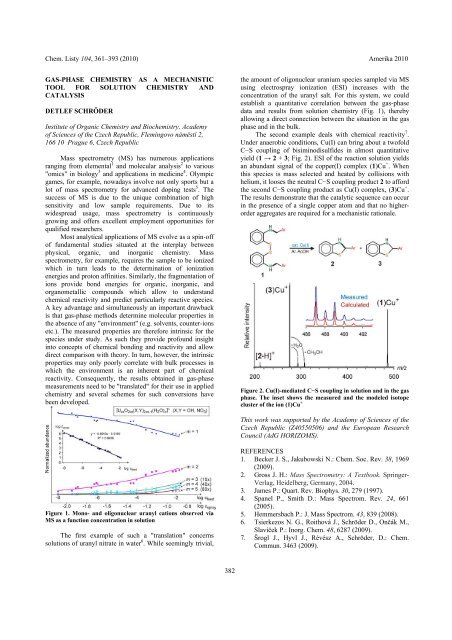
Chem. Listy 104, 361‒393 (2010) Amerika 2010GAS-PHASE CHEMISTRY AS A MECHANISTICTOOL FOR SOLUTION CHEMISTRY ANDCATALYSISDETLEF SCHRÖDERInstitute of Organic Chemistry and Biochemistry, Academyof Sciences of the Czech Republic, Flemingovo náměstí 2,166 10 Prague 6, Czech RepublicMass spectrometry (MS) has numerous applicationsranging from elemental 1 and molecular analysis 2 to various"omics" in biology 3 and applications in medicine 4 . Olympicgames, for example, nowadays involve not only sports but alot of mass spectrometry for advanced doping tests 5 . Thesuccess of MS is due to the unique combination of highsensitivity and low sample requirements. Due to itswidespread usage, mass spectrometry is continuouslygrowing and offers excellent employment opportunities forqualified researchers.Most analytical applications of MS evolve as a spin-offof fundamental studies situated at the interplay betweenphysical, organic, and inorganic chemistry. Massspectrometry, for example, requires the sample to be ionizedwhich in turn leads to the determination of ionizationenergies and proton affinities. Similarly, the fragmentation ofions provide bond energies for organic, inorganic, andorganometallic compounds which allow to understandchemical reactivity and predict particularly reactive species.A key advantage and simultaneously an important drawbackis that gas-phase methods determine molecular properties inthe absence of any "environment" (e.g. solvents, counter-ionsetc.). The measured properties are therefore intrinsic for thespecies under study. As such they provide profound insightinto concepts of chemical bonding and reactivity and allowdirect comparison with theory. In turn, however, the intrinsicproperties may only poorly correlate with bulk processes inwhich the environment is an inherent part of chemicalreactivity. Consequently, the results obtained in gas-phasemeasurements need to be "translated" for their use in appliedchemistry and several schemes for such conversions havebeen developed.the amount of oligonuclear uranium species sampled via MSusing electrospray ionization (ESI) increases with theconcentration of the uranyl salt. For this system, we couldestablish a quantitative correlation between the gas-phasedata and results from solution chemistry (Fig. 1), therebyallowing a direct connection between the situation in the gasphase and in the bulk.The second example deals with chemical reactivity 7 .Under anaerobic conditions, Cu(I) can bring about a twofoldC−S coupling of bisiminodisulfides in almost quantitativeyield (1 → 2 + 3; Fig. 2). ESI of the reaction solution yieldsan abundant signal of the copper(I) complex (1)Cu + . Whenthis species is mass selected and heated by collisions withhelium, it looses the neutral C−S coupling product 2 to affordthe second C−S coupling product as Cu(I) complex, (3)Cu + .The results demonstrate that the catalytic sequence can occurin the presence of a single copper atom and that no higherorderaggregates are required for a mechanistic rationale.Figure 2. Cu(I)-mediated C−S coupling in solution and in the gasphase. The inset shows the measured and the modeled isotopecluster of the ion (1)Cu +This work was supported by the Academy of Sciences of theCzech Republic (Z40550506) and the European ResearchCouncil (AdG HORIZOMS).Figure 1. Mono- and oligonuclear uranyl cations observed viaMS as a function concentration in solutionThe first example of such a "translation" concernssolutions of uranyl nitrate in water 6 . While seemingly trivial,REFERENCES1. Becker J. S., Jakubowski N.: Chem. Soc. Rev. 38, 1969(2009).2. Gross J. H.: Mass Spectrometry: A Textbook. Springer-Verlag, Heidelberg, Germany, 2004.3. James P.: Quart. Rev. Biophys. 30, 279 (1997).4. Spanel P., Smith D.: Mass Spectrom. Rev. 24, 661(2005).5. Hemmersbach P.: J. Mass Spectrom. 43, 839 (2008).6. Tsierkezos N. G., Roithová J., Schröder D., Ončák M.,Slavíček P.: Inorg. Chem. 48, 6287 (2009).7. Šrogl J., Hyvl J., Révész A., Schröder, D.: Chem.Commun. 3463 (2009).382
Chem. Listy 104, 361‒393 (2010) Amerika 2010KOORDINAČNÍ CHEMIE A KATALYTICKÉVYUŽITÍ HOMOLOGICKÝCHPYRIDYLFOSFINOFERROCENOVÝCH LIGANDŮPETR ŠTĚPNIČKA a,* , JIŘÍ SCHULZ a , THORSTENKLEMANN b , ULRICH SIEMELING b a IVANACÍSAŘOVÁ aa Univerzita Karlova, PřF, Katedra anorganické chemie,Hlavova 2030, 128 40 Praha 2;b Universität Kassel,Heinrich-Plett-Strasse 40, D-34132 Kasselstepnic@natur.cuni.cz, schulz@natur.cuni.czBifunkční P III ,N-donorové ligandy patří do skupinytakzvaných hemilabilních donorů 1 . Díky možnosti koordinacek atomu kovu prostřednictvím měkkého (P III ) i tvrdého(N) donorového atomu a tvorbě nesymetrických chelátovýchkomplexů nalezly tyto látky široké uplatnění v organickékatalýze 2,3 . Hemilabilní koordinace jednoho z donorovýchatomů ligandu umožňuje zároveň uvolnit koordinační místopro přistupující substrát i stabilizaci katalyzátoru v průběhukatalytického cyklu.Významnou pozici mezi P III ,N-hemilabilními donoryzaujímají pyridyl-fosfinové ligandy 4,5 , které se vyznačujízajímavými elektronickými i stérickými vlastnostmi, jenž lzecíleně modifikovat změnou povahy substituentů. Pro tentoúčel může být výhodným substituentem ferrocenové jádro,které vyniká velkou elektronovou bohatostí a dobředefinovanou geometrií.Z tohoto důvodu jsme se rozhodli studovat dvahomologické pyridyl-fosfinoferrocenové ligandy 1 (cit. 6 ) a 2,jejich koordinační chemii vůči palladiu a katalytické využitív palladiem katalyzované Suzukiho-Miyaurově reakcia kyanačních reakcích arylbromidů.FePPh 2NFe1 2PPh 2Schéma 1. Připravené pyridyl-fosfinoferrocenové ligandyTato práce vznikla za podpory grantu GAČR P207/10/0176.LITERATURA1. Bader A., Lindner E.: Coord. Chem. Rev. 108, 27(1991).2. Guiry P. J., Saunders C. P.: Adv. Synth. Catal. 346, 497(2004).3. Maggini S.: Coord. Chem. Rev. 253, 1793 (2009).4. Newkome G.R.: Chem. Rev. 93, 2067 (1993).5. Chelucci G., Orrú G., Pinna G. A.: Tetrahedron 59,9471 (2003).6. Butler I. R.: Organometallics 11, 74 (1992).NFUNKČNÍ ANALÝZA TEPLOTNĚ ZÁVISLÝCHMUTANTŮ P53JANA SLOVÁČKOVÁ a,b , DIANA GROCHOVÁ a ,JARMILA NAVRÁTILOVÁ b , JAN ŠMARDA b a JANAŠMARDOVÁ a,ba Laboratoř molekulární biologie ÚPA FN Brno, Jihlavská20, 625 00 Brno; b Ústav exp. biologie, Biologická sekce,PřF, Masarykova univerzita, Kotlářská 2, 611 37 Brnojana.slovackova@gmail.comNádorový supresor p53 hraje důležitou roliv kancerogenezi. V odpovědi na různé stresové signálytranskripční faktor p53 kontroluje důležité buněčné funkce tím,že ovlivňuje expresi svých cílových genů. Reguluje apoptózu,buněčný cyklus a genomovou integritu. U nádorů je ztrátafunkce p53 velmi často způsobena mutací genu p53. Přibližně10 % mutantů p53 je teplotně závislých, tzn. změnou teplotydochází k obnově jejich funkce. Tyto mutace mohou měnitnejenom celkovou transaktivační schopnost proteinu p53, aletaké modifikovat transaktivační schopnost ve vztahuk jednotlivým cílovým genům (tzv. diskriminativní charakter).Studovali jsme funkční vlastnosti 23 teplotně závislých(td) mutantů p53 (podrobně analyzovaných v kvasinkách 1 )v lidské nádorové linii H1299 odvozené z nemalobuněčnéhoplicního karcinomu. Sledovali jsme transaktivaci několikacílových genů p53 a potvrdili teplotní závislost a výraznýdiskriminativní charakter u 20 mutantů, kteří preferenčnětransaktivovali p21 spíše než bax. Podle transaktivačníchschopností jsme td mutanty p53 rozdělili do 4 funkčníchskupin. Obecně lze říct, že celková transaktivační aktivitamutantů nepřevyšuje aktivitu standardní varianty p53, ačkolimíra transaktivace určitých cílových genů u některýchmutantů v permisivní teplotě může být výrazně vyšší.Td mutace p53 jsou snadněji reaktivovatelné nežmutace zcela inaktivní 1‒3 . Obnovení funkce p53 může býtvyvoláno například inhibitorem CDK roskovitinem. Ačkolimechanismus účinku není zcela znám, pozorovali jsmeu některých td mutantů p53 zřetelně zesílenou apoptotickouodpověď po působení roskovitinu jak v permisivní, taki restriktivní teplotě. Funkční status p53 pravděpodobněovlivňuje míru odpovědi nádorových buněk na roskovitin,slibnou protinádorovou látku, která v současné doběpodstupuje druhou fázi klinického testování.Tato práce vznikla za podpory grantů NS/10448-3 IGA MZa MŠMT 0021622415.LITERATURA:1. Grochová D., Vaňková J., Damborský J., RavčukováB., Šmarda J., Vojtěšek B., Šmardová J.: Oncogene 27,1243 (2008).2. Boeckler F.M., Joerger A.C., Jaggi G., Rutherford T.J.,Veprintsev D.B., Fersht A.R.: Proc. Natl Acad. Sci.USA 105, 10360 (2008).3. North S., Pluquet O., Maurici D., El-Ghissassi F.,Hainaut P.: Mol. Carcinog. 33, 181 (2002).383
- Page 1 and 2: Chem. Listy 104, 361‒393 (2010) A
- Page 5 and 6: Chem. Listy 104, 361‒393 (2010) A
- Page 7 and 8: Chem. Listy 104, 361‒393 (2010) A
- Page 9 and 10: Chem. Listy 104, 361‒393 (2010) A
- Page 11 and 12: Chem. Listy 104, 361‒393 (2010) A
- Page 13 and 14: Chem. Listy 104, 361‒393 (2010) A
- Page 15 and 16: Chem. Listy 104, 361‒393 (2010) A
- Page 17 and 18: Chem. Listy 104, 361‒393 (2010) A
- Page 19 and 20: Chem. Listy 104, 361‒393 (2010) A
- Page 21: Chem. Listy 104, 361‒393 (2010) A
- Page 25 and 26: Chem. Listy 104, 361‒393 (2010) A
- Page 27 and 28: Chem. Listy 104, 361‒393 (2010) A
- Page 29 and 30: Chem. Listy 104, 361‒393 (2010) A
- Page 31 and 32: Chem. Listy 104, 361‒393 (2010) A
- Page 33: Chem. Listy 104, 361‒393 (2010) A