Swelling, compression and tribological behaviors of ...
Swelling, compression and tribological behaviors of ...
Swelling, compression and tribological behaviors of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
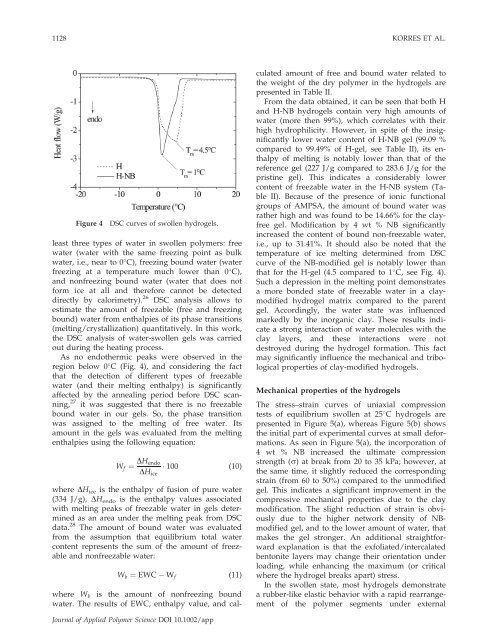
1128 KORRES ET AL.Figure 4DSC curves <strong>of</strong> swollen hydrogels.least three types <strong>of</strong> water in swollen polymers: freewater (water with the same freezing point as bulkwater, i.e., near to 0 C), freezing bound water (waterfreezing at a temperature much lower than 0 C),<strong>and</strong> nonfreezing bound water (water that does notform ice at all <strong>and</strong> therefore cannot be detecteddirectly by calorimetry). 26 DSC analysis allows toestimate the amount <strong>of</strong> freezable (free <strong>and</strong> freezingbound) water from enthalpies <strong>of</strong> its phase transitions(melting/crystallization) quantitatively. In this work,the DSC analysis <strong>of</strong> water-swollen gels was carriedout during the heating process.As no endothermic peaks were observed in theregion below 0 C (Fig. 4), <strong>and</strong> considering the factthat the detection <strong>of</strong> different types <strong>of</strong> freezablewater (<strong>and</strong> their melting enthalpy) is significantlyaffected by the annealing period before DSC scanning,27 it was suggested that there is no freezablebound water in our gels. So, the phase transitionwas assigned to the melting <strong>of</strong> free water. Itsamount in the gels was evaluated from the meltingenthalpies using the following equation:W f ¼ DH endoDH ice 100 (10)where DH ice is the enthalpy <strong>of</strong> fusion <strong>of</strong> pure water(334 J/g), DH endo is the enthalpy values associatedwith melting peaks <strong>of</strong> freezable water in gels determinedas an area under the melting peak from DSCdata. 28 The amount <strong>of</strong> bound water was evaluatedfrom the assumption that equilibrium total watercontent represents the sum <strong>of</strong> the amount <strong>of</strong> freezable<strong>and</strong> nonfreezable water:W b ¼ EWC W f (11)where W b is the amount <strong>of</strong> nonfreezing boundwater. The results <strong>of</strong> EWC, enthalpy value, <strong>and</strong> calculatedamount <strong>of</strong> free <strong>and</strong> bound water related tothe weight <strong>of</strong> the dry polymer in the hydrogels arepresented in Table II.From the data obtained, it can be seen that both H<strong>and</strong> H-NB hydrogels contain very high amounts <strong>of</strong>water (more then 99%), which correlates with theirhigh hydrophilicity. However, in spite <strong>of</strong> the insignificantlylower water content <strong>of</strong> H-NB gel (99.09 %compared to 99.49% <strong>of</strong> H-gel, see Table II), its enthalpy<strong>of</strong> melting is notably lower than that <strong>of</strong> thereference gel (227 J/g compared to 283.6 J/g for thepristine gel). This indicates a considerably lowercontent <strong>of</strong> freezable water in the H-NB system (TableII). Because <strong>of</strong> the presence <strong>of</strong> ionic functionalgroups <strong>of</strong> AMPSA, the amount <strong>of</strong> bound water wasrather high <strong>and</strong> was found to be 14.66% for the clayfreegel. Modification by 4 wt % NB significantlyincreased the content <strong>of</strong> bound non-freezable water,i.e., up to 31.41%. It should also be noted that thetemperature <strong>of</strong> ice melting determined from DSCcurve <strong>of</strong> the NB-modified gel is notably lower thanthat for the H-gel (4.5 compared to 1 C, see Fig. 4).Such a depression in the melting point demonstratesa more bonded state <strong>of</strong> freezable water in a claymodifiedhydrogel matrix compared to the parentgel. Accordingly, the water state was influencedmarkedly by the inorganic clay. These results indicatea strong interaction <strong>of</strong> water molecules with theclay layers, <strong>and</strong> these interactions were notdestroyed during the hydrogel formation. This factmay significantly influence the mechanical <strong>and</strong> <strong>tribological</strong>properties <strong>of</strong> clay-modified hydrogels.Mechanical properties <strong>of</strong> the hydrogelsThe stress–strain curves <strong>of</strong> uniaxial <strong>compression</strong>tests <strong>of</strong> equilibrium swollen at 25 C hydrogels arepresented in Figure 5(a), whereas Figure 5(b) showsthe initial part <strong>of</strong> experimental curves at small deformations.As seen in Figure 5(a), the incorporation <strong>of</strong>4 wt % NB increased the ultimate <strong>compression</strong>strength (r) at break from 20 to 35 kPa; however, atthe same time, it slightly reduced the correspondingstrain (from 60 to 50%) compared to the unmodifiedgel. This indicates a significant improvement in thecompressive mechanical properties due to the claymodification. The slight reduction <strong>of</strong> strain is obviouslydue to the higher network density <strong>of</strong> NBmodifiedgel, <strong>and</strong> to the lower amount <strong>of</strong> water, thatmakes the gel stronger. An additional straightforwardexplanation is that the exfoliated/intercalatedbentonite layers may change their orientation underloading, while enhancing the maximum (or criticalwhere the hydrogel breaks apart) stress.In the swollen state, most hydrogels demonstratea rubber-like elastic behavior with a rapid rearrangement<strong>of</strong> the polymer segments under externalJournal <strong>of</strong> Applied Polymer Science DOI 10.1002/app