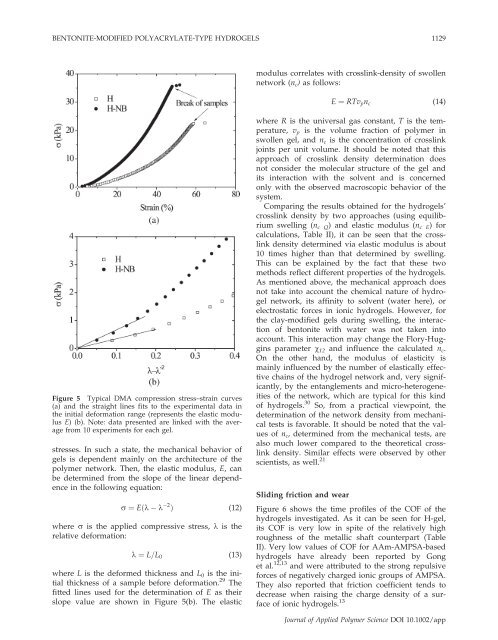
BENTONITE-MODIFIED POLYACRYLATE-TYPE HYDROGELS 1129modulus correlates with crosslink-density <strong>of</strong> swollennetwork (n c ) as follows:E ¼ RTv p n c (14)Figure 5 Typical DMA <strong>compression</strong> stress–strain curves(a) <strong>and</strong> the straight lines fits to the experimental data inthe initial deformation range (represents the elastic modulusE) (b). Note: data presented are linked with the averagefrom 10 experiments for each gel.stresses. In such a state, the mechanical behavior <strong>of</strong>gels is dependent mainly on the architecture <strong>of</strong> thepolymer network. Then, the elastic modulus, E, canbe determined from the slope <strong>of</strong> the linear dependencein the following equation:r ¼ Eðk k 2 Þ (12)where r is the applied compressive stress, k is therelative deformation:k ¼ L=L 0 (13)where L is the deformed thickness <strong>and</strong> L 0 is the initialthickness <strong>of</strong> a sample before deformation. 29 Thefitted lines used for the determination <strong>of</strong> E as theirslope value are shown in Figure 5(b). The elasticwhere R is the universal gas constant, T is the temperature,v p is the volume fraction <strong>of</strong> polymer inswollen gel, <strong>and</strong> n c is the concentration <strong>of</strong> crosslinkjoints per unit volume. It should be noted that thisapproach <strong>of</strong> crosslink density determination doesnot consider the molecular structure <strong>of</strong> the gel <strong>and</strong>its interaction with the solvent <strong>and</strong> is concernedonly with the observed macroscopic behavior <strong>of</strong> thesystem.Comparing the results obtained for the hydrogels’crosslink density by two approaches (using equilibriumswelling (n c Q ) <strong>and</strong> elastic modulus (n c E ) forcalculations, Table II), it can be seen that the crosslinkdensity determined via elastic modulus is about10 times higher than that determined by swelling.This can be explained by the fact that these twomethods reflect different properties <strong>of</strong> the hydrogels.As mentioned above, the mechanical approach doesnot take into account the chemical nature <strong>of</strong> hydrogelnetwork, its affinity to solvent (water here), orelectrostatic forces in ionic hydrogels. However, forthe clay-modified gels during swelling, the interaction<strong>of</strong> bentonite with water was not taken intoaccount. This interaction may change the Flory-Hugginsparameter v 12 <strong>and</strong> influence the calculated n c .On the other h<strong>and</strong>, the modulus <strong>of</strong> elasticity ismainly influenced by the number <strong>of</strong> elastically effectivechains <strong>of</strong> the hydrogel network <strong>and</strong>, very significantly,by the entanglements <strong>and</strong> micro-heterogeneities<strong>of</strong> the network, which are typical for this kind<strong>of</strong> hydrogels. 30 So, from a practical viewpoint, thedetermination <strong>of</strong> the network density from mechanicaltests is favorable. It should be noted that the values<strong>of</strong> n c , determined from the mechanical tests, arealso much lower compared to the theoretical crosslinkdensity. Similar effects were observed by otherscientists, as well. 21Sliding friction <strong>and</strong> wearFigure 6 shows the time pr<strong>of</strong>iles <strong>of</strong> the COF <strong>of</strong> thehydrogels investigated. As it can be seen for H-gel,its COF is very low in spite <strong>of</strong> the relatively highroughness <strong>of</strong> the metallic shaft counterpart (TableII). Very low values <strong>of</strong> COF for AAm-AMPSA-basedhydrogels have already been reported by Gonget al. 12,13 <strong>and</strong> were attributed to the strong repulsiveforces <strong>of</strong> negatively charged ionic groups <strong>of</strong> AMPSA.They also reported that friction coefficient tends todecrease when raising the charge density <strong>of</strong> a surface<strong>of</strong> ionic hydrogels. 13Journal <strong>of</strong> Applied Polymer Science DOI 10.1002/app
1130 KORRES ET AL.Figure 6 Tribological behavior <strong>of</strong> the reference (H, opensymbol) <strong>and</strong> bentonite-modified hydrgels (H-NB, filledsymbol). Solid curves represent the fits with experimentaldata.The friction <strong>of</strong> the 4 wt % NB gel was unexpectedlylow, in spite <strong>of</strong> the significantly lower swellingratio (Table II). A decrease <strong>of</strong> COF for clay-modifiedhydrogels was also observed by Haraguchi <strong>and</strong>Takada. 31 This was assigned to dangling <strong>of</strong> uncrosslinkedpolymer chain ends from exfoliated clay platelets,which affected the interface <strong>and</strong> reduced thefriction under wet conditions. On the other h<strong>and</strong>,this behavior may be related to changes in the surfaceproperties <strong>of</strong> the hydrogels. The presence <strong>of</strong>clay nanoparticles at the gels’ surface can rearrange<strong>and</strong> alter the surface polarity <strong>and</strong> morphology. Thisis accompanied by a change in the surface tension,which can reduce the friction. However, this hypothesisrequires further experiments.The specific wear rate (W S ) is remarkably lowerfor the NB-modified hydrogel when compared withthat <strong>of</strong> the reference (Table II). This suggests a reinforcingeffect <strong>of</strong> NB due to the strong adsorption <strong>of</strong>polymer chains on the filler surface, <strong>and</strong> strong H-bond interactions between the hydroxyl groups <strong>of</strong>the bentonite (at its surface) <strong>and</strong> functional groups<strong>of</strong> the copolymer network (carbonyl <strong>and</strong> aminogroups <strong>of</strong> AAm <strong>and</strong> AMPSA, respectively). All thisincreases the apparent crosslink degree. Thus,improvement in the <strong>compression</strong> mechanical properties<strong>of</strong> NB-modified hydrogel due to the nan<strong>of</strong>illerreinforcement is associated, at the same time, with ahigher resistance to sliding wear.Creep behaviorIn general, hydrogels are not simply elastic materialsbut behave viscoelastically <strong>and</strong> mechanical deformationleads to a time dependence on the appliedstress due to the movement <strong>of</strong> polymer chains segments.This movement induces an internal response,resulting in a time-dependent recovery when theexternal load is removed. Thus, the time dependence<strong>of</strong> the applied stress or strain is as important as theprediction <strong>of</strong> the material’s mechanical response.The investigation <strong>of</strong> the creep behavior makes thedetermination <strong>of</strong> the viscoelastic <strong>and</strong> viscoplasticresponses possible. In this work, the initial <strong>and</strong> NBreinforcedhydrogels were subjected to creep tests.The total deformation <strong>of</strong> hydrogels (e) after the loadcyclewas calculated as a ratio <strong>of</strong> thickness at theend <strong>of</strong> loading to the initial thickness <strong>of</strong> the samples.Additionally, the plastic (irreversible) deformation(d) was estimated as the strain value at the end <strong>of</strong>the recovery-cycle. The DMA method was applied ata relatively short load-cycle setting <strong>and</strong> TMA testswere carried out under several load-conditions withincreased durations for both load <strong>and</strong> relaxationcycles.Representative DMA creep <strong>and</strong> relaxation curvesare presented in Figure 7. At the beginning, the samplesresponded elastically to the initial mechanicalload yielding at 30 <strong>and</strong> 23% strain values for the H<strong>and</strong> H-NB gels, respectively. After 20 min <strong>of</strong> loading,the related strain (e) values reached 33 <strong>and</strong> 27%for H <strong>and</strong> H-NB, respectively (Table III). When theload was removed, the strain dropped suddenly to2.5–3% <strong>and</strong> within 3–4 min the initial thickness <strong>of</strong>the specimens was practically restored <strong>and</strong> d wasfound to be 0% for both H <strong>and</strong> H-NB gels. Such fastresponse <strong>of</strong> hydrogels to load changing conditionswas also observed by other investigators. 32Results <strong>of</strong> creep tests carried out by TMA at differentload conditions are presented in Figure 8. Thevalue <strong>of</strong> elastic (reversible) deformation (e) was evaluatedas the difference between e <strong>and</strong> d at the firstFigure 7 Compression creep <strong>and</strong> relaxation for thehydrogel samples in DMA. Notes: the applied load was 7kPa before deloading. The duration <strong>of</strong> both loading <strong>and</strong>deloading was 20 min.Journal <strong>of</strong> Applied Polymer Science DOI 10.1002/app