Instructions for Use Cautery Tip Cleaner
Instructions for Use: Cautery Tip Cleaner - Support
Instructions for Use: Cautery Tip Cleaner - Support
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
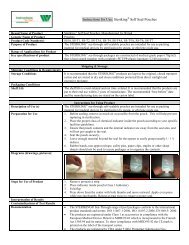
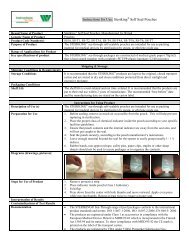
<strong>Instructions</strong> <strong>for</strong> <strong>Use</strong>: <strong>Cautery</strong> <strong>Tip</strong> <strong>Cleaner</strong>Brand Name of Product<strong>Cautery</strong> <strong>Tip</strong> <strong>Cleaner</strong>Generic Name of Product<strong>Tip</strong> <strong>Cleaner</strong>Product Code Number(s)MED6361UPurpose of ProductTo safely and effectively remove tissue and debris from surgical cautery instrument tips.Range of Applications <strong>for</strong> Product For use on surgical cautery instrument tips in the Sterile Processing DepartmentKey specifications of product Urethane foam with aluminum oxide 60 grit Latex free Non-sterileShipping Conditions & RequirementsStorage ConditionsPackaging ConditionsShelf LifeShipping & Storage<strong>Instructions</strong> <strong>for</strong> Using ProductDescription of <strong>Use</strong> (s)For removing tissue and debris from cautery instrument tips.Preparation <strong>for</strong> <strong>Use</strong>Diagrams (drawings, pictures):Steps <strong>for</strong> <strong>Use</strong> of Product 1. Consult the instrument manufacturer’s IFU <strong>for</strong> the cleaning instructions <strong>for</strong> theinstrument.2. Place <strong>Cautery</strong> <strong>Tip</strong> <strong>Cleaner</strong> Pad directly on flat surface.3. To clean, place instrument tip on pad.4. When applying pressure in a downward motion, do not allow insulation area of theinstrument come in contact with the grit. I can compromise the insulation.5. Repeat until surgical instrument tip is visibly free of tissue and debris. Checkinsulation to make sure it’s still intact.Interpretation of ResultsContraindications of Test ResultsDocumentationSpecial Warnings and Cautions Strictly <strong>for</strong> use in the sterile processing area. Not <strong>for</strong> use in the operating room.Item is not sterile. Consult the instrument manufacturer’s IFU to be sure use of the abrasive pad isindicated <strong>for</strong> use to clean the instrument.DisposalTreat as a biohazard and dispose of in compliance with facility guidelines <strong>for</strong> disposing ofbiohazard waste.Point of use:Preparation <strong>for</strong> decontamination:Disassembly <strong>Instructions</strong>:Cleaning – Manual:Cleaning – Automated:Disinfection:Drying:Maintenance, inspection, and testing:Reassembly <strong>Instructions</strong>:Packaging:Sterilization:Storage:Additional In<strong>for</strong>mation:Related Healthmark ProductsOther Product Support DocumentsReprocessing <strong>Instructions</strong>Prosys Brochure, Prosys Price List
Reference DocumentsCustomer Service contact:2012-10-11 msmithHealthmark Industries Company, Inc33671 DorekaFraser, MI 480261-586-774-7600healthmark@hmark.comhmark.com