Basem Ahmed Zoheir_Barramiya, OGR_2011.pdf
Basem Ahmed Zoheir_Barramiya, OGR_2011.pdf
Basem Ahmed Zoheir_Barramiya, OGR_2011.pdf
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ange of temperatures, commonly between 337 and 362 °C with a<br />
pronounced cluster around 350 °C. Bulk compositions, density, and<br />
isochores were calculated from microthermometric and Raman data<br />
using the Flincor program (Brown, 1989) and the equation of Bowers<br />
and Helgeson (1983) for the aqueous-carbonic inclusions. The<br />
carbonic phase is composed of a mixture of variable proportions of<br />
CH4 and CO2, an interpretation confirmed by micro-Raman spectroscopy<br />
(Fig. 9b, c). The bulk composition of the aqueous-carbonic<br />
inclusions based on the microthermometric measurements and<br />
Raman data is X CO2: 4–43 mol% (typically 25–40 mol%); X H2O: 55–<br />
96 mol% (typically 65–80 mol%); XCH4: b15 mol%; XN2: b4 mol%;and<br />
X NaCl: 1–2 mol%. CO 2 densities vary from 0.78 to 0.88 g/cm 3 ;bulk<br />
densities are mainly in the range of 0.86–0.99 g/cm 3 ; and salinity<br />
varies from 1.5 to 8.2 wt.% NaCl equiv.<br />
7.2. P–T conditions<br />
The presence of CH 4 and/or N 2 in the CO 2–H 2O–salt system may<br />
affect some microthermometric properties and can cause misestimate<br />
B. <strong>Zoheir</strong>, B. Lehmann / Ore Geology Reviews 39 (2011) 101–115<br />
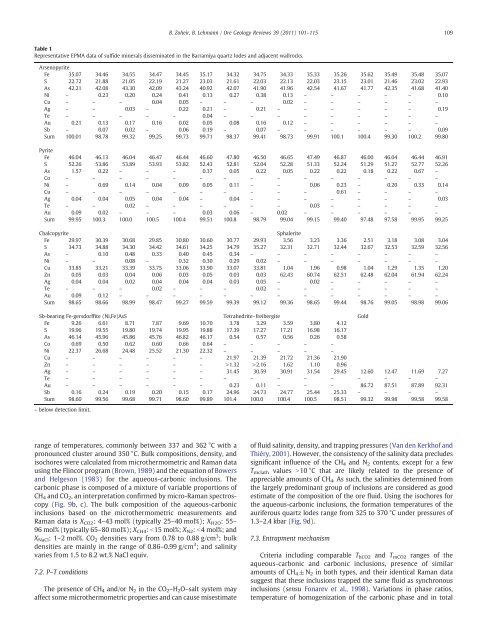
Table 1<br />
Representative EPMA data of sulfide minerals disseminated in the <strong>Barramiya</strong> quartz lodes and adjacent wallrocks.<br />
Arsenopyrite<br />
Fe 35.07 34.46 34.55 34.47 34.45 35.17 34.32 34.75 34.33 35.33 35.26 35.62 35.49 35.48 35.07<br />
S 22.72 21.88 21.05 22.19 21.27 23.03 21.61 22.03 22.13 22.03 23.15 23.01 21.46 23.02 22.93<br />
As 42.21 42.08 43.30 42.09 43.24 40.92 42.07 41.90 41.96 42.54 41.67 41.77 42.35 41.68 41.40<br />
Ni – 0.23 0.20 0.24 0.41 0.13 0.27 0.38 0.13 – – – – – 0.10<br />
Cu – – – 0.04 0.05 – – – 0.02 – – – – – –<br />
Ag – – 0.03 – 0.22 0.21 – 0.21 – – – – – – 0.19<br />
Te – – – – – 0.04 – – – – – – – – –<br />
Au 0.21 0.13 0.17 0.16 0.02 0.05 0.08 0.16 0.12 – – – – – –<br />
Sb – 0.07 0.02 – 0.06 0.19 – 0.07 – – – – – – 0.09<br />
Sum 100.01 98.78 99.32 99.25 99.73 99.71 98.37 99.41 98.73 99.91 100.1 100.4 99.30 100.2 99.80<br />
Pyrite<br />
Fe 46.04 46.13 46.04 46.47 46.44 46.60 47.80 46.50 46.65 47.49 46.87 46.00 46.04 46.44 46.91<br />
S 52.26 53.86 53.89 53.93 53.82 52.43 52.81 52.04 52.28 51.33 52.24 51.29 51.27 52.77 52.26<br />
As 1.57 0.22 – – – 0.37 0.05 0.22 0.05 0.22 0.22 0.18 0.22 0.67 –<br />
Co – – – – – – – – – – – – – – –<br />
Ni – 0.69 0.14 0.04 0.09 0.05 0.11 – – 0.06 0.23 – 0.20 0.33 0.14<br />
Cu – – – – – – – – – – 0.61 – – – –<br />
Ag 0.04 0.04 0.05 0.04 0.04 – 0.04 – – – – – – – 0.03<br />
Te – – 0.02 – – – – – – 0.03 – – – – –<br />
Au 0.09 0.02 – – – 0.03 0.06 – 0.02 – – – – – –<br />
Sum 99.95 100.3 100.0 100.5 100.4 99.51 100.8 98.79 99.04 99.15 99.40 97.48 97.58 99.95 99.25<br />
Chalcopyrite Sphalerite<br />
Fe 29.97 30.39 30.68 29.85 30.80 30.60 30.77 29.93 3.56 3.23 3.36 2.51 3.18 3.08 3.04<br />
S 34.73 34.88 34.30 34.42 34.61 34.25 34.79 35.27 32.31 32.71 32.44 32.67 32.53 32.59 32.56<br />
As – 0.10 0.48 0.33 0.40 0.45 0.34 – – – – – – – –<br />
Ni – – 0.08 – 0.32 0.30 0.29 0.02 – – – – – – –<br />
Cu 33.85 33.21 33.39 33.75 33.06 33.90 33.07 33.81 1.04 1.96 0.98 1.04 1.29 1.35 1.20<br />
Zn 0.05 0.03 0.04 0.06 0.03 0.05 0.03 0.03 62.43 60.74 62.51 62.48 62.04 61.94 62.24<br />
Ag 0.04 0.04 0.02 0.04 0.04 0.04 0.03 0.05 – 0.02 – – – – –<br />
Te – – – 0.02 – – – 0.02 – – – – – – –<br />
Au 0.09 0.12 – – – – – – – – – – – – –<br />
Sum 98.65 98.66 98.99 98.47 99.27 99.59 99.39 99.12 99.36 98.65 99.44 98.76 99.05 98.98 99.06<br />
Sb-bearing Fe-gersdorffite (Ni,Fe)AsS Tetrahedrite–freibergite Gold<br />
Fe 9.26 6.61 8.71 7.87 9.69 10.70 3.78 3.29 3.59 3.80 4.12<br />
S 19.96 19.55 19.80 19.74 19.95 19.88 17.39 17.27 17.21 16.98 16.17<br />
As 46.14 45.96 45.86 45.76 46.82 46.17 0.54 0.57 0.56 0.26 0.58<br />
Co 0.69 0.50 0.62 0.60 0.66 0.64 – – – – –<br />
Ni 22.37 26.68 24.48 25.52 21.30 22.32 – – – – –<br />
Cu – – – – – – 21.97 21.39 21.72 21.36 21.90<br />
Zn – – – – – – >1.32 >2.16 1.62 1.10 0.96<br />
Ag – – – – – – 31.45 30.59 30.91 31.54 29.45 12.60 12.47 11.69 7.27<br />
Te – – – – – – – – – – – – – – –<br />
Au – – – – – – 0.23 0.11 – – – 86.72 87.51 87.89 92.31<br />
Sb 0.16 0.24 0.19 0.20 0.15 0.17 24.96 24.73 24.77 25.44 25.33 – – – –<br />
Sum 98.60 99.56 99.68 99.71 98.60 99.89 101.4 100.0 100.4 100.5 98.51 99.32 99.98 99.58 99.58<br />
– below detection limit.<br />
of fluid salinity, density, and trapping pressures (Van den Kerkhof and<br />
Thiéry, 2001). However, the consistency of the salinity data precludes<br />
significant influence of the CH4 and N2 contents, except for a few<br />
T mclath values N10 °C that are likely related to the presence of<br />
appreciable amounts of CH4. As such, the salinities determined from<br />
the largely predominant group of inclusions are considered as good<br />
estimate of the composition of the ore fluid. Using the isochores for<br />
the aqueous-carbonic inclusions, the formation temperatures of the<br />
auriferous quartz lodes range from 325 to 370 °C under pressures of<br />
1.3–2.4 kbar (Fig. 9d).<br />
7.3. Entrapment mechanism<br />
Criteria including comparable T hCO2 and T mCO2 ranges of the<br />
aqueous-carbonic and carbonic inclusions, presence of similar<br />
amounts of CH 4 ±N 2 in both types, and their identical Raman data<br />
suggest that these inclusions trapped the same fluid as synchronous<br />
inclusions (sensu Fonarev et al., 1998). Variations in phase ratios,<br />
temperature of homogenization of the carbonic phase and in total<br />
109