International Approach for Veterinary Medicinal ... - Middle East - OIE
International Approach for Veterinary Medicinal ... - Middle East - OIE
International Approach for Veterinary Medicinal ... - Middle East - OIE
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
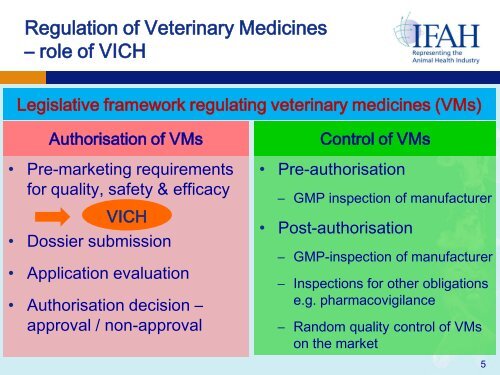
Regulation of <strong>Veterinary</strong> Medicines– role of VICHLegislative framework regulating veterinary medicines (VMs)Authorisation of VMs• Pre-marketing requirements<strong>for</strong> quality, safety & efficacyVICH• Dossier submission• Application evaluation• Authorisation decision –approval / non-approvalControl of VMs• Pre-authorisation– GMP inspection of manufacturer• Post-authorisation– GMP-inspection of manufacturer– Inspections <strong>for</strong> other obligationse.g. pharmacovigilance– Random quality control of VMson the market5