Antiemetics - BMC HealthNet Plan
Antiemetics - BMC HealthNet Plan
Antiemetics - BMC HealthNet Plan
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>BMC</strong>HP.ORG1-888-566-0008WELLSENSE.ORGCLINICAL COVERAGE GUIDELINES – <strong>Antiemetics</strong>Aloxi ® , Anzemet ® , Cesamet ® , dronabinol, Emend ® , granisetron,ondansetron, Sancuso ® , Zuplenz ®Policy ApplicabilityBoston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> Well Sense Health <strong>Plan</strong>MassHealthNew Hampshire MedicaidCommonwealth CareCommercialIntegrated Care ProgramEffective Date: 07/01/2013Policy Number: 9.104Policy Effective Date: 09/10/2003Last Review Date: 05/09/2013Approved by: Pharmacy and Therapeutics CommitteePolicy Owner/Title: Pharmacy ServicesSummary:<strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> will authorize coverage of certain antiemetic medications whenappropriate criteria are met.Description of Item or Service:Nausea and vomiting is associated with numerous medical conditions includinggastroenteritis, migraine, motion sickness, and pregnancy. Chemotherapy inducednausea and vomiting (CINV) and post-operative nausea and vomiting (PONV) are verycommon and studies to evaluate the efficacy of antiemetic drugs have mostly beenconducted in these patient populations.This guideline provides information on <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> clinical criteria and claims adjudication processing guidelines. Theuse of this guideline is not a guarantee of payment and will not determine how a specific claim(s) will be paid. Reimbursement isbased on member benefits and eligibility, medical necessity review, where applicable, coordination of benefits, adherence to <strong>BMC</strong><strong>HealthNet</strong> <strong>Plan</strong> policies, clinical coding criteria, and the <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> agreement with the rendering or dispensing provider.Reimbursement policies may be amended at <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong>’s discretion. <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> will always use the mostrecent CPT and HCPCS coding guidelines. All <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> policies are developed in accordance with state, federal andaccrediting organization guidelines and requirements, including NCQA.This document is subject to further revision in response to additional terms and requirements imposed under the Integrated CareProgram, including the ICP contract.<strong>BMC</strong>HP refers to Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> in Massachusetts and Well Sense Health <strong>Plan</strong> in New Hampshire.Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> and Well Sense Health <strong>Plan</strong> are trade names used by Boston Medical Center Health <strong>Plan</strong>,Inc.1 of 10<strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> – <strong>Antiemetics</strong>
In addition to infection and direct GI irritation, nausea and vomiting can be brought on bymany stimuli which activate the chemoreceptor trigger zone (CTZ) within the brain.Receptors which are believed to be involved in the activation of the CTZ includecholinergic, histamine, dopamine, serotonin, opiate, neurokinin, and benzodiazepinereceptors. Medications used to prevent or treat nausea and vomiting act as antagonists atthese receptor sites.For treatment of nausea and vomiting in the medical patient, the choice of antiemeticagent is generally made based on the neurotransmitters that are believed to be associatedwith a particular medical condition. Clinical trials evaluating the comparative efficacy ofantiemetic drugs in this setting are lacking. In many cases, nausea and vomiting can beproperly managed using older, less expensive agents (e.g. metoclopramide, promethazine,phenergan, diphenhydramine, meclizine).In the setting of CINV and PONV, specific treatment recommendations are based ontreatment guidelines from the American Society of Clinical Oncology (ASCO).Treatment recommendations for PONV are based on clinical trials.The American Society of Clinical Oncology (ASCO) has established a grading system todetermine the likeliness of emesis occurring with certain chemotherapy agents. ASCOhas also developed treatment guidelines based on these levels. For patients receivinghighly emetic, chemotherapy (>90% risk), including high dose cisplatin, or ananthrycycline and cyclophosphamide in combination, a three drug regimen should beinitiated, including dexamethasone, a serotonin (5-HT 3 ) antagonist, and the neurokininreceptor antagonist (NK1R) - (aprepitant or fosaprepitant). Patients who are receivingchemotherapy with moderate emetic potential (30-90%) should receive dexamethasoneand palonosetron, or another 5HT 3 antagonist when palonosetron is not available. Lowemetic regimens (10-30% risk) warrant treatment only with dexamethasone. Finally thosewith
Documentation of the following:1. A clinically relevant rationale as to why the treatment with the filmformulation of Zuplenz ® is expected to result in better efficacy thanondansetron ODT formulation (must include literature documentation)Quantity Limitations Apply – See Appendix AQuantity Limitation Override – (Duration of Approval – Maximum of 90 days, or up tothe due date whichever is earlier if hyperemesis gravidarum)All antiemetics – CINV:Documentation of the following:1. A daily dose of the requested medication that cannot be achieved withcommercially available dosage strengths and forms is required; OR2. Need for a dosage frequency greater than what is recommended by the FDA anddocumentation of the supporting rationale for the prescribed frequency; OR3. Dosage titration (up to 3 months) cannot be achieved with commercially availabledosage strengths and forms.Ondansetron – Non- CINV (including hyperemesis gravidarum):<strong>BMC</strong> <strong>HealthNet</strong> will approve prescriptions for ondansetron up to three times daily fortreatment of nausea and vomiting not related to chemotherapy, when the followingcriteria are met:Documentation of the following:1. An inadequate response or intolerance to two of the following medicationtreatment options: Antihistamine therapy (e.g. meclizine, hydroxyzine, doxylamine, ordimenhydrinate) Phenothiazine therapy (promethazine or prochlorperazine) Metoclopramide; ANDIn addition, for hyperemesis gravidarum:This guideline provides information on <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> clinical criteria and claims adjudication processing guidelines. Theuse of this guideline is not a guarantee of payment and will not determine how a specific claim(s) will be paid. Reimbursement isbased on member benefits and eligibility, medical necessity review, where applicable, coordination of benefits, adherence to <strong>BMC</strong><strong>HealthNet</strong> <strong>Plan</strong> policies, clinical coding criteria, and the <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> agreement with the rendering or dispensing provider.Reimbursement policies may be amended at <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong>’s discretion. <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> will always use the mostrecent CPT and HCPCS coding guidelines. All <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> policies are developed in accordance with state, federal andaccrediting organization guidelines and requirements, including NCQA.This document is subject to further revision in response to additional terms and requirements imposed under the Integrated CareProgram, including the ICP contract.<strong>BMC</strong>HP refers to Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> in Massachusetts and Well Sense Health <strong>Plan</strong> in New Hampshire.Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> and Well Sense Health <strong>Plan</strong> are trade names used by Boston Medical Center Health <strong>Plan</strong>,Inc.5 of 10<strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> – <strong>Antiemetics</strong>
Effective Date: 09/10/2003Dates of Review/Revision:02/01/2005 – Placed restrictions on Aloxi ® .03/10/2005 – Added Hyperemesis gravidarum as approvable indication for treatmentover 7 days for 5HT 3 blocker.03/09/2006 – Removed quantity limits on 5HT 3 blocker.07/12/2007 – P&T Annual Review - Added requirement for ondansetron prior toapproval of brand name serotonin blockers, added criteria for Cesamet ®, added quantitylimitations for selected antiemetics (see Appendix A)08/13/2007 – Removed prior authorization criteria for Aloxi ® .05/08/2008 – P&T Annual Review, removed Kytril from prior authorizationrequirements due to availability of generic granisetron, criteria for ondansetron quantitylimitation override for nausea and vomiting of pregnancy added.11/13/2008 – Added restrictions for Aloxi ® and Sancuso ® , added quantity limit overridecriteria for non-chemotherapy-related nausea and vomiting.11/12/2009 – P&T Annual Review, granisetron criteria added.11/11/2010 – P&T Annual Review, Zuplenz ® criteria added11/10/2011 – P&T Annual Review, specified indication and trial requirements fordronabinol and Cesamet ® , removed Zuplenz ® from the ondansetron QL criteria.11/08/2012 – P&T Annual Review, removed pyridoxine from trial requirement for QLrequest.Last Review Date: 05/09/2013 – Policy revision, revised criteria for Aloxi to reflectASCO 2012 guidelines, increased quantity limits for ondansetronNext Review Date: 11/14/2013Approval DatesRegulatory Approval: N/AInternal Approval:Initial approval by Pharmacy & Therapeutics Committee – September 10, 2003Authorizing entityP&T CommitteeAppendix A: Quantity Limitations for <strong>Antiemetics</strong>This guideline provides information on <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> clinical criteria and claims adjudication processing guidelines. Theuse of this guideline is not a guarantee of payment and will not determine how a specific claim(s) will be paid. Reimbursement isbased on member benefits and eligibility, medical necessity review, where applicable, coordination of benefits, adherence to <strong>BMC</strong><strong>HealthNet</strong> <strong>Plan</strong> policies, clinical coding criteria, and the <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> agreement with the rendering or dispensing provider.Reimbursement policies may be amended at <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong>’s discretion. <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> will always use the mostrecent CPT and HCPCS coding guidelines. All <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> policies are developed in accordance with state, federal andaccrediting organization guidelines and requirements, including NCQA.This document is subject to further revision in response to additional terms and requirements imposed under the Integrated CareProgram, including the ICP contract.<strong>BMC</strong>HP refers to Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> in Massachusetts and Well Sense Health <strong>Plan</strong> in New Hampshire.Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> and Well Sense Health <strong>Plan</strong> are trade names used by Boston Medical Center Health <strong>Plan</strong>,Inc.7 of 10<strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> – <strong>Antiemetics</strong>
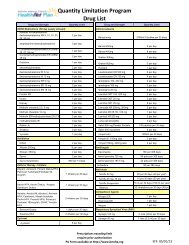
Medication Name and StrengthAnzemet ®Emend ® 40mgEmend ® 80mgEmend ® 125mgEmend ® Pak (4-125mg and 8-80mg)granisetrongranisetron oral solutiondronabinolondansetron 4mg, 8mgondansetron 24mgondansetron ODTondansetron oral solutionSancuso ®Zuplenz ® 4mg, 8mgMaximum Quantity5 per 30 days1 per 30 days8 per 30 days4 per 30 days12 capsules (4 paks) per 30 days10 per 30 days50mL per 30 days30 per 30 days30 per 30 days5 per 30 days30 per 30 days75mL per 30 days4 patches per 30 days15 per 30 daysThis guideline provides information on <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> clinical criteria and claims adjudication processing guidelines. Theuse of this guideline is not a guarantee of payment and will not determine how a specific claim(s) will be paid. Reimbursement isbased on member benefits and eligibility, medical necessity review, where applicable, coordination of benefits, adherence to <strong>BMC</strong><strong>HealthNet</strong> <strong>Plan</strong> policies, clinical coding criteria, and the <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> agreement with the rendering or dispensing provider.Reimbursement policies may be amended at <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong>’s discretion. <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> will always use the mostrecent CPT and HCPCS coding guidelines. All <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> policies are developed in accordance with state, federal andaccrediting organization guidelines and requirements, including NCQA.This document is subject to further revision in response to additional terms and requirements imposed under the Integrated CareProgram, including the ICP contract.<strong>BMC</strong>HP refers to Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> in Massachusetts and Well Sense Health <strong>Plan</strong> in New Hampshire.Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> and Well Sense Health <strong>Plan</strong> are trade names used by Boston Medical Center Health <strong>Plan</strong>,Inc.8 of 10<strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> – <strong>Antiemetics</strong>
Appendix BThis guideline provides information on <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> clinical criteria and claims adjudication processing guidelines. Theuse of this guideline is not a guarantee of payment and will not determine how a specific claim(s) will be paid. Reimbursement isbased on member benefits and eligibility, medical necessity review, where applicable, coordination of benefits, adherence to <strong>BMC</strong><strong>HealthNet</strong> <strong>Plan</strong> policies, clinical coding criteria, and the <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> agreement with the rendering or dispensing provider.Reimbursement policies may be amended at <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong>’s discretion. <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> will always use the mostrecent CPT and HCPCS coding guidelines. All <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> policies are developed in accordance with state, federal andaccrediting organization guidelines and requirements, including NCQA.This document is subject to further revision in response to additional terms and requirements imposed under the Integrated CareProgram, including the ICP contract.<strong>BMC</strong>HP refers to Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> in Massachusetts and Well Sense Health <strong>Plan</strong> in New Hampshire.Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> and Well Sense Health <strong>Plan</strong> are trade names used by Boston Medical Center Health <strong>Plan</strong>,Inc.9 of 10<strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> – <strong>Antiemetics</strong>
IMPORTANT NOTES:‣ Not all services are covered for all products or employer groups. This medical policyexpresses <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> 's determination of whether certain services or suppliesare medically necessary, experimental or investigational or cosmetic. <strong>BMC</strong> <strong>HealthNet</strong><strong>Plan</strong> has reached these conclusions based upon the regulatory status of the technologyand a review of clinical studies published in peer-reviewed medical literature. Eventhough this policy may indicate that a particular service or supply is considered coveredor not covered, this conclusion is not based upon the terms of a member’s particularbenefit plan. Each benefit plan contains its own specific provisions for coverage andexclusions. Not all services that are determined to be medically necessary willnecessarily be covered services under the terms of a member’s benefit plan. Membersand their providers need to consult the applicable benefit plan document (e.g., Evidenceof Coverage) to determine if there are any exclusions or other benefit limitationsapplicable to this service or supply. If there is a discrepancy between this medical policyand the benefit plan document, the provisions of the benefit plan document will govern. Inaddition, this policy and the benefit plan document are subject to applicable state andfederal laws that may mandate coverage for certain services and supplies.‣ To the extent applicable, this Policy and/or Procedure applies to <strong>BMC</strong>HP subcontractorsand downstream entities, if any, providing services with respect to <strong>BMC</strong>HP’s IntegratedCare Program.This guideline provides information on <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> clinical criteria and claims adjudication processing guidelines. Theuse of this guideline is not a guarantee of payment and will not determine how a specific claim(s) will be paid. Reimbursement isbased on member benefits and eligibility, medical necessity review, where applicable, coordination of benefits, adherence to <strong>BMC</strong><strong>HealthNet</strong> <strong>Plan</strong> policies, clinical coding criteria, and the <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> agreement with the rendering or dispensing provider.Reimbursement policies may be amended at <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong>’s discretion. <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> will always use the mostrecent CPT and HCPCS coding guidelines. All <strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> policies are developed in accordance with state, federal andaccrediting organization guidelines and requirements, including NCQA.This document is subject to further revision in response to additional terms and requirements imposed under the Integrated CareProgram, including the ICP contract.<strong>BMC</strong>HP refers to Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> in Massachusetts and Well Sense Health <strong>Plan</strong> in New Hampshire.Boston Medical Center <strong>HealthNet</strong> <strong>Plan</strong> and Well Sense Health <strong>Plan</strong> are trade names used by Boston Medical Center Health <strong>Plan</strong>,Inc.10 of 10<strong>BMC</strong> <strong>HealthNet</strong> <strong>Plan</strong> – <strong>Antiemetics</strong>