TAILORING DRY POWDER INHALER PERFORMANCE BY - RCPE
TAILORING DRY POWDER INHALER PERFORMANCE BY - RCPE
TAILORING DRY POWDER INHALER PERFORMANCE BY - RCPE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Tailoring Dry Powder Inhaler Performance 771<br />
fraction of the drug delivered / %<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
Mannitol 60 °C Mannitol 90 °C Mannitol 120 °C<br />
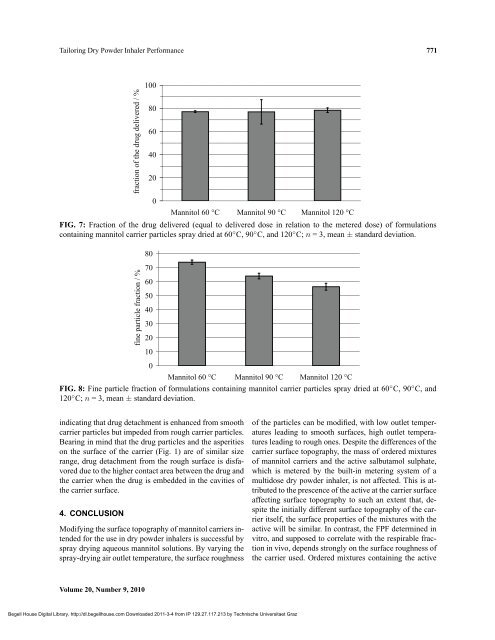
FIG. 7: Fraction of the drug delivered (equal to delivered dose in relation to the metered dose) of formulations<br />
containing mannitol carrier particles spray dried at 60 ◦ C, 90 ◦ C, and 120 ◦ C; n = 3, mean ± standard deviation.<br />
fine particle fraction / %<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
Mannitol 60 °C Mannitol 90 °C Mannitol 120 °C<br />
FIG. 8: Fine particle fraction of formulations containing mannitol carrier particles spray dried at 60◦C, 90◦C, and<br />
120◦C; n = 3, mean ± standard deviation.<br />
indicating that drug detachment is enhanced from smooth<br />
carrier particles but impeded from rough carrier particles.<br />
Bearing in mind that the drug particles and the asperities<br />
on the surface of the carrier (Fig. 1) are of similar size<br />
range, drug detachment from the rough surface is disfavored<br />
due to the higher contact area between the drug and<br />
the carrier when the drug is embedded in the cavities of<br />
the carrier surface.<br />
4. CONCLUSION<br />
Modifying the surface topography of mannitol carriers intended<br />
for the use in dry powder inhalers is successful by<br />
spray drying aqueous mannitol solutions. By varying the<br />
spray-drying air outlet temperature, the surface roughness<br />
Volume 20, Number 9, 2010<br />
Begell House Digital Library, http://dl.begellhouse.com Downloaded 2011-3-4 from IP 129.27.117.213 by Technische Universitaet Graz<br />
of the particles can be modified, with low outlet temperatures<br />
leading to smooth surfaces, high outlet temperatures<br />
leading to rough ones. Despite the differences of the<br />
carrier surface topography, the mass of ordered mixtures<br />
of mannitol carriers and the active salbutamol sulphate,<br />
which is metered by the built-in metering system of a<br />
multidose dry powder inhaler, is not affected. This is attributed<br />
to the prescence of the active at the carrier surface<br />
affecting surface topography to such an extent that, despite<br />
the initially different surface topography of the carrier<br />
itself, the surface properties of the mixtures with the<br />
active will be similar. In contrast, the FPF determined in<br />
vitro, and supposed to correlate with the respirable fraction<br />
in vivo, depends strongly on the surface roughness of<br />
the carrier used. Ordered mixtures containing the active