TerraTox - HIV-1 structure file, May 2004. Copyright ... - TerraBase Inc.
TerraTox - HIV-1 structure file, May 2004. Copyright ... - TerraBase Inc.
TerraTox - HIV-1 structure file, May 2004. Copyright ... - TerraBase Inc.
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
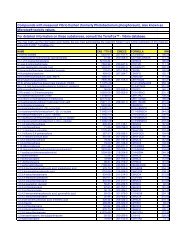
4136 C29H50N4O11SSi2<br />
4137 C29H51N5O10SSi2 TSAO deriv.; T-4126-007<br />
[1-[2',5'-bis(-O-(tert-butyldimethylsilyl)-beta-D-<br />
ribofuranosyl]-3-N-[[N-[1-(S)-<br />
(carboxy)ethyl]carbamoyl]methyl]thymine]-3'-spiro-5''-<br />
(4''-amino-1'',2''-oxathiole-2'',2''-dioxide)<br />
[C@]12(OS(=O)(=O)C=C1N)[C@@H](CO[Si](C)(C)C(C)(C)C)O[C@H]([C@@H]2O[Si](C)(C<br />
)C(C)(C)C)N3C=C(C)C(=O)N(C3=O)CC(=O)N[C@H](C)C(=O)O<br />
O1S(=O)(=O)C(CC(=O)N)=C(NCC(=O)N)[C@]12[C@@H](CO[Si](C)(C)C(C)(C)C)O[C@H]([<br />
C@@H]2O[Si](C)(C)C(C)(C)C)N3C=C(C)C(=O)N(C)C3=O<br />
4138 C30H16O8 hypericin Oc1cc(O)c2c3c(O)cc(O)c4C(=O)c5c(O)cc(C)c6c7c(C)cc(O)c(c7C8=C(c56)c34)C(=O)c1c28 548-04-9<br />
4139 C30H20N2O7<br />
2(E)-(3-hydrox-5-((E)-7-carboxy-8-hydroxyquinolin-2-<br />
yl)ystyryl)-7-carboxy-8-hydroxyquinoline<br />
Oc1c(C(=O)O)ccc2ccc(nc12)/C=C/c3cc(O)cc(/C=C/c4ccc5ccc(C(=O)O)c(O)c5n4)c3<br />
4140 C30H26O16 L-chicoric acid tetraacetate<br />
c1cc(OC(=O)C)c(OC(=O)C)cc1/C=C/C(=O)O[C@@H](C(=O)O)[C@H](C(=O)O)OC(=O)/C=<br />
C/c2ccc(OC(=O)C)c(OC(=O)C)c2<br />
4141 C30H26O16 D-chicoric acid tetraacetate<br />
c1cc(OC(=O)C)c(OC(=O)C)cc1/C=C/C(=O)O[C@H](C(=O)O)[C@@H](C(=O)O)OC(=O)/C=<br />
C/c2ccc(OC(=O)C)c(OC(=O)C)c2<br />
4142 C30H27IN2O quaternary ammonium salt; 4128-106 c1ccccc1c2ccc3CCC(=Cc3c2)C(=O)Nc4ccc(cc4)C[n+]5c(C)cccc5.[I-]<br />
4143 C30H30N2O6S cyclic urea/diaminosulfone deriv.; T-4269-004<br />
c1ccccc1CN2S(=O)(=O)N(Cc3ccccc3)[C@H](Oc4ccccc4)[C@H](O)[C@@H](O)[C@H]2Oc<br />
5ccccc5<br />
4144 C30H31IN4O5S2 cyclic sulfondiamide deriv.; T-4409-017<br />
O[C@H]1[C@@H](Cc2ccccc2)N(CC(=O)Nc4nccs4)S(=O)(=O)N(Cc4cc(I)ccc4)[C@H](Cc3c<br />
cccc3)[C@@H]1O<br />
4145 C30H32N4O5S2 cyclic sulfondiamide deriv.; T-4409-013<br />
O[C@H]1[C@@H](Cc2ccccc2)N(CC(=O)Nc4nccs4)S(=O)(=O)N(Cc4ccccc4)[C@H](Cc3ccc<br />
cc3)[C@@H]1O<br />
4146 C30H32N8O5<br />
phthalimide-[N]-(Dist-deriv); Dist-deriv= Distamycin<br />
derivative with dimethylaminopropyl end<br />
O=C(N4c1cn(C)c(c1)C(=O)Nc2cn(C)c(c2)C(=O)Nc3cnc(c3)C(=O)NCCCN(C)C)c1ccccc1C4<br />
=O<br />
4147 C30H33Cl2NO10<br />
methyl 5-[4-(4',4''-dimethoxy-3',3''-di(methoxycarbonyl)-<br />
5',5''-dichloro-diphenylmethylene)piperidine]-5-<br />
oxopentanoate<br />
c1c(Cl)c(OC)c(C(=O)OC)cc1/C(c2cc(C(=O)OC)c(OC)c(Cl)c2)=C3/CCN(C(=O)OCCCC(=O)<br />
OC)CC3<br />
4148 C30H33IN2O TAK-779 derivative; T-4053-002 c1ccccc1c2ccc3CCC(=Cc3c2)C(=O)Nc4ccc(cc4)C[N+]5(C)CCCCC5.[I-]<br />
4149 C30H33IN2O quaternary ammonium salt; 4128-102 c1ccccc1c2ccc3CCC(=Cc3c2)C(=O)Nc4ccc(cc4)C[N+]5(CCCCC5)C.[I-]<br />
4150 C30H33IN2O2 quaternary ammonium salt; 4128-109 c1cc(C)ccc1c2ccc3OCC(=Cc3c2)C(=O)Nc4ccc(cc4)C[N+]5(C)CCCCC5.[I-]<br />
4151 C30H33N3O5S <strong>HIV</strong> protease inhibitor; 4069-011 c1ccccc1CC(CC)cC2=CC(O)=C(C(=O)O2)C(C3CC3)c4cccc(c4)NS(=O)(=O)c5ncn(C)c5<br />
3-amino-2-hydroxy-4-phenylbutanoic acid deriv.; T- c12ccccc2cccc1C(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N3C[C@@H](Cl)C[C@H]3C(<br />
4152 C30H34ClN3O4 4442-038<br />
=O)NC(C)(C)C<br />
4153 C30H34ClN3O4<br />
3-amino-2-hydroxy-4-phenylbutanoic acid deriv.; T-<br />
4442-039<br />
4154 C30H34N2O6 4-aryl-1,4-dihydropyridine dimer; T-4199-007<br />
c1c2ccccc2ccc1C(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N3C[C@@H](Cl)C[C@H]3C(<br />
=O)NC(C)(C)C<br />
[C@]36(CO)[C@]2([H])[N@](C(=O)C)[C@]5([H])[C@]4(CO)[C@@]([H])(c1ccccc1)[C@@]2(<br />
CO)[C@]3([H])[N@](C(=O)C)[C@]4([H])[C@]5(CO)[C@]6([H])c7ccccc7