Intermolecular hydrogen-bonding effect on carbon-13 NMR ...
Intermolecular hydrogen-bonding effect on carbon-13 NMR ...
Intermolecular hydrogen-bonding effect on carbon-13 NMR ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
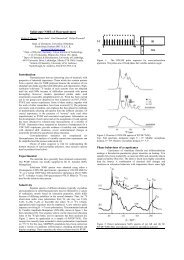
H-B<strong>on</strong>ding Effect <strong>on</strong> "C Chemical Shifts of Solid Peptides169-=2 170-u 4171 -=; 172-173-0030AAA %0027 28 29 30, 31 27 28 29 30 31N 0 LENGTH (A)N 0 LENGTH (ilFigure 3. Plots of the observed I3C chemical shifts in the solid stateagainst the N-0 <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d length (&a) for the 1-type (a) andthe 2-type (b) <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>ds. Triangles, open circles, and full circlesdenote Gly CO of the N-terminal glycyl residues, Gly CO of the sec<strong>on</strong>dglycyl residues, and Gly CO of polyglycine, respectively.Figure 2, a and b, shows schematic representati<strong>on</strong>s of the twotypes of <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>ds which exist in peptides c<strong>on</strong>sidered hereas classified by the nature of the prot<strong>on</strong>-d<strong>on</strong>ating nitrogen atom.One is denoted as the 1-type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d which is formedbetween amide >cIo and amide >N-H. The other is denotedas the 2-type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d which is formed between amide>C-O and N-terminal -NH3+. The 2-type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d exists<strong>on</strong>ly in oligopeptides with free end-groups, since such a peptideusually exists as a zwitteri<strong>on</strong> in the crystalline state, where theN-terminal is prot<strong>on</strong>ated as NH3+ and the C-terminal is deprot<strong>on</strong>atedas COO-.Figure 3, a and b, shows the plot of I3C chemical shifts of GlyCO against the N.-0 <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d length (RN-0) in the 1-typeand 2-type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>ds, respectively. The <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d<str<strong>on</strong>g>effect</str<strong>on</strong>g> <strong>on</strong> the I3C isotropic chemical shift is entirely differentbetween the two types of <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>ds.In the 1-type, a decrease of RN.-O causes a downfield shift.There exists approximately a linear relati<strong>on</strong>ship between RN.-oand the I3C chemical shifts. It is noted that not <strong>on</strong>ly in oligopeptides(dimer or trimer) but also in polypeptides ((Gly), I and11) the Gly CO chemical shifts give a similar <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>ddependence. This suggests that the I3C chemical shift of any GlyCO forming the 1-type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d is predominantly determinedby the manner of <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d. As is evident fromCIO-N and N-H--O angles, most of the 1-type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g>b<strong>on</strong>ds are neither so bent nor entirely straight, where a preferreddirecti<strong>on</strong> of the <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d approaches the directi<strong>on</strong> of theoxygen atom s$ l<strong>on</strong>e pair. The mean value of the C=%N anglesis 153'. This value is slightly larger than that of 120-<strong>13</strong>0Oobtained by averaging over the angles for N-H.-O=C type<str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>ds in <strong>13</strong>57 peptides,I0 but the angles of peptidesc<strong>on</strong>sidered here distribute with small discrepancy. Therefore, itcan be expected that the 'length <str<strong>on</strong>g>effect</str<strong>on</strong>g>" <strong>on</strong> the I3C chemical shiftin the 1-type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d is larger than the 'directi<strong>on</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g>".Further, the carb<strong>on</strong>yl carb<strong>on</strong>s of N-terminal glycine residues(indicated by triangles) are res<strong>on</strong>ated upperfield from those ofthe inner gycine residues (the sec<strong>on</strong>d residue and polypeptide areindicated by open and full circles, respectively). This may be dueto the difference of <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d length rather than the sequence<str<strong>on</strong>g>effect</str<strong>on</strong>g> of amino acids.This result is similar to that by Imashiro et al.34 that the formati<strong>on</strong>of intra- and intermolecular >C=O-H-O- type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g>b<strong>on</strong>ds of hydroxybenzaldehydes in the solid state resultsin a downfield displacement of I3C chemical hifts of the carb<strong>on</strong>ylcarb<strong>on</strong> with varying the 0-0 <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d length. Similarly,Berglund and Va~ghan~~ pointed out a similar correlati<strong>on</strong> betweenthe isotropic 'H chemical shift and 0-0 <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d distance.(34) Imashiro, F.; Maeda, S.; Takegoshi, K.; Terao, T.; Saika, A. Chem.Phys. Lerr. 1983, 99, 189.(35) Bergund, B.; Vaughan, R. W. J. Chem. Phys. 1980, 73, 2037.P.bAAJ. Am. Chem. SOC., Vol. 110, No. 11, 1988 3383H? (b) IH /cyH"-77h 0 icn91h RNIFipe 4. Molecular structure of N-acetyl-N'-methylglycine amide: (a)model A (taking the 1-type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d with two formamide molecules)and (b) model B (taking the 2-type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d with a prot<strong>on</strong>atedmethylamine molecule). The calculati<strong>on</strong> was made for thecarb<strong>on</strong> marked by asterisks.On the other hand, I3C chemical shifts of the Gly CO arelinearly displaced to upperfield with decreasing RN4 in the 2-type<str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d. The directi<strong>on</strong> of the chemical shift change isopposite to that in the 1-type. Such a c<strong>on</strong>siderable differencebetween the two types of <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>ds should be resp<strong>on</strong>siblefor the electr<strong>on</strong>ic structure of the groups participating in the<str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d. Detailed discussi<strong>on</strong> about the electr<strong>on</strong>ic structuredrawn <strong>on</strong> the basis of quantum chemical calculati<strong>on</strong> will be givenin the following secti<strong>on</strong>. A similar phenomen<strong>on</strong> was also reportedfor the <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d <str<strong>on</strong>g>effect</str<strong>on</strong>g> <strong>on</strong> nitrogen chemical shift that thedirecti<strong>on</strong> and magnitude of the chemical shift change dependcritically <strong>on</strong> the electr<strong>on</strong>ic envir<strong>on</strong>ment around nucleus.36As is seen in Figure 3a, the plots are slightly scattered fromthe dotted straight line. Such a scatter may come from thec<strong>on</strong>formati<strong>on</strong>al <str<strong>on</strong>g>effect</str<strong>on</strong>g> of the skeletal b<strong>on</strong>ds and the experimentalerrors for the determinati<strong>on</strong> of the <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d length. In spiteof the dispersity in C=O--N angles, the 2-type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>dgave a clear linearity between the I3C chemical shift and RN ...Ocompared with the 1-type. This can be attributed to the fact thatthe distributi<strong>on</strong> of distorti<strong>on</strong> angles + about the glycyl residueC,-CO b<strong>on</strong>d in the 2-type is much less than that in the 1-type.The directi<strong>on</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g> of the <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d is thought to be smallin the 2-type.I3C Shielding C<strong>on</strong>stant Calculati<strong>on</strong>. Figures Sa-d and 6a-dshow the calculated isotropic shielding c<strong>on</strong>stants (ah) and theirparamagnetic terms of tensor comp<strong>on</strong>ents (ullr uZ2, and u33) ofGly CO using the model compounds A and B (Figure 4, a andb) which corresp<strong>on</strong>d to the 1-type and 2-type <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>ds,respectively. Calculated values are all expressed in parts permilli<strong>on</strong> (ppm) with an opposite sign to that of Table I. Note thatthe negative sign for the calculated shielding c<strong>on</strong>stant denotesdeshielding, in c<strong>on</strong>trast to the positive sign of the experimentalchemical shift values. A shielding c<strong>on</strong>stant or tensor comp<strong>on</strong>entis usually represented as a sum of the diamagnetic and theparamagnetic terms. However, the behavior of the shielding tensorcan be explained by the paramagnetic term, since the diamagneticterm is isotropic.Figure 5a shows the RNa dependence of the calculated isotropicI3C shielding c<strong>on</strong>stant ( u~) of Gly CO in the 1-ty e <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g>b<strong>on</strong>d. In the regi<strong>on</strong> that RN,..o is larger than 2.6 1, uiso valuesindicate no significant RN-0 dependence in both form I and form11. This means that there exists no <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d <str<strong>on</strong>g>effect</str<strong>on</strong>g> at RN-0> 2.6 A. As shown in Figure Sa, there is significant differencein uiso between form I and form I1 in this regi<strong>on</strong>. This may comefrom the c<strong>on</strong>formati<strong>on</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g> (c<strong>on</strong>formati<strong>on</strong> change) in going fromform I to form 11. On the other hand, in the regi<strong>on</strong> that RN .ois shorter than 2.6 A, uiso values largely depend <strong>on</strong> the <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g>b<strong>on</strong>d length RN-0, that is, the <str<strong>on</strong>g>hydrogen</str<strong>on</strong>g> b<strong>on</strong>d <str<strong>on</strong>g>effect</str<strong>on</strong>g> is predominant.Therefore, the experimental data that the I3C chemicalshift values largely depend <strong>on</strong> RNq should be compared with thecalculated results at RN-.o < 2.6 A.Here, we must be c<strong>on</strong>cerned with the critical RN.4 value being2.6 A. It seems that this value of 2.6 A is somewhat short comparedwith the experimental data as shown in Figure 3. As the(36) Webb, G. A.; Witanowski, M. Proc. Indian Acad. Sci. 1985,94,241.