A review of production technologies for ... - World Health Organization
A review of production technologies for ... - World Health Organization
A review of production technologies for ... - World Health Organization
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Influenza vaccine <strong>production</strong> <strong>technologies</strong><br />
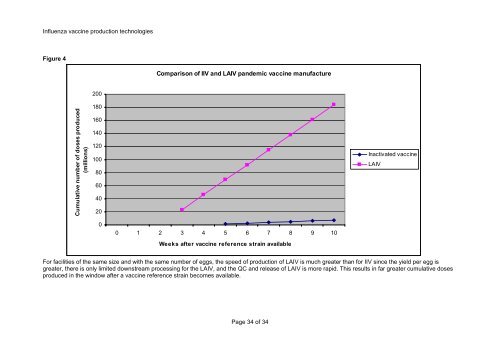
Figure 4<br />
Cumulative number <strong>of</strong> doses produced<br />
(millions)<br />
200<br />
180<br />
160<br />
140<br />
120<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
Comparison <strong>of</strong> IIV and LAIV pandemic vaccine manufacture<br />
0 1 2 3 4 5 6 7 8 9 10<br />
Weeks after vaccine reference strain available<br />
Inactivated vaccine<br />
For facilities <strong>of</strong> the same size and with the same number <strong>of</strong> eggs, the speed <strong>of</strong> <strong>production</strong> <strong>of</strong> LAIV is much greater than <strong>for</strong> IIV since the yield per egg is<br />
greater, there is only limited downstream processing <strong>for</strong> the LAIV, and the QC and release <strong>of</strong> LAIV is more rapid. This results in far greater cumulative doses<br />
produced in the window after a vaccine reference strain becomes available.<br />
Page 34 <strong>of</strong> 34<br />
LAIV