TAILORING DRY POWDER INHALER PERFORMANCE BY - RCPE
TAILORING DRY POWDER INHALER PERFORMANCE BY - RCPE
TAILORING DRY POWDER INHALER PERFORMANCE BY - RCPE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
770 Maas et al.<br />
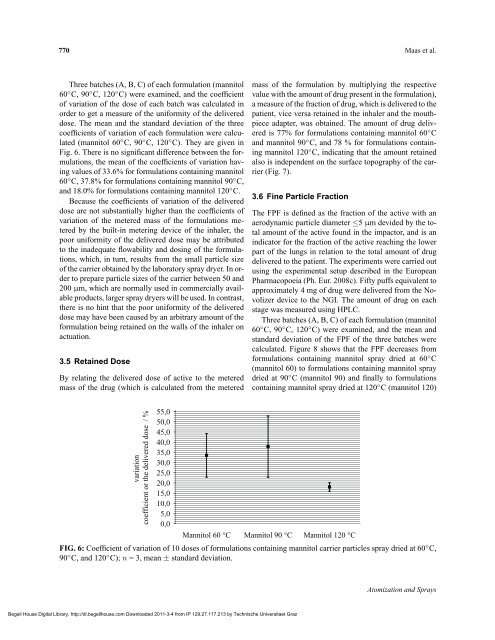
Three batches (A, B, C) of each formulation (mannitol<br />
60 ◦ C, 90 ◦ C, 120 ◦ C) were examined, and the coefficient<br />
of variation of the dose of each batch was calculated in<br />
order to get a measure of the uniformity of the delivered<br />
dose. The mean and the standard deviation of the three<br />
coefficients of variation of each formulation were calculated<br />
(mannitol 60 ◦ C, 90 ◦ C, 120 ◦ C). They are given in<br />
Fig. 6. There is no significant difference between the formulations,<br />
the mean of the coefficients of variation having<br />
values of 33.6% for formulations containing mannitol<br />
60 ◦ C, 37.8% for formulations containing mannitol 90 ◦ C,<br />
and 18.0% for formulations containing mannitol 120 ◦ C.<br />
Because the coefficients of variation of the delivered<br />
dose are not substantially higher than the coefficients of<br />
variation of the metered mass of the formulations metered<br />
by the built-in metering device of the inhaler, the<br />
poor uniformity of the delivered dose may be attributed<br />
to the inadequate flowability and dosing of the formulations,<br />
which, in turn, results from the small particle size<br />
of the carrier obtained by the laboratory spray dryer. In order<br />
to prepare particle sizes of the carrier between 50 and<br />
200 µm, which are normally used in commercially available<br />
products, larger spray dryers will be used. In contrast,<br />
there is no hint that the poor uniformity of the delivered<br />
dose may have been caused by an arbitrary amount of the<br />
formulation being retained on the walls of the inhaler on<br />
actuation.<br />
3.5 Retained Dose<br />
By relating the delivered dose of active to the metered<br />
mass of the drug (which is calculated from the metered<br />
variation<br />
coefficient or the delivered dose / %<br />
55,0<br />
50,0<br />
45,0<br />
40,0<br />
35,0<br />
30,0<br />
25,0<br />
20,0<br />
15,0<br />
10,0<br />
5,0<br />
0,0<br />
mass of the formulation by multiplying the respective<br />
value with the amount of drug present in the formulation),<br />
a measure of the fraction of drug, which is delivered to the<br />
patient, vice versa retained in the inhaler and the mouthpiece<br />
adapter, was obtained. The amount of drug delivered<br />
is 77% for formulations containing mannitol 60 ◦ C<br />
and mannitol 90 ◦ C, and 78 % for formulations containing<br />
mannitol 120 ◦ C, indicating that the amount retained<br />
also is independent on the surface topography of the carrier<br />
(Fig. 7).<br />
3.6 Fine Particle Fraction<br />
The FPF is defined as the fraction of the active with an<br />
aerodynamic particle diameter ≤5 µm devided by the total<br />
amount of the active found in the impactor, and is an<br />
indicator for the fraction of the active reaching the lower<br />
part of the lungs in relation to the total amount of drug<br />
delivered to the patient. The experiments were carried out<br />
using the experimental setup described in the European<br />
Pharmacopoeia (Ph. Eur. 2008c). Fifty puffs equivalent to<br />
approximately 4 mg of drug were delivered from the Novolizer<br />
device to the NGI. The amount of drug on each<br />
stage was measured using HPLC.<br />
Three batches (A, B, C) of each formulation (mannitol<br />
60 ◦ C, 90 ◦ C, 120 ◦ C) were examined, and the mean and<br />
standard deviation of the FPF of the three batches were<br />
calculated. Figure 8 shows that the FPF decreases from<br />
formulations containing mannitol spray dried at 60 ◦ C<br />
(mannitol 60) to formulations containing mannitol spray<br />
dried at 90 ◦ C (mannitol 90) and finally to formulations<br />
containing mannitol spray dried at 120 ◦ C (mannitol 120)<br />
Mannitol 60 °C Mannitol 90 °C Mannitol 120 °C<br />
FIG. 6: Coefficient of variation of 10 doses of formulations containing mannitol carrier particles spray dried at 60 ◦ C,<br />
90 ◦ C, and 120 ◦ C); n = 3, mean ± standard deviation.<br />
Begell House Digital Library, http://dl.begellhouse.com Downloaded 2011-3-4 from IP 129.27.117.213 by Technische Universitaet Graz<br />
Atomization and Sprays