0.16500 OXYBUTYNIN 5 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-4975-04 0.16500 OXYBUTYNIN 5 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-4975-20 0.16500 OXYBUTYNIN 5 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-4975-21 0.16500 OXYBUTYNIN 5 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-4975-28 0.16500 OXYBUTYNIN 5 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-4975-32 0.16500 OXYBUTYNIN 5 MG TABLET 0 QUALITEST EAGUL 0<strong>08</strong>32-0038-00 0.16500 OXYBUTYNIN 5 MG TABLET 0 UPSHER SMITH EAGUL 0<strong>08</strong>32-0038-10 0.16500 OXYBUTYNIN 5 MG TABLET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 0<strong>08</strong>32-0038-50 0.16500 OXYBUTYNIN 5 MG TABLET 0 UPSHER SMITH EAGUL 50111-0456-01 0.16500 OXYBUTYNIN 5 MG TABLET 0 TEVA USA EAGUL 50111-0456-02 0.16500 OXYBUTYNIN 5 MG TABLET 0 PLIVA, INC EAGUL 50111-0456-03 0.16500 OXYBUTYNIN 5 MG TABLET 0 PLIVA, INC EAGUL 68<strong>08</strong>4-0400-01 0.16500 OXYBUTYNIN 5 MG TABLET 0 AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 302LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 68<strong>08</strong>4-0400-11 0.16500 OXYBUTYNIN 5 MG TABLET 0 AHP EAGEN 00121-<strong>06</strong>71-05 0.02390 OXYBUTYNIN 5 MG/5 ML SYRUP 0 PHARMACEU ASSOC MLGEN 0<strong>06</strong>03-1491-58 0.02390 OXYBUTYNIN 5 MG/5 ML SYRUP 0 QUALITEST MLGEN 54838-0510-80 0.02390 OXYBUTYNIN 5 MG/5 ML SYRUP 0 SILARX PHARM MLGEN 60432-0092-16 0.02390 OXYBUTYNIN 5 MG/5 ML SYRUP 0 MORTON GROVE PH MLGEN 60505-60<strong>08</strong>-09 0.02390 OXYBUTYNIN 5 MG/5 ML SYRUP 0 APOTEX CORP MLBND 52544-0920-<strong>08</strong> 39.87942 OXYTROL 3.9 MG/24HR PATCH 0 ACTAVIS PHARMA, EAGEN 00245-0144-30 12.53750 PACERONE 100 MG TABLET 0 UPSHER SMITH EAGEN 00245-0144-89 12.54000 PACERONE 100 MG TABLET 0 UPSHER SMITH EAGEN 00245-0147-15 0.15390 PACERONE 200 MG TABLET 0 UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00245-0147-60 0.15390 PACERONE 200 MG TABLET 0 UPSHER SMITH EAGEN 00245-0147-89 0.15390 PACERONE 200 MG TABLET 0 UPSHER SMITH EAGEN 00245-0147-90 0.15390 PACERONE 200 MG TABLET 0 UPSHER SMITH EAGEN 00245-0145-30 11.22125 PACERONE 400 MG TABLET 0 UPSHER SMITH EAGEN 00245-0145-89 12.54000 PACERONE 400 MG TABLET 0 UPSHER SMITH EAGEN 00703-4766-01 2.33353 PACLITAXEL 100 MG/16.7 ML VIAL 0 TEVA PARENTERAL MLGEN 25021-0213-17 3.01796 PACLITAXEL 100 MG/16.7 ML VIAL 0 SAGENT PHARMACE MLGEN 55390-0114-20 3.07185 PACLITAXEL 100 MG/16.7 ML VIAL 0 BEDFORD LABS MLGEN 63323-0763-16 2.32769 PACLITAXEL 100 MG/16.7 ML VIAL 0 APP PHARMACEUTI MLGEN 00703-4767-01 2.37600 PACLITAXEL 150 MG/25 ML VIAL 0 TEVA PARENTERAL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 25021-0213-05 3.02400 PACLITAXEL 30 MG/5 ML VIAL 0 SAGENT PHARMACE MLGEN 55390-0114-05 3.<strong>06</strong>000 PACLITAXEL 30 MG/5 ML VIAL 0 BEDFORD LABS MLGEN 63323-0763-05 2.32800 PACLITAXEL 30 MG/5 ML VIAL 0 APP PHARMACEUTI MLGEN 25021-0213-50 2.34000 PACLITAXEL 300 MG/50 ML VIAL 0 SAGENT PHARMACE MLGEN 55390-0114-50 3.07800 PACLITAXEL 300 MG/50 ML VIAL 0 BEDFORD LABS MLGEN 63323-0763-50 2.32740 PACLITAXEL 300 MG/50 ML VIAL 0 APP PHARMACEUTI MLBEX 004<strong>06</strong>-9910-03 0.09990 23.23031 PAMELOR 10 MG CAPSULE G MALLINCKRODT PH EABEX 004<strong>06</strong>-9911-03 0.12210 23.69484 PAMELOR 25 MG CAPSULE G MALLINCKRODT PH EABEX 004<strong>06</strong>-9912-03 0.17010 24.15964 PAMELOR 50 MG CAPSULE G MALLINCKRODT PH EABND 55390-0127-01 18.80180 39.84000 PAMIDRONATE DISOD 30 MG VIAL 0 BEDFORD LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 55390-0129-01 74.81600 119.52000 PAMIDRONATE DISOD 90 MG VIAL 0 BEDFORD LABS EAGEN 00<strong>06</strong>9-0186-01 1.48500 PAMIDRONATE 30 MG/10 ML VIAL 0 PFIZER/NOVAPLUS MLGEN 61703-0324-18 1.07550 PAMIDRONATE 30 MG/10 ML VIAL 0 HOSPIRA MLGEN 63323-0734-10 1.48500 PAMIDRONATE 30 MG/10 ML VIAL 0 APP PHARMACEUTI MLBND 61703-0325-18 2.79295 PAMIDRONATE 60 MG/10 ML VIAL 0 HOSPIRA MLGEN 63323-0735-10 3.80010 PAMIDRONATE 90 MG/10 ML VIAL 0 APP PHARMACEUTI MLBND 10337-0<strong>06</strong>2-<strong>06</strong> 2.<strong>06</strong>450 4.58326 PAMINE FORTE 5 MG TABLET G SANDOZ EABND 10337-0<strong>06</strong>1-01 1.63200 3.42541 PAMINE 2.5 MG TABLET 0 SANDOZ EABND 50458-0342-60 2.<strong>08</strong>861 PANCREAZE DR 10,500 UNIT CAP G JANSSEN PHARM. EABND 50458-0343-60 3.35286 PANCREAZE DR 16,800 UNIT CAP G JANSSEN PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50458-0346-60 4.17589 PANCREAZE DR 21,000 UNIT CAP G JANSSEN PHARM. EABND 50458-0341-60 0.83522 PANCREAZE DR 4,200 UNIT CAP G JANSSEN PHARM. EABND 39822-0205-01 0.66076 PANCRELIPASE DR 5,000 UNIT CAP 0 X-GEN PHARMACEU EABND 00462-0153-15 5.58534 PANDEL 0.1% CREAM G SANDOZ GMBND 00462-0153-46 4.43588 PANDEL 0.1% CREAM G SANDOZ GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 303LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00462-0153-80 4.19140 PANDEL 0.1% CREAM G SANDOZ GMBND 62856-<strong>06</strong>01-22 65.98500 PANRETIN 0.1% GEL 0 EISAI INC. GMGEN 000<strong>08</strong>-<strong>06</strong><strong>06</strong>-01 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G WYETH PHARM EAGEN 00093-0011-98 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G TEVA USA EAGEN 00378-6688-77 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G MYLAN EAGEN 13668-0096-05 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G TORRENT PHARMAC EAGEN 13668-0096-30 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G TORRENT PHARMAC EAGEN 13668-0096-90 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G TORRENT PHARMAC EAGEN 45963-0569-<strong>08</strong> 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G ACTAVIS PHARMA, EAGEN 55111-0332-90 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59746-0283-90 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G CADISTA PHARMAC EAGEN 62175-0180-46 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G KREMERS URBAN EAGEN 64679-0433-02 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G WOCKHARDT USA L EAGEN 64679-0433-04 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G WOCKHARDT USA L EAGEN 65862-0559-90 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G AUROBINDO PHARM EAGEN 65862-0559-99 0.28120 PANTOPRAZOLE SOD DR 20 MG TAB G AUROBINDO PHARM EAGEN 000<strong>08</strong>-<strong>06</strong>07-01 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G WYETH PHARM EAGEN 000<strong>08</strong>-<strong>06</strong>07-04 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G WYETH PHARM EAGEN 00093-0012-98 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G TEVA USA EAGEN 00378-6689-10 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6689-77 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G MYLAN EAGEN 13668-0097-30 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G TORRENT PHARMAC EAGEN 13668-0429-05 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G TORRENT PHARMAC EAGEN 13668-0429-30 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G TORRENT PHARMAC EAGEN 13668-0429-90 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G TORRENT PHARMAC EAGEN 45963-0570-05 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G ACTAVIS PHARMA, EAGEN 45963-0570-<strong>08</strong> 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G ACTAVIS PHARMA, EAGEN 47335-0580-81 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G SUN PHARMA GLOB EAGEN 51079-0051-01 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G MYLAN INSTITUTI EAGEN 51079-0051-20 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0333-90 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G DR.REDDY'S LAB EAGEN 58517-0420-30 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G <strong>NEW</strong> HORIZON RX EAGEN 59746-0284-90 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G CADISTA PHARMAC EAGEN 62175-0181-43 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G KREMERS URBAN EAGEN 62175-0181-46 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G KREMERS URBAN EAGEN 63739-0564-10 0.25822 PANTOPRAZOLE SOD DR 40 MG TAB G MCKESSON PACKAG EAGEN 64679-0434-02 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G WOCKHARDT USA L EAGEN 64679-0434-04 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G WOCKHARDT USA L EAGEN 65862-0560-90 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G AUROBINDO PHARM EAGEN 65862-0560-99 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-<strong>06</strong>57-01 0.27585 PANTOPRAZOLE SOD DR 40 MG TAB G AHP EAGEN 68645-0400-70 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G LEGACY PHARMACE EAGEN 68645-0401-70 0.28<strong>08</strong>0 PANTOPRAZOLE SOD DR 40 MG TAB G LEGACY PHARMACE EAGEN 17478-0<strong>06</strong>9-14 2.71500 PANTOPRAZOLE SODIUM 40 MG VIAL 0 AKORN INC. EABUL 50458-<strong>06</strong>25-60 0.07570 3.36407 PARAFON FORTE DSC 500 MG CAPLT G JANSSEN PHARM. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 304LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 18860-0341-01 0.62190 PARCOPA 10 MG-100 MG ODT 0 JAZZ PHARMACEUT EAGEN 00093-7656-56 8.31825 PARICALCITOL 1 MCG CAPSULE 0 TEVA USA EAGEN 00093-7657-56 16.63524 PARICALCITOL 2 MCG CAPSULE 0 TEVA USA EAGEN 00093-7658-56 33.27075 PARICALCITOL 4 MCG CAPSULE 0 TEVA USA EABND 3<strong>06</strong>98-0017-01 5.53394 PARLODEL 2.5 MG TABLET G VALIDUS PHARMAC EABND 3<strong>06</strong>98-0017-30 5.54938 PARLODEL 2.5 MG TABLET G VALIDUS PHARMAC EABND 00078-0102-15 8.91447 PARLODEL 5 MG CAPSULE G NOVARTIS EABND 3<strong>06</strong>98-0102-30 8.91447 PARLODEL 5 MG CAPSULE G VALIDUS PHARMAC EABEX 00007-4471-20 1.64240 5.58756 PARNATE 10 MG TABLET 0 COVIS PHARMACEU EAGEN 23155-0038-01 3.<strong>06</strong>504 PAROMOMYCIN 250 MG CAPSULE G HERITAGE PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 57664-0175-<strong>08</strong> 3.<strong>06</strong>504 PAROMOMYCIN 250 MG CAPSULE G CARACO PHARM EAGEX 00378-2003-05 2.71275 PAROXETINE CR 12.5 MG TABLET G MYLAN EAGEX 00378-2003-93 2.71275 PAROXETINE CR 12.5 MG TABLET G MYLAN EAGEX 60505-3673-03 2.71575 PAROXETINE CR 12.5 MG TABLET G APOTEX CORP EAGEX 00378-2004-05 2.83075 PAROXETINE CR 25 MG TABLET G MYLAN EAGEX 00378-2004-93 2.83074 PAROXETINE CR 25 MG TABLET G MYLAN EAGEX 60505-3674-03 2.83374 PAROXETINE CR 25 MG TABLET G APOTEX CORP EAGEX 60505-3675-03 2.91900 PAROXETINE CR 37.5 MG TABLET G APOTEX CORP EAGEX 00378-2005-93 2.91575 PAROXETINE ER 37.5 MG TABLET G MYLAN EAGEX 00093-7114-98 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-7001-10 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 MYLAN EAGEX 00378-7001-93 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 MYLAN EAGEX 13107-0154-05 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 AUROBINDO PHARM EAGEX 54458-0990-10 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 INTERNATIONAL L EAGEX 59762-18<strong>08</strong>-01 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-18<strong>08</strong>-02 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-18<strong>08</strong>-03 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 GREENSTONE LLC. EAGEX 60505-0097-01 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 APOTEX CORP EAGEX 60505-0097-02 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 APOTEX CORP EAGEX 60505-0097-04 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0154-30 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0044-11 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 AHP EAGEX 68382-0097-05 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0097-<strong>06</strong> 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0097-10 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0097-16 0.13<strong>08</strong>0 PAROXETINE HCL 10 MG TABLET 0 ZYDUS PHARMACEU EAGEX 00093-7115-98 0.09650 PAROXETINE HCL 20 MG TABLET 0 TEVA USA EAGEX 00378-7002-10 0.09650 PAROXETINE HCL 20 MG TABLET 0 MYLAN EAGEX 00378-7002-93 0.09650 PAROXETINE HCL 20 MG TABLET 0 MYLAN EAGEX 13107-0155-05 0.09650 PAROXETINE HCL 20 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13107-0155-30 0.09650 PAROXETINE HCL 20 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0155-99 0.09650 PAROXETINE HCL 20 MG TABLET 0 AUROBINDO PHARM EAGEX 51079-0774-01 0.09650 PAROXETINE HCL 20 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0774-20 0.09650 PAROXETINE HCL 20 MG TABLET 0 MYLAN INSTITUTI EAGEX 54458-0989-10 0.09650 PAROXETINE HCL 20 MG TABLET 0 INTERNATIONAL L EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 305LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 58517-0160-30 0.09650 PAROXETINE HCL 20 MG TABLET 0 <strong>NEW</strong> HORIZON RX EAGEX 59762-1810-01 0.09650 PAROXETINE HCL 20 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1810-02 0.09650 PAROXETINE HCL 20 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1810-03 0.09650 PAROXETINE HCL 20 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1810-04 0.09650 PAROXETINE HCL 20 MG TABLET 0 GREENSTONE LLC. EAGEX 60505-0<strong>08</strong>3-00 0.09650 PAROXETINE HCL 20 MG TABLET 0 APOTEX CORP EAGEX 60505-0<strong>08</strong>3-01 0.09650 PAROXETINE HCL 20 MG TABLET 0 APOTEX CORP EAGEX 60505-0<strong>08</strong>3-02 0.09650 PAROXETINE HCL 20 MG TABLET 0 APOTEX CORP EAGEX 60505-0<strong>08</strong>3-04 0.09650 PAROXETINE HCL 20 MG TABLET 0 APOTEX CORP EAGEX 65862-0155-05 0.09650 PAROXETINE HCL 20 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0155-30 0.09650 PAROXETINE HCL 20 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0155-99 0.09650 PAROXETINE HCL 20 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0045-11 0.09650 PAROXETINE HCL 20 MG TABLET 0 AHP EAGEX 68382-0098-01 0.09650 PAROXETINE HCL 20 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0098-05 0.09650 PAROXETINE HCL 20 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0098-<strong>06</strong> 0.09650 PAROXETINE HCL 20 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0098-10 0.09650 PAROXETINE HCL 20 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0098-16 0.09650 PAROXETINE HCL 20 MG TABLET 0 ZYDUS PHARMACEU EAGEX 00093-7116-98 0.16970 PAROXETINE HCL 30 MG TABLET 0 TEVA USA EAGEX 00378-7003-10 0.16970 PAROXETINE HCL 30 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-7003-93 0.16970 PAROXETINE HCL 30 MG TABLET 0 MYLAN EAGEX 13107-0156-05 0.16970 PAROXETINE HCL 30 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0156-30 0.16970 PAROXETINE HCL 30 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0156-99 0.16970 PAROXETINE HCL 30 MG TABLET 0 AUROBINDO PHARM EAGEX 54458-0988-10 0.16970 PAROXETINE HCL 30 MG TABLET 0 INTERNATIONAL L EAGEX 59762-1812-01 0.16970 PAROXETINE HCL 30 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1812-02 0.16970 PAROXETINE HCL 30 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1812-03 0.16970 PAROXETINE HCL 30 MG TABLET 0 GREENSTONE LLC. EAGEX 60505-0<strong>08</strong>4-01 0.16970 PAROXETINE HCL 30 MG TABLET 0 APOTEX CORP EAGEX 60505-0<strong>08</strong>4-02 0.16970 PAROXETINE HCL 30 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-0<strong>08</strong>4-04 0.16970 PAROXETINE HCL 30 MG TABLET 0 APOTEX CORP EAGEX 65862-0156-30 0.16970 PAROXETINE HCL 30 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0156-99 0.16970 PAROXETINE HCL 30 MG TABLET 0 AUROBINDO PHARM EAGEX 68382-0099-05 0.16970 PAROXETINE HCL 30 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0099-<strong>06</strong> 0.16970 PAROXETINE HCL 30 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0099-10 0.16970 PAROXETINE HCL 30 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0099-16 0.16970 PAROXETINE HCL 30 MG TABLET 0 ZYDUS PHARMACEU EAGEX 00093-7121-98 0.17280 PAROXETINE HCL 40 MG TABLET 0 TEVA USA EAGEX 00378-7004-10 0.17280 PAROXETINE HCL 40 MG TABLET 0 MYLAN EAGEX 00378-7004-93 0.17280 PAROXETINE HCL 40 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13107-0157-05 0.17280 PAROXETINE HCL 40 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0157-30 0.17280 PAROXETINE HCL 40 MG TABLET 0 AUROBINDO PHARM EAGEX 13107-0157-99 0.17280 PAROXETINE HCL 40 MG TABLET 0 AUROBINDO PHARM EAGEX 59762-1815-01 0.17280 PAROXETINE HCL 40 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-1815-02 0.17280 PAROXETINE HCL 40 MG TABLET 0 GREENSTONE LLC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 3<strong>06</strong>LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-1815-03 0.17280 PAROXETINE HCL 40 MG TABLET 0 GREENSTONE LLC. EAGEX 60505-0101-01 0.17280 PAROXETINE HCL 40 MG TABLET 0 APOTEX CORP EAGEX 60505-0101-02 0.17280 PAROXETINE HCL 40 MG TABLET 0 APOTEX CORP EAGEX 60505-0101-04 0.17280 PAROXETINE HCL 40 MG TABLET 0 APOTEX CORP EAGEX 65862-0157-30 0.17280 PAROXETINE HCL 40 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0157-99 0.17280 PAROXETINE HCL 40 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0047-11 0.17280 PAROXETINE HCL 40 MG TABLET 0 AHP EAGEX 68382-0001-05 0.17280 PAROXETINE HCL 40 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0001-<strong>06</strong> 0.17280 PAROXETINE HCL 40 MG TABLET 0 ZYDUS PHARMACEU EAGEX 68382-0001-16 0.17280 PAROXETINE HCL 40 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 49938-0107-04 3.10226 PASER GRANULES 4 GM PACKET 0 JACOBUS PHARM. EABND 00<strong>06</strong>5-0272-25 52.86768 PATADAY 0.2% EYE DROPS 0 ALCON LABS. MLBND 00<strong>06</strong>5-0332-30 5.94007 PATANASE 0.6% NASAL SPRAY 0 ALCON LABS. GMBND 00<strong>06</strong>5-0271-05 28.98360 PATANOL 0.1% EYE DROPS G ALCON LABS. MLBEX 60505-3668-03 2.82440 4.40481 PAXIL CR 12.5 MG TABLET G APOTEX CORP EABEX 60505-3669-03 2.94840 4.59654 PAXIL CR 25 MG TABLET G APOTEX CORP EABEX 60505-3670-03 3.04780 4.73487 PAXIL CR 37.5 MG TABLET G APOTEX CORP EABEX 60505-3663-03 0.13<strong>08</strong>0 4.27671 PAXIL 10 MG TABLET G APOTEX CORP EABEX 60505-0402-05 0.89092 PAXIL 10 MG/5 ML SUSPENSION G APOTEX CORP MLBEX 60505-3664-03 0.09650 4.462<strong>08</strong> PAXIL 20 MG TABLET G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 60505-3665-03 0.16970 4.59681 PAXIL 30 MG TABLET G APOTEX CORP EABEX 60505-3666-03 0.17280 4.85660 PAXIL 40 MG TABLET G APOTEX CORP EABND 24338-0112-60 5.54869 PCE 333 MG TABLET 0 ARBOR PHARMACEU EABND 24338-0114-13 5.77680 PCE 500 MG TABLET 0 ARBOR PHARMACEU EAGEN 0<strong>08</strong>84-0396-02 1.01130 PEDI-DRI TOPICAL POWDER 0 VALEANT GMGEN 62175-0446-01 0.00234 PEG 3350 ELECTROLYTE SOLN 0 KREMERS URBAN MLGEN 00378-6669-40 0.00355 PEG-3350 AND ELECTROLYTES SOLN 0 MYLAN MLGEN 10572-0100-01 0.00309 PEG-3350 AND ELECTROLYTES SOLN 0 AFFORDABLE PHAR MLGEN 10572-0101-01 0.00309 PEG-3350 AND ELECTROLYTES SOLN 0 AFFORDABLE PHAR MLGEN 10572-0302-01 0.00360 PEG-3350 SOLUTION G AFFORDABLE PHAR ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10572-0400-01 0.00360 PEG-3350 WITH FLAVOR PACKS SOL G AFFORDABLE PHAR MLBEX 55292-<strong>06</strong>01-01 1.30492 PEGANONE 250 MG TABLET 0 RECORDATI RARE EABEX 67386-<strong>06</strong>01-01 1.30492 PEGANONE 250 MG TABLET 0 RECORDATI RARE EABND 00004-0360-30 1536.37150 PEGASYS PROCLICK 135 MCG/0.5 G GENENTECH, INC. MLBND 00004-0365-30 1536.37150 PEGASYS PROCLICK 180 MCG/0.5 G GENENTECH, INC. MLBND 00004-0350-09 768.18160 PEGASYS 180 MCG/ML VIAL G GENENTECH, INC. MLBND 00004-0352-39 3072.74300 PEGASYS 180 MCG/0.5 ML SYRINGE G GENENTECH, INC. EABND 00004-0357-30 1536.37150 PEGASYS 180 MCG/0.5 ML SYRINGE G GENENTECH, INC. MLBND 00<strong>08</strong>5-1297-01 771.86680 PEGINTRON REDIPEN 120 MCG G MERCK SHARP & D EABND 00<strong>08</strong>5-1297-02 771.90415 PEGINTRON REDIPEN 120 MCG 4PK G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>5-1370-01 810.48670 PEGINTRON REDIPEN 150 MCG G MERCK SHARP & D EABND 00<strong>08</strong>5-1370-02 810.47217 PEGINTRON REDIPEN 150 MCG 4PK G MERCK SHARP & D EABND 00<strong>08</strong>5-1323-01 700.14650 PEGINTRON REDIPEN 50 MCG G MERCK SHARP & D EABND 00<strong>08</strong>5-1323-02 700.16102 PEGINTRON REDIPEN 50 MCG 4PK G MERCK SHARP & D EABND 00<strong>08</strong>5-1316-01 735.<strong>08</strong>120 PEGINTRON REDIPEN 80 MCG G MERCK SHARP & D EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 307LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>5-1316-02 735.1<strong>08</strong>17 PEGINTRON REDIPEN 80 MCG 4PK G MERCK SHARP & D EABND 00<strong>08</strong>5-1304-01 771.86680 PEGINTRON 120 MCG KIT G MERCK SHARP & D EABND 00<strong>08</strong>5-1279-01 810.48670 PEGINTRON 150 MCG KIT G MERCK SHARP & D EABND 00<strong>08</strong>5-1368-01 700.14650 PEGINTRON 50 MCG KIT G MERCK SHARP & D EABND 00<strong>08</strong>5-1291-01 735.<strong>08</strong>120 PEGINTRON 80 MCG KIT G MERCK SHARP & D EABND 00338-1021-41 0.16832 PEN G K 1 MILLION UNIT/50 ML 0 BAXTER <strong>HEALTH</strong>CA MLBND 00338-1023-41 0.17509 PEN G K 2 MILLION UNIT/50 ML 0 BAXTER <strong>HEALTH</strong>CA MLBND 00338-1025-41 0.18186 PEN G K 3 MILLION UNIT/50 ML 0 BAXTER <strong>HEALTH</strong>CA MLBND 60793-0131-10 15.44962 PEN G 1.2 MILLION UNIT/2 ML 0 PFIZER US PHARM MLGEN 00781-6135-94 31.63125 PENICILLIN G K 5 MILLION UNIT 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-6135-95 31.63125 PENICILLIN G K 5 MILLION UNIT 0 SANDOZ EABND 00781-6153-94 47.8<strong>08</strong>00 PENICILLIN G NA 5 MILLION UNIT 0 SANDOZ EABND 00781-6153-95 47.8<strong>06</strong>34 PENICILLIN G NA 5 MILLION UNIT 0 SANDOZ EAGEN 25021-0154-20 130.34000 PENICILLIN GK 20 MILLION UNIT 0 SAGENT PHARMACE EAGEN 63323-0324-62 103.50000 PENICILLIN GK 20 MILLION UNIT 0 APP PHARMACEUTI EAGEN 00093-4125-73 0.02510 PENICILLIN VK 125 MG/5 ML SOLN 0 TEVA USA MLGEN 00093-4125-74 0.02115 PENICILLIN VK 125 MG/5 ML SOLN 0 TEVA USA MLGEN 67253-0202-10 0.02510 PENICILLIN VK 125 MG/5 ML SOLN 0 DAVA PHARMACEUT MLGEN 67253-0202-20 0.02362 PENICILLIN VK 125 MG/5 ML SOLN 0 DAVA PHARMACEUT MLGEN 00093-1172-01 0.07358 PENICILLIN VK 250 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-1172-10 0.07358 PENICILLIN VK 250 MG TABLET 0 TEVA USA EAGEN 00781-1205-01 0.07358 PENICILLIN VK 250 MG TABLET 0 SANDOZ EAGEN 00781-1205-10 0.07358 PENICILLIN VK 250 MG TABLET 0 SANDOZ EAGEN 16714-0234-01 0.07358 PENICILLIN VK 250 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0234-02 0.07358 PENICILLIN VK 250 MG TABLET 0 NORTHSTAR RX LL EAGEN 59762-1534-01 0.07358 PENICILLIN VK 250 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-1534-02 0.07358 PENICILLIN VK 250 MG TABLET 0 GREENSTONE LLC. EAGEN 65862-0175-01 0.07358 PENICILLIN VK 250 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0175-99 0.07358 PENICILLIN VK 250 MG TABLET 0 AUROBINDO PHARM EAGEN 67253-0200-10 0.07358 PENICILLIN VK 250 MG TABLET 0 DAVA PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67253-0200-11 0.07358 PENICILLIN VK 250 MG TABLET 0 DAVA PHARMACEUT EAGEN 00093-4127-73 0.02754 PENICILLIN VK 250 MG/5 ML SOLN 0 TEVA USA MLGEN 00093-4127-74 0.02336 PENICILLIN VK 250 MG/5 ML SOLN 0 TEVA USA MLGEN 67253-0203-10 0.02754 PENICILLIN VK 250 MG/5 ML SOLN 0 DAVA PHARMACEUT MLGEN 67253-0203-20 0.02625 PENICILLIN VK 250 MG/5 ML SOLN 0 DAVA PHARMACEUT MLGEN 00093-1174-01 0.16480 PENICILLIN VK 500 MG TABLET 0 TEVA USA EAGEN 00093-1174-10 0.16480 PENICILLIN VK 500 MG TABLET 0 TEVA USA EAGEN 00781-1655-01 0.16480 PENICILLIN VK 500 MG TABLET 0 SANDOZ EAGEN 00781-1655-10 0.16480 PENICILLIN VK 500 MG TABLET 0 SANDOZ EAGEN 16714-0235-01 0.16480 PENICILLIN VK 500 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0235-02 0.16480 PENICILLIN VK 500 MG TABLET 0 NORTHSTAR RX LL EAGEN 59762-1537-01 0.16480 PENICILLIN VK 500 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-1537-02 0.16480 PENICILLIN VK 500 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-1537-03 0.16480 PENICILLIN VK 500 MG TABLET 0 GREENSTONE LLC. EAGEN 65862-0176-01 0.16480 PENICILLIN VK 500 MG TABLET 0 AUROBINDO PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 3<strong>08</strong>LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0176-05 0.16480 PENICILLIN VK 500 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0176-99 0.16480 PENICILLIN VK 500 MG TABLET 0 AUROBINDO PHARM EAGEN 67253-0201-10 0.16480 PENICILLIN VK 500 MG TABLET 0 DAVA PHARMACEUT EAGEN 67253-0201-50 0.16480 PENICILLIN VK 500 MG TABLET 0 DAVA PHARMACEUT EABND 00<strong>06</strong>6-80<strong>08</strong>-02 4.80367 75.79157 PENLAC 8% SOLUTION G VALEANT MLBND 00187-5180-<strong>06</strong> 4.80367 94.73947 PENLAC 8% SOLUTION G VALEANT MLBND 23635-0310-15 1.60283 PENNSAID 1.5% SOLUTION G MALLINCKRODT BR MLBND 23635-0510-12 2.14666 PENNSAID 2% PUMP G MALLINCKRODT BR GMBND 63323-0113-10 98.35500 PENTAM 300 VIAL 0 APP PHARMACEUTI EABND 54092-0189-81 1.69883 PENTASA 250 MG CAPSULE G SHIRE US INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 54092-0191-12 3.39767 PENTASA 500 MG CAPSULE G SHIRE US INC. EAGEN 00093-5116-01 0.16943 PENTOXIFYLLINE ER 400 MG TAB 0 TEVA USA EAGEN 00093-5116-05 0.16943 PENTOXIFYLLINE ER 400 MG TAB 0 TEVA USA EAGEN 00378-0357-01 0.16943 PENTOXIFYLLINE ER 400 MG TAB 0 MYLAN EAGEN 00378-0357-05 0.16943 PENTOXIFYLLINE ER 400 MG TAB 0 MYLAN EAGEN 60505-0033-<strong>06</strong> 0.16943 PENTOXIFYLLINE ER 400 MG TAB 0 APOTEX CORP EAGEN 60505-0033-07 0.16943 PENTOXIFYLLINE ER 400 MG TAB 0 APOTEX CORP EAGEN 60505-0033-<strong>08</strong> 0.16943 PENTOXIFYLLINE ER 400 MG TAB 0 APOTEX CORP EAGEN 42998-0963-03 0.07620 PEPCID 20 MG TABLET G MARATHON PHARMA EAGEN 42998-0963-10 0.07620 PEPCID 20 MG TABLET G MARATHON PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42998-0964-03 0.07425 PEPCID 40 MG TABLET G MARATHON PHARMA EAGEN 42998-0964-10 0.07425 PEPCID 40 MG TABLET G MARATHON PHARMA EABND 65649-0211-24 1.34150 3.26372 PEPCID 40 MG/5 ML ORAL SUSP 0 SALIX PHARMACEU MLBND 49502-<strong>06</strong>05-30 4.47785 PERFOROMIST 20 MCG/2 ML SOLN G MYLAN SPECIALTY MLBND 49502-<strong>06</strong>05-61 4.47778 PERFOROMIST 20 MCG/2 ML SOLN G MYLAN SPECIALTY MLGEN 00054-0110-25 0.47388 PERINDOPRIL ERBUMINE 2 MG TAB G ROXANE LABS. EAGEN 65862-0286-01 0.47388 PERINDOPRIL ERBUMINE 2 MG TAB G AUROBINDO PHARM EAGEN 00054-0111-25 0.49490 PERINDOPRIL ERBUMINE 4 MG TAB G ROXANE LABS. EAGEN 65862-0287-01 0.49490 PERINDOPRIL ERBUMINE 4 MG TAB G AUROBINDO PHARM EAGEN 00054-0112-25 0.55070 PERINDOPRIL ERBUMINE 8 MG TAB G ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0288-01 0.55070 PERINDOPRIL ERBUMINE 8 MG TAB G AUROBINDO PHARM EAGEN 00472-0242-60 1.54687 PERMETHRIN 5% CREAM 0 ACTAVIS PHARMA, GMGEN 45802-0269-37 1.54687 PERMETHRIN 5% CREAM 0 PERRIGO CO. GMBEX 00378-0330-01 0.56630 PERPHEN-AMITRIP 2 MG-10 MG TAB 0 MYLAN EABEX 00378-0330-05 0.56637 PERPHEN-AMITRIP 2 MG-10 MG TAB 0 MYLAN EABEX 00378-0442-01 0.72110 PERPHEN-AMITRIP 2 MG-25 MG TAB 0 MYLAN EABEX 00378-0442-05 0.721<strong>08</strong> PERPHEN-AMITRIP 2 MG-25 MG TAB 0 MYLAN EABEX 00378-0042-01 0.63370 PERPHEN-AMITRIP 4 MG-10 MG TAB 0 MYLAN EABEX 00378-0574-01 0.78534 PERPHEN-AMITRIP 4 MG-25 MG TAB 0 MYLAN EABEX 00378-0574-05 0.78529 PERPHEN-AMITRIP 4 MG-25 MG TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00378-0073-01 1.29670 PERPHEN-AMITRIP 4 MG-50 MG TAB 0 MYLAN EAGEX 0<strong>06</strong>03-5<strong>06</strong>3-21 1.94962 PERPHENAZINE 16 MG TABLET 0 QUALITEST EAGEX 00781-1049-01 1.94962 PERPHENAZINE 16 MG TABLET 0 SANDOZ EAGEX 0<strong>06</strong>03-5<strong>06</strong>0-21 0.87285 PERPHENAZINE 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-5<strong>06</strong>0-28 0.82921 PERPHENAZINE 2 MG TABLET 0 QUALITEST EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 309LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-1046-01 0.87285 PERPHENAZINE 2 MG TABLET 0 SANDOZ EAGEX 0<strong>06</strong>03-5<strong>06</strong>1-21 1.19475 PERPHENAZINE 4 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-5<strong>06</strong>1-28 1.13502 PERPHENAZINE 4 MG TABLET 0 QUALITEST EAGEX 00781-1047-01 1.19475 PERPHENAZINE 4 MG TABLET 0 SANDOZ EAGEX 0<strong>06</strong>03-5<strong>06</strong>2-21 1.44915 PERPHENAZINE 8 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-5<strong>06</strong>2-28 1.37670 PERPHENAZINE 8 MG TABLET 0 QUALITEST EAGEX 00781-1048-01 1.44915 PERPHENAZINE 8 MG TABLET 0 SANDOZ EABND 00597-0017-01 0.14760 1.05825 PERSANTINE 25 MG TABLET G BOEHRINGER ING. EABND 00597-0018-01 0.17630 1.70498 PERSANTINE 50 MG TABLET G BOEHRINGER ING. EABND 00597-0019-01 0.36500 2.28117 PERSANTINE 75 MG TABLET G BOEHRINGER ING. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59767-0016-01 3.30962 PERTZYE DR 16,000 UNITS CAPS G DIGESTIVE CARE EABND 59767-0016-02 3.30964 PERTZYE DR 16,000 UNITS CAPS G DIGESTIVE CARE EABND 59767-00<strong>08</strong>-01 1.64962 PERTZYE DR 8,000 UNITS CAPSULE G DIGESTIVE CARE EABND 59767-00<strong>08</strong>-02 1.64964 PERTZYE DR 8,000 UNITS CAPSULE G DIGESTIVE CARE EABEX 68968-2010-01 7.35214 PEXEVA 10 MG TABLET G NOVEN THERAPEUT EABEX 68968-2020-01 7.64291 PEXEVA 20 MG TABLET G NOVEN THERAPEUT EABEX 68968-2030-01 7.93341 PEXEVA 30 MG TABLET G NOVEN THERAPEUT EABEX 68968-2040-01 8.22530 PEXEVA 40 MG TABLET G NOVEN THERAPEUT EAGEN 00049-0530-28 22.21500 PFIZERPEN 20 MILLION UNIT VIAL 0 PFIZER US PHARM EAGEN 00049-0520-83 7.57650 PFIZERPEN 5 MILLION UNITS VIAL 0 PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00574-7236-12 0.96120 PHENADOZ 12.5 MG SUPPOSITORY 0 WATSON LABS EAGUL 00591-2160-39 0.96120 PHENADOZ 12.5 MG SUPPOSITORY 0 ACTAVIS PHARMA, EAGUL 00574-7234-12 1.03620 PHENADOZ 25 MG SUPPOSITORY 0 WATSON LABS EAGUL 00591-2161-39 1.03620 PHENADOZ 25 MG SUPPOSITORY 0 ACTAVIS PHARMA, EAGEN 64376-<strong>08</strong>11-01 0.12200 PHENAZOPYRIDINE 100 MG TAB 0 BOCA PHARMACAL EAGEN 65162-0517-10 0.12200 PHENAZOPYRIDINE 100 MG TAB 0 AMNEAL PHARMACE EAGEN 66424-0043-01 0.12200 PHENAZOPYRIDINE 100 MG TAB 0 SDA LABS EAGEN 68<strong>08</strong>4-0292-01 0.12200 PHENAZOPYRIDINE 100 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0292-11 0.12200 PHENAZOPYRIDINE 100 MG TAB 0 AHP EAGEN 64376-<strong>08</strong>12-01 0.11640 PHENAZOPYRIDINE 200 MG TAB 0 BOCA PHARMACAL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-0520-10 0.11640 PHENAZOPYRIDINE 200 MG TAB 0 AMNEAL PHARMACE EAGEN 66424-0045-01 0.11640 PHENAZOPYRIDINE 200 MG TAB 0 SDA LABS EAGEN 66424-0045-10 0.11640 PHENAZOPYRIDINE 200 MG TAB 0 SDA LABS EAGEN 68<strong>08</strong>4-0293-01 0.11640 PHENAZOPYRIDINE 200 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0293-11 0.11640 PHENAZOPYRIDINE 200 MG TAB 0 AHP EAGEX 43386-0360-21 0.62625 PHENELZINE SULFATE 15 MG TAB 0 GAVIS PHARMACEU EAGEX 59762-0119-01 0.62625 PHENELZINE SULFATE 15 MG TAB 0 GREENSTONE LLC. EAGEX 0<strong>06</strong>41-6<strong>08</strong>2-01 0.99010 PHENERGAN 25 MG/ML AMPUL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-6<strong>08</strong>2-25 0.99010 PHENERGAN 25 MG/ML AMPUL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6<strong>08</strong>3-01 1.58700 PHENERGAN 50 MG/ML AMPUL 0 WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>41-6<strong>08</strong>3-25 1.58700 PHENERGAN 50 MG/ML AMPUL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-6<strong>08</strong>5-01 1.47<strong>08</strong>0 PHENERGAN 50 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-6<strong>08</strong>5-25 1.47<strong>08</strong>0 PHENERGAN 50 MG/ML VIAL 0 WEST-WARD,INC. MLGEN 00517-0405-25 2.25000 PHENYLEPHRINE 10 MG/ML VIAL 0 AMER. REGENT MLGEN 0<strong>06</strong>41-6142-01 10.80000 PHENYLEPHRINE 10 MG/ML VIAL 0 WEST-WARD,INC. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 310LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>41-6142-25 10.80000 PHENYLEPHRINE 10 MG/ML VIAL 0 WEST-WARD,INC. MLGEN 17478-0205-10 2.68200 PHENYLEPHRINE 10% EYE DROPS 0 AKORN INC. MLGEN 17478-0200-12 1.25900 PHENYLEPHRINE 2.5% EYE DROP 0 AKORN INC. MLGEN 17478-0200-20 2.0<strong>08</strong>50 PHENYLEPHRINE 2.5% EYE DROP 0 AKORN INC. MLGEN 242<strong>08</strong>-0740-02 2.0<strong>08</strong>50 PHENYLEPHRINE 2.5% EYE DROP 0 BAUSCH & LOMB P MLGEN 242<strong>08</strong>-0740-<strong>06</strong> 2.0<strong>08</strong>50 PHENYLEPHRINE 2.5% EYE DROP 0 BAUSCH & LOMB P MLGEN 242<strong>08</strong>-0740-59 2.0<strong>08</strong>50 PHENYLEPHRINE 2.5% EYE DROP 0 BAUSCH & LOMB P MLGEN 61314-0342-01 2.0<strong>08</strong>50 PHENYLEPHRINE 2.5% EYE DROP 0 SANDOZ MLGEN 61314-0342-02 2.0<strong>08</strong>50 PHENYLEPHRINE 2.5% EYE DROP 0 SANDOZ MLGEX 00378-2670-01 1.11352 PHENYTEK 200 MG CAPSULE 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-2670-93 1.11399 PHENYTEK 200 MG CAPSULE 0 MYLAN EAGEX 00378-3750-01 1.17830 PHENYTEK 300 MG CAPSULE 0 MYLAN EAGEX 00378-3750-93 1.17830 PHENYTEK 300 MG CAPSULE 0 MYLAN EAGEX 00378-1560-01 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 MYLAN EAGEX 00378-1560-10 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 MYLAN EAGEX 51079-0905-56 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 MYLAN INSTITUTI EAGEX 51672-4111-01 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 TARO PHARM USA EAGEX 51672-4111-03 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 TARO PHARM USA EAGEX 62756-0402-01 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 SUN PHARMACEUTI EAGEX 62756-0402-03 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64679-0720-01 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 WOCKHARDT USA L EAGEX 64679-0720-02 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 WOCKHARDT USA L EAGEX 65162-0212-10 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 AMNEAL PHARMACE EAGEX 65162-0212-11 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 AMNEAL PHARMACE EAGEX 65162-0212-50 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 AMNEAL PHARMACE EAGEX 68<strong>08</strong>4-0376-01 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 AHP EAGEX 68<strong>08</strong>4-0376-11 0.09<strong>06</strong>0 PHENYTOIN SOD EXT 100 MG CAP 0 AHP EAGEX 62756-0299-83 0.60000 PHENYTOIN SOD EXT 200 MG CAP 0 SUN PHARMACEUTI EAGEX 62756-0299-88 0.59977 PHENYTOIN SOD EXT 200 MG CAP 0 SUN PHARMACEUTI EAGEX 62756-0432-83 0.89850 PHENYTOIN SOD EXT 300 MG CAP 0 SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62756-0432-88 0.89857 PHENYTOIN SOD EXT 300 MG CAP 0 SUN PHARMACEUTI EAGEX 0<strong>06</strong>41-6138-01 0.31160 PHENYTOIN 100 MG/2 ML VIAL 0 WEST-WARD,INC./ MLGEX 0<strong>06</strong>41-6138-25 0.31160 PHENYTOIN 100 MG/2 ML VIAL 0 WEST-WARD,INC./ MLBUX 66689-0036-50 0.15210 0.32370 PHENYTOIN 100 MG/4 ML SUSP 0 VISTAPHARM MLGEX 00472-5002-<strong>08</strong> 0.<strong>08</strong>830 PHENYTOIN 125 MG/5 ML SUSP 0 ACTAVIS PHARMA, MLGEX 51672-4<strong>06</strong>9-01 0.<strong>08</strong>830 PHENYTOIN 125 MG/5 ML SUSP 0 TARO PHARM USA MLGEX 59762-0531-01 0.<strong>08</strong>830 PHENYTOIN 125 MG/5 ML SUSP 0 GREENSTONE LLC. MLGEX 60432-0131-<strong>08</strong> 0.<strong>08</strong>830 PHENYTOIN 125 MG/5 ML SUSP 0 MORTON GROVE PH MLGEX 0<strong>06</strong>41-6139-01 0.29700 PHENYTOIN 250 MG/5 ML VIAL 0 WEST-WARD,INC./ MLGEX 0<strong>06</strong>41-6139-25 0.29700 PHENYTOIN 250 MG/5 ML VIAL 0 WEST-WARD,INC./ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-5210-01 0.39472 PHENYTOIN 50 MG INFATAB 0 GREENSTONE LLC. EAGEX 00378-3850-01 0.39472 PHENYTOIN 50 MG TABLET CHEW 0 MYLAN EAGEX 00378-3850-05 0.39471 PHENYTOIN 50 MG TABLET CHEW 0 MYLAN EAGEX 51079-0129-<strong>06</strong> 0.4<strong>06</strong>50 PHENYTOIN 50 MG TABLET CHEW 0 MYLAN INSTITUTI EAGEX 0<strong>06</strong>41-0493-25 0.31160 PHENYTOIN 50 MG/ML VIAL 0 WEST-WARD,INC. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 311LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>41-2555-41 0.29700 PHENYTOIN 50 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-2555-45 0.29700 PHENYTOIN 50 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 16714-0347-01 1.12980 PHILITH 0.4-0.035 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-0347-04 1.12980 PHILITH 0.4-0.035 MG TABLET 0 NORTHSTAR RX LL EABND 00024-1535-02 0.17788 PHISOHEX 3% CLEANSER 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00024-1535-<strong>06</strong> 0.10749 PHISOHEX 3% CLEANSER 0 SAN<strong>OF</strong>I-AVENTIS MLBND 49230-<strong>06</strong>40-21 0.69898 PHOSLO 667 MG GELCAP G FRESENIUS MED EABND 49230-<strong>06</strong>43-31 0.16845 PHOSLYRA 667 MG/5 ML SOLUTION G FRESENIUS MED MLGEN 64980-0104-01 0.38077 PHOSPHA 250 NEUTRAL TABLET 0 RISING PHARM EABND 00046-1<strong>06</strong>5-05 17.66240 PHOSPHOLINE IODIDE 0.125% 0 WYETH PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00187-<strong>08</strong>44-01 15.27814 PHRENILIN FORTE CAPSULE 0 VALEANT EAGEN 00054-0056-25 0.23249 PILOCARPINE HCL 5 MG TABLET 0 ROXANE LABS. EAGEN 00115-5922-01 0.23249 PILOCARPINE HCL 5 MG TABLET 0 GLOBAL PHARM EAGEN 00228-2801-11 0.23249 PILOCARPINE HCL 5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00527-1313-01 0.23249 PILOCARPINE HCL 5 MG TABLET 0 LANNETT CO. INC EAGEN 00115-5911-01 1.12184 PILOCARPINE HCL 7.5 MG TABLET 0 GLOBAL PHARM EAGEN 00228-2837-11 1.12184 PILOCARPINE HCL 7.5 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00527-1407-01 1.12184 PILOCARPINE HCL 7.5 MG TABLET 0 LANNETT CO. INC EAGEN 61314-0203-15 4.37633 PILOCARPINE 1% EYE DROPS 0 SANDOZ MLGEN 17478-0224-12 2.35350 PILOCARPINE 2% EYE DROPS 0 AKORN INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61314-0204-15 2.54090 PILOCARPINE 2% EYE DROPS 0 SANDOZ MLGEN 61314-02<strong>06</strong>-15 4.98450 PILOCARPINE 4% EYE DROPS 0 SANDOZ MLBND 00<strong>06</strong>5-0215-35 22.72125 PILOPINE HS 4% EYE GEL 0 ALCON LABS. GMGEX 16714-0404-01 1.51100 PIMTREA 28 DAY TABLET 0 NORTHSTAR RX LL EAGEX 16714-0404-04 1.51100 PIMTREA 28 DAY TABLET 0 NORTHSTAR RX LL EABND 00378-0127-01 1.24367 PINDOLOL 10 MG TABLET G MYLAN EABND 00378-0052-01 0.91283 PINDOLOL 5 MG TABLET G MYLAN EABND 68047-0057-15 12.14566 PINNACAINE 20% OTIC DROPS 0 SIRCLE LABORATO MLGEN 00781-5634-31 9.21675 PIOGLITAZ-GLIMEPIR 30-2 MG TAB G SANDOZ EAGEN 00781-5635-31 9.21675 PIOGLITAZ-GLIMEPIR 30-4 MG TAB G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-2048-05 3.61300 PIOGLITAZONE HCL 15 MG TABLET G TEVA USA EAGEN 00093-2048-56 3.61300 PIOGLITAZONE HCL 15 MG TABLET G TEVA USA EAGEN 00093-2048-98 3.61300 PIOGLITAZONE HCL 15 MG TABLET G TEVA USA EAGEN 00378-0048-05 3.61300 PIOGLITAZONE HCL 15 MG TABLET G MYLAN EAGEN 00378-0048-77 3.61300 PIOGLITAZONE HCL 15 MG TABLET G MYLAN EAGEN 00378-0048-93 3.61300 PIOGLITAZONE HCL 15 MG TABLET G MYLAN EAGEN 00591-3205-05 3.61300 PIOGLITAZONE HCL 15 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-3205-19 3.61300 PIOGLITAZONE HCL 15 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-3205-30 3.61300 PIOGLITAZONE HCL 15 MG TABLET G ACTAVIS PHARMA, EAGEN 00781-5420-10 3.61300 PIOGLITAZONE HCL 15 MG TABLET G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-5420-31 3.61300 PIOGLITAZONE HCL 15 MG TABLET G SANDOZ EAGEN 00781-5420-92 3.61300 PIOGLITAZONE HCL 15 MG TABLET G SANDOZ EAGEN 13668-0140-05 3.61300 PIOGLITAZONE HCL 15 MG TABLET G TORRENT PHARMAC EAGEN 13668-0140-30 3.61300 PIOGLITAZONE HCL 15 MG TABLET G TORRENT PHARMAC EAGEN 13668-0140-90 3.61300 PIOGLITAZONE HCL 15 MG TABLET G TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 312LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16729-0020-10 3.61300 PIOGLITAZONE HCL 15 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0020-15 3.61300 PIOGLITAZONE HCL 15 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0020-16 3.61300 PIOGLITAZONE HCL 15 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 51991-07<strong>08</strong>-10 3.61300 PIOGLITAZONE HCL 15 MG TABLET G BRECKENRIDGE EAGEN 51991-07<strong>08</strong>-33 3.61300 PIOGLITAZONE HCL 15 MG TABLET G BRECKENRIDGE EAGEN 51991-07<strong>08</strong>-90 3.61300 PIOGLITAZONE HCL 15 MG TABLET G BRECKENRIDGE EAGEN 52343-0053-05 0.04824 PIOGLITAZONE HCL 15 MG TABLET G GEN-SOURCE RX EAGEN 52343-0053-30 0.05000 PIOGLITAZONE HCL 15 MG TABLET G GEN-SOURCE RX EAGEN 52343-0053-90 0.050<strong>08</strong> PIOGLITAZONE HCL 15 MG TABLET G GEN-SOURCE RX EAGEN 63304-0254-05 3.61300 PIOGLITAZONE HCL 15 MG TABLET G RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63304-0254-30 3.61300 PIOGLITAZONE HCL 15 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0254-90 3.61300 PIOGLITAZONE HCL 15 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0311-05 3.61300 PIOGLITAZONE HCL 15 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0311-30 3.61300 PIOGLITAZONE HCL 15 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0311-90 3.61300 PIOGLITAZONE HCL 15 MG TABLET G RANBAXY PHARMAC EAGEN 65862-0512-30 3.61300 PIOGLITAZONE HCL 15 MG TABLET G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-<strong>06</strong>49-01 0.91620 PIOGLITAZONE HCL 15 MG TABLET G AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>49-11 0.91620 PIOGLITAZONE HCL 15 MG TABLET G AHP EAGEN 00093-2047-05 3.68630 PIOGLITAZONE HCL 30 MG TABLET G TEVA USA EAGEN 00093-2047-56 3.68630 PIOGLITAZONE HCL 30 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-2047-98 3.68630 PIOGLITAZONE HCL 30 MG TABLET G TEVA USA EAGEN 00378-0228-05 3.68630 PIOGLITAZONE HCL 30 MG TABLET G MYLAN EAGEN 00378-0228-77 3.68630 PIOGLITAZONE HCL 30 MG TABLET G MYLAN EAGEN 00378-0228-93 3.68630 PIOGLITAZONE HCL 30 MG TABLET G MYLAN EAGEN 00591-32<strong>06</strong>-05 3.68630 PIOGLITAZONE HCL 30 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-32<strong>06</strong>-19 3.68630 PIOGLITAZONE HCL 30 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-32<strong>06</strong>-30 3.68630 PIOGLITAZONE HCL 30 MG TABLET G ACTAVIS PHARMA, EAGEN 00781-5421-10 3.68630 PIOGLITAZONE HCL 30 MG TABLET G SANDOZ EAGEN 00781-5421-31 3.68630 PIOGLITAZONE HCL 30 MG TABLET G SANDOZ EAGEN 00781-5421-92 3.68630 PIOGLITAZONE HCL 30 MG TABLET G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-0119-05 3.68630 PIOGLITAZONE HCL 30 MG TABLET G TORRENT PHARMAC EAGEN 13668-0119-30 3.68630 PIOGLITAZONE HCL 30 MG TABLET G TORRENT PHARMAC EAGEN 13668-0119-90 3.68630 PIOGLITAZONE HCL 30 MG TABLET G TORRENT PHARMAC EAGEN 16729-0021-10 3.68630 PIOGLITAZONE HCL 30 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0021-15 3.68630 PIOGLITAZONE HCL 30 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0021-16 3.68630 PIOGLITAZONE HCL 30 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 51079-0514-20 3.68630 PIOGLITAZONE HCL 30 MG TABLET G MYLAN INSTITUTI EAGEN 51991-0709-10 3.68630 PIOGLITAZONE HCL 30 MG TABLET G BRECKENRIDGE EAGEN 51991-0709-90 3.68630 PIOGLITAZONE HCL 30 MG TABLET G BRECKENRIDGE EAGEN 52343-0054-05 0.07377 PIOGLITAZONE HCL 30 MG TABLET G GEN-SOURCE RX EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 52343-0054-30 0.07625 PIOGLITAZONE HCL 30 MG TABLET G GEN-SOURCE RX EAGEN 52343-0054-90 0.07641 PIOGLITAZONE HCL 30 MG TABLET G GEN-SOURCE RX EAGEN 63304-0255-05 3.68630 PIOGLITAZONE HCL 30 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0255-30 3.68630 PIOGLITAZONE HCL 30 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0255-90 3.68630 PIOGLITAZONE HCL 30 MG TABLET G RANBAXY PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 313LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63304-0312-05 3.68630 PIOGLITAZONE HCL 30 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0312-30 3.68630 PIOGLITAZONE HCL 30 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0312-90 3.68630 PIOGLITAZONE HCL 30 MG TABLET G RANBAXY PHARMAC EAGEN 65862-0513-30 3.68630 PIOGLITAZONE HCL 30 MG TABLET G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-<strong>06</strong>52-01 1.42410 PIOGLITAZONE HCL 30 MG TABLET G AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>52-11 1.42410 PIOGLITAZONE HCL 30 MG TABLET G AHP EAGEN 00093-2046-05 3.99980 PIOGLITAZONE HCL 45 MG TABLET G TEVA USA EAGEN 00093-2046-56 3.99980 PIOGLITAZONE HCL 45 MG TABLET G TEVA USA EAGEN 00093-2046-98 3.99980 PIOGLITAZONE HCL 45 MG TABLET G TEVA USA EAGEN 00378-0318-05 3.99980 PIOGLITAZONE HCL 45 MG TABLET G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0318-77 3.99980 PIOGLITAZONE HCL 45 MG TABLET G MYLAN EAGEN 00378-0318-93 3.99980 PIOGLITAZONE HCL 45 MG TABLET G MYLAN EAGEN 00591-3207-05 3.99980 PIOGLITAZONE HCL 45 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-3207-19 3.99980 PIOGLITAZONE HCL 45 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-3207-30 3.99980 PIOGLITAZONE HCL 45 MG TABLET G ACTAVIS PHARMA, EAGEN 00781-5422-10 3.99980 PIOGLITAZONE HCL 45 MG TABLET G SANDOZ EAGEN 00781-5422-31 3.99980 PIOGLITAZONE HCL 45 MG TABLET G SANDOZ EAGEN 00781-5422-92 3.99980 PIOGLITAZONE HCL 45 MG TABLET G SANDOZ EAGEN 13668-0120-05 3.99980 PIOGLITAZONE HCL 45 MG TABLET G TORRENT PHARMAC EAGEN 13668-0120-30 3.99980 PIOGLITAZONE HCL 45 MG TABLET G TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-0120-90 3.99980 PIOGLITAZONE HCL 45 MG TABLET G TORRENT PHARMAC EAGEN 16729-0022-10 3.99980 PIOGLITAZONE HCL 45 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0022-15 3.99980 PIOGLITAZONE HCL 45 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0022-16 3.99980 PIOGLITAZONE HCL 45 MG TABLET G ACCORD <strong>HEALTH</strong>CA EAGEN 51079-0515-20 3.99980 PIOGLITAZONE HCL 45 MG TABLET G MYLAN INSTITUTI EAGEN 51991-0710-10 3.99980 PIOGLITAZONE HCL 45 MG TABLET G BRECKENRIDGE EAGEN 51991-0710-33 3.99980 PIOGLITAZONE HCL 45 MG TABLET G BRECKENRIDGE EAGEN 51991-0710-90 3.99980 PIOGLITAZONE HCL 45 MG TABLET G BRECKENRIDGE EAGEN 52343-0055-05 0.07911 PIOGLITAZONE HCL 45 MG TABLET G GEN-SOURCE RX EAGEN 52343-0055-30 0.<strong>08</strong>250 PIOGLITAZONE HCL 45 MG TABLET G GEN-SOURCE RX EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 52343-0055-90 0.07783 PIOGLITAZONE HCL 45 MG TABLET G GEN-SOURCE RX EAGEN 63304-0261-05 3.99980 PIOGLITAZONE HCL 45 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0261-30 3.99980 PIOGLITAZONE HCL 45 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0261-90 3.99980 PIOGLITAZONE HCL 45 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0313-05 3.99980 PIOGLITAZONE HCL 45 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0313-30 3.99980 PIOGLITAZONE HCL 45 MG TABLET G RANBAXY PHARMAC EAGEN 63304-0313-90 3.99980 PIOGLITAZONE HCL 45 MG TABLET G RANBAXY PHARMAC EAGEN 65862-0514-30 3.99980 PIOGLITAZONE HCL 45 MG TABLET G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-<strong>06</strong>60-01 1.54485 PIOGLITAZONE HCL 45 MG TABLET G AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>60-11 1.54485 PIOGLITAZONE HCL 45 MG TABLET G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-5049-<strong>06</strong> 3.00753 PIOGLITAZONE-METFORMIN 15-500 G TEVA USA EAGEN 00093-5049-86 3.00753 PIOGLITAZONE-METFORMIN 15-500 G TEVA USA EAGEN 00378-1550-91 3.00753 PIOGLITAZONE-METFORMIN 15-500 G MYLAN EAGEN 00781-5626-60 3.00753 PIOGLITAZONE-METFORMIN 15-500 G SANDOZ EAGEN 13668-0280-33 3.00753 PIOGLITAZONE-METFORMIN 15-500 G TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 314LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13668-0280-60 3.00753 PIOGLITAZONE-METFORMIN 15-500 G TORRENT PHARMAC EAGEN 65862-0525-18 3.00753 PIOGLITAZONE-METFORMIN 15-500 G AUROBINDO PHARM EAGEN 65862-0525-60 3.00753 PIOGLITAZONE-METFORMIN 15-500 G AUROBINDO PHARM EAGEN 00093-5050-<strong>06</strong> 3.00753 PIOGLITAZONE-METFORMIN 15-850 G TEVA USA EAGEN 00093-5050-86 3.00753 PIOGLITAZONE-METFORMIN 15-850 G TEVA USA EAGEN 00378-1575-91 3.00753 PIOGLITAZONE-METFORMIN 15-850 G MYLAN EAGEN 00781-5627-60 3.00753 PIOGLITAZONE-METFORMIN 15-850 G SANDOZ EAGEN 13668-0281-33 3.00753 PIOGLITAZONE-METFORMIN 15-850 G TORRENT PHARMAC EAGEN 13668-0281-60 3.00753 PIOGLITAZONE-METFORMIN 15-850 G TORRENT PHARMAC EAGEN 65862-0526-18 3.00753 PIOGLITAZONE-METFORMIN 15-850 G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0526-60 3.00753 PIOGLITAZONE-METFORMIN 15-850 G AUROBINDO PHARM EABND 00409-3374-02 4.711<strong>08</strong> PIPERACIL-TAZO 2.25 GM ADD VL 0 HOSPIRA EAGEN 00409-3383-02 3.12300 PIPERACIL-TAZOBACT 2.25 GM VL 0 HOSPIRA EAGEN 00781-3110-90 6.86000 PIPERACIL-TAZOBACT 2.25 GM VL 0 SANDOZ EAGEN 00781-3110-95 6.86000 PIPERACIL-TAZOBACT 2.25 GM VL 0 SANDOZ EAGEN 25021-0164-30 6.30000 PIPERACIL-TAZOBACT 2.25 GM VL 0 SAGENT PHARMACE EAGEN 44567-<strong>08</strong>01-10 6.59700 PIPERACIL-TAZOBACT 2.25 GM VL 0 WG CRITICAL CAR EAGEN 60505-<strong>06</strong>86-04 6.86000 PIPERACIL-TAZOBACT 2.25 GM VL 0 APOTEX CORP EABND 00409-3378-13 6.52380 PIPERACIL-TAZOBACT 3.375 GM VL 0 HOSPIRA EAGEN 00409-3385-13 4.53600 PIPERACIL-TAZOBACT 3.375 GM VL 0 HOSPIRA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3113-90 5.00110 PIPERACIL-TAZOBACT 3.375 GM VL 0 SANDOZ EAGEN 00781-3113-95 5.00110 PIPERACIL-TAZOBACT 3.375 GM VL 0 SANDOZ EAGEN 25021-0165-30 5.00110 PIPERACIL-TAZOBACT 3.375 GM VL 0 SAGENT PHARMACE EAGEN 44567-<strong>08</strong>02-10 5.00110 PIPERACIL-TAZOBACT 3.375 GM VL 0 WG CRITICAL CAR EAGEN 44567-<strong>08</strong>82-10 5.00110 PIPERACIL-TAZOBACT 3.375 GM VL 0 WG CRITICAL CAR EAGEN 60505-<strong>06</strong>87-04 5.00110 PIPERACIL-TAZOBACT 3.375 GM VL 0 APOTEX CORP EAGEN 63323-0300-30 5.00110 PIPERACIL-TAZOBACT 3.375 GM VL 0 APP PHARMACEUTI EABND 00409-3379-04 8.446<strong>08</strong> PIPERACIL-TAZOBACT 4.5 GM VIAL 0 HOSPIRA EAGEN 00409-3390-04 6.23700 PIPERACIL-TAZOBACT 4.5 GM VIAL 0 HOSPIRA EAGEN 00781-3114-91 15.32400 PIPERACIL-TAZOBACT 4.5 GM VIAL 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3114-95 15.32400 PIPERACIL-TAZOBACT 4.5 GM VIAL 0 SANDOZ EAGEN 25021-0166-48 13.05000 PIPERACIL-TAZOBACT 4.5 GM VIAL 0 SAGENT PHARMACE EAGEN 44567-<strong>08</strong>03-10 13.20300 PIPERACIL-TAZOBACT 4.5 GM VIAL 0 WG CRITICAL CAR EAGEN 60505-<strong>06</strong>88-04 15.32400 PIPERACIL-TAZOBACT 4.5 GM VIAL 0 APOTEX CORP EAGEN 44567-<strong>08</strong>04-01 95.66325 PIPERACIL-TAZOBACT 40.5 GRAM 0 WG CRITICAL CAR EAGEN 60505-0773-00 95.66325 PIPERACIL-TAZOBACT 40.5 GRAM 0 APOTEX CORP EAGEX 68180-<strong>08</strong>93-11 0.73890 PIRMELLA 1-35-28 TABLET 0 LUPIN PHARMACEU EAGEX 68180-<strong>08</strong>93-13 0.73890 PIRMELLA 1-35-28 TABLET 0 LUPIN PHARMACEU EAGEX 68180-<strong>08</strong>92-11 0.83640 PIRMELLA 7-7-7-28 TABLET 0 LUPIN PHARMACEU EAGEX 68180-<strong>08</strong>92-13 0.83640 PIRMELLA 7-7-7-28 TABLET 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00093-0756-01 0.<strong>08</strong>910 PIROXICAM 10 MG CAPSULE 0 TEVA USA EAGUL 29033-0012-01 0.<strong>08</strong>910 PIROXICAM 10 MG CAPSULE 0 NOSTRUM LABORAT EAGUL 00093-0757-01 0.11310 PIROXICAM 20 MG CAPSULE 0 TEVA USA EAGUL 00093-0757-05 0.11310 PIROXICAM 20 MG CAPSULE 0 TEVA USA EAGUL 29033-0013-01 0.11310 PIROXICAM 20 MG CAPSULE 0 NOSTRUM LABORAT EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 315LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 51285-0942-88 32.50000 33.71460 PLAN B ONE-STEP 1.5 MG TABLET 0 TEVA SPECIALTY EABND 00024-1562-10 0.1<strong>08</strong>00 3.90432 PLAQUENIL 200 MG TABLET G COVIS PHARMACEU EABND 13533-<strong>06</strong>84-17 1.21512 PLASBUMIN-25 IV SOLUTION 0 GRIFOLS THERAPE MLBND 13533-<strong>06</strong>84-21 0.90702 PLASBUMIN-25 IV SOLUTION 0 GRIFOLS THERAPE MLBND 13533-<strong>06</strong>84-72 0.90702 PLASBUMIN-25 IV SOLUTION 0 GRIFOLS THERAPE MLBND 13533-<strong>06</strong>92-17 1.21512 PLASBUMIN-25 IV SOLUTION 0 GRIFOLS THERAPE MLBND 13533-<strong>06</strong>92-21 0.90702 PLASBUMIN-25 IV SOLUTION 0 GRIFOLS THERAPE MLBND 13533-<strong>06</strong>92-72 0.90702 PLASBUMIN-25 IV SOLUTION 0 GRIFOLS THERAPE MLBND 63653-1171-01 0.13017 6.41968 PLAVIX 75 MG TABLET G BMS PRIMARYCARE EABND 63653-1171-05 0.13017 6.41971 PLAVIX 75 MG TABLET G BMS PRIMARYCARE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63653-1171-<strong>06</strong> 0.13017 6.41866 PLAVIX 75 MG TABLET G BMS PRIMARYCARE EABND 59148-0002-16 0.13595 1.77703 PLETAL 100 MG TABLET G OTSUKA AMERICA EABND 59148-0003-16 0.16538 1.77703 PLETAL 50 MG TABLET G OTSUKA AMERICA EABND 00299-6100-10 0.84660 PLIAGLIS 7%-7% CREAM 0 GALDERMA LABORA GMBND 00299-6100-35 0.99600 PLIAGLIS 7%-7% CREAM 0 GALDERMA LABORA GMGEN 42192-0321-30 1.66840 PNV-DHA S<strong>OF</strong>TGEL 0 ACELLA PHARMACE EAGEN 76439-0254-10 0.64042 PNV-FERROUS FUMARATE-DOCU-FA 0 VIRTUS PHARMACE EAGEN 42192-0320-90 1.37360 PNV-SELECT TABLET 0 ACELLA PHARMACE EAGEN 00574-<strong>06</strong>11-05 23.17500 POD<strong>OF</strong>ILOX 0.5% TOPICAL SOLN 0 PADDOCK LABS. MLGEN 00591-3204-13 21.31714 POD<strong>OF</strong>ILOX 0.5% TOPICAL SOLN 0 ACTAVIS PHARMA, ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 48102-0004-35 3.04<strong>08</strong>8 POLYCIN EYE OINTMENT 0 PERRIGO CO. GMGEN 00574-0412-02 0.03070 POLYETHYLENE GLYCOL 3350 POWD 0 PADDOCK LABS. GMGEN 00574-0412-05 0.03070 POLYETHYLENE GLYCOL 3350 POWD 0 PADDOCK LABS. GMGEN 51991-0457-57 0.03070 POLYETHYLENE GLYCOL 3350 POWD 0 BRECKENRIDGE GMGEN 51991-0457-58 0.03070 POLYETHYLENE GLYCOL 3350 POWD 0 BRECKENRIDGE GMGEN 62175-0442-15 0.03070 POLYETHYLENE GLYCOL 3350 POWD 0 KREMERS URBAN GMGEN 62175-0442-31 0.03070 POLYETHYLENE GLYCOL 3350 POWD 0 KREMERS URBAN GMGEN 25021-0117-10 9.00000 POLYMYXIN B SULFATE VIAL 0 SAGENT PHARMACE EAGEN 39822-0166-05 10.12500 POLYMYXIN B SULFATE VIAL 0 X-GEN PHARMACEU EAGEN 55390-0139-10 11.43000 POLYMYXIN B SULFATE VIAL 0 BEDFORD LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 242<strong>08</strong>-0315-10 0.63450 POLYMYXIN B-TMP EYE DROPS 0 VALEANT MLGEN 60758-09<strong>08</strong>-10 0.63450 POLYMYXIN B-TMP EYE DROPS 0 PACIFIC PHARMA MLGEN 61314-<strong>06</strong>28-10 0.63450 POLYMYXIN B-TMP EYE DROPS 0 SANDOZ MLBND 00023-7824-10 0.63450 4.97834 POLYTRIM EYE DROPS G ALLERGAN INC. MLBND 59572-0501-00 510.27271 POMALYST 1 MG CAPSULE 0 CELGENE EABND 59572-0501-21 510.27253 POMALYST 1 MG CAPSULE 0 CELGENE EABND 59572-0502-00 510.27271 POMALYST 2 MG CAPSULE 0 CELGENE EABND 59572-0502-21 510.27253 POMALYST 2 MG CAPSULE 0 CELGENE EABND 59572-0503-00 510.27271 POMALYST 3 MG CAPSULE 0 CELGENE EABND 59572-0503-21 510.27253 POMALYST 3 MG CAPSULE 0 CELGENE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59572-0504-00 510.27271 POMALYST 4 MG CAPSULE 0 CELGENE EABND 59572-0504-21 510.27253 POMALYST 4 MG CAPSULE 0 CELGENE EABND 59630-0400-30 10.78300 20.33638 PONSTEL 250 MG KAPSEALS G SHIONOGI PHARMA EAGEX 00555-9020-58 0.81150 PORTIA-28 TABLET 0 BARR EAGEN 00517-2053-25 0.15234 POTASSIUM ACET 2 MEQ/ML VIAL 0 AMER. REGENT ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 316LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00517-5024-25 0.12450 POTASSIUM ACET 4 MEQ/ML VIAL 0 AMER. REGENT MLGEN 00245-0071-11 1.23856 POTASSIUM CITRATE ER 10 MEQ TB 0 UPSHER SMITH EAGEN 44523-0415-01 1.99702 POTASSIUM CITRATE ER 15 MEQ TB 0 BIOCOMP PHARMA, EAGEN 00245-0070-11 0.60590 POTASSIUM CITRATE ER 5 MEQ TAB 0 UPSHER SMITH EAGEN 51927-14<strong>06</strong>-00 0.15000 POTASSIUM CITRATE POWDER 0 PR<strong>OF</strong>ESSIONAL CO GMGEN 00574-0181-01 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 PADDOCK LABS. EAGEN 00574-0181-05 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 PADDOCK LABS. EAGEN 00904-6<strong>06</strong>8-61 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 MAJOR PHARMACEU EAGEN 51477-0001-03 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 ZYDUS PHARMACEU EAGEN 51477-0001-04 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62037-0560-01 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0560-05 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0560-10 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0560-90 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 ACTAVIS PHARMA, EAGEN 65162-0542-10 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 AMNEAL PHARMACE EAGEN 65162-0542-11 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 AMNEAL PHARMACE EAGEN 65162-0542-50 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 AMNEAL PHARMACE EAGEN 68001-0144-00 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 BLUEPOINT LABOR EAGEN 68001-0144-03 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 BLUEPOINT LABOR EAGEN 68001-0144-<strong>08</strong> 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 BLUEPOINT LABOR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0419-01 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 AHP EAGEN 68382-0701-01 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 ZYDUS PHARMACEU EAGEN 68382-0701-05 0.59333 POTASSIUM CL ER 10 MEQ CAPSULE 0 ZYDUS PHARMACEU EAGUL 00<strong>08</strong>5-1717-01 0.25380 POTASSIUM CL ER 10 MEQ TABLET 0 SCHERING CORP G EAGEN 00245-0043-00 0.35109 POTASSIUM CL ER 10 MEQ TABLET 0 UPSHER SMITH EAGEN 00245-0043-55 0.351<strong>08</strong> POTASSIUM CL ER 10 MEQ TABLET 0 UPSHER SMITH EAGEN 00245-0243-10 0.351<strong>08</strong> POTASSIUM CL ER 10 MEQ TABLET 0 UPSHER SMITH EAGEN 00245-0243-11 0.19590 POTASSIUM CL ER 10 MEQ TABLET 0 UPSHER SMITH EAGEN 00781-1526-01 0.42345 POTASSIUM CL ER 10 MEQ TABLET 0 SANDOZ EAGEN 00781-1526-10 0.42343 POTASSIUM CL ER 10 MEQ TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00781-5710-01 0.25380 POTASSIUM CL ER 10 MEQ TABLET 0 SANDOZ EAGUL 00781-5710-10 0.25380 POTASSIUM CL ER 10 MEQ TABLET 0 SANDOZ EAGUL 62037-0710-01 0.25380 POTASSIUM CL ER 10 MEQ TABLET 0 ACTAVIS PHARMA, EAGUL 63739-0446-04 0.25380 POTASSIUM CL ER 10 MEQ TABLET 0 MCKESSON PACKAG EAGUL 63739-0446-10 0.25380 POTASSIUM CL ER 10 MEQ TABLET 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0524-01 0.45900 POTASSIUM CL ER 10 MEQ TABLET 0 AHP EAGUL 68<strong>08</strong>4-<strong>06</strong>32-01 0.25380 POTASSIUM CL ER 10 MEQ TABLET 0 AHP EAGEN 68382-<strong>06</strong>00-01 0.43882 POTASSIUM CL ER 10 MEQ TABLET 0 ZYDUS PHARMACEU EAGEN 68382-<strong>06</strong>00-10 0.41690 POTASSIUM CL ER 10 MEQ TABLET 0 ZYDUS PHARMACEU EAGEN 00<strong>08</strong>5-1718-01 0.35424 POTASSIUM CL ER 20 MEQ TABLET 0 SCHERING CORP G EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-5720-01 0.35424 POTASSIUM CL ER 20 MEQ TABLET 0 SANDOZ EAGEN 00781-5720-05 0.35424 POTASSIUM CL ER 20 MEQ TABLET 0 SANDOZ EAGEN 00781-5720-10 0.35424 POTASSIUM CL ER 20 MEQ TABLET 0 SANDOZ EAGEN 62037-0999-01 0.35424 POTASSIUM CL ER 20 MEQ TABLET 0 ACTAVIS PHARMA, EAGEN 62037-0999-05 0.35424 POTASSIUM CL ER 20 MEQ TABLET 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 317LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62037-0999-10 0.35424 POTASSIUM CL ER 20 MEQ TABLET 0 ACTAVIS PHARMA, EAGEN 63739-0447-10 0.35424 POTASSIUM CL ER 20 MEQ TABLET 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0360-01 0.35424 POTASSIUM CL ER 20 MEQ TABLET 0 AHP EAGEN 68<strong>08</strong>4-0360-11 0.35424 POTASSIUM CL ER 20 MEQ TABLET 0 AHP EABND 68382-0398-01 0.35424 0.52<strong>06</strong>5 POTASSIUM CL ER 20 MEQ TABLET 0 ZYDUS PHARMACEU EABND 68382-0398-05 0.35424 0.52<strong>06</strong>7 POTASSIUM CL ER 20 MEQ TABLET 0 ZYDUS PHARMACEU EAGEN 00574-0180-01 0.70477 POTASSIUM CL ER 8 MEQ CAPSULE 0 PADDOCK LABS. EAGEN 51477-0002-04 0.70477 POTASSIUM CL ER 8 MEQ CAPSULE 0 ZYDUS PHARMACEU EAGEN 62037-0559-01 0.70477 POTASSIUM CL ER 8 MEQ CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0559-05 0.70480 POTASSIUM CL ER 8 MEQ CAPSULE 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0702-01 0.70477 POTASSIUM CL ER 8 MEQ CAPSULE 0 ZYDUS PHARMACEU EAGEN 00245-0042-00 0.19485 POTASSIUM CL ER 8 MEQ TABLET 0 UPSHER SMITH EAGEN 00245-0042-55 0.41380 POTASSIUM CL ER 8 MEQ TABLET 0 UPSHER SMITH EAGEN 00245-0042-85 0.19492 POTASSIUM CL ER 8 MEQ TABLET 0 UPSHER SMITH EAGEN 00245-0242-10 0.41380 POTASSIUM CL ER 8 MEQ TABLET 0 UPSHER SMITH EAGEN 00245-0242-11 0.13305 POTASSIUM CL ER 8 MEQ TABLET 0 UPSHER SMITH EAGEN 00781-1516-01 0.40230 POTASSIUM CL ER 8 MEQ TABLET 0 SANDOZ EAGEN 00338-0709-48 0.02412 POTASSIUM CL 10 MEQ/100 ML SOL 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0705-41 0.04824 POTASSIUM CL 10 MEQ/50 ML SOL 0 BAXTER <strong>HEALTH</strong>CA MLGEN 0<strong>06</strong>03-1532-58 0.00700 POTASSIUM CL 10% (20 MEQ/15 ML 0 QUALITEST ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-1534-58 0.00700 POTASSIUM CL 10% (20 MEQ/15 ML 0 QUALITEST MLGEN 0<strong>06</strong>03-1535-58 0.00700 POTASSIUM CL 10% (20 MEQ/15 ML 0 QUALITEST MLGEN 00904-1007-16 0.00700 POTASSIUM CL 10% (20 MEQ/15 ML 0 MAJOR PHARMACEU MLGEN 16571-0303-16 0.00700 POTASSIUM CL 10% (40 MEQ/30 ML 0 PACK PHARMACEUT MLGEN 00264-1940-20 0.02170 POTASSIUM CL 2 MEQ/ML VIAL 0 B.BRAUN MLGEN 00409-6653-05 0.07770 POTASSIUM CL 2 MEQ/ML VIAL 0 HOSPIRA MLGEN 63323-0965-05 0.07770 POTASSIUM CL 2 MEQ/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0965-10 0.07770 POTASSIUM CL 2 MEQ/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0965-20 0.07<strong>06</strong>5 POTASSIUM CL 2 MEQ/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-0967-30 0.<strong>06</strong>600 POTASSIUM CL 2 MEQ/ML VIAL 0 APP PHARMACEUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 76439-0343-10 4.53277 POTASSIUM CL 20 MEQ PACKET 0 VIRTUS PHARMACE EAGEN 76439-0343-30 4.53300 POTASSIUM CL 20 MEQ PACKET 0 VIRTUS PHARMACE EAGEN 00338-0704-34 0.00220 POTASSIUM CL 20 MEQ-0.45% NACL 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-9257-39 0.00220 POTASSIUM CL 20 MEQ-0.45% NACL 0 HOSPIRA MLGEN 00338-0705-48 0.02412 POTASSIUM CL 20 MEQ/100 ML SOL 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0703-41 0.04824 POTASSIUM CL 20 MEQ/50 ML SOL 0 BAXTER <strong>HEALTH</strong>CA MLBND 0<strong>06</strong>03-1536-58 0.04279 0.13720 POTASSIUM CL 20% (40 MEQ/15 ML 0 QUALITEST MLBND 0<strong>06</strong>03-35<strong>08</strong>-16 0.56460 1.18579 POTASSIUM CL 25 MEQ TAB EFF 0 QUALITEST EAGEN 00338-0703-48 0.02412 POTASSIUM CL 40 MEQ/100 ML SOL 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00517-2350-25 0.11250 POTASSIUM PHOSP 150 MMOL/50 ML 0 AMER. REGENT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-2720-46 0.15110 POTASSIUM 25 MEQ TABLET EFF 0 MAJOR PHARMACEU EABND 00597-0135-54 4.84153 PRADAXA 150 MG CAPSULE 0 BOEHRINGER ING. EABND 00597-0135-60 4.84153 PRADAXA 150 MG CAPSULE 0 BOEHRINGER ING. EABND 00597-0149-54 4.84153 PRADAXA 75 MG CAPSULE 0 BOEHRINGER ING. EABND 00597-0149-60 4.84153 PRADAXA 75 MG CAPSULE 0 BOEHRINGER ING. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 318LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-5248-92 0.12987 PRAMIPEXOLE 0.125 MG TABLET 0 SANDOZ EAGEN 13668-0091-05 0.12987 PRAMIPEXOLE 0.125 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0091-90 0.12987 PRAMIPEXOLE 0.125 MG TABLET 0 TORRENT PHARMAC EAGEN 16714-0584-01 0.12987 PRAMIPEXOLE 0.125 MG TABLET 0 NORTHSTAR RX LL EAGEN 65862-<strong>06</strong>04-90 0.12987 PRAMIPEXOLE 0.125 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-<strong>06</strong>04-99 0.12987 PRAMIPEXOLE 0.125 MG TABLET 0 AUROBINDO PHARM EAGEN 68382-0196-10 0.12987 PRAMIPEXOLE 0.125 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0196-16 0.12987 PRAMIPEXOLE 0.125 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0330-90 0.12987 PRAMIPEXOLE 0.125 MG TABLET 0 GLENMARK PHARMA EAGEN 00378-1705-05 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-1705-77 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 MYLAN EAGEN 00781-5249-92 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 SANDOZ EAGEN 13668-0092-05 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0092-90 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 TORRENT PHARMAC EAGEN 16714-0585-01 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 NORTHSTAR RX LL EAGEN 51991-<strong>06</strong>29-90 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 BRECKENRIDGE EAGEN 65862-<strong>06</strong>05-90 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-<strong>06</strong>05-99 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0440-01 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0440-11 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0197-10 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0197-16 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0331-90 0.09720 PRAMIPEXOLE 0.25 MG TABLET 0 GLENMARK PHARMA EAGEN 00378-1707-05 0.09720 PRAMIPEXOLE 0.5 MG TABLET 0 MYLAN EAGEN 00378-1707-77 0.09720 PRAMIPEXOLE 0.5 MG TABLET 0 MYLAN EAGEN 00781-5250-92 0.09720 PRAMIPEXOLE 0.5 MG TABLET 0 SANDOZ EAGEN 13668-0093-05 0.09720 PRAMIPEXOLE 0.5 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0093-90 0.09720 PRAMIPEXOLE 0.5 MG TABLET 0 TORRENT PHARMAC EAGEN 16714-0586-01 0.09720 PRAMIPEXOLE 0.5 MG TABLET 0 NORTHSTAR RX LL EAGEN 65862-<strong>06</strong><strong>06</strong>-90 0.09720 PRAMIPEXOLE 0.5 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-<strong>06</strong><strong>06</strong>-99 0.09720 PRAMIPEXOLE 0.5 MG TABLET 0 AUROBINDO PHARM EAGEN 68382-0198-16 0.09720 PRAMIPEXOLE 0.5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0332-90 0.09720 PRAMIPEXOLE 0.5 MG TABLET 0 GLENMARK PHARMA EAGEN 00093-8019-98 0.09274 PRAMIPEXOLE 0.75 MG TABLET 0 TEVA USA EAGEN 00378-1713-77 0.09274 PRAMIPEXOLE 0.75 MG TABLET 0 MYLAN EAGEN 00781-5281-92 0.09274 PRAMIPEXOLE 0.75 MG TABLET 0 SANDOZ EAGEN 13668-0184-05 0.09274 PRAMIPEXOLE 0.75 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0184-90 0.09274 PRAMIPEXOLE 0.75 MG TABLET 0 TORRENT PHARMAC EAGEN 16714-0589-01 0.09274 PRAMIPEXOLE 0.75 MG TABLET 0 NORTHSTAR RX LL EAGEN 65862-<strong>06</strong>07-90 0.09274 PRAMIPEXOLE 0.75 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-<strong>06</strong>07-99 0.09274 PRAMIPEXOLE 0.75 MG TABLET 0 AUROBINDO PHARM EAGEN 00781-5251-92 0.12740 PRAMIPEXOLE 1 MG TABLET 0 SANDOZ EAGEN 13668-0094-05 0.12740 PRAMIPEXOLE 1 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0094-90 0.12740 PRAMIPEXOLE 1 MG TABLET 0 TORRENT PHARMAC EAGEN 16714-0587-01 0.12740 PRAMIPEXOLE 1 MG TABLET 0 NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 319LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51991-<strong>06</strong>31-90 0.12740 PRAMIPEXOLE 1 MG TABLET 0 BRECKENRIDGE EAGEN 65862-<strong>06</strong><strong>08</strong>-90 0.12740 PRAMIPEXOLE 1 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-<strong>06</strong><strong>08</strong>-99 0.12740 PRAMIPEXOLE 1 MG TABLET 0 AUROBINDO PHARM EAGEN 68382-0199-16 0.12740 PRAMIPEXOLE 1 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0333-90 0.12740 PRAMIPEXOLE 1 MG TABLET 0 GLENMARK PHARMA EAGEN 00378-1712-05 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 MYLAN EAGEN 00378-1712-77 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 MYLAN EAGEN 00781-5252-92 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 SANDOZ EAGEN 13668-0095-05 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 TORRENT PHARMAC EAGEN 13668-0095-90 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0588-01 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 NORTHSTAR RX LL EAGEN 51991-<strong>06</strong>32-90 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 BRECKENRIDGE EAGEN 65862-<strong>06</strong>09-90 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-<strong>06</strong>09-99 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 AUROBINDO PHARM EAGEN 68382-0200-10 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0200-16 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0334-90 0.12987 PRAMIPEXOLE 1.5 MG TABLET 0 GLENMARK PHARMA EABND 00169-0093-01 3.88240 PRANDIMET 1 MG-500 MG TABLET 0 NOVO NORDISK EABND 00169-0092-01 3.88240 PRANDIMET 2 MG-500 MG TABLET 0 NOVO NORDISK EABND 00169-0<strong>08</strong>1-81 4.26686 PRANDIN 0.5 MG TABLET G NOVO NORDISK EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00169-0<strong>08</strong>2-81 4.26686 PRANDIN 1 MG TABLET G NOVO NORDISK EABND 00169-0<strong>08</strong>4-81 4.26686 PRANDIN 2 MG TABLET G NOVO NORDISK EABUL 00003-5178-05 0.29170 3.62248 PRAVACHOL 20 MG TABLET G BMS PRIMARYCARE EABUL 00003-5194-10 0.35600 5.31578 PRAVACHOL 40 MG TABLET G BMS PRIMARYCARE EABUL 00003-5195-10 0.57530 5.31578 PRAVACHOL 80 MG TABLET G BMS PRIMARYCARE EAGUL 00093-0771-10 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 TEVA USA EAGUL 00093-0771-98 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 TEVA USA EAGUL 00378-0552-77 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 MYLAN EAGUL 00904-5891-61 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 MAJOR PHARMACEU EAGUL 54458-0927-10 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 INTERNATIONAL L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 55111-0229-05 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 DR.REDDY'S LAB EAGUL 60505-0168-05 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 APOTEX CORP EAGUL 60505-0168-09 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 APOTEX CORP EAGUL 68180-0485-02 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 LUPIN PHARMACEU EAGUL 68180-0485-09 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 LUPIN PHARMACEU EAGUL 68382-0070-05 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 ZYDUS PHARMACEU EAGUL 68382-0070-16 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 ZYDUS PHARMACEU EAGUL 68462-0195-05 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 GLENMARK PHARMA EAGUL 68462-0195-90 0.25000 PRAVASTATIN SODIUM 10 MG TAB 0 GLENMARK PHARMA EAGUL 00093-7201-10 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00093-7201-98 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 TEVA USA EAGUL 00378-0554-77 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 MYLAN EAGUL 00781-5232-92 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 SANDOZ EAGUL 00904-5892-61 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 MAJOR PHARMACEU EAGUL 16252-0527-90 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 WATSON LABS EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 320LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51079-0458-01 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 MYLAN INSTITUTI EAGUL 51079-0458-20 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 MYLAN INSTITUTI EAGUL 54458-09<strong>08</strong>-02 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 INTERNATIONAL L EAGUL 54458-0926-10 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 INTERNATIONAL L EAGUL 60505-0169-07 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 APOTEX CORP EAGUL 60505-0169-09 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 APOTEX CORP EAGUL 68180-0486-02 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 LUPIN PHARMACEU EAGUL 68180-0486-09 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 LUPIN PHARMACEU EAGUL 68382-0071-05 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 ZYDUS PHARMACEU EAGUL 68382-0071-16 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 68462-0196-05 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 GLENMARK PHARMA EAGUL 68462-0196-90 0.29170 PRAVASTATIN SODIUM 20 MG TAB 0 GLENMARK PHARMA EAGUL 00093-7202-10 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 TEVA USA EAGUL 00093-7202-98 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 TEVA USA EAGUL 00378-0557-77 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 MYLAN EAGUL 00904-5893-61 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 MAJOR PHARMACEU EAGUL 16252-0528-50 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 WATSON LABS EAGUL 16252-0528-90 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 WATSON LABS EAGUL 51079-0782-01 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 MYLAN INSTITUTI EAGUL 51079-0782-20 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 54458-0925-10 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 INTERNATIONAL L EAGUL 55111-0231-05 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 DR.REDDY'S LAB EAGUL 55111-0231-90 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 DR.REDDY'S LAB EAGUL 60505-0170-07 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 APOTEX CORP EAGUL 60505-0170-<strong>08</strong> 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 APOTEX CORP EAGUL 60505-0170-09 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 APOTEX CORP EAGUL 68180-0487-02 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 LUPIN PHARMACEU EAGUL 68180-0487-09 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 LUPIN PHARMACEU EAGUL 68382-0072-05 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 ZYDUS PHARMACEU EAGUL 68382-0072-16 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 68462-0197-05 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 GLENMARK PHARMA EAGUL 68462-0197-90 0.35600 PRAVASTATIN SODIUM 40 MG TAB 0 GLENMARK PHARMA EAGUL 00093-7270-10 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 TEVA USA EAGUL 00093-7270-98 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 TEVA USA EAGUL 00378-0553-77 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 MYLAN EAGUL 55111-0274-05 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 DR.REDDY'S LAB EAGUL 55111-0274-90 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 DR.REDDY'S LAB EAGUL 60505-1323-05 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 APOTEX CORP EAGUL 60505-1323-09 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 APOTEX CORP EAGUL 68180-0488-02 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 68180-0488-09 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 LUPIN PHARMACEU EAGUL 68382-0073-05 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 ZYDUS PHARMACEU EAGUL 68382-0073-16 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 ZYDUS PHARMACEU EAGUL 68462-0198-05 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 GLENMARK PHARMA EAGUL 68462-0198-90 0.57530 PRAVASTATIN SODIUM 80 MG TAB 0 GLENMARK PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 321LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-4<strong>06</strong>7-01 0.15510 PRAZOSIN 1 MG CAPSULE 0 TEVA USA EAGEN 00093-4<strong>06</strong>7-10 0.15510 PRAZOSIN 1 MG CAPSULE 0 TEVA USA EAGEN 00378-1101-01 0.15510 PRAZOSIN 1 MG CAPSULE 0 MYLAN EAGEN 00378-1101-10 0.15510 PRAZOSIN 1 MG CAPSULE 0 MYLAN EAGEN 51079-<strong>06</strong>30-20 0.15510 PRAZOSIN 1 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 00093-4<strong>06</strong>8-01 0.28090 PRAZOSIN 2 MG CAPSULE 0 TEVA USA EAGEN 00093-4<strong>06</strong>8-10 0.28090 PRAZOSIN 2 MG CAPSULE 0 TEVA USA EAGEN 00378-2302-01 0.28090 PRAZOSIN 2 MG CAPSULE 0 MYLAN EAGEN 00378-2302-10 0.28090 PRAZOSIN 2 MG CAPSULE 0 MYLAN EAGEN 51079-<strong>06</strong>31-20 0.28090 PRAZOSIN 2 MG CAPSULE 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-4<strong>06</strong>9-01 0.53055 PRAZOSIN 5 MG CAPSULE 0 TEVA USA EAGEN 00093-4<strong>06</strong>9-05 0.53055 PRAZOSIN 5 MG CAPSULE 0 TEVA USA EAGEN 00093-4<strong>06</strong>9-52 0.53055 PRAZOSIN 5 MG CAPSULE 0 TEVA USA EAGEN 00378-3205-01 0.53055 PRAZOSIN 5 MG CAPSULE 0 MYLAN EAGEN 00378-3205-25 0.53055 PRAZOSIN 5 MG CAPSULE 0 MYLAN EAGEN 51079-<strong>06</strong>32-20 0.49057 PRAZOSIN 5 MG CAPSULE 0 MYLAN INSTITUTI EABND 50419-<strong>08</strong>62-51 0.40500 1.14457 PRECOSE 100 MG TABLET G BAYER,PHARM DIV EABND 50419-<strong>08</strong>63-51 0.26217 0.88793 PRECOSE 25 MG TABLET G BAYER,PHARM DIV EABND 50419-<strong>08</strong>61-51 0.36460 0.95582 PRECOSE 50 MG TABLET G BAYER,PHARM DIV EABUL 11980-0180-01 1.69500 17.72880 PRED FORTE 1% EYE DROPS G ALLERGAN INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 11980-0180-05 1.69500 17.72880 PRED FORTE 1% EYE DROPS G ALLERGAN INC. MLBUL 11980-0180-10 1.69500 17.72880 PRED FORTE 1% EYE DROPS G ALLERGAN INC. MLBUL 11980-0180-15 1.69500 17.72880 PRED FORTE 1% EYE DROPS G ALLERGAN INC. MLBND 11980-0174-05 17.72880 PRED MILD 0.12% EYE DROPS 0 ALLERGAN INC. MLBND 11980-0174-10 17.72880 PRED MILD 0.12% EYE DROPS 0 ALLERGAN INC. MLBND 00023-0<strong>06</strong>6-04 25.32686 PRED-G S.O.P. EYE OINTMENT G ALLERGAN INC. GMBND 00023-01<strong>06</strong>-05 17.72880 PRED-G 1% EYE DROPS G ALLERGAN INC. MLGEN 66993-<strong>08</strong>80-15 0.71380 PREDNICARBATE 0.1% CREAM G PRASCO LABS GMGEN 66993-<strong>08</strong>80-61 0.71380 PREDNICARBATE 0.1% CREAM G PRASCO LABS GMGEN 00168-0410-15 1.13900 PREDNICARBATE 0.1% OINTMENT G SANDOZ GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00168-0410-60 0.92237 PREDNICARBATE 0.1% OINTMENT G SANDOZ GMGUL 60758-0119-05 1.69500 PREDNISOLONE AC 1% EYE DROP 0 PACIFIC PHARMA MLGUL 60758-0119-10 1.69500 PREDNISOLONE AC 1% EYE DROP 0 PACIFIC PHARMA MLGUL 60758-0119-15 1.69500 PREDNISOLONE AC 1% EYE DROP 0 PACIFIC PHARMA MLGUL 61314-<strong>06</strong>37-05 1.69500 PREDNISOLONE AC 1% EYE DROP 0 SANDOZ MLGUL 61314-<strong>06</strong>37-10 1.69500 PREDNISOLONE AC 1% EYE DROP 0 SANDOZ MLGUL 61314-<strong>06</strong>37-15 1.69500 PREDNISOLONE AC 1% EYE DROP 0 SANDOZ MLBND 00178-0582-<strong>08</strong> 0.65139 PREDNISOLONE SOD PH 25 MG/5 ML 0 MISSION PHARM. MLBND 242<strong>08</strong>-0715-10 4.70010 4.97668 PREDNISOLONE SOD 1% EYE DROP 0 VALEANT MLGEN 00093-6118-16 0.04020 PREDNISOLONE 15 MG/5 ML SOLN 0 TEVA USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-6118-87 0.04020 PREDNISOLONE 15 MG/5 ML SOLN 0 TEVA USA MLGEN 00121-0759-<strong>08</strong> 0.07700 PREDNISOLONE 15 MG/5 ML SOLN 0 PHARMACEU ASSOC MLGEN 50383-0042-24 0.04020 PREDNISOLONE 15 MG/5 ML SOLN 0 HI-TECH PHARMAC MLGEN 50383-0042-48 0.04020 PREDNISOLONE 15 MG/5 ML SOLN 0 HI-TECH PHARMAC MLGEN 60432-0212-<strong>08</strong> 0.07700 PREDNISOLONE 15 MG/5 ML SOLN 0 MORTON GROVE PH ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 322LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-1567-56 0.04020 PREDNISOLONE 15 MG/5 ML SYRUP 0 QUALITEST MLGEN 0<strong>06</strong>03-1567-58 0.04020 PREDNISOLONE 15 MG/5 ML SYRUP 0 QUALITEST MLGEN 50383-0040-04 0.48810 PREDNISOLONE 5 MG/5 ML SOLN 0 HI-TECH PHARMAC MLGEN 00054-4741-25 0.10152 PREDNISONE 1 MG TABLET 0 ROXANE LABS. EAGEN 00054-4741-31 0.10152 PREDNISONE 1 MG TABLET 0 ROXANE LABS. EAGEN 00054-8739-25 0.10152 PREDNISONE 1 MG TABLET 0 ROXANE LABS. EAGEN 0<strong>06</strong>03-5335-21 0.10152 PREDNISONE 1 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5335-32 0.10152 PREDNISONE 1 MG TABLET 0 QUALITEST EAGEN 59746-0171-<strong>06</strong> 0.10152 PREDNISONE 1 MG TABLET 0 CADISTA PHARMAC EAGEN 59746-0171-10 0.10152 PREDNISONE 1 MG TABLET 0 CADISTA PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 0<strong>06</strong>03-5338-15 0.18000 0.92486 PREDNISONE 10 MG TAB DOSE PACK 0 QUALITEST EABND 0<strong>06</strong>03-5338-31 0.18000 0.62422 PREDNISONE 10 MG TAB DOSE PACK 0 QUALITEST EAGUL 00054-0017-25 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 ROXANE LABS. EAGUL 00054-0017-29 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 ROXANE LABS. EAGUL 00143-1473-01 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1473-10 0.04725 PREDNISONE 10 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-9739-01 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-9739-10 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 WEST-WARD,INC. EAGUL 00591-5442-01 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00591-5442-05 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00591-5442-10 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 ACTAVIS PHARMA, EAGUL 0<strong>06</strong>03-5338-21 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-5338-28 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-5338-32 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 QUALITEST EAGEN 45802-0303-21 0.18000 PREDNISONE 10 MG TABLET 0 PERRIGO CO. EAGEN 45802-0303-67 0.18000 PREDNISONE 10 MG TABLET 0 PERRIGO CO. EAGUL 59746-0173-<strong>06</strong> 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 CADISTA PHARMAC EAGUL 59746-0173-10 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 CADISTA PHARMAC EAGUL 63739-0519-10 0.<strong>06</strong>150 PREDNISONE 10 MG TABLET 0 MCKESSON PACKAG EAGEN 00054-4742-25 0.11370 PREDNISONE 2.5 MG TABLET 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-8740-25 0.11370 PREDNISONE 2.5 MG TABLET 0 ROXANE LABS. EAGEN 00143-1425-01 0.04837 PREDNISONE 2.5 MG TABLET 0 WEST-WARD,INC. EAGEN 0<strong>06</strong>03-5336-21 0.11370 PREDNISONE 2.5 MG TABLET 0 QUALITEST EAGUL 00054-0018-20 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 ROXANE LABS. EAGUL 00054-0018-25 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 ROXANE LABS. EAGUL 00054-0018-29 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 ROXANE LABS. EAGUL 00143-1477-01 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1477-05 0.<strong>06</strong>270 PREDNISONE 20 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1477-10 0.07593 PREDNISONE 20 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-9738-01 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00143-9738-05 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-9738-10 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 WEST-WARD,INC. EAGUL 00591-5443-01 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00591-5443-05 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00591-5443-10 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 323LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 0<strong>06</strong>03-5339-21 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-5339-28 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-5339-32 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 QUALITEST EAGUL 59746-0175-<strong>06</strong> 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 CADISTA PHARMAC EAGUL 59746-0175-09 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 CADISTA PHARMAC EAGUL 59746-0175-10 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 CADISTA PHARMAC EAGUL 63739-0520-10 0.<strong>08</strong>040 PREDNISONE 20 MG TABLET 0 MCKESSON PACKAG EAGUL 00054-4728-25 0.02030 PREDNISONE 5 MG TABLET 0 ROXANE LABS. EAGUL 00054-4728-31 0.02030 PREDNISONE 5 MG TABLET 0 ROXANE LABS. EAGUL 00054-8724-25 0.02030 PREDNISONE 5 MG TABLET 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00143-1475-01 0.02030 PREDNISONE 5 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-1475-10 0.02030 PREDNISONE 5 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-9740-01 0.02030 PREDNISONE 5 MG TABLET 0 WEST-WARD,INC. EAGUL 00143-9740-10 0.02030 PREDNISONE 5 MG TABLET 0 WEST-WARD,INC. EAGUL 00591-5052-01 0.02030 PREDNISONE 5 MG TABLET 0 ACTAVIS PHARMA, EAGUL 00591-5052-10 0.02030 PREDNISONE 5 MG TABLET 0 ACTAVIS PHARMA, EABND 0<strong>06</strong>03-5337-15 0.22360 0.53357 PREDNISONE 5 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-5337-21 0.02030 PREDNISONE 5 MG TABLET 0 QUALITEST EABND 0<strong>06</strong>03-5337-31 0.22360 0.38716 PREDNISONE 5 MG TABLET 0 QUALITEST EAGUL 0<strong>06</strong>03-5337-32 0.02030 PREDNISONE 5 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0733-21 0.19285 PREDNISONE 5 MG TABLET 0 PERRIGO CO. EAGUL 59746-0172-<strong>06</strong> 0.02030 PREDNISONE 5 MG TABLET 0 CADISTA PHARMAC EAGUL 59746-0172-10 0.02030 PREDNISONE 5 MG TABLET 0 CADISTA PHARMAC EAGUL 63739-0518-10 0.02030 PREDNISONE 5 MG TABLET 0 MCKESSON PACKAG EABND 00054-3721-44 1.28788 PREDNISONE 5 MG/ML SOLUTION G ROXANE LABS. MLBND 00054-3722-50 0.186<strong>06</strong> PREDNISONE 5 MG/5 ML SOLUTION 0 ROXANE LABS. MLBND 00054-3722-63 0.18236 PREDNISONE 5 MG/5 ML SOLUTION 0 ROXANE LABS. MLBND 00054-8722-16 0.22331 PREDNISONE 5 MG/5 ML SOLUTION 0 ROXANE LABS. MLBND 00054-0019-25 0.33781 PREDNISONE 50 MG TABLET 0 ROXANE LABS. EABND 68220-0<strong>08</strong>4-90 2.88249 PREFERA OB TABLET 0 MEDA PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 68220-0<strong>08</strong>6-30 2.89116 PREFERA-OB ONE S<strong>OF</strong>TGEL 0 MEDA PHARMACEUT EABND 51285-0<strong>06</strong>3-90 3.29786 PREFEST TABLET 0 DURAMED/BARR EABND 00052-0315-10 76.15250 PREGNYL 10,000 UNITS VIAL 0 ORGANON PHARM. EABND 00046-<strong>08</strong>72-21 6.67458 PREMARIN VAGINAL CREAM-APPL 0 WYETH PHARM GMBND 00046-1100-81 3.01389 PREMARIN 0.3 MG TABLET 0 WYETH PHARM EABND 00046-1100-91 3.01384 PREMARIN 0.3 MG TABLET 0 WYETH PHARM EABND 00046-1101-81 3.01389 PREMARIN 0.45 MG TABLET 0 WYETH PHARM EABND 00046-1102-81 3.01389 PREMARIN 0.625 MG TABLET 0 WYETH PHARM EABND 00046-1102-91 3.01384 PREMARIN 0.625 MG TABLET 0 WYETH PHARM EABND 00046-1103-81 3.01389 PREMARIN 0.9 MG TABLET 0 WYETH PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00046-1104-81 3.01389 PREMARIN 1.25 MG TABLET 0 WYETH PHARM EABND 00046-1104-91 3.01384 PREMARIN 1.25 MG TABLET 0 WYETH PHARM EABND 00046-0749-05 167.04580 PREMARIN 25 MG VIAL 0 WYETH PHARM EABND 00046-2575-12 3.69646 PREMPHASE 0.625-5 MG TABLET 0 WYETH PHARM EABND 00046-1105-11 3.69735 PREMPRO 0.3 MG-1.5 MG TABLET 0 WYETH PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 324LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00046-11<strong>06</strong>-11 3.69735 PREMPRO 0.45-1.5 MG TABLET 0 WYETH PHARM EABND 00046-1107-11 3.69735 PREMPRO 0.625-2.5 MG TABLET 0 WYETH PHARM EABND 00046-11<strong>08</strong>-11 3.69735 PREMPRO 0.625-5 MG TABLET 0 WYETH PHARM EABND 42192-0364-60 1.40421 PRENAISSANCE NEXT-B TABLET 0 ACELLA PHARMACE EAGEN 64376-<strong>08</strong>16-01 0.10250 PRENATAL PLUS TABLET 0 BOCA PHARMACAL EAGEN 65162-<strong>06</strong>68-10 0.10250 PRENATAL PLUS TABLET 0 AMNEAL PHARMACE EAGEN 42937-07<strong>06</strong>-10 0.22500 PRENATAL 19 TABLET 0 NATIONWIDE LABO EABND 00245-0178-30 1.26650 3.40521 PRENEXA CAPSULE 0 UPSHER SMITH EABND 55566-9300-02 39.56610 PREPOPIK POWDER PACKET G FERRING PH INC EABND 64764-0541-30 2.<strong>06</strong>070 8.34537 PREVACID DR 15 MG CAPSULE G TAKEDA PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64764-0046-13 0.76950 8.34565 PREVACID DR 30 MG CAPSULE G TAKEDA PHARMACE EABND 64764-0543-11 8.34565 PREVACID 15 MG SOLUTAB G TAKEDA PHARMACE EABND 64764-0544-11 8.34565 PREVACID 30 MG SOLUTAB G TAKEDA PHARMACE EAGEN 00245-0036-42 1.10090 PREVALITE PACKET 0 UPSHER SMITH EAGEN 00245-0036-60 1.10090 PREVALITE PACKET 0 UPSHER SMITH EAGEN 00245-0036-23 0.26973 PREVALITE POWDER 0 UPSHER SMITH GMGEX 0<strong>06</strong>03-7642-17 0.49260 PREVIFEM TABLET 0 QUALITEST EABND 64764-0702-01 5.20232 PREVPAC PATIENT PACK 0 TAKEDA PHARMACE EABND 59676-0565-01 3.22604 PREZISTA 100 MG/ML SUSPENSION G JANSSEN PRODUCT MLBND 59676-0564-01 4.83907 PREZISTA 150 MG TABLET G JANSSEN PRODUCT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59676-0561-01 19.35628 PREZISTA 400 MG TABLET G JANSSEN PRODUCT EABND 59676-0562-01 19.35628 PREZISTA 600 MG TABLET G JANSSEN PRODUCT EABND 59676-0563-01 2.41953 PREZISTA 75 MG TABLET G JANSSEN PRODUCT EABND 59676-0566-30 38.71258 PREZISTA 800 MG TABLET G JANSSEN PRODUCT EABEX 00<strong>08</strong>8-2100-03 3.78324 PRIFTIN 150 MG TABLET 0 SAN<strong>OF</strong>I-AVENTIS EABND 00186-<strong>06</strong><strong>06</strong>-31 0.26514 6.<strong>08</strong>058 PRILOSEC DR 10 MG CAPSULE G ASTRAZENECA EABND 00186-<strong>06</strong><strong>06</strong>-82 0.26514 5.68716 PRILOSEC DR 10 MG CAPSULE G ASTRAZENECA EABND 00186-<strong>06</strong>10-01 6.<strong>08</strong>058 PRILOSEC DR 10 MG SUSPENSION G ASTRAZENECA EABND 00186-<strong>06</strong>25-01 6.<strong>08</strong>058 PRILOSEC DR 2.5 MG SUSPENSION G ASTRAZENECA EABND 00186-0742-31 0.<strong>08</strong>735 6.78718 PRILOSEC DR 20 MG CAPSULE G ASTRAZENECA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00186-0742-82 0.<strong>08</strong>735 6.78662 PRILOSEC DR 20 MG CAPSULE G ASTRAZENECA EABND 00186-0743-31 0.29260 10.02999 PRILOSEC DR 40 MG CAPSULE G ASTRAZENECA EABND 00186-0743-68 0.29260 10.03121 PRILOSEC DR 40 MG CAPSULE G ASTRAZENECA EABND 00186-0743-82 0.29260 9.37476 PRILOSEC DR 40 MG CAPSULE G ASTRAZENECA EABND 00024-1596-01 1.67776 PRIMAQUINE 26.3 MG TABLET 0 SAN<strong>OF</strong>I-AVENTIS EABND 000<strong>06</strong>-3514-58 5.14500 17.27694 PRIMAXIN 250 MG VIAL 0 MERCK SHARP & D EABND 000<strong>06</strong>-3551-58 18.28257 PRIMAXIN 250 MG VIAL 0 MERCK SHARP & D EABND 000<strong>06</strong>-3516-59 11.13000 32.52072 PRIMAXIN 500 MG VIAL 0 MERCK SHARP & D EABND 000<strong>06</strong>-3552-59 33.79262 PRIMAXIN 500 MG VIAL 0 MERCK SHARP & D EAGEX 00115-1031-01 0.18660 PRIMIDONE 250 MG TABLET 0 GLOBAL PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00115-1031-03 0.18660 PRIMIDONE 250 MG TABLET 0 GLOBAL PHARM EAGEX 00143-1484-01 0.18660 PRIMIDONE 250 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-1484-10 0.18660 PRIMIDONE 250 MG TABLET 0 WEST-WARD,INC. EAGEX 00527-1231-01 0.18660 PRIMIDONE 250 MG TABLET 0 LANNETT CO. INC EAGEX 00527-1231-10 0.18660 PRIMIDONE 250 MG TABLET 0 LANNETT CO. INC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 325LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00591-5321-01 0.18660 PRIMIDONE 250 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-5321-10 0.18660 PRIMIDONE 250 MG TABLET 0 ACTAVIS PHARMA, EAGEX 0<strong>06</strong>03-5372-21 0.18660 PRIMIDONE 250 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-5372-28 0.18660 PRIMIDONE 250 MG TABLET 0 QUALITEST EAGEX 65162-0545-10 0.18660 PRIMIDONE 250 MG TABLET 0 AMNEAL PHARMACE EAGEX 68<strong>08</strong>4-0203-01 0.18660 PRIMIDONE 250 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0203-11 0.18660 PRIMIDONE 250 MG TABLET 0 AHP EAGEX 00115-1030-01 0.09520 PRIMIDONE 50 MG TABLET 0 GLOBAL PHARM EAGEX 00115-1030-02 0.09520 PRIMIDONE 50 MG TABLET 0 GLOBAL PHARM EAGEX 00143-1482-01 0.09520 PRIMIDONE 50 MG TABLET 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00143-1482-05 0.09520 PRIMIDONE 50 MG TABLET 0 WEST-WARD,INC. EAGEX 00527-1301-01 0.09520 PRIMIDONE 50 MG TABLET 0 LANNETT CO. INC EAGEX 00527-1301-05 0.09520 PRIMIDONE 50 MG TABLET 0 LANNETT CO. INC EAGEX 0<strong>06</strong>03-5371-21 0.09520 PRIMIDONE 50 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-5371-28 0.09520 PRIMIDONE 50 MG TABLET 0 QUALITEST EAGEX 00904-5559-60 0.09520 PRIMIDONE 50 MG TABLET 0 MAJOR PHARMACEU EAGEX 65162-0544-10 0.09520 PRIMIDONE 50 MG TABLET 0 AMNEAL PHARMACE EAGEX 65162-0544-50 0.09520 PRIMIDONE 50 MG TABLET 0 AMNEAL PHARMACE EAGEX 68<strong>08</strong>4-0202-01 0.09520 PRIMIDONE 50 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0202-11 0.09520 PRIMIDONE 50 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 13551-0501-05 0.63171 PRIMSOL 50 MG/5 ML ORAL SOLN 0 FSC LABS MLBND 000<strong>06</strong>-01<strong>06</strong>-54 0.02687 1.26593 PRINIVIL 10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0207-54 0.03310 1.35502 PRINIVIL 20 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0019-54 0.01958 1.22600 PRINIVIL 5 MG TABLET G MERCK SHARP & D EABEX 000<strong>08</strong>-1222-01 6.41866 PRISTIQ ER 100 MG TABLET G WYETH PHARM EABEX 000<strong>08</strong>-1222-14 6.41827 PRISTIQ ER 100 MG TABLET G WYETH PHARM EABEX 000<strong>08</strong>-1222-30 6.41894 PRISTIQ ER 100 MG TABLET G WYETH PHARM EABEX 000<strong>08</strong>-1211-01 6.41866 PRISTIQ ER 50 MG TABLET G WYETH PHARM EABEX 000<strong>08</strong>-1211-14 6.41827 PRISTIQ ER 50 MG TABLET G WYETH PHARM EABEX 000<strong>08</strong>-1211-30 6.41894 PRISTIQ ER 50 MG TABLET G WYETH PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 442<strong>06</strong>-0436-05 11.91216 PRIVIGEN 10% VIAL 0 CSL BEHRING LLC MLBND 442<strong>06</strong>-0437-10 11.91216 PRIVIGEN 10% VIAL 0 CSL BEHRING LLC MLBND 442<strong>06</strong>-0438-20 11.91216 PRIVIGEN 10% VIAL 0 CSL BEHRING LLC MLBND 59310-0579-20 5.21630 PROAIR HFA 90 MCG INHALER 0 TEVA SPECIALTY GMBND 59310-0579-22 5.52975 PROAIR HFA 90 MCG INHALER 0 TEVA SPECIALTY GMGEN 00378-0156-01 0.6<strong>06</strong>87 PROBENECID 500 MG TABLET 0 MYLAN EAGEN 00527-1367-01 0.6<strong>06</strong>87 PROBENECID 500 MG TABLET 0 LANNETT CO. INC EAGEN 00527-1367-10 0.60441 PROBENECID 500 MG TABLET 0 LANNETT CO. INC EAGEN 00591-5347-01 0.6<strong>06</strong>87 PROBENECID 500 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5347-10 0.60441 PROBENECID 500 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-5325-01 0.67150 PROBENECID-COLCHICINE TABS 0 ACTAVIS PHARMA, EAGEN 64980-0149-01 0.67150 PROBENECID-COLCHICINE TABS 0 RISING PHARM EABND 00<strong>06</strong>9-2650-66 0.4<strong>08</strong>20 3.13233 PROCARDIA XL 30 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2650-72 0.4<strong>08</strong>20 3.13239 PROCARDIA XL 30 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2660-66 0.59240 5.42039 PROCARDIA XL 60 MG TABLET G PFIZER US PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 326LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>9-2660-72 0.59240 5.42050 PROCARDIA XL 60 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2670-66 1.03275 6.25405 PROCARDIA XL 90 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-2600-66 0.86986 1.39597 PROCARDIA 10 MG CAPSULE G PFIZER US PHARM EAGEN 00093-9652-01 0.07182 PROCHLORPERAZINE 10 MG TAB 0 TEVA USA EAGEN 00378-5110-01 0.07182 PROCHLORPERAZINE 10 MG TAB 0 MYLAN EAGEN 00781-5021-01 0.07182 PROCHLORPERAZINE 10 MG TAB 0 SANDOZ EAGEN 23155-0294-42 2.25400 PROCHLORPERAZINE 10 MG/2 ML VL 0 HERITAGE PHARMA MLGEN 55390-0077-10 2.07000 PROCHLORPERAZINE 10 MG/2 ML VL 0 BEDFORD LABS MLGEN 00713-0135-12 10.04812 PROCHLORPERAZINE 25 MG SUPP 0 G & W LABS. EAGEN 00093-9643-01 0.<strong>06</strong>0<strong>08</strong> PROCHLORPERAZINE 5 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5105-01 0.<strong>06</strong>0<strong>08</strong> PROCHLORPERAZINE 5 MG TABLET 0 MYLAN EAGEN 00781-5020-01 0.<strong>06</strong>0<strong>08</strong> PROCHLORPERAZINE 5 MG TABLET 0 SANDOZ EABND 55390-0077-01 2.31570 PROCHLORPERAZINE 5 MG/ML VIAL 0 BEDFORD LABS MLBND 59676-0310-01 201.98880 PROCRIT 10,000 UNITS/ML VIAL 0 JANSSEN PRODUCT MLBND 59676-0310-02 201.98880 PROCRIT 10,000 UNITS/ML VIAL 0 JANSSEN PRODUCT MLBND 59676-0312-04 201.98880 PROCRIT 10,000 UNITS/ML VIAL 0 JANSSEN PRODUCT MLBND 59676-0302-01 40.39748 PROCRIT 2,000 UNITS/ML VIAL 0 JANSSEN PRODUCT MLBND 59676-0320-00 403.97760 PROCRIT 20,000 UNITS/ML VIAL 0 JANSSEN PRODUCT MLBND 59676-0320-04 403.97760 PROCRIT 20,000 UNITS/ML VIAL 0 JANSSEN PRODUCT MLBND 59676-0303-01 60.59691 PROCRIT 3,000 UNITS/ML VIAL 0 JANSSEN PRODUCT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59676-0304-01 80.79496 PROCRIT 4,000 UNITS/ML VIAL 0 JANSSEN PRODUCT MLBND 59676-0340-01 807.95520 PROCRIT 40,000 UNITS/ML VIAL 0 JANSSEN PRODUCT MLGEN 1<strong>06</strong>31-0407-01 0.52130 PROCTOSOL-HC 2.5% CREAM 0 RANBAXY LABORAT GMGEN 64980-0301-30 0.10030 PROCTOZONE-HC 2.5% CREAM 0 RISING PHARM GMBND 49663-0001-<strong>06</strong> 62.00100 PROCYSBI DR 25 MG CAPSULE G RAPTOR PHARMACE EABND 49663-0002-25 62.00100 PROCYSBI DR 75 MG CAPSULE G RAPTOR PHARMACE EABND 68516-3202-02 0.93500 PR<strong>OF</strong>ILNINE SD 1,000 UNITS VIAL 0 GRIFOLSBND 68516-3203-02 0.93500 PR<strong>OF</strong>ILNINE SD 1,500 UNITS VIAL 0 GRIFOLSBND 68516-3201-01 0.93500 PR<strong>OF</strong>ILNINE SD 500 UNITS VIAL 0 GRIFOLSGEN 38779-0043-<strong>08</strong> 9.26250 PROGESTERONE MICRONIZED POWDER 0 MEDISCA INC. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-9725-01 2.09925 PROGESTERONE OIL 50 MG/ML VL 0 WEST-WARD,INC. MLGEN 00517-0750-01 2.09925 PROGESTERONE OIL 50 MG/ML VL 0 AMER. REGENT MLGEN 00591-3128-79 2.09925 PROGESTERONE OIL 50 MG/ML VL 0 ACTAVIS PHARMA, MLGEN 63323-0261-10 2.09925 PROGESTERONE OIL 50 MG/ML VL 0 APP PHARMACEUTI MLGEN 00093-5353-01 1.59877 PROGESTERONE 100 MG CAPSULE 0 TEVA USA EAGEN 00591-3964-01 1.59877 PROGESTERONE 100 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 17478-0766-10 1.57500 PROGESTERONE 100 MG CAPSULE 0 AKORN INC. EAGEN 00093-5354-01 3.03720 PROGESTERONE 200 MG CAPSULE 0 TEVA USA EAGEN 00591-3965-01 3.03720 PROGESTERONE 200 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 17478-0767-10 3.00000 PROGESTERONE 200 MG CAPSULE 0 AKORN INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00575-6200-30 7.83160 PROGLYCEM 50 MG/ML ORAL SUSP 0 TEVA SPECIALTY MLBND 00469-<strong>06</strong>07-73 1.97407 1.97407 PROGRAF 0.5 MG CAPSULE 0 ASTELLAS PHARMA EABND 00469-<strong>06</strong>17-11 3.94814 3.94814 PROGRAF 1 MG CAPSULE 0 ASTELLAS PHARMA EABND 00469-<strong>06</strong>17-73 3.94814 3.94814 PROGRAF 1 MG CAPSULE 0 ASTELLAS PHARMA EABND 21695-0170-00 7.9<strong>08</strong>90 7.9<strong>08</strong>90 PROGRAF 1 MG CAPSULE 0 PHYSICIAN PARTN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 327LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00469-<strong>06</strong>57-73 19.74072 19.74072 PROGRAF 5 MG CAPSULE 0 ASTELLAS PHARMA EABND 00469-3016-01 136.07352 PROGRAF 5 MG/ML AMPULE 0 ASTELLAS PHARMA MLBND 13533-0700-01 0.41500 PROLASTIN C 1,000 MG VIAL 0 GRIFOLS THERAPE EABND 13533-0700-02 0.41500 PROLASTIN C 1,000 MG VIAL 0 GRIFOLS THERAPE EABND 242<strong>08</strong>-<strong>06</strong>02-01 115.77981 PROLENSA 0.07% EYE DROPS G VALEANT MLBND 242<strong>08</strong>-<strong>06</strong>02-03 61.74923 PROLENSA 0.07% EYE DROPS G VALEANT MLBND 65483-0116-07 1804.54450 PROLEUKIN 22 MILLION UNIT VIAL 0 PROMETHEUS EABND 00007-4643-13 89.63059 PROMACTA 12.5 MG TABLET 0 GLAXOSMITHKLINE EABND 00007-4640-13 89.63059 PROMACTA 25 MG TABLET 0 GLAXOSMITHKLINE EABND 00007-4641-13 179.26146 PROMACTA 50 MG TABLET 0 GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00007-4642-13 268.89205 PROMACTA 75 MG TABLET 0 GLAXOSMITHKLINE EAGUL 45802-0758-30 0.96120 PROMETHAZINE 12.5 MG SUPPOS 0 PERRIGO CO. EAGEN 0<strong>06</strong>03-5437-21 0.<strong>08</strong>033 PROMETHAZINE 12.5 MG TABLET 0 QUALITEST EAGEN 10702-0002-01 0.<strong>08</strong>033 PROMETHAZINE 12.5 MG TABLET 0 KVK-TECH, INC. EAGEN 57664-0107-88 0.<strong>08</strong>033 PROMETHAZINE 12.5 MG TABLET 0 CARACO PHARM EAGEN 65162-0745-10 0.<strong>08</strong>033 PROMETHAZINE 12.5 MG TABLET 0 AMNEAL PHARMACE EAGEN 68001-0161-00 0.<strong>08</strong>033 PROMETHAZINE 12.5 MG TABLET 0 BLUEPOINT LABOR EAGEN 68<strong>08</strong>4-0154-01 0.<strong>08</strong>033 PROMETHAZINE 12.5 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0154-11 0.<strong>08</strong>033 PROMETHAZINE 12.5 MG TABLET 0 AHP EAGEN 68382-0040-01 0.<strong>08</strong>033 PROMETHAZINE 12.5 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 45802-0759-30 1.03620 PROMETHAZINE 25 MG SUPPOSITORY 0 PERRIGO CO. EAGEN 00591-5307-01 0.11210 PROMETHAZINE 25 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5307-10 0.11210 PROMETHAZINE 25 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-5438-21 0.11210 PROMETHAZINE 25 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5438-30 0.11210 PROMETHAZINE 25 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5438-32 0.11210 PROMETHAZINE 25 MG TABLET 0 QUALITEST EAGEN 00781-1830-01 0.11210 PROMETHAZINE 25 MG TABLET 0 SANDOZ EAGEN 00781-1830-10 0.11210 PROMETHAZINE 25 MG TABLET 0 SANDOZ EAGEN 00904-5840-61 0.11210 PROMETHAZINE 25 MG TABLET 0 MAJOR PHARMACEU EAGEN 10702-0003-01 0.11210 PROMETHAZINE 25 MG TABLET 0 KVK-TECH, INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10702-0003-10 0.11210 PROMETHAZINE 25 MG TABLET 0 KVK-TECH, INC. EAGEN 57664-01<strong>08</strong>-18 0.11210 PROMETHAZINE 25 MG TABLET 0 CARACO PHARM EAGEN 57664-01<strong>08</strong>-88 0.11210 PROMETHAZINE 25 MG TABLET 0 CARACO PHARM EAGEN 63739-0213-10 0.11210 PROMETHAZINE 25 MG TABLET 0 MCKESSON PACKAG EAGEN 65162-0521-10 0.11210 PROMETHAZINE 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0521-11 0.11210 PROMETHAZINE 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 68001-0162-00 0.11210 PROMETHAZINE 25 MG TABLET 0 BLUEPOINT LABOR EAGEN 68001-0162-<strong>08</strong> 0.11210 PROMETHAZINE 25 MG TABLET 0 BLUEPOINT LABOR EAGEN 68<strong>08</strong>4-0155-01 0.11210 PROMETHAZINE 25 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0155-11 0.11210 PROMETHAZINE 25 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0041-01 0.11210 PROMETHAZINE 25 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0041-10 0.11210 PROMETHAZINE 25 MG TABLET 0 ZYDUS PHARMACEU EAGEX 0<strong>06</strong>41-0948-31 0.75750 PROMETHAZINE 25 MG/ML AMPUL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-0948-35 0.75600 PROMETHAZINE 25 MG/ML AMPUL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-1495-31 0.99010 PROMETHAZINE 25 MG/ML AMPUL 0 WEST-WARD,INC. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 328LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>41-1495-35 0.99010 PROMETHAZINE 25 MG/ML AMPUL 0 WEST-WARD,INC. MLBND 00409-2312-02 1.04580 PROMETHAZINE 25 MG/ML SYRINGE 0 HOSPIRA MLGEX 0<strong>06</strong>41-0928-21 0.77250 PROMETHAZINE 25 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-0928-25 0.77400 PROMETHAZINE 25 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-0955-21 0.77250 PROMETHAZINE 25 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-0955-25 0.77400 PROMETHAZINE 25 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 00703-2191-04 0.84280 PROMETHAZINE 25 MG/ML VIAL 0 TEVA PARENTERAL MLGEN 0<strong>06</strong>03-5439-21 0.25380 PROMETHAZINE 50 MG TABLET 0 QUALITEST EAGEN 00781-1832-01 0.25380 PROMETHAZINE 50 MG TABLET 0 SANDOZ EAGEN 10702-0004-01 0.25380 PROMETHAZINE 50 MG TABLET 0 KVK-TECH, INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 57664-0109-88 0.25380 PROMETHAZINE 50 MG TABLET 0 CARACO PHARM EAGEN 63739-0389-10 0.25380 PROMETHAZINE 50 MG TABLET 0 MCKESSON PACKAG EAGEN 65162-0522-10 0.25380 PROMETHAZINE 50 MG TABLET 0 AMNEAL PHARMACE EAGEN 68001-0163-00 0.25380 PROMETHAZINE 50 MG TABLET 0 BLUEPOINT LABOR EAGEN 68382-0042-01 0.25380 PROMETHAZINE 50 MG TABLET 0 ZYDUS PHARMACEU EAGEN 0<strong>06</strong>41-0949-35 1.58700 PROMETHAZINE 50 MG/ML AMPUL 0 BAXTER/NOVAPLUS MLGEN 0<strong>06</strong>41-1496-31 1.58700 PROMETHAZINE 50 MG/ML AMPUL 0 WEST-WARD,INC. MLGEN 0<strong>06</strong>41-1496-35 1.58700 PROMETHAZINE 50 MG/ML AMPUL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-0929-25 1.47<strong>08</strong>0 PROMETHAZINE 50 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-0956-25 1.47<strong>08</strong>0 PROMETHAZINE 50 MG/ML VIAL 0 WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00703-2201-04 1.47<strong>08</strong>0 PROMETHAZINE 50 MG/ML VIAL 0 TEVA PARENTERAL MLGEN 0<strong>06</strong>03-1584-54 0.01512 PROMETHAZINE 6.25 MG/5 ML SYRP 0 QUALITEST MLGEN 0<strong>06</strong>03-1584-58 0.01512 PROMETHAZINE 6.25 MG/5 ML SYRP 0 QUALITEST MLGEN 50383-<strong>08</strong>01-16 0.01512 PROMETHAZINE 6.25 MG/5 ML SYRP 0 HI-TECH PHARMAC MLGEN 57664-0146-31 0.01512 PROMETHAZINE 6.25 MG/5 ML SYRP 0 CARACO PHARM MLGEN 57664-0146-34 0.01512 PROMETHAZINE 6.25 MG/5 ML SYRP 0 CARACO PHARM MLGEN 60432-<strong>06</strong><strong>08</strong>-16 0.01512 PROMETHAZINE 6.25 MG/5 ML SYRP 0 MORTON GROVE PH MLGUL 00713-0536-12 0.96120 PROMETHEGAN 12.5 MG SUPPOS 0 G & W LABS. EAGUL 00713-0526-10 1.03620 PROMETHEGAN 25 MG SUPP 0 G & W LABS. EAGUL 00713-0526-12 1.03620 PROMETHEGAN 25 MG SUPP 0 G & W LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00713-0132-12 29.68495 PROMETHEGAN 50 MG SUPPOSITORY 0 G & W LABS. EABND 00032-17<strong>08</strong>-01 1.65770 2.53838 PROMETRIUM 100 MG CAPSULE 0 ABBVIE US LLC EABND 00032-1711-01 3.13190 4.82205 PROMETRIUM 200 MG CAPSULE 0 ABBVIE US LLC EAGEN 49884-0099-05 5.12239 PROPAFENONE HCL ER 225 MG CAP 0 PAR PHARM. EAGEN 49884-0099-09 5.12233 PROPAFENONE HCL ER 225 MG CAP 0 PAR PHARM. EAGEN 49884-0210-02 6.33940 PROPAFENONE HCL SR 325 MG CAP 0 PAR PHARM. EAGEN 49884-0210-05 6.33940 PROPAFENONE HCL SR 325 MG CAP 0 PAR PHARM. EAGEN 49884-0210-09 6.33940 PROPAFENONE HCL SR 325 MG CAP 0 PAR PHARM. EAGEN 49884-0211-02 6.48962 PROPAFENONE HCL SR 425 MG CAP 0 PAR PHARM. EAGEN 49884-0211-05 6.48964 PROPAFENONE HCL SR 425 MG CAP 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0211-09 6.48958 PROPAFENONE HCL SR 425 MG CAP 0 PAR PHARM. EAGEN 00591-0582-01 0.22140 PROPAFENONE HCL 150 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-5448-21 0.22140 PROPAFENONE HCL 150 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5448-25 0.22140 PROPAFENONE HCL 150 MG TABLET 0 QUALITEST EAGEN 51079-0996-01 0.22140 PROPAFENONE HCL 150 MG TABLET 0 MYLAN INSTITUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 329LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53489-0551-01 0.22140 PROPAFENONE HCL 150 MG TABLET 0 MUTUAL PHARM CO EAGEN 63739-0509-10 0.22140 PROPAFENONE HCL 150 MG TABLET 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0361-01 0.22140 PROPAFENONE HCL 150 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0361-11 0.22140 PROPAFENONE HCL 150 MG TABLET 0 AHP EAGEN 00591-0583-01 0.39474 PROPAFENONE HCL 225 MG TAB 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-5449-21 0.39474 PROPAFENONE HCL 225 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-5449-25 0.39474 PROPAFENONE HCL 225 MG TAB 0 QUALITEST EAGEN 53489-0552-01 0.39474 PROPAFENONE HCL 225 MG TAB 0 MUTUAL PHARM CO EAGEN 63739-0510-10 0.39474 PROPAFENONE HCL 225 MG TAB 0 MCKESSON PACKAG EAGEN 0<strong>06</strong>03-5450-21 1.34096 PROPAFENONE HCL 300 MG TAB 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53489-0553-01 1.34096 PROPAFENONE HCL 300 MG TAB 0 MUTUAL PHARM CO EABND 00054-4721-25 0.66748 PROPANTHELINE 15 MG TABLET 0 ROXANE LABS. EAGEN 17478-0263-12 0.56250 PROPARACAINE 0.5% EYE DROPS 0 AKORN INC. MLGEN 242<strong>08</strong>-0730-<strong>06</strong> 0.74530 PROPARACAINE 0.5% EYE DROPS 0 VALEANT MLGEN 61314-0016-01 0.74530 PROPARACAINE 0.5% EYE DROPS 0 SANDOZ MLGEN 00228-2780-11 1.31963 PROPRANOLOL ER 120 MG CAPSULE G ACTAVIS PHARMA, EAGEN 00228-2780-50 1.31963 PROPRANOLOL ER 120 MG CAPSULE G ACTAVIS PHARMA, EAGEN 00245-0<strong>08</strong>6-10 1.31963 PROPRANOLOL ER 120 MG CAPSULE G UPSHER SMITH EAGEN 00245-0<strong>08</strong>6-11 1.31963 PROPRANOLOL ER 120 MG CAPSULE G UPSHER SMITH EAGEN 00378-6220-01 1.31963 PROPRANOLOL ER 120 MG CAPSULE G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6220-05 1.31963 PROPRANOLOL ER 120 MG CAPSULE G MYLAN EAGEN 43478-0902-88 1.31963 PROPRANOLOL ER 120 MG CAPSULE G ROUSES POINT PH EAGEN 51991-<strong>08</strong>19-01 1.31963 PROPRANOLOL ER 120 MG CAPSULE G BRECKENRIDGE EAGEN 00228-2781-11 1.62650 PROPRANOLOL ER 160 MG CAPSULE G ACTAVIS PHARMA, EAGEN 00228-2781-50 1.62650 PROPRANOLOL ER 160 MG CAPSULE G ACTAVIS PHARMA, EAGEN 00245-0<strong>08</strong>7-10 1.62650 PROPRANOLOL ER 160 MG CAPSULE G UPSHER SMITH EAGEN 00245-0<strong>08</strong>7-11 1.62650 PROPRANOLOL ER 160 MG CAPSULE G UPSHER SMITH EAGEN 00378-6260-01 1.62650 PROPRANOLOL ER 160 MG CAPSULE G MYLAN EAGEN 00378-6260-05 1.62650 PROPRANOLOL ER 160 MG CAPSULE G MYLAN EAGEN 43478-0903-88 1.62650 PROPRANOLOL ER 160 MG CAPSULE G ROUSES POINT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51991-<strong>08</strong>20-01 1.62650 PROPRANOLOL ER 160 MG CAPSULE G BRECKENRIDGE EAGEN 00228-2778-11 0.80784 PROPRANOLOL ER 60 MG CAPSULE G ACTAVIS PHARMA, EAGEN 00245-0<strong>08</strong>4-10 0.80784 PROPRANOLOL ER 60 MG CAPSULE G UPSHER SMITH EAGEN 00245-0<strong>08</strong>4-11 0.80784 PROPRANOLOL ER 60 MG CAPSULE G UPSHER SMITH EAGEN 00378-6160-01 0.80784 PROPRANOLOL ER 60 MG CAPSULE G MYLAN EAGEN 00378-6160-05 0.80784 PROPRANOLOL ER 60 MG CAPSULE G MYLAN EAGEN 43478-0900-88 0.80784 PROPRANOLOL ER 60 MG CAPSULE G ROUSES POINT PH EAGEN 51991-<strong>08</strong>17-01 0.80784 PROPRANOLOL ER 60 MG CAPSULE G BRECKENRIDGE EAGEN 00228-2779-11 1.<strong>06</strong>529 PROPRANOLOL ER 80 MG CAPSULE G ACTAVIS PHARMA, EAGEN 00228-2779-50 1.<strong>06</strong>529 PROPRANOLOL ER 80 MG CAPSULE G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00245-0<strong>08</strong>5-10 1.<strong>06</strong>529 PROPRANOLOL ER 80 MG CAPSULE G UPSHER SMITH EAGEN 00245-0<strong>08</strong>5-11 1.<strong>06</strong>529 PROPRANOLOL ER 80 MG CAPSULE G UPSHER SMITH EAGEN 00378-6180-01 1.<strong>06</strong>529 PROPRANOLOL ER 80 MG CAPSULE G MYLAN EAGEN 00378-6180-05 1.<strong>06</strong>529 PROPRANOLOL ER 80 MG CAPSULE G MYLAN EAGEN 43478-0901-88 1.<strong>06</strong>529 PROPRANOLOL ER 80 MG CAPSULE G ROUSES POINT PH EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 330LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51991-<strong>08</strong>18-01 1.<strong>06</strong>529 PROPRANOLOL ER 80 MG CAPSULE G BRECKENRIDGE EAGEN 00143-9872-01 7.50000 PROPRANOLOL 1 MG/ML VIAL 0 WEST-WARD,INC. MLGEN 00781-3777-95 7.38750 PROPRANOLOL 1 MG/ML VIAL 0 SANDOZ MLGEN 63323-<strong>06</strong>04-01 10.70250 PROPRANOLOL 1 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 00378-0182-01 0.02110 PROPRANOLOL 10 MG TABLET 0 MYLAN EAGEN 00378-0182-10 0.02110 PROPRANOLOL 10 MG TABLET 0 MYLAN EAGEN 00591-5554-01 0.02110 PROPRANOLOL 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5554-10 0.02110 PROPRANOLOL 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-5482-21 0.02110 PROPRANOLOL 10 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5482-32 0.02110 PROPRANOLOL 10 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0021-04 0.02110 PROPRANOLOL 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0021-<strong>06</strong> 0.02110 PROPRANOLOL 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0021-10 0.02110 PROPRANOLOL 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0021-12 0.02110 PROPRANOLOL 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 23155-0110-01 0.02110 PROPRANOLOL 10 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0110-10 0.02110 PROPRANOLOL 10 MG TABLET 0 HERITAGE PHARMA EAGEN 50111-0467-01 0.02110 PROPRANOLOL 10 MG TABLET 0 PLIVA, INC EAGEN 50111-0467-03 0.02110 PROPRANOLOL 10 MG TABLET 0 PLIVA, INC EAGEN 51079-0277-01 0.02110 PROPRANOLOL 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0277-20 0.02110 PROPRANOLOL 10 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0183-01 0.02230 PROPRANOLOL 20 MG TABLET 0 MYLAN EAGEN 00378-0183-10 0.02230 PROPRANOLOL 20 MG TABLET 0 MYLAN EAGEN 00591-5555-01 0.02230 PROPRANOLOL 20 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5555-10 0.02230 PROPRANOLOL 20 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-5483-02 0.02230 PROPRANOLOL 20 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5483-21 0.02230 PROPRANOLOL 20 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5483-32 0.02230 PROPRANOLOL 20 MG TABLET 0 QUALITEST EAGEN 16714-0022-04 0.02230 PROPRANOLOL 20 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0022-<strong>06</strong> 0.02230 PROPRANOLOL 20 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0022-10 0.02230 PROPRANOLOL 20 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0022-12 0.02230 PROPRANOLOL 20 MG TABLET 0 NORTHSTAR RX LL EAGEN 23155-0111-01 0.02230 PROPRANOLOL 20 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0111-10 0.02230 PROPRANOLOL 20 MG TABLET 0 HERITAGE PHARMA EAGEN 50111-0468-01 0.02230 PROPRANOLOL 20 MG TABLET 0 PLIVA, INC EAGEN 50111-0468-03 0.02230 PROPRANOLOL 20 MG TABLET 0 PLIVA, INC EAGEN 51079-0278-20 0.02230 PROPRANOLOL 20 MG TABLET 0 MYLAN INSTITUTI EABND 00054-3727-63 0.<strong>08</strong>876 PROPRANOLOL 20 MG/5 ML SOLN G ROXANE LABS. MLGEN 00378-0184-01 0.02660 PROPRANOLOL 40 MG TABLET 0 MYLAN EAGEN 00378-0184-10 0.02660 PROPRANOLOL 40 MG TABLET 0 MYLAN EAGEN 00591-5556-01 0.02660 PROPRANOLOL 40 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-5556-10 0.02660 PROPRANOLOL 40 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-5484-21 0.02660 PROPRANOLOL 40 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5484-32 0.02660 PROPRANOLOL 40 MG TABLET 0 QUALITEST EAGEN 16714-0023-04 0.02660 PROPRANOLOL 40 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0023-<strong>06</strong> 0.02660 PROPRANOLOL 40 MG TABLET 0 NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 331LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0023-10 0.02660 PROPRANOLOL 40 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0023-12 0.02660 PROPRANOLOL 40 MG TABLET 0 NORTHSTAR RX LL EAGEN 23155-0112-01 0.02660 PROPRANOLOL 40 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0112-10 0.02660 PROPRANOLOL 40 MG TABLET 0 HERITAGE PHARMA EAGEN 50111-0469-01 0.02660 PROPRANOLOL 40 MG TABLET 0 PLIVA, INC EAGEN 50111-0469-03 0.02660 PROPRANOLOL 40 MG TABLET 0 PLIVA, INC EAGEN 51079-0279-20 0.02660 PROPRANOLOL 40 MG TABLET 0 MYLAN INSTITUTI EABND 00054-3730-63 0.12682 PROPRANOLOL 40 MG/5 ML SOLN G ROXANE LABS. MLGEN 00378-0187-01 0.55170 PROPRANOLOL 60 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-5485-21 0.55170 PROPRANOLOL 60 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0024-04 0.55170 PROPRANOLOL 60 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0024-10 0.55170 PROPRANOLOL 60 MG TABLET 0 NORTHSTAR RX LL EAGEN 23155-0113-01 0.55170 PROPRANOLOL 60 MG TABLET 0 HERITAGE PHARMA EAGEN 50111-0470-01 0.55170 PROPRANOLOL 60 MG TABLET 0 TEVA USA EAGEN 00378-0185-01 0.04240 PROPRANOLOL 80 MG TABLET 0 MYLAN EAGEN 00378-0185-05 0.04240 PROPRANOLOL 80 MG TABLET 0 MYLAN EAGEN 00591-5557-01 0.04240 PROPRANOLOL 80 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5557-05 0.04240 PROPRANOLOL 80 MG TABLET 0 ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-5486-21 0.04240 PROPRANOLOL 80 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5486-28 0.04240 PROPRANOLOL 80 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0025-04 0.04240 PROPRANOLOL 80 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0025-05 0.04240 PROPRANOLOL 80 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0025-10 0.04240 PROPRANOLOL 80 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0025-11 0.04240 PROPRANOLOL 80 MG TABLET 0 NORTHSTAR RX LL EAGEN 23155-0114-01 0.04240 PROPRANOLOL 80 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0114-05 0.04240 PROPRANOLOL 80 MG TABLET 0 HERITAGE PHARMA EAGEN 50111-0471-01 0.04240 PROPRANOLOL 80 MG TABLET 0 PLIVA, INC EAGEN 50111-0471-02 0.04240 PROPRANOLOL 80 MG TABLET 0 PLIVA, INC EABUL 00378-0731-01 0.<strong>08</strong>770 0.95400 PROPRANOLOL-HCTZ 40-25 MG TAB 0 MYLAN EABUL 00378-0347-01 0.13200 1.17162 PROPRANOLOL-HCTZ 80-25 MG TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 38779-0510-<strong>08</strong> 0.12825 PROPYLENE GLYCOL LIQUID 0 MEDISCA INC. MLGEN 00143-1480-01 0.55980 PROPYLTHIOURACIL 50 MG TABLET 0 WEST-WARD,INC. EAGEN 00143-1480-10 0.53181 PROPYLTHIOURACIL 50 MG TABLET 0 WEST-WARD,INC. EABND 00228-2348-10 0.61951 PROPYLTHIOURACIL 50 MG TABLET 0 ACTAVIS PHARMA, EABND 000<strong>06</strong>-0072-31 0.18000 4.02107 PROSCAR 5 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0072-58 0.18000 4.02052 PROSCAR 5 MG TABLET G MERCK SHARP & D EABND 55566-8101-01 2.22755 PROSED-DS TABLET 0 FERRING PH INC EABND 00187-3100-10 2.11409 PROSTIGMIN 15 MG TABLET 0 VALEANT EABND 63323-0229-05 1.79280 PROTAMINE 10 MG/ML VIAL 0 APP PHARMACEUTI MLBND 63323-0229-15 1.50396 PROTAMINE 10 MG/ML VIAL 0 APP/NOVAPLUS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63323-0229-30 1.07568 PROTAMINE 10 MG/ML VIAL 0 APP PHARMACEUTI MLBND 63323-0229-35 0.89474 PROTAMINE 10 MG/ML VIAL 0 APP/NOVAPLUS MLBND 000<strong>08</strong>-<strong>08</strong>43-81 0.28120 7.00058 PROTONIX DR 20 MG TABLET G WYETH PHARM EABND 000<strong>08</strong>-<strong>08</strong>41-81 0.28<strong>08</strong>0 7.00058 PROTONIX DR 40 MG TABLET G WYETH PHARM EABND 000<strong>08</strong>-0923-55 4.98000 PROTONIX IV 40 MG VIAL 0 WYETH PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 332LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>08</strong>-0923-60 4.98000 PROTONIX IV 40 MG VIAL 0 WYETH PHARM EABND 000<strong>08</strong>-0941-01 4.98000 PROTONIX IV 40 MG VIAL 0 PFIZER/NOVAPLUS EABND 000<strong>08</strong>-0941-02 4.98000 PROTONIX IV 40 MG VIAL 0 PFIZER/NOVAPLUS EABND 000<strong>08</strong>-0941-03 4.98000 PROTONIX IV 40 MG VIAL 0 PFIZER/NOVAPLUS EABND 000<strong>08</strong>-4001-01 4.98000 PROTONIX IV 40 MG VIAL 0 WYETH PHARM EABND 000<strong>08</strong>-4001-10 4.98000 PROTONIX IV 40 MG VIAL 0 WYETH PHARM EABND 000<strong>08</strong>-4001-25 4.98000 PROTONIX IV 40 MG VIAL 0 WYETH PHARM EABND 000<strong>08</strong>-<strong>08</strong>44-01 6.61510 PROTONIX 40 MG SUSPENSION G WYETH PHARM EABND 000<strong>08</strong>-<strong>08</strong>44-02 6.61620 PROTONIX 40 MG SUSPENSION G WYETH PHARM EABND 00469-5201-11 7.05151 PROTOPIC 0.03% OINTMENT G ASTELLAS PHARMA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00469-5201-30 7.05140 PROTOPIC 0.03% OINTMENT G ASTELLAS PHARMA GMBND 00469-5201-60 7.05153 PROTOPIC 0.03% OINTMENT G ASTELLAS PHARMA GMBND 00469-5202-11 7.05151 PROTOPIC 0.1% OINTMENT G ASTELLAS PHARMA GMBND 00469-5202-30 7.05140 PROTOPIC 0.1% OINTMENT G ASTELLAS PHARMA GMBND 00469-5202-60 7.05153 PROTOPIC 0.1% OINTMENT G ASTELLAS PHARMA GMGEX 00054-0211-25 1.66779 PROTRIPTYLINE HCL 10 MG TABLET 0 ROXANE LABS. EAGEX 00555-0594-02 1.66779 PROTRIPTYLINE HCL 10 MG TABLET 0 BARR EAGEX 428<strong>06</strong>-0097-01 1.66779 PROTRIPTYLINE HCL 10 MG TABLET 0 EPIC PHARMA LLC EAGEX 50383-0960-10 1.66779 PROTRIPTYLINE HCL 10 MG TABLET 0 HI-TECH PHARMAC EAGEX 64980-0159-01 1.66779 PROTRIPTYLINE HCL 10 MG TABLET 0 RISING PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00555-0595-02 1.<strong>08</strong>486 PROTRIPTYLINE HCL 5 MG TABLET 0 BARR EAGEX 428<strong>06</strong>-0096-01 1.<strong>08</strong>486 PROTRIPTYLINE HCL 5 MG TABLET 0 EPIC PHARMA LLC EAGEX 50383-0959-10 1.<strong>08</strong>486 PROTRIPTYLINE HCL 5 MG TABLET 0 HI-TECH PHARMAC EAGEX 64980-0158-01 1.<strong>08</strong>486 PROTRIPTYLINE HCL 5 MG TABLET 0 RISING PHARM EABND 00<strong>08</strong>5-1132-01 8.52546 PROVENTIL HFA 90 MCG INHALER 0 MERCK SHARP & D GMBND 00009-0050-02 0.<strong>08</strong>168 2.49705 PROVERA 10 MG TABLET G PHARMACIA/UPJHN EABND 00009-0050-11 0.<strong>08</strong>168 2.49698 PROVERA 10 MG TABLET G PHARMACIA/UPJHN EABND 00009-0<strong>06</strong>4-04 0.<strong>06</strong>183 1.27322 PROVERA 2.5 MG TABLET G PHARMACIA/UPJHN EABND 00009-0286-03 0.07776 1.91414 PROVERA 5 MG TABLET G PHARMACIA/UPJHN EABND 52747-0504-30 0.83000 PROVIDA OB CAPSULE 0 US PHARMACEUTIC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00002-3004-75 24.92000 36.15480 PROZAC WEEKLY 90 MG CAPSULE G ELI LILLY & CO. EABEX 00777-3104-02 0.03250 7.30<strong>06</strong>8 PROZAC 10 MG PULVULE G DISTA LABS EABEX 00777-3105-02 0.02500 7.48992 PROZAC 20 MG PULVULE G DISTA LABS EABEX 00777-3105-07 0.02500 7.48992 PROZAC 20 MG PULVULE G DISTA LABS EABEX 00777-3105-30 0.02500 7.48992 PROZAC 20 MG PULVULE G DISTA LABS EABEX 00777-3107-30 0.49033 14.97984 PROZAC 40 MG PULVULE G DISTA LABS EABND 00<strong>06</strong>4-3600-45 4.18117 PRUDOXIN 5% CREAM 0 <strong>HEALTH</strong>POINT MED GMBND 00186-1988-04 4.34021 PULMICORT 0.25 MG/2 ML RESPUL 0 ASTRAZENECA MLBND 00186-1989-04 5.1<strong>08</strong>09 5.1<strong>08</strong>09 PULMICORT 0.5 MG/2 ML RESPULE 0 ASTRAZENECA MLBND 00186-1990-04 10.21702 PULMICORT 1 MG/2 ML RESPULE 0 ASTRAZENECA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00186-0916-12 169.48600 PULMICORT 180 MCG FLEXHALER G ASTRAZENECA EABND 00186-0917-<strong>06</strong> 126.57500 PULMICORT 90 MCG FLEXHALER G ASTRAZENECA EABND 50242-0100-39 34.29892 PULMOZYME 1 MG/ML AMPUL 0 GENENTECH, INC. MLBND 50242-0100-40 34.29947 PULMOZYME 1 MG/ML AMPUL 0 GENENTECH, INC. MLBND 57844-0522-<strong>06</strong> 1.07450 7.95970 PURINETHOL 50 MG TABLET G GATE PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 333LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 58914-<strong>06</strong>00-21 4.41442 PYLERA CAPSULE 0 APTALIS PHARMA EAGEX 61748-0012-01 1.51671 PYRAZINAMIDE 500 MG TABLET 0 VERSA PHARMACEU EAGEX 61748-0012-05 1.51671 PYRAZINAMIDE 500 MG TABLET 0 VERSA PHARMACEU EAGEX 61748-0012-<strong>06</strong> 1.51671 PYRAZINAMIDE 500 MG TABLET 0 VERSA PHARMACEU EAGEX 61748-0012-09 1.51671 PYRAZINAMIDE 500 MG TABLET 0 VERSA PHARMACEU EAGEX 67253-<strong>06</strong>60-10 1.51671 PYRAZINAMIDE 500 MG TABLET 0 DAVA PHARMACEUT EAGEX 67253-<strong>06</strong>60-50 0.89452 PYRAZINAMIDE 500 MG TABLET 0 DAVA PHARMACEUT EAGEN 00115-3511-01 0.28458 PYRIDOSTIGMINE BR 60 MG TABLET 0 GLOBAL PHARM EAGEN 64720-0128-10 0.28458 PYRIDOSTIGMINE BR 60 MG TABLET 0 COREPHARMA LLC EAGEN 68<strong>08</strong>4-0494-01 0.28458 PYRIDOSTIGMINE BR 60 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0494-11 0.28458 PYRIDOSTIGMINE BR 60 MG TABLET 0 AHP EAGEN 68682-0302-10 0.28458 PYRIDOSTIGMINE BR 60 MG TABLET 0 OCEANSIDE PHARM EABND 63323-0180-01 9.32720 PYRIDOXINE 100 MG/ML VIAL 0 APP PHARMACEUTI MLBND 59310-0210-12 14.28553 QNASL 80 MCG NASAL SPRAY G TEVA SPECIALTY GMBND 13310-0153-07 4.80930 6.52214 QUALAQUIN 324 MG CAPSULE G AR SCIENTIFIC EABEX 51285-0431-65 3.23973 QUARTETTE TABLET 0 TEVA SPECIALTY EAGEX 52544-0966-91 1.24580 QUASENSE 0.15-0.03 MG TABLET 0 ACTAVIS PHARMA, EAGEN 49884-0937-63 1.10090 QUESTRAN LIGHT PACKET G PAR PHARM. EAGEN 49884-0937-65 1.10090 QUESTRAN LIGHT PACKET G PAR PHARM. EAGUL 49884-0936-64 1.27670 QUESTRAN PACKET G PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 49884-0936-65 1.27670 QUESTRAN PACKET G PAR PHARM. EAGEN 49884-0936-66 0.09320 QUESTRAN POWDER G PAR PHARMACEUTI GMGEX 00054-0221-20 0.21560 QUETIAPINE FUMARATE 100 MG TAB G ROXANE LABS. EAGEX 00054-0221-25 0.21560 QUETIAPINE FUMARATE 100 MG TAB G ROXANE LABS. EAGEX 00093-8162-01 0.21560 QUETIAPINE FUMARATE 100 MG TAB G TEVA USA EAGEX 00093-8162-10 0.21560 QUETIAPINE FUMARATE 100 MG TAB G TEVA USA EAGEX 00781-5344-01 0.21560 QUETIAPINE FUMARATE 100 MG TAB G SANDOZ EAGEX 00904-6279-61 0.21560 QUETIAPINE FUMARATE 100 MG TAB G MAJOR PHARMACEU EAGEX 16714-0377-01 0.21560 QUETIAPINE FUMARATE 100 MG TAB G NORTHSTAR RX LL EAGEX 16714-0377-02 0.21560 QUETIAPINE FUMARATE 100 MG TAB G NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16729-0147-01 0.21560 QUETIAPINE FUMARATE 100 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0147-10 0.21560 QUETIAPINE FUMARATE 100 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0147-17 0.21560 QUETIAPINE FUMARATE 100 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 47335-0904-18 0.21560 QUETIAPINE FUMARATE 100 MG TAB G SUN PHARMA GLOB EAGEX 47335-0904-88 0.21560 QUETIAPINE FUMARATE 100 MG TAB G SUN PHARMA GLOB EAGEX 55111-0186-01 0.21560 QUETIAPINE FUMARATE 100 MG TAB G DR.REDDY'S LAB EAGEX 60505-3133-01 0.21560 QUETIAPINE FUMARATE 100 MG TAB G APOTEX CORP EAGEX 60505-3133-<strong>08</strong> 0.21560 QUETIAPINE FUMARATE 100 MG TAB G APOTEX CORP EAGEX 65862-0491-01 0.21560 QUETIAPINE FUMARATE 100 MG TAB G AUROBINDO PHARM EAGEX 65862-0491-99 0.21560 QUETIAPINE FUMARATE 100 MG TAB G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 67877-0250-01 0.21560 QUETIAPINE FUMARATE 100 MG TAB G ASCEND LABORATO EAGEX 67877-0250-38 0.21560 QUETIAPINE FUMARATE 100 MG TAB G ASCEND LABORATO EAGEX 68<strong>08</strong>4-0532-01 0.21560 QUETIAPINE FUMARATE 100 MG TAB G AHP EAGEX 68<strong>08</strong>4-0532-11 0.21560 QUETIAPINE FUMARATE 100 MG TAB G AHP EAGEX 68180-0447-01 0.21560 QUETIAPINE FUMARATE 100 MG TAB G LUPIN PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 334LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68180-0447-03 0.21560 QUETIAPINE FUMARATE 100 MG TAB G LUPIN PHARMACEU EAGEX 00054-0222-20 0.36383 QUETIAPINE FUMARATE 200 MG TAB G ROXANE LABS. EAGEX 00054-0222-25 0.36383 QUETIAPINE FUMARATE 200 MG TAB G ROXANE LABS. EAGEX 00093-8163-01 0.36383 QUETIAPINE FUMARATE 200 MG TAB G TEVA USA EAGEX 00093-8163-10 0.36383 QUETIAPINE FUMARATE 200 MG TAB G TEVA USA EAGEX 00781-5346-01 0.36383 QUETIAPINE FUMARATE 200 MG TAB G SANDOZ EAGEX 00904-6280-61 0.36383 QUETIAPINE FUMARATE 200 MG TAB G MAJOR PHARMACEU EAGEX 16714-0378-01 0.36383 QUETIAPINE FUMARATE 200 MG TAB G NORTHSTAR RX LL EAGEX 16714-0378-02 0.36383 QUETIAPINE FUMARATE 200 MG TAB G NORTHSTAR RX LL EAGEX 16729-0148-00 0.36383 QUETIAPINE FUMARATE 200 MG TAB G ACCORD <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16729-0148-10 0.36383 QUETIAPINE FUMARATE 200 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0148-17 0.36383 QUETIAPINE FUMARATE 200 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 47335-0905-18 0.36383 QUETIAPINE FUMARATE 200 MG TAB G SUN PHARMA GLOB EAGEX 47335-0905-88 0.36383 QUETIAPINE FUMARATE 200 MG TAB G SUN PHARMA GLOB EAGEX 55111-0189-01 0.36383 QUETIAPINE FUMARATE 200 MG TAB G DR.REDDY'S LAB EAGEX 60505-3135-01 0.36383 QUETIAPINE FUMARATE 200 MG TAB G APOTEX CORP EAGEX 60505-3135-<strong>08</strong> 0.36383 QUETIAPINE FUMARATE 200 MG TAB G APOTEX CORP EAGEX 65862-0493-01 0.36383 QUETIAPINE FUMARATE 200 MG TAB G AUROBINDO PHARM EAGEX 65862-0493-99 0.36383 QUETIAPINE FUMARATE 200 MG TAB G AUROBINDO PHARM EAGEX 67877-0246-01 0.36383 QUETIAPINE FUMARATE 200 MG TAB G ASCEND LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 67877-0246-38 0.36383 QUETIAPINE FUMARATE 200 MG TAB G ASCEND LABORATO EAGEX 68<strong>08</strong>4-0533-01 0.36383 QUETIAPINE FUMARATE 200 MG TAB G AHP EAGEX 68<strong>08</strong>4-0533-11 0.36383 QUETIAPINE FUMARATE 200 MG TAB G AHP EAGEX 68180-0448-01 0.36383 QUETIAPINE FUMARATE 200 MG TAB G LUPIN PHARMACEU EAGEX 68180-0448-02 0.36383 QUETIAPINE FUMARATE 200 MG TAB G LUPIN PHARMACEU EAGEX 00054-0220-20 0.11219 QUETIAPINE FUMARATE 25 MG TAB G ROXANE LABS. EAGEX 00054-0220-25 0.11219 QUETIAPINE FUMARATE 25 MG TAB G ROXANE LABS. EAGEX 00054-0220-31 0.11219 QUETIAPINE FUMARATE 25 MG TAB G ROXANE LABS. EAGEX 00093-8161-01 0.11219 QUETIAPINE FUMARATE 25 MG TAB G TEVA USA EAGEX 00093-8161-10 0.11219 QUETIAPINE FUMARATE 25 MG TAB G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-5343-01 0.11219 QUETIAPINE FUMARATE 25 MG TAB G SANDOZ EAGEX 00904-6277-61 0.11219 QUETIAPINE FUMARATE 25 MG TAB G MAJOR PHARMACEU EAGEX 13668-0148-10 0.11219 QUETIAPINE FUMARATE 25 MG TAB G TORRENT PHARMAC EAGEX 16714-0375-01 0.11219 QUETIAPINE FUMARATE 25 MG TAB G NORTHSTAR RX LL EAGEX 16714-0375-02 0.11219 QUETIAPINE FUMARATE 25 MG TAB G NORTHSTAR RX LL EAGEX 16729-0145-01 0.11219 QUETIAPINE FUMARATE 25 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0145-10 0.11219 QUETIAPINE FUMARATE 25 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0145-17 0.11219 QUETIAPINE FUMARATE 25 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 47335-0902-18 0.11219 QUETIAPINE FUMARATE 25 MG TAB G SUN PHARMA GLOB EAGEX 47335-0902-88 0.11219 QUETIAPINE FUMARATE 25 MG TAB G SUN PHARMA GLOB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 55111-0249-01 0.11219 QUETIAPINE FUMARATE 25 MG TAB G DR.REDDY'S LAB EAGEX 55111-0249-05 0.11219 QUETIAPINE FUMARATE 25 MG TAB G DR.REDDY'S LAB EAGEX 60505-3130-01 0.11219 QUETIAPINE FUMARATE 25 MG TAB G APOTEX CORP EAGEX 60505-3130-<strong>08</strong> 0.11219 QUETIAPINE FUMARATE 25 MG TAB G APOTEX CORP EAGEX 65862-0489-01 0.11219 QUETIAPINE FUMARATE 25 MG TAB G AUROBINDO PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 335LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0489-99 0.11219 QUETIAPINE FUMARATE 25 MG TAB G AUROBINDO PHARM EAGEX 67877-0242-01 0.11219 QUETIAPINE FUMARATE 25 MG TAB G ASCEND LABORATO EAGEX 67877-0242-10 0.11219 QUETIAPINE FUMARATE 25 MG TAB G ASCEND LABORATO EAGEX 67877-0242-38 0.11219 QUETIAPINE FUMARATE 25 MG TAB G ASCEND LABORATO EAGEX 68<strong>08</strong>4-0530-01 0.11219 QUETIAPINE FUMARATE 25 MG TAB G AHP EAGEX 68<strong>08</strong>4-0530-11 0.11219 QUETIAPINE FUMARATE 25 MG TAB G AHP EAGEX 68180-0445-01 0.11219 QUETIAPINE FUMARATE 25 MG TAB G LUPIN PHARMACEU EAGEX 68180-0445-03 0.11219 QUETIAPINE FUMARATE 25 MG TAB G LUPIN PHARMACEU EAGEX 00054-0223-20 0.45522 QUETIAPINE FUMARATE 300 MG TAB G ROXANE LABS. EAGEX 00054-0223-21 0.45522 QUETIAPINE FUMARATE 300 MG TAB G ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-8164-01 0.45522 QUETIAPINE FUMARATE 300 MG TAB G TEVA USA EAGEX 00093-8164-10 0.45522 QUETIAPINE FUMARATE 300 MG TAB G TEVA USA EAGEX 00781-5347-01 0.45522 QUETIAPINE FUMARATE 300 MG TAB G SANDOZ EAGEX 16714-0379-01 0.45522 QUETIAPINE FUMARATE 300 MG TAB G NORTHSTAR RX LL EAGEX 16714-0379-02 0.45522 QUETIAPINE FUMARATE 300 MG TAB G NORTHSTAR RX LL EAGEX 16729-0149-10 0.45522 QUETIAPINE FUMARATE 300 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0149-12 0.45522 QUETIAPINE FUMARATE 300 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0149-17 0.45522 QUETIAPINE FUMARATE 300 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 47335-09<strong>06</strong>-18 0.45522 QUETIAPINE FUMARATE 300 MG TAB G SUN PHARMA GLOB EAGEX 47335-09<strong>06</strong>-86 0.45522 QUETIAPINE FUMARATE 300 MG TAB G SUN PHARMA GLOB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 47335-09<strong>06</strong>-88 0.45522 QUETIAPINE FUMARATE 300 MG TAB G SUN PHARMA GLOB EAGEX 55111-0190-60 0.45522 QUETIAPINE FUMARATE 300 MG TAB G DR.REDDY'S LAB EAGEX 60505-3137-<strong>06</strong> 0.45522 QUETIAPINE FUMARATE 300 MG TAB G APOTEX CORP EAGEX 65862-0494-60 0.45522 QUETIAPINE FUMARATE 300 MG TAB G AUROBINDO PHARM EAGEX 65862-0494-99 0.45522 QUETIAPINE FUMARATE 300 MG TAB G AUROBINDO PHARM EAGEX 67877-0247-38 0.45522 QUETIAPINE FUMARATE 300 MG TAB G ASCEND LABORATO EAGEX 67877-0247-60 0.45522 QUETIAPINE FUMARATE 300 MG TAB G ASCEND LABORATO EAGEX 68<strong>08</strong>4-0534-01 0.45522 QUETIAPINE FUMARATE 300 MG TAB G AHP EAGEX 68<strong>08</strong>4-0534-11 0.45522 QUETIAPINE FUMARATE 300 MG TAB G AHP EAGEX 68180-0449-01 0.45522 QUETIAPINE FUMARATE 300 MG TAB G LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68180-0449-02 0.45522 QUETIAPINE FUMARATE 300 MG TAB G LUPIN PHARMACEU EAGEX 68180-0449-07 0.45522 QUETIAPINE FUMARATE 300 MG TAB G LUPIN PHARMACEU EAGEX 00054-0230-20 0.62510 QUETIAPINE FUMARATE 400 MG TAB G ROXANE LABS. EAGEX 00054-0230-25 0.62510 QUETIAPINE FUMARATE 400 MG TAB G ROXANE LABS. EAGEX 00093-8165-01 0.62510 QUETIAPINE FUMARATE 400 MG TAB G TEVA USA EAGEX 00093-8165-10 0.62510 QUETIAPINE FUMARATE 400 MG TAB G TEVA USA EAGEX 00781-5348-01 0.62510 QUETIAPINE FUMARATE 400 MG TAB G SANDOZ EAGEX 00904-6281-61 0.62510 QUETIAPINE FUMARATE 400 MG TAB G MAJOR PHARMACEU EAGEX 16714-0380-01 0.62510 QUETIAPINE FUMARATE 400 MG TAB G NORTHSTAR RX LL EAGEX 16729-0150-00 0.62510 QUETIAPINE FUMARATE 400 MG TAB G ACCORD <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16729-0150-10 0.62510 QUETIAPINE FUMARATE 400 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0150-16 0.62510 QUETIAPINE FUMARATE 400 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 47335-0907-18 0.62510 QUETIAPINE FUMARATE 400 MG TAB G SUN PHARMA GLOB EAGEX 47335-0907-88 0.62510 QUETIAPINE FUMARATE 400 MG TAB G SUN PHARMA GLOB EAGEX 55111-<strong>06</strong><strong>06</strong>-01 0.62510 QUETIAPINE FUMARATE 400 MG TAB G DR.REDDY'S LAB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 336LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-3139-01 0.62510 QUETIAPINE FUMARATE 400 MG TAB G APOTEX CORP EAGEX 65862-0495-01 0.62510 QUETIAPINE FUMARATE 400 MG TAB G AUROBINDO PHARM EAGEX 65862-0495-05 0.62510 QUETIAPINE FUMARATE 400 MG TAB G AUROBINDO PHARM EAGEX 67877-0248-01 0.62510 QUETIAPINE FUMARATE 400 MG TAB G ASCEND LABORATO EAGEX 67877-0248-38 0.62510 QUETIAPINE FUMARATE 400 MG TAB G ASCEND LABORATO EAGEX 68<strong>08</strong>4-0535-01 0.62510 QUETIAPINE FUMARATE 400 MG TAB G AHP EAGEX 68<strong>08</strong>4-0535-11 0.62510 QUETIAPINE FUMARATE 400 MG TAB G AHP EAGEX 68180-0450-01 0.62510 QUETIAPINE FUMARATE 400 MG TAB G LUPIN PHARMACEU EAGEX 68180-0450-02 0.62510 QUETIAPINE FUMARATE 400 MG TAB G LUPIN PHARMACEU EAGEX 00054-0229-20 0.20007 QUETIAPINE FUMARATE 50 MG TAB G ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00054-0229-25 0.20007 QUETIAPINE FUMARATE 50 MG TAB G ROXANE LABS. EAGEX 00054-0229-31 0.20007 QUETIAPINE FUMARATE 50 MG TAB G ROXANE LABS. EAGEX 00093-8166-01 0.20007 QUETIAPINE FUMARATE 50 MG TAB G TEVA USA EAGEX 00093-8166-10 0.20007 QUETIAPINE FUMARATE 50 MG TAB G TEVA USA EAGEX 00781-5342-01 0.20007 QUETIAPINE FUMARATE 50 MG TAB G SANDOZ EAGEX 00904-6278-61 0.20007 QUETIAPINE FUMARATE 50 MG TAB G MAJOR PHARMACEU EAGEX 13668-0149-10 0.20007 QUETIAPINE FUMARATE 50 MG TAB G TORRENT PHARMAC EAGEX 16714-0376-01 0.20007 QUETIAPINE FUMARATE 50 MG TAB G NORTHSTAR RX LL EAGEX 16714-0376-02 0.20007 QUETIAPINE FUMARATE 50 MG TAB G NORTHSTAR RX LL EAGEX 16729-0146-01 0.20007 QUETIAPINE FUMARATE 50 MG TAB G ACCORD <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16729-0146-10 0.20007 QUETIAPINE FUMARATE 50 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0146-17 0.20007 QUETIAPINE FUMARATE 50 MG TAB G ACCORD <strong>HEALTH</strong>CA EAGEX 47335-0903-18 0.20007 QUETIAPINE FUMARATE 50 MG TAB G SUN PHARMA GLOB EAGEX 47335-0903-88 0.20007 QUETIAPINE FUMARATE 50 MG TAB G SUN PHARMA GLOB EAGEX 55111-0169-01 0.20007 QUETIAPINE FUMARATE 50 MG TAB G DR.REDDY'S LAB EAGEX 55111-0169-05 0.20007 QUETIAPINE FUMARATE 50 MG TAB G DR.REDDY'S LAB EAGEX 60505-3132-01 0.20007 QUETIAPINE FUMARATE 50 MG TAB G APOTEX CORP EAGEX 60505-3132-<strong>08</strong> 0.20007 QUETIAPINE FUMARATE 50 MG TAB G APOTEX CORP EAGEX 65862-0490-01 0.20007 QUETIAPINE FUMARATE 50 MG TAB G AUROBINDO PHARM EAGEX 65862-0490-99 0.20007 QUETIAPINE FUMARATE 50 MG TAB G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 67877-0249-01 0.20007 QUETIAPINE FUMARATE 50 MG TAB G ASCEND LABORATO EAGEX 67877-0249-10 0.20007 QUETIAPINE FUMARATE 50 MG TAB G ASCEND LABORATO EAGEX 67877-0249-38 0.20007 QUETIAPINE FUMARATE 50 MG TAB G ASCEND LABORATO EAGEX 68<strong>08</strong>4-0531-01 0.20007 QUETIAPINE FUMARATE 50 MG TAB G AHP EAGEX 68<strong>08</strong>4-0531-11 0.20007 QUETIAPINE FUMARATE 50 MG TAB G AHP EAGEX 68180-0446-01 0.20007 QUETIAPINE FUMARATE 50 MG TAB G LUPIN PHARMACEU EAGEX 68180-0446-03 0.20007 QUETIAPINE FUMARATE 50 MG TAB G LUPIN PHARMACEU EAGEN 31722-0268-90 0.17290 QUINAPRIL 10 MG TABLET G CAMBER PHARMACE EAGEN 59762-5020-01 0.17290 QUINAPRIL 10 MG TABLET G GREENSTONE LLC. EAGEN 60505-0173-00 0.17290 QUINAPRIL 10 MG TABLET G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0173-01 0.17290 QUINAPRIL 10 MG TABLET G APOTEX CORP EAGEN 65862-<strong>06</strong>18-90 0.17290 QUINAPRIL 10 MG TABLET G AUROBINDO PHARM EAGEN 68180-0557-09 0.17290 QUINAPRIL 10 MG TABLET G LUPIN PHARMACEU EAGEN 31722-0269-90 0.16333 QUINAPRIL 20 MG TABLET G CAMBER PHARMACE EAGEN 59762-5021-01 0.16333 QUINAPRIL 20 MG TABLET G GREENSTONE LLC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 337LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0174-00 0.16333 QUINAPRIL 20 MG TABLET G APOTEX CORP EAGEN 60505-0174-01 0.16333 QUINAPRIL 20 MG TABLET G APOTEX CORP EAGEN 65862-<strong>06</strong>19-90 0.16333 QUINAPRIL 20 MG TABLET G AUROBINDO PHARM EAGEN 68180-0558-09 0.16333 QUINAPRIL 20 MG TABLET G LUPIN PHARMACEU EAGEN 31722-0270-90 0.16333 QUINAPRIL 40 MG TABLET G CAMBER PHARMACE EAGEN 59762-5022-01 0.16333 QUINAPRIL 40 MG TABLET G GREENSTONE LLC. EAGEN 60505-0175-00 0.16333 QUINAPRIL 40 MG TABLET G APOTEX CORP EAGEN 60505-0175-01 0.16333 QUINAPRIL 40 MG TABLET G APOTEX CORP EAGEN 65862-<strong>06</strong>20-90 0.16333 QUINAPRIL 40 MG TABLET G AUROBINDO PHARM EAGEN 68180-0559-09 0.16333 QUINAPRIL 40 MG TABLET G LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0267-90 0.16333 QUINAPRIL 5 MG TABLET G CAMBER PHARMACE EAGEN 59762-5019-01 0.16333 QUINAPRIL 5 MG TABLET G GREENSTONE LLC. EAGEN 60505-0172-00 0.16333 QUINAPRIL 5 MG TABLET G APOTEX CORP EAGEN 60505-0172-01 0.16333 QUINAPRIL 5 MG TABLET G APOTEX CORP EAGEN 65862-<strong>06</strong>17-90 0.16333 QUINAPRIL 5 MG TABLET G AUROBINDO PHARM EAGEN 68180-0556-09 0.16333 QUINAPRIL 5 MG TABLET G LUPIN PHARMACEU EAGEN 00378-0542-77 0.91749 QUINAPRIL-HCTZ 10-12.5 MG TAB G MYLAN EAGEN 31722-0374-90 0.91749 QUINAPRIL-HCTZ 10-12.5 MG TAB G CAMBER PHARMACE EAGEN 43386-0710-05 0.87165 QUINAPRIL-HCTZ 10-12.5 MG TAB G GAVIS PHARMACEU EAGEN 43386-0710-09 0.91749 QUINAPRIL-HCTZ 10-12.5 MG TAB G GAVIS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-0222-01 0.91700 QUINAPRIL-HCTZ 10-12.5 MG TAB G GREENSTONE LLC. EAGEN 60505-3409-05 0.91725 QUINAPRIL-HCTZ 10-12.5 MG TAB G APOTEX CORP EAGEN 60505-3409-09 0.91749 QUINAPRIL-HCTZ 10-12.5 MG TAB G APOTEX CORP EAGEN 65862-0161-90 0.91749 QUINAPRIL-HCTZ 10-12.5 MG TAB G AUROBINDO PHARM EAGEN 00378-0543-77 0.66150 QUINAPRIL-HCTZ 20-12.5 MG TAB G MYLAN EAGEN 31722-0375-90 0.66150 QUINAPRIL-HCTZ 20-12.5 MG TAB G CAMBER PHARMACE EAGEN 43386-0711-05 0.66150 QUINAPRIL-HCTZ 20-12.5 MG TAB G GAVIS PHARMACEU EAGEN 43386-0711-09 0.66150 QUINAPRIL-HCTZ 20-12.5 MG TAB G GAVIS PHARMACEU EAGEN 59762-0220-01 0.66150 QUINAPRIL-HCTZ 20-12.5 MG TAB G GREENSTONE LLC. EAGEN 60505-3410-05 0.66150 QUINAPRIL-HCTZ 20-12.5 MG TAB G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-3410-09 0.66150 QUINAPRIL-HCTZ 20-12.5 MG TAB G APOTEX CORP EAGEN 65862-0162-30 0.66150 QUINAPRIL-HCTZ 20-12.5 MG TAB G AUROBINDO PHARM EAGEN 65862-0162-90 0.66150 QUINAPRIL-HCTZ 20-12.5 MG TAB G AUROBINDO PHARM EAGEN 00378-0544-77 0.74560 QUINAPRIL-HCTZ 20-25 MG TAB G MYLAN EAGEN 31722-0376-90 0.74560 QUINAPRIL-HCTZ 20-25 MG TAB G CAMBER PHARMACE EAGEN 43386-0712-05 0.74560 QUINAPRIL-HCTZ 20-25 MG TAB G GAVIS PHARMACEU EAGEN 43386-0712-09 0.74560 QUINAPRIL-HCTZ 20-25 MG TAB G GAVIS PHARMACEU EAGEN 59762-0223-01 0.74560 QUINAPRIL-HCTZ 20-25 MG TAB G GREENSTONE LLC. EAGEN 60505-3411-05 0.74560 QUINAPRIL-HCTZ 20-25 MG TAB G APOTEX CORP EAGEN 60505-3411-09 0.74560 QUINAPRIL-HCTZ 20-25 MG TAB G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0163-90 0.74560 QUINAPRIL-HCTZ 20-25 MG TAB G AUROBINDO PHARM EAGEN 51079-0027-20 1.02600 QUINIDINE GLUC ER 324 MG TAB 0 MYLAN INSTITUTI EAGEN 53489-0141-01 6.79687 QUINIDINE GLUC ER 324 MG TAB 0 MUTUAL PHARM CO EABND 00002-1407-01 1.78948 QUINIDINE GLUC 80 MG/ML VIAL 0 ELI LILLY & CO. MLBND 00093-9175-01 0.87415 QUINIDINE SULF ER 300 MG TAB 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 338LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00185-4346-01 0.15750 QUINIDINE SULFATE 200 MG TAB 0 SANDOZ EAGEN 00185-4346-10 0.14647 QUINIDINE SULFATE 200 MG TAB 0 SANDOZ EAGEN 00185-1047-01 0.23500 QUINIDINE SULFATE 300 MG TAB 0 SANDOZ EAGEN 00185-1047-10 0.23500 QUINIDINE SULFATE 300 MG TAB 0 SANDOZ EAGEN 00093-3002-56 4.80930 QUININE SULFATE 324 MG CAPSULE G TEVA USA EAGEN 53489-0700-07 4.80930 QUININE SULFATE 324 MG CAPSULE G MUTUAL PHARM CO EABND 59310-0202-40 14.92759 QVAR 40 MCG ORAL INHALER G TEVA SPECIALTY GMBND 59310-0204-80 19.98487 QVAR 80 MCG ORAL INHALER G TEVA SPECIALTY GMGEN 00054-01<strong>06</strong>-25 0.17861 RAMIPRIL 1.25 MG CAPSULE 0 ROXANE LABS. EAGEN 16252-0570-30 0.17861 RAMIPRIL 1.25 MG CAPSULE 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0438-90 0.17861 RAMIPRIL 1.25 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 60505-2875-01 0.17861 RAMIPRIL 1.25 MG CAPSULE 0 APOTEX CORP EAGEN 65862-0474-01 0.17861 RAMIPRIL 1.25 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0474-30 0.17861 RAMIPRIL 1.25 MG CAPSULE 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0294-11 0.17861 RAMIPRIL 1.25 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-0294-21 0.17861 RAMIPRIL 1.25 MG CAPSULE 0 AHP EAGEN 68180-0588-01 0.17861 RAMIPRIL 1.25 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68382-0144-<strong>06</strong> 0.17861 RAMIPRIL 1.25 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 00054-0109-25 0.05890 RAMIPRIL 10 MG CAPSULE 0 ROXANE LABS. EAGEN 00054-0109-29 0.05890 RAMIPRIL 10 MG CAPSULE 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7438-01 0.05890 RAMIPRIL 10 MG CAPSULE 0 TEVA USA EAGEN 16252-0573-01 0.05890 RAMIPRIL 10 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 16252-0573-50 0.05890 RAMIPRIL 10 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 31722-0274-01 0.05890 RAMIPRIL 10 MG CAPSULE 0 CAMBER PHARMACE EAGEN 31722-0274-05 0.05890 RAMIPRIL 10 MG CAPSULE 0 CAMBER PHARMACE EAGEN 60505-2878-00 0.05890 RAMIPRIL 10 MG CAPSULE 0 APOTEX CORP EAGEN 60505-2878-01 0.05890 RAMIPRIL 10 MG CAPSULE 0 APOTEX CORP EAGEN 65862-0477-01 0.05890 RAMIPRIL 10 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0477-05 0.05890 RAMIPRIL 10 MG CAPSULE 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0268-01 0.05890 RAMIPRIL 10 MG CAPSULE 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0268-11 0.05890 RAMIPRIL 10 MG CAPSULE 0 AHP EAGEN 68180-0591-01 0.05890 RAMIPRIL 10 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68180-0591-02 0.05890 RAMIPRIL 10 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68180-0591-09 0.05890 RAMIPRIL 10 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68382-0147-01 0.05890 RAMIPRIL 10 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 68382-0147-05 0.05890 RAMIPRIL 10 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 00054-0107-25 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 ROXANE LABS. EAGEN 00054-0107-29 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 ROXANE LABS. EAGEN 00093-7436-01 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 TEVA USA EAGEN 16252-0571-01 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0272-01 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 CAMBER PHARMACE EAGEN 60505-2876-01 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 APOTEX CORP EAGEN 65862-0475-01 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0475-05 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0266-11 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 339LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0589-01 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68180-0589-02 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68180-0589-09 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68382-0145-01 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 68382-0145-05 0.04790 RAMIPRIL 2.5 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 00054-01<strong>08</strong>-25 0.05040 RAMIPRIL 5 MG CAPSULE 0 ROXANE LABS. EAGEN 00054-01<strong>08</strong>-29 0.05040 RAMIPRIL 5 MG CAPSULE 0 ROXANE LABS. EAGEN 00093-7437-01 0.05040 RAMIPRIL 5 MG CAPSULE 0 TEVA USA EAGEN 16252-0572-01 0.05040 RAMIPRIL 5 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 16252-0572-50 0.05040 RAMIPRIL 5 MG CAPSULE 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0273-01 0.05040 RAMIPRIL 5 MG CAPSULE 0 CAMBER PHARMACE EAGEN 60505-2877-01 0.05040 RAMIPRIL 5 MG CAPSULE 0 APOTEX CORP EAGEN 65862-0476-01 0.05040 RAMIPRIL 5 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0476-05 0.05040 RAMIPRIL 5 MG CAPSULE 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0267-01 0.05040 RAMIPRIL 5 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-0267-11 0.05040 RAMIPRIL 5 MG CAPSULE 0 AHP EAGEN 68180-0590-01 0.05040 RAMIPRIL 5 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68180-0590-02 0.05040 RAMIPRIL 5 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68180-0590-09 0.05040 RAMIPRIL 5 MG CAPSULE 0 LUPIN PHARMACEU EAGEN 68382-0146-01 0.05040 RAMIPRIL 5 MG CAPSULE 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 61958-1002-01 6.16621 RANEXA ER 1,000 MG TABLET 0 GILEAD SCIENCES EABND 61958-1004-01 7.04655 RANEXA ER 1,000 MG TABLET 0 GILEAD SCIENCES EABND 61958-1001-01 3.75589 RANEXA ER 500 MG TABLET 0 GILEAD SCIENCES EABND 61958-1003-01 4.29207 RANEXA ER 500 MG TABLET 0 GILEAD SCIENCES EAGEN 68382-0423-<strong>06</strong> 0.91866 RANITIDINE HCL 150 MG/6 ML VL 0 ZYDUS PHARMACEU MLGEN 55390-<strong>06</strong>16-01 0.91866 RANITIDINE HCL 25 MG/ML VIAL 0 BEDFORD LABS MLGEN 55390-<strong>06</strong>16-10 1.49850 RANITIDINE HCL 25 MG/ML VIAL 0 BEDFORD LABS MLGEN 68382-0422-02 1.40325 RANITIDINE HCL 50 MG/2 ML VIAL 0 ZYDUS PHARMACEU MLGEN 00121-0727-16 0.10464 RANITIDINE 15 MG/ML SYRUP 0 PHARMACEU ASSOC MLGEN 00472-0383-16 0.14240 RANITIDINE 15 MG/ML SYRUP 0 ACTAVIS MID ATL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-9418-58 0.14240 RANITIDINE 15 MG/ML SYRUP 0 QUALITEST MLGEN 50383-0051-16 0.14240 RANITIDINE 15 MG/ML SYRUP 0 HI-TECH PHARMAC MLGEN 54838-0550-80 0.14240 RANITIDINE 15 MG/ML SYRUP 0 SILARX PHARM MLGEN 57664-0141-34 0.14240 RANITIDINE 15 MG/ML SYRUP 0 CARACO PHARM MLGEN 60505-0351-01 0.14240 RANITIDINE 15 MG/ML SYRUP 0 APOTEX CORP MLGEN 64679-<strong>06</strong>94-01 0.14240 RANITIDINE 15 MG/ML SYRUP 0 WOCKHARDT USA L MLGEN 65162-<strong>06</strong>64-90 0.14240 RANITIDINE 15 MG/ML SYRUP 0 AMNEAL PHARMACE MLGEN 65862-0431-74 0.14240 RANITIDINE 15 MG/ML SYRUP 0 AUROBINDO PHARM MLGEN 00781-2855-05 0.68490 RANITIDINE 150 MG CAPSULE 0 SANDOZ EAGEN 00781-2855-60 0.68490 RANITIDINE 150 MG CAPSULE 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0129-05 0.68490 RANITIDINE 150 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 55111-0129-60 0.68490 RANITIDINE 150 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 00172-4357-49 0.02889 RANITIDINE 150 MG TABLET 0 TEVA USA EAGEN 00172-4357-70 0.02889 RANITIDINE 150 MG TABLET 0 TEVA USA EAGEN 00781-1883-10 0.02889 RANITIDINE 150 MG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 340LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-1883-60 0.02889 RANITIDINE 150 MG TABLET 0 SANDOZ EAGEN 49884-0544-02 0.02889 RANITIDINE 150 MG TABLET 0 PAR PHARM. EAGEN 49884-0544-10 0.02889 RANITIDINE 150 MG TABLET 0 PAR PHARM. EAGEN 53746-0253-01 0.02889 RANITIDINE 150 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0253-05 0.02889 RANITIDINE 150 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0253-10 0.02889 RANITIDINE 150 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0253-18 0.02889 RANITIDINE 150 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0253-60 0.02889 RANITIDINE 150 MG TABLET 0 AMNEAL PHARMACE EAGEN 60505-0025-04 0.02889 RANITIDINE 150 MG TABLET 0 APOTEX CORP EAGEN 60505-0025-<strong>06</strong> 0.02889 RANITIDINE 150 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0025-<strong>08</strong> 0.02889 RANITIDINE 150 MG TABLET 0 APOTEX CORP EAGEN 63739-0489-01 0.02889 RANITIDINE 150 MG TABLET 0 MCKESSON PACKAG EAGEN 63739-0489-10 0.02889 RANITIDINE 150 MG TABLET 0 MCKESSON PACKAG EAGEN 64679-0447-02 0.02889 RANITIDINE 150 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0447-04 0.02889 RANITIDINE 150 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-09<strong>06</strong>-01 0.02889 RANITIDINE 150 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-09<strong>06</strong>-03 0.02889 RANITIDINE 150 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-09<strong>06</strong>-<strong>06</strong> 0.02889 RANITIDINE 150 MG TABLET 0 WOCKHARDT USA L EAGEN 68<strong>08</strong>4-0577-01 0.02889 RANITIDINE 150 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0577-11 0.02889 RANITIDINE 150 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0248-01 0.02889 RANITIDINE 150 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0248-05 0.02889 RANITIDINE 150 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0248-60 0.02889 RANITIDINE 150 MG TABLET 0 GLENMARK PHARMA EAGEN 50383-0051-11 0.14240 RANITIDINE 150 MG/10 ML SYRUP 0 HI-TECH PHARMAC MLGEN 50383-0051-12 0.14240 RANITIDINE 150 MG/10 ML SYRUP 0 HI-TECH PHARMAC MLGEN 68094-0204-59 0.14240 RANITIDINE 150 MG/10 ML SYRUP 0 PRECISION DOSE MLGEN 00781-2865-31 1.42970 RANITIDINE 300 MG CAPSULE 0 SANDOZ EAGEN 55111-0130-01 1.42970 RANITIDINE 300 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 55111-0130-30 1.42970 RANITIDINE 300 MG CAPSULE 0 DR.REDDY'S LAB EAGUL 00172-4358-46 0.12500 RANITIDINE 300 MG TABLET 0 IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00172-4358-60 0.12500 RANITIDINE 300 MG TABLET 0 IVAX PHARMACEUT EAGUL 00781-1884-25 0.12500 RANITIDINE 300 MG TABLET 0 SANDOZ EAGUL 00781-1884-31 0.12500 RANITIDINE 300 MG TABLET 0 SANDOZ EAGUL 53746-0254-01 0.12500 RANITIDINE 300 MG TABLET 0 AMNEAL PHARMACE EAGUL 53746-0254-02 0.12500 RANITIDINE 300 MG TABLET 0 AMNEAL PHARMACE EAGUL 53746-0254-30 0.12500 RANITIDINE 300 MG TABLET 0 AMNEAL PHARMACE EAGUL 60505-0026-02 0.12500 RANITIDINE 300 MG TABLET 0 APOTEX CORP EAGUL 60505-0026-03 0.12500 RANITIDINE 300 MG TABLET 0 APOTEX CORP EAGUL 60505-0026-07 0.12500 RANITIDINE 300 MG TABLET 0 APOTEX CORP EAGUL 60505-0026-<strong>08</strong> 0.12500 RANITIDINE 300 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 64679-0742-01 0.12500 RANITIDINE 300 MG TABLET 0 WOCKHARDT USA L EAGUL 64679-0742-02 0.12500 RANITIDINE 300 MG TABLET 0 WOCKHARDT USA L EAGUL 64679-0742-04 0.12500 RANITIDINE 300 MG TABLET 0 WOCKHARDT USA L EAGUL 64679-0907-01 0.12500 RANITIDINE 300 MG TABLET 0 WOCKHARDT USA L EAGUL 64679-0907-02 0.12500 RANITIDINE 300 MG TABLET 0 WOCKHARDT USA L EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 341LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 64679-0907-04 0.12500 RANITIDINE 300 MG TABLET 0 WOCKHARDT USA L EAGUL 68462-0249-01 0.12500 RANITIDINE 300 MG TABLET 0 GLENMARK PHARMA EAGUL 68462-0249-20 0.12500 RANITIDINE 300 MG TABLET 0 GLENMARK PHARMA EAGUL 68462-0249-30 0.12500 RANITIDINE 300 MG TABLET 0 GLENMARK PHARMA EAGEN 68094-0205-58 0.14240 RANITIDINE 75 MG/5 ML SYRUP 0 PRECISION DOSE MLBND 52544-0151-30 4.68120 RAPAFLO 4 MG CAPSULE G ACTAVIS PHARMA, EABND 52544-0152-19 4.68120 RAPAFLO 8 MG CAPSULE G ACTAVIS PHARMA, EABND 52544-0152-30 4.68120 RAPAFLO 8 MG CAPSULE G ACTAVIS PHARMA, EABND 000<strong>08</strong>-1040-05 7.66139 RAPAMUNE 0.5 MG TABLET 0 WYETH PHARM EABND 000<strong>08</strong>-1041-05 15.32312 RAPAMUNE 1 MG TABLET 0 WYETH PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>08</strong>-1041-10 15.32312 RAPAMUNE 1 MG TABLET 0 WYETH PHARM EABND 000<strong>08</strong>-1030-<strong>06</strong> 15.31931 RAPAMUNE 1 MG/ML ORAL SOLN 0 WYETH PHARM MLBND 000<strong>08</strong>-1042-05 30.64625 RAPAMUNE 2 MG TABLET 0 WYETH PHARM EABND 76325-0100-04 93.37500 RAVICTI 1.1 GRAM/ML LIQUID 0 HYPERION MLBND 76325-0100-25 93.37500 RAVICTI 1.1 GRAM/ML LIQUID 0 HYPERION MLBND 75987-0020-01 30.97560 RAYOS DR 1 MG TABLET G HORIZON PHARMA EABND 75987-0021-01 30.97560 RAYOS DR 2 MG TABLET G HORIZON PHARMA EABND 75987-0022-01 30.97560 RAYOS DR 5 MG TABLET G HORIZON PHARMA EABND 50458-0388-30 2.01866 8.80132 RAZADYNE ER 16 MG CAPSULE G JANSSEN PHARM. EABND 50458-0389-30 4.98490 8.80132 RAZADYNE ER 24 MG CAPSULE G JANSSEN PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50458-0387-30 3.13700 8.80132 RAZADYNE ER 8 MG CAPSULE G JANSSEN PHARM. EABND 50458-0398-60 1.87151 4.40<strong>06</strong>6 RAZADYNE 12 MG TABLET G JANSSEN PHARM. EABND 50458-0396-60 1.87407 4.40<strong>06</strong>6 RAZADYNE 4 MG TABLET G JANSSEN PHARM. EABND 50458-0490-10 2.93355 RAZADYNE 4 MG/ML ORAL SOLUTION G JANSSEN PHARM. MLBND 50458-0397-60 1.86638 4.40<strong>06</strong>6 RAZADYNE 8 MG TABLET G JANSSEN PHARM. EABND 00<strong>08</strong>5-1194-03 1.11540 8.79493 REBETOL 200 MG CAPSULE G MERCK SHARP & D EABND 00<strong>08</strong>5-1318-01 1.93224 REBETOL 40 MG/ML SOLUTION G MERCK SHARP & D MLBND 44<strong>08</strong>7-0188-01 1146.91771 REBIF REBIDOSE TITRATION PACK G EMD SERONO, INC MLBND 44<strong>08</strong>7-3322-01 802.84240 REBIF REBIDOSE 22 MCG/0.5 ML G EMD SERONO, INC MLBND 44<strong>08</strong>7-3344-01 802.84240 REBIF REBIDOSE 44 MCG/0.5 ML G EMD SERONO, INC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 44<strong>08</strong>7-8822-01 1146.91771 REBIF TITRATION PACK G EMD SERONO, INC MLBND 44<strong>08</strong>7-0022-03 802.84240 REBIF 22 MCG/0.5 ML SYRINGE G EMD SERONO, INC MLBND 44<strong>08</strong>7-0044-03 802.84240 REBIF 44 MCG/0.5 ML SYRINGE G EMD SERONO, INC MLGEX 52544-0954-28 0.73800 RECLIPSEN 28 DAY TABLET 0 ACTAVIS PHARMA, EABND 00944-2844-10 1.<strong>08</strong>500 RECOMBINATE 1,241-1,800 UNIT V 0 BAXTER BIOSCIENBND 00944-2845-10 1.<strong>08</strong>500 RECOMBINATE 1,801-2,400 UNIT V 0 BAXTER BIOSCIENBND 00944-2841-10 1.<strong>08</strong>500 RECOMBINATE 220-400 UNIT VIAL 0 BAXTER BIOSCIENBND 00944-2842-10 1.<strong>08</strong>500 RECOMBINATE 401-800 UNIT VIAL 0 BAXTER BIOSCIENBND 00944-2843-10 1.<strong>08</strong>500 RECOMBINATE 801-1,240 UNIT VL 0 BAXTER BIOSCIENBND 58914-0301-80 13.61559 RECTIV 0.4% OINTMENT 0 APTALIS PHARMA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 62559-0166-01 0.04620 2.<strong>06</strong>205 REGLAN 10 MG TABLET G ANI PHARMACEUTI EABND 62559-0165-01 0.04280 2.<strong>06</strong>205 REGLAN 5 MG TABLET G ANI PHARMACEUTI EABND 00<strong>06</strong>4-<strong>08</strong>10-15 53.85040 REGRANEX 0.01% GEL G <strong>HEALTH</strong>POINT MED GMBND 00173-<strong>06</strong>81-01 2.93820 RELENZA 5 MG DISKHALER 0 GLAXOSMITHKLINE EABND 65649-0553-05 59.31180 RELISTOR 12 MG/0.6 ML KIT 0 SALIX PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 342LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 65649-0551-03 98.85300 RELISTOR 12 MG/0.6 ML SYRINGE 0 SALIX PHARMACEU MLBND 65649-0551-02 98.85300 RELISTOR 12 MG/0.6 ML VIAL 0 SALIX PHARMACEU MLBND 65649-0552-04 148.27950 RELISTOR 8 MG/0.4 ML SYRINGE 0 SALIX PHARMACEU MLBND 00049-2330-45 32.28423 RELPAX 20 MG TABLET G PFIZER US PHARM EABND 00049-2340-05 32.28146 RELPAX 40 MG TABLET G PFIZER US PHARM EABND 00049-2340-45 32.28423 RELPAX 40 MG TABLET G PFIZER US PHARM EABEX 00052-01<strong>06</strong>-30 0.83700 3.73555 REMERON 15 MG SOLTAB G ORGANON PHARM. EABEX 00052-0105-30 0.1<strong>08</strong>50 4.69<strong>08</strong>8 REMERON 15 MG TABLET G ORGANON PHARM. EABEX 00052-01<strong>08</strong>-30 0.86225 3.84898 REMERON 30 MG SOLTAB G ORGANON PHARM. EABEX 00052-0107-30 0.13590 4.83198 REMERON 30 MG TABLET G ORGANON PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00052-0110-30 1.12415 4.10020 REMERON 45 MG SOLTAB G ORGANON PHARM. EABEX 00052-0109-30 0.24400 4.92328 REMERON 45 MG TABLET G ORGANON PHARM. EABND 66302-0101-01 61.17100 REMODULIN 1 MG/ML VIAL 0 UNITED THERAP MLBND 66302-0110-01 611.71000 REMODULIN 10 MG/ML VIAL 0 UNITED THERAP MLBND 66302-0102-01 152.92750 REMODULIN 2.5 MG/ML VIAL 0 UNITED THERAP MLBND 66302-0105-01 305.85500 REMODULIN 5 MG/ML VIAL 0 UNITED THERAP MLBND 00327-0011-05 0.<strong>06</strong>972 RENACIDIN IRRIGATION SOLN 0 GUARDIAN CHEM. MLBND 58468-0020-01 2.18778 RENAGEL 400 MG TABLET 0 GENZYME EABND 58468-0021-01 4.37557 RENAGEL 800 MG TABLET 0 GENZYME EABND 58468-0132-01 10.49950 RENVELA 0.8 GM POWDER PACKET G GENZYME EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 58468-0132-02 10.50097 RENVELA 0.8 GM POWDER PACKET G GENZYME EABND 58468-0131-01 10.49950 RENVELA 2.4 GM POWDER PACKET G GENZYME EABND 58468-0131-02 10.50097 RENVELA 2.4 GM POWDER PACKET G GENZYME EABND 58468-0130-01 3.50032 RENVELA 800 MG TABLET 0 GENZYME EAGEN 00378-3121-01 2.49765 REPAGLINIDE 0.5 MG TABLET G MYLAN EAGEN 00378-3121-05 2.49762 REPAGLINIDE 0.5 MG TABLET G MYLAN EAGEN 00574-0240-01 0.5<strong>06</strong>25 REPAGLINIDE 0.5 MG TABLET G PADDOCK LABS. EAGEN 00781-5148-01 2.49765 REPAGLINIDE 0.5 MG TABLET G SANDOZ EAGEN 51079-0<strong>06</strong>2-03 2.80449 REPAGLINIDE 0.5 MG TABLET G MYLAN INSTITUTI EAGEN 65862-<strong>06</strong>70-01 2.49765 REPAGLINIDE 0.5 MG TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-3122-01 2.49765 REPAGLINIDE 1 MG TABLET G MYLAN EAGEN 00574-0241-01 0.5<strong>06</strong>25 REPAGLINIDE 1 MG TABLET G PADDOCK LABS. EAGEN 00781-5149-01 2.74462 REPAGLINIDE 1 MG TABLET G SANDOZ EAGEN 57664-0745-13 2.74462 REPAGLINIDE 1 MG TABLET G CARACO PHARM EAGEN 57664-0745-88 2.74462 REPAGLINIDE 1 MG TABLET G CARACO PHARM EAGEN 65862-<strong>06</strong>71-01 2.74462 REPAGLINIDE 1 MG TABLET G AUROBINDO PHARM EAGEN 00378-3123-01 2.49765 REPAGLINIDE 2 MG TABLET G MYLAN EAGEN 00574-0242-01 0.5<strong>06</strong>25 REPAGLINIDE 2 MG TABLET G PADDOCK LABS. EAGEN 00781-5150-01 2.74462 REPAGLINIDE 2 MG TABLET G SANDOZ EAGEN 57664-0747-13 2.74462 REPAGLINIDE 2 MG TABLET G CARACO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 57664-0747-88 2.74462 REPAGLINIDE 2 MG TABLET G CARACO PHARM EAGEN 65862-<strong>06</strong>72-01 2.74462 REPAGLINIDE 2 MG TABLET G AUROBINDO PHARM EABND 00007-4882-13 10.55980 13.50963 REQUIP XL 12 MG TABLET G GLAXOSMITHKLINE EABND 00007-4885-13 2.10990 2.70192 REQUIP XL 2 MG TABLET G GLAXOSMITHKLINE EABND 00007-4885-59 2.10990 2.70192 REQUIP XL 2 MG TABLET G GLAXOSMITHKLINE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 343LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00007-4887-13 4.22320 5.40385 REQUIP XL 4 MG TABLET G GLAXOSMITHKLINE EABND 00007-4887-59 4.22320 5.40394 REQUIP XL 4 MG TABLET G GLAXOSMITHKLINE EABND 00007-4883-13 6.43304 8.10578 REQUIP XL 6 MG TABLET G GLAXOSMITHKLINE EABND 00007-4888-13 5.95665 8.10578 REQUIP XL 8 MG TABLET G GLAXOSMITHKLINE EABND 00007-4890-20 0.24340 3.36150 REQUIP 0.25 MG TABLET G GLAXOSMITHKLINE EABND 00007-4891-20 0.27330 3.36150 REQUIP 0.5 MG TABLET G GLAXOSMITHKLINE EABND 00007-4892-20 0.23560 3.36150 REQUIP 1 MG TABLET G GLAXOSMITHKLINE EABND 00007-4893-20 0.15836 3.36150 REQUIP 2 MG TABLET G GLAXOSMITHKLINE EABND 00007-4895-20 0.15836 3.48666 REQUIP 3 MG TABLET G GLAXOSMITHKLINE EABND 00007-4896-20 0.17159 3.48666 REQUIP 4 MG TABLET G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00007-4894-20 0.17159 3.48666 REQUIP 5 MG TABLET G GLAXOSMITHKLINE EABND 49702-0210-17 1.76093 RESCRIPTOR 200 MG TABLET G VIIV <strong>HEALTH</strong>CARE EABND 49702-0225-17 1.99504 RESCRIPTOR 200 MG TABLET G VIIV <strong>HEALTH</strong>CARE EABND 63010-0021-18 1.65040 RESCRIPTOR 200 MG TABLET G AGOURON PHARM EABND 17350-0015-05 21.49534 RESCULA 0.15% EYE DROPS G SUCAMPO PHARMA MLBND 00264-2303-50 0.00439 RESECTISOL 5% SOLUTION 0 B.BRAUN MLBND 00185-0032-01 0.99<strong>06</strong>8 RESERPINE 0.1 MG TABLET 0 SANDOZ EABND 00185-0032-10 0.99071 RESERPINE 0.1 MG TABLET 0 SANDOZ EABND 00185-0134-01 1.36709 RESERPINE 0.25 MG TABLET 0 SANDOZ EABND 00023-9163-30 5.29180 RESTASIS 0.05% EYE EMULSION G ALLERGAN INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00023-9163-60 5.29152 RESTASIS 0.05% EYE EMULSION G ALLERGAN INC. EABND 49702-0212-48 0.12623 0.26874 RETROVIR 10 MG/ML SYRUP G VIIV <strong>HEALTH</strong>CARE MLBND 49702-0211-20 0.83457 2.68778 RETROVIR 100 MG CAPSULE G VIIV <strong>HEALTH</strong>CARE EABND 49702-0214-18 0.49572 8.<strong>06</strong>400 RETROVIR 300 MG TABLET G VIIV <strong>HEALTH</strong>CARE EABND 00<strong>06</strong>9-4190-68 0.58010 23.27144 REVATIO 20 MG TABLET G PFIZER US PHARM EAGEN 51285-0275-01 1.0<strong>08</strong>00 REVIA 50 MG TABLET 0 DURAMED/BARR EAGEN 51285-0275-02 1.0<strong>08</strong>00 REVIA 50 MG TABLET 0 DURAMED/BARR EABND 59572-0410-00 417.80216 REVLIMID 10 MG CAPSULE 0 CELGENE EABND 59572-0410-28 417.80213 REVLIMID 10 MG CAPSULE 0 CELGENE EABND 59572-0415-00 419.5<strong>06</strong>15 REVLIMID 15 MG CAPSULE 0 CELGENE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59572-0415-21 419.5<strong>06</strong>11 REVLIMID 15 MG CAPSULE 0 CELGENE EABND 59572-0402-00 410.74791 REVLIMID 2.5 MG CAPSULE 0 CELGENE EABND 59572-0402-28 410.74802 REVLIMID 2.5 MG CAPSULE 0 CELGENE EABND 59572-0420-00 423.63017 REVLIMID 20 MG CAPSULE 0 CELGENE EABND 59572-0420-21 423.63042 REVLIMID 20 MG CAPSULE 0 CELGENE EABND 59572-0425-00 423.63017 REVLIMID 25 MG CAPSULE 0 CELGENE EABND 59572-0425-21 423.63042 REVLIMID 25 MG CAPSULE 0 CELGENE EABND 59572-0405-00 410.74791 REVLIMID 5 MG CAPSULE 0 CELGENE EABND 59572-0405-28 410.74802 REVLIMID 5 MG CAPSULE 0 CELGENE EABND 00003-3623-12 18.24146 REYATAZ 100 MG CAPSULE G BMS PRIMARYCARE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00003-3624-12 19.68247 REYATAZ 150 MG CAPSULE G BMS PRIMARYCARE EABND 00003-3631-12 19.68247 REYATAZ 200 MG CAPSULE G BMS PRIMARYCARE EABND 00003-3622-12 38.99312 REYATAZ 300 MG CAPSULE G BMS PRIMARYCARE EABUL 67253-0580-42 1.26370 11.18425 RHEUMATREX 2.5 MG TABLET G DAVA PHARMACEUT EABUL 67253-0580-43 1.26370 11.16488 RHEUMATREX 2.5 MG TABLET G DAVA PHARMACEUT EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 344LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 67253-0580-44 1.26370 11.17802 RHEUMATREX 2.5 MG TABLET G DAVA PHARMACEUT EABUL 67253-0580-45 1.26370 11.18093 RHEUMATREX 2.5 MG TABLET G DAVA PHARMACEUT EABUL 67253-0580-46 1.26370 11.17802 RHEUMATREX 2.5 MG TABLET G DAVA PHARMACEUT EABND 00186-1070-<strong>08</strong> 17.21478 RHINOCORT AQUA NASAL SPRAY G ASTRAZENECA GMGEN 66435-01<strong>08</strong>-56 11.73951 RIBAPAK 200-400 MG DOSEPACK G KADMON PHARMACE EABND 66435-01<strong>08</strong>-99 12.99172 RIBAPAK 200-400 MG DOSEPACK G KADMON PHARMACE EAGEN 66435-0105-56 12.35732 RIBAPAK 400-400 MG DOSEPACK G KADMON PHARMACE EAGEN 66435-0105-99 12.35732 RIBAPAK 400-400 MG DOSEPACK G KADMON PHARMACE EAGEN 66435-01<strong>06</strong>-56 15.44678 RIBAPAK 600-400 MG DOSEPACK G KADMON PHARMACE EAGEN 66435-01<strong>06</strong>-99 15.44691 RIBAPAK 600-400 MG DOSEPACK G KADMON PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 66435-0107-56 18.53625 RIBAPAK 600-600 MG DOSEPACK G KADMON PHARMACE EAGEN 66435-0107-99 18.53598 RIBAPAK 600-600 MG DOSEPACK G KADMON PHARMACE EAGEN 66435-0101-18 1.11540 RIBASPHERE 200 MG CAPSULE G KADMON PHARMACE EAGEN 66435-0101-42 1.11540 RIBASPHERE 200 MG CAPSULE G KADMON PHARMACE EAGEN 66435-0101-56 1.11540 RIBASPHERE 200 MG CAPSULE G KADMON PHARMACE EAGEN 66435-0101-70 1.11540 RIBASPHERE 200 MG CAPSULE G KADMON PHARMACE EAGEN 66435-0101-84 1.11540 RIBASPHERE 200 MG CAPSULE G KADMON PHARMACE EAGEN 66435-0102-16 0.65250 RIBASPHERE 200 MG TABLET G KADMON PHARMACE EABND 66435-0103-56 13.67543 RIBASPHERE 400 MG TABLET G KADMON PHARMACE EABND 66435-0104-56 20.51315 RIBASPHERE 600 MG TABLET G KADMON PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7227-58 1.11540 RIBAVIRIN 200 MG CAPSULE 0 TEVA USA EAGEN 00093-7227-63 1.11540 RIBAVIRIN 200 MG CAPSULE 0 TEVA USA EAGEN 00093-7227-72 1.11540 RIBAVIRIN 200 MG CAPSULE 0 TEVA USA EAGEN 00093-7227-77 1.11540 RIBAVIRIN 200 MG CAPSULE 0 TEVA USA EAGEN 00781-2043-04 1.11540 RIBAVIRIN 200 MG CAPSULE 0 SANDOZ EAGEN 00781-2043-16 1.11540 RIBAVIRIN 200 MG CAPSULE 0 SANDOZ EAGEN 00781-2043-42 1.11540 RIBAVIRIN 200 MG CAPSULE 0 SANDOZ EAGEN 00781-2043-67 1.11540 RIBAVIRIN 200 MG CAPSULE 0 SANDOZ EAGEN 65862-0290-42 1.11540 RIBAVIRIN 200 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0290-70 1.11540 RIBAVIRIN 200 MG CAPSULE 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0290-84 1.11540 RIBAVIRIN 200 MG CAPSULE 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0179-11 1.11540 RIBAVIRIN 200 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-0179-65 1.11540 RIBAVIRIN 200 MG CAPSULE 0 AHP EAGEN 68382-0260-04 1.11540 RIBAVIRIN 200 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 68382-0260-07 1.11540 RIBAVIRIN 200 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 68382-0260-09 1.11540 RIBAVIRIN 200 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 68382-0260-12 1.11540 RIBAVIRIN 200 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 00093-7232-81 0.65250 RIBAVIRIN 200 MG TABLET 0 TEVA USA EAGEN 00781-5177-28 0.65250 RIBAVIRIN 200 MG TABLET 0 SANDOZ EAGEN 65862-0207-68 0.65250 RIBAVIRIN 200 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0150-11 0.65250 RIBAVIRIN 200 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0150-65 0.65250 RIBAVIRIN 200 MG TABLET 0 AHP EAGEN 68382-0046-03 0.65250 RIBAVIRIN 200 MG TABLET 0 ZYDUS PHARMACEU EABND 65483-0093-<strong>06</strong> 16.93<strong>08</strong>9 RIDAURA 3 MG CAPSULE 0 PROMETHEUS EABEX 00<strong>06</strong>8-0597-01 177.84410 RIFADIN IV 600 MG VIAL G SAN<strong>OF</strong>I-AVENTIS EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 345LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00<strong>06</strong>8-0510-30 1.<strong>06</strong>890 RIFADIN 150 MG CAPSULE 0 SAN<strong>OF</strong>I-AVENTIS EABEX 00<strong>06</strong>8-05<strong>08</strong>-60 0.70920 4.59128 RIFADIN 300 MG CAPSULE G SAN<strong>OF</strong>I-AVENTIS EABEX 00<strong>06</strong>8-05<strong>08</strong>-61 0.70920 4.59338 RIFADIN 300 MG CAPSULE G SAN<strong>OF</strong>I-AVENTIS EABEX 00<strong>06</strong>8-0509-60 1.53200 5.28959 RIFAMATE CAPSULE 0 SAN<strong>OF</strong>I-AVENTIS EAGEX 00<strong>06</strong>9-0141-01 58.50000 RIFAMPIN IV 600 MG VIAL 0 PFIZER/NOVAPLUS EAGEX 17478-0151-42 102.18750 RIFAMPIN IV 600 MG VIAL 0 AKORN INC. EAGEX 55390-0123-01 58.50000 RIFAMPIN IV 600 MG VIAL 0 BEDFORD LABS EAGEX 00185-<strong>08</strong>01-01 1.<strong>06</strong>890 RIFAMPIN 150 MG CAPSULE 0 SANDOZ EAGEX 00185-<strong>08</strong>01-30 1.<strong>06</strong>890 RIFAMPIN 150 MG CAPSULE 0 SANDOZ EAGEX 00527-1393-01 1.<strong>06</strong>890 RIFAMPIN 150 MG CAPSULE 0 LANNETT CO. INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00527-1393-30 1.<strong>06</strong>890 RIFAMPIN 150 MG CAPSULE 0 LANNETT CO. INC EAGEX 61748-0015-30 1.<strong>06</strong>890 RIFAMPIN 150 MG CAPSULE 0 VERSA PHARMACEU EAGEX 68<strong>08</strong>4-0357-11 1.<strong>06</strong>890 RIFAMPIN 150 MG CAPSULE 0 AHP EAGEX 68<strong>08</strong>4-0357-21 1.<strong>06</strong>890 RIFAMPIN 150 MG CAPSULE 0 AHP EAGEX 68180-<strong>06</strong>58-01 1.<strong>06</strong>890 RIFAMPIN 150 MG CAPSULE 0 LUPIN PHARMACEU EAGEX 68180-<strong>06</strong>58-<strong>06</strong> 1.<strong>06</strong>890 RIFAMPIN 150 MG CAPSULE 0 LUPIN PHARMACEU EAGEX 00185-0799-01 0.70920 RIFAMPIN 300 MG CAPSULE 0 SANDOZ EAGEX 00185-0799-05 0.70920 RIFAMPIN 300 MG CAPSULE 0 SANDOZ EAGEX 00185-0799-30 0.70920 RIFAMPIN 300 MG CAPSULE 0 SANDOZ EAGEX 00185-0799-60 0.70920 RIFAMPIN 300 MG CAPSULE 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00527-1315-01 0.70920 RIFAMPIN 300 MG CAPSULE 0 LANNETT CO. INC EAGEX 00527-1315-<strong>06</strong> 0.70920 RIFAMPIN 300 MG CAPSULE 0 LANNETT CO. INC EAGEX 00527-1315-30 0.70920 RIFAMPIN 300 MG CAPSULE 0 LANNETT CO. INC EAGEX 00904-5282-<strong>06</strong> 0.70920 RIFAMPIN 300 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 61748-0018-01 0.70920 RIFAMPIN 300 MG CAPSULE 0 VERSA PHARMACEU EAGEX 61748-0018-30 0.70920 RIFAMPIN 300 MG CAPSULE 0 VERSA PHARMACEU EAGEX 61748-0018-60 0.70920 RIFAMPIN 300 MG CAPSULE 0 VERSA PHARMACEU EAGEX 63739-0415-10 0.70920 RIFAMPIN 300 MG CAPSULE 0 MCKESSON PACKAG EAGEX 68<strong>08</strong>4-0358-01 0.70920 RIFAMPIN 300 MG CAPSULE 0 AHP EAGEX 68<strong>08</strong>4-0358-11 0.70920 RIFAMPIN 300 MG CAPSULE 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68180-<strong>06</strong>59-01 0.70920 RIFAMPIN 300 MG CAPSULE 0 LUPIN PHARMACEU EAGEX 68180-<strong>06</strong>59-<strong>06</strong> 0.70920 RIFAMPIN 300 MG CAPSULE 0 LUPIN PHARMACEU EAGEX 68180-<strong>06</strong>59-07 0.70920 RIFAMPIN 300 MG CAPSULE 0 LUPIN PHARMACEU EABEX 00<strong>08</strong>8-0576-41 3.91483 RIFATER TABLET 0 SAN<strong>OF</strong>I-AVENTIS EABND 00075-7700-60 3.26970 34.46160 RILUTEK 50 MG TABLET 0 COVIS PHARMACEU EABND 24987-0700-60 3.26970 34.46160 RILUTEK 50 MG TABLET 0 COVIS PHARMACEU EAGEN 00115-3000-13 3.26970 RILUZOLE 50 MG TABLET 0 GLOBAL PHARM EAGEN 00378-4145-91 3.26970 RILUZOLE 50 MG TABLET 0 MYLAN EAGEN 60505-3285-<strong>06</strong> 3.26970 RILUZOLE 50 MG TABLET 0 APOTEX CORP EAGEN 64980-0191-<strong>06</strong> 3.26970 RILUZOLE 50 MG TABLET 0 RISING PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0381-60 3.26970 RILUZOLE 50 MG TABLET 0 GLENMARK PHARMA EAGEN 00264-7780-00 0.00165 RINGER'S IV SOLUTION 0 B.BRAUN MLBND 1<strong>06</strong>31-02<strong>06</strong>-02 0.44644 RIOMET 500 MG/5 ML SOLUTION G RANBAXY LABORAT MLBEX 50458-0395-28 1.71360 7.32001 RISPERDAL M-TAB 0.5 MG ODT G JANSSEN PHARM. EABEX 50458-0395-30 1.71360 7.32032 RISPERDAL M-TAB 0.5 MG ODT G JANSSEN PHARM. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 346LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 50458-0315-28 8.55256 RISPERDAL M-TAB 1 MG ODT G JANSSEN PHARM. EABEX 50458-0315-30 8.55425 RISPERDAL M-TAB 1 MG ODT G JANSSEN PHARM. EABEX 50458-0325-28 3.09300 13.9<strong>06</strong><strong>06</strong> RISPERDAL M-TAB 2 MG ODT G JANSSEN PHARM. EABEX 50458-0335-28 7.47050 17.54323 RISPERDAL M-TAB 3 MG ODT G JANSSEN PHARM. EABEX 50458-0355-28 8.53357 23.56399 RISPERDAL M-TAB 4 MG ODT G JANSSEN PHARM. EABEX 50458-0301-04 0.27150 6.38436 RISPERDAL 0.25 MG TABLET G JANSSEN PHARM. EABEX 50458-0301-50 0.27150 6.38354 RISPERDAL 0.25 MG TABLET G JANSSEN PHARM. EABEX 50458-0302-<strong>06</strong> 0.29800 7.00588 RISPERDAL 0.5 MG TABLET G JANSSEN PHARM. EABEX 50458-0302-50 0.29800 7.00543 RISPERDAL 0.5 MG TABLET G JANSSEN PHARM. EABEX 50458-0300-<strong>06</strong> 0.23480 7.44814 RISPERDAL 1 MG TABLET G JANSSEN PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 50458-0300-50 0.23480 7.44712 RISPERDAL 1 MG TABLET G JANSSEN PHARM. EABEX 50458-0305-03 8.32158 RISPERDAL 1 MG/ML SOLUTION G JANSSEN PHARM. MLBEX 50458-0320-<strong>06</strong> 0.24180 12.44723 RISPERDAL 2 MG TABLET G JANSSEN PHARM. EABEX 50458-0320-50 0.24180 12.44687 RISPERDAL 2 MG TABLET G JANSSEN PHARM. EABEX 50458-0330-<strong>06</strong> 0.18040 14.61976 RISPERDAL 3 MG TABLET G JANSSEN PHARM. EABEX 50458-0330-50 0.18040 14.61885 RISPERDAL 3 MG TABLET G JANSSEN PHARM. EABEX 50458-0350-<strong>06</strong> 0.24170 19.63628 RISPERDAL 4 MG TABLET G JANSSEN PHARM. EAGEX 00378-6042-28 2.95459 RISPERIDONE 0.25 MG ODT G MYLAN EAGEX 49884-0212-52 2.95459 RISPERIDONE 0.25 MG ODT G PAR PHARM. EAGEX 49884-0212-55 2.95459 RISPERIDONE 0.25 MG ODT G PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-0221-<strong>06</strong> 0.27150 RISPERIDONE 0.25 MG TABLET G TEVA USA EAGEX 00378-3502-05 0.27150 RISPERIDONE 0.25 MG TABLET G MYLAN EAGEX 00378-3502-91 0.27150 RISPERIDONE 0.25 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-5683-20 0.27150 RISPERIDONE 0.25 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-5683-28 0.27150 RISPERIDONE 0.25 MG TABLET G QUALITEST EAGEX 00904-5973-61 0.27150 RISPERIDONE 0.25 MG TABLET G MAJOR PHARMACEU EAGEX 00904-6357-61 0.27150 RISPERIDONE 0.25 MG TABLET G MAJOR PHARMACEU EAGEX 13107-0119-05 0.27150 RISPERIDONE 0.25 MG TABLET G AUROBINDO PHARM EAGEX 13107-0119-60 0.27150 RISPERIDONE 0.25 MG TABLET G AUROBINDO PHARM EAGEX 13668-0035-05 0.27150 RISPERIDONE 0.25 MG TABLET G TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0035-60 0.27150 RISPERIDONE 0.25 MG TABLET G TORRENT PHARMAC EAGEX 50458-0590-10 0.27150 RISPERIDONE 0.25 MG TABLET G PATRIOT PHARMAC EAGEX 50458-0590-50 0.27150 RISPERIDONE 0.25 MG TABLET G PATRIOT PHARMAC EAGEX 50458-0590-60 0.27150 RISPERIDONE 0.25 MG TABLET G PATRIOT PHARMAC EAGEX 51079-0460-20 0.27150 RISPERIDONE 0.25 MG TABLET G MYLAN INSTITUTI EAGEX 51991-0316-<strong>06</strong> 0.27150 RISPERIDONE 0.25 MG TABLET G BRECKENRIDGE EAGEX 60505-2584-05 0.27150 RISPERIDONE 0.25 MG TABLET G APOTEX CORP EAGEX 60505-2584-<strong>06</strong> 0.27150 RISPERIDONE 0.25 MG TABLET G APOTEX CORP EAGEX 64679-0553-02 0.27150 RISPERIDONE 0.25 MG TABLET G WOCKHARDT USA L EAGEX 64679-0553-04 0.27150 RISPERIDONE 0.25 MG TABLET G WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0119-05 0.27150 RISPERIDONE 0.25 MG TABLET G AUROBINDO PHARM EAGEX 65862-0119-60 0.27150 RISPERIDONE 0.25 MG TABLET G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0270-01 0.27150 RISPERIDONE 0.25 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0270-11 0.27150 RISPERIDONE 0.25 MG TABLET G AHP EAGEX 68382-0112-05 0.27150 RISPERIDONE 0.25 MG TABLET G ZYDUS PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 347LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68382-0112-14 0.27150 RISPERIDONE 0.25 MG TABLET G ZYDUS PHARMACEU EAGEX 00378-6043-28 1.71360 RISPERIDONE 0.5 MG ODT G MYLAN EAGEX 00378-6043-93 1.71360 RISPERIDONE 0.5 MG ODT G MYLAN EAGEX 00781-5310-<strong>08</strong> 1.71360 RISPERIDONE 0.5 MG ODT G SANDOZ EAGEX 49884-0311-52 1.71360 RISPERIDONE 0.5 MG ODT G PAR PHARM. EAGEX 49884-0311-55 1.71360 RISPERIDONE 0.5 MG ODT G PAR PHARM. EAGEX 49884-0311-91 1.71360 RISPERIDONE 0.5 MG ODT G PAR PHARM. EAGEX 50458-<strong>06</strong>01-28 1.71360 RISPERIDONE 0.5 MG ODT G PATRIOT PHARMAC EAGEX 55111-0207-81 1.71360 RISPERIDONE 0.5 MG ODT G DR.REDDY'S LAB EAGEX 59746-0010-32 1.71360 RISPERIDONE 0.5 MG ODT G CADISTA PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68382-0154-<strong>06</strong> 1.71360 RISPERIDONE 0.5 MG ODT G ZYDUS PHARMACEU EAGEX 00093-0225-<strong>06</strong> 0.29800 RISPERIDONE 0.5 MG TABLET G TEVA USA EAGEX 00378-3505-05 0.29800 RISPERIDONE 0.5 MG TABLET G MYLAN EAGEX 00378-3505-91 0.29800 RISPERIDONE 0.5 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-5684-20 0.29800 RISPERIDONE 0.5 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-5684-28 0.29800 RISPERIDONE 0.5 MG TABLET G QUALITEST EAGEX 00904-5974-61 0.29800 RISPERIDONE 0.5 MG TABLET G MAJOR PHARMACEU EAGEX 00904-6358-61 0.29800 RISPERIDONE 0.5 MG TABLET G MAJOR PHARMACEU EAGEX 13107-0120-05 0.29800 RISPERIDONE 0.5 MG TABLET G AUROBINDO PHARM EAGEX 13107-0120-60 0.29800 RISPERIDONE 0.5 MG TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0036-05 0.29800 RISPERIDONE 0.5 MG TABLET G TORRENT PHARMAC EAGEX 13668-0036-60 0.29800 RISPERIDONE 0.5 MG TABLET G TORRENT PHARMAC EAGEX 50458-0591-10 0.29800 RISPERIDONE 0.5 MG TABLET G PATRIOT PHARMAC EAGEX 50458-0591-50 0.29800 RISPERIDONE 0.5 MG TABLET G PATRIOT PHARMAC EAGEX 50458-0591-60 0.29800 RISPERIDONE 0.5 MG TABLET G PATRIOT PHARMAC EAGEX 51079-0461-01 0.29800 RISPERIDONE 0.5 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0461-20 0.29800 RISPERIDONE 0.5 MG TABLET G MYLAN INSTITUTI EAGEX 51991-0317-<strong>06</strong> 0.29800 RISPERIDONE 0.5 MG TABLET G BRECKENRIDGE EAGEX 60505-2585-05 0.29800 RISPERIDONE 0.5 MG TABLET G APOTEX CORP EAGEX 60505-2585-<strong>06</strong> 0.29800 RISPERIDONE 0.5 MG TABLET G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64679-0554-02 0.29800 RISPERIDONE 0.5 MG TABLET G WOCKHARDT USA L EAGEX 64679-0554-04 0.29800 RISPERIDONE 0.5 MG TABLET G WOCKHARDT USA L EAGEX 65862-0120-05 0.29800 RISPERIDONE 0.5 MG TABLET G AUROBINDO PHARM EAGEX 65862-0120-60 0.29800 RISPERIDONE 0.5 MG TABLET G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0271-01 0.29800 RISPERIDONE 0.5 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0271-11 0.29800 RISPERIDONE 0.5 MG TABLET G AHP EAGEX 68382-0113-05 0.29800 RISPERIDONE 0.5 MG TABLET G ZYDUS PHARMACEU EAGEX 68382-0113-14 0.29800 RISPERIDONE 0.5 MG TABLET G ZYDUS PHARMACEU EAGEX 00378-6044-28 2.88000 RISPERIDONE 1 MG ODT G MYLAN EAGEX 00378-6044-93 2.88000 RISPERIDONE 1 MG ODT G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-5311-<strong>08</strong> 2.88000 RISPERIDONE 1 MG ODT G SANDOZ EAGEX 49884-0315-52 2.88000 RISPERIDONE 1 MG ODT G PAR PHARM. EAGEX 49884-0315-55 2.88000 RISPERIDONE 1 MG ODT G PAR PHARM. EAGEX 49884-0315-91 2.88000 RISPERIDONE 1 MG ODT G PAR PHARM. EAGEX 50458-<strong>06</strong>02-28 4.12687 RISPERIDONE 1 MG ODT G PATRIOT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 348LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0345-01 2.88000 RISPERIDONE 1 MG ODT G MYLAN INSTITUTI EAGEX 51079-0345-05 2.88000 RISPERIDONE 1 MG ODT G MYLAN INSTITUTI EAGEX 55111-02<strong>08</strong>-81 2.88000 RISPERIDONE 1 MG ODT G DR.REDDY'S LAB EAGEX 68382-0155-<strong>06</strong> 2.88000 RISPERIDONE 1 MG ODT G ZYDUS PHARMACEU EAGEX 00093-7240-<strong>06</strong> 0.23480 RISPERIDONE 1 MG TABLET G TEVA USA EAGEX 00378-3511-05 0.23480 RISPERIDONE 1 MG TABLET G MYLAN EAGEX 00378-3511-91 0.23480 RISPERIDONE 1 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-5685-20 0.23480 RISPERIDONE 1 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-5685-28 0.23480 RISPERIDONE 1 MG TABLET G QUALITEST EAGEX 00904-5975-61 0.23480 RISPERIDONE 1 MG TABLET G MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00904-6359-61 0.23480 RISPERIDONE 1 MG TABLET G MAJOR PHARMACEU EAGEX 13107-0121-05 0.23480 RISPERIDONE 1 MG TABLET G AUROBINDO PHARM EAGEX 13107-0121-60 0.23480 RISPERIDONE 1 MG TABLET G AUROBINDO PHARM EAGEX 13668-0037-05 0.23480 RISPERIDONE 1 MG TABLET G TORRENT PHARMAC EAGEX 13668-0037-60 0.23480 RISPERIDONE 1 MG TABLET G TORRENT PHARMAC EAGEX 50458-0592-10 0.23480 RISPERIDONE 1 MG TABLET G PATRIOT PHARMAC EAGEX 50458-0592-50 0.23480 RISPERIDONE 1 MG TABLET G PATRIOT PHARMAC EAGEX 50458-0592-60 0.23480 RISPERIDONE 1 MG TABLET G PATRIOT PHARMAC EAGEX 51079-0462-01 0.23480 RISPERIDONE 1 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0462-20 0.23480 RISPERIDONE 1 MG TABLET G MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0462-30 0.23480 RISPERIDONE 1 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0462-56 0.23480 RISPERIDONE 1 MG TABLET G MYLAN INSTITUTI EAGEX 51991-0318-<strong>06</strong> 0.23480 RISPERIDONE 1 MG TABLET G BRECKENRIDGE EAGEX 55111-0203-60 0.23480 RISPERIDONE 1 MG TABLET G DR.REDDY'S LAB EAGEX 60505-2586-00 0.23480 RISPERIDONE 1 MG TABLET G APOTEX CORP EAGEX 60505-2586-05 0.23480 RISPERIDONE 1 MG TABLET G APOTEX CORP EAGEX 60505-2586-<strong>06</strong> 0.23480 RISPERIDONE 1 MG TABLET G APOTEX CORP EAGEX 63739-0546-10 0.23480 RISPERIDONE 1 MG TABLET G MCKESSON PACKAG EAGEX 64679-0555-02 0.23480 RISPERIDONE 1 MG TABLET G WOCKHARDT USA L EAGEX 64679-0555-04 0.23480 RISPERIDONE 1 MG TABLET G WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0121-05 0.23480 RISPERIDONE 1 MG TABLET G AUROBINDO PHARM EAGEX 65862-0121-60 0.23480 RISPERIDONE 1 MG TABLET G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0272-01 0.23480 RISPERIDONE 1 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0272-11 0.23480 RISPERIDONE 1 MG TABLET G AHP EAGEX 68382-0114-05 0.23480 RISPERIDONE 1 MG TABLET G ZYDUS PHARMACEU EAGEX 68382-0114-14 0.23480 RISPERIDONE 1 MG TABLET G ZYDUS PHARMACEU EAGEX 00054-0<strong>06</strong>3-44 0.80730 RISPERIDONE 1 MG/ML SOLUTION G ROXANE LABS. MLGEX 0<strong>06</strong>03-9424-45 0.80730 RISPERIDONE 1 MG/ML SOLUTION G QUALITEST MLGEX 50458-0596-01 3.65349 RISPERIDONE 1 MG/ML SOLUTION G PATRIOT PHARMAC MLGEX 60505-0380-01 0.80730 RISPERIDONE 1 MG/ML SOLUTION G APOTEX CORP ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64679-<strong>06</strong>92-01 0.80730 RISPERIDONE 1 MG/ML SOLUTION G WOCKHARDT USA L MLGEX 65162-<strong>06</strong>73-84 0.80730 RISPERIDONE 1 MG/ML SOLUTION G AMNEAL PHARMACE MLGEX 65862-0167-30 0.80730 RISPERIDONE 1 MG/ML SOLUTION G AUROBINDO PHARM MLGEX 00378-6045-28 3.09300 RISPERIDONE 2 MG ODT G MYLAN EAGEX 00781-5312-<strong>08</strong> 3.09300 RISPERIDONE 2 MG ODT G SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 349LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 49884-0401-52 3.09300 RISPERIDONE 2 MG ODT G PAR PHARM. EAGEX 49884-0401-91 3.09300 RISPERIDONE 2 MG ODT G PAR PHARM. EAGEX 50458-<strong>06</strong>03-28 3.09300 RISPERIDONE 2 MG ODT G PATRIOT PHARMAC EAGEX 51079-0346-01 3.09300 RISPERIDONE 2 MG ODT G MYLAN INSTITUTI EAGEX 51079-0346-05 3.09300 RISPERIDONE 2 MG ODT G MYLAN INSTITUTI EAGEX 55111-0209-81 3.09300 RISPERIDONE 2 MG ODT G DR.REDDY'S LAB EAGEX 68382-0156-<strong>06</strong> 3.09300 RISPERIDONE 2 MG ODT G ZYDUS PHARMACEU EAGEX 00093-7241-<strong>06</strong> 0.24180 RISPERIDONE 2 MG TABLET G TEVA USA EAGEX 00378-3512-05 0.24180 RISPERIDONE 2 MG TABLET G MYLAN EAGEX 00378-3512-91 0.24180 RISPERIDONE 2 MG TABLET G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-5686-20 0.24180 RISPERIDONE 2 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-5686-28 0.24180 RISPERIDONE 2 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-5686-32 0.24180 RISPERIDONE 2 MG TABLET G QUALITEST EAGEX 00904-5976-61 0.24180 RISPERIDONE 2 MG TABLET G MAJOR PHARMACEU EAGEX 00904-6360-61 0.24180 RISPERIDONE 2 MG TABLET G MAJOR PHARMACEU EAGEX 13107-0122-05 0.24180 RISPERIDONE 2 MG TABLET G AUROBINDO PHARM EAGEX 13107-0122-60 0.24180 RISPERIDONE 2 MG TABLET G AUROBINDO PHARM EAGEX 13668-0038-05 0.24180 RISPERIDONE 2 MG TABLET G TORRENT PHARMAC EAGEX 13668-0038-60 0.24180 RISPERIDONE 2 MG TABLET G TORRENT PHARMAC EAGEX 50458-0593-10 0.24180 RISPERIDONE 2 MG TABLET G PATRIOT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 50458-0593-50 0.24180 RISPERIDONE 2 MG TABLET G PATRIOT PHARMAC EAGEX 50458-0593-60 0.24180 RISPERIDONE 2 MG TABLET G PATRIOT PHARMAC EAGEX 51079-0463-01 0.24180 RISPERIDONE 2 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0463-20 0.24180 RISPERIDONE 2 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0463-30 0.24180 RISPERIDONE 2 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0463-56 0.24180 RISPERIDONE 2 MG TABLET G MYLAN INSTITUTI EAGEX 51991-0319-<strong>06</strong> 0.24180 RISPERIDONE 2 MG TABLET G BRECKENRIDGE EAGEX 60505-2587-05 0.24180 RISPERIDONE 2 MG TABLET G APOTEX CORP EAGEX 60505-2587-<strong>06</strong> 0.24180 RISPERIDONE 2 MG TABLET G APOTEX CORP EAGEX 64679-0557-02 0.24180 RISPERIDONE 2 MG TABLET G WOCKHARDT USA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64679-0557-04 0.24180 RISPERIDONE 2 MG TABLET G WOCKHARDT USA L EAGEX 65862-0122-05 0.24180 RISPERIDONE 2 MG TABLET G AUROBINDO PHARM EAGEX 65862-0122-60 0.24180 RISPERIDONE 2 MG TABLET G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0273-01 0.24180 RISPERIDONE 2 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0273-11 0.24180 RISPERIDONE 2 MG TABLET G AHP EAGEX 68382-0115-05 0.24180 RISPERIDONE 2 MG TABLET G ZYDUS PHARMACEU EAGEX 68382-0115-14 0.24180 RISPERIDONE 2 MG TABLET G ZYDUS PHARMACEU EAGEX 00378-6046-28 7.47050 RISPERIDONE 3 MG ODT G MYLAN EAGEX 00781-5313-<strong>08</strong> 7.47050 RISPERIDONE 3 MG ODT G SANDOZ EAGEX 49884-0402-52 7.47050 RISPERIDONE 3 MG ODT G PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 49884-0402-91 7.47050 RISPERIDONE 3 MG ODT G PAR PHARM. EAGEX 50458-<strong>06</strong>04-28 7.47050 RISPERIDONE 3 MG ODT G PATRIOT PHARMAC EAGEX 51079-0347-01 7.47050 RISPERIDONE 3 MG ODT G MYLAN INSTITUTI EAGEX 51079-0347-05 7.47050 RISPERIDONE 3 MG ODT G MYLAN INSTITUTI EAGEX 55111-0470-81 7.47050 RISPERIDONE 3 MG ODT G DR.REDDY'S LAB EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 350LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-7242-<strong>06</strong> 0.18040 RISPERIDONE 3 MG TABLET G TEVA USA EAGEX 00378-3513-05 0.18040 RISPERIDONE 3 MG TABLET G MYLAN EAGEX 00378-3513-91 0.18040 RISPERIDONE 3 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-5689-20 0.18040 RISPERIDONE 3 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-5689-28 0.18040 RISPERIDONE 3 MG TABLET G QUALITEST EAGEX 00904-5977-61 0.18040 RISPERIDONE 3 MG TABLET G MAJOR PHARMACEU EAGEX 13107-0123-05 0.18040 RISPERIDONE 3 MG TABLET G AUROBINDO PHARM EAGEX 13107-0123-60 0.18040 RISPERIDONE 3 MG TABLET G AUROBINDO PHARM EAGEX 13668-0039-05 0.18040 RISPERIDONE 3 MG TABLET G TORRENT PHARMAC EAGEX 13668-0039-60 0.18040 RISPERIDONE 3 MG TABLET G TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 50458-0594-10 0.18040 RISPERIDONE 3 MG TABLET G PATRIOT PHARMAC EAGEX 50458-0594-50 0.18040 RISPERIDONE 3 MG TABLET G PATRIOT PHARMAC EAGEX 50458-0594-60 0.18040 RISPERIDONE 3 MG TABLET G PATRIOT PHARMAC EAGEX 51079-0464-01 0.18040 RISPERIDONE 3 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0464-20 0.18040 RISPERIDONE 3 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0464-30 0.18040 RISPERIDONE 3 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0464-56 0.18040 RISPERIDONE 3 MG TABLET G MYLAN INSTITUTI EAGEX 51991-0320-<strong>06</strong> 0.18040 RISPERIDONE 3 MG TABLET G BRECKENRIDGE EAGEX 60505-2588-05 0.18040 RISPERIDONE 3 MG TABLET G APOTEX CORP EAGEX 60505-2588-<strong>06</strong> 0.18040 RISPERIDONE 3 MG TABLET G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64679-0571-02 0.18040 RISPERIDONE 3 MG TABLET G WOCKHARDT USA L EAGEX 64679-0571-04 0.18040 RISPERIDONE 3 MG TABLET G WOCKHARDT USA L EAGEX 65862-0123-05 0.18040 RISPERIDONE 3 MG TABLET G AUROBINDO PHARM EAGEX 65862-0123-60 0.18040 RISPERIDONE 3 MG TABLET G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0274-01 0.18040 RISPERIDONE 3 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0274-11 0.18040 RISPERIDONE 3 MG TABLET G AHP EAGEX 68382-0116-05 0.18040 RISPERIDONE 3 MG TABLET G ZYDUS PHARMACEU EAGEX 68382-0116-14 0.18040 RISPERIDONE 3 MG TABLET G ZYDUS PHARMACEU EAGEX 00378-6047-28 8.53357 RISPERIDONE 4 MG ODT G MYLAN EAGEX 00781-5314-<strong>08</strong> 8.53357 RISPERIDONE 4 MG ODT G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 49884-0403-52 8.53357 RISPERIDONE 4 MG ODT G PAR PHARM. EAGEX 49884-0403-91 8.53357 RISPERIDONE 4 MG ODT G PAR PHARM. EAGEX 50458-<strong>06</strong>05-28 8.53357 RISPERIDONE 4 MG ODT G PATRIOT PHARMAC EAGEX 55111-0471-81 8.53357 RISPERIDONE 4 MG ODT G DR.REDDY'S LAB EAGEX 00093-7243-<strong>06</strong> 0.24170 RISPERIDONE 4 MG TABLET G TEVA USA EAGEX 00378-3514-91 0.24170 RISPERIDONE 4 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-5688-20 0.24170 RISPERIDONE 4 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-5688-28 0.24170 RISPERIDONE 4 MG TABLET G QUALITEST EAGEX 00904-5978-61 0.24170 RISPERIDONE 4 MG TABLET G MAJOR PHARMACEU EAGEX 00904-6362-61 0.24170 RISPERIDONE 4 MG TABLET G MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13107-0124-60 0.24170 RISPERIDONE 4 MG TABLET G AUROBINDO PHARM EAGEX 13668-0040-05 0.24170 RISPERIDONE 4 MG TABLET G TORRENT PHARMAC EAGEX 13668-0040-60 0.24170 RISPERIDONE 4 MG TABLET G TORRENT PHARMAC EAGEX 50458-0595-10 0.24170 RISPERIDONE 4 MG TABLET G PATRIOT PHARMAC EAGEX 50458-0595-60 0.24170 RISPERIDONE 4 MG TABLET G PATRIOT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 351LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0465-01 0.24170 RISPERIDONE 4 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0465-20 0.24170 RISPERIDONE 4 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0465-30 0.24170 RISPERIDONE 4 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0465-56 0.24170 RISPERIDONE 4 MG TABLET G MYLAN INSTITUTI EAGEX 51991-0321-<strong>06</strong> 0.24170 RISPERIDONE 4 MG TABLET G BRECKENRIDGE EAGEX 55111-02<strong>06</strong>-60 0.24170 RISPERIDONE 4 MG TABLET G DR.REDDY'S LAB EAGEX 60505-2589-<strong>06</strong> 0.24170 RISPERIDONE 4 MG TABLET G APOTEX CORP EAGEX 64679-0572-04 0.24170 RISPERIDONE 4 MG TABLET G WOCKHARDT USA L EAGEX 65862-0124-60 0.24170 RISPERIDONE 4 MG TABLET G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0277-01 0.24170 RISPERIDONE 4 MG TABLET G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0277-11 0.24170 RISPERIDONE 4 MG TABLET G AHP EAGEX 68382-0117-05 0.24170 RISPERIDONE 4 MG TABLET G ZYDUS PHARMACEU EAGEX 68382-0117-14 0.24170 RISPERIDONE 4 MG TABLET G ZYDUS PHARMACEU EABND 50242-0051-21 68.33473 RITUXAN 10 MG/ML VIAL 0 GENENTECH, INC. MLBND 50242-0053-<strong>06</strong> 68.33456 RITUXAN 10 MG/ML VIAL 0 GENENTECH, INC. MLGEN 00591-32<strong>08</strong>-60 2.04166 RIVASTIGMINE 1.5 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00781-2614-13 2.04166 RIVASTIGMINE 1.5 MG CAPSULE 0 SANDOZ EAGEN 00781-2614-60 2.04166 RIVASTIGMINE 1.5 MG CAPSULE 0 SANDOZ EAGEN 51991-0793-<strong>06</strong> 2.04166 RIVASTIGMINE 1.5 MG CAPSULE 0 BRECKENRIDGE EAGEN 55111-0352-05 2.04166 RIVASTIGMINE 1.5 MG CAPSULE 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0352-60 2.04166 RIVASTIGMINE 1.5 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 62756-0145-13 2.04166 RIVASTIGMINE 1.5 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 62756-0145-86 2.04166 RIVASTIGMINE 1.5 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 63739-0576-10 2.04166 RIVASTIGMINE 1.5 MG CAPSULE 0 MCKESSON PACKAG EAGEN 68<strong>08</strong>4-0550-01 2.04166 RIVASTIGMINE 1.5 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-0550-11 2.04166 RIVASTIGMINE 1.5 MG CAPSULE 0 AHP EAGEN 00591-3209-60 1.95740 RIVASTIGMINE 3 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00781-2615-60 1.95740 RIVASTIGMINE 3 MG CAPSULE 0 SANDOZ EAGEN 51991-0794-<strong>06</strong> 1.95740 RIVASTIGMINE 3 MG CAPSULE 0 BRECKENRIDGE EAGEN 55111-0353-05 1.95740 RIVASTIGMINE 3 MG CAPSULE 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0353-60 1.95740 RIVASTIGMINE 3 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 60505-3221-<strong>06</strong> 1.95740 RIVASTIGMINE 3 MG CAPSULE 0 APOTEX CORP EAGEN 62756-0146-13 1.95740 RIVASTIGMINE 3 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 62756-0146-86 1.95740 RIVASTIGMINE 3 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 63739-0577-10 1.95740 RIVASTIGMINE 3 MG CAPSULE 0 MCKESSON PACKAG EAGEN 00591-3210-60 2.<strong>06</strong>618 RIVASTIGMINE 4.5 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00781-2616-60 2.<strong>06</strong>618 RIVASTIGMINE 4.5 MG CAPSULE 0 SANDOZ EAGEN 51991-0795-<strong>06</strong> 2.<strong>06</strong>618 RIVASTIGMINE 4.5 MG CAPSULE 0 BRECKENRIDGE EAGEN 55111-0354-05 2.<strong>06</strong>618 RIVASTIGMINE 4.5 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 55111-0354-60 2.<strong>06</strong>618 RIVASTIGMINE 4.5 MG CAPSULE 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-3222-<strong>06</strong> 2.<strong>06</strong>618 RIVASTIGMINE 4.5 MG CAPSULE 0 APOTEX CORP EAGEN 62756-0147-13 2.<strong>06</strong>618 RIVASTIGMINE 4.5 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 62756-0147-86 2.<strong>06</strong>618 RIVASTIGMINE 4.5 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 63739-0578-10 2.<strong>06</strong>618 RIVASTIGMINE 4.5 MG CAPSULE 0 MCKESSON PACKAG EAGEN 00591-3211-60 2.04160 RIVASTIGMINE 6 MG CAPSULE 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 352LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-2617-60 2.04160 RIVASTIGMINE 6 MG CAPSULE 0 SANDOZ EAGEN 51991-0796-<strong>06</strong> 2.04160 RIVASTIGMINE 6 MG CAPSULE 0 BRECKENRIDGE EAGEN 55111-0355-05 2.04160 RIVASTIGMINE 6 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 55111-0355-60 2.04160 RIVASTIGMINE 6 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 60505-3223-<strong>06</strong> 2.04160 RIVASTIGMINE 6 MG CAPSULE 0 APOTEX CORP EAGEN 62756-0148-13 2.04160 RIVASTIGMINE 6 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 62756-0148-86 2.04160 RIVASTIGMINE 6 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 63739-0579-10 2.04160 RIVASTIGMINE 6 MG CAPSULE 0 MCKESSON PACKAG EABND 00944-3030-02 1.12500 RIXUBIS 1,000 UNIT NOMINAL 0 BAXTER BIOSCIENBND 00944-3032-02 1.12500 RIXUBIS 2,000 UNIT NOMINAL 0 BAXTER BIOSCIEN--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00944-3026-02 1.12500 RIXUBIS 250 UNIT NOMINAL 0 BAXTER BIOSCIENBND 00944-3034-02 1.12500 RIXUBIS 3,000 UNIT NOMINAL 0 BAXTER BIOSCIENBND 00944-3028-02 1.12500 RIXUBIS 500 UNIT NOMINAL 0 BAXTER BIOSCIENGEN 00378-3702-59 23.69460 RIZATRIPTAN 10 MG ODT G MYLAN EAGEN 49884-0581-52 23.69460 RIZATRIPTAN 10 MG ODT G PAR PHARM. EAGEN 49884-0581-94 23.69460 RIZATRIPTAN 10 MG ODT G PAR PHARM. EAGEN 51991-0363-78 23.69460 RIZATRIPTAN 10 MG ODT G BRECKENRIDGE EAGEN 51991-0363-99 23.69460 RIZATRIPTAN 10 MG ODT G BRECKENRIDGE EAGEN 60505-3724-01 23.69460 RIZATRIPTAN 10 MG ODT G APOTEX CORP EAGEN 65862-<strong>06</strong>26-12 23.69460 RIZATRIPTAN 10 MG ODT G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0468-<strong>06</strong> 23.69460 RIZATRIPTAN 10 MG ODT G GLENMARK PHARMA EAGEN 00378-1404-96 2.01590 RIZATRIPTAN 10 MG TABLET G MYLAN EAGEN 49884-0579-52 2.01590 RIZATRIPTAN 10 MG TABLET G PAR PHARM. EAGEN 49884-0579-94 2.01590 RIZATRIPTAN 10 MG TABLET G PAR PHARM. EAGEN 51991-0355-78 2.01590 RIZATRIPTAN 10 MG TABLET G BRECKENRIDGE EAGEN 51991-0355-99 2.01590 RIZATRIPTAN 10 MG TABLET G BRECKENRIDGE EAGEN 60505-3699-01 2.01590 RIZATRIPTAN 10 MG TABLET G APOTEX CORP EAGEN 65862-<strong>06</strong>00-12 2.01590 RIZATRIPTAN 10 MG TABLET G AUROBINDO PHARM EAGEN 68462-0466-99 2.01590 RIZATRIPTAN 10 MG TABLET G GLENMARK PHARMA EAGEN 00378-3701-59 23.69460 RIZATRIPTAN 5 MG ODT G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0580-52 23.69460 RIZATRIPTAN 5 MG ODT G PAR PHARM. EAGEN 49884-0580-94 23.69460 RIZATRIPTAN 5 MG ODT G PAR PHARM. EAGEN 51991-0362-78 23.69460 RIZATRIPTAN 5 MG ODT G BRECKENRIDGE EAGEN 51991-0362-99 23.69460 RIZATRIPTAN 5 MG ODT G BRECKENRIDGE EAGEN 60505-3723-01 23.69460 RIZATRIPTAN 5 MG ODT G APOTEX CORP EAGEN 65862-<strong>06</strong>25-12 23.69460 RIZATRIPTAN 5 MG ODT G AUROBINDO PHARM EAGEN 68462-0467-<strong>06</strong> 23.69460 RIZATRIPTAN 5 MG ODT G GLENMARK PHARMA EAGEN 00378-1403-96 1.50000 RIZATRIPTAN 5 MG TABLET G MYLAN EAGEN 49884-0578-52 1.50000 RIZATRIPTAN 5 MG TABLET G PAR PHARM. EAGEN 49884-0578-94 1.50000 RIZATRIPTAN 5 MG TABLET G PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51991-0354-78 1.50000 RIZATRIPTAN 5 MG TABLET G BRECKENRIDGE EAGEN 51991-0354-99 1.50000 RIZATRIPTAN 5 MG TABLET G BRECKENRIDGE EAGEN 60505-3698-01 1.50000 RIZATRIPTAN 5 MG TABLET G APOTEX CORP EAGEN 65862-0599-12 1.50000 RIZATRIPTAN 5 MG TABLET G AUROBINDO PHARM EAGEN 68462-0465-99 1.50000 RIZATRIPTAN 5 MG TABLET G GLENMARK PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 353LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 52244-0429-10 0.07920 1.41241 ROBAXIN 500 MG TABLET G AUXILIUM PHARM EABND 00091-7449-63 0.12530 2.32<strong>06</strong>8 ROBAXIN-750 TABLET G ACTIENT PHARMAC EABND 59630-0205-10 1.52190 8.97844 ROBINUL FORTE 2 MG TABLET G SHIONOGI PHARMA EABND 0<strong>06</strong>41-6104-01 0.64000 19.15640 ROBINUL 0.2 MG/ML VIAL G WEST-WARD,INC. MLBND 0<strong>06</strong>41-6104-25 0.64000 19.15374 ROBINUL 0.2 MG/ML VIAL G WEST-WARD,INC. MLBND 60977-0155-01 0.64000 1.27488 ROBINUL 0.2 MG/ML VIAL G WEST-WARD,INC. MLBND 60977-0155-81 0.64000 1.27820 ROBINUL 0.2 MG/ML VIAL G WEST-WARD,INC. MLBND 0<strong>06</strong>41-6105-01 0.64000 17.<strong>06</strong><strong>06</strong>5 ROBINUL 0.4 MG/2 ML VIAL G WEST-WARD,INC. MLBND 0<strong>06</strong>41-6105-25 0.64000 17.05965 ROBINUL 0.4 MG/2 ML VIAL G WEST-WARD,INC. MLBND 59630-0200-10 0.86720 5.88121 ROBINUL 1 MG TABLET G SHIONOGI PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 3<strong>06</strong>98-0143-01 0.53370 0.69653 ROCALTROL 0.25 MCG CAPSULE 0 VALIDUS PHARMAC EABND 3<strong>06</strong>98-0143-23 0.53370 1.41598 ROCALTROL 0.25 MCG CAPSULE 0 VALIDUS PHARMAC EABND 3<strong>06</strong>98-0144-01 1.40020 2.22938 ROCALTROL 0.5 MCG CAPSULE 0 VALIDUS PHARMAC EABND 3<strong>06</strong>98-0911-15 7.01660 11.88560 ROCALTROL 1 MCG/ML ORAL SOLN G VALIDUS PHARMAC MLGEN 00228-3661-03 10.26174 ROPINIROLE HCL ER 12 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-3612-30 10.26174 ROPINIROLE HCL ER 12 MG TABLET G WATSON LABS EAGEN 00781-5788-31 10.26174 ROPINIROLE HCL ER 12 MG TABLET G SANDOZ EAGEN 13811-<strong>06</strong>43-30 10.26174 ROPINIROLE HCL ER 12 MG TABLET G TRIGEN LABORATO EAGEN 55111-0728-30 10.26174 ROPINIROLE HCL ER 12 MG TABLET G DR.REDDY'S LAB EAGEN 00228-3658-03 2.05125 ROPINIROLE HCL ER 2 MG TABLET G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-3658-09 2.05133 ROPINIROLE HCL ER 2 MG TABLET G ACTAVIS PHARMA, EAGEN 00378-4090-05 2.05134 ROPINIROLE HCL ER 2 MG TABLET G MYLAN EAGEN 00378-4090-93 2.05125 ROPINIROLE HCL ER 2 MG TABLET G MYLAN EAGEN 00591-3611-19 2.05133 ROPINIROLE HCL ER 2 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-3611-30 2.05125 ROPINIROLE HCL ER 2 MG TABLET G ACTAVIS PHARMA, EAGEN 00781-5780-31 2.05125 ROPINIROLE HCL ER 2 MG TABLET G SANDOZ EAGEN 00781-5780-92 2.05133 ROPINIROLE HCL ER 2 MG TABLET G SANDOZ EAGEN 13811-<strong>06</strong>39-30 2.05125 ROPINIROLE HCL ER 2 MG TABLET G TRIGEN LABORATO EAGEN 13811-<strong>06</strong>39-90 2.05125 ROPINIROLE HCL ER 2 MG TABLET G TRIGEN LABORATO EAGEN 55111-<strong>06</strong>59-30 2.05125 ROPINIROLE HCL ER 2 MG TABLET G DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-<strong>06</strong>59-90 2.05133 ROPINIROLE HCL ER 2 MG TABLET G DR.REDDY'S LAB EAGEN 00228-3659-03 4.10250 ROPINIROLE HCL ER 4 MG TABLET G ACTAVIS PHARMA, EAGEN 00228-3659-09 4.10266 ROPINIROLE HCL ER 4 MG TABLET G ACTAVIS PHARMA, EAGEN 00378-4091-05 4.10268 ROPINIROLE HCL ER 4 MG TABLET G MYLAN EAGEN 00378-4091-93 4.10274 ROPINIROLE HCL ER 4 MG TABLET G MYLAN EAGEN 00591-3613-19 4.10266 ROPINIROLE HCL ER 4 MG TABLET G WATSON LABS EAGEN 00591-3613-30 4.10250 ROPINIROLE HCL ER 4 MG TABLET G WATSON LABS EAGEN 00781-5782-31 4.10250 ROPINIROLE HCL ER 4 MG TABLET G SANDOZ EAGEN 00781-5782-92 4.10266 ROPINIROLE HCL ER 4 MG TABLET G SANDOZ EAGEN 13811-<strong>06</strong>40-30 4.10250 ROPINIROLE HCL ER 4 MG TABLET G TRIGEN LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13811-<strong>06</strong>40-90 4.10250 ROPINIROLE HCL ER 4 MG TABLET G TRIGEN LABORATO EAGEN 55111-<strong>06</strong>61-30 4.10250 ROPINIROLE HCL ER 4 MG TABLET G DR.REDDY'S LAB EAGEN 55111-<strong>06</strong>61-90 4.10266 ROPINIROLE HCL ER 4 MG TABLET G DR.REDDY'S LAB EAGEN 00228-3640-03 6.15399 ROPINIROLE HCL ER 6 MG TABLET G ACTAVIS PHARMA, EAGEN 00378-4092-93 6.15399 ROPINIROLE HCL ER 6 MG TABLET G MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 354LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3700-30 6.15399 ROPINIROLE HCL ER 6 MG TABLET G ACTAVIS PHARMA, EAGEN 00781-5784-31 6.15399 ROPINIROLE HCL ER 6 MG TABLET G SANDOZ EAGEN 13811-<strong>06</strong>41-30 6.15399 ROPINIROLE HCL ER 6 MG TABLET G TRIGEN LABORATO EAGEN 55111-0727-30 6.15399 ROPINIROLE HCL ER 6 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0727-90 6.15399 ROPINIROLE HCL ER 6 MG TABLET G DR.REDDY'S LAB EAGEN 00228-3660-03 5.95665 ROPINIROLE HCL ER 8 MG TABLET G ACTAVIS PHARMA, EAGEN 00228-3660-09 5.95665 ROPINIROLE HCL ER 8 MG TABLET G ACTAVIS PHARMA, EAGEN 00378-4093-05 5.95665 ROPINIROLE HCL ER 8 MG TABLET G MYLAN EAGEN 00378-4093-93 5.95665 ROPINIROLE HCL ER 8 MG TABLET G MYLAN EAGEN 00591-3614-19 5.95665 ROPINIROLE HCL ER 8 MG TABLET G WATSON LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3614-30 5.95665 ROPINIROLE HCL ER 8 MG TABLET G WATSON LABS EAGEN 00781-5786-31 5.95665 ROPINIROLE HCL ER 8 MG TABLET G SANDOZ EAGEN 00781-5786-92 5.95665 ROPINIROLE HCL ER 8 MG TABLET G SANDOZ EAGEN 13811-<strong>06</strong>42-30 5.95665 ROPINIROLE HCL ER 8 MG TABLET G TRIGEN LABORATO EAGEN 13811-<strong>06</strong>42-90 5.95665 ROPINIROLE HCL ER 8 MG TABLET G TRIGEN LABORATO EAGEN 55111-<strong>06</strong>62-30 5.95665 ROPINIROLE HCL ER 8 MG TABLET G DR.REDDY'S LAB EAGEN 55111-<strong>06</strong>62-90 5.95665 ROPINIROLE HCL ER 8 MG TABLET G DR.REDDY'S LAB EAGEN 00054-0116-25 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 ROXANE LABS. EAGEN 00093-5282-01 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 TEVA USA EAGEN 00378-5525-01 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5994-61 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 MAJOR PHARMACEU EAGEN 23155-0121-01 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 HERITAGE PHARMA EAGEN 43547-0268-10 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0268-50 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 64679-0154-02 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0154-03 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 WOCKHARDT USA L EAGEN 68<strong>08</strong>4-0305-01 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0305-11 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 AHP EAGEN 68382-0338-01 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0253-01 0.24340 ROPINIROLE HCL 0.25 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0117-25 0.27330 ROPINIROLE HCL 0.5 MG TABLET 0 ROXANE LABS. EAGEN 00378-5550-01 0.27330 ROPINIROLE HCL 0.5 MG TABLET 0 MYLAN EAGEN 00378-5550-02 0.27330 ROPINIROLE HCL 0.5 MG TABLET 0 MYLAN EAGEN 23155-0122-01 0.27330 ROPINIROLE HCL 0.5 MG TABLET 0 HERITAGE PHARMA EAGEN 43547-0269-10 0.27330 ROPINIROLE HCL 0.5 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0269-50 0.27330 ROPINIROLE HCL 0.5 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 64679-0155-02 0.27330 ROPINIROLE HCL 0.5 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0155-03 0.27330 ROPINIROLE HCL 0.5 MG TABLET 0 WOCKHARDT USA L EAGEN 68382-0339-01 0.27330 ROPINIROLE HCL 0.5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0254-01 0.27330 ROPINIROLE HCL 0.5 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0118-25 0.23560 ROPINIROLE HCL 1 MG TABLET 0 ROXANE LABS. EAGEN 00378-5501-01 0.23560 ROPINIROLE HCL 1 MG TABLET 0 MYLAN EAGEN 00378-5501-02 0.23560 ROPINIROLE HCL 1 MG TABLET 0 MYLAN EAGEN 00904-5996-61 0.23560 ROPINIROLE HCL 1 MG TABLET 0 MAJOR PHARMACEU EAGEN 23155-0123-01 0.23560 ROPINIROLE HCL 1 MG TABLET 0 HERITAGE PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 355LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 43547-0270-10 0.23560 ROPINIROLE HCL 1 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0270-50 0.23560 ROPINIROLE HCL 1 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 64679-0171-02 0.23560 ROPINIROLE HCL 1 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0171-03 0.23560 ROPINIROLE HCL 1 MG TABLET 0 WOCKHARDT USA L EAGEN 68<strong>08</strong>4-0307-01 0.23560 ROPINIROLE HCL 1 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0307-11 0.23560 ROPINIROLE HCL 1 MG TABLET 0 AHP EAGEN 68382-0340-01 0.23560 ROPINIROLE HCL 1 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0255-01 0.23560 ROPINIROLE HCL 1 MG TABLET 0 GLENMARK PHARMA EAGEN 00054-0119-25 0.15836 ROPINIROLE HCL 2 MG TABLET 0 ROXANE LABS. EAGEN 00093-5285-01 0.15836 ROPINIROLE HCL 2 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5502-01 0.15836 ROPINIROLE HCL 2 MG TABLET 0 MYLAN EAGEN 00378-5502-02 0.15836 ROPINIROLE HCL 2 MG TABLET 0 MYLAN EAGEN 23155-0124-01 0.15836 ROPINIROLE HCL 2 MG TABLET 0 HERITAGE PHARMA EAGEN 43547-0271-10 0.15836 ROPINIROLE HCL 2 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0271-50 0.15836 ROPINIROLE HCL 2 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 64679-0172-02 0.15836 ROPINIROLE HCL 2 MG TABLET 0 WOCKHARDT USA L EAGEN 64679-0172-03 0.15836 ROPINIROLE HCL 2 MG TABLET 0 WOCKHARDT USA L EAGEN 68382-0341-01 0.15836 ROPINIROLE HCL 2 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0256-01 0.15836 ROPINIROLE HCL 2 MG TABLET 0 GLENMARK PHARMA EAGEN 00054-0120-25 0.15836 ROPINIROLE HCL 3 MG TABLET 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-5286-01 0.15836 ROPINIROLE HCL 3 MG TABLET 0 TEVA USA EAGEN 00378-5503-01 0.15836 ROPINIROLE HCL 3 MG TABLET 0 MYLAN EAGEN 00378-5503-02 0.15836 ROPINIROLE HCL 3 MG TABLET 0 MYLAN EAGEN 23155-0125-01 0.15836 ROPINIROLE HCL 3 MG TABLET 0 HERITAGE PHARMA EAGEN 43547-0272-10 0.15836 ROPINIROLE HCL 3 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0272-50 0.15836 ROPINIROLE HCL 3 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 64679-0174-02 0.15836 ROPINIROLE HCL 3 MG TABLET 0 WOCKHARDT USA L EAGEN 68382-0342-01 0.15836 ROPINIROLE HCL 3 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0257-01 0.15836 ROPINIROLE HCL 3 MG TABLET 0 GLENMARK PHARMA EAGEN 00054-0121-25 0.17159 ROPINIROLE HCL 4 MG TABLET 0 ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5504-01 0.17159 ROPINIROLE HCL 4 MG TABLET 0 MYLAN EAGEN 00378-5504-02 0.17159 ROPINIROLE HCL 4 MG TABLET 0 MYLAN EAGEN 23155-0126-01 0.17159 ROPINIROLE HCL 4 MG TABLET 0 HERITAGE PHARMA EAGEN 43547-0273-10 0.17159 ROPINIROLE HCL 4 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0273-50 0.17159 ROPINIROLE HCL 4 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 64679-0175-02 0.17159 ROPINIROLE HCL 4 MG TABLET 0 WOCKHARDT USA L EAGEN 68382-0343-01 0.17159 ROPINIROLE HCL 4 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68462-0258-01 0.17159 ROPINIROLE HCL 4 MG TABLET 0 GLENMARK PHARMA EAGEN 00054-0122-25 0.17159 ROPINIROLE HCL 5 MG TABLET 0 ROXANE LABS. EAGEN 00378-5505-01 0.17159 ROPINIROLE HCL 5 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5505-02 0.17159 ROPINIROLE HCL 5 MG TABLET 0 MYLAN EAGEN 43547-0274-10 0.17159 ROPINIROLE HCL 5 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 43547-0274-50 0.17159 ROPINIROLE HCL 5 MG TABLET 0 SOLCO <strong>HEALTH</strong>CAR EAGEN 64679-0177-02 0.17159 ROPINIROLE HCL 5 MG TABLET 0 WOCKHARDT USA L EAGEN 68382-0344-01 0.17159 ROPINIROLE HCL 5 MG TABLET 0 ZYDUS PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 356LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0259-01 0.17159 ROPINIROLE HCL 5 MG TABLET 0 GLENMARK PHARMA EAGUL 43538-0180-45 1.62630 ROSADAN 0.75% CREAM 0 MEDIMETRIKS PHA GMGUL 43538-0182-45 1.54170 ROSADAN 0.75% GEL 0 MEDIMETRIKS PHA GMBND 68220-0<strong>06</strong>6-05 134.10320 299.26480 ROWASA 4 GM/60 ML ENEMA KIT G MEDA PHARMACEUT EABEX 64764-<strong>08</strong>05-10 6.96112 ROZEREM 8 MG TABLET G TAKEDA PHARMACE EABEX 64764-<strong>08</strong>05-30 6.96148 ROZEREM 8 MG TABLET G TAKEDA PHARMACE EABND 59630-<strong>08</strong>25-03 2.70441 RYBIX ODT 50 MG TABLET G SHIONOGI PHARMA EABND 68453-<strong>08</strong>25-03 2.70441 RYBIX ODT 50 MG TABLET G SHIONOGI PHARMA EABND 00173-<strong>08</strong>23-18 6.80295 RYTHMOL SR 225 MG CAPSULE 0 GLAXOSMITHKLINE EABND 00173-<strong>08</strong>24-18 6.33940 8.61872 RYTHMOL SR 325 MG CAPSULE 0 GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-<strong>08</strong>26-18 8.61872 RYTHMOL SR 425 MG CAPSULE 0 GLAXOSMITHKLINE EABND 00173-0792-20 0.22140 4.02965 RYTHMOL 150 MG TABLET G GLAXOSMITHKLINE EABND 00173-0794-20 0.39474 5.29830 RYTHMOL 225 MG TABLET G GLAXOSMITHKLINE EABND 59011-0336-30 5.56690 10.01671 RYZOLT ER 300 MG TABLET G PURDUE PHARMA L EABEX 67386-0211-65 70.16936 SABRIL 500 MG POWDER PACKET G LUNDBECK INC. EABEX 67386-0111-01 70.16952 SABRIL 500 MG TABLET G LUNDBECK INC. EABEX 50419-0403-03 3.67117 SAFYRAL TABLET 0 BAYER,PHARM DIV EABND 44<strong>08</strong>7-1005-02 454.57440 SAIZEN 5 MG VIAL G EMD SERONO, INC EABND 44<strong>08</strong>7-1<strong>08</strong>0-01 727.32070 SAIZEN 8.8 MG CLICK.EASY CARTG G EMD SERONO, INC EABND 44<strong>08</strong>7-1<strong>08</strong>8-01 727.32070 SAIZEN 8.8 MG VIAL G EMD SERONO, INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 58<strong>06</strong>3-0705-10 0.23249 1.67328 SALAGEN 5 MG TABLET G EISAI INC. EABND 62856-0705-10 0.23249 1.67328 SALAGEN 5 MG TABLET G EISAI INC. EABND 62856-0775-10 1.12184 2.05176 SALAGEN 7.5 MG TABLET 0 EISAI INC. EAGEN 00264-7802-00 0.00126 SALINE 0.45% SOLN-EXCEL CON 0 B.BRAUN MLGEN 00264-7802-10 0.00180 SALINE 0.45% SOLN-EXCEL CON 0 B.BRAUN MLGEN 00264-7800-00 0.00130 SALINE 0.9% SOLN-EXCEL CONT 0 B.BRAUN MLGEN 00264-7800-10 0.00162 SALINE 0.9% SOLN-EXCEL CONT 0 B.BRAUN MLGEN 00264-7800-20 0.00162 SALINE 0.9% SOLN-EXCEL CONT 0 B.BRAUN MLGEN 42192-0365-10 0.32100 SALSALATE 500 MG TABLET 0 ACELLA PHARMACE EAGEN 64376-0507-01 0.32100 SALSALATE 500 MG TABLET 0 BOCA PHARMACAL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64376-0507-05 0.32100 SALSALATE 500 MG TABLET 0 BOCA PHARMACAL EAGEN 65162-0512-10 0.32100 SALSALATE 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0512-11 0.32100 SALSALATE 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0512-50 0.32100 SALSALATE 500 MG TABLET 0 AMNEAL PHARMACE EAGEN 42192-0366-10 0.41600 SALSALATE 750 MG TABLET 0 ACELLA PHARMACE EAGEN 64376-05<strong>08</strong>-01 0.41600 SALSALATE 750 MG TABLET 0 BOCA PHARMACAL EAGEN 64376-05<strong>08</strong>-05 0.41600 SALSALATE 750 MG TABLET 0 BOCA PHARMACAL EAGEN 65162-0513-10 0.41600 SALSALATE 750 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0513-11 0.41600 SALSALATE 750 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0513-50 0.41600 SALSALATE 750 MG TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59148-0020-50 311.25000 SAMSCA 15 MG TABLET 0 OTSUKA AMERICA EABND 59148-0021-50 311.25000 SAMSCA 30 MG TABLET 0 OTSUKA AMERICA EABND 00023-9350-30 6.21282 6.21282 SANCTURA XR 60 MG CAPSULE 0 ALLERGAN INC. EABND 00023-3513-60 2.19740 3.51905 SANCTURA 20 MG TABLET G ALLERGAN INC. EABND 42747-0726-01 394.31640 SANCUSO 3.1 MG/24 HR PATCH G PROSTRAKAN INC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 357LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0241-15 5.67500 10.07122 SANDIMMUNE 100 MG CAPSULE 0 NOVARTIS EABND 00078-0110-22 9.78038 SANDIMMUNE 100 MG/ML SOLN 0 NOVARTIS MLBND 00078-0240-15 2.52320 SANDIMMUNE 25 MG CAPSULE 0 NOVARTIS EABND 00078-0109-01 7.85<strong>06</strong>3 SANDIMMUNE 50 MG/ML AMPUL 0 NOVARTIS MLBND 00078-0109-61 7.85180 SANDIMMUNE 50 MG/ML AMPUL 0 NOVARTIS MLBND 00078-0340-61 2052.39910 SANDOSTATIN LAR 10 MG KIT 0 NOVARTIS EABND 00078-0341-61 2688.99250 SANDOSTATIN LAR 20 MG KIT 0 NOVARTIS EABND 00078-0342-61 4026.57070 SANDOSTATIN LAR 30 MG KIT 0 NOVARTIS EABND 00078-0180-01 2.87820 11.94536 SANDOSTATIN 0.05 MG/ML AMPUL 0 NOVARTIS MLBND 00078-0180-61 2.87820 11.94370 SANDOSTATIN 0.05 MG/ML AMPUL 0 NOVARTIS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0181-01 4.02220 23.17111 SANDOSTATIN 0.1 MG/ML AMPUL 0 NOVARTIS MLBND 00078-0181-61 4.02220 23.17360 SANDOSTATIN 0.1 MG/ML AMPUL 0 NOVARTIS MLBND 00078-0183-25 7.78320 47.77480 SANDOSTATIN 0.2 MG/ML VIAL G NOVARTIS MLBND 00078-0182-01 25.69680 111.75535 SANDOSTATIN 0.5 MG/ML AMPUL 0 NOVARTIS MLBND 00078-0182-61 25.69680 111.75950 SANDOSTATIN 0.5 MG/ML AMPUL 0 NOVARTIS MLBND 00078-0184-25 119.55000 235.07260 SANDOSTATIN 1 MG/ML VIAL G NOVARTIS MLBND 00<strong>06</strong>4-5010-30 5.87363 SANTYL OINTMENT 0 <strong>HEALTH</strong>POINT MED GMBEX 00052-2142-03 11.44874 SAPHRIS 10 MG TAB SL BLK CHERY G ORGANON PHARM. EABEX 00052-2142-04 11.44843 SAPHRIS 10 MG TAB SL BLK CHERY G ORGANON PHARM. EABEX 00052-0119-<strong>06</strong> 11.44874 SAPHRIS 10 MG TAB SUBLINGUAL G ORGANON PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00052-2139-03 11.44874 SAPHRIS 5 MG TAB SL BLK CHERRY G ORGANON PHARM. EABEX 00052-2139-04 11.44843 SAPHRIS 5 MG TAB SL BLK CHERRY G ORGANON PHARM. EABEX 00052-0118-<strong>06</strong> 11.44874 SAPHRIS 5 MG TABLET SUBLINGUAL G ORGANON PHARM. EABEX 00430-0210-14 0.05170 9.80052 SARAFEM 10 MG TABLET G ACTAVIS PHARMA, EABEX 00430-0220-14 0.72553 9.80052 SARAFEM 20 MG TABLET G ACTAVIS PHARMA, EABND 00456-1500-55 2.82502 SAVELLA TITRATION PACK G FOREST PHARMACE EABND 00456-1510-60 2.82476 SAVELLA 100 MG TABLET G FOREST PHARMACE EABND 00456-1512-60 2.82476 SAVELLA 12.5 MG TABLET G FOREST PHARMACE EABND 00456-1525-60 2.82476 SAVELLA 25 MG TABLET G FOREST PHARMACE EABND 00456-1550-60 2.82476 SAVELLA 50 MG TABLET G FOREST PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63323-0268-01 7.91820 SCOPOLAMINE 0.4 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 13925-0117-01 0.25460 SE-NATAL 19 CHEWABLE TABLET 0 SETON PHARMACEU EAGEN 13925-0116-01 0.21740 SE-NATAL 19 TABLET 0 SETON PHARMACEU EABEX 51285-0<strong>08</strong>7-87 2.97158 SEASONIQUE 0.15-0.03-0.01 TAB 0 DURAMED/BARR EABND 67857-0700-01 0.25124 3.61149 SECTRAL 200 MG CAPSULE G PROMIUS PHARMA EABND 67857-0701-01 0.29580 4.80171 SECTRAL 400 MG CAPSULE G PROMIUS PHARMA EABND 0<strong>06</strong>42-0077-90 1.34626 SELECT-OB CAPLET 0 EVERETT EAGEN 60505-0055-01 1.72625 SELEGILINE HCL 5 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0055-02 1.63993 SELEGILINE HCL 5 MG CAPSULE 0 APOTEX CORP EAGEN 67253-0700-<strong>06</strong> 1.72800 SELEGILINE HCL 5 MG CAPSULE 0 DAVA PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-9290-91 0.76580 SELEGILINE HCL 5 MG TABLET 0 MYLAN EAGUL 60505-3438-03 0.76580 SELEGILINE HCL 5 MG TABLET 0 APOTEX CORP EAGUL 60505-3438-<strong>08</strong> 0.76580 SELEGILINE HCL 5 MG TABLET 0 APOTEX CORP EABND 00<strong>06</strong>9-<strong>08</strong>07-60 15.55613 SELZENTRY 150 MG TABLET G PFIZER US PHARM EABND 49702-0215-18 15.55613 SELZENTRY 150 MG TABLET G VIIV <strong>HEALTH</strong>CARE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 358LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 49702-0223-18 18.830<strong>06</strong> SELZENTRY 150 MG TABLET G VIIV <strong>HEALTH</strong>CARE EABND 00<strong>06</strong>9-<strong>08</strong><strong>08</strong>-60 15.55613 SELZENTRY 300 MG TABLET G PFIZER US PHARM EABND 49702-0216-18 15.55613 SELZENTRY 300 MG TABLET G VIIV <strong>HEALTH</strong>CARE EABND 49702-0224-18 18.830<strong>06</strong> SELZENTRY 300 MG TABLET G VIIV <strong>HEALTH</strong>CARE EABND 55513-0073-30 16.70292 SENSIPAR 30 MG TABLET 0 AMGEN EABND 55513-0074-30 33.40584 SENSIPAR 60 MG TABLET 0 AMGEN EABND 55513-0075-30 50.1<strong>08</strong>76 SENSIPAR 90 MG TABLET 0 AMGEN EABND 00173-0520-00 4.27212 SEREVENT DISKUS 50 MCG G GLAXOSMITHKLINE EABND 00173-0521-00 3.37727 SEREVENT DISKUS 50 MCG G GLAXOSMITHKLINE EABEX 13845-1200-03 7.47000 SEROMYCIN 250 MG CAPSULE 0 THE CHAO CENTER EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 44<strong>08</strong>7-8090-<strong>06</strong> 1.02<strong>06</strong>0 10.17995 SEROPHENE 50 MG TABLET G EMD SERONO, INC EABEX 00310-0281-60 12.59178 SEROQUEL XR 150 MG TABLET G ASTRAZENECA EABEX 00310-0282-60 13.859<strong>06</strong> SEROQUEL XR 200 MG TABLET G ASTRAZENECA EABEX 00310-0283-60 18.17091 SEROQUEL XR 300 MG TABLET G ASTRAZENECA EABEX 00310-0284-60 21.35534 SEROQUEL XR 400 MG TABLET G ASTRAZENECA EABEX 00310-0280-60 7.01239 SEROQUEL XR 50 MG TABLET G ASTRAZENECA EABEX 00310-0271-10 0.21560 6.64033 SEROQUEL 100 MG TABLET G ASTRAZENECA EABEX 00310-0272-10 0.36383 12.52719 SEROQUEL 200 MG TABLET G ASTRAZENECA EABEX 00310-0275-10 0.11219 3.86954 SEROQUEL 25 MG TABLET G ASTRAZENECA EABEX 00310-0275-34 0.11219 3.68573 SEROQUEL 25 MG TABLET G ASTRAZENECA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00310-0274-60 0.45522 16.42542 SEROQUEL 300 MG TABLET G ASTRAZENECA EABEX 00310-0279-10 0.62510 19.30347 SEROQUEL 400 MG TABLET G ASTRAZENECA EABEX 00310-0279-39 0.62510 19.30347 SEROQUEL 400 MG TABLET G ASTRAZENECA EABEX 00310-0278-10 0.20007 6.35863 SEROQUEL 50 MG TABLET G ASTRAZENECA EABEX 00310-0278-34 0.20007 6.05597 SEROQUEL 50 MG TABLET G ASTRAZENECA EABEX 00310-0278-39 0.20007 6.35863 SEROQUEL 50 MG TABLET G ASTRAZENECA EABND 44<strong>08</strong>7-0004-01 245.13220 SEROSTIM 4 MG VIAL N EMD SERONO, INC EABND 44<strong>08</strong>7-0004-07 245.13576 SEROSTIM 4 MG VIAL N EMD SERONO, INC EABND 44<strong>08</strong>7-0005-01 3<strong>06</strong>.41940 SEROSTIM 5 MG VIAL N EMD SERONO, INC EABND 44<strong>08</strong>7-0005-07 3<strong>06</strong>.41940 SEROSTIM 5 MG VIAL N EMD SERONO, INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 44<strong>08</strong>7-00<strong>06</strong>-01 367.7<strong>06</strong>60 SEROSTIM 6 MG VIAL N EMD SERONO, INC EABND 44<strong>08</strong>7-00<strong>06</strong>-07 367.70303 SEROSTIM 6 MG VIAL N EMD SERONO, INC EAGEX 00143-9654-05 0.07304 SERTRALINE HCL 100 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-9654-09 0.07304 SERTRALINE HCL 100 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-9654-30 0.07304 SERTRALINE HCL 100 MG TABLET 0 WEST-WARD,INC. EAGEX 00185-0265-30 0.07304 SERTRALINE HCL 100 MG TABLET 0 SANDOZ EAGEX 00378-4188-01 0.07304 SERTRALINE HCL 100 MG TABLET 0 MYLAN EAGEX 00378-4188-05 0.07304 SERTRALINE HCL 100 MG TABLET 0 MYLAN EAGEX 00378-8127-01 0.07304 SERTRALINE HCL 100 MG TABLET 0 MYLAN EAGEX 00378-8127-05 0.07304 SERTRALINE HCL 100 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16714-<strong>06</strong>13-01 0.07304 SERTRALINE HCL 100 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>13-04 0.07304 SERTRALINE HCL 100 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>13-05 0.07304 SERTRALINE HCL 100 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>13-<strong>06</strong> 0.07304 SERTRALINE HCL 100 MG TABLET 0 NORTHSTAR RX LL EAGEX 31722-0214-05 0.07304 SERTRALINE HCL 100 MG TABLET 0 CAMBER PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 359LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 31722-0214-30 0.07304 SERTRALINE HCL 100 MG TABLET 0 CAMBER PHARMACE EAGEX 31722-0214-90 0.07304 SERTRALINE HCL 100 MG TABLET 0 CAMBER PHARMACE EAGEX 51079-0151-20 0.07304 SERTRALINE HCL 100 MG TABLET 0 MYLAN INSTITUTI EAGEX 54458-0945-10 0.07304 SERTRALINE HCL 100 MG TABLET 0 INTERNATIONAL L EAGEX 59762-4910-01 0.07304 SERTRALINE HCL 100 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-4910-02 0.07304 SERTRALINE HCL 100 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-4910-04 0.07304 SERTRALINE HCL 100 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-4910-05 0.07304 SERTRALINE HCL 100 MG TABLET 0 GREENSTONE LLC. EAGEX 60505-0182-03 0.07304 SERTRALINE HCL 100 MG TABLET 0 APOTEX CORP EAGEX 60505-0182-<strong>08</strong> 0.07304 SERTRALINE HCL 100 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0013-05 0.07304 SERTRALINE HCL 100 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0013-30 0.07304 SERTRALINE HCL 100 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0182-01 0.07304 SERTRALINE HCL 100 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0182-11 0.07304 SERTRALINE HCL 100 MG TABLET 0 AHP EAGEX 68180-0353-02 0.07304 SERTRALINE HCL 100 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0353-05 0.07304 SERTRALINE HCL 100 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0353-<strong>06</strong> 0.07304 SERTRALINE HCL 100 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0353-09 0.07304 SERTRALINE HCL 100 MG TABLET 0 LUPIN PHARMACEU EAGEX 68645-0426-54 0.07304 SERTRALINE HCL 100 MG TABLET 0 LEGACY PHARMACE EAGEX 76282-0214-01 0.07304 SERTRALINE HCL 100 MG TABLET 0 EXELAN PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 76282-0214-05 0.07304 SERTRALINE HCL 100 MG TABLET 0 EXELAN PHARMACE EAGEX 00143-9656-09 0.04428 SERTRALINE HCL 25 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-9656-30 0.04428 SERTRALINE HCL 25 MG TABLET 0 WEST-WARD,INC. EAGEX 00378-8011-01 0.04428 SERTRALINE HCL 25 MG TABLET 0 MYLAN EAGEX 00378-8011-05 0.04428 SERTRALINE HCL 25 MG TABLET 0 MYLAN EAGEX 16714-<strong>06</strong>11-01 0.04428 SERTRALINE HCL 25 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>11-04 0.04428 SERTRALINE HCL 25 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>11-05 0.04428 SERTRALINE HCL 25 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>11-<strong>06</strong> 0.04428 SERTRALINE HCL 25 MG TABLET 0 NORTHSTAR RX LL EAGEX 31722-0212-05 0.04428 SERTRALINE HCL 25 MG TABLET 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 31722-0212-30 0.04428 SERTRALINE HCL 25 MG TABLET 0 CAMBER PHARMACE EAGEX 31722-0212-90 0.04428 SERTRALINE HCL 25 MG TABLET 0 CAMBER PHARMACE EAGEX 51079-0149-20 0.04428 SERTRALINE HCL 25 MG TABLET 0 MYLAN INSTITUTI EAGEX 54458-0947-10 0.04428 SERTRALINE HCL 25 MG TABLET 0 INTERNATIONAL L EAGEX 59762-4960-01 0.04428 SERTRALINE HCL 25 MG TABLET 0 GREENSTONE LLC. EAGEX 60505-0180-03 0.04428 SERTRALINE HCL 25 MG TABLET 0 APOTEX CORP EAGEX 60505-0180-<strong>08</strong> 0.04428 SERTRALINE HCL 25 MG TABLET 0 APOTEX CORP EAGEX 65862-0011-05 0.04428 SERTRALINE HCL 25 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0011-30 0.04428 SERTRALINE HCL 25 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0180-01 0.04428 SERTRALINE HCL 25 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0180-11 0.04428 SERTRALINE HCL 25 MG TABLET 0 AHP EAGEX 68180-0351-<strong>06</strong> 0.04428 SERTRALINE HCL 25 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0351-09 0.04428 SERTRALINE HCL 25 MG TABLET 0 LUPIN PHARMACEU EAGEX 68645-0424-54 0.04428 SERTRALINE HCL 25 MG TABLET 0 LEGACY PHARMACE EAGEX 76282-0212-01 0.04428 SERTRALINE HCL 25 MG TABLET 0 EXELAN PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 360LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 76282-0212-05 0.04428 SERTRALINE HCL 25 MG TABLET 0 EXELAN PHARMACE EAGEX 00143-9655-05 0.05373 SERTRALINE HCL 50 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-9655-09 0.05373 SERTRALINE HCL 50 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-9655-30 0.05373 SERTRALINE HCL 50 MG TABLET 0 WEST-WARD,INC. EAGEX 00378-8121-01 0.05373 SERTRALINE HCL 50 MG TABLET 0 MYLAN EAGEX 00378-8121-05 0.05373 SERTRALINE HCL 50 MG TABLET 0 MYLAN EAGEX 16714-<strong>06</strong>12-01 0.05373 SERTRALINE HCL 50 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>12-04 0.05373 SERTRALINE HCL 50 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>12-05 0.05373 SERTRALINE HCL 50 MG TABLET 0 NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>12-<strong>06</strong> 0.05373 SERTRALINE HCL 50 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 31722-0213-05 0.05373 SERTRALINE HCL 50 MG TABLET 0 CAMBER PHARMACE EAGEX 31722-0213-30 0.05373 SERTRALINE HCL 50 MG TABLET 0 CAMBER PHARMACE EAGEX 31722-0213-90 0.05373 SERTRALINE HCL 50 MG TABLET 0 CAMBER PHARMACE EAGEX 51079-0150-20 0.05373 SERTRALINE HCL 50 MG TABLET 0 MYLAN INSTITUTI EAGEX 54458-0913-02 0.05373 SERTRALINE HCL 50 MG TABLET 0 INTERNATIONAL L EAGEX 54458-0944-10 0.05373 SERTRALINE HCL 50 MG TABLET 0 INTERNATIONAL L EAGEX 59762-4900-01 0.05373 SERTRALINE HCL 50 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-4900-02 0.05373 SERTRALINE HCL 50 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-4900-04 0.05373 SERTRALINE HCL 50 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-4900-05 0.05373 SERTRALINE HCL 50 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-0181-03 0.05373 SERTRALINE HCL 50 MG TABLET 0 APOTEX CORP EAGEX 60505-0181-<strong>08</strong> 0.05373 SERTRALINE HCL 50 MG TABLET 0 APOTEX CORP EAGEX 65862-0012-05 0.05373 SERTRALINE HCL 50 MG TABLET 0 AUROBINDO PHARM EAGEX 65862-0012-30 0.05373 SERTRALINE HCL 50 MG TABLET 0 AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0181-01 0.05373 SERTRALINE HCL 50 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0181-11 0.05373 SERTRALINE HCL 50 MG TABLET 0 AHP EAGEX 68180-0352-02 0.05373 SERTRALINE HCL 50 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0352-05 0.05373 SERTRALINE HCL 50 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0352-<strong>06</strong> 0.05373 SERTRALINE HCL 50 MG TABLET 0 LUPIN PHARMACEU EAGEX 68180-0352-09 0.05373 SERTRALINE HCL 50 MG TABLET 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68645-0425-54 0.05373 SERTRALINE HCL 50 MG TABLET 0 LEGACY PHARMACE EAGEX 76282-0213-01 0.05373 SERTRALINE HCL 50 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-0213-05 0.05373 SERTRALINE HCL 50 MG TABLET 0 EXELAN PHARMACE EAGEX 76282-0213-30 0.05373 SERTRALINE HCL 50 MG TABLET 0 EXELAN PHARMACE EAGEX 16714-<strong>06</strong>01-01 0.67680 SERTRALINE 20 MG/ML ORAL CONC 0 NORTHSTAR RX LL MLGEX 16714-<strong>06</strong>01-02 0.67680 SERTRALINE 20 MG/ML ORAL CONC 0 NORTHSTAR RX LL MLGEX 59762-4940-01 0.67680 SERTRALINE 20 MG/ML ORAL CONC 0 GREENSTONE LLC. MLGEN 60258-0151-01 0.11022 SF 1.1% GEL 0 CYPRESS PHARM. GMGEN 60258-0150-01 0.12249 SF 5000 PLUS CREAM 0 CYPRESS PHARM. GMGEN 00093-5517-98 0.58010 SILDENAFIL 20 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-1657-77 0.58010 SILDENAFIL 20 MG TABLET G MYLAN EAGEN 00591-3780-19 0.58010 SILDENAFIL 20 MG TABLET G ACTAVIS PHARMA, EAGEN 13668-0185-05 0.58010 SILDENAFIL 20 MG TABLET G TORRENT PHARMAC EAGEN 13668-0185-90 0.58010 SILDENAFIL 20 MG TABLET G TORRENT PHARMAC EAGEN 16714-0338-01 0.58010 SILDENAFIL 20 MG TABLET G NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 361LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0372-90 0.58010 SILDENAFIL 20 MG TABLET G DR.REDDY'S LAB EAGEN 59762-0033-01 0.58010 SILDENAFIL 20 MG TABLET G GREENSTONE LLC. EAGEN 60505-3404-05 0.58010 SILDENAFIL 20 MG TABLET G APOTEX CORP EAGEN 60505-3404-09 0.58010 SILDENAFIL 20 MG TABLET G APOTEX CORP EAGEN 65162-0351-09 0.58010 SILDENAFIL 20 MG TABLET G AMNEAL PHARMACE EABEX 42847-0103-03 5.48270 SILENOR 3 MG TABLET G SOMAXON PHARMAC EABEX 42847-0103-10 7.10961 SILENOR 3 MG TABLET G PERNIX THERAPEU EABEX 42847-0103-30 7.48383 SILENOR 3 MG TABLET G PERNIX THERAPEU EABEX 42847-01<strong>06</strong>-03 5.48270 SILENOR 6 MG TABLET G SOMAXON PHARMAC EABEX 42847-01<strong>06</strong>-10 7.10961 SILENOR 6 MG TABLET G PERNIX THERAPEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 42847-01<strong>06</strong>-30 7.48383 SILENOR 6 MG TABLET G PERNIX THERAPEU EABUL 61570-0131-20 0.<strong>06</strong>280 0.41417 SILVADENE 1% CREAM G MONARCH PHRM GMBUL 61570-0131-40 0.<strong>06</strong>280 0.14661 SILVADENE 1% CREAM G MONARCH PHRM GMBUL 61570-0131-50 0.<strong>06</strong>280 0.27456 SILVADENE 1% CREAM G MONARCH PHRM GMBUL 61570-0131-85 0.<strong>06</strong>280 0.28718 SILVADENE 1% CREAM G MONARCH PHRM GMBUL 61570-0131-98 0.<strong>06</strong>280 0.11473 SILVADENE 1% CREAM G MONARCH PHRM GMGUL 00591-<strong>08</strong>10-46 0.<strong>06</strong>280 SILVER SULFADIAZINE 1% CREAM 0 ACTAVIS PHARMA, GMGUL 00591-<strong>08</strong>10-55 0.<strong>06</strong>280 SILVER SULFADIAZINE 1% CREAM 0 ACTAVIS PHARMA, GMGUL 00591-<strong>08</strong>10-83 0.<strong>06</strong>280 SILVER SULFADIAZINE 1% CREAM 0 ACTAVIS PHARMA, GMGUL 00591-<strong>08</strong>10-85 0.<strong>06</strong>280 SILVER SULFADIAZINE 1% CREAM 0 ACTAVIS PHARMA, GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 67877-0124-05 0.<strong>06</strong>280 SILVER SULFADIAZINE 1% CREAM 0 ASCEND LABORATO GMGUL 67877-0124-20 0.<strong>06</strong>280 SILVER SULFADIAZINE 1% CREAM 0 ASCEND LABORATO GMGUL 67877-0124-25 0.<strong>06</strong>280 SILVER SULFADIAZINE 1% CREAM 0 ASCEND LABORATO GMGUL 67877-0124-40 0.<strong>06</strong>280 SILVER SULFADIAZINE 1% CREAM 0 ASCEND LABORATO GMGUL 67877-0124-50 0.<strong>06</strong>280 SILVER SULFADIAZINE 1% CREAM 0 ASCEND LABORATO GMGUL 67877-0124-85 0.<strong>06</strong>280 SILVER SULFADIAZINE 1% CREAM 0 ASCEND LABORATO GMBND 00<strong>06</strong>5-4147-27 11.56605 SIMBRINZA 1%-0.2% EYE DROPS G ALCON LABS. MLBND 00074-3455-90 6.91048 SIMCOR 1,000-20 MG TABLET 0 ABBVIE US LLC EABND 00074-3457-90 6.91048 SIMCOR 1,000-40 MG TABLET 0 ABBVIE US LLC EABND 00074-3312-90 3.90727 SIMCOR 500-20 MG TABLET 0 ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00074-3459-90 3.90727 SIMCOR 500-40 MG TABLET 0 ABBVIE US LLC EABND 00074-3315-90 5.57317 SIMCOR 750-20 MG TABLET 0 ABBVIE US LLC EABND 57894-0071-02 3104.67310 SIMPONI 100 MG/ML PEN INJECTOR G JANSSEN BIOTECH MLBND 57894-0071-01 3104.67310 SIMPONI 100 MG/ML SYRINGE G JANSSEN BIOTECH MLBND 57894-0070-02 5399.43220 SIMPONI 50 MG/0.5 ML PEN INJEC G JANSSEN BIOTECH MLBND 57894-0070-01 5399.43220 SIMPONI 50 MG/0.5 ML SYRINGE G JANSSEN BIOTECH MLGEN 00093-7153-10 0.03497 SIMVASTATIN 10 MG TABLET 0 TEVA USA EAGEN 00093-7153-98 0.03497 SIMVASTATIN 10 MG TABLET 0 TEVA USA EAGEN 00904-5800-61 0.03497 SIMVASTATIN 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 16714-<strong>06</strong>82-01 0.03497 SIMVASTATIN 10 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-<strong>06</strong>82-02 0.03497 SIMVASTATIN 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>82-03 0.03497 SIMVASTATIN 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 16729-0004-10 0.03497 SIMVASTATIN 10 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0004-15 0.03497 SIMVASTATIN 10 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0004-17 0.03497 SIMVASTATIN 10 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 362LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42571-0010-05 0.03497 SIMVASTATIN 10 MG TABLET 0 MICRO LABS USA, EAGEN 51079-0454-01 0.03497 SIMVASTATIN 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0454-20 0.03497 SIMVASTATIN 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 52343-0022-30 0.02424 SIMVASTATIN 10 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0022-45 0.02233 SIMVASTATIN 10 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0022-90 0.01850 SIMVASTATIN 10 MG TABLET 0 GEN-SOURCE RX EAGEN 54458-0900-10 0.03497 SIMVASTATIN 10 MG TABLET 0 INTERNATIONAL L EAGEN 54458-0934-10 0.03497 SIMVASTATIN 10 MG TABLET 0 INTERNATIONAL L EAGEN 55111-0198-05 0.03497 SIMVASTATIN 10 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0198-30 0.03497 SIMVASTATIN 10 MG TABLET 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0198-90 0.03497 SIMVASTATIN 10 MG TABLET 0 DR.REDDY'S LAB EAGEN 65862-0051-26 0.03497 SIMVASTATIN 10 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0051-30 0.03497 SIMVASTATIN 10 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0051-90 0.03497 SIMVASTATIN 10 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0051-99 0.03497 SIMVASTATIN 10 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0478-01 0.03497 SIMVASTATIN 10 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0478-02 0.03497 SIMVASTATIN 10 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0478-03 0.03497 SIMVASTATIN 10 MG TABLET 0 LUPIN PHARMACEU EAGEN 68382-0<strong>06</strong>6-05 0.03497 SIMVASTATIN 10 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0<strong>06</strong>6-10 0.03497 SIMVASTATIN 10 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0<strong>06</strong>6-16 0.03497 SIMVASTATIN 10 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00093-7154-10 0.02943 SIMVASTATIN 20 MG TABLET 0 TEVA USA EAGEN 00093-7154-56 0.02943 SIMVASTATIN 20 MG TABLET 0 TEVA USA EAGEN 00093-7154-98 0.02943 SIMVASTATIN 20 MG TABLET 0 TEVA USA EAGEN 00904-5801-61 0.02943 SIMVASTATIN 20 MG TABLET 0 MAJOR PHARMACEU EAGEN 16714-<strong>06</strong>83-01 0.02943 SIMVASTATIN 20 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>83-02 0.02943 SIMVASTATIN 20 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>83-03 0.02943 SIMVASTATIN 20 MG TABLET 0 NORTHSTAR RX LL EAGEN 16729-0005-10 0.02943 SIMVASTATIN 20 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0005-15 0.02943 SIMVASTATIN 20 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16729-0005-17 0.02943 SIMVASTATIN 20 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 51079-0455-01 0.02943 SIMVASTATIN 20 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0455-20 0.02943 SIMVASTATIN 20 MG TABLET 0 MYLAN INSTITUTI EAGEN 52343-0023-30 0.02943 SIMVASTATIN 20 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0023-45 0.02943 SIMVASTATIN 20 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0023-90 0.02758 SIMVASTATIN 20 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0023-99 0.01866 SIMVASTATIN 20 MG TABLET 0 GEN-SOURCE RX EAGEN 54458-<strong>08</strong>99-10 0.02943 SIMVASTATIN 20 MG TABLET 0 INTERNATIONAL L EAGEN 54458-0933-10 0.02943 SIMVASTATIN 20 MG TABLET 0 INTERNATIONAL L EAGEN 55111-0199-05 0.02943 SIMVASTATIN 20 MG TABLET 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0199-10 0.02943 SIMVASTATIN 20 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0199-90 0.02943 SIMVASTATIN 20 MG TABLET 0 DR.REDDY'S LAB EAGEN 63304-0791-10 0.02943 SIMVASTATIN 20 MG TABLET 0 RANBAXY PHARMAC EAGEN 63739-0437-04 0.02943 SIMVASTATIN 20 MG TABLET 0 MCKESSON PACKAG EAGEN 63739-0572-10 0.02943 SIMVASTATIN 20 MG TABLET 0 MCKESSON PACKAG EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 363LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-0052-26 0.02943 SIMVASTATIN 20 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0052-30 0.02943 SIMVASTATIN 20 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0052-90 0.02943 SIMVASTATIN 20 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0052-99 0.02943 SIMVASTATIN 20 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0479-01 0.02943 SIMVASTATIN 20 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0479-02 0.02943 SIMVASTATIN 20 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0479-03 0.02943 SIMVASTATIN 20 MG TABLET 0 LUPIN PHARMACEU EAGEN 68382-0<strong>06</strong>7-05 0.02943 SIMVASTATIN 20 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0<strong>06</strong>7-10 0.02943 SIMVASTATIN 20 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0<strong>06</strong>7-16 0.02943 SIMVASTATIN 20 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68645-0261-54 0.02943 SIMVASTATIN 20 MG TABLET 0 LEGACY PHARMACE EAGEN 68645-0470-54 0.02943 SIMVASTATIN 20 MG TABLET 0 LEGACY PHARMACE EAGEN 00093-7155-10 0.04577 SIMVASTATIN 40 MG TABLET 0 TEVA USA EAGEN 00093-7155-98 0.04577 SIMVASTATIN 40 MG TABLET 0 TEVA USA EAGEN 00904-5802-61 0.04577 SIMVASTATIN 40 MG TABLET 0 MAJOR PHARMACEU EAGEN 16714-<strong>06</strong>84-01 0.04577 SIMVASTATIN 40 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>84-02 0.04577 SIMVASTATIN 40 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>84-03 0.04577 SIMVASTATIN 40 MG TABLET 0 NORTHSTAR RX LL EAGEN 16729-00<strong>06</strong>-10 0.04577 SIMVASTATIN 40 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-00<strong>06</strong>-15 0.04577 SIMVASTATIN 40 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16729-00<strong>06</strong>-17 0.04577 SIMVASTATIN 40 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 51079-0456-01 0.04577 SIMVASTATIN 40 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0456-20 0.04577 SIMVASTATIN 40 MG TABLET 0 MYLAN INSTITUTI EAGEN 52343-0024-30 0.04050 SIMVASTATIN 40 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0024-45 0.03883 SIMVASTATIN 40 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0024-90 0.035<strong>08</strong> SIMVASTATIN 40 MG TABLET 0 GEN-SOURCE RX EAGEN 54458-<strong>08</strong>94-10 0.04577 SIMVASTATIN 40 MG TABLET 0 INTERNATIONAL L EAGEN 54458-0932-10 0.04577 SIMVASTATIN 40 MG TABLET 0 INTERNATIONAL L EAGEN 55111-0200-05 0.04577 SIMVASTATIN 40 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0200-10 0.04577 SIMVASTATIN 40 MG TABLET 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0200-30 0.04577 SIMVASTATIN 40 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0200-90 0.04577 SIMVASTATIN 40 MG TABLET 0 DR.REDDY'S LAB EAGEN 63304-0792-10 0.04577 SIMVASTATIN 40 MG TABLET 0 RANBAXY PHARMAC EAGEN 63739-0573-10 0.04577 SIMVASTATIN 40 MG TABLET 0 MCKESSON PACKAG EAGEN 65862-0053-22 0.04577 SIMVASTATIN 40 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0053-30 0.04577 SIMVASTATIN 40 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0053-90 0.04577 SIMVASTATIN 40 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0053-99 0.04577 SIMVASTATIN 40 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0480-01 0.04577 SIMVASTATIN 40 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0480-02 0.04577 SIMVASTATIN 40 MG TABLET 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68180-0480-03 0.04577 SIMVASTATIN 40 MG TABLET 0 LUPIN PHARMACEU EAGEN 68382-0<strong>06</strong>8-05 0.04577 SIMVASTATIN 40 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0<strong>06</strong>8-10 0.04577 SIMVASTATIN 40 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0<strong>06</strong>8-16 0.04577 SIMVASTATIN 40 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68645-0262-54 0.04577 SIMVASTATIN 40 MG TABLET 0 LEGACY PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 364LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68645-0471-54 0.04577 SIMVASTATIN 40 MG TABLET 0 LEGACY PHARMACE EAGEN 00093-7152-98 0.04104 SIMVASTATIN 5 MG TABLET 0 TEVA USA EAGEN 16714-<strong>06</strong>81-01 0.04104 SIMVASTATIN 5 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>81-02 0.04104 SIMVASTATIN 5 MG TABLET 0 NORTHSTAR RX LL EAGEN 52343-0021-30 0.02424 SIMVASTATIN 5 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0021-90 0.01842 SIMVASTATIN 5 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0021-99 0.01548 SIMVASTATIN 5 MG TABLET 0 GEN-SOURCE RX EAGEN 55111-0197-05 0.04104 SIMVASTATIN 5 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0197-30 0.04104 SIMVASTATIN 5 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0197-90 0.04104 SIMVASTATIN 5 MG TABLET 0 DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63739-0570-10 0.04104 SIMVASTATIN 5 MG TABLET 0 MCKESSON PACKAG EAGEN 65862-0050-30 0.04104 SIMVASTATIN 5 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0050-90 0.04104 SIMVASTATIN 5 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0482-<strong>06</strong> 0.04104 SIMVASTATIN 5 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0482-09 0.04104 SIMVASTATIN 5 MG TABLET 0 LUPIN PHARMACEU EAGEN 68382-0<strong>06</strong>5-10 0.04104 SIMVASTATIN 5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0<strong>06</strong>5-16 0.04104 SIMVASTATIN 5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00093-7156-10 0.10071 SIMVASTATIN 80 MG TABLET 0 TEVA USA EAGEN 00093-7156-98 0.10071 SIMVASTATIN 80 MG TABLET 0 TEVA USA EAGEN 16714-<strong>06</strong>85-01 0.10071 SIMVASTATIN 80 MG TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-<strong>06</strong>85-02 0.10071 SIMVASTATIN 80 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>85-03 0.10071 SIMVASTATIN 80 MG TABLET 0 NORTHSTAR RX LL EAGEN 16729-0007-10 0.10071 SIMVASTATIN 80 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0007-15 0.10071 SIMVASTATIN 80 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 16729-0007-17 0.10071 SIMVASTATIN 80 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEN 42571-0<strong>08</strong>0-05 0.10071 SIMVASTATIN 80 MG TABLET 0 MICRO LABS USA, EAGEN 52343-0025-30 0.05300 SIMVASTATIN 80 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0025-45 0.05933 SIMVASTATIN 80 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0025-90 0.04842 SIMVASTATIN 80 MG TABLET 0 GEN-SOURCE RX EAGEN 52343-0025-99 0.04576 SIMVASTATIN 80 MG TABLET 0 GEN-SOURCE RX EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-0268-05 0.10071 SIMVASTATIN 80 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0268-30 0.10071 SIMVASTATIN 80 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0268-90 0.10071 SIMVASTATIN 80 MG TABLET 0 DR.REDDY'S LAB EAGEN 65862-0054-30 0.10071 SIMVASTATIN 80 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0054-39 0.10071 SIMVASTATIN 80 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0054-90 0.10071 SIMVASTATIN 80 MG TABLET 0 AUROBINDO PHARM EAGEN 65862-0054-99 0.10071 SIMVASTATIN 80 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0481-01 0.10071 SIMVASTATIN 80 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0481-02 0.10071 SIMVASTATIN 80 MG TABLET 0 LUPIN PHARMACEU EAGEN 68180-0481-03 0.10071 SIMVASTATIN 80 MG TABLET 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0<strong>06</strong>9-05 0.10071 SIMVASTATIN 80 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0<strong>06</strong>9-10 0.10071 SIMVASTATIN 80 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0<strong>06</strong>9-16 0.10071 SIMVASTATIN 80 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68645-0263-54 0.10071 SIMVASTATIN 80 MG TABLET 0 LEGACY PHARMACE EAGEN 68645-0472-54 0.10071 SIMVASTATIN 80 MG TABLET 0 LEGACY PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 365LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-3918-68 0.33075 1.14755 SINEMET CR 25-100 TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-3919-68 0.64260 2.21095 SINEMET CR 50-200 TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-3915-68 0.14364 0.9<strong>08</strong>76 SINEMET 10-100 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-3916-68 0.13240 1.02604 SINEMET 25-100 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-3917-68 0.18170 1.30741 SINEMET 25-250 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0117-31 0.34250 5.21738 SINGULAIR 10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0117-54 0.34250 5.21673 SINGULAIR 10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-9117-31 0.34250 5.85122 SINGULAIR 10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-9117-54 0.34250 5.85039 SINGULAIR 10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-9117-80 0.34250 5.85045 SINGULAIR 10 MG TABLET G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-3841-30 5.85122 5.85122 SINGULAIR 4 MG GRANULES G MERCK SHARP & D EABND 000<strong>06</strong>-0711-31 0.39290 5.21738 SINGULAIR 4 MG TABLET CHEW G MERCK SHARP & D EABND 000<strong>06</strong>-0711-54 0.39290 5.21673 SINGULAIR 4 MG TABLET CHEW G MERCK SHARP & D EABND 000<strong>06</strong>-1711-31 0.39290 5.85122 SINGULAIR 4 MG TABLET CHEW G MERCK SHARP & D EABND 000<strong>06</strong>-1711-54 0.39290 5.85039 SINGULAIR 4 MG TABLET CHEW G MERCK SHARP & D EABND 000<strong>06</strong>-0275-31 0.38840 5.21738 SINGULAIR 5 MG TABLET CHEW G MERCK SHARP & D EABND 000<strong>06</strong>-0275-54 0.38840 5.21673 SINGULAIR 5 MG TABLET CHEW G MERCK SHARP & D EABND 000<strong>06</strong>-9275-31 0.38840 5.85122 SINGULAIR 5 MG TABLET CHEW G MERCK SHARP & D EABND 000<strong>06</strong>-9275-54 0.38840 5.85039 SINGULAIR 5 MG TABLET CHEW G MERCK SHARP & D EABND 000<strong>06</strong>-9275-82 0.38840 5.85049 SINGULAIR 5 MG TABLET CHEW G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-1001-01 6.23070 SIROLIMUS 0.5 MG TABLET 0 GREENSTONE LLC. EAGEN 68382-0520-01 6.49035 SIROLIMUS 0.5 MG TABLET 0 ZYDUS PHARMACEU EABEX 59676-0701-01 158.93616 SIRTURO 100 MG TABLET 0 JANSSEN PRODUCT EABND 60793-0136-01 3.91280 5.07320 SKELAXIN 800 MG TABLET G PFIZER US PHARM EABND 60793-0136-05 3.91280 5.07324 SKELAXIN 800 MG TABLET G PFIZER US PHARM EABND 49281-0183-71 2.19531 SKLICE 0.5% LOTION 0 SAN<strong>OF</strong>I-PASTEUR GMGEN 00121-0595-16 0.00700 SOD CITRATE-CITRIC ACID SOLN 0 PHARMACEU ASSOC MLGEN 00591-0149-87 5.15220 SOD FER GLUC CPLX 62.5 MG/5 ML 0 ACTAVIS PHARMA, MLGEN 00591-25<strong>08</strong>-26 5.15220 SOD FER GLUC CPLX 62.5 MG/5 ML 0 ACTAVIS PHARMA, MLGEN 00591-25<strong>08</strong>-87 5.15220 SOD FER GLUC CPLX 62.5 MG/5 ML 0 ACTAVIS PHARMA, ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00574-2003-02 0.11718 SOD POLYSTYREN SULF 15 G/60 ML 0 PADDOCK LABS. MLGEN 00574-2003-16 0.11718 SOD POLYSTYREN SULF 15 G/60 ML 0 PADDOCK LABS. MLGEN 00517-2096-25 0.15234 SODIUM ACETATE 2 MEQ/ML VIAL 0 AMER. REGENT MLGEN 00517-5023-25 0.11250 SODIUM ACETATE 4 MEQ/ML VIAL 0 AMER. REGENT MLGEN 63323-0032-61 0.07929 SODIUM ACETATE 4 MEQ/ML VIAL 0 APP PHARMACEUTI MLGEN 38779-0551-<strong>08</strong> 0.29925 SODIUM BENZOATE POWDER 0 MEDISCA INC. GMBND 63323-0026-05 0.50796 SODIUM BICARB 4.2% VIAL 0 APP PHARMACEUTI MLGEN 00409-6637-34 0.11160 SODIUM BICARB 8.4% ABBOJECT 0 HOSPIRA MLGEN 76329-3352-01 0.1<strong>08</strong>00 SODIUM BICARB 8.4% SYRINGE 0 INTERNATIONAL M MLGEN 00409-6625-02 0.02170 SODIUM BICARB 8.4% VIAL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00338-0043-04 0.00177 SODIUM CHLORIDE 0.45% SOLN 0 BAXTER <strong>HEALTH</strong>CA MLBND 00409-7132-02 0.01<strong>06</strong>7 SODIUM CHLORIDE 0.45% SOLN 0 HOSPIRA MLGEN 00409-7985-02 0.00180 SODIUM CHLORIDE 0.45% SOLN 0 HOSPIRA MLGEN 00409-7985-09 0.00156 SODIUM CHLORIDE 0.45% SOLN 0 HOSPIRA MLGEN 00487-9302-01 0.03260 SODIUM CHLORIDE 0.9% INHAL VL 0 NEPHRON CORP ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 366LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00264-2201-00 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 B.BRAUN MLGEN 00264-2201-10 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 B.BRAUN MLGEN 00264-2201-50 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 B.BRAUN MLGEN 00264-2201-70 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 B.BRAUN MLGEN 00338-0047-27 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0047-29 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0047-44 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0047-46 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0047-47 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0048-02 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 BAXTER <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00338-0048-03 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0048-04 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0050-47 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-6138-03 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 HOSPIRA MLGEN 00409-6138-22 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 HOSPIRA MLGEN 00409-7138-09 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 HOSPIRA MLGEN 00409-7138-36 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 HOSPIRA MLGEN 00409-7972-05 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 HOSPIRA MLGEN 00409-7972-07 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 HOSPIRA MLGEN 00409-7972-<strong>08</strong> 0.00135 SODIUM CHLORIDE 0.9% IRRIG. 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-7101-02 0.00980 SODIUM CHLORIDE 0.9% SOLN 0 HOSPIRA MLBND 00409-7101-66 0.03585 SODIUM CHLORIDE 0.9% SOLN 0 HOSPIRA MLBND 00409-7101-67 0.01822 SODIUM CHLORIDE 0.9% SOLN 0 HOSPIRA MLBND 00338-0553-11 0.09900 SODIUM CHLORIDE 0.9% SOLN. 0 BAXTER <strong>HEALTH</strong>CA MLBND 00338-0553-18 0.03600 0.04950 SODIUM CHLORIDE 0.9% SOLN. 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00264-1800-31 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 B.BRAUN MLGEN 00264-1800-32 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 B.BRAUN MLGEN 00264-4001-55 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 B.BRAUN MLGEN 00338-0049-01 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0049-02 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 BAXTER <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00338-0049-03 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0049-04 0.00153 SODIUM CHLORIDE 0.9% SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0049-11 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0049-18 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0049-31 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0049-38 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0049-48 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-6304-02 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-6304-03 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-1583-01 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-7983-02 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 HOSPIRA MLGEN 00409-7983-03 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 HOSPIRA MLGEN 00409-7983-09 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 HOSPIRA MLGEN 00409-7983-53 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 HOSPIRA MLGEN 00409-7983-61 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 HOSPIRA ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 367LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-7984-20 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 HOSPIRA MLGEN 00409-7984-23 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 HOSPIRA MLGEN 00409-7984-36 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 HOSPIRA MLGEN 00409-7984-37 0.00162 SODIUM CHLORIDE 0.9% SOLUTION 0 HOSPIRA MLGEN 00409-1918-32 0.39600 SODIUM CHLORIDE 0.9% SYRINGE 0 HOSPIRA MLGEN 00409-1918-33 0.31500 SODIUM CHLORIDE 0.9% SYRINGE 0 HOSPIRA MLGEN 00409-1918-35 0.18000 SODIUM CHLORIDE 0.9% SYRINGE 0 HOSPIRA MLGEN 00409-2102-02 0.05040 SODIUM CHLORIDE 0.9% VIAL 0 HOSPIRA MLGEN 00409-2102-05 0.05040 SODIUM CHLORIDE 0.9% VIAL 0 HOSPIRA MLGEN 00409-4888-10 0.05040 SODIUM CHLORIDE 0.9% VIAL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-4888-12 0.05040 SODIUM CHLORIDE 0.9% VIAL 0 HOSPIRA MLGEN 00409-4888-20 0.04275 SODIUM CHLORIDE 0.9% VIAL 0 HOSPIRA MLGEN 00409-4888-50 0.03168 SODIUM CHLORIDE 0.9% VIAL 0 HOSPIRA MLGEN 63323-0186-00 0.05040 SODIUM CHLORIDE 0.9% VIAL 0 APP PHARMACEUTI MLGEN 63323-0186-02 0.05040 SODIUM CHLORIDE 0.9% VIAL 0 APP PHARMACEUTI MLGEN 63323-0186-10 0.05040 SODIUM CHLORIDE 0.9% VIAL 0 APP PHARMACEUTI MLGEN 63323-0186-20 0.05040 SODIUM CHLORIDE 0.9% VIAL 0 APP PHARMACEUTI MLGEN 00264-7805-10 0.00750 SODIUM CHLORIDE 3% IV SOLN 0 B.BRAUN MLGEN 00338-0054-03 0.00417 SODIUM CHLORIDE 3% IV SOLN 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-1130-02 0.01656 SODIUM CHLORIDE 4 MEQ/ML VL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-1141-02 0.01830 SODIUM CHLORIDE 4 MEQ/ML VL 0 HOSPIRA MLGEN 00517-2930-25 0.01830 SODIUM CHLORIDE 4 MEQ/ML VL 0 AMER. REGENT MLGEN 63323-0<strong>08</strong>8-61 0.01830 SODIUM CHLORIDE 4 MEQ/ML VL 0 APP PHARMACEUTI MLGEN 00264-78<strong>06</strong>-10 0.00338 SODIUM CHLORIDE 5% IV SOLN 0 B.BRAUN MLGEN 00409-6657-73 0.03150 SODIUM CL 2.5 MEQ/ML VIAL 0 HOSPIRA MLGEN 63323-0139-40 0.04050 SODIUM CL 2.5 MEQ/ML VIAL 0 APP PHARMACEUTI MLBND 25010-0210-27 1244.33600 SODIUM EDECRIN 50 MG VIAL 0 VALEANT EAGEN 44946-1032-<strong>08</strong> 0.12760 SODIUM FLUORIDE 0.5 MG/ML DROP 0 SANCILIO & COMP MLGEN 51862-0165-50 0.12760 SODIUM FLUORIDE 0.5 MG/ML DROP 0 LIBERTAS PHARMA MLGEN 51927-1237-00 0.15000 SODIUM HYDROXIDE GRANULES 0 PR<strong>OF</strong>ESSIONAL CO GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42794-0<strong>08</strong>6-14 16.83459 SODIUM PHENYLBUTYRATE POWDER 0 SIGMAPHARM LABO GMGEN 00517-3450-25 0.11250 SODIUM PHOSPHATE 3MM/ML VIAL 0 AMER. REGENT MLGEN 46287-0012-16 0.23127 SODIUM POLYSTYRENE SULF PWD 0 CAROLINA MED. GMBND 10337-<strong>08</strong>03-01 11.48637 SOLARAZE 3% GEL G SANDOZ GMBND 99207-0467-30 31.43016 SOLODYN ER 105 MG TABLET G VALEANT EABND 99207-0464-30 31.43016 SOLODYN ER 115 MG TABLET G VALEANT EABND 99207-0465-30 31.43016 SOLODYN ER 55 MG TABLET G VALEANT EABND 99207-0463-30 31.43016 SOLODYN ER 65 MG TABLET G VALEANT EABND 99207-0466-30 31.43016 SOLODYN ER 80 MG TABLET G VALEANT EABND 13632-0123-01 0.99600 SOLTAMOX 10 MG/5 ML SOLN 0 DARA BIOSCIENCE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-0920-03 29.63930 SOLU-CORTEF 1,000 MG ACT-O-VL 0 PHARMACIA/UPJHN EABND 00009-0005-01 46.02350 SOLU-CORTEF 1,000 MG VIAL 0 PFIZER US PHARM EABND 00009-0900-13 4.02550 SOLU-CORTEF 100 MG ACT-O-VL 0 PHARMACIA/UPJHN EABND 00009-0900-20 4.02782 SOLU-CORTEF 100 MG ACT-O-VL 0 PHARMACIA/UPJHN EABND 00009-0011-03 6.24160 SOLU-CORTEF 100 MG VIAL 0 PFIZER US PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 368LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-0011-04 6.24857 SOLU-CORTEF 100 MG VIAL 0 PFIZER US PHARM EABND 00009-<strong>08</strong>25-01 4.83890 SOLU-CORTEF 100 MG VIAL G PHARMACIA/UPJHN EABND 00009-0909-<strong>08</strong> 7.12140 SOLU-CORTEF 250 MG ACT-O-VL 0 PHARMACIA/UPJHN EABND 00009-0912-05 15.80320 SOLU-CORTEF 500 MG ACT-O-VL G PHARMACIA/UPJHN EABND 00009-<strong>06</strong>98-01 17.45800 23.42260 SOLU-MEDROL 1 GM VIAL 0 PHARMACIA/UPJHN EABND 00009-0018-20 31.77240 SOLU-MEDROL 1,000 MG VIAL 0 PFIZER US PHARM EABND 00009-0047-22 5.32594 SOLU-MEDROL 125 MG VIAL 0 PFIZER US PHARM EABND 00009-0047-26 3.46000 5.32594 SOLU-MEDROL 125 MG VIAL 0 PFIZER/NOVAPLUS EABND 00009-0047-27 5.32860 SOLU-MEDROL 125 MG VIAL 0 PFIZER/NOVAPLUS EABND 00009-0796-01 51.15290 SOLU-MEDROL 2,000 MG VIAL 0 PHARMACIA/UPJHN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-0039-28 3.303<strong>06</strong> SOLU-MEDROL 40 MG VIAL 0 PFIZER US PHARM EABND 00009-0039-32 1.95000 3.303<strong>06</strong> SOLU-MEDROL 40 MG VIAL 0 PFIZER/NOVAPLUS EABND 00009-0039-33 3.30340 SOLU-MEDROL 40 MG VIAL 0 PFIZER/NOVAPLUS EABND 00009-0003-02 20.03620 SOLU-MEDROL 500 MG VIAL 0 PFIZER US PHARM EABND 00009-0758-01 8.23500 12.36700 SOLU-MEDROL 500 MG VIAL 0 PHARMACIA/UPJHN EABND 15054-0120-01 8920.17600 SOMATULINE 120 MG/0.5 ML SYRGE 0 IPSEN BIOPHARMA MLBND 15054-0<strong>06</strong>0-01 15159.12000 SOMATULINE 60 MG/0.2 ML SYRING 0 IPSEN BIOPHARMA MLBND 15054-0090-01 11151.88000 SOMATULINE 90 MG/0.3 ML SYRING 0 IPSEN BIOPHARMA MLBND 00009-5176-01 118.10900 SOMAVERT 10 MG VIAL 0 PHARMACIA/UPJHN EABND 00009-5176-02 128.73300 SOMAVERT 10 MG VIAL 0 PHARMACIA/UPJHN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-5178-01 168.73070 SOMAVERT 15 MG VIAL 0 PHARMACIA/UPJHN EABND 00009-5178-02 193.11610 SOMAVERT 15 MG VIAL 0 PHARMACIA/UPJHN EABND 00009-5180-01 236.20970 SOMAVERT 20 MG VIAL 0 PHARMACIA/UPJHN EABND 00009-5180-02 257.46600 SOMAVERT 20 MG VIAL 0 PHARMACIA/UPJHN EABND 00145-0090-25 30.78912 30.78912 SORIATANE 10 MG CAPSULE 0 STIEFEL LABS. EABND 00145-3817-03 37.95092 SORIATANE 17.5 MG CAPSULE 0 STIEFEL LABS. EABND 00145-0091-25 37.95092 37.95092 SORIATANE 25 MG CAPSULE 0 STIEFEL LABS. EABND 00145-2130-<strong>06</strong> 7.43528 SORILUX 0.005% FOAM G GLAXOSMITHKLINE GMBND 00145-2130-07 7.10729 SORILUX 0.005% FOAM G GLAXOSMITHKLINE GMGEN 00378-5124-01 0.19548 SOTALOL AF 120 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0223-01 0.19548 SOTALOL AF 120 MG TABLET 0 APOTEX CORP EAGEN 00378-5125-01 0.47500 SOTALOL AF 160 MG TABLET 0 MYLAN EAGEN 60505-0224-01 0.47500 SOTALOL AF 160 MG TABLET 0 APOTEX CORP EAGEN 00378-5123-01 0.09504 SOTALOL AF 80 MG TABLET 0 MYLAN EAGEN 60505-0222-01 0.09504 SOTALOL AF 80 MG TABLET 0 APOTEX CORP EAGEN 00093-1<strong>06</strong>0-01 0.19548 SOTALOL 120 MG TABLET 0 TEVA USA EAGEN 00185-0170-01 0.19548 SOTALOL 120 MG TABLET 0 SANDOZ EAGEN 00185-0170-09 0.19548 SOTALOL 120 MG TABLET 0 SANDOZ EAGEN 0<strong>06</strong>03-5770-21 0.19548 SOTALOL 120 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5770-25 0.19548 SOTALOL 120 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0159-00 0.19548 SOTALOL 120 MG TABLET 0 APOTEX CORP EAGEN 00093-1<strong>06</strong>2-01 0.47500 SOTALOL 160 MG TABLET 0 TEVA USA EAGEN 00185-0177-01 0.47500 SOTALOL 160 MG TABLET 0 SANDOZ EAGEN 00185-0177-09 0.47500 SOTALOL 160 MG TABLET 0 SANDOZ EAGEN 0<strong>06</strong>03-5771-21 0.47500 SOTALOL 160 MG TABLET 0 QUALITEST EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 369LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0<strong>08</strong>1-00 0.47500 SOTALOL 160 MG TABLET 0 APOTEX CORP EAGEN 00093-1<strong>06</strong>3-01 0.36680 SOTALOL 240 MG TABLET 0 TEVA USA EAGEN 00185-0174-01 0.36680 SOTALOL 240 MG TABLET 0 SANDOZ EAGEN 00185-0174-09 0.36680 SOTALOL 240 MG TABLET 0 SANDOZ EAGEN 60505-0<strong>08</strong>2-00 0.36680 SOTALOL 240 MG TABLET 0 APOTEX CORP EAGEN 00093-1<strong>06</strong>1-01 0.09504 SOTALOL 80 MG TABLET 0 TEVA USA EAGEN 00185-0171-01 0.09504 SOTALOL 80 MG TABLET 0 SANDOZ EAGEN 00185-0171-05 0.09504 SOTALOL 80 MG TABLET 0 SANDOZ EAGEN 00185-0171-09 0.09504 SOTALOL 80 MG TABLET 0 SANDOZ EAGEN 0<strong>06</strong>03-5769-21 0.09504 SOTALOL 80 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-5769-28 0.09504 SOTALOL 80 MG TABLET 0 QUALITEST EAGEN 60505-0<strong>08</strong>0-00 0.09504 SOTALOL 80 MG TABLET 0 APOTEX CORP EAGEN 1<strong>06</strong>31-0585-31 6.50525 SOTRET 20 MG CAPSULE 0 RANBAXY LABORAT EABND 61958-1501-01 996.00000 SOVALDI 400 MG TABLET G GILEAD SCIENCES EABND 10122-<strong>08</strong>01-20 14.81882 SPECTRACEF 200 MG DOSE PACK TB G METHAPHARM INC EAGEN 44183-0900-04 1.54956 SPINOSAD 0.9% TOPICAL SUSP 0 MACOVEN PHARMAC MLBND 00597-0075-41 9.32754 SPIRIVA 18 MCG CP-HANDIHALER 0 BOEHRINGER ING. EABND 00597-0075-47 9.32754 SPIRIVA 18 MCG CP-HANDIHALER 0 BOEHRINGER ING. EABND 00597-0075-75 15.68866 SPIRIVA 18 MCG CP-HANDIHALER 0 BOEHRINGER ING. EAGEN 00228-2673-11 0.45550 SPIRONOLACTONE 100 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-2673-50 0.45550 SPIRONOLACTONE 100 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00378-0437-01 0.45550 SPIRONOLACTONE 100 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-5765-21 0.45550 SPIRONOLACTONE 100 MG TABLET 0 QUALITEST EAGEN 51079-0980-01 0.45550 SPIRONOLACTONE 100 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0980-20 0.45550 SPIRONOLACTONE 100 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0329-01 0.45550 SPIRONOLACTONE 100 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0329-05 0.45550 SPIRONOLACTONE 100 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0329-<strong>06</strong> 0.45550 SPIRONOLACTONE 100 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0329-07 0.45550 SPIRONOLACTONE 100 MG TABLET 0 MUTUAL PHARM CO EAGEN 53746-0515-01 0.45550 SPIRONOLACTONE 100 MG TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-5013-01 0.45550 SPIRONOLACTONE 100 MG TABLET 0 GREENSTONE LLC. EAGEN 65162-0515-10 0.45550 SPIRONOLACTONE 100 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0515-50 0.45550 SPIRONOLACTONE 100 MG TABLET 0 AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-02<strong>08</strong>-01 0.45550 SPIRONOLACTONE 100 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-02<strong>08</strong>-11 0.45550 SPIRONOLACTONE 100 MG TABLET 0 AHP EAGEN 00228-2803-11 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00228-2803-50 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00378-2146-01 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 MYLAN EAGEN 00378-2146-05 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-5763-21 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-5763-28 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5763-30 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5763-32 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 QUALITEST EAGEN 00781-1599-01 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 SANDOZ EAGEN 00781-1599-05 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 370LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-1599-10 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 SANDOZ EAGEN 51079-0103-20 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0103-63 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0143-01 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0143-05 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0143-10 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 MUTUAL PHARM CO EAGEN 53746-0511-01 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0511-05 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0511-10 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 59762-5011-01 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-5011-02 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 GREENSTONE LLC. EAGEN 63739-0544-10 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 MCKESSON PACKAG EAGEN 65162-0511-10 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0511-11 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0511-50 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-02<strong>06</strong>-01 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-02<strong>06</strong>-11 0.<strong>06</strong>510 SPIRONOLACTONE 25 MG TABLET 0 AHP EAGEN 00228-2672-11 0.24800 SPIRONOLACTONE 50 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00228-2672-50 0.24800 SPIRONOLACTONE 50 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00378-0243-01 0.24800 SPIRONOLACTONE 50 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0243-05 0.24800 SPIRONOLACTONE 50 MG TABLET 0 MYLAN EAGEN 00378-0243-93 0.24800 SPIRONOLACTONE 50 MG TABLET 0 MYLAN EAGEN 0<strong>06</strong>03-5764-21 0.24800 SPIRONOLACTONE 50 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5764-28 0.24800 SPIRONOLACTONE 50 MG TABLET 0 QUALITEST EAGEN 51079-0979-01 0.24800 SPIRONOLACTONE 50 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0979-20 0.24800 SPIRONOLACTONE 50 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0328-01 0.24800 SPIRONOLACTONE 50 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0328-05 0.24800 SPIRONOLACTONE 50 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0328-<strong>06</strong> 0.24800 SPIRONOLACTONE 50 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0328-07 0.24800 SPIRONOLACTONE 50 MG TABLET 0 MUTUAL PHARM CO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53746-0514-01 0.24800 SPIRONOLACTONE 50 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0514-05 0.24800 SPIRONOLACTONE 50 MG TABLET 0 AMNEAL PHARMACE EAGEN 59762-5012-01 0.24800 SPIRONOLACTONE 50 MG TABLET 0 GREENSTONE LLC. EAGEN 63739-0545-10 0.24800 SPIRONOLACTONE 50 MG TABLET 0 MCKESSON PACKAG EAGEN 65162-0514-10 0.24800 SPIRONOLACTONE 50 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0514-50 0.24800 SPIRONOLACTONE 50 MG TABLET 0 AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-0207-01 0.24800 SPIRONOLACTONE 50 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0207-11 0.24800 SPIRONOLACTONE 50 MG TABLET 0 AHP EAGUL 00378-0141-01 0.34630 SPIRONOLACTONE-HCTZ 25-25 TAB 0 MYLAN EAGUL 00378-0141-05 0.34630 SPIRONOLACTONE-HCTZ 25-25 TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 51079-0104-20 0.34630 SPIRONOLACTONE-HCTZ 25-25 TAB 0 MYLAN INSTITUTI EAGUL 53489-0144-01 0.34630 SPIRONOLACTONE-HCTZ 25-25 TAB 0 MUTUAL PHARM CO EAGUL 53489-0144-05 0.34630 SPIRONOLACTONE-HCTZ 25-25 TAB 0 MUTUAL PHARM CO EAGUL 53489-0144-10 0.34630 SPIRONOLACTONE-HCTZ 25-25 TAB 0 MUTUAL PHARM CO EAGUL 59762-5014-01 0.34630 SPIRONOLACTONE-HCTZ 25-25 TAB 0 GREENSTONE LLC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 371LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50458-0295-15 1.62613 SPORANOX 10 MG/ML SOLUTION G JANSSEN PHARM. MLBND 50458-0290-01 7.27350 18.39390 SPORANOX 100 MG CAPSULE G JANSSEN PHARM. EABND 50458-0290-04 7.27350 18.39390 SPORANOX 100 MG CAPSULE G JANSSEN PHARM. EABND 50458-0290-28 7.27350 18.44497 SPORANOX 100 MG CAPSULE G JANSSEN PHARM. EAGEX 00555-9016-58 0.49260 SPRINTEC 28 DAY TABLET 0 BARR EABND 00517-8880-01 32.86800 SPRIX 15.75 MG NASAL SPRAY G AMER. REGENT EABND 00517-8880-05 32.86800 SPRIX 15.75 MG NASAL SPRAY G AMER. REGENT EABND 00003-<strong>08</strong>52-22 293.47444 SPRYCEL 100 MG TABLET 0 BMS PRIMARYCARE EABND 00003-<strong>08</strong>57-22 293.47444 SPRYCEL 140 MG TABLET 0 BMS PRIMARYCARE EABND 00003-0527-11 78.39709 SPRYCEL 20 MG TABLET 0 BMS PRIMARYCARE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00003-0528-11 156.79391 SPRYCEL 50 MG TABLET 0 BMS PRIMARYCARE EABND 00003-0524-11 156.79391 SPRYCEL 70 MG TABLET 0 BMS PRIMARYCARE EABND 00003-<strong>08</strong>55-22 293.47444 SPRYCEL 80 MG TABLET 0 BMS PRIMARYCARE EAGEN 00054-0379-63 0.09000 SPS 15 GM/60 ML SUSPENSION 0 ROXANE LABS. MLGEN 46287-00<strong>06</strong>-01 0.11718 SPS 15 GM/60 ML SUSPENSION 0 CAROLINA MED. MLGEN 46287-00<strong>06</strong>-60 0.11718 SPS 15 GM/60 ML SUSPENSION 0 CAROLINA MED. MLGEN 00054-0379-50 0.27562 SPS 30 GM/120 ML ENEMA 0 ROXANE LABS. MLBND 00054-0379-55 0.18301 SPS 50 GM/200 ML ENEMA 0 ROXANE LABS. MLGEX 52544-0967-28 0.85280 SRONYX 0.10-0.02 MG TABLET 0 ACTAVIS PHARMA, EABUL 43598-0210-25 0.<strong>06</strong>280 0.29846 SSD 1% CREAM G DR.REDDY'S LAB GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 43598-0210-40 0.<strong>06</strong>280 0.13195 SSD 1% CREAM G DR.REDDY'S LAB GMBUL 43598-0210-50 0.<strong>06</strong>280 0.24700 SSD 1% CREAM G DR.REDDY'S LAB GMBUL 43598-0210-55 0.<strong>06</strong>280 0.24700 SSD 1% CREAM G DR.REDDY'S LAB GMBUL 43598-0210-85 0.<strong>06</strong>280 0.25847 SSD 1% CREAM G DR.REDDY'S LAB GMBUL 49884-<strong>06</strong>00-40 0.<strong>06</strong>280 0.07277 SSD 1% CREAM G PAR PHARM. GMBND 00245-0003-<strong>08</strong> 0.61868 SSKI 1 GM/ML SOLUTION 0 UPSHER SMITH MLBND 00245-0003-31 0.62443 SSKI 1 GM/ML SOLUTION 0 UPSHER SMITH MLBND 00078-04<strong>08</strong>-05 3.09100 4.79540 STALEVO 100 TABLET 0 NOVARTIS EABND 00078-0545-05 3.09100 4.79540 STALEVO 125 TABLET 0 NOVARTIS EABND 00078-0409-05 3.80401 4.79540 STALEVO 150 TABLET 0 NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0527-05 2.71958 4.79540 STALEVO 200 TABLET 0 NOVARTIS EABND 00078-0407-05 3.09100 4.79540 STALEVO 50 TABLET 0 NOVARTIS EABND 00078-0544-05 3.09100 4.79540 STALEVO 75 TABLET 0 NOVARTIS EABND 60258-0159-10 0.04135 STANNOUS FLUOR 0.63% RINSE 0 CYPRESS PHARM. GMBND 00078-0352-05 1.21270 2.45132 STARLIX 120 MG TABLET G NOVARTIS EABND 00078-0351-05 0.85830 2.35952 STARLIX 60 MG TABLET G NOVARTIS EAGEN 42799-0113-01 0.23814 STAVUDINE 1 MG/ML SOLUTION G EDENBRIDGE PHAR MLGEN 64376-0133-02 0.23814 STAVUDINE 1 MG/ML SOLUTION G BOCA PHARMACAL MLGEN 00378-5040-91 1.<strong>08</strong>405 STAVUDINE 15 MG CAPSULE G MYLAN EAGEN 31722-0515-60 1.<strong>08</strong>405 STAVUDINE 15 MG CAPSULE G CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5041-91 0.98888 STAVUDINE 20 MG CAPSULE G MYLAN EAGEN 31722-0516-60 0.98888 STAVUDINE 20 MG CAPSULE G CAMBER PHARMACE EAGEN 00378-5042-91 1.04193 STAVUDINE 30 MG CAPSULE G MYLAN EAGEN 31722-0517-60 1.04193 STAVUDINE 30 MG CAPSULE G CAMBER PHARMACE EAGEN 00378-5043-91 0.92760 STAVUDINE 40 MG CAPSULE G MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 372LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0518-60 0.92760 STAVUDINE 40 MG CAPSULE G CAMBER PHARMACE EABEX 68968-3125-01 1.27239 STAVZOR DR 125 MG CAPSULE 0 NOVEN THERAPEUT EABEX 68968-3250-01 2.49904 STAVZOR DR 250 MG CAPSULE 0 NOVEN THERAPEUT EABEX 68968-3500-01 4.6<strong>08</strong>07 STAVZOR DR 500 MG CAPSULE 0 NOVEN THERAPEUT EAGEN 00264-7850-00 0.00203 STERILE WATER FOR INJECTION 0 B.BRAUN MLGEN 00264-7850-10 0.00203 STERILE WATER FOR INJECTION 0 B.BRAUN MLGEN 00264-7850-20 0.00203 STERILE WATER FOR INJECTION 0 B.BRAUN MLGEN 00338-0013-04 0.00203 STERILE WATER FOR INJECTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0013-<strong>06</strong> 0.00203 STERILE WATER FOR INJECTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0013-<strong>08</strong> 0.00203 STERILE WATER FOR INJECTION 0 BAXTER <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00338-0013-29 0.00203 STERILE WATER FOR INJECTION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-1590-02 0.00203 STERILE WATER FOR INJECTION 0 HOSPIRA MLGEN 00409-7990-09 0.00186 STERILE WATER FOR INJECTION 0 HOSPIRA MLGEN 00264-2101-00 0.00135 STERILE WATER FOR IRRIGATION 0 B.BRAUN MLGEN 00264-2101-10 0.00135 STERILE WATER FOR IRRIGATION 0 B.BRAUN MLGEN 00264-2101-50 0.00129 STERILE WATER FOR IRRIGATION 0 B.BRAUN MLGEN 00264-2101-70 0.00135 STERILE WATER FOR IRRIGATION 0 B.BRAUN MLGEN 00338-0003-44 0.00135 STERILE WATER FOR IRRIGATION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0003-46 0.00135 STERILE WATER FOR IRRIGATION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0003-47 0.00135 STERILE WATER FOR IRRIGATION 0 BAXTER <strong>HEALTH</strong>CA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00338-0004-02 0.00135 STERILE WATER FOR IRRIGATION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0004-03 0.00135 STERILE WATER FOR IRRIGATION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0004-04 0.00135 STERILE WATER FOR IRRIGATION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00338-0004-05 0.00135 STERILE WATER FOR IRRIGATION 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-6139-03 0.00135 STERILE WATER FOR IRRIGATION 0 HOSPIRA MLGEN 00409-6139-22 0.00135 STERILE WATER FOR IRRIGATION 0 HOSPIRA MLGEN 00409-7139-09 0.00135 STERILE WATER FOR IRRIGATION 0 HOSPIRA MLGEN 00409-7139-36 0.00135 STERILE WATER FOR IRRIGATION 0 HOSPIRA MLGEN 00409-7973-05 0.00135 STERILE WATER FOR IRRIGATION 0 HOSPIRA MLGEN 00409-7973-07 0.00135 STERILE WATER FOR IRRIGATION 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-7973-<strong>08</strong> 0.00135 STERILE WATER FOR IRRIGATION 0 HOSPIRA MLBND 00053-6871-00 226.68960 STIMATE 1.5 MG/ML NASAL SPRAY 0 CSL BEHRING LLC MLBND 50419-0171-03 122.22797 STIVARGA 40 MG TABLET 0 BAYER,PHARM DIV EABND 00002-3227-30 7.38036 STRATTERA 10 MG CAPSULE 0 ELI LILLY & CO. EABND 00002-3251-30 8.65524 STRATTERA 100 MG CAPSULE 0 ELI LILLY & CO. EABND 00002-3238-30 7.38036 STRATTERA 18 MG CAPSULE 0 ELI LILLY & CO. EABND 00002-3228-30 7.38036 STRATTERA 25 MG CAPSULE 0 ELI LILLY & CO. EABND 00002-3229-30 8.01282 STRATTERA 40 MG CAPSULE G ELI LILLY & CO. EABND 00002-3239-30 8.01282 STRATTERA 60 MG CAPSULE 0 ELI LILLY & CO. EABND 00002-3250-30 8.65524 STRATTERA 80 MG CAPSULE 0 ELI LILLY & CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 39822-07<strong>06</strong>-02 18.67500 STREPTOMYCIN SULF 1 GM VIAL 0 X-GEN PHARMACEU EABND 61958-1201-01 81.58070 STRIBILD TABLET G GILEAD SCIENCES EABND 000<strong>06</strong>-0032-20 4.63389 STROMECTOL 3 MG TABLET 0 MERCK SHARP & D EAGEN 00093-2210-01 0.22450 SUCRALFATE 1 GM TABLET 0 TEVA USA EAGEN 00093-2210-05 0.22450 SUCRALFATE 1 GM TABLET 0 TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 373LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-2210-98 0.22450 SUCRALFATE 1 GM TABLET 0 TEVA USA EAGEN 00591-0780-01 0.22450 SUCRALFATE 1 GM TABLET 0 ACTAVIS PHARMA, EAGEN 00591-0780-05 0.22450 SUCRALFATE 1 GM TABLET 0 ACTAVIS PHARMA, EAGEN 29033-0003-01 0.22450 SUCRALFATE 1 GM TABLET 0 NOSTRUM LABORAT EAGEN 29033-0003-05 0.22450 SUCRALFATE 1 GM TABLET 0 NOSTRUM LABORAT EAGEN 51079-0753-20 0.22450 SUCRALFATE 1 GM TABLET 0 MYLAN INSTITUTI EAGEN 68<strong>08</strong>4-0593-01 0.22450 SUCRALFATE 1 GM TABLET 0 AHP EAGEN 66689-0790-01 0.34275 SUCRALFATE 1 GM/10 ML SUSP 0 VISTAPHARM MLGEN 68094-0171-59 0.36692 SUCRALFATE 1 GM/10 ML SUSP 0 PRECISION DOSE MLBND 59630-0501-10 13.177<strong>08</strong> SULAR ER 17 MG TABLET G SHIONOGI PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59630-0503-10 13.177<strong>08</strong> SULAR ER 34 MG TABLET G SHIONOGI PHARMA EABND 59630-0500-10 13.177<strong>08</strong> SULAR ER 8.5 MG TABLET G SHIONOGI PHARMA EAGEN 242<strong>08</strong>-0317-05 2.47333 SULF-PRED 10-0.23% EYE DROPS 0 VALEANT MLGEN 242<strong>08</strong>-0317-10 1.87500 SULF-PRED 10-0.23% EYE DROPS 0 VALEANT MLGEN 61314-0297-05 2.43750 SULF-PRED 10-0.25% EYE DROPS 0 SANDOZ MLGEN 61314-0297-10 1.88250 SULF-PRED 10-0.25% EYE DROPS 0 SANDOZ MLGUL 242<strong>08</strong>-<strong>06</strong>70-04 0.16900 SULFACETAMIDE 10% EYE DROPS 0 VALEANT MLGUL 61314-0701-01 0.16900 SULFACETAMIDE 10% EYE DROPS 0 SANDOZ MLBND 48102-0103-35 15.61822 SULFACETAMIDE 10% EYE OINTMENT G PERRIGO CO. GMBND 00185-0757-01 3.55837 SULFADIAZINE 500 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00185-0757-10 3.416<strong>08</strong> SULFADIAZINE 500 MG TABLET 0 SANDOZ EAGEN 0<strong>06</strong>03-5781-21 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5781-28 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 QUALITEST EAGEN 00904-2725-40 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 MAJOR PHARMACEU EAGEN 00904-2725-60 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 MAJOR PHARMACEU EAGEN 51079-0128-01 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 MYLAN INSTITUTI EAGEN 51079-0128-20 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 MYLAN INSTITUTI EAGEN 53489-0146-01 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 MUTUAL PHARM CO EAGEN 53489-0146-05 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 MUTUAL PHARM CO EAGEN 53746-0272-01 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53746-0272-05 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 AMNEAL PHARMACE EAGEN 58517-0140-20 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 <strong>NEW</strong> HORIZON RX EAGEN 65862-0420-01 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 AUROBINDO PHARM EAGEN 65862-0420-05 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0230-01 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 AHP EAGEN 68<strong>08</strong>4-0230-11 0.<strong>06</strong>548 SULFAMETHOXAZOLE-TMP DS TABLET 0 AHP EAGEN 0<strong>06</strong>03-5780-21 0.<strong>08</strong>046 SULFAMETHOXAZOLE-TMP SS TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5780-28 0.<strong>08</strong>046 SULFAMETHOXAZOLE-TMP SS TABLET 0 QUALITEST EAGEN 53489-0145-01 0.<strong>08</strong>046 SULFAMETHOXAZOLE-TMP SS TABLET 0 MUTUAL PHARM CO EAGEN 53489-0145-05 0.<strong>08</strong>046 SULFAMETHOXAZOLE-TMP SS TABLET 0 MUTUAL PHARM CO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53746-0271-01 0.<strong>08</strong>046 SULFAMETHOXAZOLE-TMP SS TABLET 0 AMNEAL PHARMACE EAGEN 53746-0271-05 0.<strong>08</strong>046 SULFAMETHOXAZOLE-TMP SS TABLET 0 AMNEAL PHARMACE EAGEN 65862-0419-01 0.<strong>08</strong>046 SULFAMETHOXAZOLE-TMP SS TABLET 0 AUROBINDO PHARM EAGEN 65862-0419-05 0.<strong>08</strong>046 SULFAMETHOXAZOLE-TMP SS TABLET 0 AUROBINDO PHARM EAGEN 00121-4793-20 0.07235 SULFAMETHOXAZOLE-TMP SUSP 0 PHARMACEU ASSOC ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 374LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50383-<strong>08</strong>23-16 0.07235 SULFAMETHOXAZOLE-TMP SUSP 0 HI-TECH PHARMAC MLGEN 50383-<strong>08</strong>24-16 0.07235 SULFAMETHOXAZOLE-TMP SUSP 0 HI-TECH PHARMAC MLBND 50383-<strong>08</strong>24-20 0.16600 SULFAMETHOXAZOLE-TMP SUSP 0 HI-TECH PHARMAC MLBND 50383-<strong>08</strong>24-21 0.16600 SULFAMETHOXAZOLE-TMP SUSP 0 HI-TECH PHARMAC MLGEN 65862-0496-47 0.07235 SULFAMETHOXAZOLE-TMP SUSP 0 AUROBINDO PHARM MLBND 00703-9503-03 0.92528 SULFAMETHOXAZOLE-TMP VIAL 0 TEVA PARENTERAL MLBND 00703-9514-03 0.92520 SULFAMETHOXAZOLE-TMP VIAL 0 TEVA PARENTERAL MLBND 00703-9526-01 0.92517 SULFAMETHOXAZOLE-TMP VIAL 0 TEVA PARENTERAL MLBND 51079-<strong>06</strong>24-85 148.29112 SULFAMYLON POWDER PACKET 0 MYLAN INSTITUTI EABND 51079-<strong>06</strong>23-81 0.57792 SULFAMYLON 8.5% CREAM 0 MYLAN INSTITUTI GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 51079-<strong>06</strong>23-82 0.55552 SULFAMYLON 8.5% CREAM 0 MYLAN INSTITUTI GMBND 51079-<strong>06</strong>23-83 0.49781 SULFAMYLON 8.5% CREAM 0 MYLAN INSTITUTI GMGEN 51927-4450-00 1.09500 SULFANILAMIDE POWDER 0 PR<strong>OF</strong>ESSIONAL CO GMGEN 59762-0104-01 0.24030 SULFASALAZINE DR 500 MG TAB 0 GREENSTONE LLC. EAGEN 59762-0104-02 0.24030 SULFASALAZINE DR 500 MG TAB 0 GREENSTONE LLC. EAGEN 49452-7523-02 1.80075 SULFASALAZINE POWDER 0 SPECTRUM GMGEN 00591-0796-01 0.15600 SULFASALAZINE 500 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-0796-05 0.15600 SULFASALAZINE 500 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-0796-10 0.15600 SULFASALAZINE 500 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00904-1152-40 0.15600 SULFASALAZINE 500 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-1152-60 0.15600 SULFASALAZINE 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 59762-5000-01 0.15600 SULFASALAZINE 500 MG TABLET 0 GREENSTONE LLC. EAGEN 59762-5000-02 0.15600 SULFASALAZINE 500 MG TABLET 0 GREENSTONE LLC. EABND 54879-0007-16 0.07235 0.07791 SULFATRIM PEDIATRIC SUSPENSION 0 STI PHARMA, LLC MLGEN 0<strong>06</strong>03-5803-21 0.24030 SULFAZINE EC 500 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-5803-25 0.24030 SULFAZINE EC 500 MG TAB 0 QUALITEST EAGEN 0<strong>06</strong>03-5801-04 0.15600 SULFAZINE 500 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5801-21 0.15600 SULFAZINE 500 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5801-28 0.15600 SULFAZINE 500 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-5801-32 0.15600 SULFAZINE 500 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0427-01 0.17699 SULINDAC 150 MG TABLET 0 MYLAN EAGEN 00591-5661-01 0.17699 SULINDAC 150 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5661-05 0.17699 SULINDAC 150 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00904-6216-40 0.17699 SULINDAC 150 MG TABLET 0 MAJOR PHARMACEU EAGEN 23155-0005-01 0.17699 SULINDAC 150 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-0005-05 0.17699 SULINDAC 150 MG TABLET 0 HERITAGE PHARMA EAGEN 428<strong>06</strong>-0018-01 0.17699 SULINDAC 150 MG TABLET 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0018-05 0.17699 SULINDAC 150 MG TABLET 0 EPIC PHARMA LLC EAGEN 53489-0478-01 0.17699 SULINDAC 150 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0478-05 0.17699 SULINDAC 150 MG TABLET 0 MUTUAL PHARM CO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0531-01 0.16430 SULINDAC 200 MG TABLET 0 MYLAN EAGEN 00591-5660-01 0.16430 SULINDAC 200 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-5660-05 0.16430 SULINDAC 200 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00904-6217-40 0.16430 SULINDAC 200 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6217-60 0.16430 SULINDAC 200 MG TABLET 0 MAJOR PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 375LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 23155-00<strong>06</strong>-01 0.16430 SULINDAC 200 MG TABLET 0 HERITAGE PHARMA EAGEN 23155-00<strong>06</strong>-05 0.16430 SULINDAC 200 MG TABLET 0 HERITAGE PHARMA EAGEN 428<strong>06</strong>-0011-01 0.16430 SULINDAC 200 MG TABLET 0 EPIC PHARMA LLC EAGEN 428<strong>06</strong>-0011-05 0.16430 SULINDAC 200 MG TABLET 0 EPIC PHARMA LLC EAGEN 51079-<strong>06</strong>67-20 0.16430 SULINDAC 200 MG TABLET 0 MYLAN INSTITUTI EAGEN 53489-0479-01 0.16430 SULINDAC 200 MG TABLET 0 MUTUAL PHARM CO EAGEN 53489-0479-05 0.16430 SULINDAC 200 MG TABLET 0 MUTUAL PHARM CO EAGEN 00093-0224-19 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G TEVA USA EAGEN 00093-0224-90 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G TEVA USA EAGEN 00378-5632-59 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16252-0592-99 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G ACTAVIS PHARMA, EAGEN 16714-0533-11 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G NORTHSTAR RX LL EAGEN 55111-0293-09 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0293-36 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0293-90 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G DR.REDDY'S LAB EAGEN 59762-1852-09 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G GREENSTONE LLC. EAGEN 62756-0522-69 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G SUN PHARMACEUTI EAGEN 62756-0522-88 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G SUN PHARMACEUTI EAGEN 63304-0099-19 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G RANBAXY PHARMAC EAGEN 65862-0148-36 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0341-97 1.00790 SUMATRIPTAN SUCC 100 MG TABLET G AHP EAGEN 00378-5630-59 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G MYLAN EAGEN 16252-0590-99 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G ACTAVIS PHARMA, EAGEN 16714-0531-11 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G NORTHSTAR RX LL EAGEN 55111-0291-09 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0291-36 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0291-90 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G DR.REDDY'S LAB EAGEN 59762-1850-09 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G GREENSTONE LLC. EAGEN 62756-0520-69 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G SUN PHARMACEUTI EAGEN 62756-0520-88 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63304-0097-19 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G RANBAXY PHARMAC EAGEN 65862-0146-36 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0339-97 0.84470 SUMATRIPTAN SUCC 25 MG TABLET G AHP EAGEN 00378-5631-59 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G MYLAN EAGEN 16252-0591-99 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G ACTAVIS PHARMA, EAGEN 16714-0532-11 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G NORTHSTAR RX LL EAGEN 55111-0292-09 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0292-36 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0292-90 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G DR.REDDY'S LAB EAGEN 59762-1851-09 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62756-0521-69 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G SUN PHARMACEUTI EAGEN 62756-0521-88 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G SUN PHARMACEUTI EAGEN 63304-0098-19 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G RANBAXY PHARMAC EAGEN 65862-0147-36 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0340-97 1.15000 SUMATRIPTAN SUCC 50 MG TABLET G AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 376LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-6523-<strong>06</strong> 35.82500 SUMATRIPTAN 20 MG NASAL SPRAY G SANDOZ EAGEN 00781-6523-86 35.82500 SUMATRIPTAN 20 MG NASAL SPRAY G SANDOZ EAGEN 00781-3170-07 147.55200 SUMATRIPTAN 4 MG/0.5 ML CART 0 SANDOZ MLGEN 00781-3169-07 155.78400 SUMATRIPTAN 4 MG/0.5 ML INJECT 0 SANDOZ MLGEN 00781-6524-<strong>06</strong> 31.88<strong>06</strong>0 SUMATRIPTAN 5 MG NASAL SPRAY G SANDOZ EAGEN 00781-6524-86 31.88<strong>06</strong>0 SUMATRIPTAN 5 MG NASAL SPRAY G SANDOZ EAGEN 00781-3172-07 147.52000 SUMATRIPTAN 6 MG/0.5 ML INJECT 0 SANDOZ MLGEN 47335-0276-41 138.30000 SUMATRIPTAN 6 MG/0.5 ML INJECT 0 SUN PHARMA GLOB MLGEN 00781-3173-07 131.17000 SUMATRIPTAN 6 MG/0.5 ML REFILL 0 SANDOZ MLBND 25021-0703-70 69.72000 SUMATRIPTAN 6 MG/0.5 ML SYRNG 0 SAGENT PHARMACE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00143-9638-01 43.20000 SUMATRIPTAN 6 MG/0.5 ML VIAL 0 WEST-WARD,INC. MLGEN 00143-9638-05 43.20000 SUMATRIPTAN 6 MG/0.5 ML VIAL 0 WEST-WARD,INC. MLGEN 00703-7351-02 75.98740 SUMATRIPTAN 6 MG/0.5 ML VIAL 0 TEVA PARENTERAL MLGEN 00781-3174-14 75.98740 SUMATRIPTAN 6 MG/0.5 ML VIAL 0 SANDOZ MLGEN 00781-3174-71 75.98740 SUMATRIPTAN 6 MG/0.5 ML VIAL 0 SANDOZ MLGEN 25021-0703-60 46.80000 SUMATRIPTAN 6 MG/0.5 ML VIAL 0 SAGENT PHARMACE MLGEN 42023-0121-05 32.40000 SUMATRIPTAN 6 MG/0.5 ML VIAL 0 JHP PHARMACEUTI MLGEN 55150-0173-01 75.98740 SUMATRIPTAN 6 MG/0.5 ML VIAL 0 AUROMEDICS PHAR MLGEN 63323-0273-01 75.98740 SUMATRIPTAN 6 MG/0.5 ML VIAL 0 APP PHARMACEUTI MLGEN 64679-0728-01 75.98740 SUMATRIPTAN 6 MG/0.5 ML VIAL 0 WOCKHARDT USA L ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 43376-01<strong>06</strong>-01 196.99220 SUMAVEL DOSEPRO 6 MG/0.5 ML G ZOGENIX, INC. MLBND 43376-01<strong>06</strong>-<strong>06</strong> 196.98943 SUMAVEL DOSEPRO 6 MG/0.5 ML G ZOGENIX, INC. MLBND 27437-0203-11 14.88356 SUPRAX 100 MG TABLET CHEWABLE 0 LUPIN PHARMA EABND 68180-0202-03 2.97671 SUPRAX 100 MG/5 ML SUSPENSION 0 LUPIN PHARMA MLBND 27437-0205-11 29.76214 SUPRAX 200 MG TABLET CHEWABLE 0 LUPIN PHARMA EABND 27437-02<strong>06</strong>-02 5.95165 SUPRAX 200 MG/5 ML SUSPENSION 0 LUPIN PHARMA MLBND 27437-02<strong>06</strong>-03 5.95242 SUPRAX 200 MG/5 ML SUSPENSION 0 LUPIN PHARMA MLBND 27437-0201-<strong>08</strong> 17.57807 SUPRAX 400 MG TABLET 0 LUPIN PHARMA EABND 52268-0012-01 0.20149 SUPREP BOWEL PREP KIT G BRAINTREE LABS. MLBEX 51285-0554-02 3.64580 7.77145 SURMONTIL 100 MG CAPSULE 0 DURAMED/BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 51285-0538-02 3.37<strong>08</strong>7 SURMONTIL 25 MG CAPSULE 0 DURAMED/BARR EABEX 51285-0539-02 5.51402 SURMONTIL 50 MG CAPSULE 0 DURAMED/BARR EABND 00056-0474-92 7.95<strong>08</strong>4 SUSTIVA 200 MG CAPSULE G BMS EABND 00056-0470-30 1.98895 SUSTIVA 50 MG CAPSULE G BMS EABND 00056-0510-30 23.85254 SUSTIVA 600 MG TABLET G BMS EABND 00<strong>06</strong>9-0550-38 114.41935 SUTENT 12.5 MG CAPSULE 0 PFIZER US PHARM EABND 00<strong>06</strong>9-0770-38 228.83841 SUTENT 25 MG CAPSULE 0 PFIZER US PHARM EABND 00<strong>06</strong>9-0980-38 422.04136 SUTENT 50 MG CAPSULE 0 PFIZER US PHARM EAGEX 00781-5658-15 2.05392 SYEDA 28 TABLET 0 SANDOZ EABND 00<strong>08</strong>5-1388-01 1154.97820 SYLATRON 296 MCG KIT 0 MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>5-1388-02 4619.88790 SYLATRON 296 MCG 4-PACK 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1287-02 1732.45900 SYLATRON 444 MCG KIT 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1287-03 6929.83600 SYLATRON 444 MCG 4-PACK 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1312-01 3464.92630 SYLATRON 888 MCG KIT 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1312-02 13099.89830 SYLATRON 888 MCG 4-PACK 0 MERCK SHARP & D EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 377LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64543-0114-01 0.40950 SYMAX FASTABS 0.125 MG TABLET 0 CAPELLON EABND 00186-0370-20 24.83815 SYMBICORT 160-4.5 MCG INHALER G ASTRAZENECA GMBND 00186-0370-28 28.69310 SYMBICORT 160-4.5 MCG INHALER G ASTRAZENECA GMBND 00186-0372-20 21.73053 SYMBICORT 80-4.5 MCG INHALER G ASTRAZENECA GMBND 00186-0372-28 21.68946 SYMBICORT 80-4.5 MCG INHALER G ASTRAZENECA GMBEX 00002-3232-30 20.12916 20.12916 SYMBYAX 12-25 MG CAPSULE G ELI LILLY & CO. EABEX 00002-3234-30 20.12916 20.12916 SYMBYAX 12-50 MG CAPSULE G ELI LILLY & CO. EABEX 00002-3230-30 9.77076 9.77076 SYMBYAX 3-25 MG CAPSULE G ELI LILLY & CO. EABEX 00002-3231-30 13.35636 13.35636 SYMBYAX 6-25 MG CAPSULE G ELI LILLY & CO. EABEX 00002-3233-30 13.35636 13.35636 SYMBYAX 6-50 MG CAPSULE G ELI LILLY & CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 66780-0121-02 109.85203 SYMLINPEN 120 PEN INJECTOR G AMYLIN PHARMACE MLBND 66780-0115-02 161.88596 SYMLINPEN 60 PEN INJECTOR G AMYLIN PHARMACE MLBND 60574-4113-01 2458.8<strong>08</strong>60 SYNAGIS 100 MG/1 ML VIAL G MEDIMMUNE, INC. MLBND 60574-4114-01 2604.25780 SYNAGIS 50 MG/0.5 ML VIAL G MEDIMMUNE, INC. MLBND 43538-0920-60 2.45000 4.43745 SYNALAR 0.01% SOLUTION G MEDIMETRIKS PHA MLBND 43538-0920-90 2.45000 2.95821 SYNALAR 0.01% SOLUTION G MEDIMETRIKS PHA MLBND 43538-0900-12 1.38645 1.69098 SYNALAR 0.025% CREAM G MEDIMETRIKS PHA GMBND 43538-0910-12 1.38705 1.69098 SYNALAR 0.025% OINTMENT G MEDIMETRIKS PHA GMBND 00025-0166-<strong>08</strong> 180.23761 SYNAREL 2 MG/ML NASAL SPRAY 0 PHARMACIA/UPJHN MLBND 61570-0260-10 236.19725 SYNERCID 500 MG VIAL 0 MONARCH PHRM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00074-6624-19 0.29850 0.85158 SYNTHROID 100 MCG TABLET 0 ABBVIE US LLC EABUL 00074-6624-90 0.29850 0.85158 SYNTHROID 100 MCG TABLET 0 ABBVIE US LLC EABUL 00074-9296-19 0.34430 0.86314 SYNTHROID 112 MCG TABLET 0 ABBVIE US LLC EABUL 00074-9296-90 0.34430 0.86320 SYNTHROID 112 MCG TABLET 0 ABBVIE US LLC EABUL 00074-7<strong>06</strong>8-19 0.34950 0.87500 SYNTHROID 125 MCG TABLET 0 ABBVIE US LLC EABUL 00074-7<strong>06</strong>8-90 0.34950 0.87500 SYNTHROID 125 MCG TABLET 0 ABBVIE US LLC EABND 00074-3727-90 0.36020 0.89510 SYNTHROID 137 MCG TABLET 0 ABBVIE US LLC EABUL 00074-7<strong>06</strong>9-19 0.36000 0.90123 SYNTHROID 150 MCG TABLET 0 ABBVIE US LLC EABUL 00074-7<strong>06</strong>9-90 0.36000 0.90128 SYNTHROID 150 MCG TABLET 0 ABBVIE US LLC EABUL 00074-7070-19 0.42750 0.99863 SYNTHROID 175 MCG TABLET 0 ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00074-7070-90 0.42750 0.99867 SYNTHROID 175 MCG TABLET 0 ABBVIE US LLC EABND 00074-7148-19 0.43551 1.00072 SYNTHROID 200 MCG TABLET 0 ABBVIE US LLC EABND 00074-7148-90 0.43551 1.00079 SYNTHROID 200 MCG TABLET 0 ABBVIE US LLC EABUL 00074-4341-19 0.23180 0.76989 SYNTHROID 25 MCG TABLET 0 ABBVIE US LLC EABUL 00074-4341-90 0.23180 0.76987 SYNTHROID 25 MCG TABLET 0 ABBVIE US LLC EABND 00074-7149-19 0.58580 1.19935 SYNTHROID 300 MCG TABLET 0 ABBVIE US LLC EABND 00074-7149-90 0.58580 1.19925 SYNTHROID 300 MCG TABLET 0 ABBVIE US LLC EABUL 00074-4552-19 0.26330 0.80578 SYNTHROID 50 MCG TABLET 0 ABBVIE US LLC EABUL 00074-4552-90 0.26330 0.80583 SYNTHROID 50 MCG TABLET 0 ABBVIE US LLC EABUL 00074-5182-19 0.29100 0.83116 SYNTHROID 75 MCG TABLET 0 ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00074-5182-90 0.29100 0.83101 SYNTHROID 75 MCG TABLET 0 ABBVIE US LLC EABUL 00074-6594-19 0.29550 0.84573 SYNTHROID 88 MCG TABLET 0 ABBVIE US LLC EABUL 00074-6594-90 0.29550 0.84586 SYNTHROID 88 MCG TABLET 0 ABBVIE US LLC EABND 25010-0710-15 105.<strong>08</strong>762 SYPRINE 250 MG CAPSULE 0 VALEANT EABND 00173-<strong>08</strong>80-25 10.00050 TABLOID 40 MG TABLET 0 GLAXOSMITHKLINE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 378LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 76388-<strong>08</strong>80-25 12.50976 TABLOID 40 MG TABLET 0 PRASCO LABS EABND 50222-0227-04 12.12630 TACLONEX OINTMENT G LEO PHARMA INC. GMBND 50222-0227-81 10.71696 TACLONEX OINTMENT G LEO PHARMA INC. GMBND 50222-0501-<strong>06</strong> 10.88696 TACLONEX 0.005%-0.<strong>06</strong>4% SUSPENS G LEO PHARMA INC. GMBND 50222-0501-66 10.88696 TACLONEX 0.005%-0.<strong>06</strong>4% SUSPENS G LEO PHARMA INC. GMGEN 00378-2045-01 1.67235 TACROLIMUS 0.5 MG CAPSULE G MYLAN EAGEN 00378-2045-05 1.67235 TACROLIMUS 0.5 MG CAPSULE G MYLAN EAGEN 00781-2102-01 1.67235 TACROLIMUS 0.5 MG CAPSULE G SANDOZ EAGEN 00781-9302-01 1.67235 TACROLIMUS 0.5 MG CAPSULE G SANDOZ/NOVAPLUS EAGEN 16729-0041-01 1.67235 TACROLIMUS 0.5 MG CAPSULE G ACCORD <strong>HEALTH</strong>CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50742-0207-01 1.67235 TACROLIMUS 0.5 MG CAPSULE G INGENUS PHARMAC EAGEN 51079-<strong>08</strong>17-01 1.67235 TACROLIMUS 0.5 MG CAPSULE G MYLAN INSTITUTI EAGEN 51079-<strong>08</strong>17-20 1.67235 TACROLIMUS 0.5 MG CAPSULE G MYLAN INSTITUTI EAGEN 55111-0525-01 1.67235 TACROLIMUS 0.5 MG CAPSULE G DR.REDDY'S LAB EAGEN 62175-0380-37 1.67235 TACROLIMUS 0.5 MG CAPSULE G KREMERS URBAN EAGEN 68<strong>08</strong>4-0449-01 1.67235 TACROLIMUS 0.5 MG CAPSULE G AHP EAGEN 68<strong>08</strong>4-0449-11 1.67235 TACROLIMUS 0.5 MG CAPSULE G AHP EAGEN 00378-2046-01 3.34462 TACROLIMUS 1 MG CAPSULE G MYLAN EAGEN 00378-2046-05 3.34462 TACROLIMUS 1 MG CAPSULE G MYLAN EAGEN 00781-2103-01 3.34462 TACROLIMUS 1 MG CAPSULE G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-9303-01 3.34462 TACROLIMUS 1 MG CAPSULE G SANDOZ/NOVAPLUS EAGEN 16729-0042-01 3.34462 TACROLIMUS 1 MG CAPSULE G ACCORD <strong>HEALTH</strong>CA EAGEN 50742-02<strong>08</strong>-01 3.34462 TACROLIMUS 1 MG CAPSULE G INGENUS PHARMAC EAGEN 51079-<strong>08</strong>18-01 3.34462 TACROLIMUS 1 MG CAPSULE G MYLAN INSTITUTI EAGEN 51079-<strong>08</strong>18-20 3.34462 TACROLIMUS 1 MG CAPSULE G MYLAN INSTITUTI EAGEN 55111-0526-01 3.34462 TACROLIMUS 1 MG CAPSULE G DR.REDDY'S LAB EAGEN 62175-0381-37 3.34462 TACROLIMUS 1 MG CAPSULE G KREMERS URBAN EAGEN 68<strong>08</strong>4-0450-01 3.34462 TACROLIMUS 1 MG CAPSULE G AHP EAGEN 68<strong>08</strong>4-0450-11 3.34462 TACROLIMUS 1 MG CAPSULE G AHP EAGEN 00378-2047-01 16.72312 TACROLIMUS 5 MG CAPSULE G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-2104-01 16.72312 TACROLIMUS 5 MG CAPSULE G SANDOZ EAGEN 00781-9304-01 16.72312 TACROLIMUS 5 MG CAPSULE G SANDOZ/NOVAPLUS EAGEN 16729-0043-01 16.72312 TACROLIMUS 5 MG CAPSULE G ACCORD <strong>HEALTH</strong>CA EAGEN 50742-0209-01 16.72312 TACROLIMUS 5 MG CAPSULE G INGENUS PHARMAC EAGEN 51079-0028-01 16.72312 TACROLIMUS 5 MG CAPSULE G MYLAN INSTITUTI EAGEN 51079-0028-20 16.72312 TACROLIMUS 5 MG CAPSULE G MYLAN INSTITUTI EAGEN 55111-0527-01 16.72312 TACROLIMUS 5 MG CAPSULE G DR.REDDY'S LAB EAGEN 62175-0382-37 16.72312 TACROLIMUS 5 MG CAPSULE G KREMERS URBAN EAGEN 68<strong>08</strong>4-0451-01 16.72312 TACROLIMUS 5 MG CAPSULE G AHP EAGEN 68<strong>08</strong>4-0451-11 16.72312 TACROLIMUS 5 MG CAPSULE G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-<strong>08</strong>46-<strong>08</strong> 44.15890 TAFINLAR 50 MG CAPSULE 0 GLAXOSMITHKLINE EABND 00173-<strong>08</strong>47-<strong>08</strong> 66.23400 TAFINLAR 75 MG CAPSULE 0 GLAXOSMITHKLINE EABND 00004-<strong>08</strong>02-85 10.10940 TAMIFLU 30 MG GELCAP 0 ROCHE LABS. EABND 00004-<strong>08</strong>01-85 10.10940 TAMIFLU 45 MG GELCAP 0 ROCHE LABS. EABND 00004-<strong>08</strong>20-09 1.83664 TAMIFLU 6 MG/ML SUSPENSION 0 GENENTECH, INC. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 379LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00004-<strong>08</strong>22-05 1.83664 TAMIFLU 6 MG/ML SUSPENSION 0 GENENTECH, INC. MLBND 00004-<strong>08</strong>00-85 11.01991 TAMIFLU 75 MG CAPSULE 0 ROCHE LABS. EAGEN 00093-0784-05 0.19642 TAMOXIFEN 10 MG TABLET 0 TEVA USA EAGEN 00093-0784-<strong>06</strong> 0.19642 TAMOXIFEN 10 MG TABLET 0 TEVA USA EAGEN 00093-0784-10 0.19642 TAMOXIFEN 10 MG TABLET 0 TEVA USA EAGEN 00093-0784-86 0.19642 TAMOXIFEN 10 MG TABLET 0 TEVA USA EAGEN 00378-0144-91 0.19642 TAMOXIFEN 10 MG TABLET 0 MYLAN EAGEN 00591-2472-18 0.19642 TAMOXIFEN 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-2472-60 0.19642 TAMOXIFEN 10 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00093-0782-01 0.43497 TAMOXIFEN 20 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-0782-05 0.43497 TAMOXIFEN 20 MG TABLET 0 TEVA USA EAGEN 00093-0782-10 0.43497 TAMOXIFEN 20 MG TABLET 0 TEVA USA EAGEN 00093-0782-56 0.43497 TAMOXIFEN 20 MG TABLET 0 TEVA USA EAGEN 00378-0274-01 0.43497 TAMOXIFEN 20 MG TABLET 0 MYLAN EAGEN 00378-0274-93 0.43497 TAMOXIFEN 20 MG TABLET 0 MYLAN EAGEN 00591-2473-19 0.43497 TAMOXIFEN 20 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-2473-30 0.43497 TAMOXIFEN 20 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00093-7338-01 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 TEVA USA EAGEN 00115-8211-01 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 GLOBAL PHARM EAGEN 00115-8211-03 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 GLOBAL PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-2996-11 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00228-2996-50 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00378-2500-10 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MYLAN EAGEN 00378-2500-77 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MYLAN EAGEN 00781-2076-01 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 SANDOZ EAGEN 00904-6156-61 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 10370-0169-01 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 PAR PHARM. EAGEN 10370-0169-05 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 PAR PHARM. EAGEN 51079-0294-01 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 51079-0294-17 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0294-19 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 51079-0294-20 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 51079-0294-30 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 51079-0294-56 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MYLAN INSTITUTI EAGEN 62756-0160-13 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 62756-0160-81 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 62756-0160-88 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 SUN PHARMACEUTI EAGEN 63739-<strong>08</strong>11-41 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MCKESSON PACKAG EAGEN 63739-<strong>08</strong>11-43 0.14758 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MCKESSON PACKAG EAGEN 63739-<strong>08</strong>11-45 0.13779 TAMSULOSIN HCL 0.4 MG CAPSULE 0 MCKESSON PACKAG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0516-02 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 WOCKHARDT USA L EAGEN 64679-0516-03 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 WOCKHARDT USA L EAGEN 65862-0598-01 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 AUROBINDO PHARM EAGEN 65862-0598-05 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0407-01 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 380LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0407-11 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 AHP EAGEN 68382-0132-01 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 68382-0132-10 0.17210 TAMSULOSIN HCL 0.4 MG CAPSULE 0 ZYDUS PHARMACEU EAGEN 60793-0105-01 0.30250 TAPAZOLE 10 MG TABLET 0 PFIZER US PHARM EAGEN 60793-0104-01 0.15800 TAPAZOLE 5 MG TABLET 0 PFIZER US PHARM EABND 50242-0<strong>06</strong>3-01 182.33993 TARCEVA 100 MG TABLET 0 GENENTECH, INC. EABND 50242-0<strong>06</strong>4-01 2<strong>06</strong>.23950 TARCEVA 150 MG TABLET 0 GENENTECH, INC. EABND 50242-0<strong>06</strong>2-01 66.38561 TARCEVA 25 MG TABLET 0 GENENTECH, INC. EABND 62856-<strong>06</strong>04-22 66.01322 TARGRETIN 1% GEL 0 VALEANT GMBND 62856-<strong>06</strong>02-10 76.02351 TARGRETIN 75 MG S<strong>OF</strong>TGEL 0 VALEANT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00074-3288-13 1.99730 4.23192 TARKA ER 1-240 MG TABLET 0 ABBVIE US LLC EABND 00074-3287-13 2.44670 4.23192 TARKA ER 2-180 MG TABLET 0 ABBVIE US LLC EABND 00074-3289-13 2.44670 4.23192 TARKA ER 2-240 MG TABLET 0 ABBVIE US LLC EABND 00074-3290-13 2.44670 4.23192 TARKA ER 4-240 MG TABLET 0 ABBVIE US LLC EABND 13811-0569-30 0.25090 0.35939 TARON-BC TABLET 0 TRIGEN LABORATO EAGEN 13811-0536-30 0.61520 TARON-C DHA CAPSULE 0 TRIGEN LABORATO EABND 13811-0543-30 1.41874 TARON-PREX PRENATAL DHA CAP 0 TRIGEN LABORATO EABND 00078-0592-51 73.72593 TASIGNA 150 MG CAPSULE 0 NOVARTIS EABND 00078-0592-87 73.72593 TASIGNA 150 MG CAPSULE 0 NOVARTIS EABND 00078-0526-51 73.72563 TASIGNA 200 MG CAPSULE 0 NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0526-87 73.72593 TASIGNA 200 MG CAPSULE 0 NOVARTIS EABND 00075-8003-01 112.69875 473.79720 TAXOTERE 20 MG/ML VIAL 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00075-8004-04 112.82431 473.78682 TAXOTERE 80 MG/4 ML VIAL 0 SAN<strong>OF</strong>I-AVENTIS MLGEN 00409-5092-16 3.53700 TAZICEF 1 GM ADD-VANTAGE VIAL 0 HOSPIRA EAGEN 00409-5092-52 3.56400 TAZICEF 1 GM ADD-VANTAGE VIAL 0 HOSPIRA/NOVA+ EAGEN 00409-5<strong>08</strong>2-16 3.53700 TAZICEF 1 GRAM VIAL 0 HOSPIRA EAGEN 00409-5<strong>08</strong>2-52 2.97900 TAZICEF 1 GRAM VIAL 0 HOSPIRA/NOVA+ EAGEN 00409-5093-11 9.05400 TAZICEF 2 GM ADD-VANTAGE 0 HOSPIRA EAGEN 00409-5093-51 8.63100 TAZICEF 2 GM ADD-VANTAGE 0 HOSPIRA/NOVA+ EAGEN 00409-5<strong>08</strong>4-11 8.22600 TAZICEF 2 GRAM VIAL 0 HOSPIRA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-5<strong>08</strong>4-51 8.<strong>08</strong>200 TAZICEF 2 GRAM VIAL 0 HOSPIRA/NOVA+ EAGEN 00409-5<strong>08</strong>6-11 22.41900 TAZICEF 6 GRAM VIAL 0 HOSPIRA EAGEN 00409-5<strong>08</strong>6-51 17.36100 TAZICEF 6 GRAM VIAL 0 HOSPIRA/NOVA+ EABND 00023-9155-30 8.10937 TAZORAC 0.05% CREAM G ALLERGAN INC. GMBND 00023-9155-60 8.10771 TAZORAC 0.05% CREAM G ALLERGAN INC. GMBND 00023-8335-03 8.10937 TAZORAC 0.05% GEL G ALLERGAN INC. GMBND 00023-8335-10 8.10727 TAZORAC 0.05% GEL G ALLERGAN INC. GMBND 00023-9156-30 8.61512 TAZORAC 0.1% CREAM G ALLERGAN INC. GMBND 00023-9156-60 8.61374 TAZORAC 0.1% CREAM G ALLERGAN INC. GMBND 00023-0042-03 8.61512 TAZORAC 0.1% GEL G ALLERGAN INC. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00023-0042-10 8.61440 TAZORAC 0.1% GEL G ALLERGAN INC. GMGEN 62037-<strong>06</strong>96-30 0.62210 TAZTIA XT 120 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-<strong>06</strong>96-90 0.62210 TAZTIA XT 120 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-<strong>06</strong>97-30 0.81330 TAZTIA XT 180 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-<strong>06</strong>97-90 0.81330 TAZTIA XT 180 MG CAPSULE 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 381LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62037-<strong>06</strong>98-30 1.16285 TAZTIA XT 240 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-<strong>06</strong>98-90 1.16285 TAZTIA XT 240 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-<strong>06</strong>99-30 1.38010 TAZTIA XT 300 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-<strong>06</strong>99-90 1.38010 TAZTIA XT 300 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0700-30 1.54115 TAZTIA XT 360 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 62037-0700-90 1.54115 TAZTIA XT 360 MG CAPSULE 0 ACTAVIS PHARMA, EABND 644<strong>06</strong>-0005-01 81.81428 TECFIDERA DR 120 MG CAPSULE G BIOGEN-IDEC EABND 644<strong>06</strong>-00<strong>06</strong>-02 81.80480 TECFIDERA DR 240 MG CAPSULE G BIOGEN-IDEC EABND 644<strong>06</strong>-0007-03 81.80480 TECFIDERA STARTER PACK G BIOGEN-IDEC EABND 00456-0400-01 57.25340 TEFLARO 400 MG VIAL 0 FOREST PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00456-0400-10 57.25174 TEFLARO 400 MG VIAL 0 FOREST PHARMACE EABND 00456-<strong>06</strong>00-01 57.25340 TEFLARO 600 MG VIAL 0 FOREST PHARMACE EABND 00456-<strong>06</strong>00-10 57.25174 TEFLARO 600 MG VIAL 0 FOREST PHARMACE EABEX 00078-0510-05 0.76426 TEGRETOL XR 100 MG TABLET 0 NOVARTIS EABEX 00078-0511-05 1.52529 1.52529 TEGRETOL XR 200 MG TABLET 0 NOVARTIS EABEX 00078-0512-05 3.04834 3.04834 TEGRETOL XR 400 MG TABLET 0 NOVARTIS EABEX 00078-0492-05 0.<strong>06</strong>840 0.56755 TEGRETOL 100 MG TABLET CHEW 0 NOVARTIS EABUX 00078-05<strong>08</strong>-83 0.21456 0.21456 TEGRETOL 100 MG/5 ML SUSP 0 NOVARTIS MLBEX 00078-0509-05 0.04960 1.499<strong>06</strong> TEGRETOL 200 MG TABLET G NOVARTIS EABND 00078-<strong>06</strong>04-15 3.44311 TEKAMLO 150 MG-10 MG TABLET G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-<strong>06</strong>03-15 3.44311 TEKAMLO 150 MG-5 MG TABLET G NOVARTIS EABND 00078-<strong>06</strong><strong>06</strong>-15 4.34532 TEKAMLO 300 MG-10 MG TABLET G NOVARTIS EABND 00078-<strong>06</strong>05-15 4.34532 TEKAMLO 300 MG-5 MG TABLET G NOVARTIS EABND 00078-0521-15 3.45169 TEKTURNA HCT 150-12.5 MG TAB G NOVARTIS EABND 00078-0522-15 3.45169 TEKTURNA HCT 150-25 MG TABLET G NOVARTIS EABND 00078-0523-15 4.35445 TEKTURNA HCT 300-12.5 MG TAB G NOVARTIS EABND 00078-0524-15 4.35445 TEKTURNA HCT 300-25 MG TABLET G NOVARTIS EABND 00078-0485-15 3.45169 TEKTURNA 150 MG TABLET G NOVARTIS EABND 00078-0485-35 3.45147 TEKTURNA 150 MG TABLET G NOVARTIS EABND 00078-0486-15 4.35445 TEKTURNA 300 MG TABLET G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0486-35 4.35434 TEKTURNA 300 MG TABLET G NOVARTIS EABND 00<strong>08</strong>5-1366-01 223.61563 TEMODAR 100 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1366-02 223.61196 TEMODAR 100 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1366-03 223.61196 TEMODAR 100 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1366-04 223.61563 TEMODAR 100 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1425-01 313.05940 TEMODAR 140 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1425-02 313.<strong>06</strong>058 TEMODAR 140 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1425-03 313.05940 TEMODAR 140 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1425-04 313.<strong>06</strong>058 TEMODAR 140 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1430-01 402.50352 TEMODAR 180 MG CAPSULE 0 MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>5-1430-03 402.50352 TEMODAR 180 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1430-04 402.50553 TEMODAR 180 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1519-01 44.72336 TEMODAR 20 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1519-02 44.72870 TEMODAR 20 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1519-03 44.72870 TEMODAR 20 MG CAPSULE 0 MERCK SHARP & D EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 382LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>5-1519-04 44.72336 TEMODAR 20 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1417-01 559.18594 559.18594 TEMODAR 250 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1417-02 559.18594 559.18594 TEMODAR 250 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-1248-01 8.0<strong>06</strong>18 TEMODAR 5 MG CAPSULE 0 SCHERING CORP. EABND 00<strong>08</strong>5-1248-03 8.43991 TEMODAR 5 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-3004-01 11.23761 TEMODAR 5 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-3004-02 11.102<strong>08</strong> TEMODAR 5 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-3004-03 11.102<strong>08</strong> TEMODAR 5 MG CAPSULE 0 MERCK SHARP & D EABND 00<strong>08</strong>5-3004-04 11.23761 TEMODAR 5 MG CAPSULE 0 MERCK SHARP & D EABND 00462-0301-60 0.35970 4.25790 TEMOVATE EMOLLIENT 0.05% CRM G SANDOZ GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00462-0163-30 0.18250 5.39638 TEMOVATE 0.05% CREAM G SANDOZ GMBUL 10337-0163-30 0.18250 5.93588 TEMOVATE 0.05% CREAM G SANDOZ GMBUL 10337-0163-60 0.18250 5.31144 TEMOVATE 0.05% CREAM G SANDOZ GMBUL 00462-0162-15 0.19400 6.54261 TEMOVATE 0.05% OINTMENT G SANDOZ GMBUL 10337-0162-15 0.19400 7.19665 TEMOVATE 0.05% OINTMENT G SANDOZ GMBUL 10337-0162-30 0.19400 6.01224 TEMOVATE 0.05% OINTMENT G SANDOZ GMGEN 00093-7601-41 181.85625 TEMOZOLOMIDE 100 MG CAPSULE G TEVA USA EAGEN 00093-7601-57 181.85250 TEMOZOLOMIDE 100 MG CAPSULE G TEVA USA EAGEN 00781-2693-44 189.43339 TEMOZOLOMIDE 100 MG CAPSULE G SANDOZ EAGEN 00781-2693-75 189.43050 TEMOZOLOMIDE 100 MG CAPSULE G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7638-41 254.59767 TEMOZOLOMIDE 140 MG CAPSULE G TEVA USA EAGEN 00093-7638-57 254.59650 TEMOZOLOMIDE 140 MG CAPSULE G TEVA USA EAGEN 00781-2694-44 265.20589 TEMOZOLOMIDE 140 MG CAPSULE G SANDOZ EAGEN 00781-2694-75 265.20450 TEMOZOLOMIDE 140 MG CAPSULE G SANDOZ EAGEN 00093-7639-41 327.33910 TEMOZOLOMIDE 180 MG CAPSULE G TEVA USA EAGEN 00093-7639-57 327.33750 TEMOZOLOMIDE 180 MG CAPSULE G TEVA USA EAGEN 00781-2695-44 340.97785 TEMOZOLOMIDE 180 MG CAPSULE G SANDOZ EAGEN 00781-2695-75 340.97700 TEMOZOLOMIDE 180 MG CAPSULE G SANDOZ EAGEN 00093-7600-41 36.37125 TEMOZOLOMIDE 20 MG CAPSULE G TEVA USA EAGEN 00093-7600-57 36.37650 TEMOZOLOMIDE 20 MG CAPSULE G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-2692-44 37.88678 TEMOZOLOMIDE 20 MG CAPSULE G SANDOZ EAGEN 00781-2692-75 37.89150 TEMOZOLOMIDE 20 MG CAPSULE G SANDOZ EAGEN 00093-7602-57 454.75950 TEMOZOLOMIDE 250 MG CAPSULE G TEVA USA EAGEN 00781-2696-75 473.70900 TEMOZOLOMIDE 250 MG CAPSULE G SANDOZ EAGEN 00093-7599-41 9.13928 TEMOZOLOMIDE 5 MG CAPSULE G TEVA USA EAGEN 00093-7599-57 9.02850 TEMOZOLOMIDE 5 MG CAPSULE G TEVA USA EAGEN 00781-2691-44 9.52017 TEMOZOLOMIDE 5 MG CAPSULE G SANDOZ EAGEN 00781-2691-75 9.40500 TEMOZOLOMIDE 5 MG CAPSULE G SANDOZ EABND 67857-0705-01 0.<strong>08</strong>180 2.39471 TENEX 1 MG TABLET G PROMIUS PHARMA EABND 67857-0705-05 0.<strong>08</strong>180 2.02717 TENEX 1 MG TABLET G PROMIUS PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 67857-07<strong>06</strong>-01 0.11800 3.55688 TENEX 2 MG TABLET G PROMIUS PHARMA EABND 44567-0507-01 159.16<strong>08</strong>0 TENIPOSIDE 50 MG/5 ML AMPULE 0 WG CRITICAL CAR MLBND 00310-0117-10 0.19967 2.41613 TENORETIC 100 TABLET G ASTRAZENECA EABND 00310-0115-10 0.07000 1.72142 TENORETIC 50 TABLET G ASTRAZENECA EABND 00310-0101-10 0.02590 2.350<strong>06</strong> TENORMIN 100 MG TABLET G ASTRAZENECA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 383LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00310-0107-10 0.01340 1.53541 TENORMIN 25 MG TABLET G ASTRAZENECA EABND 00310-0105-10 0.01530 1.56629 TENORMIN 50 MG TABLET G ASTRAZENECA EABUL 00<strong>06</strong>2-5356-01 1.98680 2.19078 TERAZOL 3 CREAM 0 ORTHO PHARM. GMBND 00<strong>06</strong>2-5351-01 13.10000 14.60523 TERAZOL 3 80 MG SUPPOSITORY G ORTHO PHARM. EABND 00<strong>06</strong>2-5350-01 0.72470 0.97368 TERAZOL 7 CREAM G ORTHO PHARM. GMGEN 00093-4336-01 0.05562 TERAZOSIN 1 MG CAPSULE 0 TEVA USA EAGEN 00093-4336-05 0.05562 TERAZOSIN 1 MG CAPSULE 0 TEVA USA EAGEN 00378-2260-01 0.05562 TERAZOSIN 1 MG CAPSULE 0 MYLAN EAGEN 00781-2051-01 0.05562 TERAZOSIN 1 MG CAPSULE 0 SANDOZ EAGEN 00781-2051-05 0.05562 TERAZOSIN 1 MG CAPSULE 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6126-61 0.05562 TERAZOSIN 1 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 59746-0383-<strong>06</strong> 0.05562 TERAZOSIN 1 MG CAPSULE 0 CADISTA PHARMAC EAGEN 59746-0383-09 0.05562 TERAZOSIN 1 MG CAPSULE 0 CADISTA PHARMAC EAGEN 59746-0383-10 0.05562 TERAZOSIN 1 MG CAPSULE 0 CADISTA PHARMAC EAGEN 60505-0115-00 0.05562 TERAZOSIN 1 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0115-05 0.05562 TERAZOSIN 1 MG CAPSULE 0 APOTEX CORP EAGEN 00093-4339-01 0.<strong>06</strong>939 TERAZOSIN 10 MG CAPSULE 0 TEVA USA EAGEN 00093-4339-05 0.<strong>06</strong>939 TERAZOSIN 10 MG CAPSULE 0 TEVA USA EAGEN 00378-1570-01 0.<strong>06</strong>939 TERAZOSIN 10 MG CAPSULE 0 MYLAN EAGEN 00781-2054-01 0.<strong>06</strong>939 TERAZOSIN 10 MG CAPSULE 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-2054-05 0.<strong>06</strong>939 TERAZOSIN 10 MG CAPSULE 0 SANDOZ EAGEN 59746-0386-<strong>06</strong> 0.<strong>06</strong>939 TERAZOSIN 10 MG CAPSULE 0 CADISTA PHARMAC EAGEN 59746-0386-09 0.<strong>06</strong>939 TERAZOSIN 10 MG CAPSULE 0 CADISTA PHARMAC EAGEN 59746-0386-10 0.<strong>06</strong>939 TERAZOSIN 10 MG CAPSULE 0 CADISTA PHARMAC EAGEN 60505-0118-00 0.<strong>06</strong>939 TERAZOSIN 10 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0118-05 0.<strong>06</strong>939 TERAZOSIN 10 MG CAPSULE 0 APOTEX CORP EAGEN 00093-4337-01 0.05616 TERAZOSIN 2 MG CAPSULE 0 TEVA USA EAGEN 00093-4337-10 0.05616 TERAZOSIN 2 MG CAPSULE 0 TEVA USA EAGEN 00378-2264-01 0.05616 TERAZOSIN 2 MG CAPSULE 0 MYLAN EAGEN 00781-2052-01 0.05616 TERAZOSIN 2 MG CAPSULE 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-2052-05 0.05616 TERAZOSIN 2 MG CAPSULE 0 SANDOZ EAGEN 00904-6127-61 0.05616 TERAZOSIN 2 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 59746-0384-<strong>06</strong> 0.05616 TERAZOSIN 2 MG CAPSULE 0 CADISTA PHARMAC EAGEN 59746-0384-09 0.05616 TERAZOSIN 2 MG CAPSULE 0 CADISTA PHARMAC EAGEN 59746-0384-10 0.05616 TERAZOSIN 2 MG CAPSULE 0 CADISTA PHARMAC EAGEN 60505-0116-00 0.05616 TERAZOSIN 2 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0116-05 0.05616 TERAZOSIN 2 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0116-07 0.05616 TERAZOSIN 2 MG CAPSULE 0 APOTEX CORP EAGEN 00093-4338-01 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 TEVA USA EAGEN 00093-4338-10 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-2268-01 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 MYLAN EAGEN 00781-2053-01 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 SANDOZ EAGEN 00781-2053-05 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 SANDOZ EAGEN 00904-6128-61 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 59746-0385-<strong>06</strong> 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 CADISTA PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 384LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59746-0385-09 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 CADISTA PHARMAC EAGEN 59746-0385-10 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 CADISTA PHARMAC EAGEN 60505-0117-00 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0117-05 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 APOTEX CORP EAGEN 60505-0117-07 0.<strong>06</strong>318 TERAZOSIN 5 MG CAPSULE 0 APOTEX CORP EAGEN 38779-2481-04 17.95500 TERBINAFINE HCL POWDER 0 MEDISCA INC. GMGEN 16714-0501-01 0.14554 TERBINAFINE HCL 250 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0501-02 0.14554 TERBINAFINE HCL 250 MG TABLET 0 NORTHSTAR RX LL EAGEN 31722-0209-01 0.14554 TERBINAFINE HCL 250 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0209-30 0.14554 TERBINAFINE HCL 250 MG TABLET 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51991-0526-01 0.14554 TERBINAFINE HCL 250 MG TABLET 0 BRECKENRIDGE EAGEN 51991-0526-33 0.14554 TERBINAFINE HCL 250 MG TABLET 0 BRECKENRIDGE EAGEN 55111-0250-30 0.14554 TERBINAFINE HCL 250 MG TABLET 0 DR.REDDY'S LAB EAGEN 55111-0250-90 0.14554 TERBINAFINE HCL 250 MG TABLET 0 DR.REDDY'S LAB EAGEN 60505-2572-01 0.14554 TERBINAFINE HCL 250 MG TABLET 0 APOTEX CORP EAGEN 60505-2572-03 0.14554 TERBINAFINE HCL 250 MG TABLET 0 APOTEX CORP EAGEN 65862-0079-30 0.14554 TERBINAFINE HCL 250 MG TABLET 0 AUROBINDO PHARM EAGEN 76282-0209-30 0.14554 TERBINAFINE HCL 250 MG TABLET 0 EXELAN PHARMACE EAGEN 00143-9746-10 3.60000 TERBUTALINE SULF 1 MG/ML VIAL 0 WEST-WARD,INC. MLGEN 55390-0101-10 3.60000 TERBUTALINE SULF 1 MG/ML VIAL 0 BEDFORD LABS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00115-2611-01 0.24700 TERBUTALINE SULFATE 2.5 MG TAB 0 GLOBAL PHARM EAGEN 00527-1318-01 0.24700 TERBUTALINE SULFATE 2.5 MG TAB 0 LANNETT CO. INC EAGEN 68<strong>08</strong>4-0256-11 0.24700 TERBUTALINE SULFATE 2.5 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0256-21 0.24700 TERBUTALINE SULFATE 2.5 MG TAB 0 AHP EAGEN 00115-2622-01 0.46657 TERBUTALINE SULFATE 5 MG TAB 0 GLOBAL PHARM EAGEN 00527-1311-01 0.67450 TERBUTALINE SULFATE 5 MG TAB 0 LANNETT CO. INC EAGEN 68<strong>08</strong>4-0257-11 0.67450 TERBUTALINE SULFATE 5 MG TAB 0 AHP EAGEN 68<strong>08</strong>4-0257-21 0.67450 TERBUTALINE SULFATE 5 MG TAB 0 AHP EAGEN 00168-0346-46 0.71667 TERCONAZOLE 0.4% CREAM 0 SANDOZ GMGEN 00591-3196-89 0.68217 TERCONAZOLE 0.4% CREAM 0 ACTAVIS PHARMA, GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-1304-<strong>06</strong> 0.72470 TERCONAZOLE 0.4% CREAM 0 TARO PHARM USA GMGEN 00591-3197-52 1.53487 TERCONAZOLE 0.8% CREAM 0 ACTAVIS PHARMA, GMGEN 51672-1302-00 1.53487 TERCONAZOLE 0.8% CREAM 0 TARO PHARM USA GMGEN 45802-0717-<strong>08</strong> 13.10000 TERCONAZOLE 80 MG SUPPOSITORY 0 PERRIGO CO. EABND 00<strong>06</strong>9-0122-01 0.09140 1.23504 TESSALON PERLE 100 MG CAP G PFIZER US PHARM EABND 00<strong>06</strong>5-0741-12 4.14827 TETRACAINE 0.5% EYE DROPS 0 ALCON SURGICAL MLGEN 242<strong>08</strong>-0920-64 0.34160 TETRACAINE 0.5% EYE DROPS 0 VALEANT MLGEN 00172-2416-60 0.05100 TETRACYCLINE 250 MG CAPSULE 0 IVAX PHARMACEUT EAGEN 00172-2416-80 0.04162 TETRACYCLINE 250 MG CAPSULE 0 IVAX PHARMACEUT EAGEN 00172-2407-60 0.<strong>08</strong>850 TETRACYCLINE 500 MG CAPSULE 0 IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00172-2407-80 0.<strong>06</strong>697 TETRACYCLINE 500 MG CAPSULE 0 IVAX PHARMACEUT EABND 57844-0713-19 268.1<strong>06</strong>60 TEV-TROPIN 5 MG VIAL G GATE PHARM EABND 00074-3015-11 4.45270 TEVETEN HCT 600-12.5 MG TAB G ABBVIE US LLC EABND 00074-3020-11 4.45270 TEVETEN HCT 600-25 MG TAB G ABBVIE US LLC EABND 00074-3040-11 4.19830 TEVETEN 600 MG TABLET G ABBVIE US LLC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 385LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00178-0455-01 4.93020 TEXACORT 2.5% SOLUTION G MISSION PHARM. MLBND 59572-0210-15 260.43740 THALOMID 100 MG CAPSULE 0 CELGENE EABND 59572-0210-95 260.43715 THALOMID 100 MG CAPSULE 0 CELGENE EABND 59572-0215-13 278.47152 THALOMID 150 MG CAPSULE 0 CELGENE EABND 59572-0215-93 278.47174 THALOMID 150 MG CAPSULE 0 CELGENE EABND 59572-0220-16 296.51779 THALOMID 200 MG CAPSULE 0 CELGENE EABND 59572-0220-96 296.51789 THALOMID 200 MG CAPSULE 0 CELGENE EABND 59572-0205-14 160.44671 THALOMID 50 MG CAPSULE 0 CELGENE EABND 59572-0205-17 176.48290 THALOMID 50 MG CAPSULE 0 CELGENE EABND 59572-0205-94 160.44617 THALOMID 50 MG CAPSULE 0 CELGENE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59572-0205-97 176.48622 THALOMID 50 MG CAPSULE 0 CELGENE EABND 50474-0100-01 1.23802 THEO-24 ER 100 MG CAPSULE 0 AUXILIUM PHARM EABND 50474-0200-01 1.84002 THEO-24 ER 200 MG CAPSULE 0 AUXILIUM PHARM EABND 50474-0300-01 2.26141 THEO-24 ER 300 MG CAPSULE 0 AUXILIUM PHARM EABND 50474-0300-50 2.14367 THEO-24 ER 300 MG CAPSULE 0 AUXILIUM PHARM EABND 50474-0400-01 3.18205 THEO-24 ER 400 MG CAPSULE 0 AUXILIUM PHARM EAGEN 00904-5887-61 0.11550 THEOPHYLLINE ER 100 MG TABLET 0 MAJOR PHARMACEU EAGEN 50111-0483-01 0.11550 THEOPHYLLINE ER 100 MG TABLET 0 PLIVA, INC EAGEN 50111-0483-02 0.11550 THEOPHYLLINE ER 100 MG TABLET 0 PLIVA, INC EAGEN 00904-5888-61 0.17604 THEOPHYLLINE ER 200 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50111-0482-01 0.17604 THEOPHYLLINE ER 200 MG TABLET 0 PLIVA, INC EAGEN 50111-0482-02 0.17604 THEOPHYLLINE ER 200 MG TABLET 0 PLIVA, INC EAGEN 50111-0482-03 0.17604 THEOPHYLLINE ER 200 MG TABLET 0 PLIVA, INC EAGEN 00904-5889-61 0.19450 THEOPHYLLINE ER 300 MG TAB 0 MAJOR PHARMACEU EAGEN 23155-0<strong>06</strong>2-01 0.19450 THEOPHYLLINE ER 300 MG TAB 0 HERITAGE PHARMA EAGEN 50111-0459-01 0.19450 THEOPHYLLINE ER 300 MG TAB 0 PLIVA, INC EAGEN 50111-0459-02 0.19450 THEOPHYLLINE ER 300 MG TAB 0 PLIVA, INC EAGEN 50111-0459-03 0.19450 THEOPHYLLINE ER 300 MG TAB 0 PLIVA, INC EAGEN 29033-0001-01 1.01820 THEOPHYLLINE ER 400 MG TABLET 0 NOSTRUM LABORAT EAGEN 42858-0701-01 1.01820 THEOPHYLLINE ER 400 MG TABLET 0 RHODES PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0380-01 1.01820 THEOPHYLLINE ER 400 MG TABLET 0 GLENMARK PHARMA EAGEN 23155-0<strong>06</strong>3-01 0.51202 THEOPHYLLINE ER 450 MG TAB 0 HERITAGE PHARMA EAGEN 50111-0518-01 0.51202 THEOPHYLLINE ER 450 MG TAB 0 PLIVA, INC EAGEN 29033-0002-01 1.47127 THEOPHYLLINE ER 600 MG TABLET 0 NOSTRUM LABORAT EAGEN 42858-0702-01 1.47127 THEOPHYLLINE ER 600 MG TABLET 0 RHODES PHARMACE EAGEN 68462-0356-01 1.47127 THEOPHYLLINE ER 600 MG TABLET 0 GLENMARK PHARMA EABND 00264-9554-10 0.0<strong>08</strong>65 THEOPHYLLINE 400 MG/500 ML D5W 0 B.BRAUN MLBND 00121-4794-15 0.15440 0.69280 THEOPHYLLINE 80 MG/15 ML SOLN 0 PHARMACEU ASSOC MLGEN 51862-0131-16 0.13167 THEOPHYLLINE 80 MG/15 ML SOLN 0 LIBERTAS PHARMA MLGEN 54838-0556-80 0.15435 THEOPHYLLINE 80 MG/15 ML SOLN 0 SILARX PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0013-02 4.26750 THIAMINE 100 MG/ML VIAL 0 APP PHARMACEUTI MLBND 00178-0900-01 1.25496 THIOLA 100 MG TABLET 0 MISSION PHARM. EAGEX 00378-<strong>06</strong>12-01 0.20518 THIORIDAZINE 10 MG TABLET 0 MYLAN EAGEX 51079-0565-20 0.20518 THIORIDAZINE 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 53489-0148-01 0.18832 THIORIDAZINE 10 MG TABLET 0 MUTUAL PHARM CO EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 386LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 53489-0148-10 0.18834 THIORIDAZINE 10 MG TABLET 0 MUTUAL PHARM CO EAGEX 00378-<strong>06</strong>18-01 0.4<strong>06</strong>00 THIORIDAZINE 100 MG TABLET 0 MYLAN EAGEX 51079-0580-20 0.4<strong>06</strong>00 THIORIDAZINE 100 MG TABLET 0 MYLAN INSTITUTI EAGEX 53489-0500-01 0.37717 THIORIDAZINE 100 MG TABLET 0 MUTUAL PHARM CO EAGEX 00378-<strong>06</strong>14-01 0.28653 THIORIDAZINE 25 MG TABLET 0 MYLAN EAGEX 51079-0566-20 0.28653 THIORIDAZINE 25 MG TABLET 0 MYLAN INSTITUTI EAGEX 53489-0149-01 0.26482 THIORIDAZINE 25 MG TABLET 0 MUTUAL PHARM CO EAGEX 53489-0149-10 0.26484 THIORIDAZINE 25 MG TABLET 0 MUTUAL PHARM CO EAGEX 00378-<strong>06</strong>16-01 0.35328 THIORIDAZINE 50 MG TABLET 0 MYLAN EAGEX 51079-0567-20 0.35328 THIORIDAZINE 50 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 53489-0150-01 0.33120 THIORIDAZINE 50 MG TABLET 0 MUTUAL PHARM CO EAGEX 53489-0150-10 0.33122 THIORIDAZINE 50 MG TABLET 0 MUTUAL PHARM CO EABND 55390-0030-10 592.62000 THIOTEPA 15 MG VIAL 0 BEDFORD LABS EAGUX 00378-1001-01 0.13880 THIOTHIXENE 1 MG CAPSULE 0 MYLAN EAGUX 00781-2226-01 0.13880 THIOTHIXENE 1 MG CAPSULE 0 SANDOZ EAGUX 00378-5010-01 0.4<strong>06</strong>50 THIOTHIXENE 10 MG CAPSULE 0 MYLAN EAGUX 00781-2229-01 0.4<strong>06</strong>50 THIOTHIXENE 10 MG CAPSULE 0 SANDOZ EAGUX 51079-0589-20 0.4<strong>06</strong>50 THIOTHIXENE 10 MG CAPSULE 0 MYLAN INSTITUTI EAGUX 00378-2002-01 0.18600 THIOTHIXENE 2 MG CAPSULE 0 MYLAN EAGUX 00781-2227-01 0.18600 THIOTHIXENE 2 MG CAPSULE 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 51079-0587-20 0.18600 THIOTHIXENE 2 MG CAPSULE 0 MYLAN INSTITUTI EAGUX 00378-3005-01 0.29630 THIOTHIXENE 5 MG CAPSULE 0 MYLAN EAGUX 00781-2228-01 0.29630 THIOTHIXENE 5 MG CAPSULE 0 SANDOZ EAGUX 51079-0588-20 0.29630 THIOTHIXENE 5 MG CAPSULE 0 MYLAN INSTITUTI EABND 00456-0050-01 0.63129 THYROLAR-1 STRENGTH TABLET 0 FOREST PHARMACE EABND 00456-0045-01 0.50505 THYROLAR-1/2 STRENGTH TAB 0 FOREST PHARMACE EABND 00456-0040-01 0.455<strong>08</strong> THYROLAR-1/4 STRENGTH TAB 0 FOREST PHARMACE EABND 00456-0055-01 0.74235 THYROLAR-2 STRENGTH TABLET 0 FOREST PHARMACE EABND 00456-0<strong>06</strong>0-01 0.90743 THYROLAR-3 STRENGTH TABLET 0 FOREST PHARMACE EAGEX 55253-<strong>06</strong>00-30 5.02200 TIAGABINE HCL 2 MG TABLET G CEPHALON,INC.-T EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62756-0200-83 5.00000 TIAGABINE HCL 2 MG TABLET G SUN PHARMACEUTI EAGEX 55253-<strong>06</strong>01-30 5.02200 TIAGABINE HCL 4 MG TABLET G CEPHALON,INC.-T EAGEX 62756-0224-83 5.00000 TIAGABINE HCL 4 MG TABLET G SUN PHARMACEUTI EABND 00456-2612-00 0.62210 1.04481 TIAZAC ER 120 MG CAPSULE G FOREST PHARMACE EABND 00456-2612-30 0.62210 1.3<strong>06</strong>69 TIAZAC ER 120 MG CAPSULE G FOREST PHARMACE EABND 00456-2612-90 0.62210 1.18367 TIAZAC ER 120 MG CAPSULE G FOREST PHARMACE EABND 00456-2613-00 0.81330 1.26<strong>08</strong>6 TIAZAC ER 180 MG CAPSULE G FOREST PHARMACE EABND 00456-2613-30 0.81330 1.57755 TIAZAC ER 180 MG CAPSULE G FOREST PHARMACE EABND 00456-2613-90 0.81330 1.42852 TIAZAC ER 180 MG CAPSULE G FOREST PHARMACE EABND 00456-2614-00 1.16285 1.80703 TIAZAC ER 240 MG CAPSULE G FOREST PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00456-2614-30 1.16285 2.23823 TIAZAC ER 240 MG CAPSULE G FOREST PHARMACE EABND 00456-2614-90 1.16285 2.02732 TIAZAC ER 240 MG CAPSULE G FOREST PHARMACE EABND 00456-2615-00 1.38010 2.35265 TIAZAC ER 300 MG CAPSULE G FOREST PHARMACE EABND 00456-2615-30 1.38010 2.90112 TIAZAC ER 300 MG CAPSULE G FOREST PHARMACE EABND 00456-2615-90 1.38010 2.62630 TIAZAC ER 300 MG CAPSULE G FOREST PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 387LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00456-2616-00 1.54115 2.37549 TIAZAC ER 360 MG CAPSULE G FOREST PHARMACE EABND 00456-2616-30 1.54115 2.95646 TIAZAC ER 360 MG CAPSULE G FOREST PHARMACE EABND 00456-2616-90 1.54115 2.67684 TIAZAC ER 360 MG CAPSULE G FOREST PHARMACE EABND 00456-2617-00 1.76920 2.51350 TIAZAC ER 420 MG CAPSULE G FOREST PHARMACE EABND 00456-2617-30 1.76920 3.09922 TIAZAC ER 420 MG CAPSULE G FOREST PHARMACE EABND 00456-2617-90 1.76920 2.80549 TIAZAC ER 420 MG CAPSULE G FOREST PHARMACE EAGUL 00093-0154-01 0.27320 TICLOPIDINE 250 MG TABLET G TEVA USA EAGUL 57664-0327-<strong>06</strong> 0.27320 TICLOPIDINE 250 MG TABLET G CARACO PHARM EAGUL 57664-0327-13 0.27320 TICLOPIDINE 250 MG TABLET G CARACO PHARM EAGUL 57664-0327-83 0.27320 TICLOPIDINE 250 MG TABLET G CARACO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 57664-0327-86 0.27320 TICLOPIDINE 250 MG TABLET G CARACO PHARM EAGUL 60505-0027-02 0.27320 TICLOPIDINE 250 MG TABLET G APOTEX CORP EAGUL 60505-0027-04 0.27320 TICLOPIDINE 250 MG TABLET G APOTEX CORP EAGUL 60505-0027-07 0.27320 TICLOPIDINE 250 MG TABLET G APOTEX CORP EABND 42023-0118-01 10.86802 TIGAN 100 MG/ML VIAL 0 JHP PHARMACEUTI MLBND 42023-0119-25 12.679<strong>08</strong> TIGAN 100 MG/ML VIAL 0 JHP PHARMACEUTI MLBUL 61570-0079-01 1.01930 2.30715 TIGAN 300 MG CAPSULE 0 MONARCH PHRM EABND 00<strong>06</strong>9-5800-60 4.33702 TIKOSYN 125 MCG CAPSULE 0 PFIZER US PHARM EABND 00<strong>06</strong>9-5810-60 4.33702 TIKOSYN 250 MCG CAPSULE 0 PFIZER US PHARM EABND 00<strong>06</strong>9-5810-61 TIKOSYN 250 MCG CAPSULE 0 PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>9-5820-60 4.33702 TIKOSYN 500 MCG CAPSULE 0 PFIZER US PHARM EAGEX 52544-0143-31 1.514<strong>08</strong> TILIA FE 28 TABLET 0 ACTAVIS PHARMA, EABND 00029-6571-26 13.28000 TIMENTIN 3.1 GM VIAL 0 GLAXOSMITHKLINE EABND 00029-6571-31 0.15362 TIMENTIN 3.1 GM/100 ML ISO 0 GLAXOSMITHKLINE MLBND 00029-6579-21 132.43480 TIMENTIN 31 GM BULK VIAL 0 GLAXOSMITHKLINE EABND 00378-0221-01 0.69288 TIMOLOL MALEATE 10 MG TABLET G MYLAN EABND 00378-0715-01 1.27836 TIMOLOL MALEATE 20 MG TABLET G MYLAN EABND 00378-0055-01 0.560<strong>08</strong> TIMOLOL MALEATE 5 MG TABLET G MYLAN EAGEN 16571-0140-10 0.53171 TIMOLOL 0.25% EYE DROPS 0 PACK PHARMACEUT MLGEN 16571-0140-15 0.53171 TIMOLOL 0.25% EYE DROPS 0 PACK PHARMACEUT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16571-0140-50 0.53171 TIMOLOL 0.25% EYE DROPS 0 PACK PHARMACEUT MLGEN 60505-0552-02 0.53171 TIMOLOL 0.25% EYE DROPS 0 APOTEX CORP MLGEN 60505-0552-03 0.53171 TIMOLOL 0.25% EYE DROPS 0 APOTEX CORP MLGEN 60505-0552-04 0.53171 TIMOLOL 0.25% EYE DROPS 0 APOTEX CORP MLGEN 60505-0597-01 0.53171 TIMOLOL 0.25% EYE DROPS 0 APOTEX CORP MLGEN 60505-0597-02 0.53171 TIMOLOL 0.25% EYE DROPS 0 APOTEX CORP MLGEN 60505-0597-03 0.53171 TIMOLOL 0.25% EYE DROPS 0 APOTEX CORP MLGEN 60758-<strong>08</strong>02-05 0.53171 TIMOLOL 0.25% EYE DROPS 0 PACIFIC PHARMA MLGEN 60758-<strong>08</strong>02-10 0.53171 TIMOLOL 0.25% EYE DROPS 0 PACIFIC PHARMA MLGEN 60758-<strong>08</strong>02-15 0.53171 TIMOLOL 0.25% EYE DROPS 0 PACIFIC PHARMA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61314-0226-05 0.53171 TIMOLOL 0.25% EYE DROPS 0 SANDOZ MLGEN 61314-0226-10 0.53171 TIMOLOL 0.25% EYE DROPS 0 SANDOZ MLGEN 61314-0226-15 0.53171 TIMOLOL 0.25% EYE DROPS 0 SANDOZ MLGEN 25010-<strong>08</strong>16-56 7.40750 TIMOLOL 0.25% GEL-SOLUTION 0 VALEANT MLGEN 61314-0224-05 7.40750 TIMOLOL 0.25% GFS GEL-SOLUTION 0 SANDOZ ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 388LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16571-0141-10 0.67590 TIMOLOL 0.5% EYE DROPS 0 PACK PHARMACEUT MLGEN 16571-0141-15 0.67590 TIMOLOL 0.5% EYE DROPS 0 PACK PHARMACEUT MLGEN 16571-0141-50 0.67590 TIMOLOL 0.5% EYE DROPS 0 PACK PHARMACEUT MLGEN 17478-0288-10 0.67590 TIMOLOL 0.5% EYE DROPS 0 AKORN INC. MLGEN 17478-0288-11 0.55500 TIMOLOL 0.5% EYE DROPS 0 AKORN INC. MLGEN 17478-0288-12 0.56000 TIMOLOL 0.5% EYE DROPS 0 AKORN INC. MLGEN 50383-0021-05 0.67590 TIMOLOL 0.5% EYE DROPS 0 HI-TECH PHARMAC MLGEN 50383-0021-10 0.67590 TIMOLOL 0.5% EYE DROPS 0 HI-TECH PHARMAC MLGEN 50383-0021-15 0.67590 TIMOLOL 0.5% EYE DROPS 0 HI-TECH PHARMAC MLGEN 60505-0551-02 0.67590 TIMOLOL 0.5% EYE DROPS 0 APOTEX CORP ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0551-03 0.67590 TIMOLOL 0.5% EYE DROPS 0 APOTEX CORP MLGEN 60505-0551-04 0.67590 TIMOLOL 0.5% EYE DROPS 0 APOTEX CORP MLGEN 60505-0598-01 0.67590 TIMOLOL 0.5% EYE DROPS 0 APOTEX CORP MLGEN 60505-0598-02 0.67590 TIMOLOL 0.5% EYE DROPS 0 APOTEX CORP MLGEN 60505-0598-03 0.67590 TIMOLOL 0.5% EYE DROPS 0 APOTEX CORP MLGEN 60758-<strong>08</strong>01-05 0.67590 TIMOLOL 0.5% EYE DROPS 0 PACIFIC PHARMA MLGEN 60758-<strong>08</strong>01-10 0.67590 TIMOLOL 0.5% EYE DROPS 0 PACIFIC PHARMA MLGEN 60758-<strong>08</strong>01-15 0.67590 TIMOLOL 0.5% EYE DROPS 0 PACIFIC PHARMA MLGEN 61314-0227-05 0.67590 TIMOLOL 0.5% EYE DROPS 0 SANDOZ MLGEN 61314-0227-10 0.67590 TIMOLOL 0.5% EYE DROPS 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61314-0227-15 0.67590 TIMOLOL 0.5% EYE DROPS 0 SANDOZ MLGEN 25010-<strong>08</strong>17-56 8.27310 TIMOLOL 0.5% GEL-SOLUTION 0 VALEANT MLGEN 61314-0225-05 8.27310 TIMOLOL 0.5% GFS GEL-SOLUTION 0 SANDOZ MLBND 25010-<strong>08</strong>12-56 0.53171 23.6<strong>08</strong>52 TIMOPTIC 0.25% EYE DROPS G VALEANT MLBND 25010-<strong>08</strong>14-66 4.72836 TIMOPTIC 0.25% OCUDOSE DROP G VALEANT EABND 25010-<strong>08</strong>13-16 0.67590 16.36760 TIMOPTIC 0.5% EYE DROPS G VALEANT MLBND 25010-<strong>08</strong>13-56 0.67590 25.54740 TIMOPTIC 0.5% EYE DROPS G VALEANT MLBND 25010-<strong>08</strong>15-66 5.39182 TIMOPTIC 0.5% OCUDOSE DROP G VALEANT EABND 25010-<strong>08</strong>10-56 7.40750 27.62074 TIMOPTIC-XE 0.25% EYE SOLN G VALEANT MLBND 25010-<strong>08</strong>11-56 8.27310 30.26678 TIMOPTIC-XE 0.5% EYE SOLN G VALEANT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00178-8250-40 3.97880 7.1<strong>08</strong>12 TINDAMAX 250 MG TABLET G MISSION PHARM. EABND 00178-8500-20 5.20120 14.21624 TINDAMAX 500 MG TABLET G MISSION PHARM. EABND 00178-8500-60 5.20120 13.16324 TINDAMAX 500 MG TABLET G MISSION PHARM. EAGEN 00054-0347-16 3.8<strong>06</strong>25 TINIDAZOLE 250 MG TABLET G ROXANE LABS. EAGEN 43386-0550-04 3.97880 TINIDAZOLE 250 MG TABLET G GAVIS PHARMACEU EAGEN 44523-0425-40 3.97880 TINIDAZOLE 250 MG TABLET G BIOCOMP PHARMA, EAGEN 00054-0348-07 5.20120 TINIDAZOLE 500 MG TABLET G ROXANE LABS. EAGEN 00054-0348-21 5.20120 TINIDAZOLE 500 MG TABLET G ROXANE LABS. EAGEN 43386-0551-02 5.20120 TINIDAZOLE 500 MG TABLET G GAVIS PHARMACEU EAGEN 43386-0551-<strong>06</strong> 5.20120 TINIDAZOLE 500 MG TABLET G GAVIS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 44523-0450-20 5.20120 TINIDAZOLE 500 MG TABLET G BIOCOMP PHARMA, EAGEN 44523-0450-60 5.20120 TINIDAZOLE 500 MG TABLET G BIOCOMP PHARMA, EABND 24090-0495-84 2.49711 TIROSINT 100 MCG CAPSULE 0 AKRIMAX PHARMAC EABND 24090-0496-84 2.49711 TIROSINT 112 MCG CAPSULE 0 AKRIMAX PHARMAC EABND 24090-0497-84 2.49711 TIROSINT 125 MCG CAPSULE 0 AKRIMAX PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 389LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 24090-0490-84 2.49711 TIROSINT 13 MCG CAPSULE 0 AKRIMAX PHARMAC EABND 24090-0498-84 2.49711 TIROSINT 137 MCG CAPSULE 0 AKRIMAX PHARMAC EABND 24090-0499-84 2.49711 TIROSINT 150 MCG CAPSULE 0 AKRIMAX PHARMAC EABND 24090-0491-84 2.49711 TIROSINT 25 MCG CAPSULE 0 AKRIMAX PHARMAC EABND 24090-0492-84 2.49711 TIROSINT 50 MCG CAPSULE 0 AKRIMAX PHARMAC EABND 24090-0493-84 2.49711 TIROSINT 75 MCG CAPSULE 0 AKRIMAX PHARMAC EABND 24090-0494-84 2.49711 TIROSINT 88 MCG CAPSULE 0 AKRIMAX PHARMAC EABND 49702-0228-13 40.93532 TIVICAY 50 MG TABLET G VIIV <strong>HEALTH</strong>CARE EAGEN 00378-1665-19 1.79640 TIZANIDINE HCL 2 MG CAPSULE G MYLAN EAGEN 00591-2788-86 1.79640 TIZANIDINE HCL 2 MG CAPSULE G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-2648-07 1.79640 TIZANIDINE HCL 2 MG CAPSULE G APOTEX CORP EAGUL 00185-0034-10 0.26000 TIZANIDINE HCL 2 MG TABLET 0 SANDOZ EAGUL 00185-0034-51 0.26000 TIZANIDINE HCL 2 MG TABLET 0 SANDOZ EAGUL 00378-0722-19 0.26000 TIZANIDINE HCL 2 MG TABLET 0 MYLAN EAGUL 55111-0179-10 0.26000 TIZANIDINE HCL 2 MG TABLET 0 DR.REDDY'S LAB EAGUL 55111-0179-15 0.26000 TIZANIDINE HCL 2 MG TABLET 0 DR.REDDY'S LAB EAGUL 57664-0502-13 0.26000 TIZANIDINE HCL 2 MG TABLET 0 CARACO PHARM EAGUL 57664-0502-18 0.26000 TIZANIDINE HCL 2 MG TABLET 0 CARACO PHARM EAGUL 57664-0502-83 0.26000 TIZANIDINE HCL 2 MG TABLET 0 CARACO PHARM EAGUL 57664-0502-88 0.26000 TIZANIDINE HCL 2 MG TABLET 0 CARACO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 57664-0502-89 0.26000 TIZANIDINE HCL 2 MG TABLET 0 CARACO PHARM EAGUL 60505-0251-02 0.26000 TIZANIDINE HCL 2 MG TABLET 0 APOTEX CORP EAGUL 60505-0251-03 0.26000 TIZANIDINE HCL 2 MG TABLET 0 APOTEX CORP EAGEN 00378-1666-19 2.27730 TIZANIDINE HCL 4 MG CAPSULE G MYLAN EAGEN 00591-2789-86 2.27730 TIZANIDINE HCL 4 MG CAPSULE G ACTAVIS PHARMA, EAGEN 60505-2649-07 2.27730 TIZANIDINE HCL 4 MG CAPSULE G APOTEX CORP EAGUL 00185-4400-10 0.32000 TIZANIDINE HCL 4 MG TABLET 0 SANDOZ EAGUL 00185-4400-23 0.32000 TIZANIDINE HCL 4 MG TABLET 0 SANDOZ EAGUL 00185-4400-51 0.32000 TIZANIDINE HCL 4 MG TABLET 0 SANDOZ EAGUL 00378-0724-19 0.32000 TIZANIDINE HCL 4 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00904-6193-61 0.32000 TIZANIDINE HCL 4 MG TABLET 0 MAJOR PHARMACEU EAGUL 51079-0998-01 0.32000 TIZANIDINE HCL 4 MG TABLET 0 MYLAN INSTITUTI EAGUL 55111-0180-10 0.32000 TIZANIDINE HCL 4 MG TABLET 0 DR.REDDY'S LAB EAGUL 55111-0180-15 0.32000 TIZANIDINE HCL 4 MG TABLET 0 DR.REDDY'S LAB EAGUL 57664-0503-13 0.32000 TIZANIDINE HCL 4 MG TABLET 0 CARACO PHARM EAGUL 57664-0503-18 0.32000 TIZANIDINE HCL 4 MG TABLET 0 CARACO PHARM EAGUL 57664-0503-83 0.32000 TIZANIDINE HCL 4 MG TABLET 0 CARACO PHARM EAGUL 57664-0503-89 0.32000 TIZANIDINE HCL 4 MG TABLET 0 CARACO PHARM EAGUL 58517-0380-30 0.32000 TIZANIDINE HCL 4 MG TABLET 0 <strong>NEW</strong> HORIZON RX EAGUL 60505-0252-02 0.32000 TIZANIDINE HCL 4 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 60505-0252-03 0.32000 TIZANIDINE HCL 4 MG TABLET 0 APOTEX CORP EAGUL 68<strong>08</strong>4-0013-01 0.32000 TIZANIDINE HCL 4 MG TABLET 0 AHP EAGUL 68<strong>08</strong>4-0013-11 0.32000 TIZANIDINE HCL 4 MG TABLET 0 AHP EAGUL 68<strong>08</strong>4-<strong>06</strong>45-01 0.32000 TIZANIDINE HCL 4 MG TABLET 0 AHP EAGEN 00378-1667-19 3.86619 TIZANIDINE HCL 6 MG CAPSULE G MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 390LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-2790-86 3.86619 TIZANIDINE HCL 6 MG CAPSULE G ACTAVIS PHARMA, EAGEN 60505-2650-07 3.86619 TIZANIDINE HCL 6 MG CAPSULE G APOTEX CORP EAGEN 13811-<strong>06</strong>00-30 2.11449 TL-SELECT CAPSULE 0 TRIGEN LABORATO EABND 13811-0507-30 2.34170 TL-SELECT DHA S<strong>OF</strong>TGEL 0 TRIGEN LABORATO EABND 00078-<strong>06</strong>30-11 29.688<strong>06</strong> TOBI PODHALER 28 MG INHALE CAP 0 NOVARTIS EABND 00078-<strong>06</strong>30-35 29.68795 TOBI PODHALER 28 MG INHALE CAP 0 NOVARTIS EABND 00078-<strong>06</strong>30-56 29.68791 TOBI PODHALER 28 MG INHALE CAP 0 NOVARTIS EABND 00078-0494-61 23.75128 TOBI 300 MG/5 ML SOLUTION 0 NOVARTIS MLBND 00078-0494-71 23.75035 TOBI 300 MG/5 ML SOLUTION 0 NOVARTIS MLBND 00<strong>06</strong>5-<strong>06</strong>47-05 26.53344 26.53344 TOBRADEX EYE DROPS 0 ALCON LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>5-<strong>06</strong>47-10 26.52348 26.52348 TOBRADEX EYE DROPS 0 ALCON LABS. MLBND 00<strong>06</strong>5-<strong>06</strong>47-25 26.47368 26.47368 TOBRADEX EYE DROPS 0 ALCON LABS. MLBND 00<strong>06</strong>5-<strong>06</strong>48-35 49.<strong>06</strong>011 TOBRADEX EYE OINTMENT 0 ALCON LABS. GMBND 00<strong>06</strong>5-<strong>06</strong>52-05 23.56536 TOBRADEX ST EYE DROPS G ALCON LABS. MLGUL 17478-0290-10 0.67200 TOBRAMYCIN 0.3% EYE DROPS 0 AKORN INC. MLGUL 242<strong>08</strong>-0290-05 0.67200 TOBRAMYCIN 0.3% EYE DROPS 0 VALEANT MLGUL 61314-<strong>06</strong>43-05 0.67200 TOBRAMYCIN 0.3% EYE DROPS 0 SANDOZ MLGEN 39822-0412-01 74.31250 TOBRAMYCIN 1.2 GM VIAL 0 X-GEN PHARMACEU EAGEN 39822-0412-<strong>06</strong> 74.31250 TOBRAMYCIN 1.2 GM VIAL 0 X-GEN PHARMACEU EAGEN 63323-0303-51 74.31250 TOBRAMYCIN 1.2 GM VIAL 0 APP PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0303-55 69.93000 TOBRAMYCIN 1.2 GM VIAL 0 APP/NOVAPLUS EAGEN 63323-0305-02 1.19800 TOBRAMYCIN 10 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 00093-4<strong>08</strong>5-63 19.31579 TOBRAMYCIN 300 MG/5 ML AMPULE G TEVA USA MLGEN 00409-3590-02 0.50970 TOBRAMYCIN 40 MG/ML VIAL 0 HOSPIRA MLGEN 00703-9402-04 0.56640 TOBRAMYCIN 40 MG/ML VIAL 0 TEVA PARENTERAL MLGEN 00703-9416-01 0.56640 TOBRAMYCIN 40 MG/ML VIAL 0 TEVA PARENTERAL MLGEN 63323-03<strong>06</strong>-02 0.56640 TOBRAMYCIN 40 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-03<strong>06</strong>-30 0.56640 TOBRAMYCIN 40 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 63323-03<strong>06</strong>-55 0.5<strong>08</strong>50 TOBRAMYCIN 40 MG/ML VIAL 0 APP/NOVAPLUS MLGEN 63323-03<strong>06</strong>-56 0.46590 TOBRAMYCIN 40 MG/ML VIAL 0 APP/NOVAPLUS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0307-51 0.56640 TOBRAMYCIN 40 MG/ML VIAL 0 APP PHARMACEUTI MLBND 00409-3469-13 0.17131 TOBRAMYCIN 60 MG/50 ML NS 0 HOSPIRA MLBND 00409-3470-23 0.09581 TOBRAMYCIN 80 MG/100 ML NS 0 HOSPIRA MLGEN 00<strong>06</strong>9-0301-01 0.56640 TOBRAMYCIN 80 MG/2 ML VIAL 0 PFIZER/NOVAPLUS MLGEN 00<strong>06</strong>9-0301-02 0.56640 TOBRAMYCIN 80 MG/2 ML VIAL 0 PFIZER/NOVAPLUS MLGEN 00409-3578-01 0.56640 TOBRAMYCIN 80 MG/2 ML VIAL 0 HOSPIRA MLGEN 242<strong>08</strong>-0295-05 19.62600 TOBRAMYCIN-DEXAMETH OPHTH SUSP G VALEANT MLGEN 242<strong>08</strong>-0295-10 19.62225 TOBRAMYCIN-DEXAMETH OPHTH SUSP G VALEANT MLGEN 242<strong>08</strong>-0295-25 19.57800 TOBRAMYCIN-DEXAMETH OPHTH SUSP G VALEANT MLGEN 61314-<strong>06</strong>47-05 19.63350 TOBRAMYCIN-DEXAMETH OPHTH SUSP G SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61314-<strong>06</strong>47-10 19.62600 TOBRAMYCIN-DEXAMETH OPHTH SUSP G SANDOZ MLGEN 61314-<strong>06</strong>47-25 19.59300 TOBRAMYCIN-DEXAMETH OPHTH SUSP G SANDOZ MLGUL 00<strong>06</strong>5-<strong>06</strong>43-05 0.67200 TOBREX 0.3% EYE DROPS G ALCON LABS. MLBND 00<strong>06</strong>5-<strong>06</strong>44-35 28.17257 TOBREX 0.3% EYE OINTMENT G ALCON LABS. GMGEX 004<strong>06</strong>-9920-03 0.12825 T<strong>OF</strong>RANIL 10 MG TABLET 0 MALLINCKRODT PH EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 391LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 004<strong>06</strong>-9921-03 0.17280 T<strong>OF</strong>RANIL 25 MG TABLET 0 MALLINCKRODT PH EAGEX 004<strong>06</strong>-9922-03 0.23420 T<strong>OF</strong>RANIL 50 MG TABLET 0 MALLINCKRODT PH EABEX 004<strong>06</strong>-9924-03 11.56432 18.76823 T<strong>OF</strong>RANIL-PM 100 MG CAPSULE 0 MALLINCKRODT BR EABEX 004<strong>06</strong>-9925-03 9.20420 19.13288 T<strong>OF</strong>RANIL-PM 125 MG CAPSULE 0 MALLINCKRODT BR EABEX 004<strong>06</strong>-9926-03 11.35617 19.49697 T<strong>OF</strong>RANIL-PM 150 MG CAPSULE 0 MALLINCKRODT BR EABEX 004<strong>06</strong>-9923-03 8.26440 18.40386 T<strong>OF</strong>RANIL-PM 75 MG CAPSULE 0 MALLINCKRODT BR EAGEN 00378-0217-01 1.21267 TOLAZAMIDE 250 MG TABLET G MYLAN EAGEN 68<strong>08</strong>4-0556-11 1.41390 TOLAZAMIDE 250 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0556-21 1.41390 TOLAZAMIDE 250 MG TABLET G AHP EABND 00378-0551-01 0.62100 2.02686 TOLAZAMIDE 500 MG TABLET G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00378-0215-01 0.17340 0.97848 TOLBUTAMIDE 500 MG TABLET G MYLAN EABND 00378-0215-05 0.17340 0.97838 TOLBUTAMIDE 500 MG TABLET G MYLAN EABND 53489-05<strong>06</strong>-01 0.61200 0.62250 TOLMETIN SODIUM 200 MG TAB G MUTUAL PHARM CO EAGEN 00093-2075-01 1.03130 TOLMETIN SODIUM 400 MG CAP G TEVA USA EAGEN 00378-5200-01 1.03130 TOLMETIN SODIUM 400 MG CAP G MYLAN EABND 00378-0313-01 3.29933 TOLMETIN SODIUM 600 MG TAB G MYLAN EAGEN 00093-2056-05 1.11132 TOLTERODINE TARTRATE 1 MG TAB G TEVA USA EAGEN 00093-2056-<strong>06</strong> 1.11132 TOLTERODINE TARTRATE 1 MG TAB G TEVA USA EAGEN 00093-2056-42 1.11132 TOLTERODINE TARTRATE 1 MG TAB G TEVA USA EAGEN 00378-5445-91 1.11132 TOLTERODINE TARTRATE 1 MG TAB G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-0170-01 1.11132 TOLTERODINE TARTRATE 1 MG TAB G GREENSTONE LLC. EAGEN 59762-0170-<strong>06</strong> 1.11132 TOLTERODINE TARTRATE 1 MG TAB G GREENSTONE LLC. EAGEN 60505-3527-05 1.11132 TOLTERODINE TARTRATE 1 MG TAB G APOTEX CORP EAGEN 60505-3527-<strong>06</strong> 1.11132 TOLTERODINE TARTRATE 1 MG TAB G APOTEX CORP EAGEN 00093-2055-05 1.14050 TOLTERODINE TARTRATE 2 MG TAB G TEVA USA EAGEN 00093-2055-<strong>06</strong> 1.14050 TOLTERODINE TARTRATE 2 MG TAB G TEVA USA EAGEN 00093-2055-42 1.14050 TOLTERODINE TARTRATE 2 MG TAB G TEVA USA EAGEN 00378-5446-05 1.14050 TOLTERODINE TARTRATE 2 MG TAB G MYLAN EAGEN 00378-5446-91 1.14050 TOLTERODINE TARTRATE 2 MG TAB G MYLAN EAGEN 59762-<strong>08</strong>00-02 1.14050 TOLTERODINE TARTRATE 2 MG TAB G GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-<strong>08</strong>00-<strong>06</strong> 1.14050 TOLTERODINE TARTRATE 2 MG TAB G GREENSTONE LLC. EAGEN 60505-3528-05 1.14050 TOLTERODINE TARTRATE 2 MG TAB G APOTEX CORP EAGEN 60505-3528-<strong>06</strong> 1.14050 TOLTERODINE TARTRATE 2 MG TAB G APOTEX CORP EABEX 50458-<strong>06</strong>41-65 0.16420 10.78404 TOPAMAX 100 MG TABLET G JANSSEN PHARM. EABEX 50458-<strong>06</strong>47-65 0.53330 3.74191 TOPAMAX 15 MG SPRINKLE CAP G JANSSEN PHARM. EABEX 50458-<strong>06</strong>42-65 0.11970 12.62457 TOPAMAX 200 MG TABLET G JANSSEN PHARM. EABEX 50458-<strong>06</strong>45-65 0.61590 4.52364 TOPAMAX 25 MG SPRINKLE CAP G JANSSEN PHARM. EABEX 50458-<strong>06</strong>39-65 0.<strong>08</strong>330 3.95661 TOPAMAX 25 MG TABLET G JANSSEN PHARM. EABEX 50458-<strong>06</strong>40-65 0.103<strong>08</strong> 7.89662 TOPAMAX 50 MG TABLET G JANSSEN PHARM. EABND 51672-5205-03 2.25180 4.13962 TOPICORT 0.05% CREAM G TARO PHARM USA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 51672-5205-07 2.12<strong>06</strong>5 TOPICORT 0.05% CREAM G TARO PHARM USA GMBND 51672-5205-<strong>08</strong> 1.83264 TOPICORT 0.05% CREAM G TARO PHARM USA GMGEN 51672-5202-01 3.97076 TOPICORT 0.05% GEL G TARO PHARM USA GMGEN 51672-5202-03 3.72975 TOPICORT 0.05% GEL G TARO PHARM USA GMBND 51672-5263-01 3.90930 TOPICORT 0.05% OINTMENT G TARO PHARM USA GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 392LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 51672-5263-03 4.34671 TOPICORT 0.05% OINTMENT G TARO PHARM USA GMBND 51672-5263-07 2.67193 TOPICORT 0.05% OINTMENT G TARO PHARM USA GMGEN 51672-5204-01 1.57780 TOPICORT 0.25% CREAM G TARO PHARM USA GMGEN 51672-5204-03 1.57780 TOPICORT 0.25% CREAM G TARO PHARM USA GMGEN 51672-5204-07 1.57780 TOPICORT 0.25% CREAM G TARO PHARM USA GMGEN 51672-5204-09 1.57780 TOPICORT 0.25% CREAM G TARO PHARM USA GMGEN 51672-5203-01 4.89099 TOPICORT 0.25% OINTMENT G TARO PHARM USA GMGEN 51672-5203-03 4.66787 TOPICORT 0.25% OINTMENT G TARO PHARM USA GMGEN 51672-5203-07 2.58622 TOPICORT 0.25% OINTMENT G TARO PHARM USA GMBND 51672-5281-07 4.54615 TOPICORT 0.25% SPRAY G TARO PHARM USA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00245-0713-60 0.16420 TOPIRAGEN 100 MG TABLET G UPSHER SMITH EAGEX 00245-0714-60 0.11970 TOPIRAGEN 200 MG TABLET G UPSHER SMITH EAGEX 00245-0711-60 0.<strong>08</strong>330 TOPIRAGEN 25 MG TABLET G UPSHER SMITH EAGEX 00245-0712-60 0.103<strong>08</strong> TOPIRAGEN 50 MG TABLET G UPSHER SMITH EAGEX 00093-7219-<strong>06</strong> 0.16420 TOPIRAMATE 100 MG TABLET G TEVA USA EAGEX 00093-7219-10 0.16420 TOPIRAMATE 100 MG TABLET G TEVA USA EAGEX 00378-6103-05 0.16420 TOPIRAMATE 100 MG TABLET G MYLAN EAGEX 00378-6103-91 0.16420 TOPIRAMATE 100 MG TABLET G MYLAN EAGEX 13668-0033-05 0.16420 TOPIRAMATE 100 MG TABLET G TORRENT PHARMAC EAGEX 13668-0033-60 0.16420 TOPIRAMATE 100 MG TABLET G TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 31722-0280-05 0.16420 TOPIRAMATE 100 MG TABLET G CAMBER PHARMACE EAGEX 31722-0280-10 0.16420 TOPIRAMATE 100 MG TABLET G CAMBER PHARMACE EAGEX 31722-0280-60 0.16420 TOPIRAMATE 100 MG TABLET G CAMBER PHARMACE EAGEX 51079-0728-01 0.16420 TOPIRAMATE 100 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0728-20 0.16420 TOPIRAMATE 100 MG TABLET G MYLAN INSTITUTI EAGEX 60505-2762-05 0.16420 TOPIRAMATE 100 MG TABLET G APOTEX CORP EAGEX 60505-2762-<strong>06</strong> 0.16420 TOPIRAMATE 100 MG TABLET G APOTEX CORP EAGEX 62756-0711-13 0.16420 TOPIRAMATE 100 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0711-86 0.16420 TOPIRAMATE 100 MG TABLET G SUN PHARMACEUTI EAGEX 65862-0173-60 0.16420 TOPIRAMATE 100 MG TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0344-01 0.16420 TOPIRAMATE 100 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0344-11 0.16420 TOPIRAMATE 100 MG TABLET G AHP EAGEX 68382-0140-05 0.16420 TOPIRAMATE 100 MG TABLET G ZYDUS PHARMACEU EAGEX 68382-0140-14 0.16420 TOPIRAMATE 100 MG TABLET G ZYDUS PHARMACEU EAGEX 68462-0109-10 0.16420 TOPIRAMATE 100 MG TABLET G GLENMARK PHARMA EAGEX 68462-0109-60 0.16420 TOPIRAMATE 100 MG TABLET G GLENMARK PHARMA EAGEX 00093-7335-<strong>06</strong> 0.53330 TOPIRAMATE 15 MG SPRINKLE CAP G TEVA USA EAGEX 16252-0568-60 0.53330 TOPIRAMATE 15 MG SPRINKLE CAP G ACTAVIS PHARMA, EAGEX 68382-0004-14 0.53330 TOPIRAMATE 15 MG SPRINKLE CAP G ZYDUS PHARMACEU EAGEX 00093-7220-<strong>06</strong> 0.11970 TOPIRAMATE 200 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-7220-10 0.11970 TOPIRAMATE 200 MG TABLET G TEVA USA EAGEX 00378-6105-05 0.11970 TOPIRAMATE 200 MG TABLET G MYLAN EAGEX 00378-6105-91 0.11970 TOPIRAMATE 200 MG TABLET G MYLAN EAGEX 13668-0034-05 0.11970 TOPIRAMATE 200 MG TABLET G TORRENT PHARMAC EAGEX 13668-0034-60 0.11970 TOPIRAMATE 200 MG TABLET G TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 393LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 31722-0281-05 0.11970 TOPIRAMATE 200 MG TABLET G CAMBER PHARMACE EAGEX 31722-0281-10 0.11970 TOPIRAMATE 200 MG TABLET G CAMBER PHARMACE EAGEX 31722-0281-60 0.11970 TOPIRAMATE 200 MG TABLET G CAMBER PHARMACE EAGEX 60505-2763-05 0.11970 TOPIRAMATE 200 MG TABLET G APOTEX CORP EAGEX 60505-2763-<strong>06</strong> 0.11970 TOPIRAMATE 200 MG TABLET G APOTEX CORP EAGEX 62756-0712-13 0.11970 TOPIRAMATE 200 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0712-86 0.11970 TOPIRAMATE 200 MG TABLET G SUN PHARMACEUTI EAGEX 65862-0174-60 0.11970 TOPIRAMATE 200 MG TABLET G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0345-11 0.11970 TOPIRAMATE 200 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0345-21 0.11970 TOPIRAMATE 200 MG TABLET G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68382-0141-05 0.11970 TOPIRAMATE 200 MG TABLET G ZYDUS PHARMACEU EAGEX 68382-0141-14 0.11970 TOPIRAMATE 200 MG TABLET G ZYDUS PHARMACEU EAGEX 68462-0110-10 0.11970 TOPIRAMATE 200 MG TABLET G GLENMARK PHARMA EAGEX 68462-0110-60 0.11970 TOPIRAMATE 200 MG TABLET G GLENMARK PHARMA EAGEX 00093-7336-<strong>06</strong> 0.61590 TOPIRAMATE 25 MG SPRINKLE CAP G TEVA USA EAGEX 00781-2276-60 0.61590 TOPIRAMATE 25 MG SPRINKLE CAP G SANDOZ EAGEX 16252-0569-60 0.61590 TOPIRAMATE 25 MG SPRINKLE CAP G ACTAVIS PHARMA, EAGEX 68382-0005-14 0.61590 TOPIRAMATE 25 MG SPRINKLE CAP G ZYDUS PHARMACEU EAGEX 00093-0155-<strong>06</strong> 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G TEVA USA EAGEX 00093-0155-10 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-6101-05 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G MYLAN EAGEX 00378-6101-91 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G MYLAN EAGEX 13668-0031-05 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G TORRENT PHARMAC EAGEX 13668-0031-60 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G TORRENT PHARMAC EAGEX 31722-0278-05 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G CAMBER PHARMACE EAGEX 31722-0278-10 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G CAMBER PHARMACE EAGEX 31722-0278-60 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G CAMBER PHARMACE EAGEX 51079-0726-01 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0726-20 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G MYLAN INSTITUTI EAGEX 60505-2760-05 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-2760-<strong>06</strong> 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G APOTEX CORP EAGEX 62756-0707-13 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0707-86 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G SUN PHARMACEUTI EAGEX 65862-0171-60 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0342-01 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0342-11 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G AHP EAGEX 68382-0138-05 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G ZYDUS PHARMACEU EAGEX 68382-0138-14 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G ZYDUS PHARMACEU EAGEX 68462-01<strong>08</strong>-10 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G GLENMARK PHARMA EAGEX 68462-01<strong>08</strong>-60 0.<strong>08</strong>330 TOPIRAMATE 25 MG TABLET G GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-7540-<strong>06</strong> 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G TEVA USA EAGEX 00093-7540-10 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G TEVA USA EAGEX 00378-6102-05 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G MYLAN EAGEX 00378-6102-91 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G MYLAN EAGEX 13668-0032-05 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 394LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0032-60 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G TORRENT PHARMAC EAGEX 31722-0279-05 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G CAMBER PHARMACE EAGEX 31722-0279-10 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G CAMBER PHARMACE EAGEX 31722-0279-60 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G CAMBER PHARMACE EAGEX 51079-0727-01 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0727-20 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G MYLAN INSTITUTI EAGEX 60505-2761-05 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G APOTEX CORP EAGEX 60505-2761-<strong>06</strong> 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G APOTEX CORP EAGEX 62756-0710-13 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G SUN PHARMACEUTI EAGEX 62756-0710-86 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G SUN PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0172-60 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0343-11 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0343-21 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G AHP EAGEX 68382-0139-05 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G ZYDUS PHARMACEU EAGEX 68382-0139-14 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G ZYDUS PHARMACEU EAGEX 68462-0153-10 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G GLENMARK PHARMA EAGEX 68462-0153-60 0.103<strong>08</strong> TOPIRAMATE 50 MG TABLET G GLENMARK PHARMA EAGEN 00703-5657-01 1.36770 TOPOSAR 1,000 MG/50 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-5653-01 1.68600 TOPOSAR 100 MG/5 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-5656-01 1.36770 TOPOSAR 500 MG/25 ML VIAL 0 TEVA PARENTERAL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00186-1092-05 1.78948 1.78948 TOPROL XL 100 MG TABLET G ASTRAZENECA EABND 00186-1094-05 2.847<strong>06</strong> 2.847<strong>06</strong> TOPROL XL 200 MG TABLET 0 ASTRAZENECA EABND 00186-1<strong>08</strong>8-05 1.19071 1.19071 TOPROL XL 25 MG TABLET G ASTRAZENECA EABND 00186-1<strong>08</strong>8-39 1.19071 1.19071 TOPROL XL 25 MG TABLET G ASTRAZENECA EABND 00186-1090-05 1.19071 1.19071 TOPROL XL 50 MG TABLET G ASTRAZENECA EAGEN 0<strong>06</strong>03-6135-21 0.10351 TORSEMIDE 10 MG TABLET 0 QUALITEST EAGEN 31722-0530-01 0.10351 TORSEMIDE 10 MG TABLET 0 CAMBER PHARMACE EAGEN 50111-0916-01 0.10351 TORSEMIDE 10 MG TABLET 0 TEVA USA EAGEN 59762-1701-01 0.10351 TORSEMIDE 10 MG TABLET 0 GREENSTONE LLC. EAGEN 65862-0126-01 0.10351 TORSEMIDE 10 MG TABLET 0 AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-6137-21 0.48030 TORSEMIDE 100 MG TABLET 0 QUALITEST EAGEN 31722-0532-01 0.48030 TORSEMIDE 100 MG TABLET 0 CAMBER PHARMACE EAGEN 50111-0918-01 0.48030 TORSEMIDE 100 MG TABLET 0 TEVA USA EAGEN 59762-1703-01 0.48030 TORSEMIDE 100 MG TABLET 0 GREENSTONE LLC. EAGEN 60505-0235-01 0.48030 TORSEMIDE 100 MG TABLET 0 APOTEX CORP EAGEN 65862-0128-01 0.48030 TORSEMIDE 100 MG TABLET 0 AUROBINDO PHARM EAGEN 00054-0077-25 0.12730 TORSEMIDE 20 MG TABLET 0 ROXANE LABS. EAGEN 00054-0077-29 0.12730 TORSEMIDE 20 MG TABLET 0 ROXANE LABS. EAGEN 0<strong>06</strong>03-6136-21 0.12730 TORSEMIDE 20 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-6136-28 0.12730 TORSEMIDE 20 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0531-01 0.12730 TORSEMIDE 20 MG TABLET 0 CAMBER PHARMACE EAGEN 50111-0917-01 0.12730 TORSEMIDE 20 MG TABLET 0 TEVA USA EAGEN 50111-0917-03 0.12730 TORSEMIDE 20 MG TABLET 0 PLIVA, INC EAGEN 59762-1702-01 0.12730 TORSEMIDE 20 MG TABLET 0 GREENSTONE LLC. EAGEN 65862-0127-01 0.12730 TORSEMIDE 20 MG TABLET 0 AUROBINDO PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 395LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0539-01 0.12730 TORSEMIDE 20 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0539-11 0.12730 TORSEMIDE 20 MG TABLET 0 AHP EABND 00517-0770-10 3.78895 TORSEMIDE 20 MG/2 ML VIAL 0 AMER. REGENT MLGEN 0<strong>06</strong>03-6134-21 0.12240 TORSEMIDE 5 MG TABLET 0 QUALITEST EAGEN 31722-0529-01 0.12240 TORSEMIDE 5 MG TABLET 0 CAMBER PHARMACE EAGEN 50111-0915-01 0.12240 TORSEMIDE 5 MG TABLET 0 PLIVA, INC EAGEN 59762-1700-01 0.12240 TORSEMIDE 5 MG TABLET 0 GREENSTONE LLC. EAGEN 65862-0125-01 0.12240 TORSEMIDE 5 MG TABLET 0 AUROBINDO PHARM EABND 00517-0771-10 2.138<strong>08</strong> TORSEMIDE 50 MG/5 ML VIAL 0 AMER. REGENT MLBND 00<strong>06</strong>9-0242-30 6.03216 TOVIAZ ER 4 MG TABLET G PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>9-0244-30 6.03216 TOVIAZ ER 8 MG TABLET 0 PFIZER US PHARM EABND 00517-9310-25 0.42330 TRACE ELEMENTS-4 VIAL 0 AMER. REGENT MLBND 66215-0102-<strong>06</strong> 117.03000 TRACLEER 125 MG TABLET 0 ACTELION PHARMA EABND 66215-0101-<strong>06</strong> 117.03000 TRACLEER 62.5 MG TABLET 0 ACTELION PHARMA EABND 00597-0140-30 9.42382 TRADJENTA 5 MG TABLET G BOEHRINGER ING. EABND 00597-0140-61 9.42307 TRADJENTA 5 MG TABLET G BOEHRINGER ING. EABND 00597-0140-90 9.423<strong>08</strong> TRADJENTA 5 MG TABLET G BOEHRINGER ING. EAGEN 10370-0221-11 1.9<strong>08</strong>20 TRAMADOL ER 100 MG TABLET G PAR PHARM. EAGEN 47335-0531-83 1.9<strong>08</strong>20 TRAMADOL ER 100 MG TABLET G SUN PHARMA GLOB EAGEN 47335-0531-88 1.9<strong>08</strong>20 TRAMADOL ER 100 MG TABLET G SUN PHARMA GLOB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10370-0222-11 2.58290 TRAMADOL ER 200 MG TABLET G PAR PHARM. EAGEN 47335-0533-83 2.58290 TRAMADOL ER 200 MG TABLET G SUN PHARMA GLOB EAGEN 47335-0533-88 2.58290 TRAMADOL ER 200 MG TABLET G SUN PHARMA GLOB EAGEN 10370-0223-11 5.56690 TRAMADOL ER 300 MG TABLET G PAR PHARM. EAGEN 47335-0537-83 5.56690 TRAMADOL ER 300 MG TABLET G SUN PHARMA GLOB EAGEN 47335-0537-88 5.56690 TRAMADOL ER 300 MG TABLET G SUN PHARMA GLOB EAGEN 10147-0901-03 2.64130 TRAMADOL HCL ER 100 MG TABLET G PATRIOT PHARMAC EAGEN 47335-<strong>08</strong>59-83 2.64130 TRAMADOL HCL ER 100 MG TABLET G SUN PHARMA GLOB EAGEN 49884-<strong>08</strong>21-11 2.64130 TRAMADOL HCL ER 100 MG TABLET G PAR PHARM. EAGEN 68180-0383-<strong>06</strong> 2.64130 TRAMADOL HCL ER 100 MG TABLET G LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 10147-0902-03 4.36840 TRAMADOL HCL ER 200 MG TABLET G PATRIOT PHARMAC EAGEN 47335-<strong>08</strong>60-83 4.36840 TRAMADOL HCL ER 200 MG TABLET G SUN PHARMA GLOB EAGEN 49884-<strong>08</strong>22-11 4.36840 TRAMADOL HCL ER 200 MG TABLET G PAR PHARM. EAGEN 68180-0384-<strong>06</strong> 4.36840 TRAMADOL HCL ER 200 MG TABLET G LUPIN PHARMACEU EAGEN 10147-0903-03 5.09040 TRAMADOL HCL ER 300 MG TABLET G PATRIOT PHARMAC EAGEN 47335-<strong>08</strong>61-83 5.09040 TRAMADOL HCL ER 300 MG TABLET G SUN PHARMA GLOB EAGEN 49884-<strong>08</strong>23-11 5.09040 TRAMADOL HCL ER 300 MG TABLET G PAR PHARM. EAGEN 68180-0385-<strong>06</strong> 5.09040 TRAMADOL HCL ER 300 MG TABLET G LUPIN PHARMACEU EAGEN 00093-0058-01 0.02150 TRAMADOL HCL 50 MG TABLET G TEVA USA EAGEN 00093-0058-05 0.02150 TRAMADOL HCL 50 MG TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-4151-01 0.02150 TRAMADOL HCL 50 MG TABLET G MYLAN EAGEN 00378-4151-05 0.02150 TRAMADOL HCL 50 MG TABLET G MYLAN EAGEN 00904-6365-61 0.02150 TRAMADOL HCL 50 MG TABLET G MAJOR PHARMACEU EAGEN 16714-0111-04 0.02150 TRAMADOL HCL 50 MG TABLET G NORTHSTAR RX LL EAGEN 16714-0111-05 0.02150 TRAMADOL HCL 50 MG TABLET G NORTHSTAR RX LL EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 396LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16714-0111-<strong>06</strong> 0.02150 TRAMADOL HCL 50 MG TABLET G NORTHSTAR RX LL EAGEN 16714-0111-10 0.02150 TRAMADOL HCL 50 MG TABLET G NORTHSTAR RX LL EAGEN 16714-0111-11 0.02150 TRAMADOL HCL 50 MG TABLET G NORTHSTAR RX LL EAGEN 16714-0111-12 0.02150 TRAMADOL HCL 50 MG TABLET G NORTHSTAR RX LL EAGEN 51079-0991-30 0.02150 TRAMADOL HCL 50 MG TABLET G MYLAN INSTITUTI EAGEN 51079-0991-56 0.02150 TRAMADOL HCL 50 MG TABLET G MYLAN INSTITUTI EAGEN 57664-0377-<strong>08</strong> 0.02150 TRAMADOL HCL 50 MG TABLET G CARACO PHARM EAGEN 57664-0377-13 0.02150 TRAMADOL HCL 50 MG TABLET G CARACO PHARM EAGEN 57664-0377-18 0.02150 TRAMADOL HCL 50 MG TABLET G CARACO PHARM EAGEN 60505-0171-01 0.02150 TRAMADOL HCL 50 MG TABLET G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0171-02 0.02150 TRAMADOL HCL 50 MG TABLET G APOTEX CORP EAGEN 60505-0171-<strong>08</strong> 0.02150 TRAMADOL HCL 50 MG TABLET G APOTEX CORP EAGEN 62584-0559-01 0.02150 TRAMADOL HCL 50 MG TABLET G AHP EAGEN 62584-0559-11 0.02150 TRAMADOL HCL 50 MG TABLET G AHP EAGEN 63739-<strong>06</strong>71-10 0.02150 TRAMADOL HCL 50 MG TABLET G MCKESSON PACKAG EAGEN 65162-<strong>06</strong>27-10 0.02150 TRAMADOL HCL 50 MG TABLET G AMNEAL PHARMACE EAGEN 65162-<strong>06</strong>27-11 0.02150 TRAMADOL HCL 50 MG TABLET G AMNEAL PHARMACE EAGEN 65162-<strong>06</strong>27-50 0.02150 TRAMADOL HCL 50 MG TABLET G AMNEAL PHARMACE EAGEN 68382-0319-01 0.02150 TRAMADOL HCL 50 MG TABLET G ZYDUS PHARMACEU EAGEN 68382-0319-10 0.02150 TRAMADOL HCL 50 MG TABLET G ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00172-6359-10 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G IVAX PHARMACEUT EAGEN 00172-6359-60 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G IVAX PHARMACEUT EAGEN 00172-6359-70 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G IVAX PHARMACEUT EAGEN 00378-8<strong>08</strong>8-01 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G MYLAN EAGEN 00378-8<strong>08</strong>8-05 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G MYLAN EAGEN 42571-0119-01 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G MICRO LABS USA, EAGEN 42571-0119-05 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G MICRO LABS USA, EAGEN 49884-0946-01 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G PAR PHARM. EAGEN 49884-0946-05 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G PAR PHARM. EAGEN 57664-0537-13 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G CARACO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 57664-0537-18 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G CARACO PHARM EAGEN 57664-0537-88 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G CARACO PHARM EAGEN 65162-<strong>06</strong>17-10 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G AMNEAL PHARMACE EAGEN 65162-<strong>06</strong>17-11 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G AMNEAL PHARMACE EAGEN 65162-<strong>06</strong>17-50 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-0496-01 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G AHP EAGEN 68382-0334-01 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G ZYDUS PHARMACEU EAGEN 68382-0334-05 0.18280 TRAMADOL-ACETAMINOPHN 37.5-325 G ZYDUS PHARMACEU EABUL 65483-0391-10 0.21570 0.62059 TRANDATE 100 MG TABLET G PROMETHEUS EABUL 65483-0392-10 0.35820 1.04696 TRANDATE 200 MG TABLET G PROMETHEUS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 65483-0393-10 0.53630 1.<strong>06</strong>057 TRANDATE 300 MG TABLET G PROMETHEUS EAGEN 00093-7325-01 0.33669 TRANDOLAPRIL 1 MG TABLET 0 TEVA USA EAGEN 16252-0541-30 0.33669 TRANDOLAPRIL 1 MG TABLET 0 ACTAVIS PHARMA, EAGEN 65862-0164-01 0.33669 TRANDOLAPRIL 1 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0566-01 0.33669 TRANDOLAPRIL 1 MG TABLET 0 LUPIN PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 397LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-7326-01 0.33669 TRANDOLAPRIL 2 MG TABLET 0 TEVA USA EAGEN 16252-0542-90 0.33669 TRANDOLAPRIL 2 MG TABLET 0 ACTAVIS PHARMA, EAGEN 65862-0165-01 0.33669 TRANDOLAPRIL 2 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0567-01 0.33669 TRANDOLAPRIL 2 MG TABLET 0 LUPIN PHARMACEU EAGEN 00093-7327-01 0.33588 TRANDOLAPRIL 4 MG TABLET 0 TEVA USA EAGEN 16252-0543-90 0.33588 TRANDOLAPRIL 4 MG TABLET 0 ACTAVIS PHARMA, EAGEN 65862-0166-01 0.33588 TRANDOLAPRIL 4 MG TABLET 0 AUROBINDO PHARM EAGEN 68180-0568-01 0.33588 TRANDOLAPRIL 4 MG TABLET 0 LUPIN PHARMACEU EAGEN 00591-3720-30 3.91500 TRANEXAMIC ACID 650 MG TABLET 0 ACTAVIS PHARMA, EAGEN 66993-0121-30 3.91074 TRANEXAMIC ACID 650 MG TABLET 0 PRASCO LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>7-4345-04 15.16202 TRANSDERM-SCOP 1.5 MG/72HR 0 NOVARTIS CONSUM EABND 10019-0553-01 15.88620 TRANSDERM-SCOP 1.5 MG/72HR 0 BAXTER <strong>HEALTH</strong>CA EABND 10019-0553-02 15.88620 TRANSDERM-SCOP 1.5 MG/72HR 0 BAXTER <strong>HEALTH</strong>CA EABND 10019-0553-88 15.88620 TRANSDERM-SCOP 1.5 MG/72HR 0 BAXTER <strong>HEALTH</strong>CA EAGEX 49884-0032-01 1.64240 TRANYLCYPROMINE SULF 10 MG TAB 0 PAR PHARM. EAGEX 64980-0183-01 1.64240 TRANYLCYPROMINE SULF 10 MG TAB 0 RISING PHARM EABND 00<strong>06</strong>5-0260-05 40.68660 TRAVATAN Z 0.004% EYE DROP G ALCON LABS. MLBND 00<strong>06</strong>5-0260-25 40.69656 TRAVATAN Z 0.004% EYE DROP G ALCON LABS. MLBND 49884-0044-48 34.54460 TRAVOPROST 0.004% EYE DROP G PAR PHARM. MLBND 49884-0044-63 34.54460 TRAVOPROST 0.004% EYE DROP G PAR PHARM. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-6161-02 0.04740 TRAZODONE 100 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6161-04 0.04740 TRAZODONE 100 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6161-16 0.04740 TRAZODONE 100 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6161-20 0.04740 TRAZODONE 100 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6161-21 0.04740 TRAZODONE 100 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6161-28 0.04740 TRAZODONE 100 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6161-32 0.04740 TRAZODONE 100 MG TABLET 0 QUALITEST EAGEX 13668-0331-01 0.04740 TRAZODONE 100 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0331-05 0.04740 TRAZODONE 100 MG TABLET 0 TORRENT PHARMAC EAGEX 50111-0434-01 0.04740 TRAZODONE 100 MG TABLET 0 TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 50111-0434-02 0.04740 TRAZODONE 100 MG TABLET 0 PLIVA, INC EAGEX 50111-0434-03 0.04740 TRAZODONE 100 MG TABLET 0 PLIVA, INC EAGEX 53489-0511-01 0.04740 TRAZODONE 100 MG TABLET 0 MUTUAL PHARM CO EAGEX 60505-2654-00 0.04740 TRAZODONE 100 MG TABLET 0 APOTEX CORP EAGEX 60505-2654-01 0.04740 TRAZODONE 100 MG TABLET 0 APOTEX CORP EAGEX 60505-2654-05 0.04740 TRAZODONE 100 MG TABLET 0 APOTEX CORP EAGEX 60505-2654-07 0.04740 TRAZODONE 100 MG TABLET 0 APOTEX CORP EAGEX 68<strong>08</strong>4-0125-01 0.04740 TRAZODONE 100 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0125-11 0.04740 TRAZODONE 100 MG TABLET 0 AHP EAGEX 68645-0452-70 0.04740 TRAZODONE 100 MG TABLET 0 LEGACY PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0332-01 0.1<strong>06</strong>65 TRAZODONE 150 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0332-05 0.1<strong>06</strong>65 TRAZODONE 150 MG TABLET 0 TORRENT PHARMAC EAGEX 50111-0441-01 0.1<strong>06</strong>65 TRAZODONE 150 MG TABLET 0 TEVA USA EAGEX 50111-0441-02 0.1<strong>06</strong>65 TRAZODONE 150 MG TABLET 0 PLIVA, INC EAGEX 53489-0517-01 0.1<strong>06</strong>65 TRAZODONE 150 MG TABLET 0 MUTUAL PHARM CO EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 398LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-2655-01 0.1<strong>06</strong>65 TRAZODONE 150 MG TABLET 0 APOTEX CORP EAGEX 60505-2655-05 0.1<strong>06</strong>65 TRAZODONE 150 MG TABLET 0 APOTEX CORP EAGEX 60505-2655-07 0.1<strong>06</strong>65 TRAZODONE 150 MG TABLET 0 APOTEX CORP EAGEX 68<strong>08</strong>4-<strong>06</strong><strong>08</strong>-01 0.1<strong>06</strong>65 TRAZODONE 150 MG TABLET 0 AHP EAGEX 00555-0733-02 3.19200 TRAZODONE 300 MG TABLET 0 BARR EAGEX 13668-0333-01 3.19200 TRAZODONE 300 MG TABLET 0 TORRENT PHARMAC EAGEX 60505-2659-01 3.19200 TRAZODONE 300 MG TABLET 0 APOTEX CORP EAGEX 0<strong>06</strong>03-6160-02 0.02460 TRAZODONE 50 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6160-13 0.02460 TRAZODONE 50 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6160-16 0.02460 TRAZODONE 50 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-6160-20 0.02460 TRAZODONE 50 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6160-21 0.02460 TRAZODONE 50 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6160-28 0.02460 TRAZODONE 50 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6160-32 0.02460 TRAZODONE 50 MG TABLET 0 QUALITEST EAGEX 13668-0330-01 0.02460 TRAZODONE 50 MG TABLET 0 TORRENT PHARMAC EAGEX 13668-0330-05 0.02460 TRAZODONE 50 MG TABLET 0 TORRENT PHARMAC EAGEX 50111-0433-01 0.02460 TRAZODONE 50 MG TABLET 0 TEVA USA EAGEX 50111-0433-02 0.02460 TRAZODONE 50 MG TABLET 0 PLIVA, INC EAGEX 50111-0433-03 0.02460 TRAZODONE 50 MG TABLET 0 PLIVA, INC EAGEX 53489-0510-01 0.02460 TRAZODONE 50 MG TABLET 0 MUTUAL PHARM CO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-2653-01 0.02460 TRAZODONE 50 MG TABLET 0 APOTEX CORP EAGEX 60505-2653-05 0.02460 TRAZODONE 50 MG TABLET 0 APOTEX CORP EAGEX 60505-2653-07 0.02460 TRAZODONE 50 MG TABLET 0 APOTEX CORP EAGEX 68<strong>08</strong>4-0124-01 0.02460 TRAZODONE 50 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0124-11 0.02460 TRAZODONE 50 MG TABLET 0 AHP EAGEX 68645-0451-70 0.02460 TRAZODONE 50 MG TABLET 0 LEGACY PHARMACE EABEX 000<strong>08</strong>-4117-01 3.50334 TRECATOR 250 MG TABLET 0 WYETH PHARM EAGEN 00555-<strong>08</strong><strong>08</strong>-02 20.81330 TRETINOIN 10 MG CAPSULE 0 BARR EAGEN 10370-0268-01 20.81330 TRETINOIN 10 MG CAPSULE 0 PAR PHARM. EAGEN 68<strong>08</strong>4-0075-11 20.81330 TRETINOIN 10 MG CAPSULE 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0075-21 20.81330 TRETINOIN 10 MG CAPSULE 0 AHP EABND 51285-0368-01 21.76370 TREXALL 10 MG TABLET 0 DURAMED/BARR EABND 51285-0369-01 32.64611 TREXALL 15 MG TABLET 0 DURAMED/BARR EABND 51285-0366-01 10.88157 TREXALL 5 MG TABLET 0 DURAMED/BARR EABND 51285-0367-01 16.32278 TREXALL 7.5 MG TABLET 0 DURAMED/BARR EABND 00173-0750-00 23.74261 TREXIMET 85-500 MG TABLET G GLAXOSMITHKLINE EABND 00173-0750-49 27.69<strong>06</strong>4 TREXIMET 85-500 MG TABLET G GLAXOSMITHKLINE EAGEX 00555-9032-70 1.514<strong>08</strong> TRI-LEGEST FE-28 DAY TABLET 0 BARR EAGEX 16714-0363-01 0.46850 TRI-LINYAH TABLET 0 NORTHSTAR RX LL EAGEX 16714-0363-04 0.46850 TRI-LINYAH TABLET 0 NORTHSTAR RX LL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 52544-0274-28 0.99160 2.41031 TRI-NORINYL 28 TABLET G ACTAVIS PHARMA, EAGEX 0<strong>06</strong>03-7663-17 0.46850 TRI-PREVIFEM TABLET 0 QUALITEST EAGEX 00555-9018-58 0.46850 TRI-SPRINTEC TABLET 0 BARR EAGEN 0<strong>06</strong>03-1785-47 0.14840 TRI-VIT-FLUOR 0.25 MG/ML DROP 0 QUALITEST MLGEN 44946-1035-<strong>08</strong> 0.29610 TRI-VIT-FLUOR 0.25 MG/ML DROP 0 SANCILIO & COMP ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 399LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-1786-47 0.22650 TRI-VIT-FLUOR 0.5 MG/ML DROP 0 QUALITEST MLGEN 44946-1036-<strong>08</strong> 0.29610 TRI-VIT-FLUOR 0.5 MG/ML DROP 0 SANCILIO & COMP MLBND 0<strong>06</strong>03-1787-47 0.21950 0.32751 TRI-VIT-FLUOR-IRON 0.25 MG/ML 0 QUALITEST MLGEN 13811-0529-90 0.12490 TRIADVANCE TABLET 0 TRIGEN LABORATO EAGUL 00168-0003-15 0.03750 TRIAMCINOLONE 0.025% CREAM 0 SANDOZ GMGUL 00168-0003-80 0.03750 TRIAMCINOLONE 0.025% CREAM 0 SANDOZ GMGUL 0<strong>06</strong>03-7861-74 0.03750 TRIAMCINOLONE 0.025% CREAM 0 QUALITEST GMGUL 0<strong>06</strong>03-7861-90 0.03750 TRIAMCINOLONE 0.025% CREAM 0 QUALITEST GMGUL 45802-0<strong>06</strong>3-35 0.03750 TRIAMCINOLONE 0.025% CREAM 0 PERRIGO CO. GMGUL 45802-0<strong>06</strong>3-36 0.03750 TRIAMCINOLONE 0.025% CREAM 0 PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00168-0336-60 0.41820 TRIAMCINOLONE 0.025% LOTION 0 SANDOZ MLGEN 60432-0560-60 0.41820 TRIAMCINOLONE 0.025% LOTION 0 MORTON GROVE PH MLGEN 61748-0219-60 0.41820 TRIAMCINOLONE 0.025% LOTION 0 VERSA PHARMACEU MLGEN 00168-0005-80 0.10453 TRIAMCINOLONE 0.025% OINT 0 SANDOZ GMGEN 45802-0054-35 0.3<strong>06</strong>00 TRIAMCINOLONE 0.025% OINT 0 PERRIGO CO. GMGEN 45802-0054-36 0.09478 TRIAMCINOLONE 0.025% OINT 0 PERRIGO CO. GMGUL 00168-0004-15 0.04690 TRIAMCINOLONE 0.1% CREAM 0 SANDOZ GMGUL 00168-0004-16 0.04690 TRIAMCINOLONE 0.1% CREAM 0 SANDOZ GMGUL 00168-0004-80 0.04690 TRIAMCINOLONE 0.1% CREAM 0 SANDOZ GMGUL 0<strong>06</strong>03-7862-74 0.04690 TRIAMCINOLONE 0.1% CREAM 0 QUALITEST GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 0<strong>06</strong>03-7862-90 0.04690 TRIAMCINOLONE 0.1% CREAM 0 QUALITEST GMGUL 45802-0<strong>06</strong>4-05 0.04690 TRIAMCINOLONE 0.1% CREAM 0 PERRIGO CO. GMGUL 45802-0<strong>06</strong>4-35 0.04690 TRIAMCINOLONE 0.1% CREAM 0 PERRIGO CO. GMGUL 45802-0<strong>06</strong>4-36 0.04690 TRIAMCINOLONE 0.1% CREAM 0 PERRIGO CO. GMGUL 51672-1282-01 0.04690 TRIAMCINOLONE 0.1% CREAM 0 TARO PHARM USA GMGUL 51672-1282-02 0.04690 TRIAMCINOLONE 0.1% CREAM 0 TARO PHARM USA GMGUL 51672-1282-<strong>08</strong> 0.04690 TRIAMCINOLONE 0.1% CREAM 0 TARO PHARM USA GMGUL 67877-0251-15 0.04690 TRIAMCINOLONE 0.1% CREAM 0 ASCEND LABORATO GMGUL 67877-0251-30 0.04690 TRIAMCINOLONE 0.1% CREAM 0 ASCEND LABORATO GMGUL 67877-0251-45 0.04690 TRIAMCINOLONE 0.1% CREAM 0 ASCEND LABORATO GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 67877-0251-80 0.04690 TRIAMCINOLONE 0.1% CREAM 0 ASCEND LABORATO GMGEN 00168-0337-60 1.01074 TRIAMCINOLONE 0.1% LOTION 0 SANDOZ MLGEN 0<strong>06</strong>03-7864-49 1.01074 TRIAMCINOLONE 0.1% LOTION 0 QUALITEST MLGEN 60432-0561-60 0.53025 TRIAMCINOLONE 0.1% LOTION 0 MORTON GROVE PH MLGEN 61748-0220-60 0.84999 TRIAMCINOLONE 0.1% LOTION 0 VERSA PHARMACEU MLGUL 00168-00<strong>06</strong>-15 0.05020 TRIAMCINOLONE 0.1% OINTMENT 0 SANDOZ GMGEN 00168-00<strong>06</strong>-16 0.04960 TRIAMCINOLONE 0.1% OINTMENT 0 SANDOZ GMGUL 00168-00<strong>06</strong>-80 0.05020 TRIAMCINOLONE 0.1% OINTMENT 0 SANDOZ GMGUL 45802-0055-05 0.05020 TRIAMCINOLONE 0.1% OINTMENT 0 PERRIGO CO. GMGUL 45802-0055-35 0.05020 TRIAMCINOLONE 0.1% OINTMENT 0 PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 45802-0055-36 0.05020 TRIAMCINOLONE 0.1% OINTMENT 0 PERRIGO CO. GMGEN 51672-1267-05 9.00100 TRIAMCINOLONE 0.1% PASTE 0 TARO PHARM USA GMGEN 64980-0320-05 9.00100 TRIAMCINOLONE 0.1% PASTE 0 RISING PHARM GMGUL 00168-0002-15 0.23700 TRIAMCINOLONE 0.5% CREAM 0 SANDOZ GMGUL 45802-0<strong>06</strong>5-35 0.23700 TRIAMCINOLONE 0.5% CREAM 0 PERRIGO CO. GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 400LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 45802-0049-35 0.55886 TRIAMCINOLONE 0.5% OINTMENT 0 PERRIGO CO. GMGEN 00093-2<strong>08</strong>5-17 5.28410 TRIAMCINOLONE 55 MCG NASAL SPR G TEVA USA GMGEN 00955-1710-16 5.28410 TRIAMCINOLONE 55 MCG NASAL SPR G WINTHROP US GMGEN 00378-2537-01 0.31027 TRIAMTERENE-HCTZ 37.5-25 MG CP 0 MYLAN EAGEN 00378-2537-10 0.30098 TRIAMTERENE-HCTZ 37.5-25 MG CP 0 MYLAN EAGEN 00527-1632-01 0.31027 TRIAMTERENE-HCTZ 37.5-25 MG CP 0 LANNETT CO. INC EAGEN 00527-1632-10 0.30098 TRIAMTERENE-HCTZ 37.5-25 MG CP 0 LANNETT CO. INC EAGEN 00781-2074-01 0.28147 TRIAMTERENE-HCTZ 37.5-25 MG CP 0 SANDOZ EAGEN 00781-2074-10 0.27051 TRIAMTERENE-HCTZ 37.5-25 MG CP 0 SANDOZ EAGUL 00378-1352-01 0.16830 TRIAMTERENE-HCTZ 37.5-25 MG TB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00378-1352-05 0.16830 TRIAMTERENE-HCTZ 37.5-25 MG TB 0 MYLAN EAGUL 00591-0424-01 0.16830 TRIAMTERENE-HCTZ 37.5-25 MG TB 0 ACTAVIS PHARMA, EAGUL 00591-0424-05 0.16830 TRIAMTERENE-HCTZ 37.5-25 MG TB 0 ACTAVIS PHARMA, EAGUL 00781-1123-01 0.16830 TRIAMTERENE-HCTZ 37.5-25 MG TB 0 SANDOZ EAGUL 00781-1123-05 0.16830 TRIAMTERENE-HCTZ 37.5-25 MG TB 0 SANDOZ EAGUL 60505-2656-01 0.16830 TRIAMTERENE-HCTZ 37.5-25 MG TB 0 APOTEX CORP EAGUL 60505-2656-05 0.16830 TRIAMTERENE-HCTZ 37.5-25 MG TB 0 APOTEX CORP EABND 00781-2715-01 1.72374 TRIAMTERENE-HCTZ 50-25 MG CAP 0 SANDOZ EAGUL 00378-1355-01 0.04880 TRIAMTERENE-HCTZ 75-50 MG TAB 0 MYLAN EAGUL 00378-1355-05 0.04880 TRIAMTERENE-HCTZ 75-50 MG TAB 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00591-0348-01 0.04880 TRIAMTERENE-HCTZ 75-50 MG TAB 0 ACTAVIS PHARMA, EAGUL 00591-0348-05 0.04880 TRIAMTERENE-HCTZ 75-50 MG TAB 0 ACTAVIS PHARMA, EAGUL 00591-0348-10 0.04880 TRIAMTERENE-HCTZ 75-50 MG TAB 0 ACTAVIS PHARMA, EAGUL 00781-10<strong>08</strong>-01 0.04880 TRIAMTERENE-HCTZ 75-50 MG TAB 0 SANDOZ EAGUL 00781-10<strong>08</strong>-05 0.04880 TRIAMTERENE-HCTZ 75-50 MG TAB 0 SANDOZ EAGUL 51079-0433-20 0.04880 TRIAMTERENE-HCTZ 75-50 MG TAB 0 MYLAN INSTITUTI EAGUL 60505-2657-01 0.04880 TRIAMTERENE-HCTZ 75-50 MG TAB 0 APOTEX CORP EAGUL 60505-2657-05 0.04880 TRIAMTERENE-HCTZ 75-50 MG TAB 0 APOTEX CORP EABND 00245-0136-17 4.73344 TRIANEX 0.05% OINTMENT G UPSHER SMITH GMBND 65597-0114-30 4.42224 TRIBENZOR 20-5-12.5 MG TABLET G DAIICHI SANKYO, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 65597-0114-90 4.42224 TRIBENZOR 20-5-12.5 MG TABLET G DAIICHI SANKYO, EABND 65597-0117-30 5.59752 TRIBENZOR 40-10-12.5 MG TABLET G DAIICHI SANKYO, EABND 65597-0117-90 5.59752 TRIBENZOR 40-10-12.5 MG TABLET G DAIICHI SANKYO, EABND 65597-0118-30 5.59752 TRIBENZOR 40-10-25 MG TABLET G DAIICHI SANKYO, EABND 65597-0118-90 5.59752 TRIBENZOR 40-10-25 MG TABLET G DAIICHI SANKYO, EABND 65597-0115-30 5.59752 TRIBENZOR 40-5-12.5 MG TABLET G DAIICHI SANKYO, EABND 65597-0115-90 5.59752 TRIBENZOR 40-5-12.5 MG TABLET G DAIICHI SANKYO, EABND 65597-0116-30 5.59752 TRIBENZOR 40-5-25 MG TABLET G DAIICHI SANKYO, EABND 65597-0116-90 5.59752 TRIBENZOR 40-5-25 MG TABLET G DAIICHI SANKYO, EABND 67112-0401-90 1.74189 TRICARE PRENATAL DHA ONE SFTGL 0 MEDECOR PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 67112-0101-00 1.00322 TRICARE PRENATAL TABLET 0 MEDECOR PHARMA EAGEN 00121-<strong>06</strong>77-16 0.01940 TRICITRATES ORAL SOLUTION 0 PHARMACEU ASSOC MLBND 00074-6123-90 6.38094 6.38094 TRICOR 145 MG TABLET 0 ABBVIE US LLC EABND 00074-6122-90 2.12692 2.12692 TRICOR 48 MG TABLET 0 ABBVIE US LLC EAGEX 00378-2401-01 0.59986 TRIFLUOPERAZINE 1 MG TABLET 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 401LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-1030-01 0.59986 TRIFLUOPERAZINE 1 MG TABLET 0 SANDOZ EAGEX 00378-2410-01 1.67825 TRIFLUOPERAZINE 10 MG TABLET 0 MYLAN EAGEX 00781-1036-01 1.67825 TRIFLUOPERAZINE 10 MG TABLET 0 SANDOZ EAGEX 00378-2402-01 0.88396 TRIFLUOPERAZINE 2 MG TABLET 0 MYLAN EAGEX 00781-1032-01 0.88396 TRIFLUOPERAZINE 2 MG TABLET 0 SANDOZ EAGEX 00378-2405-01 1.11338 TRIFLUOPERAZINE 5 MG TABLET 0 MYLAN EAGEX 00781-1034-01 1.11338 TRIFLUOPERAZINE 5 MG TABLET 0 SANDOZ EAGEX 51079-0574-20 1.11338 TRIFLUOPERAZINE 5 MG TABLET 0 MYLAN INSTITUTI EAGEN 59762-0040-01 17.65600 TRIFLURIDINE 1% EYE DROPS 0 GREENSTONE LLC. MLGEN 61314-0044-75 17.65600 TRIFLURIDINE 1% EYE DROPS 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59630-0485-30 6.63170 TRIGLIDE 160 MG TABLET G SHIONOGI PHARMA EAGEX 00143-1764-01 0.05778 TRIHEXYPHENIDYL 2 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-1764-10 0.05778 TRIHEXYPHENIDYL 2 MG TABLET 0 WEST-WARD,INC. EAGEX 00591-5335-01 0.05778 TRIHEXYPHENIDYL 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-5335-10 0.05778 TRIHEXYPHENIDYL 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 0<strong>06</strong>03-6240-21 0.05778 TRIHEXYPHENIDYL 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6240-32 0.05778 TRIHEXYPHENIDYL 2 MG TABLET 0 QUALITEST EAGEX 16571-0160-10 0.05778 TRIHEXYPHENIDYL 2 MG TABLET 0 PACK PHARMACEUT EAGEX 16571-0160-11 0.05778 TRIHEXYPHENIDYL 2 MG TABLET 0 PACK PHARMACEUT EAGEX 62584-<strong>08</strong>86-11 0.05778 TRIHEXYPHENIDYL 2 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00121-<strong>06</strong>58-16 0.04617 TRIHEXYPHENIDYL 2 MG/5 ML ELX 0 PHARMACEU ASSOC MLGEX 61748-0054-16 0.04617 TRIHEXYPHENIDYL 2 MG/5 ML ELX 0 VERSA PHARMACEU MLGEX 00143-1763-01 0.16599 TRIHEXYPHENIDYL 5 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-1763-10 0.16599 TRIHEXYPHENIDYL 5 MG TABLET 0 WEST-WARD,INC. EAGEX 00591-5337-01 0.16599 TRIHEXYPHENIDYL 5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-5337-10 0.16599 TRIHEXYPHENIDYL 5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 0<strong>06</strong>03-6241-21 0.16599 TRIHEXYPHENIDYL 5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-6241-32 0.16599 TRIHEXYPHENIDYL 5 MG TABLET 0 QUALITEST EAGEX 16571-0161-10 0.16599 TRIHEXYPHENIDYL 5 MG TABLET 0 PACK PHARMACEUT EAGEX 16571-0161-11 0.16599 TRIHEXYPHENIDYL 5 MG TABLET 0 PACK PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62584-<strong>08</strong>87-11 0.16599 TRIHEXYPHENIDYL 5 MG TABLET 0 AHP EABEX 00078-0456-05 0.13840 2.72613 TRILEPTAL 150 MG TABLET G NOVARTIS EABEX 00078-0337-05 0.27660 4.97900 TRILEPTAL 300 MG TABLET G NOVARTIS EABEX 00078-0357-52 1.0<strong>08</strong>21 TRILEPTAL 300 MG/5 ML SUSP 0 NOVARTIS MLBEX 00078-0457-05 0.55320 9.15116 TRILEPTAL 600 MG TABLET G NOVARTIS EABND 00074-9189-90 6.<strong>08</strong>297 6.<strong>08</strong>297 TRILIPIX DR 135 MG CAPSULE 0 ABBVIE US LLC EABND 00074-9642-90 2.02759 2.02759 TRILIPIX DR 45 MG CAPSULE 0 ABBVIE US LLC EAGEN 51525-6831-04 0.00360 TRILYTE WITH FLAVOR PACKETS G WALLACE PHARMAC MLGEN 68220-0131-04 0.00360 TRILYTE WITH FLAVOR PACKETS G WALLACE PHARMAC MLGUL 43386-<strong>06</strong>60-24 1.01930 TRIMETHOBENZAMIDE 300 MG CAP 0 GAVIS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 43386-<strong>06</strong>60-26 1.01930 TRIMETHOBENZAMIDE 300 MG CAP 0 GAVIS PHARMACEU EAGUL 53489-0376-01 1.01930 TRIMETHOBENZAMIDE 300 MG CAP 0 MUTUAL PHARM CO EAGEN 00093-2158-01 0.31230 TRIMETHOPRIM 100 MG TABLET 0 TEVA USA EAGEN 00591-5571-01 0.31230 TRIMETHOPRIM 100 MG TABLET 0 ACTAVIS PHARMA, EAGEN 43386-0330-01 0.31230 TRIMETHOPRIM 100 MG TABLET 0 GAVIS PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 402LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 45963-0295-30 3.64580 TRIMIPRAMINE MALEATE 100 MG CP 0 ACTAVIS PHARMA, EAGEX 45963-0293-30 1.53300 TRIMIPRAMINE MALEATE 25 MG CAP 0 ACTAVIS PHARMA, EAGEX 45963-0294-30 2.50780 TRIMIPRAMINE MALEATE 50 MG CAP 0 ACTAVIS PHARMA, EAGEN 13811-<strong>06</strong>14-90 0.27125 TRINATAL GT TABLET 0 TRIGEN LABORATO EAGEN 13811-0007-10 0.19510 TRINATAL RX 1 TABLET 0 TRIGEN LABORATO EAGEN 13811-<strong>06</strong>15-10 0.07900 TRINATAL ULTRA TABLET 0 TRIGEN LABORATO EABEX 52544-0248-28 0.46850 1.16556 TRINESSA TABLET 0 ACTAVIS PHARMA, EAGEN 64376-<strong>08</strong>23-50 0.14840 TRIPLE-VIT W-FLUOR 0.25 MG/ML 0 BOCA PHARMACAL MLGEN 13811-0558-30 1.17380 TRIVEEN-PRX RNF CAPSULE 0 TRIGEN LABORATO EAGEX 52544-0291-28 0.73260 TRIVORA-28 TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 49702-0217-18 26.72102 TRIZIVIR TABLET G VIIV <strong>HEALTH</strong>CARE EABEX 17772-0103-15 13.81120 TROKENDI XR 100 MG CAPSULE G SUPERNUS PHARMA EABEX 17772-0104-15 18.89<strong>08</strong>0 TROKENDI XR 200 MG CAPSULE G SUPERNUS PHARMA EABEX 17772-0101-15 5.34520 TROKENDI XR 25 MG CAPSULE G SUPERNUS PHARMA EABEX 17772-0102-15 6.97200 TROKENDI XR 50 MG CAPSULE G SUPERNUS PHARMA EAGUL 242<strong>08</strong>-0590-64 0.65500 TROPICAMIDE 0.5% EYE DROPS 0 VALEANT MLGUL 61314-0354-01 0.65500 TROPICAMIDE 0.5% EYE DROPS 0 SANDOZ MLGUL 242<strong>08</strong>-0585-59 0.70000 TROPICAMIDE 1% EYE DROPS 0 VALEANT MLGUL 242<strong>08</strong>-0585-64 0.70000 TROPICAMIDE 1% EYE DROPS 0 VALEANT MLGUL 61314-0355-01 0.70000 TROPICAMIDE 1% EYE DROPS 0 SANDOZ ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 61314-0355-02 0.70000 TROPICAMIDE 1% EYE DROPS 0 SANDOZ MLGEN 00574-0118-30 5.05250 TROSPIUM CHLORIDE ER 60 MG CAP G PADDOCK LABS. EAGEN 00591-3636-30 5.05250 TROSPIUM CHLORIDE ER 60 MG CAP G ACTAVIS PHARMA, EAGEN 00574-0145-60 2.12825 TROSPIUM CHLORIDE 20 MG TABLET G PADDOCK LABS. EAGEN 60505-3454-<strong>06</strong> 2.19740 TROSPIUM CHLORIDE 20 MG TABLET G APOTEX CORP EAGEN 68462-0461-60 2.12825 TROSPIUM CHLORIDE 20 MG TABLET G GLENMARK PHARMA EABND 000<strong>06</strong>-3519-36 2.27450 7.63932 TRUSOPT 2% EYE DROPS 0 MERCK SHARP & D MLBND 61958-0701-01 42.60390 TRUVADA 200 MG-300 MG TABLET G GILEAD SCIENCES EABND 00456-<strong>08</strong>00-31 117.52800 TUDORZA PRESSAIR 400 MCG INH G FOREST PHARMACE EABND 00456-<strong>08</strong>00-60 235.04770 TUDORZA PRESSAIR 400 MCG INH G FOREST PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00597-0125-37 5.25915 TWYNSTA 40-10 MG TABLET G BOEHRINGER ING. EABND 00597-0124-37 5.25915 TWYNSTA 40-5 MG TABLET G BOEHRINGER ING. EABND 00597-0127-37 5.25915 TWYNSTA 80-10 MG TABLET G BOEHRINGER ING. EABND 00597-0126-37 5.25915 TWYNSTA 80-5 MG TABLET G BOEHRINGER ING. EABND 000<strong>08</strong>-4990-01 61.00500 TYGACIL 50 MG VIAL 0 WYETH PHARM EABND 000<strong>08</strong>-4990-02 61.00002 TYGACIL 50 MG VIAL 0 WYETH PHARM EABND 000<strong>08</strong>-4990-19 95.21760 TYGACIL 50 MG VIAL 0 WYETH PHARM EABND 000<strong>08</strong>-4990-20 95.21594 TYGACIL 50 MG VIAL 0 WYETH PHARM EABND 00173-0752-00 33.03538 TYKERB 250 MG TABLET 0 GLAXOSMITHKLINE EABND 66302-02<strong>06</strong>-02 159.67891 TYVASO INHALATION REFILL KIT 0 UNITED THERAP ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 66302-02<strong>06</strong>-01 178.07792 TYVASO INHALATION STARTER KIT 0 UNITED THERAP MLBND 66302-02<strong>06</strong>-03 159.70344 TYVASO 1.74 MG/2.9 ML SOLUTION 0 UNITED THERAP MLBND 00078-0538-15 32.35561 TYZEKA 600 MG TABLET 0 NOVARTIS EABND 59630-0780-<strong>08</strong> 0.23<strong>08</strong>6 ULESFIA 5% LOTION 0 ZYLERA GMBND 59630-0780-88 0.49360 ULESFIA 5% LOTION 0 ZYLERA GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 403LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64764-0918-30 7.11005 ULORIC 40 MG TABLET G TAKEDA PHARMACE EABND 64764-<strong>06</strong>77-30 7.11005 ULORIC 80 MG TABLET G TAKEDA PHARMACE EAGEN 13811-0049-30 0.87380 ULTIMATECARE ONE CAPSULE 0 TRIGEN LABORATO EABND 13811-0050-30 1.35622 ULTIMATECARE ONE NF CAPSULE 0 TRIGEN LABORATO EABND 50458-<strong>06</strong>50-60 0.18280 2.<strong>06</strong>877 ULTRACET TABLET G JANSSEN PHARM. EABND 50458-<strong>06</strong>53-30 2.64130 5.64400 ULTRAM ER 100 MG TABLET G JANSSEN PHARM. EABND 50458-<strong>06</strong>55-30 4.36840 9.33418 ULTRAM ER 200 MG TABLET G JANSSEN PHARM. EABND 50458-<strong>06</strong>57-30 5.09040 13.02325 ULTRAM ER 300 MG TABLET G JANSSEN PHARM. EABND 50458-<strong>06</strong>59-60 0.02150 2.23436 ULTRAM 50 MG TABLET G JANSSEN PHARM. EABUL 1<strong>06</strong>31-0103-50 0.48000 7.17551 ULTRAVATE 0.05% CREAM G RANBAXY LABORAT GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 1<strong>06</strong>31-0102-50 0.53250 7.17551 ULTRAVATE 0.05% OINTMENT G RANBAXY LABORAT GMBND 58914-0003-10 2.49913 ULTRESA DR 13,800 UNIT CAPSULE G APTALIS PHARMA EABND 58914-0019-10 3.69798 ULTRESA DR 20,700 UNIT CAPSULE G APTALIS PHARMA EABND 58914-0005-10 4.54117 ULTRESA DR 23,000 UNIT CAPSULE G APTALIS PHARMA EABND 00049-0013-81 2.88850 UNASYN 1.5 GM VIAL G PFIZER US PHARM EABND 00049-0013-83 2.88850 7.56794 UNASYN 1.5 GM VIAL G PFIZER US PHARM EABND 00049-0024-28 32.39420 71.44640 UNASYN 15 GM VIAL G PFIZER US PHARM EABND 00049-0014-83 3.36825 14.28845 UNASYN 3 GM VIAL G PFIZER US PHARM EABND 00091-3720-01 0.64787 2.49141 UNIRETIC 15-12.5 TABLET G UCB PHARMA EABND 00091-3725-01 0.64787 2.49141 UNIRETIC 15-25 MG TABLET G UCB PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00091-3712-01 0.64787 2.49141 UNIRETIC 7.5-12.5 MG TABLET G UCB PHARMA EABUL 00527-1374-01 0.29850 0.56315 UNITHROID 100 MCG TABLET 0 LANNETT CO. INC EABUL 00527-1375-01 0.34430 0.65213 UNITHROID 112 MCG TABLET 0 LANNETT CO. INC EABUL 00527-1376-01 0.34950 0.66126 UNITHROID 125 MCG TABLET 0 LANNETT CO. INC EABUL 00527-1377-01 0.36000 0.68134 UNITHROID 150 MCG TABLET 0 LANNETT CO. INC EABUL 00527-1378-01 0.42750 0.81223 UNITHROID 175 MCG TABLET 0 LANNETT CO. INC EABND 00527-1379-01 0.43551 0.81389 UNITHROID 200 MCG TABLET 0 LANNETT CO. INC EABUL 00527-1370-01 0.23180 0.43550 UNITHROID 25 MCG TABLET 0 LANNETT CO. INC EABUL 00527-1371-01 0.26330 0.49650 UNITHROID 50 MCG TABLET 0 LANNETT CO. INC EABUL 00527-1372-01 0.29100 0.54937 UNITHROID 75 MCG TABLET 0 LANNETT CO. INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00527-1373-01 0.29550 0.55925 UNITHROID 88 MCG TABLET 0 LANNETT CO. INC EABND 00091-3715-01 0.67163 2.71459 UNIVASC 15 MG TABLET G UCB PHARMA EABND 00091-3707-01 0.70362 2.59126 UNIVASC 7.5 MG TABLET G UCB PHARMA EAGEN 51285-<strong>06</strong>90-02 0.20360 URECHOLINE 10 MG TABLET 0 DURAMED/BARR EAGEN 51285-<strong>06</strong>91-02 0.17890 URECHOLINE 25 MG TABLET 0 DURAMED/BARR EAGEN 51285-<strong>06</strong>97-02 0.10746 URECHOLINE 5 MG TABLET 0 DURAMED/BARR EAGEN 51285-<strong>06</strong>92-02 0.43899 URECHOLINE 50 MG TABLET 0 DURAMED/BARR EABND 66663-0219-01 4.50339 URELLE TABLET 0 MEDA PHARMACEUT EABND 00178-<strong>06</strong>15-01 2.48311 UROCIT-K ER 15 MEQ TABLET 0 MISSION PHARM. EABND 00178-<strong>06</strong>10-01 0.89849 1.53135 UROCIT-K SR 10 MEQ TABLET G MISSION PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00178-<strong>06</strong>00-01 0.60590 1.09261 UROCIT-K SR 5 MEQ TABLET G MISSION PHARM. EABND 00486-1114-01 0.50422 UROQID-ACID NO.2 500-500 TB 0 BEACH PRODUCTS EABND 00024-4200-10 0.25370 12.27072 UROXATRAL 10 MG TABLET G COVIS PHARMACEU EABND 58914-0790-10 3.72660 5.21821 URSO FORTE 500 MG TABLET 0 APTALIS PHARMA EABND 58914-0785-10 1.71670 2.94467 URSO 250 MG TABLET G APTALIS PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 404LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-2368-01 1.71670 URSODIOL 250 MG TABLET 0 ACTAVIS PHARMA, EAGEN 49884-0412-01 1.71670 URSODIOL 250 MG TABLET 0 PAR PHARM. EAGEN 66993-0405-02 1.71670 URSODIOL 250 MG TABLET 0 PRASCO LABS EAGEN 68462-0473-01 1.71670 URSODIOL 250 MG TABLET 0 GLENMARK PHARMA EAGEN 00527-1326-01 0.37380 URSODIOL 300 MG CAPSULE 0 LANNETT CO. INC EAGEN 00591-3159-01 0.37380 URSODIOL 300 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00904-6221-60 0.37380 URSODIOL 300 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 428<strong>06</strong>-0503-01 0.37380 URSODIOL 300 MG CAPSULE 0 EPIC PHARMA LLC EAGEN 68<strong>08</strong>4-0213-01 0.37380 URSODIOL 300 MG CAPSULE 0 AHP EAGEN 68<strong>08</strong>4-0213-11 0.37380 URSODIOL 300 MG CAPSULE 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-2369-01 3.56505 URSODIOL 500 MG TABLET 0 ACTAVIS PHARMA, EAGEN 49884-0413-01 3.56505 URSODIOL 500 MG TABLET 0 PAR PHARM. EAGEN 66993-04<strong>06</strong>-02 3.56505 URSODIOL 500 MG TABLET 0 PRASCO LABS EAGEN 68462-0474-01 3.56505 URSODIOL 500 MG TABLET 0 GLENMARK PHARMA EABND 00169-5176-03 10.69662 VAGIFEM 10 MCG VAGINAL TAB 0 NOVO NORDISK EABND 00169-5176-04 10.69685 VAGIFEM 10 MCG VAGINAL TAB 0 NOVO NORDISK EAGEN 00054-0115-13 9.49200 VALACYCLOVIR HCL 1 GRAM TABLET G ROXANE LABS. EAGEN 00054-0115-27 9.49200 VALACYCLOVIR HCL 1 GRAM TABLET G ROXANE LABS. EAGEN 00093-7259-56 9.49200 VALACYCLOVIR HCL 1 GRAM TABLET G TEVA USA EAGEN 00093-7259-98 9.47724 VALACYCLOVIR HCL 1 GRAM TABLET G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-4276-05 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G MYLAN EAGEN 00378-4276-77 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G MYLAN EAGEN 00378-4276-93 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G MYLAN EAGEN 00591-3249-30 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G ACTAVIS PHARMA, EAGEN 00781-5209-31 9.49200 VALACYCLOVIR HCL 1 GRAM TABLET G SANDOZ EAGEN 00781-5209-92 9.49224 VALACYCLOVIR HCL 1 GRAM TABLET G SANDOZ EAGEN 16714-<strong>06</strong>97-01 9.47100 VALACYCLOVIR HCL 1 GRAM TABLET G NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>97-02 9.47940 VALACYCLOVIR HCL 1 GRAM TABLET G NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>97-03 9.47941 VALACYCLOVIR HCL 1 GRAM TABLET G NORTHSTAR RX LL EAGEN 45963-0559-<strong>08</strong> 9.48166 VALACYCLOVIR HCL 1 GRAM TABLET G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45963-0559-30 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G ACTAVIS PHARMA, EAGEN 51660-0905-30 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G OHM LABS. EAGEN 52343-0052-30 0.33174 VALACYCLOVIR HCL 1 GRAM TABLET G GEN-SOURCE RX EAGEN 52343-0052-90 0.331<strong>08</strong> VALACYCLOVIR HCL 1 GRAM TABLET G GEN-SOURCE RX EAGEN 55111-0553-05 9.28770 VALACYCLOVIR HCL 1 GRAM TABLET G DR.REDDY'S LAB EAGEN 55111-0553-30 9.49200 VALACYCLOVIR HCL 1 GRAM TABLET G DR.REDDY'S LAB EAGEN 55111-0553-90 9.47724 VALACYCLOVIR HCL 1 GRAM TABLET G DR.REDDY'S LAB EAGEN 59746-0325-30 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G CADISTA PHARMAC EAGEN 59746-0325-37 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G CADISTA PHARMAC EAGEN 59762-8398-01 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59762-8398-<strong>06</strong> 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G GREENSTONE LLC. EAGEN 63304-0905-30 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G RANBAXY PHARMAC EAGEN 63739-0552-10 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G MCKESSON PACKAG EAGEN 64679-0153-02 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G WOCKHARDT USA L EAGEN 64679-0153-03 9.48166 VALACYCLOVIR HCL 1 GRAM TABLET G WOCKHARDT USA L EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 405LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64679-0153-04 9.48166 VALACYCLOVIR HCL 1 GRAM TABLET G WOCKHARDT USA L EAGEN 65862-0449-30 9.28125 VALACYCLOVIR HCL 1 GRAM TABLET G AUROBINDO PHARM EAGEN 65862-0449-90 9.28125 VALACYCLOVIR HCL 1 GRAM TABLET G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0409-11 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G AHP EAGEN 68<strong>08</strong>4-0409-21 9.48150 VALACYCLOVIR HCL 1 GRAM TABLET G AHP EAGEN 00054-0114-13 5.42400 VALACYCLOVIR HCL 500 MG TABLET G ROXANE LABS. EAGEN 00054-0114-27 5.42400 VALACYCLOVIR HCL 500 MG TABLET G ROXANE LABS. EAGEN 00093-7258-56 5.42400 VALACYCLOVIR HCL 500 MG TABLET G TEVA USA EAGEN 00093-7258-98 5.42358 VALACYCLOVIR HCL 500 MG TABLET G TEVA USA EAGEN 00378-4275-05 5.41800 VALACYCLOVIR HCL 500 MG TABLET G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-4275-77 5.41800 VALACYCLOVIR HCL 500 MG TABLET G MYLAN EAGEN 00378-4275-93 5.41800 VALACYCLOVIR HCL 500 MG TABLET G MYLAN EAGEN 00591-3248-19 5.25549 VALACYCLOVIR HCL 500 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-3248-30 5.41800 VALACYCLOVIR HCL 500 MG TABLET G ACTAVIS PHARMA, EAGEN 00781-52<strong>08</strong>-31 5.42400 VALACYCLOVIR HCL 500 MG TABLET G SANDOZ EAGEN 00781-52<strong>08</strong>-92 5.42358 VALACYCLOVIR HCL 500 MG TABLET G SANDOZ EAGEN 16714-<strong>06</strong>98-01 5.42100 VALACYCLOVIR HCL 500 MG TABLET G NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>98-02 5.42<strong>08</strong>5 VALACYCLOVIR HCL 500 MG TABLET G NORTHSTAR RX LL EAGEN 16714-<strong>06</strong>98-03 5.42<strong>08</strong>3 VALACYCLOVIR HCL 500 MG TABLET G NORTHSTAR RX LL EAGEN 45963-0558-<strong>08</strong> 5.41749 VALACYCLOVIR HCL 500 MG TABLET G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45963-0558-30 5.41800 VALACYCLOVIR HCL 500 MG TABLET G ACTAVIS PHARMA, EAGEN 51079-0093-01 5.41800 VALACYCLOVIR HCL 500 MG TABLET G MYLAN INSTITUTI EAGEN 51079-0093-03 5.41800 VALACYCLOVIR HCL 500 MG TABLET G MYLAN INSTITUTI EAGEN 51660-0904-30 5.41800 VALACYCLOVIR HCL 500 MG TABLET G OHM LABS. EAGEN 52343-0051-30 0.17100 VALACYCLOVIR HCL 500 MG TABLET G GEN-SOURCE RX EAGEN 52343-0051-90 0.17049 VALACYCLOVIR HCL 500 MG TABLET G GEN-SOURCE RX EAGEN 55111-0552-05 5.31511 VALACYCLOVIR HCL 500 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0552-30 5.42400 VALACYCLOVIR HCL 500 MG TABLET G DR.REDDY'S LAB EAGEN 55111-0552-90 5.42358 VALACYCLOVIR HCL 500 MG TABLET G DR.REDDY'S LAB EAGEN 59746-0324-30 5.41800 VALACYCLOVIR HCL 500 MG TABLET G CADISTA PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 59746-0324-37 5.41800 VALACYCLOVIR HCL 500 MG TABLET G CADISTA PHARMAC EAGEN 59762-8399-01 5.41800 VALACYCLOVIR HCL 500 MG TABLET G GREENSTONE LLC. EAGEN 59762-8399-<strong>06</strong> 5.41800 VALACYCLOVIR HCL 500 MG TABLET G GREENSTONE LLC. EAGEN 63304-0904-30 5.41800 VALACYCLOVIR HCL 500 MG TABLET G RANBAXY PHARMAC EAGEN 63739-0525-10 5.41807 VALACYCLOVIR HCL 500 MG TABLET G MCKESSON PACKAG EAGEN 64679-0152-01 5.41800 VALACYCLOVIR HCL 500 MG TABLET G WOCKHARDT USA L EAGEN 64679-0152-03 5.41800 VALACYCLOVIR HCL 500 MG TABLET G WOCKHARDT USA L EAGEN 64679-0152-04 5.41750 VALACYCLOVIR HCL 500 MG TABLET G WOCKHARDT USA L EAGEN 65862-0448-30 5.30349 VALACYCLOVIR HCL 500 MG TABLET G AUROBINDO PHARM EAGEN 65862-0448-90 5.30349 VALACYCLOVIR HCL 500 MG TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-04<strong>08</strong>-01 5.41807 VALACYCLOVIR HCL 500 MG TABLET G AHP EAGEN 68<strong>08</strong>4-04<strong>08</strong>-11 5.41807 VALACYCLOVIR HCL 500 MG TABLET G AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>33-01 2.97997 VALACYCLOVIR HCL 500 MG TABLET G AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>33-11 2.97997 VALACYCLOVIR HCL 500 MG TABLET G AHP EABND 66215-0016-60 48.14000 VALCHLOR 0.016% GEL 0 ACTELION PHARMA GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 4<strong>06</strong>LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00004-0038-22 64.14142 VALCYTE 450 MG TABLET 0 GENENTECH, INC. EABND 00004-0039-09 8.82356 VALCYTE 50 MG/ML SOLUTION 0 GENENTECH, INC. MLGEX 00143-9637-01 3.11160 VALPROATE SOD 500 MG/5 ML VL 0 WEST-WARD,INC./ MLGEX 00143-9637-10 3.11160 VALPROATE SOD 500 MG/5 ML VL 0 WEST-WARD,INC./ MLGEX 55390-0007-10 0.64800 VALPROATE SOD 500 MG/5 ML VL 0 BEDFORD LABS MLGEX 63323-0494-05 1.20510 VALPROATE SOD 500 MG/5 ML VL 0 APP PHARMACEUTI MLGEX 00591-4012-01 0.16830 VALPROIC ACID 250 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 0<strong>08</strong>32-10<strong>08</strong>-00 0.16830 VALPROIC ACID 250 MG CAPSULE 0 UPSHER SMITH EAGEX 50111-<strong>08</strong>52-01 0.16830 VALPROIC ACID 250 MG CAPSULE 0 PLIVA, INC EAGEX 51079-0298-<strong>08</strong> 0.16830 VALPROIC ACID 250 MG CAPSULE 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0298-56 0.16830 VALPROIC ACID 250 MG CAPSULE 0 MYLAN INSTITUTI EAGEX 62756-<strong>08</strong>73-88 0.16830 VALPROIC ACID 250 MG CAPSULE 0 SUN PHARMACEUTI EAGEX 00121-<strong>06</strong>75-16 0.01660 VALPROIC ACID 250 MG/5 ML SOLN 0 PHARMACEU ASSOC MLGUX 00121-4675-55 0.05940 VALPROIC ACID 250 MG/5 ML SOLN 0 PHARMACEU ASSOC MLGEX 00591-0426-16 0.01660 VALPROIC ACID 250 MG/5 ML SOLN 0 ACTAVIS PHARMA, MLGEX 0<strong>06</strong>03-1841-58 0.01660 VALPROIC ACID 250 MG/5 ML SOLN 0 QUALITEST MLGUX 50383-0792-<strong>06</strong> 0.05940 VALPROIC ACID 250 MG/5 ML SOLN 0 HI-TECH PHARMAC MLGUX 50383-0792-07 0.05940 VALPROIC ACID 250 MG/5 ML SOLN 0 HI-TECH PHARMAC MLGEX 50383-0792-16 0.01660 VALPROIC ACID 250 MG/5 ML SOLN 0 HI-TECH PHARMAC MLGEX 57664-0124-34 0.01660 VALPROIC ACID 250 MG/5 ML SOLN 0 CARACO PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60432-<strong>06</strong>21-16 0.01660 VALPROIC ACID 250 MG/5 ML SOLN 0 MORTON GROVE PH MLGUX 68094-0193-59 0.05940 VALPROIC ACID 250 MG/5 ML SOLN 0 PRECISION DOSE MLGUX 68094-0193-62 0.05940 VALPROIC ACID 250 MG/5 ML SOLN 0 PRECISION DOSE MLGUX 00121-4675-10 0.05940 VALPROIC ACID 500 MG/10 ML SOL 0 PHARMACEU ASSOC MLGUX 50383-0792-12 0.05940 VALPROIC ACID 500 MG/10 ML SOL 0 HI-TECH PHARMAC MLGUX 68094-0701-61 0.05940 VALPROIC ACID 500 MG/10 ML SOL 0 PRECISION DOSE MLGEN 00378-6322-77 3.20533 VALSARTAN-HCTZ 160-12.5 MG TAB G MYLAN EAGEN 00591-2316-10 3.20533 VALSARTAN-HCTZ 160-12.5 MG TAB G ACTAVIS PHARMA, EAGEN 00591-2316-19 3.20533 VALSARTAN-HCTZ 160-12.5 MG TAB G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-6346-02 3.20533 VALSARTAN-HCTZ 160-12.5 MG TAB G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-6346-28 3.20530 VALSARTAN-HCTZ 160-12.5 MG TAB G QUALITEST EAGEN 00781-5949-92 3.2<strong>08</strong>92 VALSARTAN-HCTZ 160-12.5 MG TAB G SANDOZ EAGEN 51079-0193-03 3.53574 VALSARTAN-HCTZ 160-12.5 MG TAB G MYLAN INSTITUTI EAGEN 60505-3807-09 3.2<strong>08</strong>92 VALSARTAN-HCTZ 160-12.5 MG TAB G APOTEX CORP EAGEN 65862-0548-90 3.20933 VALSARTAN-HCTZ 160-12.5 MG TAB G AUROBINDO PHARM EAGEN 65862-0548-99 3.2<strong>08</strong>90 VALSARTAN-HCTZ 160-12.5 MG TAB G AUROBINDO PHARM EAGEN 68180-0104-02 3.20530 VALSARTAN-HCTZ 160-12.5 MG TAB G LUPIN PHARMACEU EAGEN 68180-0104-09 3.20533 VALSARTAN-HCTZ 160-12.5 MG TAB G LUPIN PHARMACEU EAGEN 00378-6323-77 3.63500 VALSARTAN-HCTZ 160-25 MG TAB G MYLAN EAGEN 00591-2317-10 3.63500 VALSARTAN-HCTZ 160-25 MG TAB G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-2317-19 3.63500 VALSARTAN-HCTZ 160-25 MG TAB G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-6347-02 3.63500 VALSARTAN-HCTZ 160-25 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-6347-28 3.63502 VALSARTAN-HCTZ 160-25 MG TAB G QUALITEST EAGEN 00781-5950-92 3.639<strong>08</strong> VALSARTAN-HCTZ 160-25 MG TAB G SANDOZ EAGEN 51079-0194-03 4.00950 VALSARTAN-HCTZ 160-25 MG TAB G MYLAN INSTITUTI EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 407LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-38<strong>08</strong>-09 3.639<strong>08</strong> VALSARTAN-HCTZ 160-25 MG TAB G APOTEX CORP EAGEN 65862-0549-90 3.63950 VALSARTAN-HCTZ 160-25 MG TAB G AUROBINDO PHARM EAGEN 65862-0549-99 3.63918 VALSARTAN-HCTZ 160-25 MG TAB G AUROBINDO PHARM EAGEN 68180-0105-02 3.63502 VALSARTAN-HCTZ 160-25 MG TAB G LUPIN PHARMACEU EAGEN 68180-0105-09 3.63500 VALSARTAN-HCTZ 160-25 MG TAB G LUPIN PHARMACEU EAGEN 00378-6324-77 4.<strong>06</strong>1<strong>08</strong> VALSARTAN-HCTZ 320-12.5 MG TAB G MYLAN EAGEN 00591-2318-19 4.<strong>06</strong>1<strong>08</strong> VALSARTAN-HCTZ 320-12.5 MG TAB G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-6348-02 4.<strong>06</strong>1<strong>08</strong> VALSARTAN-HCTZ 320-12.5 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-6348-28 4.<strong>06</strong>1<strong>08</strong> VALSARTAN-HCTZ 320-12.5 MG TAB G QUALITEST EAGEN 00781-5951-92 4.<strong>06</strong>558 VALSARTAN-HCTZ 320-12.5 MG TAB G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-3809-09 4.<strong>06</strong>558 VALSARTAN-HCTZ 320-12.5 MG TAB G APOTEX CORP EAGEN 65862-0550-05 4.<strong>06</strong>1<strong>08</strong> VALSARTAN-HCTZ 320-12.5 MG TAB G AUROBINDO PHARM EAGEN 65862-0550-90 4.<strong>06</strong>599 VALSARTAN-HCTZ 320-12.5 MG TAB G AUROBINDO PHARM EAGEN 68180-0101-02 4.<strong>06</strong>1<strong>08</strong> VALSARTAN-HCTZ 320-12.5 MG TAB G LUPIN PHARMACEU EAGEN 68180-0101-09 4.<strong>06</strong>1<strong>08</strong> VALSARTAN-HCTZ 320-12.5 MG TAB G LUPIN PHARMACEU EAGEN 00378-6325-77 4.60725 VALSARTAN-HCTZ 320-25 MG TAB G MYLAN EAGEN 00591-2319-19 4.60725 VALSARTAN-HCTZ 320-25 MG TAB G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-6349-02 4.60725 VALSARTAN-HCTZ 320-25 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-6349-28 4.60722 VALSARTAN-HCTZ 320-25 MG TAB G QUALITEST EAGEN 00781-5952-92 4.61233 VALSARTAN-HCTZ 320-25 MG TAB G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-3810-09 4.61233 VALSARTAN-HCTZ 320-25 MG TAB G APOTEX CORP EAGEN 65862-0551-05 4.60722 VALSARTAN-HCTZ 320-25 MG TAB G AUROBINDO PHARM EAGEN 65862-0551-90 4.61274 VALSARTAN-HCTZ 320-25 MG TAB G AUROBINDO PHARM EAGEN 68180-0102-02 4.60722 VALSARTAN-HCTZ 320-25 MG TAB G LUPIN PHARMACEU EAGEN 68180-0102-09 4.60725 VALSARTAN-HCTZ 320-25 MG TAB G LUPIN PHARMACEU EAGEN 00378-6321-77 2.94600 VALSARTAN-HCTZ 80-12.5 MG TAB G MYLAN EAGEN 00591-2315-10 2.94600 VALSARTAN-HCTZ 80-12.5 MG TAB G ACTAVIS PHARMA, EAGEN 00591-2315-19 2.94600 VALSARTAN-HCTZ 80-12.5 MG TAB G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-6345-02 2.94600 VALSARTAN-HCTZ 80-12.5 MG TAB G QUALITEST EAGEN 0<strong>06</strong>03-6345-28 2.94603 VALSARTAN-HCTZ 80-12.5 MG TAB G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-5948-92 2.94924 VALSARTAN-HCTZ 80-12.5 MG TAB G SANDOZ EAGEN 51079-0192-03 3.24950 VALSARTAN-HCTZ 80-12.5 MG TAB G MYLAN INSTITUTI EAGEN 60505-38<strong>06</strong>-09 2.94924 VALSARTAN-HCTZ 80-12.5 MG TAB G APOTEX CORP EAGEN 65862-0547-90 2.94966 VALSARTAN-HCTZ 80-12.5 MG TAB G AUROBINDO PHARM EAGEN 65862-0547-99 2.94933 VALSARTAN-HCTZ 80-12.5 MG TAB G AUROBINDO PHARM EAGEN 68180-0103-02 2.94603 VALSARTAN-HCTZ 80-12.5 MG TAB G LUPIN PHARMACEU EAGEN 68180-0103-09 2.94600 VALSARTAN-HCTZ 80-12.5 MG TAB G LUPIN PHARMACEU EABND 00173-0565-04 11.67173 11.67173 VALTREX 1 GM CAPLET 0 GLAXOSMITHKLINE EABND 00173-0565-10 11.67192 11.67192 VALTREX 1 GM CAPLET 0 GLAXOSMITHKLINE EABND 00173-0933-<strong>08</strong> 6.66960 6.66960 VALTREX 500 MG CAPLET 0 GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-0933-10 6.66895 6.66895 VALTREX 500 MG CAPLET 0 GLAXOSMITHKLINE EABND 66593-3125-02 28.90309 28.90309 VANCOCIN HCL 125 MG PULVULE 0 VIROPHARMA INCO EABND 66593-3126-02 53.28600 53.28600 VANCOCIN HCL 250 MG PULVULE 0 VIROPHARMA INCO EABND 00338-3552-48 0.11473 VANCOMYCIN HCL 1G/200 ML BAG 0 BAXTER <strong>HEALTH</strong>CA MLGEN 00409-6510-01 35.91000 VANCOMYCIN HCL 10 GM VIAL 0 HOSPIRA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 4<strong>08</strong>LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00409-6510-49 35.91000 VANCOMYCIN HCL 10 GM VIAL 0 HOSPIRA/NOVA+ EAGEN 63323-0314-61 154.80000 VANCOMYCIN HCL 10 GM VIAL 0 APP PHARMACEUTI EAGEN 67457-0342-10 54.18000 VANCOMYCIN HCL 10 GM VIAL 0 MYLAN INSTITUTI EAGEN 00591-3560-15 23.50537 VANCOMYCIN HCL 125 MG CAPSULE G ACTAVIS PHARMA, EAGEN 17478-0741-02 23.49375 VANCOMYCIN HCL 125 MG CAPSULE G AKORN INC. EAGEN 47781-0729-02 23.47912 VANCOMYCIN HCL 125 MG CAPSULE G ALVOGEN INC EAGEN 63323-0338-20 26.10000 VANCOMYCIN HCL 125 MG CAPSULE G APP PHARMACEUTI EAGEN 63323-0338-22 9.00000 VANCOMYCIN HCL 125 MG CAPSULE G APP/NOVAPLUS EAGEN 66993-0210-19 23.47950 VANCOMYCIN HCL 125 MG CAPSULE G PRASCO LABS EAGEN 00591-3561-15 43.33500 VANCOMYCIN HCL 250 MG CAPSULE G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 17478-0742-02 43.32375 VANCOMYCIN HCL 250 MG CAPSULE G AKORN INC. EAGEN 47781-0730-02 43.28662 VANCOMYCIN HCL 250 MG CAPSULE G ALVOGEN INC EAGEN 63323-0339-20 48.10500 VANCOMYCIN HCL 250 MG CAPSULE G APP PHARMACEUTI EAGEN 63323-0339-22 16.20000 VANCOMYCIN HCL 250 MG CAPSULE G APP/NOVAPLUS EAGEN 66993-0211-19 43.28700 VANCOMYCIN HCL 250 MG CAPSULE G PRASCO LABS EAGEN 00<strong>06</strong>9-2590-01 19.64025 VANCOMYCIN HCL 5 GM VIAL 0 PFIZER US PHARM EAGEN 00409-6509-01 18.43500 VANCOMYCIN HCL 5 GM VIAL 0 HOSPIRA EAGEN 00409-6509-49 17.72250 VANCOMYCIN HCL 5 GM VIAL 0 HOSPIRA EAGEN 25021-0157-99 19.64025 VANCOMYCIN HCL 5 GM VIAL 0 SAGENT PHARMACE EAGEN 63323-0295-61 19.64025 VANCOMYCIN HCL 5 GM VIAL 0 APP PHARMACEUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 67457-0341-05 19.64025 VANCOMYCIN HCL 5 GM VIAL 0 MYLAN INSTITUTI EABND 00409-6535-01 5.21904 VANCOMYCIN 1 GM ADD-VAN VIAL 0 HOSPIRA EAGEN 00409-6533-01 3.54600 VANCOMYCIN 1 GM VIAL 0 HOSPIRA EAGEN 00409-6533-49 3.31200 VANCOMYCIN 1 GM VIAL 0 HOSPIRA/NOVA+ EAGEN 00409-6533-61 3.94000 VANCOMYCIN 1 GM VIAL 0 HOSPIRA EAGEN 00781-3188-80 3.94000 VANCOMYCIN 1 GM VIAL 0 SANDOZ EAGEN 63323-0284-20 3.94000 VANCOMYCIN 1 GM VIAL 0 APP PHARMACEUTI EAGEN 67457-0222-20 3.94000 VANCOMYCIN 1 GM VIAL 0 MYLAN INSTITUTI EAGEN 67457-0340-01 3.94000 VANCOMYCIN 1 GM VIAL 0 MYLAN INSTITUTI EABND 00409-6534-01 3.34656 VANCOMYCIN 500 MG A-V VIAL 0 HOSPIRA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-6534-49 2.719<strong>08</strong> VANCOMYCIN 500 MG A-V VIAL 0 HOSPIRA/NOVA+ EAGEN 00<strong>06</strong>9-2587-03 2.46960 VANCOMYCIN 500 MG VIAL 0 PFIZER US PHARM EAGEN 00<strong>06</strong>9-2587-10 2.46960 VANCOMYCIN 500 MG VIAL 0 PFIZER US PHARM EAGEN 00409-4332-01 2.26800 VANCOMYCIN 500 MG VIAL 0 HOSPIRA EAGEN 00409-4332-49 1.98900 VANCOMYCIN 500 MG VIAL 0 HOSPIRA/NOVA+ EAGEN 00781-3187-70 2.46960 VANCOMYCIN 500 MG VIAL 0 SANDOZ EAGEN 63323-0221-10 2.46960 VANCOMYCIN 500 MG VIAL 0 APP PHARMACEUTI EAGEN 67457-0225-10 2.46960 VANCOMYCIN 500 MG VIAL 0 MYLAN INSTITUTI EAGEN 67457-0339-50 2.46960 VANCOMYCIN 500 MG VIAL 0 MYLAN INSTITUTI EABND 00409-6531-01 3.48600 VANCOMYCIN 750 MG VIAL 0 HOSPIRA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00338-3580-48 0.<strong>08</strong>021 VANCOMYCIN 750 MG/150 ML BAG 0 BAXTER <strong>HEALTH</strong>CA MLBND 00338-3551-48 0.<strong>06</strong>573 VANCOMYCIN-D5W 500 MG/100 ML 0 BAXTER <strong>HEALTH</strong>CA MLBND 00245-<strong>08</strong>60-70 0.39970 VANDAZOLE VAGINAL 0.75% GEL 0 UPSHER SMITH GMBND 99207-0525-10 17.25044 VANOS 0.1% CREAM G VALEANT GMBND 99207-0525-30 21.07674 VANOS 0.1% CREAM G VALEANT GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 409LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 99207-0525-60 18.15818 VANOS 0.1% CREAM G VALEANT GMBND 52937-0001-20 1.61884 VASCEPA 1 GM CAPSULE G AMARIN PHARMA I EABND 00187-0146-01 0.11813 4.83516 VASERETIC 10-25 MG TABLET G VALEANT EABND 64455-0146-01 0.11813 3.11565 VASERETIC 10-25 MG TABLET G VALEANT EABUL 00187-0142-10 0.07320 4.95710 VASOTEC 10 MG TABLET G VALEANT EABUL 00187-0142-90 0.07320 4.95749 VASOTEC 10 MG TABLET G VALEANT EABUL 00187-0140-30 0.04730 3.886<strong>06</strong> VASOTEC 2.5 MG TABLET G VALEANT EABUL 00187-0140-90 0.04730 3.886<strong>06</strong> VASOTEC 2.5 MG TABLET G VALEANT EABND 00187-0143-30 0.04470 7.05361 VASOTEC 20 MG TABLET G VALEANT EABND 64455-0143-10 0.04470 4.54500 VASOTEC 20 MG TABLET G VALEANT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00187-0141-30 0.05700 4.5<strong>08</strong>00 VASOTEC 5 MG TABLET G VALEANT EABUL 64455-0141-10 0.05700 3.15103 VASOTEC 5 MG TABLET G VALEANT EABUL 64455-0141-90 0.05700 3.15104 VASOTEC 5 MG TABLET G VALEANT EABND 00299-2012-10 6.48147 VECTICAL 3 MCG/G OINTMENT G GALDERMA LABORA GMBND 66215-0403-01 22.46810 VELETRI 0.5 MG VIAL 0 ACTELION PHARMA EABND 66215-0401-01 37.76500 VELETRI 1.5 MG VIAL 0 ACTELION PHARMA EABND 66215-0402-01 44.93620 VELETRI 1.5 MG VIAL 0 ACTELION PHARMA EAGEX 00555-9051-67 0.84780 VELIVET 28 DAY TABLET 0 BARR EABND 49230-<strong>06</strong>45-51 9.46200 VELPHORO 500 MG CHEWABLE TAB G FRESENIUS MED EAGEN 13811-0026-10 0.1<strong>06</strong>90 VENATAL-FA TABLET 0 TRIGEN LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-7386-05 0.74240 VENLAFAXINE HCL ER 150 MG CAP G TEVA USA EAGEX 00093-7386-56 0.74240 VENLAFAXINE HCL ER 150 MG CAP G TEVA USA EAGEX 00093-7386-98 0.74240 VENLAFAXINE HCL ER 150 MG CAP G TEVA USA EAGEX 00904-6248-61 0.74240 VENLAFAXINE HCL ER 150 MG CAP G MAJOR PHARMACEU EAGEX 13668-0020-01 0.74240 VENLAFAXINE HCL ER 150 MG CAP G TORRENT PHARMAC EAGEX 13668-0020-05 0.74240 VENLAFAXINE HCL ER 150 MG CAP G TORRENT PHARMAC EAGEX 13668-0020-30 0.74240 VENLAFAXINE HCL ER 150 MG CAP G TORRENT PHARMAC EAGEX 13668-0020-90 0.74240 VENLAFAXINE HCL ER 150 MG CAP G TORRENT PHARMAC EAGEX 59762-0182-01 0.74240 VENLAFAXINE HCL ER 150 MG CAP G GREENSTONE LLC. EAGEX 59762-0182-02 0.74240 VENLAFAXINE HCL ER 150 MG CAP G GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-0182-04 0.74240 VENLAFAXINE HCL ER 150 MG CAP G GREENSTONE LLC. EAGEX 60505-3780-03 0.74240 VENLAFAXINE HCL ER 150 MG CAP G APOTEX CORP EAGEX 60505-3780-09 0.74240 VENLAFAXINE HCL ER 150 MG CAP G APOTEX CORP EAGEX 63739-0512-10 0.32347 VENLAFAXINE HCL ER 150 MG CAP G MCKESSON PACKAG EAGEX 64679-0718-01 0.74240 VENLAFAXINE HCL ER 150 MG CAP G WOCKHARDT USA L EAGEX 64679-0718-04 0.74240 VENLAFAXINE HCL ER 150 MG CAP G WOCKHARDT USA L EAGEX 64679-0718-05 0.74240 VENLAFAXINE HCL ER 150 MG CAP G WOCKHARDT USA L EAGEX 65862-0529-30 0.74240 VENLAFAXINE HCL ER 150 MG CAP G AUROBINDO PHARM EAGEX 65862-0529-90 0.74240 VENLAFAXINE HCL ER 150 MG CAP G AUROBINDO PHARM EAGEX 65862-<strong>06</strong>97-01 0.74240 VENLAFAXINE HCL ER 150 MG CAP G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-<strong>06</strong>97-05 0.74240 VENLAFAXINE HCL ER 150 MG CAP G AUROBINDO PHARM EAGEX 65862-<strong>06</strong>97-30 0.74240 VENLAFAXINE HCL ER 150 MG CAP G AUROBINDO PHARM EAGEX 65862-<strong>06</strong>97-90 0.74240 VENLAFAXINE HCL ER 150 MG CAP G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0486-01 0.74240 VENLAFAXINE HCL ER 150 MG CAP G AHP EAGEX 68<strong>08</strong>4-0486-11 0.74240 VENLAFAXINE HCL ER 150 MG CAP G AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 410LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68382-0036-<strong>06</strong> 0.74240 VENLAFAXINE HCL ER 150 MG CAP G ZYDUS PHARMACEU EAGEX 68382-0036-10 0.74240 VENLAFAXINE HCL ER 150 MG CAP G ZYDUS PHARMACEU EAGEX 68382-0036-16 0.74240 VENLAFAXINE HCL ER 150 MG CAP G ZYDUS PHARMACEU EAGEX 00131-3267-32 2.49160 VENLAFAXINE HCL ER 150 MG TAB G UCB PHARMA EAGEX 00131-3267-46 2.49160 VENLAFAXINE HCL ER 150 MG TAB G UCB PHARMA EAGEX 41616-0758-81 2.49160 VENLAFAXINE HCL ER 150 MG TAB G SUN PHARMA GLOB EAGEX 41616-0758-83 2.49160 VENLAFAXINE HCL ER 150 MG TAB G SUN PHARMA GLOB EAGEX 65580-0303-03 2.49160 VENLAFAXINE HCL ER 150 MG TAB G UCB PHARMA EAGEX 65580-0303-09 2.49160 VENLAFAXINE HCL ER 150 MG TAB G UCB PHARMA EABEX 00131-3268-32 6.51240 7.28020 VENLAFAXINE HCL ER 225 MG TAB G UCB PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00131-3268-46 6.51240 7.28011 VENLAFAXINE HCL ER 225 MG TAB G UCB PHARMA EABEX 65580-0304-03 6.51240 10.22560 VENLAFAXINE HCL ER 225 MG TAB G UCB PHARMA EABEX 65580-0304-09 6.51240 10.22560 VENLAFAXINE HCL ER 225 MG TAB G UCB PHARMA EAGEX 00093-7384-05 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G TEVA USA EAGEX 00093-7384-56 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G TEVA USA EAGEX 00093-7384-98 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G TEVA USA EAGEX 00904-6246-61 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G MAJOR PHARMACEU EAGEX 13668-0018-01 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G TORRENT PHARMAC EAGEX 13668-0018-05 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G TORRENT PHARMAC EAGEX 13668-0018-30 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0018-90 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G TORRENT PHARMAC EAGEX 59762-0180-01 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G GREENSTONE LLC. EAGEX 59762-0180-02 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G GREENSTONE LLC. EAGEX 59762-0180-04 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G GREENSTONE LLC. EAGEX 60505-3778-03 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G APOTEX CORP EAGEX 60505-3778-09 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G APOTEX CORP EAGEX 64679-0716-01 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G WOCKHARDT USA L EAGEX 64679-0716-04 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G WOCKHARDT USA L EAGEX 64679-0716-05 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G WOCKHARDT USA L EAGEX 65862-0527-01 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0527-30 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G AUROBINDO PHARM EAGEX 65862-0527-90 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G AUROBINDO PHARM EAGEX 65862-0527-99 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0484-01 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G AHP EAGEX 68<strong>08</strong>4-0484-11 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G AHP EAGEX 68<strong>08</strong>4-<strong>06</strong>98-01 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G AHP EAGEX 68<strong>08</strong>4-<strong>06</strong>98-11 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G AHP EAGEX 68382-0034-<strong>06</strong> 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G ZYDUS PHARMACEU EAGEX 68382-0034-10 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G ZYDUS PHARMACEU EAGEX 68382-0034-16 0.29270 VENLAFAXINE HCL ER 37.5 MG CAP G ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00131-3265-32 2.55849 VENLAFAXINE HCL ER 37.5 MG TAB G UCB PHARMA EAGEX 00131-3265-46 2.55833 VENLAFAXINE HCL ER 37.5 MG TAB G UCB PHARMA EAGEX 41616-0760-81 2.55750 VENLAFAXINE HCL ER 37.5 MG TAB G SUN PHARMA GLOB EAGEX 41616-0760-83 2.55750 VENLAFAXINE HCL ER 37.5 MG TAB G SUN PHARMA GLOB EAGEX 65580-0301-03 2.72800 VENLAFAXINE HCL ER 37.5 MG TAB G UCB PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 411LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65580-0301-09 2.72800 VENLAFAXINE HCL ER 37.5 MG TAB G UCB PHARMA EAGEX 00093-7385-05 0.34670 VENLAFAXINE HCL ER 75 MG CAP G TEVA USA EAGEX 00093-7385-56 0.34670 VENLAFAXINE HCL ER 75 MG CAP G TEVA USA EAGEX 00093-7385-98 0.34670 VENLAFAXINE HCL ER 75 MG CAP G TEVA USA EAGEX 00904-6247-61 0.34670 VENLAFAXINE HCL ER 75 MG CAP G MAJOR PHARMACEU EAGEX 13668-0019-01 0.34670 VENLAFAXINE HCL ER 75 MG CAP G TORRENT PHARMAC EAGEX 13668-0019-05 0.34670 VENLAFAXINE HCL ER 75 MG CAP G TORRENT PHARMAC EAGEX 13668-0019-30 0.34670 VENLAFAXINE HCL ER 75 MG CAP G TORRENT PHARMAC EAGEX 13668-0019-90 0.34670 VENLAFAXINE HCL ER 75 MG CAP G TORRENT PHARMAC EAGEX 59762-0181-01 0.34670 VENLAFAXINE HCL ER 75 MG CAP G GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-0181-02 0.34670 VENLAFAXINE HCL ER 75 MG CAP G GREENSTONE LLC. EAGEX 59762-0181-03 0.34670 VENLAFAXINE HCL ER 75 MG CAP G GREENSTONE LLC. EAGEX 59762-0181-04 0.34670 VENLAFAXINE HCL ER 75 MG CAP G GREENSTONE LLC. EAGEX 60505-3779-03 0.34670 VENLAFAXINE HCL ER 75 MG CAP G APOTEX CORP EAGEX 60505-3779-09 0.34670 VENLAFAXINE HCL ER 75 MG CAP G APOTEX CORP EAGEX 64679-0717-01 0.34670 VENLAFAXINE HCL ER 75 MG CAP G WOCKHARDT USA L EAGEX 64679-0717-04 0.34670 VENLAFAXINE HCL ER 75 MG CAP G WOCKHARDT USA L EAGEX 64679-0717-05 0.34670 VENLAFAXINE HCL ER 75 MG CAP G WOCKHARDT USA L EAGEX 65862-0528-01 0.34670 VENLAFAXINE HCL ER 75 MG CAP G AUROBINDO PHARM EAGEX 65862-0528-30 0.34670 VENLAFAXINE HCL ER 75 MG CAP G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0528-90 0.34670 VENLAFAXINE HCL ER 75 MG CAP G AUROBINDO PHARM EAGEX 65862-0528-99 0.34670 VENLAFAXINE HCL ER 75 MG CAP G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0485-01 0.34670 VENLAFAXINE HCL ER 75 MG CAP G AHP EAGEX 68<strong>08</strong>4-0485-11 0.34670 VENLAFAXINE HCL ER 75 MG CAP G AHP EAGEX 68382-0035-<strong>06</strong> 0.34670 VENLAFAXINE HCL ER 75 MG CAP G ZYDUS PHARMACEU EAGEX 68382-0035-10 0.34670 VENLAFAXINE HCL ER 75 MG CAP G ZYDUS PHARMACEU EAGEX 68382-0035-16 0.34670 VENLAFAXINE HCL ER 75 MG CAP G ZYDUS PHARMACEU EAGEX 00131-3266-32 2.86449 VENLAFAXINE HCL ER 75 MG TAB G UCB PHARMA EAGEX 00131-3266-46 2.86449 VENLAFAXINE HCL ER 75 MG TAB G UCB PHARMA EAGEX 41616-0759-81 2.86250 VENLAFAXINE HCL ER 75 MG TAB G SUN PHARMA GLOB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 41616-0759-83 2.86250 VENLAFAXINE HCL ER 75 MG TAB G SUN PHARMA GLOB EAGEX 65580-0302-03 3.05340 VENLAFAXINE HCL ER 75 MG TAB G UCB PHARMA EAGEX 65580-0302-09 3.05340 VENLAFAXINE HCL ER 75 MG TAB G UCB PHARMA EAGEX 00093-7383-01 0.58190 VENLAFAXINE HCL 100 MG TABLET G TEVA USA EAGEX 00378-4885-01 0.58190 VENLAFAXINE HCL 100 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-6151-21 0.58190 VENLAFAXINE HCL 100 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6151-25 0.58190 VENLAFAXINE HCL 100 MG TABLET G QUALITEST EAGEX 16714-0315-01 0.58190 VENLAFAXINE HCL 100 MG TABLET G NORTHSTAR RX LL EAGEX 23155-0250-01 0.58190 VENLAFAXINE HCL 100 MG TABLET G HERITAGE PHARMA EAGEX 23155-0250-09 0.58190 VENLAFAXINE HCL 100 MG TABLET G HERITAGE PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 55111-0549-90 0.58190 VENLAFAXINE HCL 100 MG TABLET G DR.REDDY'S LAB EAGEX 65162-0305-09 0.58190 VENLAFAXINE HCL 100 MG TABLET G AMNEAL PHARMACE EAGEX 65862-04<strong>08</strong>-01 0.58190 VENLAFAXINE HCL 100 MG TABLET G AUROBINDO PHARM EAGEX 65862-04<strong>08</strong>-20 0.58190 VENLAFAXINE HCL 100 MG TABLET G AUROBINDO PHARM EAGEX 68001-0156-00 0.58190 VENLAFAXINE HCL 100 MG TABLET G BLUEPOINT LABOR EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 412LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68382-0101-01 0.58190 VENLAFAXINE HCL 100 MG TABLET G ZYDUS PHARMACEU EAGEX 00093-0199-01 0.41120 VENLAFAXINE HCL 25 MG TABLET G TEVA USA EAGEX 00378-4881-01 0.41120 VENLAFAXINE HCL 25 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-6156-21 0.41120 VENLAFAXINE HCL 25 MG TABLET G QUALITEST EAGEX 16714-0311-01 0.41120 VENLAFAXINE HCL 25 MG TABLET G NORTHSTAR RX LL EAGEX 23155-0246-01 0.41120 VENLAFAXINE HCL 25 MG TABLET G HERITAGE PHARMA EAGEX 23155-0246-09 0.41120 VENLAFAXINE HCL 25 MG TABLET G HERITAGE PHARMA EAGEX 55111-0545-90 0.41120 VENLAFAXINE HCL 25 MG TABLET G DR.REDDY'S LAB EAGEX 65162-0300-09 0.41120 VENLAFAXINE HCL 25 MG TABLET G AMNEAL PHARMACE EAGEX 65862-0404-01 0.41120 VENLAFAXINE HCL 25 MG TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-0404-60 0.41120 VENLAFAXINE HCL 25 MG TABLET G AUROBINDO PHARM EAGEX 68001-0157-00 0.41120 VENLAFAXINE HCL 25 MG TABLET G BLUEPOINT LABOR EAGEX 68382-0018-01 0.41120 VENLAFAXINE HCL 25 MG TABLET G ZYDUS PHARMACEU EAGEX 00093-7380-01 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G TEVA USA EAGEX 00378-4882-01 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-6157-21 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6157-25 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G QUALITEST EAGEX 16714-0312-01 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G NORTHSTAR RX LL EAGEX 23155-0247-01 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G HERITAGE PHARMA EAGEX 23155-0247-09 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G HERITAGE PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0480-01 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0480-20 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G MYLAN INSTITUTI EAGEX 55111-0546-90 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G DR.REDDY'S LAB EAGEX 65162-0302-09 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G AMNEAL PHARMACE EAGEX 65862-0405-01 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G AUROBINDO PHARM EAGEX 65862-0405-60 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G AUROBINDO PHARM EAGEX 65862-0405-90 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G AUROBINDO PHARM EAGEX 68001-0158-00 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G BLUEPOINT LABOR EAGEX 68<strong>08</strong>4-0330-01 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0330-11 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68382-0019-01 0.37570 VENLAFAXINE HCL 37.5 MG TABLET G ZYDUS PHARMACEU EAGEX 00093-7381-01 0.39782 VENLAFAXINE HCL 50 MG TABLET G TEVA USA EAGEX 00378-4883-01 0.39782 VENLAFAXINE HCL 50 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-6149-21 0.39782 VENLAFAXINE HCL 50 MG TABLET G QUALITEST EAGEX 16714-0313-01 0.39782 VENLAFAXINE HCL 50 MG TABLET G NORTHSTAR RX LL EAGEX 23155-0248-01 0.39782 VENLAFAXINE HCL 50 MG TABLET G HERITAGE PHARMA EAGEX 23155-0248-09 0.39782 VENLAFAXINE HCL 50 MG TABLET G HERITAGE PHARMA EAGEX 55111-0547-90 0.39782 VENLAFAXINE HCL 50 MG TABLET G DR.REDDY'S LAB EAGEX 65162-03<strong>06</strong>-09 0.39782 VENLAFAXINE HCL 50 MG TABLET G AMNEAL PHARMACE EAGEX 65862-04<strong>06</strong>-01 0.39782 VENLAFAXINE HCL 50 MG TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65862-04<strong>06</strong>-30 0.39782 VENLAFAXINE HCL 50 MG TABLET G AUROBINDO PHARM EAGEX 65862-04<strong>06</strong>-90 0.39782 VENLAFAXINE HCL 50 MG TABLET G AUROBINDO PHARM EAGEX 68001-0159-00 0.39782 VENLAFAXINE HCL 50 MG TABLET G BLUEPOINT LABOR EAGEX 68382-0020-01 0.39782 VENLAFAXINE HCL 50 MG TABLET G ZYDUS PHARMACEU EAGEX 00093-7382-01 0.33680 VENLAFAXINE HCL 75 MG TABLET G TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 413LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-4884-01 0.33680 VENLAFAXINE HCL 75 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-6150-16 0.33680 VENLAFAXINE HCL 75 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6150-21 0.33680 VENLAFAXINE HCL 75 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6150-25 0.33680 VENLAFAXINE HCL 75 MG TABLET G QUALITEST EAGEX 16714-0314-01 0.33680 VENLAFAXINE HCL 75 MG TABLET G NORTHSTAR RX LL EAGEX 23155-0249-01 0.33680 VENLAFAXINE HCL 75 MG TABLET G HERITAGE PHARMA EAGEX 23155-0249-09 0.33680 VENLAFAXINE HCL 75 MG TABLET G HERITAGE PHARMA EAGEX 51079-0482-01 0.33680 VENLAFAXINE HCL 75 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0482-20 0.33680 VENLAFAXINE HCL 75 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0482-30 0.33680 VENLAFAXINE HCL 75 MG TABLET G MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0482-56 0.33680 VENLAFAXINE HCL 75 MG TABLET G MYLAN INSTITUTI EAGEX 55111-0548-90 0.33680 VENLAFAXINE HCL 75 MG TABLET G DR.REDDY'S LAB EAGEX 65162-0307-09 0.33680 VENLAFAXINE HCL 75 MG TABLET G AMNEAL PHARMACE EAGEX 65862-0407-01 0.33680 VENLAFAXINE HCL 75 MG TABLET G AUROBINDO PHARM EAGEX 65862-0407-30 0.33680 VENLAFAXINE HCL 75 MG TABLET G AUROBINDO PHARM EAGEX 68001-0160-00 0.33680 VENLAFAXINE HCL 75 MG TABLET G BLUEPOINT LABOR EAGEX 68<strong>08</strong>4-0331-01 0.33680 VENLAFAXINE HCL 75 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0331-11 0.33680 VENLAFAXINE HCL 75 MG TABLET G AHP EAGEX 68382-0021-01 0.33680 VENLAFAXINE HCL 75 MG TABLET G ZYDUS PHARMACEU EABND 00517-2340-10 9.96000 VEN<strong>OF</strong>ER 100 MG/5 ML VIAL 0 AMER. REGENT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00517-2340-25 9.96000 VEN<strong>OF</strong>ER 100 MG/5 ML VIAL 0 AMER. REGENT MLBND 49230-0534-10 7.17120 VEN<strong>OF</strong>ER 100 MG/5 ML VIAL 0 FRESENIUS MED MLBND 49230-0534-25 7.17120 VEN<strong>OF</strong>ER 100 MG/5 ML VIAL 0 FRESENIUS MED MLBND 00517-2310-05 9.96000 VEN<strong>OF</strong>ER 200 MG/10 ML VIAL 0 AMER. REGENT MLBND 00517-2325-10 9.87700 VEN<strong>OF</strong>ER 50 MG/2.5 ML VIAL 0 AMER. REGENT MLBND 49230-0530-10 7.17120 VEN<strong>OF</strong>ER 50 MG/2.5 ML VIAL 0 FRESENIUS MED MLBND 66215-0302-00 84.16200 VENTAVIS 10 MCG/1 ML SOLUTION 0 ACTELION PHARMA MLBND 66215-0302-30 84.16200 VENTAVIS 10 MCG/1 ML SOLUTION 0 ACTELION PHARMA MLBND 66215-0303-00 84.16200 VENTAVIS 20 MCG/1 ML SOLUTION 0 ACTELION PHARMA MLBND 66215-0303-30 84.16200 VENTAVIS 20 MCG/1 ML SOLUTION 0 ACTELION PHARMA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-<strong>06</strong>82-20 2.36<strong>08</strong>8 VENTOLIN HFA 90 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-<strong>06</strong>82-21 2.11753 VENTOLIN HFA 90 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-<strong>06</strong>82-24 2.11753 VENTOLIN HFA 90 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-<strong>06</strong>82-54 2.11753 VENTOLIN HFA 90 MCG INHALER G GLAXOSMITHKLINE GMBND 00173-0753-00 11.95864 VERAMYST 27.5 MCG NASAL SPRAY G GLAXOSMITHKLINE GMGEN 00378-6201-01 1.24133 VERAPAMIL ER PM 100 MG CAPSULE 0 MYLAN EAGEN 62175-0485-37 1.24133 VERAPAMIL ER PM 100 MG CAPSULE 0 KREMERS URBAN EAGEN 00378-6202-01 1.599<strong>08</strong> VERAPAMIL ER PM 200 MG CAPSULE 0 MYLAN EAGEN 00378-6202-05 1.599<strong>08</strong> VERAPAMIL ER PM 200 MG CAPSULE 0 MYLAN EAGEN 62175-0486-37 1.599<strong>08</strong> VERAPAMIL ER PM 200 MG CAPSULE 0 KREMERS URBAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6203-01 2.44620 VERAPAMIL ER PM 300 MG CAPSULE 0 MYLAN EAGEN 00378-6203-05 2.44620 VERAPAMIL ER PM 300 MG CAPSULE 0 MYLAN EAGEN 62175-0487-37 2.44620 VERAPAMIL ER PM 300 MG CAPSULE 0 KREMERS URBAN EAGEN 00378-6320-01 0.74519 VERAPAMIL ER 120 MG CAPSULE 0 MYLAN EAGEN 00378-6320-93 0.74519 VERAPAMIL ER 120 MG CAPSULE 0 MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 414LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-2880-01 0.74519 VERAPAMIL ER 120 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00172-4285-60 0.27419 VERAPAMIL ER 120 MG TABLET 0 IVAX PHARMACEUT EAGEN 00378-2120-01 0.27419 VERAPAMIL ER 120 MG TABLET 0 MYLAN EAGEN 00378-2120-93 0.27419 VERAPAMIL ER 120 MG TABLET 0 MYLAN EAGEN 57664-0116-88 0.27419 VERAPAMIL ER 120 MG TABLET 0 CARACO PHARM EAGEN 60505-2740-01 0.27419 VERAPAMIL ER 120 MG TABLET 0 APOTEX CORP EAGEN 68<strong>08</strong>4-<strong>06</strong>65-01 0.27419 VERAPAMIL ER 120 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>65-11 0.27419 VERAPAMIL ER 120 MG TABLET 0 AHP EAGEN 68462-0292-01 0.27419 VERAPAMIL ER 120 MG TABLET 0 GLENMARK PHARMA EAGEN 00378-6380-01 0.77645 VERAPAMIL ER 180 MG CAPSULE 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6380-93 0.77645 VERAPAMIL ER 180 MG CAPSULE 0 MYLAN EAGEN 00591-2882-01 0.77645 VERAPAMIL ER 180 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00172-4286-60 0.17037 VERAPAMIL ER 180 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-4286-70 0.17037 VERAPAMIL ER 180 MG TABLET 0 IVAX PHARMACEUT EAGEN 00378-2180-01 0.17037 VERAPAMIL ER 180 MG TABLET 0 MYLAN EAGEN 00378-2180-05 0.17037 VERAPAMIL ER 180 MG TABLET 0 MYLAN EAGEN 57664-0117-13 0.17037 VERAPAMIL ER 180 MG TABLET 0 CARACO PHARM EAGEN 57664-0117-88 0.17037 VERAPAMIL ER 180 MG TABLET 0 CARACO PHARM EAGEN 60505-2741-01 0.17037 VERAPAMIL ER 180 MG TABLET 0 APOTEX CORP EAGEN 60505-2741-05 0.17037 VERAPAMIL ER 180 MG TABLET 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-<strong>06</strong>74-01 0.17037 VERAPAMIL ER 180 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>74-11 0.17037 VERAPAMIL ER 180 MG TABLET 0 AHP EAGEN 68462-0293-01 0.17037 VERAPAMIL ER 180 MG TABLET 0 GLENMARK PHARMA EAGEN 68462-0293-05 0.17037 VERAPAMIL ER 180 MG TABLET 0 GLENMARK PHARMA EAGEN 00378-6440-01 0.87759 VERAPAMIL ER 240 MG CAPSULE 0 MYLAN EAGEN 00378-6440-93 0.87759 VERAPAMIL ER 240 MG CAPSULE 0 MYLAN EAGEN 00591-2884-01 0.87759 VERAPAMIL ER 240 MG CAPSULE 0 ACTAVIS PHARMA, EAGEN 00172-4280-60 0.14594 VERAPAMIL ER 240 MG TABLET 0 IVAX PHARMACEUT EAGEN 00172-4280-70 0.14594 VERAPAMIL ER 240 MG TABLET 0 IVAX PHARMACEUT EAGEN 00378-1411-01 0.14594 VERAPAMIL ER 240 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-1411-05 0.14594 VERAPAMIL ER 240 MG TABLET 0 MYLAN EAGEN 00378-1411-77 0.14594 VERAPAMIL ER 240 MG TABLET 0 MYLAN EAGEN 51079-<strong>08</strong>69-30 0.14594 VERAPAMIL ER 240 MG TABLET 0 MYLAN INSTITUTI EAGEN 57664-0118-13 0.14594 VERAPAMIL ER 240 MG TABLET 0 CARACO PHARM EAGEN 57664-0118-88 0.14594 VERAPAMIL ER 240 MG TABLET 0 CARACO PHARM EAGEN 60505-2742-01 0.14594 VERAPAMIL ER 240 MG TABLET 0 APOTEX CORP EAGEN 60505-2742-05 0.14594 VERAPAMIL ER 240 MG TABLET 0 APOTEX CORP EAGEN 68<strong>08</strong>4-<strong>06</strong>82-01 0.14594 VERAPAMIL ER 240 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>82-11 0.14594 VERAPAMIL ER 240 MG TABLET 0 AHP EAGEN 68462-0260-01 0.14594 VERAPAMIL ER 240 MG TABLET 0 GLENMARK PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68462-0260-05 0.14594 VERAPAMIL ER 240 MG TABLET 0 GLENMARK PHARMA EAGEN 00378-0772-01 0.<strong>08</strong>630 VERAPAMIL 120 MG TABLET 0 MYLAN EAGEN 00378-0772-05 0.<strong>08</strong>630 VERAPAMIL 120 MG TABLET 0 MYLAN EAGEN 00591-0345-01 0.<strong>08</strong>630 VERAPAMIL 120 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-0345-05 0.<strong>08</strong>630 VERAPAMIL 120 MG TABLET 0 ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 415LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-0345-10 0.<strong>08</strong>630 VERAPAMIL 120 MG TABLET 0 ACTAVIS PHARMA, EAGEN 23155-0027-01 0.<strong>08</strong>630 VERAPAMIL 120 MG TABLET 0 HERITAGE PHARMA EAGEN 00591-2886-01 1.50970 VERAPAMIL 360 MG CAP PELLET 0 ACTAVIS PHARMA, EABND 00591-0404-01 0.18580 0.22957 VERAPAMIL 40 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00378-0512-01 0.05630 VERAPAMIL 80 MG TABLET 0 MYLAN EAGEN 00591-0343-01 0.05630 VERAPAMIL 80 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-0343-05 0.05630 VERAPAMIL 80 MG TABLET 0 ACTAVIS PHARMA, EAGEN 00591-0343-10 0.05630 VERAPAMIL 80 MG TABLET 0 ACTAVIS PHARMA, EAGEN 23155-0026-01 0.05630 VERAPAMIL 80 MG TABLET 0 HERITAGE PHARMA EABND 63032-0111-00 5.22900 VERDESO 0.05% FOAM G AQUA PHARMACEUT GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63032-0111-50 5.57760 VERDESO 0.05% FOAM G AQUA PHARMACEUT GMBND 10337-0450-03 29.71704 VEREGEN 15% OINTMENT 0 DOAK DERM. GMBND 10337-0450-15 24.78656 VEREGEN 15% OINTMENT 0 DOAK DERM. GMBND 00091-4<strong>08</strong>5-01 1.24133 3.88207 VERELAN PM 100 MG CAP PELLET 0 UCB PHARMA EABND 62175-0570-37 1.24133 3.88207 VERELAN PM 100 MG CAP PELLET 0 KREMERS URBAN EABND 00091-4<strong>08</strong>6-01 1.599<strong>08</strong> 4.99992 VERELAN PM 200 MG CAP PELLET 0 UCB PHARMA EABND 62175-0571-37 1.599<strong>08</strong> 4.99992 VERELAN PM 200 MG CAP PELLET 0 KREMERS URBAN EABND 00091-4<strong>08</strong>7-01 2.44620 7.26922 VERELAN PM 300 MG CAP PELLET 0 UCB PHARMA EABND 62175-0572-37 2.44620 7.26922 VERELAN PM 300 MG CAP PELLET 0 KREMERS URBAN EABND 00091-2490-23 0.74519 4.79939 VERELAN 120 MG CAP PELLET G UCB PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 62175-0580-37 0.74519 4.79939 VERELAN 120 MG CAP PELLET G KREMERS URBAN EABND 00091-2489-23 0.77645 5.02631 VERELAN 180 MG CAP PELLET G UCB PHARMA EABND 62175-0581-37 0.77645 5.02631 VERELAN 180 MG CAP PELLET G KREMERS URBAN EABND 00091-2491-23 0.87759 5.67230 VERELAN 240 MG CAP PELLET G UCB PHARMA EABND 62175-0582-37 0.87759 5.67230 VERELAN 240 MG CAP PELLET G KREMERS URBAN EABND 00091-2495-23 1.50970 8.33834 VERELAN 360 MG CAP PELLET 0 UCB PHARMA EABND 62175-0583-37 1.50970 8.33834 VERELAN 360 MG CAP PELLET 0 KREMERS URBAN EABND 63717-0915-<strong>08</strong> 0.84047 VERIPRED 20 20 MG/5 ML SOLN G HAWTHORN PHARM MLBEX 18860-0121-01 7.31205 VERSACLOZ 50 MG/ML SUSPENSION G JAZZ PHARMACEUT MLBND 51248-0151-01 6.73655 VESICARE 10 MG TABLET 0 ASTELLAS PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 51248-0151-03 6.73646 VESICARE 10 MG TABLET 0 ASTELLAS PHARMA EABND 51248-0150-01 6.73655 VESICARE 5 MG TABLET G ASTELLAS PHARMA EABND 51248-0150-03 6.73646 VESICARE 5 MG TABLET G ASTELLAS PHARMA EAGEX 52544-0982-28 1.89669 VESTURA 3 MG-0.02 MG TABLET 0 ACTAVIS PHARMA, EAGEX 52544-0982-31 1.89669 VESTURA 3 MG-0.02 MG TABLET 0 ACTAVIS PHARMA, EABND 00<strong>06</strong>5-<strong>06</strong>27-03 8.944<strong>08</strong> VEXOL 1% EYE DROPS 0 ALCON LABS. MLBND 00<strong>06</strong>5-<strong>06</strong>27-07 9.79<strong>06</strong>8 VEXOL 1% EYE DROPS 0 ALCON LABS. MLBND 00049-3190-01 148.59490 VFEND IV 200 MG VIAL 0 PFIZER/NOVAPLUS EABND 00049-3190-28 148.59490 VFEND IV 200 MG VIAL 0 PFIZER US PHARM EABND 00049-3190-38 148.59490 VFEND IV 200 MG VIAL 0 PFIZER/AMERINET EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00049-4190-01 148.59490 VFEND IV 200 MG VIAL 0 PFIZER US PHARM EABND 00049-3180-30 27.54718 60.71726 VFEND 200 MG TABLET 0 PFIZER US PHARM EABND 00049-3160-44 10.77197 13.90017 VFEND 40 MG/ML SUSPENSION 0 PFIZER US PHARM MLBND 00049-3170-30 7.92500 15.17904 VFEND 50 MG TABLET 0 PFIZER US PHARM EABND 00469-3525-30 62.55710 VIBATIV 250 MG VIAL 0 ASTELLAS PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 416LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00469-3575-50 186.45950 VIBATIV 750 MG VIAL 0 ASTELLAS PHARMA EABND 52118-0001-01 297.80400 VIBATIV 750 MG VIAL 0 THERAVANCE, INC EABUL 00<strong>06</strong>9-0950-50 0.14910 5.98762 VIBRAMYCIN 100 MG CAPSULE G PFIZER US PHARM EABND 00<strong>06</strong>9-0970-65 0.38456 VIBRAMYCIN 25 MG/5 ML SUSP G PFIZER US PHARM MLBND 00<strong>06</strong>9-0971-93 0.59569 VIBRAMYCIN 50 MG/5 ML SYRUP G PFIZER US PHARM MLBND 00169-4<strong>06</strong>0-12 59.28136 VICTOZA 2-PAK 18 MG/3 ML PEN G NOVO NORDISK MLBND 00169-4<strong>06</strong>0-13 59.28228 VICTOZA 3-PAK 18 MG/3 ML PEN G NOVO NORDISK MLBND 00<strong>08</strong>5-0314-02 19.82133 VICTRELIS 200 MG CAPSULE G MERCK SHARP & D EABND 59572-0102-01 395.28000 560.48240 VIDAZA 100 MG VIAL 0 CELGENE EABND 67211-0102-01 395.28000 484.<strong>06</strong>430 VIDAZA 100 MG VIAL 0 CELGENE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>08</strong>7-6671-17 2.72370 4.48255 VIDEX EC 125 MG CAPSULE G BMS ONCO/IMMUN EABND 00<strong>08</strong>7-6672-17 4.35740 7.17009 VIDEX EC 200 MG CAPSULE G BMS ONCO/IMMUN EABND 00<strong>08</strong>7-6673-17 5.55240 9.13774 VIDEX EC 250 MG CAPSULE G BMS ONCO/IMMUN EABND 00<strong>08</strong>7-6674-17 8.67230 14.27157 VIDEX EC 400 MG CAPSULE G BMS ONCO/IMMUN EABND 00<strong>08</strong>7-6632-41 0.55195 VIDEX 2 GM PEDIATRIC SOLN G BMS ONCO/IMMUN MLBND 00<strong>08</strong>7-6633-41 0.60249 VIDEX 4 GM PEDIATRIC SOLN G BMS ONCO/IMMUN MLBND 00<strong>06</strong>5-4013-03 34.99280 VIGAMOX 0.5% EYE DROPS G ALCON LABS. MLBEX 00456-1100-31 4.97889 VIIBRYD TITRATION PACK G FOREST PHARMACE EABEX 00456-1110-30 4.97889 VIIBRYD 10 MG TABLET G FOREST PHARMACE EABEX 00456-1120-30 4.97889 VIIBRYD 20 MG TABLET G FOREST PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00456-1140-30 4.97889 VIIBRYD 40 MG TABLET G FOREST PHARMACE EABND 00186-0510-60 1.90470 VIMOVO DR 375-20 MG TABLET G ASTRAZENECA EABND 75987-0031-04 13.26672 VIMOVO DR 375-20 MG TABLET G HORIZON PHARMA EABND 00186-0520-60 1.90470 VIMOVO DR 500-20 MG TABLET G ASTRAZENECA EABND 75987-0030-04 13.26672 VIMOVO DR 500-20 MG TABLET G HORIZON PHARMA EAGEN 51991-<strong>06</strong>17-90 0.27300 VINACAL PRENATAL TABLET 0 BRECKENRIDGE EAGEN 51991-0159-90 0.27125 VINATE GT TABLET 0 BRECKENRIDGE EABND 51991-0178-01 0.30436 VINATE II TABLET 0 BRECKENRIDGE EAGEN 51991-0566-01 0.19510 VINATE ONE TABLET 0 BRECKENRIDGE EABND 51991-0584-33 1.02200 VINATE PN CARE TABLET 0 BRECKENRIDGE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51991-0155-01 0.13380 VINATE-M TABLET 0 BRECKENRIDGE EABND 55390-0091-10 13.74480 VINBLASTINE SULF 10 MG VIAL 0 BEDFORD LABS EABND 63323-0278-10 3.300<strong>08</strong> VINBLASTINE 1 MG/ML VIAL 0 APP PHARMACEUTI MLGEN 00703-4412-11 13.54500 VINCASAR PFS 2 MG/2 ML VIAL 0 TEVA PARENTERAL MLGEN 00703-4402-11 13.54500 VINCRISTINE 1 MG/ML VIAL 0 TEVA PARENTERAL MLGEN 61703-0309-<strong>06</strong> 5.10000 VINCRISTINE 1 MG/ML VIAL 0 HOSPIRA MLGEN 61703-0309-16 4.81875 VINCRISTINE 2 MG/2 ML VIAL 0 HOSPIRA MLGEN 00703-4182-01 18.00000 VINORELBINE 10 MG/ML VIAL 0 TEVA PARENTERAL MLGEN 55390-0<strong>06</strong>9-01 27.36000 VINORELBINE 10 MG/ML VIAL 0 BEDFORD LABS MLGEN 61703-0341-<strong>06</strong> 14.59500 VINORELBINE 10 MG/ML VIAL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00703-4183-01 18.00000 VINORELBINE 50 MG/5 ML VIAL 0 TEVA PARENTERAL MLGEN 55390-0070-01 25.56000 VINORELBINE 50 MG/5 ML VIAL 0 BEDFORD LABS MLGEN 66758-0045-02 43.41150 VINORELBINE 50 MG/5 ML VIAL 0 SANDOZ INC. MLBND 58914-0112-10 2.42127 VIOKACE 10,440-39,150 UNITS TB G APTALIS PHARMA EABND 58914-0117-10 4.77681 VIOKACE 20,880-78,300 UNITS TB G APTALIS PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 417LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68462-0318-29 1.51100 VIORELE 28 DAY TABLET 0 GLENMARK PHARMA EABND 63010-0010-30 3.02603 VIRACEPT 250 MG TABLET G VIIV <strong>HEALTH</strong>CARE EABND 63010-0027-70 7.56510 VIRACEPT 625 MG TABLET G VIIV <strong>HEALTH</strong>CARE EABND 00597-0123-30 20.84738 VIRAMUNE XR 400 MG TABLET G BOEHRINGER ING. EABND 00597-0046-46 0.17415 11.23997 VIRAMUNE 200 MG TABLET G BOEHRINGER ING. EABND 00597-0046-60 0.17415 11.23888 VIRAMUNE 200 MG TABLET G BOEHRINGER ING. EABND 00597-0047-24 0.6<strong>08</strong>45 VIRAMUNE 50 MG/5 ML SUSP G BOEHRINGER ING. MLBND 00187-0007-14 6283.72250 VIRAZOLE 6 GM VIAL 0 VALEANT EABND 61958-0403-01 7.90298 VIREAD POWDER G GILEAD SCIENCES GMBND 61958-0404-01 26.86516 VIREAD 150 MG TABLET G GILEAD SCIENCES EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 61958-0405-01 26.86516 VIREAD 200 MG TABLET G GILEAD SCIENCES EABND 61958-04<strong>06</strong>-01 26.86516 VIREAD 250 MG TABLET G GILEAD SCIENCES EABND 61958-0401-01 28.98719 VIREAD 300 MG TABLET G GILEAD SCIENCES EABND 61570-0037-75 17.65600 25.27294 VIROPTIC 1% EYE DROPS G MONARCH PHRM MLGEN 76439-0340-30 1.66840 VIRT-PN DHA S<strong>OF</strong>TGEL 0 VIRTUS PHARMACE EAGEN 76439-0249-30 2.13624 VIRT-SELECT CAPSULE 0 VIRTUS PHARMACE EABEX 00<strong>06</strong>9-5410-66 0.<strong>06</strong>670 1.96145 VISTARIL 25 MG CAPSULE G PFIZER US PHARM EABEX 00<strong>06</strong>9-5420-66 0.<strong>08</strong>750 2.39156 VISTARIL 50 MG CAPSULE G PFIZER US PHARM EABND 61958-0101-01 142.<strong>08</strong>000 147.4<strong>08</strong>00 VISTIDE 75 MG/ML VIAL 0 GILEAD SCIENCES MLGEN 00574-0194-51 0.28026 VIT D2 1.25 MG (50,000 UNIT) 0 PADDOCK LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00955-0250-50 0.28026 VIT D2 1.25 MG (50,000 UNIT) 0 WINTHROP US EAGEN 50111-0990-01 0.28026 VIT D2 1.25 MG (50,000 UNIT) 0 BARR EAGEN 51991-<strong>06</strong>04-01 0.28026 VIT D2 1.25 MG (50,000 UNIT) 0 BRECKENRIDGE EAGEN 57664-0136-88 0.28026 VIT D2 1.25 MG (50,000 UNIT) 0 CARACO PHARM EAGEN 64980-0157-01 0.28026 VIT D2 1.25 MG (50,000 UNIT) 0 RISING PHARM EAGEN 68<strong>08</strong>4-0463-01 0.28026 VIT D2 1.25 MG (50,000 UNIT) 0 AHP EAGEN 68<strong>08</strong>4-0463-11 0.28026 VIT D2 1.25 MG (50,000 UNIT) 0 AHP EABND 0<strong>06</strong>42-0079-12 0.32460 1.21495 VITAFOL-OB CAPLET 0 EVERETT EABND 0<strong>06</strong>42-0070-30 2.54810 VITAFOL-ONE CAPSULE 0 EVERETT EABND 0<strong>06</strong>42-0078-12 0.89889 VITAFOL-PN CAPLET 0 EVERETT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-9157-01 7.68912 VITAMIN K 1 MG/0.5 ML AMPUL 0 HOSPIRA MLBND 00409-9158-01 11.40420 VITAMIN K 10 MG/ML AMPUL 0 HOSPIRA MLBND 64661-0<strong>08</strong>0-30 1.63120 3.31972 VIVA DHA PRENATAL S<strong>OF</strong>TGEL 0 JAYMAC PHARMA EAGEX 51285-0594-02 1.66779 VIVACTIL 10 MG TABLET 0 DURAMED/BARR EAGEX 51285-0595-02 1.<strong>08</strong>486 VIVACTIL 5 MG TABLET 0 DURAMED/BARR EABND 00078-0365-45 10.46076 VIVELLE-DOT 0.025 MG PATCH 0 NOVARTIS EABND 00078-0343-45 10.45972 VIVELLE-DOT 0.0375 MG PATCH 0 NOVARTIS EABND 00078-0344-45 10.46491 VIVELLE-DOT 0.05 MG PATCH 0 NOVARTIS EABND 00078-0345-45 10.48047 VIVELLE-DOT 0.075 MG PATCH 0 NOVARTIS EABND 00078-0346-45 10.48117 VIVELLE-DOT 0.1 MG PATCH 0 NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13811-0514-10 0.14050 VOL-NATE TABLET 0 TRIGEN LABORATO EAGEN 13811-0519-01 0.293<strong>08</strong> VOL-PLUS TABLET 0 TRIGEN LABORATO EAGEN 13811-0519-10 0.53287 VOL-PLUS TABLET 0 TRIGEN LABORATO EAGEN 13811-0519-50 0.293<strong>08</strong> VOL-PLUS TABLET 0 TRIGEN LABORATO EAGEN 13811-0516-90 0.11500 VOL-TAB RX TABLET 0 TRIGEN LABORATO EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 418LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63481-<strong>06</strong>84-47 0.36370 VOLTAREN 1% GEL 0 ENDO PHARM INC. GMBND 00078-0446-05 0.51910 9.36281 VOLTAREN-XR 100 MG TABLET G NOVARTIS EAGEN 00093-5290-56 27.54718 VORICONAZOLE 200 MG TABLET 0 TEVA USA EAGEN 00378-1640-93 27.54718 VORICONAZOLE 200 MG TABLET 0 MYLAN EAGEN 00781-5668-31 27.54718 VORICONAZOLE 200 MG TABLET 0 SANDOZ EAGEN 51079-0165-01 27.54718 VORICONAZOLE 200 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0165-03 27.54718 VORICONAZOLE 200 MG TABLET 0 MYLAN INSTITUTI EAGEN 59762-0930-02 27.54718 VORICONAZOLE 200 MG TABLET 0 GREENSTONE LLC. EAGEN 68<strong>08</strong>4-0538-25 27.54718 VORICONAZOLE 200 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0538-95 27.54718 VORICONAZOLE 200 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-6270-45 10.55300 VORICONAZOLE 40 MG/ML SUSP 0 MYLAN MLGEN 59762-0935-03 10.55300 VORICONAZOLE 40 MG/ML SUSP 0 GREENSTONE LLC. MLGEN 00093-5289-56 7.92500 VORICONAZOLE 50 MG TABLET 0 TEVA USA EAGEN 00378-1626-93 7.92500 VORICONAZOLE 50 MG TABLET 0 MYLAN EAGEN 00781-5667-31 7.92500 VORICONAZOLE 50 MG TABLET 0 SANDOZ EAGEN 51079-0164-01 7.92500 VORICONAZOLE 50 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0164-03 7.92500 VORICONAZOLE 50 MG TABLET 0 MYLAN INSTITUTI EAGEN 59762-0925-01 7.92500 VORICONAZOLE 50 MG TABLET 0 GREENSTONE LLC. EAGEN 68<strong>08</strong>4-<strong>06</strong>42-21 7.92500 VORICONAZOLE 50 MG TABLET 0 AHP EABND 00095-0201-10 11.62240 17.84500 VOSOL HC EAR DROPS G ECR PHARM. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68774-<strong>06</strong>00-01 1.16532 VOSPIRE ER 4 MG TABLET 0 DAVA P EAGEN 68774-<strong>06</strong>01-01 2.18511 VOSPIRE ER 8 MG TABLET 0 DAVA P EABND 00173-<strong>08</strong>04-09 63.91649 VOTRIENT 200 MG TABLET 0 GLAXOSMITHKLINE EAGEN 76439-0237-90 1.57583 VP-HEME OB TABLET 0 VIRTUS PHARMACE EABND 76439-0223-30 1.75655 VP-PNV-DHA CAPSULE 0 VIRTUS PHARMACE EABND 54092-0701-04 1371.49200 VPRIV 400 UNITS VIAL 0 SHIRE US INC. EABND 00015-3075-19 62.50730 VUMON 50 MG/5 ML AMPULE 0 BMS ONCO/IMMUN MLBND 00145-0002-04 7.47<strong>08</strong>3 VUSION OINTMENT G PRESTIUM PHARMA GMGEX 68180-<strong>08</strong>75-13 1.12980 VYFEMLA 28 TABLET 0 LUPIN PHARMACEU EABND 66582-0311-31 6.07836 VYTORIN 10-10 MG TABLET G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 66582-0311-54 6.07762 VYTORIN 10-10 MG TABLET G MERCK SHARP & D EABND 66582-0311-82 6.07755 VYTORIN 10-10 MG TABLET G MERCK SHARP & D EABND 66582-0312-31 6.07836 VYTORIN 10-20 MG TABLET G MERCK SHARP & D EABND 66582-0312-54 6.07762 VYTORIN 10-20 MG TABLET G MERCK SHARP & D EABND 66582-0312-87 6.07756 VYTORIN 10-20 MG TABLET G MERCK SHARP & D EABND 66582-0313-31 6.07836 VYTORIN 10-40 MG TABLET G MERCK SHARP & D EABND 66582-0313-54 6.07762 VYTORIN 10-40 MG TABLET G MERCK SHARP & D EABND 66582-0313-74 6.07755 VYTORIN 10-40 MG TABLET G MERCK SHARP & D EABND 66582-0313-86 6.07756 VYTORIN 10-40 MG TABLET G MERCK SHARP & D EABND 66582-0315-31 6.07836 VYTORIN 10-80 MG TABLET G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 66582-0315-54 6.07762 VYTORIN 10-80 MG TABLET G MERCK SHARP & D EABND 66582-0315-66 6.07755 VYTORIN 10-80 MG TABLET G MERCK SHARP & D EABND 66582-0315-74 6.07755 VYTORIN 10-80 MG TABLET G MERCK SHARP & D EAGEN 00555-<strong>08</strong>31-02 0.<strong>06</strong>520 WARFARIN SODIUM 1 MG TABLET 0 BARR EAGEN 00555-<strong>08</strong>31-05 0.<strong>06</strong>520 WARFARIN SODIUM 1 MG TABLET 0 BARR EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 419LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0327-01 0.<strong>06</strong>520 WARFARIN SODIUM 1 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0327-10 0.<strong>06</strong>520 WARFARIN SODIUM 1 MG TABLET 0 CAMBER PHARMACE EAGEN 51672-4027-01 0.<strong>06</strong>520 WARFARIN SODIUM 1 MG TABLET 0 TARO PHARM USA EAGEN 51672-4027-03 0.<strong>06</strong>520 WARFARIN SODIUM 1 MG TABLET 0 TARO PHARM USA EAGEN 51672-4027-07 0.<strong>06</strong>520 WARFARIN SODIUM 1 MG TABLET 0 TARO PHARM USA EAGEN 65162-0761-10 0.<strong>06</strong>520 WARFARIN SODIUM 1 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0761-11 0.<strong>06</strong>520 WARFARIN SODIUM 1 MG TABLET 0 AMNEAL PHARMACE EAGEN 68382-0052-01 0.<strong>06</strong>520 WARFARIN SODIUM 1 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0052-10 0.<strong>06</strong>520 WARFARIN SODIUM 1 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00555-<strong>08</strong>35-02 0.07180 WARFARIN SODIUM 10 MG TABLET 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0335-01 0.07180 WARFARIN SODIUM 10 MG TABLET 0 CAMBER PHARMACE EAGEN 51672-4035-01 0.07180 WARFARIN SODIUM 10 MG TABLET 0 TARO PHARM USA EAGEN 51672-4035-03 0.07180 WARFARIN SODIUM 10 MG TABLET 0 TARO PHARM USA EAGEN 65162-0769-10 0.07180 WARFARIN SODIUM 10 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0769-11 0.07180 WARFARIN SODIUM 10 MG TABLET 0 AMNEAL PHARMACE EAGEN 68382-0059-01 0.07180 WARFARIN SODIUM 10 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00555-<strong>08</strong>69-02 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 BARR EAGEN 00555-<strong>08</strong>69-05 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 BARR EAGEN 31722-0328-01 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0328-10 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-4028-01 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 TARO PHARM USA EAGEN 51672-4028-03 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 TARO PHARM USA EAGEN 51672-4028-07 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 TARO PHARM USA EAGEN 62584-0984-01 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 AHP EAGEN 62584-0984-11 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 AHP EAGEN 65162-0762-10 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0762-11 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 AMNEAL PHARMACE EAGEN 68382-0053-01 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0053-10 0.<strong>06</strong>520 WARFARIN SODIUM 2 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00555-<strong>08</strong>32-02 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00555-<strong>08</strong>32-05 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 BARR EAGEN 31722-0329-01 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0329-10 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 CAMBER PHARMACE EAGEN 51672-4029-01 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 TARO PHARM USA EAGEN 51672-4029-03 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 TARO PHARM USA EAGEN 51672-4029-07 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 TARO PHARM USA EAGEN 65162-0763-10 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0763-11 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-0027-01 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0027-11 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0<strong>06</strong>4-01 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0<strong>06</strong>4-10 0.<strong>06</strong>520 WARFARIN SODIUM 2.5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00555-0925-02 0.<strong>06</strong>520 WARFARIN SODIUM 3 MG TABLET 0 BARR EAGEN 31722-0330-01 0.<strong>06</strong>520 WARFARIN SODIUM 3 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0330-10 0.<strong>06</strong>520 WARFARIN SODIUM 3 MG TABLET 0 CAMBER PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 420LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-4030-01 0.<strong>06</strong>520 WARFARIN SODIUM 3 MG TABLET 0 TARO PHARM USA EAGEN 51672-4030-03 0.<strong>06</strong>520 WARFARIN SODIUM 3 MG TABLET 0 TARO PHARM USA EAGEN 51672-4030-07 0.<strong>06</strong>520 WARFARIN SODIUM 3 MG TABLET 0 TARO PHARM USA EAGEN 65162-0764-10 0.<strong>06</strong>520 WARFARIN SODIUM 3 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0764-11 0.<strong>06</strong>520 WARFARIN SODIUM 3 MG TABLET 0 AMNEAL PHARMACE EAGEN 68382-0054-01 0.<strong>06</strong>520 WARFARIN SODIUM 3 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0054-10 0.<strong>06</strong>520 WARFARIN SODIUM 3 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00555-<strong>08</strong>74-02 0.<strong>06</strong>520 WARFARIN SODIUM 4 MG TABLET 0 BARR EAGEN 00555-<strong>08</strong>74-05 0.<strong>06</strong>520 WARFARIN SODIUM 4 MG TABLET 0 BARR EAGEN 31722-0331-01 0.<strong>06</strong>520 WARFARIN SODIUM 4 MG TABLET 0 CAMBER PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0331-10 0.<strong>06</strong>520 WARFARIN SODIUM 4 MG TABLET 0 CAMBER PHARMACE EAGEN 51672-4031-01 0.<strong>06</strong>520 WARFARIN SODIUM 4 MG TABLET 0 TARO PHARM USA EAGEN 51672-4031-03 0.<strong>06</strong>520 WARFARIN SODIUM 4 MG TABLET 0 TARO PHARM USA EAGEN 51672-4031-07 0.<strong>06</strong>520 WARFARIN SODIUM 4 MG TABLET 0 TARO PHARM USA EAGEN 65162-0765-10 0.<strong>06</strong>520 WARFARIN SODIUM 4 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0765-11 0.<strong>06</strong>520 WARFARIN SODIUM 4 MG TABLET 0 AMNEAL PHARMACE EAGEN 68382-0055-01 0.<strong>06</strong>520 WARFARIN SODIUM 4 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0055-10 0.<strong>06</strong>520 WARFARIN SODIUM 4 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00555-<strong>08</strong>33-02 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 BARR EAGEN 00555-<strong>08</strong>33-05 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 31722-0332-01 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 CAMBER PHARMACE EAGEN 31722-0332-10 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 CAMBER PHARMACE EAGEN 51672-4032-01 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 TARO PHARM USA EAGEN 51672-4032-03 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 TARO PHARM USA EAGEN 51672-4032-07 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 TARO PHARM USA EAGEN 58517-0360-30 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 <strong>NEW</strong> HORIZON RX EAGEN 62584-0994-01 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 AHP EAGEN 62584-0994-11 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 AHP EAGEN 65162-0766-10 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0766-11 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68382-0056-01 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0056-10 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 68382-0056-16 0.<strong>06</strong>520 WARFARIN SODIUM 5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00555-0926-02 0.07180 WARFARIN SODIUM 6 MG TABLET 0 BARR EAGEN 31722-0333-01 0.07180 WARFARIN SODIUM 6 MG TABLET 0 CAMBER PHARMACE EAGEN 51672-4033-01 0.07180 WARFARIN SODIUM 6 MG TABLET 0 TARO PHARM USA EAGEN 51672-4033-03 0.07180 WARFARIN SODIUM 6 MG TABLET 0 TARO PHARM USA EAGEN 65162-0767-10 0.07180 WARFARIN SODIUM 6 MG TABLET 0 AMNEAL PHARMACE EAGEN 65162-0767-11 0.07180 WARFARIN SODIUM 6 MG TABLET 0 AMNEAL PHARMACE EAGEN 68382-0057-01 0.07180 WARFARIN SODIUM 6 MG TABLET 0 ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00555-<strong>08</strong>34-02 0.07200 WARFARIN SODIUM 7.5 MG TABLET 0 BARR EAGEN 31722-0334-01 0.07200 WARFARIN SODIUM 7.5 MG TABLET 0 CAMBER PHARMACE EAGEN 51672-4034-01 0.07200 WARFARIN SODIUM 7.5 MG TABLET 0 TARO PHARM USA EAGEN 51672-4034-03 0.07200 WARFARIN SODIUM 7.5 MG TABLET 0 TARO PHARM USA EAGEN 65162-0768-10 0.07200 WARFARIN SODIUM 7.5 MG TABLET 0 AMNEAL PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 421LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65162-0768-11 0.07200 WARFARIN SODIUM 7.5 MG TABLET 0 AMNEAL PHARMACE EAGEN 68382-0058-01 0.07200 WARFARIN SODIUM 7.5 MG TABLET 0 ZYDUS PHARMACEU EAGEN 00409-4887-10 0.04720 WATER FOR INJECTION VIAL 0 HOSPIRA MLGEN 00409-4887-20 0.04720 WATER FOR INJECTION VIAL 0 HOSPIRA MLGEN 00409-4887-50 0.03492 WATER FOR INJECTION VIAL 0 HOSPIRA MLGEN 00409-4887-99 0.03195 WATER FOR INJECTION VIAL 0 HOSPIRA MLGEN 00517-3010-25 0.04720 WATER FOR INJECTION VIAL 0 AMER. REGENT MLGEN 00517-3020-25 0.04720 WATER FOR INJECTION VIAL 0 AMER. REGENT MLGEN 00517-3050-25 0.02520 WATER FOR INJECTION VIAL 0 AMER. REGENT MLGEN 63323-0185-00 0.030<strong>06</strong> WATER FOR INJECTION VIAL 0 APP PHARMACEUTI ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63323-0185-05 0.04720 WATER FOR INJECTION VIAL 0 APP PHARMACEUTI MLGEN 63323-0185-10 0.04720 WATER FOR INJECTION VIAL 0 APP PHARMACEUTI MLGEN 63323-0185-20 0.04720 WATER FOR INJECTION VIAL 0 APP PHARMACEUTI MLGEN 63323-0185-50 0.03744 WATER FOR INJECTION VIAL 0 APP PHARMACEUTI MLBND 65597-0902-30 11.11536 WELCHOL 3.75G PACKET G DAIICHI SANKYO, EABND 65597-0701-18 1.85256 WELCHOL 625 MG TABLET G DAIICHI SANKYO, EABEX 00173-0947-55 0.24840 3.79393 WELLBUTRIN SR 100 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0135-55 0.28390 4.<strong>06</strong>602 WELLBUTRIN SR 150 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0722-00 0.49170 7.55<strong>06</strong>5 WELLBUTRIN SR 200 MG TABLET G GLAXOSMITHKLINE EABEX 00187-0730-30 0.65250 10.87438 WELLBUTRIN XL 150 MG TABLET G VALEANT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00187-0730-90 0.65250 10.87466 WELLBUTRIN XL 150 MG TABLET G VALEANT EABEX 00187-0731-30 0.71766 14.35429 WELLBUTRIN XL 300 MG TABLET G VALEANT EABEX 64455-0731-30 0.71766 9.84767 WELLBUTRIN XL 300 MG TABLET G VALEANT EABEX 00173-0178-55 0.36100 2.94525 WELLBUTRIN 100 MG TABLET G GLAXOSMITHKLINE EABEX 00173-0177-55 2.2<strong>08</strong>46 WELLBUTRIN 75 MG TABLET G GLAXOSMITHKLINE EAGEX 16714-0370-01 0.43098 WERA 0.5/0.035 MG 28 TABLET 0 NORTHSTAR RX LL EABND 67467-0182-02 0.85500 WILATE 1,000-1,000 UNIT KIT 0 OCTAPHARMA USABND 67467-0182-01 0.85500 WILATE 500-500 UNIT KIT 0 OCTAPHARMA USABEX 68180-<strong>08</strong>98-11 2.16920 WYMZYA FE CHEWABLE TABLET 0 LUPIN PHARMACEU EABEX 68180-<strong>08</strong>98-13 2.16920 WYMZYA FE CHEWABLE TABLET 0 LUPIN PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00013-8303-01 3.66296 47.44280 XALATAN 0.005% EYE DROPS G PHARMACIA/UPJHN MLBND 00013-8303-04 3.66296 47.44280 XALATAN 0.005% EYE DROPS G PHARMACIA/UPJHN MLBND 00<strong>06</strong>9-8141-20 189.48402 XALKORI 200 MG CAPSULE 0 PFIZER US PHARM EABND 00<strong>06</strong>9-8140-20 189.48402 XALKORI 250 MG CAPSULE 0 PFIZER US PHARM EABND 50458-0580-10 8.81<strong>06</strong>1 XARELTO 10 MG TABLET 0 JANSSEN PHARM. EABND 50458-0580-30 8.81072 XARELTO 10 MG TABLET 0 JANSSEN PHARM. EABND 50458-0578-10 8.81<strong>06</strong>1 XARELTO 15 MG TABLET 0 JANSSEN PHARM. EABND 50458-0578-30 8.81072 XARELTO 15 MG TABLET 0 JANSSEN PHARM. EABND 50458-0578-90 8.81<strong>06</strong>3 XARELTO 15 MG TABLET 0 JANSSEN PHARM. EABND 50458-0579-10 8.81<strong>06</strong>1 XARELTO 20 MG TABLET 0 JANSSEN PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50458-0579-30 8.81072 XARELTO 20 MG TABLET 0 JANSSEN PHARM. EABND 50458-0579-90 8.81<strong>06</strong>3 XARELTO 20 MG TABLET 0 JANSSEN PHARM. EABND 00<strong>06</strong>9-1001-01 36.46909 XELJANZ 5 MG TABLET G PFIZER US PHARM EABND 00004-1100-20 10.82417 XELODA 150 MG TABLET 0 GENENTECH, INC. EABND 00004-1101-50 36.07609 XELODA 500 MG TABLET 0 GENENTECH, INC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 422LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 67386-0421-01 55.92762 XENAZINE 12.5 MG TABLET 0 LUNDBECK INC. EABND 67386-0422-01 111.85517 XENAZINE 25 MG TABLET 0 LUNDBECK INC. EABND 00037-0501-05 59.38816 XERESE 5%-1% CREAM G VALEANT GMBND 00187-5104-01 70.55830 XERESE 5%-1% CREAM G VALEANT GMBND 65649-0301-03 13.31264 XIFAXAN 200 MG TABLET G SALIX PHARMACEU EABND 65649-0301-41 13.31237 XIFAXAN 200 MG TABLET G SALIX PHARMACEU EABND 65649-0303-02 21.93634 XIFAXAN 550 MG TABLET G SALIX PHARMACEU EABND 63402-0515-30 3.25720 6.18516 XOPENEX CONC 1.25 MG/0.5 ML G SUNOVION PHARMA EABND 63402-0510-01 3.41628 XOPENEX HFA 45 MCG INHALER G SUNOVION PHARMA GMBND 63402-0511-24 0.82107 2.<strong>06</strong>174 XOPENEX 0.31 MG/3 ML SOLUTION G SUNOVION PHARMA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63402-0512-24 1.01350 2.<strong>06</strong>174 XOPENEX 0.63 MG/3 ML SOLUTION G SUNOVION PHARMA MLBND 63402-0513-24 1.03760 2.<strong>06</strong>174 XOPENEX 1.25 MG/3 ML SOLUTION G SUNOVION PHARMA MLBND 00469-0125-99 65.48326 XTANDI 40 MG CAPSULE 0 ASTELLAS PHARMA EABND 63323-0497-50 0.<strong>08</strong>289 0.35922 XYLOCAINE 4% SOLUTION 0 APP PHARMACEUTI MLBND 58394-0024-03 1.44420 XYNTHA SOL<strong>OF</strong>USE 1,000 UNIT KIT 0 WYETH PHARMBND 58394-0025-03 1.44420 XYNTHA SOL<strong>OF</strong>USE 2,000 UNIT KIT 0 WYETH PHARMBND 58394-0022-03 1.44420 XYNTHA SOL<strong>OF</strong>USE 250 UNIT KIT 0 WYETH PHARMBND 58394-0023-03 1.44420 XYNTHA SOL<strong>OF</strong>USE 500 UNIT KIT 0 WYETH PHARMBND 58394-0014-01 1.44420 XYNTHA 1,000 UNIT KIT 0 WYETH PHARMBND 58394-0015-01 1.44420 XYNTHA 2,000 UNIT KIT 0 WYETH PHARM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 58394-0012-01 1.44420 XYNTHA 250 UNIT KIT 0 WYETH PHARMBND 58394-0016-03 1.44420 XYNTHA 3,000 UNIT SYRINGE KIT 0 WYETH PHARMBND 58394-0013-01 1.44420 XYNTHA 500 UNIT KIT 0 WYETH PHARMBND 00024-5801-21 0.48890 0.57511 XYZAL 2.5 MG/5 ML SOLUTION G SAN<strong>OF</strong>I-AVENTIS MLBND 00024-5800-90 0.23112 2.83740 XYZAL 5 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABEX 50419-0402-03 2.<strong>08</strong>130 2.76567 YASMIN 28 TABLET 0 BAYER,PHARM DIV EABEX 50419-0405-03 2.04356 2.76567 YAZ 28 TABLET 0 BAYER,PHARM DIV EAGEN 49884-0303-02 1.34199 ZAFIRLUKAST 10 MG TABLET G PAR PHARM. EAGEN 55111-<strong>06</strong>25-60 1.62150 ZAFIRLUKAST 10 MG TABLET G DR.REDDY'S LAB EAGEN 49884-0304-02 1.34199 ZAFIRLUKAST 20 MG TABLET G PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55111-<strong>06</strong>26-60 1.62150 ZAFIRLUKAST 20 MG TABLET G DR.REDDY'S LAB EABND 10144-<strong>06</strong>02-15 1.79640 2.50056 ZANAFLEX 2 MG CAPSULE G ACORDA THERAPEU EABND 10144-<strong>06</strong>04-15 2.27730 3.16954 ZANAFLEX 4 MG CAPSULE G ACORDA THERAPEU EABUL 10144-0594-15 0.32000 2.35996 ZANAFLEX 4 MG TABLET G ACORDA THERAPEU EABND 10144-<strong>06</strong><strong>06</strong>-15 4.20920 4.75401 ZANAFLEX 6 MG CAPSULE G ACORDA THERAPEU EABND 24987-0364-01 0.91866 1.25620 ZANTAC 1,000 MG/40 ML VIAL G COVIS PHARMACEU MLBND 00173-0383-54 0.14240 0.71118 ZANTAC 15 MG/ML SYRUP G GLAXOSMITHKLINE MLBND 00173-0344-14 0.02889 4.35612 ZANTAC 150 MG TABLET G GLAXOSMITHKLINE EABND 00173-0344-42 0.02889 4.35735 ZANTAC 150 MG TABLET G GLAXOSMITHKLINE EABND 00173-0363-01 0.91866 1.28096 ZANTAC 150 MG/6 ML VIAL 0 COVIS PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 24987-0363-01 0.91866 1.28096 ZANTAC 150 MG/6 ML VIAL 0 COVIS PHARMACEU MLBND 00173-0362-38 1.65626 ZANTAC 25 MG/ML VIAL G COVIS PHARMACEU MLBND 00173-0363-00 0.91866 1.25620 ZANTAC 25 MG/ML VIAL 0 COVIS PHARMACEU MLBUL 00173-0393-40 0.12500 7.90934 ZANTAC 300 MG TABLET G GLAXOSMITHKLINE EABND 00173-0441-00 0.10789 ZANTAC 50 MG/50 ML PLAST-BAG 0 COVIS PHARMACEU ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 423LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 52544-0981-31 2.05401 ZARAH TABLET 0 ACTAVIS PHARMA, EABEX 00071-0237-24 0.93650 2.05781 ZARONTIN 250 MG CAPSULE 0 PFIZER US PHARM EAGEX 00071-2418-23 0.22000 ZARONTIN 250 MG/5 ML SOLUTION 0 PFIZER US PHARM MLBND 53014-0975-71 0.80444 2.76074 ZAROXOLYN 2.5 MG TABLET G UCB PHARMA EABND 53014-<strong>08</strong>50-71 0.91467 3.13756 ZAROXOLYN 5 MG TABLET G UCB PHARMA EAGEN 13811-0580-30 1.66840 ZATEAN-PN DHA CAPSULE 0 TRIGEN LABORATO EAGEN 13811-0582-30 1.56810 ZATEAN-PN PLUS S<strong>OF</strong>TGEL 0 TRIGEN LABORATO EAGEN 13811-0581-90 1.37360 ZATEAN-PN TABLET 0 TRIGEN LABORATO EABND 66215-0201-18 242.36000 ZAVESCA 100 MG CAPSULE 0 ACTELION PHARMA EABND 66215-0201-90 242.36000 ZAVESCA 100 MG CAPSULE 0 ACTELION PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 51285-0<strong>06</strong>1-01 0.45846 4.48<strong>06</strong>1 ZEBETA 10 MG TABLET G DURAMED/BARR EABND 51285-0<strong>06</strong>0-01 0.45846 4.48<strong>06</strong>1 ZEBETA 5 MG TABLET G DURAMED/BARR EAGEN 59630-0170-11 2.23335 ZEBUTAL CAPSULE 0 SHIONOGI PHARMA EAGEN 683<strong>08</strong>-0554-10 0.48480 ZEBUTAL 50-325-40 MG CAPSULE 0 MIDLOTHIAN LABO EABND 00187-0453-02 13.45098 ZELAPAR 1.25 MG ODT TABLET 0 VALEANT EABND 50242-0090-01 45.03<strong>08</strong>2 ZELBORAF 240 MG TABLET 0 GENENTECH, INC. EABND 00053-7201-02 0.48970 ZEMAIRA 1,000 MG VIAL 0 CSL BEHRING LLC EABND 00074-4317-30 11.24041 ZEMPLAR 1 MCG CAPSULE 0 ABBVIE US LLC EABND 00074-4314-30 22.48027 ZEMPLAR 2 MCG CAPSULE 0 ABBVIE US LLC EABND 00074-4637-01 6.03576 ZEMPLAR 2 MCG/ML VIAL 0 ABBVIE US LLC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00074-4315-30 44.96<strong>08</strong>2 ZEMPLAR 4 MCG CAPSULE 0 ABBVIE US LLC EABND 00074-1658-01 15.<strong>08</strong>940 ZEMPLAR 5 MCG/ML VIAL 0 ABBVIE US LLC MLGEN 55111-0135-81 7.36020 ZENATANE 10 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 55111-0136-81 8.43370 ZENATANE 20 MG CAPSULE 0 DR.REDDY'S LAB EAGEN 55111-0137-81 8.98670 ZENATANE 40 MG CAPSULE 0 DR.REDDY'S LAB EAGEX 52544-0292-31 1.96017 ZENCHENT FE TABLET CHEWABLE 0 ACTAVIS PHARMA, EAGEX 65162-0347-84 2.39830 ZENCHENT FE TABLET CHEWABLE 0 AMNEAL PHARMACE EAGEX 52544-0953-28 1.12980 ZENCHENT 0.4 MG-35 MCG TABLET 0 ACTAVIS PHARMA, EABND 42865-0101-02 1.99150 ZENPEP DR 10,000 UNITS CAPSULE 0 APTALIS PHARMA EABND 42865-0102-02 2.87636 ZENPEP DR 15,000 UNITS CAPSULE 0 APTALIS PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 42865-0103-02 3.90722 ZENPEP DR 20,000 UNITS CAPSULE 0 APTALIS PHARMA EABND 42865-0105-02 4.83499 ZENPEP DR 25,000 UNITS CAPSULE 0 APTALIS PHARMA EABND 42865-0104-02 1.05592 ZENPEP DR 3,000 UNITS CAPSULE 0 APTALIS PHARMA EABND 42865-0100-02 1.00745 ZENPEP DR 5,000 UNITS CAPSULE 0 APTALIS PHARMA EAGEX 00093-2090-28 1.96017 ZEOSA CHEWABLE TABLET 0 TEVA USA EAGEX 00093-2090-58 1.96017 ZEOSA CHEWABLE TABLET 0 TEVA USA EABND 00003-1968-01 0.23814 0.42632 ZERIT 1 MG/ML SOLUTION G BMS ONCO/IMMUN MLBND 00003-1964-01 1.<strong>08</strong>405 6.80157 ZERIT 15 MG CAPSULE G BMS ONCO/IMMUN EABND 00003-1965-01 0.98888 7.07257 ZERIT 20 MG CAPSULE G BMS ONCO/IMMUN EABND 00003-1966-01 1.04193 7.51288 ZERIT 30 MG CAPSULE G BMS ONCO/IMMUN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00003-1967-01 0.92760 7.65149 ZERIT 40 MG CAPSULE G BMS ONCO/IMMUN EABND 00310-0141-11 0.03186 1.45523 ZESTORETIC 10-12.5 MG TABLET G ASTRAZENECA EABND 00310-0142-11 0.04170 1.57525 ZESTORETIC 20-12.5 MG TABLET G ASTRAZENECA EABND 00310-0145-11 0.05120 1.59418 ZESTORETIC 20-25 MG TABLET G ASTRAZENECA EABND 00310-0131-11 0.02687 1.31638 ZESTRIL 10 MG TABLET G ASTRAZENECA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 424LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00310-0135-11 0.01810 0.85041 ZESTRIL 2.5 MG TABLET G ASTRAZENECA EABND 00310-0132-11 0.03310 1.40942 ZESTRIL 20 MG TABLET G ASTRAZENECA EABND 00310-0133-11 0.09113 1.99548 ZESTRIL 30 MG TABLET G ASTRAZENECA EABND 00310-0134-11 0.<strong>06</strong>872 2.<strong>06</strong>113 ZESTRIL 40 MG TABLET G ASTRAZENECA EABND 00310-0130-11 0.01958 1.27496 ZESTRIL 5 MG TABLET G ASTRAZENECA EABND 66582-0414-31 6.13370 ZETIA 10 MG TABLET G MERCK SHARP & D EABND 66582-0414-54 6.134<strong>06</strong> ZETIA 10 MG TABLET G MERCK SHARP & D EABND 66582-0414-74 6.13414 ZETIA 10 MG TABLET G MERCK SHARP & D EABND 66582-0414-76 6.13414 ZETIA 10 MG TABLET G MERCK SHARP & D EABND 63402-0737-60 23.84004 ZETONNA 37 MCG NASAL SPRAY G SUNOVION PHARMA GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 51285-0040-01 0.<strong>06</strong>480 4.48<strong>06</strong>1 ZIAC 10-6.25 MG TABLET G DURAMED/BARR EABND 51285-0047-02 0.<strong>06</strong>386 4.48042 ZIAC 2.5-6.25 MG TABLET G DURAMED/BARR EABND 51285-0050-02 0.<strong>06</strong>480 4.48042 ZIAC 5-6.25 MG TABLET G DURAMED/BARR EABND 49702-0222-48 0.60946 ZIAGEN 20 MG/ML SOLUTION G VIIV <strong>HEALTH</strong>CARE MLBND 49702-0221-18 9.27344 9.27344 ZIAGEN 300 MG TABLET G VIIV <strong>HEALTH</strong>CARE EAGEN 52343-0044-01 0.59850 ZIDOVUDINE 100 MG CAPSULE G GEN-SOURCE RX EAGEN 64376-0128-01 0.83457 ZIDOVUDINE 100 MG CAPSULE G BOCA PHARMACAL EAGEN 65862-0107-01 0.83457 ZIDOVUDINE 100 MG CAPSULE G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0461-11 0.83457 ZIDOVUDINE 100 MG CAPSULE G AHP EAGEN 68<strong>08</strong>4-0461-21 0.83457 ZIDOVUDINE 100 MG CAPSULE G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-61<strong>06</strong>-91 0.49572 ZIDOVUDINE 300 MG TABLET G MYLAN EAGEN 31722-0509-60 0.49572 ZIDOVUDINE 300 MG TABLET G CAMBER PHARMACE EAGEN 52343-0045-60 0.17250 ZIDOVUDINE 300 MG TABLET G GEN-SOURCE RX EAGEN 65862-0024-60 0.49572 ZIDOVUDINE 300 MG TABLET G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0462-11 0.49572 ZIDOVUDINE 300 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0462-21 0.49572 ZIDOVUDINE 300 MG TABLET G AHP EAGEN 64376-0129-23 0.12623 ZIDOVUDINE 50 MG/5 ML SYRUP G BOCA PHARMACAL MLGEN 65862-0048-24 0.12623 ZIDOVUDINE 50 MG/5 ML SYRUP G AUROBINDO PHARM MLBND 00173-0437-00 11.56854 ZINACEF 1.5 GM ADD-VANT VIAL 0 COVIS PHARMACEU EABND 00173-0354-10 4.<strong>06</strong>037 11.16516 ZINACEF 1.5 GM VIAL G COVIS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 24987-0354-10 4.<strong>06</strong>037 11.16516 ZINACEF 1.5 GM VIAL G COVIS PHARMACEU EABND 00173-0425-00 0.30959 ZINACEF 1.5 GRAM/50 ML 0 COVIS PHARMACEU MLBND 24987-0425-00 0.34030 ZINACEF 1.5 GRAM/50 ML 0 COVIS PHARMACEU MLBND 00173-0400-00 18.82500 54.73296 ZINACEF 7.5 GM VIAL G COVIS PHARMACEU EABND 00173-0436-00 6.01218 ZINACEF 750 MG ADD-VANT VIAL 0 COVIS PHARMACEU EABND 24987-0436-00 6.69312 ZINACEF 750 MG TWISTVIAL 0 COVIS PHARMACEU EABND 00173-0352-10 2.00260 5.61744 ZINACEF 750 MG VIAL G COVIS PHARMACEU EABND 24987-0352-10 2.00260 5.61744 ZINACEF 750 MG VIAL G COVIS PHARMACEU EABND 00517-8105-25 0.79480 ZINC SULFATE 25 MG/5 ML VIAL 0 AMER. REGENT MLBND 000<strong>06</strong>-3931-30 3.44588 ZIOPTAN 0.0015% EYE DROPS G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-3931-54 3.44579 ZIOPTAN 0.0015% EYE DROPS G MERCK SHARP & D EAGEX 00781-2164-60 3.37750 ZIPRASIDONE HCL 20 MG CAPSULE G SANDOZ EAGEX 00904-6269-<strong>08</strong> 3.37750 ZIPRASIDONE HCL 20 MG CAPSULE G MAJOR PHARMACEU EAGEX 55111-0256-60 3.37750 ZIPRASIDONE HCL 20 MG CAPSULE G DR.REDDY'S LAB EAGEX 59762-2001-01 3.37750 ZIPRASIDONE HCL 20 MG CAPSULE G GREENSTONE LLC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 425LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-2528-<strong>06</strong> 3.37750 ZIPRASIDONE HCL 20 MG CAPSULE G APOTEX CORP EAGEX 64679-0991-02 3.37750 ZIPRASIDONE HCL 20 MG CAPSULE G WOCKHARDT USA L EAGEX 64679-0991-04 3.37750 ZIPRASIDONE HCL 20 MG CAPSULE G WOCKHARDT USA L EAGEX 68<strong>08</strong>4-0581-09 3.37750 ZIPRASIDONE HCL 20 MG CAPSULE G AHP EAGEX 68<strong>08</strong>4-0581-11 3.37750 ZIPRASIDONE HCL 20 MG CAPSULE G AHP EAGEX 68180-0331-07 3.37750 ZIPRASIDONE HCL 20 MG CAPSULE G LUPIN PHARMACEU EAGEX 00781-2166-60 2.39850 ZIPRASIDONE HCL 40 MG CAPSULE G SANDOZ EAGEX 00904-6270-<strong>08</strong> 2.39850 ZIPRASIDONE HCL 40 MG CAPSULE G MAJOR PHARMACEU EAGEX 55111-0257-60 2.39850 ZIPRASIDONE HCL 40 MG CAPSULE G DR.REDDY'S LAB EAGEX 59762-2002-01 2.39850 ZIPRASIDONE HCL 40 MG CAPSULE G GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-2529-<strong>06</strong> 2.39850 ZIPRASIDONE HCL 40 MG CAPSULE G APOTEX CORP EAGEX 64679-0992-02 2.39850 ZIPRASIDONE HCL 40 MG CAPSULE G WOCKHARDT USA L EAGEX 64679-0992-04 2.39850 ZIPRASIDONE HCL 40 MG CAPSULE G WOCKHARDT USA L EAGEX 68<strong>08</strong>4-0582-09 2.39850 ZIPRASIDONE HCL 40 MG CAPSULE G AHP EAGEX 68<strong>08</strong>4-0582-11 2.39850 ZIPRASIDONE HCL 40 MG CAPSULE G AHP EAGEX 68180-0332-07 2.39850 ZIPRASIDONE HCL 40 MG CAPSULE G LUPIN PHARMACEU EAGEX 00781-2167-60 3.85300 ZIPRASIDONE HCL 60 MG CAPSULE G SANDOZ EAGEX 00904-6271-<strong>08</strong> 3.85300 ZIPRASIDONE HCL 60 MG CAPSULE G MAJOR PHARMACEU EAGEX 55111-0258-60 3.85300 ZIPRASIDONE HCL 60 MG CAPSULE G DR.REDDY'S LAB EAGEX 59762-2003-01 3.85300 ZIPRASIDONE HCL 60 MG CAPSULE G GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-2530-<strong>06</strong> 3.85300 ZIPRASIDONE HCL 60 MG CAPSULE G APOTEX CORP EAGEX 64679-0993-02 3.85300 ZIPRASIDONE HCL 60 MG CAPSULE G WOCKHARDT USA L EAGEX 64679-0993-04 3.85300 ZIPRASIDONE HCL 60 MG CAPSULE G WOCKHARDT USA L EAGEX 68<strong>08</strong>4-0583-09 3.85300 ZIPRASIDONE HCL 60 MG CAPSULE G AHP EAGEX 68<strong>08</strong>4-0583-11 3.85300 ZIPRASIDONE HCL 60 MG CAPSULE G AHP EAGEX 68180-0333-07 3.85300 ZIPRASIDONE HCL 60 MG CAPSULE G LUPIN PHARMACEU EAGEX 00781-2168-60 4.26398 ZIPRASIDONE HCL 80 MG CAPSULE G SANDOZ EAGEX 00904-6272-<strong>08</strong> 4.26398 ZIPRASIDONE HCL 80 MG CAPSULE G MAJOR PHARMACEU EAGEX 55111-0259-60 4.26398 ZIPRASIDONE HCL 80 MG CAPSULE G DR.REDDY'S LAB EAGEX 59762-2004-01 4.26398 ZIPRASIDONE HCL 80 MG CAPSULE G GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-2004-02 4.26398 ZIPRASIDONE HCL 80 MG CAPSULE G GREENSTONE LLC. EAGEX 60505-2531-<strong>06</strong> 4.26398 ZIPRASIDONE HCL 80 MG CAPSULE G APOTEX CORP EAGEX 64679-0994-02 4.26398 ZIPRASIDONE HCL 80 MG CAPSULE G WOCKHARDT USA L EAGEX 64679-0994-04 4.26398 ZIPRASIDONE HCL 80 MG CAPSULE G WOCKHARDT USA L EAGEX 68<strong>08</strong>4-0584-09 4.26398 ZIPRASIDONE HCL 80 MG CAPSULE G AHP EAGEX 68<strong>08</strong>4-0584-11 4.26398 ZIPRASIDONE HCL 80 MG CAPSULE G AHP EAGEX 68180-0334-07 4.26398 ZIPRASIDONE HCL 80 MG CAPSULE G LUPIN PHARMACEU EABND 242<strong>08</strong>-0535-35 46.98464 ZIRGAN 0.15% OPHTHALMIC GEL 0 VALEANT GMBND 00<strong>06</strong>9-3150-14 3.91500 28.54702 ZITHROMAX I.V. 500 MG VIAL G PFIZER US PHARM EABND 00<strong>06</strong>9-3150-83 3.91500 5.97600 ZITHROMAX I.V. 500 MG VIAL G PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>9-3070-75 4.<strong>06</strong>795 32.12837 ZITHROMAX TRI-PAK 500 MG TAB G PFIZER US PHARM EABND 00<strong>06</strong>9-3051-07 57.48912 ZITHROMAX 1 GM POWDER PACKET 0 PFIZER US PHARM EABND 00<strong>06</strong>9-3051-75 57.48580 ZITHROMAX 1 GM POWDER PACKET 0 PFIZER US PHARM EABND 00<strong>06</strong>9-3110-19 0.76230 4.53567 ZITHROMAX 100 MG/5 ML SUSP G PFIZER US PHARM MLBND 00<strong>06</strong>9-3120-19 0.79380 4.53567 ZITHROMAX 200 MG/5 ML SUSP G PFIZER US PHARM ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 426LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>9-3130-19 0.79380 3.02378 ZITHROMAX 200 MG/5 ML SUSP G PFIZER US PHARM MLBND 00<strong>06</strong>9-3140-19 0.79380 2.26783 ZITHROMAX 200 MG/5 ML SUSP G PFIZER US PHARM MLBND 00<strong>06</strong>9-3<strong>06</strong>0-30 0.61650 16.<strong>06</strong>575 ZITHROMAX 250 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-3<strong>06</strong>0-75 0.61650 16.<strong>06</strong>418 ZITHROMAX 250 MG Z-PAK TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-3070-30 4.<strong>06</strong>795 32.13<strong>06</strong>8 ZITHROMAX 500 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-3<strong>08</strong>0-30 3.38405 38.55709 ZITHROMAX 600 MG TABLET G PFIZER US PHARM EABND 00<strong>06</strong>9-4170-34 1<strong>06</strong>.53880 ZMAX 2 G/60 ML ORAL SUSPENSION 0 PFIZER US PHARM EABND 000<strong>06</strong>-0735-31 0.03497 3.55433 ZOCOR 10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0735-54 0.03497 3.55507 ZOCOR 10 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0740-31 0.02943 6.20231 ZOCOR 20 MG TABLET G MERCK SHARP & D EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 000<strong>06</strong>-0740-54 0.02943 6.20268 ZOCOR 20 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0749-31 0.04577 6.20231 ZOCOR 40 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0749-54 0.04577 6.20268 ZOCOR 40 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0726-31 0.04104 2.65240 ZOCOR 5 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0543-31 0.10071 6.20231 ZOCOR 80 MG TABLET G MERCK SHARP & D EABND 000<strong>06</strong>-0543-54 0.10071 6.20268 ZOCOR 80 MG TABLET G MERCK SHARP & D EABND 00173-0569-00 0.67914 20.81003 Z<strong>OF</strong>RAN ODT 4 MG TABLET G GLAXOSMITHKLINE EABND 00173-0570-00 1.04450 34.66246 Z<strong>OF</strong>RAN ODT 8 MG TABLET G GLAXOSMITHKLINE EABND 00173-0442-00 10.64<strong>06</strong>0 Z<strong>OF</strong>RAN 2 MG/ML VIAL G GLAXOSMITHKLINE MLBND 00173-0446-00 0.21070 22.05974 Z<strong>OF</strong>RAN 4 MG TABLET G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00173-0489-00 1.45720 4.47768 Z<strong>OF</strong>RAN 4 MG/5 ML ORAL SOLN G GLAXOSMITHKLINE MLBND 00173-0447-00 0.19040 36.74382 Z<strong>OF</strong>RAN 8 MG TABLET G GLAXOSMITHKLINE EABND 00173-0447-04 0.19040 36.73580 Z<strong>OF</strong>RAN 8 MG TABLET G GLAXOSMITHKLINE EAGEN 00143-9642-01 54.00000 ZOLEDRONIC ACID 4 MG/5 ML VIAL 0 WEST-WARD,INC. MLGEN 43598-0330-11 60.00000 ZOLEDRONIC ACID 4 MG/5 ML VIAL 0 DR.REDDY'S LAB MLGEN 60505-6110-00 60.00000 ZOLEDRONIC ACID 4 MG/5 ML VIAL 0 APOTEX CORP MLBND 000<strong>06</strong>-0568-40 91.879<strong>06</strong> ZOLINZA 100 MG CAPSULE 0 MERCK SHARP & D EAGEN 00115-<strong>06</strong>91-51 37.59624 ZOLMITRIPTAN 2.5 MG ODT G GLOBAL PHARM EAGEN 60505-3718-00 38.01750 ZOLMITRIPTAN 2.5 MG ODT G APOTEX CORP EAGEN 00115-<strong>06</strong>71-51 37.59624 ZOLMITRIPTAN 2.5 MG TABLET G GLOBAL PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-4260-56 37.97625 ZOLMITRIPTAN 2.5 MG TABLET G MYLAN EAGEN 60505-3693-00 38.01750 ZOLMITRIPTAN 2.5 MG TABLET G APOTEX CORP EAGEN 68462-0497-76 39.69810 ZOLMITRIPTAN 2.5 MG TABLET G GLENMARK PHARMA EAGEN 00115-<strong>06</strong>92-50 11.18950 ZOLMITRIPTAN 5 MG ODT G GLOBAL PHARM EAGEN 60505-3719-00 11.18950 ZOLMITRIPTAN 5 MG ODT G APOTEX CORP EAGEN 00115-<strong>06</strong>72-50 8.93930 ZOLMITRIPTAN 5 MG TABLET G GLOBAL PHARM EAGEN 00378-4261-53 8.93930 ZOLMITRIPTAN 5 MG TABLET G MYLAN EAGEN 60505-3694-00 8.93930 ZOLMITRIPTAN 5 MG TABLET G APOTEX CORP EAGEN 68462-0498-33 8.93930 ZOLMITRIPTAN 5 MG TABLET G GLENMARK PHARMA EABEX 00049-4910-30 0.07304 5.39887 ZOL<strong>OF</strong>T 100 MG TABLET G PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00049-4940-23 0.67680 2.13227 ZOL<strong>OF</strong>T 20 MG/ML ORAL CONC G PFIZER US PHARM MLBEX 00049-4960-30 0.04428 5.39887 ZOL<strong>OF</strong>T 25 MG TABLET G PFIZER US PHARM EABEX 00049-4900-30 0.05373 5.39887 ZOL<strong>OF</strong>T 50 MG TABLET G PFIZER US PHARM EABND 00078-0590-61 8.92025 ZOMETA 4 MG/100 ML INJECTION 0 NOVARTIS MLBND 00078-0387-25 60.00000 178.40518 ZOMETA 4 MG/5 ML VIAL 0 NOVARTIS ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 427LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64896-<strong>06</strong>91-51 40.10170 63.09383 ZOMIG ZMT 2.5 MG TABLET G IMPAX PHARMACEU EABND 00310-0213-21 11.18950 26.36356 ZOMIG ZMT 5 MG TABLET G ASTRAZENECA EABND 64896-<strong>06</strong>92-50 11.18950 63.09383 ZOMIG ZMT 5 MG TABLET G IMPAX PHARMACEU EABND 64896-<strong>06</strong>82-51 47.72361 ZOMIG 2.5 MG NASAL SPRAY G IMPAX PHARMACEU EABND 00310-0210-20 24.32176 ZOMIG 2.5 MG TABLET G ASTRAZENECA EABND 64896-<strong>06</strong>71-51 39.69810 63.09383 ZOMIG 2.5 MG TABLET G IMPAX PHARMACEU EABND 00310-02<strong>08</strong>-60 35.21136 ZOMIG 5 MG NASAL SPRAY G ASTRAZENECA EABND 64896-<strong>06</strong>81-51 47.72361 ZOMIG 5 MG NASAL SPRAY G IMPAX PHARMACEU EABND 00310-0211-25 8.93930 26.86986 ZOMIG 5 MG TABLET G ASTRAZENECA EABND 64896-<strong>06</strong>72-50 8.93930 63.09383 ZOMIG 5 MG TABLET G IMPAX PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 10337-<strong>08</strong>04-03 7.24866 ZONALON 5% CREAM 0 DOAK DERM. GMBND 10337-<strong>08</strong>04-45 6.55404 ZONALON 5% CREAM 0 DOAK DERM. GMBND 68025-0058-10 2.57964 ZONATUSS 150 MG CAPSULE 0 VERTICAL PHARM EABEX 62856-<strong>06</strong>80-10 0.40700 5.90130 ZONEGRAN 100 MG CAPSULE G EISAI INC. EABEX 62856-<strong>06</strong>81-10 0.14157 1.478<strong>06</strong> ZONEGRAN 25 MG CAPSULE G EISAI INC. EAGEX 00378-6727-01 0.40700 ZONISAMIDE 100 MG CAPSULE 0 MYLAN EAGEX 00378-6727-05 0.40700 ZONISAMIDE 100 MG CAPSULE 0 MYLAN EAGEX 31722-0228-01 0.40700 ZONISAMIDE 100 MG CAPSULE 0 CAMBER PHARMACE EAGEX 51079-0768-20 0.40700 ZONISAMIDE 100 MG CAPSULE 0 MYLAN INSTITUTI EAGEX 60505-2547-01 0.40700 ZONISAMIDE 100 MG CAPSULE 0 APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 62756-0260-02 0.40700 ZONISAMIDE 100 MG CAPSULE 0 SUN PHARMACEUTI EAGEX 64679-0990-01 0.40700 ZONISAMIDE 100 MG CAPSULE 0 WOCKHARDT USA L EAGEX 64720-0179-10 0.40700 ZONISAMIDE 100 MG CAPSULE 0 COREPHARMA LLC EAGEX 64720-0179-50 0.40700 ZONISAMIDE 100 MG CAPSULE 0 COREPHARMA LLC EAGEX 68<strong>08</strong>4-0183-01 0.40700 ZONISAMIDE 100 MG CAPSULE 0 AHP EAGEX 68<strong>08</strong>4-0183-11 0.40700 ZONISAMIDE 100 MG CAPSULE 0 AHP EAGEX 68462-0130-01 0.40700 ZONISAMIDE 100 MG CAPSULE 0 GLENMARK PHARMA EAGEX 68462-0130-05 0.40700 ZONISAMIDE 100 MG CAPSULE 0 GLENMARK PHARMA EAGEX 76282-0228-01 0.40700 ZONISAMIDE 100 MG CAPSULE 0 EXELAN PHARMACE EAGEX 00378-6725-01 0.14157 ZONISAMIDE 25 MG CAPSULE 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-2545-01 0.14157 ZONISAMIDE 25 MG CAPSULE 0 APOTEX CORP EAGEX 62756-0258-02 0.14157 ZONISAMIDE 25 MG CAPSULE 0 SUN PHARMACEUTI EAGEX 64679-0945-01 0.14157 ZONISAMIDE 25 MG CAPSULE 0 WOCKHARDT USA L EAGEX 64720-0177-10 0.14157 ZONISAMIDE 25 MG CAPSULE 0 COREPHARMA LLC EAGEX 68462-0128-01 0.14157 ZONISAMIDE 25 MG CAPSULE 0 GLENMARK PHARMA EAGUX 00378-6726-01 0.21120 ZONISAMIDE 50 MG CAPSULE 0 MYLAN EAGUX 60505-2546-01 0.21120 ZONISAMIDE 50 MG CAPSULE 0 APOTEX CORP EAGUX 62756-0259-02 0.21120 ZONISAMIDE 50 MG CAPSULE 0 SUN PHARMACEUTI EAGUX 64679-0946-01 0.21120 ZONISAMIDE 50 MG CAPSULE 0 WOCKHARDT USA L EAGUX 64720-0178-10 0.21120 ZONISAMIDE 50 MG CAPSULE 0 COREPHARMA LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 68462-0129-01 0.21120 ZONISAMIDE 50 MG CAPSULE 0 GLENMARK PHARMA EABND 44<strong>08</strong>7-3388-07 944.27202 ZORBTIVE 8.8 MG VIAL G EMD SERONO, INC EABND 00078-0417-20 6.59503 ZORTRESS 0.25 MG TABLET 0 NOVARTIS EABND 00078-0414-20 13.190<strong>08</strong> ZORTRESS 0.5 MG TABLET 0 NOVARTIS EABND 00078-0415-20 19.78485 ZORTRESS 0.75 MG TABLET 0 NOVARTIS EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 428LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 42211-0203-29 2.49000 ZORVOLEX 18 MG CAPSULE G IROKO PHARMACEU EABND 42211-0204-29 2.49000 ZORVOLEX 35 MG CAPSULE G IROKO PHARMACEU EABND 002<strong>06</strong>-2409-02 0.30390 ZOSYN 2.25 GM/50 ML GALAXY BAG 0 PFIZER/NOVAPLUS MLBND 002<strong>06</strong>-8860-01 0.30394 ZOSYN 2.25 GM/50 ML GALAXY BAG 0 WYETH PHARM MLBND 002<strong>06</strong>-2100-01 6.77280 ZOSYN 2.25 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-2100-02 6.77280 ZOSYN 2.25 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-2404-02 6.86000 12.66912 ZOSYN 2.25 GRAM VIAL 0 PFIZER/NOVAPLUS EABND 002<strong>06</strong>-2501-01 6.77280 ZOSYN 2.25 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-2501-10 6.77280 ZOSYN 2.25 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-8852-<strong>08</strong> 6.86000 12.66580 ZOSYN 2.25 GRAM VIAL 0 WYETH PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 002<strong>06</strong>-8852-16 6.86000 12.66912 ZOSYN 2.25 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-2411-01 0.45596 ZOSYN 3.375 GM/50 ML GALAXY 0 PFIZER/NOVAPLUS MLBND 002<strong>06</strong>-2411-02 0.45596 ZOSYN 3.375 GM/50 ML GALAXY 0 PFIZER/NOVAPLUS MLBND 002<strong>06</strong>-8861-01 0.45600 ZOSYN 3.375 GM/50 ML GALAXY 0 WYETH PHARM MLBND 002<strong>06</strong>-8861-02 0.45596 ZOSYN 3.375 GM/50 ML GALAXY 0 WYETH PHARM MLBND 002<strong>06</strong>-2405-02 5.00110 19.00700 ZOSYN 3.375 GRAM VIAL 0 PFIZER/NOVAPLUS EABND 002<strong>06</strong>-3100-01 5.00110 10.15920 ZOSYN 3.375 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-3100-02 5.00110 10.15920 ZOSYN 3.375 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-3501-01 5.00110 10.15920 ZOSYN 3.375 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-3501-10 5.00110 10.15920 ZOSYN 3.375 GRAM VIAL 0 WYETH PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 002<strong>06</strong>-8854-<strong>08</strong> 5.00110 19.00700 ZOSYN 3.375 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-8854-16 5.00110 19.00700 ZOSYN 3.375 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-2413-01 0.28892 ZOSYN 4.5 GM/100 ML GALAXY BAG 0 PFIZER/NOVAPLUS MLBND 002<strong>06</strong>-2413-02 0.28892 ZOSYN 4.5 GM/100 ML GALAXY BAG 0 PFIZER/NOVAPLUS MLBND 002<strong>06</strong>-8862-01 0.28892 ZOSYN 4.5 GM/100 ML GALAXY BAG 0 WYETH PHARM MLBND 002<strong>06</strong>-8862-02 0.28892 ZOSYN 4.5 GM/100 ML GALAXY BAG 0 WYETH PHARM MLBND 002<strong>06</strong>-24<strong>08</strong>-01 15.32400 24.07166 ZOSYN 4.5 GRAM VIAL 0 PFIZER/NOVAPLUS EABND 002<strong>06</strong>-24<strong>08</strong>-02 15.32400 24.07166 ZOSYN 4.5 GRAM VIAL 0 PFIZER/NOVAPLUS EABND 002<strong>06</strong>-4100-01 13.54560 ZOSYN 4.5 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-4100-02 13.54560 ZOSYN 4.5 GRAM VIAL 0 WYETH PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 002<strong>06</strong>-4501-01 13.54560 ZOSYN 4.5 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-4501-10 13.54560 ZOSYN 4.5 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-8855-<strong>08</strong> 15.32400 24.07000 ZOSYN 4.5 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-8855-16 15.32400 24.07166 ZOSYN 4.5 GRAM VIAL 0 WYETH PHARM EABND 002<strong>06</strong>-2416-01 95.66325 228.14210 ZOSYN 40.5 GRAM BULK VIAL 0 PFIZER/NOVAPLUS EABND 002<strong>06</strong>-8859-10 95.66325 228.14210 ZOSYN 40.5 GRAM BULK VIAL 0 WYETH PHARM EAGEX 52544-0383-28 0.77620 ZOVIA 1-35E TABLET 0 ACTAVIS PHARMA, EABEX 52544-0384-28 0.89890 0.98681 ZOVIA 1-50E TABLET 0 ACTAVIS PHARMA, EABUL 00173-0991-55 0.14780 4.28163 ZOVIRAX 200 MG CAPSULE G PRESTIUM PHARMA EABND 00173-0953-96 0.19040 0.74996 ZOVIRAX 200 MG/5 ML SUSP G PRESTIUM PHARMA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUL 00173-0949-55 0.23340 8.30962 ZOVIRAX 400 MG TABLET G PRESTIUM PHARMA EABND 00187-0994-45 102.27260 ZOVIRAX 5% CREAM G VALEANT GMBND 64455-0994-45 40.94556 ZOVIRAX 5% CREAM G VALEANT GMBND 00187-0993-95 21.33330 28.22774 ZOVIRAX 5% OINTMENT G VALEANT GMBND 64455-0993-94 11.1<strong>08</strong>72 ZOVIRAX 5% OINTMENT G VALEANT GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 429LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 01 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64455-0993-95 21.33330 22.33474 ZOVIRAX 5% OINTMENT G VALEANT GMBND 00173-0945-55 0.30250 16.15719 ZOVIRAX 800 MG TABLET G PRESTIUM PHARMA EABND 00173-0556-01 0.43350 3.09658 ZYBAN SR 150 MG TABLET G GLAXOSMITHKLINE EABND 00173-0556-02 0.43350 3.09658 ZYBAN SR 150 MG TABLET G GLAXOSMITHKLINE EABND 99207-0276-75 119.76014 ZYCLARA 2.5% CREAM PUMP 0 VALEANT GMBND 99207-0270-28 32.07861 ZYCLARA 3.75% CREAM 0 VALEANT EABND 99207-0271-75 119.76014 ZYCLARA 3.75% CREAM PUMP 0 VALEANT GMBND 10122-0902-12 18.04849 ZYFLO CR 600 MG TABLET 0 CORNERSTONE THE EABND 10122-0901-12 18.04849 ZYFLO 600 MG FILMTAB 0 CORNERSTONE THE EABND 242<strong>08</strong>-0358-05 33.88<strong>06</strong>0 ZYLET EYE DROPS G VALEANT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 242<strong>08</strong>-0358-10 33.87977 ZYLET EYE DROPS G VALEANT MLBND 65483-0991-10 0.03416 1.32335 ZYLOPRIM 100 MG TABLET G PROMETHEUS EABND 65483-0993-10 0.05900 3.62461 ZYLOPRIM 300 MG TABLET G PROMETHEUS EABND 65483-0993-50 0.05900 0.94372 ZYLOPRIM 300 MG TABLET G PROMETHEUS EABND 00023-3615-25 46.63936 ZYMAXID 0.5% EYE DROPS G ALLERGAN INC. MLBEX 00002-4454-85 5.54000 19.34232 ZYPREXA ZYDIS 10 MG TABLET G ELI LILLY & CO. EABEX 00002-4455-85 8.15000 28.52544 ZYPREXA ZYDIS 15 MG TABLET G ELI LILLY & CO. EABEX 00002-4456-85 23.73792 37.7<strong>08</strong>56 ZYPREXA ZYDIS 20 MG TABLET G ELI LILLY & CO. EABEX 00002-4453-85 9.68338 13.16407 ZYPREXA ZYDIS 5 MG TABLET G ELI LILLY & CO. EABEX 00002-4117-04 0.56070 18.36624 ZYPREXA 10 MG TABLET G ELI LILLY & CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00002-4117-30 0.56070 18.36624 ZYPREXA 10 MG TABLET G ELI LILLY & CO. EABEX 00002-4415-04 0.84150 27.54936 ZYPREXA 15 MG TABLET G ELI LILLY & CO. EABEX 00002-4415-30 0.84150 27.54936 ZYPREXA 15 MG TABLET G ELI LILLY & CO. EABEX 00002-4112-30 0.30740 10.32658 ZYPREXA 2.5 MG TABLET G ELI LILLY & CO. EABEX 00002-4420-04 1.09400 36.73248 ZYPREXA 20 MG TABLET G ELI LILLY & CO. EABEX 00002-4420-30 1.09400 36.73248 ZYPREXA 20 MG TABLET G ELI LILLY & CO. EABEX 00002-4115-30 0.36360 12.18799 ZYPREXA 5 MG TABLET G ELI LILLY & CO. EABEX 00002-4116-30 0.45270 14.83237 ZYPREXA 7.5 MG TABLET G ELI LILLY & CO. EABND 57894-0150-12 56.74371 ZYTIGA 250 MG TABLET 0 JANSSEN BIOTECH EABND 00009-5136-01 4.25053 ZYVOX 100 MG/5 ML SUSPENSION G PHARMACIA/UPJHN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00009-5137-01 0.65570 ZYVOX 200 MG/100 ML IV SOLN G PHARMACIA/UPJHN MLBND 00009-5135-02 127.51<strong>08</strong>2 ZYVOX 600 MG TABLET G PHARMACIA/UPJHN EABND 00009-5140-01 0.43721 ZYVOX 600 MG/300 ML IV SOLN G PHARMACIA/UPJHN MLBND 00187-<strong>06</strong>51-42 29.38283 8-MOP 10 MG CAPSULE 0 VALEANT EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 430LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 42747-0221-04 30.17465 ABSTRAL 100 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 42747-0221-32 30.17283 ABSTRAL 100 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 57881-0331-04 30.17465 ABSTRAL 100 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 57881-0331-32 30.17283 ABSTRAL 100 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 42747-0222-04 37.89780 ABSTRAL 200 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 42747-0222-32 37.89676 ABSTRAL 200 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 57881-0332-04 37.89780 ABSTRAL 200 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 57881-0332-32 37.89676 ABSTRAL 200 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 42747-0223-04 45.44457 ABSTRAL 300 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 42747-0223-32 45.44561 ABSTRAL 300 MCG TAB SUBLINGUAL G GALENA BIOPHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 57881-0333-04 45.44457 ABSTRAL 300 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 57881-0333-32 45.44561 ABSTRAL 300 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 42747-0224-04 54.67002 ABSTRAL 400 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 42747-0224-32 54.67054 ABSTRAL 400 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 57881-0334-04 54.67002 ABSTRAL 400 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 57881-0334-32 54.67054 ABSTRAL 400 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 42747-0226-04 70.41512 ABSTRAL 600 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 42747-0226-32 70.414<strong>08</strong> ABSTRAL 600 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 57881-0336-04 70.41512 ABSTRAL 600 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 57881-0336-32 70.414<strong>08</strong> ABSTRAL 600 MCG TAB SUBLINGUAL G GALENA BIOPHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 42747-0228-04 86.12495 ABSTRAL 800 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 42747-0228-32 86.12495 ABSTRAL 800 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 57881-0338-04 86.12495 ABSTRAL 800 MCG TAB SUBLINGUAL G GALENA BIOPHARM EABND 63459-0512-30 31.71670 107.93320 ACTIQ 1,200 MCG LOZENGE G TEVA USA EABND 63459-0516-30 35.20160 133.13200 ACTIQ 1,600 MCG LOZENGE G TEVA USA EABND 63459-0502-01 10.87125 45.21840 ACTIQ 200 MCG LOZENGE G TEVA USA EABND 63459-0502-30 10.87125 45.21840 ACTIQ 200 MCG LOZENGE G TEVA USA EABND 63459-0504-30 13.77322 57.27000 ACTIQ 400 MCG LOZENGE G TEVA USA EABND 63459-05<strong>06</strong>-30 16.86995 70.11840 ACTIQ 600 MCG LOZENGE G TEVA USA EABND 63459-05<strong>08</strong>-30 24.39546 83.03320 ACTIQ 800 MCG LOZENGE G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 54092-0383-01 7.09442 7.09442 ADDERALL XR 10 MG CAPSULE G SHIRE US INC. EABND 54092-0385-01 7.09442 7.09442 ADDERALL XR 15 MG CAPSULE G SHIRE US INC. EABND 54092-0387-01 7.09442 7.09442 ADDERALL XR 20 MG CAPSULE G SHIRE US INC. EABND 54092-0389-01 7.09442 7.09442 ADDERALL XR 25 MG CAPSULE G SHIRE US INC. EABND 54092-0391-01 7.09442 7.09442 ADDERALL XR 30 MG CAPSULE G SHIRE US INC. EABND 54092-0381-01 7.09442 7.09442 ADDERALL XR 5 MG CAPSULE G SHIRE US INC. EABND 00555-0764-02 0.92700 4.58840 ADDERALL 10 MG TABLET G BARR EAGEN 57844-0110-01 0.92700 ADDERALL 10 MG TABLET G TEVA USA EABND 00555-0765-02 1.13141 4.58840 ADDERALL 12.5 MG TABLET G BARR EABND 00555-0766-02 1.<strong>06</strong>650 4.58840 ADDERALL 15 MG TABLET G BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00555-0767-02 1.<strong>06</strong>580 4.58840 ADDERALL 20 MG TABLET G BARR EAGEN 57844-0120-01 1.<strong>06</strong>580 ADDERALL 20 MG TABLET G TEVA USA EABND 00555-0768-02 0.90070 4.58840 ADDERALL 30 MG TABLET G BARR EAGEN 57844-0130-01 0.90070 ADDERALL 30 MG TABLET G TEVA USA EABND 00555-0762-02 0.92700 4.58840 ADDERALL 5 MG TABLET G BARR EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 431LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00555-0763-02 0.98130 4.58840 ADDERALL 7.5 MG TABLET G BARR EAGEN 00185-0111-01 0.92700 AMPHETAMINE SALTS 10 MG TAB G SANDOZ EAGEN 00555-0972-02 0.92700 AMPHETAMINE SALTS 10 MG TAB G TEVA USA EAGEN 64720-0132-10 0.92700 AMPHETAMINE SALTS 10 MG TAB G COREPHARMA LLC EAGEN 00555-0776-02 1.13141 AMPHETAMINE SALTS 12.5 MG TB G TEVA USA EAGEN 00555-0777-02 1.<strong>06</strong>650 AMPHETAMINE SALTS 15 MG TAB G TEVA USA EAGEN 00185-0401-01 1.<strong>06</strong>580 AMPHETAMINE SALTS 20 MG TABLET G SANDOZ EAGEN 00555-0973-02 1.<strong>06</strong>580 AMPHETAMINE SALTS 20 MG TABLET G TEVA USA EAGEN 64720-0135-10 1.<strong>06</strong>580 AMPHETAMINE SALTS 20 MG TABLET G COREPHARMA LLC EAGEN 00185-0404-01 0.90070 AMPHETAMINE SALTS 30 MG TAB G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00555-0974-02 0.90070 AMPHETAMINE SALTS 30 MG TAB G TEVA USA EAGEN 64720-0136-10 0.90070 AMPHETAMINE SALTS 30 MG TAB G COREPHARMA LLC EAGEN 00185-0<strong>08</strong>4-01 0.92700 AMPHETAMINE SALTS 5 MG TAB G SANDOZ EAGEN 00555-0971-02 0.92700 AMPHETAMINE SALTS 5 MG TAB G BARR EAGEN 64720-0130-10 0.92700 AMPHETAMINE SALTS 5 MG TAB G COREPHARMA LLC EABND 00555-0775-02 0.98130 1.42262 AMPHETAMINE SALTS 7.5 MG TAB G BARR EABND 60793-<strong>06</strong><strong>08</strong>-01 18.20347 AVINZA 120 MG CAPSULE G PFIZER US PHARM EABND 60793-<strong>06</strong>05-01 5.28394 AVINZA 30 MG CAPSULE G PFIZER US PHARM EABND 60793-<strong>06</strong>03-01 7.83470 AVINZA 45 MG CAPSULE G PFIZER US PHARM EABND 60793-<strong>06</strong><strong>06</strong>-01 10.26<strong>08</strong>7 AVINZA 60 MG CAPSULE G PFIZER US PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 60793-<strong>06</strong>04-01 13.05772 AVINZA 75 MG CAPSULE G PFIZER US PHARM EABND 60793-<strong>06</strong>07-01 15.42804 AVINZA 90 MG CAPSULE G PFIZER US PHARM EABND 00574-7045-12 21.14771 BELLADONNA-OPIUM 16.2-30 SUPP G PADDOCK LABS. EABND 00574-7040-12 25.7<strong>08</strong>56 BELLADONNA-OPIUM 16.2-60 SUPP 0 PADDOCK LABS. EABND 00037-1221-50 27.81562 CESAMET 1 MG CAPSULE 0 MEDA PHARMACEUT EABND 00054-0243-24 0.47915 CODEINE SULFATE 15 MG TABLET G ROXANE LABS. EABND 00054-0244-25 0.36190 0.51584 CODEINE SULFATE 30 MG TABLET G ROXANE LABS. EABND 51224-0300-10 0.21321 CODEINE SULFATE 30 MG/5 ML SOL G TAGI PHARMA MLBND 00054-0245-25 0.94478 CODEINE SULFATE 60 MG TABLET G ROXANE LABS. EABND 50458-0585-01 4.97744 7.57275 CONCERTA ER 18 MG TABLET G JANSSEN PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50458-0588-01 5.10220 7.76265 CONCERTA ER 27 MG TABLET G JANSSEN PHARM. EABND 50458-0586-01 5.26290 8.00701 CONCERTA ER 36 MG TABLET G JANSSEN PHARM. EABND 50458-0587-01 5.72660 8.71251 CONCERTA ER 54 MG TABLET G JANSSEN PHARM. EAGEN 004<strong>06</strong>-8961-01 3.56990 D-AMPHETAMINE ER 10 MG CAPSULE G MALLINCKRODT PH EAGEN 00555-0955-02 3.56990 D-AMPHETAMINE ER 10 MG CAPSULE G BARR EAGEN 54505-0328-09 3.56990 D-AMPHETAMINE ER 10 MG CAPSULE G LINEAGE THERAPE EAGEN 64720-0328-09 3.56990 D-AMPHETAMINE ER 10 MG CAPSULE G COREPHARMA LLC EAGEN 004<strong>06</strong>-8962-01 4.55200 D-AMPHETAMINE ER 15 MG CAPSULE G MALLINCKRODT PH EAGEN 00555-0956-02 4.55200 D-AMPHETAMINE ER 15 MG CAPSULE G BARR EAGEN 54505-0329-09 4.55200 D-AMPHETAMINE ER 15 MG CAPSULE G LINEAGE THERAPE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64720-0329-09 4.55200 D-AMPHETAMINE ER 15 MG CAPSULE G COREPHARMA LLC EAGEN 004<strong>06</strong>-8960-01 2.85788 D-AMPHETAMINE ER 5 MG CAPSULE G MALLINCKRODT PH EAGEN 00555-0954-02 2.85788 D-AMPHETAMINE ER 5 MG CAPSULE G BARR EAGEN 54505-0327-09 2.85788 D-AMPHETAMINE ER 5 MG CAPSULE G LINEAGE THERAPE EAGEN 64720-0327-09 2.85788 D-AMPHETAMINE ER 5 MG CAPSULE G COREPHARMA LLC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 432LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 68968-5552-03 7.58149 DAYTRANA 10 MG/9 HR PATCH G NOVEN THERAPEUT EABND 68968-5553-03 7.58149 DAYTRANA 15 MG/9 HR PATCH G NOVEN THERAPEUT EABND 68968-5554-03 7.58149 DAYTRANA 20 MG/9 HOUR PATCH G NOVEN THERAPEUT EABND 68968-5555-03 7.58149 DAYTRANA 30 MG/9 HOUR PATCH G NOVEN THERAPEUT EABND 00024-0337-04 0.28998 3.07498 DEMEROL 100 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00024-0337-05 0.28998 3.93959 DEMEROL 100 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABND 00409-1180-69 4.37244 DEMEROL 100 MG/ML SYRINGE G HOSPIRA MLBND 00409-1201-20 4.00350 DEMEROL 100 MG/ML VIAL G HOSPIRA MLBND 00409-1176-30 4.52184 DEMEROL 25 MG/ML SYRINGE G HOSPIRA MLBND 00024-0335-05 0.20588 2.07134 DEMEROL 50 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-1203-01 3.<strong>08</strong>760 DEMEROL 50 MG/ML AMPUL G HOSPIRA MLBND 00409-1253-01 2.77884 DEMEROL 50 MG/ML AMPUL G HOSPIRA MLBND 00409-1255-02 1.45416 DEMEROL 50 MG/ML AMPUL G HOSPIRA MLBND 00409-1178-30 4.66128 DEMEROL 50 MG/ML SYRINGE G HOSPIRA MLBND 00409-1179-30 4.37244 DEMEROL 75 MG/ML SYRINGE G HOSPIRA MLBND 00409-1254-01 1.78616 DEMEROL 75 MG/1.5 ML AMPUL 0 HOSPIRA MLBND 55292-0102-01 3.59268 5.73745 DESOXYN 5 MG TABLET G RECORDATI RARE EABND 67386-0102-01 3.59268 4.58998 DESOXYN 5 MG TABLET G RECORDATI RARE EABND 52054-0513-09 3.56990 11.20509 DEXEDRINE SPANSULE 10 MG G AMEDRA PHARMACE EABND 52054-0514-09 4.55200 11.20509 DEXEDRINE SPANSULE 15 MG G AMEDRA PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 52054-0512-09 2.85788 11.20509 DEXEDRINE SPANSULE 5 MG G AMEDRA PHARMACE EAGEN 00781-2684-01 5.77972 DEXMETHYLPHENIDATE ER 15 MG CP G SANDOZ EAGEN 49884-0428-01 5.55622 DEXMETHYLPHENIDATE ER 15 MG CP G PAR PHARM. EAGEN 00378-4<strong>08</strong>4-01 5.34682 DEXMETHYLPHENIDATE ER 30 MG CP G MYLAN EAGEN 49884-0430-01 5.83417 DEXMETHYLPHENIDATE ER 30 MG CP G PAR PHARM. EAGEN 00093-5562-01 6.12637 DEXMETHYLPHENIDATE ER 40 MG CP G TEVA USA EAGEN 00093-5277-01 1.05000 DEXMETHYLPHENIDATE 10 MG TAB G TEVA USA EAGEN 00093-5275-01 0.51225 DEXMETHYLPHENIDATE 2.5 MG TAB G TEVA USA EAGEN 00093-5276-01 0.73027 DEXMETHYLPHENIDATE 5 MG TAB G TEVA USA EAGEN 00115-1329-01 4.59862 DEXTROAMP-AMPHET ER 10 MG CAP G GLOBAL PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-3059-11 5.28735 DEXTROAMP-AMPHET ER 10 MG CAP G ACTAVIS PHARMA, EAGEN 00555-0787-02 4.59862 DEXTROAMP-AMPHET ER 10 MG CAP G TEVA USA EAGEN 00115-1330-01 4.59862 DEXTROAMP-AMPHET ER 15 MG CAP G GLOBAL PHARM EAGEN 00228-3<strong>06</strong>3-11 5.28735 DEXTROAMP-AMPHET ER 15 MG CAP G ACTAVIS PHARMA, EAGEN 00555-0791-02 4.59862 DEXTROAMP-AMPHET ER 15 MG CAP G TEVA USA EAGEN 00115-1331-01 4.59862 DEXTROAMP-AMPHET ER 20 MG CAP G GLOBAL PHARM EAGEN 00228-3<strong>06</strong>0-11 5.28735 DEXTROAMP-AMPHET ER 20 MG CAP G ACTAVIS PHARMA, EAGEN 00555-0788-02 4.59862 DEXTROAMP-AMPHET ER 20 MG CAP G TEVA USA EAGEN 00115-1332-01 4.59862 DEXTROAMP-AMPHET ER 25 MG CAP G GLOBAL PHARM EAGEN 00228-3<strong>06</strong>4-11 5.28735 DEXTROAMP-AMPHET ER 25 MG CAP G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00555-0792-02 4.59862 DEXTROAMP-AMPHET ER 25 MG CAP G TEVA USA EAGEN 00115-1333-01 4.59862 DEXTROAMP-AMPHET ER 30 MG CAP G GLOBAL PHARM EAGEN 00228-3<strong>06</strong>1-11 5.28735 DEXTROAMP-AMPHET ER 30 MG CAP G ACTAVIS PHARMA, EAGEN 00555-0789-02 4.59862 DEXTROAMP-AMPHET ER 30 MG CAP G TEVA USA EAGEN 00115-1328-01 4.59862 DEXTROAMP-AMPHET ER 5 MG CAP G GLOBAL PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 433LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-3<strong>06</strong>2-11 5.28735 DEXTROAMP-AMPHET ER 5 MG CAP G ACTAVIS PHARMA, EAGEN 00555-0790-02 4.59862 DEXTROAMP-AMPHET ER 5 MG CAP G TEVA USA EAGUL 004<strong>06</strong>-8959-01 0.34350 DEXTROAMPHETAMINE 10 MG TAB G MALLINCKRODT PH EAGUL 00555-0953-02 0.34350 DEXTROAMPHETAMINE 10 MG TAB G BARR EAGUL 13107-0036-01 0.34350 DEXTROAMPHETAMINE 10 MG TAB G AUROBINDO PHARM EAGUL 52536-0510-01 0.34350 DEXTROAMPHETAMINE 10 MG TAB G WILSHIRE PHARMA EAGEN 004<strong>06</strong>-8958-01 2.17500 DEXTROAMPHETAMINE 5 MG TAB G MALLINCKRODT PH EAGEN 00555-0952-02 2.17500 DEXTROAMPHETAMINE 5 MG TAB G BARR EAGEN 13107-0035-01 2.17500 DEXTROAMPHETAMINE 5 MG TAB G AUROBINDO PHARM EAGEN 52536-0500-01 2.18250 DEXTROAMPHETAMINE 5 MG TAB G WILSHIRE PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 278<strong>08</strong>-0<strong>08</strong>5-01 1.36839 DEXTROAMPHETAMINE 5 MG/5 ML G TRIS PHARMA INC MLGEN 76181-0002-25 1.36839 DEXTROAMPHETAMINE 5 MG/5 ML G TALEC PHARMA MLBND 59011-0451-01 0.31345 0.49965 DILAUDID 1 MG/ML LIQUID G PURDUE PHARMA L MLBND 59011-0452-01 0.09491 1.36236 DILAUDID 2 MG TABLET G PURDUE PHARMA L EABND 59011-0452-10 0.09491 1.07758 DILAUDID 2 MG TABLET G PURDUE PHARMA L EABND 59011-0442-10 1.50<strong>06</strong>4 DILAUDID 2 MG/ML AMPUL G PURDUE PHARMA L MLBND 59011-0442-25 1.42892 DILAUDID 2 MG/ML AMPUL G PURDUE PHARMA L MLBND 59011-0454-01 0.11350 2.07226 DILAUDID 4 MG TABLET G PURDUE PHARMA L EABND 59011-0454-05 0.11350 1.67573 DILAUDID 4 MG TABLET G PURDUE PHARMA L EABND 59011-0454-10 0.11350 1.75901 DILAUDID 4 MG TABLET G PURDUE PHARMA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59011-0444-10 1.81770 DILAUDID 4 MG/ML AMPUL G PURDUE PHARMA L MLBND 59011-0458-10 3.20147 DILAUDID 8 MG TABLET G PURDUE PHARMA L EABND 59011-0445-01 2.00240 4.39153 DILAUDID-HP 10 MG/ML AMPUL G PURDUE PHARMA L MLBND 59011-0445-05 2.00240 4.17091 DILAUDID-HP 10 MG/ML AMPUL G PURDUE PHARMA L MLBND 59011-0445-50 1.72872 4.28578 DILAUDID-HP 10 MG/ML VIAL G PURDUE PHARMA L MLBND 59011-0446-25 95.28400 DILAUDID-HP 250 MG VIAL G PURDUE PHARMA L EABND 50458-0094-05 17.72190 111.<strong>08</strong>222 DURAGESIC 100 MCG/HR PATCH G JANSSEN PHARM. EABND 50458-0090-05 14.02500 24.85684 DURAGESIC 12 MCG/HR PATCH G JANSSEN PHARM. EABND 50458-0091-05 4.61000 30.01114 DURAGESIC 25 MCG/HR PATCH G JANSSEN PHARM. EABND 50458-0092-05 15.13260 54.87130 DURAGESIC 50 MCG/HR PATCH G JANSSEN PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50458-0093-05 13.66400 83.69554 DURAGESIC 75 MCG/HR PATCH G JANSSEN PHARM. EABND 60977-0017-01 0.75696 DURAMORPH 1 MG/ML AMPUL G WEST-WARD,INC. MLGEN 60951-0712-70 0.82685 ENDOCET 10-325 MG TABLET G QUALITEST EAGUL 60951-<strong>06</strong>02-70 0.23400 ENDOCET 5-325 TABLET G QUALITEST EAGUL 60951-<strong>06</strong>02-85 0.23400 ENDOCET 5-325 TABLET G QUALITEST EAGEN 60951-0700-70 2.03655 ENDOCET 7.5-325 MG TABLET G QUALITEST EABND 60951-0310-70 0.98072 ENDODAN 4.83-325 MG TABLET G QUALITEST EABND 23635-0412-01 16.911<strong>08</strong> EXALGO ER 12 MG TABLET G MALLINCKRODT BR EABND 23635-0416-01 22.54827 EXALGO ER 16 MG TABLET G MALLINCKRODT BR EABND 23635-0432-01 45.09639 EXALGO ER 32 MG TABLET G MALLINCKRODT BR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 23635-04<strong>08</strong>-01 11.27413 EXALGO ER 8 MG TABLET G MALLINCKRODT BR EAGEN 004<strong>06</strong>-9212-30 31.71670 FENTANYL CIT OTFC 1,200 MCG G MALLINCKRODT PH EAGEN 49884-0463-52 31.71670 FENTANYL CIT OTFC 1,200 MCG G PAR PHARM. EAGEN 49884-0463-55 31.71670 FENTANYL CIT OTFC 1,200 MCG G PAR PHARM. EAGEN 55253-0074-01 31.71670 FENTANYL CIT OTFC 1,200 MCG G ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 434LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55253-0074-30 31.71670 FENTANYL CIT OTFC 1,200 MCG G ACTAVIS PHARMA, EAGEN 004<strong>06</strong>-9216-30 35.20160 FENTANYL CIT OTFC 1,600 MCG G MALLINCKRODT PH EAGEN 49884-0464-52 35.20160 FENTANYL CIT OTFC 1,600 MCG G PAR PHARM. EAGEN 49884-0464-55 35.20160 FENTANYL CIT OTFC 1,600 MCG G PAR PHARM. EAGEN 55253-0075-01 35.20160 FENTANYL CIT OTFC 1,600 MCG G ACTAVIS PHARMA, EAGEN 55253-0075-30 35.20160 FENTANYL CIT OTFC 1,600 MCG G ACTAVIS PHARMA, EAGEN 004<strong>06</strong>-9202-30 10.87125 FENTANYL CITRATE OTFC 200 MCG G MALLINCKRODT PH EAGEN 49884-0459-52 10.87125 FENTANYL CITRATE OTFC 200 MCG G PAR PHARM. EAGEN 49884-0459-55 10.87125 FENTANYL CITRATE OTFC 200 MCG G PAR PHARM. EAGEN 55253-0070-01 10.87125 FENTANYL CITRATE OTFC 200 MCG G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 55253-0070-30 10.87125 FENTANYL CITRATE OTFC 200 MCG G ACTAVIS PHARMA, EAGEN 49884-0460-52 13.77322 FENTANYL CITRATE OTFC 400 MCG G PAR PHARM. EAGEN 49884-0460-55 13.77322 FENTANYL CITRATE OTFC 400 MCG G PAR PHARM. EAGEN 55253-0071-01 13.77322 FENTANYL CITRATE OTFC 400 MCG G ACTAVIS PHARMA, EAGEN 55253-0071-30 13.77322 FENTANYL CITRATE OTFC 400 MCG G ACTAVIS PHARMA, EAGEN 004<strong>06</strong>-92<strong>06</strong>-30 16.86995 FENTANYL CITRATE OTFC 600 MCG G MALLINCKRODT PH EAGEN 49884-0461-52 16.86995 FENTANYL CITRATE OTFC 600 MCG G PAR PHARM. EAGEN 49884-0461-55 16.86995 FENTANYL CITRATE OTFC 600 MCG G PAR PHARM. EAGEN 55253-0072-01 16.86995 FENTANYL CITRATE OTFC 600 MCG G ACTAVIS PHARMA, EAGEN 004<strong>06</strong>-92<strong>08</strong>-30 24.39546 FENTANYL CITRATE OTFC 800 MCG G MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-0462-52 24.39546 FENTANYL CITRATE OTFC 800 MCG G PAR PHARM. EAGEN 49884-0462-55 24.39546 FENTANYL CITRATE OTFC 800 MCG G PAR PHARM. EAGEN 55253-0073-01 24.39546 FENTANYL CITRATE OTFC 800 MCG G ACTAVIS PHARMA, EAGEN 55253-0073-30 24.39546 FENTANYL CITRATE OTFC 800 MCG G ACTAVIS PHARMA, EAGEN 00409-9093-32 0.09330 FENTANYL 0.05 MG/ML AMPUL G HOSPIRA MLGEN 00409-9093-38 0.07200 FENTANYL 0.05 MG/ML AMPUL G HOSPIRA MLGEN 10019-0033-72 0.09330 FENTANYL 0.05 MG/ML AMPUL G WEST-WARD,INC. MLGEN 00409-9094-61 0.<strong>08</strong>316 FENTANYL 0.05 MG/ML VIAL G HOSPIRA MLGEN 10019-0037-83 0.17280 FENTANYL 0.05 MG/ML VIAL G WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6026-01 0.09330 FENTANYL 1,000 MCG/20 ML AMPUL G WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>41-6026-05 0.09330 FENTANYL 1,000 MCG/20 ML AMPUL G WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6029-01 0.2<strong>08</strong>80 FENTANYL 1,000 MCG/20 ML VIAL G WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6029-25 0.2<strong>08</strong>80 FENTANYL 1,000 MCG/20 ML VIAL G WEST-WARD,INC. MLGEN 00093-6903-45 17.72190 FENTANYL 100 MCG/HR PATCH G TEVA USA EAGEN 00245-0423-05 17.72190 FENTANYL 100 MCG/HR PATCH G UPSHER SMITH EAGEN 00245-0423-89 17.72190 FENTANYL 100 MCG/HR PATCH G UPSHER SMITH EAGEN 00378-9124-16 17.72190 FENTANYL 100 MCG/HR PATCH G MYLAN EAGEN 00378-9124-98 17.72190 FENTANYL 100 MCG/HR PATCH G MYLAN EAGEN 004<strong>06</strong>-9000-76 17.72190 FENTANYL 100 MCG/HR PATCH G MALLINCKRODT PH EAGEN 00591-3214-72 17.72190 FENTANYL 100 MCG/HR PATCH G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3603-54 17.72190 FENTANYL 100 MCG/HR PATCH G ACTAVIS PHARMA, EAGEN 00781-7244-55 17.72190 FENTANYL 100 MCG/HR PATCH G SANDOZ EAGEN 49884-0764-78 17.72190 FENTANYL 100 MCG/HR PATCH G PAR PHARM. EAGEN 60505-7004-00 17.72190 FENTANYL 100 MCG/HR PATCH G APOTEX CORP EAGEN 60505-7004-02 17.72190 FENTANYL 100 MCG/HR PATCH G APOTEX CORP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 435LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-7009-00 17.72190 FENTANYL 100 MCG/HR PATCH G APOTEX CORP EAGEN 60505-7009-02 17.72190 FENTANYL 100 MCG/HR PATCH G APOTEX CORP EAGEN 67767-0123-18 17.72190 FENTANYL 100 MCG/HR PATCH G PAR PHARM. EAGEN 0<strong>06</strong>41-6024-01 0.09330 FENTANYL 100 MCG/2 ML AMPUL G WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6024-10 0.09330 FENTANYL 100 MCG/2 ML AMPUL G WEST-WARD,INC. MLGEN 00378-9119-16 14.02500 FENTANYL 12 MCG/HR PATCH G MYLAN EAGEN 00378-9119-98 14.02500 FENTANYL 12 MCG/HR PATCH G MYLAN EAGEN 00781-7240-55 14.02500 FENTANYL 12 MCG/HR PATCH G SANDOZ EAGEN 00093-6900-45 4.61000 FENTANYL 25 MCG/HR PATCH G TEVA USA EAGEN 00245-0420-05 4.61000 FENTANYL 25 MCG/HR PATCH G UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-9121-16 4.61000 FENTANYL 25 MCG/HR PATCH G MYLAN EAGEN 00378-9121-98 4.61000 FENTANYL 25 MCG/HR PATCH G MYLAN EAGEN 004<strong>06</strong>-9025-76 4.61000 FENTANYL 25 MCG/HR PATCH G MALLINCKRODT PH EAGEN 00591-3198-72 4.61000 FENTANYL 25 MCG/HR PATCH G ACTAVIS PHARMA, EAGEN 00591-3600-54 4.61000 FENTANYL 25 MCG/HR PATCH G ACTAVIS PHARMA, EAGEN 00781-7241-55 4.61000 FENTANYL 25 MCG/HR PATCH G SANDOZ EAGEN 49884-0761-52 4.61000 FENTANYL 25 MCG/HR PATCH G PAR PHARM. EAGEN 49884-0761-78 4.61000 FENTANYL 25 MCG/HR PATCH G PAR PHARM. EAGEN 60505-7001-02 4.61000 FENTANYL 25 MCG/HR PATCH G APOTEX CORP EAGEN 60505-70<strong>06</strong>-00 4.61000 FENTANYL 25 MCG/HR PATCH G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-70<strong>06</strong>-02 4.61000 FENTANYL 25 MCG/HR PATCH G APOTEX CORP EAGEN 67767-0120-18 4.61000 FENTANYL 25 MCG/HR PATCH G PAR PHARM. EAGEN 0<strong>06</strong>41-6025-01 0.09330 FENTANYL 250 MCG/5 ML AMPUL G WEST-WARD,INC. MLGEN 0<strong>06</strong>41-6025-10 0.09330 FENTANYL 250 MCG/5 ML AMPUL G WEST-WARD,INC. MLGEN 00093-6901-45 15.13260 FENTANYL 50 MCG/HR PATCH G TEVA USA EAGEN 00245-0421-05 15.13260 FENTANYL 50 MCG/HR PATCH G UPSHER SMITH EAGEN 00378-9122-16 15.13260 FENTANYL 50 MCG/HR PATCH G MYLAN EAGEN 00378-9122-98 15.13260 FENTANYL 50 MCG/HR PATCH G MYLAN EAGEN 004<strong>06</strong>-9050-76 15.13260 FENTANYL 50 MCG/HR PATCH G MALLINCKRODT PH EAGEN 00591-3212-72 15.13260 FENTANYL 50 MCG/HR PATCH G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3601-54 15.13260 FENTANYL 50 MCG/HR PATCH G ACTAVIS PHARMA, EAGEN 00781-7242-55 15.13260 FENTANYL 50 MCG/HR PATCH G SANDOZ EAGEN 49884-0762-78 15.13260 FENTANYL 50 MCG/HR PATCH G PAR PHARM. EAGEN 60505-7002-02 15.13260 FENTANYL 50 MCG/HR PATCH G APOTEX CORP EAGEN 60505-7007-00 15.13260 FENTANYL 50 MCG/HR PATCH G APOTEX CORP EAGEN 60505-7007-02 15.13260 FENTANYL 50 MCG/HR PATCH G APOTEX CORP EAGEN 67767-0121-18 15.13260 FENTANYL 50 MCG/HR PATCH G PAR PHARM. EAGEN 00093-6902-45 13.66400 FENTANYL 75 MCG/HR PATCH G TEVA USA EAGEN 00245-0422-05 13.66400 FENTANYL 75 MCG/HR PATCH G UPSHER SMITH EAGEN 00245-0422-89 13.66400 FENTANYL 75 MCG/HR PATCH G UPSHER SMITH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-9123-16 13.66400 FENTANYL 75 MCG/HR PATCH G MYLAN EAGEN 00378-9123-98 13.66400 FENTANYL 75 MCG/HR PATCH G MYLAN EAGEN 004<strong>06</strong>-9075-76 13.66400 FENTANYL 75 MCG/HR PATCH G MALLINCKRODT PH EAGEN 00591-3213-72 13.66400 FENTANYL 75 MCG/HR PATCH G ACTAVIS PHARMA, EAGEN 00591-3602-54 13.66400 FENTANYL 75 MCG/HR PATCH G ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 436LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-7243-55 13.66400 FENTANYL 75 MCG/HR PATCH G SANDOZ EAGEN 49884-0763-78 13.66400 FENTANYL 75 MCG/HR PATCH G PAR PHARM. EAGEN 60505-7003-02 13.66400 FENTANYL 75 MCG/HR PATCH G APOTEX CORP EAGEN 60505-70<strong>08</strong>-00 13.66400 FENTANYL 75 MCG/HR PATCH G APOTEX CORP EAGEN 60505-70<strong>08</strong>-02 13.66400 FENTANYL 75 MCG/HR PATCH G APOTEX CORP EAGEN 67767-0122-18 13.66400 FENTANYL 75 MCG/HR PATCH G PAR PHARM. EABND 63459-0541-28 31.51628 FENTORA 100 MCG BUCCAL TABLET G CEPHALON,INC.-T EABND 63459-0542-28 39.80442 FENTORA 200 MCG BUCCAL TABLET G CEPHALON,INC.-T EABND 63459-0544-28 57.76800 FENTORA 400 MCG BUCCAL TABLET G CEPHALON,INC.-T EABND 63459-0546-28 75.02013 FENTORA 600 MCG BUCCAL TABLET G CEPHALON,INC.-T EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63459-0548-28 92.41457 FENTORA 800 MCG BUCCAL TABLET G CEPHALON,INC.-T EABND 00078-0431-05 7.30159 FOCALIN XR 10 MG CAPSULE G NOVARTIS EABND 00078-0493-05 6.83247 FOCALIN XR 15 MG CAPSULE G NOVARTIS EABND 00078-0432-05 7.5<strong>08</strong>84 FOCALIN XR 20 MG CAPSULE G NOVARTIS EABND 00078-<strong>06</strong><strong>08</strong>-05 7.88441 FOCALIN XR 25 MG CAPSULE G NOVARTIS EABND 00078-0433-05 6.58190 FOCALIN XR 30 MG CAPSULE G NOVARTIS EABND 00078-<strong>06</strong>09-05 8.27891 FOCALIN XR 35 MG CAPSULE G NOVARTIS EABND 00078-0434-05 7.53316 FOCALIN XR 40 MG CAPSULE G NOVARTIS EABND 00078-0430-05 7.19751 FOCALIN XR 5 MG CAPSULE G NOVARTIS EABND 00078-0382-05 1.29189 FOCALIN 10 MG TABLET G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0380-05 0.63013 FOCALIN 2.5 MG TABLET G NOVARTIS EABND 00078-0381-05 0.89830 FOCALIN 5 MG TABLET G NOVARTIS EAGEN 38779-0731-<strong>06</strong> 320.62500 HYDROMORPHONE HCL POWDER G MEDISCA INC. GMBND 00409-1283-05 4.58160 HYDROMORPHONE 0.5 MG/0.5 ML G HOSPIRA MLGEN 00054-0386-63 0.29987 HYDROMORPHONE 1 MG/ML SOLUTION G ROXANE LABS. MLBND 00409-1283-10 2.07168 HYDROMORPHONE 1 MG/ML SYRINGE G HOSPIRA MLGEN 00409-2634-01 1.72872 HYDROMORPHONE 10 MG/ML VIAL G HOSPIRA MLGEN 00703-0018-01 1.72872 HYDROMORPHONE 10 MG/ML VIAL G TEVA PARENTERAL MLGEN 00703-0110-03 1.72872 HYDROMORPHONE 10 MG/ML VIAL G TEVA PARENTERAL MLGEN 004<strong>06</strong>-3243-01 0.09491 HYDROMORPHONE 2 MG TABLET G MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00527-1353-01 0.09491 HYDROMORPHONE 2 MG TABLET G LANNETT CO. INC EAGEN 42858-0301-01 0.09491 HYDROMORPHONE 2 MG TABLET G RHODES PHARMACE EAGEN 68<strong>08</strong>4-0423-01 0.09491 HYDROMORPHONE 2 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0423-11 0.09491 HYDROMORPHONE 2 MG TABLET G AHP EABND 00409-1312-10 2.23104 HYDROMORPHONE 2 MG/ML SYRINGE G HOSPIRA MLBND 00409-1312-30 2.23104 HYDROMORPHONE 2 MG/ML SYRINGE G HOSPIRA MLGEN 0<strong>06</strong>41-0121-25 1.29600 HYDROMORPHONE 2 MG/ML VIAL G WEST-WARD,INC. MLGEN 0<strong>06</strong>41-2341-41 0.58500 HYDROMORPHONE 2 MG/ML VIAL G WEST-WARD,INC. MLBND 00574-7224-<strong>06</strong> 10.18133 HYDROMORPHONE 3 MG SUPPOS G PADDOCK LABS. EAGEN 00054-0264-24 0.11350 HYDROMORPHONE 4 MG TABLET G ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0264-25 0.11350 HYDROMORPHONE 4 MG TABLET G ROXANE LABS. EAGEN 004<strong>06</strong>-3244-01 0.11350 HYDROMORPHONE 4 MG TABLET G MALLINCKRODT PH EAGEN 00527-1354-01 0.11350 HYDROMORPHONE 4 MG TABLET G LANNETT CO. INC EAGEN 42858-0302-01 0.11350 HYDROMORPHONE 4 MG TABLET G RHODES PHARMACE EAGEN 42858-0302-50 0.11350 HYDROMORPHONE 4 MG TABLET G RHODES PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 437LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0472-01 0.11350 HYDROMORPHONE 4 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0472-11 0.11350 HYDROMORPHONE 4 MG TABLET G AHP EABND 00409-1304-31 2.07168 HYDROMORPHONE 4 MG/ML SYRIN G HOSPIRA MLGEN 42858-0304-16 0.29987 HYDROMORPHONE 5 MG/5 ML SOLN G RHODES PHARMACE MLGEN 00409-2634-05 1.45620 HYDROMORPHONE 50 MG/5 ML VIAL G HOSPIRA MLGEN 00703-0113-03 1.69560 HYDROMORPHONE 50 MG/5 ML VIAL G TEVA PARENTERAL MLGEN 00409-2634-50 1.58760 HYDROMORPHONE 500 MG/50 ML VIA G HOSPIRA MLGEN 00054-0265-25 0.98947 HYDROMORPHONE 8 MG TABLET G ROXANE LABS. EAGEN 004<strong>06</strong>-3249-01 1.03815 HYDROMORPHONE 8 MG TABLET G MALLINCKRODT PH EAGEN 00527-1355-01 0.29025 HYDROMORPHONE 8 MG TABLET G LANNETT CO. INC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42858-0303-01 0.29025 HYDROMORPHONE 8 MG TABLET G RHODES PHARMACE EAGEN 51224-0102-50 0.29025 HYDROMORPHONE 8 MG TABLET G TAGI PHARMA EABND 60977-0114-01 6.47400 INFUMORPH 10 MG/ML AMPUL P-F G WEST-WARD,INC. MLBND 60977-0114-74 6.47400 INFUMORPH 10 MG/ML AMPUL P-F G WEST-WARD,INC. MLBND 60977-0115-01 10.95600 INFUMORPH 25 MG/ML AMPUL P-F G WEST-WARD,INC. MLBND 60977-0115-74 10.95600 INFUMORPH 25 MG/ML AMPUL P-F G WEST-WARD,INC. MLBND 46987-0410-11 5.05968 5.05968 KADIAN ER 10 MG CAPSULE G ACTAVIS PHARMA, EABND 46987-0324-11 19.97976 19.97976 KADIAN ER 100 MG CAPSULE G ACTAVIS PHARMA, EABND 46987-0329-11 26.41392 KADIAN ER 130 MG CAPSULE G ACTAVIS PHARMA, EABND 46987-0330-11 30.47760 KADIAN ER 150 MG CAPSULE G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 46987-0322-11 5.58756 5.58756 KADIAN ER 20 MG CAPSULE G ACTAVIS PHARMA, EABND 46987-0377-11 41.05512 KADIAN ER 200 MG CAPSULE G ACTAVIS PHARMA, EABND 46987-0325-11 6.07560 6.07560 KADIAN ER 30 MG CAPSULE G ACTAVIS PHARMA, EABND 46987-0327-11 8.10744 KADIAN ER 40 MG CAPSULE G ACTAVIS PHARMA, EABND 46987-0323-11 10.15920 10.15920 KADIAN ER 50 MG CAPSULE G ACTAVIS PHARMA, EABND 46987-0326-11 12.16116 12.16116 KADIAN ER 60 MG CAPSULE G ACTAVIS PHARMA, EABND 46987-0328-11 14.18304 KADIAN ER 70 MG CAPSULE G ACTAVIS PHARMA, EABND 46987-0412-11 16.19496 16.19496 KADIAN ER 80 MG CAPSULE G ACTAVIS PHARMA, EABND 00054-0438-25 1.77719 LEVORPHANOL 2 MG TABLET G ROXANE LABS. EABND 59630-0994-10 7.13243 MAGNACET 10 MG-400 MG TABLET G SHIONOGI PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 68453-0994-10 5.94371 MAGNACET 10 MG-400 MG TABLET G SHIONOGI PHARMA EABND 59630-0992-10 7.13243 MAGNACET 5 MG-400 MG TABLET G SHIONOGI PHARMA EABND 59630-0993-10 7.13243 MAGNACET 7.5 MG-400 MG TABLET G SHIONOGI PHARMA EABND 00409-6030-04 0.25597 MEPERIDINE 10 MG/ML CARTRDGE G HOSPIRA MLGEN 00054-4596-25 0.28998 MEPERIDINE 100 MG TABLET G ROXANE LABS. EAGEN 00555-0382-02 0.28998 MEPERIDINE 100 MG TABLET G BARR EAGEN 428<strong>06</strong>-0051-01 0.28998 MEPERIDINE 100 MG TABLET G EPIC PHARMA LLC EABND 0<strong>06</strong>41-6054-01 1.36120 MEPERIDINE 100 MG/ML VIAL G WEST-WARD,INC. MLBND 0<strong>06</strong>41-6054-25 1.36452 MEPERIDINE 100 MG/ML VIAL G WEST-WARD,INC. MLBND 0<strong>06</strong>41-6052-01 1.19520 MEPERIDINE 25 MG/ML VIAL G WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 0<strong>06</strong>41-6052-25 1.19520 MEPERIDINE 25 MG/ML VIAL G WEST-WARD,INC. MLGEN 00054-4595-25 0.20588 MEPERIDINE 50 MG TABLET G ROXANE LABS. EAGEN 00054-8595-11 0.20588 MEPERIDINE 50 MG TABLET G ROXANE LABS. EAGEN 00555-0381-02 0.20588 MEPERIDINE 50 MG TABLET G BARR EAGEN 0<strong>06</strong>03-4415-21 0.20588 MEPERIDINE 50 MG TABLET G QUALITEST EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 438LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 428<strong>06</strong>-0050-01 0.20588 MEPERIDINE 50 MG TABLET G EPIC PHARMA LLC EABND 0<strong>06</strong>41-6053-01 1.24500 MEPERIDINE 50 MG/ML VIAL G WEST-WARD,INC. MLBND 0<strong>06</strong>41-6053-25 1.24500 MEPERIDINE 50 MG/ML VIAL G WEST-WARD,INC. MLBND 00054-3545-63 0.20789 MEPERIDINE 50 MG/5 ML SOLUTION G ROXANE LABS. MLGEN 0<strong>06</strong>03-4416-21 0.28998 MEPERITAB 100 MG TABLET G QUALITEST EABND 53014-0579-07 4.39210 6.24002 METADATE CD 10 MG CAPSULE G UCB PHARMA EABND 53014-0580-07 4.39210 6.24002 METADATE CD 20 MG CAPSULE G UCB PHARMA EABND 53014-0581-07 4.39210 6.24002 METADATE CD 30 MG CAPSULE G UCB PHARMA EABND 53014-0582-07 6.02460 8.55929 METADATE CD 40 MG CAPSULE G UCB PHARMA EABND 53014-0583-07 7.40240 10.51693 METADATE CD 50 MG CAPSULE G UCB PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 53014-0584-07 7.40240 10.51693 METADATE CD 60 MG CAPSULE G UCB PHARMA EAGEN 53014-0594-07 1.62547 METADATE ER 20 MG TABLET G UCB PHARMA EAGEN 00054-4571-25 0.10503 METHADONE HCL 10 MG TABLET G ROXANE LABS. EAGEN 00054-8554-24 0.10503 METHADONE HCL 10 MG TABLET G ROXANE LABS. EAGEN 004<strong>06</strong>-5771-01 0.10503 METHADONE HCL 10 MG TABLET G MALLINCKRODT PH EAGEN 004<strong>06</strong>-5771-62 0.10503 METHADONE HCL 10 MG TABLET G MALLINCKRODT PH EAGEN 66689-<strong>08</strong>10-10 0.10503 METHADONE HCL 10 MG TABLET G VISTAPHARM EAGEN 67877-0116-01 0.10503 METHADONE HCL 10 MG TABLET G ASCEND LABORATO EABND 67457-0217-20 7.73975 METHADONE HCL 10 MG/ML VIAL G MYLAN INSTITUTI MLGEN 00054-4570-25 0.<strong>06</strong>652 METHADONE HCL 5 MG TABLET G ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-8553-24 0.07182 METHADONE HCL 5 MG TABLET G ROXANE LABS. EAGEN 004<strong>06</strong>-5755-01 0.<strong>06</strong>510 METHADONE HCL 5 MG TABLET G MALLINCKRODT PH EAGEN 004<strong>06</strong>-5755-62 0.07182 METHADONE HCL 5 MG TABLET G MALLINCKRODT PH EAGEN 00054-3553-44 0.62640 METHADONE INTENSOL 10 MG/ML G ROXANE LABS. MLGEN 00054-0391-68 0.07844 METHADONE 10 MG/ML ORAL CONC G ROXANE LABS. MLGEN 00054-0392-68 0.07844 METHADONE 10 MG/ML ORAL CONC G ROXANE LABS. MLGEN 66689-<strong>06</strong>94-30 0.62640 METHADONE 10 MG/ML ORAL CONC G VISTAPHARM MLGEN 66689-<strong>06</strong>94-39 0.<strong>06</strong><strong>08</strong>8 METHADONE 10 MG/ML ORAL CONC G VISTAPHARM MLGEN 66689-<strong>06</strong>94-79 0.<strong>06</strong><strong>08</strong>8 METHADONE 10 MG/ML ORAL CONC G VISTAPHARM MLGEN 66689-<strong>06</strong>95-79 0.<strong>06</strong><strong>08</strong>8 METHADONE 10 MG/ML ORAL CONC G VISTAPHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-3556-63 0.10360 METHADONE 10 MG/5 ML SOLUTION G ROXANE LABS. MLGEN 66689-0712-16 0.10980 METHADONE 10 MG/5 ML SOLUTION G VISTAPHARM MLGEN 00054-3555-63 0.05982 METHADONE 5 MG/5 ML SOLUTION G ROXANE LABS. MLGEN 66689-0711-16 0.07020 METHADONE 5 MG/5 ML SOLUTION G VISTAPHARM MLBND 004<strong>06</strong>-0527-10 0.<strong>08</strong>680 METHADOSE 10 MG/ML ORAL CONC G MALLINCKRODT PH MLBND 004<strong>06</strong>-8725-10 0.<strong>08</strong>680 METHADOSE 10 MG/ML ORAL CONC G MALLINCKRODT PH MLGEN 00378-8115-01 3.43687 METHAMPHETAMINE 5 MG TABLET G MYLAN EABND 59630-0762-10 5.93524 METHYLIN 10 MG CHEWABLE TABLET G SHIONOGI PHARMA EABND 59630-0755-50 1.18801 METHYLIN 10 MG/5 ML SOLUTION 0 SHIONOGI PHARMA MLBND 59630-0760-10 2.91462 METHYLIN 2.5 MG CHEWABLE TAB G SHIONOGI PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59630-0761-10 4.16328 METHYLIN 5 MG CHEWABLE TABLET G SHIONOGI PHARMA EABND 59630-0750-50 0.83333 METHYLIN 5 MG/5 ML SOLUTION 0 SHIONOGI PHARMA MLGEN 00093-5295-01 4.20157 METHYLPHENIDATE CD 10 MG CAP G TEVA USA EAGEN 62175-0151-37 4.32810 METHYLPHENIDATE CD 10 MG CAP G KREMERS URBAN EAGEN 00093-5296-01 4.20157 METHYLPHENIDATE CD 20 MG CAP G TEVA USA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 439LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62175-0152-37 4.32810 METHYLPHENIDATE CD 20 MG CAP G KREMERS URBAN EAGEN 00093-5297-01 4.20157 METHYLPHENIDATE CD 30 MG CAP G TEVA USA EAGEN 62175-0153-37 4.32810 METHYLPHENIDATE CD 30 MG CAP G KREMERS URBAN EAGEN 00093-5298-01 5.76330 METHYLPHENIDATE CD 40 MG CAP G TEVA USA EAGEN 62175-0154-37 5.33640 METHYLPHENIDATE CD 40 MG CAP G KREMERS URBAN EAGEN 00093-5292-01 7.<strong>08</strong>142 METHYLPHENIDATE CD 50 MG CAP G TEVA USA EAGEN 62175-0155-37 7.29450 METHYLPHENIDATE CD 50 MG CAP G KREMERS URBAN EAGEN 00093-5293-01 7.<strong>08</strong>142 METHYLPHENIDATE CD 60 MG CAP G TEVA USA EAGEN 62175-0156-37 7.29450 METHYLPHENIDATE CD 60 MG CAP G KREMERS URBAN EABND 004<strong>06</strong>-1445-01 1.26512 1.42685 METHYLPHENIDATE ER 10 MG TAB G MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-2715-01 4.66635 METHYLPHENIDATE ER 18 MG TAB G ACTAVIS PHARMA, EAGEN 62175-0310-37 4.66635 METHYLPHENIDATE ER 18 MG TAB G KREMERS URBAN EAGEN 00093-5346-01 3.70380 METHYLPHENIDATE ER 20 MG CAP G TEVA USA EAGEN 004<strong>06</strong>-1473-01 1.85407 METHYLPHENIDATE ER 20 MG TAB G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0127-01 4.78335 METHYLPHENIDATE ER 27 MG TAB G MALLINCKRODT PH EAGEN 00591-2716-01 4.78335 METHYLPHENIDATE ER 27 MG TAB G ACTAVIS PHARMA, EAGEN 62175-0311-37 4.78335 METHYLPHENIDATE ER 27 MG TAB G KREMERS URBAN EAGEN 00093-5347-01 3.78810 METHYLPHENIDATE ER 30 MG CAP G TEVA USA EAGEN 004<strong>06</strong>-0136-01 4.93395 METHYLPHENIDATE ER 36 MG TAB G MALLINCKRODT PH EAGEN 00591-2717-01 4.93395 METHYLPHENIDATE ER 36 MG TAB G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62175-0312-37 4.93395 METHYLPHENIDATE ER 36 MG TAB G KREMERS URBAN EAGEN 00093-5348-01 3.89332 METHYLPHENIDATE ER 40 MG CAP G TEVA USA EAGEN 004<strong>06</strong>-0154-01 5.36872 METHYLPHENIDATE ER 54 MG TAB G MALLINCKRODT PH EAGEN 00591-2718-01 5.36872 METHYLPHENIDATE ER 54 MG TAB G ACTAVIS PHARMA, EAGEN 62175-0313-37 5.36872 METHYLPHENIDATE ER 54 MG TAB G KREMERS URBAN EAGEN 67767-0200-01 4.24492 METHYLPHENIDATE LA 20 MG CAP G ACTAVIS PHARMA, EAGEN 67767-0201-01 4.35255 METHYLPHENIDATE LA 30 MG CAP G ACTAVIS PHARMA, EAGEN 67767-0202-01 4.47345 METHYLPHENIDATE LA 40 MG CAP G ACTAVIS PHARMA, EAGEN 00781-5754-01 1.87492 METHYLPHENIDATE SR 20 MG TAB G SANDOZ EAGUL 004<strong>06</strong>-1144-01 0.30<strong>06</strong>0 METHYLPHENIDATE 10 MG TABLET G MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 004<strong>06</strong>-1144-10 0.30<strong>06</strong>0 METHYLPHENIDATE 10 MG TABLET G MALLINCKRODT PH EAGUL 00591-5883-01 0.30<strong>06</strong>0 METHYLPHENIDATE 10 MG TABLET G ACTAVIS PHARMA, EAGUL 00781-5749-01 0.30<strong>06</strong>0 METHYLPHENIDATE 10 MG TABLET G SANDOZ EAGUL 53014-0530-07 0.30<strong>06</strong>0 METHYLPHENIDATE 10 MG TABLET G UCB PHARMA EAGUL 57664-0229-88 0.30<strong>06</strong>0 METHYLPHENIDATE 10 MG TABLET G CARACO PHARM EAGEN 51991-0713-50 0.96573 METHYLPHENIDATE 10 MG/5 ML SOL 0 BRECKENRIDGE MLGUL 004<strong>06</strong>-1146-01 0.33090 METHYLPHENIDATE 20 MG TABLET G MALLINCKRODT PH EAGUL 004<strong>06</strong>-1146-10 0.33090 METHYLPHENIDATE 20 MG TABLET G MALLINCKRODT PH EAGUL 00591-5884-01 0.33090 METHYLPHENIDATE 20 MG TABLET G ACTAVIS PHARMA, EAGUL 00781-5753-01 0.33090 METHYLPHENIDATE 20 MG TABLET G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 53014-0532-07 0.33090 METHYLPHENIDATE 20 MG TABLET G UCB PHARMA EAGUL 57664-0230-88 0.33090 METHYLPHENIDATE 20 MG TABLET G CARACO PHARM EAGUL 004<strong>06</strong>-1142-01 0.22530 METHYLPHENIDATE 5 MG TABLET G MALLINCKRODT PH EAGUL 004<strong>06</strong>-1142-10 0.22530 METHYLPHENIDATE 5 MG TABLET G MALLINCKRODT PH EAGUL 00591-5882-01 0.22530 METHYLPHENIDATE 5 MG TABLET G ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 440LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00781-5748-01 0.22530 METHYLPHENIDATE 5 MG TABLET G SANDOZ EAGUL 53014-0531-07 0.22530 METHYLPHENIDATE 5 MG TABLET G UCB PHARMA EAGUL 57664-0228-88 0.22530 METHYLPHENIDATE 5 MG TABLET G CARACO PHARM EAGEN 51991-0712-50 0.67741 METHYLPHENIDATE 5 MG/5 ML SOLN 0 BRECKENRIDGE MLGEN 00378-2661-01 0.83320 MORPHINE SULF ER 100 MG TABLET G MYLAN EAGEN 004<strong>06</strong>-8390-01 0.83320 MORPHINE SULF ER 100 MG TABLET G MALLINCKRODT PH EAGEN 42858-<strong>08</strong>04-01 0.83320 MORPHINE SULF ER 100 MG TABLET G RHODES PHARMACE EAGEN 60951-<strong>06</strong>58-70 0.83320 MORPHINE SULF ER 100 MG TABLET G QUALITEST EAGEN 68<strong>08</strong>4-0160-01 0.83320 MORPHINE SULF ER 100 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0160-11 0.83320 MORPHINE SULF ER 100 MG TABLET G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-2658-01 0.51772 MORPHINE SULF ER 15 MG TABLET G MYLAN EAGEN 004<strong>06</strong>-8315-01 0.51772 MORPHINE SULF ER 15 MG TABLET G MALLINCKRODT PH EAGEN 42858-<strong>08</strong>01-01 0.51772 MORPHINE SULF ER 15 MG TABLET G RHODES PHARMACE EAGEN 60951-<strong>06</strong>52-70 0.51772 MORPHINE SULF ER 15 MG TABLET G QUALITEST EAGEN 68<strong>08</strong>4-0157-01 0.51772 MORPHINE SULF ER 15 MG TABLET G AHP EAGEN 00378-2662-01 1.29554 MORPHINE SULF ER 200 MG TABLET G MYLAN EAGEN 004<strong>06</strong>-8320-01 1.29554 MORPHINE SULF ER 200 MG TABLET G MALLINCKRODT PH EAGEN 60951-<strong>06</strong>59-70 1.29554 MORPHINE SULF ER 200 MG TABLET G QUALITEST EAGEN 00378-2659-01 1.27380 MORPHINE SULF ER 30 MG TABLET G MYLAN EAGEN 004<strong>06</strong>-8330-01 2.37892 MORPHINE SULF ER 30 MG TABLET G MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42858-<strong>08</strong>02-01 2.37892 MORPHINE SULF ER 30 MG TABLET G RHODES PHARMACE EAGEN 60951-<strong>06</strong>53-70 1.27095 MORPHINE SULF ER 30 MG TABLET G QUALITEST EAGEN 68<strong>08</strong>4-0158-01 1.20554 MORPHINE SULF ER 30 MG TABLET G AHP EAGEN 00378-2660-01 2.31331 MORPHINE SULF ER 60 MG TABLET G MYLAN EAGEN 004<strong>06</strong>-8380-01 2.31331 MORPHINE SULF ER 60 MG TABLET G MALLINCKRODT PH EAGEN 60951-<strong>06</strong>55-70 2.31331 MORPHINE SULF ER 60 MG TABLET G QUALITEST EAGEN 68<strong>08</strong>4-0159-01 2.31331 MORPHINE SULF ER 60 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0159-11 2.31331 MORPHINE SULF ER 60 MG TABLET G AHP EAGEN 00054-0237-49 0.04614 MORPHINE SULF 10 MG/5 ML SOLN G ROXANE LABS. MLGEN 00054-0237-55 0.04614 MORPHINE SULF 10 MG/5 ML SOLN G ROXANE LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0237-63 0.04614 MORPHINE SULF 10 MG/5 ML SOLN G ROXANE LABS. MLGEN 66689-0032-04 0.04614 MORPHINE SULF 10 MG/5 ML SOLN G VISTAPHARM MLGEN 66689-0032-16 0.04614 MORPHINE SULF 10 MG/5 ML SOLN G VISTAPHARM MLGEN 00054-0404-41 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G ROXANE LABS. MLGEN 00054-0404-44 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G ROXANE LABS. MLGEN 00054-0404-50 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G ROXANE LABS. MLGEN 004<strong>06</strong>-8003-12 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G MALLINCKRODT PH MLGEN 004<strong>06</strong>-8003-15 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G MALLINCKRODT PH MLGEN 004<strong>06</strong>-8003-24 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G MALLINCKRODT PH MLGEN 004<strong>06</strong>-8003-30 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G MALLINCKRODT PH ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00527-1425-35 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G LANNETT CO. INC MLGEN 00527-1425-36 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G LANNETT CO. INC MLGEN 00527-1425-62 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G LANNETT CO. INC MLGEN 00527-1425-63 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G LANNETT CO. INC MLGEN 00574-0153-12 0.28050 MORPHINE SULF 100 MG/5 ML SOLN G PADDOCK LABS. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 441LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00574-0153-30 0.29230 MORPHINE SULF 100 MG/5 ML SOLN G PADDOCK LABS. MLGEN 00054-0238-49 0.<strong>08</strong>374 MORPHINE SULF 20 MG/5 ML SOLN G ROXANE LABS. MLGEN 00054-0238-63 0.<strong>08</strong>374 MORPHINE SULF 20 MG/5 ML SOLN G ROXANE LABS. MLGEN 66689-0033-04 0.<strong>08</strong>374 MORPHINE SULF 20 MG/5 ML SOLN G VISTAPHARM MLGEN 66689-0033-16 0.07110 MORPHINE SULF 20 MG/5 ML SOLN G VISTAPHARM MLGEN 00228-3501-11 3.54907 MORPHINE SULFATE ER 10 MG CAP G ACTAVIS PHARMA, EAGEN 0<strong>08</strong>32-0225-00 3.54907 MORPHINE SULFATE ER 10 MG CAP G UPSHER SMITH EAGEN 0<strong>08</strong>32-0225-50 3.54909 MORPHINE SULFATE ER 10 MG CAP G UPSHER SMITH EAGEN 00228-3507-11 14.23972 MORPHINE SULFATE ER 100 MG CAP G ACTAVIS PHARMA, EAGEN 00591-3453-01 14.25555 MORPHINE SULFATE ER 100 MG CAP G WATSON LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>08</strong>32-0233-00 14.25555 MORPHINE SULFATE ER 100 MG CAP G UPSHER SMITH EAGEN 0<strong>08</strong>32-0233-50 14.25553 MORPHINE SULFATE ER 100 MG CAP G UPSHER SMITH EAGEN 49884-<strong>06</strong>70-01 14.25555 MORPHINE SULFATE ER 100 MG CAP G PAR PHARM. EAGEN 49884-<strong>08</strong>38-01 14.25555 MORPHINE SULFATE ER 100 MG CAP G PAR PHARM. EAGEN 00228-3093-11 14.80402 MORPHINE SULFATE ER 120 MG CAP G ACTAVIS PHARMA, EAGEN 00228-3502-11 3.91732 MORPHINE SULFATE ER 20 MG CAP G ACTAVIS PHARMA, EAGEN 0<strong>08</strong>32-0226-00 3.92167 MORPHINE SULFATE ER 20 MG CAP G UPSHER SMITH EAGEN 0<strong>08</strong>32-0226-50 3.92166 MORPHINE SULFATE ER 20 MG CAP G UPSHER SMITH EAGEN 49884-<strong>06</strong>65-01 3.92167 MORPHINE SULFATE ER 20 MG CAP G PAR PHARM. EAGEN 49884-<strong>08</strong>33-01 3.92167 MORPHINE SULFATE ER 20 MG CAP G PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-3090-11 4.29720 MORPHINE SULFATE ER 30 MG CAP G ACTAVIS PHARMA, EAGEN 00228-3503-11 4.26052 MORPHINE SULFATE ER 30 MG CAP G ACTAVIS PHARMA, EAGEN 00591-3450-01 4.26525 MORPHINE SULFATE ER 30 MG CAP G WATSON LABS EAGEN 0<strong>08</strong>32-0227-00 4.26525 MORPHINE SULFATE ER 30 MG CAP G UPSHER SMITH EAGEN 0<strong>08</strong>32-0227-50 4.26525 MORPHINE SULFATE ER 30 MG CAP G UPSHER SMITH EAGEN 49884-<strong>06</strong>66-01 4.26525 MORPHINE SULFATE ER 30 MG CAP G PAR PHARM. EAGEN 49884-<strong>08</strong>34-01 4.26525 MORPHINE SULFATE ER 30 MG CAP G PAR PHARM. EAGEN 00228-3116-11 6.37162 MORPHINE SULFATE ER 45 MG CAP G ACTAVIS PHARMA, EAGEN 00228-3504-11 7.11997 MORPHINE SULFATE ER 50 MG CAP G ACTAVIS PHARMA, EAGEN 00591-3451-01 7.12785 MORPHINE SULFATE ER 50 MG CAP G WATSON LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>08</strong>32-0228-00 7.12785 MORPHINE SULFATE ER 50 MG CAP G UPSHER SMITH EAGEN 0<strong>08</strong>32-0228-50 7.12782 MORPHINE SULFATE ER 50 MG CAP G UPSHER SMITH EAGEN 49884-<strong>06</strong>67-01 7.12785 MORPHINE SULFATE ER 50 MG CAP G PAR PHARM. EAGEN 49884-<strong>08</strong>35-01 7.12785 MORPHINE SULFATE ER 50 MG CAP G PAR PHARM. EAGEN 00228-3091-11 8.34472 MORPHINE SULFATE ER 60 MG CAP G ACTAVIS PHARMA, EAGEN 00228-3505-11 8.52090 MORPHINE SULFATE ER 60 MG CAP G ACTAVIS PHARMA, EAGEN 0<strong>08</strong>32-0229-00 8.53035 MORPHINE SULFATE ER 60 MG CAP G UPSHER SMITH EAGEN 0<strong>08</strong>32-0229-50 8.53032 MORPHINE SULFATE ER 60 MG CAP G UPSHER SMITH EAGEN 49884-<strong>06</strong>68-01 8.53035 MORPHINE SULFATE ER 60 MG CAP G PAR PHARM. EAGEN 49884-<strong>08</strong>36-01 8.53035 MORPHINE SULFATE ER 60 MG CAP G PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-3117-11 10.61925 MORPHINE SULFATE ER 75 MG CAP G ACTAVIS PHARMA, EAGEN 00228-35<strong>06</strong>-11 11.35177 MORPHINE SULFATE ER 80 MG CAP G ACTAVIS PHARMA, EAGEN 00591-3576-01 11.36437 MORPHINE SULFATE ER 80 MG CAP G WATSON LABS EAGEN 0<strong>08</strong>32-0230-00 11.36437 MORPHINE SULFATE ER 80 MG CAP G UPSHER SMITH EAGEN 0<strong>08</strong>32-0230-50 11.36437 MORPHINE SULFATE ER 80 MG CAP G UPSHER SMITH EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 442LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49884-<strong>06</strong>69-01 11.36437 MORPHINE SULFATE ER 80 MG CAP G PAR PHARM. EAGEN 49884-<strong>08</strong>37-01 11.36437 MORPHINE SULFATE ER 80 MG CAP G PAR PHARM. EAGEN 00228-3092-11 12.54690 MORPHINE SULFATE ER 90 MG CAP G ACTAVIS PHARMA, EABND 00054-0235-25 0.20243 MORPHINE SULFATE IR 15 MG TAB G ROXANE LABS. EABND 00054-0236-25 0.34486 MORPHINE SULFATE IR 30 MG TAB G ROXANE LABS. EAGEN 004<strong>06</strong>-1521-55 7.95750 MORPHINE SULFATE POWDER G MALLINCKRODT PH GMGEN 38779-<strong>06</strong>73-05 78.37500 MORPHINE SULFATE POWDER G MEDISCA INC. GMBND 00409-2029-02 0.25232 MORPHINE SULFATE 1 MG/ML VIAL G HOSPIRA MLBND 00409-1135-02 0.68<strong>06</strong>0 MORPHINE SULFATE 25 MG/ML VIAL G HOSPIRA MLBND 00409-1134-03 0.55817 MORPHINE SULFATE 50 MG/ML VIAL G HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00409-1134-05 0.47442 MORPHINE SULFATE 50 MG/ML VIAL G HOSPIRA MLBND 00409-1261-30 1.62348 MORPHINE 10 MG/ML SYRINGE G HOSPIRA MLBND 0<strong>06</strong>41-6072-01 0.39840 MORPHINE 300 MG/20 ML VIAL G WEST-WARD,INC. MLBND 59011-0261-25 3.91444 MS CONTIN CR 30 MG TABLET G PURDUE PHARMA L EABND 59011-0263-10 0.83320 11.30924 MS CONTIN 100 MG TABLET G PURDUE PHARMA L EABND 59011-0264-10 1.29554 20.71140 MS CONTIN 200 MG TABLET G PURDUE PHARMA L EABND 59011-0262-10 2.31331 7.63849 MS CONTIN 60 MG TABLET G PURDUE PHARMA L EABND 50458-<strong>08</strong>61-01 4.95399 NUCYNTA ER 100 MG TABLET G JANSSEN PHARM. EABND 50458-<strong>08</strong>62-01 6.37716 NUCYNTA ER 150 MG TABLET G JANSSEN PHARM. EABND 50458-<strong>08</strong>63-01 8.12044 NUCYNTA ER 200 MG TABLET G JANSSEN PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 50458-<strong>08</strong>64-01 8.12044 NUCYNTA ER 250 MG TABLET G JANSSEN PHARM. EABND 50458-<strong>08</strong>60-01 2.68104 NUCYNTA ER 50 MG TABLET G JANSSEN PHARM. EABND 50458-<strong>08</strong>40-04 3.71898 NUCYNTA 100 MG TABLET G JANSSEN PHARM. EABND 50458-<strong>08</strong>20-04 2.38583 NUCYNTA 50 MG TABLET G JANSSEN PHARM. EABND 50458-<strong>08</strong>30-04 2.79286 NUCYNTA 75 MG TABLET G JANSSEN PHARM. EABND 00037-5120-30 61.38624 ONSOLIS 1,200 MCG SOLUBLE FILM G MEDA PHARMACEUT EABND 00037-5200-30 21.35175 ONSOLIS 200 MCG SOLUBLE FILM G MEDA PHARMACEUT EABND 00037-5400-30 31.34135 ONSOLIS 400 MCG SOLUBLE FILM G MEDA PHARMACEUT EABND 00037-5600-30 41.36913 ONSOLIS 600 MCG SOLUBLE FILM G MEDA PHARMACEUT EABND 00037-5800-30 51.35846 ONSOLIS 800 MCG SOLUBLE FILM G MEDA PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63481-<strong>08</strong>14-01 3.9<strong>08</strong>88 OPANA ER 10 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>14-20 3.9<strong>08</strong>88 OPANA ER 10 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>14-60 3.81329 OPANA ER 10 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>15-01 5.42031 OPANA ER 15 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>15-20 5.42031 OPANA ER 15 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>15-60 5.28820 OPANA ER 15 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>16-01 6.93257 OPANA ER 20 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>16-20 6.93257 OPANA ER 20 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>16-60 6.76352 OPANA ER 20 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>17-01 9.97826 OPANA ER 30 MG TABLET G ENDO PHARM INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63481-<strong>08</strong>17-20 9.97826 OPANA ER 30 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>17-60 9.73492 OPANA ER 30 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>18-01 13.02436 OPANA ER 40 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>18-20 13.02436 OPANA ER 40 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>18-60 12.7<strong>06</strong>47 OPANA ER 40 MG TABLET G ENDO PHARM INC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 443LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63481-<strong>08</strong>12-01 2.03599 OPANA ER 5 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>12-20 2.03599 OPANA ER 5 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>12-60 1.98591 OPANA ER 5 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>13-01 2.97264 OPANA ER 7.5 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>13-20 2.97264 OPANA ER 7.5 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>08</strong>13-60 2.89946 OPANA ER 7.5 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>06</strong>24-10 2.59458 OPANA 1 MG/ML INJ AMPULE G ENDO PHARM INC. MLBND 63481-<strong>06</strong>13-70 4.61370 5.72517 OPANA 10 MG TABLET G ENDO PHARM INC. EABND 63481-<strong>06</strong>12-70 2.11613 3.15317 OPANA 5 MG TABLET G ENDO PHARM INC. EAGEN 42799-0217-01 4.71<strong>08</strong>9 OPIUM TINCTURE 10 MG/ML 0 EDENBRIDGE PHAR ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42799-0217-02 4.70091 OPIUM TINCTURE 10 MG/ML 0 EDENBRIDGE PHAR MLGEN 42998-0203-02 5.02000 OPIUM TINCTURE 10 MG/ML 0 MARATHON PHARMA MLBND 60793-0525-01 2.91031 OXECTA 5 MG TABLET G KING PHARM EABND 60793-0526-01 2.91031 OXECTA 7.5 MG TABLET G KING PHARM EAGEN 00378-7103-01 1.53682 OXYCODON-ACETAMINOPHEN 2.5-325 G MYLAN EAGEN 0<strong>06</strong>03-4978-21 1.53682 OXYCODON-ACETAMINOPHEN 2.5-325 G QUALITEST EAGEN 00228-2982-11 0.77550 OXYCODON-ACETAMINOPHEN 7.5-325 G ACTAVIS PHARMA, EAGEN 00378-7105-01 1.02052 OXYCODON-ACETAMINOPHEN 7.5-325 G MYLAN EAGEN 004<strong>06</strong>-0522-01 2.03655 OXYCODON-ACETAMINOPHEN 7.5-325 G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0522-62 2.03655 OXYCODON-ACETAMINOPHEN 7.5-325 G MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-0933-01 2.03655 OXYCODON-ACETAMINOPHEN 7.5-325 G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-4979-21 0.77550 OXYCODON-ACETAMINOPHEN 7.5-325 G QUALITEST EAGEN 0<strong>06</strong>03-4979-28 0.77550 OXYCODON-ACETAMINOPHEN 7.5-325 G QUALITEST EAGEN 13107-0045-01 0.77550 OXYCODON-ACETAMINOPHEN 7.5-325 G AUROBINDO PHARM EAGEN 13107-0045-05 0.77550 OXYCODON-ACETAMINOPHEN 7.5-325 G AUROBINDO PHARM EAGEN 47781-0229-01 0.77550 OXYCODON-ACETAMINOPHEN 7.5-325 G ALVOGEN INC EAGEN 47781-0229-05 0.77550 OXYCODON-ACETAMINOPHEN 7.5-325 G ALVOGEN INC EAGEN 68<strong>08</strong>4-0379-01 2.<strong>06</strong>250 OXYCODON-ACETAMINOPHEN 7.5-325 G AHP EAGEN 68<strong>08</strong>4-0379-11 2.<strong>06</strong>250 OXYCODON-ACETAMINOPHEN 7.5-325 G AHP EAGEN 004<strong>06</strong>-0582-01 0.79537 OXYCODON-ACETAMINOPHEN 7.5-500 G MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-<strong>08</strong>24-01 0.78975 OXYCODON-ACETAMINOPHEN 7.5-500 G ACTAVIS PHARMA, EAGEN 53746-0205-01 0.79537 OXYCODON-ACETAMINOPHEN 7.5-500 G AMNEAL PHARMACE EAGUL 00527-1426-36 0.95000 OXYCODONE CONC 20 MG/ML SOLN G LANNETT CO. INC MLGEN 10702-0056-01 0.25300 OXYCODONE HCL 10 MG TABLET G KVK-TECH, INC. EAGEN 68<strong>08</strong>4-0048-01 0.25300 OXYCODONE HCL 10 MG TABLET G AHP EAGEN 68<strong>08</strong>4-0048-11 0.25300 OXYCODONE HCL 10 MG TABLET G AHP EAGEN 68382-0794-01 0.25300 OXYCODONE HCL 10 MG TABLET G ZYDUS PHARMACEU EAGUL 00054-0522-44 0.95000 OXYCODONE HCL 100 MG/5 ML SOLN G ROXANE LABS. MLGUL 66689-0025-30 0.95000 OXYCODONE HCL 100 MG/5 ML SOLN G VISTAPHARM MLGUL 68462-0347-37 0.95000 OXYCODONE HCL 100 MG/5 ML SOLN G GLENMARK PHARMA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00228-2878-11 0.66950 OXYCODONE HCL 15 MG TABLET G ACTAVIS PHARMA, EAGUL 00378-6113-01 0.66950 OXYCODONE HCL 15 MG TABLET G MYLAN EAGUL 004<strong>06</strong>-8515-01 0.66950 OXYCODONE HCL 15 MG TABLET G MALLINCKRODT PH EAGUL 0<strong>06</strong>03-4991-21 0.66950 OXYCODONE HCL 15 MG TABLET G QUALITEST EAGUL 0<strong>06</strong>03-4991-28 0.66950 OXYCODONE HCL 15 MG TABLET G QUALITEST EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 444LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 10702-00<strong>08</strong>-01 0.66950 OXYCODONE HCL 15 MG TABLET G KVK-TECH, INC. EAGUL 13107-0056-01 0.66950 OXYCODONE HCL 15 MG TABLET G AUROBINDO PHARM EAGUL 47781-0264-01 0.66950 OXYCODONE HCL 15 MG TABLET G ALVOGEN INC EAGUL 47781-0264-05 0.66950 OXYCODONE HCL 15 MG TABLET G ALVOGEN INC EAGUL 57664-0187-88 0.66950 OXYCODONE HCL 15 MG TABLET G CARACO PHARM EAGUL 68<strong>08</strong>4-0184-01 0.66950 OXYCODONE HCL 15 MG TABLET G AHP EAGUL 68<strong>08</strong>4-0184-11 0.66950 OXYCODONE HCL 15 MG TABLET G AHP EAGUL 68382-0795-01 0.66950 OXYCODONE HCL 15 MG TABLET G ZYDUS PHARMACEU EAGEN 10702-0057-01 0.37841 OXYCODONE HCL 20 MG TABLET G KVK-TECH, INC. EAGEN 68382-0796-01 0.37841 OXYCODONE HCL 20 MG TABLET G ZYDUS PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 00228-2879-11 1.30940 OXYCODONE HCL 30 MG TABLET G ACTAVIS PHARMA, EAGUL 00378-6114-01 1.30940 OXYCODONE HCL 30 MG TABLET G MYLAN EAGUL 004<strong>06</strong>-8530-01 1.30940 OXYCODONE HCL 30 MG TABLET G MALLINCKRODT PH EAGUL 0<strong>06</strong>03-4992-21 1.30940 OXYCODONE HCL 30 MG TABLET G QUALITEST EAGUL 0<strong>06</strong>03-4992-28 1.30940 OXYCODONE HCL 30 MG TABLET G QUALITEST EAGUL 10702-0009-01 1.30940 OXYCODONE HCL 30 MG TABLET G KVK-TECH, INC. EAGEN 13107-0057-01 1.15529 OXYCODONE HCL 30 MG TABLET G AUROBINDO PHARM EAGEN 47781-0265-01 1.15529 OXYCODONE HCL 30 MG TABLET G ALVOGEN INC EAGEN 47781-0265-05 1.15529 OXYCODONE HCL 30 MG TABLET G ALVOGEN INC EAGUL 57664-0224-88 1.30940 OXYCODONE HCL 30 MG TABLET G CARACO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 68<strong>08</strong>4-0185-01 1.30940 OXYCODONE HCL 30 MG TABLET G AHP EAGUL 68<strong>08</strong>4-0185-11 1.30940 OXYCODONE HCL 30 MG TABLET G AHP EAGEN 68382-0797-01 1.15529 OXYCODONE HCL 30 MG TABLET G ZYDUS PHARMACEU EAGUL 004<strong>06</strong>-0554-01 0.21380 OXYCODONE HCL 5 MG CAPSULE G MALLINCKRODT PH EAGUL 683<strong>08</strong>-0145-01 0.21380 OXYCODONE HCL 5 MG CAPSULE G MIDLOTHIAN LABO EAGUL 00378-6112-01 0.23990 OXYCODONE HCL 5 MG TABLET G MYLAN EAGUL 004<strong>06</strong>-0552-01 0.23990 OXYCODONE HCL 5 MG TABLET G MALLINCKRODT PH EAGUL 0<strong>06</strong>03-4990-21 0.23990 OXYCODONE HCL 5 MG TABLET G QUALITEST EAGUL 0<strong>06</strong>03-4990-28 0.23990 OXYCODONE HCL 5 MG TABLET G QUALITEST EAGUL 10702-0018-01 0.23990 OXYCODONE HCL 5 MG TABLET G KVK-TECH, INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 13107-0055-01 0.23990 OXYCODONE HCL 5 MG TABLET G AUROBINDO PHARM EAGUL 47781-0263-01 0.23990 OXYCODONE HCL 5 MG TABLET G ALVOGEN INC EAGUL 47781-0263-05 0.23990 OXYCODONE HCL 5 MG TABLET G ALVOGEN INC EAGUL 57664-0223-88 0.23990 OXYCODONE HCL 5 MG TABLET G CARACO PHARM EAGUL 68<strong>08</strong>4-0354-01 0.23990 OXYCODONE HCL 5 MG TABLET G AHP EAGUL 68<strong>08</strong>4-0354-11 0.23990 OXYCODONE HCL 5 MG TABLET G AHP EAGUL 683<strong>08</strong>-0505-47 0.23990 OXYCODONE HCL 5 MG TABLET G MIDLOTHIAN LABO EAGUL 68382-0793-01 0.23990 OXYCODONE HCL 5 MG TABLET G ZYDUS PHARMACEU EAGEN 00054-0523-41 0.67200 OXYCODONE HCL 5 MG/5 ML SOLN G ROXANE LABS. MLGEN 00054-0523-63 0.21375 OXYCODONE HCL 5 MG/5 ML SOLN G ROXANE LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64950-0354-50 0.20520 OXYCODONE HCL 5 MG/5 ML SOLN G LEHIGH VALLEY T MLGEN 66689-0403-16 0.21375 OXYCODONE HCL 5 MG/5 ML SOLN G VISTAPHARM MLGEN 00228-2983-11 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G ACTAVIS PHARMA, EAGEN 00378-71<strong>06</strong>-01 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G MYLAN EAGEN 004<strong>06</strong>-0523-01 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G MALLINCKRODT PH EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 445LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 004<strong>06</strong>-0523-62 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G MALLINCKRODT PH EAGEN 00591-0932-01 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-4982-21 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G QUALITEST EAGEN 0<strong>06</strong>03-4982-28 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G QUALITEST EAGEN 13107-0046-01 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G AUROBINDO PHARM EAGEN 13107-0046-05 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G AUROBINDO PHARM EAGEN 47781-0230-01 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G ALVOGEN INC EAGEN 47781-0230-05 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G ALVOGEN INC EAGEN 53746-0204-01 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-0378-01 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 683<strong>08</strong>-0480-47 0.82685 OXYCODONE-ACETAMINOPHEN 10-325 G MIDLOTHIAN LABO EAGEN 00591-<strong>08</strong>25-01 1.03260 OXYCODONE-ACETAMINOPHEN 10-650 G ACTAVIS PHARMA, EAGEN 53746-02<strong>06</strong>-01 1.09237 OXYCODONE-ACETAMINOPHEN 10-650 G AMNEAL PHARMACE EAGUL 00228-2981-50 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G ACTAVIS PHARMA, EAGUL 00378-7104-01 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G MYLAN EAGUL 004<strong>06</strong>-0512-01 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G MALLINCKRODT PH EAGUL 004<strong>06</strong>-0512-05 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G MALLINCKRODT PH EAGUL 004<strong>06</strong>-0512-62 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G MALLINCKRODT PH EAGUL 00591-0749-01 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G ACTAVIS PHARMA, EAGUL 00591-0749-05 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 0<strong>06</strong>03-4998-21 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G QUALITEST EAGUL 0<strong>06</strong>03-4998-28 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G QUALITEST EAGUL 13107-0044-01 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G AUROBINDO PHARM EAGUL 13107-0044-05 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G AUROBINDO PHARM EAGUL 53746-0203-01 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G AMNEAL PHARMACE EAGUL 53746-0203-05 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G AMNEAL PHARMACE EAGUL 68<strong>08</strong>4-0355-01 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G AHP EAGUL 68<strong>08</strong>4-0355-11 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G AHP EAGUL 683<strong>08</strong>-0405-47 0.23400 OXYCODONE-ACETAMINOPHEN 5-325 G MIDLOTHIAN LABO EAGUL 00054-2795-25 0.32300 OXYCODONE-ACETAMINOPHEN 5-500 G ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 004<strong>06</strong>-0532-01 0.30187 OXYCODONE-ACETAMINOPHEN 5-500 G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0532-05 0.27169 OXYCODONE-ACETAMINOPHEN 5-500 G MALLINCKRODT PH EAGUL 00555-<strong>06</strong>58-02 0.32300 OXYCODONE-ACETAMINOPHEN 5-500 G BARR EAGUL 00591-0737-01 0.32300 OXYCODONE-ACETAMINOPHEN 5-500 G WATSON LABS EAGEN 00378-6117-01 0.88620 OXYCODONE-ASPIRIN 4.83-325 MG G MYLAN EAGEN 00591-3551-01 0.85305 OXYCODONE-ASPIRIN 4.83-325 MG G ACTAVIS PHARMA, EAGEN 00228-4029-11 0.84900 OXYCODONE-IBUPR<strong>OF</strong>EN 5-400 TAB G ACTAVIS PHARMA, EAGEN 00555-0778-02 0.84900 OXYCODONE-IBUPR<strong>OF</strong>EN 5-400 TAB G BARR EABND 59011-0410-10 2.27195 OXYCONTIN 10 MG TABLET G PURDUE PHARMA L EABND 59011-0415-10 3.40250 OXYCONTIN 15 MG TABLET G PURDUE PHARMA L EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59011-0420-10 4.34762 OXYCONTIN 20 MG TABLET G PURDUE PHARMA L EABND 59011-0420-20 4.45917 OXYCONTIN 20 MG TABLET G PURDUE PHARMA L EABND 59011-0430-10 6.15362 OXYCONTIN 30 MG TABLET G PURDUE PHARMA L EABND 59011-0440-10 7.71418 OXYCONTIN 40 MG TABLET G PURDUE PHARMA L EABND 59011-0460-10 11.22550 OXYCONTIN 60 MG TABLET G PURDUE PHARMA L EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 446LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59011-0480-10 14.5<strong>06</strong>65 OXYCONTIN 80 MG TABLET G PURDUE PHARMA L EAGEN 00228-3228-<strong>06</strong> 2.22760 OXYMORPHONE HCL ER 10 MG TAB G ACTAVIS PHARMA, EAGEN 00228-3228-11 2.22760 OXYMORPHONE HCL ER 10 MG TAB G ACTAVIS PHARMA, EAGEN 00115-1316-01 3.62017 OXYMORPHONE HCL ER 15 MG TAB G GLOBAL PHARM EAGEN 00115-1316-13 3.62025 OXYMORPHONE HCL ER 15 MG TAB G GLOBAL PHARM EAGEN 00228-3262-<strong>06</strong> 3.87812 OXYMORPHONE HCL ER 15 MG TAB G ACTAVIS PHARMA, EAGEN 00228-3262-11 3.87817 OXYMORPHONE HCL ER 15 MG TAB G ACTAVIS PHARMA, EAGEN 00115-1233-01 4.62997 OXYMORPHONE HCL ER 20 MG TAB G GLOBAL PHARM EAGEN 00115-1233-13 4.62999 OXYMORPHONE HCL ER 20 MG TAB G GLOBAL PHARM EAGEN 00228-3229-<strong>06</strong> 4.62999 OXYMORPHONE HCL ER 20 MG TAB G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-3229-11 4.62997 OXYMORPHONE HCL ER 20 MG TAB G ACTAVIS PHARMA, EAGEN 00115-1317-01 6.66405 OXYMORPHONE HCL ER 30 MG TAB G GLOBAL PHARM EAGEN 00115-1317-13 6.66412 OXYMORPHONE HCL ER 30 MG TAB G GLOBAL PHARM EAGEN 00228-3263-<strong>06</strong> 6.66399 OXYMORPHONE HCL ER 30 MG TAB G ACTAVIS PHARMA, EAGEN 00228-3263-11 6.66405 OXYMORPHONE HCL ER 30 MG TAB G ACTAVIS PHARMA, EAGEN 00115-1234-01 8.69835 OXYMORPHONE HCL ER 40 MG TAB G GLOBAL PHARM EAGEN 00115-1234-13 8.69825 OXYMORPHONE HCL ER 40 MG TAB G GLOBAL PHARM EAGEN 00228-3230-<strong>06</strong> 8.69837 OXYMORPHONE HCL ER 40 MG TAB G ACTAVIS PHARMA, EAGEN 00228-3230-11 8.69835 OXYMORPHONE HCL ER 40 MG TAB G ACTAVIS PHARMA, EAGEN 00228-3227-<strong>06</strong> 1.16004 OXYMORPHONE HCL ER 5 MG TABLET G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00228-3227-11 1.16004 OXYMORPHONE HCL ER 5 MG TABLET G ACTAVIS PHARMA, EAGEN 00115-1315-01 1.72487 OXYMORPHONE HCL ER 7.5 MG TAB G GLOBAL PHARM EAGEN 00115-1315-13 1.72487 OXYMORPHONE HCL ER 7.5 MG TAB G GLOBAL PHARM EAGEN 00228-3261-<strong>06</strong> 1.72487 OXYMORPHONE HCL ER 7.5 MG TAB G ACTAVIS PHARMA, EAGEN 00228-3261-11 1.72487 OXYMORPHONE HCL ER 7.5 MG TAB G ACTAVIS PHARMA, EAGEN 00054-0284-25 4.41315 OXYMORPHONE HCL 10 MG TABLET G ROXANE LABS. EAGEN 00093-5862-01 4.40917 OXYMORPHONE HCL 10 MG TABLET G TEVA USA EAGEN 004<strong>06</strong>-1010-01 4.40917 OXYMORPHONE HCL 10 MG TABLET G MALLINCKRODT PH EAGEN 10702-0071-01 4.61370 OXYMORPHONE HCL 10 MG TABLET G KVK-TECH, INC. EAGEN 60951-0795-70 4.01197 OXYMORPHONE HCL 10 MG TABLET G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-0283-25 2.11613 OXYMORPHONE HCL 5 MG TABLET G ROXANE LABS. EAGEN 00093-5861-01 2.11613 OXYMORPHONE HCL 5 MG TABLET G TEVA USA EAGEN 004<strong>06</strong>-1009-01 2.11613 OXYMORPHONE HCL 5 MG TABLET G MALLINCKRODT PH EAGEN 10702-0070-01 2.11613 OXYMORPHONE HCL 5 MG TABLET G KVK-TECH, INC. EAGEN 60951-0794-70 2.11613 OXYMORPHONE HCL 5 MG TABLET G QUALITEST EAGEN 63481-<strong>06</strong>29-70 0.82685 PERCOCET 10-325 MG TABLET G ENDO PHARM INC. EAGEN 63481-<strong>06</strong>27-70 1.6<strong>06</strong>51 PERCOCET 2.5-325 MG TABLET G ENDO PHARM INC. EAGUL 63481-<strong>06</strong>23-70 0.23400 PERCOCET 5-325 MG TABLET G ENDO PHARM INC. EAGUL 63481-<strong>06</strong>23-85 0.23400 PERCOCET 5-325 MG TABLET G ENDO PHARM INC. EAGEN 63481-<strong>06</strong>28-70 5.18865 PERCOCET 7.5-325 MG TABLET G ENDO PHARM INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63481-0121-70 1.34858 PERCODAN TABLET G ENDO PHARM INC. EABND 24090-<strong>06</strong>83-85 8.11380 PRIMLEV 10-300 MG TABLET G AKRIMAX PHARMAC EABND 24090-<strong>06</strong>83-88 8.11383 PRIMLEV 10-300 MG TABLET G AKRIMAX PHARMAC EABND 24090-<strong>06</strong>81-85 8.11380 PRIMLEV 5-300 MG TABLET G AKRIMAX PHARMAC EABND 24090-<strong>06</strong>81-88 8.11383 PRIMLEV 5-300 MG TABLET G AKRIMAX PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 447LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 24090-<strong>06</strong>82-85 8.11380 PRIMLEV 7.5-300 MG TABLET G AKRIMAX PHARMAC EABND 24090-<strong>06</strong>82-88 8.11383 PRIMLEV 7.5-300 MG TABLET G AKRIMAX PHARMAC EAGEN 13551-0701-05 1.45962 PROCENTRA 5 MG/5 ML SOLUTION G FSC LABS MLBND 24478-0190-10 3.05025 QUILLIVANT XR 25 MG/5 ML SUSP G PFIZER US PHARM MLBND 24478-0200-20 1.52512 QUILLIVANT XR 25 MG/5 ML SUSP G PFIZER US PHARM MLBND 24478-0205-25 1.22010 QUILLIVANT XR 25 MG/5 ML SUSP G PFIZER US PHARM MLBND 24478-0210-30 1.01675 QUILLIVANT XR 25 MG/5 ML SUSP G PFIZER US PHARM MLBND 00078-0424-05 6.01252 RITALIN LA 10 MG CAPSULE G NOVARTIS EABND 00078-0370-05 4.24492 6.01252 RITALIN LA 20 MG CAPSULE G NOVARTIS EABND 00078-0371-05 4.36863 6.14947 RITALIN LA 30 MG CAPSULE G NOVARTIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00078-0372-05 4.51593 6.32028 RITALIN LA 40 MG CAPSULE G NOVARTIS EABND 00078-0442-05 2.21319 RITALIN SR 20 MG TABLET G NOVARTIS EABUL 00078-0440-05 0.30<strong>06</strong>0 0.93275 RITALIN 10 MG TABLET G NOVARTIS EABUL 00078-0441-05 0.33090 1.34119 RITALIN 20 MG TABLET G NOVARTIS EABUL 00078-0439-05 0.22530 0.65428 RITALIN 5 MG TABLET G NOVARTIS EABND 00054-3686-63 0.10205 ROXICET 5-325 ORAL SOLUTION G ROXANE LABS. MLGUL 00054-4650-25 0.23400 ROXICET 5-325 TABLET G ROXANE LABS. EAGUL 00054-4650-29 0.23400 ROXICET 5-325 TABLET G ROXANE LABS. EAGUL 00054-8650-24 0.23400 ROXICET 5-325 TABLET G ROXANE LABS. EABND 23635-0582-10 1.15529 5.70309 ROXICODONE 30 MG TABLET G MALLINCKRODT BR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 42998-<strong>06</strong>79-01 15.35118 SECONAL SODIUM 100 MG CAPSULE 0 MARATHON PHARMA EABND 20482-0012-15 62.34960 SUBSYS 1,200 MCG SPRAY G INSYS THERAPEUT EABND 20482-0012-30 56.17246 SUBSYS 1,200 MCG SPRAY G INSYS THERAPEUT EABND 20482-0016-15 79.57044 SUBSYS 1,600 MCG SPRAY G INSYS THERAPEUT EABND 20482-0016-30 71.68599 SUBSYS 1,600 MCG SPRAY G INSYS THERAPEUT EABND 20482-0001-01 27.80500 SUBSYS 100 MCG SPRAY G INSYS THERAPEUT EABND 20482-0001-10 30.87517 SUBSYS 100 MCG SPRAY G INSYS THERAPEUT EABND 20482-0001-30 27.81025 SUBSYS 100 MCG SPRAY G INSYS THERAPEUT EABND 20482-0002-01 38.99340 SUBSYS 200 MCG SPRAY G INSYS THERAPEUT EABND 20482-0002-10 38.99340 SUBSYS 200 MCG SPRAY G INSYS THERAPEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 20482-0002-30 38.99340 SUBSYS 200 MCG SPRAY G INSYS THERAPEUT EABND 20482-0004-01 56.6<strong>06</strong>00 SUBSYS 400 MCG SPRAY G INSYS THERAPEUT EABND 20482-0004-10 56.60268 SUBSYS 400 MCG SPRAY G INSYS THERAPEUT EABND 20482-0004-30 56.60268 SUBSYS 400 MCG SPRAY G INSYS THERAPEUT EABND 20482-00<strong>06</strong>-01 73.49650 SUBSYS 600 MCG SPRAY G INSYS THERAPEUT EABND 20482-00<strong>06</strong>-30 73.49484 SUBSYS 600 MCG SPRAY G INSYS THERAPEUT EABND 20482-00<strong>08</strong>-01 90.53640 SUBSYS 800 MCG SPRAY G INSYS THERAPEUT EABND 20482-00<strong>08</strong>-30 90.53640 SUBSYS 800 MCG SPRAY G INSYS THERAPEUT EABND 59417-0102-10 6.60754 VYVANSE 20 MG CAPSULE G SHIRE US INC. EABND 59417-0103-10 6.60754 VYVANSE 30 MG CAPSULE G SHIRE US INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 59417-0104-10 6.60754 VYVANSE 40 MG CAPSULE G SHIRE US INC. EABND 59417-0105-10 6.60754 VYVANSE 50 MG CAPSULE G SHIRE US INC. EABND 59417-01<strong>06</strong>-10 6.60754 VYVANSE 60 MG CAPSULE G SHIRE US INC. EABND 59417-0107-10 6.60754 VYVANSE 70 MG CAPSULE G SHIRE US INC. EAGUL 24338-<strong>08</strong>53-10 0.34350 ZENZEDI 10 MG TABLET G ARBOR PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 448LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 03 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 24338-<strong>08</strong>50-10 4.29276 ZENZEDI 2.5 MG TABLET G ARBOR PHARMACEU EAGEN 24338-<strong>08</strong>51-10 3.87900 ZENZEDI 5 MG TABLET G ARBOR PHARMACEU EABND 24338-<strong>08</strong>52-10 4.29276 ZENZEDI 7.5 MG TABLET G ARBOR PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 449LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 04 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 64376-<strong>06</strong>11-01 1.04110 1.39772 ACETAMINOPH-CAFF-DIHYDROCODEIN G BOCA PHARMACAL EABND 64376-<strong>06</strong>11-31 1.04110 1.44<strong>08</strong>8 ACETAMINOPH-CAFF-DIHYDROCODEIN G BOCA PHARMACAL EAGEN 00093-0050-01 0.14499 ACETAMINOPHEN-COD #2 TABLET G TEVA USA EAGEN 004<strong>06</strong>-0483-01 0.14499 ACETAMINOPHEN-COD #2 TABLET G MALLINCKRODT PH EAGEN 0<strong>06</strong>03-2337-21 0.14499 ACETAMINOPHEN-COD #2 TABLET G QUALITEST EAGEN 13107-0058-01 0.14499 ACETAMINOPHEN-COD #2 TABLET G AUROBINDO PHARM EAGEN 00093-0150-01 0.13020 ACETAMINOPHEN-COD #3 TABLET G TEVA USA EAGEN 00093-0150-10 0.13020 ACETAMINOPHEN-COD #3 TABLET G TEVA USA EAGEN 004<strong>06</strong>-0484-01 0.13020 ACETAMINOPHEN-COD #3 TABLET G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0484-03 0.13020 ACETAMINOPHEN-COD #3 TABLET G MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 004<strong>06</strong>-0484-10 0.13020 ACETAMINOPHEN-COD #3 TABLET G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0484-20 0.13020 ACETAMINOPHEN-COD #3 TABLET G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0484-50 0.13020 ACETAMINOPHEN-COD #3 TABLET G MALLINCKRODT PH EAGEN 0<strong>06</strong>03-2338-02 0.13020 ACETAMINOPHEN-COD #3 TABLET G QUALITEST EAGEN 0<strong>06</strong>03-2338-04 0.13020 ACETAMINOPHEN-COD #3 TABLET G QUALITEST EAGEN 0<strong>06</strong>03-2338-16 0.13020 ACETAMINOPHEN-COD #3 TABLET G QUALITEST EAGEN 0<strong>06</strong>03-2338-20 0.13020 ACETAMINOPHEN-COD #3 TABLET G QUALITEST EAGEN 0<strong>06</strong>03-2338-21 0.13020 ACETAMINOPHEN-COD #3 TABLET G QUALITEST EAGEN 0<strong>06</strong>03-2338-22 0.13020 ACETAMINOPHEN-COD #3 TABLET G QUALITEST EAGEN 0<strong>06</strong>03-2338-32 0.13020 ACETAMINOPHEN-COD #3 TABLET G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13107-0059-01 0.13020 ACETAMINOPHEN-COD #3 TABLET G AUROBINDO PHARM EAGEN 13107-0059-99 0.13020 ACETAMINOPHEN-COD #3 TABLET G AUROBINDO PHARM EAGEN 51079-0161-20 0.13020 ACETAMINOPHEN-COD #3 TABLET G MYLAN INSTITUTI EAGEN 51079-0161-21 0.13020 ACETAMINOPHEN-COD #3 TABLET G MYLAN INSTITUTI EAGEN 51079-0161-99 0.13020 ACETAMINOPHEN-COD #3 TABLET G MYLAN INSTITUTI EAGEN 63739-0004-10 0.13020 ACETAMINOPHEN-COD #3 TABLET G MCKESSON PACKAG EAGEN 64720-0304-11 0.13020 ACETAMINOPHEN-COD #3 TABLET G COREPHARMA LLC EAGEN 65162-0033-10 0.13020 ACETAMINOPHEN-COD #3 TABLET G AMNEAL PHARMACE EAGEN 65162-0033-11 0.13020 ACETAMINOPHEN-COD #3 TABLET G AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-0372-01 0.12562 ACETAMINOPHEN-COD #3 TABLET G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0372-11 0.12562 ACETAMINOPHEN-COD #3 TABLET G AHP EAGEN 00093-0350-01 0.22990 ACETAMINOPHEN-COD #4 TABLET G TEVA USA EAGEN 00093-0350-05 0.22990 ACETAMINOPHEN-COD #4 TABLET G TEVA USA EAGEN 00093-0350-10 0.22990 ACETAMINOPHEN-COD #4 TABLET G TEVA USA EAGEN 004<strong>06</strong>-0485-01 0.22990 ACETAMINOPHEN-COD #4 TABLET G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0485-05 0.22990 ACETAMINOPHEN-COD #4 TABLET G MALLINCKRODT PH EAGEN 0<strong>06</strong>03-2339-21 0.22990 ACETAMINOPHEN-COD #4 TABLET G QUALITEST EAGEN 0<strong>06</strong>03-2339-28 0.22990 ACETAMINOPHEN-COD #4 TABLET G QUALITEST EAGEN 13107-0<strong>06</strong>0-01 0.22990 ACETAMINOPHEN-COD #4 TABLET G AUROBINDO PHARM EAGEN 13107-0<strong>06</strong>0-05 0.22990 ACETAMINOPHEN-COD #4 TABLET G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 13107-0<strong>06</strong>0-99 0.22990 ACETAMINOPHEN-COD #4 TABLET G AUROBINDO PHARM EAGEN 64720-0305-10 0.22990 ACETAMINOPHEN-COD #4 TABLET G COREPHARMA LLC EAGEN 64720-0305-50 0.22990 ACETAMINOPHEN-COD #4 TABLET G COREPHARMA LLC EAGEN 68<strong>08</strong>4-0373-01 0.15937 ACETAMINOPHEN-COD #4 TABLET G AHP EAGEN 68<strong>08</strong>4-0373-11 0.15937 ACETAMINOPHEN-COD #4 TABLET G AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 450LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 04 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 68220-0055-10 30.13198 ANADROL-50 TABLET G MEDA PHARMACEUT EABND 52544-0076-60 6.210<strong>06</strong> ANDRODERM 2 MG/24HR PATCH G ACTAVIS PHARMA, EABND 52544-0077-30 12.42012 ANDRODERM 4 MG/24HR PATCH G ACTAVIS PHARMA, EABND 00051-8462-33 5.01574 ANDROGEL 1.62% GEL PUMP G ABBVIE US LLC GMBND 00051-8462-31 10.<strong>06</strong>380 ANDROGEL 1.62%(1.25G) GEL PCKT G ABBVIE US LLC GMBND 00051-8462-30 5.17145 ANDROGEL 1.62%(2.5G) GEL PCKT G ABBVIE US LLC GMBND 00051-8488-88 2.58584 ANDROGEL 1% GEL PUMP G ABBVIE US LLC GMBND 00051-8425-30 5.03201 ANDROGEL 1%(2.5G) GEL PACKET G ABBVIE US LLC GMBND 00051-8450-30 2.58584 ANDROGEL 1%(5G) GEL PACKET G ABBVIE US LLC GMBND 00187-0902-01 61.01994 ANDROID 10 MG CAPSULE G VALEANT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 0<strong>08</strong>32-0<strong>08</strong>6-00 4.79607 ANDROXY 10 MG TABLET G UPSHER SMITH EAGEN 51991-0074-01 1.75350 ASCOMP WITH CODEINE CAPSULE G BRECKENRIDGE EAGEN 51991-0074-05 1.75350 ASCOMP WITH CODEINE CAPSULE G BRECKENRIDGE EAGEN 57664-0419-88 1.38562 ASPIRIN-CAFF-DIHYDROCODEIN CAP G CARACO PHARM EABND 00002-1975-90 4.34975 AXIRON 30 MG/ACTUATION SOLN G ELI LILLY & CO. MLBND 12496-0757-01 2.83290 12.09144 BUPRENEX 0.3 MG/ML AMPUL 0 RECKITT BENCKIS MLGEN 00228-3155-03 7.82450 BUPRENORPHIN-NALOXON 8-2 MG TB G ACTAVIS PHARMA, EAGEN 004<strong>06</strong>-1924-03 7.81599 BUPRENORPHIN-NALOXON 8-2 MG TB G MALLINCKRODT PH EAGEN 65162-0415-03 7.81599 BUPRENORPHIN-NALOXON 8-2 MG TB G AMNEAL PHARMACE EAGEN 00054-0176-13 2.39490 BUPRENORPHINE 2 MG TABLET SL G ROXANE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00093-5378-56 2.39490 BUPRENORPHINE 2 MG TABLET SL G TEVA USA EAGEN 50383-0924-93 2.39490 BUPRENORPHINE 2 MG TABLET SL G HI-TECH PHARMAC EAGEN 00054-0177-13 2.47000 BUPRENORPHINE 8 MG TABLET SL G ROXANE LABS. EAGEN 00093-5379-56 2.47000 BUPRENORPHINE 8 MG TABLET SL G TEVA USA EAGEN 50383-0930-93 2.47000 BUPRENORPHINE 8 MG TABLET SL G HI-TECH PHARMAC EAGEN 00228-3154-03 4.36599 BUPRENORPHN-NALOXN 2-0.5 MG TB G ACTAVIS PHARMA, EAGEN 004<strong>06</strong>-1923-03 4.36925 BUPRENORPHN-NALOXN 2-0.5 MG TB G MALLINCKRODT PH EAGEN 65162-0416-03 4.36925 BUPRENORPHN-NALOXN 2-0.5 MG TB G AMNEAL PHARMACE EAGEN 0<strong>06</strong>03-2545-21 0.26422 BUTALB-ACETAMIN-CAFF 50-500-40 0 QUALITEST EAGEN 0<strong>06</strong>03-2545-28 0.26422 BUTALB-ACETAMIN-CAFF 50-500-40 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00591-2641-01 4.25790 BUTALB-ACETAMINOPH-CAFF-CODEIN G ACTAVIS PHARMA, EAGEN 00143-3000-01 0.28715 BUTALB-CAFF-ACETAMINOPH-CODEIN G WEST-WARD,INC. EAGEN 00591-3220-01 0.28715 BUTALB-CAFF-ACETAMINOPH-CODEIN G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-2553-21 0.28715 BUTALB-CAFF-ACETAMINOPH-CODEIN G QUALITEST EAGEN 16590-0470-30 0.28715 BUTALB-CAFF-ACETAMINOPH-CODEIN G STAT RX USA EAGEN 51991-0073-01 0.28715 BUTALB-CAFF-ACETAMINOPH-CODEIN G BRECKENRIDGE EAGEN 00527-1312-01 1.75350 BUTALBITAL COMP-CODEINE #3 CAP G LANNETT CO. INC EAGEN 00591-3546-01 1.75350 BUTALBITAL COMP-CODEINE #3 CAP G ACTAVIS PHARMA, EAGEN 00591-3546-05 1.75350 BUTALBITAL COMP-CODEINE #3 CAP G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-2548-21 0.13030 BUTALBITAL COMPOUND TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00527-1552-01 0.53518 BUTALBITAL-ASA-CAFFEINE CAP 0 LANNETT CO. INC EAGEN 00591-3219-01 0.53518 BUTALBITAL-ASA-CAFFEINE CAP 0 ACTAVIS PHARMA, EABND 00143-1785-01 0.13030 0.58307 BUTALBITAL-ASA-CAFFEINE TABLET 0 WEST-WARD,INC. EABND 00143-1785-10 0.13030 0.54987 BUTALBITAL-ASA-CAFFEINE TABLET 0 WEST-WARD,INC. EABND 00143-1785-30 0.13030 0.64740 BUTALBITAL-ASA-CAFFEINE TABLET 0 WEST-WARD,INC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 451LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 04 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00037-0113-60 3.52<strong>06</strong>1 BUTISOL SODIUM 30 MG TABLET 0 MEDA PHARMACEUT EABEX 00037-0110-16 1.04960 BUTISOL SODIUM 30 MG/5 ML ELX 0 MEDA PHARMACEUT MLBEX 00037-0114-60 4.90729 BUTISOL SODIUM 50 MG TABLET 0 MEDA PHARMACEUT EABND 59011-0751-04 60.<strong>08</strong>577 BUTRANS 10 MCG/HR PATCH G PURDUE PHARMA L EABND 59011-0752-04 1<strong>06</strong>.37487 BUTRANS 20 MCG/HR PATCH G PURDUE PHARMA L EABND 59011-0750-04 40.05995 BUTRANS 5 MCG/HR PATCH G PURDUE PHARMA L EAGUL 00185-0749-01 1.83750 CARISOPRODOL CPD-CODEINE TAB G SANDOZ EAGEN 64980-0176-01 1.55010 CARISOPRODOL-ASPIRIN-CODEIN TB G RISING PHARM EAGEN 00131-2104-37 0.03650 CO-GESIC 5-500 TABLET G UCB PHARMA EAGEN 00009-0347-02 4.14000 DEPO-TESTOSTERONE 100 MG/ML VL G PHARMACIA/UPJHN ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00009-0417-01 15.41850 DEPO-TESTOSTERONE 200 MG/ML G PHARMACIA/UPJHN MLGEN 00009-0417-02 10.45275 DEPO-TESTOSTERONE 200 MG/ML G PHARMACIA/UPJHN MLGEN 00378-8172-91 16.89225 DRONABINOL 10 MG CAPSULE G MYLAN EAGEN 00591-3593-60 16.89225 DRONABINOL 10 MG CAPSULE G ACTAVIS PHARMA, EAGEN 49884-<strong>08</strong>69-02 16.89125 DRONABINOL 10 MG CAPSULE G PAR PHARM. EAGEN 00378-8170-91 4.41987 DRONABINOL 2.5 MG CAPSULE G MYLAN EAGEN 00591-3591-60 4.41987 DRONABINOL 2.5 MG CAPSULE G ACTAVIS PHARMA, EAGEN 49884-<strong>08</strong>67-02 4.41962 DRONABINOL 2.5 MG CAPSULE G PAR PHARM. EAGEN 68<strong>08</strong>4-0174-01 4.41960 DRONABINOL 2.5 MG CAPSULE G AHP EAGEN 68<strong>08</strong>4-0174-11 4.41960 DRONABINOL 2.5 MG CAPSULE G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-8171-91 9.19862 DRONABINOL 5 MG CAPSULE G MYLAN EAGEN 00591-3592-60 9.19862 DRONABINOL 5 MG CAPSULE G ACTAVIS PHARMA, EAGEN 49884-<strong>08</strong>68-02 9.19812 DRONABINOL 5 MG CAPSULE G PAR PHARM. EAGEN 68<strong>08</strong>4-0175-11 9.19824 DRONABINOL 5 MG CAPSULE G AHP EAGEN 68<strong>08</strong>4-0175-21 9.19824 DRONABINOL 5 MG CAPSULE G AHP EAGEN 52544-0957-01 0.<strong>06</strong>030 FIORICET 50-325-40 MG TABLET 0 ACTAVIS PHARMA, EAGEN 52544-0957-05 0.<strong>06</strong>030 FIORICET 50-325-40 MG TABLET 0 ACTAVIS PHARMA, EABND 52544-0958-01 0.28715 4.54790 FIORICET-COD 30-50-325-40 CAP G ACTAVIS PHARMA, EABND 52544-0<strong>08</strong>2-01 4.73100 FIORICET-COD 50-300-40-30 CAP G ACTAVIS PHARMA, EABND 52544-0955-01 0.53518 2.16995 FIORINAL 50-325-40 MG CAPSULE 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 52544-0956-01 1.75350 4.54790 FIORINAL-COD 30-50-325-40 CAP G ACTAVIS PHARMA, EABND 63481-0183-16 5.89438 FORTESTA 10 MG GEL PUMP G ENDO PHARM INC. GMBEX 62856-0280-30 18.88416 FYCOMPA 10 MG TABLET G EISAI INC. EABEX 62856-0282-30 18.88416 FYCOMPA 12 MG TABLET G EISAI INC. EABEX 62856-0272-30 9.442<strong>08</strong> FYCOMPA 2 MG TABLET G EISAI INC. EABEX 62856-0274-30 18.88416 FYCOMPA 4 MG TABLET G EISAI INC. EABEX 62856-0276-30 18.88416 FYCOMPA 6 MG TABLET G EISAI INC. EABEX 62856-0278-30 18.88416 FYCOMPA 8 MG TABLET G EISAI INC. EAGEN 76014-0001-25 0.17770 HYCET 7.5 MG-325 MG/15 ML SOLN G ECLAT PHARMACEU MLGEN 00121-0772-04 0.17770 HYDROCODON-ACETAMIN 7.5-325/15 G PHARMACEU ASSOC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00121-4772-15 0.21673 HYDROCODON-ACETAMIN 7.5-325/15 G PHARMACEU ASSOC MLGEN 64376-<strong>06</strong>40-16 0.17770 HYDROCODON-ACETAMIN 7.5-325/15 G BOCA PHARMACAL MLGEN 64376-<strong>06</strong>40-40 0.17770 HYDROCODON-ACETAMIN 7.5-325/15 G BOCA PHARMACAL MLGEN 66689-0023-16 0.09133 HYDROCODON-ACETAMIN 7.5-325/15 G VISTAPHARM MLBND 00121-<strong>06</strong>55-04 0.03470 0.05240 HYDROCODON-ACETAMIN 7.5-500/15 G PHARMACEU ASSOC ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 452LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 04 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00121-<strong>06</strong>55-16 0.03470 0.10219 HYDROCODON-ACETAMIN 7.5-500/15 G PHARMACEU ASSOC MLGEN 00591-0388-01 0.18875 HYDROCODON-ACETAMINOPH 2.5-500 G ACTAVIS PHARMA, EAGEN 64376-<strong>06</strong>49-01 1.51510 HYDROCODON-ACETAMINOPH 7.5-300 G BOCA PHARMACAL EAGEN 64376-<strong>06</strong>49-05 1.51510 HYDROCODON-ACETAMINOPH 7.5-300 G BOCA PHARMACAL EAGEN 004<strong>06</strong>-0366-01 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0366-05 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G MALLINCKRODT PH EAGEN 00591-2605-01 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G ACTAVIS PHARMA, EAGEN 00591-2605-05 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G ACTAVIS PHARMA, EAGEN 00591-3203-01 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G WATSON LABS EAGEN 0<strong>06</strong>03-3891-02 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-3891-21 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G QUALITEST EAGEN 0<strong>06</strong>03-3891-22 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G QUALITEST EAGEN 0<strong>06</strong>03-3891-28 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G QUALITEST EAGEN 0<strong>06</strong>03-3891-32 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G QUALITEST EAGEN 13107-0020-01 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G AUROBINDO PHARM EAGEN 13107-0020-05 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G AUROBINDO PHARM EAGEN 51079-0778-01 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G MYLAN INSTITUTI EAGEN 51079-0778-21 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G MYLAN INSTITUTI EAGEN 57664-0170-13 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G CARACO PHARM EAGEN 57664-0170-88 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G CARACO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-<strong>06</strong>01-01 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G AHP EAGEN 68<strong>08</strong>4-<strong>06</strong>01-11 0.19510 HYDROCODON-ACETAMINOPH 7.5-325 G AHP EAGEN 004<strong>06</strong>-0358-01 0.32130 HYDROCODON-ACETAMINOPH 7.5-500 G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0358-05 0.25920 HYDROCODON-ACETAMINOPH 7.5-500 G MALLINCKRODT PH EAGEN 00591-0385-01 0.42907 HYDROCODON-ACETAMINOPH 7.5-500 G ACTAVIS PHARMA, EAGEN 00591-0385-05 0.38619 HYDROCODON-ACETAMINOPH 7.5-500 G ACTAVIS PHARMA, EAGEN 53746-0112-01 0.42907 HYDROCODON-ACETAMINOPH 7.5-500 G AMNEAL PHARMACE EAGEN 53746-0112-05 0.38619 HYDROCODON-ACETAMINOPH 7.5-500 G AMNEAL PHARMACE EAGEN 53746-0112-10 0.34757 HYDROCODON-ACETAMINOPH 7.5-500 G AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-0145-01 0.17812 HYDROCODON-ACETAMINOPH 7.5-500 G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0145-11 0.17812 HYDROCODON-ACETAMINOPH 7.5-500 G AHP EAGEN 004<strong>06</strong>-0359-01 0.03861 HYDROCODON-ACETAMINOPH 7.5-650 G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0359-05 0.03861 HYDROCODON-ACETAMINOPH 7.5-650 G MALLINCKRODT PH EAGEN 00591-0502-01 0.03861 HYDROCODON-ACETAMINOPH 7.5-650 G WATSON LABS EAGEN 00591-0502-05 0.03861 HYDROCODON-ACETAMINOPH 7.5-650 G WATSON LABS EAGEN 00591-2611-01 0.03861 HYDROCODON-ACETAMINOPH 7.5-650 G ACTAVIS PHARMA, EAGEN 53746-0113-01 0.03861 HYDROCODON-ACETAMINOPH 7.5-650 G AMNEAL PHARMACE EAGEN 53746-0113-05 0.03861 HYDROCODON-ACETAMINOPH 7.5-650 G AMNEAL PHARMACE EAGEN 004<strong>06</strong>-0360-01 0.05400 HYDROCODON-ACETAMINOPH 7.5-750 G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0360-05 0.05400 HYDROCODON-ACETAMINOPH 7.5-750 G MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 004<strong>06</strong>-0360-09 0.05400 HYDROCODON-ACETAMINOPH 7.5-750 G MALLINCKRODT PH EAGEN 00591-0387-01 0.05400 HYDROCODON-ACETAMINOPH 7.5-750 G ACTAVIS PHARMA, EAGEN 00591-0387-05 0.05400 HYDROCODON-ACETAMINOPH 7.5-750 G ACTAVIS PHARMA, EAGEN 00904-7632-61 0.05400 HYDROCODON-ACETAMINOPH 7.5-750 G MAJOR PHARMACEU EAGEN 53746-0118-01 0.05400 HYDROCODON-ACETAMINOPH 7.5-750 G AMNEAL PHARMACE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 453LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 04 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53746-0118-05 0.05400 HYDROCODON-ACETAMINOPH 7.5-750 G AMNEAL PHARMACE EAGEN 53746-0118-10 0.05400 HYDROCODON-ACETAMINOPH 7.5-750 G AMNEAL PHARMACE EABND 68<strong>08</strong>4-0144-01 0.05400 0.14525 HYDROCODON-ACETAMINOPH 7.5-750 G AHP EABND 68<strong>08</strong>4-0144-11 0.05400 0.14525 HYDROCODON-ACETAMINOPH 7.5-750 G AHP EAGEN 64376-<strong>06</strong>48-01 1.38380 HYDROCODON-ACETAMINOPHEN 5-300 G BOCA PHARMACAL EAGEN 64376-<strong>06</strong>48-05 1.38380 HYDROCODON-ACETAMINOPHEN 5-300 G BOCA PHARMACAL EAGEN 004<strong>06</strong>-0365-01 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0365-05 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G MALLINCKRODT PH EAGEN 00591-3202-01 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G ACTAVIS PHARMA, EAGEN 00591-3202-05 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-3890-02 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G QUALITEST EAGEN 0<strong>06</strong>03-3890-04 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G QUALITEST EAGEN 0<strong>06</strong>03-3890-16 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G QUALITEST EAGEN 0<strong>06</strong>03-3890-20 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G QUALITEST EAGEN 0<strong>06</strong>03-3890-21 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G QUALITEST EAGEN 0<strong>06</strong>03-3890-22 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G QUALITEST EAGEN 0<strong>06</strong>03-3890-28 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G QUALITEST EAGEN 0<strong>06</strong>03-3890-32 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G QUALITEST EAGEN 13107-0019-01 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G AUROBINDO PHARM EAGEN 13107-0019-05 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0777-01 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G MYLAN INSTITUTI EAGEN 51079-0777-21 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G MYLAN INSTITUTI EAGEN 53746-0109-01 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G AMNEAL PHARMACE EAGEN 53746-0109-05 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G AMNEAL PHARMACE EAGEN 53746-0109-10 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G AMNEAL PHARMACE EAGEN 57664-0126-13 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G CARACO PHARM EAGEN 57664-0126-88 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G CARACO PHARM EAGEN 68<strong>08</strong>4-0368-01 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G AHP EAGEN 68<strong>08</strong>4-0368-11 0.13050 HYDROCODON-ACETAMINOPHEN 5-325 G AHP EAGEN 004<strong>06</strong>-0357-01 0.03650 HYDROCODON-ACETAMINOPHEN 5-500 G MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 004<strong>06</strong>-0357-05 0.03650 HYDROCODON-ACETAMINOPHEN 5-500 G MALLINCKRODT PH EAGEN 00591-0349-01 0.03650 HYDROCODON-ACETAMINOPHEN 5-500 G ACTAVIS PHARMA, EAGEN 00591-0349-05 0.03650 HYDROCODON-ACETAMINOPHEN 5-500 G ACTAVIS PHARMA, EAGEN 53746-0111-01 0.03650 HYDROCODON-ACETAMINOPHEN 5-500 G AMNEAL PHARMACE EAGEN 53746-0111-05 0.03650 HYDROCODON-ACETAMINOPHEN 5-500 G AMNEAL PHARMACE EAGEN 53746-0111-10 0.03650 HYDROCODON-ACETAMINOPHEN 5-500 G AMNEAL PHARMACE EAGEN 62584-0738-11 0.03650 HYDROCODON-ACETAMINOPHEN 5-500 G AHP EAGEN 63739-0455-01 0.03650 HYDROCODON-ACETAMINOPHEN 5-500 G MCKESSON PACKAG EAGEN 63739-0455-10 0.03650 HYDROCODON-ACETAMINOPHEN 5-500 G MCKESSON PACKAG EAGEN 10914-0552-01 1.79497 HYDROCODON-ACETAMINOPHN 10-300 G SHIONOGI PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64376-<strong>06</strong>43-01 1.95460 HYDROCODON-ACETAMINOPHN 10-300 G BOCA PHARMACAL EAGEN 64376-<strong>06</strong>43-05 1.95460 HYDROCODON-ACETAMINOPHN 10-300 G BOCA PHARMACAL EAGEN 004<strong>06</strong>-0367-01 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0367-05 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G MALLINCKRODT PH EAGEN 00591-<strong>08</strong>53-01 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G ACTAVIS PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 454LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 04 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-<strong>08</strong>53-05 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G WATSON LABS EAGEN 00591-2612-01 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G ACTAVIS PHARMA, EAGEN 00591-2612-05 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-3887-02 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G QUALITEST EAGEN 0<strong>06</strong>03-3887-04 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G QUALITEST EAGEN 0<strong>06</strong>03-3887-12 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G QUALITEST EAGEN 0<strong>06</strong>03-3887-20 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G QUALITEST EAGEN 0<strong>06</strong>03-3887-21 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G QUALITEST EAGEN 0<strong>06</strong>03-3887-22 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G QUALITEST EAGEN 0<strong>06</strong>03-3887-26 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-3887-28 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G QUALITEST EAGEN 0<strong>06</strong>03-3887-32 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G QUALITEST EAGEN 13107-0021-01 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G AUROBINDO PHARM EAGEN 13107-0021-05 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G AUROBINDO PHARM EAGEN 13107-0021-99 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G AUROBINDO PHARM EAGEN 51079-0779-01 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G MYLAN INSTITUTI EAGEN 51079-0779-21 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G MYLAN INSTITUTI EAGEN 53746-0110-01 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G AMNEAL PHARMACE EAGEN 53746-0110-05 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G AMNEAL PHARMACE EAGEN 53746-0110-10 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G AMNEAL PHARMACE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 57664-0176-13 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G CARACO PHARM EAGEN 57664-0176-88 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G CARACO PHARM EAGEN 68<strong>08</strong>4-0100-01 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G AHP EAGEN 68<strong>08</strong>4-0100-11 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G AHP EAGEN 68<strong>08</strong>4-0353-01 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G AHP EAGEN 68<strong>08</strong>4-0353-11 0.13325 HYDROCODON-ACETAMINOPHN 10-325 G AHP EAGEN 004<strong>06</strong>-0363-01 0.24870 HYDROCODON-ACETAMINOPHN 10-500 G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0363-05 0.24870 HYDROCODON-ACETAMINOPHN 10-500 G MALLINCKRODT PH EAGEN 00591-0540-01 0.24870 HYDROCODON-ACETAMINOPHN 10-500 G WATSON LABS EAGEN 00591-2609-01 0.24870 HYDROCODON-ACETAMINOPHN 10-500 G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-2609-05 0.24870 HYDROCODON-ACETAMINOPHN 10-500 G ACTAVIS PHARMA, EAGEN 53746-0119-01 0.24870 HYDROCODON-ACETAMINOPHN 10-500 G AMNEAL PHARMACE EAGEN 53746-0119-05 0.24870 HYDROCODON-ACETAMINOPHN 10-500 G AMNEAL PHARMACE EAGEN 53746-0119-10 0.24870 HYDROCODON-ACETAMINOPHN 10-500 G AMNEAL PHARMACE EAGEN 68<strong>08</strong>4-0362-01 0.24870 HYDROCODON-ACETAMINOPHN 10-500 G AHP EAGEN 004<strong>06</strong>-0361-01 0.07<strong>06</strong>1 HYDROCODON-ACETAMINOPHN 10-650 G MALLINCKRODT PH EAGEN 004<strong>06</strong>-0361-05 0.07<strong>06</strong>1 HYDROCODON-ACETAMINOPHN 10-650 G MALLINCKRODT PH EAGEN 00591-0503-01 0.07<strong>06</strong>1 HYDROCODON-ACETAMINOPHN 10-650 G WATSON LABS EAGEN 00591-0503-05 0.07<strong>06</strong>1 HYDROCODON-ACETAMINOPHN 10-650 G WATSON LABS EAGEN 00591-2610-01 0.07<strong>06</strong>1 HYDROCODON-ACETAMINOPHN 10-650 G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-2610-05 0.07<strong>06</strong>1 HYDROCODON-ACETAMINOPHN 10-650 G ACTAVIS PHARMA, EAGEN 53746-0114-01 0.07<strong>06</strong>1 HYDROCODON-ACETAMINOPHN 10-650 G AMNEAL PHARMACE EAGEN 53746-0114-05 0.07<strong>06</strong>1 HYDROCODON-ACETAMINOPHN 10-650 G AMNEAL PHARMACE EAGEN 53746-0114-10 0.07<strong>06</strong>1 HYDROCODON-ACETAMINOPHN 10-650 G AMNEAL PHARMACE EAGEN 004<strong>06</strong>-0362-01 0.20345 HYDROCODON-ACETAMINOPHN 10-660 G MALLINCKRODT PH EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 455LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 04 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-0517-01 0.20345 HYDROCODON-ACETAMINOPHN 10-660 G ACTAVIS PHARMA, EAGEN 004<strong>06</strong>-0364-01 0.49<strong>08</strong>0 HYDROCODON-ACETAMINOPHN 10-750 G MALLINCKRODT PH EAGEN 00591-2607-01 0.49<strong>08</strong>0 HYDROCODON-ACETAMINOPHN 10-750 G ACTAVIS PHARMA, EAGEN 00591-3228-01 0.49<strong>08</strong>0 HYDROCODON-ACETAMINOPHN 10-750 G ACTAVIS PHARMA, EAGEN 53746-0117-01 0.69590 HYDROCODONE-IBUPR<strong>OF</strong>EN 10-200 G AMNEAL PHARMACE EAGEN 53746-0116-01 2.19097 HYDROCODONE-IBUPR<strong>OF</strong>EN 2.5-200 G AMNEAL PHARMACE EAGEN 53746-0146-01 2.28<strong>06</strong>0 HYDROCODONE-IBUPR<strong>OF</strong>EN 5-200 MG G AMNEAL PHARMACE EAGEN 00093-5161-01 0.23369 HYDROCODONE-IBUPR<strong>OF</strong>EN 7.5-200 G TEVA USA EAGEN 0<strong>06</strong>03-3897-21 0.23369 HYDROCODONE-IBUPR<strong>OF</strong>EN 7.5-200 G QUALITEST EAGEN 0<strong>06</strong>03-3897-28 0.23369 HYDROCODONE-IBUPR<strong>OF</strong>EN 7.5-200 G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53746-0145-01 0.23369 HYDROCODONE-IBUPR<strong>OF</strong>EN 7.5-200 G AMNEAL PHARMACE EAGEN 62037-0524-01 0.23369 HYDROCODONE-IBUPR<strong>OF</strong>EN 7.5-200 G ACTAVIS PHARMA, EAGEN 62037-0524-05 0.23369 HYDROCODONE-IBUPR<strong>OF</strong>EN 7.5-200 G ACTAVIS PHARMA, EAGEN 50991-0579-01 0.69590 IBUDONE 10-200 MG TABLET G POLY PHARM. EAGEN 50991-0578-01 0.66930 IBUDONE 5-200 MG TABLET G POLY PHARM. EAGEN 38779-1754-04 24.93750 KETAMINE HCL POWDER 0 MEDISCA INC. GMGEN 38779-1754-05 24.93750 KETAMINE HCL POWDER 0 MEDISCA INC. GMGEN 38779-1754-09 24.93750 KETAMINE HCL POWDER 0 MEDISCA INC. GMBND 00095-9090-16 0.38692 LORTAB 10 MG-300 MG/15 ML ELXR G ECR PHARM. MLGEN 50474-0910-01 0.24870 LORTAB 10-500 TABLET G UCB PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 50474-0910-50 0.24870 LORTAB 10-500 TABLET G UCB PHARMA EAGEN 50474-0902-01 0.03650 LORTAB 5-500 TABLET G UCB PHARMA EAGEN 50474-0902-50 0.03650 LORTAB 5-500 TABLET G UCB PHARMA EAGEN 50474-0909-16 0.03470 LORTAB 7.5-500 MG/15 ML ELIXIR G UCB PHARMA MLGUL 50474-0907-01 0.64260 LORTAB 7.5-500 TABLET G UCB PHARMA EAGUL 50474-0907-50 0.64260 LORTAB 7.5-500 TABLET G UCB PHARMA EABND 00051-0023-21 36.05271 36.05271 MARINOL 10 MG CAPSULE G ABBVIE US LLC EABND 00051-0021-21 9.43309 9.43309 MARINOL 2.5 MG CAPSULE G ABBVIE US LLC EABND 00051-0022-21 19.63267 19.63267 MARINOL 5 MG CAPSULE G ABBVIE US LLC EABND 52544-0163-01 0.49<strong>08</strong>0 1.90783 MAXIDONE 10-750 MG TABLET G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 52544-<strong>06</strong>34-01 0.49<strong>08</strong>0 1.90783 MAXIDONE 10-750 MG TABLET G ACTAVIS PHARMA, EABND 00115-7037-01 8.64669 METHITEST 10 MG TABLET G GLOBAL PHARM EAGEN 52544-0161-01 0.13325 NORCO 10-325 TABLET G ACTAVIS PHARMA, EAGEN 52544-0161-05 0.13325 NORCO 10-325 TABLET G ACTAVIS PHARMA, EAGEN 52544-0539-01 0.13325 NORCO 10-325 TABLET G WATSON PHARMA EAGEN 52544-0913-01 0.13050 NORCO 5-325 TABLET G ACTAVIS PHARMA, EAGEN 52544-0162-01 0.19510 NORCO 7.5-325 TABLET G ACTAVIS PHARMA, EAGEN 52544-0729-01 0.19510 NORCO 7.5-325 TABLET G WATSON PHARMA EABND 00095-7029-01 1.99200 ORBIVAN CAPSULE 0 ECR PHARM. EAGEN 00185-0272-60 8.80290 OXANDROLONE 10 MG TABLET G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00245-0272-<strong>06</strong> 8.80290 OXANDROLONE 10 MG TABLET G UPSHER SMITH EAGEN 00591-3545-60 8.80290 OXANDROLONE 10 MG TABLET G WATSON LABS EAGEN 49884-0302-02 8.80290 OXANDROLONE 10 MG TABLET G PAR PHARM. EAGEN 00185-0271-01 3.14483 OXANDROLONE 2.5 MG TABLET G SANDOZ EAGEN 00245-0271-11 3.14483 OXANDROLONE 2.5 MG TABLET G UPSHER SMITH EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 456LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 04 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3544-01 3.14483 OXANDROLONE 2.5 MG TABLET G ACTAVIS PHARMA, EAGEN 49884-0301-01 3.14483 OXANDROLONE 2.5 MG TABLET G PAR PHARM. EABND 50383-<strong>08</strong>55-16 0.55002 PAREGORIC LIQUID 0 HI-TECH PHARMAC MLBUL 58407-0091-01 0.19430 0.36262 STAGESIC 5-500 CAPSULE G MAGNA PHARM EABND 12496-1212-03 14.02036 SUBOXONE 12 MG-3 MG SL FILM G RECKITT BENCKIS EABND 12496-1202-01 3.91760 SUBOXONE 2 MG-0.5 MG SL FILM G RECKITT BENCKIS EABND 12496-1202-03 3.91262 SUBOXONE 2 MG-0.5 MG SL FILM G RECKITT BENCKIS EABND 12496-1283-02 4.56483 5.36844 SUBOXONE 2 MG-0.5 MG TABLET SL G RECKITT BENCKIS EABND 12496-1204-03 7.01018 SUBOXONE 4 MG-1 MG SL FILM G RECKITT BENCKIS EABND 12496-12<strong>08</strong>-01 7.01350 SUBOXONE 8 MG-2 MG SL FILM G RECKITT BENCKIS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 12496-12<strong>08</strong>-03 7.01018 SUBOXONE 8 MG-2 MG SL FILM G RECKITT BENCKIS EABND 12496-13<strong>06</strong>-02 8.17400 9.62136 SUBOXONE 8 MG-2 MG TABLET SL G RECKITT BENCKIS EABND 497<strong>08</strong>-0419-88 1.7<strong>06</strong><strong>06</strong> SYNALGOS-DC CAPSULE G CARACO PHARMA I EABND 66887-0001-05 2.54251 TESTIM 1% (50MG) GEL G AUXILIUM PHARM GMGEN 62756-0017-40 4.14000 TESTOSTERON CYP 1,000 MG/10 ML G SUN PHARMACEUTI MLGEN 00591-3223-79 7.60425 TESTOSTERON CYP 2,000 MG/10 ML G ACTAVIS PHARMA, MLGEN 62756-0016-40 8.46375 TESTOSTERON CYP 2,000 MG/10 ML G SUN PHARMACEUTI MLGEN 00781-3073-70 4.14000 TESTOSTERONE CYP 100 MG/ML G SANDOZ MLGEN 00574-<strong>08</strong>20-01 15.41850 TESTOSTERONE CYP 200 MG/ML G PADDOCK LABS. MLGEN 00574-<strong>08</strong>20-10 7.60425 TESTOSTERONE CYP 200 MG/ML G PADDOCK LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00781-3074-70 8.46450 TESTOSTERONE CYP 200 MG/ML G SANDOZ MLGEN 00781-3074-71 15.41850 TESTOSTERONE CYP 200 MG/ML G SANDOZ MLGEN 62756-0015-40 15.41850 TESTOSTERONE CYP 200 MG/ML G SUN PHARMACEUTI MLGEN 00591-3221-26 12.74250 TESTOSTERONE ENAN 200 MG/ML G ACTAVIS PHARMA, MLBND 00187-0901-01 61.01994 TESTRED 10 MG CAPSULE G VALEANT EAGEN 50458-0513-60 0.13020 TYLENOL WITH CODEINE #3 TABLET G JANSSEN PHARM. EAGEN 50458-0513-80 0.13020 TYLENOL WITH CODEINE #3 TABLET G JANSSEN PHARM. EAGEN 50458-0515-70 0.22990 TYLENOL WITH CODEINE #4 TABLET G JANSSEN PHARM. EAGEN 00074-3043-13 1.51510 VICODIN ES 7.5-300 MG TABLET G ABBVIE US LLC EAGEN 00074-3043-53 1.51510 VICODIN ES 7.5-300 MG TABLET G ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00074-1973-14 0.05400 VICODIN ES 7.5-750 MG TABLET G ABBVIE US LLC EAGEN 00074-1973-54 0.05400 VICODIN ES 7.5-750 MG TABLET G ABBVIE US LLC EAGEN 00074-3054-13 1.95460 VICODIN HP 10-300 MG TABLET G ABBVIE US LLC EAGEN 00074-3054-53 1.95460 VICODIN HP 10-300 MG TABLET G ABBVIE US LLC EAGEN 00074-2274-14 0.20345 VICODIN HP 10-660 MG TABLET G ABBVIE US LLC EAGEN 00074-2274-54 0.20345 VICODIN HP 10-660 MG TABLET G ABBVIE US LLC EAGEN 00074-3041-13 1.38380 VICODIN 5-300 MG TABLET G ABBVIE US LLC EAGEN 00074-3041-53 1.38380 VICODIN 5-300 MG TABLET G ABBVIE US LLC EAGEN 00074-1949-14 0.03650 VICODIN 5-500 TABLET G ABBVIE US LLC EABND 00074-2277-14 0.23369 3.32298 VICOPR<strong>OF</strong>EN 200-7.5 MG TAB G ABBVIE US LLC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00074-2277-54 0.23369 3.09040 VICOPR<strong>OF</strong>EN 200-7.5 MG TAB G ABBVIE US LLC EAGEN 59630-0911-10 1.95460 XODOL 10-300 TABLET G SHIONOGI PHARMA EAGEN 68453-0911-10 1.95460 XODOL 10-300 TABLET G SHIONOGI PHARMA EAGEN 59630-0912-10 1.38380 XODOL 5-300 TABLET G SHIONOGI PHARMA EAGEN 59630-0913-10 1.51510 XODOL 7.5-300 MG TABLET G SHIONOGI PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 457LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 04 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 68727-0100-01 15.93996 XYREM 500 MG/ML ORAL SOLUTION G JAZZ PHARMACEUT MLBND 63717-<strong>08</strong>95-16 0.30378 ZAMICET 10-325 MG/15 ML SOLN G HAWTHORN PHARM MLGEN 68453-0170-10 1.61925 ZEBUTAL CAPSULE 0 SHIONOGI PHARMA EABND 00095-<strong>06</strong>74-16 0.38955 ZOLVIT 10 MG-300 MG/15 ML SOLN G ECR PHARM. MLBND 54123-0914-30 3.50260 ZUBSOLV 1.4-0.36 MG TABLET SL G OREXO US, INC. EABND 54123-0957-30 7.01018 ZUBSOLV 5.7-1.4 MG TABLET SL G OREXO US, INC. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 458LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00093-5450-<strong>06</strong> 0.57645 ALPRAZOLAM ER 0.5 MG TABLET 0 TEVA USA EAGEX 00228-3<strong>08</strong>3-<strong>06</strong> 0.57645 ALPRAZOLAM ER 0.5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00378-5021-91 0.57645 ALPRAZOLAM ER 0.5 MG TABLET 0 MYLAN EAGEX 64980-0140-<strong>06</strong> 0.57645 ALPRAZOLAM ER 0.5 MG TABLET 0 RISING PHARM EAGEX 65162-<strong>08</strong>09-<strong>06</strong> 0.57645 ALPRAZOLAM ER 0.5 MG TABLET 0 AMNEAL PHARMACE EAGEX 65862-0454-60 0.57645 ALPRAZOLAM ER 0.5 MG TABLET 0 AUROBINDO PHARM EAGEX 00093-5451-<strong>06</strong> 0.45250 ALPRAZOLAM ER 1 MG TABLET 0 TEVA USA EAGEX 00228-3<strong>08</strong>4-<strong>06</strong> 0.45250 ALPRAZOLAM ER 1 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00378-5022-91 0.45250 ALPRAZOLAM ER 1 MG TABLET 0 MYLAN EAGEX 64980-0141-<strong>06</strong> 0.45250 ALPRAZOLAM ER 1 MG TABLET 0 RISING PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65162-<strong>08</strong>10-<strong>06</strong> 0.45250 ALPRAZOLAM ER 1 MG TABLET 0 AMNEAL PHARMACE EAGEX 65862-0455-60 0.45250 ALPRAZOLAM ER 1 MG TABLET 0 AUROBINDO PHARM EAGEX 00093-5452-<strong>06</strong> 0.55458 ALPRAZOLAM ER 2 MG TABLET 0 TEVA USA EAGEX 00228-3<strong>08</strong>7-<strong>06</strong> 0.55458 ALPRAZOLAM ER 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 64980-0142-<strong>06</strong> 0.55458 ALPRAZOLAM ER 2 MG TABLET 0 RISING PHARM EAGEX 65162-<strong>08</strong>12-<strong>06</strong> 0.55458 ALPRAZOLAM ER 2 MG TABLET 0 AMNEAL PHARMACE EAGEX 65862-0456-60 0.55458 ALPRAZOLAM ER 2 MG TABLET 0 AUROBINDO PHARM EAGEX 00093-5453-<strong>06</strong> 1.81545 ALPRAZOLAM ER 3 MG TABLET 0 TEVA USA EAGEX 00228-3<strong>08</strong>6-<strong>06</strong> 1.81545 ALPRAZOLAM ER 3 MG TABLET 0 ACTAVIS PHARMA, EAGEX 64980-0143-<strong>06</strong> 1.81545 ALPRAZOLAM ER 3 MG TABLET 0 RISING PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 65162-<strong>08</strong>13-<strong>06</strong> 1.81545 ALPRAZOLAM ER 3 MG TABLET 0 AMNEAL PHARMACE EAGEX 65862-0457-60 1.81545 ALPRAZOLAM ER 3 MG TABLET 0 AUROBINDO PHARM EAGEX 49884-0110-52 1.05740 ALPRAZOLAM ODT 0.25 MG TAB 0 PAR PHARM. EAGEX 49884-0110-74 1.05740 ALPRAZOLAM ODT 0.25 MG TAB 0 PAR PHARM. EAGEX 49884-0111-52 1.31750 ALPRAZOLAM ODT 0.5 MG TAB 0 PAR PHARM. EAGEX 49884-0111-74 1.31750 ALPRAZOLAM ODT 0.5 MG TAB 0 PAR PHARM. EAGEX 49884-0213-52 1.89165 ALPRAZOLAM ODT 1 MG TAB 0 PAR PHARM. EAGEX 49884-0213-74 1.89165 ALPRAZOLAM ODT 1 MG TAB 0 PAR PHARM. EAGEX 49884-0214-52 2.74200 ALPRAZOLAM ODT 2 MG TAB 0 PAR PHARM. EAGEX 49884-0214-74 2.74200 ALPRAZOLAM ODT 2 MG TAB 0 PAR PHARM. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-0057-01 0.57645 ALPRAZOLAM XR 0.5 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-0059-01 0.45250 ALPRAZOLAM XR 1 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-0<strong>06</strong>6-01 0.55458 ALPRAZOLAM XR 2 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-0<strong>06</strong>8-01 1.81545 ALPRAZOLAM XR 3 MG TABLET 0 GREENSTONE LLC. EAGEX 00228-4019-11 1.05740 ALPRAZOLAM 0.25 MG ODT 0 ACTAVIS PHARMA, EAGEX 00228-2027-10 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00228-2027-50 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00228-2027-96 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00378-4001-77 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 MYLAN EAGEX 0<strong>06</strong>03-2127-21 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-2127-28 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2127-32 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 QUALITEST EAGEX 00781-1<strong>06</strong>1-01 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 SANDOZ EAGEX 00781-1<strong>06</strong>1-05 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 SANDOZ EAGEX 00781-1<strong>06</strong>1-10 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 459LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00904-5858-61 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 MAJOR PHARMACEU EAGEX 47335-<strong>06</strong>03-13 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 SUN PHARMA GLOB EAGEX 47335-<strong>06</strong>03-18 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 SUN PHARMA GLOB EAGEX 47335-<strong>06</strong>03-88 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 SUN PHARMA GLOB EAGEX 51079-0788-20 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 MYLAN INSTITUTI EAGEX 59762-3719-01 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-3719-03 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-3719-04 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 GREENSTONE LLC. EAGEX 64376-<strong>06</strong>30-01 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-<strong>06</strong>30-05 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 BOCA PHARMACAL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64376-<strong>06</strong>30-10 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 BOCA PHARMACAL EAGEX 67253-0900-09 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 DAVA PHARMACEUT EAGEX 67253-0900-10 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 DAVA PHARMACEUT EAGEX 67253-0900-11 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 DAVA PHARMACEUT EAGEX 67253-0900-50 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 DAVA PHARMACEUT EAGEX 68<strong>08</strong>4-0018-11 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-<strong>06</strong>47-01 0.04170 ALPRAZOLAM 0.25 MG TABLET 0 AHP EAGEX 00228-4022-11 1.31750 ALPRAZOLAM 0.5 MG ODT 0 ACTAVIS PHARMA, EAGEX 00228-2029-10 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00228-2029-50 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00228-2029-96 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00378-4003-01 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 MYLAN EAGEX 00378-4003-05 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 MYLAN EAGEX 00378-4003-77 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 MYLAN EAGEX 0<strong>06</strong>03-2128-21 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2128-28 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2128-32 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 QUALITEST EAGEX 00781-1077-01 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 SANDOZ EAGEX 00781-1077-05 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 SANDOZ EAGEX 00781-1077-10 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00904-5859-61 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 MAJOR PHARMACEU EAGEX 47335-<strong>06</strong>04-13 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 SUN PHARMA GLOB EAGEX 47335-<strong>06</strong>04-18 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 SUN PHARMA GLOB EAGEX 47335-<strong>06</strong>04-88 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 SUN PHARMA GLOB EAGEX 51079-0789-20 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 MYLAN INSTITUTI EAGEX 59762-3720-01 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-3720-03 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-3720-04 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 GREENSTONE LLC. EAGEX 64376-<strong>06</strong>31-01 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-<strong>06</strong>31-05 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 BOCA PHARMACAL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64376-<strong>06</strong>31-10 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 BOCA PHARMACAL EAGEX 67253-0901-10 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 DAVA PHARMACEUT EAGEX 67253-0901-11 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 DAVA PHARMACEUT EAGEX 67253-0901-50 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 DAVA PHARMACEUT EAGEX 68<strong>08</strong>4-0019-11 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 460LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-<strong>06</strong>72-01 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-<strong>06</strong>72-11 0.04051 ALPRAZOLAM 0.5 MG TABLET 0 AHP EAGEX 00228-4024-11 1.99300 ALPRAZOLAM 1 MG ODT 0 ACTAVIS PHARMA, EAGEX 00228-2031-10 0.04750 ALPRAZOLAM 1 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00228-2031-50 0.04750 ALPRAZOLAM 1 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00228-2031-96 0.04750 ALPRAZOLAM 1 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00378-4005-01 0.04750 ALPRAZOLAM 1 MG TABLET 0 MYLAN EAGEX 00378-4005-05 0.04750 ALPRAZOLAM 1 MG TABLET 0 MYLAN EAGEX 00378-4005-77 0.04750 ALPRAZOLAM 1 MG TABLET 0 MYLAN EAGEX 0<strong>06</strong>03-2129-21 0.04750 ALPRAZOLAM 1 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-2129-28 0.04750 ALPRAZOLAM 1 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2129-32 0.04750 ALPRAZOLAM 1 MG TABLET 0 QUALITEST EAGEX 00781-1079-01 0.04750 ALPRAZOLAM 1 MG TABLET 0 SANDOZ EAGEX 00781-1079-05 0.04750 ALPRAZOLAM 1 MG TABLET 0 SANDOZ EAGEX 00781-1079-10 0.04750 ALPRAZOLAM 1 MG TABLET 0 SANDOZ EAGEX 47335-<strong>06</strong>05-13 0.04750 ALPRAZOLAM 1 MG TABLET 0 SUN PHARMA GLOB EAGEX 47335-<strong>06</strong>05-18 0.04750 ALPRAZOLAM 1 MG TABLET 0 SUN PHARMA GLOB EAGEX 47335-<strong>06</strong>05-88 0.04750 ALPRAZOLAM 1 MG TABLET 0 SUN PHARMA GLOB EAGEX 51079-0790-20 0.04750 ALPRAZOLAM 1 MG TABLET 0 MYLAN INSTITUTI EAGEX 59762-3721-01 0.04750 ALPRAZOLAM 1 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-3721-03 0.04750 ALPRAZOLAM 1 MG TABLET 0 GREENSTONE LLC. EAGEX 59762-3721-04 0.04750 ALPRAZOLAM 1 MG TABLET 0 GREENSTONE LLC. EAGEX 64376-<strong>06</strong>32-01 0.04750 ALPRAZOLAM 1 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-<strong>06</strong>32-05 0.04750 ALPRAZOLAM 1 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-<strong>06</strong>32-10 0.04750 ALPRAZOLAM 1 MG TABLET 0 BOCA PHARMACAL EAGEX 67253-0902-10 0.04750 ALPRAZOLAM 1 MG TABLET 0 DAVA PHARMACEUT EAGEX 67253-0902-11 0.04750 ALPRAZOLAM 1 MG TABLET 0 DAVA PHARMACEUT EAGEX 67253-0902-50 0.04750 ALPRAZOLAM 1 MG TABLET 0 DAVA PHARMACEUT EAGEX 68<strong>08</strong>4-0020-11 0.04750 ALPRAZOLAM 1 MG TABLET 0 AHP EABEX 00054-3<strong>06</strong>8-44 2.46814 ALPRAZOLAM 1 MG/ML ORAL CONC 0 ROXANE LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00228-4025-11 2.74200 ALPRAZOLAM 2 MG ODT 0 ACTAVIS PHARMA, EAGEX 00228-2039-10 0.04040 ALPRAZOLAM 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00228-2039-50 0.04040 ALPRAZOLAM 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00378-4007-01 0.04040 ALPRAZOLAM 2 MG TABLET 0 MYLAN EAGEX 0<strong>06</strong>03-2130-21 0.04040 ALPRAZOLAM 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2130-28 0.04040 ALPRAZOLAM 2 MG TABLET 0 QUALITEST EAGEX 00781-1<strong>08</strong>9-01 0.04040 ALPRAZOLAM 2 MG TABLET 0 SANDOZ EAGEX 00781-1<strong>08</strong>9-05 0.04040 ALPRAZOLAM 2 MG TABLET 0 SANDOZ EAGEX 47335-<strong>06</strong><strong>06</strong>-88 0.04040 ALPRAZOLAM 2 MG TABLET 0 SUN PHARMA GLOB EAGEX 59762-3722-01 0.04040 ALPRAZOLAM 2 MG TABLET 0 GREENSTONE LLC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 59762-3722-03 0.04040 ALPRAZOLAM 2 MG TABLET 0 GREENSTONE LLC. EAGEX 64376-<strong>06</strong>33-01 0.04040 ALPRAZOLAM 2 MG TABLET 0 BOCA PHARMACAL EAGEX 64376-<strong>06</strong>33-05 0.04040 ALPRAZOLAM 2 MG TABLET 0 BOCA PHARMACAL EAGEX 67253-0903-10 0.04040 ALPRAZOLAM 2 MG TABLET 0 DAVA PHARMACEUT EAGEX 67253-0903-50 0.04040 ALPRAZOLAM 2 MG TABLET 0 DAVA PHARMACEUT EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 461LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00024-5521-31 2.62500 9.87716 AMBIEN CR 12.5 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABEX 00024-5501-31 1.55510 9.87716 AMBIEN CR 6.25 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABEX 00024-5421-31 0.02687 9.87716 AMBIEN 10 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABEX 00024-5421-50 0.02687 9.87711 AMBIEN 10 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABEX 00024-5401-31 0.02580 9.87716 AMBIEN 5 MG TABLET G SAN<strong>OF</strong>I-AVENTIS EABEX 00187-0<strong>06</strong>3-01 0.02740 6.28<strong>06</strong>1 ATIVAN 0.5 MG TABLET G VALEANT EABEX 64455-0<strong>06</strong>3-01 0.02740 6.28<strong>06</strong>1 ATIVAN 0.5 MG TABLET G VALEANT EABEX 00187-0<strong>06</strong>4-01 0.02940 8.38988 ATIVAN 1 MG TABLET G VALEANT EABEX 00187-0<strong>06</strong>4-10 0.02940 8.21704 ATIVAN 1 MG TABLET G VALEANT EABEX 64455-0<strong>06</strong>4-01 0.02940 6.99158 ATIVAN 1 MG TABLET G VALEANT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 64455-0<strong>06</strong>4-10 0.02940 8.21704 ATIVAN 1 MG TABLET G VALEANT EABEX 00187-0<strong>06</strong>5-01 0.04910 13.37113 ATIVAN 2 MG TABLET G VALEANT EABEX 64455-0<strong>06</strong>5-01 0.04910 11.14258 ATIVAN 2 MG TABLET G VALEANT EABEX 0<strong>06</strong>41-6001-01 0.74600 1.723<strong>08</strong> ATIVAN 2 MG/ML VIAL G WEST-WARD,INC. MLBEX 0<strong>06</strong>41-6001-25 0.74600 1.723<strong>08</strong> ATIVAN 2 MG/ML VIAL G WEST-WARD,INC. MLBEX 0<strong>06</strong>41-6003-01 1.14240 2.39040 ATIVAN 4 MG/ML VIAL G WEST-WARD,INC. MLBEX 0<strong>06</strong>41-6003-25 1.14240 2.39040 ATIVAN 4 MG/ML VIAL G WEST-WARD,INC. MLGEN 00054-3090-36 4.54129 BUTORPHANOL 10 MG/ML SPRAY G ROXANE LABS. MLGEN 00378-9639-43 4.54129 BUTORPHANOL 10 MG/ML SPRAY G MYLAN MLGEN 60505-<strong>08</strong>13-01 4.54129 BUTORPHANOL 10 MG/ML SPRAY G APOTEX CORP ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUL 23155-0145-01 0.27<strong>08</strong>0 CARISOPRODL-ASPIRIN 200-325 MG G HERITAGE PHARMA EAGUL 64980-0175-01 0.27<strong>08</strong>0 CARISOPRODL-ASPIRIN 200-325 MG G RISING PHARM EAGUL 00185-0724-01 0.27<strong>08</strong>0 CARISOPRODOL COMPOUND TAB G SANDOZ EAGUL 00185-0724-05 0.27<strong>08</strong>0 CARISOPRODOL COMPOUND TAB G SANDOZ EAGEN 51525-5901-01 2.47942 CARISOPRODOL 250 MG TABLET G WALLACE PHARMAC EAGEN 00143-1176-01 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G WEST-WARD,INC. EAGEN 00143-1176-05 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G WEST-WARD,INC. EAGEN 00143-1176-10 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G WEST-WARD,INC. EAGEN 00591-5513-01 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-5513-05 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-5513-10 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G ACTAVIS PHARMA, EAGEN 0<strong>06</strong>03-2582-21 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G QUALITEST EAGEN 0<strong>06</strong>03-2582-28 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G QUALITEST EAGEN 0<strong>06</strong>03-2582-32 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G QUALITEST EAGEN 51079-<strong>08</strong>19-01 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G MYLAN INSTITUTI EAGEN 51079-<strong>08</strong>19-20 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G MYLAN INSTITUTI EAGEN 62756-0446-02 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G SUN PHARMACEUTI EAGEN 62756-0446-04 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G SUN PHARMACEUTI EAGEN 62756-0446-05 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G SUN PHARMACEUTI EAGEN 63739-0049-10 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G MCKESSON PACKAG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 64980-0174-01 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G RISING PHARM EAGEN 64980-0174-05 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G RISING PHARM EAGEN 64980-0174-10 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G RISING PHARM EAGEN 65862-0158-01 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G AUROBINDO PHARM EAGEN 68<strong>08</strong>4-0380-01 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G AHP EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 462LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68<strong>08</strong>4-0380-11 0.<strong>06</strong>399 CARISOPRODOL 350 MG TABLET G AHP EABEX 00378-0211-01 0.68540 0.97865 CHLORDIAZEPO-AMITRIPTYL 5-12.5 0 MYLAN EABEX 00378-0211-05 0.68540 0.97863 CHLORDIAZEPO-AMITRIPTYL 5-12.5 0 MYLAN EABEX 00378-0277-01 0.96700 1.38078 CHLORDIAZEPOX-AMITRIPTYL 10-25 0 MYLAN EABEX 00378-0277-05 0.96700 1.38<strong>08</strong>0 CHLORDIAZEPOX-AMITRIPTYL 10-25 0 MYLAN EAGEX 00555-0033-02 0.07360 CHLORDIAZEPOXIDE 10 MG CAPSULE 0 BARR EAGEX 00555-0033-05 0.07360 CHLORDIAZEPOXIDE 10 MG CAPSULE 0 BARR EAGEX 43547-0252-10 0.07360 CHLORDIAZEPOXIDE 10 MG CAPSULE 0 SOLCO <strong>HEALTH</strong>CAR EAGEX 51079-0375-20 0.07360 CHLORDIAZEPOXIDE 10 MG CAPSULE 0 MYLAN INSTITUTI EAGEX 00555-0159-02 0.<strong>08</strong>090 CHLORDIAZEPOXIDE 25 MG CAPSULE 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00555-0159-04 0.<strong>08</strong>090 CHLORDIAZEPOXIDE 25 MG CAPSULE 0 BARR EAGEX 43547-0253-10 0.<strong>08</strong>090 CHLORDIAZEPOXIDE 25 MG CAPSULE 0 SOLCO <strong>HEALTH</strong>CAR EAGEX 51079-0141-20 0.<strong>08</strong>090 CHLORDIAZEPOXIDE 25 MG CAPSULE 0 MYLAN INSTITUTI EAGEX 00555-0158-02 0.09260 CHLORDIAZEPOXIDE 5 MG CAPSULE 0 BARR EAGEX 00555-0158-04 0.09260 CHLORDIAZEPOXIDE 5 MG CAPSULE 0 BARR EAGEX 43547-0251-10 0.09260 CHLORDIAZEPOXIDE 5 MG CAPSULE 0 SOLCO <strong>HEALTH</strong>CAR EAGEX 51079-0374-20 0.09260 CHLORDIAZEPOXIDE 5 MG CAPSULE 0 MYLAN INSTITUTI EAGEX 00555-0094-96 0.9<strong>08</strong>00 CLONAZEPAM 0.125 MG DIS TAB 0 BARR EAGEX 49884-03<strong>06</strong>-02 0.9<strong>08</strong>00 CLONAZEPAM 0.125 MG DIS TAB 0 PAR PHARM. EAGEX 00555-0095-96 0.84630 CLONAZEPAM 0.25 MG ODT 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 49884-0307-02 0.84630 CLONAZEPAM 0.25 MG ODT 0 PAR PHARM. EAGEX 00555-0096-96 0.82430 CLONAZEPAM 0.5 MG DIS TABLET 0 BARR EAGEX 49884-03<strong>08</strong>-02 0.82430 CLONAZEPAM 0.5 MG DIS TABLET 0 PAR PHARM. EAGEX 00093-<strong>08</strong>32-01 0.01660 CLONAZEPAM 0.5 MG TABLET 0 TEVA USA EAGEX 00093-<strong>08</strong>32-05 0.01660 CLONAZEPAM 0.5 MG TABLET 0 TEVA USA EAGEX 00093-<strong>08</strong>32-10 0.01660 CLONAZEPAM 0.5 MG TABLET 0 TEVA USA EAGEX 00185-0<strong>06</strong>3-01 0.01660 CLONAZEPAM 0.5 MG TABLET 0 SANDOZ EAGEX 00185-0<strong>06</strong>3-05 0.01660 CLONAZEPAM 0.5 MG TABLET 0 SANDOZ EAGEX 00185-0<strong>06</strong>3-10 0.01660 CLONAZEPAM 0.5 MG TABLET 0 SANDOZ EAGEX 00228-3003-11 0.01660 CLONAZEPAM 0.5 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00228-3003-50 0.01660 CLONAZEPAM 0.5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00378-1910-01 0.01660 CLONAZEPAM 0.5 MG TABLET 0 MYLAN EAGEX 00378-1910-10 0.01660 CLONAZEPAM 0.5 MG TABLET 0 MYLAN EAGEX 00378-1910-77 0.01660 CLONAZEPAM 0.5 MG TABLET 0 MYLAN EAGEX 0<strong>06</strong>03-2948-02 0.01660 CLONAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2948-13 0.01660 CLONAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2948-16 0.01660 CLONAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2948-20 0.01660 CLONAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2948-21 0.01660 CLONAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2948-22 0.01660 CLONAZEPAM 0.5 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-2948-26 0.01660 CLONAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2948-28 0.01660 CLONAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2948-32 0.01660 CLONAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2948-60 0.01660 CLONAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 00904-6101-61 0.01660 CLONAZEPAM 0.5 MG TABLET 0 MAJOR PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 463LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 16729-0136-00 0.01660 CLONAZEPAM 0.5 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0136-16 0.01660 CLONAZEPAM 0.5 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 51079-<strong>08</strong>81-56 0.01660 CLONAZEPAM 0.5 MG TABLET 0 MYLAN INSTITUTI EAGEX 57664-0273-<strong>08</strong> 0.01660 CLONAZEPAM 0.5 MG TABLET 0 CARACO PHARM EAGEX 57664-0273-13 0.01660 CLONAZEPAM 0.5 MG TABLET 0 CARACO PHARM EAGEX 57664-0273-18 0.01660 CLONAZEPAM 0.5 MG TABLET 0 CARACO PHARM EAGEX 60505-0<strong>06</strong>6-01 0.01660 CLONAZEPAM 0.5 MG TABLET 0 APOTEX CORP EAGEX 60505-0<strong>06</strong>6-03 0.01660 CLONAZEPAM 0.5 MG TABLET 0 APOTEX CORP EAGEX 63739-0263-10 0.01660 CLONAZEPAM 0.5 MG TABLET 0 MCKESSON PACKAG EAGEX 00555-0097-96 1.05610 CLONAZEPAM 1 MG DIS TABLET 0 BARR EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 49884-0309-02 1.05610 CLONAZEPAM 1 MG DIS TABLET 0 PAR PHARM. EAGEX 00093-<strong>08</strong>33-01 0.02630 CLONAZEPAM 1 MG TABLET 0 TEVA USA EAGEX 00093-<strong>08</strong>33-05 0.02630 CLONAZEPAM 1 MG TABLET 0 TEVA USA EAGEX 00093-<strong>08</strong>33-10 0.02630 CLONAZEPAM 1 MG TABLET 0 TEVA USA EAGEX 00185-0<strong>06</strong>4-01 0.02630 CLONAZEPAM 1 MG TABLET 0 SANDOZ EAGEX 00185-0<strong>06</strong>4-05 0.02630 CLONAZEPAM 1 MG TABLET 0 SANDOZ EAGEX 00185-0<strong>06</strong>4-10 0.02630 CLONAZEPAM 1 MG TABLET 0 SANDOZ EAGEX 00228-3004-11 0.02630 CLONAZEPAM 1 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00228-3004-50 0.02630 CLONAZEPAM 1 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00378-1912-01 0.02630 CLONAZEPAM 1 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-1912-10 0.02630 CLONAZEPAM 1 MG TABLET 0 MYLAN EAGEX 00378-1912-77 0.02630 CLONAZEPAM 1 MG TABLET 0 MYLAN EAGEX 0<strong>06</strong>03-2949-02 0.02630 CLONAZEPAM 1 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2949-13 0.02630 CLONAZEPAM 1 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2949-16 0.02630 CLONAZEPAM 1 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2949-20 0.02630 CLONAZEPAM 1 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2949-21 0.02630 CLONAZEPAM 1 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2949-22 0.02630 CLONAZEPAM 1 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2949-28 0.02630 CLONAZEPAM 1 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2949-32 0.02630 CLONAZEPAM 1 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-2949-60 0.02630 CLONAZEPAM 1 MG TABLET 0 QUALITEST EAGEX 00904-6102-61 0.02630 CLONAZEPAM 1 MG TABLET 0 MAJOR PHARMACEU EAGEX 16729-0137-00 0.02630 CLONAZEPAM 1 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0137-16 0.02630 CLONAZEPAM 1 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 57664-0274-<strong>08</strong> 0.02630 CLONAZEPAM 1 MG TABLET 0 CARACO PHARM EAGEX 57664-0274-13 0.02630 CLONAZEPAM 1 MG TABLET 0 CARACO PHARM EAGEX 57664-0274-18 0.02630 CLONAZEPAM 1 MG TABLET 0 CARACO PHARM EAGEX 60505-0<strong>06</strong>7-01 0.02630 CLONAZEPAM 1 MG TABLET 0 APOTEX CORP EAGEX 60505-0<strong>06</strong>7-03 0.02630 CLONAZEPAM 1 MG TABLET 0 APOTEX CORP EAGEX 63739-0264-10 0.02630 CLONAZEPAM 1 MG TABLET 0 MCKESSON PACKAG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00555-0098-96 1.43559 CLONAZEPAM 2 MG ODT 0 BARR EAGEX 49884-0310-02 1.43559 CLONAZEPAM 2 MG ODT 0 PAR PHARM. EAGEX 00093-<strong>08</strong>34-01 0.04270 CLONAZEPAM 2 MG TABLET 0 TEVA USA EAGEX 00093-<strong>08</strong>34-05 0.04270 CLONAZEPAM 2 MG TABLET 0 TEVA USA EAGEX 00185-0<strong>06</strong>5-01 0.04270 CLONAZEPAM 2 MG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 464LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00185-0<strong>06</strong>5-05 0.04270 CLONAZEPAM 2 MG TABLET 0 SANDOZ EAGEX 00185-0<strong>06</strong>5-10 0.04270 CLONAZEPAM 2 MG TABLET 0 SANDOZ EAGEX 00228-3005-11 0.04270 CLONAZEPAM 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00228-3005-50 0.04270 CLONAZEPAM 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00378-1914-01 0.04270 CLONAZEPAM 2 MG TABLET 0 MYLAN EAGEX 00378-1914-05 0.04270 CLONAZEPAM 2 MG TABLET 0 MYLAN EAGEX 0<strong>06</strong>03-2950-02 0.04270 CLONAZEPAM 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2950-16 0.04270 CLONAZEPAM 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2950-20 0.04270 CLONAZEPAM 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-2950-21 0.04270 CLONAZEPAM 2 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-2950-28 0.04270 CLONAZEPAM 2 MG TABLET 0 QUALITEST EAGEX 00904-6103-61 0.04270 CLONAZEPAM 2 MG TABLET 0 MAJOR PHARMACEU EAGEX 16729-0138-00 0.04270 CLONAZEPAM 2 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 16729-0138-16 0.04270 CLONAZEPAM 2 MG TABLET 0 ACCORD <strong>HEALTH</strong>CA EAGEX 57664-0275-<strong>08</strong> 0.04270 CLONAZEPAM 2 MG TABLET 0 CARACO PHARM EAGEX 57664-0275-13 0.04270 CLONAZEPAM 2 MG TABLET 0 CARACO PHARM EAGEX 57664-0275-18 0.04270 CLONAZEPAM 2 MG TABLET 0 CARACO PHARM EAGEX 60505-0<strong>06</strong>8-01 0.04270 CLONAZEPAM 2 MG TABLET 0 APOTEX CORP EAGEX 60505-0<strong>06</strong>8-03 0.04270 CLONAZEPAM 2 MG TABLET 0 APOTEX CORP EAGUX 00378-0070-01 0.27540 CLORAZEPATE 15 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 51672-4044-01 0.27540 CLORAZEPATE 15 MG TABLET 0 TARO PHARM USA EAGUX 00378-0030-01 0.13770 CLORAZEPATE 3.75 MG TABLET 0 MYLAN EAGUX 00378-0030-05 0.13770 CLORAZEPATE 3.75 MG TABLET 0 MYLAN EAGUX 51672-4042-01 0.13770 CLORAZEPATE 3.75 MG TABLET 0 TARO PHARM USA EAGUX 51672-4042-02 0.13770 CLORAZEPATE 3.75 MG TABLET 0 TARO PHARM USA EAGUX 00378-0040-01 0.19470 CLORAZEPATE 7.5 MG TABLET 0 MYLAN EAGUX 00378-0040-05 0.19470 CLORAZEPATE 7.5 MG TABLET 0 MYLAN EAGUX 51672-4043-01 0.19470 CLORAZEPATE 7.5 MG TABLET 0 TARO PHARM USA EAGUX 51672-4043-02 0.19470 CLORAZEPATE 7.5 MG TABLET 0 TARO PHARM USA EAGUX 63304-0553-01 0.19470 CLORAZEPATE 7.5 MG TABLET 0 RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00187-<strong>06</strong>59-20 3<strong>08</strong>.05450 DIASTAT ACUDIAL 12.5-15-20 MG 0 VALEANT EABEX 00187-<strong>06</strong>58-20 3<strong>08</strong>.05450 DIASTAT ACUDIAL 5-7.5-10 MG KT 0 VALEANT EABEX 66490-<strong>06</strong>50-20 259.69040 DIASTAT 2.5 MG PEDI SYSTEM 0 VALEANT EAGEX 51927-1014-00 6.42750 DIAZEPAM POWDER 0 PR<strong>OF</strong>ESSIONAL CO GMGEX 00093-6138-32 250.53000 DIAZEPAM 10 MG RECTAL GEL G TEVA USA EAGEX 00172-3927-60 0.03310 DIAZEPAM 10 MG TABLET 0 IVAX PHARMACEUT EAGEX 00172-3927-70 0.03310 DIAZEPAM 10 MG TABLET 0 IVAX PHARMACEUT EAGEX 00172-3927-80 0.03310 DIAZEPAM 10 MG TABLET 0 TEVA USA EAGEX 00378-0477-01 0.03310 DIAZEPAM 10 MG TABLET 0 MYLAN EAGEX 00378-0477-05 0.03310 DIAZEPAM 10 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00591-5620-01 0.03310 DIAZEPAM 10 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-5620-05 0.03310 DIAZEPAM 10 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-5620-10 0.03310 DIAZEPAM 10 MG TABLET 0 ACTAVIS PHARMA, EAGEX 0<strong>06</strong>03-3215-02 0.03310 DIAZEPAM 10 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3215-16 0.03310 DIAZEPAM 10 MG TABLET 0 QUALITEST EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 465LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-3215-20 0.03310 DIAZEPAM 10 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3215-21 0.03310 DIAZEPAM 10 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3215-22 0.03310 DIAZEPAM 10 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3215-28 0.03310 DIAZEPAM 10 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3215-32 0.03310 DIAZEPAM 10 MG TABLET 0 QUALITEST EAGEX 51079-0286-20 0.03310 DIAZEPAM 10 MG TABLET 0 MYLAN INSTITUTI EAGEX 00172-3925-60 0.02440 DIAZEPAM 2 MG TABLET 0 IVAX PHARMACEUT EAGEX 00172-3925-70 0.02440 DIAZEPAM 2 MG TABLET 0 IVAX PHARMACEUT EAGEX 00378-0271-01 0.02440 DIAZEPAM 2 MG TABLET 0 MYLAN EAGEX 00378-0271-05 0.02440 DIAZEPAM 2 MG TABLET 0 MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00591-5621-01 0.02440 DIAZEPAM 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-5621-05 0.02440 DIAZEPAM 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-5621-10 0.02440 DIAZEPAM 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 0<strong>06</strong>03-3213-16 0.02440 DIAZEPAM 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3213-20 0.02440 DIAZEPAM 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3213-21 0.02440 DIAZEPAM 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3213-28 0.02440 DIAZEPAM 2 MG TABLET 0 QUALITEST EAGEX 51079-0284-20 0.02440 DIAZEPAM 2 MG TABLET 0 MYLAN INSTITUTI EAGEX 00093-6137-32 211.19250 DIAZEPAM 2.5 MG RECTAL GEL G TEVA USA EAGEX 00093-6139-32 250.53000 DIAZEPAM 20 MG RECTAL GEL G TEVA USA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00172-3926-60 0.02240 DIAZEPAM 5 MG TABLET 0 IVAX PHARMACEUT EAGEX 00172-3926-70 0.02240 DIAZEPAM 5 MG TABLET 0 IVAX PHARMACEUT EAGEX 00172-3926-80 0.02240 DIAZEPAM 5 MG TABLET 0 TEVA USA EAGEX 00378-0345-01 0.02240 DIAZEPAM 5 MG TABLET 0 MYLAN EAGEX 00378-0345-05 0.02240 DIAZEPAM 5 MG TABLET 0 MYLAN EAGEX 00591-5619-01 0.02240 DIAZEPAM 5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-5619-05 0.02240 DIAZEPAM 5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-5619-10 0.02240 DIAZEPAM 5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 0<strong>06</strong>03-3214-02 0.02240 DIAZEPAM 5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3214-16 0.02240 DIAZEPAM 5 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-3214-20 0.02240 DIAZEPAM 5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3214-21 0.02240 DIAZEPAM 5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3214-22 0.02240 DIAZEPAM 5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3214-28 0.02240 DIAZEPAM 5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-3214-32 0.02240 DIAZEPAM 5 MG TABLET 0 QUALITEST EAGEX 00904-5880-61 0.02240 DIAZEPAM 5 MG TABLET 0 MAJOR PHARMACEU EAGEX 51079-0285-20 0.02240 DIAZEPAM 5 MG TABLET 0 MYLAN INSTITUTI EAGEX 63739-0073-10 0.02240 DIAZEPAM 5 MG TABLET 0 MCKESSON PACKAG EAGEX 68<strong>08</strong>4-0359-01 0.02240 DIAZEPAM 5 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0359-11 0.02240 DIAZEPAM 5 MG TABLET 0 AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00054-3185-44 1.03418 DIAZEPAM 5 MG/ML ORAL CONC 0 ROXANE LABS. MLGEX 00409-1273-32 4.33160 DIAZEPAM 5 MG/ML SYRINGE 0 HOSPIRA MLBEX 00409-3213-12 1.88244 DIAZEPAM 5 MG/ML VIAL 0 HOSPIRA MLGEX 68094-0750-59 0.68970 DIAZEPAM 5 MG/5 ML ORAL SOLN 0 PRECISION DOSE MLGEX 68094-0750-62 0.68970 DIAZEPAM 5 MG/5 ML ORAL SOLN 0 PRECISION DOSE ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 466LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00054-3188-63 0.11259 DIAZEPAM 5 MG/5 ML SOLUTION 0 ROXANE LABS. MLBEX 59547-0310-19 8.96400 DORAL 15 MG TABLET G NURO PHARMA INC EABEX 63004-7734-01 3.82107 DORAL 15 MG TABLET G QUESTCOR EABEX 00037-6010-30 6.83228 EDLUAR 10 MG SL TABLET G MEDA PHARMACEUT EABEX 00037-6050-30 6.83228 EDLUAR 5 MG SL TABLET G MEDA PHARMACEUT EAGEX 00591-0744-01 0.31347 ESTAZOLAM 1 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00093-0130-01 0.34344 ESTAZOLAM 2 MG TABLET 0 TEVA USA EAGEX 00591-0745-01 0.34344 ESTAZOLAM 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00143-3367-01 0.<strong>06</strong>890 FLURAZEPAM 15 MG CAPSULE 0 WEST-WARD,INC. EAGEX 00143-3367-05 0.<strong>06</strong>890 FLURAZEPAM 15 MG CAPSULE 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-4415-01 0.<strong>06</strong>890 FLURAZEPAM 15 MG CAPSULE 0 MYLAN EAGEX 00143-3370-01 0.<strong>08</strong>469 FLURAZEPAM 30 MG CAPSULE 0 WEST-WARD,INC. EAGEX 00143-3370-05 0.<strong>08</strong>469 FLURAZEPAM 30 MG CAPSULE 0 WEST-WARD,INC. EAGEX 00378-4430-01 0.<strong>08</strong>469 FLURAZEPAM 30 MG CAPSULE 0 MYLAN EABUX 00009-0017-59 0.32510 2.84158 HALCION 0.25 MG TABLET G PHARMACIA/UPJHN EABEX 59011-0256-30 7.56157 INTERMEZZO 1.75 MG TAB SUBLING G PURDUE PHARMA L EABEX 59011-0255-30 7.56157 INTERMEZZO 3.5 MG TAB SUBLING G PURDUE PHARMA L EABEX 00004-0<strong>06</strong>8-01 0.01660 2.03748 KLONOPIN 0.5 MG TABLET G ROCHE LABS. EABEX 00004-0<strong>06</strong>8-32 0.01660 1.97656 KLONOPIN 0.5 MG TABLET G ROCHE LABS. EABEX 00004-0058-01 0.02630 2.32424 KLONOPIN 1 MG TABLET G ROCHE LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00004-0058-32 0.02630 2.25430 KLONOPIN 1 MG TABLET G ROCHE LABS. EABEX 00004-0098-01 0.04270 3.22048 KLONOPIN 2 MG TABLET G ROCHE LABS. EAGEX 00054-3532-44 0.60916 LORAZEPAM INTENSOL 2 MG/ML 0 ROXANE LABS. MLGEX 00228-2057-10 0.02740 LORAZEPAM 0.5 MG TABLET 0 SANDOZ EAGEX 00228-2057-50 0.02740 LORAZEPAM 0.5 MG TABLET 0 SANDOZ EAGEX 00378-2321-01 0.02740 LORAZEPAM 0.5 MG TABLET 0 MYLAN EAGEX 00378-2321-05 0.02740 LORAZEPAM 0.5 MG TABLET 0 MYLAN EAGEX 00591-0240-01 0.02740 LORAZEPAM 0.5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-0240-05 0.02740 LORAZEPAM 0.5 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-0240-10 0.02740 LORAZEPAM 0.5 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-4246-21 0.02740 LORAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-4246-28 0.02740 LORAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-4246-32 0.02740 LORAZEPAM 0.5 MG TABLET 0 QUALITEST EAGEX 00781-5404-01 0.02740 LORAZEPAM 0.5 MG TABLET 0 SANDOZ EAGEX 00781-5404-05 0.02740 LORAZEPAM 0.5 MG TABLET 0 SANDOZ EAGEX 00904-6007-60 0.02740 LORAZEPAM 0.5 MG TABLET 0 MAJOR PHARMACEU EAGEX 51079-0417-20 0.02740 LORAZEPAM 0.5 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0417-21 0.02740 LORAZEPAM 0.5 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0417-56 0.02740 LORAZEPAM 0.5 MG TABLET 0 MYLAN INSTITUTI EAGEX 63304-0772-01 0.02740 LORAZEPAM 0.5 MG TABLET 0 RANBAXY PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 63304-0772-05 0.02740 LORAZEPAM 0.5 MG TABLET 0 RANBAXY PHARMAC EAGEX 63304-0772-90 0.02740 LORAZEPAM 0.5 MG TABLET 0 RANBAXY PHARMAC EAGEX 63739-0499-10 0.02740 LORAZEPAM 0.5 MG TABLET 0 MCKESSON PACKAG EAGEX 64125-0904-01 0.02740 LORAZEPAM 0.5 MG TABLET 0 EXCELLIUM PHARM EAGEX 64125-0904-05 0.02740 LORAZEPAM 0.5 MG TABLET 0 EXCELLIUM PHARM EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 467LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64125-0904-10 0.02740 LORAZEPAM 0.5 MG TABLET 0 EXCELLIUM PHARM EAGEX 68<strong>08</strong>4-0<strong>08</strong>8-01 0.02740 LORAZEPAM 0.5 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0<strong>08</strong>8-11 0.02740 LORAZEPAM 0.5 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0412-01 0.02740 LORAZEPAM 0.5 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0412-11 0.02740 LORAZEPAM 0.5 MG TABLET 0 AHP EAGEX 00228-2059-10 0.02940 LORAZEPAM 1 MG TABLET 0 SANDOZ EAGEX 00228-2059-50 0.02940 LORAZEPAM 1 MG TABLET 0 SANDOZ EAGEX 00378-2457-01 0.02940 LORAZEPAM 1 MG TABLET 0 MYLAN EAGEX 00378-2457-10 0.02940 LORAZEPAM 1 MG TABLET 0 MYLAN EAGEX 00591-0241-01 0.02940 LORAZEPAM 1 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00591-0241-05 0.02940 LORAZEPAM 1 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-0241-10 0.02940 LORAZEPAM 1 MG TABLET 0 ACTAVIS PHARMA, EAGEX 0<strong>06</strong>03-4247-21 0.02940 LORAZEPAM 1 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-4247-28 0.02940 LORAZEPAM 1 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-4247-32 0.02940 LORAZEPAM 1 MG TABLET 0 QUALITEST EAGEX 00781-54<strong>06</strong>-01 0.02940 LORAZEPAM 1 MG TABLET 0 SANDOZ EAGEX 00781-54<strong>06</strong>-05 0.02940 LORAZEPAM 1 MG TABLET 0 SANDOZ EAGEX 00904-60<strong>08</strong>-60 0.02940 LORAZEPAM 1 MG TABLET 0 MAJOR PHARMACEU EAGEX 51079-0386-20 0.02940 LORAZEPAM 1 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0386-21 0.02940 LORAZEPAM 1 MG TABLET 0 MYLAN INSTITUTI EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 51079-0386-56 0.02940 LORAZEPAM 1 MG TABLET 0 MYLAN INSTITUTI EAGEX 63304-0773-01 0.02940 LORAZEPAM 1 MG TABLET 0 RANBAXY PHARMAC EAGEX 63304-0773-05 0.02940 LORAZEPAM 1 MG TABLET 0 RANBAXY PHARMAC EAGEX 63304-0773-10 0.02940 LORAZEPAM 1 MG TABLET 0 RANBAXY PHARMAC EAGEX 63304-0773-90 0.02940 LORAZEPAM 1 MG TABLET 0 RANBAXY PHARMAC EAGEX 63739-0155-10 0.02940 LORAZEPAM 1 MG TABLET 0 MCKESSON PACKAG EAGEX 63739-0500-10 0.02940 LORAZEPAM 1 MG TABLET 0 MCKESSON PACKAG EAGEX 64125-0905-01 0.02940 LORAZEPAM 1 MG TABLET 0 EXCELLIUM PHARM EAGEX 64125-0905-05 0.02940 LORAZEPAM 1 MG TABLET 0 EXCELLIUM PHARM EAGEX 64125-0905-10 0.02940 LORAZEPAM 1 MG TABLET 0 EXCELLIUM PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0<strong>08</strong>9-01 0.02940 LORAZEPAM 1 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0<strong>08</strong>9-11 0.02940 LORAZEPAM 1 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0413-01 0.02940 LORAZEPAM 1 MG TABLET 0 AHP EAGEX 00093-4822-10 0.04910 LORAZEPAM 2 MG TABLET 0 TEVA USA EAGEX 00228-2<strong>06</strong>3-10 0.04910 LORAZEPAM 2 MG TABLET 0 SANDOZ EAGEX 00228-2<strong>06</strong>3-50 0.04910 LORAZEPAM 2 MG TABLET 0 SANDOZ EAGEX 00378-2777-01 0.04910 LORAZEPAM 2 MG TABLET 0 MYLAN EAGEX 00378-2777-05 0.04910 LORAZEPAM 2 MG TABLET 0 MYLAN EAGEX 00591-0242-01 0.04910 LORAZEPAM 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 00591-0242-05 0.04910 LORAZEPAM 2 MG TABLET 0 ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00591-0242-10 0.04910 LORAZEPAM 2 MG TABLET 0 ACTAVIS PHARMA, EAGEX 0<strong>06</strong>03-4248-21 0.04910 LORAZEPAM 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-4248-28 0.04910 LORAZEPAM 2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-4248-32 0.04910 LORAZEPAM 2 MG TABLET 0 QUALITEST EAGEX 00781-54<strong>08</strong>-01 0.04910 LORAZEPAM 2 MG TABLET 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 468LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-54<strong>08</strong>-05 0.04910 LORAZEPAM 2 MG TABLET 0 SANDOZ EAGEX 00904-6009-60 0.04910 LORAZEPAM 2 MG TABLET 0 MAJOR PHARMACEU EAGEX 51079-0387-20 0.04910 LORAZEPAM 2 MG TABLET 0 MYLAN INSTITUTI EAGEX 51079-0387-21 0.04910 LORAZEPAM 2 MG TABLET 0 MYLAN INSTITUTI EAGEX 63304-0774-01 0.04910 LORAZEPAM 2 MG TABLET 0 RANBAXY PHARMAC EAGEX 63304-0774-05 0.04910 LORAZEPAM 2 MG TABLET 0 RANBAXY PHARMAC EAGEX 63304-0774-90 0.04910 LORAZEPAM 2 MG TABLET 0 RANBAXY PHARMAC EAGEX 63739-0156-10 0.04910 LORAZEPAM 2 MG TABLET 0 MCKESSON PACKAG EAGEX 63739-0501-10 0.04910 LORAZEPAM 2 MG TABLET 0 MCKESSON PACKAG EAGEX 64125-09<strong>06</strong>-01 0.04910 LORAZEPAM 2 MG TABLET 0 EXCELLIUM PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 64125-09<strong>06</strong>-05 0.04910 LORAZEPAM 2 MG TABLET 0 EXCELLIUM PHARM EAGEX 64125-09<strong>06</strong>-10 0.04910 LORAZEPAM 2 MG TABLET 0 EXCELLIUM PHARM EAGEX 68<strong>08</strong>4-0090-01 0.04910 LORAZEPAM 2 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0090-11 0.04910 LORAZEPAM 2 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0414-01 0.04910 LORAZEPAM 2 MG TABLET 0 AHP EAGEX 68<strong>08</strong>4-0414-11 0.04910 LORAZEPAM 2 MG TABLET 0 AHP EAGEX 00574-0163-30 0.60916 LORAZEPAM 2 MG/ML ORAL CONCENT 0 PADDOCK LABS. MLGEX 50383-0705-30 0.60916 LORAZEPAM 2 MG/ML ORAL CONCENT 0 HI-TECH PHARMAC MLGEX 65162-<strong>06</strong>87-84 0.60916 LORAZEPAM 2 MG/ML ORAL CONCENT 0 AMNEAL PHARMACE MLGEX 00409-6778-02 0.74600 LORAZEPAM 2 MG/ML VIAL 0 HOSPIRA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>41-6044-01 0.67500 LORAZEPAM 2 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-6044-25 0.67500 LORAZEPAM 2 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-6048-01 0.67500 LORAZEPAM 2 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-6048-25 0.67500 LORAZEPAM 2 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 17478-0040-01 0.74600 LORAZEPAM 2 MG/ML VIAL 0 AKORN INC. MLGEX 76329-8261-01 0.74600 LORAZEPAM 2 MG/ML VIAL 0 INTERNATIONAL M MLGEX 0<strong>06</strong>41-6045-01 1.14240 LORAZEPAM 4 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-6045-25 1.14240 LORAZEPAM 4 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-6049-01 1.14240 LORAZEPAM 4 MG/ML VIAL 0 WEST-WARD,INC. MLGEX 0<strong>06</strong>41-6049-25 1.14240 LORAZEPAM 4 MG/ML VIAL 0 WEST-WARD,INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 63402-0190-30 10.76676 LUNESTA 1 MG TABLET G SUNOVION PHARMA EABEX 63402-0191-10 10.76676 LUNESTA 2 MG TABLET G SUNOVION PHARMA EABEX 63402-0193-10 10.76676 LUNESTA 3 MG TABLET G SUNOVION PHARMA EAGEX 00591-5239-01 1.05015 MEPROBAMATE 200 MG TABLET 0 WATSON LABS EAGEX 23155-0128-01 5.15662 MEPROBAMATE 200 MG TABLET 0 HERITAGE PHARMA EAGEX 55111-<strong>06</strong>40-01 5.15625 MEPROBAMATE 200 MG TABLET 0 DR.REDDY'S LAB EAGEX 00591-5238-01 1.37332 MEPROBAMATE 400 MG TABLET 0 WATSON LABS EAGEX 23155-0129-01 6.12400 MEPROBAMATE 400 MG TABLET 0 HERITAGE PHARMA EAGEX 55111-<strong>06</strong>41-01 6.12400 MEPROBAMATE 400 MG TABLET 0 DR.REDDY'S LAB EAGEN 00378-5573-01 11.07887 MODAFINIL 100 MG TABLET G MYLAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-5573-05 11.07887 MODAFINIL 100 MG TABLET G MYLAN EAGEN 00378-5573-93 11.07887 MODAFINIL 100 MG TABLET G MYLAN EAGEN 00591-3499-01 11.07887 MODAFINIL 100 MG TABLET G ACTAVIS PHARMA, EAGEN 00591-3499-30 11.07887 MODAFINIL 100 MG TABLET G ACTAVIS PHARMA, EAGEN 42043-0160-01 11.07887 MODAFINIL 100 MG TABLET G KARALEX PHARMA, EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 469LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 42043-0160-03 11.07887 MODAFINIL 100 MG TABLET G KARALEX PHARMA, EAGEN 49884-0534-11 11.07887 MODAFINIL 100 MG TABLET G PAR PHARM. EAGEN 51079-0561-03 11.07887 MODAFINIL 100 MG TABLET G MYLAN INSTITUTI EAGEN 55253-<strong>08</strong>01-30 11.07887 MODAFINIL 100 MG TABLET G CEPHALON,INC.-T EAGEN 55253-<strong>08</strong>01-90 11.07887 MODAFINIL 100 MG TABLET G CEPHALON,INC.-T EAGEN 60505-2526-03 11.07887 MODAFINIL 100 MG TABLET G APOTEX CORP EAGEN 65862-<strong>06</strong>01-01 11.07887 MODAFINIL 100 MG TABLET G AUROBINDO PHARM EAGEN 00378-5575-05 15.67995 MODAFINIL 200 MG TABLET G MYLAN EAGEN 00378-5575-93 15.67995 MODAFINIL 200 MG TABLET G MYLAN EAGEN 00591-3500-01 15.67995 MODAFINIL 200 MG TABLET G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00591-3500-30 15.67995 MODAFINIL 200 MG TABLET G ACTAVIS PHARMA, EAGEN 42043-0161-01 15.67995 MODAFINIL 200 MG TABLET G KARALEX PHARMA, EAGEN 42043-0161-03 15.67995 MODAFINIL 200 MG TABLET G KARALEX PHARMA, EAGEN 49884-0535-09 15.67995 MODAFINIL 200 MG TABLET G PAR PHARM. EAGEN 49884-0535-11 15.67995 MODAFINIL 200 MG TABLET G PAR PHARM. EAGEN 51079-0562-03 15.67995 MODAFINIL 200 MG TABLET G MYLAN INSTITUTI EAGEN 55253-<strong>08</strong>02-30 15.67995 MODAFINIL 200 MG TABLET G CEPHALON,INC.-T EAGEN 55253-<strong>08</strong>02-90 15.67995 MODAFINIL 200 MG TABLET G CEPHALON,INC.-T EAGEN 60505-2527-01 15.67995 MODAFINIL 200 MG TABLET G APOTEX CORP EAGEN 60505-2527-03 15.67995 MODAFINIL 200 MG TABLET G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 65862-<strong>06</strong>02-01 15.67995 MODAFINIL 200 MG TABLET G AUROBINDO PHARM EABND 00187-0500-02 6.84990 MOT<strong>OF</strong>EN TABLET 0 VALEANT EABEX 18860-0321-01 1.05740 3.37129 NIRAVAM 0.25 MG ODT 0 JAZZ PHARMACEUT EABEX 18860-0322-01 1.31750 4.83026 NIRAVAM 0.5 MG ODT 0 JAZZ PHARMACEUT EABEX 18860-0323-01 1.99300 6.44453 NIRAVAM 1 MG ODT G JAZZ PHARMACEUT EABEX 18860-0324-01 2.74200 10.95782 NIRAVAM 2 MG ODT G JAZZ PHARMACEUT EABND 63459-0215-30 16.69960 NUVIGIL 150 MG TABLET G CEPHALON,INC.-T EABND 63459-0225-30 16.69960 NUVIGIL 250 MG TABLET G CEPHALON,INC.-T EABND 63459-0205-30 5.54440 NUVIGIL 50 MG TABLET G CEPHALON,INC.-T EABEX 67386-0311-01 7.36176 ONFI 10 MG TABLET G LUNDBECK INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 67386-0314-01 8.<strong>06</strong>112 ONFI 10 MG TABLET G LUNDBECK INC. EABEX 67386-0313-21 4.38980 ONFI 2.5 MG/ML SUSPENSION G LUNDBECK INC. MLBEX 67386-0312-01 14.72337 ONFI 20 MG TABLET G LUNDBECK INC. EABEX 67386-0315-01 16.122<strong>08</strong> ONFI 20 MG TABLET G LUNDBECK INC. EABEX 67386-0310-01 3.68<strong>08</strong>0 ONFI 5 MG TABLET G LUNDBECK INC. EAGUX 00172-4804-60 0.53630 OXAZEPAM 10 MG CAPSULE 0 IVAX PHARMACEUT EAGUX 00172-4804-70 0.53630 OXAZEPAM 10 MG CAPSULE 0 IVAX PHARMACEUT EAGUX 00228-2<strong>06</strong>7-10 0.53630 OXAZEPAM 10 MG CAPSULE 0 ACTAVIS PHARMA, EAGUX 00228-2<strong>06</strong>7-50 0.53630 OXAZEPAM 10 MG CAPSULE 0 ACTAVIS PHARMA, EAGUX 00781-2809-01 0.53630 OXAZEPAM 10 MG CAPSULE 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 00172-4805-60 0.57090 OXAZEPAM 15 MG CAPSULE 0 IVAX PHARMACEUT EAGUX 00172-4805-70 0.57090 OXAZEPAM 15 MG CAPSULE 0 IVAX PHARMACEUT EAGUX 00228-2<strong>06</strong>9-10 0.57090 OXAZEPAM 15 MG CAPSULE 0 ACTAVIS PHARMA, EAGUX 00228-2<strong>06</strong>9-50 0.57090 OXAZEPAM 15 MG CAPSULE 0 ACTAVIS PHARMA, EAGUX 00781-2810-01 0.57090 OXAZEPAM 15 MG CAPSULE 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 470LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00172-48<strong>06</strong>-60 1.22992 OXAZEPAM 30 MG CAPSULE 0 IVAX PHARMACEUT EAGUX 00228-2073-10 1.23370 OXAZEPAM 30 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00781-2811-01 1.19265 OXAZEPAM 30 MG CAPSULE 0 SANDOZ EAGUL 00591-0396-01 0.85170 PENTAZOCIN-ACETAMINOPHN 25-650 G ACTAVIS PHARMA, EABUL 43386-<strong>06</strong>70-01 0.85170 1.26658 PENTAZOCIN-ACETAMINOPHN 25-650 G GAVIS PHARMACEU EAGEN 00591-0395-01 0.97530 PENTAZOCINE-NALOXONE TABLET G ACTAVIS PHARMA, EAGEN 43386-<strong>06</strong>80-01 1.26187 PENTAZOCINE-NALOXONE TABLET G GAVIS PHARMACEU EAGEX 00143-1458-01 0.10485 PHENOBARBITAL 100 MG TABLET 0 WEST-WARD,INC. EABEX 00143-1458-05 0.15712 0.3<strong>08</strong>76 PHENOBARBITAL 100 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-1458-10 0.09433 PHENOBARBITAL 100 MG TABLET 0 WEST-WARD,INC. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 0<strong>06</strong>41-0477-25 28.68480 PHENOBARBITAL 130 MG/ML VIAL 0 WEST-WARD,INC. MLBEX 00143-1445-05 0.13944 PHENOBARBITAL 15 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-1445-10 0.04312 PHENOBARBITAL 15 MG TABLET 0 WEST-WARD,INC. EAGEX 0<strong>06</strong>03-5165-21 0.13056 PHENOBARBITAL 16.2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-5165-32 0.13056 PHENOBARBITAL 16.2 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-15<strong>08</strong>-58 0.14556 PHENOBARBITAL 20 MG/5 ML ELIX 0 QUALITEST MLGEX 16571-0330-16 0.15856 PHENOBARBITAL 20 MG/5 ML SOLN 0 PACK PHARMACEUT MLBEX 00143-1450-05 0.17529 PHENOBARBITAL 30 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-1450-10 0.05391 PHENOBARBITAL 30 MG TABLET 0 WEST-WARD,INC. EAGEX 0<strong>06</strong>03-5166-02 0.21567 PHENOBARBITAL 32.4 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 0<strong>06</strong>03-5166-16 0.21650 PHENOBARBITAL 32.4 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-5166-20 0.21662 PHENOBARBITAL 32.4 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-5166-21 0.21682 PHENOBARBITAL 32.4 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-5166-22 0.21587 PHENOBARBITAL 32.4 MG TABLET 0 QUALITEST EAGEX 0<strong>06</strong>03-5166-32 0.2<strong>06</strong>13 PHENOBARBITAL 32.4 MG TABLET 0 QUALITEST EAGEX 63739-0201-10 0.34747 PHENOBARBITAL 32.4 MG TABLET 0 MCKESSON PACKAG EABEX 00143-1455-05 0.21912 PHENOBARBITAL 60 MG TABLET 0 WEST-WARD,INC. EAGEX 00143-1455-10 0.<strong>06</strong>738 PHENOBARBITAL 60 MG TABLET 0 WEST-WARD,INC. EABEX 0<strong>06</strong>03-5167-21 0.30070 PHENOBARBITAL 64.8 MG TABLET 0 QUALITEST EABEX 0<strong>06</strong>03-5167-32 0.28526 PHENOBARBITAL 64.8 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 0<strong>06</strong>41-0476-21 10.83980 PHENOBARBITAL 65 MG/ML VIAL 0 WEST-WARD,INC. MLBEX 0<strong>06</strong>41-0476-25 10.83648 PHENOBARBITAL 65 MG/ML VIAL 0 WEST-WARD,INC. MLBEX 0<strong>06</strong>03-5168-21 0.42396 PHENOBARBITAL 97.2 MG TABLET 0 QUALITEST EABEX 0<strong>06</strong>03-5168-32 0.40197 PHENOBARBITAL 97.2 MG TABLET 0 QUALITEST EABND 63459-0101-01 11.07887 24.35220 PROVIGIL 100 MG TABLET G CEPHALON,INC.-T EABND 63459-0101-30 11.07887 24.40200 PROVIGIL 100 MG TABLET G CEPHALON,INC.-T EABND 63459-0201-01 15.67995 36.84204 PROVIGIL 200 MG TABLET G CEPHALON,INC.-T EABND 63459-0201-30 15.67995 36.85200 PROVIGIL 200 MG TABLET G CEPHALON,INC.-T EABEX 004<strong>06</strong>-9916-01 0.07007 12.91654 RESTORIL 15 MG CAPSULE G MALLINCKRODT PH EABEX 004<strong>06</strong>-9914-03 5.84050 13.15909 RESTORIL 22.5 MG CAPSULE G MALLINCKRODT PH EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 004<strong>06</strong>-9917-01 0.10350 13.40400 RESTORIL 30 MG CAPSULE G MALLINCKRODT PH EABEX 004<strong>06</strong>-9915-01 4.61945 12.43821 RESTORIL 7.5 MG CAPSULE G MALLINCKRODT PH EABEX 004<strong>06</strong>-9915-03 4.61945 12.43810 RESTORIL 7.5 MG CAPSULE G MALLINCKRODT PH EABND 00037-2250-10 4.81018 SOMA 250 MG TABLET G MEDA PHARMACEUT EABND 00037-2250-30 4.80929 SOMA 250 MG TABLET G MEDA PHARMACEUT EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 471LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00037-2001-01 0.<strong>06</strong>399 7.01557 SOMA 350 MG TABLET G MEDA PHARMACEUT EABEX 60793-0146-01 0.25<strong>08</strong>3 5.47<strong>08</strong>6 SONATA 10 MG CAPSULE G PFIZER US PHARM EABEX 60793-0145-01 0.25043 5.32569 SONATA 5 MG CAPSULE G PFIZER US PHARM EABND 00409-1941-01 123.13548 TALWIN 30 MG/ML AMPUL 0 HOSPIRA MLGEX 00228-2076-10 0.07007 TEMAZEPAM 15 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00228-2076-50 0.07007 TEMAZEPAM 15 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00378-4010-01 0.07007 TEMAZEPAM 15 MG CAPSULE 0 MYLAN EAGEX 00378-4010-05 0.07007 TEMAZEPAM 15 MG CAPSULE 0 MYLAN EAGEX 00378-4010-77 0.07007 TEMAZEPAM 15 MG CAPSULE 0 MYLAN EAGEX 00781-2201-01 0.07007 TEMAZEPAM 15 MG CAPSULE 0 SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-2201-05 0.07007 TEMAZEPAM 15 MG CAPSULE 0 SANDOZ EAGEX 51079-0418-20 0.07007 TEMAZEPAM 15 MG CAPSULE 0 MYLAN INSTITUTI EAGEX 51079-0418-21 0.07007 TEMAZEPAM 15 MG CAPSULE 0 MYLAN INSTITUTI EAGEX 63739-0231-10 0.07007 TEMAZEPAM 15 MG CAPSULE 0 MCKESSON PACKAG EAGEX 67877-0146-01 0.07007 TEMAZEPAM 15 MG CAPSULE 0 ASCEND LABORATO EAGEX 67877-0146-05 0.07007 TEMAZEPAM 15 MG CAPSULE 0 ASCEND LABORATO EAGEX 00378-3120-93 5.84050 TEMAZEPAM 22.5 MG CAPSULE G MYLAN EAGEX 004<strong>06</strong>-9959-03 5.84050 TEMAZEPAM 22.5 MG CAPSULE G MALLINCKRODT PH EAGEX 53489-<strong>06</strong>50-07 5.84050 TEMAZEPAM 22.5 MG CAPSULE G MUTUAL PHARM CO EAGEX 67877-0149-30 5.84050 TEMAZEPAM 22.5 MG CAPSULE G ASCEND LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00228-2077-10 0.10350 TEMAZEPAM 30 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00228-2077-50 0.10350 TEMAZEPAM 30 MG CAPSULE 0 ACTAVIS PHARMA, EAGEX 00378-5050-01 0.10350 TEMAZEPAM 30 MG CAPSULE 0 MYLAN EAGEX 00378-5050-05 0.10350 TEMAZEPAM 30 MG CAPSULE 0 MYLAN EAGEX 00378-5050-77 0.10350 TEMAZEPAM 30 MG CAPSULE 0 MYLAN EAGEX 00781-2202-01 0.10350 TEMAZEPAM 30 MG CAPSULE 0 SANDOZ EAGEX 00781-2202-05 0.10350 TEMAZEPAM 30 MG CAPSULE 0 SANDOZ EAGEX 51079-0419-20 0.10350 TEMAZEPAM 30 MG CAPSULE 0 MYLAN INSTITUTI EAGEX 67877-0147-01 0.10350 TEMAZEPAM 30 MG CAPSULE 0 ASCEND LABORATO EAGEX 67877-0147-05 0.10350 TEMAZEPAM 30 MG CAPSULE 0 ASCEND LABORATO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-3110-01 4.61945 TEMAZEPAM 7.5 MG CAPSULE G MYLAN EAGEX 004<strong>06</strong>-9960-01 4.61945 TEMAZEPAM 7.5 MG CAPSULE G MALLINCKRODT PH EAGEX 53489-<strong>06</strong>48-01 4.61945 TEMAZEPAM 7.5 MG CAPSULE G MUTUAL PHARM CO EAGEX 67877-0148-01 4.61945 TEMAZEPAM 7.5 MG CAPSULE G ASCEND LABORATO EAGEX 68<strong>08</strong>4-0549-21 4.61945 TEMAZEPAM 7.5 MG CAPSULE G AHP EABUX 55292-0303-01 0.27540 5.42471 TRANXENE T-TAB 15 MG G RECORDATI RARE EABUX 67386-0303-01 0.27540 5.42471 TRANXENE T-TAB 15 MG G RECORDATI RARE EABUX 55292-0301-01 0.13770 3.21392 TRANXENE T-TAB 3.75 MG G RECORDATI RARE EABUX 67386-0301-01 0.13770 3.21392 TRANXENE T-TAB 3.75 MG G RECORDATI RARE EABUX 55292-0302-01 0.19470 3.99844 TRANXENE T-TAB 7.5 MG G RECORDATI RARE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BUX 67386-0302-01 0.19470 3.99844 TRANXENE T-TAB 7.5 MG G RECORDATI RARE EAGUX 00054-4858-51 0.30120 TRIAZOLAM 0.125 MG TABLET G ROXANE LABS. EAGUX 59762-3717-04 0.30120 TRIAZOLAM 0.125 MG TABLET G GREENSTONE LLC. EAGUX 00054-4859-29 0.32510 TRIAZOLAM 0.25 MG TABLET G ROXANE LABS. EAGUX 00054-4859-51 0.32510 TRIAZOLAM 0.25 MG TABLET G ROXANE LABS. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 472LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GUX 59762-3718-03 0.32510 TRIAZOLAM 0.25 MG TABLET G GREENSTONE LLC. EAGUX 59762-3718-04 0.32510 TRIAZOLAM 0.25 MG TABLET G GREENSTONE LLC. EABEX 00009-0057-07 0.57645 3.87761 XANAX XR 0.5 MG TABLET G PHARMACIA/UPJHN EABEX 00009-0059-07 0.45250 4.82479 XANAX XR 1 MG TABLET G PHARMACIA/UPJHN EABEX 00009-0<strong>06</strong>6-07 0.55458 6.40400 XANAX XR 2 MG TABLET G PHARMACIA/UPJHN EABEX 00009-0<strong>06</strong>8-07 1.81545 9.60490 XANAX XR 3 MG TABLET G PHARMACIA/UPJHN EABEX 00009-0029-01 0.04170 1.87248 XANAX 0.25 MG TABLET G PHARMACIA/UPJHN EABEX 00009-0029-02 0.04170 1.87241 XANAX 0.25 MG TABLET G PHARMACIA/UPJHN EABEX 00009-0055-01 0.04051 2.33271 XANAX 0.5 MG TABLET G PHARMACIA/UPJHN EABEX 00009-0055-03 0.04051 2.33276 XANAX 0.5 MG TABLET G PHARMACIA/UPJHN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00009-0090-01 0.04750 3.11258 XANAX 1 MG TABLET G PHARMACIA/UPJHN EABEX 00009-0090-04 0.04750 3.11263 XANAX 1 MG TABLET G PHARMACIA/UPJHN EABEX 00009-0094-01 0.04040 5.29232 XANAX 2 MG TABLET G PHARMACIA/UPJHN EAGEX 00054-0<strong>08</strong>5-25 0.25<strong>08</strong>3 ZALEPLON 10 MG CAPSULE G ROXANE LABS. EAGEX 00093-5269-01 0.25<strong>08</strong>3 ZALEPLON 10 MG CAPSULE G TEVA USA EAGEX 00378-6810-01 0.25<strong>08</strong>3 ZALEPLON 10 MG CAPSULE G MYLAN EAGEX 16714-0561-02 0.25<strong>08</strong>3 ZALEPLON 10 MG CAPSULE G NORTHSTAR RX LL EAGEX 29300-0132-01 0.25<strong>08</strong>3 ZALEPLON 10 MG CAPSULE G UNICHEM PHARMAC EAGEX 64720-0323-10 0.25<strong>08</strong>3 ZALEPLON 10 MG CAPSULE G COREPHARMA LLC EAGEX 65862-0215-01 0.25<strong>08</strong>3 ZALEPLON 10 MG CAPSULE G AUROBINDO PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00054-0<strong>08</strong>4-25 0.25043 ZALEPLON 5 MG CAPSULE G ROXANE LABS. EAGEX 00093-5268-01 0.25043 ZALEPLON 5 MG CAPSULE G TEVA USA EAGEX 00378-6805-01 0.25043 ZALEPLON 5 MG CAPSULE G MYLAN EAGEX 16714-0551-02 0.25043 ZALEPLON 5 MG CAPSULE G NORTHSTAR RX LL EAGEX 29300-0131-01 0.25043 ZALEPLON 5 MG CAPSULE G UNICHEM PHARMAC EAGEX 64720-0322-10 0.25043 ZALEPLON 5 MG CAPSULE G COREPHARMA LLC EAGEX 65862-0214-01 0.25043 ZALEPLON 5 MG CAPSULE G AUROBINDO PHARM EAGEX 67877-0210-01 0.25043 ZALEPLON 5 MG CAPSULE G ASCEND LABORATO EAGEX 00228-3482-11 2.62500 ZOLPIDEM TART ER 12.5 MG TAB G ACTAVIS PHARMA, EAGEX 00228-3482-50 2.62500 ZOLPIDEM TART ER 12.5 MG TAB G ACTAVIS PHARMA, EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-5316-01 2.62500 ZOLPIDEM TART ER 12.5 MG TAB G SANDOZ EAGEX 00781-5316-05 2.62500 ZOLPIDEM TART ER 12.5 MG TAB G SANDOZ EAGEX 00955-1703-10 2.62500 ZOLPIDEM TART ER 12.5 MG TAB G WINTHROP US EAGEX 10370-0116-10 2.62500 ZOLPIDEM TART ER 12.5 MG TAB G PAR PHARM. EAGEX 10370-0116-50 2.62500 ZOLPIDEM TART ER 12.5 MG TAB G PAR PHARM. EAGEX 68<strong>08</strong>4-0523-11 2.62500 ZOLPIDEM TART ER 12.5 MG TAB G AHP EAGEX 68<strong>08</strong>4-0523-21 2.62500 ZOLPIDEM TART ER 12.5 MG TAB G AHP EAGEX 00228-3481-11 1.55510 ZOLPIDEM TART ER 6.25 MG TAB G ACTAVIS PHARMA, EAGEX 00228-3481-50 1.55510 ZOLPIDEM TART ER 6.25 MG TAB G ACTAVIS PHARMA, EAGEX 00781-5315-01 1.55510 ZOLPIDEM TART ER 6.25 MG TAB G SANDOZ EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-5315-05 1.55510 ZOLPIDEM TART ER 6.25 MG TAB G SANDOZ EAGEX 00955-1702-10 1.55510 ZOLPIDEM TART ER 6.25 MG TAB G WINTHROP US EAGEX 10370-0117-10 1.55510 ZOLPIDEM TART ER 6.25 MG TAB G PAR PHARM. EAGEX 00093-0074-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G TEVA USA EAGEX 00378-5310-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G MYLAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 473LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00378-5310-05 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-6469-13 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6469-20 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6469-21 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6469-28 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6469-32 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G QUALITEST EAGEX 00781-5318-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G SANDOZ EAGEX 00781-5318-10 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G SANDOZ EAGEX 00904-6<strong>08</strong>3-61 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G MAJOR PHARMACEU EAGEX 13668-00<strong>08</strong>-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G TORRENT PHARMAC EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-00<strong>08</strong>-05 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G TORRENT PHARMAC EAGEX 13668-00<strong>08</strong>-10 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G TORRENT PHARMAC EAGEX 13668-00<strong>08</strong>-15 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G TORRENT PHARMAC EAGEX 13668-00<strong>08</strong>-71 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G TORRENT PHARMAC EAGEX 13668-00<strong>08</strong>-90 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G TORRENT PHARMAC EAGEX 16714-<strong>06</strong>22-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>22-02 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G NORTHSTAR RX LL EAGEX 51079-0725-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0725-20 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G MYLAN INSTITUTI EAGEX 55111-0479-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G DR.REDDY'S LAB EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 55111-0479-05 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G DR.REDDY'S LAB EAGEX 60505-2605-00 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G APOTEX CORP EAGEX 60505-2605-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G APOTEX CORP EAGEX 60505-2605-<strong>08</strong> 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G APOTEX CORP EAGEX 63739-0526-10 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G MCKESSON PACKAG EAGEX 64679-0715-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G WOCKHARDT USA L EAGEX 64679-0715-04 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G WOCKHARDT USA L EAGEX 65862-0160-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G AUROBINDO PHARM EAGEX 65862-0160-05 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0200-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 68<strong>08</strong>4-0226-01 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0226-11 0.02687 ZOLPIDEM TARTRATE 10 MG TABLET G AHP EAGEX 00093-0073-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G TEVA USA EAGEX 00378-5305-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G MYLAN EAGEX 00378-5305-05 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G MYLAN EAGEX 0<strong>06</strong>03-6468-09 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6468-16 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6468-21 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6468-28 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G QUALITEST EAGEX 0<strong>06</strong>03-6468-32 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00781-5317-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G SANDOZ EAGEX 00781-5317-10 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G SANDOZ EAGEX 00904-6<strong>08</strong>2-61 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G MAJOR PHARMACEU EAGEX 13668-0007-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G TORRENT PHARMAC EAGEX 13668-0007-05 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G TORRENT PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 474LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 05 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 13668-0007-10 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G TORRENT PHARMAC EAGEX 13668-0007-71 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G TORRENT PHARMAC EAGEX 16714-<strong>06</strong>21-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G NORTHSTAR RX LL EAGEX 16714-<strong>06</strong>21-02 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G NORTHSTAR RX LL EAGEX 51079-0724-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G MYLAN INSTITUTI EAGEX 51079-0724-20 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G MYLAN INSTITUTI EAGEX 55111-0478-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G DR.REDDY'S LAB EAGEX 55111-0478-05 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G DR.REDDY'S LAB EAGEX 60505-2604-00 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G APOTEX CORP EAGEX 60505-2604-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G APOTEX CORP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 60505-2604-<strong>08</strong> 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G APOTEX CORP EAGEX 64679-0714-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G WOCKHARDT USA L EAGEX 64679-0714-04 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G WOCKHARDT USA L EAGEX 65862-0159-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G AUROBINDO PHARM EAGEX 65862-0159-05 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G AUROBINDO PHARM EAGEX 68<strong>08</strong>4-0189-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0225-01 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G AHP EAGEX 68<strong>08</strong>4-0225-11 0.02580 ZOLPIDEM TARTRATE 5 MG TABLET G AHP EABEX 00095-0950-05 8.53713 ZOLPIMIST 5 MG ORAL SPRAY G ECR PHARM. ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 475LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: <strong>06</strong> PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00121-0504-04 0.01499 ACETAMINOPHEN-CODEINE SOLUTION G PHARMACEU ASSOC MLGEN 00121-0504-16 0.01499 ACETAMINOPHEN-CODEINE SOLUTION G PHARMACEU ASSOC MLGEN 0<strong>06</strong>03-1020-58 0.01499 ACETAMINOPHEN-CODEINE SOLUTION G QUALITEST MLGEN 0<strong>06</strong>03-9013-54 0.01499 ACETAMINOPHEN-CODEINE SOLUTION G QUALITEST MLGEN 0<strong>06</strong>03-9013-58 0.01499 ACETAMINOPHEN-CODEINE SOLUTION G QUALITEST MLGEN 50383-0079-16 0.01499 ACETAMINOPHEN-CODEINE SOLUTION G HI-TECH PHARMAC MLGEN 60432-0245-04 0.01499 ACETAMINOPHEN-CODEINE SOLUTION G MORTON GROVE PH MLGEN 60432-0245-16 0.01499 ACETAMINOPHEN-CODEINE SOLUTION G MORTON GROVE PH MLBND 00187-0003-01 1.35797 CAPITAL WITH CODEINE SUSP G VALEANT MLBND 00054-3194-46 0.11110 0.14095 DIPHENOXYLAT-ATROP 2.5-0.025/5 0 ROXANE LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00378-0415-01 0.12620 DIPHENOXYLATE-ATROP 2.5-0.025 0 MYLAN EAGEN 00378-0415-10 0.12620 DIPHENOXYLATE-ATROP 2.5-0.025 0 MYLAN EAGEN 00378-0415-77 0.12620 DIPHENOXYLATE-ATROP 2.5-0.025 0 MYLAN EAGEN 51079-0<strong>06</strong>7-20 0.12620 DIPHENOXYLATE-ATROP 2.5-0.025 0 MYLAN INSTITUTI EAGEN 59762-1<strong>06</strong>1-01 0.12620 DIPHENOXYLATE-ATROP 2.5-0.025 0 GREENSTONE LLC. EAGEN 59762-1<strong>06</strong>1-02 0.12620 DIPHENOXYLATE-ATROP 2.5-0.025 0 GREENSTONE LLC. EABND 00025-0<strong>06</strong>1-31 0.12620 1.43714 LOMOTIL 2.5-0.025 MG TABLET G PFIZER US PHARM EABEX 00071-1015-68 3.99266 LYRICA 100 MG CAPSULE G PFIZER US PHARM EABEX 00071-1016-68 3.99266 LYRICA 150 MG CAPSULE G PFIZER US PHARM EABEX 00071-1020-01 1.<strong>06</strong>357 LYRICA 20 MG/ML ORAL SOLUTION G PFIZER US PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00071-1017-68 3.99266 LYRICA 200 MG CAPSULE G PFIZER US PHARM EABEX 00071-1019-68 3.99266 LYRICA 225 MG CAPSULE G PFIZER US PHARM EABEX 00071-1012-68 3.99266 LYRICA 25 MG CAPSULE G PFIZER US PHARM EABEX 00071-1018-68 3.99266 LYRICA 300 MG CAPSULE G PFIZER US PHARM EABEX 00071-1013-68 3.99266 LYRICA 50 MG CAPSULE G PFIZER US PHARM EABEX 00071-1014-68 3.99266 LYRICA 75 MG CAPSULE G PFIZER US PHARM EABEX 00173-<strong>08</strong>12-59 7.81011 POTIGA 200 MG TABLET G GLAXOSMITHKLINE EABEX 00173-<strong>08</strong>13-59 7.81011 POTIGA 300 MG TABLET G GLAXOSMITHKLINE EABEX 00173-<strong>08</strong>14-59 7.81011 POTIGA 400 MG TABLET G GLAXOSMITHKLINE EABEX 00173-<strong>08</strong>10-59 3.90505 POTIGA 50 MG TABLET G GLAXOSMITHKLINE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BEX 00131-5410-70 1.02566 VIMPAT 10 MG/ML SOLUTION G UCB PHARMA MLBEX 00131-5410-71 1.19237 VIMPAT 10 MG/ML SOLUTION G UCB PHARMA MLBEX 00131-2478-35 9.60725 VIMPAT 100 MG TABLET G UCB PHARMA EABEX 00131-2478-60 10.56866 VIMPAT 100 MG TABLET G UCB PHARMA EABEX 00131-2479-35 10.17469 VIMPAT 150 MG TABLET G UCB PHARMA EABEX 00131-2479-60 11.19282 VIMPAT 150 MG TABLET G UCB PHARMA EABEX 00131-2480-35 10.17760 VIMPAT 200 MG TABLET G UCB PHARMA EABEX 00131-2480-60 11.19642 VIMPAT 200 MG TABLET G UCB PHARMA EABEX 00131-2477-35 6.14517 VIMPAT 50 MG TABLET G UCB PHARMA EABEX 00131-2477-60 6.76020 VIMPAT 50 MG TABLET G UCB PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 476LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00135-0200-01 9.46500 ABREVA 10% CREAM 0 GSK CONSUMER HE GMBND 00135-0200-03 9.46500 ABREVA 10% CREAM 0 GSK CONSUMER HE GMBND 00713-0118-01 0.44527 ACEPHEN 120 MG SUPPOSITORY 0 G & W LABS. EABND 00713-0118-12 0.44527 ACEPHEN 120 MG SUPPOSITORY 0 G & W LABS. EABND 00713-0118-50 0.44527 ACEPHEN 120 MG SUPPOSITORY 0 G & W LABS. EAGEN 00713-0164-01 0.47151 ACEPHEN 325 MG SUPPOSITORY 0 G & W LABS. EAGEN 00713-0164-12 0.47151 ACEPHEN 325 MG SUPPOSITORY 0 G & W LABS. EAGEN 00713-0164-50 0.47151 ACEPHEN 325 MG SUPPOSITORY 0 G & W LABS. EAGEN 00713-0165-01 0.52417 ACEPHEN 650 MG SUPPOSITORY 0 G & W LABS. EAGEN 00713-0165-12 0.52417 ACEPHEN 650 MG SUPPOSITORY 0 G & W LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00713-0165-50 0.52417 ACEPHEN 650 MG SUPPOSITORY 0 G & W LABS. EAGEN 45802-0732-30 0.44527 ACETAMINOPHEN 120 MG SUPPOS 0 PERRIGO CO. EAGEN 45802-0732-33 0.44527 ACETAMINOPHEN 120 MG SUPPOS 0 PERRIGO CO. EAGEN 00536-1320-12 0.47151 ACETAMINOPHEN 325 MG SUPPOS 0 RUGBY EAGEN 63739-0440-01 0.02968 ACETAMINOPHEN 325 MG TABLET 0 MCKESSON PACKAG EABND 00121-<strong>06</strong>57-11 0.02340 ACETAMINOPHEN 325 MG/10.15 ML 0 PHARMACEU ASSOC MLGEN 10267-1190-01 0.02807 ACETAMINOPHEN 500 MG CAPLET 0 CONTRACT PHARM EAGEN 10267-1190-04 0.02807 ACETAMINOPHEN 500 MG CAPLET 0 CONTRACT PHARM EAGEN 10267-1190-05 0.02807 ACETAMINOPHEN 500 MG CAPLET 0 CONTRACT PHARM EAGEN 10267-1190-<strong>06</strong> 0.02807 ACETAMINOPHEN 500 MG CAPLET 0 CONTRACT PHARM EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51645-0705-01 0.02807 ACETAMINOPHEN 500 MG CAPLET 0 PLUS PHARMA,INC EAGEN 51645-0705-10 0.02807 ACETAMINOPHEN 500 MG CAPLET 0 PLUS PHARMA,INC EAGEN 63739-0439-01 0.02807 ACETAMINOPHEN 500 MG CAPLET 0 MCKESSON PACKAG EAGEN 51645-07<strong>06</strong>-01 0.02807 ACETAMINOPHEN 500 MG TABLET 0 PLUS PHARMA,INC EAGEN 51645-07<strong>06</strong>-10 0.02807 ACETAMINOPHEN 500 MG TABLET 0 PLUS PHARMA,INC EABND 60258-0050-<strong>08</strong> 0.02340 ACETAMINOPHEN 500 MG/5 ML LIQ 0 CYPRESS PHARM. MLGEN 00536-1260-12 0.52417 ACETAMINOPHEN 650 MG SUPPOS 0 RUGBY EAGEN 45802-0730-30 0.52417 ACETAMINOPHEN 650 MG SUPPOS 0 PERRIGO CO. EAGEN 45802-0730-32 0.52417 ACETAMINOPHEN 650 MG SUPPOS 0 PERRIGO CO. EAGEN 45802-0730-33 0.52417 ACETAMINOPHEN 650 MG SUPPOS 0 PERRIGO CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00121-<strong>06</strong>57-21 0.02340 ACETAMINOPHEN 650 MG/20.3 ML 0 PHARMACEU ASSOC MLGEN 63868-0486-25 0.07620 ACID CONTROLLER 20 MG TABLET 0 CHAIN DRUG EAGEN 00904-7727-14 0.0<strong>08</strong>70 ACID GONE ANTACID LIQUID 0 MAJOR PHARMACEU MLGEN 00113-0141-65 0.13110 ACID REDUCER 10 MG TABLET 0 PERRIGO CO. EAGEN 24385-0255-65 0.13110 ACID REDUCER 10 MG TABLET 0 AMERISOURCEBERG EAGEN 36800-0141-65 0.13110 ACID REDUCER 10 MG TABLET 0 TOPCO EAGEN 36800-0141-72 0.13110 ACID REDUCER 10 MG TABLET 0 TOPCO EAGEN 37205-<strong>06</strong>14-65 0.13110 ACID REDUCER 10 MG TABLET 0 LEADER EAGEN 00113-0194-02 0.07620 ACID REDUCER 20 MG TABLET 0 PERRIGO CO. EAGEN 24385-0385-51 0.07620 ACID REDUCER 20 MG TABLET 0 AMERISOURCEBERG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24385-0385-63 0.07620 ACID REDUCER 20 MG TABLET 0 AMERISOURCEBERG EAGEN 24385-0385-71 0.07620 ACID REDUCER 20 MG TABLET 0 AMERISOURCEBERG EAGEN 36800-0194-02 0.07620 ACID REDUCER 20 MG TABLET 0 TOPCO EAGEN 36800-0194-51 0.07620 ACID REDUCER 20 MG TABLET 0 TOPCO EAGEN 36800-0194-71 0.07620 ACID REDUCER 20 MG TABLET 0 TOPCO EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 477LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-<strong>08</strong>61-63 0.07620 ACID REDUCER 20 MG TABLET 0 LEADER EAGEN 00113-0271-39 0.10993 ACID REDUCER 75 MG TABLET 0 PERRIGO CO. EAGEN 00113-0271-72 0.10993 ACID REDUCER 75 MG TABLET 0 PERRIGO CO. EAGEN 00031-8736-12 0.02680 ADULT ROBITUSSIN PEAK COLD LIQ 0 WYETH CONSUMER MLGEN 00031-8736-18 0.02680 ADULT ROBITUSSIN PEAK COLD LIQ 0 WYETH CONSUMER MLGEN 00031-8736-42 0.02680 ADULT ROBITUSSIN PEAK COLD LIQ 0 WYETH CONSUMER MLGEN 00113-<strong>08</strong>51-40 0.0<strong>08</strong>70 ADVANCED ANTACID LIQUID 0 PERRIGO CO. MLBND 00573-0164-40 0.<strong>06</strong>470 ADVIL PM CAPLET 0 WYETH CONSUMER EABND 00573-0164-43 0.<strong>06</strong>470 ADVIL PM CAPLET 0 WYETH CONSUMER EABND 00573-0160-20 0.05567 ADVIL 200 MG CAPLET 0 WYETH CONSUMER EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00573-0160-30 0.05567 ADVIL 200 MG CAPLET 0 WYETH CONSUMER EABND 00573-0160-40 0.05567 ADVIL 200 MG CAPLET 0 WYETH CONSUMER EABND 00573-0161-51 0.05567 ADVIL 200 MG CAPLET 0 WYETH CONSUMER EABND 00573-0165-20 0.05567 ADVIL 200 MG GEL CAPLET 0 WYETH CONSUMER EABND 00573-0165-30 0.05567 ADVIL 200 MG GEL CAPLET 0 WYETH CONSUMER EABND 00573-0165-40 0.05567 ADVIL 200 MG GEL CAPLET 0 WYETH CONSUMER EABND 00573-0169-20 0.<strong>06</strong>470 ADVIL 200 MG LIQUI-GEL CAPSULE 0 WYETH CONSUMER EABND 00573-0150-20 0.05567 ADVIL 200 MG TABLET 0 WYETH CONSUMER EABND 00573-0150-30 0.05567 ADVIL 200 MG TABLET 0 WYETH CONSUMER EABND 00573-0150-40 0.05567 ADVIL 200 MG TABLET 0 WYETH CONSUMER EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00573-0151-10 0.05567 ADVIL 200 MG TABLET 0 WYETH CONSUMER EABND 00573-0154-98 0.05567 ADVIL 200 MG TABLET 0 WYETH CONSUMER EAGEN 17478-0<strong>06</strong>2-35 1.37180 AKWA TEARS OINTMENT 0 AKORN INC. GMGEN 00573-2660-12 0.56431 ALAVERT D-12 ALLERGY-SINUS TAB G WYETH CONSUMER EAGEN 00573-2660-24 0.56431 ALAVERT D-12 ALLERGY-SINUS TAB G WYETH CONSUMER EAGEN 49614-0234-26 0.05700 ALL DAY ALLERGY 1 MG/ML SYRUP 0 MEDICINE SHOP MLGEN 00113-9458-13 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 PERRIGO CO. EAGEN 00113-9458-39 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 PERRIGO CO. EAGEN 00113-9458-66 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 PERRIGO CO. EAGEN 00904-5852-43 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5852-46 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5852-89 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 24385-0998-65 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 AMERISOURCEBERG EAGEN 24385-0998-74 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 AMERISOURCEBERG EAGEN 24385-0998-75 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 AMERISOURCEBERG EAGEN 36800-0458-13 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 TOPCO EAGEN 36800-0458-39 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 TOPCO EAGEN 36800-0458-47 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 TOPCO EAGEN 36800-0458-66 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 TOPCO EAGEN 36800-0458-72 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0458-87 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 TOPCO EAGEN 36800-0458-95 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 TOPCO EAGEN 37205-<strong>08</strong>20-70 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 LEADER EAGEN 37205-<strong>08</strong>20-75 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 LEADER EAGEN 37205-<strong>08</strong>20-76 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 LEADER EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 478LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-<strong>08</strong>25-70 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 LEADER EAGEN 37205-<strong>08</strong>25-74 0.12903 ALL DAY ALLERGY 10 MG TABLET 0 LEADER EAGEN 00113-0176-53 0.61830 ALL DAY ALLERGY-D TABLET G PERRIGO CO. EAGEN 00113-0176-62 0.61830 ALL DAY ALLERGY-D TABLET G PERRIGO CO. EAGEN 00904-5831-12 0.61830 ALL DAY ALLERGY-D TABLET G MAJOR PHARMACEU EAGEN 24385-0175-53 0.61830 ALL DAY ALLERGY-D TABLET G AMERISOURCEBERG EAGEN 36800-0176-62 0.61830 ALL DAY ALLERGY-D TABLET G TOPCO EABND 00536-0370-97 0.02680 ALLER-CHLOR SYRUP 0 RUGBY MLBND 00536-3467-01 0.02360 ALLER-CHLOR 4 MG TABLET 0 RUGBY EABND 00536-3467-10 0.02360 ALLER-CHLOR 4 MG TABLET 0 RUGBY EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00536-3467-35 0.<strong>08</strong>580 ALLER-CHLOR 4 MG TABLET 0 RUGBY EAGEN 00113-0165-52 0.61380 ALLERGY & CONGEST RELIEF TB G PERRIGO CO. EAGEN 37205-<strong>08</strong>27-62 0.61830 ALLERGY D-12 TABLET G LEADER EAGEN 49614-0235-62 0.61830 ALLERGY D-12 TABLET G MEDICINE SHOP EAGEN 37205-0348-52 0.61380 ALLERGY RELIEF D-24 TABLET G LEADER EAGEN 37205-0348-88 0.61380 ALLERGY RELIEF D-24 TABLET G LEADER EAGEN 37205-0378-26 0.04749 ALLERGY RELIEF SYRUP 0 LEADER MLGEN 00113-<strong>06</strong>12-39 0.09890 ALLERGY RELIEF 10 MG TABLET 0 PERRIGO CO. EAGEN 36800-<strong>06</strong>12-46 0.09890 ALLERGY RELIEF 10 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>12-65 0.09890 ALLERGY RELIEF 10 MG TABLET 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-<strong>06</strong>12-72 0.09890 ALLERGY RELIEF 10 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>12-76 0.09890 ALLERGY RELIEF 10 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>12-87 0.09890 ALLERGY RELIEF 10 MG TABLET 0 TOPCO EAGEN 49614-0170-60 0.09890 ALLERGY RELIEF 10 MG TABLET 0 MEDICINE SHOP EAGEN 49614-0170-65 0.09890 ALLERGY RELIEF 10 MG TABLET 0 MEDICINE SHOP EAGEN 46122-0040-22 0.61666 ALLERGY RELIEF 180 MG TABLET G AMERISOURCEBERG EAGEN 46122-0040-61 0.61666 ALLERGY RELIEF 180 MG TABLET G AMERISOURCEBERG EAGEN 46122-0040-65 0.61666 ALLERGY RELIEF 180 MG TABLET G AMERISOURCEBERG EAGEN 46122-0040-75 0.61666 ALLERGY RELIEF 180 MG TABLET G AMERISOURCEBERG EAGEN 24385-0531-26 0.04749 ALLERGY RELIEF 5 MG/5 ML SOLN 0 AMERISOURCEBERG ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51660-0724-15 0.61380 ALLERGY RELIEF-NASAL DECONG TB G OHM LABS. EAGEN 00904-5728-15 0.09890 ALLERGY 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5728-89 0.09890 ALLERGY 10 MG TABLET 0 MAJOR PHARMACEU EAGEX 36800-0462-62 0.03330 ALLERGY 25 MG CAPSULE 0 TOPCO EAGEX 36800-0462-67 0.03330 ALLERGY 25 MG CAPSULE 0 TOPCO EAGEX 36800-0462-78 0.03330 ALLERGY 25 MG CAPSULE 0 TOPCO EAGEX 36800-0479-62 0.03330 ALLERGY 25 MG TABLET 0 TOPCO EAGEX 36800-0479-67 0.03330 ALLERGY 25 MG TABLET 0 TOPCO EAGEX 36800-0479-78 0.03330 ALLERGY 25 MG TABLET 0 TOPCO EAGEX 36800-0479-79 0.03330 ALLERGY 25 MG TABLET 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00113-0463-62 0.<strong>08</strong>580 ALLERGY 4 MG TABLET 0 PERRIGO CO. EAGEN 00904-0012-24 0.<strong>08</strong>580 ALLERGY 4 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-0012-59 0.02360 ALLERGY 4 MG TABLET 0 MAJOR PHARMACEU EAGEN 36800-0463-62 0.<strong>08</strong>580 ALLERGY 4 MG TABLET 0 TOPCO EAGEN 37205-0215-62 0.<strong>08</strong>580 ALLERGY 4 MG TABLET 0 LEADER EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 479LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0215-78 0.02360 ALLERGY 4 MG TABLET 0 LEADER EAGEN 24385-0351-52 0.61380 ALLERGY-CONGEST RELIEF ER TAB G AMERISOURCEBERG EAGEN 00113-0165-22 0.61380 ALLERGY-CONGESTION RELF ER TAB G PERRIGO CO. EAGEN 49483-0242-10 0.02360 ALLERGY-TIME 4 MG TABLET 0 TIME-CAP LABS EABND 58605-0400-01 0.15291 ALLFEN 400 MG TABLET 0 MCR/AMERICAN PH EABND 00536-0025-83 0.0<strong>08</strong>70 ALMACONE LIQUID 0 RUGBY MLBND 00536-3504-01 0.03690 ALMACONE TABLET CHEWABLE 0 RUGBY EAGEN 00536-0015-83 0.0<strong>08</strong>70 ALMACONE-2 LIQUID 0 RUGBY MLBND 00536-0091-85 0.01450 ALUMINUM HYDROXIDE GEL 0 RUGBY MLGEN 00904-2621-70 0.02950 ANIMAL SHAPES TABLET CHEW 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0535-40 0.0<strong>08</strong>70 ANTACID ANTI-GAS LIQUID 0 LEADER MLGEN 46122-0007-41 0.04333 ANTACID EX-STR 750 MG TAB CHEW 0 AMERISOURCEBERG EAGEN 62107-0020-11 0.0<strong>08</strong>70 ANTACID LIQUID 0 PRIME MARKETING MLGEN 37205-0536-40 0.0<strong>08</strong>70 ANTACID MAXIMUM STRENGTH LIQ 0 LEADER MLGEN 00113-0357-40 0.0<strong>08</strong>70 ANTACID PLUS ANTI-GAS RELF LIQ 0 PERRIGO CO. MLGEN 36800-0357-40 0.0<strong>08</strong>70 ANTACID PLUS ANTI-GAS RELF LIQ 0 TOPCO MLGEN 36800-<strong>08</strong>51-40 0.0<strong>08</strong>70 ANTACID PLUS ANTI-GAS RELF LIQ 0 TOPCO MLGEN 00113-0588-40 0.0<strong>08</strong>70 ANTACID PLUS ANTI-GAS SUSP 0 PERRIGO CO. MLBND 62107-0021-11 0.0<strong>08</strong>70 ANTACID PLUS E-S LIQUID 0 PRIME MARKETING MLGEN 24385-0356-40 0.0<strong>08</strong>70 ANTACID SUSPENSION 0 AMERISOURCEBERG ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0340-40 0.0<strong>08</strong>70 ANTACID SUSPENSION 0 TOPCO MLGEN 49614-0361-40 0.0<strong>08</strong>70 ANTACID SUSPENSION 0 MEDICINE SHOP MLGEN 24385-01<strong>06</strong>-80 0.04333 ANTACID XTRA STRENGTH CHEW TAB 0 AMERISOURCEBERG EAGEN 37205-0205-80 0.04333 ANTACID XTRA STRENGTH CHEW TAB 0 LEADER EAGEN 00113-0478-47 0.03531 ANTACID 500 MG CHEWABLE TABLET 0 PERRIGO CO. EAGEN 37205-0200-47 0.03531 ANTACID 500 MG CHEWABLE TABLET 0 LEADER EAGEN 37205-0530-40 0.0<strong>08</strong>70 ANTACID-ANTIGAS LIQUID 0 LEADER MLGEN 00113-0340-40 0.0<strong>08</strong>70 ANTACID-SIMETHICONE LIQUID 0 PERRIGO CO. MLGEN 49614-0358-40 0.0<strong>08</strong>70 ANTACID-SIMETHICONE LIQUID 0 MEDICINE SHOP MLGEN 00113-0224-53 0.23958 ANTI-DIARRHEAL 2 MG CAPLET 0 PERRIGO CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00113-0224-89 0.23958 ANTI-DIARRHEAL 2 MG CAPLET 0 PERRIGO CO. EAGEN 00113-0224-91 0.23958 ANTI-DIARRHEAL 2 MG CAPLET 0 PERRIGO CO. EAGEN 00904-7725-12 0.23958 ANTI-DIARRHEAL 2 MG CAPLET 0 MAJOR PHARMACEU EAGEN 00904-7725-24 0.23958 ANTI-DIARRHEAL 2 MG CAPLET 0 MAJOR PHARMACEU EAGEN 24385-0386-89 0.23958 ANTI-DIARRHEAL 2 MG CAPLET 0 AMERISOURCEBERG EAGEN 24385-0554-67 0.23958 ANTI-DIARRHEAL 2 MG CAPLET 0 AMERISOURCEBERG EAGEN 37205-0370-53 0.23958 ANTI-DIARRHEAL 2 MG CAPLET 0 LEADER EAGEN 37205-0370-67 0.23958 ANTI-DIARRHEAL 2 MG CAPLET 0 LEADER EAGEN 37205-0370-89 0.23958 ANTI-DIARRHEAL 2 MG CAPLET 0 LEADER EAGEN 49614-0554-53 0.23958 ANTI-DIARRHEAL 2 MG CAPLET 0 MEDICINE SHOP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0224-53 0.23958 ANTI-DIARRHEAL 2 MG TABLET 0 TOPCO EAGEN 36800-0224-62 0.23958 ANTI-DIARRHEAL 2 MG TABLET 0 TOPCO EAGEN 36800-0224-80 0.23958 ANTI-DIARRHEAL 2 MG TABLET 0 TOPCO EAGEN 36800-0224-91 0.23958 ANTI-DIARRHEAL 2 MG TABLET 0 TOPCO EAGEN 00536-5150-26 0.05013 ANTI-FUNGAL 1% POWDER 0 RUGBY GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 480LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00113-0541-64 0.<strong>08</strong>144 ANTI-ITCH 1% CREAM 0 PERRIGO CO. GMBND 45802-0276-03 0.10370 ANTI-ITCH 1% OINTMENT 0 PERRIGO CO. GMGEN 24385-0205-03 0.11290 ANTIFUNGAL 1% CREAM 0 AMERISOURCEBERG GMGEN 00904-0250-24 0.09827 APRODINE TABLET 0 MAJOR PHARMACEU EABND 58914-0214-60 0.37733 AQUADEKS PEDIATRIC LIQUID 0 APTALIS PHARMA MLBND 58914-0011-<strong>06</strong> 0.55505 AQUADEKS S<strong>OF</strong>TGEL 0 APTALIS PHARMA EAGEN 36800-0544-62 0.04260 ARTHRITIS PAIN ER 650 MG CAPLT 0 TOPCO EAGEN 36800-0544-71 0.04260 ARTHRITIS PAIN ER 650 MG CAPLT 0 TOPCO EAGEN 36800-0544-78 0.04260 ARTHRITIS PAIN ER 650 MG CAPLT 0 TOPCO EAGEN 36800-0966-47 0.04260 ARTHRITIS PAIN ER 650 MG CAPLT 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0034-71 0.04260 ARTHRITIS PAIN ER 650 MG CAPLT 0 LEADER EAGEN 37205-0034-78 0.04260 ARTHRITIS PAIN ER 650 MG CAPLT 0 LEADER EAGEN 49614-0473-71 0.04260 ARTHRITIS PAIN ER 650 MG CAPLT 0 MEDICINE SHOP EAGEN 49614-0473-78 0.04260 ARTHRITIS PAIN ER 650 MG CAPLT 0 MEDICINE SHOP EAGEN 51660-0333-01 0.04260 ARTHRITIS PAIN ER 650 MG CAPLT 0 OHM LABS. EAGEN 63868-0<strong>08</strong>9-50 0.04260 ARTHRITIS PAIN ER 650 MG CAPLT 0 CHAIN DRUG EAGEN 24385-<strong>06</strong>29-78 0.04260 ARTHRITIS PAIN RELF ER 650 MG 0 AMERISOURCEBERG EABND 24385-00<strong>06</strong>-05 0.26403 ARTIFICIAL TEARS DROPS 0 AMERISOURCEBERG MLBND 00536-6550-91 1.37180 ARTIFICIAL TEARS EYE OINT 0 RUGBY GMGEN 00536-1970-72 0.27930 ARTIFICIAL TEARS 1.4 % DROPS 0 RUGBY ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62107-0027-26 0.02781 ASPIR EC 81 MG TABLET 0 PRIME MARKETING EAGEN 62107-0027-32 0.02781 ASPIR EC 81 MG TABLET 0 PRIME MARKETING EAGEN 00904-7704-18 0.02781 ASPIR-LOW EC 81 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7704-70 0.02781 ASPIR-LOW EC 81 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7704-80 0.02781 ASPIR-LOW EC 81 MG TABLET 0 MAJOR PHARMACEU EAGEN 00536-3313-01 0.02128 ASPIRIN EC 325 MG TABLET 0 RUGBY EAGEN 00536-3313-10 0.02128 ASPIRIN EC 325 MG TABLET 0 RUGBY EAGEN 0<strong>06</strong>03-0168-21 0.02128 ASPIRIN EC 325 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-0169-21 0.02128 ASPIRIN EC 325 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-0169-32 0.02128 ASPIRIN EC 325 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-2011-59 0.02128 ASPIRIN EC 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-2013-60 0.02128 ASPIRIN EC 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-2013-80 0.02128 ASPIRIN EC 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 16103-0357-<strong>08</strong> 0.02128 ASPIRIN EC 325 MG TABLET 0 PHARBEST PHARMA EAGEN 16103-0357-11 0.02128 ASPIRIN EC 325 MG TABLET 0 PHARBEST PHARMA EAGEN 24385-0429-02 0.02128 ASPIRIN EC 325 MG TABLET 0 AMERISOURCEBERG EAGEN 36800-0429-02 0.02128 ASPIRIN EC 325 MG TABLET 0 TOPCO EAGEN 37205-0429-96 0.02128 ASPIRIN EC 325 MG TABLET 0 LEADER EAGEN 63739-0023-01 0.02128 ASPIRIN EC 325 MG TABLET 0 MCKESSON PACKAG EAGEN 00536-3<strong>08</strong>6-10 0.02781 ASPIRIN EC 81 MG TABLET 0 RUGBY EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00536-3<strong>08</strong>6-41 0.02781 ASPIRIN EC 81 MG TABLET 0 RUGBY EAGEN 0<strong>06</strong>03-0026-22 0.02781 ASPIRIN EC 81 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-0026-32 0.02781 ASPIRIN EC 81 MG TABLET 0 QUALITEST EAGEN 10267-2404-01 0.02781 ASPIRIN EC 81 MG TABLET 0 CONTRACT PHARM EAGEN 16103-0356-09 0.02781 ASPIRIN EC 81 MG TABLET 0 PHARBEST PHARMA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 481LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16103-0356-11 0.02781 ASPIRIN EC 81 MG TABLET 0 PHARBEST PHARMA EAGEN 24385-0541-87 0.02781 ASPIRIN EC 81 MG TABLET 0 AMERISOURCEBERG EAGEN 37205-0510-76 0.02781 ASPIRIN EC 81 MG TABLET 0 LEADER EAGEN 37205-0510-87 0.02781 ASPIRIN EC 81 MG TABLET 0 LEADER EAGEN 63739-0522-10 0.02781 ASPIRIN EC 81 MG TABLET 0 MCKESSON PACKAG EABND 00574-7034-12 0.2<strong>08</strong>00 ASPIRIN 300 MG SUPPOSITORY 0 PADDOCK LABS. EAGEN 00113-0416-78 0.01740 ASPIRIN 325 MG COATED TABLET 0 PERRIGO CO. EAGEN 00536-3305-01 0.01740 ASPIRIN 325 MG TABLET 0 RUGBY EAGEN 00536-3305-10 0.01740 ASPIRIN 325 MG TABLET 0 RUGBY EAGEN 0<strong>06</strong>03-0031-21 0.01740 ASPIRIN 325 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-2009-40 0.01740 ASPIRIN 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-2009-60 0.01740 ASPIRIN 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-2019-59 0.01740 ASPIRIN 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-2019-80 0.01740 ASPIRIN 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 37205-0145-78 0.01740 ASPIRIN 325 MG TABLET 0 LEADER EAGEN 37205-0145-87 0.01740 ASPIRIN 325 MG TABLET 0 LEADER EABND 00574-7036-12 0.32<strong>06</strong>0 ASPIRIN 600 MG SUPPOSITORY 0 PADDOCK LABS. EAGEN 00113-0467-68 0.04820 ASPIRIN 81 MG CHEWABLE TABLET 0 PERRIGO CO. EAGEN 00536-3297-36 0.04820 ASPIRIN 81 MG CHEWABLE TABLET 0 RUGBY EAGEN 00904-4040-73 0.04820 ASPIRIN 81 MG CHEWABLE TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6288-89 0.04820 ASPIRIN 81 MG CHEWABLE TABLET 0 MAJOR PHARMACEU EAGEN 36800-0467-68 0.04820 ASPIRIN 81 MG CHEWABLE TABLET 0 TOPCO EAGEN 37205-07<strong>08</strong>-68 0.04820 ASPIRIN 81 MG CHEWABLE TABLET 0 LEADER EAGEN 49483-0334-63 0.04820 ASPIRIN 81 MG CHEWABLE TABLET 0 TIME-CAP LABS EAGEN 63739-0434-01 0.04820 ASPIRIN 81 MG CHEWABLE TABLET 0 MCKESSON PACKAG EAGEN 63739-0434-03 0.04820 ASPIRIN 81 MG CHEWABLE TABLET 0 MCKESSON PACKAG EAGEN 16103-0366-05 0.04820 ASPIRIN 81 MG TABLET CHEW 0 PHARBEST PHARMA EAGEN 45802-0032-01 0.14930 ATHLETE'S FOOT 1% CREAM 0 PERRIGO CO. GMGEN 45802-0032-03 0.14930 ATHLETE'S FOOT 1% CREAM 0 PERRIGO CO. GMBND 00225-0382-80 0.07570 AYR SALINE 0.65% NOSE DROPS 0 B.F ASCHER & CO ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00225-0380-80 0.07570 AYR SALINE 0.65% NOSE SPRAY 0 B.F ASCHER & CO MLGEN 00904-4181-60 0.05040 B-COMPLEX WITH B12 TABLET 0 MAJOR PHARMACEU EABND 00225-0550-50 0.07570 BABY AYR SALINE 0.65% DROPS 0 B.F ASCHER & CO MLBND 00168-0111-09 0.<strong>08</strong>290 BACITRACIN ZINC OINTMENT 0 SANDOZ EAGEN 00168-0011-04 0.<strong>08</strong>290 BACITRACIN ZN 500 UNIT/GM OINT 0 SANDOZ GMGEN 00168-0011-16 0.<strong>08</strong>290 BACITRACIN ZN 500 UNIT/GM OINT 0 SANDOZ GMGEN 00168-0011-31 0.<strong>08</strong>290 BACITRACIN ZN 500 UNIT/GM OINT 0 SANDOZ GMGEN 00168-0011-35 0.<strong>08</strong>290 BACITRACIN ZN 500 UNIT/GM OINT 0 SANDOZ GMGEN 0<strong>06</strong>03-0441-50 0.<strong>06</strong>579 BACITRACIN ZN 500 UNIT/GM OINT 0 QUALITEST GMGEN 24385-0<strong>06</strong>0-03 0.<strong>06</strong>579 BACITRACIN ZN 500 UNIT/GM OINT 0 AMERISOURCEBERG GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-2075-01 0.<strong>08</strong>290 BACITRACIN ZN 500 UNIT/GM OINT 0 TARO PHARM USA GMGEN 00713-0280-31 0.<strong>06</strong>579 BACITRACIN 500 UNIT/GM OINTMNT 0 G & W LABS. GMGEN 37205-0275-10 0.<strong>06</strong>579 BACITRACIN 500 UNIT/GM OINTMNT 0 LEADER GMGEN 45802-0<strong>06</strong>0-01 0.<strong>06</strong>579 BACITRACIN 500 UNIT/GM OINTMNT 0 PERRIGO CO. GMGEN 45802-0<strong>06</strong>0-03 0.<strong>06</strong>579 BACITRACIN 500 UNIT/GM OINTMNT 0 PERRIGO CO. GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 482LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 45802-0<strong>06</strong>0-70 0.<strong>08</strong>290 BACITRACIN 500 UNIT/GM OINTMNT 0 PERRIGO CO. EABND 00904-3192-51 0.05040 BALANCED B-100 TABLET 0 MAJOR PHARMACEU EAGEN 00904-3177-60 0.05040 BALANCED B-50 TABLET 0 MAJOR PHARMACEU EAGEX 00904-1228-20 0.02118 BANOPHEN ALLERGY 12.5 MG/5 ML 0 MAJOR PHARMACEU MLGEX 00904-5174-16 0.02118 BANOPHEN 12.5 MG/5 ML ELIXIR 0 MAJOR PHARMACEU MLGEX 00904-2035-24 0.03330 BANOPHEN 25 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-53<strong>06</strong>-60 0.03330 BANOPHEN 25 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-53<strong>06</strong>-80 0.03330 BANOPHEN 25 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-5307-60 0.05760 BANOPHEN 50 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 00904-5307-80 0.05760 BANOPHEN 50 MG CAPSULE 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00536-4092-56 0.<strong>08</strong>540 BENZOYL PEROXIDE 10% GEL 0 RUGBY GMBND 00536-<strong>08</strong>15-95 0.09520 BENZOYL PEROXIDE 10% LOTION 0 RUGBY MLGEN 00536-4<strong>08</strong>9-56 0.<strong>08</strong>030 BENZOYL PEROXIDE 5% GEL 0 RUGBY GMBND 00536-<strong>08</strong>10-95 0.07160 BENZOYL PEROXIDE 5% LOTION 0 RUGBY MLBND 00<strong>06</strong>5-0419-28 0.27930 BION TEARS EYE DROPS 0 ALCON CONSUMER EAGEN 00904-5433-60 0.02950 BIOTIN 300 MCG TABLET 0 MAJOR PHARMACEU EAGEN 40985-0271-16 BIOTIN 5,000 MCG CAPSULE G 21ST CENTURY HE EAGEN 24385-0903-78 0.03100 BISA-LAX EC 5 MG TABLET 0 AMERISOURCEBERG EABND 00713-0109-01 0.19997 BISAC-EVAC 10 MG SUPPOSITORY 0 G & W LABS. EABND 00713-0109-05 0.19997 BISAC-EVAC 10 MG SUPPOSITORY 0 G & W LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00713-0109-<strong>08</strong> 0.19997 BISAC-EVAC 10 MG SUPPOSITORY 0 G & W LABS. EABND 00713-0109-10 0.19997 BISAC-EVAC 10 MG SUPPOSITORY 0 G & W LABS. EABND 00713-0109-12 0.19997 BISAC-EVAC 10 MG SUPPOSITORY 0 G & W LABS. EABND 00713-0109-50 0.19997 BISAC-EVAC 10 MG SUPPOSITORY 0 G & W LABS. EAGEN 00536-3381-01 0.03100 BISACODYL EC 5 MG TABLET 0 RUGBY EAGEN 00536-3381-10 0.03100 BISACODYL EC 5 MG TABLET 0 RUGBY EAGEN 00904-7927-60 0.03100 BISACODYL EC 5 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7927-61 0.03100 BISACODYL EC 5 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7927-80 0.03100 BISACODYL EC 5 MG TABLET 0 MAJOR PHARMACEU EAGEN 16103-0367-<strong>08</strong> 0.03100 BISACODYL EC 5 MG TABLET 0 PHARBEST PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0128-63 0.03100 BISACODYL EC 5 MG TABLET 0 LEADER EAGEN 00536-1355-01 0.19997 BISACODYL 10 MG SUPPOSITORY 0 RUGBY EAGEN 00536-1355-12 0.19997 BISACODYL 10 MG SUPPOSITORY 0 RUGBY EAGEN 00574-7050-12 0.19997 BISACODYL 10 MG SUPPOSITORY 0 PADDOCK LABS. EAGEN 00574-7050-50 0.19997 BISACODYL 10 MG SUPPOSITORY 0 PADDOCK LABS. EAGEN 37205-0102-53 0.19997 BISACODYL 10 MG SUPPOSITORY 0 LEADER EAGEN 45802-0572-42 0.19997 BISACODYL 10 MG SUPPOSITORY 0 PERRIGO CO. EAGEN 45802-0572-78 0.19997 BISACODYL 10 MG SUPPOSITORY 0 PERRIGO CO. EAGEN 45802-0710-30 0.19997 BISACODYL 10 MG SUPPOSITORY 0 PERRIGO CO. EAGEN 45802-0710-32 0.19997 BISACODYL 10 MG SUPPOSITORY 0 PERRIGO CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0710-33 0.19997 BISACODYL 10 MG SUPPOSITORY 0 PERRIGO CO. EAGEN 00904-5058-12 0.19997 BISCOLAX 10 MG SUPPOSITORY 0 MAJOR PHARMACEU EAGEN 00904-5058-60 0.19997 BISCOLAX 10 MG SUPPOSITORY 0 MAJOR PHARMACEU EAGEN 00904-1313-09 0.01889 BISMATROL SUSPENSION 0 MAJOR PHARMACEU MLGEN 00904-1315-46 0.13647 BISMATROL TABLET CHEW 0 MAJOR PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 483LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00193-1465-50 1.07800 BREEZE 2 DISC TEST STRIP 0 BAYER <strong>HEALTH</strong>CAR EAGEN 00193-1466-21 1.05325 BREEZE 2 DISC TEST STRIP 0 BAYER <strong>HEALTH</strong>CAR EAGEN 00193-1440-01 22.00000 BREEZE 2 METER 0 BAYER <strong>HEALTH</strong>CAR EABND 54838-0136-40 0.02680 BROTAPP DM LIQUID 0 SILARX PHARM MLBND 54838-0136-70 0.02140 BROTAPP DM LIQUID 0 SILARX PHARM MLBND 54838-0125-40 0.01790 BROTAPP LIQUID 0 SILARX PHARM MLBND 54838-0125-70 0.01790 BROTAPP LIQUID 0 SILARX PHARM MLBND 54838-0125-80 0.01790 BROTAPP LIQUID 0 SILARX PHARM MLGEN 00536-4742-97 0.03531 CAL-GEST 500 MG TABLET CHEW 0 RUGBY EAGEN 00182-4141-26 0.04140 CALCARB 600 WITH VIT D TAB 0 IVAX PHARMACEUT EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5272-60 0.05230 CALCITRATE + VIT D CAPLET 0 MAJOR PHARMACEU EAGEN 00904-5<strong>06</strong>2-60 0.05230 CALCITRATE 200 MG (950 MG) TAB 0 MAJOR PHARMACEU EAGEN 16103-0369-07 0.04140 CALCIUM + VITAMIN D TABLET 0 PHARBEST PHARMA EAGEN 16103-0369-11 0.04140 CALCIUM + VITAMIN D TABLET 0 PHARBEST PHARMA EAGEN 00113-0595-23 0.02190 CALCIUM ANTACID 1,000 MG TAB 0 PERRIGO CO. EAGEN 37205-0333-69 0.02190 CALCIUM ANTACID 1,000 MG TAB 0 LEADER EAGEN 00113-0485-47 0.03531 CALCIUM ANTACID 500 MG CHW TAB 0 PERRIGO CO. EAGEN 00904-1257-92 0.03531 CALCIUM ANTACID 500 MG CHW TAB 0 MAJOR PHARMACEU EAGEN 00904-1258-92 0.03531 CALCIUM ANTACID 500 MG CHW TAB 0 MAJOR PHARMACEU EAGEN 36800-0485-47 0.03531 CALCIUM ANTACID 500 MG CHW TAB 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00054-3117-63 0.02<strong>06</strong>2 CALCIUM CARB 1,250 MG/5 ML SUS 0 ROXANE LABS. MLGEN 00121-0766-16 0.02<strong>06</strong>2 CALCIUM CARB 1,250 MG/5 ML SUS 0 PHARMACEU ASSOC MLGEN 00121-4766-05 0.02<strong>06</strong>2 CALCIUM CARB 1,250 MG/5 ML SUS 0 PHARMACEU ASSOC MLGEN 00054-4120-25 0.04138 CALCIUM CARB 500 (1,250) MG TB 0 ROXANE LABS. EAGEN 00054-8120-25 0.04138 CALCIUM CARBONATE 1.25 GM TAB 0 ROXANE LABS. EABND 00536-3414-10 0.01499 CALCIUM CARBONATE 648 MG TAB 0 RUGBY EABND 00054-0262-20 0.05230 CALCIUM GLUCONATE 500 MG TAB 0 ROXANE LABS. EABND 00054-0262-25 0.05230 CALCIUM GLUCONATE 500 MG TAB 0 ROXANE LABS. EAGEN 00904-3238-52 0.05230 CALCIUM 500 MG CHEWABLE TABLET 0 MAJOR PHARMACEU EAGEN 37205-0392-87 0.04140 CALCIUM 600 + VIT D CAPLET 0 LEADER EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-<strong>08</strong>29-72 0.04140 CALCIUM 600 + VIT D TABLET 0 LEADER EAGEN 00904-5856-52 0.04140 CALCIUM 600 + VIT D 200 TABLET 0 MAJOR PHARMACEU EAGEN 00904-5856-92 0.04140 CALCIUM 600 + VIT D 200 TABLET 0 MAJOR PHARMACEU EAGEN 00904-3233-52 0.04140 CALCIUM 600 + VIT D 400 TABLET 0 MAJOR PHARMACEU EAGEN 00904-3233-92 0.04140 CALCIUM 600 + VIT D 400 TABLET 0 MAJOR PHARMACEU EAGEN 00904-3233-93 0.04140 CALCIUM 600 + VIT D 400 TABLET 0 MAJOR PHARMACEU EAGEN 00904-3232-52 0.04140 CALCIUM 600 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-3232-61 0.04140 CALCIUM 600 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-3232-92 0.04140 CALCIUM 600 MG TABLET 0 MAJOR PHARMACEU EAGEN 16103-0368-07 0.04140 CALCIUM 600 MG TABLET 0 PHARBEST PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62107-0042-<strong>06</strong> 0.04140 CALCIUM 600 MG TABLET 0 PRIME MARKETING EABND 00005-5509-24 0.04140 CALTRATE 600 + D TABLET 0 WYETH CONSUMER EABND 00005-5509-25 0.04140 CALTRATE 600 + D TABLET 0 WYETH CONSUMER EABND 00005-5567-19 0.05230 CALTRATE 600+D PLUS TAB CHEW 0 WYETH CONSUMER EAGEN 53329-0079-64 0.11529 CARRINGTON ANTIFUNGAL 2% CREAM 0 MEDLINE INDUS. GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 484LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 0<strong>08</strong>84-2893-01 0.73356 CASTELLANI PAINT MODIFIED 0 VALEANT MLBND 0<strong>08</strong>84-2993-01 0.73356 CASTELLANI PAINT MODIFIED 0 VALEANT MLGEN 54838-0010-70 0.01880 CENTAMIN LIQUID 0 SILARX PHARM MLBND 00005-4528-35 0.02950 CENTRUM CHEWABLE TABLET 0 WYETH CONSUMER EABND 00904-5486-52 0.05040 CERTAVITE SR-ANTIOXIDANT TAB 0 MAJOR PHARMACEU EABND 00904-5486-89 0.05040 CERTAVITE SR-ANTIOXIDANT TAB 0 MAJOR PHARMACEU EAGEN 00904-5023-09 0.01880 CERTAVITE-ANTIOXIDANT LIQUID 0 MAJOR PHARMACEU MLGEN 00904-2641-13 0.05040 CERTAVITE-ANTIOXIDANT TABLET 0 MAJOR PHARMACEU EAGEN 00904-2641-46 0.05040 CERTAVITE-ANTIOXIDANT TABLET 0 MAJOR PHARMACEU EAGEN 00904-2641-72 0.05040 CERTAVITE-ANTIOXIDANT TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-2<strong>08</strong>8-<strong>08</strong> 0.05700 CETIRIZINE HCL 1 MG/ML SOLN 0 TARO PHARM USA MLGEN 45802-0974-26 0.05700 CETIRIZINE HCL 1 MG/ML SYRUP 0 PERRIGO CO. MLGEN 51672-2102-<strong>08</strong> 0.05700 CETIRIZINE HCL 1 MG/1 ML SOLN 0 TARO PHARM USA MLGEN 00378-3637-01 0.12903 CETIRIZINE HCL 10 MG TABLET 0 MYLAN EAGEN 00378-3637-05 0.12903 CETIRIZINE HCL 10 MG TABLET 0 MYLAN EAGEN 00536-4<strong>08</strong>8-07 0.12903 CETIRIZINE HCL 10 MG TABLET 0 RUGBY EAGEN 00536-4<strong>08</strong>8-11 0.12903 CETIRIZINE HCL 10 MG TABLET 0 RUGBY EAGEN 00536-4<strong>08</strong>8-88 0.12903 CETIRIZINE HCL 10 MG TABLET 0 RUGBY EAGEN 00781-1684-01 0.12903 CETIRIZINE HCL 10 MG TABLET 0 SANDOZ EAGEN 00904-5852-61 0.12903 CETIRIZINE HCL 10 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16571-0402-10 0.12903 CETIRIZINE HCL 10 MG TABLET 0 PACK PHARMACEUT EAGEN 16571-0402-50 0.12903 CETIRIZINE HCL 10 MG TABLET 0 PACK PHARMACEUT EAGEN 16714-0271-02 0.12903 CETIRIZINE HCL 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 16714-0271-03 0.12903 CETIRIZINE HCL 10 MG TABLET 0 NORTHSTAR RX LL EAGEN 45802-0919-39 0.12903 CETIRIZINE HCL 10 MG TABLET 0 PERRIGO CO. EAGEN 45802-0919-87 0.12903 CETIRIZINE HCL 10 MG TABLET 0 PERRIGO CO. EAGEN 51079-0597-01 0.12903 CETIRIZINE HCL 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 51079-0597-20 0.12903 CETIRIZINE HCL 10 MG TABLET 0 MYLAN INSTITUTI EAGEN 51660-0938-30 0.12903 CETIRIZINE HCL 10 MG TABLET 0 OHM LABS. EAGEN 51660-0938-31 0.12903 CETIRIZINE HCL 10 MG TABLET 0 OHM LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51660-0938-54 0.12903 CETIRIZINE HCL 10 MG TABLET 0 OHM LABS. EAGEN 51660-0938-90 0.12903 CETIRIZINE HCL 10 MG TABLET 0 OHM LABS. EAGEN 55111-<strong>06</strong>99-90 0.12903 CETIRIZINE HCL 10 MG TABLET 0 DR.REDDY'S LAB EAGEN 60505-2633-01 0.12903 CETIRIZINE HCL 10 MG TABLET 0 APOTEX CORP EAGEN 60505-2633-<strong>08</strong> 0.12903 CETIRIZINE HCL 10 MG TABLET 0 APOTEX CORP EAGEN 00378-3635-01 0.16300 CETIRIZINE HCL 5 MG TABLET 0 MYLAN EAGEN 00781-1683-01 0.16300 CETIRIZINE HCL 5 MG TABLET 0 SANDOZ EAGEN 16571-0401-10 0.16300 CETIRIZINE HCL 5 MG TABLET 0 PACK PHARMACEUT EAGEN 60505-2632-01 0.16300 CETIRIZINE HCL 5 MG TABLET 0 APOTEX CORP EAGEN 68094-0720-59 0.<strong>08</strong>924 CETIRIZINE HCL 5 MG/5 ML SOLN G PRECISION DOSE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68094-0720-62 0.<strong>08</strong>924 CETIRIZINE HCL 5 MG/5 ML SOLN G PRECISION DOSE MLBND 00121-4780-05 0.<strong>08</strong>924 CETIRIZINE HCL 5 MG/5 ML SYRUP G PHARMACEU ASSOC MLGEN 45802-0721-62 0.61830 CETIRIZINE-PSE ER 5-120 MG TAB G PERRIGO CO. EAGEN 00395-2662-16 0.03530 CHERRY SYRUP 0 HUMCO LAB. MLGEN 37205-<strong>08</strong>74-71 0.20760 CHEST CONGESTION RELIEF PE 0 LEADER EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 485LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24385-<strong>06</strong>02-71 0.15291 CHEST CONGESTION RELIEF TAB 0 AMERISOURCEBERG EAGEN 37205-0476-71 0.15291 CHEST CONGESTION RELIEF TABLET 0 LEADER EAGEN 24385-0026-71 0.02680 CHEST CONGST-COUGH RELIEF TAB 0 AMERISOURCEBERG EAGEN 24385-0925-71 0.20760 CHEST-SINUS CONGST RLF TABLET 0 AMERISOURCEBERG EAGEN 00113-0974-26 0.05700 CHILD ALL DAY ALLERGY 1 MG/ML 0 PERRIGO CO. MLGEN 00904-5828-20 0.05700 CHILD ALL DAY ALLERGY 1 MG/ML 0 MAJOR PHARMACEU MLGEN 24385-0188-26 0.05700 CHILD ALL DAY ALLERGY 1 MG/ML 0 AMERISOURCEBERG MLGEN 36800-0974-26 0.05700 CHILD ALL DAY ALLERGY 1 MG/ML 0 TOPCO MLGEN 46122-0020-26 0.05700 CHILD ALL DAY ALLERGY 1 MG/ML 0 AMERISOURCEBERG MLGEN 0<strong>06</strong>03-0024-36 0.04820 CHILD ASPIRIN 81 MG CHEW TAB 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24385-0278-68 0.04820 CHILD ASPIRIN 81 MG CHEW TAB 0 AMERISOURCEBERG EAGEN 37205-0467-68 0.04820 CHILD ASPIRIN 81 MG CHEW TAB 0 LEADER EAGEN 62107-0026-36 0.04820 CHILD ASPIRIN 81 MG CHEW TAB 0 PRIME MARKETING EAGEN 47335-0344-83 CHILD CETIRIZINE 10 MG CHEW TB G SUN PHARMA GLOB EAGEN 00904-0536-60 0.02950 CHILD CHEW + IRON TAB CHEW 0 MAJOR PHARMACEU EAGEN 00904-0536-70 0.02950 CHILD CHEW + IRON TAB CHEW 0 MAJOR PHARMACEU EAGEN 00904-2621-60 0.02950 CHILD CHEW VITAMIN TABLET 0 MAJOR PHARMACEU EAGEN 50383-<strong>06</strong>27-50 0.10<strong>08</strong>0 CHILD FERROUS SULFATE 15 MG/ML 0 HI-TECH PHARMAC MLGEN 36800-0579-53 0.44527 CHILD FEVER REDUCER 120 MG SUP 0 TOPCO EAGEN 37205-0282-26 0.04567 CHILD IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 LEADER ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-2092-<strong>08</strong> 0.04749 CHILD LORATADINE 5 MG/5 ML SYR 0 TARO PHARM USA MLGEN 00113-0419-26 0.02680 CHILD MUCUS RELIEF COUGH LIQ 0 PERRIGO CO. MLGEN 24385-0985-26 0.02680 CHILD MUCUS RELIEF COUGH LIQ 0 AMERISOURCEBERG MLGEN 36800-0419-26 0.02680 CHILD MUCUS RELIEF COUGH LIQ 0 TOPCO MLGEN 37205-0993-26 0.02680 CHILD MUCUS RELIEF COUGH LIQ 0 LEADER MLGEN 00113-0212-26 0.02330 CHILD PAIN & FEVER 160 MG/5 ML 0 PERRIGO CO. MLBND 00536-0122-85 0.02044 CHILD PAIN & FEVER 160 MG/5 ML 0 RUGBY MLBND 00536-0122-97 0.02044 CHILD PAIN & FEVER 160 MG/5 ML 0 RUGBY MLGEN 37205-05<strong>08</strong>-26 0.02330 CHILD PAIN & FEVER 160 MG/5 ML 0 LEADER MLGEN 37205-0518-26 0.02330 CHILD PAIN & FEVER 160 MG/5 ML 0 LEADER ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0717-26 0.02330 CHILD PAIN & FEVER 160 MG/5 ML 0 LEADER MLGEN 00113-0105-26 0.02330 CHILD PAIN RLF 160 MG/5 ML SUS 0 PERRIGO CO. MLGEN 36800-0105-26 0.02330 CHILD PAIN RLF 160 MG/5 ML SUS 0 TOPCO MLGEN 36800-0130-26 0.02330 CHILD PAIN RLF 160 MG/5 ML SUS 0 TOPCO MLGEN 36800-0175-26 0.02330 CHILD PAIN RLF 160 MG/5 ML SUS 0 TOPCO MLGEN 46122-0019-26 0.02330 CHILD PAIN RLF 160 MG/5 ML SUS 0 AMERISOURCEBERG MLGEN 00536-3233-07 0.07070 CHILD PAIN-FEVER 80 MG TAB CHW 0 RUGBY EAGEN 62107-0053-30 0.07070 CHILD TACTINAL 80 MG TAB CHW 0 PRIME MARKETING EAGEN 00<strong>06</strong>7-6431-04 0.01816 CHILD TRIAMINIC FEVER SYRUP 0 NOVARTIS CONSUM MLGEN 62107-0048-01 0.02950 CHILD VITAMIN-IRON TAB CHEW 0 PRIME MARKETING EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62107-0048-10 0.02950 CHILD VITAMIN-IRON TAB CHEW 0 PRIME MARKETING EAGEX 36800-0379-26 0.02118 CHILD'S ALLERGY 12.5 MG/5 ML 0 TOPCO MLGEX 36800-0379-34 0.02118 CHILD'S ALLERGY 12.5 MG/5 ML 0 TOPCO MLBND 11523-4328-01 0.85000 CHILD'S CLARITIN 5 MG TAB CHEW 0 MSD CONSUMER CA EABND 11523-4328-02 0.85000 CHILD'S CLARITIN 5 MG TAB CHEW 0 MSD CONSUMER CA EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 486LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 11523-4328-03 0.85000 CHILD'S CLARITIN 5 MG TAB CHEW 0 MSD CONSUMER CA EABND 11523-7198-01 0.85000 CHILD'S CLARITIN 5 MG TAB CHEW 0 MSD CONSUMER CA EABND 11523-7198-04 0.85000 CHILD'S CLARITIN 5 MG TAB CHEW 0 MSD CONSUMER CA EAGEN 63824-0280-64 0.01900 CHILD'S MUCINEX 100 MG/5 ML LQ 0 RECKITT BENCKIS MLGEN 37205-0516-65 0.07070 CHILD'S PAIN RELIEVER RAPID TB 0 LEADER EAGEN 24385-0105-26 0.02330 CHILD'S PAIN RELIEVER SUSP 0 AMERISOURCEBERG MLGEN 24385-0130-26 0.02330 CHILD'S PAIN RELIEVER SUSP 0 AMERISOURCEBERG MLGEN 24385-0146-26 0.02330 CHILD'S PAIN RELIEVER SUSP 0 AMERISOURCEBERG MLGEN 00113-0166-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 PERRIGO CO. MLGEN 00113-<strong>06</strong>60-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 PERRIGO CO. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00113-<strong>08</strong>97-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 PERRIGO CO. MLGEN 00113-<strong>08</strong>97-34 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 PERRIGO CO. MLGEN 00904-5309-09 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 MAJOR PHARMACEU MLGEN 00904-5309-20 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 MAJOR PHARMACEU MLGEN 00904-5577-20 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 MAJOR PHARMACEU MLGEN 24385-0361-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 AMERISOURCEBERG MLGEN 24385-0361-34 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 AMERISOURCEBERG MLGEN 24385-0372-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 AMERISOURCEBERG MLGEN 24385-0905-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 AMERISOURCEBERG MLGEN 24385-0905-34 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 AMERISOURCEBERG ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0283-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 LEADER MLGEN 37205-<strong>06</strong>43-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 LEADER MLGEN 37205-<strong>06</strong>60-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 LEADER MLGEN 37205-<strong>08</strong>48-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 LEADER MLGEN 45802-<strong>08</strong>97-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 PERRIGO CO. MLGEN 46122-0110-26 0.04567 CHILDREN IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 AMERISOURCEBERG MLGEN 00573-0171-30 0.04567 CHILDREN'S ADVIL 100 MG/5 ML 0 WYETH CONSUMER MLGEN 00573-0174-30 0.04567 CHILDREN'S ADVIL 100 MG/5 ML 0 WYETH CONSUMER MLGEN 00573-0290-01 0.04567 CHILDREN'S ADVIL 100 MG/5 ML 0 WYETH CONSUMER MLGEN 36800-0987-26 0.02680 CHILDREN'S COLD & COUGH ELIXIR 0 TOPCO ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00113-09<strong>06</strong>-26 0.02140 CHILDREN'S COLD-ALLERGY ELIXIR 0 PERRIGO CO. MLGEN 36800-09<strong>06</strong>-26 0.02140 CHILDREN'S COLD-ALLERGY ELIXIR 0 TOPCO MLBND 00904-5791-46 0.07070 CHILDREN'S MAPAP 80 MG RAPID 0 MAJOR PHARMACEU EABND 63824-0274-64 0.01900 CHILDREN'S MUCINEX COUGH LIQ 0 RECKITT BENCKIS MLBND 63824-0281-64 0.02680 CHILDREN'S MUCINEX COUGH LIQ 0 RECKITT BENCKIS MLGEN 00113-0288-26 0.02680 CHILDREN'S MUCUS RELIEF LIQ 0 PERRIGO CO. MLGEN 36800-0288-26 0.01900 CHILDREN'S MUCUS RELIEF LIQ 0 TOPCO MLGEN 54838-0144-40 0.01816 CHILDREN'S SILAPAP ELIXIR 0 SILARX PHARM MLGEN 54838-0144-70 0.01816 CHILDREN'S SILAPAP ELIXIR 0 SILARX PHARM MLGEN 54838-0144-80 0.01816 CHILDREN'S SILAPAP ELIXIR 0 SILARX PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 54838-0104-40 0.01827 CHILDREN'S SILFEDRINE LIQ 0 SILARX PHARM MLGEN 54838-0104-70 0.01827 CHILDREN'S SILFEDRINE LIQ 0 SILARX PHARM MLGEN 62107-0047-01 0.02950 CHILDRENS CHEW VITAMIN TAB 0 PRIME MARKETING EAGEN 62107-0047-10 0.02950 CHILDRENS CHEW VITAMIN TAB 0 PRIME MARKETING EABND 00135-0<strong>08</strong>9-69 0.01970 CITRUCEL POWDER 0 GSK CONSUMER HE GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 487LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00135-0<strong>08</strong>9-71 0.01970 CITRUCEL POWDER 0 GSK CONSUMER HE GMBND 00<strong>06</strong>8-0420-17 0.01970 CITRUCEL POWDER S-F 0 GSK CONSUMER HE GMBND 00135-0090-70 0.01970 CITRUCEL POWDER S-F 0 GSK CONSUMER HE GMBND 00135-0090-74 0.01970 CITRUCEL POWDER S-F 0 GSK CONSUMER HE GMBND 11523-7160-01 0.09890 CLARITIN 10 MG TABLET 0 MSD CONSUMER CA EABND 11523-7160-02 0.09890 CLARITIN 10 MG TABLET 0 MSD CONSUMER CA EABND 11523-7160-03 0.09890 CLARITIN 10 MG TABLET 0 MSD CONSUMER CA EABND 11523-7160-05 0.09890 CLARITIN 10 MG TABLET 0 MSD CONSUMER CA EABND 11523-7237-01 0.09890 CLARITIN 10 MG TABLET 0 MSD CONSUMER CA EABND 11523-7237-03 0.09890 CLARITIN 10 MG TABLET 0 MSD CONSUMER CA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 11523-7237-05 0.09890 CLARITIN 10 MG TABLET 0 MSD CONSUMER CA EABND 11523-7237-<strong>06</strong> 0.09890 CLARITIN 10 MG TABLET 0 MSD CONSUMER CA EABND 11523-7163-01 0.04749 CLARITIN 5 MG/5 ML SYRUP 0 S-P <strong>HEALTH</strong>CARE MLBND 11523-7197-01 0.04749 CLARITIN 5 MG/5 ML SYRUP 0 S-P <strong>HEALTH</strong>CARE MLBND 11523-7162-01 0.56431 CLARITIN-D 12 HOUR TABLET G S-P <strong>HEALTH</strong>CARE EABND 11523-7162-03 0.56431 CLARITIN-D 12 HOUR TABLET G S-P <strong>HEALTH</strong>CARE EABND 11523-4332-01 0.61380 CLARITIN-D 24 HOUR TABLET G MSD CONSUMER CA EABND 11523-4332-02 0.61380 CLARITIN-D 24 HOUR TABLET G MSD CONSUMER CA EABND 11523-7161-01 0.61380 CLARITIN-D 24 HOUR TABLET G S-P <strong>HEALTH</strong>CARE EABND 11523-7161-02 0.61380 CLARITIN-D 24 HOUR TABLET G S-P <strong>HEALTH</strong>CARE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0181-04 0.03193 CLEARLAX POWDER 0 TOPCO GMGEN 36800-03<strong>06</strong>-01 0.03193 CLEARLAX POWDER 0 TOPCO GMGEN 36800-03<strong>06</strong>-02 0.03193 CLEARLAX POWDER 0 TOPCO GMGEN 36800-03<strong>06</strong>-03 0.03193 CLEARLAX POWDER 0 TOPCO GMGEN 36800-03<strong>06</strong>-04 0.03193 CLEARLAX POWDER 0 TOPCO GMGEN 37205-<strong>06</strong>12-71 0.03193 CLEARLAX POWDER 0 LEADER GMGEN 37205-<strong>06</strong>12-72 0.03193 CLEARLAX POWDER 0 LEADER GMGEN 37205-<strong>06</strong>12-73 0.03193 CLEARLAX POWDER 0 LEADER GMGEN 46122-0014-33 0.03193 CLEARLAX POWDER 0 AMERISOURCEBERG GMGEN 46122-0014-71 0.03193 CLEARLAX POWDER 0 AMERISOURCEBERG GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-7822-36 0.11290 CLOTRIM ANTIFUNGAL CREAM 0 MAJOR PHARMACEU GMGEN 51672-2003-<strong>06</strong> 0.12054 CLOTRIM 1% VAGINAL CREAM 0 TARO PHARM USA GMGEN 00904-7822-31 0.11290 CLOTRIMAZOLE 1% CREAM 0 MAJOR PHARMACEU GMGEN 37205-0160-10 0.11290 CLOTRIMAZOLE 1% CREAM 0 LEADER GMGEN 45802-0434-01 0.11290 CLOTRIMAZOLE 1% CREAM 0 PERRIGO CO. GMGEN 45802-0434-11 0.11290 CLOTRIMAZOLE 1% CREAM 0 PERRIGO CO. GMGEN 51672-2002-01 0.11290 CLOTRIMAZOLE 1% CREAM 0 TARO PHARM USA GMGEN 51672-2002-02 0.11290 CLOTRIMAZOLE 1% CREAM 0 TARO PHARM USA GMBND 0<strong>06</strong>03-0728-54 0.02680 CODITUSS DM SYRUP 0 QUALITEST MLGEN 24385-0517-26 0.02160 COLD & ALLERGY ELIXIR 0 AMERISOURCEBERG ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24385-0517-34 0.02160 COLD & ALLERGY ELIXIR 0 AMERISOURCEBERG MLGEN 37205-0931-26 0.02140 COLD & ALLERGY ELIXIR 0 LEADER MLGEN 24385-0924-62 0.10098 COLD & ALLERGY PE TABLET 0 AMERISOURCEBERG EAGEN 24385-0519-26 0.02680 COLD & COUGH ELIXIR 0 AMERISOURCEBERG MLBND 24385-0195-62 0.20760 COLD HEAD CONGESTION CAPLET 0 AMERISOURCEBERG EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 488LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 37205-0958-62 0.20760 COLD HEAD CONGESTION CAPLET 0 LEADER EAGEN 10267-1007-<strong>06</strong> 0.09827 COLD, ALLERGY & SINUS TABLET 0 CONTRACT PHARM EAGEX 37205-0277-62 0.03330 COMPLETE ALLERGY 25 MG CAP 0 LEADER EAGEX 37205-0277-78 0.03330 COMPLETE ALLERGY 25 MG CAP 0 LEADER EAGEX 37205-0270-62 0.03330 COMPLETE ALLERGY 25 MG TAB 0 LEADER EAGEX 37205-0270-78 0.03330 COMPLETE ALLERGY 25 MG TAB 0 LEADER EAGEN 62107-0041-13 0.05040 COMPLETE MULTIVITAMIN TAB 0 PRIME MARKETING EAGEN 62107-0045-<strong>06</strong> 0.05400 COMPLETE SENIOR TABLET 0 PRIME MARKETING EAGEN 00225-0580-<strong>06</strong> 0.20760 CONGESTAC TABLET 0 B.F ASCHER & CO EAGEN 00225-0580-<strong>08</strong> 0.20760 CONGESTAC TABLET 0 B.F ASCHER & CO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00193-7151-01 16.50000 CONTOUR METER 0 BAYER <strong>HEALTH</strong>CAR EAGEN 00193-7252-01 16.50000 CONTOUR NEXT EZ METER 0 BAYER <strong>HEALTH</strong>CAR EAGEN 00193-7377-01 16.50000 CONTOUR NEXT METER 0 BAYER <strong>HEALTH</strong>CAR EAGEN 00193-7311-50 0.72600 CONTOUR NEXT STRIPS 0 BAYER <strong>HEALTH</strong>CAR EAGEN 00193-7312-21 0.72600 CONTOUR NEXT STRIPS 0 BAYER <strong>HEALTH</strong>CAR EAGEN 00193-7411-01 26.40000 CONTOUR NEXT USB METER 0 BAYER <strong>HEALTH</strong>CAR EAGEN 00193-7<strong>08</strong>0-50 1.14400 CONTOUR TEST STRIPS 0 BAYER <strong>HEALTH</strong>CAR EAGEN 00193-7090-21 1.07250 CONTOUR TEST STRIPS 0 BAYER <strong>HEALTH</strong>CAR EAGEN 00193-7393-01 26.40000 CONTOUR USB METER 0 BAYER <strong>HEALTH</strong>CAR EAGEN 00904-5817-44 0.02760 COUGH & COLD TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00536-0970-97 0.01624 COUGH SYRUP 0 RUGBY MLBND 00536-<strong>08</strong>25-97 0.01900 COUGH SYRUP 100 MG/5 ML 0 RUGBY MLGEN 37205-0466-72 0.10221 COUGHTAB 200 MG TABLET 0 LEADER EAGEN 57782-0397-26 0.44653 CROMOLYN SODIUM NASAL SPRAY 0 VALEANT MLGEN 00904-3865-75 0.07570 DEEP SEA 0.65% NOSE SPRAY 0 MAJOR PHARMACEU MLGEN 54838-0138-40 0.01900 DIABETIC SILTUSSIN DAS-NA LIQ 0 SILARX PHARM MLGEN 54838-0139-40 0.02680 DIABETIC SILTUSSIN-DM LIQUID 0 SILARX PHARM MLGEN 00904-5782-20 0.02680 DIMAPHEN DM ELIXIR 0 MAJOR PHARMACEU MLGEN 00904-5781-09 0.02160 DIMAPHEN ELIXIR 0 MAJOR PHARMACEU MLGEN 00904-5781-20 0.02160 DIMAPHEN ELIXIR 0 MAJOR PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-3327-21 0.05538 DIMENHYDRINATE 50 MG TABLET 0 QUALITEST EABND 00031-2235-13 0.02140 DIMETAPP COLD & ALLERGY ELIXIR 0 WYETH CONSUMER MLBND 00031-2235-19 0.02140 DIMETAPP COLD & ALLERGY ELIXIR 0 WYETH CONSUMER MLGEN 00031-2247-13 0.02680 DIMETAPP COLD & CONGEST LIQUID 0 WYETH CONSUMER MLBND 00031-2234-19 0.02680 DIMETAPP COLD & COUGH LIQUID 0 WYETH CONSUMER MLBND 00031-2234-18 0.02140 DIMETAPP DM COLD & COUGH ELIX 0 WYETH CONSUMER MLBND 00031-2238-13 0.02680 DIMETAPP LONG-ACTING COUGH LIQ 0 WYETH CONSUMER MLBND 00536-0590-85 0.01200 DIOCTO 50 MG/5 ML LIQUID 0 RUGBY MLBND 00536-<strong>06</strong>00-85 0.00990 DIOCTO 60 MG/15 ML SYRUP 0 RUGBY MLBND 00536-1001-85 0.00990 DIOCTO 60 MG/15 ML SYRUP 0 RUGBY ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 24385-0462-78 0.03330 DIPHEDRYL ALLERGY CAPSULE 0 AMERISOURCEBERG EAGEX 00536-0770-85 0.02118 DIPHENHIST 12.5 MG/5 ML SOLN 0 RUGBY MLGEX 00536-0770-97 0.02118 DIPHENHIST 12.5 MG/5 ML SOLN 0 RUGBY MLBEX 00536-3594-01 0.03330 DIPHENHIST 25 MG CAPSULE 0 RUGBY EAGEX 00185-<strong>06</strong>48-01 0.03330 DIPHENHYDRAMINE 25 MG CAPSULE 0 SANDOZ EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 489LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 00185-<strong>06</strong>48-10 0.03330 DIPHENHYDRAMINE 25 MG CAPSULE 0 SANDOZ EAGEX 0<strong>06</strong>03-3339-21 0.03330 DIPHENHYDRAMINE 25 MG CAPSULE 0 QUALITEST EAGEX 0<strong>06</strong>03-3339-32 0.03330 DIPHENHYDRAMINE 25 MG CAPSULE 0 QUALITEST EAGEX 00904-53<strong>06</strong>-61 0.03330 DIPHENHYDRAMINE 25 MG CAPSULE 0 MAJOR PHARMACEU EAGEX 10267-<strong>08</strong>35-01 0.03330 DIPHENHYDRAMINE 25 MG CAPSULE 0 CONTRACT PHARM EAGEX 10267-<strong>08</strong>35-04 0.03330 DIPHENHYDRAMINE 25 MG CAPSULE 0 CONTRACT PHARM EAGEX 00185-<strong>06</strong>49-01 0.<strong>08</strong>136 DIPHENHYDRAMINE 50 MG CAPSULE 0 SANDOZ EAGEX 00185-<strong>06</strong>49-10 0.05241 DIPHENHYDRAMINE 50 MG CAPSULE 0 SANDOZ EAGEX 0<strong>06</strong>03-3340-21 0.<strong>08</strong>136 DIPHENHYDRAMINE 50 MG CAPSULE 0 QUALITEST EAGEX 0<strong>06</strong>03-3340-32 0.<strong>08</strong>136 DIPHENHYDRAMINE 50 MG CAPSULE 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 10267-<strong>08</strong>36-01 0.03780 DIPHENHYDRAMINE 50 MG CAPSULE 0 CONTRACT PHARM EAGEX 10267-<strong>08</strong>36-04 0.01890 DIPHENHYDRAMINE 50 MG CAPSULE 0 CONTRACT PHARM EAGEN 0<strong>06</strong>03-0150-21 0.03020 DOC-Q-LACE 100 MG S<strong>OF</strong>TGEL 0 QUALITEST EAGEN 0<strong>06</strong>03-0150-32 0.03020 DOC-Q-LACE 100 MG S<strong>OF</strong>TGEL 0 QUALITEST EAGEN 0<strong>06</strong>03-0747-58 0.00990 DOC-Q-LACE 60 MG/15 ML SYRUP 0 QUALITEST MLGEN 0<strong>06</strong>03-0149-21 0.04150 DOC-Q-LAX TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-0149-32 0.04150 DOC-Q-LAX TABLET 0 QUALITEST EAGEN 50383-0771-10 0.01200 DOCU LIQUID 100 MG/10 ML 0 HI-TECH PHARMAC MLGEN 50383-0771-11 0.01200 DOCU LIQUID 100 MG/10 ML 0 HI-TECH PHARMAC MLGEN 50383-0771-16 0.01200 DOCU LIQUID 50 MG/5 ML 0 HI-TECH PHARMAC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24385-0435-78 0.05000 DOCUSATE CAL 240 MG S<strong>OF</strong>TGEL 0 AMERISOURCEBERG EAGEN 00536-3756-01 0.03020 DOCUSATE SODIUM 100 MG CAPSULE 0 RUGBY EAGEN 00536-3756-10 0.03020 DOCUSATE SODIUM 100 MG CAPSULE 0 RUGBY EAGEN 63739-0478-01 0.03020 DOCUSATE SODIUM 100 MG CAPSULE 0 MCKESSON PACKAG EAGEN 63739-0478-10 0.03020 DOCUSATE SODIUM 100 MG CAPSULE 0 MCKESSON PACKAG EAGEN 16103-0384-11 0.03020 DOCUSATE SODIUM 100 MG S<strong>OF</strong>TGEL 0 PHARBEST PHARMA EAGEN 16103-0384-99 0.03020 DOCUSATE SODIUM 100 MG S<strong>OF</strong>TGEL 0 PHARBEST PHARMA EAGEN 45802-0486-78 0.03020 DOCUSATE SODIUM 100 MG S<strong>OF</strong>TGEL 0 PERRIGO CO. EAGEN 62584-<strong>06</strong>83-11 0.03020 DOCUSATE SODIUM 100 MG S<strong>OF</strong>TGEL 0 AHP EAGEN 00121-0544-10 0.01200 DOCUSATE SODIUM 50 MG/5 ML LIQ 0 PHARMACEU ASSOC ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62107-0033-01 0.03020 DOCUSIL 100 MG S<strong>OF</strong>TGEL 0 PRIME MARKETING EAGEN 62107-0033-10 0.03020 DOCUSIL 100 MG S<strong>OF</strong>TGEL 0 PRIME MARKETING EABND 17433-9878-05 2.99000 DOCUSOL MINI-ENEMA 0 ALLIANCE LABS, EABND 00904-2244-61 0.03020 DOK 100 MG CAPSULE 0 MAJOR PHARMACEU EABND 00904-7889-59 0.03020 DOK 100 MG CAPSULE 0 MAJOR PHARMACEU EABND 00904-7889-60 0.03020 DOK 100 MG CAPSULE 0 MAJOR PHARMACEU EABND 00904-7889-80 0.03020 DOK 100 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 00904-5869-60 0.03520 DOK 100 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-2051-12 0.05538 DRIMINATE 50 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-2051-59 0.05538 DRIMINATE 50 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00024-0391-02 1.33366 DRISDOL 8,000 UNITS/ML DROPS 0 SAN<strong>OF</strong>I-AVENTIS MLBND 00573-1191-20 0.13520 DRISTAN LONG LASTING MIST 0 WYETH CONSUMER MLGEN 62107-0030-01 0.03100 DUCODYL EC 5 MG TABLET 0 PRIME MARKETING EAGEN 62107-0030-10 0.03100 DUCODYL EC 5 MG TABLET 0 PRIME MARKETING EABND 00485-0023-01 0.05040 ECEE PLUS TABLET 0 EDWARDS PHARMAC EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 490LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00135-0014-27 0.02128 ECOTRIN EC 325 MG TABLET 0 GSK CONSUMER HE EAGEN 00135-0117-01 0.02781 ECOTRIN EC 81 MG TABLET 0 GSK CONSUMER HE EAGEN 00135-0117-02 0.02781 ECOTRIN EC 81 MG TABLET 0 GSK CONSUMER HE EAGEN 00135-0117-88 0.02781 ECOTRIN EC 81 MG TABLET 0 GSK CONSUMER HE EAGEN 62107-0028-01 0.02128 ECPIRIN EC 325 MG TABLET 0 PRIME MARKETING EAGEN 62107-0028-32 0.02128 ECPIRIN EC 325 MG TABLET 0 PRIME MARKETING EAGEN 00485-0254-01 0.10098 ED-A-HIST 4 MG-10 MG TABLET 0 EDWARDS PHARMAC EAGEN 00259-0351-16 0.01880 ELDERTONIC ELIXIR 0 MERZ MLGEN 68047-0143-16 0.02140 ENDAC<strong>OF</strong>-DM LIQUID 0 LARKEN LABS MLGEN 00536-7415-51 0.0<strong>06</strong>21 ENEMA 0 RUGBY ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24385-0039-28 0.0<strong>06</strong>21 ENEMA 0 AMERISOURCEBERG MLGEN 49614-0331-27 0.0<strong>06</strong>21 ENEMA 0 MEDICINE SHOP MLGEN 49614-0331-28 0.0<strong>06</strong>21 ENEMA 0 MEDICINE SHOP MLBND 00904-6320-78 0.0<strong>06</strong>21 ENEMA READY TO USE 0 MAJOR PHARMACEU MLGEN 37205-0030-36 0.0<strong>06</strong>21 ENEMA TWIN PACK 0 LEADER MLBND 17433-9876-03 0.39593 ENEMEEZ MINI ENEMA 0 ALLIANCE LABS, MLBND 17433-9877-03 0.40489 ENEMEEZ PLUS MINI ENEMA 0 ALLIANCE LABS, MLBND 00<strong>08</strong>7-0265-24 0.00340 ENFALYTE NURSETTE SOLUTION 0 MJ NUTRITIONAL MLGEN 43199-0015-60 1.33366 ERGOCALCIFEROL ORAL SOLUTION 0 COUNTY LINE PHA MLGEN 76439-0234-60 1.33366 ERGOCALCIFEROL ORAL SOLUTION 0 VIRTUS PHARMACE ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>7-0016-90 0.03010 EX-LAX MAX RELIEF PILLS 0 NOVARTIS CONSUM EAGEN 00536-0970-85 0.01624 EXTRA ACTION COUGH SYRUP 0 RUGBY MLGEN 00172-2662-46 0.13110 FAMOTIDINE 10 MG TABLET 0 TEVA USA EAGEN 00172-2662-48 0.13110 FAMOTIDINE 10 MG TABLET 0 TEVA USA EAGEN 00172-2662-72 0.13110 FAMOTIDINE 10 MG TABLET 0 TEVA USA EAGEN 00904-0260-80 0.05040 FARBEE WITH C CAPLET 0 MAJOR PHARMACEU EABND 00<strong>08</strong>7-0740-02 0.10<strong>08</strong>0 FER-IN-SOL 15 MG/ML DROPS 0 MJ NUTRITIONAL MLBND 00904-5477-60 0.02170 FERATE 28 MG TABLET 0 MAJOR PHARMACEU EAGEN 00024-1015-10 0.02170 FERGON 27 MG TABLET 0 BAYER INC. EAGEN 00904-1465-16 0.01090 FEROSUL 220 MG/5 ML ELIXIR 0 MAJOR PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-7591-82 0.02170 FEROSUL 325 MG TABLET 0 MAJOR PHARMACEU EABND 0<strong>08</strong>13-0012-<strong>06</strong> 0.02170 FERRETTS 325 MG TABLET 0 PHARMICS EAGEN 51991-0203-11 0.19377 FERREX 150 CAPSULE 0 BRECKENRIDGE EABND 51991-0703-90 1.05600 FERREX 150 PLUS CAPSULE 0 BRECKENRIDGE EAGEN 49483-0<strong>06</strong>3-01 0.02170 FERRO-TIME 325 MG TABLET 0 TIME-CAP LABS EAGEN 51991-0181-42 0.02170 FERROCITE TABLET 0 BRECKENRIDGE EABND 00574-05<strong>08</strong>-01 0.07254 FERROUS GLUCONATE 324 MG TAB 0 PADDOCK LABS. EABND 00574-05<strong>08</strong>-10 0.07254 FERROUS GLUCONATE 324 MG TAB 0 PADDOCK LABS. EABND 00574-05<strong>08</strong>-11 0.07254 FERROUS GLUCONATE 324 MG TAB 0 PADDOCK LABS. EABND 00904-2137-61 0.02170 FERROUS GLUCONATE 324 MG TAB 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00574-<strong>06</strong><strong>08</strong>-01 0.02170 FERROUS SULF EC 324 MG TABLET 0 PADDOCK LABS. EABND 00574-<strong>06</strong><strong>08</strong>-10 0.02170 FERROUS SULF EC 324 MG TABLET 0 PADDOCK LABS. EABND 00574-<strong>06</strong><strong>08</strong>-11 0.02170 FERROUS SULF EC 324 MG TABLET 0 PADDOCK LABS. EABND 00245-01<strong>08</strong>-01 0.02170 FERROUS SULF EC 325 MG TABLET 0 UPSHER SMITH EABND 00245-01<strong>08</strong>-89 0.02170 FERROUS SULF EC 325 MG TABLET 0 UPSHER SMITH EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 491LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6<strong>06</strong>0-50 0.10<strong>08</strong>0 FERROUS SULF 15 MG IRON/ML DRP 0 MAJOR PHARMACEU MLGEN 54838-0011-50 0.10<strong>08</strong>0 FERROUS SULF 15 MG IRON/ML DRP 0 SILARX PHARM MLGEN 00536-<strong>06</strong>50-85 0.01090 FERROUS SULF 220 MG/5 ML ELIX 0 RUGBY MLGEN 0<strong>06</strong>03-0763-58 0.01090 FERROUS SULF 220 MG/5 ML ELIX 0 QUALITEST MLGEN 54838-0001-80 0.01090 FERROUS SULF 220 MG/5 ML ELIX 0 SILARX PHARM MLBND 00121-0530-05 0.01090 FERROUS SULF 300 MG/5 ML LIQ 0 PHARMACEU ASSOC MLGEN 0<strong>06</strong>03-0179-29 0.02170 FERROUS SULFATE 325 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>77-0070-01 0.02170 FERROUS SULFATE 325 MG TABLET 0 URL PHARMA EAGEN 0<strong>06</strong>77-0070-10 0.02170 FERROUS SULFATE 325 MG TABLET 0 URL PHARMA EAGEN 0<strong>06</strong>77-0071-01 0.02170 FERROUS SULFATE 325 MG TABLET 0 URL PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>77-0071-10 0.02170 FERROUS SULFATE 325 MG TABLET 0 URL PHARMA EAGEN 00904-7590-82 0.02170 FERROUS SULFATE 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7591-60 0.02170 FERROUS SULFATE 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7591-61 0.02170 FERROUS SULFATE 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7591-80 0.02170 FERROUS SULFATE 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 10267-0950-01 0.02170 FERROUS SULFATE 325 MG TABLET 0 CONTRACT PHARM EAGEN 10267-0950-04 0.02170 FERROUS SULFATE 325 MG TABLET 0 CONTRACT PHARM EAGEN 49483-0<strong>06</strong>3-10 0.02170 FERROUS SULFATE 325 MG TABLET 0 TIME-CAP LABS EAGEN 49483-0<strong>06</strong>4-10 0.02170 FERROUS SULFATE 325 MG TABLET 0 TIME-CAP LABS EAGEN 64376-<strong>08</strong>09-10 0.02170 FERROUS SULFATE 325 MG TABLET 0 BOCA PHARMACAL EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62107-0044-01 0.02170 FERROUSUL 325 MG TABLET 0 PRIME MARKETING EAGEN 00378-0782-05 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MYLAN EAGEN 00378-0782-93 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MYLAN EAGEN 00904-6214-18 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MAJOR PHARMACEU EAGEN 00904-6214-46 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MAJOR PHARMACEU EAGEN 00904-6214-48 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MAJOR PHARMACEU EAGEN 00904-6214-52 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MAJOR PHARMACEU EAGEN 00904-6214-89 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MAJOR PHARMACEU EAGEN 00904-6311-18 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MAJOR PHARMACEU EAGEN 00904-6311-46 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6311-52 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MAJOR PHARMACEU EAGEN 00904-6311-89 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MAJOR PHARMACEU EAGEN 36800-0571-13 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G TOPCO EAGEN 45802-0571-78 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G PERRIGO CO. EAGEN 51079-0548-01 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MYLAN INSTITUTI EAGEN 51079-0548-20 0.61666 FEX<strong>OF</strong>ENADINE HCL 180 MG TABLET G MYLAN INSTITUTI EAGEN 00378-0781-05 0.54333 FEX<strong>OF</strong>ENADINE HCL 60 MG TABLET G MYLAN EAGEN 00378-0781-91 0.54333 FEX<strong>OF</strong>ENADINE HCL 60 MG TABLET G MYLAN EAGEN 36800-0425-53 0.54333 FEX<strong>OF</strong>ENADINE HCL 60 MG TABLET G TOPCO EAGEN 45802-0425-78 0.54333 FEX<strong>OF</strong>ENADINE HCL 60 MG TABLET G PERRIGO CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51079-0547-20 0.54333 FEX<strong>OF</strong>ENADINE HCL 60 MG TABLET G MYLAN INSTITUTI EAGEN 36800-0477-75 0.09133 FIBER LAXATIVE 625 MG CAPLET 0 TOPCO EAGEN 37205-0213-75 0.09133 FIBER LAXATIVE 625 MG TABLET 0 LEADER EAGEN 00904-2500-91 0.09133 FIBER TABLET 0 MAJOR PHARMACEU EAGEN 24385-0125-76 0.09133 FIBER TABS 0 AMERISOURCEBERG EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 492LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00904-5675-16 0.01970 FIBER THERAPY POWDER 0 MAJOR PHARMACEU GMBND 00904-5788-77 0.01528 FIBER THERAPY POWDER 0 MAJOR PHARMACEU GMGEN 24385-0466-78 0.09983 FIBER THERAPY 500 MG CAPLET 0 AMERISOURCEBERG EAGEN 00536-43<strong>06</strong>-05 0.09133 FIBER-LAX CAPTABS 0 RUGBY EAGEN 00536-43<strong>06</strong>-<strong>08</strong> 0.09133 FIBER-LAX CAPTABS 0 RUGBY EAGEN 00536-43<strong>06</strong>-11 0.09133 FIBER-LAX CAPTABS 0 RUGBY EABND 00005-2500-02 0.09133 FIBERCON 625 MG CAPLET 0 WYETH CONSUMER EABND 00005-2500-33 0.09133 FIBERCON 625 MG CAPLET 0 WYETH CONSUMER EABND 00132-0704-02 0.03100 FLEET BISACODYL EC 5 MG TAB 0 FLEET,C.B. CO. EABND 00132-0703-36 0.03162 FLEET BISACODYL 10 MG ENEMA 0 FLEET,C.B. CO. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00132-0201-42 0.0<strong>06</strong>21 FLEET ENEMA 0 FLEET,C.B. CO. MLBND 00132-0202-20 0.0<strong>08</strong>67 FLEET PEDIA-LAX ENEMA 0 FLEET,C.B. CO. MLGEN 24385-0397-40 0.0<strong>08</strong>70 FOAMING ANTACID LIQUID 0 AMERISOURCEBERG MLBND 24385-0126-78 0.04250 FOAMING ANTACID TABLET CHEW 0 AMERISOURCEBERG EAGEN 00904-3197-60 0.01890 FOLIC ACID 400 MCG TABLET 0 MAJOR PHARMACEU EAGEN 00904-3197-70 0.01890 FOLIC ACID 400 MCG TABLET 0 MAJOR PHARMACEU EAGEN 37205-0188-85 0.01890 FOLIC ACID 400 MCG TABLET 0 LEADER EABND 00178-0031-12 0.05040 FOSFREE TABLET 0 MISSION PHARM. EABND 00178-0031-60 0.05040 FOSFREE TABLET 0 MISSION PHARM. EAGEN 99073-0709-14 16.50000 FREESTYLE FREEDOM LITE METER 0 ABBOTT DIABETES EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 99073-0711-43 41.80000 FREESTYLE INSULINX GLUCOSE SYS 0 ABBOTT DIABETES EAGEN 99073-0712-31 1.53230 FREESTYLE INSULINX TEST STRIP 0 ABBOTT DIABETES EAGEN 99073-0712-27 1.53230 FREESTYLE INSULINX TEST STRIPS 0 ABBOTT DIABETES EAGEN 99073-07<strong>08</strong>-05 16.50000 FREESTYLE LITE METER 0 ABBOTT DIABETES EAGEN 99073-07<strong>08</strong>-22 1.42098 FREESTYLE LITE TEST STRIP 0 ABBOTT DIABETES EAGEN 99073-07<strong>08</strong>-27 1.37291 FREESTYLE LITE TEST STRIP 0 ABBOTT DIABETES EAGEN 99073-0120-50 1.42098 FREESTYLE TEST STRIPS 0 ABBOTT DIABETES EAGEN 99073-0121-01 1.37291 FREESTYLE TEST STRIPS 0 ABBOTT DIABETES EAGEN 43386-0312-<strong>08</strong> 0.03193 GAVILAX POWDER 0 GAVIS PHARMACEU GMGEN 43386-0312-14 0.03193 GAVILAX POWDER 0 GAVIS PHARMACEU GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00135-0098-26 0.03690 GAVISCON ES TABLET CHEW 0 GSK CONSUMER HE EABND 00135-0095-41 0.0<strong>08</strong>40 GAVISCON EXTRA STRENGTH LIQ 0 GSK CONSUMER HE MLBND 00135-0094-41 0.0<strong>08</strong>70 GAVISCON LIQUID 0 GSK CONSUMER HE MLBND 00135-0096-26 0.04250 GAVISCON 80-14.2 MG TAB CHEW 0 GSK CONSUMER HE EABND 00078-0425-16 0.27930 GENTEAL GEL DROPS 0 ALCON LABS. MLBND 00078-0425-24 0.27930 GENTEAL GEL DROPS 0 ALCON LABS. MLBND 00078-0518-24 0.27930 GENTEAL MILD-MODERATE EYE DROP 0 ALCON LABS. MLBND 00078-0429-57 0.87698 GENTEAL SEVERE 0.3% EYE GEL 0 ALCON LABS. GMBND 00078-0429-97 0.87698 GENTEAL SEVERE 0.3% EYE GEL 0 ALCON LABS. GMGEN 00904-5414-16 0.01880 GERAVIM LIQUID 0 MAJOR PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 54838-0007-80 0.01880 GERIATON LIQUID 0 SILARX PHARM MLGEN 00904-5896-15 0.15207 GLUCOSE 4 GRAM TABLET CHEW 0 MAJOR PHARMACEU EABND 00574-0<strong>06</strong>9-15 0.13600 GLUTOSE 15 GEL 0 PADDOCK LABS. GMBND 00574-0<strong>06</strong>9-30 0.13600 GLUTOSE 15 GEL 0 PADDOCK LABS. GMBND 00574-0070-15 0.13600 GLUTOSE 15 GEL 0 PADDOCK LABS. GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 493LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00574-0070-30 0.13600 GLUTOSE 15 GEL 0 PADDOCK LABS. GMBND 00574-0<strong>06</strong>9-45 0.13600 GLUTOSE 45 GEL 0 PADDOCK LABS. GMGEN 62175-0190-07 0.03193 GLYCOLAX POWDER 0 KREMERS URBAN GMGEN 62175-0190-15 0.03193 GLYCOLAX POWDER 0 KREMERS URBAN GMGEN 62175-0190-31 0.03193 GLYCOLAX POWDER 0 KREMERS URBAN GMGEN 24385-0175-62 0.61830 GNP ALL DAY ALLERGY-D TABLET G AMERISOURCEBERG EAGEN 45802-0498-78 0.37626 GUAIFENESIN ER 600 MG TABLET 0 PERRIGO CO. EAGEN 00536-<strong>08</strong>25-85 0.01310 GUAIFENESIN 100 MG/5 ML SYRUP 0 RUGBY MLGEN 68<strong>08</strong>4-0430-98 <strong>HEALTH</strong>YLAX POWDER PACKET G AHP EAGEN 68<strong>08</strong>4-0430-99 <strong>HEALTH</strong>YLAX POWDER PACKET G AHP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5529-52 0.13110 HEARTBURN RELIEF 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5529-87 0.13110 HEARTBURN RELIEF 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 36800-0047-02 0.07430 HEARTBURN RELIEF 150 MG TAB 0 TOPCO EAGEN 36800-0047-71 0.07430 HEARTBURN RELIEF 150 MG TAB 0 TOPCO EAGEN 00904-6282-41 0.75170 HEARTBURN RELIEF 24 HR DR CAP G MAJOR PHARMACEU EAGEN 00904-6282-71 0.75170 HEARTBURN RELIEF 24 HR DR CAP G MAJOR PHARMACEU EAGEN 62011-0144-02 0.10993 HM ACID REDUCER 75 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0092-01 0.02680 HM ADT TUSSIN COUGH CONG DM LQ 0 <strong>HEALTH</strong> MART MLGEN 62011-0<strong>08</strong>9-02 0.02680 HM ADULT TUSSIN CHEST CONG LIQ 0 <strong>HEALTH</strong> MART MLGEN 62011-0091-01 0.01624 HM ADULT TUSSIN DM SYRUP 0 <strong>HEALTH</strong> MART ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62011-0091-02 0.01624 HM ADULT TUSSIN DM SYRUP 0 <strong>HEALTH</strong> MART MLGEN 62011-0122-01 0.0<strong>08</strong>70 HM ADV ANTACID-ANTIGAS SUSP 0 <strong>HEALTH</strong> MART MLGEN 62011-0052-01 0.12903 HM ALL DAY ALLERGY 10 MG TAB 0 <strong>HEALTH</strong> MART EAGEN 62011-0055-01 0.61830 HM ALLERGY COMPLETE-D TABLET G <strong>HEALTH</strong> MART EAGEN 62011-0074-03 0.09890 HM ALLERGY RELIEF 10 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0059-01 0.<strong>08</strong>580 HM ALLERGY RELIEF 4 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0071-01 0.61380 HM ALLERGY RLF-NASAL DECONG TB G <strong>HEALTH</strong> MART EAGEX 62011-0056-03 0.03330 HM ALLERGY 25 MG CAPSULE 0 <strong>HEALTH</strong> MART EAGEX 62011-0058-03 0.03330 HM ALLERGY 25 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0149-01 0.0<strong>08</strong>70 HM ANTACID ANTI-GAS SUSPENSION 0 <strong>HEALTH</strong> MART ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62011-0147-01 0.0<strong>08</strong>70 HM ANTACID-ANTIGAS SUSPENSION 0 <strong>HEALTH</strong> MART MLGEN 62011-0148-01 0.0<strong>08</strong>70 HM ANTACID-ANTIGAS SUSPENSION 0 <strong>HEALTH</strong> MART MLGEN 62011-0150-01 0.23958 HM ANTI-DIARRHEAL 2 MG CAPLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0150-02 0.23958 HM ANTI-DIARRHEAL 2 MG CAPLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0150-03 0.23958 HM ANTI-DIARRHEAL 2 MG CAPLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0026-01 0.04260 HM ARTHRITIS PAIN ER 650 MG 0 <strong>HEALTH</strong> MART EAGEN 62011-0040-01 0.02128 HM ASPIRIN EC 325 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0003-01 0.02781 HM ASPIRIN EC 81 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0020-01 0.01740 HM ASPIRIN 325 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0041-01 0.01740 HM ASPIRIN 325 MG TABLET 0 <strong>HEALTH</strong> MART EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62011-0021-01 0.04820 HM ASPIRIN 81 MG CHEWABLE TAB 0 <strong>HEALTH</strong> MART EAGEN 62011-0028-01 0.04820 HM ASPIRIN 81 MG CHEWABLE TAB 0 <strong>HEALTH</strong> MART EAGEN 62011-0094-01 0.<strong>08</strong>290 HM BACITRACIN ZN 500 UNIT/GM 0 <strong>HEALTH</strong> MART GMGEN 62011-0132-01 0.03531 HM CAL ANTACID 500 MG CHEW TAB 0 <strong>HEALTH</strong> MART EAGEN 62011-0131-01 0.04333 HM CAL ANTACID 750 MG CHEW TAB 0 <strong>HEALTH</strong> MART EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 494LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62011-0<strong>06</strong>0-01 0.15291 HM CHEST CONGEST RLF 400 MG TB 0 <strong>HEALTH</strong> MART EAGEX 62011-0057-01 0.02118 HM CHILD ALLERGY 12.5 MG/5 ML 0 <strong>HEALTH</strong> MART MLGEN 62011-0093-01 0.05700 HM CHILD CETIRIZINE 1 MG/ML 0 <strong>HEALTH</strong> MART MLGEN 62011-0072-01 0.04749 HM CHILD LORATADINE 5 MG/5 ML 0 <strong>HEALTH</strong> MART MLGEN 62011-0029-01 0.02330 HM CHILD PAIN RLF 160 MG/5 ML 0 <strong>HEALTH</strong> MART MLGEN 62011-0010-01 0.04567 HM CHLD IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 <strong>HEALTH</strong> MART MLGEN 62011-0011-01 0.04567 HM CHLD IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 <strong>HEALTH</strong> MART MLGEN 62011-0030-01 0.04567 HM CHLD IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 <strong>HEALTH</strong> MART MLGEN 62011-0022-01 0.02330 HM CHLD PAIN-FEVER 160 MG/5 ML 0 <strong>HEALTH</strong> MART MLGEN 62011-0183-01 0.02330 HM CHLD PAIN-FEVER 160 MG/5 ML 0 <strong>HEALTH</strong> MART ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62011-0153-01 0.03193 HM CLEARLAX POWDER 0 <strong>HEALTH</strong> MART GMGEN 62011-0153-02 0.03193 HM CLEARLAX POWDER 0 <strong>HEALTH</strong> MART GMGEN 62011-0142-01 0.13110 HM FAMOTIDINE 10 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0<strong>06</strong>7-01 0.61666 HM FEX<strong>OF</strong>ENADINE HCL 180 MG TAB G <strong>HEALTH</strong> MART EAGEN 62011-0<strong>06</strong>7-02 0.61666 HM FEX<strong>OF</strong>ENADINE HCL 180 MG TAB G <strong>HEALTH</strong> MART EAGEN 62011-0<strong>06</strong>8-01 0.54333 HM FEX<strong>OF</strong>ENADINE HCL 60 MG TAB G <strong>HEALTH</strong> MART EAGEN 62011-0137-01 0.01631 HM FIBER POWDER 0 <strong>HEALTH</strong> MART GMGEN 62011-0042-02 0.15207 HM GLUCOSE 4 GRAM TABLET CHEW 0 <strong>HEALTH</strong> MART EAGEN 62011-0043-02 0.15207 HM GLUCOSE 4 GRAM TABLET CHEW 0 <strong>HEALTH</strong> MART EAGEN 62011-0095-01 0.<strong>08</strong>144 HM HYDROCORTISONE 1% CREAM 0 <strong>HEALTH</strong> MART GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62011-0096-01 0.<strong>08</strong>144 HM HYDROCORTISONE 1% CREAM 0 <strong>HEALTH</strong> MART GMGEN 62011-0015-01 0.05567 HM IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0015-02 0.05567 HM IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0016-01 0.<strong>06</strong>470 HM IBUPR<strong>OF</strong>EN 200 MG CAPSULE 0 <strong>HEALTH</strong> MART EAGEN 62011-0013-01 0.05567 HM IBUPR<strong>OF</strong>EN 200 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0014-01 0.05567 HM IBUPR<strong>OF</strong>EN 200 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0014-03 0.05567 HM IBUPR<strong>OF</strong>EN 200 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0039-01 0.05567 HM IBUPR<strong>OF</strong>EN 200 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0001-01 0.02330 HM INFNT PAIN & FEVER 160 MG/5 0 <strong>HEALTH</strong> MART MLGEN 62011-0168-01 0.75170 HM LANSOPRAZOLE DR 15 MG CAP G <strong>HEALTH</strong> MART EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62011-0168-02 0.75170 HM LANSOPRAZOLE DR 15 MG CAP G <strong>HEALTH</strong> MART EAGEN 62011-0168-03 0.75170 HM LANSOPRAZOLE DR 15 MG CAP G <strong>HEALTH</strong> MART EAGEN 62011-0112-01 0.13501 HM LICE TREATMENT 1% LOTION 0 <strong>HEALTH</strong> MART MLGEN 62011-0019-01 0.02781 HM LOW DOSE ASPIRIN EC 81 MG 0 <strong>HEALTH</strong> MART EAGEN 62011-0203-01 0.27930 HM LUBRICAT PLUS 0.5% EYE DRPS 0 <strong>HEALTH</strong> MART EAGEN 62011-0076-01 0.37626 HM MUCUS ER 600 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0076-02 0.37626 HM MUCUS ER 600 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0078-01 0.07353 HM NASAL DECONGEST 30 MG TAB 0 <strong>HEALTH</strong> MART EAGEN 62011-0050-01 1.77934 HM NICOTINE 7 MG/24HR PATCH 0 <strong>HEALTH</strong> MART EAGEN 62011-0157-02 0.54542 HM OMEPRAZOLE DR 20 MG TABLET G <strong>HEALTH</strong> MART EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62011-0157-03 0.54542 HM OMEPRAZOLE DR 20 MG TABLET G <strong>HEALTH</strong> MART EAGEN 62011-0023-01 0.02807 HM PAIN RELIEF 500 MG CAPLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0023-03 0.02807 HM PAIN RELIEF 500 MG CAPLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0034-01 0.02807 HM PAIN RELIEF 500 MG CAPLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0027-01 0.02807 HM PAIN RELIEF 500 MG TABLET 0 <strong>HEALTH</strong> MART EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 495LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62011-0032-01 0.02968 HM PAIN RELIEVER 325 MG TABLET 0 <strong>HEALTH</strong> MART EAGEN 62011-0<strong>08</strong>6-01 0.07570 HM SALINE 0.65% NASAL SPRAY 0 <strong>HEALTH</strong> MART MLBND 00002-8730-01 20.98600 HUMULIN N 100 UNITS/ML PEN 0 ELI LILLY & CO. MLBND 00002-8730-59 20.98600 HUMULIN N 100 UNITS/ML PEN 0 ELI LILLY & CO. MLBND 00002-8315-01 10.05640 HUMULIN N 100 UNITS/ML VIAL 0 ELI LILLY & CO. MLBND 00002-8215-01 10.05640 HUMULIN R 100 UNITS/ML VIAL 0 ELI LILLY & CO. MLBND 00002-8215-17 10.05700 HUMULIN R 100 UNITS/ML VIAL 0 ELI LILLY & CO. MLBND 00002-8770-59 20.98600 HUMULIN 70-30 PEN 0 ELI LILLY & CO. MLBND 00002-8715-01 10.05640 HUMULIN 70-30 VIAL 0 ELI LILLY & CO. MLGEN 00536-5105-97 0.<strong>06</strong>764 HYDRO SKIN 1% LOTION 0 RUGBY ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 51672-2<strong>06</strong>3-02 0.<strong>08</strong>144 HYDROCORTISONE PLUS 1% CREAM 0 TARO PHARM USA GMGEN 00168-0154-<strong>08</strong> 0.<strong>08</strong>144 HYDROCORTISONE 1% CREAM 0 SANDOZ GMGEN 00168-0154-31 0.<strong>08</strong>144 HYDROCORTISONE 1% CREAM 0 SANDOZ GMGEN 00472-0339-56 0.<strong>08</strong>144 HYDROCORTISONE 1% CREAM 0 ACTAVIS PHARMA, GMGEN 00472-0343-56 0.<strong>08</strong>144 HYDROCORTISONE 1% CREAM 0 ACTAVIS PHARMA, GMGEN 0<strong>06</strong>03-0535-50 0.<strong>08</strong>144 HYDROCORTISONE 1% CREAM 0 QUALITEST GMGEN 00904-7623-31 0.<strong>08</strong>144 HYDROCORTISONE 1% CREAM 0 MAJOR PHARMACEU GMBND 24385-0274-03 0.<strong>08</strong>150 HYDROCORTISONE 1% CREAM 0 AMERISOURCEBERG GMGEN 37205-0162-10 0.<strong>08</strong>144 HYDROCORTISONE 1% CREAM 0 LEADER GMGEN 45802-0438-03 0.<strong>08</strong>144 HYDROCORTISONE 1% CREAM 0 PERRIGO CO. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 45802-0438-05 0.<strong>08</strong>144 HYDROCORTISONE 1% CREAM 0 PERRIGO CO. GMGEN 51672-2<strong>06</strong>9-02 0.<strong>08</strong>144 HYDROCORTISONE 1% CREAM 0 TARO PHARM USA GMGEN 24385-0283-<strong>06</strong> 0.<strong>06</strong>764 HYDROCORTISONE 1% LOTION 0 AMERISOURCEBERG MLGEN 00168-0181-31 0.10370 HYDROCORTISONE 1% OINTMENT 0 SANDOZ GMGEN 00472-0345-56 0.10370 HYDROCORTISONE 1% OINTMENT 0 ACTAVIS PHARMA, GMBND 24385-0276-03 0.10370 HYDROCORTISONE 1% OINTMENT 0 AMERISOURCEBERG GMGEN 51672-2018-02 0.10370 HYDROCORTISONE 1% OINTMENT 0 TARO PHARM USA GMBND 00536-51<strong>08</strong>-95 0.<strong>08</strong>144 HYDROSKIN 1% CREAM 0 RUGBY GMGEN 00472-1255-94 0.04567 IBUPR<strong>OF</strong>EN 100 MG/5 ML SUSP 0 ACTAVIS PHARMA, MLGEN 00113-0517-71 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 PERRIGO CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00113-<strong>06</strong>47-62 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 PERRIGO CO. EAGEN 00113-<strong>06</strong>47-71 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 PERRIGO CO. EAGEN 00113-<strong>06</strong>47-78 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 PERRIGO CO. EAGEN 00904-5323-24 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 MAJOR PHARMACEU EAGEN 00904-7912-51 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 MAJOR PHARMACEU EAGEN 00904-7912-59 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 MAJOR PHARMACEU EAGEN 24385-0058-78 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 AMERISOURCEBERG EAGEN 24385-0058-82 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 AMERISOURCEBERG EAGEN 36800-0183-83 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 TOPCO EAGEN 36800-0517-71 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0517-78 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 TOPCO EAGEN 37205-0341-71 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 LEADER EAGEN 37205-0341-78 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 LEADER EAGEN 37205-<strong>06</strong>05-71 0.05567 IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 LEADER EAGEN 00113-<strong>06</strong>04-62 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 PERRIGO CO. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 496LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00113-<strong>06</strong>04-71 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 PERRIGO CO. EAGEN 00113-<strong>06</strong>04-78 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 PERRIGO CO. EAGEN 00113-<strong>06</strong>04-90 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 PERRIGO CO. EAGEN 00113-<strong>06</strong>04-93 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 PERRIGO CO. EAGEN 00536-3587-01 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 RUGBY EAGEN 00536-3587-02 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 RUGBY EAGEN 00536-3587-<strong>06</strong> 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 RUGBY EAGEN 00904-7912-24 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7914-51 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7914-59 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-7914-61 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7915-24 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7915-40 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7915-51 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7915-59 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-7915-70 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 MAJOR PHARMACEU EAGEN 24385-0059-78 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 AMERISOURCEBERG EAGEN 24385-<strong>06</strong>04-85 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 AMERISOURCEBERG EAGEN 36800-0074-71 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EAGEN 36800-0074-78 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-<strong>06</strong>04-62 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>04-71 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>04-78 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>04-85 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>04-90 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>04-93 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>47-62 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>47-71 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>47-78 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EAGEN 36800-<strong>06</strong>47-90 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0345-71 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 LEADER EAGEN 37205-0345-78 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 LEADER EAGEN 37205-0350-71 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 LEADER EAGEN 37205-0350-85 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 LEADER EAGEN 37205-0350-90 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 LEADER EAGEN 53746-0140-01 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 AMNEAL PHARMACE EAGEN 53746-0140-10 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 AMNEAL PHARMACE EAGEN 63739-0134-01 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 MCKESSON PACKAG EAGEN 63868-0983-50 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 CHAIN DRUG EAGEN 66424-0995-10 0.05567 IBUPR<strong>OF</strong>EN 200 MG TABLET 0 SDA LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 68094-0503-59 0.04567 IBUPR<strong>OF</strong>EN 200 MG/10 ML SUSP 0 PRECISION DOSE MLGEN 68094-0503-61 0.04567 IBUPR<strong>OF</strong>EN 200 MG/10 ML SUSP 0 PRECISION DOSE MLGEN 68094-0503-62 0.04567 IBUPR<strong>OF</strong>EN 200 MG/10 ML SUSP 0 PRECISION DOSE MLBND 00<strong>06</strong>5-8040-82 0.02950 ICAPS MV TABLET 0 ALCON CONSUMER EABND 00<strong>06</strong>5-8040-83 0.02950 ICAPS MV TABLET 0 ALCON CONSUMER EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 497LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63044-0203-01 0.19377 IFEREX 150 CAPSULE 0 NNODUM CORP EAGEN 36800-00<strong>08</strong>-05 0.15883 INFANT PAIN RLF 80 MG/0.8 ML 0 TOPCO MLGEN 36800-0289-05 0.15883 INFANT'S PAIN RLF 80 MG/0.8 ML 0 TOPCO MLGEN 36800-0289-10 0.15883 INFANT'S PAIN RLF 80 MG/0.8 ML 0 TOPCO MLGEN 24385-0289-10 0.18396 INFANTS PAIN RELIEF W-O ASA 0 AMERISOURCEBERG MLGEN 00187-0746-33 0.13600 INSTA-GLUCOSE 40% GEL 0 VALEANT GMGEN 53329-0<strong>08</strong>0-57 0.11529 INZO ANTIFUNGAL 2% CREAM 0 MEDLINE INDUS. GMGEN 53329-0<strong>08</strong>0-58 0.11529 INZO ANTIFUNGAL 2% CREAM 0 MEDLINE INDUS. GMGEN 37205-0413-96 0.02170 IRON 325 MG TABLET 0 LEADER EAGEN 00904-5779-60 0.05000 KAO-TIN 240 MG S<strong>OF</strong>TGEL 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00536-2310-97 0.02680 KIDKARE COUGH & COLD LIQUID 0 RUGBY MLGEN 00224-1856-<strong>06</strong> 0.01331 KONSYL EASY MIX FORMULA POWDER 0 KONSYL PHARM. GMGEN 00224-0500-90 0.09133 KONSYL FIBER 625 MG TABLET 0 KONSYL PHARM. EAGEN 00224-1841-03 0.01295 KONSYL ORANGE POWDER 0 KONSYL PHARM. GMGEN 00224-1801-<strong>06</strong> 0.01331 KONSYL POWDER 0 KONSYL PHARM. GMGEN 00224-1801-07 0.01331 KONSYL POWDER 0 KONSYL PHARM. GMBND 00224-1801-24 0.01970 KONSYL 6 GM PACKET 0 KONSYL PHARM. EAGEN 00224-1822-03 0.01482 KONSYL-D POWDER 0 KONSYL PHARM. GMGEN 00224-1822-07 0.01482 KONSYL-D POWDER 0 KONSYL PHARM. GMGEN 38779-0315-<strong>08</strong> LACTOSE MONOHYDRATE POWDER 0 MEDISCA INC. GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00<strong>06</strong>7-6114-46 0.05013 LAMISIL AF DEFENSE 1% POWDER 0 NOVARTIS CONSUM GMGEN 00<strong>06</strong>7-3998-30 0.33487 LAMISIL AT 1% CREAM 0 NOVARTIS CONSUM GMGEN 00536-4122-88 0.75170 LANSOPRAZOLE DR 15 MG CAPSULE G RUGBY EAGEN 36800-0019-14 0.75170 LANSOPRAZOLE DR 15 MG CAPSULE G TOPCO EAGEN 36800-0019-42 0.75170 LANSOPRAZOLE DR 15 MG CAPSULE G TOPCO EAGEN 36800-0117-03 0.75170 LANSOPRAZOLE DR 15 MG CAPSULE G TOPCO EAGEN 45802-0245-01 0.75170 LANSOPRAZOLE DR 15 MG CAPSULE G PERRIGO CO. EAGEN 45802-0245-02 0.75170 LANSOPRAZOLE DR 15 MG CAPSULE G PERRIGO CO. EAGEN 46122-0107-04 0.75170 LANSOPRAZOLE DR 15 MG CAPSULE G AMERISOURCEBERG EAGEN 24385-0903-63 0.03100 LAXATIVE EC 5 MG TABLET 0 AMERISOURCEBERG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0298-65 0.03010 LAXATIVE FEMININE 5 MG TAB 0 LEADER EAGEN 15127-0109-12 0.19997 LAXATIVE 10 MG SUPPOSITORY 0 SELECT BRAND EAGEN 24385-0107-53 0.19997 LAXATIVE 10 MG SUPPOSITORY 0 AMERISOURCEBERG EAGEN 36800-0<strong>08</strong>6-63 0.03010 LAXATIVE 5 MG TABLET 0 TOPCO EAGEN 37205-0477-78 0.04260 LEADER 8-HR PAIN RELIEF 650 MG 0 LEADER EAGEN 00113-<strong>08</strong>66-26 0.05462 LICE KILLING SHAMPOO 0 PERRIGO CO. MLGEN 00904-2528-20 0.05462 LICE KILLING SHAMPOO 0 MAJOR PHARMACEU MLGEN 37205-0165-26 0.05462 LICE KILLING SHAMPOO 0 LEADER MLGEN 24385-0116-03 0.05462 LICE TREATMENT SHAMPOO 0 AMERISOURCEBERG MLGEN 64543-0350-90 0.20760 LIQUIBID D-R TABLET 0 CAPELLON EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24385-0357-40 0.0<strong>08</strong>70 LIQUID ANTACID SUSPENSION 0 AMERISOURCEBERG MLGEN 00904-5017-35 0.27930 LIQUITEARS 1.4 % DROPS 0 MAJOR PHARMACEU MLBND 50383-<strong>06</strong>18-04 0.02830 LOPERAMIDE 1 MG/5 ML LIQUID 0 HI-TECH PHARMAC MLGEN 54838-0558-40 0.04749 LORATADINE ALLERGY 5 MG/5 ML 0 SILARX PHARM MLGEN 54838-0554-40 0.04749 LORATADINE HIVES 5 MG/5 ML 0 SILARX PHARM ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 498LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00<strong>06</strong>7-<strong>06</strong>74-30 0.09890 LORATADINE 10 MG TABLET 0 NOVARTIS CONSUM EAGEN 00781-5077-01 0.09890 LORATADINE 10 MG TABLET 0 SANDOZ EAGEN 00781-5077-64 0.09890 LORATADINE 10 MG TABLET 0 SANDOZ EAGEN 00781-5077-76 0.09890 LORATADINE 10 MG TABLET 0 SANDOZ EAGEN 00904-6074-61 0.09890 LORATADINE 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 24385-0471-52 0.09890 LORATADINE 10 MG TABLET 0 AMERISOURCEBERG EAGEN 24385-0471-65 0.09890 LORATADINE 10 MG TABLET 0 AMERISOURCEBERG EAGEN 24385-0471-78 0.09890 LORATADINE 10 MG TABLET 0 AMERISOURCEBERG EAGEN 24385-0471-99 0.09890 LORATADINE 10 MG TABLET 0 AMERISOURCEBERG EAGEN 37205-0346-47 0.09890 LORATADINE 10 MG TABLET 0 LEADER EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0346-52 0.09890 LORATADINE 10 MG TABLET 0 LEADER EAGEN 37205-0346-60 0.09890 LORATADINE 10 MG TABLET 0 LEADER EAGEN 37205-0346-65 0.09890 LORATADINE 10 MG TABLET 0 LEADER EAGEN 37205-0346-72 0.09890 LORATADINE 10 MG TABLET 0 LEADER EAGEN 45802-<strong>06</strong>50-65 0.09890 LORATADINE 10 MG TABLET 0 PERRIGO CO. EAGEN 45802-<strong>06</strong>50-78 0.09890 LORATADINE 10 MG TABLET 0 PERRIGO CO. EAGEN 45802-<strong>06</strong>50-87 0.09890 LORATADINE 10 MG TABLET 0 PERRIGO CO. EAGEN 51660-0526-01 0.09890 LORATADINE 10 MG TABLET 0 OHM LABS. EAGEN 51660-0526-05 0.09890 LORATADINE 10 MG TABLET 0 OHM LABS. EAGEN 51660-0526-31 0.09890 LORATADINE 10 MG TABLET 0 OHM LABS. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 60505-0147-01 0.09890 LORATADINE 10 MG TABLET 0 APOTEX CORP EAGEN 60505-0147-<strong>08</strong> 0.09890 LORATADINE 10 MG TABLET 0 APOTEX CORP EAGEN 68<strong>08</strong>4-0248-01 0.09890 LORATADINE 10 MG TABLET 0 AHP EAGEN 68<strong>08</strong>4-0248-11 0.09890 LORATADINE 10 MG TABLET 0 AHP EAGEN 00904-6234-20 0.04749 LORATADINE 5 MG/5 ML SOLN 0 MAJOR PHARMACEU MLGEN 24385-0543-26 0.04749 LORATADINE 5 MG/5 ML SYRUP 0 AMERISOURCEBERG MLGEN 51672-2073-<strong>08</strong> 0.04749 LORATADINE 5 MG/5 ML SYRUP 0 TARO PHARM USA MLGEN 51672-2<strong>08</strong>5-<strong>08</strong> 0.04749 LORATADINE 5 MG/5 ML SYRUP 0 TARO PHARM USA MLGEN 45802-01<strong>06</strong>-39 0.56431 LORATADINE-D 12 HOUR TABLET G PERRIGO CO. EAGEN 45802-01<strong>06</strong>-52 0.56431 LORATADINE-D 12 HOUR TABLET G PERRIGO CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 46122-0109-52 0.56431 LORATADINE-D 12 HOUR TABLET G AMERISOURCEBERG EAGEN 00904-5833-15 0.61380 LORATADINE-D 24HR TABLET G MAJOR PHARMACEU EAGEN 00904-5833-48 0.61380 LORATADINE-D 24HR TABLET G MAJOR PHARMACEU EAGEN 46122-02<strong>06</strong>-22 0.61380 LORATADINE-D 24HR TABLET G AMERISOURCEBERG EAGEN 37205-<strong>06</strong>36-05 0.41340 LUBRICANT EYE DROPS 0 LEADER MLGEN 46122-0195-65 0.27930 LUBRICATING PLUS 0.5% EYE DRPS 0 AMERISOURCEBERG EAGEN 00904-5168-38 1.37180 LUBRIFRESH PM OINTMENT 0 MAJOR PHARMACEU GMBND 00<strong>06</strong>7-6281-44 0.0<strong>08</strong>70 MAALOX ADVANCED SUSPENSION 0 NOVARTIS CONSUM MLBND 00<strong>06</strong>7-6281-62 0.0<strong>08</strong>70 MAALOX ADVANCED SUSPENSION 0 NOVARTIS CONSUM MLBND 00<strong>06</strong>7-6281-71 0.0<strong>08</strong>70 MAALOX ADVANCED SUSPENSION 0 NOVARTIS CONSUM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>7-6279-35 0.1<strong>06</strong>50 MAALOX ADVANCED TAB CHEW 0 NOVARTIS CONSUM EAGEN 00904-7911-52 0.<strong>08</strong>800 MAG DELAY 64 MG TABLET 0 MAJOR PHARMACEU EABND 00121-1761-30 0.0<strong>08</strong>70 MAG-AL PLUS SUSPENSION 0 PHARMACEU ASSOC MLBND 59528-05<strong>08</strong>-05 0.05500 MAGNEBIND 300 TABLET 0 NEPHRO-TECH EAGEN 60258-0171-01 0.05500 MAGNESIUM OXIDE 400 MG TABLET 0 CYPRESS PHARM. EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 499LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 76439-0217-12 0.05500 MAGNESIUM OXIDE 400 MG TABLET 0 VIRTUS PHARMACE EABND 0<strong>06</strong>03-0213-21 0.05500 MAGNESIUM OXIDE 420 MG TABLET 0 QUALITEST EAGEN 00904-4239-60 0.03931 MAGNESIUM OXIDE 500 MG TABLET 0 MAJOR PHARMACEU EABND 37205-0351-78 0.03635 MAGNESIUM 250 MG TABLET 0 LEADER EABND 00256-0172-01 0.05500 MAGONATE 27 MG TABLET 0 VALEANT EABND 00187-5267-01 0.26325 MAGONATE 54 MG/5 ML LIQUID 0 VALEANT MLBND 00256-0184-07 0.<strong>06</strong>050 MAGONATE 54 MG/5 ML LIQUID 0 VALEANT MLGEN 68585-0005-75 0.<strong>08</strong>800 MAG64 DR 64 MG TABLET 0 RISING PHARM EAGEN 00904-5769-60 0.04260 MAPAP ARTHRITIS ER 650 MG CPLT 0 MAJOR PHARMACEU EAGEN 00904-1985-00 0.02044 MAPAP 160 MG/5 ML ELIXIR 0 MAJOR PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-1985-16 0.01816 MAPAP 160 MG/5 ML ELIXIR 0 MAJOR PHARMACEU MLGEN 00904-1985-20 0.02044 MAPAP 160 MG/5 ML ELIXIR 0 MAJOR PHARMACEU MLGEN 00904-1982-51 0.02968 MAPAP 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-1982-59 0.02968 MAPAP 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-1982-60 0.02968 MAPAP 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-1982-61 0.02968 MAPAP 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-1982-80 0.02968 MAPAP 325 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-1983-24 0.02807 MAPAP 500 MG CAPLET 0 MAJOR PHARMACEU EAGEN 00904-1983-40 0.02807 MAPAP 500 MG CAPLET 0 MAJOR PHARMACEU EAGEN 00904-1983-51 0.02807 MAPAP 500 MG CAPLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-1983-59 0.02807 MAPAP 500 MG CAPLET 0 MAJOR PHARMACEU EAGEN 00904-1983-80 0.02807 MAPAP 500 MG CAPLET 0 MAJOR PHARMACEU EAGEN 00904-1983-94 0.02807 MAPAP 500 MG CAPLET 0 MAJOR PHARMACEU EAGEN 00904-1987-60 0.03878 MAPAP 500 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 00904-5816-51 0.02807 MAPAP 500 MG GELCAP 0 MAJOR PHARMACEU EAGEN 00904-5816-60 0.02807 MAPAP 500 MG GELCAP 0 MAJOR PHARMACEU EAGEN 00904-1988-59 0.02807 MAPAP 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-1988-60 0.02807 MAPAP 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-1988-61 0.02807 MAPAP 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-1988-80 0.02807 MAPAP 500 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5256-46 0.07070 MAPAP 80 MG TABLET CHEW 0 MAJOR PHARMACEU EAGEN 00536-3985-01 0.04621 MECLIZINE 12.5 MG TABLET 0 RUGBY EAGEN 00536-3985-10 0.04621 MECLIZINE 12.5 MG TABLET 0 RUGBY EAGEN 00781-1345-01 0.04621 MECLIZINE 12.5 MG TABLET 0 SANDOZ EAGEN 00781-1375-01 0.<strong>08</strong>384 MECLIZINE 25 MG TABLET 0 SANDOZ EAGEN 00536-3990-01 0.04580 MECLIZINE 25 MG TABLET CHEW 0 RUGBY EAGEN 13811-<strong>06</strong>48-10 0.04580 MECLIZINE 25 MG TABLET CHEW 0 TRIGEN LABORATO EAGEN 49614-0123-00 0.02807 MEDI-TABS 500 MG CAPLET 0 MEDICINE SHOP EAGEN 49614-0123-71 0.02807 MEDI-TABS 500 MG CAPLET 0 MEDICINE SHOP EAGEN 49614-0123-78 0.02807 MEDI-TABS 500 MG CAPLET 0 MEDICINE SHOP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49614-0188-78 0.02807 MEDI-TABS 500 MG GELTAB 0 MEDICINE SHOP EAGEN 49614-0360-26 0.02680 MEDI-TUSSIN DM DIABETIC LIQ 0 MEDICINE SHOP MLBND 00394-0130-12 0.37000 MERIBIN 5 MG CAPSULE G MERICON EAGEN 38779-0<strong>08</strong>5-05 METHYLCELLULOSE 1,500 CPS PWD 0 MEDISCA INC. GMGEN 00904-0004-14 0.0<strong>08</strong>70 MI ACID SUSPENSION 0 MAJOR PHARMACEU ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 500LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-0005-14 0.0<strong>08</strong>70 MI ACID SUSPENSION 0 MAJOR PHARMACEU MLBND 00904-5115-71 0.03690 MI-ACID DS TABLET 0 MAJOR PHARMACEU EAGEN 00472-0730-63 0.13054 MICONAZOLE NITRATE 2% CREAM 0 ACTAVIS PHARMA, GMGEN 00472-0735-42 0.11529 MICONAZOLE NITRATE 2% CREAM 0 ACTAVIS PHARMA, GMGEN 00472-0735-56 0.11529 MICONAZOLE NITRATE 2% CREAM 0 ACTAVIS PHARMA, GMGEN 51672-2001-01 0.11529 MICONAZOLE NITRATE 2% CREAM 0 TARO PHARM USA GMGEN 51672-2001-02 0.11529 MICONAZOLE NITRATE 2% CREAM 0 TARO PHARM USA GMGEN 51672-2035-<strong>06</strong> 0.13054 MICONAZOLE NITRATE 2% CREAM 0 TARO PHARM USA GMGEN 00713-0197-57 0.80714 MICONAZOLE 100 MG VAG SUPP 0 G & W LABS. EAGEN 00904-7734-45 0.13054 MICONAZOLE 7 CREAM 0 MAJOR PHARMACEU GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-7734-57 0.13054 MICONAZOLE 7 CREAM 0 MAJOR PHARMACEU GMGEN 24385-0590-29 0.13054 MICONAZOLE 7 CREAM 0 AMERISOURCEBERG GMGEN 00472-1736-07 0.80714 MICONAZOLE 7 100 MG VAG SUPP 0 ACTAVIS PHARMA, EAGEN 62107-0023-11 0.0<strong>08</strong>70 MILANTEX DBL-STRENGTH LIQ 0 PRIME MARKETING MLGEN 62107-0022-11 0.0<strong>08</strong>70 MILANTEX LIQUID 0 PRIME MARKETING MLGEN 00113-0332-40 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 PERRIGO CO. MLGEN 00113-0396-40 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 PERRIGO CO. MLGEN 00121-0431-30 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 PHARMACEU ASSOC MLGEN 00536-2470-83 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 RUGBY MLGEN 00536-2470-85 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 RUGBY ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00574-0018-16 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 PADDOCK LABS. MLGEN 00904-0788-14 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 MAJOR PHARMACEU MLGEN 00904-0789-14 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 MAJOR PHARMACEU MLGEN 15127-<strong>08</strong>33-73 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 SELECT BRAND MLGEN 15127-<strong>08</strong>35-73 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 SELECT BRAND MLGEN 24385-0332-40 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 AMERISOURCEBERG MLGEN 24385-0396-40 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 AMERISOURCEBERG MLGEN 24385-<strong>06</strong><strong>08</strong>-40 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 AMERISOURCEBERG MLGEN 36800-0949-40 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 TOPCO MLGEN 37205-<strong>08</strong>33-40 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 LEADER ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-<strong>08</strong>34-40 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 LEADER MLGEN 62107-0024-11 0.00799 MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 PRIME MARKETING MLGEN 37205-<strong>08</strong>31-43 0.0<strong>08</strong>10 MINERAL OIL 0 LEADER MLBND 00574-<strong>06</strong>18-16 0.0<strong>08</strong>10 MINERAL OIL LAXATIVE 0 PADDOCK LABS. MLGEN 49483-0330-10 0.02781 MINIPRIN EC 81 MG TABLET 0 TIME-CAP LABS EAGEN 49483-0330-12 0.02781 MINIPRIN EC 81 MG TABLET 0 TIME-CAP LABS EAGEN 00904-0478-60 0.03690 MINTOX PLUS TABLET CHEWABLE 0 MAJOR PHARMACEU EAGEN 00904-5721-14 0.0<strong>08</strong>70 MINTOX SUSPENSION 0 MAJOR PHARMACEU MLBND 11523-7234-04 0.03193 MIRALAX POWDER 0 S-P <strong>HEALTH</strong>CARE GMGEN 24385-0004-12 0.05538 MOTION SICKNESS 50 MG TABLET 0 AMERISOURCEBERG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00485-0250-01 0.20760 MUCAPHED TABLET 0 EDWARDS PHARMAC EABND 63824-0072-35 0.20760 MUCINEX DM ER 1,200-60 MG TAB 0 RECKITT BENCKIS EABND 63824-0056-32 0.496<strong>08</strong> MUCINEX DM ER 600-30 MG TABLET 0 RECKITT BENCKIS EABND 63824-0056-34 0.496<strong>08</strong> MUCINEX DM ER 600-30 MG TABLET 0 RECKITT BENCKIS EABND 63824-00<strong>08</strong>-10 0.37626 MUCINEX ER 600 MG TABLET 0 RECKITT BENCKIS EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 501LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 63824-00<strong>08</strong>-15 0.37626 MUCINEX ER 600 MG TABLET 0 RECKITT BENCKIS EABND 63824-00<strong>08</strong>-20 0.37626 MUCINEX ER 600 MG TABLET 0 RECKITT BENCKIS EABND 63824-00<strong>08</strong>-32 0.37626 MUCINEX ER 600 MG TABLET 0 RECKITT BENCKIS EABND 63824-00<strong>08</strong>-34 0.37626 MUCINEX ER 600 MG TABLET 0 RECKITT BENCKIS EABND 63824-00<strong>08</strong>-40 0.37626 MUCINEX ER 600 MG TABLET 0 RECKITT BENCKIS EABND 63824-00<strong>08</strong>-50 0.37626 MUCINEX ER 600 MG TABLET 0 RECKITT BENCKIS EABND 63824-00<strong>08</strong>-61 0.37626 MUCINEX ER 600 MG TABLET 0 RECKITT BENCKIS EABND 63824-00<strong>08</strong>-62 0.37626 MUCINEX ER 600 MG TABLET 0 RECKITT BENCKIS EABND 63824-0018-66 0.02680 MUCINEX FAST-MAX DM MAX LIQUID 0 RECKITT BENCKIS MLBND 63824-0123-75 0.13520 MUCINEX FULL FORCE NASAL SPRAY 0 RECKITT BENCKIS ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63824-0122-75 0.13520 MUCINEX MOISTURE SMART NAS SPR 0 RECKITT BENCKIS MLGEN 49483-0280-<strong>06</strong> 0.02680 MUCOSA DM TABLET 0 TIME-CAP LABS EAGEN 00904-6013-46 0.02680 MUCUS RELIEF DM TABLET 0 MAJOR PHARMACEU EAGEN 00904-6233-46 0.02680 MUCUS RELIEF DM TABLET 0 MAJOR PHARMACEU EAGEN 00904-6233-52 0.02680 MUCUS RELIEF DM TABLET 0 MAJOR PHARMACEU EABND 00904-5792-46 0.20760 MUCUS RELIEF SINUS TABLET 0 MAJOR PHARMACEU EABND 00904-5792-52 0.20760 MUCUS RELIEF SINUS TABLET 0 MAJOR PHARMACEU EAGEN 00904-6232-46 0.15291 MUCUS RELIEF 400 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6232-52 0.15291 MUCUS RELIEF 400 MG TABLET 0 MAJOR PHARMACEU EAGEN 54838-00<strong>08</strong>-70 0.01880 MULTI-DELYN LIQUID 0 SILARX PHARM ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 54838-0009-70 0.01880 MULTI-DELYN WITH IRON LIQUID 0 SILARX PHARM MLBND 242<strong>08</strong>-0276-15 0.43000 MURO-128 2% EYE DROPS 0 VALEANT MLBND 242<strong>08</strong>-0277-15 0.58422 MURO-128 5% EYE DROPS 0 VALEANT MLBND 242<strong>08</strong>-0277-30 0.58422 MURO-128 5% EYE DROPS 0 VALEANT MLBND 242<strong>08</strong>-0385-55 2.57100 MURO-128 5% EYE OINTMENT 0 VALEANT GMBND 242<strong>08</strong>-0385-56 2.57100 MURO-128 5% EYE OINTMENT 0 VALEANT GMBND 00536-1850-97 0.02038 NASAL DECON(P-EPHED)30 MG/5 ML 0 RUGBY MLGEN 00113-0094-23 0.10400 NASAL DECONGESTANT PE 10 MG TB 0 PERRIGO CO. EAGEN 00113-0094-68 0.10400 NASAL DECONGESTANT PE 10 MG TB 0 PERRIGO CO. EAGEN 00113-0094-89 0.10400 NASAL DECONGESTANT PE 10 MG TB 0 PERRIGO CO. EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0094-23 0.10400 NASAL DECONGESTANT PE 10 MG TB 0 TOPCO EAGEN 36800-0094-47 0.10400 NASAL DECONGESTANT PE 10 MG TB 0 TOPCO EAGEN 36800-0094-68 0.10400 NASAL DECONGESTANT PE 10 MG TB 0 TOPCO EAGEN 36800-0094-89 0.10400 NASAL DECONGESTANT PE 10 MG TB 0 TOPCO EAGEN 37205-0473-69 0.10400 NASAL DECONGESTANT PE 10 MG TB 0 LEADER EAGEN 37205-0473-89 0.10400 NASAL DECONGESTANT PE 10 MG TB 0 LEADER EAGEN 51672-2030-03 0.13520 NASAL DECONGESTANT 0.05% SPRAY 0 TARO PHARM USA MLGEN 51672-2030-05 0.13520 NASAL DECONGESTANT 0.05% SPRAY 0 TARO PHARM USA MLGEN 36800-0432-62 0.07353 NASAL DECONGESTANT 30 MG TAB 0 TOPCO EAGEN 36800-0432-67 0.07353 NASAL DECONGESTANT 30 MG TAB 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0<strong>06</strong>7-10 0.13520 NASAL SPRAY X-MOIST 0 LEADER MLGEN 00113-0304-10 0.13520 NASAL SPRAY 0.05% 0 PERRIGO CO. MLGEN 36800-0<strong>06</strong>5-10 0.13520 NASAL SPRAY 0.05% 0 TOPCO MLGEN 36800-0304-10 0.13520 NASAL SPRAY 0.05% 0 TOPCO MLGEN 36800-0388-10 0.13520 NASAL SPRAY 0.05% 0 TOPCO ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 502LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-<strong>08</strong>17-10 0.13520 NASAL SPRAY 0.05% 0 TOPCO MLGEN 00904-5018-35 0.27930 NATURAL BALANCE TEARS DROPS 0 MAJOR PHARMACEU MLGEN 00904-5199-65 0.01482 NATURAL FIBER LAX POWDER 0 MAJOR PHARMACEU GMGEN 00904-5199-66 0.01970 NATURAL FIBER LAX POWDER 0 MAJOR PHARMACEU GMGEN 00904-5200-65 0.01295 NATURAL FIBER LAX POWDER 0 MAJOR PHARMACEU GMGEN 00904-5200-66 0.01295 NATURAL FIBER LAX POWDER 0 MAJOR PHARMACEU GMBND 0<strong>06</strong>03-0993-89 0.01972 NATURAL VEGETABLE FIBER POWDER 0 QUALITEST GMGEN 0<strong>06</strong>03-0994-63 0.01631 NATURAL VEGETABLE FIBER POWDER 0 QUALITEST GMGEN 00536-6237-72 0.27930 NATURE'S TEARS DROPS 0 RUGBY MLBND 00024-1754-01 0.13520 NEO-SYNEPHRINE 12 HOUR SPRAY 0 BAYER INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-4342-60 0.02830 NIACIN TR 500 MG CAPLET 0 MAJOR PHARMACEU EAGEN 00904-4342-70 0.02830 NIACIN TR 500 MG CAPLET 0 MAJOR PHARMACEU EAGEN 00904-<strong>06</strong>31-60 0.02830 NIACIN TR 500 MG CAPSULE 0 MAJOR PHARMACEU EAGEN 00904-2271-60 0.02790 NIACIN 100 MG TABLET 0 MAJOR PHARMACEU EAGEN 10267-0012-01 0.02790 NIACIN 100 MG TABLET 0 CONTRACT PHARM EAGEN 10267-0012-04 0.02790 NIACIN 100 MG TABLET 0 CONTRACT PHARM EAGEN 00904-2270-60 0.02470 NIACIN 50 MG CAPLET 0 MAJOR PHARMACEU EAGEN 00182-4405-01 0.02830 NIACIN 500 MG TABLET 0 IVAX PHARMACEUT EAGEN 00904-2272-60 0.02830 NIACIN 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-2272-80 0.02830 NIACIN 500 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-4202-60 0.02830 NIACINAMIDE 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00135-0195-02 1.84253 NICODERM CQ 14 MG/24HR PATCH 0 GSK CONSUMER HE EAGEN 00135-0195-05 1.84253 NICODERM CQ 14 MG/24HR PATCH 0 GSK CONSUMER HE EAGEN 00135-0145-02 2.04183 NICODERM CQ 21 MG/24HR PATCH 0 GSK CONSUMER HE EAGEN 00135-0194-01 2.04183 NICODERM CQ 21 MG/24HR PATCH 0 GSK CONSUMER HE EAGEN 00135-0194-02 2.04183 NICODERM CQ 21 MG/24HR PATCH 0 GSK CONSUMER HE EAGEN 00135-0194-05 2.04183 NICODERM CQ 21 MG/24HR PATCH 0 GSK CONSUMER HE EAGEN 00135-0196-02 1.77934 NICODERM CQ 7 MG/24HR PATCH 0 GSK CONSUMER HE EAGEN 00135-0196-05 1.77934 NICODERM CQ 7 MG/24HR PATCH 0 GSK CONSUMER HE EAGEN 00904-5734-11 0.20128 NICORELIEF 2 MG GUM 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5734-51 0.20128 NICORELIEF 2 MG GUM 0 MAJOR PHARMACEU EAGEN 00904-5736-11 0.20128 NICORELIEF 2 MG GUM 0 MAJOR PHARMACEU EAGEN 00904-5736-51 0.20128 NICORELIEF 2 MG GUM 0 MAJOR PHARMACEU EAGEN 00904-5735-11 0.25968 NICORELIEF 4 MG GUM 0 MAJOR PHARMACEU EAGEN 00904-5735-51 0.25968 NICORELIEF 4 MG GUM 0 MAJOR PHARMACEU EAGEN 00904-5737-11 0.25968 NICORELIEF 4 MG GUM 0 MAJOR PHARMACEU EAGEN 00904-5737-51 0.25968 NICORELIEF 4 MG GUM 0 MAJOR PHARMACEU EABND 00135-0157-07 0.20128 NICORETTE 2 MG CHEWING GUM 0 GSK CONSUMER HE EABND 00135-0225-03 0.20128 NICORETTE 2 MG CHEWING GUM 0 GSK CONSUMER HE EABND 00135-0229-05 0.20128 NICORETTE 2 MG CHEWING GUM 0 GSK CONSUMER HE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00135-0241-02 0.20128 NICORETTE 2 MG CHEWING GUM 0 GSK CONSUMER HE EABND 00135-0241-05 0.20128 NICORETTE 2 MG CHEWING GUM 0 GSK CONSUMER HE EABND 00135-0241-<strong>06</strong> 0.20128 NICORETTE 2 MG CHEWING GUM 0 GSK CONSUMER HE EABND 00135-0466-01 0.20128 NICORETTE 2 MG CHEWING GUM 0 GSK CONSUMER HE EABND 00135-0466-02 0.20128 NICORETTE 2 MG CHEWING GUM 0 GSK CONSUMER HE EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 503LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00135-0466-05 0.20128 NICORETTE 2 MG CHEWING GUM 0 GSK CONSUMER HE EABND 00135-0474-02 0.20128 NICORETTE 2 MG CHEWING GUM 0 GSK CONSUMER HE EABND 00135-05<strong>08</strong>-02 0.45300 NICORETTE 2 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-05<strong>08</strong>-03 0.45300 NICORETTE 2 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0510-01 0.45300 NICORETTE 2 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0510-02 0.45300 NICORETTE 2 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0510-05 0.45300 NICORETTE 2 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0512-01 0.45300 NICORETTE 2 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0512-05 0.45300 NICORETTE 2 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0514-03 0.45300 NICORETTE 2 MG LOZENGE 0 GSK CONSUMER HE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00135-0158-07 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EAGEN 00135-0158-11 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EAGEN 00135-0226-02 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EAGEN 00135-0226-03 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EAGEN 00135-0230-04 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EAGEN 00135-0242-02 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EAGEN 00135-0242-05 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EAGEN 00135-0242-<strong>06</strong> 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EAGEN 00135-0467-02 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EAGEN 00135-0467-05 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00135-0475-02 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EAGEN 00135-0475-05 0.25968 NICORETTE 4 MG CHEWING GUM 0 GSK CONSUMER HE EABND 00135-0509-02 0.45300 NICORETTE 4 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0509-03 0.45300 NICORETTE 4 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0511-01 0.45300 NICORETTE 4 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0511-02 NICORETTE 4 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0511-05 0.45300 NICORETTE 4 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0513-01 0.45300 NICORETTE 4 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0513-05 0.45300 NICORETTE 4 MG LOZENGE 0 GSK CONSUMER HE EABND 00135-0515-03 0.45300 NICORETTE 4 MG LOZENGE 0 GSK CONSUMER HE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00<strong>06</strong>7-5125-07 1.84253 NICOTINE 14 MG/24HR PATCH 0 NOVARTIS CONSUM EAGEN 00<strong>06</strong>7-5125-14 1.84253 NICOTINE 14 MG/24HR PATCH 0 NOVARTIS CONSUM EAGEN 00536-5895-88 1.84253 NICOTINE 14 MG/24HR PATCH 0 RUGBY EAGEN 37205-0361-74 1.84253 NICOTINE 14 MG/24HR PATCH 0 LEADER EAGEN 00113-02<strong>06</strong>-25 0.20128 NICOTINE 2 MG CHEWING GUM 0 PERRIGO CO. EAGEN 00536-1362-<strong>06</strong> 0.20128 NICOTINE 2 MG CHEWING GUM 0 RUGBY EAGEN 00536-1362-23 0.20128 NICOTINE 2 MG CHEWING GUM 0 RUGBY EAGEN 00536-3029-<strong>06</strong> 0.20128 NICOTINE 2 MG CHEWING GUM 0 RUGBY EAGEN 00536-3029-23 0.20128 NICOTINE 2 MG CHEWING GUM 0 RUGBY EAGEN 00536-3029-34 0.20128 NICOTINE 2 MG CHEWING GUM 0 RUGBY EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00536-3112-01 0.20128 NICOTINE 2 MG CHEWING GUM 0 RUGBY EAGEN 00536-3112-37 0.20128 NICOTINE 2 MG CHEWING GUM 0 RUGBY EAGEN 00536-3386-01 0.20128 NICOTINE 2 MG CHEWING GUM 0 RUGBY EAGEN 00536-3386-37 0.20128 NICOTINE 2 MG CHEWING GUM 0 RUGBY EAGEN 00536-3404-01 0.20128 NICOTINE 2 MG CHEWING GUM 0 RUGBY EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 504LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00536-3404-37 0.20128 NICOTINE 2 MG CHEWING GUM 0 RUGBY EAGEN 24385-0170-58 0.20128 NICOTINE 2 MG CHEWING GUM 0 AMERISOURCEBERG EAGEN 36800-0029-25 0.20128 NICOTINE 2 MG CHEWING GUM 0 TOPCO EAGEN 36800-02<strong>06</strong>-25 0.20128 NICOTINE 2 MG CHEWING GUM 0 TOPCO EAGEN 36800-0456-78 0.20128 NICOTINE 2 MG CHEWING GUM 0 TOPCO EAGEN 37205-0967-58 0.20128 NICOTINE 2 MG CHEWING GUM 0 LEADER EAGEN 46122-0173-20 0.20128 NICOTINE 2 MG CHEWING GUM 0 AMERISOURCEBERG EAGEN 00113-0344-05 NICOTINE 2 MG LOZENGE 0 PERRIGO CO. EAGEN 24385-0975-67 0.55600 NICOTINE 2 MG LOZENGE 0 AMERISOURCEBERG EAGEN 36800-0344-05 0.45300 NICOTINE 2 MG LOZENGE 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00<strong>06</strong>7-5126-07 2.04183 NICOTINE 21 MG/24HR PATCH 0 NOVARTIS CONSUM EAGEN 00<strong>06</strong>7-5126-14 2.04183 NICOTINE 21 MG/24HR PATCH 0 NOVARTIS CONSUM EAGEN 00536-5896-88 2.04183 NICOTINE 21 MG/24HR PATCH 0 RUGBY EAGEN 37205-0358-74 2.04183 NICOTINE 21 MG/24HR PATCH 0 LEADER EAGEN 00113-0422-25 0.25968 NICOTINE 4 MG CHEWING GUM 0 PERRIGO CO. EAGEN 00113-0532-78 0.25968 NICOTINE 4 MG CHEWING GUM 0 PERRIGO CO. EAGEN 00536-1372-23 0.25968 NICOTINE 4 MG CHEWING GUM 0 RUGBY EAGEN 00536-3030-<strong>06</strong> 0.25968 NICOTINE 4 MG CHEWING GUM 0 RUGBY EAGEN 00536-3030-23 0.25968 NICOTINE 4 MG CHEWING GUM 0 RUGBY EAGEN 00536-3113-01 0.25968 NICOTINE 4 MG CHEWING GUM 0 RUGBY EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00536-3113-37 0.25968 NICOTINE 4 MG CHEWING GUM 0 RUGBY EAGEN 00536-3387-01 0.25968 NICOTINE 4 MG CHEWING GUM 0 RUGBY EAGEN 00536-3387-37 0.25968 NICOTINE 4 MG CHEWING GUM 0 RUGBY EAGEN 00536-3405-01 0.25968 NICOTINE 4 MG CHEWING GUM 0 RUGBY EAGEN 00536-3405-37 0.25968 NICOTINE 4 MG CHEWING GUM 0 RUGBY EAGEN 24385-0171-58 0.25968 NICOTINE 4 MG CHEWING GUM 0 AMERISOURCEBERG EAGEN 36800-0170-25 0.25968 NICOTINE 4 MG CHEWING GUM 0 TOPCO EAGEN 36800-0170-71 0.25968 NICOTINE 4 MG CHEWING GUM 0 TOPCO EAGEN 36800-0422-71 0.25968 NICOTINE 4 MG CHEWING GUM 0 TOPCO EAGEN 36800-0532-78 0.25968 NICOTINE 4 MG CHEWING GUM 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-<strong>08</strong>54-78 0.25968 NICOTINE 4 MG CHEWING GUM 0 TOPCO EAGEN 00113-<strong>08</strong>73-05 0.45300 NICOTINE 4 MG LOZENGE 0 PERRIGO CO. EAGEN 24385-0976-67 0.45300 NICOTINE 4 MG LOZENGE 0 AMERISOURCEBERG EAGEN 36800-<strong>08</strong>73-05 0.45300 NICOTINE 4 MG LOZENGE 0 TOPCO EAGEN 00<strong>06</strong>7-5124-07 1.77934 NICOTINE 7 MG/24HR PATCH 0 NOVARTIS CONSUM EAGEN 00<strong>06</strong>7-5124-14 1.77934 NICOTINE 7 MG/24HR PATCH 0 NOVARTIS CONSUM EAGEN 00536-5894-88 1.77934 NICOTINE 7 MG/24HR PATCH 0 RUGBY EAGEN 37205-0363-74 1.77934 NICOTINE 7 MG/24HR PATCH 0 LEADER EAGEN 49614-0146-62 0.03330 NIGHT TIME SLEEP 25 MG CAPLT 0 MEDICINE SHOP EAGEN 24385-0431-78 0.03330 NIGHTTIME SLEEP AID CPLT 0 AMERISOURCEBERG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00113-0431-62 0.03330 NIGHTTIME SLEEP AID 25 MG CPLT 0 PERRIGO CO. EAGEN 24385-0431-26 0.03330 NIGHTTIME SLEEP AID 25 MG CPLT 0 AMERISOURCEBERG EAGEN 36800-0431-62 0.03330 NIGHTTIME SLEEP AID 25 MG CPLT 0 TOPCO EAGEN 36800-0431-67 0.03330 NIGHTTIME SLEEP AID 25 MG CPLT 0 TOPCO EAGEN 24385-0405-72 0.02807 NON-ASPIRIN 500 MG TABLET 0 AMERISOURCEBERG EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 505LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5728-87 0.09890 NON-DROWSY ALLERGY 10 MG TAB 0 MAJOR PHARMACEU EAGEN 63162-0518-30 0.14376 NORTEMP 80 MG/0.8 ML DROP 0 BALLAY PHARM MLBND 00169-1834-11 10.04020 NOVOLIN N 100 UNITS/ML VIAL 0 NOVO NORDISK MLBND 00169-1833-11 10.04020 NOVOLIN R 100 UNITS/ML VIAL 0 NOVO NORDISK MLBND 00169-1837-11 10.04020 NOVOLIN 70-30 100 UNIT/ML VIAL 0 NOVO NORDISK MLBND 00536-5005-72 0.13520 NRS-NASAL RELIEF NOSE SPRAY 0 RUGBY MLBND 00187-5260-01 0.07570 OCEAN 0.65% NASAL SPRAY 0 VALEANT MLBND 00187-5260-03 0.07570 OCEAN 0.65% NASAL SPRAY 0 VALEANT MLBND 00256-0152-18 0.07570 OCEAN 0.65% NASAL SPRAY 0 VALEANT MLBND 00256-0152-25 0.07570 OCEAN 0.65% NASAL SPRAY 0 VALEANT ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00256-0152-01 0.07570 OCEAN 0.65% NOSE SPRAY 0 VALEANT MLGEN 242<strong>08</strong>-0387-60 0.05040 OCUVITE WITH LUTEIN TABLET 0 BAUSCH & LOMB C EAGEN 00113-0915-30 0.54542 OMEPRAZOLE DR 20 MG TABLET G PERRIGO CO. EAGEN 00113-0915-55 0.54542 OMEPRAZOLE DR 20 MG TABLET G PERRIGO CO. EAGEN 00904-5834-41 0.54542 OMEPRAZOLE DR 20 MG TABLET G MAJOR PHARMACEU EAGEN 00904-5834-42 0.54542 OMEPRAZOLE DR 20 MG TABLET G MAJOR PHARMACEU EAGEN 00904-5834-71 0.54542 OMEPRAZOLE DR 20 MG TABLET G MAJOR PHARMACEU EAGEN 36800-0915-03 0.54542 OMEPRAZOLE DR 20 MG TABLET G TOPCO EAGEN 36800-0915-30 0.54542 OMEPRAZOLE DR 20 MG TABLET G TOPCO EAGEN 36800-0915-55 0.54542 OMEPRAZOLE DR 20 MG TABLET G TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0915-74 0.54542 OMEPRAZOLE DR 20 MG TABLET G TOPCO EAGEN 37205-<strong>08</strong>37-<strong>06</strong> 0.54542 OMEPRAZOLE DR 20 MG TABLET G LEADER EAGEN 37205-<strong>08</strong>37-15 0.54542 OMEPRAZOLE DR 20 MG TABLET G LEADER EAGEN 37205-<strong>08</strong>37-66 0.54542 OMEPRAZOLE DR 20 MG TABLET G LEADER EAGEN 37205-<strong>08</strong>37-74 0.54542 OMEPRAZOLE DR 20 MG TABLET G LEADER EAGEN 45802-<strong>08</strong>88-30 0.54542 OMEPRAZOLE DR 20 MG TABLET G PERRIGO CO. EAGEN 45802-<strong>08</strong>88-55 0.54542 OMEPRAZOLE DR 20 MG TABLET G PERRIGO CO. EAGEN 46122-0029-03 0.54542 OMEPRAZOLE DR 20 MG TABLET G AMERISOURCEBERG EAGEN 46122-0029-04 0.54542 OMEPRAZOLE DR 20 MG TABLET G AMERISOURCEBERG EAGEN 46122-0029-74 0.54542 OMEPRAZOLE DR 20 MG TABLET G AMERISOURCEBERG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62107-0039-01 0.02950 ONCE DAILY TABLET 0 PRIME MARKETING EAGEN 62107-0039-10 0.02950 ONCE DAILY TABLET 0 PRIME MARKETING EAGEN 62107-0040-01 0.05040 ONCE DAILY WITH IRON TABLET 0 PRIME MARKETING EAGEN 62107-0040-10 0.05040 ONCE DAILY WITH IRON TABLET 0 PRIME MARKETING EAGEN 00178-0550-01 0.05040 ONCOVITE TABLET 0 MISSION PHARM. EAGEN 46122-0120-78 0.02950 ONE DAILY ESSENTIAL TABLET 0 AMERISOURCEBERG EAGEN 46122-0120-85 0.02950 ONE DAILY ESSENTIAL TABLET 0 AMERISOURCEBERG EAGEN 37205-0<strong>08</strong>0-82 0.02950 ONE DAILY TABLET 0 LEADER EAGEN 37205-0352-78 0.02950 ONE DAILY TABLET 0 LEADER EAGEN 53885-0247-01 60.50000 ONE TOUCH ULTRA SYSTEM KIT 0 LIFESCAN EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53885-0244-50 1.30240 ONE TOUCH ULTRA TEST STRIPS 0 LIFESCAN EAGEN 53885-0245-10 1.30240 ONE TOUCH ULTRA TEST STRIPS 0 LIFESCAN EAGEN 53885-0994-25 1.30240 ONE TOUCH ULTRA TEST STRIPS 0 LIFESCAN EAGEN 53885-0448-01 16.50000 ONE TOUCH ULTRA 2 GLUCOSE SYST 0 LIFESCAN EAGEN 53885-02<strong>08</strong>-01 16.50000 ONE TOUCH ULTRAMINI METER 0 LIFESCAN EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 5<strong>06</strong>LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 53885-0419-01 16.50000 ONE TOUCH ULTRAMINI METER 0 LIFESCAN EAGEN 53885-0420-01 16.50000 ONE TOUCH ULTRAMINI METER 0 LIFESCAN EAGEN 53885-0911-01 16.50000 ONE TOUCH ULTRAMINI METER 0 LIFESCAN EAGEN 53885-0912-01 16.50000 ONE TOUCH ULTRAMINI METER 0 LIFESCAN EAGEN 53885-0267-01 25.30000 ONE TOUCH VERIO IQ METER 0 LIFESCAN EAGEN 53885-0270-25 1.42340 ONE TOUCH VERIO TEST STRIP 0 LIFESCAN EAGEN 53885-0271-50 1.42340 ONE TOUCH VERIO TEST STRIP 0 LIFESCAN EAGEN 53885-0272-10 1.42340 ONE TOUCH VERIO TEST STRIP 0 LIFESCAN EAGEN 00574-0312-16 0.04016 ORA-BLEND SF SUSPENSION 0 PADDOCK LABS. MLGEN 00574-0311-16 0.04016 ORA-BLEND SUSPENSION 0 PADDOCK LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00574-0303-16 0.04016 ORA-PLUS SUSPENDING VEHICLE 0 PADDOCK LABS. MLGEN 00574-0304-16 0.05620 ORA-SWEET ORAL SYRUP 0 PADDOCK LABS. MLGEN 00574-0302-16 0.03563 ORA-SWEET-SF SYRUP 0 PADDOCK LABS. MLGEN 00536-7817-<strong>08</strong> 0.04373 OYSCO 500+D TABLET 0 RUGBY EAGEN 00536-7817-10 0.04373 OYSCO 500+D TABLET 0 RUGBY EAGEN 00536-41<strong>06</strong>-02 0.04138 OYSCO-500 TABLET 0 RUGBY EAGEN 00536-41<strong>06</strong>-<strong>08</strong> 0.04138 OYSCO-500 TABLET 0 RUGBY EAGEN 16103-0361-11 0.04373 OYSTER SHELL CALCIUM +D TABLET 0 PHARBEST PHARMA EAGEN 00904-1883-52 0.04138 OYSTER SHELL CALCIUM 500 MG TB 0 MAJOR PHARMACEU EAGEN 00904-1883-61 0.04138 OYSTER SHELL CALCIUM 500 MG TB 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-1883-72 0.04138 OYSTER SHELL CALCIUM 500 MG TB 0 MAJOR PHARMACEU EAGEN 00904-1883-80 0.04138 OYSTER SHELL CALCIUM 500 MG TB 0 MAJOR PHARMACEU EAGEN 00904-1883-92 0.04138 OYSTER SHELL CALCIUM 500 MG TB 0 MAJOR PHARMACEU EAGEN 16103-0360-11 0.04138 OYSTER SHELL CALCIUM 500 MG TB 0 PHARBEST PHARMA EAGEN 16103-0360-99 0.04138 OYSTER SHELL CALCIUM 500 MG TB 0 PHARBEST PHARMA EAGEN 62107-0049-01 0.04138 OYSTER SHELL CALCIUM 500 MG TB 0 PRIME MARKETING EAGEN 62107-0049-05 0.04138 OYSTER SHELL CALCIUM 500 MG TB 0 PRIME MARKETING EAGEN 00904-5460-52 0.04373 OYSTER SHELL CALCIUM-VIT D TAB 0 MAJOR PHARMACEU EAGEN 00904-5460-61 0.04373 OYSTER SHELL CALCIUM-VIT D TAB 0 MAJOR PHARMACEU EAGEN 00904-5460-72 0.04373 OYSTER SHELL CALCIUM-VIT D TAB 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5460-80 0.04373 OYSTER SHELL CALCIUM-VIT D TAB 0 MAJOR PHARMACEU EAGEN 00904-5460-92 0.04373 OYSTER SHELL CALCIUM-VIT D TAB 0 MAJOR PHARMACEU EAGEN 37205-0<strong>08</strong>3-87 0.03765 OYSTER SHELL CALCIUM-VIT D TAB 0 LEADER EAGEN 62107-0075-<strong>06</strong> 0.04373 OYSTER SHELL CALCIUM-VIT D TAB 0 PRIME MARKETING EABND 00536-3222-01 0.02968 PAIN & FEVER 325 MG TABLET 0 RUGBY EABND 00536-3222-10 0.02968 PAIN & FEVER 325 MG TABLET 0 RUGBY EABND 00536-3218-01 0.02807 PAIN & FEVER 500 MG CAPLET 0 RUGBY EABND 00536-3218-10 0.02807 PAIN & FEVER 500 MG CAPLET 0 RUGBY EABND 00536-3231-01 0.02807 PAIN & FEVER 500 MG TABLET 0 RUGBY EABND 00536-3231-10 0.02807 PAIN & FEVER 500 MG TABLET 0 RUGBY EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00536-1936-72 0.15883 PAIN & FEVER 80 MG/0.8 ML DROP 0 RUGBY MLGEN 00113-0217-71 0.04260 PAIN RELIEF ER 650 MG CAPLET 0 PERRIGO CO. EAGEN 00113-0544-62 0.04260 PAIN RELIEF ER 650 MG CAPLET 0 PERRIGO CO. EAGEN 00113-0544-71 0.04260 PAIN RELIEF ER 650 MG CAPLET 0 PERRIGO CO. EAGEN 24385-<strong>06</strong>29-71 0.04260 PAIN RELIEF ER 650 MG CAPLET 0 AMERISOURCEBERG EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 507LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0217-78 0.04260 PAIN RELIEF ER 650 MG CAPLET 0 TOPCO EAGEN 00113-0403-78 0.02968 PAIN RELIEF 325 MG TABLET 0 PERRIGO CO. EAGEN 36800-0403-78 0.02968 PAIN RELIEF 325 MG TABLET 0 TOPCO EAGEN 00113-0484-62 0.02807 PAIN RELIEF 500 MG CAPLET 0 PERRIGO CO. EAGEN 00113-0484-71 0.02807 PAIN RELIEF 500 MG CAPLET 0 PERRIGO CO. EAGEN 00113-0484-78 0.02807 PAIN RELIEF 500 MG CAPLET 0 PERRIGO CO. EAGEN 00113-0484-90 0.02807 PAIN RELIEF 500 MG CAPLET 0 PERRIGO CO. EAGEN 24385-0484-25 0.02807 PAIN RELIEF 500 MG CAPLET 0 AMERISOURCEBERG EAGEN 24385-0484-47 0.02807 PAIN RELIEF 500 MG CAPLET 0 AMERISOURCEBERG EAGEN 36800-0484-62 0.02807 PAIN RELIEF 500 MG CAPLET 0 TOPCO EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0484-71 0.02807 PAIN RELIEF 500 MG CAPLET 0 TOPCO EAGEN 36800-0484-78 0.02807 PAIN RELIEF 500 MG CAPLET 0 TOPCO EAGEN 36800-0484-90 0.02807 PAIN RELIEF 500 MG CAPLET 0 TOPCO EAGEN 36800-0046-62 0.02807 PAIN RELIEF 500 MG GELCAP 0 TOPCO EAGEN 36800-0046-71 0.02807 PAIN RELIEF 500 MG GELCAP 0 TOPCO EAGEN 36800-0046-78 0.02807 PAIN RELIEF 500 MG GELCAP 0 TOPCO EAGEN 36800-0046-83 0.02807 PAIN RELIEF 500 MG GELCAP 0 TOPCO EAGEN 00113-0227-71 0.02807 PAIN RELIEF 500 MG TABLET 0 PERRIGO CO. EAGEN 00113-0405-72 0.02807 PAIN RELIEF 500 MG TABLET 0 PERRIGO CO. EAGEN 16103-0376-<strong>08</strong> 0.02807 PAIN RELIEF 500 MG TABLET 0 PHARBEST PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0227-71 0.02807 PAIN RELIEF 500 MG TABLET 0 TOPCO EAGEN 36800-0227-78 0.02807 PAIN RELIEF 500 MG TABLET 0 TOPCO EAGEN 36800-0405-72 0.02807 PAIN RELIEF 500 MG TABLET 0 TOPCO EAGEN 36800-0405-78 0.02807 PAIN RELIEF 500 MG TABLET 0 TOPCO EAGEN 24385-0484-71 0.02807 PAIN RELIEVER 500 MG CAPLET 0 AMERISOURCEBERG EAGEN 24385-0484-78 0.02807 PAIN RELIEVER 500 MG CAPLET 0 AMERISOURCEBERG EAGEN 24385-0484-90 0.02807 PAIN RELIEVER 500 MG CAPLET 0 AMERISOURCEBERG EAGEN 24385-<strong>06</strong>18-71 0.02807 PAIN RELIEVER 500 MG CAPLET 0 AMERISOURCEBERG EAGEN 37205-0594-90 0.02807 PAIN RELIEVER 500 MG CAPLET 0 LEADER EAGEN 37205-0980-71 0.03878 PAIN RELIEVER 500 MG GELCAP 0 LEADER EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0980-78 0.03878 PAIN RELIEVER 500 MG GELCAP 0 LEADER EAGEN 24385-0145-71 0.02807 PAIN RELIEVER 500 MG TABLET 0 AMERISOURCEBERG EAGEN 37205-0035-78 0.02807 PAIN RELIEVER 500 MG TABLET 0 LEADER EAGEN 46122-0178-78 0.02807 PAIN RELIEVER 500 MG TABLET 0 AMERISOURCEBERG EAGEN 49614-0168-71 0.02807 PAIN RELIEVER 500 MG TABLET 0 MEDICINE SHOP EAGEN 49614-0168-78 0.02807 PAIN RELIEVER 500 MG TABLET 0 MEDICINE SHOP EAGEN 00904-5050-20 0.02680 PEDIA RELIEF COUGH-COLD LIQUID 0 MAJOR PHARMACEU MLBND 00074-5498-20 0.00340 PEDIALYTE ELECTROLYTE SINGLES 0 ABBOTT NUTRITIO MLBND 00074-0245-01 0.00340 PEDIALYTE FREEZER POPS 0 ABBOTT NUTRITIO MLBND 00074-0240-01 0.00340 PEDIALYTE SOLUTION 0 ABBOTT NUTRITIO ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00074-5175-30 0.00340 PEDIALYTE SOLUTION 0 ABBOTT NUTRITIO MLBND 00074-6470-32 0.00340 PEDIALYTE SOLUTION 0 ABBOTT NUTRITIO MLBND 00074-6471-32 0.00340 PEDIALYTE SOLUTION 0 ABBOTT NUTRITIO MLGEN 54838-0115-40 0.02680 PEDIATRIC COUGH-COLD LIQUID 0 SILARX PHARM MLGEN 00904-5118-69 0.00340 PEDIATRIC ELECTROLYTE SOLUTION 0 MAJOR PHARMACEU ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 5<strong>08</strong>LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5768-69 0.00340 PEDIATRIC ELECTROLYTE SOLUTION 0 MAJOR PHARMACEU MLGEN 00536-1810-59 0.01889 PEPTIC RELIEF SUSPENSION 0 RUGBY MLGEN 00536-4301-07 0.13647 PEPTIC RELIEF TABLET CHEW 0 RUGBY EAGEN 00472-5242-67 0.13501 PERMETHRIN 1% LOTION 0 ACTAVIS PHARMA, MLGEN 00472-5242-69 0.13501 PERMETHRIN 1% LOTION 0 ACTAVIS PHARMA, MLGEN 16103-0346-<strong>08</strong> 0.<strong>08</strong>580 PHARBECHLOR 4 MG TABLET 0 PHARBEST PHARMA EAGEX 16103-0348-11 0.03330 PHARBEDRYL 25 MG CAPSULE 0 PHARBEST PHARMA EAGEN 16103-0353-07 0.02968 PHARBETOL 325 MG TABLET 0 PHARBEST PHARMA EAGEN 16103-0353-<strong>08</strong> 0.02968 PHARBETOL 325 MG TABLET 0 PHARBEST PHARMA EAGEN 16103-0353-99 0.02968 PHARBETOL 325 MG TABLET 0 PHARBEST PHARMA EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 16103-0350-04 0.02807 PHARBETOL 500 MG CAPLET 0 PHARBEST PHARMA EAGEN 16103-0350-<strong>08</strong> 0.02807 PHARBETOL 500 MG CAPLET 0 PHARBEST PHARMA EAGEN 16103-0350-11 0.02807 PHARBETOL 500 MG CAPLET 0 PHARBEST PHARMA EABND 0<strong>06</strong>03-1520-54 0.02680 PHENYLHISTINE DH LIQUID 0 QUALITEST MLBND 00904-3535-78 0.0<strong>06</strong>21 PHOSPHATE ENEMA 0 MAJOR PHARMACEU MLBND 00904-5666-75 0.<strong>06</strong>954 PHOSPHATE LAXATIVE 0 MAJOR PHARMACEU MLGEN 37205-0720-65 0.13647 PINK BISMUTH TABLET CHEW 0 LEADER EABEX 51285-0943-88 23.40000 PLAN B ONE-STEP 1.5 MG TABLET 0 DURAMED/BARR EABND 00<strong>08</strong>7-0402-03 0.14540 POLY-VI-SOL DROPS 0 MJ NUTRITIONAL MLGEN 00904-5099-50 0.14540 POLY-VITA DROPS 0 MAJOR PHARMACEU ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5100-50 0.14540 POLY-VITA WITH IRON DROPS 0 MAJOR PHARMACEU MLGEN 00574-0309-16 0.03339 POLYBASE OINTMENT 0 PADDOCK LABS. GMGEN 00904-6025-61 0.04640 POLYETHYLENE GLYCOL 3350 POWD G MAJOR PHARMACEU EAGEN 00904-6025-77 0.03193 POLYETHYLENE GLYCOL 3350 POWD 0 MAJOR PHARMACEU GMGEN 00904-6025-84 0.03193 POLYETHYLENE GLYCOL 3350 POWD 0 MAJOR PHARMACEU GMGEN 45802-<strong>08</strong>68-01 0.03193 POLYETHYLENE GLYCOL 3350 POWD 0 PERRIGO CO. GMGEN 45802-<strong>08</strong>68-02 0.03193 POLYETHYLENE GLYCOL 3350 POWD 0 PERRIGO CO. GMGEN 45802-<strong>08</strong>68-03 0.03193 POLYETHYLENE GLYCOL 3350 POWD 0 PERRIGO CO. GMGEN 57599-8814-01 16.50000 PRECISION XTRA MONITOR 0 ABBOTT DIABETES EAGEN 57599-9728-04 1.42098 PRECISION XTRA TEST STRIPS 0 ABBOTT DIABETES EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 57599-9877-05 1.37291 PRECISION XTRA TEST STRIPS 0 ABBOTT DIABETES EAGEN 00904-5313-46 0.<strong>06</strong>040 PRENATAL TABLET 0 MAJOR PHARMACEU EAGEN 00904-5313-60 0.<strong>06</strong>040 PRENATAL TABLET 0 MAJOR PHARMACEU EAGEN 51645-<strong>08</strong>37-01 0.<strong>06</strong>040 PRENATAL TABLET 0 PLUS PHARMA,INC EAGEN 62107-0<strong>06</strong>3-01 0.<strong>06</strong>040 PRENATAL TABLET 0 PRIME MARKETING EAGEN 37205-0395-82 0.<strong>06</strong>040 PRENATAL VITAMIN TABLET 0 LEADER EABND 00<strong>06</strong>7-6286-14 0.75170 PREVACID 24HR DR 15 MG CAPSULE G NOVARTIS CONSUM EABND 00<strong>06</strong>7-6286-28 0.75170 PREVACID 24HR DR 15 MG CAPSULE G NOVARTIS CONSUM EABND 00<strong>06</strong>7-6286-42 0.75170 PREVACID 24HR DR 15 MG CAPSULE G NOVARTIS CONSUM EABND 37000-0455-02 0.75170 PRILOSEC OTC 20.6 MG TABLET G PROCTER&GAMBLE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 37000-0455-03 0.75170 PRILOSEC OTC 20.6 MG TABLET G PROCTER&GAMBLE EABND 37000-0455-04 0.75170 PRILOSEC OTC 20.6 MG TABLET G PROCTER&GAMBLE EAGEN 00904-7735-18 0.05040 PROSIGHT TABLET 0 MAJOR PHARMACEU EAGEN 00904-7735-52 0.05040 PROSIGHT TABLET 0 MAJOR PHARMACEU EAGEN 62107-0002-01 0.05567 PROVIL 200 MG TABLET 0 PRIME MARKETING EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 509LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00536-1850-85 0.02038 PSEUDOEPHED 30 MG/5 ML SOLN 0 RUGBY MLGEN 10267-1004-<strong>06</strong> 0.07353 PSEUDOEPHEDRINE 30 MG TABLET 0 CONTRACT PHARM EAGEN 37205-0445-80 0.07353 PSEUDOEPHEDRINE 30 MG TABLET 0 LEADER EAGEN 45802-0432-62 0.07353 PSEUDOEPHEDRINE 30 MG TABLET 0 PERRIGO CO. EABND 37205-0285-16 0.09360 PYRETHRIN LICE TREATMENT 0 LEADER MLGEN 38779-1556-04 36.37390 PYRIDOXAL-5-PHOSPHATE POWDER 0 MEDISCA INC. GMGEN 61748-0092-01 0.03100 PYRIDOXINE 25 MG TABLET 0 VERSA PHARMACEU EAGEN 61748-0092-10 0.03100 PYRIDOXINE 25 MG TABLET 0 VERSA PHARMACEU EAGEN 61748-0092-30 0.03100 PYRIDOXINE 25 MG TABLET 0 VERSA PHARMACEU EAGEN 61748-0095-01 0.03390 PYRIDOXINE 50 MG TABLET 0 VERSA PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 61748-0095-10 0.03390 PYRIDOXINE 50 MG TABLET 0 VERSA PHARMACEU EAGEN 61748-0095-30 0.03390 PYRIDOXINE 50 MG TABLET 0 VERSA PHARMACEU EAGEX 0<strong>06</strong>03-<strong>08</strong>23-54 0.02118 Q-DRYL 12.5 MG/5 ML LIQUID 0 QUALITEST MLGEX 0<strong>06</strong>03-<strong>08</strong>23-58 0.02118 Q-DRYL 12.5 MG/5 ML LIQUID 0 QUALITEST MLGEX 0<strong>06</strong>03-<strong>08</strong>23-81 0.02118 Q-DRYL 12.5 MG/5 ML LIQUID 0 QUALITEST MLGEX 0<strong>06</strong>03-<strong>08</strong>23-94 0.02118 Q-DRYL 12.5 MG/5 ML LIQUID 0 QUALITEST MLGEN 0<strong>06</strong>03-<strong>08</strong>41-54 0.02330 Q-PAP CHILDREN'S SUSPENSION 0 QUALITEST MLGEN 0<strong>06</strong>03-<strong>08</strong>42-54 0.02330 Q-PAP CHILDREN'S SUSPENSION 0 QUALITEST MLGEN 0<strong>06</strong>03-<strong>08</strong>43-54 0.02330 Q-PAP CHILDREN'S SUSPENSION 0 QUALITEST MLGEN 0<strong>06</strong>03-0268-29 0.02807 Q-PAP EX-STR 500 MG TABLET 0 QUALITEST EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-0268-32 0.02807 Q-PAP EX-STR 500 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-<strong>08</strong>39-58 0.01816 Q-PAP 160 MG/5 ML LIQUID 0 QUALITEST MLGEN 0<strong>06</strong>03-<strong>08</strong>39-94 0.01816 Q-PAP 160 MG/5 ML LIQUID 0 QUALITEST MLGEN 0<strong>06</strong>03-<strong>08</strong>40-94 0.01816 Q-PAP 160 MG/5 ML LIQUID 0 QUALITEST MLGEN 0<strong>06</strong>03-0263-29 0.02968 Q-PAP 325 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-0263-32 0.02968 Q-PAP 325 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-<strong>08</strong>38-73 0.14376 Q-PAP 80 MG/0.8 ML DROPS 0 QUALITEST MLGEN 0<strong>06</strong>03-<strong>08</strong>55-81 0.01624 Q-TUSSIN DM SYRUP 0 QUALITEST MLGEN 0<strong>06</strong>03-<strong>08</strong>57-58 0.01310 Q-TUSSIN 100 MG/5 ML SOLUTION 0 QUALITEST MLGEN 0<strong>06</strong>03-<strong>08</strong>57-81 0.01310 Q-TUSSIN 100 MG/5 ML SOLUTION 0 QUALITEST ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-<strong>08</strong>57-94 0.01900 Q-TUSSIN 100 MG/5 ML SOLUTION 0 QUALITEST MLGEN 0<strong>06</strong>03-<strong>08</strong>55-58 0.01624 Q-TUSSIN-DM SYRUP 0 QUALITEST MLGEN 0<strong>06</strong>03-<strong>08</strong>55-94 0.01624 Q-TUSSIN-DM SYRUP 0 QUALITEST MLGEN 63868-0712-57 0.0<strong>08</strong>70 QC ANTACID SUSPENSION 0 CHAIN DRUG MLGEN 63868-<strong>06</strong>94-57 0.0<strong>08</strong>70 QC ANTACID-ANTIGAS SUSPENSION 0 CHAIN DRUG MLGEN 63868-0338-12 0.23958 QC ANTI-DIARRHEAL 2 MG CAPLET 0 CHAIN DRUG EAGEN 63868-0338-24 0.23958 QC ANTI-DIARRHEAL 2 MG CAPLET 0 CHAIN DRUG EAGEN 63868-0338-60 0.23958 QC ANTI-DIARRHEAL 2 MG CAPLET 0 CHAIN DRUG EAGEN 63868-0<strong>08</strong>9-01 0.04260 QC ARTHRITIS PAIN ER 650 MG 0 CHAIN DRUG EAGEN 63868-0352-10 0.03600 QC ASPIRIN 325 MG TABLET 0 CHAIN DRUG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63868-0364-12 0.33487 QC ATHLETE'S FOOT 1% CREAM 0 CHAIN DRUG GMGEN 63868-0916-28 0.<strong>06</strong>579 QC BACITRACIN 500 UNIT/GM OINT 0 CHAIN DRUG GMGEX 63868-<strong>08</strong>23-54 0.02118 QC CHILD ALLERGY 12.5 MG/5 ML 0 CHAIN DRUG MLGEN 63868-0241-36 0.04820 QC CHILD ASPIRIN 81 MG CHW TAB 0 CHAIN DRUG EAGEN 63868-0758-18 0.04567 QC CHILD IBUPR<strong>OF</strong>EN 100 MG/5 ML 0 CHAIN DRUG ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 510LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63868-0175-26 0.02330 QC CHILD PAIN RLF 160 MG/5 ML 0 CHAIN DRUG MLGEN 63868-<strong>08</strong>26-24 0.<strong>08</strong>580 QC CHLORPHENIRAMINE 4 MG TAB 0 CHAIN DRUG EAGEX 63868-0500-01 0.03330 QC COMPLETE ALLERGY 25 MG CPLT 0 CHAIN DRUG EAGEN 63868-0530-90 0.09133 QC FIBERLAX 625 MG CAPLET 0 CHAIN DRUG EAGEN 63868-0328-25 0.03100 QC GENTLE LAXATIVE EC 5 MG TAB 0 CHAIN DRUG EAGEN 63868-0791-01 0.05567 QC IBUPR<strong>OF</strong>EN IB 200 MG CAPLET 0 CHAIN DRUG EAGEN 63868-0790-01 0.05567 QC IBUPR<strong>OF</strong>EN IB 200 MG TABLET 0 CHAIN DRUG EAGEN 63868-0756-18 0.04567 QC IBUPR<strong>OF</strong>EN 100 MG/5 ML SUSP 0 CHAIN DRUG MLGEN 63868-0341-10 0.05567 QC IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 CHAIN DRUG EAGEN 63868-0341-24 0.05567 QC IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 CHAIN DRUG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63868-0341-50 0.05567 QC IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 CHAIN DRUG EAGEN 63868-0983-09 0.05567 QC IBUPR<strong>OF</strong>EN 200 MG TABLET 0 CHAIN DRUG EAGEN 63868-0151-10 0.09890 QC LORATADINE 10 MG TABLET 0 CHAIN DRUG EAGEN 63868-0151-30 0.09890 QC LORATADINE 10 MG TABLET 0 CHAIN DRUG EAGEN 63868-0310-12 0.00799 QC MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 CHAIN DRUG MLGEN 63868-0056-30 0.13520 QC NASAL RELIEF 0.05% SPRAY 0 CHAIN DRUG MLGEN 63868-<strong>06</strong>11-32 0.03330 QC NIGHTTIME SLEEP 25 MG TAB 0 CHAIN DRUG EAGEN 63868-0507-01 0.02807 QC NON-ASPIRIN PAIN RELIEF TB 0 CHAIN DRUG EAGEN 63868-0<strong>08</strong>8-01 0.02807 QC NON-ASPIRIN 500 MG CAPLET 0 CHAIN DRUG EAGEN 63868-0<strong>08</strong>8-03 0.02807 QC NON-ASPIRIN 500 MG CAPLET 0 CHAIN DRUG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63868-0<strong>08</strong>8-24 0.02807 QC NON-ASPIRIN 500 MG CAPLET 0 CHAIN DRUG EAGEN 63868-0<strong>08</strong>8-50 0.02807 QC NON-ASPIRIN 500 MG CAPLET 0 CHAIN DRUG EAGEN 63868-0503-50 0.02807 QC NON-ASPIRIN 500 MG CAPLET 0 CHAIN DRUG EAGEN 63868-0987-10 0.02807 QC NON-ASPIRIN 500 MG GELCAP 0 CHAIN DRUG EAGEN 63868-0315-10 0.02807 QC NON-ASPIRIN 500 MG TABLET 0 CHAIN DRUG EAGEN 63868-0315-60 0.02807 QC NON-ASPIRIN 500 MG TABLET 0 CHAIN DRUG EAGEN 63868-0989-30 0.13647 QC PINK BISMUTH TABLET CHEW 0 CHAIN DRUG EAGEN 63868-0380-45 0.0<strong>06</strong>21 QC READY TO USE ENEMA 0 CHAIN DRUG MLGEN 63868-0380-90 0.0<strong>06</strong>21 QC READY TO USE ENEMA 0 CHAIN DRUG MLGEN 63868-0789-24 0.03330 QC REST SIMPLY 25 MG CAPLET 0 CHAIN DRUG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 63868-0263-01 0.03135 QC SENNA LAXATIVE 8.6 MG TAB 0 CHAIN DRUG EAGEN 63868-0104-46 0.14930 QC TOLNAFTATE 1% CREAM 0 CHAIN DRUG GMGEN 63868-0244-04 0.02680 QC TUSSIN CF LIQUID 0 CHAIN DRUG MLGEN 63868-0244-<strong>08</strong> 0.02680 QC TUSSIN CF LIQUID 0 CHAIN DRUG MLGEN 63868-<strong>08</strong>55-54 0.01624 QC TUSSIN DM SYRUP 0 CHAIN DRUG MLGEN 63868-<strong>08</strong>55-56 0.01624 QC TUSSIN DM SYRUP 0 CHAIN DRUG MLGEN 63868-<strong>08</strong>57-54 0.01900 QC TUSSIN 100 MG/5 ML LIQUID 0 CHAIN DRUG MLGEN 63868-<strong>08</strong>57-56 0.01310 QC TUSSIN 100 MG/5 ML LIQUID 0 CHAIN DRUG MLGEX 0<strong>06</strong>03-<strong>08</strong>60-54 0.02831 QUENALIN 12.5 MG/5 ML SYRUP 0 QUALITEST MLGEN 00904-5832-24 0.07430 RANITIDINE 150 MG TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5832-51 0.07430 RANITIDINE 150 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6350-24 0.02889 RANITIDINE 150 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6350-51 0.02889 RANITIDINE 150 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5818-46 0.10993 RANITIDINE 75 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-6349-46 0.10993 RANITIDINE 75 MG TABLET 0 MAJOR PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 511LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6349-52 0.10993 RANITIDINE 75 MG TABLET 0 MAJOR PHARMACEU EAGEN 37205-0531-65 0.10993 RANITIDINE 75 MG TABLET 0 LEADER EAGEN 37205-0531-72 0.10993 RANITIDINE 75 MG TABLET 0 LEADER EAGEN 49614-0220-65 0.10993 RANITIDINE 75 MG TABLET 0 MEDICINE SHOP EABND 00023-4554-30 0.27930 REFRESH CELLUVISC 1% EYE DROPS 0 ALLERGAN INC. EABND 00023-05<strong>06</strong>-01 0.27930 REFRESH CLASSIC EYE DROPS 0 ALLERGAN INC. EABND 00023-05<strong>06</strong>-50 0.27930 REFRESH CLASSIC EYE DROPS 0 ALLERGAN INC. EABND 00023-0312-04 1.37180 REFRESH LACRI-LUBE OINTMENT 0 ALLERGAN INC. GMBND 00023-0312-07 1.37180 REFRESH LACRI-LUBE OINTMENT 0 ALLERGAN INC. GMBND 00023-3240-15 0.27930 REFRESH OPTIVE EYE DROPS 0 ALLERGAN INC. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00023-3416-30 0.27930 REFRESH OPTIVE SENSITIVE DROPS 0 ALLERGAN INC. EABND 00023-3416-60 0.27930 REFRESH OPTIVE SENSITIVE DROPS 0 ALLERGAN INC. EABND 00023-0403-30 0.27930 REFRESH PLUS 0.5% EYE DROPS 0 ALLERGAN INC. EABND 00023-0403-50 0.27930 REFRESH PLUS 0.5% EYE DROPS 0 ALLERGAN INC. EABND 00023-0403-70 0.27930 REFRESH PLUS 0.5% EYE DROPS 0 ALLERGAN INC. EABND 00023-5487-30 0.27930 REFRESH PLUS 0.5% EYE DROPS 0 ALLERGAN INC. EABND 00023-0798-15 0.27930 REFRESH TEARS 0.5% EYE DROPS 0 ALLERGAN INC. MLGEN 00536-1875-16 0.01331 REGULOID LAXATIVE POWDER 0 RUGBY GMGEN 00536-1875-79 0.01331 REGULOID LAXATIVE POWDER 0 RUGBY GMGEN 00536-1881-16 0.01331 REGULOID LAXATIVE POWDER 0 RUGBY GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00536-1881-79 0.01331 REGULOID LAXATIVE POWDER 0 RUGBY GMGEN 00536-4444-54 0.01295 REGULOID POWDER 0 RUGBY GMGEN 00536-4444-89 0.01295 REGULOID POWDER 0 RUGBY GMGEN 00536-4445-54 0.01295 REGULOID POWDER ORANGE 0 RUGBY GMGEN 00536-4445-89 0.01295 REGULOID POWDER ORANGE 0 RUGBY GMBND 00002-8315-91 2.21020 RELION HUMULIN N 100 UNIT/ML 0 ELI LILLY & CO. MLBND 00002-8215-91 2.21020 RELION HUMULIN R 100 UNIT/ML 0 ELI LILLY & CO. MLBND 00002-8715-91 2.21020 RELION HUMULIN 70-30 VIAL 0 ELI LILLY & CO. MLBND 00169-1834-02 10.04020 RELION NOVOLIN N 100 UNIT/ML 0 NOVO NORDISK-WA MLBND 00169-1833-02 10.04020 RELION NOVOLIN R 100 UNIT/ML 0 NOVO NORDISK-WA ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00169-1837-02 10.04020 RELION NOVOLIN 70-30 VIAL 0 NOVO NORDISK-WA MLGEN 37205-0194-62 0.03330 RESTFULLY SLEEP CAPLET 0 LEADER EAGEN 00904-63<strong>06</strong>-20 0.02680 ROBAFEN DM COUGH LIQUID 0 MAJOR PHARMACEU MLGEN 00904-0<strong>06</strong>1-00 0.01900 ROBAFEN 100 MG/5 ML SYRUP 0 MAJOR PHARMACEU MLGEN 00904-0<strong>06</strong>1-09 0.01310 ROBAFEN 100 MG/5 ML SYRUP 0 MAJOR PHARMACEU MLGEN 00904-0<strong>06</strong>1-16 0.01310 ROBAFEN 100 MG/5 ML SYRUP 0 MAJOR PHARMACEU MLGEN 00904-0053-09 0.01624 ROBAFEN-DM SYRUP 0 MAJOR PHARMACEU MLGEN 00904-0053-16 0.01624 ROBAFEN-DM SYRUP 0 MAJOR PHARMACEU MLGEN 00904-0053-20 0.01624 ROBAFEN-DM SYRUP 0 MAJOR PHARMACEU MLBND 00031-8693-12 0.02680 ROBITUSSIN LONG-ACTING LIQ 0 WYETH CONSUMER ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00536-1945-83 0.0<strong>08</strong>70 RULOX SUSPENSION 0 RUGBY MLGEN 00536-25<strong>06</strong>-76 0.07570 SALINE MIST 0.65% NOSE SPRY 0 RUGBY MLGEX 15127-0283-24 0.03330 SB ALLERGY MEDICINE 25 MG CAP 0 SELECT BRAND EAGEN 15127-0909-14 0.12903 SB ALLERGY 10 MG TABLET 0 SELECT BRAND EAGEN 15127-0909-30 0.12903 SB ALLERGY 10 MG TABLET 0 SELECT BRAND EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 512LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 15127-0909-90 0.12903 SB ALLERGY 10 MG TABLET 0 SELECT BRAND EAGEN 15127-0745-52 0.0<strong>08</strong>70 SB ANTACID ANTI-GAS D-S LIQ 0 SELECT BRAND MLGEN 15127-0207-37 0.04333 SB ANTACID XTRA STR CHEW TAB 0 SELECT BRAND EAGEN 15127-0210-24 0.03531 SB ANTACID 500 MG CHEW TABLET 0 SELECT BRAND EAGEN 15127-0211-24 0.03531 SB ANTACID 500 MG CHEW TABLET 0 SELECT BRAND EAGEN 15127-<strong>08</strong>83-52 0.0<strong>08</strong>70 SB ANTACID-ANTIGAS LIQUID 0 SELECT BRAND MLGEN 15127-0338-12 0.23958 SB ANTI-DIARRHEA 2 MG CAPLET 0 SELECT BRAND EAGEN 15127-0338-66 0.23958 SB ANTI-DIARRHEA 2 MG CAPLET 0 SELECT BRAND EAGEN 15127-0011-05 0.02128 SB ASPIRIN EC 325 MG TABLET 0 SELECT BRAND EAGEN 15127-0760-<strong>08</strong> 0.02128 SB ASPIRIN EC 325 MG TABLET 0 SELECT BRAND EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 15127-0738-01 0.01740 SB ASPIRIN 325 MG TABLET 0 SELECT BRAND EAGEN 15127-0738-05 0.01740 SB ASPIRIN 325 MG TABLET 0 SELECT BRAND EAGEN 15127-0738-10 0.01740 SB ASPIRIN 325 MG TABLET 0 SELECT BRAND EAGEN 15127-0738-21 0.01740 SB ASPIRIN 325 MG TABLET 0 SELECT BRAND EAGEN 15127-0178-07 0.03100 SB BISACODYL EC 5 MG TABLET 0 SELECT BRAND EAGEN 15127-0180-<strong>08</strong> 0.13647 SB BISMUTH CHEWABLE TABLET 0 SELECT BRAND EAGEN 15127-0550-68 0.01889 SB BISMUTH REGULAR LIQUID 0 SELECT BRAND MLGEN 15127-0016-01 0.02360 SB CHLORPHENIRAMINE 4 MG TAB 0 SELECT BRAND EAGEN 15127-<strong>08</strong>21-09 0.<strong>08</strong>580 SB CHLORPHENIRAMINE 4 MG TAB 0 SELECT BRAND EAGEN 15127-<strong>08</strong>64-<strong>08</strong> 0.02336 SB COUGH CONTROL DM MAX LIQUID 0 SELECT BRAND ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 15127-0940-44 0.01900 SB COUGH CONTROL SYRUP 0 SELECT BRAND MLGEN 15127-0940-48 0.01310 SB COUGH CONTROL SYRUP 0 SELECT BRAND MLGEN 15127-0288-10 0.03020 SB DOCUSATE SOD 100 MG CAPSULE 0 SELECT BRAND EAGEN 15127-0213-20 0.09133 SB FIBER LAXATIVE 625 MG TAB 0 SELECT BRAND EAGEN 15127-0335-01 0.05567 SB IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 SELECT BRAND EAGEN 15127-0335-24 0.05567 SB IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 SELECT BRAND EAGEN 15127-0907-24 0.05567 SB IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 SELECT BRAND EAGEN 15127-0907-50 0.05567 SB IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 SELECT BRAND EAGEN 15127-0312-01 0.05567 SB IBUPR<strong>OF</strong>EN 200 MG TABLET 0 SELECT BRAND EAGEN 15127-0312-24 0.05567 SB IBUPR<strong>OF</strong>EN 200 MG TABLET 0 SELECT BRAND EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 15127-0312-50 0.05567 SB IBUPR<strong>OF</strong>EN 200 MG TABLET 0 SELECT BRAND EAGEN 15127-0905-50 0.05567 SB IBUPR<strong>OF</strong>EN 200 MG TABLET 0 SELECT BRAND EAGEN 49348-0443-34 0.05462 SB LICE KILLING SHAMPOO 0 SUNMARK MLGEN 15127-0715-01 0.09890 SB LORATADINE 10 MG TABLET 0 SELECT BRAND EAGEN 15127-0262-12 0.05538 SB MOTION SICKNESS 50 MG TAB 0 SELECT BRAND EAGEN 15127-<strong>08</strong>04-13 0.01295 SB NATURAL FIBER LAX POWDER 0 SELECT BRAND GMGEN 15127-<strong>08</strong>04-19 0.01295 SB NATURAL FIBER LAX POWDER 0 SELECT BRAND GMGEN 15127-0072-<strong>08</strong> 0.02968 SB NON-ASPIRIN 325 MG TABLET 0 SELECT BRAND EAGEN 15127-0072-24 0.02968 SB NON-ASPIRIN 325 MG TABLET 0 SELECT BRAND EAGEN 15127-0735-<strong>08</strong> 0.02807 SB NON-ASPIRIN 500 MG CAPLET 0 SELECT BRAND EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 15127-0735-09 0.02807 SB NON-ASPIRIN 500 MG CAPLET 0 SELECT BRAND EAGEN 15127-0735-16 0.02807 SB NON-ASPIRIN 500 MG CAPLET 0 SELECT BRAND EAGEN 15127-0730-<strong>06</strong> 0.02807 SB NON-ASPIRIN 500 MG TABLET 0 SELECT BRAND EAGEN 15127-0730-21 0.02807 SB NON-ASPIRIN 500 MG TABLET 0 SELECT BRAND EAGEN 15127-<strong>08</strong>30-04 0.07070 SB NON-ASPIRIN 80 MG TAB CHW 0 SELECT BRAND EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 513LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 15127-<strong>08</strong>32-04 0.07070 SB NON-ASPIRIN 80 MG TAB CHW 0 SELECT BRAND EAGEN 15127-0735-05 0.02807 SB PAIN RELIEVER 500 MG CAPLET 0 SELECT BRAND EAGEN 15127-0258-16 0.03330 SB SLEEP TABLET 0 SELECT BRAND EAGEN 15127-0304-05 0.13520 SB 12HR NASAL SPRAY 0.05% 0 SELECT BRAND MLGEN 15127-0304-10 0.13520 SB 12HR NASAL SPRAY 0.05% 0 SELECT BRAND MLGEN 00536-5904-01 0.03135 SENEXON TABLET 0 RUGBY EAGEN 00536-5904-10 0.03135 SENEXON TABLET 0 RUGBY EAGEN 00536-1275-59 0.05474 SENEXON 8.8 MG/5 ML LIQUID 0 RUGBY MLGEN 00536-4<strong>08</strong>6-01 0.04150 SENEXON-S TABLET 0 RUGBY EAGEN 00536-4<strong>08</strong>6-10 0.04150 SENEXON-S TABLET 0 RUGBY EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-5165-59 0.03135 SENNA LAXATIVE 8.6 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5165-80 0.03135 SENNA LAXATIVE 8.6 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5512-52 0.04150 SENNA PLUS TABLET 0 MAJOR PHARMACEU EAGEN 00904-5512-80 0.04150 SENNA PLUS TABLET 0 MAJOR PHARMACEU EAGEN 24385-0505-72 0.04150 SENNA PLUS TABLET 0 AMERISOURCEBERG EAGEN 51645-<strong>08</strong>50-01 0.04150 SENNA PLUS TABLET 0 PLUS PHARMA,INC EAGEN 51645-<strong>08</strong>50-10 0.04150 SENNA PLUS TABLET 0 PLUS PHARMA,INC EAGEN 68<strong>08</strong>4-0050-11 0.04150 SENNA PLUS TABLET 0 AHP EAGEN 00904-5165-61 0.03135 SENNA 8.6 MG TABLET 0 MAJOR PHARMACEU EAGEN 37205-0241-78 0.03135 SENNA 8.6 MG TABLET 0 LEADER EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6289-09 0.05474 SENNA 8.8 MG/5 ML SYRUP 0 MAJOR PHARMACEU MLGEN 00536-0355-01 0.04150 SENNA-DOCUSATE SODIUM TABLET 0 RUGBY EAGEN 00182-1093-01 0.03135 SENNA-GEN NF TABLET 0 IVAX PHARMACEUT EAGEN 00182-1093-10 0.03135 SENNA-GEN NF TABLET 0 IVAX PHARMACEUT EAGEN 0<strong>06</strong>03-0282-21 0.03135 SENNA-LAX 8.6 MG TABLET 0 QUALITEST EAGEN 0<strong>06</strong>03-0282-32 0.03135 SENNA-LAX 8.6 MG TABLET 0 QUALITEST EAGEN 24385-0404-78 0.03135 SENNA-LAX 8.6 MG TABLET 0 AMERISOURCEBERG EAGEN 63739-0432-01 0.04150 SENNA-TIME S TABLET 0 MCKESSON PACKAG EAGEN 49483-0<strong>08</strong>0-01 0.03135 SENNA-TIME 8.6 MG TABLET 0 TIME-CAP LABS EAGEN 49483-0<strong>08</strong>0-10 0.03135 SENNA-TIME 8.6 MG TABLET 0 TIME-CAP LABS EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 0<strong>06</strong>03-0283-32 0.04150 SENNALAX-S TABLET 0 QUALITEST EAGEN 62107-0031-01 0.03135 SENNO TABLET 0 PRIME MARKETING EAGEN 54838-0116-80 0.01200 SILACE 50 MG/5 ML LIQUID 0 SILARX PHARM MLGEN 54838-0107-80 0.00990 SILACE 60 MG/15 ML SYRUP 0 SILARX PHARM MLGEX 54838-0135-40 0.02118 SILADRYL 12.5 MG/5 ML LIQUID 0 SILARX PHARM MLGEX 54838-0135-70 0.02118 SILADRYL 12.5 MG/5 ML LIQUID 0 SILARX PHARM MLGEX 54838-0135-80 0.02118 SILADRYL 12.5 MG/5 ML LIQUID 0 SILARX PHARM MLBND 54838-0145-15 0.18396 SILAPAP INFANT'S DROPS 0 SILARX PHARM MLBND 54838-0145-30 0.18396 SILAPAP INFANT'S DROPS 0 SILARX PHARM MLGEN 51927-9009-00 SILICA GEL POWDER 0 PR<strong>OF</strong>ESSIONAL CO GM--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 54838-0154-40 0.02831 SILPHEN COUGH SYRUP 0 SILARX PHARM MLGEX 54838-0154-70 0.02831 SILPHEN COUGH SYRUP 0 SILARX PHARM MLGEX 54838-0154-80 0.02831 SILPHEN COUGH SYRUP 0 SILARX PHARM MLGEN 54838-0209-40 0.01624 SILTUSSIN DM COUGH SYRUP 0 SILARX PHARM MLGEN 54838-0209-70 0.01624 SILTUSSIN DM COUGH SYRUP 0 SILARX PHARM ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 514LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 54838-0209-80 0.01624 SILTUSSIN DM COUGH SYRUP 0 SILARX PHARM MLGEN 54838-0133-40 0.02680 SILTUSSIN DM DAS LIQUID 0 SILARX PHARM MLGEN 54838-0117-40 0.01900 SILTUSSIN SA 100 MG/5 ML SYR 0 SILARX PHARM MLGEN 54838-0117-70 0.01310 SILTUSSIN SA 100 MG/5 ML SYR 0 SILARX PHARM MLGEN 54838-0117-80 0.01310 SILTUSSIN SA 100 MG/5 ML SYR 0 SILARX PHARM MLGEN 38779-1779-<strong>08</strong> 0.57000 SIMPLE SYRUP 0 MEDISCA INC. MLGEN 24385-0961-62 0.10098 SINUS & ALLERGY PE TABLET 0 AMERISOURCEBERG EAGEN 36800-<strong>06</strong>88-05 0.13520 SINUS RELIEF 0.05% NASAL SPRAY 0 TOPCO MLBND 00904-4274-51 0.03330 SLEEP TABS 25 MG TABLET 0 MAJOR PHARMACEU EABND 67618-0107-60 0.05500 SLOW-MAG 71.5 MG TABLET 0 PURDUE PROD LP EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-0442-12 0.13110 SM ACID REDUCER 10 MG TABLET 0 SUNMARK EAGEN 49348-0442-44 0.13110 SM ACID REDUCER 10 MG TABLET 0 SUNMARK EAGEN 49348-0246-44 0.20443 SM ACID REDUCER 200 MG TABLET 0 SUNMARK EAGEN 49348-0733-12 0.10993 SM ACID REDUCER 75 MG TABLET 0 SUNMARK EAGEN 49348-0733-44 0.10993 SM ACID REDUCER 75 MG TABLET 0 SUNMARK EAGEN 49348-0939-44 0.12903 SM ALL DAY ALLERGY 10 MG TAB 0 SUNMARK EAGEN 49348-0984-46 0.12903 SM ALL DAY ALLERGY 10 MG TAB 0 SUNMARK EAGEN 49348-<strong>08</strong>51-04 0.61830 SM ALL DAY ALLERGY-D TABLET G SUNMARK EAGEX 49348-0045-34 0.02118 SM ALLERGY RELIEF 12.5 MG/5 ML 0 SUNMARK MLGEX 49348-0045-37 0.02118 SM ALLERGY RELIEF 12.5 MG/5 ML 0 SUNMARK ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEX 49348-0971-10 0.03330 SM ALLERGY RELIEF 25 MG CAP 0 SUNMARK EAGEN 49348-0025-04 0.<strong>08</strong>580 SM ALLERGY 4-HR 4 MG TABLET 0 SUNMARK EAGEN 49348-0025-10 0.02360 SM ALLERGY 4-HR 4 MG TABLET 0 SUNMARK EAGEN 49348-0035-39 0.0<strong>08</strong>70 SM ANTACID ANTI-GAS LIQUID 0 SUNMARK MLGEN 49348-0019-39 0.0<strong>08</strong>70 SM ANTACID SUSPENSION 0 SUNMARK MLGEN 49348-0744-39 0.0<strong>08</strong>70 SM ANTACID SUSPENSION 0 SUNMARK MLGEN 49348-<strong>08</strong>65-13 0.04333 SM ANTACID XTRA STR CHEW TAB 0 SUNMARK EAGEN 49348-0169-21 0.03531 SM ANTACID 500 MG CHEW TABLET 0 SUNMARK EAGEN 49348-0170-21 0.03531 SM ANTACID 500 MG CHEW TABLET 0 SUNMARK EAGEN 49348-0020-39 0.0<strong>08</strong>70 SM ANTACID-ANTIGAS LIQUID 0 SUNMARK ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-0529-02 0.23958 SM ANTI-DIARRHEAL 2 MG CAPLET 0 SUNMARK EAGEN 49348-0529-04 0.23958 SM ANTI-DIARRHEAL 2 MG CAPLET 0 SUNMARK EAGEN 49348-0529-<strong>08</strong> 0.23958 SM ANTI-DIARRHEAL 2 MG CAPLET 0 SUNMARK EAGEN 49348-0529-34 0.23958 SM ANTI-DIARRHEAL 2 MG CAPLET 0 SUNMARK EAGEN 49348-0154-72 0.<strong>06</strong>579 SM ANTIBIOTIC 500 UNIT/GM OINT 0 SUNMARK GMGEN 49348-0155-29 0.14930 SM ANTIFUNGAL 1% CREAM 0 SUNMARK GMGEN 49348-0279-72 0.11290 SM ANTIFUNGAL 1% CREAM 0 SUNMARK GMGEN 49348-0921-09 0.04260 SM ARTHRITIS PAIN ER 650 MG 0 SUNMARK EAGEN 49348-0921-10 0.04260 SM ARTHRITIS PAIN ER 650 MG 0 SUNMARK EABND 49348-<strong>06</strong>99-29 0.26403 SM ARTIFICIAL TEARS 0 SUNMARK ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-<strong>06</strong>53-15 0.02781 SM ASPIRIN EC 81 MG TABLET 0 SUNMARK EAGEN 49348-0001-10 0.01740 SM ASPIRIN 325 MG TABLET 0 SUNMARK EAGEN 49348-0001-14 0.01740 SM ASPIRIN 325 MG TABLET 0 SUNMARK EAGEN 49348-0001-23 0.01740 SM ASPIRIN 325 MG TABLET 0 SUNMARK EAGEN 49348-0498-07 0.04820 SM ASPIRIN 81 MG CHEWABLE TAB 0 SUNMARK EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 515LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-0790-72 0.33487 SM ATHLETE'S 1% FOOT CREAM 0 SUNMARK GMGEN 49348-0561-34 0.04333 SM CAL ANTACID 750 MG CHEW TAB 0 SUNMARK EAGEN 49348-0954-34 0.04333 SM CAL ANTACID 750 MG CHEW TAB 0 SUNMARK EAGEN 49348-0264-43 0.03765 SM CALCIUM 500 + VIT D 400 TAB 0 SUNMARK EAGEN 49348-0774-09 0.20760 SM CHEST CONG RELIEF PE CAPLET 0 SUNMARK EAGEN 49348-0728-09 0.02680 SM CHEST CONGEST RLF DM CAPLET 0 SUNMARK EAGEN 49348-0729-09 0.15291 SM CHEST CONGESTION RELIEF CAP 0 SUNMARK EAGEN 49348-0934-34 0.05700 SM CHILD ALL DAY ALLER 1 MG/ML 0 SUNMARK MLGEN 49348-0191-07 0.04820 SM CHILD ASPIRIN 81 MG CHW TAB 0 SUNMARK EAGEN 49348-0757-07 0.04820 SM CHILD ASPIRIN 81 MG CHW TAB 0 SUNMARK EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-0777-34 0.02160 SM CHILD COLD- ALLERGY LIQUID 0 SUNMARK MLGEN 49348-0777-37 0.02140 SM CHILD COLD- ALLERGY LIQUID 0 SUNMARK MLGEN 49348-0266-34 0.02330 SM CHILD'S PAIN RELIEVER SUSP 0 SUNMARK MLGEN 49348-0797-34 0.02330 SM CHILD'S PAIN RELIEVER SUSP 0 SUNMARK MLGEN 49348-<strong>08</strong>88-34 0.02330 SM CHILD'S PAIN RELIEVER SUSP 0 SUNMARK MLGEN 49348-<strong>08</strong>93-50 0.03193 SM CLEARLAX POWDER 0 SUNMARK GMGEN 49348-<strong>08</strong>93-70 0.03193 SM CLEARLAX POWDER 0 SUNMARK GMGEN 49348-<strong>08</strong>93-92 0.03193 SM CLEARLAX POWDER 0 SUNMARK GMGEN 49348-0715-04 0.10098 SM COLD & ALLERGY PE TABLET 0 SUNMARK EAGEN 49348-0541-10 0.09983 SM FIBER LAXATIVE 500 MG CPLT 0 SUNMARK EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-0759-13 0.09133 SM FIBER LAXATIVE 625 MG TAB 0 SUNMARK EABND 49348-0047-65 0.01970 SM FIBER POWDER 0 SUNMARK GMGEN 49348-0166-65 0.01482 SM FIBER POWDER 0 SUNMARK GMGEN 49348-0166-93 0.01482 SM FIBER POWDER 0 SUNMARK GMGEN 49348-0599-05 0.03100 SM GENTLE LAXATIVE EC 5 MG TAB 0 SUNMARK EAGEN 49348-0913-01 0.15207 SM GLUCOSE 4 GRAM TAB CHEW 0 SUNMARK EAGEN 49348-0914-09 0.15207 SM GLUCOSE 4 GRAM TAB CHEW 0 SUNMARK EAGEN 49348-0915-01 0.15207 SM GLUCOSE 4 GRAM TAB CHEW 0 SUNMARK EAGEN 49348-0916-09 0.15207 SM GLUCOSE 4 GRAM TAB CHEW 0 SUNMARK EAGEN 49348-0247-09 0.20443 SM HEARTBURN RELIEF 200 MG TAB 0 SUNMARK EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-0435-78 0.<strong>08</strong>144 SM HYDROCORTISONE 1% CREAM 0 SUNMARK GMGEN 49348-0521-72 0.<strong>08</strong>144 SM HYDROCORTISONE 1% CREAM 0 SUNMARK GMGEN 49348-0522-72 0.10370 SM HYDROCORTISONE 1% OINTMENT 0 SUNMARK GMGEN 49348-0727-09 0.05567 SM IBUPR<strong>OF</strong>EN IB 200 MG CAPLET 0 SUNMARK EAGEN 49348-0727-10 0.05567 SM IBUPR<strong>OF</strong>EN IB 200 MG CAPLET 0 SUNMARK EAGEN 49348-<strong>08</strong>29-09 0.05567 SM IBUPR<strong>OF</strong>EN IB 200 MG TABLET 0 SUNMARK EAGEN 49348-<strong>08</strong>29-10 0.05567 SM IBUPR<strong>OF</strong>EN IB 200 MG TABLET 0 SUNMARK EAGEN 49348-0229-34 0.04567 SM IBUPR<strong>OF</strong>EN 100 MG/5 ML SUSP 0 SUNMARK MLGEN 49348-0229-37 0.04567 SM IBUPR<strong>OF</strong>EN 100 MG/5 ML SUSP 0 SUNMARK MLGEN 49348-0499-34 0.04567 SM IBUPR<strong>OF</strong>EN 100 MG/5 ML SUSP 0 SUNMARK ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-0500-34 0.04567 SM IBUPR<strong>OF</strong>EN 100 MG/5 ML SUSP 0 SUNMARK MLGEN 49348-0196-09 0.05567 SM IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 SUNMARK EAGEN 49348-0196-10 0.05567 SM IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 SUNMARK EAGEN 49348-0196-19 0.05567 SM IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 SUNMARK EAGEN 49348-0196-35 0.05567 SM IBUPR<strong>OF</strong>EN 200 MG CAPLET 0 SUNMARK EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 516LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-07<strong>06</strong>-04 0.05567 SM IBUPR<strong>OF</strong>EN 200 MG TABLET 0 SUNMARK EAGEN 49348-07<strong>06</strong>-09 0.05567 SM IBUPR<strong>OF</strong>EN 200 MG TABLET 0 SUNMARK EAGEN 49348-07<strong>06</strong>-10 0.05567 SM IBUPR<strong>OF</strong>EN 200 MG TABLET 0 SUNMARK EAGEN 49348-07<strong>06</strong>-14 0.05567 SM IBUPR<strong>OF</strong>EN 200 MG TABLET 0 SUNMARK EAGEN 49348-07<strong>06</strong>-19 0.05567 SM IBUPR<strong>OF</strong>EN 200 MG TABLET 0 SUNMARK EAGEN 49348-0460-30 0.13501 SM LICE TREATMENT PERMETHRIN 0 SUNMARK MLGEN 49348-0460-34 0.13501 SM LICE TREATMENT PERMETHRIN 0 SUNMARK MLGEN 49348-0543-01 0.61380 SM LORATA-DINE D 24HR TABLET G SUNMARK EAGEN 49348-0543-57 0.61380 SM LORATA-DINE D 24HR TABLET G SUNMARK EAGEN 49348-<strong>08</strong>18-01 0.09890 SM LORATADINE 10 MG TABLET 0 SUNMARK EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-<strong>08</strong>18-12 0.09890 SM LORATADINE 10 MG TABLET 0 SUNMARK EAGEN 49348-<strong>08</strong>18-13 0.09890 SM LORATADINE 10 MG TABLET 0 SUNMARK EAGEN 49348-<strong>08</strong>18-44 0.09890 SM LORATADINE 10 MG TABLET 0 SUNMARK EAGEN 49348-<strong>06</strong>36-34 0.04749 SM LORATADINE 5 MG/5 ML SYRUP 0 SUNMARK MLGEN 49348-<strong>08</strong>72-77 0.13054 SM MICONAZOLE NITRATE 2% CREAM 0 SUNMARK GMGEN 49348-0530-77 0.13054 SM MICONAZOLE 7 CREAM 0 SUNMARK GMGEN 49348-<strong>08</strong>33-61 0.80714 SM MICONAZOLE 7 100 MG VAG SUP 0 SUNMARK EAGEN 49348-<strong>06</strong>80-39 0.00799 SM MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 SUNMARK MLGEN 49348-<strong>06</strong>87-39 0.00799 SM MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 SUNMARK MLGEN 49348-<strong>06</strong>88-39 0.00799 SM MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 SUNMARK ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-<strong>06</strong>88-44 0.00799 SM MILK <strong>OF</strong> MAGNESIA SUSPENSION 0 SUNMARK MLGEN 49348-<strong>08</strong>04-38 0.0<strong>08</strong>10 SM MINERAL OIL HEAVY 0 SUNMARK MLGEN 49348-0070-02 0.05538 SM MOTION SICKNESS 50 MG TAB 0 SUNMARK EAGEN 49348-<strong>08</strong>28-34 0.02680 SM MUCUS RELIEF COUGH LIQUID 0 SUNMARK MLGEN 49348-0024-04 0.07353 SM NASAL DECONGEST 30 MG TAB 0 SUNMARK EAGEN 49348-0024-34 0.07353 SM NASAL DECONGEST 30 MG TAB 0 SUNMARK EAGEN 49348-0028-27 0.13520 SM NASAL SPRAY 0.05% 0 SUNMARK MLGEN 49348-0276-27 0.13520 SM NASAL SPRAY 0.05% 0 SUNMARK MLGEN 49348-0532-12 0.04150 SM NAT LAX PLUS STOOL S<strong>OF</strong>TENER 0 SUNMARK EAGEN 49348-0573-<strong>08</strong> 0.20128 SM NICOTINE 2 MG CHEWING GUM 0 SUNMARK EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-0573-36 0.20128 SM NICOTINE 2 MG CHEWING GUM 0 SUNMARK EAGEN 49348-<strong>06</strong>91-36 0.20128 SM NICOTINE 2 MG CHEWING GUM 0 SUNMARK EAGEN 49348-0787-10 0.20128 SM NICOTINE 2 MG CHEWING GUM 0 SUNMARK EAGEN 49348-0572-<strong>08</strong> 0.25968 SM NICOTINE 4 MG CHEWING GUM 0 SUNMARK EAGEN 49348-0572-36 0.25968 SM NICOTINE 4 MG CHEWING GUM 0 SUNMARK EAGEN 49348-<strong>06</strong>92-36 0.25968 SM NICOTINE 4 MG CHEWING GUM 0 SUNMARK EAGEN 49348-0788-10 0.25968 SM NICOTINE 4 MG CHEWING GUM 0 SUNMARK EAGEN 00<strong>06</strong>7-6128-14 1.77934 SM NICOTINE 7 MG/24HR PATCH 0 SUNMARK EAGEN 49348-<strong>08</strong>46-46 0.54542 SM OMEPRAZOLE DR 20 MG TABLET G SUNMARK EAGEN 49348-<strong>08</strong>46-61 0.54542 SM OMEPRAZOLE DR 20 MG TABLET G SUNMARK EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-<strong>08</strong>46-78 0.54542 SM OMEPRAZOLE DR 20 MG TABLET G SUNMARK EAGEN 49348-0042-09 0.02807 SM PAIN RELIEVER 500 MG CAPLET 0 SUNMARK EAGEN 49348-0042-10 0.02807 SM PAIN RELIEVER 500 MG CAPLET 0 SUNMARK EAGEN 49348-0042-14 0.02807 SM PAIN RELIEVER 500 MG CAPLET 0 SUNMARK EAGEN 49348-0730-09 0.02807 SM PAIN RELIEVER 500 MG TABLET 0 SUNMARK EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 517LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-0730-10 0.02807 SM PAIN RELIEVER 500 MG TABLET 0 SUNMARK EAGEN 49348-0998-10 0.02807 SM PAIN RELIEVER 500 MG TABLET 0 SUNMARK EAGEN 49348-0356-25 0.07570 SM SALINE 0.65% NASAL SPRAY 0 SUNMARK MLGEN 49348-0<strong>06</strong>2-10 0.03135 SM SENNA LAXATIVE 8.6 MG TAB 0 SUNMARK EAGEN 49348-0716-04 0.10098 SM SINUS & ALLERGY PE TABLET 0 SUNMARK EAGEN 49348-0471-04 0.03330 SM SLEEP AID NIGHT TIME CAPLET 0 SUNMARK EAGEN 49348-0922-37 0.01889 SM STOMACH RELIEF 262 MG/15 ML 0 SUNMARK MLGEN 49348-0953-44 0.13647 SM STOMACH RLF 262 MG CHEW TAB 0 SUNMARK EAGEN 49348-<strong>06</strong>16-10 0.03020 SM STOOL S<strong>OF</strong>TENER 100 MG SFTGL 0 SUNMARK EAGEN 49348-0737-34 0.02680 SM TUSSIN CF SYRUP 0 SUNMARK ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-0737-37 0.02680 SM TUSSIN CF SYRUP 0 SUNMARK MLGEN 49348-0017-34 0.01624 SM TUSSIN DM SYRUP 0 SUNMARK MLGEN 49348-0017-37 0.01624 SM TUSSIN DM SYRUP 0 SUNMARK MLGEN 49348-0017-39 0.01624 SM TUSSIN DM SYRUP 0 SUNMARK MLGEN 49348-<strong>08</strong>61-34 0.01624 SM TUSSIN DM SYRUP 0 SUNMARK MLGEN 49348-<strong>08</strong>61-37 0.01624 SM TUSSIN DM SYRUP 0 SUNMARK MLGEN 49348-0278-34 0.01900 SM TUSSIN 100 MG/5 ML LIQUID 0 SUNMARK MLGEN 49348-0278-37 0.01310 SM TUSSIN 100 MG/5 ML LIQUID 0 SUNMARK MLGEN 49348-0<strong>08</strong>8-10 0.04100 SM VITAMIN B-1 100 MG TABLET 0 SUNMARK EAGEN 49348-0924-09 0.04260 SM 8 HOUR PAIN RELIEF 650 MG 0 SUNMARK EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 49348-0924-10 0.04260 SM 8 HOUR PAIN RELIEF 650 MG 0 SUNMARK EAGEN 00536-4544-10 0.01352 SODIUM BICARB 650 MG TABLET 0 RUGBY EAGEN 0<strong>06</strong>77-0131-10 0.01352 SODIUM BICARB 650 MG TABLET 0 URL PHARMA EAGEN 64980-0182-10 0.01352 SODIUM BICARB 650 MG TABLET 0 RISING PHARM EAGEN 00487-9301-03 0.04468 SODIUM CHLORIDE 0.9% INHAL VL 0 NEPHRON CORP MLGEN 00904-5314-35 0.58422 SODIUM CHLORIDE 5% EYE DROP 0 MAJOR PHARMACEU MLGEN 17478-<strong>06</strong>23-12 0.58422 SODIUM CHLORIDE 5% EYE DROP 0 AKORN INC. MLGEN 00904-5315-38 2.57100 SODIUM CHLORIDE 5% EYE OINT 0 MAJOR PHARMACEU GMGEN 17478-<strong>06</strong>22-35 2.57100 SODIUM CHLORIDE 5% EYE OINT 0 AKORN INC. GMGEN 00135-0057-07 0.03330 SOMINEX 25 MG TABLET 0 GSK CONSUMER HE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0302-34 0.01889 STOMACH RLF 262 MG/15 ML SUSP 0 TOPCO MLGEN 36800-0302-40 0.01889 STOMACH RLF 262 MG/15 ML SUSP 0 TOPCO MLGEN 24385-0495-72 0.04150 STOOL S<strong>OF</strong>T-STIMULANT LAX TAB 0 AMERISOURCEBERG EAGEN 24385-0469-43 0.00990 STOOL S<strong>OF</strong>TENER SYRUP 0 AMERISOURCEBERG MLGEN 00113-0486-72 0.03020 STOOL S<strong>OF</strong>TENER 100 MG CAPSULE 0 PERRIGO CO. EABND 66424-0030-10 0.03020 STOOL S<strong>OF</strong>TENER 100 MG CAPSULE 0 SDA LABS EAGEN 36800-0486-72 0.03020 STOOL S<strong>OF</strong>TENER 100 MG S<strong>OF</strong>TGEL 0 TOPCO EAGEN 36800-0486-78 0.03020 STOOL S<strong>OF</strong>TENER 100 MG S<strong>OF</strong>TGEL 0 TOPCO EAGEN 46122-0231-72 0.03020 STOOL S<strong>OF</strong>TENER 100 MG S<strong>OF</strong>TGEL 0 AMERISOURCEBERG EAGEN 46122-0231-78 0.03020 STOOL S<strong>OF</strong>TENER 100 MG S<strong>OF</strong>TGEL 0 AMERISOURCEBERG EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24385-0468-43 0.01200 STOOL S<strong>OF</strong>TENER 50 MG/5 ML LIQ 0 AMERISOURCEBERG MLGEN 24385-0495-78 0.04150 STOOL S<strong>OF</strong>TENER-LAXATIVE TABLET 0 AMERISOURCEBERG EABND 00904-0262-52 0.05040 STRESS FORMULA TABLET 0 MAJOR PHARMACEU EABND 00904-0263-52 0.05040 STRESS FORMULA WITH IRON TAB 0 MAJOR PHARMACEU EABND 00904-2727-52 0.05040 STRESS FORMULA WITH ZINC TAB 0 MAJOR PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 518LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00245-0133-89 0.05040 STRESS 600 WITH ZINC TAB 0 UPSHER SMITH EAGEN 00904-5733-49 0.10400 SUDOGEST PE 10 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5351-96 0.20760 SUDOGEST SINUS & ALLERGY TAB 0 MAJOR PHARMACEU EAGEN 00904-5053-24 0.07353 SUDOGEST 30 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-5053-59 0.07353 SUDOGEST 30 MG TABLET 0 MAJOR PHARMACEU EAGEN 49348-0<strong>08</strong>6-10 0.05040 SUPER B COMPLEX-C CAPLET 0 SUNMARK EAGEN 00904-2253-60 0.05040 SUPERPLEX-T TABLET 0 MAJOR PHARMACEU EAGEN 24385-0432-62 0.07353 SUPHEDRIN 30 MG TABLET 0 AMERISOURCEBERG EAGEN 63868-0146-24 0.07353 SUPHEDRINE SINUS CONG 30 MG TB 0 CHAIN DRUG EAGEN 00574-0307-16 0.04016 SUSPENDOL-S LIQUID 0 PADDOCK LABS. ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00<strong>06</strong>5-0429-30 0.41340 SYSTANE 0.3-0.4% EYE DROPS 0 ALCON CONSUMER MLBND 00<strong>06</strong>5-0429-67 0.41340 SYSTANE 0.3-0.4% EYE DROPS 0 ALCON CONSUMER MLBND 00<strong>06</strong>5-0431-32 0.41340 SYSTANE 0.3-0.4% EYE DROPS 0 ALCON CONSUMER EAGEN 00904-0530-46 0.02950 TAB-A-VITE TABLET 0 MAJOR PHARMACEU EAGEN 00904-0530-60 0.02950 TAB-A-VITE TABLET 0 MAJOR PHARMACEU EAGEN 00904-0530-61 0.02950 TAB-A-VITE TABLET 0 MAJOR PHARMACEU EAGEN 00904-0530-80 0.02950 TAB-A-VITE TABLET 0 MAJOR PHARMACEU EAGEN 00904-0531-60 0.05040 TAB-A-VITE WITH IRON TABLET 0 MAJOR PHARMACEU EAGEN 00904-0531-80 0.05040 TAB-A-VITE WITH IRON TABLET 0 MAJOR PHARMACEU EAGEN 62107-0052-01 0.02968 TACTINAL 325 MG TABLET 0 PRIME MARKETING EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62107-0052-10 0.02968 TACTINAL 325 MG TABLET 0 PRIME MARKETING EAGEN 62107-0051-01 0.02807 TACTINAL 500 MG CAPLET 0 PRIME MARKETING EAGEN 62107-0051-10 0.02807 TACTINAL 500 MG CAPLET 0 PRIME MARKETING EAGEN 62107-0050-01 0.02807 TACTINAL 500 MG TABLET 0 PRIME MARKETING EAGEN 62107-0050-10 0.02807 TACTINAL 500 MG TABLET 0 PRIME MARKETING EABND 00<strong>06</strong>5-0426-22 0.23830 TEARS NATURALE FORTE DROPS 0 ALCON CONSUMER MLBND 00<strong>06</strong>5-0426-23 0.23830 TEARS NATURALE FORTE DROPS 0 ALCON CONSUMER MLBND 00<strong>06</strong>5-0416-25 0.27930 TEARS NATURALE FREE DROPS 0 ALCON CONSUMER EABND 00<strong>06</strong>5-0418-15 0.27930 TEARS NATURALE-II EYE DROPS 0 ALCON CONSUMER MLBND 00<strong>06</strong>5-0418-30 0.27930 TEARS NATURALE-II EYE DROPS 0 ALCON CONSUMER ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 24385-0524-03 0.33487 TERBINAFINE 1% CREAM 0 AMERISOURCEBERG GMGEN 24385-0524-05 0.33487 TERBINAFINE 1% CREAM 0 AMERISOURCEBERG GMGEN 51672-2<strong>08</strong>0-01 0.33487 TERBINAFINE 1% CREAM 0 TARO PHARM USA GMGEN 51672-2<strong>08</strong>0-02 0.33487 TERBINAFINE 1% CREAM 0 TARO PHARM USA GMGEN 62107-0<strong>06</strong>6-01 0.05040 THERA CAPLET 0 PRIME MARKETING EAGEN 62107-0<strong>06</strong>6-05 0.05040 THERA CAPLET 0 PRIME MARKETING EABND 00904-5492-61 0.05040 THERA M PLUS TABLET 0 MAJOR PHARMACEU EABND 00904-0539-13 0.05040 THERA TABLET 0 MAJOR PHARMACEU EABND 00904-0539-61 0.05040 THERA TABLET 0 MAJOR PHARMACEU EABND 00904-0539-80 0.05040 THERA TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 62107-0038-05 0.05040 THERA-M CAPLET 0 PRIME MARKETING EAGEN 62107-0038-13 0.05040 THERA-M CAPLET 0 PRIME MARKETING EABND 00904-5492-13 0.05040 THERA-M TABLET 0 MAJOR PHARMACEU EABND 00904-5492-80 0.05040 THERA-M TABLET 0 MAJOR PHARMACEU EAGEN 37205-0197-10 0.14930 TOLNAFTATE AF 1% CREAM 0 LEADER GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 519LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-0722-36 0.14930 TOLNAFTATE 1% CREAM 0 MAJOR PHARMACEU GMGEN 51672-2020-01 0.14930 TOLNAFTATE 1% CREAM 0 TARO PHARM USA GMGEN 51672-2020-02 0.14930 TOLNAFTATE 1% CREAM 0 TARO PHARM USA GMGEN 00904-0726-45 0.05013 TOLNAFTATE 1% POWDER 0 MAJOR PHARMACEU GMBND 45802-0033-85 0.22930 TOLNAFTATE 1% SOLUTION 0 PERRIGO CO. MLGEN 00904-0260-13 0.05040 TOTAL B WITH VIT C CAPLET 0 MAJOR PHARMACEU EAGEN 63868-0160-12 0.05538 TRAVEL SICKNESS 50 MG TABLET 0 CHAIN DRUG EAGEN 00904-2015-59 0.03420 TRI-BUFFERED ASPIRIN 325 MG 0 MAJOR PHARMACEU EABND 00<strong>08</strong>7-0403-03 0.13500 TRI-VI-SOL DROPS 0 MJ NUTRITIONAL MLBND 00<strong>08</strong>7-0453-03 0.13500 TRI-VI-SOL-IRON DROPS 0 MJ NUTRITIONAL ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-6274-50 0.13500 TRI-VITA DROPS 0 MAJOR PHARMACEU MLBND 00135-0074-22 0.04333 TUMS E-X TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0074-24 0.04333 TUMS E-X TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0074-25 0.04333 TUMS E-X TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0074-46 0.04333 TUMS E-X TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0076-25 0.04333 TUMS E-X TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0140-01 0.04333 TUMS E-X TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0140-03 0.04333 TUMS E-X TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0154-05 0.04333 TUMS E-X TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0178-02 0.04333 TUMS E-X TABLET CHEWABLE 0 GSK CONSUMER HE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00135-0178-03 0.04333 TUMS E-X TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0178-<strong>08</strong> 0.04333 TUMS E-X TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0522-03 0.03531 TUMS FRESHERS ANTACID CHEW TAB 0 GSK CONSUMER HE EABND 00135-0469-01 0.04333 TUMS KIDS 300 MG (750) CHEWTAB 0 GSK CONSUMER HE EABND 00135-0243-02 0.04333 TUMS SMOOTHIES CHEW TABLET 0 GSK CONSUMER HE EABND 00135-0245-02 0.04333 TUMS SMOOTHIES CHEW TABLET 0 GSK CONSUMER HE EABND 00135-0456-03 0.04333 TUMS SMOOTHIES CHEW TABLET 0 GSK CONSUMER HE EABND 00135-0070-03 0.03531 TUMS TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0070-27 0.03531 TUMS TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0070-48 0.03531 TUMS TABLET CHEWABLE 0 GSK CONSUMER HE EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------BND 00135-0071-27 0.03531 TUMS TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0071-48 0.03531 TUMS TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0118-14 0.02190 TUMS ULTRA TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0118-83 0.02190 TUMS ULTRA TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0180-14 0.02190 TUMS ULTRA TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0181-02 0.02190 TUMS ULTRA TABLET CHEWABLE 0 GSK CONSUMER HE EABND 00135-0181-14 0.02190 TUMS ULTRA TABLET CHEWABLE 0 GSK CONSUMER HE EAGEN 54859-0505-04 0.02680 TUSNEL DIABETIC LIQUID 0 LLORENS PHARM MLGEN 36800-0516-26 0.02680 TUSSIN CF COUGH & COLD LIQ 0 TOPCO MLGEN 36800-0516-34 0.02680 TUSSIN CF COUGH & COLD LIQ 0 TOPCO ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00113-0516-26 0.02680 TUSSIN CF COUGH & COLD SYRUP 0 PERRIGO CO. MLGEN 00113-0516-34 0.02680 TUSSIN CF COUGH & COLD SYRUP 0 PERRIGO CO. MLGEN 24385-0904-26 0.02680 TUSSIN CF COUGH & COLD SYRUP 0 AMERISOURCEBERG MLGEN 24385-0904-34 0.02680 TUSSIN CF COUGH & COLD SYRUP 0 AMERISOURCEBERG MLGEN 37205-0709-26 0.02680 TUSSIN CF COUGH & COLD SYRUP 0 LEADER ML** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 520LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 37205-0709-34 0.02680 TUSSIN CF LIQUID 0 LEADER MLGEN 49614-0321-34 0.02680 TUSSIN CF LIQUID 0 MEDICINE SHOP MLGEN 00113-0359-34 0.01624 TUSSIN DM COUGH & CHEST LIQUID 0 PERRIGO CO. MLGEN 00113-0359-26 0.01624 TUSSIN DM COUGH SYRUP 0 PERRIGO CO. MLGEN 36800-0359-26 0.02680 TUSSIN DM LIQUID 0 TOPCO MLGEN 36800-0359-34 0.02680 TUSSIN DM LIQUID 0 TOPCO MLGEN 36800-0359-40 0.02680 TUSSIN DM LIQUID 0 TOPCO MLGEN 36800-0578-26 0.02680 TUSSIN DM LIQUID 0 TOPCO MLGEN 37205-0712-26 0.02680 TUSSIN DM LIQUID 0 LEADER MLGEN 36800-0799-26 0.02336 TUSSIN DM MAX LIQUID 0 TOPCO ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 36800-0799-34 0.02336 TUSSIN DM MAX LIQUID 0 TOPCO MLGEN 46122-0017-26 0.02336 TUSSIN DM MAX LIQUID 0 AMERISOURCEBERG MLGEN 46122-0017-34 0.02336 TUSSIN DM MAX LIQUID 0 AMERISOURCEBERG MLGEN 24385-0359-26 0.01624 TUSSIN DM SYRUP 0 AMERISOURCEBERG MLGEN 24385-0359-34 0.01624 TUSSIN DM SYRUP 0 AMERISOURCEBERG MLGEN 37205-0970-26 0.01624 TUSSIN DM SYRUP 0 LEADER MLGEN 37205-0970-34 0.01624 TUSSIN DM SYRUP 0 LEADER MLGEN 49348-0307-34 0.01910 TUSSIN HONEY SYRUP 0 SUNMARK MLGEN 37205-0960-26 0.01900 TUSSIN MUCUS-CONGEST 100 MG/5 0 LEADER MLGEN 37205-0960-34 0.01310 TUSSIN MUCUS-CONGEST 100 MG/5 0 LEADER ML--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00113-0310-26 0.01900 TUSSIN 100 MG/5 ML SYRUP 0 PERRIGO CO. MLGEN 00113-0310-34 0.01310 TUSSIN 100 MG/5 ML SYRUP 0 PERRIGO CO. MLGEN 24385-0310-26 0.01900 TUSSIN 100 MG/5 ML SYRUP 0 AMERISOURCEBERG MLGEN 24385-0310-34 0.01310 TUSSIN 100 MG/5 ML SYRUP 0 AMERISOURCEBERG MLGEN 49348-0<strong>08</strong>1-10 0.03300 V-R VIT C 250 MG TABLET CHEW 0 SUNMARK EAGEN 49348-0<strong>08</strong>2-10 0.04670 V-R VIT C 500 MG TABLET CHEW 0 SUNMARK EAGEN 49348-0092-10 0.04440 V-R VITAMIN B-6 100 MG TABLET 0 SUNMARK EAGEN 0<strong>06</strong>03-0992-63 0.01331 VEGETABLE LAXATIVE POWDER 0 QUALITEST GMGEN 00904-2<strong>08</strong>5-60 0.03500 VITAMIN A 10,000 UNITS CAPSULE 0 MAJOR PHARMACEU EAGEN 00904-5042-60 0.05040 VITAMIN AND MINERALS TABLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-0544-60 0.04100 VITAMIN B-1 100 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-0544-80 0.04100 VITAMIN B-1 100 MG TABLET 0 MAJOR PHARMACEU EAGEN 62107-0059-01 0.04100 VITAMIN B-1 100 MG TABLET 0 PRIME MARKETING EAGEN 62107-0<strong>06</strong>0-01 0.03390 VITAMIN B-1 50 MG TABLET 0 PRIME MARKETING EAGEN 00904-0518-60 0.04440 VITAMIN B-6 100 MG TABLET 0 MAJOR PHARMACEU EAGEN 51645-0910-01 0.04440 VITAMIN B-6 100 MG TABLET 0 PLUS PHARMA,INC EAGEN 62107-0<strong>06</strong>1-01 0.04440 VITAMIN B-6 100 MG TABLET 0 PRIME MARKETING EAGEN 00904-0520-60 0.03390 VITAMIN B-6 50 MG TABLET 0 MAJOR PHARMACEU EAGEN 51645-0909-01 0.03390 VITAMIN B-6 50 MG TABLET 0 PLUS PHARMA,INC EAGEN 00904-0528-60 0.04670 VITAMIN C TR 500 MG CAPLET 0 MAJOR PHARMACEU EA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-0522-60 0.03300 VITAMIN C 250 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-0525-60 0.03300 VITAMIN C 250 MG TABLET CHEW 0 MAJOR PHARMACEU EAGEN 37205-0<strong>08</strong>9-90 0.04670 VITAMIN C 500 MG CAPLET 0 LEADER EAGEN 49348-0<strong>08</strong>4-10 0.04670 VITAMIN C 500 MG CAPSULE SA 0 SUNMARK EAGEN 00904-0523-60 0.04670 VITAMIN C 500 MG TABLET 0 MAJOR PHARMACEU EA** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT
<strong>NEW</strong> <strong>YORK</strong> <strong>STATE</strong> <strong>DEPARTMENT</strong> <strong>OF</strong> <strong>HEALTH</strong> 02/28/2014 PAGE: 521LIST <strong>OF</strong> MEDICAID REIMBURSABLE DRUGSRX TYPE: 07 PRICING ERRORS ARE NOT REIMBURSABLE PRICES EFFECTIVE 02/28/2014LTM BASISIND NDC CODE MRA COST COST ALTERNATE FORMULARY DESCRIPTION PA CD LABELER <strong>OF</strong> MRA--- ------------- ------------ -------------- -------------------------------------------------- ----- ------------------ ----------GEN 00904-0523-61 0.04670 VITAMIN C 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-0523-72 0.04670 VITAMIN C 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 00904-0523-80 0.04670 VITAMIN C 500 MG TABLET 0 MAJOR PHARMACEU EAGEN 62107-0046-01 0.04670 VITAMIN C 500 MG TABLET 0 PRIME MARKETING EAGEN 62107-0046-10 0.04670 VITAMIN C 500 MG TABLET 0 PRIME MARKETING EAGEN 00904-0526-60 0.04670 VITAMIN C 500 MG TABLET CHEW 0 MAJOR PHARMACEU EAGEN 63868-0499-30 0.03100 WOMAN'S LAXATIVE EC 5 MG TAB 0 CHAIN DRUG EAGEN 36800-0174-65 0.03010 WOMEN'S LAXATIVE 5 MG TABLET 0 TOPCO EAGEN 00168-0<strong>06</strong>2-02 0.05619 ZINC OXIDE 20% OINTMENT 0 SANDOZ GMGEN 00168-0<strong>06</strong>2-31 0.05619 ZINC OXIDE 20% OINTMENT 0 SANDOZ GM** PRIOR APPROVAL CODES:PA code "0" = PA not required; PA code "N" = PA requiredPA code "G" = PA required for Non Preferred drugs OR drugs not meeting clinical criteria (FQD, STEP) OR drugs inClinical Drug Review Program, the Brand Less than Generic Program or the Mandatory Generic Program*** OTC, SUPPLY AND COMPOUND PRODUCTS LISTING AT BACK <strong>OF</strong> REPORT