Pompe's disease - RePub - Erasmus Universiteit Rotterdam
Pompe's disease - RePub - Erasmus Universiteit Rotterdam
Pompe's disease - RePub - Erasmus Universiteit Rotterdam
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 1<br />
muscles in approximately 25% of cases (93, 131-133). Daily exercise may also improve the<br />
condition of the patient but should not be exhaustive. The variation of <strong>disease</strong> severity and<br />
clinical course of patients with Pompe’s <strong>disease</strong> complicates the evaluation of the effect of any<br />
form of therapy.<br />
At present ERT is the most promising as the preliminary results of the clinical trials suggest<br />
that patients with both the early as well as the late onset form of <strong>disease</strong> can benefi t from this<br />
type of therapy. The application of gene therapy is still in the pre-clinical phase and dependent<br />
on the production of safe and effective vectors for gene delivery.<br />
Gene therapy<br />
Amalfi tano et al. and Pauly et al. (134, 135) showed that a single intravenous injection of a<br />
modifi ed adenoviral vector, expressing human acid α-glucosidase, resulted in effi cient hepatic<br />
transduction and expression of acid α-glucosidase. High levels of acid α-glucosisdase activity<br />
were also found in the plasma of the treated animals. The plasma enzyme secreted by the<br />
hepatocytes can potentially restore the enzyme defi ciency in other tissues via endocytosis.<br />
Local administration of adenoviral gene constructs by intra-muscular injection in three days<br />
old mice led to a 50-fold elevation of acid α-glucosidase activity in the muscles at the site of<br />
injection. Importantly the vector RNA was also detected in the hind limb muscle, the heart<br />
and the liver for as long as 6 months (136). A similar experiment was performed in acid<br />
maltase defi cient quails (137) and the results were more or less the same.<br />
Intra-muscular and intra-cardial delivery of acid α-glucosidase gene constructs with a<br />
recombinant adeno-associated vector resulted in near normal enzyme activities and at least<br />
a partial restoration of muscle strength in the soleus muscle of KO mice up to 6 months after<br />
treatment (138). Martin-Touaux et al. performed a similar experiment injecting an acid αglucosidase<br />
expressing adenoviral vector in the gastrocnemius muscle of KO mouse neonates.<br />
Strong expression was detected at the site of injection but activity was also found in heart and<br />
more distant skeletal muscles.<br />
All gene therapy studies use a single injection while expression of the gene is still transient.<br />
The problem of immune response arising with repeated dosing, especially when using<br />
adenovirus-derived vectors, has to be overcome.<br />
Enzyme replacement therapy<br />
The studies towards enzyme replacement therapy in Pompe’s <strong>disease</strong> were aimed to use the<br />
mannose 6-phosphate receptor to target the acid α-glucosidase to the muscle and heart (139).<br />
Once the proof of principle was obtained, efforts were devoted to the large-scale production of<br />
recombinant human enzyme. The two production systems that were successfully developed<br />
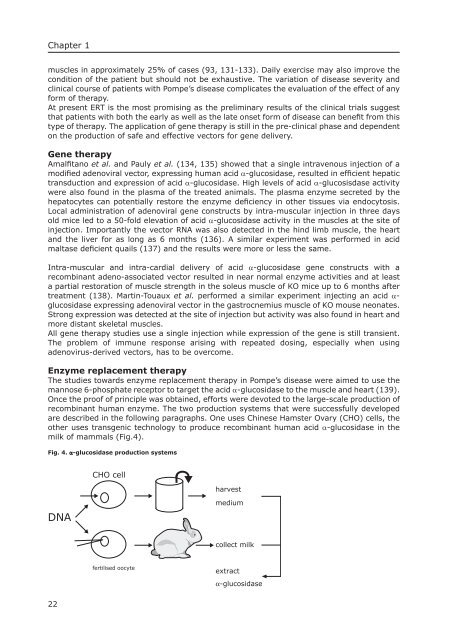
are described in the following paragraphs. One uses Chinese Hamster Ovary (CHO) cells, the<br />
other uses transgenic technology to produce recombinant human acid α-glucosidase in the<br />
milk of mammals (Fig.4).<br />
Fig. 4. α-glucosidase production systems<br />
DNA<br />
22<br />
CHO cell<br />
fertilised oocyte<br />
harvest<br />
medium<br />
collect milk<br />
extract<br />
α-glucosidase