TerraTox - HIV-1 structure file, May 2004. Copyright ... - TerraBase Inc.
TerraTox - HIV-1 structure file, May 2004. Copyright ... - TerraBase Inc.
TerraTox - HIV-1 structure file, May 2004. Copyright ... - TerraBase Inc.
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
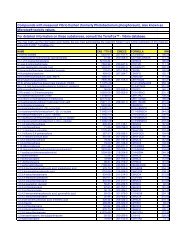
4115 C29H38N2O5 <strong>HIV</strong>-1 protease inhibitor; T-4842-020<br />
CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@](Cc2ccccc2)([H])C(=O)N[C@@<br />
H]3[C@H](O)CC=C3<br />
4116 C29H39NO6 <strong>HIV</strong>-1 protease inhibitor; T-4842-023<br />
CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@](Cc2ccccc2)([H])C(=O)[C@@H]<br />
(C(C)C)C(=O)O<br />
4117 C29H39N3O3 E917 c1ccccc1Oc2ccc(cc2)CN2CCC3(CC2)N(CCCC)C(=O)C(CC(C)C)NC3=O<br />
4118 C29H40N4O5S<br />
4-[(Z)-(ethoxyimino)[4-(methylsulfonyl)phenyl]methyl]-1'-<br />
[(2,4-dimethyl-3-piperidinyl)carbonyl]-4'-methyl-1,4'- c1cc(S(=O)(=O)C)ccc1/C(=N\OCC)C2CCN(CC2)C3(C)CCN(CC3)C(=O)c4c(C)cc[n+]([Obipiperidine<br />
N-oxide<br />
])c4C<br />
4119 C29H40N8O2 piperidine deriv.; T-4694-023 C1CN(C)CCN1C(=O)Nc2cc3cc(nc3cc2)C(=O)N4CCC(CC4)N(CC)c5ncccc5NCC<br />
4120 C29H41N5O2 E916 n1c(c2ccccc2)ncc1CN2CCC3(CC2)N(CCCC)C(=O)C(CC4CCCCC4)NC3=O<br />
4121 C29H41N5O5 piperidine deriv.; T-4694-002 c1c(OCCOCCOCCO)ccc2cc(nc12)C(=O)N3CCC(CC3)N(C)c4ncccc4NC(C)C<br />
O[C@H]1[C@@H](Cc2ccccc2)N(CCC(C)C)C(=O)N(CCC(C)C)[C@H](Cc3ccccc3)[C@@H]<br />
1O<br />
4122 C29H42N2O3 cyclic urea deriv.; T-4100-018<br />
4123 C29H42N2O3 cyclic urea deriv.; T-4100-011 O[C@H]1[C@@H](Cc2ccccc2)N(CCCCC)C(=O)N(CCCCC)[C@H](Cc3ccccc3)[C@@H]1O<br />
4124 C29H42N2O4 L-364,505 deriv.; T-4328-013 CC(C)(C)OC(=O)N[C@H](Cc2ccccc2)[C@H](O)C[C@H](Cc2ccccc2)C(=O)NCCC(C)C<br />
O[C@H]1[C@@H](Cc2ccccc2)N(CCOCCOC)C(=O)N(CCOCCOC)[C@H](Cc3ccccc3)[C@<br />
4125 C29H42N2O7 cyclic urea deriv.; T-4100-016<br />
@H]1O<br />
4126 C29H42N8O3S piperidine deriv.; T-4694-026 C1CN(C)CCN1S(=O)(=O)Nc2cc3cc(nc3cc2)C(=O)N4CCC(CC4)N(CC)c5ncccc5NC(C)C<br />
4127 C29H43N5O7S hydroxyethylhydrazine deriv.; T-4391-711<br />
COC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc2sccc2)NC(=O)<br />
[C@@H](C(C)C)NC(=O)OC<br />
4128 C29H44O6 NSC 382025<br />
C[C@]1(C(=O)O)[C@H](O)[C@H](O)C[C@]2(C)[C@@]3([H])CC=C4[C@]5([H])CC(C)(C)C<br />
C[C@]5(C(=O)O)CC[C@@]4([H])[C@]3(C)CC[C@]12[H]<br />
4129 C29H46O4 plantanic acid<br />
C1(C)(C)[C@](O)([H])CC[C@]2(C)[C@@]3([H])CC[C@]4([H])[C@@]5([H])[C@]([H])(C(C)=<br />
O)CC[C@]5(C(=O)O)CC[C@@]4(C)[C@]3(C)CC[C@@]12([H])<br />
4130 C29H47N3O4 <strong>HIV</strong>-1 protease inhibitor; T-4842-046<br />
CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN4[C@H](C(=O)NC(C)(C)C)C[C@]5([H]<br />
)CCCC[C@]5([H])C4<br />
4131 C29H47N3O8S2Si2 TSAO deriv.; T-4126-174<br />
O1S(=O)(=O)C(c4ccsc4)=C(N)[C@]12[C@@H](CO[Si](C)(C)C(C)(C)C)O[C@H]([C@@H]2<br />
O[Si](C)(C)C(C)(C)C)N3C=C(C)C(=O)N(C)C3=O<br />
4132 C29H49N3O10SSi2 TSAO deriv.; T-4126-171<br />
O1S(=O)(=O)C(/C=C/C(=O)OC)=C(N)[C@]12[C@@H](CO[Si](C)(C)C(C)(C)C)O[C@H]([C@<br />
@H]2O[Si](C)(C)C(C)(C)C)N3C=C(C)C(=O)N(C)C3=O<br />
4133 C29H49N3O10SSi2 TSAO deriv.; T-4126-152<br />
O1S(=O)(=O)C(/C=C/C(=O)OCC)=C(N)[C@]12[C@@H](CO[Si](C)(C)C(C)(C)C)O[C@H]([C<br />
@@H]2O[Si](C)(C)C(C)(C)C)N3C=C(C)C(=O)NC3=O<br />
4134 C29H49N5O7 2-ethoxyhydrazine deriv.; T-4416-002<br />
c1ccccc1C[C@H](NC(=O)[C@H](C(C)C)NC(=O)OC)[C@@H](O)CN(CCC(C)C)NC(=O)[C@<br />
H](C(C)C)NC(=O)OC<br />
COC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(CCC(C)C)NC(=O)<br />
4135 C29H49N5O7 hydroxyethylhydrazine deriv.; T-4391-703<br />
[C@@H](C(C)C)NC(=O)OC