A review of - ukelg
A review of - ukelg
A review of - ukelg
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
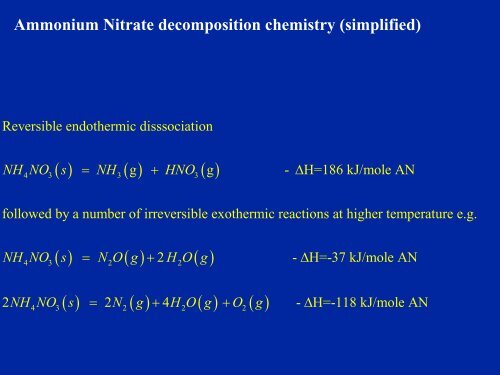
Ammonium Nitrate decomposition chemistry (simplified)Reversible endothermic disssociation( ) ( ) ( )NH NO s = NH g + HNO g - ΔH=186 kJ/mole AN4 3 3 3followed by a number <strong>of</strong> irreversible exothermic reactions at higher temperature e.g.( ) = ( ) + 2 ( )NH NO s N O g H O g4 3 2 2- ΔH=-37 kJ/mole AN( ) ( ) ( ) ( )2NH NO s = 2N g + 4 H O g + O g - ΔH=-118 kJ/mole AN4 3 2 2 2