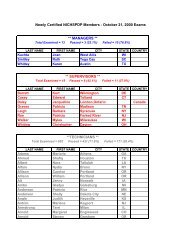
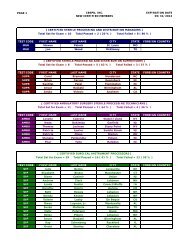
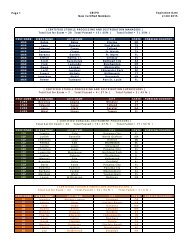
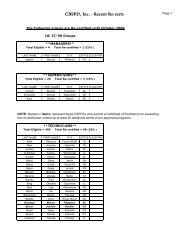
Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
Hospitals: Ethylene Oxide Sterilizers - US Environmental Protection ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
73618 Federal Register / Vol. 72, No. 248 / Friday, December 28, 2007 / Rules and Regulationspwalker on PROD1PC71 with RULESnecessity in hospitals. The commenterexplained that the medical devicesprocessed by ethylene oxide areexpensive and that hospitals can onlyafford minimal amounts on hand. Thecommenter further explained that someof the medical devices are old andcannot be replaced. The commenternoted that these devices are typicallyutilized in surgical areas. Thecommenter stated that EPA’s rationalemakes clear that existing ethylene oxideemission control technology will notprovide the type of cost-benefit neededto justify new hospital investment in thecontrol devices. The commenter notedthat the cost of add-on control wouldinclude not just the cost of the device,but also the cost of installation, facilitymodification, annual testing of controldevices, and utility and maintenance.Response: CAA section 112(d)(5)provides that, with respect to areasource categories listed pursuant toCAA section 112(c), the Administratormay, in lieu of MACT, promulgatestandards or requirements whichprovide for the use of GACT. Asexplained in the preamble to theproposed rule, EPA is issuing thestandards for the hospital sterilizers areasource category under CAA section112(d)(5).In determining what constitutesGACT for a particular area sourcecategory, EPA evaluates the controltechnologies and management practicesthat reduce HAP emissions and aregenerally available for the area sourcecategory. The legislative historysupporting CAA section 112(d)(5)provides that EPA may consider costs indetermining what constitutes GACT forthe area source category. 5In considering costs, the commenterswho recommended add-on controlfocused mainly on the actual costs tohospitals and asserted that such controlis likely not too costly if many hospitalsare using it under existing State or localrequirements. As we stated in thepreamble to the proposed rule, EPArecognizes that over half of the hospitalsuse add-on controls. However, theactual cost to individual hospitals is butone cost factor that we considered inthis rulemaking. We also noted that thetotal annualized cost for add-on5 Additional information on the definition of‘‘generally available control technologies ormanagement practices’’ (GACT) is found in theSenate report on the 1990 amendments to the CAA(S. Rep. No. 101–228, 101st Cong. 1st session. 171–172). That report states that GACT is to encompass:* * * methods, practices and techniques which arecommercially available and appropriate forapplication by the sources in the categoryconsidering economic impacts and the technicalcapabilities of the firms to operate and maintain theemissions control systems.controls, which we estimated to be $8.5million, exceeds the total annualizedcost for the management practice, whichwe estimated to range from $32,000 to$61,000, by more than 100 fold. Inaddition, we considered the costeffectivenessof the add-on controls.See, e.g., Husquavarna AB v. EPA, 439U.S. App. DC 118, 254 F.3d 195, 201(DC Cir. 2001) (finding EPA’s decisionto consider costs on a per ton ofemissions removed basis reasonablebecause CAA section 213 did notmandate a specific method of costanalysis). EPA’s cost analysis for theadd-on controls showed poor costeffectiveness.Specifically, EPA’s costeffectivenessestimate for add-oncontrols was $200,000 per ton ofethylene oxide reduced. This costeffectivenessexcludes monitoring,recordkeeping, and reporting costs.We also considered alternatives toethylene oxide sterilization, as onecommenter suggested. We learned fromseveral commenters that, althoughethylene oxide sterilization in hospitalshas declined, it remains a necessity forcertain medical devices that cannot beeasily replaced or sterilized by othermeans. We agree with these commentersthat, in light of the declined level ofethylene oxide sterilization and the lackof alternatives for sterilizing certainunique and expensive medical devices,the benefit of requiring add-on controlis outweighed by the various costsassociated with such control. Based onthe foregoing, we determined that addoncontrols do not represent GACT forthis area source category.One commenter argued that EPArequired add-on control in the HMIWIstandard that were not necessarily costeffectiveand that EPA should take thesame approach in this final rule. 6 TheHMIWI standard, however, waspromulgated pursuant to section 129 ofthe CAA, which requires that EPAestablish standards that reflect theMACT. Consistent with therequirements of CAA section 129, EPAissued the original HMIWI standardsbased on MACT. CAA section 129(a)(2)does not allow EPA to consider costs insetting the floor for control. By contrast,EPA is issuing this final rule pursuantto CAA section 112(d)(5), which allowsEPA to consider costs, including costeffectiveness,in establishing GACT.Thus, the HMIWI rule is not relevant,6 40 CFR part 60, subpart Ce—EmissionGuidelines and Compliance Times for Hospital/Medical/Infectious Waste Incinerators (constructedon or before June 20, 1996).40 CFR part 60, subpart Ec—Standards ofPerformance for Hospital/Medical/Infectious WasteIncinerators for Which Construction is CommencedAfter June 20, 1996.VerDate Aug2005 23:53 Dec 27, 2007 Jkt 214001 PO 00000 Frm 00046 Fmt 4700 Sfmt 4700 E:\FR\FM\28DER1.SGM 28DER1because in that rule, EPA, by statute,could not consider costs.2. Best Available Control Technology(BACT) or MACTComment: One commenter stated that,because ethylene oxide is a knownhuman carcinogen, its emissions shouldbe controlled using the BACT. Thecommenter stated alternatively that, dueto the widespread use of control onhospital sterilizers, the MACT floorlevel of control would be add-oncontrols under CAA section 112(d)(2).The commenter stated that, based on theexperience in its State, the MACT floorand associated recordkeeping arefeasible and prudent and, therefore,none of EPA’s proposals are inaccordance with legal requirements. Thecommenter claimed that the proposedNESHAP must be revised to representMACT floor of add-on emission controland recordkeeping as required by law.Response: CAA section 112(c)(2)requires that EPA establish emissionstandards under CAA section 112(d) forthe categories listed under CAA section112(c), including area source categorieslisted pursuant to CAA section112(c)(3). As mentioned above, EPAmay issue standards for listed areasource categories based on MACT (CAAsection 112(d)(2)) or GACT (CAAsection 112(d)(5)). CAA Section 112(d)does not contain a standard based onBACT. Therefore, EPA rejects thecommenter’s request to require the useof BACT because such standard is notauthorized by the CAA.The commenter also arguedalternatively that neither of EPA’sproposed alternatives was in accordancewith legal requirements and that EPAmust issue a MACT standard as requiredby law. The commenter, however, didnot identify any legal requirement thatallegedly is not satisfied by EPA’sproposed alternatives or requires EPA toissue a MACT standard for the HospitalSterilizer Area Source category. On thecontrary, the commenter noted that‘‘EPA is ’exercising discretion’ inpromulgating standards or requirementsunder section 112(d)(5) of the CAA.’’Although the commenter acknowledgedthat EPA has discretion under CAAsection 112(d)(5) to issue a GACTstandard in lieu of a MACT standard forlisted area source categories, it claimedthat, based on its State’s experiencewith regulating and controlling ethyleneoxide emissions from hospitalsterilizers, the MACT floor andassociated recordkeeping are feasibleand prudent. The commenter arguedthat, therefore, neither of EPA’sproposals is acceptable in accordancewith legal requirements and that EPA