The Electrical experimenter
The Electrical experimenter
The Electrical experimenter
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
May, 1917 THE ELECTRICAL EXPERIMENTER<br />
I.\<br />
ACIDS, BASES, AND SALTS.<br />
this lesson vvc shall take- up the<br />
study of the .various acids and chariicteristics.<br />
<strong>The</strong>se form one of the<br />
most important studies in the realm<br />
(if chemistry. A resume of the general<br />
properties of acids are briefly as folliuvs<br />
:<br />
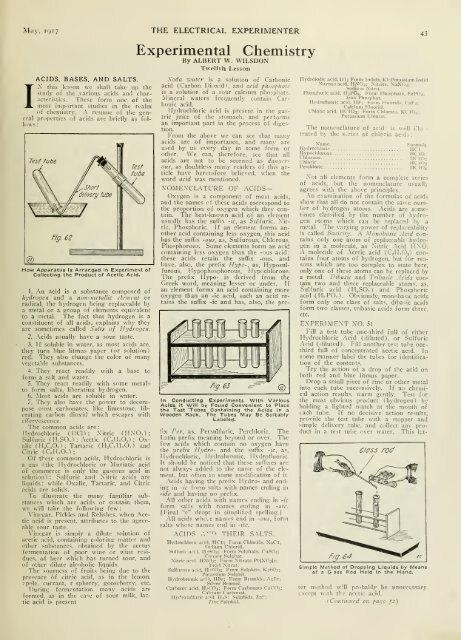
Test tutje<br />
\0^<br />
@<br />
F'9-<br />
•<br />
rest<br />
. tube<br />
/L Short 1fV<br />
''^delivery tube<br />
WJiw<br />
62<br />
How Apparatus Is Arranged In Experiment of<br />
Collecting the Product of Acetic Acid.<br />
1. An acid is a substance composed of<br />
hxdrogcn and a iioit-iiiclallic clciiicnt or<br />
radical, the hydrogen being replaceable by<br />
a metal or a group of elements equivalent<br />
to a metal. <strong>The</strong> fact that hydrogen is a<br />
constituent of all acids, explains why they<br />
are sometimes called Salts of Hydrogen.<br />
2. .Acids usually have a sour taste.<br />
3. If soluble in water, as most acids are.<br />
they turn blue litmus paper (or solution)<br />
red. <strong>The</strong>y also change the color of many<br />
vegetable substances.<br />
4. <strong>The</strong>y react readily with a base to<br />
form a salt and water.<br />
5. <strong>The</strong>y react readily with some inetals<br />
to form salts, liberating hydrogen.<br />
6.<br />
7.<br />
Most acids are<br />
<strong>The</strong>y also have<br />
soluble in water.<br />
the power to decompose<br />
most carbonates, like limestone, liberating<br />
carbon dioxid which escapes with<br />
effervescence.<br />
<strong>The</strong> common acids are:<br />
Hvdrochloric (HCH: Xitric (HNC) ;<br />
Sulfuric (H,SOJ: .Acetic (CJT.O,) ; Oxalic<br />
(HAO,") : Tartaric (njC,ll„0,) and<br />
Citric (CoHsO;-);<br />
Of tbese common acids. Hydrochloric is<br />
a cas (the Hydrochloric or Muriatic acid<br />
of commerce is only the gaseous acid in<br />
solution) : Sulfuric and Xitric acids are<br />
liquids : while Oxalic, Tartaric, and Citric<br />
acids<br />
To<br />
are solids.<br />
illustrate the many familiar substances<br />
which are acids or contain tlieni,<br />
we will take the following few<br />
Vinegar, Pickles and Relishes, when .Acetic<br />
acid is present, attributes to the agreeable<br />
sour taste.<br />
N'inegar is simply a dilute solution of<br />
acetic acid, containing coloring matter and<br />
other substances, obtained by the acetus<br />
fermentation of poor wine or wine residues,<br />
of beer which has turned sour, and<br />
of other dilute alcoholic liquids.<br />
<strong>The</strong> sourness of fruits being due to the<br />
presence of citric acid, as in the lemon,<br />
rpnle. currant, r spberry, gooseberrv,<br />
Durinf; fermentation many acids<br />
etc.<br />
are<br />
formed, as in the ca':e of sour milk, lactic<br />
acid is present<br />
Experimental Chemistry<br />
By ALBERT W. WILSDON<br />
Twelfth Lesson<br />
Soda water is a solution of Carbonic<br />
acid (Carbon Dioxid), and acid phosphate<br />
is a solution of a sour calcium pliosphatc.<br />
.Mineral waters frequently contain Carbonic<br />
acid.<br />
Hydrochloric acid is present in tlie gastric<br />
juice of tlie stomach, and performs<br />
an important part in the process of digestion.<br />
b'rom the above we can see that many<br />
acids are of importance, and many are<br />
used by us every day in some form or<br />
otlier. We can, therefore, see that all<br />
acids are not to be scorned as dangerous,<br />
as doubtless many readers of this article<br />
have heretofore believed, when the<br />
word acid was mentioned.<br />
XOMEXCLATURE OF ACIDS—<br />
Oxygen is a component of most acids,<br />
and the names ol these acids correspond to<br />
the proportion of oxygen which they contain.<br />
<strong>The</strong> best-known acid of an element<br />
usually has the suffix -ic, as Sulfuric. Nitric.<br />
Phosphoric. If an element forms another<br />
acid containing less oxygen, this acid<br />
has the suflnx -ous, as, Sulfurous, Chlorous,<br />
Phosphorous. Some elements form an acid<br />
containing less oxygen than the -ous acid:<br />
these acids retain the suffix -ous, and<br />
liave, also, the prefix Hypo-, as, Hyposulfurous,<br />
Hypophosphorous, Hypochlorous.<br />
<strong>The</strong> prefix Hypo- is derived from the<br />
Greek word, meaning lesser or under. If<br />
an element forms an acid containing more<br />
oxygen than an -ic acid, such an acid retains<br />
the suffix -ic and has, also, the pre-<br />
In Conducting Experiments With Various<br />
Acids it Will be Found Convenient to Place<br />
the Test Tubes Containing the Acids In a<br />
Wooden Rack. <strong>The</strong> Tubes May Be Suitably<br />
Labeled.<br />
fix Per, as, Persulfuric, Perchloric. <strong>The</strong><br />
Latin prefi.x meaning beyond or over. <strong>The</strong><br />
few acids wliich contain no oxygen have<br />
the prefix Hydro- and the suffi.x -ic. as,<br />
I lydrochloric, Hydrobromic, Hydrofluoric.<br />
It should be noticed that these suftixes arc<br />
not always added to the name of the element,<br />
but often to some modification of it,<br />
.\cids having the prefix Hydro- and ending<br />
in -ic form salts with names ending in<br />
-ide and having no prefix.<br />
.All other acids with names ending in -iV<br />
form salts with names enilin,g in -ate.<br />
[I'inal "e" dropt in simplified spelling.]<br />
.AH acids who:e names end in -ous, form<br />
salts whose names end in -ite.<br />
ACIDS /v:-'!^ THEIR SALTS.<br />
Hydrochloric acid. HCl; Form Clilorids. NaCl;<br />
Po'lium Chlorid.<br />
Sulfuric aci I. H^SOj; Form Sulphats. CUSO4;<br />
Co'ipcr .'^ulpliat.<br />
Nitric aril. llNO.i; Form Xitrats PbtNOslj;<br />
1 eai Nitrat.<br />
Sulfuroin acirl. H''-"0,i: Form .Sulphits. KrSOj;<br />
Potassium Fulphit.<br />
Hydrobromic acil. HBr: Form bromide, .AfiBr:<br />
Silver Broinid.<br />
Carbonic acid. I^.'CO.i; Form Carbonats CaC03;<br />
Calcium Carbonat.<br />
Hydro^ulfuric arid. H2S; Sulphids. ZnF;<br />
7inc Sulphid.<br />
43<br />
Hydroiodic acid.HI; Form lodids, KI;Potas3iumIodid<br />
Nitrous acid. H.VOa; .Nitrits. NaNOa;<br />
Sodium Nitrit.<br />
Phosphoric acid. lljPOa; Form Phosphats. FeP04;<br />
Iron Phospliat.<br />
Hydrofluoric acid, HF: I'orm Fluorids, CaFj;<br />
Calcium Fluorid.<br />
Chloric acid, HCIO3; Form Chlorals. KClOj;<br />
Potassium Chloral.<br />
<strong>The</strong> nomenclature of acid; is well i'.lu -<br />
trated Ijy the s-ries of chlo.-in acids:<br />
Name. Formula.<br />
Hydrochloric HCl<br />
Hypochlorous HCIO<br />
Chlorous HCI02<br />
Chloric HCIO3<br />
Perchloric HCIO4<br />
Xot all elements form a comp!ete series<br />
of acids, but the nomenclature usually<br />
agrees with the above principles.<br />
-An examination of tlie formulas of acids<br />
show that all do not contain the san.e number<br />
of hydrogen atoms. .Acids are sometimes<br />
classified by the number of hydrogen<br />
?toms which can be replaced by a<br />
metal. <strong>The</strong> varying power of replaceab'ility<br />
is called Basicity. A Monobasic Acid contains<br />
only one atom of replaceable hydrogen<br />
in a molecule, as Xitric .Acid H.XO.<br />
A molecule of -Acetic acid (CjH,0,) contains<br />
four atoms of hydrogen, but for reasons<br />
which are too complex to state here,<br />
only one of these atoms can be replaced by<br />
a metal. Dibasic and Tribasic Acids contain<br />
two and three replaceable atoms, as.<br />
Sulfuric acid (HjSO,) and Phosphoric<br />
acid (H., PO,). Obviously, monobasic acids<br />
form only one class of salts, dibasic acids<br />
form two classes, tribasic acids form three,<br />
etc.<br />
EXPERIMEXT NO. 51<br />
ImII a test tube one-third full of either<br />
Hydrochloric -Acid (diluted), or Sulfuric<br />
.Acid (diluted). Fill another test tube onethird<br />
full of concentrated acetic acid. In<br />
some manner label the tubes for identification<br />
of the contents.<br />
Try the action of a drop of tb.e acid on<br />
both red and blue litmus paper.<br />
Drop a small piece of zinc or other metal<br />
into each tube successively. If no chemical<br />
action results, warm gently. Test for<br />
the most obvious product (hydrogen") by<br />
holding a lighted match at the mouth of<br />
each tube. If no decisive action results,<br />
provide the test tube with a stopper and<br />
simple delivery tube, and collect any product<br />
in a test tube over water. This lat-<br />
Slmple Method of Dropping Liquids by Means<br />
of a G ass Rod Held In the Hand.<br />
ter method will probably be unnecessary<br />
except with the acetic acid.<br />
(Continued or. page 52)