Instructions for Use Steriking Self Seal Pouches
Instructions for Use: Steriking Self Seal Pouches - Support
Instructions for Use: Steriking Self Seal Pouches - Support
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
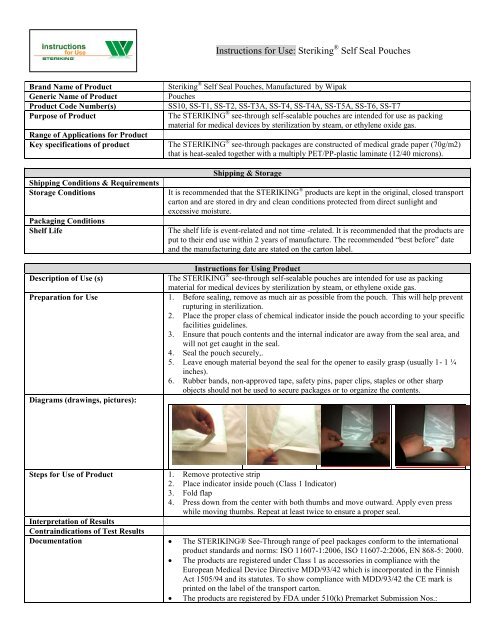
<strong>Instructions</strong> <strong>for</strong> <strong>Use</strong>: <strong>Steriking</strong> ® <strong>Self</strong> <strong>Seal</strong> <strong>Pouches</strong>Brand Name of ProductGeneric Name of ProductProduct Code Number(s)Purpose of ProductRange of Applications <strong>for</strong> ProductKey specifications of productShipping Conditions & RequirementsStorage ConditionsPackaging ConditionsShelf Life<strong>Steriking</strong> ® <strong>Self</strong> <strong>Seal</strong> <strong>Pouches</strong>, Manufactured by Wipak<strong>Pouches</strong>SS10, SS-T1, SS-T2, SS-T3A, SS-T4, SS-T4A, SS-T5A, SS-T6, SS-T7The STERIKING ® see-through self-sealable pouches are intended <strong>for</strong> use as packingmaterial <strong>for</strong> medical devices by sterilization by steam, or ethylene oxide gas.The STERIKING ® see-through packages are constructed of medical grade paper (70g/m2)that is heat-sealed together with a multiply PET/PP-plastic laminate (12/40 microns).Shipping & StorageIt is recommended that the STERIKING ® products are kept in the original, closed transportcarton and are stored in dry and clean conditions protected from direct sunlight andexcessive moisture.The shelf life is event-related and not time -related. It is recommended that the products areput to their end use within 2 years of manufacture. The recommended “best be<strong>for</strong>e” dateand the manufacturing date are stated on the carton label.<strong>Instructions</strong> <strong>for</strong> Using ProductDescription of <strong>Use</strong> (s)The STERIKING ® see-through self-sealable pouches are intended <strong>for</strong> use as packingmaterial <strong>for</strong> medical devices by sterilization by steam, or ethylene oxide gas.Preparation <strong>for</strong> <strong>Use</strong> 1. Be<strong>for</strong>e sealing, remove as much air as possible from the pouch. This will help preventrupturing in sterilization.2. Place the proper class of chemical indicator inside the pouch according to your specificfacilities guidelines.3. Ensure that pouch contents and the internal indicator are away from the seal area, andwill not get caught in the seal.4. <strong>Seal</strong> the pouch securely,.5. Leave enough material beyond the seal <strong>for</strong> the opener to easily grasp (usually 1- 1 ¼inches).6. Rubber bands, non-approved tape, safety pins, paper clips, staples or other sharpobjects should not be used to secure packages or to organize the contents.Diagrams (drawings, pictures):Steps <strong>for</strong> <strong>Use</strong> of Product 1. Remove protective strip2. Place indicator inside pouch (Class 1 Indicator)3. Fold flap4. Press down from the center with both thumbs and move outward. Apply even presswhile moving thumbs. Repeat at least twice to ensure a proper seal.Interpretation of ResultsContraindications of Test ResultsDocumentation The STERIKING® See-Through range of peel packages con<strong>for</strong>m to the internationalproduct standards and norms: ISO 11607-1:2006, ISO 11607-2:2006, EN 868-5: 2000. The products are registered under Class 1 as accessories in compliance with theEuropean Medical Device Directive MDD/93/42 which is incorporated in the FinnishAct 1505/94 and its statutes. To show compliance with MDD/93/42 the CE mark isprinted on the label of the transport carton. The products are registered by FDA under 510(k) Premarket Submission Nos.:
Special Warnings and CautionsDisposalK803293, K953776 and K973827. Wipak Oy is certified to ISO 9001:2000; ISO14001: 2004; OHSAS 18001: 1999; ISO 22000: 2005 and DS 3027: 2002. STERIKING® sterilization packages are designed, validated, and manufactured to suittheir intended purposes.The STERIKING® standard range of see-through packages is not suitable <strong>for</strong> sterilizationby irradiation or by hot, dry air at the temperatures over 140 ºC.Please refer to the local/national regulations regarding waste disposal.Reprocessing <strong>Instructions</strong>Point of use:Preparation <strong>for</strong> decontamination:Disassembly <strong>Instructions</strong>:Cleaning – Manual:Cleaning – Automated:Disinfection:Drying:Maintenance, inspection, and testing:Reassembly <strong>Instructions</strong>:Packaging:Sterilization: Compatible with steam and EO sterilization May be used in standard steam cycles and extended sterilization cycles (validation dataavailable online). Be sure to arrange pouches in such a way that there is minimal to no contact betweenpouches. If touching, arrange so that paper side is facing plastic side. Chemical Indicators con<strong>for</strong>m to ISO 11140-1:2005 class 1: Process indicators. Steamindicator changes color from blue to dark brown/black and EO gas indicator from pinkto yellow on the outside of the pouch.Storage:The sterilized products are sorted <strong>for</strong> storage or delivery to the wards. The products shouldbe stored in a dust-free place protected from sunlight, preferably in closed cabinets. It isrecommended that the room climate has a humidity of 40% to 60% and a temperature of15-25ºC.Additional In<strong>for</strong>mation:The products are <strong>for</strong> single use only.Related Healthmark ProductsOther Product Support DocumentsReference DocumentsCustomer Service contact:2013-11-08 msmithSterilization Packaging Brochure, Sterilization Packaging Price ListUS Distributor:Healthmark Industries Company, Inc33671 DorekaFraser, MI 480261-586-774-7600healthmark@hmark.comhmark.com