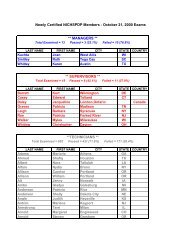
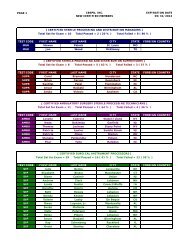
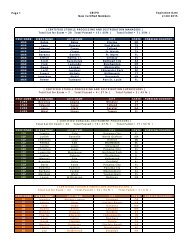
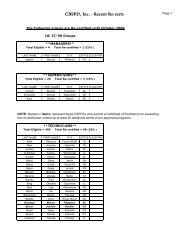
Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Federal Register / Vol. 72, No. 248 / Friday, December 28, 2007 / Rules and Regulations73615pwalker on PROD1PC71 with RULESof patients’ medical needs instead of byEPA. However, to assure that hospitalsrun sterilizers in full loads exceptduring medically necessarycircumstances, the final rule requiresthat facilities document and maintainrecords of every sterilization cycle,including each partially loadedsterilization, and confirm that it wasmedically necessary.Comment: One commenter noted thatmany university hospitals develop newand unique surgical procedures anddevices that may need to be sterilized inpartial loads to comply with the morestringent requirements for sterilizing anew instrument.Response: We believe that it ismedically necessary to allow hospitalsto sterilize medical devices that areunder research and developmentwithout a full load. The novelty oruniqueness of the design in someinstances require different sterilizingparameters than those used for regularmedical devices. In addition, unlikemedical devices that are regularly usedfor patient care, new and experimentalmedical devices that are under researchand development do not haveestablished or known sterilizationcycles. Therefore, they may compromisethe effectiveness of sterilizing otherdevices in the same loads. However,hospitals generally do not possessenough medical devices that are underresearch and development to fully loada sterilizer. To avoid compromising thesterilization process of medical devicesregularly used for patient care, webelieve that it is medically necessary toallow hospitals to sterilize medicaldevices that are under research anddevelopment in separate and partialloads. <strong>Hospitals</strong> may invoke the medicalnecessity exception in the final rulewhen sterilizing devices that are underresearch and development.4. National or UrbanComment: Three commentersrecommended that EPA apply this rulenationwide. Two of the commentersnoted that hospital parking areas aretypically close to the hospital and thatvisitors and employees are, therefore,exposed to emissions from hospitalethylene oxide sterilizers regardless ofthe hospital’s location (i.e., urban orrural). One commenter stated that theimpacts of ethylene oxide emissions arelocalized and would be similar for mosturban and rural areas. According to thecommenter, hospitals are typicallylocated in residential areas, whether ornot they are in urban areas, and thatpopulations residing nearby wouldlikely be exposed to the ethylene oxideemissions from a hospital ethyleneoxide sterilization facility. Anothercommenter further stated that hospitalsclearly serve more sensitive populationswho could be more susceptible toimpacts from exposure to ethyleneoxide. The commenter similarly notedthat the impacts of ethylene oxideemissions are very local and would beroughly the same for both urban andrural areas, except perhaps for hospitalslocated in areas with a high populationdensity.Two commenters noted that the cost(of controlling a sterilizer) to a facilityis the same for a rural hospital and anurban hospital. The commenters statedthat, because the cost and impact are thesame, there does not appear to be anyrationale for treating rural hospitalsdifferently from urban hospitals.Response: We agree that a nationwideapproach is appropriate given the factsand circumstances of this particular areasource category. A rule of nationwideapplicability is particularly appropriatehere because requiring controlsnationwide provides for equitableemission reductions. Control costs arenot expected to differ in rural versusurban settings, therefore, the control’scost-effectiveness is the same, andeconomic impacts are equallydistributed. Furthermore, becausehospitals are generally located indensely populated areas, we expectnegligible difference in the scope of thisrule’s coverage between a national andan urban (i.e., Urban-1 and Urban-2areas) rule. 4 We have received nocomments recommending that we limitthis rule’s applicability only to hospitalsin Urban-1 and Urban-2 areas.5. Compliance DateComment: One commenter stated thatEPA’s proposal that a source complywith the management practices within 1year after the effective date of the finalrule may not be a sufficient period oftime. The commenter stated that twoscenarios could result for medicalfacilities under the managementpractice alternative. According to thecommenter, one scenario could be thatmedical facilities may need to purchasesmaller ethylene oxide sterilizers to turnaround medical instrumentation andequipment without having to purchasemore of these medical items, and this4 In the Integrated Urban Strategy, EPA defined‘‘urban areas’’ to include Urban-1 and Urban-2areas. (64 FR 38724). The Urban-1 and Urban-2definitions are based on the United States CensusBureau’s most current decennial census data.Urban-1 areas are counties with metropolitanstatistical areas with a population greater than250,000. Urban-2 counties are all other countieswhere more than 50 percent of the population isdesignated urban by the United States CensusBureau.VerDate Aug2005 23:53 Dec 27, 2007 Jkt 214001 PO 00000 Frm 00043 Fmt 4700 Sfmt 4700 E:\FR\FM\28DER1.SGM 28DER1could involve construction projects/costs to make ready additional space toaccommodate the new sterilizers. Thecommenter stated that the otherscenario could be that medical facilitiesmay need to purchase additionalmedical instrumentation and equipmentto allow for sufficient availability whilewaiting for enough items to accumulateto run a full load in an ethylene oxidesterilizer. The commenter suggested thatEPA consider the costs of additionalethylene oxide sterilizer equipment andrelated construction, as well as theadditional medical instrumentation andequipment costs in any proposed ruleby EPA.Response: EPA does not believe thatthe management practice requirement inAlternative 1 will result in either of thescenarios described above. Themanagement practice requires sterilizingfull loads except during medicallynecessary circumstances, i.e., necessaryto protect human health. As discussedabove, this exception to runningsterilizers in full loads is based onpatient needs. Under the final rule,whether a medically necessarycircumstance exists must be determinedby an authorized hospital personnel.The final rule, however, requires onlythat the hospital personnel considerwhether sterilizing a partial load isnecessary to protect human health; thepersonnel are not required to considerwhether there are viable alternatives torunning a partial load, such aspurchasing additional sterilizerequipment or medical devices, beforeinvoking the exception to themanagement practice requirement.Therefore, we do not expect any needfor construction and/or capitalexpenditures associated with such newpurchases, as the commenter suggested.We have received no other commentssuggesting that hospitals may havedifficulty achieving compliance withthe management practice alternativewithin 1 year, as we proposed. We,therefore, retain the 1-year compliancedeadline in the final rule.6. RecordkeepingComment: In the proposed rule, EPAsolicited comments on whether torequire recordkeeping under Alternative1. We received six comments onrecordkeeping. One commenter askedthat EPA specify what recordkeepingwould entail if less than full loads wererun and what EPA would propose to bedone with these records. Anothercommenter stated that, regardless of thesize of the load, all items sterilized arerecorded following the Association forthe Advancement of MedicalInstrumentation standard, <strong>Ethylene</strong>