Research Report - Nikolaus-Fiebiger-Zentrum für Molekulare Medizin
Research Report - Nikolaus-Fiebiger-Zentrum für Molekulare Medizin
Research Report - Nikolaus-Fiebiger-Zentrum für Molekulare Medizin
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Increased TGF-� signaling in the cardiovascular system has also been found in mice<br />
hypomorphic for fibulin-4 and in the patient positive FBLN4 mutation. Recently, we<br />
found fibulin-4 expression also in fetal growth plate cartilage. Preliminary data on<br />
Fbln4 null mice showing reduced bone mass and increased bone fragility, and<br />
mutations found in human patients indicate that fibulin-4 may be also involved in<br />
skeletal development. The goal of this study is to obtain further insight into molecular<br />
changes causing the observed of skeletal alterations in fibulin-4 deficient mice such<br />
as limb contracture, detached distal phalanges and reduced bone mass.<br />
Results:<br />
Biochemical analyses on bone revealed that collagen I from fibulin-4 null mice was<br />
less cross-linked compared with those from wild type and heterozygous mice.<br />
Reduction of proteolytic activation of lysyl oxidase (LOX) which is essential for<br />
collagen crosslinking was detected not only in osteoblast culture but also in<br />
chondrocyte and fibroblast culture from fibulin-4 null mice. The mechanism how<br />
fibulin-4 is involved in LOX activation will be elucidated in vitro. It is still necessary to<br />
quantify the crosslink markers hyroxylyslpyridinoline (HP)/lysylpyridinoline (LP) of<br />
some more samples.<br />
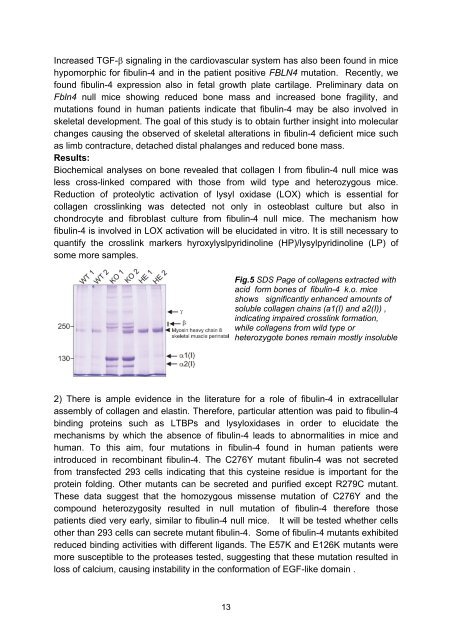
Fig.5 SDS Page of collagens extracted with<br />
acid form bones of fibulin-4 k.o. mice<br />
shows significantly enhanced amounts of<br />
soluble collagen chains (a1(I) and a2(I)) ,<br />
indicating impaired crosslink formation,<br />
while collagens from wild type or<br />
heterozygote bones remain mostly insoluble<br />
2) There is ample evidence in the literature for a role of fibulin-4 in extracellular<br />
assembly of collagen and elastin. Therefore, particular attention was paid to fibulin-4<br />
binding proteins such as LTBPs and lysyloxidases in order to elucidate the<br />
mechanisms by which the absence of fibulin-4 leads to abnormalities in mice and<br />
human. To this aim, four mutations in fibulin-4 found in human patients were<br />
introduced in recombinant fibulin-4. The C276Y mutant fibulin-4 was not secreted<br />
from transfected 293 cells indicating that this cysteine residue is important for the<br />
protein folding. Other mutants can be secreted and purified except R279C mutant.<br />
These data suggest that the homozygous missense mutation of C276Y and the<br />
compound heterozygosity resulted in null mutation of fibulin-4 therefore those<br />
patients died very early, similar to fibulin-4 null mice. It will be tested whether cells<br />
other than 293 cells can secrete mutant fibulin-4. Some of fibulin-4 mutants exhibited<br />
reduced binding activities with different ligands. The E57K and E126K mutants were<br />
more susceptible to the proteases tested, suggesting that these mutation resulted in<br />
loss of calcium, causing instability in the conformation of EGF-like domain .<br />
13