European Union centralised procedure for marketing ... - INCT-Inofar
European Union centralised procedure for marketing ... - INCT-Inofar
European Union centralised procedure for marketing ... - INCT-Inofar
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
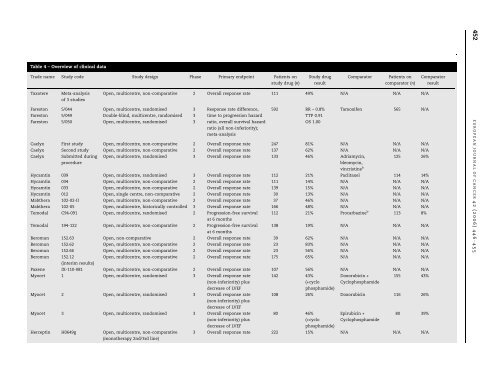
Table 4 – Overview of clinical dataTrade name Study code Study design Phase Primary endpoint Patients onstudy drug (n)TaxotereMeta-analysisof 3 studiesStudy drugresultComparatorPatients oncomparator (n)Open, multicentre, non-comparative 2 Overall response rate 111 49% N/A N/A N/AFarestonFarestonFareston5/0445/0495/050Open, multicentre, randomisedDouble-blind, multicentre, randomisedOpen, multicentre, randomised333Response rate difference,time to progression hazardratio, overall survival hazardratio (all non-inferiority);meta-analysis592 RR – 0.8%TTP 0.91OS 1.00Tamoxifen 565 N/ACaelyx First study Open, multicentre, non-comparative 2 Overall response rate 247 81% N/A N/A N/ACaelyx Second study Open, multicentre, non-comparative 2 Overall response rate 137 62% N/A N/A N/ACaelyx Submitted during<strong>procedure</strong>Open, multicentre, randomised 3 Overall response rate 133 46% Adriamycin,bleomycin,vincristine b 125 26%Hycamtin 039 Open, multicentre, randomised 3 Overall response rate 112 21% Paclitaxel 114 14%Hycamtin 034 Open, multicentre, non-comparative 2 Overall response rate 111 14% N/A N/A N/AHycamtin 033 Open, multicentre, non-comparative 2 Overall response rate 139 15% N/A N/A N/AHycamtin 012 Open, single centre, non-comparative 2 Overall response rate 30 13% N/A N/A N/AMabthera 102-02-II Open, multicentre, non-comparative 2 Overall response rate 37 46% N/A N/A N/AMabthera 102-05 Open, multicentre, historically controlled 3 Overall response rate 166 48% N/A N/A N/ATemodal C94-091 Open, multicentre, randomised 2 Progression-free survivalat 6 months112 21% Procarbazine b 113 8%Temodal 194-122 Open, multicentre, non-comparative 2 Progression-free survivalat 6 months138 19% N/A N/A N/ABeromun 152.63 Open, non-comparative 2 Overall response rate 39 62% N/A N/A N/ABeromun 152.62 Open, multicentre, non-comparative 2 Overall response rate 23 83% N/A N/A N/ABeromun 152.66 Open, multicentre, non-comparative 2 Overall response rate 23 56% N/A N/A N/ABeromun 152.12(interim results)Open, multicentre, non-comparative 2 Overall response rate 175 65% N/A N/A N/APaxene IX-110-081 Open, multicentre, non-comparative 2 Overall response rate 107 56% N/A N/A N/AMyocet 1 Open, multicentre, randomised 3 Overall response rate(non-inferiority) plusdecrease of LVEF142 43%(+cyclophosphamide)Doxorubicin +Cyclophosphamide155 43%Myocet 2 Open, multicentre, randomised 3 Overall response rate(non-inferiority) plusdecrease of LVEFMyocet 3 Open, multicentre, randomised 3 Overall response rate(non-inferiority) plusdecrease of LVEFHerceptin H0649g Open, multicentre, non-comparative(monotherapy 2nd/3rd line)108 26% Doxorubicin 116 26%80 46%(+cyclophosphamide)Epirubicin +Cyclophosphamide80 39%3 Overall response rate 222 15% N/A N/A N/AComparatorresult452 E U R O P E A N J O U R NA L O F CA N C E R42 (2006) 446– 455