Closing Remarks Newsletter - Summer 2009 - Gore Medical
Closing Remarks Newsletter - Summer 2009 - Gore Medical
Closing Remarks Newsletter - Summer 2009 - Gore Medical
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
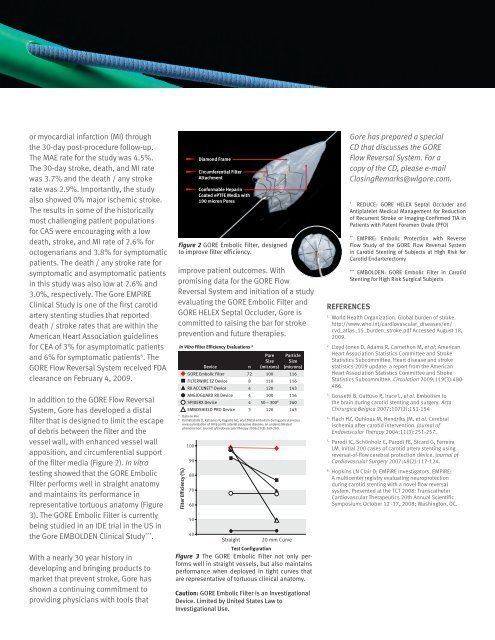
or myocardial infarction (MI) throughthe 30-day post-procedure follow-up.The MAE rate for the study was 4.5%.The 30-day stroke, death, and MI ratewas 3.7% and the death / any strokerate was 2.9%. Importantly, the studyalso showed 0% major ischemic stroke.The results in some of the historicallymost challenging patient populationsfor CAS were encouraging with a lowdeath, stroke, and MI rate of 2.6% foroctogenarians and 3.8% for symptomaticpatients. The death / any stroke rate forsymptomatic and asymptomatic patientsin this study was also low at 2.6% and3.0%, respectively. The <strong>Gore</strong> EMPiREClinical Study is one of the first carotidartery stenting studies that reporteddeath / stroke rates that are within theAmerican Heart Association guidelinesfor CEA of 3% for asymptomatic patientsand 6% for symptomatic patients 6 . TheGORE Flow Reversal System received FDAclearance on February 4, <strong>2009</strong>.In addition to the GORE Flow ReversalSystem, <strong>Gore</strong> has developed a distalfilter that is designed to limit the escapeof debris between the filter and thevessel wall, with enhanced vessel wallapposition, and circumferential supportof the filter media (Figure 2). In vitrotesting showed that the GORE EmbolicFilter performs well in straight anatomyand maintains its performance inrepresentative tortuous anatomy (Figure3). The GORE Embolic Filter is currentlybeing studied in an IDE trial in the US inthe <strong>Gore</strong> EMBOLDEN Clinical Study *** .With a nearly 30 year history indeveloping and bringing products tomarket that prevent stroke, <strong>Gore</strong> hasshown a continuing commitment toproviding physicians with tools thatDiamond FrameCircumferential FilterAttachmentConformable HeparinCoated ePTFE Media with100 micron PoresFigure 2 GORE Embolic Filter, designedto improve filter efficiency.improve patient outcomes. Withpromising data for the GORE FlowReversal System and initiation of a studyevaluating the GORE Embolic Filter andGORE HELEX Septal Occluder, <strong>Gore</strong> iscommitted to raising the bar for strokeprevention and future therapies.In Vitro Filter Efficiency Evaluations AFilter Efficiency (%)100908070605040DeviceStraight20 mm CurveTest ConfigurationFigure 3 The GORE Embolic Filter not only performswell in GORE straight Embolic vessels, FIlter but ACCUNET also maintainsDeviceperformanceFILTERWIREwhen deployedEZ DeviceinANGIOGUARDtight curves thatSpideRXDeviceare representative of tortuous clinical anatomy.Embro Sheild ProCaution: GORE Embolic Filter is an InvestigationalDevice. Limited by United States Law toInvestigational Use.nPoreSize(microns)ParticleSize(microns)GORE Embolic Filter 72 100 116FILTERWIRE EZ Device 8 110 116RX ACCUNET ® Device 4 120 143ANGIOGUARD RX Device 4 100 116SPIDERX Device 4 50 – 300 B 240EmboShield PRO Device 3 120 143A Data on FileB Karnabatidis D, Katsanos K, Kagadis GC, et al.Distal embolism during percutaneousrevascularization of infra-aortic arterial occlusive disease: an underestimatedphenomenon. Journal of Endovascular Therapy 2006;13(3):269-280.<strong>Gore</strong> has prepared a specialCD that discusses the GOREFlow Reversal System. For acopy of the CD, please e-mail<strong>Closing</strong><strong>Remarks</strong>@wlgore.com.*REDUCE: GORE HELEX Septal Occluder andAntiplatelet <strong>Medical</strong> Management for Reductionof Recurrent Stroke or Imaging-Confirmed TIA inPatients with Patent Foramen Ovale (PFO)**EMPIRE: Embolic Protection with ReverseFlow Study of the GORE Flow Reversal Systemin Carotid Stenting of Subjects at High Risk forCarotid Endarterectomy***EMBOLDEN: GORE Embolic Filter in CarotidStenting for High Risk Surgical SubjectsREFERENCES1.World Health Organization. Global burden of stroke.http://www.who.int/cardiovascular_diseases/en/cvd_atlas_15_burden_stroke.pdf Accessed August 18,<strong>2009</strong>.2.Lloyd-Jones D, Adams R, Carnethon M, et al; AmericanHeart Association Statistics Committee and StrokeStatistics Subcommittee. Heart disease and strokestatistics-<strong>2009</strong> update: a report from the AmericanHeart Association Statistics Committee and StrokeStatistics Subcommittee. Circulation <strong>2009</strong>;119(3):480-486.3.Gossetti B, Gattuso R, Irace L, et al. Embolism tothe brain during carotid stenting and surgery. ActaChirurgica Belgica 2007;107(2):151-154 .4.Flach HZ, Ouhlous M, Hendriks JM, et al. Cerebralischemia after carotid intervention. Journal ofEndovascular Therapy 2004;11(3):251-257.5.Parodi JC, Schönholz C, Parodi FE, Sicard G, FerreiraLM. Initial 200 cases of carotid artery stenting usingreversal-of-flow cerebral protection device. Journal ofCardiovascular Surgery 2007;48(2):117-124.6.Hopkins LN Clair D; EMPiRE Investigators. EMPiRE:A multicenter registry evaluating neuroprotectionduring carotid stenting with a novel flow reversalsystem. Presented at the TCT 2008: TranscatheterCardiovascular Therapeutics 20th Annual ScientificSymposium; October 12 -17, 2008; Washington, DC.