Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
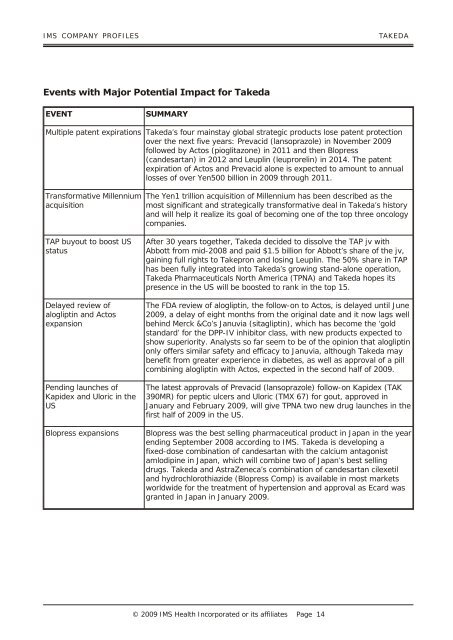
<strong>IMS</strong> COM PANY PRO FILES TAKEDA<br />
Events with Major Potential Impact for Takeda<br />
EVENT SUMMARY<br />
Multiple patent expirations Takeda’s four mainstay global strategic products lose patent protection<br />
over the next five years: Prevacid (lansoprazole) in November 2009<br />
followed by Actos (pioglitazone) in 2011 and then Blopress<br />
(candesartan) in 2012 and Leuplin (leuprorelin) in 2014. The patent<br />
expiration of Actos and Prevacid alone is expected to amount to annual<br />
losses of over Yen500 billion in 2009 through 2011.<br />
Transformative Millennium<br />
acquisition<br />
TAP buyout to boost US<br />
status<br />
Delayed review of<br />
alogliptin and Actos<br />
expansion<br />
Pending launches of<br />
Kapidex and Uloric in the<br />
US<br />
The Yen1 trillion acquisition of Millennium has been described as the<br />
most significant and strategically transformative deal in Takeda’s history<br />
and will help it realize its goal of becoming one of the top three oncology<br />
companies.<br />
After 30 years together, Takeda decided to dissolve the TAP jv with<br />
Abbott from mid-2008 and paid $1.5 billion for Abbott’s share of the jv,<br />
gaining full rights to Takepron and losing Leuplin. The 50% share in TAP<br />
has been fully integrated into Takeda’s growing stand-alone operation,<br />
Takeda Pharmaceuticals North America (TPNA) and Takeda hopes its<br />
presence in the US will be boosted to rank in the top 15.<br />
The FDA review of alogliptin, the follow-on to Actos, is delayed until June<br />
2009, a delay of eight months from the original date and it now lags well<br />
behind Merck &Co’s Januvia (sitagliptin), which has become the ‘gold<br />
standard’ for the DPP-IV inhibitor class, with new products expected to<br />
show superiority. Analysts so far seem to be of the opinion that alogliptin<br />
only offers similar safety and efficacy to Januvia, although Takeda may<br />
benefit from greater experience in diabetes, as well as approval of a pill<br />
combining alogliptin with Actos, expected in the second half of 2009.<br />
The latest approvals of Prevacid (lansoprazole) follow-on Kapidex (TAK<br />
390MR) for peptic ulcers and Uloric (TMX 67) for gout, approved in<br />
January and February 2009, will give TPNA two new drug launches in the<br />
first half of 2009 in the US.<br />
Blopress expansions Blopress was the best selling pharmaceutical product in Japan in the year<br />
ending September 2008 according to <strong>IMS</strong>. Takeda is developing a<br />
fixed-dose combination of candesartan with the calcium antagonist<br />
amlodipine in Japan, which will combine two of Japan’s best selling<br />
drugs. Takeda and AstraZeneca’s combination of candesartan cilexetil<br />
and hydrochlorothiazide (Blopress Comp) is available in most markets<br />
worldwide for the treatment of hypertension and approval as Ecard was<br />
granted in Japan in January 2009.<br />
© 2009 <strong>IMS</strong> Health In cor po rated or its af fil i ates Page 14