Physician-to-Physician Presentation - Gore Medical
Physician-to-Physician Presentation - Gore Medical
Physician-to-Physician Presentation - Gore Medical
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
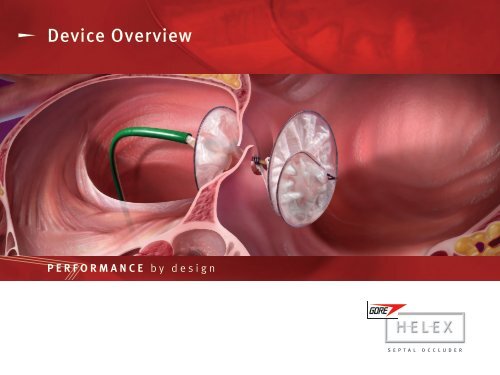
IndicationThe GORE HELEX Septal Occluder is a permanently implanted prosthesis indicated for the percutaneous,transcatheter closure of atrial septal defects (ASDs), such as ostium secundum and Patent ForamenOvale.
Product AttributesCompliant DesignCircular ShapeFlat ProfileePTFE Occlusion MembraneCircumferential NitinolSupport Frame
Device ComponentsePTFECentral EyeletNitinol FrameRight Atrial EyeletDelivery Catheter
Locking the DevicePre-LockPost-LockRight Atrial EyeletRetrieval CordMandrelExtended Lock Loop(In Mandrel)Left AtrialMandrel FlareRetrieval CordMandrelRight Atrial EyeletLeft Atrial EyeletRadiopaque TipCenter EyeletRadiopaque TipLock LoopCenter EyeletMandrel is pulled backLock loop forms
Flat Profile and Low Metal Mass
Flexible and CompliantFluoroscopy at one month follow-upin patient with deficient anteriorsuperior rimDevice observed splaying aorta
Inert and BiocompatibleePTFE proven in more than25 million clinical implantsReduced thrombogenicityPorosity engineered for controlledtissue responseAllows formation of functionalintimal liningAfter three months in canine model
Easily RetrievableRetrieval cord maintains connectionbetween device and delivery systemAllows tension-free confirmationof device position after lock release,and easy retrieval if necessary
Hydrophilic CoatingHydrophilic coating facilitatesimmediate wetting of membraneDryWetClear echo visibility of the device
Animal DataIn vivo tissue responsedemonstrating flat profile,conformance <strong>to</strong> the septum,and non-thrombogenicOccluder material.Left Atrial ViewRight Atrial Side ViewRetrieval after threemonths incanine model
US Clinical Trial Data SummaryFeasibility Pivotal * Continued AccessSubjects Enrolled 63 143 196Median Age (years) 11 (0.5-66) 7 (1-72) 5.4 (0.8 – 58)Successful Implant86.4% of attemptedimplants (51 / 59)88.1% of attemptedimplants (119 / 135)85.6% of attemptedimplants (137 / 160)Adverse Events 3.9% 5.9% 2.2%Clnically Successful94.6% 98.1% 99.1%OcclusionComposite Clinical Success 89.5% 91.9% 96.6%*Two arm study comparing surgical closure <strong>to</strong> device closureClinically Successful Occlusion: No residual leak or insignificant residual leakComposite Clinical Success: Clinically successful occlusion AND no major adverse eventThree US clinical studies were conducted <strong>to</strong> evaluate the GORE HELEX Septal Occluder. These studies were performedwith the original delivery system. The product described here is the same Occluder with a modified delivery system.Please note that the modified delivery system was not evaluated under the original US clinical study.
US Clinical Trial Key LearningsGORE HELEX Septal Occluder is safe and effective for the closure of ASDsNo instances of perforation of cardiac structuresNo instances of thrombus on the deviceOptimal results when 2:1 device diameter <strong>to</strong> defect diameter ratio is used <strong>to</strong>close defects up <strong>to</strong> 18 mmClinical Trial DataDevice <strong>to</strong> Defect RatioAccess< 1.61.6 – 2.02.0 or greaterClinical Closure 92.6% 96.3% 100.0%Embolization Rate 3.0% 4.2% 0.0%Composite Success 80.0% 90.0% 95.0%*Information comes from Latson, et al., Analysis of fac<strong>to</strong>rs related <strong>to</strong> successful transcatheter closure of secundum atrial septal defects usingthe HELEX septal occluder. American Heart Journal 2006;151:1129.e7-1129.e11.
GORE HELEX Septal Occluder SizingThe GORE HELEX Septal Occludercomes in five sizes(15 mm – 35 mm)It is recommended for ASDsand PFOs measuring up <strong>to</strong> 18 mmWhen choosing the appropriatesize, a 2:1 device diameter <strong>to</strong>defect diameter ratiois recommended
INDICATIONS FOR USE OUTSIDE THE US: The GORE HELEX Septal Occluder is a permanentlyimplanted prosthesis indicated for the percutaneous, transcatheter closure of atrial septal defects(ASDs), such as ostium secundum and Patent Foramen Ovale. Refer <strong>to</strong> Instructions for Use atgoremedical.com for a complete description of all contraindications, warnings, precautions andadverse events.Products listed may not be available in all markets.GORE, HELEX, and designs are trademarks of W. L. <strong>Gore</strong> & Associates.© 2009 W. L. <strong>Gore</strong> & Associates, Inc. AL0049-EU1 JUNE 2009