BCA table lecture
BCA table lecture
BCA table lecture
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
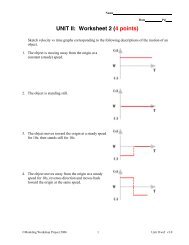
Percent Yield problems• Change moles to grams to finish.• How many grams of aluminum iodide will form (theoreticalyield) when 13.5 g of aluminum reacts with excess iodine?• 0.50 mol AlI 3 x 407.71 g1 mol200 grams of AlI 3 will form= 203.86 g = 2.0 x 10 2 gPercent Yield problems• If given the actual amount formed in lab –calculate % yield• How many grams of aluminum iodide will form (theoreticalyield) when 13.5 g of aluminum reacts with excess iodine?What is the percent yield if only 100 grams was formed inthe lab?• % Yield = actual yield/theoretical yield x 100• =100/200 x 100% = 50.% yieldOnly moles go in the <strong>BCA</strong> <strong>table</strong>Limiting reactant problems• The balanced equation deals with how many, nothow much.• If given mass of reactants for products, convert tomoles first, then use the <strong>table</strong>.• <strong>BCA</strong> approach distinguishes between what you start with andwhat reacts.• When 0.50 mole of aluminum reacts with 0.72 mole of iodine to formaluminum iodide, how many moles of the excess reactant will remain?How many moles of aluminum iodide will be formed?2 Al + 3 I 2 ----> 2 AlI 3Before: 0.50 0.72 0ChangeAfter4