Report in English with a French summary - KCE
Report in English with a French summary - KCE
Report in English with a French summary - KCE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
8 Sp<strong>in</strong>e technology <strong>KCE</strong> reports vol.39<br />
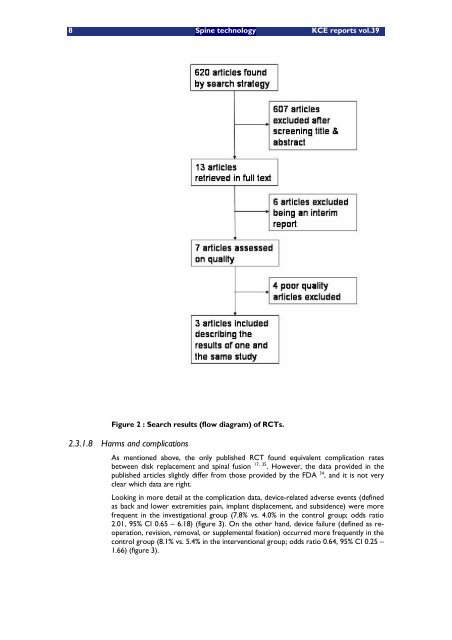
Figure 2 : Search results (flow diagram) of RCTs.<br />
2.3.1.8 Harms and complications<br />
As mentioned above, the only published RCT found equivalent complication rates<br />
between disk replacement and sp<strong>in</strong>al fusion 17, 25 . However, the data provided <strong>in</strong> the<br />
published articles slightly differ from those provided by the FDA 34 , and it is not very<br />
clear which data are right.<br />
Look<strong>in</strong>g <strong>in</strong> more detail at the complication data, device-related adverse events (def<strong>in</strong>ed<br />
as back and lower extremities pa<strong>in</strong>, implant displacement, and subsidence) were more<br />
frequent <strong>in</strong> the <strong>in</strong>vestigational group (7.8% vs. 4.0% <strong>in</strong> the control group; odds ratio<br />
2.01, 95% CI 0.65 6.18) (figure 3). On the other hand, device failure (def<strong>in</strong>ed as reoperation,<br />
revision, removal, or supplemental fixation) occurred more frequently <strong>in</strong> the<br />
control group (8.1% vs. 5.4% <strong>in</strong> the <strong>in</strong>terventional group; odds ratio 0.64, 95% CI 0.25<br />
1.66) (figure 3).