Ï - ADDI
Ï - ADDI
Ï - ADDI
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
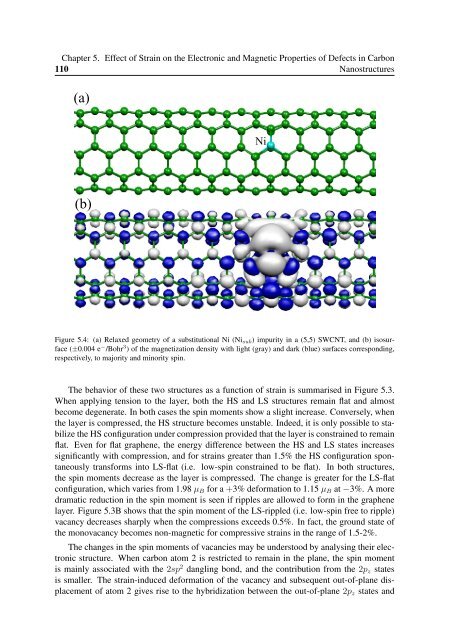
Chapter 5. Effect of Strain on the Electronic and Magnetic Properties of Defects in Carbon110Nanostructures(a)Ni(b)Figure 5.4: (a) Relaxed geometry of a substitutional Ni (Ni sub ) impurity in a (5,5) SWCNT, and (b) isosurface(±0.004 e − /Bohr 3 ) of the magnetization density with light (gray) and dark (blue) surfaces corresponding,respectively, to majority and minority spin.The behavior of these two structures as a function of strain is summarised in Figure 5.3.When applying tension to the layer, both the HS and LS structures remain flat and almostbecome degenerate. In both cases the spin moments show a slight increase. Conversely, whenthe layer is compressed, the HS structure becomes unstable. Indeed, it is only possible to stabilizethe HS configuration under compression provided that the layer is constrained to remainflat. Even for flat graphene, the energy difference between the HS and LS states increasessignificantly with compression, and for strains greater than 1.5% the HS configuration spontaneouslytransforms into LS-flat (i.e. low-spin constrained to be flat). In both structures,the spin moments decrease as the layer is compressed. The change is greater for the LS-flatconfiguration, which varies from 1.98 µ B for a +3% deformation to 1.15 µ B at −3%. A moredramatic reduction in the spin moment is seen if ripples are allowed to form in the graphenelayer. Figure 5.3B shows that the spin moment of the LS-rippled (i.e. low-spin free to ripple)vacancy decreases sharply when the compressions exceeds 0.5%. In fact, the ground state ofthe monovacancy becomes non-magnetic for compressive strains in the range of 1.5-2%.The changes in the spin moments of vacancies may be understood by analysing their electronicstructure. When carbon atom 2 is restricted to remain in the plane, the spin momentis mainly associated with the 2sp 2 dangling bond, and the contribution from the 2p z statesis smaller. The strain-induced deformation of the vacancy and subsequent out-of-plane displacementof atom 2 gives rise to the hybridization between the out-of-plane 2p z states and