Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
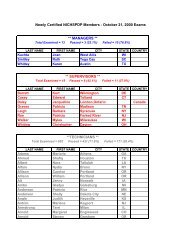
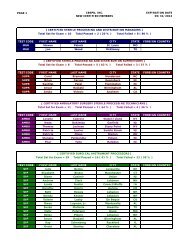
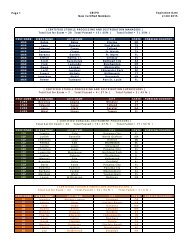
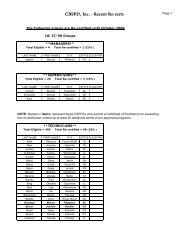
73616 Federal Register / Vol. 72, No. 248 / Friday, December 28, 2007 / Rules and Regulationspwalker on PROD1PC71 with RULES<strong>Oxide</strong> Sterilization in Health CareFacilities: Safety and Effectiveness,ANSI/AAMI ST 41:1999. According tothe commenter, the sterilizer recordsunder this standard include thefollowing: Load or lot number; itemdescription and quantity; thedepartment; the name of the sterilizeroperator; aeration time and temperature;results of the biological monitoring(which is processed with each load toensure that sterilization has occurred);chemical indicator results; and reportsof nonresponsive chemical indicators.Two commenters stated that hospitalskeep a record of each load they run fortraceability. Two commenters statedthat hospitals could probably add a fewmore items of information to theirrecords to comply with EPA’srequirements. These commentersrecommended that EPA’s recordkeepingrequirements be consistent withhospitals’ current practice inmaintaining records of sterilized loads.Two commenters indicated that someState programs require keepingsterilization records, and onecommenter stated that some States haverequired such recordkeeping for manyyears. The commenters indicated thatsome hospitals keep such recordsthrough computerized recordkeepingsystems while others use handwrittenrecords. The commenters believed thatthese requirements are not likely to beoverly burdensome or costly to thefacilities.Response: In light of the commentsindicating that hospitals are alreadykeeping records of each sterilizationcycle and that such recordkeepingprovisions are not overly costly orburdensome, we are requiring affectedfacilities to keep sterilization records inthe final rule. Specifically, the final rulerequires that a facility record the dateand time of each sterilization cycle,whether each sterilization cyclecontains a full load of items, and, foreach partial load, state that it wasmedically necessary. Based oninformation provided during thecomment period, we believe that thisrecordkeeping requirement is consistentwith hospitals’ current practice. We alsobelieve the time required to keep theserecords would be offset by the timesaved by the reduced cycles run.7. Title V PermittingComment: One commenter favoredtitle V permitting. The commenterstated that, by requiring title V permits,title V funds could be used to assurecompliance. The commenter noted that,according to an EPA Regional office,title V funds cannot be used for non-titleV programs. The commenter stated thatif, from a national perspective, EPAprefers to exempt area sources such asthese from title V permitting, EPAshould explain the level of effort theyexpect from State and local agencies,and develop a funding mechanism forthat effort. The commenter further notedthat, in this case, the commenter’s Statealready has operating permits foraffected facilities and that there wouldbe little cost involved in updating thesepermits to reflect the Federal ruleduring the normal permit renewalprocess.Response: As discussed in thepreamble to the proposed rule, EPAconsidered four factors in determiningwhether title V is ‘‘unnecessarilyburdensome’’ for a particular areasource category. Based on itsconsideration of these factors, EPAconcluded that the requirements of titleV would be unnecessarily burdensomefor area source hospital ethylene oxidesterilization facilities. Among otherthings, EPA concluded that title Vpermitting would not result insignificant improvements to thecompliance requirements for thehospital ethylene oxide sterilizationarea source category and that title Vpermitting would likely impose asignificant burden on facilities withinthe source category, some of which aresmall businesses. The Agency alsofound that, for many facilities, the costof obtaining a title V permit may farexceed the cost of complying with thefinal rule without significant gains incompliance. EPA further determinedthat the proposed exemptions from titleV would not adversely affect publichealth, welfare, and the environment.Although the commenter advocatestitle V permitting, the commenter failedto address EPA’s application of the fourfactors described above, and itsconclusion that the proposedexemptions would not adversely affectpublic health, welfare, and theenvironment. Indeed, none of thecommenters disagreed with any ofEPA’s proposed findings describedabove and in the proposed rule thatserved as the basis for the proposed titleV exemption.Instead of challenging EPA’sapplication of the four factors relevantto determining whether title Vrequirements would be unnecessarilyburdensome on a particular area sourcecategory, the commenter focuses on thefact that, in its State, area sourcehospital sterilizers have State operatingpermits and that adding therequirements of this rule to thosepermits would involve little costs. Thefact that title V permitting may not beburdensome or costly in one State doesVerDate Aug2005 23:53 Dec 27, 2007 Jkt 214001 PO 00000 Frm 00044 Fmt 4700 Sfmt 4700 E:\FR\FM\28DER1.SGM 28DER1not reflect the burden or costsassociated with title V permittingnationwide. Once again, the commenterhas not identified any flaws in EPA’sapplication of the four factor testdescribed above, which involve anassessment of the costs of title Vreporting for the entire source category.Therefore, for the reasons discussedabove and in the proposed rule, we areexempting area source hospital ethyleneoxide sterilization facilities from therequirements of title V in this final rule.The commenter apparently favoredtitle V permitting based on its belief that‘‘by requiring title V permits, EPAwould allow title V funds to be used toassure compliance.’’ The commenterrequested that EPA explain the level ofState and local efforts that may beinvolved in implementing and enforcingthe requirements of the final rule anddevelop a funding mechanism for thateffort. We expect such effort to beminimal. We believe that themanagement practice and the associatedrecordkeeping requirements in this finalrule are straightforward and can,therefore, be easily implemented andenforced. Further, according to thecomments received, the managementpractice requirement is consistent withhospital practices and hospitals arealready keeping records of sterilizationcycles. In light of the above, we do notanticipate that State and local agencieswould need to spend a significant levelof effort to implement and enforce thisrule. EPA, however, remains committedto working with State and local agenciesto implement this rule. State and localagencies that receive grants forcontinuing air programs under CAAsection 105 should work with theirproject officers to determine whatresources are necessary to implementand enforce this area source standard.EPA will continue to provide theresources appropriated for CAA section105 grants consistent with the statuteand the allotment formula developedpursuant to the statute.Comment: One commenter agreedwith EPA’s proposal that title V permitsare not necessary for area sources. Thecommenter noted that some hospitals,however, already have or are covered bytitle V permits, and that any rulemakinghas the potential to impose additionalpermit modification costs. Thecommenter asserted that EPA shouldminimize title V permitting cost impactsby adding a provision in this rule statingthat an existing title V permit does nothave to be reopened or revised toaddress the requirements of this ruleuntil the next time the permit isrenewed, reopened, or revised foranother reason. The commenter