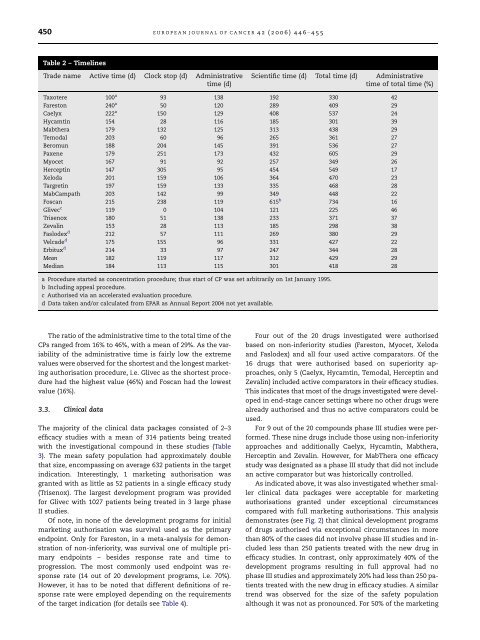
E U RO P E A N J O U R NA L O F CA N C E R42 (2006) 446– 455 449• For products authorised in 2004 all data were takenand/or calculated from the EPAR, as the Annual Report2004 was not yet available.• Additionally, <strong>for</strong> each <strong>marketing</strong> authorisation <strong>procedure</strong>the proportion of the administrative time comparedwith the total time of the CP was calculatedand given as a percentage value.3. Clinical data: in<strong>for</strong>mation on the clinical data packages,such as the size of the patient populations as well asthe clinical data regarding study design and phase, primaryendpoints, comparator(s) and numbers of patients,were extracted from the ‘Scientific discussion’ moduleof the EPARs as published on the homepage of theEMEA. 7The clinical data were used to investigate whether those<strong>marketing</strong> authorisation <strong>procedure</strong>s per<strong>for</strong>med under exceptionalcircumstances had reduced data requirements comparedwith those that were not per<strong>for</strong>med underexceptional circumstances. For this purpose the data of threedifferent parameters were classified as follows:• Availability of phase III studies in the clinical developmentprogram.• Size of the efficacy population: 250 patients or more versusless than 250.• Size of the safety population: 500 patients or more versusless than 500 patients.For the two groups of drugs, i.e. those authorised viaexceptional circumstances versus those with a full <strong>marketing</strong>authorisation, the percentage of drugs falling into eachclass was calculated and the data were analyseddescriptively.Only 1 of the 20 drugs reviewed by the CPMP thus far, i.e.Glivec, was reviewed in an accelerated evaluation <strong>procedure</strong>.As a consequence, Glivec is the product that has the shortesttotal time <strong>for</strong> the EU <strong>marketing</strong> authorisation <strong>procedure</strong>(225 d) among all oncology drugs investigated (<strong>for</strong> details seebelow).3.2. Timelines of the initial <strong>marketing</strong> authorisation<strong>procedure</strong>3.2.1. Total timeThe shortest total time of all oncology drugs investigated wasobserved <strong>for</strong> Glivec, at 225 d, whereas the longest total timewas observed <strong>for</strong> Foscan, at 734 d. However, these 734 d includeda so-called appeal <strong>procedure</strong> because the CPMP hadinitially issued a negative opinion on Foscan that was subsequentlyrevised to become a positive opinion based – amongother factors – on certain label changes. The mean as wellas the median total time is in the range 13–14 months (429and 418 d, respectively; see Table 2).3.2.2. Active timeThe active time <strong>for</strong> scientific evaluation of the <strong>marketing</strong>authorisation application by the authorities is below 210 din 75% of cases, which is the target defined by EU legislation.8 In 20% of cases it was slightly higher. In only 1 casethe benchmark of 210 d was clearly missed (Fareston). An activetime considerably below 150 d was only achieved in 2cases: <strong>for</strong> Glivec, which had qualified <strong>for</strong> accelerated evaluation,and <strong>for</strong> Taxotere, where the short active time can be explainedby the fact that the review had already started underthe <strong>for</strong>mer concentration <strong>procedure</strong> in 1994, but only the reviewtime under the CP starting on 1st January 1995 wascalculated.3. Results3.1. Procedural in<strong>for</strong>mationAs outlined above, there are three main mechanisms available,which may be used to facilitate the development as wellas the <strong>marketing</strong> authorisation process of oncology drugs inthe EU: orphan drug legislation, exceptional circumstancesprovision and the accelerated evaluation <strong>procedure</strong>. For eachof the 20 oncology drugs investigated, in<strong>for</strong>mation is providedregarding these mechanisms in Table 1.Two of the 20 oncology drugs investigated were granted orphandrug status be<strong>for</strong>e their <strong>marketing</strong> authorisation <strong>procedure</strong>started (Glivec and Trisenox).In fact, 7 (35%) of the 20 oncology drugs investigated havebeen authorised under exceptional circumstances. Six out of10 (60%) drugs authorised since the beginning of 2001 wereauthorised under these conditions. Based on additional clinicaldata submitted by the applicant, Taxotere has meanwhilereceived full approval. As the other drugs have only beenauthorised during the past three years they are still regardedas authorised under exceptional circumstances and have tofulfil post-<strong>marketing</strong> obligations in order to achieve full approvalstatus.3.2.3. Clock-stop timeThe mean clock-stop time was 119 d, with the majority ofproducts having a clock-stop time of 3–9 months. By far thelongest clock-stop time (305 d) was observed <strong>for</strong> Herceptin,because the applicant had to implement during this time asingle-dose vial instead of the proposed multi-dose vial. Theauthorities had requested this change because benzylalcoholwhich was used as a conserving agent <strong>for</strong> the multi-dose vialis not allowed as a conserving agent <strong>for</strong> intravenous productsin the EU.3.2.4. Scientific timeOn average, the scientific time was 312 d, ranging from 121 to615 d. A scientific time of 615 d was needed <strong>for</strong> Foscan as anappeal <strong>procedure</strong> was required be<strong>for</strong>e the CPMP issued apositive opinion (see above).3.2.5. Administrative timeThe mean administrative time <strong>for</strong> the investigated oncologyCPs was 117 d and ranged from 92 up to 173 d. Thus, in nocase the administrative process was completed within thetarget time frame of 90 d. The maximum time of 173 d wasobserved <strong>for</strong> Paxene where the decision-making processwas temporarily suspended.
450 E U R O P E A N J O U R NA L O F CA N C E R42 (2006) 446– 455Table 2 – TimelinesTrade name Active time (d) Clock stop (d) Administrativetime (d)Scientific time (d) Total time (d) Administrativetime of total time (%)Taxotere 100 a 93 138 192 330 42Fareston 240 a 50 120 289 409 29Caelyx 222 a 150 129 408 537 24Hycamtin 154 28 116 185 301 39Mabthera 179 132 125 313 438 29Temodal 203 60 96 265 361 27Beromun 188 204 145 391 536 27Paxene 179 251 173 432 605 29Myocet 167 91 92 257 349 26Herceptin 147 305 95 454 549 17Xeloda 201 159 106 364 470 23Targretin 197 159 133 335 468 28MabCampath 203 142 99 349 448 22Foscan 215 238 119 615 b 734 16Glivec c 119 0 104 121 225 46Trisenox 180 51 138 233 371 37Zevalin 153 28 113 185 298 38Faslodex d 212 57 111 269 380 29Velcade d 175 155 96 331 427 22Erbitux d 214 33 97 247 344 28Mean 182 119 117 312 429 29Median 184 113 115 301 418 28a Procedure started as concentration <strong>procedure</strong>; thus start of CP was set arbitrarily on 1st January 1995.b Including appeal <strong>procedure</strong>.c Authorised via an accelerated evaluation <strong>procedure</strong>.d Data taken and/or calculated from EPAR as Annual Report 2004 not yet available.The ratio of the administrative time to the total time of theCPs ranged from 16% to 46%, with a mean of 29%. As the variabilityof the administrative time is fairly low the extremevalues were observed <strong>for</strong> the shortest and the longest <strong>marketing</strong>authorisation <strong>procedure</strong>, i.e. Glivec as the shortest <strong>procedure</strong>had the highest value (46%) and Foscan had the lowestvalue (16%).3.3. Clinical dataThe majority of the clinical data packages consisted of 2–3efficacy studies with a mean of 314 patients being treatedwith the investigational compound in these studies (Table3). The mean safety population had approximately doublethat size, encompassing on average 632 patients in the targetindication. Interestingly, 1 <strong>marketing</strong> authorisation wasgranted with as little as 52 patients in a single efficacy study(Trisenox). The largest development program was provided<strong>for</strong> Glivec with 1027 patients being treated in 3 large phaseII studies.Of note, in none of the development programs <strong>for</strong> initial<strong>marketing</strong> authorisation was survival used as the primaryendpoint. Only <strong>for</strong> Fareston, in a meta-analysis <strong>for</strong> demonstrationof non-inferiority, was survival one of multiple primaryendpoints – besides response rate and time toprogression. The most commonly used endpoint was responserate (14 out of 20 development programs, i.e. 70%).However, it has to be noted that different definitions of responserate were employed depending on the requirementsof the target indication (<strong>for</strong> details see Table 4).Four out of the 20 drugs investigated were authorisedbased on non-inferiority studies (Fareston, Myocet, Xelodaand Faslodex) and all four used active comparators. Of the16 drugs that were authorised based on superiority approaches,only 5 (Caelyx, Hycamtin, Temodal, Herceptin andZevalin) included active comparators in their efficacy studies.This indicates that most of the drugs investigated were developedin end-stage cancer settings where no other drugs werealready authorised and thus no active comparators could beused.For 9 out of the 20 compounds phase III studies were per<strong>for</strong>med.These nine drugs include those using non-inferiorityapproaches and additionally Caelyx, Hycamtin, Mabthera,Herceptin and Zevalin. However, <strong>for</strong> MabThera one efficacystudy was designated as a phase III study that did not includean active comparator but was historically controlled.As indicated above, it was also investigated whether smallerclinical data packages were acceptable <strong>for</strong> <strong>marketing</strong>authorisations granted under exceptional circumstancescompared with full <strong>marketing</strong> authorisations. This analysisdemonstrates (see Fig. 2) that clinical development programsof drugs authorised via exceptional circumstances in morethan 80% of the cases did not involve phase III studies and includedless than 250 patients treated with the new drug inefficacy studies. In contrast, only approximately 40% of thedevelopment programs resulting in full approval had nophase III studies and approximately 20% had less than 250 patientstreated with the new drug in efficacy studies. A similartrend was observed <strong>for</strong> the size of the safety populationalthough it was not as pronounced. For 50% of the <strong>marketing</strong>