Swissmedic Vigilance News
Edition 31 – November 2023
Edition 31 – November 2023
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Statistical review 2022<br />
Pharmacovigilance: Human medicinal products<br />
<strong>Swissmedic</strong> evaluates safety signals associated with<br />
medicinal products and vaccines on the basis of reports<br />
of adverse drug reactions (ADRs) from within<br />
Switzerland. If its investigations confirm a new<br />
risk, <strong>Swissmedic</strong> initiates the necessary actions (for<br />
example amending the medicinal product information),<br />
often after first consulting its international<br />
partner authorities. As part of the pharmacovigilance<br />
network, all reports from medical professionals<br />
and, in increasing numbers, patients are<br />
entered in the national database and evaluated by<br />
specialists. Some are also assessed on <strong>Swissmedic</strong>’s<br />
behalf at six regional pharmacovigilance centres<br />
(RPVCs). Pharmaceutical companies also submit a<br />
large number of reports of adverse reactions from<br />
within Switzerland to <strong>Swissmedic</strong>.<br />
Activities<br />
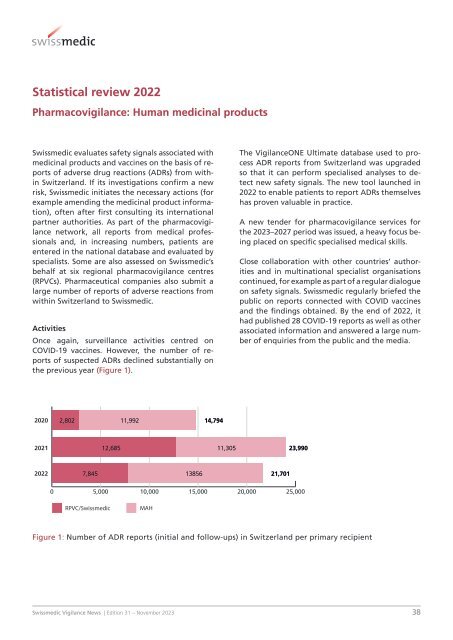
Once again, surveillance activities centred on<br />
COVID-19 vaccines. However, the number of reports<br />
of suspected ADRs declined substantially on<br />
the previous year (Figure 1).<br />
The <strong>Vigilance</strong>ONE Ultimate database used to process<br />
ADR reports from Switzerland was upgraded<br />
so that it can perform specialised analyses to detect<br />
new safety signals. The new tool launched in<br />
2022 to enable patients to report ADRs themselves<br />
has proven valuable in practice.<br />
A new tender for pharmacovigilance services for<br />
the 2023–2027 period was issued, a heavy focus being<br />
placed on specific specialised medical skills.<br />
Close collaboration with other countries’ authorities<br />
and in multinational specialist organisations<br />
continued, for example as part of a regular dialogue<br />
on safety signals. <strong>Swissmedic</strong> regularly briefed the<br />
public on reports connected with COVID vaccines<br />
and the findings obtained. By the end of 2022, it<br />
had published 28 COVID-19 reports as well as other<br />
associated information and answered a large number<br />
of enquiries from the public and the media.<br />
2020 2,802 11,992 14,794<br />
2021<br />
12,685 11,305 23,990<br />
2022<br />
7,845 13856 21,701<br />
0 5,000 10,000 15,000 20,000 25,000<br />
RPVC/<strong>Swissmedic</strong><br />
MAH<br />
Figure 1: Number of ADR reports (initial and follow-ups) in Switzerland per primary recipient<br />
<strong>Swissmedic</strong> <strong>Vigilance</strong> <strong>News</strong> | Edition 31 – November 2023<br />
38