TerraTox - HIV-1 structure file, May 2004. Copyright ... - TerraBase Inc.
TerraTox - HIV-1 structure file, May 2004. Copyright ... - TerraBase Inc.
TerraTox - HIV-1 structure file, May 2004. Copyright ... - TerraBase Inc.
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
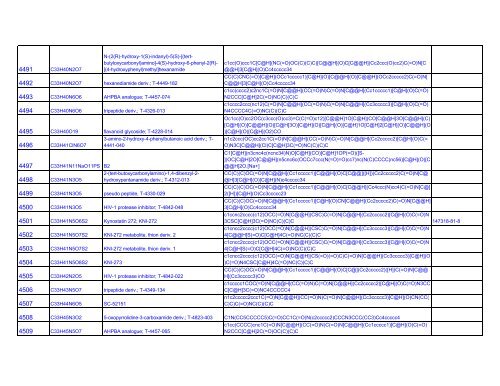
4491 C33H40N2O7<br />
N-(2(R)-hydroxy-1(S)-indanyl)-5(S)-[(tert-<br />
butyloxycarbonyl)amino]-4(S)-hydroxy-6-phenyl-2(R)-<br />
[(4-hydroxyphenyl)methyl]hexanamide<br />
4492 C33H40N2O7 hexanediamide deriv.; T-4449-182<br />
4493 C33H40N6O6 AHPBA analogue; T-4457-074<br />
4494 C33H40N6O6 tripeptide deriv.; T-4326-013<br />
4495 C33H40O19 flavanoid glycoside; T-4228-014<br />
3-amino-2-hydroxy-4-phenylbutanoic acid deriv.; T-<br />
4496 C33H41ClN6O7 4441-040<br />
4497 C33H41N11NaO11PS B2<br />
2-(tert-butoxycarbonylamino)-1,4-dibenzyl-2-<br />
4498 C33H41N3O5 hydroxypentanamide deriv.; T-4312-013<br />
4499 C33H41N3O5 pseudo peptide, T-4330-029<br />
4500 C33H41N3O5 <strong>HIV</strong>-1 protease inhibitor; T-4842-048<br />
4501 C33H41N5O6S2 Kynostatin 272; KNI-272<br />
4502 C33H41N5O7S2 KNI-272 metabolite, thion deriv. 2<br />
4503 C33H41N5O7S2 KNI-272 metabolite, thion deriv. 1<br />
4504 C33H41N5O8S2 KNI-273<br />
4505 C33H42N2O5 <strong>HIV</strong>-1 protease inhibitor; T-4842-022<br />
4506 C33H43N5O7 tripeptide deriv.; T-4349-134<br />
4507 C33H44N6O5 SC-52151<br />
c1cc(O)ccc1C[C@H](NC(=O)OC(C)(C)C)[C@@H](O)C[C@@H](Cc2ccc(O)cc2)C(=O)N[C<br />
@@H]3[C@H](O)Cc4ccccc34<br />
CC(C)CNC(=O)[C@H](OCc1ccccc1)[C@H](O)[C@@H](O)[C@@H](OCc2ccccc2)C(=O)N[<br />
C@@H]3[C@H](O)Cc4ccccc34<br />
c1cc(cccc2)c2nc1C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)<br />
N2CCC[C@H]2C(=O)NC(C)(C)C<br />
c1cccc2ccc(nc12)C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](Cc3ccccc3)[C@H](O)C(=O)<br />
N4CCCC4C(=O)NC(C)(C)C<br />
Oc1cc(O)cc2OC(c3ccc(O)cc3)=C(C(=O)c12)[C@@H]1O[C@H](CO[C@@H]3O[C@@H](C)<br />
[C@H](O)[C@@H](O)[C@H]3O)[C@H](O)[C@H](O)[C@H]1O[C@H]2[C@H](O)[C@@H](O<br />
)[C@H](O)[C@H](O2)CO<br />
n1c2ccc(OC)cc2cc1C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=<br />
O)N3C[C@@H](Cl)C[C@H]3C(=O)NC(C)(C)C<br />
C1[C@H](n3cnc4c(ncnc34)N)O[C@H](CO)[C@H]1OP(=O)([S-<br />
])OC[C@H]2O[C@@H](n5cnc6c(OCCc7ccc(N(=O)=O)cc7)nc(N(C)CCCC)nc56)[C@H](O)[C<br />
@@H]2O.[Na+]<br />
CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@]([H])(Cc2ccccc2)C(=O)N[C@<br />
@H]3[C@H](O)[C@H](N)c4ccccc34<br />
CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc4ccc(N)cc4)C(=O)N[C@]<br />
2([H])[C@H](O)Cc3ccccc23<br />
CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](Cc2ccccc2)C(=O)N[C@@H]<br />
3[C@H](O)Cc4ccccc34<br />
c1ccnc2cccc(c12)OCC(=O)N[C@@H](CSC)C(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N<br />
3CSC[C@H]3C(=O)NC(C)(C)C 147318-81-8<br />
c1cncc2cccc(c12)OCC(=O)N[C@@H](CSC)C(=O)N[C@@H](Cc3ccccc3)[C@H](O)C(=O)N<br />
4[C@@H]S(=O)C[C@H]4C(=O)NC(C)(C)C<br />
c1cncc2cccc(c12)OCC(=O)N[C@@H](CSC)C(=O)N[C@@H](Cc3ccccc3)[C@H](O)C(=O)N<br />
4[C@H]S(=O)C[C@H]4C(=O)NC(C)(C)C<br />
c1cncc2cccc(c12)OCC(=O)N[C@@H](CS(=O)(=O)C)C(=O)N[C@@H](Cc3ccccc3)[C@H](O<br />
)C(=O)N4CSC[C@H]4C(=O)NC(C)(C)C<br />
CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@](Cc2ccccc2)([H])C(=O)N[C@@<br />
H](Cc3ccccc3)CO<br />
c1ccccc1COC(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N3CC<br />
C[C@H]3C(=O)NC4CCCCC4<br />
n1c2ccccc2ccc1C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(<br />
C)C)C(=O)NC(C)(C)C<br />
4508 C33H45N3O2 5-oxopyrrolidine-3-carboxamide deriv.; T-4823-403 C1N(CC5CCCCC5)C(=O)CC1C(=O)N(c2ccccc2)CCCN3CCC(CC3)Cc4ccccc4<br />
c1cc(CCCC)cnc1C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)<br />
4509 C33H45N5O7 AHPBA analogue; T-4457-065<br />
N2CCC[C@H]2C(=O)OC(C)(C)C