Created in the image of God
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
CREATED IN THE IMAGE OF GOD<br />
M.M.NINAN<br />
somewhere else <strong>in</strong> <strong>the</strong> universe, it will also be carbon based. This assumption is referred to by critics<br />
as carbon chauv<strong>in</strong>ism.<br />
Characteristics <strong>of</strong> carbon as a basis for life<br />
The two most important characteristics <strong>of</strong> carbon as a basis for <strong>the</strong> chemistry <strong>of</strong> life are that it has four<br />
valence bonds and that <strong>the</strong> energy required mak<strong>in</strong>g or break<strong>in</strong>g a bond is just at an appropriate level<br />
for build<strong>in</strong>g molecules which are not only stable, but also reactive.<br />
The fact that carbon atoms bond readily to o<strong>the</strong>r carbon atoms allows for <strong>the</strong> build<strong>in</strong>g <strong>of</strong> arbitrarily<br />
long complex molecules and polymers.<br />
There are not many o<strong>the</strong>r elements which even appear to be promis<strong>in</strong>g candidates for support<strong>in</strong>g life -<br />
for example, processes such as metabolism - but <strong>the</strong> most frequently suggested alternative is silicon.<br />
This is <strong>in</strong> <strong>the</strong> same group <strong>in</strong> <strong>the</strong> Periodic Table <strong>of</strong> elements and <strong>the</strong>refore also has four valence bonds.<br />
It also bonds to itself, but generally <strong>in</strong> <strong>the</strong> form <strong>of</strong> crystal lattices ra<strong>the</strong>r than long cha<strong>in</strong>s. Silicon<br />
compounds are generally stable but do not support <strong>the</strong> ability readily to re-comb<strong>in</strong>e <strong>in</strong> different<br />
permutations <strong>in</strong> a manner that would plausibly support lifelike processes.<br />
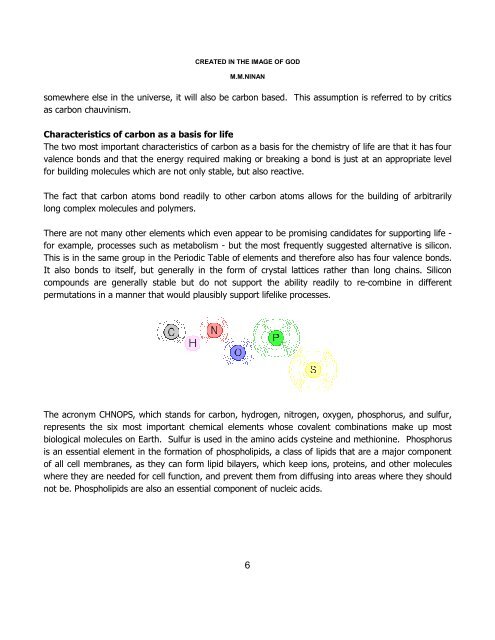
The acronym CHNOPS, which stands for carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur,<br />
represents <strong>the</strong> six most important chemical elements whose covalent comb<strong>in</strong>ations make up most<br />
biological molecules on Earth. Sulfur is used <strong>in</strong> <strong>the</strong> am<strong>in</strong>o acids cyste<strong>in</strong>e and methion<strong>in</strong>e. Phosphorus<br />
is an essential element <strong>in</strong> <strong>the</strong> formation <strong>of</strong> phospholipids, a class <strong>of</strong> lipids that are a major component<br />
<strong>of</strong> all cell membranes, as <strong>the</strong>y can form lipid bilayers, which keep ions, prote<strong>in</strong>s, and o<strong>the</strong>r molecules<br />
where <strong>the</strong>y are needed for cell function, and prevent <strong>the</strong>m from diffus<strong>in</strong>g <strong>in</strong>to areas where <strong>the</strong>y should<br />
not be. Phospholipids are also an essential component <strong>of</strong> nucleic acids.<br />
6