2. PROTOLYTICKÉ REAKCE
2. PROTOLYTICKÉ REAKCE
2. PROTOLYTICKÉ REAKCE
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
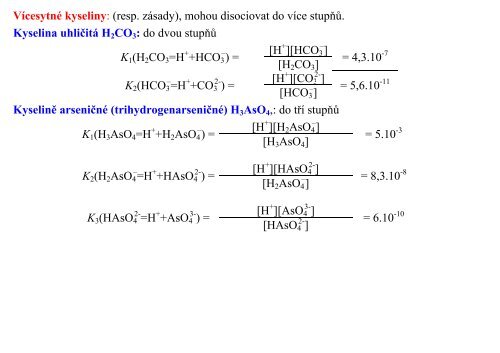
Vícesytné kyseliny: (resp. zásady), mohou disociovat do více stupňů.<br />
Kyselina uhličitá H2CO3: do dvou stupňů<br />
K1(H2CO3=H + +HCO3 – ) =<br />
K2(HCO3 – =H + +CO3 2- ) =<br />
[H + ][HCO3 – ]<br />
= 4,3.10 -7<br />
[H2CO3]<br />
[H + ][CO3 2- ] -11<br />
= 5,6.10<br />
[HCO3 – ]<br />
Kyselině arseničné (trihydrogenarseničné) H3AsO4,: do tří stupňů<br />
K1(H3AsO4=H + +H2AsO4 – ) =<br />
K2(H2AsO4 – =H + +HAsO4 2- ) =<br />
K3(HAsO4 2- =H + +AsO4 3- ) =<br />
[H + ][H2AsO4 – ]<br />
[H3AsO4]<br />
[H + ][HAsO4 2- ]<br />
[H2AsO4 – ]<br />
[H + ][AsO4 3- ]<br />
[HAsO4 2- ]<br />
= 5.10 -3<br />
= 8,3.10 -8<br />
= 6.10 -10