A review of production technologies for ... - World Health Organization
A review of production technologies for ... - World Health Organization
A review of production technologies for ... - World Health Organization
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Influenza vaccine <strong>production</strong> <strong>technologies</strong><br />
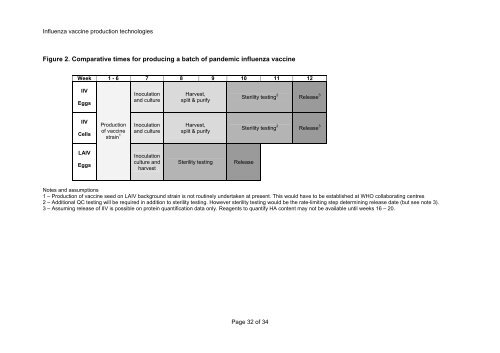
Figure 2. Comparative times <strong>for</strong> producing a batch <strong>of</strong> pandemic influenza vaccine<br />
Week 1 - 6 7 8 9 10 11 12<br />
IIV<br />
Eggs<br />
IIV<br />
Cells<br />
LAIV<br />
Eggs<br />
Production<br />
<strong>of</strong> vaccine<br />
strain 1<br />
Inoculation<br />
and culture<br />
Inoculation<br />
and culture<br />
Inoculation<br />
culture and<br />
harvest<br />
Harvest,<br />
split & purify<br />
Harvest,<br />
split & purify<br />
Sterility testing Release<br />
Sterility testing 2<br />
Sterility testing 2<br />
Release 3<br />
Release 3<br />
Notes and assumptions<br />
1 – Production <strong>of</strong> vaccine seed on LAIV background strain is not routinely undertaken at present. This would have to be established at WHO collaborating centres<br />
2 – Additional QC testing will be required in addition to sterility testing. However sterility testing would be the rate-limiting step determining release date (but see note 3).<br />
3 – Assuming release <strong>of</strong> IIV is possible on protein quantification data only. Reagents to quantify HA content may not be available until weeks 16 – 20.<br />
Page 32 <strong>of</strong> 34