Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
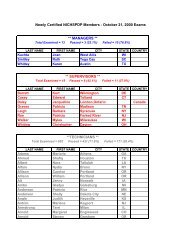
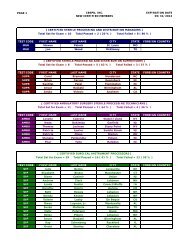
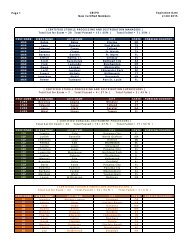
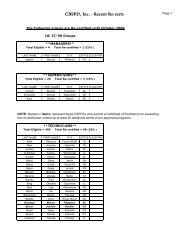
Federal Register / Vol. 72, No. 248 / Friday, December 28, 2007 / Rules and Regulations73613pwalker on PROD1PC71 with RULESrequired compliance certificationdescribed above. The final rule does notrequire ongoing reporting.Except for hospital ethylene oxidesterilization facilities that demonstratecompliance by using add-on controls,affected sources must maintain on siterecords of the date and time of eachsterilization operation. If less than a fullload is sterilized due to medicalnecessity, the operator must record thisas well. These sterilization records mustbe kept in a form suitable and readilyavailable for expeditious review. Theymust be kept for 5 years and at least themost recent 2 years on site.IV. Exemption of Certain Area SourceCategories From Title V PermittingRequirementsSection 502(a) of the CAA providesthat the Administrator may exempt anarea source category from title V if hedetermines that compliance with title Vrequirements is ‘‘impracticable,infeasible, or unnecessarilyburdensome’’ on an area sourcecategory. See CAA section 502(a). InDecember 2005, in a nationalrulemaking, EPA interpreted the term‘‘unnecessarily burdensome’’ in CAAsection 502 and developed a four-factorbalancing test for determining whethertitle V is unnecessarily burdensome fora particular area source category, suchthat an exemption from title V isappropriate. See 70 FR 75320, December19, 2005 (Exemption Rule).The four factors that EPA identified inthe Exemption Rule for determiningwhether title V is ‘‘unnecessarilyburdensome’’ on a particular area sourcecategory include: (1) whether title Vwould result in significantimprovements to the compliancerequirements, including monitoring,recordkeeping, and reporting, that areproposed for an area source category (70FR 75323); (2) whether title Vpermitting would impose significantburdens on the area source category andwhether the burdens would beaggravated by any difficulty the sourcesmay have in obtaining assistance frompermitting agencies (70 FR 75324); (3)whether the costs of title V permittingfor the area source category would bejustified, taking into consideration anypotential gains in compliance likely tooccur for such sources (70 FR 75325);and (4) whether there areimplementation and enforcementprograms in place that are sufficient toassure compliance with the NESHAP forthe area source category, without relyingon title V permits (70 FR 75326).In discussing the above factors in theExemption Rule, we explained that weconsidered on ‘‘a case-by-case basis theextent to which one or more of the fourfactors supported title V exemptions fora given source category, and then weassessed whether considered togetherthose factors demonstrated thatcompliance with title V requirementswould be ‘unnecessarily burdensome’on the category, consistent with section502(a) of the Act.’’ See 70 FR 75323.Thus, in the Exemption Rule, weexplained that not all of the four factorsmust weigh in favor of exemption forEPA to determine that title V isunnecessarily burdensome for aparticular area source category. Instead,the factors are to be considered incombination, and EPA determineswhether the factors, taken together,support an exemption from title V for aparticular source category.In the Exemption Rule, in addition todetermining whether compliance withtitle V requirements would beunnecessarily burdensome on thehospital sterilizer area source category,we considered, consistent with theguidance provided by the legislativehistory of CAA section 502(a), whetherexempting the Hospital Sterilizer AreaSource category would adversely affectpublic health, welfare, or theenvironment. See 70 FR 15254–15255,March 25, 2005.In the proposed rule, we evaluated thefour factors described above in relationto the Hospital Sterilizer Area Sourcecategory and explained our proposedconclusion that the factors collectivelydemonstrated that compliance with titleV requirements would be unnecessarilyburdensome for the source category.Among other things, we explained inthe preamble to the proposed rule, thattitle V permitting would not result insignificant improvements to thecompliance requirements for theHospital Sterilizer Area Source category.In the proposal, we further explainedthat title V permitting may impose asignificant burden on facilities withinthis source category, some of which aresmall businesses. We explained that, formany facilities, the cost of obtaining atitle V permit may far exceed the cost ofcomplying with the final rule withoutsignificant gains in compliance. Basedon the above analysis, we proposed thattitle V permitting would be‘‘unnecessarily burdensome’’ forhospital sterilizer area sources. We alsoproposed that the exemptions from titleV would not adversely affect publichealth, welfare, and the environment.In response to the proposed rule, wereceived two comments concerning theproposed title V exemption. However,as discussed in more detail in sectionV.A.7 of this preamble, neithercomment addressed the above-VerDate Aug2005 23:53 Dec 27, 2007 Jkt 214001 PO 00000 Frm 00041 Fmt 4700 Sfmt 4700 E:\FR\FM\28DER1.SGM 28DER1mentioned factors that we considered inproposing the title V exemption.Accordingly, our assessment of thesefactors remains unchanged in light ofthese comments. We, therefore, finalizethe proposed exemption for the HospitalSterilizer Area Source category in thisrule. Hospital sterilizer area sources arenot required to obtain title V permitssolely for purposes of being the subjectof this final NESHAP; however, if theyare otherwise required to obtain title Vpermits, such requirements are notaffected by this exemption.V. Summary of Comments andResponsesThe hospital sterilizer area source rulewas proposed on November 6, 2006 (71FR 64907). The 60-day comment periodended on January 5, 2007, and wereceived a total of 10 comment letters onthe proposed NESHAP. Comments werereceived from one industry tradeassociation, a representative of oneaffected facility, representatives fromtwo affected Federal agencies, onesterilant manufacturer, three State andlocal air pollution control agencies, oneState agency association, and oneprivate citizen. This final rule reflectsour consideration of all of the commentsreceived on the proposed action. Thissection summarizes the significantcomments received on the proposedNESHAP and our response thereto. Asummary of all of the minor commentsand EPA’s response thereto arepresented here in this preamble and inthe Response to Comments Document(RTC Document), which is available inDocket No. EPA–HQ–OAR–2005–0171.A. Proposed Alternative 1: ManagementPractice1. Management Practice ApproachComment: Two commenterssupported promulgation of themanagement practice approach, i.e.,Regulatory Alternative 1. One of thecommenters noted that EPA recognizesthat, by minimizing ethylene oxide usewith the management practice, hospitalethylene oxide sterilization facilitiesalso minimize ethylene oxide emissions.Both commenters expressed that theproposed management practicealternative ensures that hospitalssterilize the most number of medicaldevices per pounds of ethylene oxideemitted, and it is consistent withhospital practices.Two commenters stated that themanagement practice is common sense.One commenter argued that EPA’sproposed GACT were neither acceptablenor consistent with legal requirements.Another commenter stated that EPA’s