No. 30 - Glatt
No. 30 - Glatt
No. 30 - Glatt
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
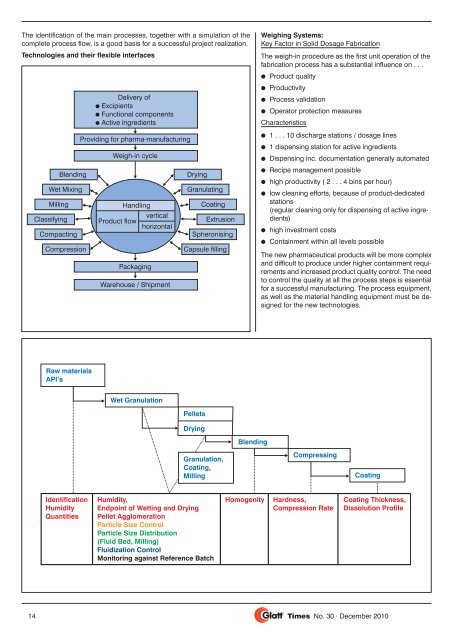
The identification of the main processes, together with a simulation of the<br />
complete process flow, is a good basis for a successful project realization.<br />
Technologies and their flexible interfaces<br />
14<br />
Milling<br />
Classifying<br />
Blending<br />
Wet Mixing<br />
Compacting<br />
Compression<br />
Raw materials<br />
API’s<br />
Identification<br />
Humidity<br />
Quantities<br />
Delivery of<br />
l Excipients<br />
l Functional components<br />
l Active ingredients<br />
Providing for pharma-manufacturing<br />
Weigh-in cycle<br />
Product flow vertical<br />
Handling<br />
horizontal<br />
Packaging<br />
Warehouse / Shipment<br />
Wet Granulation<br />
Drying<br />
Granulating<br />
Coating<br />
Extrusion<br />
Spheronising<br />
Capsule filling<br />
Pellets<br />
Drying<br />
Granulation,<br />
Coating,<br />
Milling<br />
Humidity,<br />
Endpoint of Wetting and Drying<br />
Pellet Agglomeration<br />
Particle Size Control<br />
Particle Size Distribution<br />
(Fluid Bed, Milling)<br />
Fluidization Control<br />
Monitoring against Reference Batch<br />
Blending<br />
Weighing Systems:<br />
Key Factor in Solid Dosage Fabrication<br />
The weigh-in procedure as the first unit operation of the<br />
fabrication process has a substantial influence on . . .<br />
l Product quality<br />
l Productivity<br />
l Process validation<br />
l Operator protection measures<br />
Characteristics<br />
l 1 . . . 10 discharge stations / dosage lines<br />
l 1 dispensing station for active ingredients<br />
l Dispensing inc. documentation generally automated<br />
l Recipe management possible<br />
l high productivity ( 2 . . . 4 bins per hour)<br />
l low cleaning efforts, because of product-dedicated<br />
stations<br />
(regular cleaning only for dispensing of active ingredients)<br />
l high investment costs<br />
l Containment within all levels possible<br />
The new pharmaceutical products will be more complex<br />
and difficult to produce under higher containment requirements<br />
and increased product quality control. The need<br />
to control the quality at all the process steps is essential<br />
for a successful manufacturing. The process equipment,<br />
as well as the material handling equipment must be designed<br />
for the new technologies.<br />
Compressing<br />
Homogenity Hardness,<br />
Compression Rate<br />
Coating<br />
Coating Thickness,<br />
Dissolution Profile<br />
Times <strong>No</strong>. <strong>30</strong> · December 2010