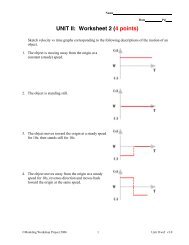
BCA table lecture
BCA table lecture
BCA table lecture
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Focus on mole relationshipsFocus on mole relationships• Step 2: fill in the before line2 H 2 S + 3 O 2 ----> 2 SO 2 + 2 H 2 OBefore: 2.4 xs 0 0ChangeAfter• Assume more than enough O 2 to react• Step 3: use ratio of coefficients to determinechange2 H 2 S + 3 O 2 ----> 2 SO 2 + 2 H 2 OBefore: 2.4 xs 0 0Change –2.4 –3.6 +2.4 +2.4After• Reactants are consumed (-), products accumulate (+)Emphasize that change and finalstate are not equivalentComplete calculations on the side• Step 4: Complete the <strong>table</strong>2 H 2 S + 3 O 2 ----> 2 SO 2 + 2 H 2 OBefore: 2.4 xs 0 0Change –2.4 –3.6 +2.4 +2.4After 0 xs 2.4 2.4• In this case, desired answer is in moles• If mass is required, convert moles to grams in theusual way3.6 moles O 2×32 .0 g1mole =115 g2