Speciifiicatiion STERIKING LT-Blueline Pouches and Reels
Specification - Support
Specification - Support
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
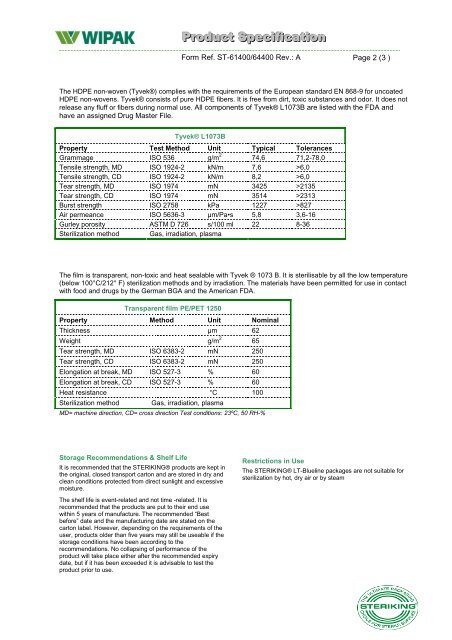
Product <strong>Speciifiicatiion</strong>Form Ref. ST-61400/64400 Rev.: A Page 2 (3 )The HDPE non-woven (Tyvek®) complies with the requirements of the European st<strong>and</strong>ard EN 868-9 for uncoatedHDPE non-wovens. Tyvek® consists of pure HDPE fibers. It is free from dirt, toxic substances <strong>and</strong> odor. It does notrelease any fluff or fibers during normal use. All components of Tyvek® L1073B are listed with the FDA <strong>and</strong>have an assigned Drug Master File.Tyvek® L1073BProperty Test Method Unit Typical TolerancesGrammage ISO 536 g/m 2 74,6 71,2-78,0Tensile strength, MD ISO 1924-2 kN/m 7,6 >6,0Tensile strength, CD ISO 1924-2 kN/m 8,2 >6,0Tear strength, MD ISO 1974 mN 3425 >2135Tear strength, CD ISO 1974 mN 3514 >2313Burst strength ISO 2758 kPa 1227 >827Air permeance ISO 5636-3 μm/Pa•s 5,8 3,6-16Gurley porosity ASTM D 726 s/100 ml 22 8-36Sterilization method Gas, irradiation, plasmaThe film is transparent, non-toxic <strong>and</strong> heat sealable with Tyvek ® 1073 B. It is sterilisable by all the low temperature(below 100°C/212° F) sterilization methods <strong>and</strong> by irradiation. The materials have been permitted for use in contactwith food <strong>and</strong> drugs by the German BGA <strong>and</strong> the American FDA.Transparent film PE/PET 1250Property Method Unit NominalThickness μm 62Weight g/m 2 65Tear strength, MD ISO 6383-2 mN 250Tear strength, CD ISO 6383-2 mN 250Elongation at break, MD ISO 527-3 % 60Elongation at break, CD ISO 527-3 % 60Heat resistance °C 100Sterilization method Gas, irradiation, plasmaMD= machine direction, CD= cross direction Test conditions: 23ºC, 50 RH-%Storage Recommendations & Shelf LifeIt is recommended that the <strong>STERIKING</strong>® products are kept inthe original, closed transport carton <strong>and</strong> are stored in dry <strong>and</strong>clean conditions protected from direct sunlight <strong>and</strong> excessivemoisture.Restrictions in UseThe <strong>STERIKING</strong>® <strong>LT</strong>-<strong>Blueline</strong> packages are not suitable forsterilization by hot, dry air or by steamThe shelf life is event-related <strong>and</strong> not time -related. It isrecommended that the products are put to their end usewithin 5 years of manufacture. The recommended “Bestbefore” date <strong>and</strong> the manufacturing date are stated on thecarton label. However, depending on the requirements of theuser, products older than five years may still be useable if thestorage conditions have been according to therecommendations. No collapsing of performance of theproduct will take place either after the recommended expirydate, but if it has been exceeded it is advisable to test theproduct prior to use.