PDF (361 K) - Laboratoire Matière et Systèmes Complexes - Paris 7
PDF (361 K) - Laboratoire Matière et Systèmes Complexes - Paris 7
PDF (361 K) - Laboratoire Matière et Systèmes Complexes - Paris 7
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Fig. 5 Calculated shapes of the vesicle inside the cylindrical nanochannel<br />
in a symm<strong>et</strong>ry plane (x,z). Top: G ¼ 0 (no adhesion) for the<br />
reduced tensions s ¼ 0, s ¼ 10, s ¼ 1 10 2 , s ¼ 1 10 3 , s ¼ 1 10 5 (from<br />
top to bottom). Bottom: G ¼ 2 for the same reduced tensions, however<br />
from bottom to top. In both cases, for s ¼ 1 10 5 the shape is almost<br />
a half-sphere.<br />
tension increases, the vesicle tends to a sphere of radius equal to<br />
the radius of the channel—except within a small region of size<br />
xO(k/s) close the channel walls.<br />
The deviation of the shape from a spherical cap of radius<br />
equal to the radius of the channel can be characterized by the<br />
normalized height h ¼ z(r ¼ 0)/a of the top of the vesicle with<br />
respect to the d<strong>et</strong>achment point (see Fig. 6). Increasing the<br />
adhesion energy at fixed tension reduces the height of the<br />
vesicle. When the adhesion energy G equals k/(2a 2 )(G ¼ 1/2),<br />
the vesicle becomes exactly a half-sphere with radius equal to<br />
the channel radius, whatever the tension. Indeed, according to<br />
eqn (11), for G ¼ k/(2a 2 ) the curvature at the d<strong>et</strong>achment point is<br />
1/a and a perfect sphere is always a solution of the shape<br />
equations. In this case, the pressure drop is related to the<br />
tension by the ordinary Laplace law; indeed, the curvature<br />
terms in eqn (2) vanish. At adhesion energies higher than k/(2a 2 )<br />
(G > 1/2), the vesicle develops an oblate shape that tends to<br />
a sphere as the tension increases (see Fig. 6 and bottom curves<br />
of Fig. 5).<br />
Fig. 6 The normalized height h of the top of the vesicle (with respect to<br />
the d<strong>et</strong>achment point) in the cylindrical nanochannel, as a function of the<br />
reduced tension s for different normalized adhesion energies G (G ¼ 0, G<br />
¼ 0.2, G ¼ 0.5, G ¼ 2, G ¼ 10). For G ¼ 1/2, the vesicle is a perfect halfsphere<br />
of radius a whatever the tension.<br />
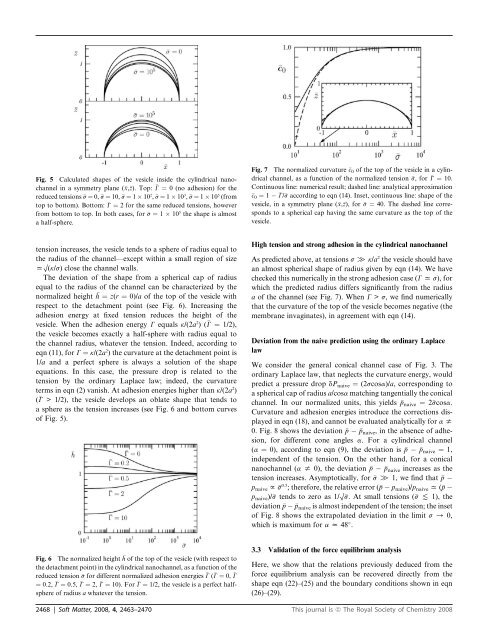
Fig. 7 The normalized curvature c 0 of the top of the vesicle in a cylindrical<br />
channel, as a function of the normalized tension s, for G ¼ 10.<br />
Continuous line: numerical result; dashed line: analytical approximation<br />
c0 ¼ 1 G/s according to eqn (14). Ins<strong>et</strong>, continuous line: shape of the<br />
vesicle, in a symm<strong>et</strong>ry plane (x,z), for s ¼ 40. The dashed line corresponds<br />
to a spherical cap having the same curvature as the top of the<br />
vesicle.<br />
High tension and strong adhesion in the cylindrical nanochannel<br />
As predicted above, at tensions s [ k/a 2 the vesicle should have<br />
an almost spherical shape of radius given by eqn (14). We have<br />
checked this numerically in the strong adhesion case (G x s), for<br />
which the predicted radius differs significantly from the radius<br />
a of the channel (see Fig. 7). When G > s, we find numerically<br />
that the curvature of the top of the vesicle becomes negative (the<br />
membrane invaginates), in agreement with eqn (14).<br />
Deviation from the naive prediction using the ordinary Laplace<br />
law<br />
We consider the general conical channel case of Fig. 3. The<br />
ordinary Laplace law, that neglects the curvature energy, would<br />
predict a pressure drop dPnaive ¼ (2scosa)/a, corresponding to<br />
a spherical cap of radius a/cosa matching tangentially the conical<br />
channel. In our normalized units, this yields pnaive ¼ 2scosa.<br />
Curvature and adhesion energies introduce the corrections displayed<br />
in eqn (18), and cannot be evaluated analytically for a s<br />
0. Fig. 8 shows the deviation p p naive, in the absence of adhesion,<br />
for different cone angles a. For a cylindrical channel<br />
(a ¼ 0), according to eqn (9), the deviation is p p naive ¼ 1,<br />
independent of the tension. On the other hand, for a conical<br />
nanochannel (a s 0), the deviation p pnaive increases as the<br />
tension increases. Asymptotically, for s [ 1, we find that p<br />
pnaive f s 0.5 ; therefore, the relative error (p pnaive)/pnaive x (p<br />
pnaive)/s tends to zero as 1/Os. At small tensions (s ( 1), the<br />
deviation p pnaive is almost independent of the tension; the ins<strong>et</strong><br />
of Fig. 8 shows the extrapolated deviation in the limit s / 0,<br />
which is maximum for a x 48 .<br />
3.3 Validation of the force equilibrium analysis<br />
Here, we show that the relations previously deduced from the<br />
force equilibrium analysis can be recovered directly from the<br />
shape eqn (22)–(25) and the boundary conditions shown in eqn<br />
(26)–(29).<br />
2468 | Soft Matter, 2008, 4, 2463–2470 This journal is ª The Royal Soci<strong>et</strong>y of Chemistry 2008