TAILORING DRY POWDER INHALER PERFORMANCE BY - RCPE
TAILORING DRY POWDER INHALER PERFORMANCE BY - RCPE
TAILORING DRY POWDER INHALER PERFORMANCE BY - RCPE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Tailoring Dry Powder Inhaler Performance 769<br />
90 ◦ C, and 8.9 mg for formulations containing mannitol<br />
120 ◦ C.<br />
In addition, the uniformity of dosing was evaluated by<br />
calculating the coefficient of variation of the mass of each<br />
batch (A, B, C). The mean and the standard deviation<br />
of the three coefficients of variation of each formulation<br />
(mannitol 60 ◦ C, 90 ◦ C, 120 ◦ C) were calculated. The coefficients<br />
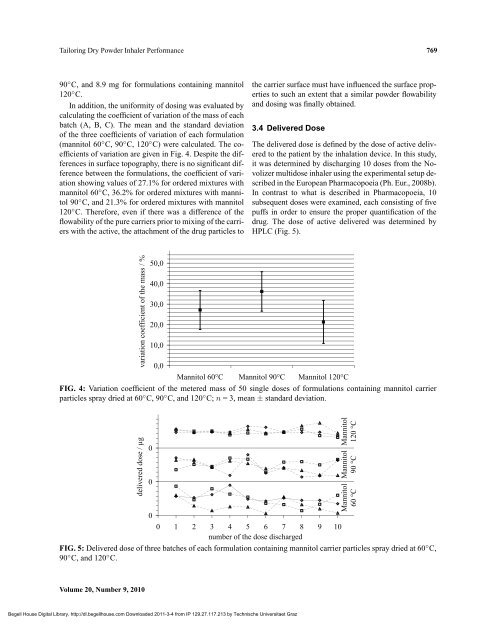
of variation are given in Fig. 4. Despite the differences<br />
in surface topography, there is no significant difference<br />
between the formulations, the coefficient of variation<br />
showing values of 27.1% for ordered mixtures with<br />
mannitol 60 ◦ C, 36.2% for ordered mixtures with mannitol<br />
90 ◦ C, and 21.3% for ordered mixtures with mannitol<br />
120 ◦ C. Therefore, even if there was a difference of the<br />
flowability of the pure carriers prior to mixing of the carriers<br />
with the active, the attachment of the drug particles to<br />
variation coefficient of the mass / %<br />
50,0<br />
40,0<br />
30,0<br />
20,0<br />
10,0<br />
the carrier surface must have influenced the surface properties<br />
to such an extent that a similar powder flowability<br />
and dosing was finally obtained.<br />
3.4 Delivered Dose<br />
The delivered dose is defined by the dose of active delivered<br />
to the patient by the inhalation device. In this study,<br />
it was determined by discharging 10 doses from the Novolizer<br />
multidose inhaler using the experimental setup described<br />
in the European Pharmacopoeia (Ph. Eur., 2008b).<br />
In contrast to what is described in Pharmacopoeia, 10<br />
subsequent doses were examined, each consisting of five<br />
puffs in order to ensure the proper quantification of the<br />
drug. The dose of active delivered was determined by<br />
HPLC (Fig. 5).<br />
0,0<br />
Mannitol 60°C Mannitol 90°C Mannitol 120°C<br />
FIG. 4: Variation coefficient of the metered mass of 50 single doses of formulations containing mannitol carrier<br />
particles spray dried at 60◦C, 90◦C, and 120◦C; n = 3, mean ± standard deviation.<br />
delivered dose / µg<br />
0<br />
0<br />
0<br />
0 1 2 3 4 5 6 7 8 9 10<br />
number of the dose discharged<br />
FIG. 5: Delivered dose of three batches of each formulation containing mannitol carrier particles spray dried at 60◦C, 90◦C, and 120◦C. Volume 20, Number 9, 2010<br />
Begell House Digital Library, http://dl.begellhouse.com Downloaded 2011-3-4 from IP 129.27.117.213 by Technische Universitaet Graz<br />
Mannitol<br />
120 °C<br />
Mannitol<br />
90 °C<br />
Mannitol<br />
60 °C